
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 31 March 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.852042
This article is part of the Research Topic Neuropharmacological Targets for Depressive Disorders and Seizures View all 5 articles
Epilepsy and depression are both serious and potentially disabling conditions which often coexist—bidirectional relationship between the two disorders has been observed. Comorbidity between depression and epilepsy can be attributed to: underlying common pathophysiological mechanisms, psychiatric side effect of antiepileptic medications and psychological response to stress in people with chronic, neurological condition. Despite high prevalence of depressive symptoms in patients with epilepsy, current evidence of the effectiveness of antidepressant therapy in this group of patients is very limited. Vortioxetine is an antidepressant with multimodal activity, very good treatment tolerability, low risk of inducing pharmacokinetic interactions, relative safety of treatment in patients with somatic comorbidities, low risk of causing: sedation, sexual dysfunctions and metabolic side effects. Vortioxetine seems to be a promising treatment option for depressed patients with cognitive dysfunctions, anhedonia and anxiety. In this case series, we report nine cases of patients with epilepsy and depressive symptoms treated with vortioxetine. Seven cases are patients with secondary focal and generalized epilepsy and two with unclassified epilepsy. Three patients presented with depressive episode in the course of bipolar disorder and six patients had depressive symptoms due to organic mood disorder. The dose range of vortioxetine was between 10 and 20 mg. In all of the presented cases effectiveness and tolerability of treatment were very good. Remission of depressive symptoms was achieved in all patients. No epilepsy seizures after switch to vortioxetine were observed in seven cases. In two patients seizures occurred during the first months of vortioxetine treatment but this most probably was due to suboptimal antiepileptic treatment—satisfactory seizure control was achieved after optimization of antiepileptic pharmacotherapy. Vortioxetine was discontinued in two of the presented cases due to pregnancy planning. The duration of observation period during vortioxetine therapy ranged from 2 to 48 months. In conclusion, vortioxetine can be a promising treatment option in patients with epilepsy and comorbid depressive symptoms.
Epilepsy is a chronic, heterogenous neurological condition affecting more than 50 million people globally (Elger et al., 2017). It is defined by any of the following: (Elger et al., 2017) At least two unprovoked (or reflex) seizures occurring >24 h apart; (Fisher et al., 2014) one unprovoked (or reflex) seizure and at least 60% probability of further seizures, occurring over the next 10 years; (Josephson et al., 2017) diagnosis of an epilepsy syndrome (Fisher et al., 2014). Psychiatric disorders, especially depression, are considered to be prevalent comorbidities in patients with epilepsy. The risk of developing depression seems to be increased around twofold in epilepsy (compared to the general population) (Tellez-Zenteno et al., 2007; Josephson et al., 2017). Data considering the prevalence of depression in epilepsy patients is inconsistent, with results ranging from around 6% to more than 50% (Bosak et al., 2012; Bosak et al., 2015a; Błaszczyk and Czuczwar, 2016; Elger et al., 2017)—higher prevalence is observed in drug-refractory than in well-controlled epilepsy (Bosak et al., 2012; Garcia et al., 2015; Tao and Wang, 2016) The inter-ictal form is the most common manifestation of depression in epilepsy patients (Bosak et al., 2012; Błaszczyk and Czuczwar, 2016). Clinical presentation of mood disorders in this group of patients can be different from typical depressive episode—symptoms such as irritability, low frustration tolerance, mood lability, anxiety and fatigue are prevalent (Kwon and Park, 2014). Sometimes terms “interictal dysphoric disorder” or “dysthymic-like disorders of epilepsy” are used in the literature to describe mood disorders in epilepsy (Bosak et al., 2012; Tao and Wang, 2016).
Interestingly, bidirectional relationship between depression and epilepsy has been observed. Depression is associated with around 2.5 times increased hazard of epilepsy (Josephson et al., 2017), although some studies reported that the risk of developing epilepsy in patients with a history of depression can be even up to seven-fold higher (compared to the general population) (Kanner, 2009; Bosak et al., 2012; Błaszczyk and Czuczwar, 2016). Furthermore, more than two-fold increase in the incidence of treatment-resistant epilepsy has been observed in patients with preceding depression (Hitiris et al., 2007). Comorbid depression in epilepsy is also associated with reduced quality of life, poorer treatment adherence, premature mortality and higher risk of unemployment (Johnson et al., 2004; Taylor et al., 2011; Fazel et al., 2013; Garcia et al., 2015; Keezer et al., 2016; Henning et al., 2019). Lifetime history of psychiatric disorders may be a predictor of poor outcome after epilepsy surgery (Kanner et al., 2009). It is worth mentioning that people with epilepsy have even 5-fold increased risk of suicide (compared to controls) and this can be mediated by psychiatric disorders (Bosak et al., 2015b; Bosak et al., 2016; Hesdorffer et al., 2016).
