- 1Department of Gynecology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Standardization of Traditional Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 4State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Department of Cardiovascular Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 6Health Science Center, Shenzhen University, Shenzhen, China
- 7Department of Gynecology, Peking Union Medical College Hospital, Beijing, China
Importance: The incidence of dyslipidemia increases after menopause. Menopause hormone therapy (MHT) is recommended for menopause related disease. However, it is benefit for lipid profiles is inconclusive.
Objective: To conduct a systematic review and meta-analysis of randomized controlled trials to evaluate the effects of MHT on lipid profile in postmenopausal women.
Evidence Review: Related articles were searched on PubMed/Medline, EMBASE, Web of Science, and Cochrane Library databases from inception to December 2020. Data extraction and quality evaluation were performed independently by two reviewers. The methodological quality was assessed using the “Cochrane Risk of Bias checklist”.
Results: Seventy-three eligible studies were selected. The results showed that MHT significantly decreased the levels of TC (WMD: −0.43, 95% CI: −0.53 to −0.33), LDL-C (WMD: −0.47, 95% CI: −0.55 to −0.40) and LP (a) (WMD: −49.46, 95% CI: −64.27 to −34.64) compared with placebo or no treatment. Oral MHT led to a significantly higher TG compared with transdermal MHT (WMD: 0.12, 95% CI: 0.04–0.21). The benefits of low dose MHT on TG was also concluded when comparing with conventional-dose estrogen (WMD: −0.18, 95% CI: −0.32 to −0.03). The results also showed that conventional MHT significantly decreased LDL-C (WMD: −0.35, 95% CI: −0.50 to −0.19), but increase TG (WMD: 0.42, 95%CI: 0.18–0.65) compared with tibolone. When comparing with the different MHT regimens, estrogen (E) + progesterone (P) regimen significantly increased TC (WMD: 0.15, 95% CI: 0.09 to 0.20), LDL-C (WMD: 0.12, 95% CI: 0.07–0.17) and Lp(a) (WMD: 44.58, 95% CI:28.09–61.06) compared with estrogen alone.
Conclusion and Relevance: MHT plays a positive role in lipid profile in postmenopausal women, meanwhile for women with hypertriglyceridemia, low doses or transdermal MHT or tibolone would be a safer choice. Moreover, E + P regimen might blunt the benefit of estrogen on the lipid profile.
Clinical Trial Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018092924], identifier [No. CRD42018092924].
Introduction
Several studies have shown that menopause transition is associated with an unfavorable effect on lipid profile, accompanying with an increase in the levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and lipoprotein (a) [LP (a)], and sometimes with a decrease in the level of high-density lipoprotein cholesterol (HDL-C) (Anagnostis et al., 2015; Anagnostis et al., 2016). It is well-known that an unfavorable lipid profile plays a crucial role in the development and progression of cardiovascular disease (CVD) (McQueen et al., 2008; Lee et al., 2017), which is the leading cause of morbidity and mortality in postmenopausal women (Tandon et al., 2010).
Menopause signifies the permanent cessation of menstruation, resulting from loss of ovarian follicular activity and deficiency of estrogen. As postmenopausal women have significantly higher levels of LDL-C and TC than premenopausal women (Ambikairajah et al., 2019), estrogen has been found to play a protective role by regulating lipid metabolism. In this frame, estrogen-based menopause hormone therapy (MHT) could influence lipid profile in postmenopausal women. It has been reported that MHT is the most effective treatment for menopause-related symptoms caused by the loss of estrogen (Baber et al., 2016). Besides, MHT has been shown to have a favorable risk–benefit ratio for women without dyslipidemia who underwent treatment at the age under 60 years old or within 10 years after menopause onset (2019 Surveillance of Menopause, 2019). A meta-analysis conducted in 2001 concluded that MHT could decrease the levels of TC and LDL-C, and increase HDL-C level (Godsland, 2001). A review performed in 2017 showed that MHT significantly decreased LP (a) concentration (Anagnostis et al., 2017). Some studies have shown that MHT negatively influences TG level (Mercuro et al., 2003; Nii et al., 2016). However, a study conducted in 2016 indicated that TG level was lower in MHT group than that in non-MHT group (Ki et al., 2016). Pu et al. pointed out that hormone therapy with 17β-estradiol provided more benefits for decreasing TG level, while conjugated equine estrogen (CEE) showed a better effect on reducing the levels of both HDL-C and LDL-C (Pu et al., 2017). To date, long-term effects of MHT or different routes of administration of estrogen on the lipid profile were scarcely reported. In addition, it has been shown that both dosage and type of progestogen are of great importance for the lipoprotein fractions (Odmark et al., 2004). The Women’s Health Initiative (WHI) study demonstrated that CEEs with medroxyprogesterone acetate (MPA) had an increased risk of developing coronary heart disease (CHD) by 18%, while the CEE was not associated with an increased risk of CHD, raising a question concerning the safety of progestogen (Manson et al., 2013; Manson et al., 2017). But few meta-analyses have concentrated on the effects of progestogen on lipid profile. Given these limitations, an updated meta-analysis is precious to indicate the effects of MHT on the lipid profile. The present study aimed to systematically review and analyze data from randomized controlled trials (RCTs)to find out the effects of MHT concerning factors, including duration of therapy, route of administration, dosage, and types of regimens [estrogen-alone (E-alone) or estrogen plus progestogen (E + P)], on lipid profile in menopausal women.
Methods
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement checklist (Moher et al., 2009), and that was registered on PROSPERO (Registration No. CRD42018092924).
Study Selection
PubMed/Medline, EMBASE, Web of Science, and Cochrane Library databases were comprehensively and systematically searched from inception to 31 December 2020, for studies published in English. The main search items were as follows: (“Menopause Hormone Therapy” OR “hormone therapy” OR “estrogen therapy” OR “estradiol therapy”) AND [“TC” OR “TG” OR “LDL” OR “HDL” OR “LP (a)” OR “lipid” AND (“postmenopausal women” OR “menopausal women” OR “menopause” OR “peri-menopausal women”). This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement checklist (Moher et al., 2009), and that was registered on PROSPERO (Registration No. CRD42018092924). Two authors screened and evaluated all the abstracts and potentially eligible articles, any discrepancies between reviewers in the study selection were resolved via consultation with a third reviewer.
Articles that meet the following requirements were included: 1) original RCTs that were published in English; 2) administration of MTH for postmenopausal concerning factors, such as duration of therapy, route of administration, dosage, and types of regimens (E-alone or estrogen E + P); 3) inclusion of placebo, no treatment or non-MHT as a control group. For different regimens, regarding the effects of different types of estrogen on lipid profile, the same type of estrogen was required in 2 groups; 4) reporting the levels of TC, TG, LDL, HDL or Lp (a) as the outcome measures for lipid profile, and data were available directly from articles or could be calculated by mathematical formulas. The unit of TC, TG, LDL, and HDL was uniformly converted to mmol/L, and the unit of Lp(a) was converted to mg/L. As tibolone can alleviate menopause symptoms, studies that compared the effects of tibolone with MHT on lipid profile were included, while studies that concentrated on only the effects of tibolone were excluded from this review.
Data Extraction and Quality Assessment
Data extraction of the studies included: 1) basic data of retrieved articles (title, the first author’s full name, year of publication, journal, etc.); 2)study design; 3) participants’ demographic characteristics (age, number of cases, etc.); 4)inclusion and exclusion criteria particularly for each article; 5) MHT-based data (name, dose, route of administration, the duration of treatment and type of regimen); 6) data related to control group (name, dose, route of administration, duration, type of regimen, etc.); 7) Serum lipid profiles. The data that provided baseline values and percentage changes after treatment only, which was unable to be converted into averages and standard deviations would be excluded. If raw data is needed, the corresponding author would be contacted to get more details. The Cochrane Risk of Bias check list (Higgins et al., 2011) was used to evaluate the risk of bias of randomized clinical trials.
Statistical Analysis
Data analyzed was performed with the Cochrane Collaboration Review Manager (version 5.2) software, each outcome was expressed as mean ± standard deviation (SD). Heterogeneity among studies was estimated by I2 statistic. If I2 ≥ 50%, the random-effects model was used to perform the analysis; Otherwise, the fixed-effects model was utilized. We used the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Ver. 6.2) to resolve the post-treatment data in some trials (Higgins et al., 2021). Millimoles per liter (mmol/L) will be used to measure TC, TG, LDL, and HDL while milligrams per liter (mg/L) were used to measure Lp(a).
Results
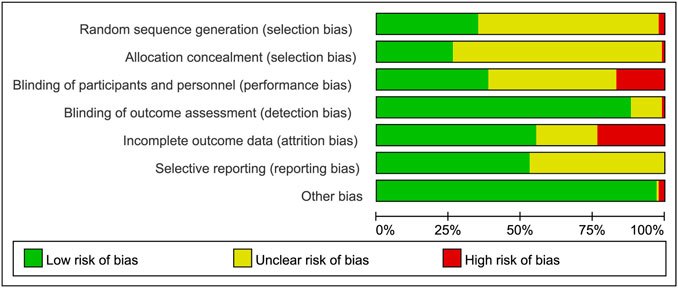
A total of 9,497 records were searched through database, after removal of duplicates, 6,784 articles were screened full-text and finally 73 articles were included in this meta-analysis (Figure 1). Clinical characteristics of included-articles were described in Table 1. The details for risk of bias are available in Figure 2 and Figure 3.
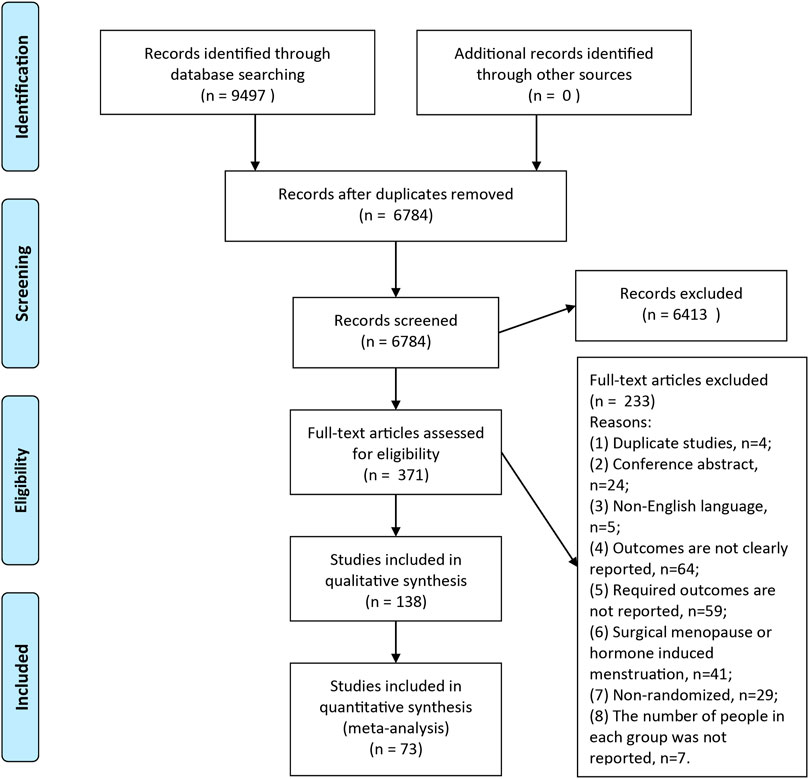
FIGURE 1. Flow Diagram. A total of 6,784 articles were retrieved, and 73 articles were included in the current meta-analysis.
