- 1Clinical Research Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
- 2Department of Pharmacy, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
The Fuyou (Fy) formula is an in-hospital preparation consisting of traditional Chinese medicine (TCM) that has been used for treating precocious puberty (PP) for more than 20 years. In this study, we aimed to clarify the effect of the Fy formula and its major components on PP. To confirm the effect of the Fy formula on the release of hypothalamic gonadotropin-releasing hormone (GnRH), GT1-7 cells were treated with estrogen to build the model group and subsequently treated with the Fy formula and its major components to explore their effects on the secretion of GnRH. The level of GnRH in GT1-7 cells was determined using enzyme-linked immunosorbent assay. The results illustrated that, compared to the model group, the Fy formula inhibited the release of GnRH. In addition, the expression levels of proteins related to GnRH secretion, including GnRH, gonadotropin-releasing hormone receptor (GnRHR), Kiss-1 metastasis-suppressor (Kiss1), G-protein coupled receptor 54 (GPR54), estrogen receptor α (ERα), insulin-like growth factor-1 (IGF-1), and insulin-like growth factor-1 receptor (IGF-1R), were detected by real-time polymerase chain reaction (RT-qPCR). The results demonstrated that the Fy formula significantly reduced the level of GnRH secretion in the GT1-7 cell lines compared with the model group. Moreover, it significantly downregulated the expression of GnRH, GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R. In summary, our results indicate that the Fy formula and its major components may inhibit the effects of estrogen, which alleviates PP through transcriptional regulation of target genes.
Introduction
Precocious puberty (PP) is an endocrine disorder that is defined as puberty starting before the age of 9 in boys and eight in girls. Observational data from Europe show that breast development begins before the age of 8 years in 5% of girls. In China, the incidence of PP is approximately 1/50,000–10,000 population, and the male-to-female ratio is approximately 1:5–10 (Dong et al., 2019). The causes of pathological PP are normally categorized into central precocious puberty (CPP) and peripheral precocious puberty (PPP). Idiopathic CPP is the most common form of CPP, originating from the early activation of the hypothalamic-pituitary-gonadal (HPG) axis with pulsatile secretion of hypothalamic gonadotropin-releasing hormone (GnRH). Approximately 74% of girls with CPP have the idiopathic form (Bradley et al., 2020). CPP may also occur secondary to tumors involving the hypothalamus and congenital defects in neuronal migration, resulting in a heterotopic mass of GnRH-secreting neurons acting as an ectopic GnRH pulse generator. In the remaining situations, disruption of a normal inhibitory restraint on the onset of puberty is caused by an extensive variety of insults to the central nervous system (CNS) (Eugster and Pescovitz, 2001). These include hypothalamic tumors, cerebral malformations involving the hypothalamus, and congenital brain disorders, infections, or acquired injuries. PPP is often related to increased sex steroid levels independent of GnRH. It can be caused by virilizing tumors, including adrenal tumors, gonadal tumors, or human chorionic gonadotropin (hCG)-secreting germ cell tumors. Neoclassic tissues can then lead to an increase in androgen or estrogen production (Latronico et al., 2016). Familial male precocious puberty (FMPP) is caused by an activating mutation in the luteinizing hormone (LH) receptor gene. The activating mutation leads to the continuous activation of adenylate cyclase, resulting in gonadal autonomic hyperfunction. Congenital adrenal hyperplasia may lead to the excessive production of adrenal androgens. McCune-Albright syndrome and recurrent autonomous ovarian cysts caused by somatic activating mutations in the GNAS gene lead to an increase in the signal transduction of the GnRH signaling pathway. Children with PPP can easily develop CPP due to their early bone age and long-term hyperestrogenemia (Carel and Léger, 2008).
Puberty onset is thought to integrate diverse genetic and environmental signals (Leka-Emiri et al., 2017). The hypothalamic secretion of GnRH has been established as a pivotal pathway for initiating puberty onset. The synthesis and secretion of GnRH neurons in the hypothalamus are essential for the regulation of hormonal cascade effects, including pituitary gonadotropin release, ovarian maturation, and estrogen production. All of these hormonal events are necessary for normal sexual maturation and reproductive function. The release of GnRH activates the synthesis and secretion of LH and follicle-stimulating hormone (FSH) from the anterior pituitary, thus leading to the stimulation of gonadal function (Carel et al., 2004). Briefly, LH initiates the growth and ovulation of the corpus luteum in girls and release of androgen in boys. FSH mediates the formation and maturation of ovarian follicles in girls and spermatogenesis in boys, inducing secondary sexual characteristics (Kanasaki et al., 2017). Reproductive control of the HPG axis also facilitates negative gonadal feedback. GnRH is not the only hormone involved in puberty onset but is the most important factor identified to date. Therefore, the regulation of GnRH secretion and expression is critical for the pathogenesis of PP.
The pharmacological therapy for PP includes GnRH analogs (GnRHas), GnRH antagonists, and traditional Chinese medicine (TCM) Fy formula. GnRHas are the gold-standard management for CPP, as they provide continuous stimulation of pituitary gonadotrophs, resulting in the downregulation of the HPG axis and thus leading to decreased secretion of LH and FSH (Eugster and Pescovitz, 2001). Numerous studies have demonstrated that the use of GnRHas results in the stabilization of pubertal symptoms. Local side effects include pain at the injection site, sterile abscesses, and implant site reactions (Aguirre and Eugster, 2018). Other side effects include headache, hot flashes, decreased bone density, and vaginal bleeding (Fuqua, 2013). Several GnRHas have been synthesized and are currently under investigation in clinical trials. They exhibit high-affinity binding to the human GnRH receptor (GnRHR), leading to a rapid decrease in gonadal sex steroids to castrate levels; however, the detailed mechanism for this is still under investigation (M. Chen and Eugster, 2015).