Comorbidity between depression and epilepsy can be attributed to different underlying common pathophysiological mechanisms. Some of the suggested ones are: neuroinflammation, structural and functional brain abnormalities (in temporal and prefrontal areas), alterations in neurotransmission (particularly GABA, glutamate, serotonin, noradrenaline, dopamine transmission), hyperactivity in hypothalamic-pituitary-adrenal axis, shared genetic susceptibility (Bosak et al., 2012; Błaszczyk and Czuczwar, 2016; Mazarati and Sankar, 2016; Tao and Wang, 2016). Besides, a few antiseizure medications are known to potentially induce or exacerbate depressive symptoms, e.g., levetiracetam, topiramate, vigabatrin, barbiturates and zonisamide (Bosak et al., 2012; Błaszczyk and Czuczwar, 2016; Chen et al., 2017). Finally, depressive symptoms could potentially be an element of psychological response to stress, stigmatization and isolation due to living with epilepsy (Błaszczyk and Czuczwar, 2016).
Data considering efficacy and safety of antidepressants in treatment of depressive symptoms in epilepsy patients are relatively scarce—only a few randomized controlled trials have been published so far (Elger et al., 2017). According to the Cochrane Review from 2021, current evidence on the effectiveness of treatment with antidepressant medications in patients with epilepsy is very limited (Maguire et al., 2021). Relatively the largest amount of data refers to SSRIs (SSRI—Selective Serotonin Reuptake Inhibitors) which seem to be safe and effective (although heterogeneity of the results is significant) (Mula, 2019). Despite previous fears of a potential seizure-provoking action of antidepressants, data from more thorough analyses indicate that treatment with these medications is rather safe (with exceptions for bupropion, clomipramine and maprotiline) (Alper et al., 2007; Steinert and Fröscher, 2018; Mula, 2019). Moreover, a summary of FDA data from clinical trials showed that the incidence of seizures was lower in patients receiving antidepressants than placebo (standardized incidence ratio = 0.48) (Alper et al., 2007).
One of the latest therapeutic options for treatment of depressive symptoms is the multimodal antidepressant vortioxetine which has a very favorable safety and tolerance profile and a relatively low risk of drug interactions (Chen et al., 2018). Therefore, it seems reasonable to consider vortioxetine as a therapeutic option in patients with epilepsy and depressive symptoms.
Preclinical studies mostly indicate that vortioxetine may reduce seizure activity. Vortioxetine has been observed to reduce the number of epileptic discharges in penicillin-induced epilepsy model and to play a role in controlling epileptiform activity in pentylenetetrazole-induced kindling model in rats (Ögün et al., 2019; Taskiran and Unal, 2021). One study has shown proconvulsant activity of vortioxetine in genetic absence epileptic WAG/Rij rats (Aygun and Ayyildiz, 2021).
To the best of our knowledge, only one case of a patient with epilepsy (symptomatic occipital lobe epilepsy) successfully treated with vortioxetine has been published so far (Onder et al., 2018). In this case series, we report nine cases of patients with epilepsy and depressive symptoms treated with vortioxetine.
All nine cases are outpatients treated in the Department of Psychiatry, University Hospital in Cracow (Poland) during the last 5 years. The cases were identified by thorough analysis of records from patient visits since 2015 until September 2021. Relevant socio-demographic and clinical data are presented in Table 1.