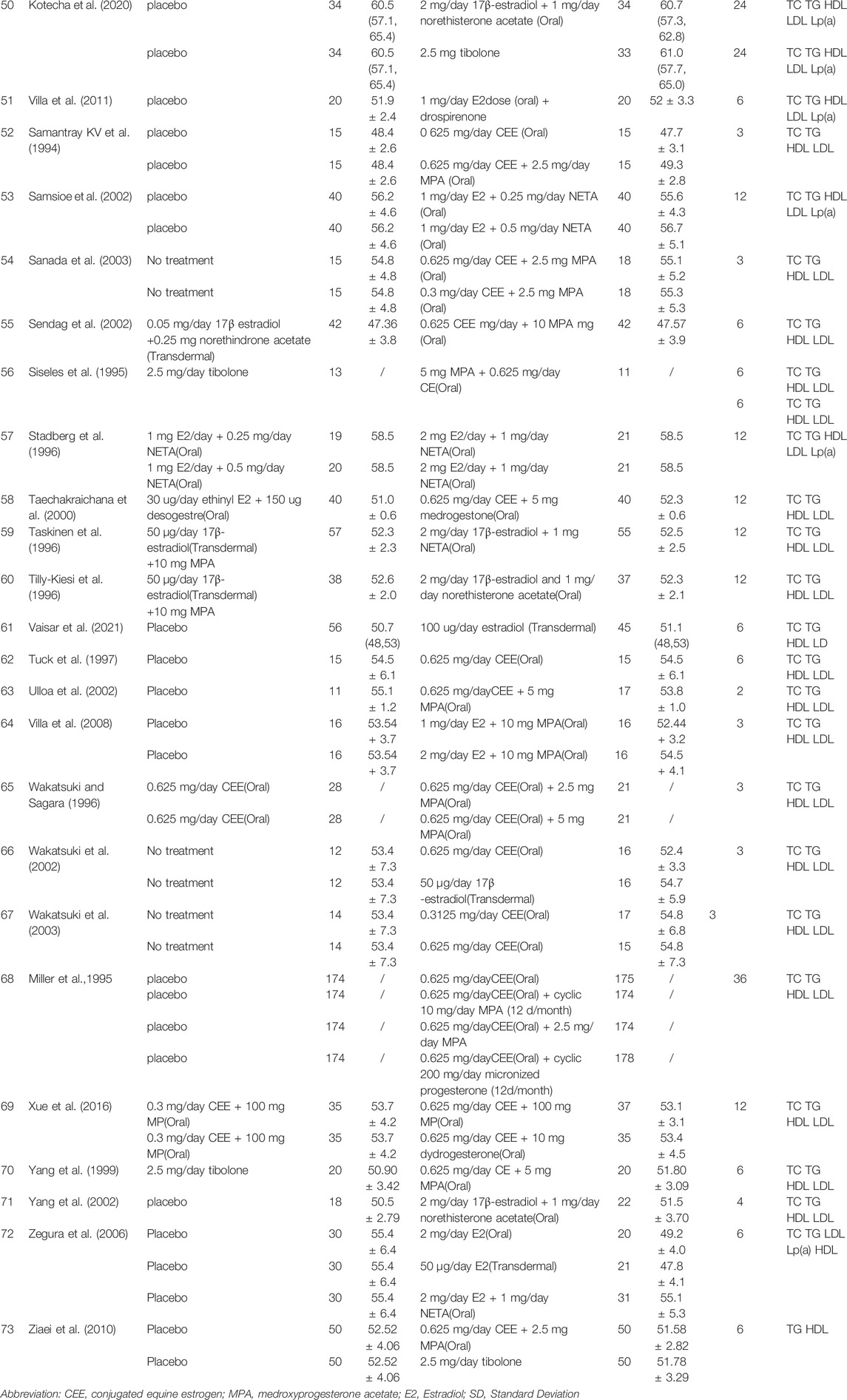
TABLE 1. Baseline characteristics and clinical outcomes of menopausal women with menopause hormone therapy.
Comparing the Effects of MHT on Lipid Profile With Placebo or no Treatment
Forty-seven studies (Cheng et al., 1993; Munk-Jensen et al., 1994; Samantray KV et al., 1994; Conard et al., 1995; Miller et al.,1995; Binder et al., 1996; Draper et al., 1996; Haines et al., 1996; Milner et al., 1996; Perrone et al., 1996; Conard et al., 1997; Heikkinen et al., 1997; Tuck et al., 1997; Espeland et al., 1998; Meschia et al., 1998; Lewis-Barned et al., 1999; Mijatovic et al., 1999; Davidson et al., 2000; Seed et al., 2000; Bunyavejchevin and Limpaphayom, 2001; Gräser et al., 2001; Luyer et al., 2001; Teede et al., 2001; Duvernoy et al., 2002; Samsioe et al., 2002; Ulloa et al., 2002; Wakatsuki et al., 2002; Yang et al., 2002; Hemelaar et al., 2003; Jirapinyo et al., 2003; Oral and Ozbaşar, 2003; Sanada et al., 2003; Wakatsuki et al., 2003; Stevenson et al., 2004; Bukowska et al., 2005; Zegura et al., 2006; Demirol et al., 2007; Fernandes et al., 2008; Villa et al., 2008; Ziaei et al., 2010; Cayan et al., 2011; Villa et al., 2011; Terauchi et al., 2012; Labos et al., 2013; Gregersen et al., 2019; Kotecha et al., 2020; Vaisar et al., 2021) compared the effects of MHT therapy and placebo on blood lipids. The duration of MHT was classified into the following periods: < 3 months, 3–5 months, 6–12 months, 13–24 months, and >24 months. For articles that evaluated the effects of MHT on lipid profile at multiple time points, the result in each time point was included as separate data.
The meta-analysis of data demonstrated that intake MHT could significantly reduce the serum TC (Miller et al., 1995; Binder et al., 1996; Bunyavejchevin and Limpaphayom, 2001; Cayan et al., 2011; Cheng et al., 1993; Conard et al., 1995; Conard et al., 1997; Davidson et al., 2000; Draper et al., 1996; Duvernoy et al., 2002; Fernandes et al., 2008; Gräser et al., 2001; Gregersen et al., 2019; Haines et al., 1996) (WMD: −0.43, 95% CI: −0.53 to −0.33, I2 = 93%) (Figure 4A) and LDL (Miller et al., 1995; Binder et al., 1996; Bunyavejchevin and Limpaphayom, 2001; Cayan et al., 2011; Cheng et al., 1993; Conard et al., 1995; Conard et al., 1997; Davidson et al., 2000; Draper et al., 1996; Duvernoy et al., 2002; Fernandes et al., 2008; Gräser et al., 2001; Gregersen et al., 2019; Haines et al., 1996) (WMD: −0.47, 95% CI: −0.55 to −0.40, I2 = 87%) throughout almost all treatment duration (Figure 4B). Except the duration between half year to 1 year (WMD: −0.08, 95% CI: −0.13 to −0.03), there was no significant difference in reducing TG (Cheng et al., 1993; Conard et al., 1995; Miller et al., 1995; Binder et al., 1996; Conard et al., 1997; Davidson et al., 2000; Bunyavejchevin and Limpaphayom, 2001; Bukowska et al., 2005; Cayan et al., 2011), (Duvernoy et al., 2002), (Haines et al., 1996; Heikkinen et al., 1997; Gräser et al., 2001; Fernandes et al., 2008; Gregersen et al., 2019) between the two groups (WMD: −0.00, 95% CI: −0.06 to 0.05, I2 = 84%) (Figure 4C). While come to Lp(a) (Bukowska et al., 2005; Conard et al., 1995; Conard et al., 1997; Davidson et al., 2000; Demirol et al., 2007; Espeland et al., 1998; Gregersen et al., 2019; Haines et al., 1996; Hemelaar et al., 2003; Kotecha et al., 2020; Meschia et al., 1998; Mijatovic et al., 1999; Milner et al., 1996; Samsioe et al., 2002), the results showed that MHT could remarkably decrease Lp(a) (WMD: −49.46, 95% CI: −64.27 to −34.64, I2 = 89%) (Figure 4E). However, the similar trend was only observed in periods of 6–12 months and >24 months. Data from 43 studies suggested an ignorable change in HDL (Miller et al., 1995; Binder et al., 1996; Bukowska et al., 2005; Bunyavejchevin and Limpaphayom, 2001; Cayan et al., 2011; Cheng et al., 1993; Conard et al., 1995; Conard et al., 1997; Davidson et al., 2000; Draper et al., 1996; Duvernoy et al., 2002; Fernandes et al., 2008; Gräser et al., 2001; Gregersen et al., 2019) (WMD: −0.00, 95% CI: −0.05to 0.05, I2 = 94%) (Figure 4D).
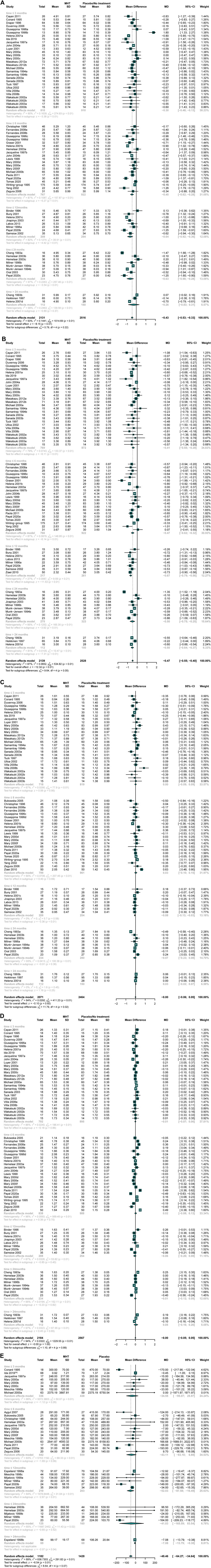
FIGURE 4. Comparing MHT wih placebo or no treatment. The treatment duration was classified into the following periods in each lipid index: < 3 months, 3–5 months, 6–12 months, 13–24 months, and >24 months. MHT led to a significant reduction in TC concentration, LDL-C concentration and Lp(a) concentration compared with placebo or no treatment. (A) TC concentration; (B) LDL-C concentration; (C) TG concentration; (D) HDL-C concentration; (E) Lp(a) concentration.
Comparing the Effects of Oral MHT With Transdermal MHT
A total of 16 studies (Hemelaar et al., 2003; Bukowska et al., 2005; Meschia et al., 1998; Perrone et al., 1996; Seed et al., 2000; Zegura et al., 2006; Casanova et al., 2015; Casanova et al., 2009; Castelo-Branco et al., 2007; Faguer de Moustier et al., 1989; Hemelaar et al., 2006; Lahdenperä et al., 1996; Sendag et al., 2002; Taskinen et al., 1996; Tilly-Kiesi et al., 1996; Abbas et al., 2004) that enrolled 670 participants in oral MHT group and 676 in transdermal MHT group were analyzed. When comparing the effects between 2 groups, the result indicated that oral MHT could significantly decreased LDL-C (Hemelaar et al., 2003; Meschia et al., 1998; Perrone et al., 1996; Seed et al., 2000; Casanova et al., 2015; Zegura et al., 2006; Casanova et al., 2009; Castelo-Branco et al., 2007; Faguer de Moustier et al., 1989; Hemelaar et al., 2006; Lahdenperä et al., 1996; Sendag et al., 2002; Taskinen et al., 1996; Tilly-Kiesi et al., 1996) (WMD: 0.23, 95%CI: −0.31 to −0.14, I2 = 28%) (Figure 5B) while there was no significant difference in TC (Hemelaar et al., 2003; Meschia et al., 1998; Perrone et al., 1996; Seed et al., 2000; Casanova et al., 2015; Casanova et al., 2009; Zegura et al., 2006; Castelo-Branco et al., 2007; Faguer de Moustier et al., 1989; Hemelaar et al., 2006; Lahdenperä et al., 1996; Sendag et al., 2002; Taskinen et al., 1996; Tilly-Kiesi et al., 1996) (WMD: −0.13, 95% CI: −0.30 to 0.04, I2 = 69%) (Figure 5A). However, the result revealed that oral MHT may significantly increase TG (Bukowska et al., 2005; Hemelaar et al., 2003; Perrone et al., 1996; Seed et al., 2000; Casanova et al., 2015; Casanova et al., 2009; Zegura et al., 2006; Castelo-Branco et al., 2007; Faguer de Moustier et al., 1989; Hemelaar et al., 2006; Lahdenperä et al., 1996; Sendag et al., 2002; Taskinen et al., 1996; Tilly-Kiesi et al., 1996) (WMD: 0.12, 95% CI: 0.04 to 0.21, I2 = 50%) (Figure 5C), while both HDL (Bukowska et al., 2005; Hemelaar et al., 2003; Perrone et al., 1996; Seed et al., 2000; Casanova et al., 2015; Casanova et al., 2009; Zegura et al., 2006; Castelo-Branco et al., 2007; Faguer de Moustier et al., 1989; Hemelaar et al., 2006; Lahdenperä et al., 1996; Sendag et al., 2002; Taskinen et al., 1996; Abbas et al., 2004) (WMD: -0.02, 95% CI: −0.10 to 0.06, I2 = 84%) (Figure 5D) and Lp(a) (Meschia et al., 1998; Seed et al., 2000; Hemelaar et al., 2003; Bukowska et al., 2005; Hemelaar et al., 2006; Zegura et al., 2006) (WMD: 5.04, 95% CI: −20.32 to 30.41, I2 = 0%) had no significance (Figure 5E).