TCM treating PP includes Zhibai Dihuang wan, Dabuyin wan and Fy formula. Zhibai Dihuang wan and Dabuyin wan were applied to yin deficiency, fire hyperactivity syndrome, phlegm dampness stagnation syndrome, liver depression and fire transformation syndrome. However, the recommendation level for the use of these two medicines are low, and there is no indication in the drug instruction. At present, there is no Chinese patent medicine with definite curative effect for treating PP are commercially available. The Fy formula is an in-hospital preparation used at Beijing Children’s Hospital, and it is composed of TCM and was developed by pediatric gynecologists according to the pathogenesis, etiology, and physical characteristics of children with PP. It was approved by the National Medical Products Administration (NMPA) as a compound preparation for TCM in hospitals in 2001. In the study of Fy formula treatment of 60 female with PP, it has been showed that the total effective rate is 83.3%. The changes of 60 cases before and after treatment were breast nucleus index, blood E2 level, number of positive cases of vaginal cell smear, bone age, uterine and follicular volume. The treatment can improve the symptoms of liver depression, yin deficiency and fire hyperactivity, reduce the level of estrogen and delay the speed of bone age maturation (Liu et al., 2009). It has also been reported that the Fy formula is able to regulate early pubertal symptoms, reducing the size of the mammary nucleus and effectively controlling estrogen levels and bone age. It has also been shown that the Fy formula exerts an inhibitory effect on female ovarian cysts complicated by PP, leading to a reduction in E2 levels and postponing the rate of bone maturation with no evident adverse effects (Pan et al., 2019).
It has been showed that the Fy formula induces downregulation of the mRNA expression of kiss1, GPR54, and GnRH in female rats (Bai et al., 2020). A previous integrated pharmacological study on the mechanism of Fy formula in treating PP demonstrated that it can effectively reduce the levels of FSH, LH, and E2 in Sprague-Dawley rats (Guo et al., 2021b). Also, the TCM-chemical component-target-pathway study based on integrated pharmacology illustrated that ERα, ERβ, IGF, and IGF1 are associated with PP, so these can be potential therapeutic targets for PP (Guo et al., 2021a). Therefore, in this study, we aimed to explore the effects of the Fy formula on the secretion of GnRH and expression of related genes in the treatment of PP.
Materials and Methods
Materials and Reagents
Dulbecco’s modified Eagle medium (DMEM) was purchased from Corning (NY, United States). Fetal bovine serum (FBS) was obtained from Gibco (Grand Island, NY). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO, United States). Penicillin-streptomycin and 0.25% trypsin-EDTA were purchased from MacGene (Beijing, China). Mycoplasma Prevention Reagent (MycAway™) was obtained from Yeasen Biotech (Hong Kong, China). Phosphate-buffered saline (PBS) was purchased from Solarbio (Beijing, China).
The TCM standards estradiol (E2, serial number: 100,182-21,906, purity: 96.3%), quercetin (serial number: 100081-201610, purity: 99.90%), and luteolin (serial number: 111520-202006, purity: 94.40%) were purchased from the National Institutes for Food and Drug Control (Beijing, China). Apigenin (serial number: B20981-20 mg, purity: 98.00%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). The reagents were dissolved in DMSO (Sigma-Aldrich) and stored at 4°C.
Fy Formula Preparation
The Fy formula is an in-hospital preparation obtained by mixing the following 12 herbs: Prunella vulgarism L (Xiakucao), Carapax Trionycis (Cubiejia), Gentiana scabra Bunge (Longdan), Chrysanthemum morifolium (Ramat.) Hemsl (Juhua), Lycium chinense Mill (Digupi), Alisma plantago-aquatica L (Zexie), Scrophularia ningpoensis Hemsl (Xuanshen), Paeonia suffruticosa Andrews (Mudanpi), Rehmannia glutinosa (Gaertn.) DC (Shengdihunag), Hordeum vulgare L (Maiya), Concha oetreae (Muli), and Thallus laminariae (Kunbu) at the ratio of 1.5:1:0.6:0.6:1:1:1.5:0.6:1.2:2:3:1. All herbs were purchased from the Beijing Bencao Fangyuan Pharmaceutical Group Co. Ltd., and the Fy formula was prepared by the Preparation Center of Beijing Children’s Hospital (approval number: Z20053679; lot number: 20201202).
Cell Cultures
The GT1-7 cell line (mouse GnRH neuronal cell line) was kindly provided by Prof. P. Mellon (University of California, San Diego, CA, United States). GT1-7 cells were grown in a monolayer culture in DMEM (Corning) supplemented with 10% FBS (Gibco), 1% penicillin-streptomycin (Macgene), and 0.5% Mycoplasma Prevention Reagent (Yeasen Biotech). The cultures were incubated at 37°C in an atmosphere of 5% CO2 in a 25 mm flask (Corning) for 2 days after seeding, with a medium change at 24 h. The cells were then washed twice with PBS and digested with 0.25% trypsin-EDTA (MacGene). When more than half of the cells were observed to become round under a microscope, serum-containing medium was added to terminate the digestion. After obtaining a single-cell suspension, the cells were cultured in an incubator and inoculated three times for subsequent experiments. The cells were treated with different treatments in serum-free medium (SFM) for 24 h depending on the experiment.
CCK-8 Assay
Cell counting kit-8 (CCK-8) was purchased from Solarbio (Beijing, China). To assay the toxicity of the Fy formula, quercetin, luteolin, apigenin, and GT1-7 cells were seeded in 96-well plates at a density of 2.0×105 cells/well. After 24 h of incubation, the cells were pretreated with 100 pmol/L E2 in SFM overnight. The cells were then incubated with 75, 150, 225, 300, 450, and 525 μg/ml of Fy formula or 5, 10, 15, 20, 30, and 40 μmol/ml of quercetin, luteolin, and apigenin separately in SFM for 24 h. Subsequently, the cells were treated with 10 μL of CCK-8 solution (Gibco) for 1 h at 37°C and 5% CO2. The optical density (OD) was determined by measuring the absorbance at 450 nm using a microplate reader (BioTek Synergy, United States).
