- 1Drug and Herbal Research Centre, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 2National Pharmaceutical Regulatory Agency, Petaling Jaya, Malaysia
Botanical drug products consist of complex phytochemical constituents that vary based on various factors that substantially produce different pharmacological activities and possible side effects. Marantodes pumilum (Blume) Kuntze (Primulaceae) is one of the most popular Malay traditional botanical drugs and widely recognized for its medicinal use. Many studies have been conducted focusing on the identification of bioactive substances, pharmacological and toxicological activities in its specific varieties but less comprehensive study on M. pumilum authentication. Lack of quality control (QC) measurement assessment may cause different quality issues on M. pumilum containing products like adulteration by pharmaceutical substances, substitution, contamination, misidentification with toxic plant species, which may be detrimental to consumers’ health and safety. This systematic literature review aims to provide an overview of the current scenario on the quality control of botanical drug products as determined by pharmacopoeia requirements specifically for M. pumilum authentication or identification. A systematic search for peer-reviewed publications to document literature search for M. pumilum authentication was performed using four electronic databases: Web of Science, PubMed, Scopus and ScienceDirect for related studies from January 2010 to December 2021. The research studies published in English and related articles for identification or authentication of M. pumilum were the main inclusion criteria in this review. A total 122 articles were identified, whereby 33 articles met the inclusion criteria. Macroscopy, microscopy, chemical fingerprinting techniques using chromatography, spectroscopy and hyphenated techniques, and genetic-based fingerprinting using DNA barcoding method have been used to identify M. pumilum and to distinguish between different varieties and plant parts. The study concluded that a combination of approaches is necessary for authenticating botanical drug substances and products containing M. pumilum to assure the quality, safety, and efficacy of marketed botanical drug products, particularly those with therapeutic claims.
Introduction
Since ancient times, botanical drugs have been employed in the daily lives of the world population due to their medicinal efficacy in promoting well-being and health. Around 80% of the world’s population consumes botanical drugs as health supplements since they are thought to be effective in disease management and have been recognized as safe for decades owing to their natural origin. Despite the widespread use of botanical drug products for a long time, problems with quality control persist. The increased demand for botanical drug products may expose them to various types of adulteration, such as substitution, contamination, or the use of fillers, all of which represent a threat to the health and safety of consumers (Techen et al., 2014; Abubakar et al., 2018). In fact, different countries define botanical drug products differently and use different systems for registering, licensing, dispensing, manufacturing, and trading them to ensure their safety, efficacy, and quality. As a result, there is a disparity in registration requirements between countries and variation in the botanical drugs quality.
Recognizing the need, for the past few decades, WHO has consistently issued various guidelines and policies related to botanical drugs, such as Guidelines for the Assessment of Herbal Medicines, Good Agricultural and Collection Practices (GACP) and Quality Control Methods for Medicinal Plant Materials, with the goal of standardizing and harmonizing botanical drugs regulation globally (Yadav and Dixit, 2008). Currently, botanical drug products must meet pharmacopoeia identification requirements for organoleptic evaluation (touch, smell, sight, and taste), macroscopic evaluation (shape, color, and texture), microscopic assessment and chemical fingerprint techniques such as chromatography and spectroscopy (Li et al., 2020). Additionally, the British Pharmacopoeia has included DNA barcoding as a means of identifying botanical drugs (Heinrich and Anagnostou, 2017). In fact, Malaysia’s regulatory body has consistently adopted various international guidelines issued by the World Health Organization (WHO), Medicines and Healthcare Products Regulatory Agency (MHRA), European Medicines Agency (EMA), Therapeutic Goods Administration (TGA), and Food and Drug Administration (FDA) to strengthen the registration requirements for botanical drug products since 1992. It is vital to identify and authenticate botanical drugs and products utilizing a variety of approaches, whether during the final product phase for clinical study evaluation or throughout product development for the market (Smillie and Khan, 2010).
Due to the open online market, the botanical drug-based industry has also piqued the interest of Asian countries. Malaysia’s forest is home to a diverse array of medicinal plants with a great potential for use in the botanical drugs industry. Malaysia’s botanical drugs domestic market was expected to grow at a 15% annual rate from RM7 billion in 2010 to around RM29 billion by 2020 (Fadzil et al., 2018). The increase in the number of botanical drug products registered with the National Pharmaceutical Regulatory Agency (NPRA) demonstrates the growing demand for botanical drug products (Fadzil et al., 2018). Realizing the huge economic opportunities in the local botanical drugs industry and the requirements that need to be complied with, the agricultural National Key Economic Areas (NKEA) Entry Point Project 1 (EPP1) was focused on potential growth that might contribute to Malaysia’s gross national income (GNI). Due to their potential therapeutic properties, Malaysia’s government has identified 11 important plants, including Eurycoma longifolia Jack (Simaroubaceae), Marantodes pumilum (Blume) Kuntze (Primulaceae), Andrographis paniculata (Burm.f.) Nees (Acanthaceae), and others, to be commercialized as high-value botanical drug products. M. pumilum, locally known as Kacip Fatimah, is widely spread in Southeast Asian tropical forests and is well-known for its medicinal properties. It is a member of the Primulaceae family and was formally recognized as a member of the family Myrsinaceae and known as Labisia pumila (Blume) Fern.-Vill (WFO, 2022). In various parts of Malaysia, M. pumilum is referred to as kachip patimah, selusuh fatimah, rumput siti fatimah, akar fatimah, kachit fatimah, pokok pinggang, rumput palis, tadah matahari, mata pelandok rimba, bunga belangkas hutan (Chua et al., 2011) and sangkoh (Iban) (Abdullah et al., 2013). There are eight M. pumilum varieties and only three varieties; var. alata (Scheff.) Mez., var. pumila and var. lanceolata (Scheff.) Mez. are widely distributed in Malaysia rain forest and have attracted the researcher’s interest thus far (Sunarno, 2005; Chua et al., 2012). The three varieties can be distinguished by their petioles and leaf characteristics. M. pumilum var. alata has red veins and broad winged petioles, whereas var. pumila has an emarginate winged petiole and an ovate leaf blade, and var. lanceolata has a long, non-winged or terete petiole (Figure 1). However, due to the close macromorphological features, it was extremely difficult to visually separate them based on petiole characteristics, particularly when the petioles were not fully formed (Abdullah et al., 2012).

FIGURE 1. Voucher specimens of (A) Marantodes pumilum var. alata, (B) Marantodes pumilum var. pumila and (C) Marantodes pumilum var. lanceolata (obtained from the Kepong Herbarium of Forest Research Institute Malaysia).
Traditionally, indigenous women of the Malay Archipelago consumed water decoctions of M. pumilum to aid in labor and delivery, while the botanical drug is believed to tone the abdominal muscles, assist in tightening the birth canal, and enhance overall body strength during postpartum (Zakaria and Mohd, 1994). Additionally, ancient communities employed the M. pumilum to treat diarrhoea, rheumatism, gonorrhea and flatulence (Burkill, 1966). Numerous research has shown that M. pumilum has a wide range of pharmacological activities, including antibacterial, antifungal, anti-inflammatory, cytotoxicity, antioxidative, xanthine oxidase inhibitory, phytoestrogenic, anticarcinogenic, anti-aging, anti-hyperuricemia, anti-osteoporotic, anti-obesity, cardioprotective effect, and uterotonic (Norhaiza et al., 2009; Choi et al., 2010; Karimi et al., 2011, 2013; Pihie et al., 2011; Jamal et al., 2012; Mamat et al., 2014; Pandey et al., 2014; Dianita et al., 2016; Hairi et al., 2018; Wan Omar et al., 2019; Aladdin et al., 2020; Rahmi et al., 2020).
Phytochemical constituents found in M. pumilum varieties include flavonoids, phenolics, methyl gallate, carotenoids, ascorbic acids, fatty acids, saponins, alkenyl compounds and benzoquinone derivatives (Ahmad et al., 2018). Many variables influence the phytochemical constituents, such as environmental factors, species varieties and plant parts. The variation in phytochemical contents between batches often results in markedly variable pharmacological actions and probable side effects (Liang et al., 2004; Goodarzi et al., 2013). According to Karimi et al. (2011), the phytochemical constituent presence and abundance differs between M. pumilum varieties and plant parts. The study indicated that gallic acid was highest in var. alata leaves, followed by var. lanceolata leaves and var. pumila leaves. Several studies have established that different M. pumilum species and plant parts possess distinct pharmacological properties, including phytoestrogenic activity of var. alata leaves (Giaze et al., 2018; Hairi et al., 2018), xanthine oxidase inhibitory activity of var. pumila leaves (Aladdin et al., 2020) and anti-inflammatory effect of var. pumila roots (Rahmi et al., 2020).
Due to the wide range of phytochemicals found in this plant that can contribute to different pharmacological effects and side effects, majority of research on M. pumilum has focused on the bioactive substances, pharmacological and toxicological activities and less studies conducted specifically on its identification and authentication. As such, the aim of this review is to present an overview of the current state of authentication for M. pumilum in the global botanical drug products industry.