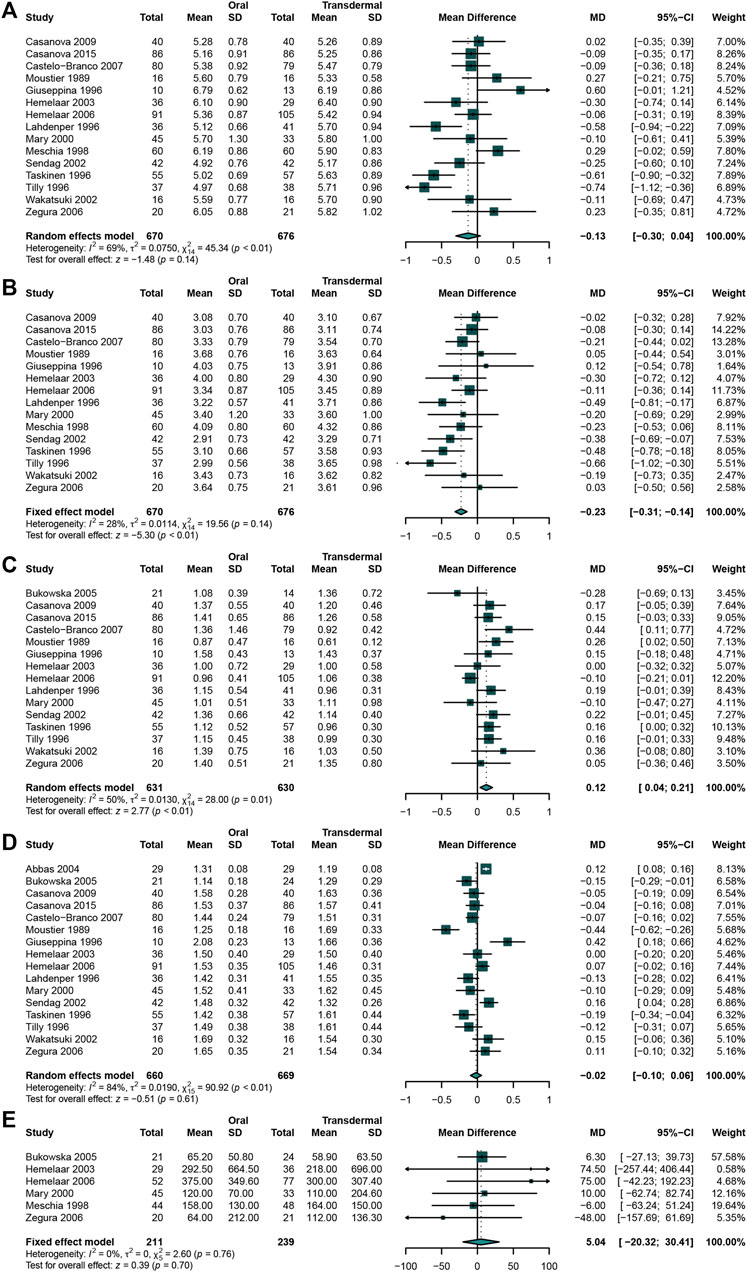
FIGURE 5. Comparing oral estrogen with transdermal estrogen Oral MHT significantly decreased LDL-C concentration and increased TG concentration compared with that in transdermal MHT group. (A) TC concentration; (B) LDL-C concentration; (C) TG concentration; (D) HDL-C concentration; (E) Lp(a) concentration.
Comparing the Effects of a Low-Dose Estrogen With a Conventional-Dose of Estrogen
The studies were classified according to the dosage of estrogen. A total of 10 studies (Cheng et al., 1993; Stadberg et al., 1996; Taechakraichana et al., 2000; Sanada et al., 2003; Wakatsuki et al., 2003; de Kraker et al., 2004; Koh et al., 2004; Christodoulakos et al., 2006; Villa et al., 2008; Xue et al., 2016)that enrolled 584 participants in low-dose estrogen group and 594 in conventional dose estrogen group were analyzed. 1mg/day or less of Estradiol valerate or 17 β-estradiol, 0.3 mg/day or less of conjugated estrogens were defined as low dose estrogen.
The meta-analysis result showed that the low-dose estrogen led to a significant reduction in TG (Cheng et al., 1993; Sanada et al., 2003; Villa et al., 2008; Wakatsuki et al., 2003; Christodoulakos et al., 2006; Stadberg et al., 1996; Koh et al., 2004; Taechakraichana et al., 2000; Xue et al., 2016) (WMD: −0.18, 95% CI: −0.32 to −0.03, I2 = 93%) (Figure 6C) and HDL-C (Cheng et al., 1993; Sanada et al., 2003; Villa et al., 2008; Wakatsuki et al., 2003; Christodoulakos et al., 2006; de Kraker et al., 2004; Stadberg et al., 1996; Koh et al., 2004; Taechakraichana et al., 2000; Xue et al., 2016) (WMD: −0.05, 95% CI: −0.07 to −0.04, I2 = 36%) (Figure 6D) comparing with the conventional-dose estrogen. There was no significant on TC (Cheng et al., 1993; Sanada et al., 2003; Villa et al., 2008; Wakatsuki et al., 2003; Abbas et al., 2004; Christodoulakos et al., 2006; de Kraker et al., 2004; Stadberg et al., 1996; Koh et al., 2004; Taechakraichana et al., 2000) (WMD: −0.11, 95% CI: −0.26 to 0.04, I2 = 86%) (Figure 6A) and LDL-C (Cheng et al., 1993; Stadberg et al., 1996; Taechakraichana et al., 2000; Sanada et al., 2003; Wakatsuki et al., 2003; de Kraker et al., 2004; Koh et al., 2004; Christodoulakos et al., 2006; Villa et al., 2008; Xue et al., 2016) (WMD: 0.06, 95% CI: −0.17 to 0.29, I2 = 96%) (Figure 6B). Because of only one study evaluated the effects of different doses on Lp(a), meta-analysis was not carried out.
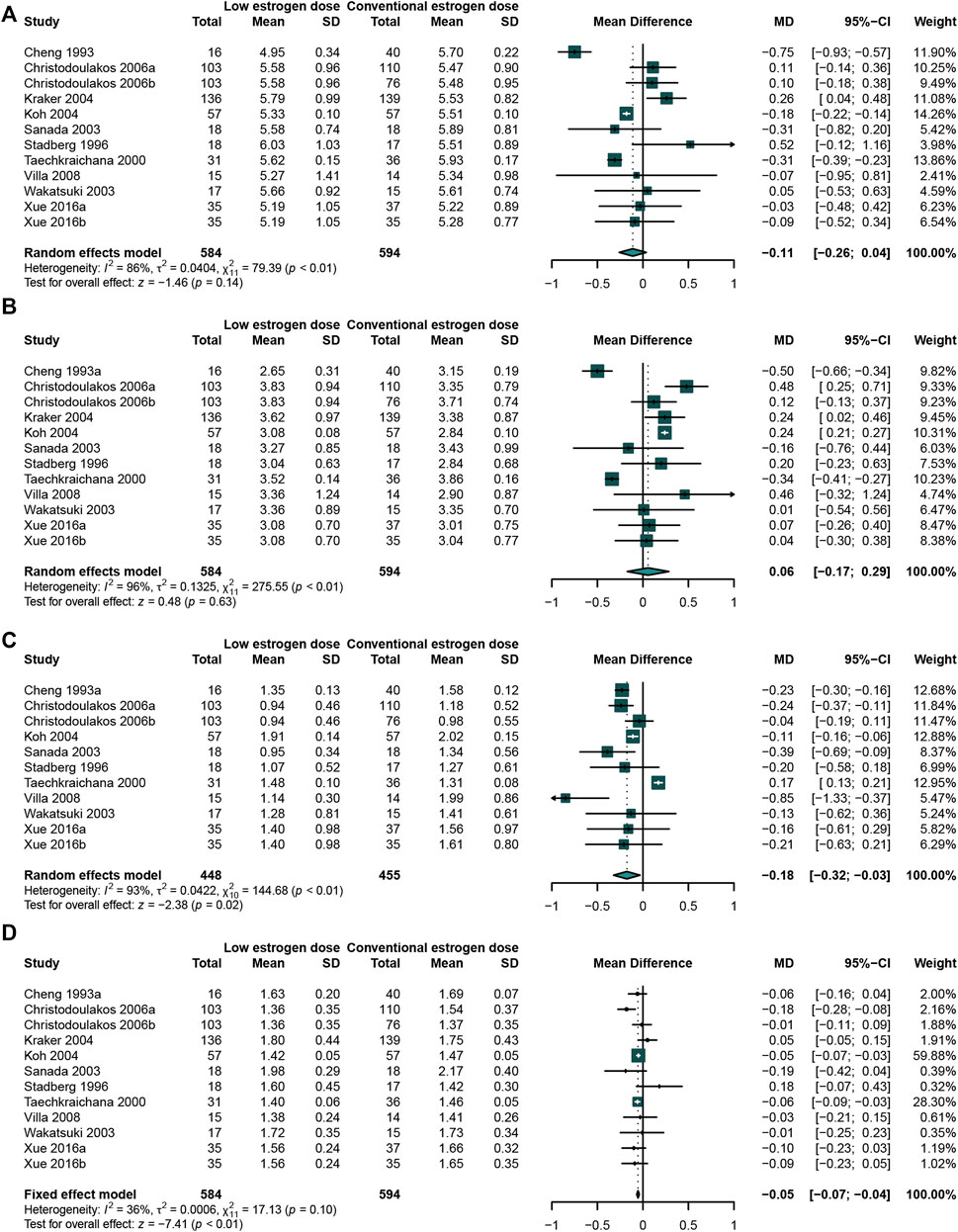
FIGURE 6. Studies comparing low-dose estrogen with conventional-dose estrogen. A low-dose estrogen led to a significant reduction in TG concentration compared with a conventional-dose estrogen. (A) TC concentration; (B) LDL-C concentration; (C) TG concentration; (D) HDL-C concentration; (E) Lp(a) concentration.
Comparing the Effects of Conventional MHT With Tibolone
As tibolone is widely used in mitigating the menopause symptoms, it is necessary to compare the effects of conventional MHT therapy with tibolone on lipids profile. A total of 13 studies (Cayan et al., 2011; Kotecha et al., 2020; Milner et al., 1996; Ziaei et al., 2010; Christodoulakos et al., 2006; Castelo-Branco et al., 1999; Farish et al., 1999; Koh et al., 2003; Koh et al., 2005; Mendoza et al., 2002; Pan et al., 2002; Siseles et al., 1995; Yang et al., 1999)that enrolled 646 participants in conventional MHT group and 828 in tibolone group were analyzed. The outcomes of meta-analysis presented the significantly increasing TG (Cayan et al., 2011; Kotecha et al., 2020; Milner et al., 1996; Ziaei et al., 2010; Christodoulakos et al., 2006; Castelo-Branco et al., 1999; Farish et al., 1999; Koh et al., 2003; Koh et al., 2005; Mendoza et al., 2002; Pan et al., 2002; Siseles et al., 1995; Yang et al., 1999) (WMD:0.42, 95%CI: 0.18 to 0.65, I2 = 98%) (Figure 7C) and HDL-C (Cayan et al., 2011; Kotecha et al., 2020; Milner et al., 1996; Ziaei et al., 2010; Christodoulakos et al., 2006; Castelo-Branco et al., 1999; Farish et al., 1999; Koh et al., 2003; Koh et al., 2005; Mendoza et al., 2002; Pan et al., 2002; Siseles et al., 1995; Yang et al., 1999) (WMD: 0.36, 95% CI: 0.27 to 0.45, I2 = 95%) (Figure 7D) concentration while significantly decreasing LDL-C (Cayan et al., 2011; Kotecha et al., 2020; Milner et al., 1996; Ziaei et al., 2010; Christodoulakos et al., 2006; Castelo-Branco et al., 1999; Farish et al., 1999; Koh et al., 2003; Koh et al., 2005; Mendoza et al., 2002; Pan et al., 2002; Siseles et al., 1995; Yang et al., 1999) (WMD: −0.35, 95% CI: −0.50 to −0.19, I2 = 87%) (Figure 7B) concentration in conventional MHT group. No significant difference was identified in TC (Cayan et al., 2011; Kotecha et al., 2020; Milner et al., 1996; Christodoulakos et al., 2006; Castelo-Branco et al., 1999; Farish et al., 1999; Koh et al., 2003; Koh et al., 2005; Mendoza et al., 2002; Pan et al., 2002; Siseles et al., 1995; Yang et al., 1999) (WMD: 0.15, 95% CI: −0.15 to 0.44, I2 = 96%) (Figure 7A) and Lp(a) (Milner et al., 1996; Farish et al., 1999; Demirol et al., 2007; Kotecha et al., 2020) (WMD: −18.31, 95% CI: −51.84 to 15.22, I2 = 56%) (Figure 7E) concentration between two groups.