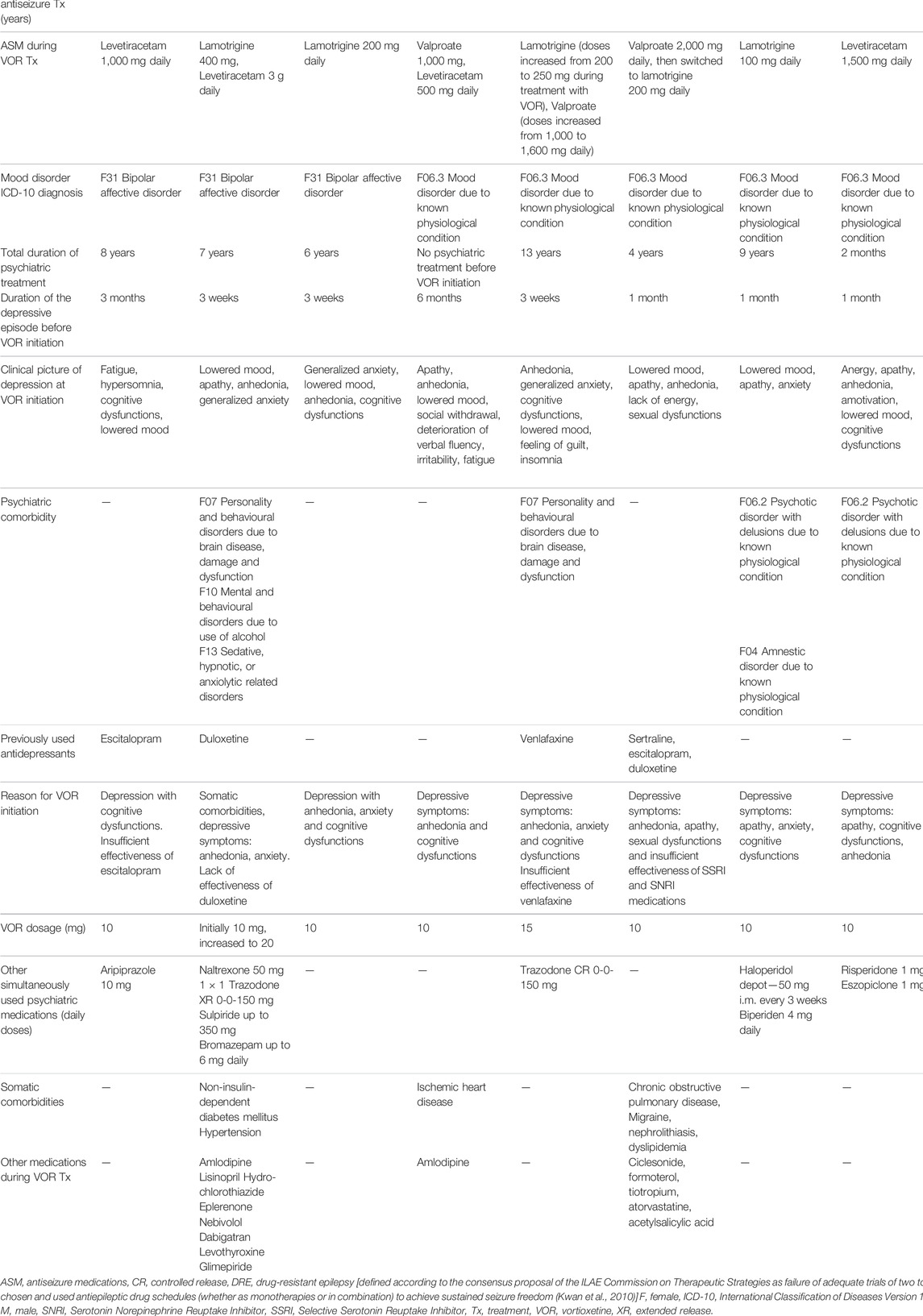
TABLE 1. Presentation of nine cases of patients with depressive symptoms and epilepsy treated with vortioxetine.
The presented group of cases includes five female and four male patients, aged between 24 and 77 year-old. Seven cases are patients with secondary focal and generalized epilepsy and two with unclassified epilepsy, with mostly satisfactory control of the neurological disease. In most cases (5/9) lamotrigine was used as part of the antiseizure pharmacotherapy, but four patients were treated with levetiracetam and one with topiramate—medications with, as already mentioned in the introduction, know potential to induce psychiatric side effects. However, due to good antiseizure effect, the attending neurologists decided to maintain treatment with the use of these medications. Before starting treatment with vortioxetine three patients presented with depressive episode in the course of bipolar disorder and six patients had depressive symptoms due to organic mood disorder (i.e., due to known physiological condition). Vortioxetine was the first line of antidepressant treatment in four patients, second line in four patients and the fourth line in one case. Vortioxetine was preferred either because of the clinical presentation (depression with anhedonia, anxiety and cognitive dysfunctions—in all patients), insufficient effectiveness of previously used antidepressants (in five patients) or somatic comorbidities and high risk of drug interactions due to polytherapy (in three patient). In the subgroup of patients with bipolar disorder, vortioxetine was chosen after analysis of the history of previous lack of antidepressant effectiveness of other agents (such as mood-stabilizers or antipsychotics) and taking into consideration relative safety of different pharmacological options when treating patients with epilepsy. Moreover, results of a naturalistic, open-label, add-on study of vortioxetine in bipolar depression indicate that this medication can be an efficient and well-tolerated therapeutic option in bipolar patients (Siwek et al., 2021a). It is also worth noticing that two out of three bipolar patients in our case series were treated with lamotrigine which was used not only due to its antiseizure but also antidepressant properties.