Materials and Methods
Search Strategy
A systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Moher et al., 2009). A search strategy based on a combination of relevant keywords and Boolean operators was used [ALL = (authentication or identification or quality control or chemical profiling or fingerprint) and (marantodes pumilum or labisia pumila or kacip fatimah)] for Web of Science database and [(“authentication” OR “identification” OR “quality control” OR “chemical profiling” OR “fingerprint”) AND (“marantodes pumilum” OR “labisia pumila” OR “kacip Fatimah”)] for PubMed, Scopus and ScienceDirect databases. Following the search conducted on 18 November 2021, the option “search alert” was selected to receive weekly updates for all four literature databases. All the selected articles were saved in Mendeley Desktop Version 1.19.8 (2008–2020) reference manager.
Selection Process and Criteria
Identification: Database searches identified 606 records (WoS = 22, PubMed = 4, Scopus = 499 and ScienceDirect = 81).
Screening: Articles were screened manually in four stages. Initially, articles that were published as a review, book chapter, or conference proceeding were excluded. Secondly, articles that were published between January 2010 and December 2021 were considered. Thirdly, articles without data or information on the identification or authentication of Marantodes pumilum (Kacip Fatimah) as botanical drug substances or botanical drug products were omitted. Following screening, 552 articles were deleted. Finally, duplicate entries were removed from the databases, leaving 37 eligible articles.
Eligibility: A total of 37 full-text papers were evaluated and screened for eligibility using the following criteria:
1. The tested sample was required to be made up of botanical drug substances or botanical drug products. A variety of scientific names, that is, Labisia pumila (synonym), Marantodes pumilum, and the common name Kacip Fatimah were accepted for the review.
2. The details of the tested sample, including the collection site, plant species, plant parts and sample processing, were clearly documented and described.
3. All pertinent methodologies within the scope of the studies were accepted. The tested samples were authenticated using several techniques, including macroscopic and microscopic methods, chemical fingerprinting, genetic fingerprinting, and phytochemical analysis.
Included: 33 peer-reviewed articles were included in the systematic review of the literature, as described in Figure 2. The flowchart was made in accordance with PRISMA guidelines (Moher et al., 2009).
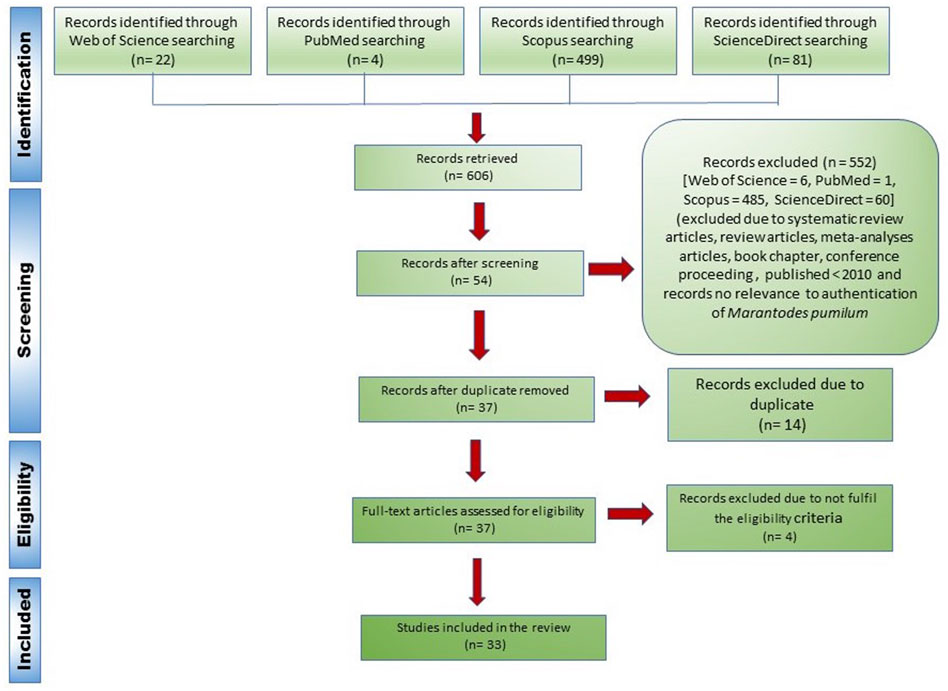
FIGURE 2. Flowchart of the article search process according to the PRISMA guidelines (Moher et al., 2009).
Results
All selected articles were published between January 2010 and December 2021 (Table 1). Thirty-three full-text articles met the inclusion criteria and were classified as research articles in this review. Majority of the included full-text articles were conducted locally at various universities and institutions in Malaysia, while six were conducted abroad in China (study 24), Sweden (studies 1 and 5), the United States of America (studies 6 and 7) and India (study 2). Even though the genus Labisia was reclassified as Marantodes in 2012, 81% (n = 27) of publications cited Labisia pumila rather than Marantodes pumilum. Four studies were conducted on all three common varieties of M. pumilum var. alata, var. pumila, and var. lanceolata; one study used M. pumilum var. alata and var. pumila, sixteen studies focused exclusively on M. pumilum var. alata, one study used only M. pumilum var. pumila and ten studies made no mention of the M. pumilum variety. On average, 70% of publications indicated the variety used in the study, with the remaining 30% of articles lacking identification at variety level. Apart from that, 60.6% (n = 20) studies used M. pumilum leaves, whereas other studies used whole plants (21.2%, n = 7), leaves and stem/roots (15.15%, n = 5), and stem-roots (3%, n = 1). According to the review, (72.7%, n = 24) majority of the researchers used wild plant sources rather than cultivated sources.
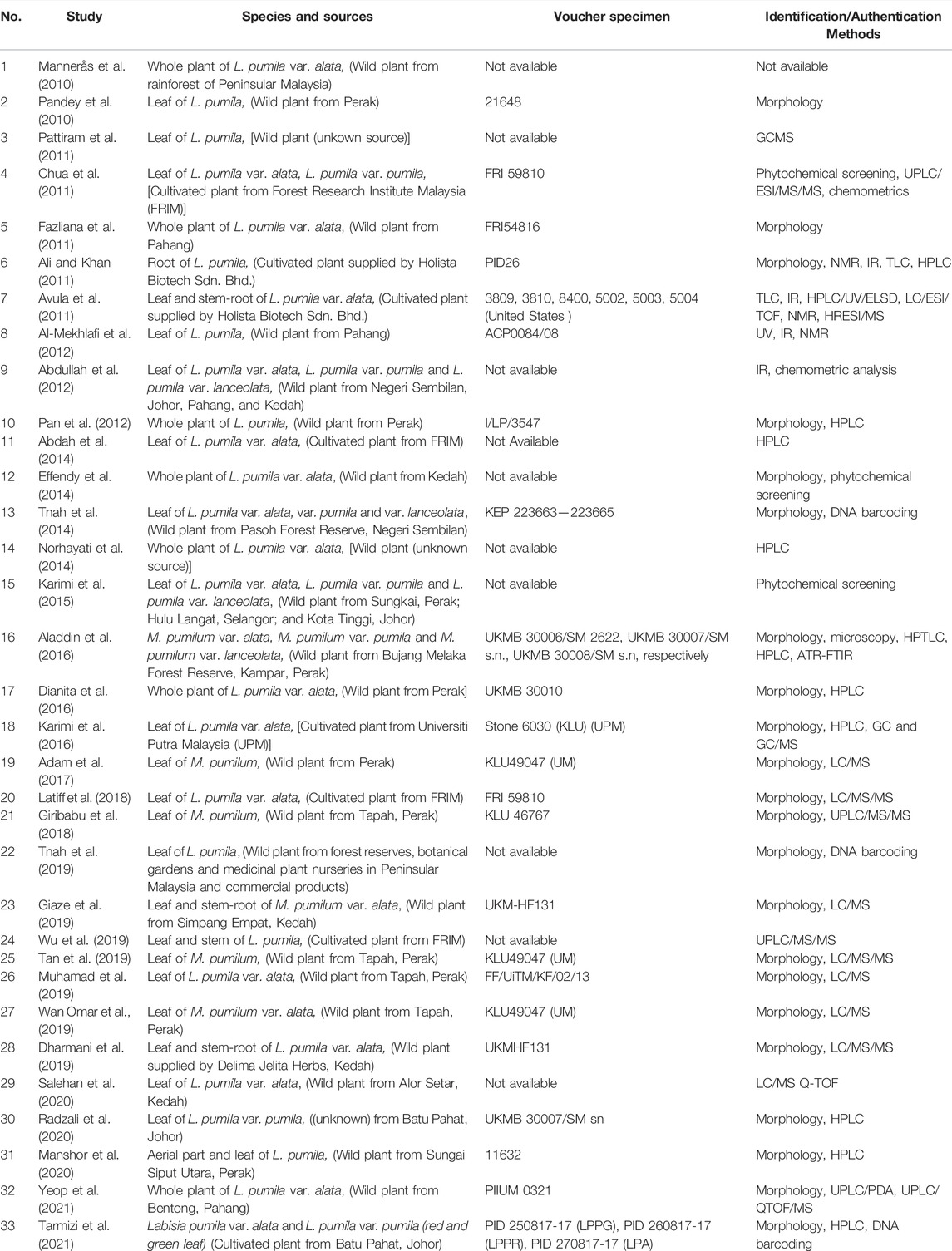
TABLE 1. Authentication and identification methods of Marantodes pumilum botanical drug substances and commercial products.