ELISA
To determine the concentration of GnRH, GT1-7 cells were seeded in 24-well plates. The cells were treated with E2, Fy, quercetin, luteolin, and apigenin, as previously described. After treatment, the supernatants were collected. Relative GnRH concentrations in the supernatant were determined using the mouse GnRH ELISA kit from mlbio (Shanghai, China) with serial dilutions of 80, 40, 20, 10, 5, and 0 mIU/mL as a standard curve.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
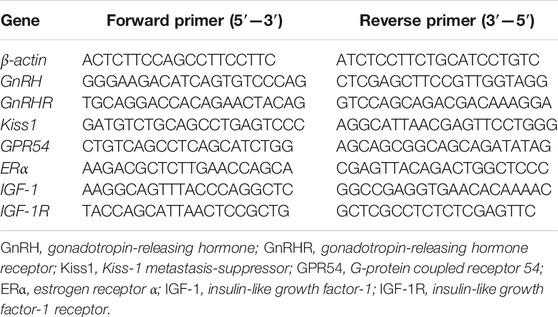
The cells were seeded in 6-well plates and treated with E2, Fy, and TCM, as described previously. At the end of the treatment, total RNA was extracted from GT1-7 cells using an RNA Easy Fast Tissue/Cell Kit (TIANGEN BIOTECH Co., Ltd, Beijing, China) according to the manufacturer’s protocol. The RNA samples were quantified using a microplate reader (BioTek Synergy, United States) at 260/280 nm. First-strand cDNA was prepared using 2 μg RNA reverse-transcribed with a FastKing RT Kit (TIANGEN BIOTECH Co., Ltd, Beijing, China). The synthesized first-strand cDNA was stored at −80°C until use. mRNA expression was analyzed using a 7500 Fast Real-Time PCR system (Applied Biosystems, United States) with the following thermocycling conditions: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 60 s. Single-stranded oligonucleotide primer sets were designed (Tianyi Huiyuan, Beijing, China) to target β-actin, Erα, Kiss1, GPR45, GnRH, GnRHR, IGF1, and IGF1R. The primer sequences used for qRT-PCR are listed in Table 1. Data were analyzed using the 2ΔΔCt method, and mRNA expression was normalized to that of β-actin.
Statistical Analysis
All experiments were performed in triplicate independently and data were expressed as mean ± SD. Significant differences were analyzed by one-way analysis of variance (ANOVA), and the data were plotted using Prism7 software (GraphPad software, Inc., San Diego, United States). Statistical significance was determined using p < 0.05.
Results
Composition Analysis of the Fy Formula
The TCM components of the Fy formula are listed in Table 2. Prunella vulgaris L. and Carapax trionycis exert effects on the liver that can nourish yin, moderate heat, and relieve congestion. Gentiana scabra Bunge, Chrysanthemum morifolium (Ramat.) Hemsl, Lycium chinense Mill, Alisma plantago-aquatica L., Scroophularia ningpoensis Hemsl, Paeonia suffruticosa Andrews, and Rehmannia glutinosa (Gaertn.) DCs can nourish yin, eliminate dampness, and cool blood. Hordeum vulgare L, Concha oetreae and Thallus laminariae act on the liver to relieve congestion and are used as adjuvants. As previously reported, five compounds were recognized with the HPLC-MS/MS method from Fy formula including Luteolin, Quercetin, Apigenin, Kaempferol and Emodin. Also, the concentration of these five target components in Fy Formula were determined using preliminary LC-MS/MS method, the concentration of Luteolin, Quercetin and Apigenin are much higher than Kaempferol and Emodin in Fy formula. Therefore, the compounds with higher concentration were selected in the experiment. (Guo et al., 2021a). We aimed to determine the effects of the Fy formula and its major components, including luteolin, quercetin, and apigenin, on GnRH secretion and related gene expression in PP treatment.
Effects of Luteolin, Apigenin, Quercetin, and the Fy Formula on the Proliferation of GT1-7 Cells
The nontoxic concentrations of Fy and its major chemical components in GT1-7 cells were evaluated based on cell viability. The CCK-8 assay was performed to examine the proliferation of GT1-7 cells following treatment with different concentrations of luteolin, apigenin, quercetin, and Fy. As demonstrated in Figure 1, the GT1-7 cells treated with high concentrations of the Fy formula and its major components showed reduced cell proliferation activity compared to the control group cells. The cell proliferation activity of the GT1-7 cells was not significantly different at concentrations of 5 and 10 μM for luteolin, apigenin, and quercetin and concentrations of 450 μg/ml and 525 μg/ml for the Fy formula compared to the control group. To ensure that the Fy formula was administered at a concentration sufficient to exert the desired effect, the final concentrations of 10 μM and 525 μg/ml were selected for use in subsequent experiments.