The dose range of vortioxetine was between 10 and 20 mg. In all presented cases remission of depressive symptoms was achieved and tolerability of the treatment was good. Time needed to reach remission ranged from 3 weeks to 3 months. No epilepsy seizures after introduction of vortioxetine were observed in seven cases. In two cases seizures occurred during the observation period. In one patient (Patient 5) epileptic seizure appeared after 7 months of the treatment and this could have been attributed to inadequately low doses of lamotrigine (which was previously used in doses up to 600 mg with optimal seizure control, but during the first months of vortioxetine treatment daily dose of lamotrigine was reduced by neurologists to only 200 mg)—after lamotrigine dose adjustments no seizures were observed for the following 5 months of observation. In another patient (Patient 6) epileptic seizure occurred after 2 months of vortioxetine treatment—antiseizure medication was switched afterwards (from valproate to lamotrigine) with optimal control: no other epileptic seizure was observed during nearly 4 following years of antidepressant treatment. It is worth noticing that possibly in these two cases no optimal control of epileptic seizures was reached prior to vortioxetine treatment—both patients had seizure episodes requiring hospitalization shortly before vortioxetine initiation. Vortioxetine was discontinued in two of the presented cases due to pregnancy planning, taking into consideration scarcity of data regarding safety of vortioxetine use in pregnant women. Duration of observation period ranged from 2 to 48 months.
Table 2 presents clinical data on the effectiveness and tolerability of treatment with vortioxetine.
In the presented cases vortioxetine was very well tolerated and effective (remission of depressive symptoms was reached in all patients), despite the fact that all patients were treated with polytherapy and three had somatic comorbidities (apart from epilepsy). It is worth mentioning that the majority of cases included patients with symptomatic epilepsy due to brain lesion (after stroke, cardiac arrest, brain injury or neurosurgery)—current data on effectiveness and tolerability of antidepressants in patients with depression following brain damage (due to, e.g., stroke or traumatic brain injury) is limited, which makes any reports of successful treatment of depressive episodes in this group of patients especially valuable (Qin et al., 2018; Kreitzer et al., 2019). Besides, two of the presented cases were elderly patients (74 and 77 year-old)—both reached remission in 2 months and tolerated vortioxetine very well, even though possible reduced tolerability and effectiveness of antidepressants in patients over the age of 65 has been observed (Cleare et al., 2015).
As already mentioned in the introduction, current evidence on the treatment of depression in epilepsy is limited and inconclusive. According to the available clinical recommendations, SSRI and SNRI (Serotonin Norepinephrine Reuptake Inhibitors) are the first-line pharmacological treatment options, while tricyclic antidepressants or NDRI (norepinephrine-dopamine reuptake inhibitors) should be avoided (Elger et al., 2017; Mula, 2019).
Vortioxetine is a relatively new antidepressant (approved by FDA—Food and Drug Administration, in 2013) with multimodal activity—it is an inhibitor of serotonin transporter, an agonist of 5HT1A receptor, a partial agonist of 5HT1B receptor, and an antagonist of 5HT1D, 5HT3 and 5HT7 receptors. Antidepressant efficacy has been demonstrated in doses 5–20 mg daily. It is metabolized primarily by CYP2D6 isoenzyme of P450 cytochrome (to inactive metabolites). Vortioxetine does not seem to impact activity of the liver enzymes in a clinically relevant way which potentially lowers the risk of pharmacokinetic drug interactions (Chen et al., 2018). Results of the network meta-analysis comparing efficacy and acceptability of 21 antidepressants in patients with MDD (Major Depressive Disorder) indicate that vortioxetine was in the group of more effective and more tolerable agents (Cipriani et al., 2018). Data from clinical trials has shown that vortioxetine carries relatively (compared to other antidepressants) low risk of inducing sexual dysfunctions, weight gain and sleep disturbance (Jacobsen et al., 2015; Jacobsen et al., 2016; D’Agostino et al., 2015; Liguori et al., 2019; Baldwin et al., 2016a). Besides, efficacy of vortioxetine on anhedonia and anxiety symptoms in patients with MDD has been observed (Baldwin et al., 2016b; Cao et al., 2019). Vortioxetine also appears to have relatively good tolerability and safety profile in depressed elderly patients (Borhannejad et al., 2020; Danielak, 2021).
Vortioxetine seems to be a promising treatment option for cognitive dysfunctions in MDD patients—it has been observed to improve cognition (both objectively and subjectively) independently of the alleviation of depression severity and to induce greater improvement in different cognitive domains compared to other antidepressants (Bennabi et al., 2019). Moreover, it has been demonstrated that vortioxetine added to a mood stabilizing agents can be an effective and well-tolerated treatment option in patients with bipolar depression (Siwek et al., 2021a). Finally, it is worth remembering that vortioxetine withdrawal can be associated with occurrence of discontinuation symptoms (although, contrary to the majority of SSRI and SNRI medications, the risk of withdrawal syndrome seems to be low) (Siwek et al., 2021b).