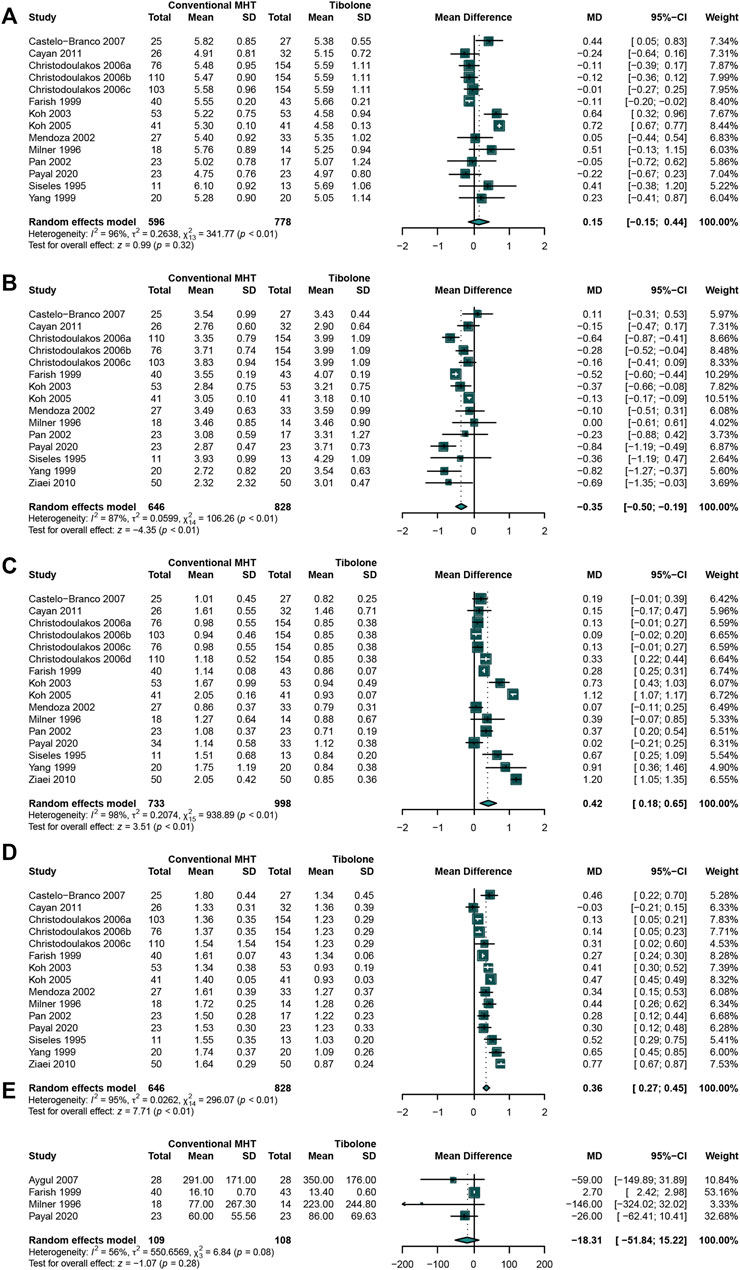
FIGURE 7. Studies comparing conventional MHT with Tibolone. The conventional MHT could decrease LDL-C concentration, increase TG concentration and HDL-C concentration compared with Tibolone. (A) TC concentration; (B) LDL-C concentration; (C) TG concentration; (D) HDL-C concentration; (E) Lp(a) concentration.
Comparing the Effects of Estrogen alone (E-Alone) With Estrogen–Progestogen(E + P) Regimen
In total, 8 studies (Samantray KV et al., 1994; Miller et al., 1995; Farish et al., 1996; Wakatsuki and Sagara, 1996; Davidson et al., 2000; Hemelaar et al., 2003; Zegura et al., 2006; Fernandes et al., 2008) that enrolled 836 participants in E-alone group and 818 in E + P group met the criteria of eligibility. The micronized progesterone was used in 2 studies as separate group (Miller et al., 1995; Espeland et al., 1998) and synthetic progestogen was utilized in all these 8 studies.
The results revealed that E + P regimen significantly increased the concentration of TC (Miller et al., 1995; Davidson et al., 2000; Fernandes et al., 2008; Hemelaar et al., 2003; Samantray KV et al., 1994; Zegura et al., 2006; Farish et al., 1996; Wakatsuki and Sagara, 1996) (WMD: 0.15, 95% CI: 0.09 to 0.20, I2 = 18%) (Figure 8A), LDL-C (Miller et al., 1995; Fernandes et al., 2008; Hemelaar et al., 2003; Samantray KV et al., 1994; Zegura et al., 2006; Farish et al., 1996; Wakatsuki and Sagara, 1996) (WMD: 0.12, 95% CI: 0.07 to 0.17, I2 = 29%) (Figure 8B), HDL-C (Miller et al., 1995; Fernandes et al., 2008; Hemelaar et al., 2003; Samantray KV et al., 1994; Zegura et al., 2006; Farish et al., 1996; Wakatsuki and Sagara, 1996) (WMD: 0.10, 95% CI: 0.03 to 0.18, I2 = 87%) (Figure 8D), and Lp(a) (Farish et al., 1996; Espeland et al., 1998; Davidson et al., 2000; Hemelaar et al., 2003; Zegura et al., 2006) (WMD: 44.58, 95% CI:28.09 to 61.06, I2 = 90%) (Figure 8E) concentration compared with E-alone. No significant difference was found in TG (Miller et al., 1995; Fernandes et al., 2008; Hemelaar et al., 2003; Samantray KV et al., 1994; Zegura et al., 2006; Farish et al., 1996; Wakatsuki and Sagara, 1996) concentration between these two groups (WMD: 0.05, 95% CI: −0.04 to 0.13, I2 = 64%) (Figure 8C).
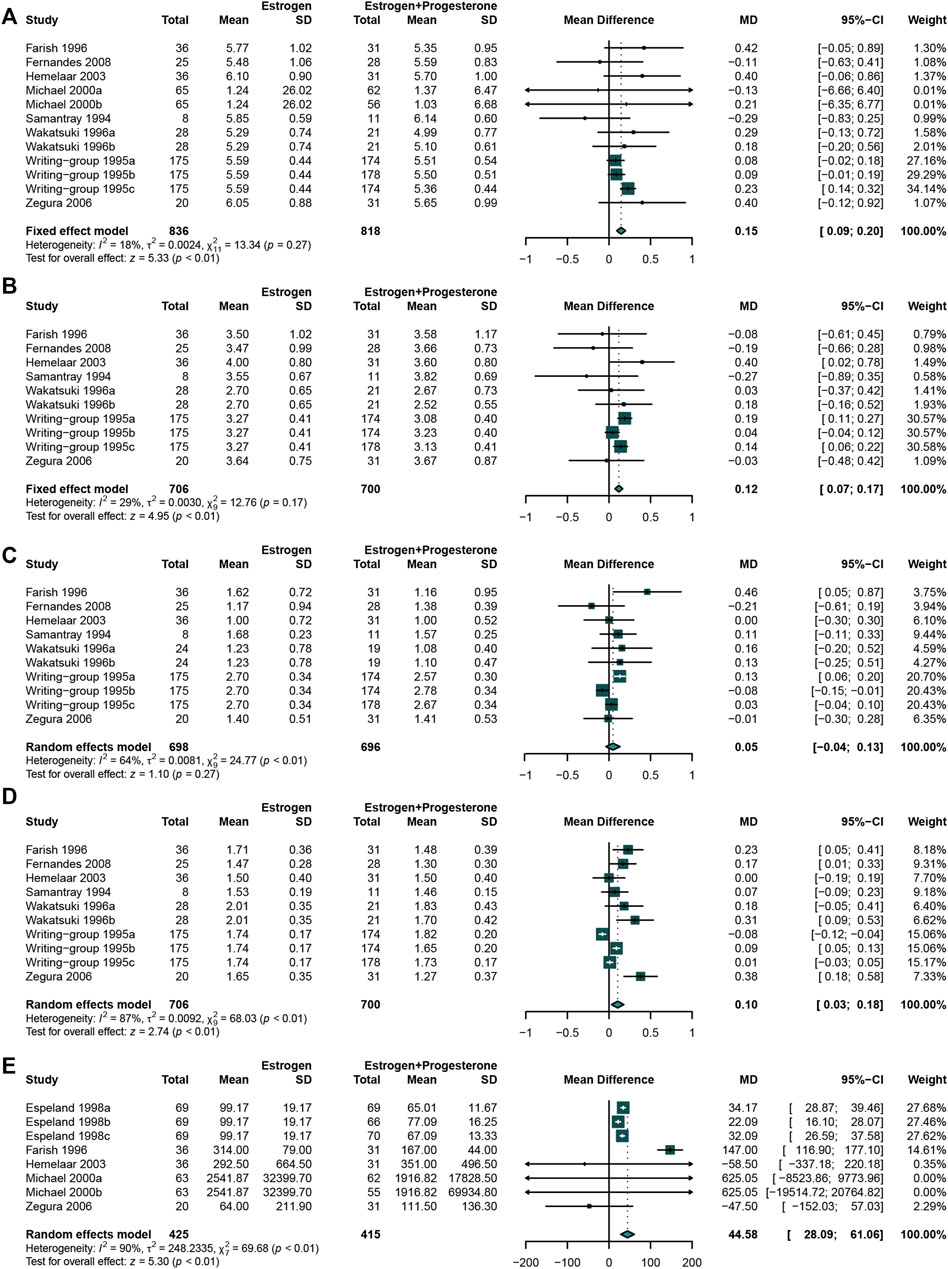
FIGURE 8. Studies comparing estrogen alone with estrogen plus progestogen regimen. The estrogen plus progestogen regimen could significantly increased TC, LDL-C, HDL-C, and Lp(a) concentration compared with estrogen alone. (A) TC concentration; (B) LDL-C concentration; (C) TG concentration; (D) HDL-C concentration; (E) Lp(a) concentration.
Sensitivity Analysis and Publication Bias Assessment
Considering that most of the pooled outcomes had an I2 greater than 50%, one-by-one exclusion was performed as a sensitivity analysis to confirm the robustness of the outcomes. While omitting the study de Kraker 2004 (de Kraker et al., 2004), low-dose estrogen seems to decrease TC significantly (MD: −0.17,95% CI: −0.31 to −0.02) (Figure 9). The cause of unstable results may be attributed to the difference type of estrogen used in this study. Also, an unstable result was found in TG of comparing E-alone and E + P regimen. When study of writing−group 1995 (Miller et al., 1995) was excluded, E + P group could significantly higher TG (MD: 0.08, 95% CI: 0.01–0.15) (Figure 10) than Estrogen alone. The longer period of using MPA may be a source of instability. Egger test and funnel plots suggested that there was little indication of publication bias in studies with more than 10 trials (Figure 11).