Notably, all investigations included in this review employed at least one approach for identifying or authenticating M. pumilum. Generally, about (66.70%, n = 22) of the study conducted morphological tests on the M. pumilum samples and 67.7% (n = 23) of the 33 study included the voucher specimen number for the test samples except for studies 1, 3, 9, 11, 12, 14, 15, 22, 24, and 29. Multiple sources of M. pumilum from different areas in Malaysia were used in 18.2% (n = 6) of research conducted abroad. Botanists authenticated most test samples based on morphological identification, except for those used in studies 1 and 24 that were conducted in China and Sweden, respectively, due to the lack of information on morphological identification and voucher specimen for M. pumilum in the articles compared to other studies. Qualified botanists in public educational institutions and government research institutions performed the plant authentication.
The researchers’ choice of approaches for detecting phytochemical characteristics varies according to the sensitivity of the procedures. Most studies employed chemical fingerprinting techniques (58.46%, n = 38), followed by macroscopic and microscopic techniques (33.85%, n = 22), genetic fingerprinting techniques (4.6%, n = 3) and chemical tests (3.07%, n = 2) (Figure 3). Less sensitive techniques such as ultraviolet (UV) spectroscopy, thin layer chromatography (TLC), high performance thin layer chromatography (HPTLC) and Fourier transform infrared (FTIR) spectroscopy were complemented by high-end instruments such as liquid chromatography-mass spectrometry (LC/MS/MS) or nuclear magnetic resonance (NMR). Among the chromatographic techniques, HPLC is the mostly used method (30.30%, n = 10), followed by HPTLC/TLC (12.12%, n = 4) and gas chromatography (GC) (3.03%, n = 1). Over the past 10 years, a total of 36.6% (n = 12) studies have applied the hyphenated chromatographic and mass spectrometric techniques in their research.

FIGURE 3. Marantodes pumilum identification and authentication techniques reported between 2010 and 2021.
Two studies (12 and 15) used phytochemical screening, whereas study 4 combined phytochemical analysis with a more advanced instrument, ultra-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC/ESI/MS/MS), to identify the nine flavanols and nine phenolics in various fractions of M. pumilum. Additionally, the study 4 used a chemometric approach such as principal component analysis (PCA) to demonstrate the similarities and differences in phytochemical profiles of different fractions. Chemometrics was also used in conjunction with the chemical fingerprinting technique in study 9, which reported on the use of macroscopy, IR spectroscopy, and chemometric analysis as a powerful technique for differentiating 84 test samples from seven different locations in Peninsular Malaysia. The first method for simultaneous determination of triterpenes, saponins and alkenated-phenolics in the leaves, stems, and roots of M. pumilum var. alata was developed in study 7 using high performance liquid chromatography-ultraviolet-evaporative light scattering detector (HPLC-UV-ELSD) in conjunction with other structure elucidation techniques such as TLC, IR, liquid chromatography coupled with electrospray ionization quadrupole time of flight mass spectrometry (LC/ESI/TOF), NMR and high resolution electrospray ionization mass spectrometry (HRESI/MS). Spectroscopic and chemical analyses were used in study 6 to elucidate the structures of alkyl phenols and saponins found in the roots of M. pumilum. Three investigations (studies 13, 22. and 33) used DNA fingerprinting to identify M. pumilum.
Table 2 summarises selected characteristic features derived from the 33 articles used to distinguish M. pumilum varities using various analytical techniques.
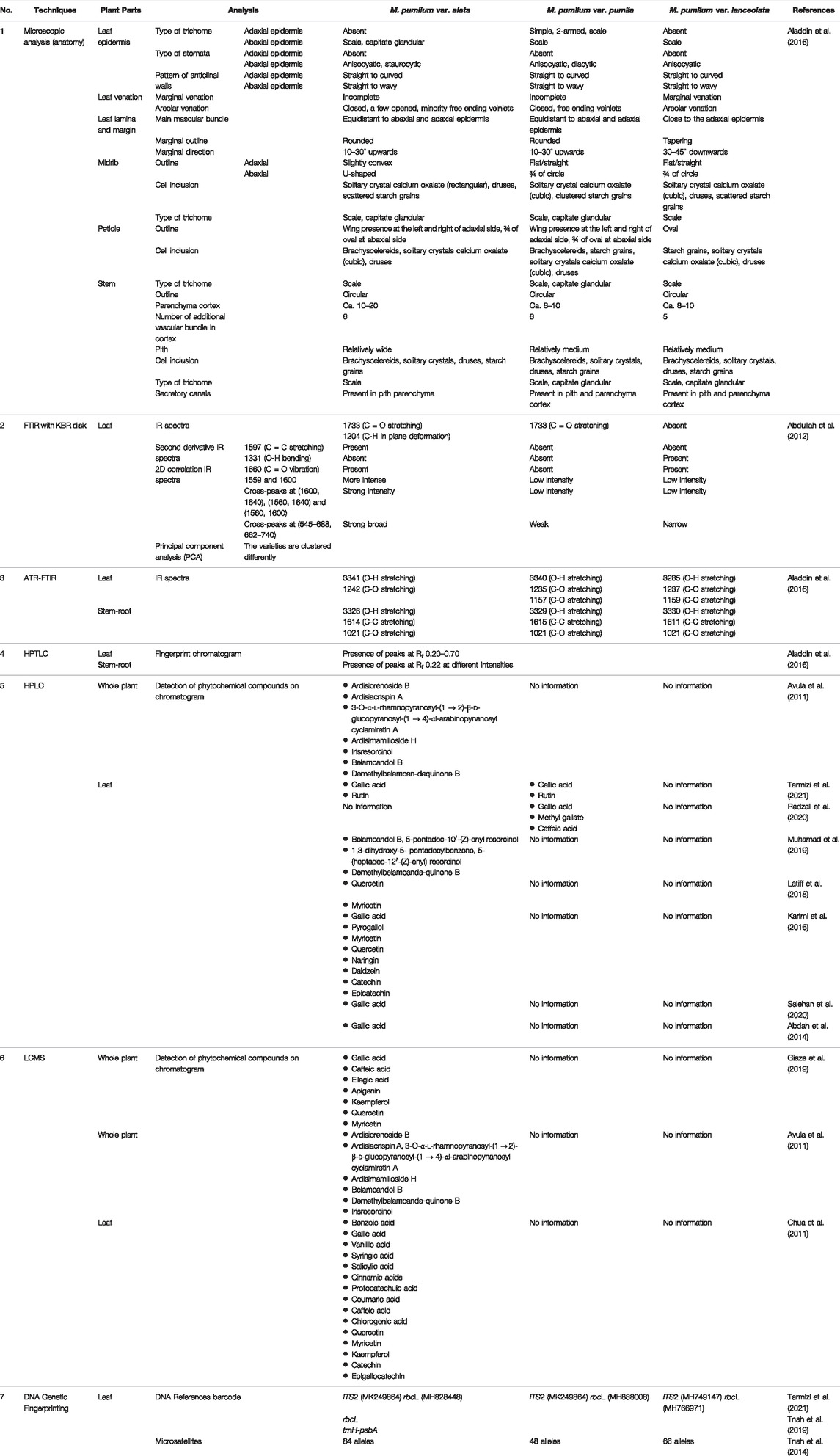
TABLE 2. Characteristic features to distinguish Marantodes pumilum varieties based on different analytical techniques.
Discussion
Between 2016 and 2020, almost 3,000 botanical drug products were registered, increasing by 16% and accounting for 50% of all registered products in Malaysia within 5 years (NPRA, 2020). The figures indicate the significant growth of botanical drug products in the Malaysian market because of persistent client demand. Authentication of botanical drugs is critical to ensuring the consistency of their quality, safety, and efficacy prior to production. Numerous experts have emphasized the importance of certification of botanical drugs to assure their purity and safety. As a result, rigorous authentication must be performed to ascertain the quality and safety of botanical drug products prior to registration approval. Standardization and quality control of raw materials must follow the pharmacopoeia-defined process of identification and authentication.
The systematic review assessed current quality control trends for M. pumilum in the botanical drug research and development setting, as described by pharmacopoeia. In general, the 33 peer-reviewed articles demonstrate that a variety of techniques have been used to identify and authenticate different varieties and plant parts of M. pumilum. Most of the articles reviewed collected wild M. pumilum specimens. The continued reliance on raw materials derived from wild resources, whether for research or commercial purposes, will eventually deplete the supply of M. pumilum. This has become a source of concern for botanical drugs suppliers, as wild M. pumilum is known to grow slowly in its natural habitat. As a result, several research institutions have conducted extensive tissue culture breeding for M. pumilum to keep up with the expanding market demand (Tarmizi et al., 2021).
Additionally, it is discovered that most of the reviewed studies employed at least one method of identification, and that awareness of the requirement has gradually increased since 2010. Effective identification and authentication tools are critical for monitoring the source of high-demand raw materials to avoid undesirable activities such as adulteration of raw materials, which negatively impacts the quality of botanical drug products. As recently reported, this approach was widely used for ginseng products, supplements, commercial botanical drug products, and Kadsura crude drugs (Liu et al., 2019; Ichim et al., 2020; Ichim and de Boer, 2021).