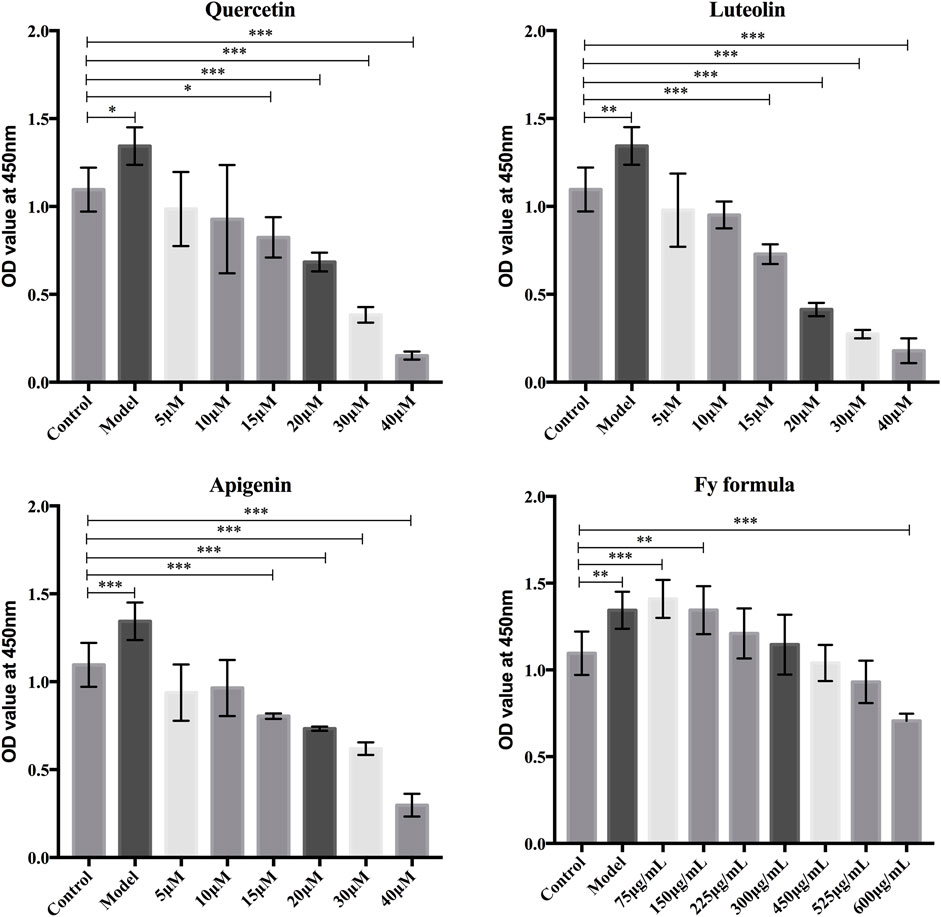
FIGURE 1. Effects of luteolin, apigenin, quercetin, and the Fy formula, at different concentrations, on GT1-7 cells. The CCK-8 assay was conducted to determine cell proliferation in the GT1-7 cells after treatment with luteolin, apigenin, quercetin, and the Fy formula at different concentrations.
The Fy Formula, Luteolin, Apigenin, and Quercetin Inhibit GnRH Secretion in GT1-7 Cells
After pretreatment of the GT1-7 cells with E2, ELISA was performed to determine the level of GnRH in the GT1-7 cells. The GnRH concentration in the culture medium is shown in Figure 2. Comparing the model and control groups, the E2 treatment resulted in an increase in GnRH secretion. Moreover, treatment with the Fy formula, luteolin, apigenin, and quercetin led to a significant reduction in the concentration of GnRH in the culture medium and thus inhibited the increased level of GnRH secretion caused by E2 in GT1-7 cells.
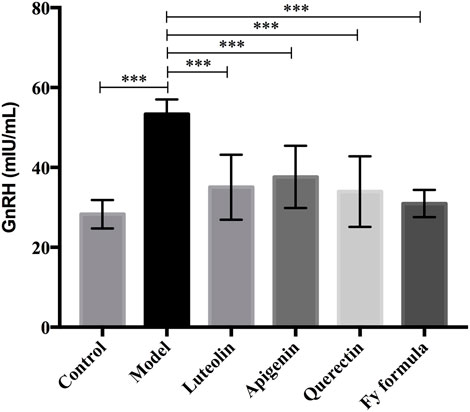
FIGURE 2. Effects of luteolin, apigenin, quercetin, and the Fy formula on GnRH secretion. Cells pretreated with E2 stimulate the secretion of GnRH, and the level of GnRH secretion decreases when coincubated with luteolin, apigenin, quercetin, and the Fy formula. ***p < 0.0001 compared to the model group.
The Fy Formula, Luteolin, Apigenin, and Quercetin Inhibit GnRH, GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R Expression in GT1-7 Cells
To further analyze whether the potential molecular mechanism of the Fy formula on GnRH secretion in GT1-7 cells is via the GnRH receptor, E2 receptor, Kiss1/GPR54 signaling pathway, or IGF-1, the mRNA expression levels of these genes were quantified by RT-qPCR (Figure 3). GT1-7 cells treated with E2 showed significantly upregulated gene expression of GnRH, GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R. In contrast, GT1-7 cells treated with the Fy formula, luteolin, apigenin, and quercetin showed downregulated expression of all genes involved in GnRH secretion. These results indicate that the effect of the Fy formula is mediated by inhibiting the expression of GnRH itself, as well as GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R, which are related to GnRH secretion.