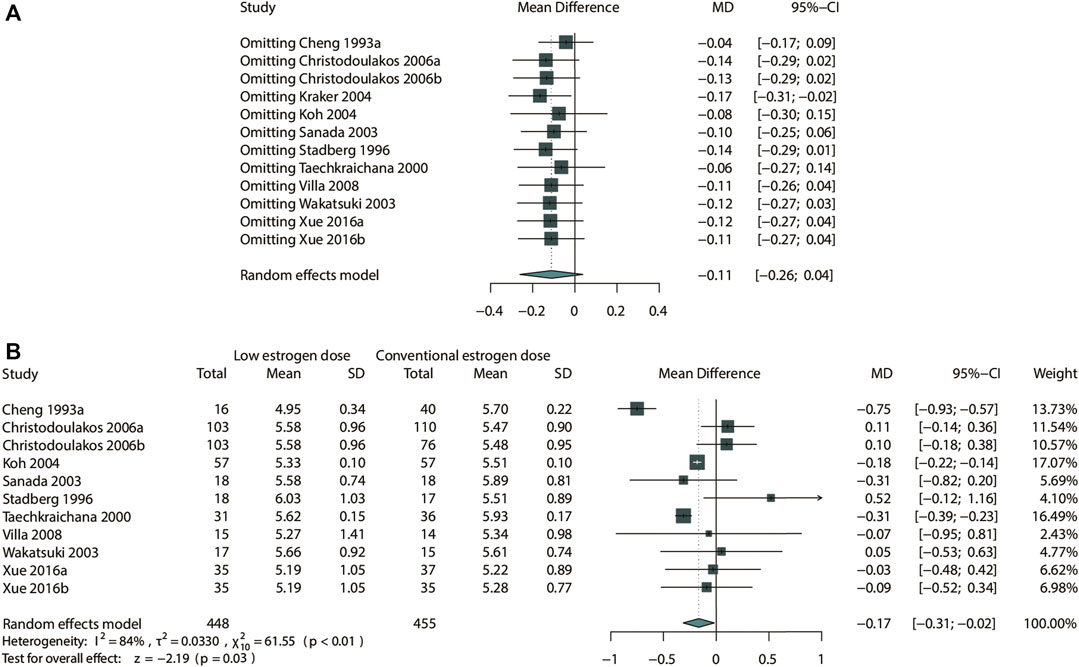
FIGURE 9. Sensitivity analysis for TC in the subgroup of low-dose estrogen. Sensitivity analysis suggested that while omitting the study Kraker 2004, low-dose estrogen could decrease TC significantly (MD: −0.17 mmol/L, 95% CI: −0.31 to −0.02 mmol/L). (A) Sensitivity analysis; (B) forrest plot after omitted study Kraker 2004.
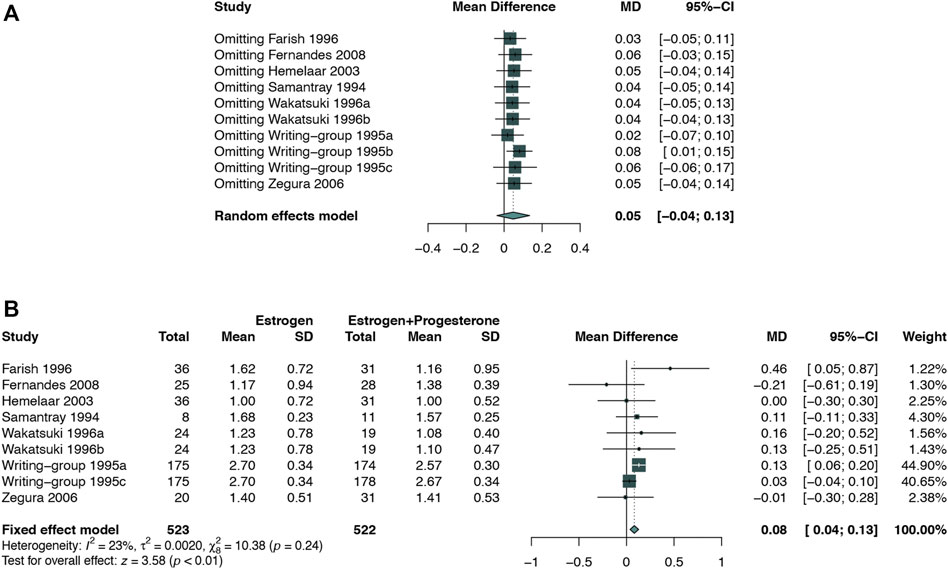
FIGURE 10. Sensitivity analysis for TG in the subgroup of estrogen alone vs. E + P regimen. Sensitivity analysis suggested that while omitting one group of the study Writing−group 1995b, E + P group cause a significantly higher TG (WMD: 0.08 mmol/L, 95% CI: 0.01–0.15 mmol/L) than Estrogen alone. (A) Sensitivity analysis; (B) forest plot after omitted study Writing−group 1995b.
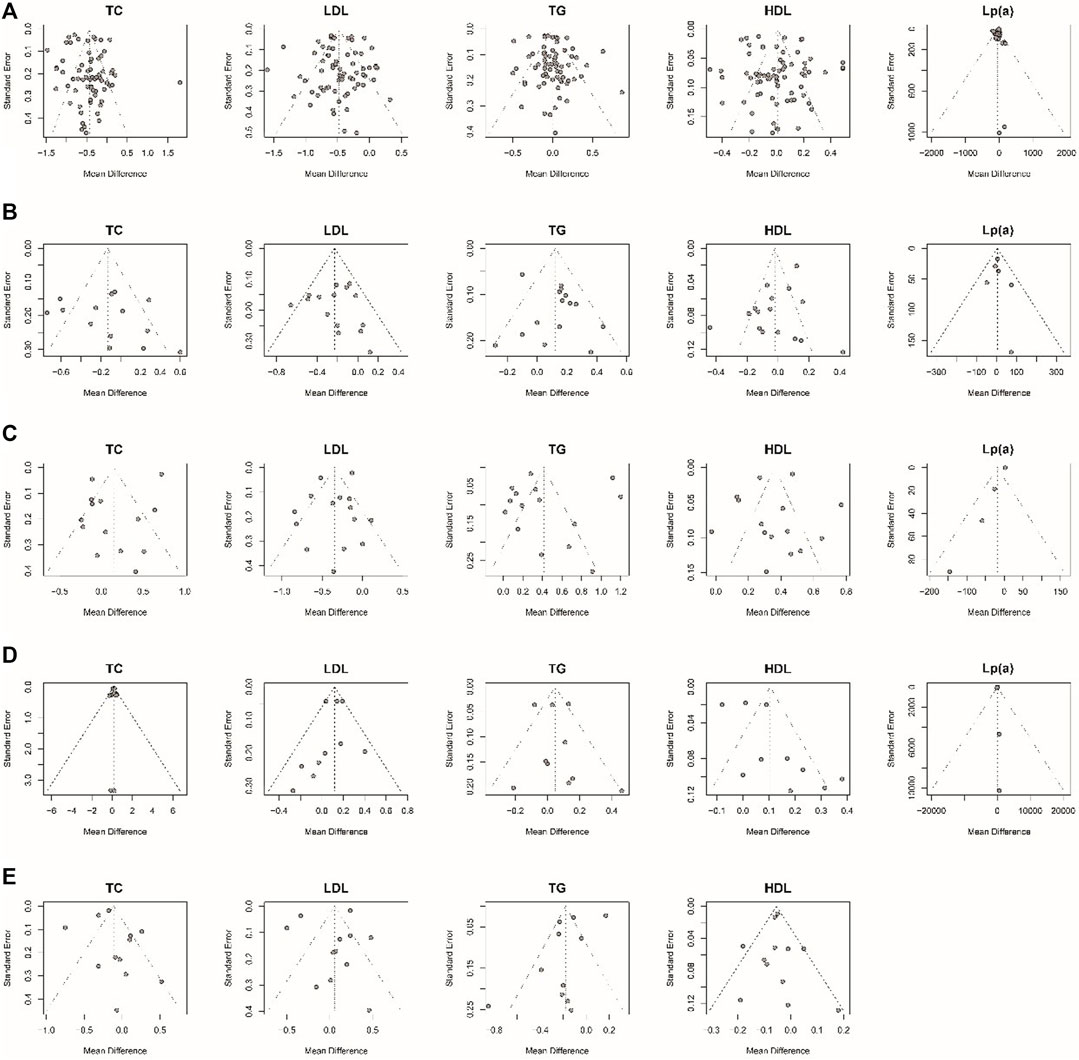
FIGURE 11. Funnel plots examining publication bias. The Egger test suggested that there was no evidence of publication bias in studies with more than 10 articles. (A) MHT vs. Placebo or no treatment; (B) oral MHT vs. transdermal MHT; (C) Conventional MHT vs. Tibolone; (D) Estrogen vs. Estrogen-Progestogen; (E) Low-dose MHT vs. Conventional MHT.
Discussion
Endogenous Sex Hormones and CVD Risk for Women
Endogenous sex hormones are involved in the pathogenesis of cardiovascular disease (CVD) in women. Studies have shown that estradiol (E2), the major form of ovarian estrogen before menopause, plays an active role in metabolic actions (Franck et al., 2013). Higher estrone levels were related to a higher brachial flow-mediated dilation (ie, better endothelial function) (Thurston et al., 2018). After menopause there is a drastic change in the endogenous hormonal milieu, with a decrease in estradiol. And the circulating estrone (E1) levels are relatively higher than E2. E1 is produced mostly by the conversion of androgens in peripheral tissues, and could be also converted from E2 by 17 β⁃ Hydroxysteroid dehydrogenase, E1 secretion also decreased after menopause and was equivalent to nearly 1/3 before menopause (Qureshi et al., 2020). Studies showed that higher E1 is associated with more stable plaque (Cortés Yamnia et al., 2020) and better endothelial function (Thurston et al., 2018), lower levels of E1 have been associated with increased all-cause mortality among postmenopausal women (de Padua Mansur et al., 20122012), which proved the importance of estrogen on CVD. In addition to E1 and E2, sex hormone binding globulin (SHBG) and testosterone (T) may be associated with future risk of CVD also. One study showed that a more androgenic hormone profile (i.e., higher levels of free T and lower levels of SHBG) was associated with greater Coronary Artery Calcium (CAC) progression up to 10 years in postmenopausal women (Subramanya et al., 2019). In summary, as deficiency of endogenous estrogen after menopause and the importance of estrogen for CVD, the exogenous estrogen based MHT should be benefit for CVD and related high-risk factors in theory.
The Effects of MHT on Lipid Profile in Postmenopausal Women
Our systematic review indicated that compared with placebo or no treatment, MHT could significantly decrease the concentrations of TC, LDL-C, and Lp (a). Lp(a) is an independent risk factor for CVD and recurrent ischemic stroke (Nordestgaard et al., 2010), the previous study showed the similar result of MHT on Lp(a) with us (van Dam-Nolen et al., 2021). As for the TG concentration, previous study had showed that MHT could significantly increase it (Stevenson et al., 2015). However, no significant difference in TG between two groups was found in our study. Hence, generally speaking, MHT was associated with favorable changes in lipid parameters whether short-term or long-term using in postmenopausal women.
The bioavailability of oral estrogen is mainly low due to first-pass metabolism, which may result in adverse reactions that influence the risk of CVD. Transdermal MHT is more appropriate for cases with a high-risk of CVD or dyslipidemia than oral agents. The results of our study showed that oral MHT significantly increased TG concentration compared with transdermal MHT. In addition, a meta-analysis conducted in 2006 revealed that oral MHT adversely affected C-reactive protein (CRP) level (Ambikairajah et al., 2019). Therefore, for women with hypertriglyceridemia or other high-risk factors of CVD, transdermal route is recommended. However, oral MHT is associated with positive effects in LDL-C concentration in our study. As we know, the LDL-C concentration is the main risk factor for the occurrence and development of atherosclerosis, and was regarded as an important index to assess the risk of atherosclerotic CVD (ASCVD) (Stone et al., 2013; Jacobson et al., 2015). Hence, for women without any risk of CVD or hypertriglyceridemia, oral MHT could possibly provide greater benefits.
Considering the safety factor, the minimum effective dose of estrogen was recommanded (Menopause Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association, 2018). However, whether the low-dose MHT could achieve the same effects on lipid profile as conventional-dose MHT is still confused. One study indicated that low-dose MHT was associated with higher levels of TC and LDL-C, lower TG level (Casanova et al., 2015). Our study showed the similar benefit on TG in low-dose MHT group, but no significant difference in TC and LDL-C levels between two groups. Furthermore, low-dose MHT was found could decrease HDL-C level. Epidemiologically, a low plasma level of HDL-C was associated with an increased risk of ischemic CVD (Haase et al., 2012). Taken together, the advantage of low-dose MHT on lipid profile was possibly only confined to the TG level.
Tibolone is a synthetic hormone with estrogenic, progestogenic, and androgenic properties, and was widely used for alleviating menopausal symptoms in postmenopausal women. Tibolone has shown promising effects on improving depression and libido, and does not increase breast density (Cummings et al., 2008). As for its effects on lipid profile, a meta-analysis10 conducted in 2017 concluded that there was no significant difference between conventional MHT and Tibolone in Lp(a) concentration, which is similar to our findings. While conventional MHT was found with lower LDL-C level and higher HDL-C level compared with Tibolone, while higher TG concentration. It is suggested that tibolone is more beneficial on TG concentration.