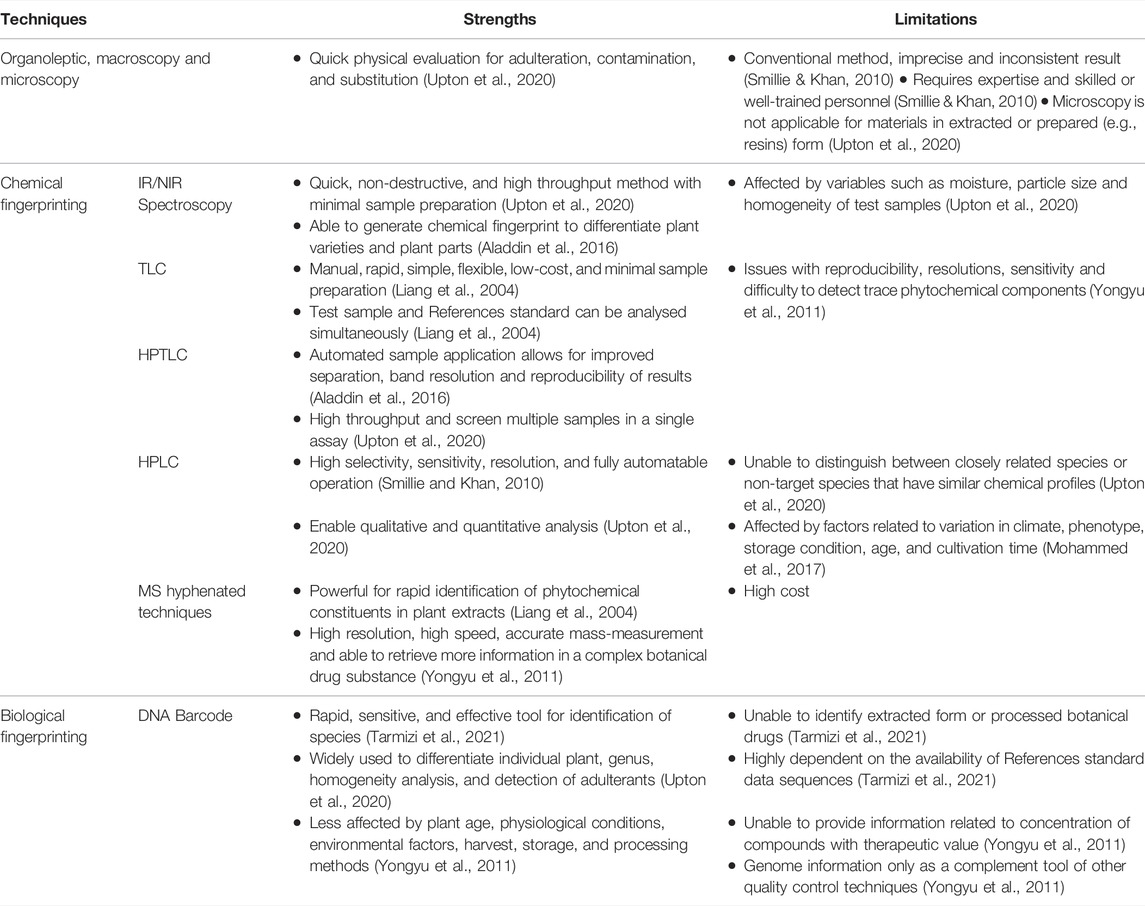
Organoleptic evaluation, macroscopy, and microscopy are the first three fundamental principal methods of identification and authentication used to ensure the quality of botanical drugs (World Health Organization, 2011). Due to the morphological and phytochemical complexity of interspecific hybrids, within-species variation, and the difficulty associated with recognizing species in some plant genera, voucher specimens are critical for organoleptic and macroscopic verification of source material utilized in botanical drugs research (Eisenman et al., 2012). From this review, ten of the articles did not provide information on voucher specimens of M. pumilum used in the studies. Lack of adequate voucher specimens has resulted in major issues, such as the inability to duplicate crucial experimental results and the incorrect assignment of phytochemical and pharmacological data to the correct genus and species. Authentication is especially important for M. pumilum since previous studies have shown that different varieties, plant parts and types of extracts have varying phytochemical compositions and pharmacological actions (Karimi et al., 2015; Aladdin et al., 2020; Rahmi et al., 2020). Thus, prior to producing botanical drug products containing M. pumilum for a particular intended pharmacological action, accurate plant variety and plant part identification must be performed. Adhering to the pharmacopoeia monograph specification for identification tests of botanical drugs would confirm the material’s authenticity. Microscopic examination enables primary species identification, as well as the detection of adulteration, contamination, and substitution of botanical drugs (Upton et al., 2020) (Table 3). Aladdin et al. (2016) reported the first effective distinction of three M. pumilum varieties and plant parts utilizing microscopic approach. The microscopical characteristics, such as stomata, trichomes, stem and leaf margin, petiole, midrib, vascular system, anticlinal walls, secretory canals, and cell inclusion, can all be used to differentiate and identify each variety of M. pumilum and its plant part (Table 2). As previously reported, 41% of 508 botanical drug products sold in 13 countries that were microscopically authenticated were found to be adulterated (Ichim et al., 2020). However, macroscopic, and microscopic analyses alone are insufficient for reliably identifying plant species and determining their quality (Smillie & Khan 2010; Srirama et al., 2017). Plant tissues with little or no cellular variations, processed materials, and extensive dehydration of plants can eliminate diagnostic features, making analysis challenging with these methods (Smillie and Khan, 2010). The issues are compounded by the lack of appropriate reference material and a scarcity of qualified taxonomists (Ichim et al., 2020). Therefore, additional identification techniques should be included to verify the authenticity of plant materials.
Numerous papers describe the use of fingerprint profiling for botanical drugs identification and authentication by spectroscopy and chromatography. Guo (2017) demonstrated that evaluating the botanical drugs quality based on a single or specified markers overlooks the synergistic effects of the multi-phytochemical components of botanical drugs, suggesting that a holistic quality assessment approach using chemical fingerprinting method is relevant. In a prior work, fingerprints of different plant parts of Panax notoginseng (Burkill) F.H. Chen (Araliaceae) were generated using a combination of near infrared spectroscopy (NIR), HPLC, UPLC, and capillary electrophoresis (CE) (Zhu et al., 2014). Aladdin et al. (2016) have reported the usage of a more simple, convenient, and non-destructive phytochemical fingerprinting technique, namely attenuated total reflectance-FTIR (ATR-FTIR) without the usage of KBr (Table 2). The work was an advance on a prior study that used multi-step infrared spectroscopy and a KBR disc (Abdullah et al., 2012). Even though the method without KBr has a lower resolution than the method with KBr disc (Zou et al., 2005), the results of the different profiles between the three M. pumilum varieties and plant parts clearly demonstrated that the ATR-FTIR fingerprinting technique can be used to determine the identity and quality control of M. pumilum raw materials. However, this technique alone may be best suited for a single authentic plant ingredient because it may be difficult to gain accurate information of the phytochemical compositions and to detect adulterants based only on the chemical functional groups from the infrared spectrum. Moisture, particle size, and homogeneity of test samples all have an impact on analytical precision (Upton et al., 2020) (Table 3).
The phytochemical fingerprint profile of botanical drugs generates a large amount of data in the form of chromatograms or spectra, making it nearly impossible for the analyst to visually inspect each data point and exploit the useful chemical information contained in the fingerprint data via univariate analysis. As a result, a multivariate data analysis technique was developed to analyze chemical fingerprinting data to eliminate or reduce undesired sources of variation caused by various variables or instrumental responses from the analytical techniques, as well as to extract useful and meaningful information from the fingerprint data (Gad et al., 2013; Huang et al., 2016). Chemometric techniques have been widely used in quality control of botanical drug products due to their capacity to tackle a variety of problems in a variety of domains, including similarity analysis and exploratory learning. Apart from that, the chemometric approach can analyze a variety of data, both qualitatively and quantitatively, via a classification algorithm and a multivariate calibration algorithm (Li et al., 2020). The combination of chemometric techniques such as principal component analysis (PCA) and multilayer perceptron classifier (MLPC) modelling with ATR-FTIR has been reported as a rapid and effective method for botanical drugs quality evaluation of Gastrodia elata Blume (Orchidaceae) powder (Zhan et al., 2022). From this systematic review study, Abdullah et al. (2012) successfully characterized leaves of three M. pumilum varieties using IR and chemometrics (Table 2), whereas Chua et al. (2011) used an integrated approach of chemical fingerprinting with UPLC-ESI-MS/MS and chemometric technique to differentiate M. pumilum var. alata and var. pumila leaves based on the compositions of nine flavonols, two flavanols, and nine phenols.