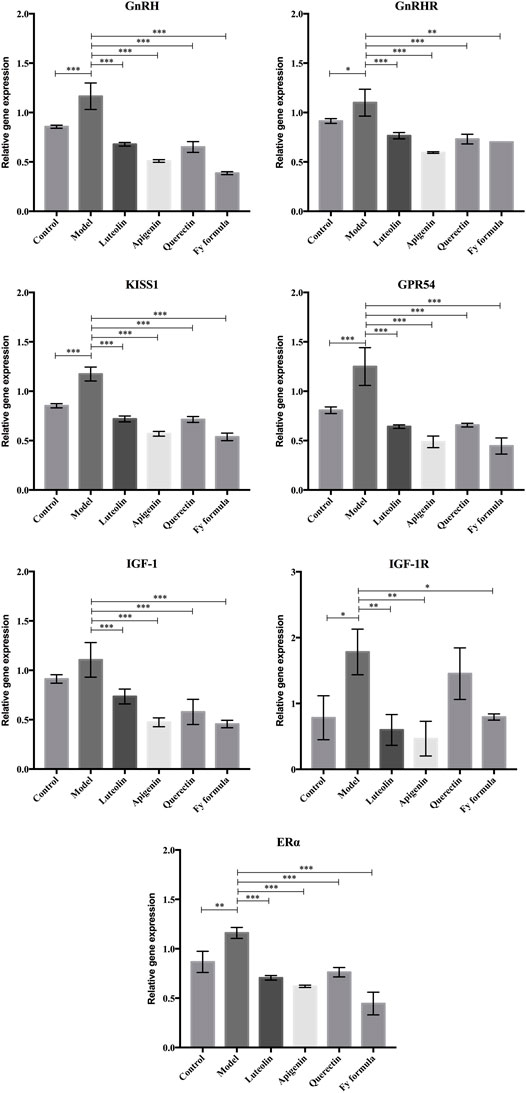
FIGURE 3. Effects of luteolin, apigenin, quercetin, and the Fy formula on the expression of GnRH, GnRHRc, Kiss1, GPR54, ERα IGF-1, and IGF-1R, which are involved in GnRH secretion. *p < 0.05, **p < 0.001, and ***p < 0.0001 compared to the model group.
Discussion
In our previous study, five major chemical components of the Fy formula were identified using HPLC-MS/MS: luteolin, quercetin, apigenin, kaempferol, and emodin (Guo et al., 2021b). Three compounds with higher concentration were selected in the experiment including Luteolin, Quercetin and Apigenin. The association of ERα, ERβ, IGF, and IGF1 with PP are identified by “TCM-chemical component-target-pathway” study based on integrated pharmacology, which indicated that these proteins can be the potential targets for treating PP (Guo et al., 2021b). Moreover, it has been reported that the treatment with Fy formula can result in a significantly reduction in the level of E2, LH and FSH in Sprague-Dawley rats (Guo et al., 2021b). Furthermore, it has been illustrated that the Fy formula is able to downregulate the expression of Kiss1, GPR54, and GnRH in female rats (Bai et al., 2020).
As an in-hospital preparation at Beijing Children’s Hospital, the Fy formula has been used for the treatment of PP for more than 20 years. Clinical data illustrated that the Fy formula significantly reduced the level of estrogen in the blood serum of patients. It can also delay bone maturation and decrease the mammary gland size in women with PP. Furthermore, TCM research has revealed that the herbs used in the preparation of the Fy formula have intervention effects on ovarian cysts in girls complicated with PP. Taken together, these findings indicate the clinical benefits of the Fy formula, which is an advantageous therapeutic approach owing to its low cost. However, the mechanism of action of the Fy formula in treating PP has not been fully clarified.
GT1-7 cells are a valuable GnRH-expressing cell model with a number of characteristics common to normal GnRH neurons. In addition, GT1-7 cells express a number of genes relevant to reproduction, circadian rhythm, and energy homeostasis, including GnRH, Kiss1, and GPR54. Estrogen has been proven to be the main regulator of GnRH neuronal function in the female brain, which possesses a bimodal effect on the hypothalamic–pituitary axis. In GT1-7 cells, estrogen exerts a stimulatory effect at low concentrations (Qian et al., 2020) and an inhibitory effect at high concentration on the secretion of GnRH and gonadotropin (Roy et al., 1999). It has been suggested that the binding sites for E2 in the plasma membrane of GT1-7 cells share structural homology with classical estrogen receptors (ERs) at their carboxy-terminal domain (Morales et al., 2007). To investigate the effects of the Fy formula on GnRH secretion and related gene expression in PP treatment, we incubated GT1-7 cells with E2 (100 pmol/L) for 24 h, followed by the Fy formula, luteolin, apigenin, or quercetin for another 24 h. Our results showed that E2 treatment increased the release of GnRH from the GT1-7 cells and established a PP model in GT1-7 cells. However, the level of GnRH in the GT1-7 cells was decreased by treatment with the Fy formula, luteolin, apigenin, and quercetin compared to that in the model group. This suggests that the Fy formula can significantly reduce GnRH secretion.
To determine the role of potential targets associated with GnRH secretion in the effects of the Fy formula on PP, gene expression was analyzed. Our results showed that GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R mRNA levels were higher in the model group than in the control group. We also found that, compared to the model group, the Fy formula significantly downregulated the expression of GnRH secretion-related genes, including GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R, in GT1-7 cells. Taken together, these results indicate that the Fy formula and its major components, including luteolin, apigenin, and quercetin, are able to inhibit GnRH secretion in GT1-7 cells by inhibiting the expression of related genes, weakening the binding between signaling molecules and their receptors, and ultimately reducing the pulsed secretion of GnRH, thus reducing the activation of downstream pituitary and gonadal development and alleviating the symptoms of PP.
GnRH is a decapeptide that serves as a vital element in the regulation of the reproductive cycle and sexual maturation. GnRH drives the release of pituitary gonadotropic hormones, including LH, FSH, and gonadotropin, by interacting with pituitary gonadotropes through binding to its high-affinity receptor GnRHR on the cell surface (Tzoupis et al., 2020). GnRHR belongs to the G protein-coupled receptor family (GPCRs) that is characterized by seven transmembrane domains. It has been demonstrated that GnRHR gene expression is dependent on GnRH pulse in rat pituitary cultures, with increased mRNA expression levels being observed under high pulse frequency. In response to varying GnRH pulses, GnRHR appears to differentially activate multiple distinct signaling pathways implicated in the synthesis of both LH and FSH (Stamatiades and Kaiser, 2018). In our study, it was shown that the Fy formula and its major components could significantly suppress the expression of both GnRH and GnRHR, indicating that the Fy formula may delay pituitary gonadotropic hormone release and alleviate PP.