Seizures were very rarely observed in the published results of clinical trials of vortioxetine in MDD patients—only one randomized, double-blind 8-week trial reported 1 case of seizure occurrence in a patient receiving 5 mg of vortioxetine (out of 153 patients in this group and compared to no seizures observed in the placebo arm) (Mahableshwarkar et al., 2013).
Data considering use of vortioxetine in epilepsy patients is scarce. To the best of our knowledge, only one case of a 52-year-old female patient with secondary epilepsy and depressive symptoms has been presented—the authors reported excellent response to vortioxetine (in dose of 20 mg daily) and, interestingly, reduction of visual scotomas after initiation of antidepressant treatment (Onder et al., 2018). As patients with epilepsy often suffer from somatic and psychiatric comorbidities and thus may frequently require polytherapy, vortioxetine as a relatively safe, well-tolerated antidepressant with low risk of inducing pharmacokinetic interactions seems to be a valid treatment option in this group of patients (Baldwin et al., 2016a; Bosak et al., 2019). Besides, patients with epilepsy commonly suffer from cognitive impairment and it would be interesting to find out whether cognition enhancing properties of vortioxetine may additionally improve effects of treatment with this medication (Holmes, 2015).
Vortioxetine, as an effective and relatively well-tolerated antidepressant with potentially lower risk of pharmacokinetic drug interactions and additional cognitive benefits in depression, seems to be a promising treatment option for patients with epilepsy and depressive symptoms (especially in the view of current limited evidence on the effectiveness of antidepressants in epilepsy patients). We have presented 9 cases of patients successfully treated with this medication—symptomatic remission was achieved with good treatment tolerance. Further studies regarding vortioxetine use in larger populations of patients with epilepsy and depression are definitely needed in order to broaden our knowledge and improve clinical management of this group of patients.
The data analyzed in this study is subject to the following licenses/restrictions: The dataset contains clinical information about outpatients and cannot be openly available due to privacy restrictions. Requests to access these datasets should be directed to marcin.siwek@uj.edu.pl.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization: MS, AG, MB, and DD. Data acquisition: MS, MB, and DD. Formal analysis: MS, AG, and MB. Supervision: DD. Writing−original draft: AG and MS. Writing−review & editing: MS, AG, MB, and DD.
Additional funding for the publication fee from Jagiellonian University in Cracow.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alper, K., Schwartz, K. A., Kolts, R. L., and Khan, A. (2007). Seizure Incidence in Psychopharmacological Clinical Trials: An Analysis of Food and Drug Administration (FDA) Summary Basis of Approval Reports. Biol. Psychiatry 62 (4), 345–354. doi:10.1016/j.biopsych.2006.09.023
Aygun, H., and Ayyildiz, M. (2021). Vortioxetine Increases Absence-like Seizures in WAG/Rij Rats but Decreases Penicillin- and Pentylenetetrazole-Induced Seizures in Wistar Rats. Epilepsy Behav. 116, 107797. doi:10.1016/j.yebeh.2021.107797
Baldwin, D. S., Chrones, L., Florea, I., Nielsen, R., Nomikos, G. G., Palo, W., et al. (2016). The Safety and Tolerability of Vortioxetine: Analysis of Data from Randomized Placebo-Controlled Trials and Open-Label Extension Studies. J. Psychopharmacol. 30 (3), 242–252. doi:10.1177/0269881116628440
Baldwin, D. S., Florea, I., Jacobsen, P. L., Zhong, W., and Nomikos, G. G. (2016). A Meta-Analysis of the Efficacy of Vortioxetine in Patients with Major Depressive Disorder (MDD) and High Levels of Anxiety Symptoms. J. Affect Disord. 206, 140–150. doi:10.1016/j.jad.2016.07.015
Bennabi, D., Haffen, E., and Van Waes, V. (2019). Vortioxetine for Cognitive Enhancement in Major Depression: From Animal Models to Clinical Research. Front. Psychiatry 1010, 771. doi:10.3389/fpsyt.2019.00771
Błaszczyk, B., and Czuczwar, S. J. (2016). Epilepsy Coexisting with Depression. Pharmacol. Rep. 68 (5), 1084–1092. doi:10.1016/j.pharep.2016.06.011
Borhannejad, F., Shariati, B., Naderi, S., Shalbafan, M., Mortezaei, A., Sahebolzamani, E., et al. (2020). Comparison of Vortioxetine and Sertraline for Treatment of Major Depressive Disorder in Elderly Patients: A Double-Blind Randomized Trial. J. Clin. Pharm. Ther. 45 (4), 804–811. doi:10.1111/jcpt.13177
Bosak, M., Dudek, D., and Siwek, M. (2012). Depresja U Chorych Z Padaczka [Depression in Patients with Epilepsy]. Psychiatr. Pol. 46 (5), 891–902.