TLC technique has been widely recognized as a preliminary screening approach to HPLC, owing to its ability to rapidly generate a fingerprint of varied plant materials in a single, simple, and low-cost analysis while producing high sample throughput. TLC is used for preliminary screening or identification of phytochemical components that provide the plant’s unique fingerprint (Liang et al., 2004). The HPTLC technique, when combined with automated sample application and densitometric scanning, offers numerous advantages, including high throughput samples, low analytical costs, and the ability to separate test samples and reference standards concurrently (Upton et al., 2020) (Table 3). These methods are described in most international pharmacopoeias, including the British Pharmacopoeia, the United States Pharmacopeia, and the Chinese Pharmacopoeia. There are numerous studies that used TLC and HPTLC for authentication such as ginseng, Radix Puerariae and M. pumilum (Xie et al., 2006; Siyumbwa, 2015). Aladdin et al. (2016) reported that the HPTLC fingerprint profiles of three varieties of M. pumilum leaves and stem-roots gathered from a wild source at a single location were somewhat comparable with varying intensities, implying the presence of identical phytochemicals in varying concentrations (Table 2). According to Karimi et al. (2015), total phenolics, total flavonoids, and fatty acid concentration also varied between M. pumilum var. alata, var. pumila, and var. lanceolata leaves obtained from three separate locations from the wild. The presence of identical compounds with varying compositions complicates accurate authentication of commercial plant materials, as phytochemical compound concentrations are influenced by a variety of extrinsic factors, such as geographic (latitude, altitude, and soil type), climatic (light, temperature, rainfall, and atmospheric compositions) and agricultural practice (cultivation, harvesting and processing methods, and storage conditions), as well as intrinsic factors, such as plant age, genetics, chemotypes and botanical parts (Liang et al., 2004; Yongyu et al., 2011). M. pumilum has been reported to contain potential pharmacologically active phytochemicals such as quercetin, myricetin, kaempferol, naringin, rutin, apigenin, catechin, epigallocatechin, pyrogallol, gallic acid, ascorbic acid, salicylic acid, syringic acid, vanillic acid, protocatechuic acid, coumaric acid, caffeic acid, chlorogenic acid, daidzein, genistein, β-carotene, anthocyanins, demethylbelamcandaquinone B, and 3,7-dihydroxy-5-methoxy-4,8-dimethyl-isocoumarin (Norhaiza et al., 2009; Ali and Khan, 2011; Chua et al., 2011; Ehsan et al., 2011; Hairi et al., 2018; Wu et al., 2018; Aladdin et al., 2020). Gallic acid and caffeic acid were identified as the major phenolic acids in the methanol extracts of three M. pumilum varieties (Karimi et al., 2011). Due to their widespread presence in other plants, the two compounds cannot be considered a unique biomarker for M. pumilum. Hence, the diverse class of phytochemicals necessitates the use of more sensitive approaches for M. pumilum identification and authentication.
Liquid chromatography is one of the most efficient analytical techniques for phytochemical profiling since the stationary phase column, mobile phase gradient system and detector can all be modified to suit the analysis of a variety of phytochemical components. For the development of a validated analytical method, statistically significant representative set of plant samples from multiple populations is used to establish a fingerprint profile, whereas reference standards, whether commercially available, extracted, or isolated, are necessary (Smillie and Khan, 2010). In this review, Avula et al. (2011) used HPLC to assess the phytochemicals content isolated from M. pumilum var. alata leaves, stems, and roots (Table 2), whereas Aladdin et al. (2016) distinguished three varieties of M. pumilum and their plant parts based on fingerprinting profile. However, both studies utilized only botanical drugs obtained from a single location, indicating that additional research is necessary. Closely related plant species with similar chemical profiles will not be discriminated using HPLC (Table 3). Researchers have used hyphenated chromatographic and mass spectrometric techniques, such as liquid chromatography-mass spectrometry (LC/MS), gas chromatography-mass spectrometry (GC/MS), and capillary electrophoresis-mass spectrometry (CE/MS), to authenticate plant materials. In this systematic review study, LC/MS (Giaze et al., 2019), LC/MS/MS (Dharmani et al., 2019), UPLC/MS/MS (Wu et al., 2019), and LC/ESI/TOF (Avula et al., 2011) were used to determine phytochemical compositions of different M. pumilum plant parts, whereas UPLC-ESI/MS/MS was used to detect components between M. pumilum var. alata and var. pumila leaves (Chua et al., 2011). To date, the combination of chromatography and mass spectrometry is the most advanced approach and is often used by researchers to analyze the composition of botanical drugs qualitatively and quantitatively to determine the consistency of their quality. Mass spectrometry imaging may be used to visually assess quality variations (Wei et al., 2020). However, the cost of the hyphenated equipment is a significant constraint (Table 3).
The researchers are currently interested in the other tool for botanical drugs authentication using genetic fingerprinting techniques. DNA barcoding enabled a rapid examination of the botanical drugs composition and was found to be an effective technique for authenticating dried and powdered plant materials for quality control purposes (Gesto-Borroto et al., 2021). Tnah et al. (2014) were the first to report the use of a genetic-based fingerprint technique to differentiate leaves of three M. pumilum varieties collected in the wild, followed by another investigation of botanical drug products containing M. pumilum (Tnah et al., 2019) (Table 2). According to the latter study, DNA barcoding was able to detect 56.7% of 30 selected botanical drug products containing M. pumilum and were found to be authentic, 10% were substituted with other plant taxa, and 6.7% were contaminated. DNA information was not detected in 26.6% of botanical drug products due to the low concentration or degradation of the processed botanical drug preparations. The study reported the authenticity of a product containing a single ingredient of M. pumilum, however, for a product containing a mixture of M. pumilum and Querqus lusitanica Lam. (Fagaceae) had no DNA sequence. Additionally, a recent study by Tarmizi et al. (2021) effectively used DNA barcoding and HPLC techniques for the identification of cultivated M. pumilum var. alata and var. pumila leaves, as well as investigation of the authenticity of botanical drug products containing M. pumilum. However, nine of test samples (60%) reported not amplifiable due to the lack or low concentration of DNA recovered from degradation. This demonstrated that the primary limitation of the current DNA barcoding approach is its inability to identify M. pumilum at the variety level due to inaccuracies in selecting the targeted sequence, use of a large barcode size and DNA degradation in processed materials (Table 3). As a result, DNA barcoding may not be suitable as a stand-alone method of identification of processed botanical drug products and should be combined with additional authentication techniques such as morphological features and chemical analyses.
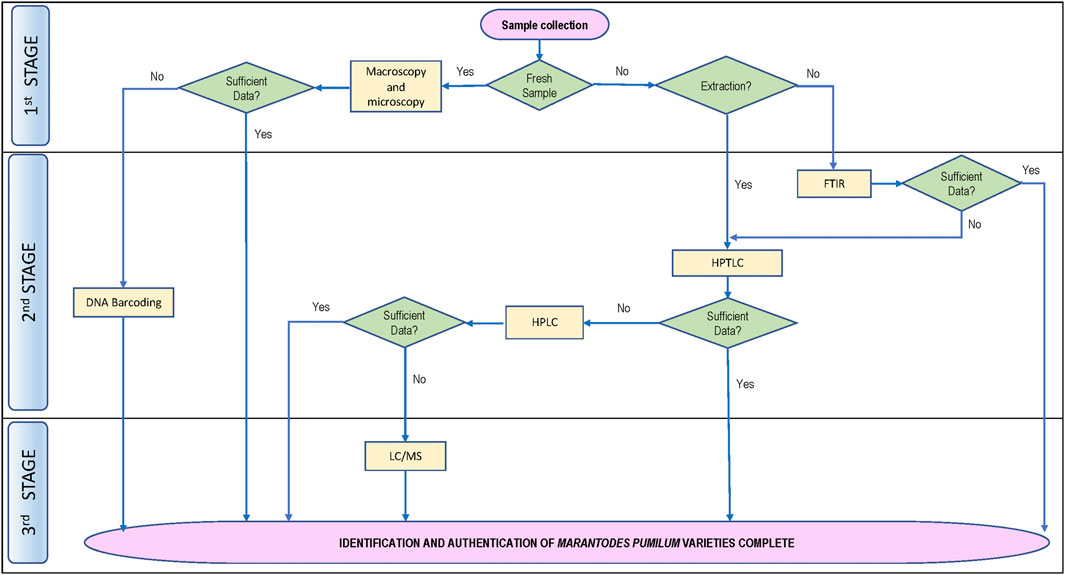
The various techniques used to identify M. pumilum varieties and plant parts in the articles reviewed in this study (Table 1, 2), such as macroscopy, microscopy, chromatography, spectroscopy and chemometrics, suggest that a combination of approaches is required to authenticate botanical drug substances and products. However, the existing reports did not address phytochemical variation of a plant variety or plant part collected from various locations, differences between those collected from the wild sources and those collected from cultivated sources, as well as adulteration with other plant parts or varieties. When producing a standardized botanical drug product, obtaining botanical drug substance from a cultivated plantation location rather than the wild will assure plant homogeneity. Phytochemical indicators are frequently used to standardize botanical drug products. A guideline for selecting marker substances for quality control of botanical drug is provided by the World Health Organization (2017). To facilitate correct identification and authentication of M. pumilum utilized in botanical drug products development, it is vital to identify the phytochemical constituents with known therapeutic activity. As shown in Figure 4, a flow procedure for M. pumilum authentication up to the variety level is proposed based on an existing approach.