Recent research has demonstrated that signaling by kisspeptin through its receptor, G protein-coupled receptor GPR54 (also called Kiss1R), is the most potent stimulator of GnRH-induced gonadotropin release (Chan et al., 2009 and; Blaustein, 2010). Kisspeptin is encoded by the KISS1 gene, which contacts GnRH neurons within the hypothalamus and induces GnRH release by binding to its receptor, GPR54 (Mayer and Boehm, 2011). Subsequently, GnRH reaches the pituitary gland through portal circulation and initiates the secretion of pituitary gonadotropins (Trevisan et al., 2018). Kisspeptin treatment in immature rodents and primates was able to induce activation of the gonadotropic axis and precocious pubertal development (Kanasaki et al., 2017). Serum kisspeptin-54 levels were higher in girls with CPP than in prepubescent controls, implying that kisspeptin secretion may stimulate the onset of puberty (C.-Y. Chen et al., 2013). It has also been reported that in GPR54-overexpressing GT1-7 cells, intracellular signaling, such as extracellular signal-regulated kinase (ERK) activation and protein kinase A (PKA) signaling pathways, were activated, resulting in increased GnRH receptor expression in response to kisspeptin (Kanasaki et al., 2017 and; Kang et al., 2009). Therefore, the development of kisspeptin antagonists may be a new approach for treating PP. In our results, we found that the Fy formula can target kisspeptin and its receptor GPR54 by suppressing their mRNA expression, thus inhibiting their activity.
IGF-1 is an important somatotropic hormone that mediates the regulation of the reproductive axis. In addition, it has emerged as a prime candidate for having a significant role in the onset of puberty. IGF-1 may promote the secretion of prepubertal GnRH, and the level of IGF-1 increases in the circulation as puberty approaches, which can advance the timing of puberty (Dees et al., 2021). Multiple findings suggested that IGF-1 may prime pituitary gonadotrophs and stimulate the synthesis of GnRHR and FSH during puberty onset in prepubertal salmon. Also, IGF-1 enhances pituitary gonadotropic hormone release, which accelerates puberty onset in rats (Luckenbach et al., 2010). More recent findings have depicted a later action of IGF-1 in regulating the synthesis and release of kisspeptin. It has been reported that IGF-1 activates kiss-1 in female rats, expressing kisspeptins that are involved in the secretion of pituitary gonadotropins at puberty, as previously described (Hiney et al., 2009). Furthermore, girls with CPP have remarkably higher levels of IGF-1 and insulin than healthy girls (Sørensen et al., 2012 and; Kanety et al., 1996). Our research on GT1-7 cell lines further corroborated that the Fy formula appears to downregulate the expression of IGF-1 and its receptor, inhibiting their activity in pubertal development and hence relieving PP.
With respect to the E2, several studies have reported that it alters pulsatile GnRH secretion through the binding and activation of ERs (Thomas and Dong, 2006). The ER is a member of the nuclear receptor superfamily that participates in the transcriptional regulation of multiple genes. The classic ER signaling pathway is initiated by the binding of estrogen to its receptor. Two isoforms of ER have been described: ERα and ERβ. This leads to receptor dimerization and subsequent combination with the estrogen response element located on the promoter of target genes, which finally activates gene transcription (Radovick et al., 2012). ERα may contribute to the feedback regulation of kisspeptin expression during pubertal development (Clarkson, 2013), which is associated with the restraint of GnRH release prior to puberty onset, followed by enhanced initiation of GnRH secretion and thus prompt reproductive maturation throughout puberty (Mayer et al., 2010). In contrast, ERβ may directly participate in estrogen regulation by regulating neuronal activity, gene expression, and pulsatile secretion of GnRH (Wolfe and Wu, 2012 and; Fixemer et al., 2003). Our research suggests that the Fy formula can inhibit this effect, demonstrating that the gene expression levels of both ERα and kiss-1 are repressed by the TCM components of the Fy formula.
However, this study remains few limitations that need to be considered. First of all, the effects of Fy formula against PP have been identified in vitro, the effects of Fy formula in treating PP in vivo still need to be verified in the future by comparing the level of GnRH secretion and gene expression before and after the treatment of Fy formula. Secondly, the mechanism of protein interactions in treating PP still need to be further clarified.
Conclusion
In conclusion, we have shown evidence of inhibitory effect of Fy formula in GnRH secretion in GT1-7 cell lines and have shown that Fy formula down regulates GnRH gene expression in vitro. The Fy formula also suppresses the expression level in all genes that involved in the GnRH secretion including GnRHR, Kiss1, GPR54, ERα, IGF-1, and IGF-1R, and hence delay the pituitary gonadotropic hormone release and alleviating the symptoms of PP.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
CG designed and performed the experiments. YZ performed the experiments and wrote the manuscript. MZ designed the primers used. NS provided technical support. QD analyzed the data. YY and HH helped construct the illustrations. QW and YL revised the manuscript.
Funding
This study was supported by the Beijing Traditional Chinese Medicine Science and Technology Development Fund Project (grant no. QN-2020-26).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Prof. P. Mellon and Prof. T. Feng for supplying the GT1-7 cell lines.