Progestogens are indicated as a part of systemic hormone therapy in women with an intact uterus, preventing estrogen-induced endometrial hyperplasia and cancer during estrogen exposure. However, an increased risk of CHD in women receiving estrogen plus progestogen therapy rather than in those receiving CEE alone was reported (Falkeborn et al., 1992). Thus, it should be indicated whether progestogen contributes to adverse outcomes of CVD. However, no large-scale RCT has evaluated the lipid profile according to the type of progestogen used. A previous observational study revealed that the addition of progestogens blunts the lipid-related effects (Shufelt and Manson, 2021), and a meta-analysis performed in 2017 indicated that there was no significant difference in the reduction of Lp(a) concentration by E-alone compared with E + P (Anagnostis et al., 2017). The results in our study showed that E + P regimen weakened the benefits of estrogen mono-therapy. However, it should be noted that the progestogens included in our analysis were mainly composed of synthetic progestogen, and further research is required to explore whether natural progesterone could positively influence lipid profile.
Except for routine MHT, selective estrogen receptor modulators (SERMs), such as tamoxifen and raloxifene, are widely used for patients with breast cancer or osteoporosis. SERMs mimic estrogen action in certain tissues while opposing it in others. The effect of SERMs on lipids profile is also an issue worthy of attention. The meta-analysis had showed that tamoxifen can alter the lipid profile in females, particularly by decreasing TC, LDL-C and HDL–C (Alomar et al., 2022). Rraloxifene can increase HDL-C and lower LDL-C and TC (Yang et al., 2021). Thus, SERMs is beneficial to blood lipids in general.
In addition, although the result showed the positive effects of MHT on lipid profile, it needs to be emphasized that MHT is not recommended as first-line therapy for dyslipidemia or for reducing the risk of cardiovascular disease (Panagiotis et al., 2020). For postmenopausal women with carotid atherosclerosis, the prospective study had showed that total estradiol was associated with presence of vulnerable carotid plaque as well as increased risk of stroke (Glisic et al., 2018). Therefore, it is recommended to start MHT in women <60 years of age or <10 years since menopause for the beneficial effects on CVD outcomes (2019 Surveillance of Menopause, 2019; El Khoudary et al., 2020).
For dyslipidemia, the most commonly used medication is HMG-CoA reductase inhibitors (ie, statins). Statin therapy can also have effects on gonada steroidogenesis, since this process requires cholesterol as a biochemical substrate. LDL-C has been shown to be a preferential precursor for the production of ovarian steroid hormones (Grummer and Carroll, 1988). However, no reduction in E2 or E1 in postmenopausal women taking statins, despite a significant decrease in their LDL-C levels (Bairey Merz et al., 2002). But there are many studies showing an association between statin treatment and a reduction in testosterone levels (Stamerra et al., 2021). For polycystic ovary syndrome (PCOS) women, statins could decrease testosterone and Luteinizing hormone (LH)/Follicle stimulating hormone (FSH) ratio (Seyam et al., 2017), which is beneficial in treatment of PCOS. However, the role of statins for primary prevention in postmenopausal women is debated (Cangemi et al., 2017). Evidence-based data of statins for the reduction of CVD events and all-cause mortality in primary prevention in postmenopausal women is needed (El Khoudary et al., 2020).
Limitations
The limitations of the present study should be pointed out. Firstly, among the eligible studies, few studies were specifically designed to evaluate the effects of MHT on lipid profile as the primary outcome, restricting the generalization of our findings. Secondly, the lipid profile at baseline in the majority of the included studies was almost normal, while it remained elusive whether MHT would have the similar effects on lipid profile in women with dyslipidemia. Thirdly, owing to the small sample size, the comparison between the effects of different types of progestogen on lipid profile was not comprehensively performed. Therefore, further research needs to be conducted to eliminate the above-mentioned limitations and to confirm our findings.
Conclusion
This meta-analysis indicated that MHT plays a positive role in lipid profile in postmenopausal women. Oral MHT was more effective in reducing LDL-C level than transdermal MHT, while it increased TG concentration. E + P regimen might blunt the benefit of estrogen on lipid profile.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GN, QY, and XL conceived and designed the study. GN and XY developed the search strategy and data extraction form and drafted the paper. Articles searching, search result screening, data extraction, and risk of bias assessment were performed by XY, WL, QG, and JW. Data verification and analysis were carried out by YW, JL and HY. XY and XL drew the figures and the table. QY provided methodological perspectives and revised the paper.
Funding
National Nature Science Foundation of China (81804132 and 82174161), Natural Science Foundation of Guangdong Province (2021A1515012573), Science and Technology Foundation of Guangzhou City (202102010257), the TCM Research Fund of Guangdong Provincial Hospital of Chinese Medicine (YN2019ML04) (YN10101912), the Research Fund for Bajian Talents of Guangdong Provincial Hospital of Chinese Medicine (No. BJ2022KY09).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, A., Fadel, P. J., Wang, Z., Arbique, D., Jialal, I., and Vongpatanasin, W. (2004). Contrasting Effects of Oral versus Transdermal Estrogen on Serum Amyloid A (SAA) and High-Density Lipoprotein-SAA in Postmenopausal Women. Arterioscler Thromb. Vasc. Biol. 24 (10), e164–7. doi:10.1161/01.ATV.0000140198.16664.8e
Alomar, S. A., Prabahar, M. A. K., Arafah, O. A., Almarshood, F., Baradwan, S., Aboudi, S. A. S., et al. (2022). The Effect of Tamoxifen on the Lipid Profile in Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Exp. Gerontol. 159, 111680. doi:10.1016/j.exger.2021.111680
Ambikairajah, A., Walsh, E., and Cherbuin, N. (2019). Lipid Profile Differences during Menopause: a Review with Meta-Analysis. Menopause 26 (11), 1327–1333. doi:10.1097/GME.0000000000001403
Anagnostis, P., Galanis, P., Chatzistergiou, V., Stevenson, J. C., Godsland, I. F., Lambrinoudaki, I., et al. (2017). The Effect of Hormone Replacement Therapy and Tibolone on Lipoprotein (A) Concentrations in Postmenopausal Women: A Systematic Review and Meta-Analysis. Maturitas 99, 27–36. doi:10.1016/j.maturitas.2017.02.009
Anagnostis, P., Stevenson, J. C., Crook, D., Johnston, D. G., and Godsland, I. F. (2016). Effects of Gender, Age and Menopausal Status on Serum Apolipoprotein Concentrations. Clin. Endocrinol. (Oxf) 85 (5), 733–740. doi:10.1111/cen.13085
Anagnostis, P., Stevenson, J. C., Crook, D., Johnston, D. G., and Godsland, I. F. (2015). Effects of Menopause, Gender and Age on Lipids and High-Density Lipoprotein Cholesterol Subfractions. Maturitas 81 (1), 62–68. doi:10.1016/j.maturitas.2015.02.262
Baber, R. J., Panay, N., and Fenton, A. (2016). 2016 IMS Recommendations on Women's Midlife Health and Menopause Hormone Therapy. Climacteric 19 (2), 109–150. doi:10.3109/13697137.2015.1129166
Bairey Merz, C. N., Olson, M. B., Johnson, B. D., Bittner, V., Hodgson, T. K., Berga, S. L., et al. (2002). Cholesterol-lowering Medication, Cholesterol Level, and Reproductive Hormones in Women: the Women's Ischemia Syndrome Evaluation (WISE). Am. J. Med. 113, 723–727. doi:10.1016/s0002-9343(02)01366-9
Binder, E. F., Birge, S. J., and Kohrt, W. M. (1996). Effects of Endurance Exercise and Hormone Replacement Therapy on Serum Lipids in Older Women. J. Am. Geriatr. Soc. 44 (3), 231–236. doi:10.1111/j.1532-5415.1996.tb00907.x
Bukowska, H., Stanosz, S., Zochowska, E., Millo, B., Sieja, K., Chełstowski, K., et al. (2005). Does the Type of Hormone Replacement Therapy Affect Lipoprotein (A), Homocysteine, and C-Reactive Protein Levels in Postmenopausal Women? Metabolism 54 (1), 72–78. doi:10.1016/j.metabol.2004.07.015
Bunyavejchevin, S., and Limpaphayom, K. K. (2001). The Metabolic and Bone Density Effects of Continuous Combined 17-beta Estradiol and Noresthisterone Acetate Treatments in Thai Postmenopausal Women: a Double-Blind Placebo-Controlled Trial. J. Med. Assoc. Thai 84 (1), 45–53.
Cangemi, R., Romiti, G. F., Campolongo, G., Ruscio, E., Sciomer, S., Gianfrilli, D., et al. (2017). Gender Related Differences in Treatment and Response to Statins in Primary and Secondary Cardiovascular Prevention: The Never-Ending Debate. Pharmacol. Res. 117, 148–155. doi:10.1016/j.phrs.2016.12.027
Casanova, G., Bossardi Ramos, R., Ziegelmann, P., and Spritzer, P. M. (2015). Effects of Low-Dose versus Placebo or Conventional-Dose Postmenopausal Hormone Therapy on Variables Related to Cardiovascular Risk: a Systematic Review and Meta-Analyses of Randomized Clinical Trials. J. Clin. Endocrinol. Metab. 100 (3), 1028–1037. doi:10.1210/jc.2014-3301
Casanova, G., dos Reis, A. M., and Spritzer, P. M. (2015). Low-dose Oral or Non-oral Hormone Therapy: Effects on C-Reactive Protein and Atrial Natriuretic Peptide in Menopause. Climacteric 18 (1), 86–93. doi:10.3109/13697137.2014.940309
Casanova, G., Radavelli, S., Lhullier, F., and Spritzer, P. M. (2009). Effects of Nonoral Estradiol-Micronized Progesterone or Low-Dose Oral Estradiol-Drospirenone Therapy on Metabolic Variables and Markers of Endothelial Function in Early Postmenopause. Fertil. Steril 92 (2), 605–612. doi:10.1016/j.fertnstert.2008.06.049
Castelo-Branco, C., Casals, E., Figueras, F., Sanjuan, A., Vicente, J. J., Balasch, J., et al. (1999). Two-year Prospective and Comparative Study on the Effects of Tibolone on Lipid Pattern, Behavior of Apolipoproteins AI and B. Menopause 6 (2), 92–97. doi:10.1097/00042192-199906020-00004
Castelo-Branco, C., Palacios, S., Vázquez, F., Villero, J., Ferrer, J., Ascaso, C., et al. (2007). Effects on Serum Lipid and Leptin Levels of Three Different Doses of Norethisterone Continuously Combined with a Fixed Dose of 17 beta-Estradiol for Nasal Administration in Postmenopausal Women: a Controlled, Double-Blind Study. Fertil. Steril 88 (2), 383–389. doi:10.1016/j.fertnstert.2006.11.142
Cayan, F., Gen, R., Akbay, E., Dilek, U., and Dilek, S. (2011). The Effect of Hormone Therapy and Tibolone on Glucose and Lipid Metabolism in Healthy Postmenopausal Women. Turk Geriatri Dergisi 14, 19–25.
Cheng, G. J., Liu, J. L., Zhang, Q., Fan, W., Ye, H. F., Wang, Z. Q., et al. (1993). Nylestriol Replacement Therapy in Postmenopausal Women. A Three-Year Prospective Study. Chin. Med. J. (Engl) 106 (12), 911–916.