Bosak, M., Dudek, D., Siwek, M., and Szczudlik, A. (2015). Subtypes of Interictal Depressive Disorders According to ICD-10 in Patients with Epilepsy. Neurol. Neurochir Pol. 49 (2), 90–94. doi:10.1016/j.pjnns.2015.01.008
Bosak, M., Kowalik, M., Mołek, P., and Słowik, A. (2019). Somatic Comorbidity in Polish Patients with Epilepsy. Pol. Arch. Intern. Med. 129 (5), 303–307. doi:10.20452/pamw.14794
Bosak, M., Turaj, W., Dudek, D., Siwek, M., and Szczudlik, A. (2015). Depressogenic Medications and Other Risk Factors for Depression Among Polish Patients with Epilepsy. Neuropsychiatr. Dis. Treat. 11, 2509–2517. doi:10.2147/NDT.S91538
Bosak, M., Turaj, W., Dudek, D., Siwek, M., and Szczudlik, A. (2016). Suicidality and its Determinants Among Polish Patients with Epilepsy. Neurol. Neurochir Pol. 50 (6), 432–438. doi:10.1016/j.pjnns.2016.07.009
Cao, B., Park, C., Subramaniapillai, M., Lee, Y., Iacobucci, M., Mansur, R. B., et al. (2019). The Efficacy of Vortioxetine on Anhedonia in Patients with Major Depressive Disorder. Front. Psychiatry 10, 17. doi:10.3389/fpsyt.2019.00017
Chen, B., Choi, H., Hirsch, L. J., Katz, A., Legge, A., Buchsbaum, R., et al. (2017). Psychiatric and Behavioral Side Effects of Antiepileptic Drugs in Adults with Epilepsy. Epilepsy Behav. 76, 24–31. doi:10.1016/j.yebeh.2017.08.039
Chen, G., Højer, A.-M., Areberg, J., and Nomikos, G. (2018). Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clin. Pharmacokinet. 57, 673–686. doi:10.1007/s40262-017-0612-7
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: a Systematic Review and Network Meta-Analysis. Lancet 391 (10128), 1357–1366. doi:10.1016/S0140-6736(17)32802-7
Cleare, A., Pariante, C. M., Young, A. H., Anderson, I. M., Christmas, D., Cowen, P. J., et al. (2015). Evidence-based Guidelines for Treating Depressive Disorders with Antidepressants: A Revision of the 2008 British Association for Psychopharmacology Guidelines. J. Psychopharmacol. 29 (5), 459–525. doi:10.1177/0269881115581093
D’Agostino, A., English, C. D., and Rey, J. A. (2015). Vortioxetine (Brintellix): A New Serotonergic Antidepressant. P T 40 (1), 36–40.
Danielak, D. (2021). Vortioxetine in Management of Major Depressive Disorder - a Favorable Alternative for Elderly Patients? Expert Opin. Pharmacother. 22 (9), 1167–1177. doi:10.1080/14656566.2021.1880567
Elger, C. E., Johnston, S. A., and Hoppe, C. (2017). Diagnosing and Treating Depression in Epilepsy. Seizure 44, 184–193. doi:10.1016/j.seizure.2016.10.018
Fazel, S., Wolf, A., Långström, N., Newton, C. R., and Lichtenstein, P. (2013). Premature Mortality in Epilepsy and the Role of Psychiatric Comorbidity: A Total Population Study. Lancet 382 (9905), 1646–1654. doi:10.1016/S0140-6736(13)60899-5
Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., et al. (2014). ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 55 (4), 475–482. doi:10.1111/epi.12550
Fisher, R. S., Cross, J. H., French, J. A., Higurashi, N., Hirsch, E., Jansen, F. E., et al. (2017). Operational Classification of Seizure Types by the International League against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58 (4), 522–530. doi:10.1111/epi.13670
Garcia, M. E., Garcia-Morales, I., and Gil-Nagel, A. (2015). Prevalence of Depressive Symptoms and Their Impact on Quality of Life in Patients with Drug-Resistant Focal Epilepsy (IMDYVA Study). Epilepsy Res. 110, 157–165. doi:10.1016/j.eplepsyres.2014.11.003
Henning, O., Lossius, M. I., Lima, M., Mevåg, M., Villagran, A., Nakken, K. O., et al. (2019). Refractory Epilepsy and Nonadherence to Drug Treatment. Epilepsia Open 4 (4), 618–623. doi:10.1002/epi4.12367