References
Aguirre, R. S., and Eugster, E. A. (2018). Central Precocious Puberty: From Genetics to Treatment. Best Pract. Res. Clin. Endocrinol. Metab. 32 (4), 343–354. doi:10.1016/j.beem.2018.05.008
Bai, G. L., Hu, K. L., Huan, Y., Wang, X., Lei, L., Zhang, M., et al. (2020). The Traditional Chinese Medicine Fuyou Formula Alleviates Precocious Puberty by Inhibiting GPR54/GnRH in the Hypothalamus. Front. Pharmacol. 11, 596525. doi:10.3389/fphar.2020.596525
Blaustein, J. D. (2010). The Year in Neuroendocrinology. Mol. Endocrinol. 24 (1), 252–260. doi:10.1210/me.2009-0350
Bradley, S. H., Lawrence, N., Steele, C., and Mohamed, Z. (2020). Precocious Puberty. BMJ 368, l6597. doi:10.1136/bmj.l6597
Carel, J. C., Lahlou, N., Roger, M., and Chaussain, J. L. (2004). Precocious Puberty and Statural Growth. Hum. Reprod. Update 10 (2), 135–147. doi:10.1093/humupd/dmh012
Carel, J. C., and Léger, J. (2008). Clinical Practice. Precocious Puberty. N. Engl. J. Med. 358 (22), 2366–2377. doi:10.1056/NEJMcp0800459
Chan, Y. M., Broder-Fingert, S., Wong, K. M., and Seminara, S. B. (2009). Kisspeptin/Gpr54-independent Gonadotrophin-Releasing Hormone Activity in Kiss1 and Gpr54 Mutant Mice. J. Neuroendocrinol 21 (12), 1015–1023. doi:10.1111/j.1365-2826.2009.01926.x
Chen, C. Y., Chou, Y. Y., Wu, Y. M., Lin, C. C., Lin, S. J., and Lee, C. C. (2013). Phthalates May Promote Female Puberty by Increasing Kisspeptin Activity. Hum. Reprod. 28 (10), 2765–2773. doi:10.1093/humrep/det325
Chen, M., and Eugster, E. A. (2015). Central Precocious Puberty: Update on Diagnosis and Treatment. Paediatr. Drugs 17 (4), 273–281. doi:10.1007/s40272-015-0130-8
Clarkson, J. (2013). Effects of Estradiol on Kisspeptin Neurons during Puberty. Front. Neuroendocrinol 34 (2), 120–131. doi:10.1016/j.yfrne.2013.02.002
Dees, W. L., Hiney, J. K., and Srivastava, V. K. (2021). IGF-1 Influences Gonadotropin-Releasing Hormone Regulation of Puberty. Neuroendocrinology 111 (12), 1151–1163. doi:10.1159/000514217
Dong, G., Zhang, J., Yang, Z., Feng, X., Li, J., Li, D., et al. (2019). The Association of Gut Microbiota with Idiopathic Central Precocious Puberty in Girls. Front. Endocrinol. (Lausanne) 10, 941. doi:10.3389/fendo.2019.00941
Eugster, E. A., and Pescovitz, O. H. (2001). Advances in the Treatment of Precocious Puberty. Expert Opin. Investig. Drugs 10 (9), 1623–1630. doi:10.1517/13543784.10.9.1623
Fixemer, T., Remberger, K., and Bonkhoff, H. (2003). Differential Expression of the Estrogen Receptor Beta (ERbeta) in Human Prostate Tissue, Premalignant Changes, and in Primary, Metastatic, and Recurrent Prostatic Adenocarcinoma. Prostate 54 (2), 79–87. doi:10.1002/pros.10171
Fuqua, J. S. (2013). Treatment and Outcomes of Precocious Puberty: an Update. J. Clin. Endocrinol. Metab. 98 (6), 2198–2207. doi:10.1210/jc.2013-1024
Guo, C., Sun, N., Hu, K., Bai, G., Zhang, M., Wang, Q., et al. (2021b). Integrated Pharmacological Analysis on the Mechanism of Fuyou Formula in Treating Precocious Puberty. Front. Pharmacol. 12, 649732. doi:10.3389/fphar.2021.649732
Guo, C., Liu, J., Zhang, M., Wang, Q., Ding, Q., and Zhao, L. (2021a). Study on the "traditional Chinese Medicine - Chemical Component - Target - Pathway" of Fuyou Mixture in the Treatment of Precocious Puberty Based on Integrated Pharmacology. Chin. J. Clin. Pharmacol. 37 (5), 572–575.
Hiney, J. K., Srivastava, V. K., Pine, M. D., and Les Dees, W. (2009). Insulin-like Growth Factor-I Activates KiSS-1 Gene Expression in the Brain of the Prepubertal Female Rat. Endocrinology 150 (1), 376–384. doi:10.1210/en.2008-0954
Kanasaki, H., Oride, A., Mijiddorj, T., Sukhbaatar, U., and Kyo, S. (2017). How Is GnRH Regulated in GnRH-Producing Neurons? Studies Using GT1-7 Cells as a GnRH-Producing Cell Model. Gen. Comp. Endocrinol. 247, 138–142. doi:10.1016/j.ygcen.2017.01.025
Kanety, H., Karasik, A., Pariente, C., and Kauschansky, A. (1996). Insulin-like Growth Factor-I and IGF Binding Protein-3 Remain High after GnRH Analogue Therapy in Girls with central Precocious Puberty. Clin. Endocrinol. (Oxf) 45 (1), 7–12. doi:10.1111/j.1365-2265.1996.tb02053.x
Kang, K., Lee, S. B., Jung, S. H., Cha, K. H., Park, W. D., Sohn, Y. C., et al. (2009). Tectoridin, a Poor Ligand of Estrogen Receptor Alpha, Exerts its Estrogenic Effects via an ERK-dependent Pathway. Mol. Cell 27 (3), 351–357. doi:10.1007/s10059-009-0045-8