Christodoulakos, G. E., Lambrinoudaki, I. V., Economou, E. V., Papadias, C., Panoulis, C. P., Kouskouni, E. E., et al. (2006). Differential Effect of Hormone Therapy and Tibolone on Lipids, Lipoproteins, and the Atherogenic index of Plasma. J. Cardiovasc. Pharmacol. 47 (4), 542–548. doi:10.1097/01.fjc.0000211747.16573.d5
Conard, J., Basdevant, A., Thomas, J. L., Ochsenbein, E., Denis, C., Guyene, T. T., et al. (1995). Cardiovascular Risk Factors and Combined Estrogen-Progestin Replacement Therapy: a Placebo-Controlled Study with Nomegestrol Acetate and Estradiol. Fertil. Steril 64 (5), 957–962. doi:10.1016/s0015-0282(16)57909-6
Conard, J., Gompel, A., Pelissier, C., Mirabel, C., and Basdevant, A. (1997). Fibrinogen and Plasminogen Modifications during Oral Estradiol Replacement Therapy. Fertil. Steril 68 (3), 449–453. doi:10.1016/s0015-0282(97)00220-3
Cortés Yamnia, I., Barinas-Mitchell, E., Suder Egnot, N., Bhasin, S., Jasuja, R., Santoro, N., et al. (2020). Associations of Endogenous Sex Hormones with Carotid Plaque Burden and Characteristics in Midlife Women.[J]. J. Clin. Endocrinol. Metab. 105. undefined. doi:10.1210/clinem/dgz327
Cummings, S. R., Ettinger, B., Delmas, P. D., Kenemans, P., Stathopoulos, V., Verweij, P., et al. (2008). The Effects of Tibolone in Older Postmenopausal Women. N. Engl. J. Med. 359 (7), 697–708. doi:10.1056/NEJMoa0800743
Davidson, M. H., Maki, K. C., Marx, P., Maki, A. C., Cyrowski, M. S., Nanavati, N., et al. (2000). Effects of Continuous Estrogen and Estrogen-Progestin Replacement Regimens on Cardiovascular Risk Markers in Postmenopausal Women. Arch. Intern. Med. 160 (21), 3315–3325. doi:10.1001/archinte.160.21.3315
de Kraker, A. T., Kenemans, P., Smolders, R. G., Kroeks, M. V., and van der Mooren, M. J. (2004). The Effects of 17 Beta-Oestradiol Plus Dydrogesterone Compared with Conjugated Equine Oestrogens Plus Medroxyprogesterone Acetate on Lipids, Apolipoproteins and Lipoprotein(a). Maturitas 49 (3), 253–263. doi:10.1016/j.maturitas.2004.05.006
de Padua Mansur, A., Silva, T. C., Takada, J. Y., Avakian, S. D., Strunz, C. M., Machado César, L. A., et al. (201220122). Long-term Prospective Study of the Influence of Estrone Levels on Events in Postmenopausal Women with or at High Risk for Coronary Artery Disease. ScientificWorldJournal 2012 (-6-4), 363595. doi:10.1100/2012/363595
Demirol, A., Guven, S., Guvendag Guven, E. S., Kirazli, S., Gurgan, T., and Ayhan, A. (2007). Comparison of the Effects of Tibolone and Estrogen Therapy on Hemostasis in Surgical Menopause: a Randomized, Double-Blind, Placebo-Controlled Study. Fertil. Steril 87 (4), 842–848. doi:10.1016/j.fertnstert.2006.08.090
Draper, M. W., Flowers, D. E., Huster, W. J., Neild, J. A., Harper, K. D., and Arnaud, C. (1996). A Controlled Trial of Raloxifene (LY139481) HCl: Impact on Bone Turnover and Serum Lipid Profile in Healthy Postmenopausal Women. J. Bone Miner Res. 11 (6), 835–842. doi:10.1002/jbmr.5650110615
Duvernoy, C. S., Rose, P. A., Kim, H. M., Kehrer, C., and Brook, R. D. (2002). Combined Continuous Ethinyl Estradiol/norethindrone Acetate Does Not Improve Forearm Blood Flow in Postmenopausal Women at Risk for Cardiovascular Events: a Pilot Study. J. Womens Health (Larchmt) 16 (7), 963–970. doi:10.1089/jwh.2006.0321
El Khoudary, S. R., Aggarwal, B., Beckie, T. M., Hodis, H. N., Johnson, A. E., Langer, R. D., et al. (2020). Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 142, e506–e532. doi:10.1161/CIR.0000000000000912
Espeland, M. A., Marcovina, S. M., Miller, V., Wood, P. D., Wasilauskas, C., Sherwin, R., et al. (1998). Effect of Postmenopausal Hormone Therapy on Lipoprotein(a) Concentration. PEPI Investigators. Postmenopausal Estrogen/Progestin Interventions. Circulation 97 (10), 979–986. doi:10.1161/01.cir.97.10.979
Faguer de Moustier, B., Conard, J., Guyene, T. T., Sitt, Y., Denys, I., Arnoux-Rouveyre, M., et al. (1989). Comparative Metabolic Study of Percutaneous versus Oral Micronized 17 Beta-Oestradiol in Replacement Therapy. Maturitas 11 (4), 275–286. doi:10.1016/0378-5122(89)90024-8
Falkeborn, M., Persson, I., Adami, H. O., Bergström, R., Eaker, E., Lithell, H., et al. (1992). The Risk of Acute Myocardial Infarction after Oestrogen and Oestrogen-Progestogen Replacement. Br. J. Obstet. Gynaecol. 99 (10), 821–828. doi:10.1111/j.1471-0528.1992.tb14414.x
Farish, E., Barnes, J. F., Fletcher, C. D., Ekevall, K., Calder, A., and Hart, D. M. (1999). Effects of Tibolone on Serum Lipoprotein and Apolipoprotein Levels Compared with a Cyclical Estrogen/progestogen Regimen. Menopause 6 (2), 98–104. doi:10.1097/00042192-199906020-00005
Farish, E., Spowart, K., Barnes, J. F., Fletcher, C. D., Calder, A., Brown, A., et al. (1996). Effects of Postmenopausal Hormone Replacement Therapy on Lipoproteins Including Lipoprotein(a) and LDL Subfractions. Atherosclerosis 126 (1), 77–84. doi:10.1016/0021-9150(96)05895-9
Fernandes, C. E., Pompei, L. M., Machado, R. B., Ferreira, J. A., Melo, N. R., and Peixoto, S. (2008). Effects of Estradiol and Norethisterone on Lipids, Insulin Resistance and Carotid Flow. Maturitas 59 (3), 249–258. doi:10.1016/j.maturitas.2008.02.001
Franck, M. J., Clegg, D. J., and Hevener, A. L. (2013). The Role of Estrogens in Control of Energy Balance and Glucose homeostasis.[J]. Endocr. Rev. (3), 309–338.
Glisic, M., Mujaj, B., Rueda-Ochoa, O. L., Asllanaj, E., Laven, J. S. E., Kavousi, M., et al. (2018). Associations of Endogenous Estradiol and Testosterone Levels with Plaque Composition and Risk of Stroke in Subjects with Carotid Atherosclerosis. Circ. Res. 122, 97–105. doi:10.1161/CIRCRESAHA.117.311681
Godsland, I. F. (2001). Effects of Postmenopausal Hormone Replacement Therapy on Lipid, Lipoprotein, and Apolipoprotein (A) Concentrations: Analysis of Studies Published from 1974-2000. Fertil. Steril 75 (5), 898–915. doi:10.1016/s0015-0282(01)01699-5
Gräser, T., Müller, A., Druckman, R., and Oettel, M. (2001). Effects of a Combination of 2 MG Estradiol Valerate and 3 MG Dienogest on Coagulation, Lipid Profile, and Glucose Metabolism in Postmenopausal Women. Drugs of Today 37, 87–99.
Gregersen, I., Høibraaten, E., Holven, K. B., Løvdahl, L., Ueland, T., Mowinckel, M. C., et al. (2019). Effect of Hormone Replacement Therapy on Atherogenic Lipid Profile in Postmenopausal Women. Thromb. Res. 184, 1–7. doi:10.1016/j.thromres.2019.10.005
Grummer, R. R., and Carroll, D. J. (1988). A Review of Lipoprotein Cholesterol Metabolism: Importance to Ovarian Function. J. Anim. Sci. 66, 3160–3173. doi:10.2527/jas1988.66123160x
Haase, C. L., Tybjærg-Hansen, A., Qayyum, A. A., Schou, J., Nordestgaard, B. G., and Frikke-Schmidt, R. (2012). LCAT, HDL Cholesterol and Ischemic Cardiovascular Disease: a Mendelian Randomization Study of HDL Cholesterol in 54,500 Individuals. J. Clin. Endocrinol. Metab. 97 (2), E248–E256. doi:10.1210/jc.2011-1846
Haines, C., Chung, T., Chang, A., Masarei, J., Tomlinson, B., and Wong, E. (1996). Effect of Oral Estradiol on Lp(a) and Other Lipoproteins in Postmenopausal Women. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Arch. Intern. Med. 156 (8), 866–872. doi:10.1001/archinte.156.8.866
Heikkinen, A. M., Tuppurainen, M. T., Niskanen, L., Komulainen, M., Penttilä, I., and Saarikoski, S. (1997). Long-term Vitamin D3 Supplementation May Have Adverse Effects on Serum Lipids during Postmenopausal Hormone Replacement Therapy. Eur. J. Endocrinol. 137 (5), 495–502. doi:10.1530/eje.0.1370495
Hemelaar, M., Kenemans, P., de Bie, L., van de Weijer, P. H., and van der Mooren, M. J. (2006). Intranasal Continuous Combined 17 Beta-Estradiol/norethisterone Therapy Improves the Lipid Profile in Healthy Postmenopausal Women. Fertil. Steril 85 (4), 979–988. doi:10.1016/j.fertnstert.2005.09.036
Hemelaar, M., van der Mooren, M. J., Mijatovic, V., Bouman, A. A., Schijf, C. P., Kroeks, M. V., et al. (2003). Oral, More Than Transdermal, Estrogen Therapy Improves Lipids and Lipoprotein(a) in Postmenopausal Women: a Randomized, Placebo-Controlled Study. Menopause 10 (6), 550–558. doi:10.1097/01.GME.0000064866.58809.E5
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Jacobson, T. A., Ito, M. K., Maki, K. C., Orringer, C. E., Bays, H. E., Jones, P. H., et al. (2015). National Lipid Association Recommendations for Patient-Centered Management of Dyslipidemia: Part 1--full Report. J. Clin. Lipidol. 9 (2), 129–169. doi:10.1016/j.jacl.2015.02.003
Jirapinyo, M., Theppisai, U., Manonai, J., Suchartwatnachai, C., and Jorgensen, L. N. (2003). Effect of Combined Oral Estrogen/progestogen Preparation (Kliogest) on Bone mineral Density, Plasma Lipids and Postmenopausal Symptoms in HRT-Naïve Thai Women. Acta Obstet. Gynecol. Scand. 82 (9), 857–866. doi:10.1034/j.1600-0412.2003.00185.x
J. P. T. T. J. Higgins, J. Chandler, M. Cumpston, T. Li, M. J. Page, and V. A. Welch (Editors) (2021). Cochrane Handbook for Systematic Reviews of Interventions (Cochrane. (updated February 2021).version 6.2.