Conclusion
This review found that no one technique for authenticating M. pumilum botanical drug substances and products can be used. Each technique has its own distinct interpretation of the plant, ranging from simple morphological characteristics to a more comprehensive comprehension of the M. pumilum’s phytochemical constituents. Developing proper authentication procedures is critical for the development and manufacturing of botanical drugs, whether for clinical trials or before the product reaches the consumer. Thus, additional research is necessary to determine the most effective authentication techniques for differentiating the varieties of M. pumilum and their plant parts to ensure that the correct species is used in the manufacturing process of botanical drug products and to avoid adulterations that could pose a health risk to consumers.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
II is a Phd. candidate who conducted a systematic literature search, assessed the findings and prepared a systematic literature review draft. JJ, MS, and NZ contributed ideas for the systematic literature review design and reviewed the manuscript. JJ is the project leader and revised the manuscript.
Funding
The study was supported by the UKM research grant scheme (GUP 2021-007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our gratitude to Universiti Kebangsaan Malaysia for the financial support and the usage of library facilities.
References
Abdah, S. N. M., Sarmidi, M. R., Yaakob, H. Y., and Ware, I. (2014). Fractionation of Labisia pumila Using Solid-phase Extraction for Extraction of Gallic Acid. J. Teknol. 69 (4), 65–68. doi:10.11113/jt.v69.3176
Abdullah, F., Ling, S. K., Man, S., Tan, A. L., Tan, H. P., and Abdullah, Z. (2012). Characterization and Identification of Labisia pumila by Multi-Steps Infrared Spectroscopy. Vib. Spectrosc. 62, 200–206. doi:10.1016/j.vibspec.2012.06.004
Abdullah, N., Chermahini, S. H., Chua, L. S., and Sarmidi, M. R. (2013). Labisia pumila: A Review on its Traditional, Phytochemical and Biological Uses. World Appl. Sci. J. 27 (10), 1297–1306. doi:10.5829/idosi.wasj.2013.27.10.1391
Abubakar, B. M., Salleh, F. M., Shamsir Omar, M. S., and Wagiran, A. (2018). Assessing Product Adulteration of Eurycoma longifolia (Tongkat Ali) Herbal Medicinal Product Using DNA Barcoding and HPLC Analysis. Pharm. Biol. 56 (1), 368–377. doi:10.1080/13880209.2018.1479869
Adam, S. H., Giribabu, N., Bakar, N. M. A., and Salleh, N. (2017). Marantodes pumilum (Kacip Fatimah) Enhances Iin-Vvitro Glucose Uptake in 3T3-L1 Adipocyte Cells and Reduces Pancreatic Complications in Streptozotocin-Nicotinamide Induced Male Diabetic Rats. Biomed. Pharmacother. 96, 716–726. doi:10.1016/j.biopha.2017.10.042
Ahmad, S. U., Azam, A., Shuid, A. N., and Mohamed, I. N. (2018). Antioxidant and Anti-inflammatory Activities of Marantodes pumilum (Blume) Kuntze and Their Relationship with the Phytochemical Content. Rec. Nat. Prod. 12 (6), 518–534. doi:10.25135/rnp.58.17.11.188
Al-Mekhlafi, N. A., Shaari, K., Abas, F., Kneer, R., Jeyaraj, E. J., Stanslas, J., et al. (2012). Alkenylresorcinols and Cytotoxic Activity of the Constituents Isolated from Labisia pumila. Phytochemistry 80, 42–49. doi:10.1016/j.phytochem.2012.04.008
Aladdin, N.-A., Jamal, J. A., Talip, N., Hamsani, N. A. M., Rahman, M. R. A., Sabandar, C. W., et al. (2016). Comparative Study of Three Marantodes pumilum Varieties by Microscopy, Spectroscopy and Chromatography. Rev. Bras. Farmacogn. 26 (1), 1–14. doi:10.1016/j.bjp.2015.10.002
Aladdin, N. A., Husain, K., Jalil, J., Sabandar, C. W., and Jamal, J. A. (2020). Xanthine Oxidase Inhibitory Activity of a New Isocoumarin Obtained from Marantodes pumilum var. pumila Leaves. BMC Complement. Med. Ther. 20 (1), 324–412. doi:10.1186/s12906-020-03119-8
Ali, Z., and Khan, I. A. (2011). Alkyl Phenols and Saponins from the Roots of Labisia pumila (Kacip Fatimah). Phytochemistry 72 (16), 2075–2080. doi:10.1016/j.phytochem.2011.06.014
Avula, B., Wang, Y. H., Ali, Z., Smillie, T. J., and Khan, I. A. (2011). Quantitative Determination of Triperpene Saponins and Alkenated-Phenolics from Labisia pumila Using an LC-UV/ELSD Method and Confirmation by LC-ESI-TOF. Planta Med. 77 (15), 1742–1748. doi:10.1055/s-0030-1271037
Burkill, I. H. (1966). A Dictionary of the Economic Products of the Malay Peninsula. Kuala Lumpur: Ministry of Agriculture and Cooperatives.
Choi, H. K., Kim, D. H., Kim, J. W., Ngadiran, S., Sarmidi, M. R., and Park, C. S. (2010). Labisia pumila Extract Protects Skin Cells from Photoaging Caused by UVB Irradiation. J. Biosci. Bioeng. 109 (3), 291–296. doi:10.1016/j.jbiosc.2009.08.478
Chua, L. S., Latiff, N. A., Lee, S. Y., Lee, C. T., Sarmidi, M. R., and Aziz, R. A. (2011). Flavonoids and Phenolic Acids from Labisia pumila (Kacip Fatimah). Food Chem. 127, 1186–1192. doi:10.1016/j.foodchem.2011.01.122
Chua, L. S., Lee, S. Y., Abdullah, N., and Sarmidi, M. R. (2012). Review on Labisia pumila (Kacip Fatimah): Bioactive Phytochemicals and Skin Collagen Synthesis Promoting Herb. Fitoterapia 83 (8), 1322–1335. doi:10.1016/j.fitote.2012.04.002
Dharmani, M., Kamarulzaman, K., Giribabu, N., Choy, K. W., Zuhaida, M. Z., Aladdin, N. A., et al. (2019). Effect of Marantodes pumilum Blume (Kuntze) var.Alata on β-cell Function and Insulin Signaling in Ovariectomised Diabetic Rats. Phytomedicine 65, 153101. doi:10.1016/j.phymed.2019.153101
Dianita, R., Jantan, I., Jalil, J., and Amran, A. Z. (2016). Effects of Labisia pumila var. alata Extracts on the Lipid Profile, Serum Antioxidant Status and Abdominal Aorta of High-Cholesterol Diet Rats. Phytomedicine 23 (8), 810–817. doi:10.1016/j.phymed.2016.04.004
Effendy, N. M., Khamis, M. F., Soelaiman, I. N., and Shuid, A. N. (2014). The Effects of Labisia pumila on Postmenopausal Osteoporotic Rat Model: Dose and Time-dependent Micro-CT Analysis. J. Xray Sci. Technol. 22 (4), 503–518. doi:10.3233/XST-140441
Ehsan, K., Hawa, Z. E. J., and Sahida, A. (2011). Phytochemical Analysis and Antimicrobial Activities of Methanolic Extracts of Leaf, Stem and Root from Different Varieties of Labisia pumila Benth. Molecules 16, 4438–4450.
Eisenman, S. W., Tucker, A. O., and Struwe, L. (2012). Voucher Specimens Are Essential for Documenting Source Material Used in Medicinal Plant Investigations. JAMP 1 (1), 30–43.