Latronico, A. C., Brito, V. N., and Carel, J. C. (2016). Causes, Diagnosis, and Treatment of central Precocious Puberty. Lancet Diabetes Endocrinol. 4 (3), 265–274. doi:10.1016/s2213-8587(15)00380-0
Leka-Emiri, S., Chrousos, G. P., and Kanaka-Gantenbein, C. (2017). The Mystery of Puberty Initiation: Genetics and Epigenetics of Idiopathic central Precocious Puberty (ICPP). J. Endocrinol. Invest. 40 (8), 789–802. doi:10.1007/s40618-017-0627-9
Liu, H., Liu, J., and Liu, G. (2009). Clinical Study on 60 Cases of True Precocious Puberty in Girls Treated with Fuyou Mixture. Beijing Traditional Chin. Med. 28 (8), 596525. doi:10.3389/fphar.2020.596525
Luckenbach, J. A., Dickey, J. T., and Swanson, P. (2010). Regulation of Pituitary GnRH Receptor and Gonadotropin Subunits by IGF1 and GnRH in Prepubertal Male Coho salmon. Gen. Comp. Endocrinol. 167 (3), 387–396. doi:10.1016/j.ygcen.2009.09.010
Mayer, C., Acosta-Martinez, M., Dubois, S. L., Wolfe, A., Radovick, S., Boehm, U., et al. (2010). Timing and Completion of Puberty in Female Mice Depend on Estrogen Receptor Alpha-Signaling in Kisspeptin Neurons. Proc. Natl. Acad. Sci. U S A. 107 (52), 22693–22698. doi:10.1073/pnas.1012406108
Mayer, C., and Boehm, U. (2011). Female Reproductive Maturation in the Absence of kisspeptin/GPR54 Signaling. Nat. Neurosci. 14 (6), 704–710. doi:10.1038/nn.2818
Morales, A., Gonzalez, M., Marin, R., Diaz, M., and Alonso, R. (2007). Estrogen Inhibition of Norepinephrine Responsiveness Is Initiated at the Plasma Membrane of GnRH-Producing GT1-7 Cells. J. Endocrinol. 194 (1), 193–200. doi:10.1677/joe-06-0001
Pan, Y., Liu, J., and Liu, H. (2019). Clinical Observation on Treatment of Ovarian Cyst Complicated with Precocious Puberty in Girls with Chinese Herbal Medicine Fuyou Mixture. Beijing J. Trad. Chin. Med. 38 (07), 700–703. doi:10.16025/j.1674-1307.2019.07.021
Qian, F., Shi, N., and Zhou, H. (2020). Estrogen Can Promote the Expression of Genes Related to Precocious Puberty in GT1-7 Mouse Hypothalamic GnRH Neuronal Cell Line via Activating G Protein-Coupled Estrogen Receptor. Gen. Physiol. Biophys. 39 (1), 27–36. doi:10.4149/gpb_2019049
Radovick, S., Levine, J. E., and Wolfe, A. (2012). Estrogenic Regulation of the GnRH Neuron. Front. Endocrinol. (Lausanne) 3, 52. doi:10.3389/fendo.2012.00052
Roy, D., Angelini, N. L., and Belsham, D. D. (1999). Estrogen Directly Respresses Gonadotropin-Releasing Hormone (GnRH) Gene Expression in Estrogen Receptor-Alpha (ERalpha)- and ERbeta-Expressing GT1-7 GnRH Neurons. Endocrinology 140 (11), 5045–5053. doi:10.1210/endo.140.11.7117
Sørensen, K., Aksglaede, L., Petersen, J. H., Andersson, A. M., and Juul, A. (2012). Serum IGF1 and Insulin Levels in Girls with normal and Precocious Puberty. Eur. J. Endocrinol. 166 (5), 903–910. doi:10.1530/eje-12-0106
Stamatiades, G. A., and Kaiser, U. B. (2018). Gonadotropin Regulation by Pulsatile GnRH: Signaling and Gene Expression. Mol. Cel Endocrinol 463, 131–141. doi:10.1016/j.mce.2017.10.015
Thomas, P., and Dong, J. (2006). Binding and Activation of the Seven-Transmembrane Estrogen Receptor GPR30 by Environmental Estrogens: a Potential Novel Mechanism of Endocrine Disruption. J. Steroid Biochem. Mol. Biol. 102 (1-5), 175–179. doi:10.1016/j.jsbmb.2006.09.017
Trevisan, C. M., Montagna, E., de Oliveira, R., Christofolini, D. M., Barbosa, C. P., Crandall, K. A., et al. (2018). Kisspeptin/GPR54 System: What Do We Know about its Role in Human Reproduction? Cell Physiol Biochem 49 (4), 1259–1276. doi:10.1159/000493406
Tzoupis, H., Nteli, A., Androutsou, M. E., and Tselios, T. (2020). Gonadotropin-Releasing Hormone and GnRH Receptor: Structure, Function and Drug Development. Curr. Med. Chem. 27 (36), 6136–6158. doi:10.2174/0929867326666190712165444
Keywords: precocious puberty, fuyou formula, GnRH, gene expression, KISS1, ER
Citation: Zhang Y, Sun N, Zhang M, Ding Q, Wang Q, Liang Y, He H, Yang Y and Guo C (2022) Effects of Fuyou Formula on GnRH Secretion and Related Gene Expression in Treating Precocious Puberty. Front. Pharmacol. 13:852550. doi: 10.3389/fphar.2022.852550
Received: 11 January 2022; Accepted: 03 February 2022;
Published: 11 March 2022.
Edited by:
Jian Gao, Shanghai Children’s Medical Center, ChinaReviewed by:
Hui Zhao, Capital Medical University, ChinaMao Chenmei, Children’s Hospital of Soochow University, China
Copyright © 2022 Zhang, Sun, Zhang, Ding, Wang, Liang, He, Yang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyan Guo, Z3VvY2h1bnlhbjIwNUAxNjMuY29t
 Yi Zhang
Yi Zhang Ning Sun1
Ning Sun1 Chunyan Guo
Chunyan Guo