Hesdorffer, D. C., Ishihara, L., Webb, D. J., Mynepalli, L., Galwey, N. W., and Hauser, W. A. (2016). Occurrence and Recurrence of Attempted Suicide Among People with Epilepsy. JAMA Psychiatry 73 (1), 80–86. doi:10.1001/jamapsychiatry.2015.2516
Hitiris, N., Mohanraj, R., Norrie, J., Sills, G. J., and Brodie, M. J. (2007). Predictors of Pharmacoresistant Epilepsy. Epilepsy Res. 75 (2-3), 192–196. doi:10.1016/j.eplepsyres.2007.06.003
Holmes, G. L. (2015). Cognitive Impairment in Epilepsy: The Role of Network Abnormalities. Epileptic Disord. 17 (2), 101–116. doi:10.1684/epd.2015.0739
Jacobsen, P. L., Mahableshwarkar, A. R., Chen, Y., Chrones, L., and Clayton, A. H. (2015). Effect of Vortioxetine vs. Escitalopram on Sexual Functioning in Adults with Well-Treated Major Depressive Disorder Experiencing SSRI-Induced Sexual Dysfunction. J. Sex. Med. 12 (10), 2036–2048. doi:10.1111/jsm.12980
Jacobsen, P. L., Mahableshwarkar, A. R., Palo, W. A., Chen, Y., Dragheim, M., and Clayton, A. H. (2016). Treatment-emergent Sexual Dysfunction in Randomized Trials of Vortioxetine for Major Depressive Disorder or Generalized Anxiety Disorder: A Pooled Analysis. CNS Spectr. 21 (5), 367–378. doi:10.1017/S1092852915000553
Johnson, E. K., Jones, J. E., Seidenberg, M., and Hermann, B. P. (2004). The Relative Impact of Anxiety, Depression, and Clinical Seizure Features on Health-Related Quality of Life in Epilepsy. Epilepsia 45 (5), 544–550. doi:10.1111/j.0013-9580.2004.47003.x
Josephson, C. B., Lowerison, M., Vallerand, I., Sajobi, T. T., Patten, S., Jette, N., et al. (2017). Association of Depression and Treated Depression with Epilepsy and Seizure Outcomes: A Multicohort Analysis. JAMA Neurol. 74 (5), 533–539. doi:10.1001/jamaneurol.2016.5042
Kanner, A. M., Byrne, R., Chicharro, A., Wuu, J., and Frey, M. (2009). A Lifetime Psychiatric History Predicts a Worse Seizure Outcome Following Temporal Lobectomy. Neurology 72 (9), 793–799. doi:10.1212/01.wnl.0000343850.85763.9c
Kanner, A. M. (2009). Suicidality and Epilepsy: A Complex Relationship that Remains Misunderstood and Underestimated. Epilepsy Curr. 9 (3), 63–66. doi:10.1111/j.1535-7511.2009.01294.x
Keezer, M. R., Sisodiya, S. M., and Sander, J. W. (2016). Comorbidities of Epilepsy: Current Concepts and Future Perspectives. Lancet Neurol. 15 (1), 106–115. doi:10.1016/S1474-4422(15)00225-2
Kreitzer, N., Ancona, R., Mccullumsmith, C., Kurowski, B. G., Foreman, B., Ngwenya, L. B., et al. (2019). The Effect of Antidepressants on Depression after Traumatic Brain Injury: A Meta-Analysis. J. Head Trauma Rehabil. 34 (3), E47–E54. doi:10.1097/HTR.0000000000000439
Kwan, P., Arzimanoglou, A., Berg, A. T., Brodie, M. J., Hauser, W. A., Mathern, G., et al. (2010). Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51, 1069–1077. doi:10.1111/j.1528-1167.2009.02397.x
Kwon, O. Y., and Park, S. P. (2014). Depression and Anxiety in People with Epilepsy. J. Clin. Neurol. 10 (3), 175–188. doi:10.3988/jcn.2014.10.3.175
Liguori, C., Ferini-Strambi, L., Izzi, F., Mari, L., Manfredi, N., D'Elia, A., et al. (2019). Preliminary Evidence that Vortioxetine May Improve Sleep Quality in Depressed Patients with Insomnia: a Retrospective Questionnaire Analysis. Br. J. Clin. Pharmacol. 85 (1), 240–244. doi:10.1111/bcp.13772
Maguire, M. J., Weston, J., Singh, J., and Marson, A. G. (2021). Antidepressants for People with Epilepsy and Depression. Cochrane Database Syst. Rev. 4, CD010682. doi:10.1002/14651858.CD010682.pub2