Fadzil, N. F., Wagiran, A., Salleh, F. M., Shamsiah, A., and Izham, N. H. M. (2018). Authenticity Testing and Detection of Eurycoma longifolia in Commercial Herbal Products Using Bar-High Resolution Melting Analysis. Genes 9 (8), 408. doi:10.3390/genes9080408
Fazliana, M., Ramos, N. L., Luthje, P., Sekikubo, M., and Holm, A. (2011). Labisia pumila var. alata Reduces Bacterial Load by Inducing Uroepithelial Cell Apoptosis. J. Ethnopharmacol. 136 (1), 111–116. doi:10.1016/jep.2011.04.01810.1016/j.jep.2011.04.018
Gad, H. A., El-Ahmady, S. H., Abou-Shoer, M. I., and Al-Azizi, M. M. (2013). Application of Chemometrics in Authentication of Herbal Medicines: A Review. Phytochem. Anal. 24 (1). doi:10.1002/pca.2378
Gesto-Borroto, R., Medina-Jiménez, K., Lorence, A., and Villarreal, M. L. (2021). Application of DNA Barcoding for Quality Control of Herbal Drugs and Their Phytopharmaceuticals. Rev. Bras. Farmacogn. 31, 127–141. doi:10.1007/s43450-021-00128-7
Giaze, T. R., Shuid, A. N., Soelaiman, I. N., Mohamed, N., Jamal, J. A., and Fauzi, M. B. (2019). Comparative Anti-osteoporotic Properties of the Leaves and Roots of Marantodes pumilum var. alata in Postmenopausal Rat Model. J. Tradit. Complement. Med. 9 (4), 393–400. doi:10.1016/j.jtcme.2019.01.002
Giaze, T. R., Shuid, A. N., Soelaiman, I. N., Muhammad, N., Fauzi, M. B., and Arlamsyah, A. Z. (2018). Marantodes pumilum Leaves Promote Repair of Osteoporotic Fracture in Postmenopausal Sprague-Dawley Rats. Int. J. Pharmacol. 14 (7), 973–980. doi:10.3923/ijp.2018.973.980
Giribabu, N., Karim, K., and Salleh, N. (2018). Effects of Marantodes pumilum (Kacip Fatimah) on Vaginal pH and Expression of Vacoular ATPase and Carbonic Anhydrase in the Vagina of Sex-Steroid Deficient Female Rats. Phytomedicine 49, 95–105. doi:10.1016/j.phymed.2018.05.018
Goodarzi, M., Russel, P. J., and Heyden, Y. V. (2013). Similarity Analyses of Chromatographic Herbal Fingerprints: A Review. Anal. Chim. Acta 804, 16–28. doi:10.1016/j.aca.2013.09.017
Guo, D. (2017). Quality Marker Concept Inspires the Quality Research of Traditional Chinese Medicines. Chin. Herb. Med. 9 (1), 1–2. doi:10.1016/S1674-6384(17)60069-8
Hairi, H. A., Jamal, J. A., Aladdin, N. A., Husain, K., Sofi, N. S. M., Mohamed, N. I., et al. (2018). Demethylbelamcandaquinone B (Dmcq B) Is the Active Compound of Marantodes pumilum var. alata (Blume) Kuntze with Osteoanabolic Activities. Molecules 23 (7), 1–19. doi:10.3390/molecules23071686
Heinrich, M., and Anagnostou, S. (2017). From Pharmacognosia to DNA-Based Medicinal Plant Authentication – Pharmacognosy through the Centuries. Planta Med. 83 (14-15), 1110–1116. doi:10.1055/s-0043-108999
Huang, Y., Wu, Z., Su, R., Ruan, G., Du, F., and Li, G. (2016). Current Application of Chemometrics in Traditional Chinese Herbal Medicine Research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1026, 27–35. doi:10.1016/j.jchromb.2015.12.050
Ichim, M. C., and de Boer, H. J. (2021). A Review of Authenticity and Authentication of Commercial Ginseng Herbal Medicines and Food Supplements. Front. Pharmacol. 11, 2185. doi:10.3389/fphar.2020.612071
Ichim, M. C., Hasser, A., and Nick, P. (2020). Microscopic Authentication of Commercial Herbal Products in the Globalized Market: Potential and Limitations. Front. Pharmacol. 11, 876. doi:10.3389/fphar.2020.00876
Jamal, J. A., Ramli, N., Stanslas, J., and Husain, K. (2012). Estrogenic Activity of Selected Myrsinaceae Species in MCF-7 Human Breast Cancer Cells. Int. J. Pharm. Pharm. Sci. 4 (4), 547–553.
Karimi, E., Jaafar, H. Z. E., and Ahmad, S. (2013). Antifungal, Anti-inflammatory and Cytotoxicity Activities of Three Varieties of Labisia pumila Benth: From Microwave Obtained Extracts. BMC Complement. Altern. Med. 13, 1–20. doi:10.1186/1472-6882-13-20
Karimi, E., Jaafar, H. Z. E., and Ahmad, S. (2011). Phytochemical Analysis and Antimicrobial Activities of Methanolic Extracts of Leaf, Stem and Root from Different Varieties of Labisa pumila Benth. Molecules 16 (6), 4438–4450. doi:10.3390/molecules16064438
Karimi, E., Jaafar, H. Z. E., and Ghasemzadeh, A. (2016). Chemical Composition, Antioxidant and Anticancer Potential of Labisia pumila Variety alata under CO2 Enrichment. NJAS - Wagen. J. Life Sci. 78, 85–91. doi:10.1016/j.njas.2016.05.002
Karimi, E., Jaafar, H. Z. E., Ghasemzadeh, A., and Ebrahimi, M. (2015). Fatty Acid Composition, Antioxidant and Antibacterial Properties of the Microwave Aqueous Extract of Three Varieties of Labisia pumila Benth. Biol. Res. 48, 1–6. doi:10.1186/0717-6287-48-9
Latiff, N. A., Chua, L. S., Sarmidi, M. R., Ware, I., and Rashid, S. N. A. A. (2018). Liquid Chromatography Tandem Mass Spectrometry for the Detection and Validation of Quercetin-3-O-Rutinoside and Myricetin from Fractionated Labisia pumila var. alata. Malays. J. Anal. Sci. 22 (5), 817–827.
Li, Y., Shen, Y., Yao, C. L., and Guo, D-A. (2020). Quality Assessment of Herbal Medicines Based on Chemical Fingerprints Combined with Chemometrics Approach: A Review. J. Pharm. Biomed. Anal. 185, 113215. doi:10.1016/j.jpba.2020.22321510.1016/j.jpba.2020.113215
Liang, Y. Z., Peishan, X., and Chan, K. (2004). Quality Control of Herbal Medicines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 812, 53–70. doi:10.1016/j.jchromb.2004.08.041
Liu, J., Wei, X., Zhang, X., Qi, Y., Zhang, B., Liu, H., et al. (2019). A Comprehensive Comparative Study for the Authentication of the Kadsura Crude Drug. Front. Pharmacol. 9, 1576. doi:10.3389/fphar.2018.01576
Mamat, N., Jamal, J. A., Jantan, I., and Husain, K. (2014). Xanthine Oxidase Inhibitory and DPPH Radical Scavenging Activities of Some Primulaceae Species. Sains Malays 43 (12), 1827–1833. doi:10.17576/jsm-2014-4312-03
Mannerås, L., Fazliana, M., Nazaimoon, W. M. W., Lonn, M., Gu, H. F., and Ostenson, C. G. (2010). Beneficial Metabolic Effects of the Malaysian Herb Labisia pumila var. alata in a Rat Model of Polycystic Ovary Syndrome. J. Ethnopharmacol. 127 (2), 346–351. doi:10.1016/j.jep.2009.10.032
Manshor, N. M., Razali, N., Jusoh, R. R., Asmawi, M. Z., Mohamed, N., and Zainol, S. (2020). Vasorelaxant Effect of Water Fraction of Labisia pumila and its Mechanisms in Spontaneously Hypertensive Rats Aortic Ring Preparation. Int. J. Cardiol. Hypertens. 4, 100024. doi:10.1016/j.ijchy.2020.100024
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6 (7). doi:10.1371/journal.pmed.100009710.1136/bmj.b2535
Muhamad, M., Choo, C. Y., Hasuda, T., and Hitotsuyanagi, Y. (2019). Estrogenic Phytochemical from Labisia pumila (Myrsinaceae) with Selectivity towards Estrogen Receptor Alpha and Beta Subtypes. Fitoterapia 137, 104256. doi:10.1016/j.fitote.2019.104256
Norhaiza, M., Maziah, M., and Hakiman, M. (2009). Antioxidative Properties of Leaf Extracts of a Popular Malaysian Herb, Labisia pumila. J. Med. Plants Res. 3 (4), 217–223.
Norhayati, M. N., George, A., Hazlina, N. H. N., Azidah, A. K., Indiana, H. I., and Law, K. S. (2014). Efficacy and Safety of Labisia pumila var. alata Water Extract Among Pre- and Postmenopausal Women. J. Med. Food 17 (8), 929–938. doi:10.1089/jmf.2013.2953
NPRA, (2020). Annual Report 2020. Published on the Internet. Avilable from https://npra.gov.my/index.php/en/informationen/annual-reports/npra-annual-reports.html. Accessed at 03 June 2021
Pan, Y., Tiong, K. H., Rashid, B. A. A., Ismail, Z., Ismail, R., and Mak, J. W. (2012). Inhibitory Effects of Cytochrome P450 Enzymes CYP2C8, CYP2C9, CYP2C19 and CYP3A4 by Labisia pumila Extracts. J. Ethnopharmacol. 143 (2), 586–591. doi:10.1016/j.jep.2012.07.024
Pandey, A., Bani, S., Sangwan, P., and Koul, S. (2010). Selective Th1 Upregulation by Ethyl Acetate Fraction of Labisia pumila. J. Ethnopharmacol. 132 (1), 309–315. doi:10.1016/j.jep.2010.08.039
Pandey, A., Sarang, B., and Sangwan, P. L. (2014). Anti-obesity Potential of Gallic Acid from Labisia pumila, through Augmentation of Adipokines in High Fat Diet Induced Obesity in C57BL/6 Mice. Adv. Res. 2 (10), 556–570. doi:10.9734/air/2014/10182
Pattiram, P. D., Lasekan, O., Tan, C. P., and Zaidul, I. S. M. (2011). Identification of the Aroma-Active Constituents of the Essential Oils of Water Dropwort (Oenanthe javanica) and “Kacip Fatimah” (Labisia pumila). Int. Food Res. J. 18 (3), 1021–1026.
Pihie, A. H. L., Othman, F., and Zakaria, Z. A. (2011). Anticarcinogenic Activity of Labisia pumila against 7,12- Dimethylbenz (A) Anthracene (DMBA)/croton Oil-Induced Mouse Skin Carcinogenesis. Afr. J. Pharm. Pharmacol. 5 (7), 823–832. doi:10.5897/AJPP11.140
Radzali, S. A., Markom, M., and Saleh, N. M. (2020). Co-solvent Selection for Supercritical Fluid Extraction (SFE) of Phenolic Compounds from Labisia pumila. Molecules 25 (24), 1–15. doi:10.3390/molecules25245859
Rahmi, E. P., Kumolosasi, E., Jalil, J., Husain, K., Buang, F., Abd. Razak, A. F., et al. (2020). Anti-hyperuricemic and Anti-inflammatory Effects of Marantodes pumilum as Potential Treatment for Gout. Front. Pharmacol. 11, 289. doi:10.3389/fphar.2020.00289
Salehan, N. A. M., Naila, A., Ajit, A., and Sulaiman, A. Z. (2020). Effect of Solid to Water Ratio, Time and Temperature on Aqueous Extraction of Gallic Acid from Labisia pumila var. alata of Malaysia. Pak. J. Sci. Ind. Res. B Biol. Sci. 63 (2), 93–99. doi:10.52763/pjsir.biol.sci.63.2.2020.93.99
Siyumbwa, S., Kamran, S., Akowuah, G., Teo, S. S., Amini, F., and Patrick, N. (2015). The HPTLC Validated Method Development for the Quantification of Naringin from the Partially Purified Labisia pumila Dichloromethane Extract. J. Chromatogr. Sep. Tech. 6 (4), 271–274. doi:10.4172/2157-7064.1000271
Smillie, T. J., and Khan, I. A. (2010). A Comprehensive Approach to Identifying and Authenticating Botanical Products. Clin. Pharmacol. Ther. 87 (2), 175–185. doi:10.1038/clpt.2009.287
Srirama, R., Kumar, J. U. S., Seethapathy, G. S., Newmaster, S. G., Ragupathy, S., and Ganeshaiah, K. N. (2017). Species Adulteration in the Herbal Trade: Causes, Consequences and Mitigation. Drug Saf. 40 (8), 651–661. doi:10.1007/s40264-017-0527-0
Sunarno, B. (2005). Revision of the Genus Labisia (Myrsinaceae). Blumea J. Plant Taxon. Plant Geogr. 50 (3), 579–597. doi:10.3767/000651905x622879
Tan, N. A. S., Giribabu, N., Karim, K., Nymathulla, S., and Salleh, N. (2019). Intravaginal Treatment with Marantodes pumilum (Kacip Fatimah) Ameliorates Vaginal Atrophy in Rats with Post-menopausal Condition. J. Ethnopharmacol. 236, 9–20. doi:10.1016/j.jep.2019.02.027
Tarmizi, A. A. A., Wagiran, A., Mohd Salleh, F., Chua, L. S., Abdulllah, F. I., and Hasham, R. (2021). Integrated Approach for Species Identification and Quality Analysis for Labisia pumila Using DNA Barcoding and HPLC. Plants 10, 717. doi:10.3390/plants10040717
Techen, N., Parveen, I., Zhiqiang, P., and Khan, I. A. (2014). DNA Barcoding of Medicinal Plant Material for Identification. Curr. Opin. Biotechnol. 25, 103–110. doi:10.1016/j.copbio.2013.0901010.1016/j.copbio.2013.09.010
Tnah, L. H., Lee, C. T., Lee, S. L., Ng, C. H., and Ng, K. K. S. (2014). Development of Microsatellites in Labisia pumila (Myrsinaceae), an Economically Important Malaysian Herb. Appl. Plant Sc. 2 (6), 1400019. doi:10.3732/apps.1400019
Tnah, L. H., Lee, S. L., Tan, A. L., Lee, C. T., Ng, K. K. S., and Ng, C. H. (2019). DNA Barcode Database of Common Herbal Plants in the Tropics: a Resource for Herbal Product Authentication. Food control. 95, 318–326. doi:10.1016/j.foodcont.2018.08.022
Upton, R., David, B., Stefan, G., and Glasl, S. (2020). Botanical Ingredient Identification and Quality Assessment: Strengths and Limitations of Analytical Techniques. Phytochem. Rev. 19 (5), 1157–1177. doi:10.1007/s11101-019-09625-z
Wan Omar, W. F. N., Giribabu, N., Karim, K., and Salleh, N. (2019). Marantodes pumilum (Blume) Kuntze (Kacip Fatimah) Stimulates Uterine Contraction in Rats in Post-partum Period. J. Ethnopharmacol. 245, 112175. doi:10.1016/j.jep.2019.112175
Wei, X. C., Cao, B., Luo, C. H., Huang, H. Z., Tan, P., Xu, X. R., et al. (2020). Recent Advances of Novel Technologies for Quality Consistency Assessment of Natural Herbal Medicines and Preparations. Chin. Med. (United Kingdom) 15 (1), 1–24. doi:10.1186/s13020-020-00335-9
WFO (2022). Marantodes pumilum (Blume) Kuntze. Available at: http://www.worldfloraonline.org/taxon/wfo-0001039157 (Accessed on Apr 21, 2022).
World Health Organization (2017). “WHO Guidelines for Selecting Marker Substances of Herbal Origin for Quality Control of Herbal Medicines,” in WHO Expert Committee on Specifications for Pharmaceutical Preparation: Fifty First Report. Annex 1. WHO Technical Report Series, No. 1003 (Geneva: World Health Organization).
World Health Organization (2011).Quality Control Methods for Herbal Materials. Geneva: World Health Organization.
Wu, H., Xi, H., Lai, F., Ma, J., Chen, W., and Liu, H. (2019). Cellular Antioxidant Activity and Caco-2 Cell Uptake Characteristics of Flavone Extracts from Labisia pumila. Int. J. Food Sci. Technol. 54 (2), 536–549. doi:10.1111/ijfs.13968
Wu, H., Xi, H., Lai, F., Ma, J., and Liu, H. (2018). Chemical and Cellular Antioxidant Activity of Flavone Extracts of Labisia pumila before and after In Vitro Gastrointestinal Digestion. RSC Adv. 8, 12116–12126. doi:10.1039/c8ra00142a
Xie, P., Chen, S., Liang, Y., Wang, X., Tian, R., and Upton, R. (2006). Chromatographic Fingerprint Analysis-A Rational Approach for Quality Assessment of Traditional Chinese Herbal Medicine. J. Chromatogr. A 1112 (1–2), 171–180. doi:10.1016/j.chroma.2005.12.091
Yadav, N. P., and Dixit, V. K. (2008). Recent Approaches in Herbal Drug Standardization. Int. J. Integr. Biol. 2 (3), 195–203.
Yeop, A., Sandanasamy, J., Pang, S. F., and Gimbun, J. (2021). Stability and Controlled Release Enhancement of Labisia pumila’s Polyphenols. Food Biosci. 41, 101025. doi:10.1016/j.fbio.2021.101025
Yongyu, Z., Shujun, S., Wenyu, W., Huijuan, C., and Jianbing, W. (2011). Quality Control Method for Herbal Medicine - Chemical fingerprint Analysis. Quality Control of Herbal Medicines and Related Areas, 171–194.
Zhan, W., Yang, X., Lu, G., Deng, Y., and Yang, L. (2022). A Rapid Quality Grade Discrimination Method for Gastrodia elata Powder Using ATR-FTIR and Chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 264, 120189. doi:10.1016/j.saa.2021.120189
Zhu, J., Fan, X., Cheng, Y., Agarwal, R., Moore, C. M. V., and Chen, S. T. (2014). Chemometric Analysis for Identification of Botanical Raw Materials for Pharmaceutical Use: A Case Study Using Panax notoginseng. PLoS ONE 9 (1), 1–10. doi:10.1371/journal.pone.0087462
Keywords: authentication, identification, fingerprinting, Marantodes pumilum, quality control
Citation: Ibrahim IS, Mohd Said M, Mohammad Zainoor N and Jamal JA (2022) Authentication of Marantodes pumilum (Blume) Kuntze: A Systematic Review. Front. Pharmacol. 13:855384. doi: 10.3389/fphar.2022.855384
Received: 15 January 2022; Accepted: 27 April 2022;
Published: 08 June 2022.
Edited by:
Abdul Rohman, Gadjah Mada University, IndonesiaReviewed by:
Faezah Mohd Salleh, University of Technology Malaysia, MalaysiaKwabena F.M. Opuni, University of Ghana, Ghana
Copyright © 2022 Ibrahim, Mohd Said, Mohammad Zainoor and Jamal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jamia Azdina Jamal, amFtaWFAdWttLmVkdS5teQ==
 Ida Syazrina Ibrahim
Ida Syazrina Ibrahim Mazlina Mohd Said
Mazlina Mohd Said Noraida Mohammad Zainoor
Noraida Mohammad Zainoor Jamia Azdina Jamal
Jamia Azdina Jamal