Mahableshwarkar, A. R., Jacobsen, P. L., and Chen, Y. (2013). A Randomized, Double-Blind Trial of 2.5 Mg and 5 Mg Vortioxetine (Lu AA21004) versus Placebo for 8 Weeks in Adults with Major Depressive Disorder. Curr. Med. Res. Opin. 29 (3), 217–226. doi:10.1185/03007995.2012.761600
Mazarati, A., and Sankar, R. (2016). Common Mechanisms Underlying Epileptogenesis and the Comorbidities of Epilepsy. Cold Spring Harb Perspect. Med. 6 (7), a022798. doi:10.1101/cshperspect.a022798
Mula, M. (2019). Developments in Depression in Epilepsy: Screening, Diagnosis, and Treatment. Expert Rev. Neurother 19 (3), 269–276. doi:10.1080/14737175.2019.1585244
Ögün, M. N., Çetinkaya, A., and Beyazçiçek, E. (2019). The Effect of Vortioxetine on Penicillin-Induced Epileptiform Activity in Rats. Arq Neuropsiquiatr 77 (6), 412–417. doi:10.1590/0004-282X20190064
Onder, H., Coskun, A., and Goksungur, M. T. (2018). Recovery of Visual Scotomas by Vortioxetine in a Patient with Symptomatic Occipital Lobe Epilepsy. Ann. Indian Acad. Neurol. 21 (1), 88–90. doi:10.4103/aian.AIAN_291_17
Qin, B., Chen, H., Gao, W., Zhao, L. B., Zhao, M. J., Qin, H. X., et al. (2018). Efficacy, Acceptability, and Tolerability of Antidepressant Treatments for Patients with post-stroke Depression: A Network Meta-Analysis. Braz. J. Med. Biol. Res. 51 (7), e7218. doi:10.1590/1414-431x20187218
Scheffer, I. E., Berkovic, S., Capovilla, G., Connolly, M. B., French, J., Guilhoto, L., et al. (2017). ILAE Classification of the Epilepsies: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58 (4), 512–521. doi:10.1111/epi.13709
Siwek, M., Chrobak, A., Sołtys, Z., Dudek, D., Krupa, A., and Rybakowski, J. (2021). A Naturalistic, 24-week, Open-Label, Add-On Study of Vortioxetine in Bipolar Depression. Psychiatr. Pol. ONLINE FIRST Nr 222: 1–14. doi:10.12740/pp/onlinefirst/132962
Siwek, M., Chrobak, A. A., Gorostowicz, A., Krupa, A. J., and Dudek, D. (2021). Withdrawal Symptoms Following Discontinuation of Vortioxetine-Retrospective Chart Review. Pharmaceuticals (Basel) 14 (5), 451. doi:10.3390/ph14050451
Steinert, T., and Fröscher, W. (2018). Epileptic Seizures under Antidepressive Drug Treatment: Systematic Review. Pharmacopsychiatry 51 (4), 121–135. doi:10.1055/s-0043-117962
Tao, K., and Wang, X. (2016). The Comorbidity of Epilepsy and Depression: Diagnosis and Treatment. Expert Rev. Neurother 16 (11), 1321–1333. doi:10.1080/14737175.2016.1204233
Taskiran, M., and Unal, G. (2021). Vortioxetine Suppresses Epileptiform Activity and Cognition Deficits in a Chronic PTZ-Induced Kindling Rat Model. Epileptic Disord. 23 (6), 893–900. doi:10.1684/epd.2021.1344
Taylor, R. S., Sander, J. W., Taylor, R. J., and Baker, G. A. (2011). Predictors of Health-Related Quality of Life and Costs in Adults with Epilepsy: A Systematic Review. Epilepsia 52 (12), 2168–2180. doi:10.1111/j.1528-1167.2011.03213.x
Keywords: vortioxetine, depression, bipolar disorder, seizure, antidepressants, epilepsy, mood disorders, depressive symptoms
Citation: Siwek M, Gorostowicz A, Bosak M and Dudek D (2022) Case Report: Vortioxetine in the Treatment of Depressive Symptoms in Patients With Epilepsy—Case Series. Front. Pharmacol. 13:852042. doi: 10.3389/fphar.2022.852042
Received: 10 January 2022; Accepted: 11 March 2022;
Published: 31 March 2022.
Edited by:
Aleksandra Szopa, Medical University of Lublin, PolandReviewed by:
Hatice Aygun, Tokat Gaziosmanpaşa University, TurkeyCopyright © 2022 Siwek, Gorostowicz, Bosak and Dudek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcin Siwek, marcin.siwek@uj.edu.pl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.