Ki, E. Y., Hur, S. Y., Park, J. S., Do Han, K., and Park, Y. G. (2016). Differences in the Lipid Profile and Hormone Replacement Therapy Use in Korean Postmenopausal Women: the Korea National Health and Nutrition Examination Survey (KNHANES) 2010-2012. Arch. Gynecol. Obstet. 294 (1), 165–173. doi:10.1007/s00404-015-3982-9
Koh, K. K., Ahn, J. Y., Jin, D. K., Yoon, B. K., Kim, H. S., Kim, D. S., et al. (2003). Significant Differential Effects of Hormone Therapy or Tibolone on Markers of Cardiovascular Disease in Postmenopausal Women: a Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Arterioscler Thromb. Vasc. Biol. 23 (10), 1889–1894. doi:10.1161/01.ATV.0000091502.96745.95
Koh, K. K., Han, S. H., Shin, M. S., Ahn, J. Y., Lee, Y., and Shin, E. K. (2005). Significant Differential Effects of Lower Doses of Hormone Therapy or Tibolone on Markers of Cardiovascular Disease in post-menopausal Women: a Randomized, Double-Blind, Crossover Study. Eur. Heart J. 26 (14), 1362–1368. doi:10.1093/eurheartj/ehi311
Koh, K. K., Shin, M. S., Sakuma, I., Ahn, J. Y., Jin, D. K., Kim, H. S., et al. (2004). Effects of Conventional or Lower Doses of Hormone Replacement Therapy in Postmenopausal Women. Arterioscler Thromb. Vasc. Biol. 24 (8), 1516–1521. doi:10.1161/01.ATV.0000133683.65877.bc
Kotecha, P. T., Godsland, I. F., Crook, D., and Stevenson, J. C. (2020). Effects of Tibolone or Continuous Combined Oestradiol and Norethisterone Acetate on Lipids, High-Density Lipoprotein Subfractions and Apolipoproteins in Postmenopausal Women in a Two-Year, Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Endocrinol. (Oxf) 92 (4), 303–311. doi:10.1111/cen.14155
Labos, G., Trakakis, E., Pliatsika, P., Augoulea, A., Vaggopoulos, V., Basios, G., et al. (2013). Efficacy and Safety of DT56a Compared to Hormone Therapy in Greek post-menopausal Women. J. Endocrinol. Invest. 36 (7), 521–526. doi:10.3275/8926
Lahdenperä, S., Puolakka, J., Pyörälä, T., Luotola, H., and Taskinen, M. R. (1996). Effects of Postmenopausal Estrogen/progestin Replacement Therapy on LDL Particles; Comparison of Transdermal and Oral Treatment Regimens. Atherosclerosis 122 (2), 153–162. doi:10.1016/0021-9150(95)05728-5
Lee, J. S., Chang, P. Y., Zhang, Y., Kizer, J. R., Best, L. G., and Howard, B. V. (2017). Triglyceride and HDL-C Dyslipidemia and Risks of Coronary Heart Disease and Ischemic Stroke by Glycemic Dysregulation Status: The Strong Heart Study. Diabetes care 40 (4), 529–537. doi:10.2337/dc16-1958
Lewis-Barned, N. J., Sutherland, W. H., Walker, R. J., Walker, H. L., De Jong, S. A., Edwards, E. A., et al. (1999). Plasma Cholesterol Esterification and Transfer, the Menopause, and Hormone Replacement Therapy in Women. J. Clin. Endocrinol. Metab. 84 (10), 3534–3538. doi:10.1210/jcem.84.10.6022
Luyer, M. D., Khosla, S., Owen, W. G., and Miller, V. M. (2001). Prospective Randomized Study of Effects of Unopposed Estrogen Replacement Therapy on Markers of Coagulation and Inflammation in Postmenopausal Women. J. Clin. Endocrinol. Metab. 86 (8), 3629–3634. doi:10.1210/jcem.86.8.7768
Manson, J. E., Aragaki, A. K., Rossouw, J. E., Anderson, G. L., Prentice, R. L., LaCroix, A. Z., et al. (2017). Menopausal Hormone Therapy and Long-Term All-Cause and Cause-specific Mortality: The Women's Health Initiative Randomized Trials. Jama 318 (10), 927–938. doi:10.1001/jama.2017.11217
Manson, J. E., Chlebowski, R. T., Stefanick, M. L., Aragaki, A. K., Rossouw, J. E., Prentice, R. L., et al. (2013). Menopausal Hormone Therapy and Health Outcomes during the Intervention and Extended Poststopping Phases of the Women's Health Initiative Randomized Trials. Jama 310 (13), 1353–1368. doi:10.1001/jama.2013.278040
McQueen, M. J., Hawken, S., Wang, X., Ounpuu, S., Sniderman, A., Probstfield, J., et al. (2008). Lipids, Lipoproteins, and Apolipoproteins as Risk Markers of Myocardial Infarction in 52 Countries (The INTERHEART Study): a Case-Control Study. Lancet 372 (9634), 224–233. doi:10.1016/S0140-6736(08)61076-4
Mendoza, N., Pisón, J. A., Fernández, M., Sánchez, M. C., Malde, J., and Miranda, J. A. (2002). Prospective, Randomised Study with Three HRT Regimens in Postmenopausal Women with an Intact Uterus. Maturitas 41 (4), 289–298. doi:10.1016/s0378-5122(01)00298-5
Menopause Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association (2018). Chinese Guideline on Menopause Management and Menopause Hormone Therapy (2018). Zhonghua fu chan ke za zhi 53 (11), 729–739. doi:10.3760/cma.j.issn.0529-567x.2018.11.001
Mercuro, G., Vitale, C., Fini, M., Zoncu, S., Leonardo, F., and Rosano, G. M. (2003). Lipid Profiles and Endothelial Function with Low-Dose Hormone Replacement Therapy in Postmenopausal Women at Risk for Coronary Artery Disease: a Randomized Trial. Int. J. Cardiol. 89 (2-3), 257–265. doi:10.1016/s0167-5273(02)00505-3
Meschia, M., Bruschi, F., Soma, M., Amicarelli, F., Paoletti, R., and Crosignani, P. (1998). Effects of Oral and Transdermal Hormone Replacement Therapy on Lipoprotein(A) and Lipids: a Randomized Controlled Trial. Menopause 5 (3), 157–162. doi:10.1097/00042192-199805030-00005
Mijatovic, V., Kenemans, P., and van der Moore, M. J. (1999). Postmenopausal Oestradiol-Dydrogesterone Therapy Favourably Affects Fibrinolysis and Lp(a) in Healthy Women. Fibrinolysis and Proteolysis 13 (4), 177–183. doi:10.1016/s0268-9499(99)90069-3
Miller, V. T., LaRosa, J., Barnabei, V., Kessler, C., Levin, G., and Smith-Roth, A. (1995). Effects of Estrogen or Estrogen/progestin Regimens on Heart Disease Risk Factors in Postmenopausal Women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. Jama 273 (3), 199–208. doi:10.1001/jama.1995.03520270033028
Milner, M. H., Sinnott, M. M., Cooke, T. M., Kelly, A., McGill, T., and Harrison, R. F. (1996). A 2-year Study of Lipid and Lipoprotein Changes in Postmenopausal Women with Tibolone and Estrogen-Progestin. Obstet. Gynecol. 87 (4), 593–599. doi:10.1016/0029-7844(95)00468-8
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Munk-Jensen, N., Ulrich, L. G., Obel, E. B., Nielsen, S. P., Edwards, D., and Meinertz, H. (1994). Continuous Combined and Sequential Estradiol and Norethindrone Acetate Treatment of Postmenopausal Women: Effect of Plasma Lipoproteins in a Two-Year Placebo-Controlled Trial. Am. J. Obstet. Gynecol. 171 (1), 132–138. doi:10.1016/0002-9378(94)90458-8
Nii, S., Shinohara, K., Matsushita, H., Noguchi, Y., Watanabe, K., and Wakatsuki, A. (2016). Hepatic Effects of Estrogen on Plasma Distribution of Small Dense Low-Density Lipoprotein and Free Radical Production in Postmenopausal Women. J. Atheroscler. Thromb. 23 (7), 810–818. doi:10.5551/jat.33175
Nordestgaard, B. G., Chapman, M. J., Ray, K., Borén, J., Andreotti, F., Watts, G. F., et al. (2010). Lipoprotein(a) as a Cardiovascular Risk Factor: Current Status. Eur. Heart J. 31, 2844–2853. doi:10.1093/eurheartj/ehq386
Odmark, I. S., Bäckström, T., Haeger, M., Jonsson, B., and Bixo, M. (2004). Effects of Continuous Combined Conjugated Estrogen/medroxyprogesterone Acetate and 17beta-Estadiol/norethisterone Acetate on Lipids and Lipoproteins. Maturitas 48 (2), 137–146. doi:10.1016/j.maturitas.2003.08.004
Oral, B., and Ozbaşar, D. (2003). The Effect of Sodium Monofluorophosphate Therapy on Lipid and Lipoprotein Metabolism in Postmenopausal Women. Eur. J. Obstet. Gynecol. Reprod. Biol. 107 (2), 180–184. doi:10.1016/s0301-2115(02)00404-9
Pan, H. A., Wang, S. T., Chen, C. H., Pai, M. C., Wu, M. H., and Huang, K. E. (2002). Flow Resistance in Carotid and Middle Cerebral Arteries in Postmenopausal Women: a Comparative Study of Tibolone and Continuous Combined Hormone Replacement Therapy. Climacteric 5 (3), 259–265. doi:10.1080/cmt.5.3.259.265
Panagiotis, P., Johannes, B., Cano, A., Ceausu, L., Chedraui, P., Durmusoglu, F., et al. (2020). Menopause Symptom Management in Women with Dyslipidemias: An EMAS Clinical Guide. Maturitas 135, 82–88. doi:10.1016/j.maturitas.2020.03.007
Perrone, G., Stefanutti, C., Galoppi, P., Anelli, G., Capri, O., Lucani, G., et al. (1996). Effect of Oral and Transdermal Hormone Replacement Therapy on Lipid Profile and Lp(a) Level in Menopausal Women with Hypercholesterolemia. Int. J. Fertil. Menopausal Stud. 41 (6), 509–515.
Pu, D., Tan, R., Yu, Q., and Wu, J. (2017). Metabolic Syndrome in Menopause and Associated Factors: a Meta-Analysis. Climacteric 20 (6), 583–591. doi:10.1080/13697137.2017.1386649
Qureshi, R., Picon-Ruiz, M., Aurrekoetxea-Rodriguez, I., Nunes de Paiva, V., D'Amico, M., Yoon, H., et al. (2020). The Major Pre- and Postmenopausal Estrogens Play Opposing Roles in Obesity-Driven Mammary Inflammation and Breast Cancer Development. Cell Metab 31 (6), 1154–e9. doi:10.1016/j.cmet.2020.05.008
Samantray Kv, M., Majumdar, S., Khunnu, B., Ray, S., and Sehgal, A. (1994). Hormone Replacement Therapy in Postmenopausal Women, 28. German National Library of Medicine, 62–69.
Samsioe, G., Li, C., Borgfeldt, C., Wilawan, K., Aberg, A., and Larsen, S. (2002). Changes in Lipid and Lipoprotein Profile in Postmenopausal Women Receiving Low-Dose Combinations of 17beta-Estradiol and Norethisterone Acetate. Menopause 9 (5), 335–342. doi:10.1097/00042192-200209000-00006
Sanada, M., Higashi, Y., Nakagawa, K., Tsuda, M., Kodama, I., Kimura, M., et al. (2003). A Comparison of Low-Dose and Standard-Dose Oral Estrogen on Forearm Endothelial Function in Early Postmenopausal Women. J. Clin. Endocrinol. Metab. 88 (3), 1303–1309. doi:10.1210/jc.2002-021147
Seed, M., Sands, R. H., McLaren, M., Kirk, G., and Darko, D. (2000). The Effect of Hormone Replacement Therapy and Route of Administration on Selected Cardiovascular Risk Factors in post-menopausal Women. Fam. Pract. 17 (6), 497–507. doi:10.1093/fampra/17.6.497
Sendag, F., Karadadas, N., Ozsener, S., and Bilgin, O. (2002). Effects of Sequential Combined Transdermal and Oral Hormone Replacement Therapies on Serum Lipid and Lipoproteins in Postmenopausal Women. Arch. Gynecol. Obstet. 266 (1), 38–43. doi:10.1007/pl00007497
Seyam, E., Gelany, S. A., Gelany, A. A. A., Ahmed, A., Youseff, A. M., Ibrahim, E. M., et al. (2017). Evaluation of Prolonged Use of Statins on the Clinical and Biochemical Abnormalities and Ovulation Dysfunction in Single Young Women with Polycystic Ovary Syndrome[J]. Gynecol. Endocrinol., 1–8.
Shufelt, C. L., and Manson, J. E. (2021). Menopausal Hormone Therapy and Cardiovascular Disease: The Role of Formulation, Dose, and Route of Delivery. J. Clin. Endocrinol. Metab. 106 (5), 1245–1254. doi:10.1210/clinem/dgab042
Siseles, N. O., Halperin, H., Benencia, H. J., Berg, G., Pilnik, S., Mesch, V., et al. (1995). A Comparative Study of Two Hormone Replacement Therapy Regimens on Safety and Efficacy Variables. Maturitas 21 (3), 201–210. doi:10.1016/0378-5122(94)00889-f
Stadberg, E., Mattsson, L.-A., and Uvebrant, M. (1996). Low Doses of 17β-Estradiol and Norethisterone Acetate as Continuous Combined Replacement Therapy in Postmenopausal Women: Lipid Metabolic Effects. Menopause 3 (2), 90–96. doi:10.1097/00042192-199603020-00006
Stamerra, C. A., Ferri, P. C., Giorgini, P., Reiner, Z., Johnston, T. P., and Sahebkar, A. (2021). Statin Therapy and Sex Hormones. Eur. J. Pharmacol. 890, 173745. doi:10.1016/j.ejphar.2020.173745
Stevenson, J. C., Chines, A., Pan, K., Ryan, K. A., and Mirkin, S. (2015). A Pooled Analysis of the Effects of Conjugated Estrogens/Bazedoxifene on Lipid Parameters in Postmenopausal Women from the Selective Estrogens, Menopause, and Response to Therapy (SMART) Trials. J. Clin. Endocrinol. Metab. 100 (6), 2329–2338. doi:10.1210/jc.2014-2649
Stevenson, J. C., Oladipo, A., Manassiev, N., Whitehead, M. I., Guilford, S., and Proudler, A. J. (2004). Randomized Trial of Effect of Transdermal Continuous Combined Hormone Replacement Therapy on Cardiovascular Risk Markers. Br. J. Haematol. 124 (6), 802–808. doi:10.1111/j.1365-2141.2004.04846.x
Stone, N. J., Robinson, J. G., Lichtenstein, A. H., Bairey Merz, C. N., Blum, C. B., Eckel, R. H., et al. (2013). 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63 (25 Pt B), 2889–2934. doi:10.1016/j.jacc.2013.11.002
Subramanya, V., Zhao, D., Ouyang, P., Ying, W., Vaidya, D., Ndumele, C. E., et al. (2019). Association of Endogenous Sex Hormone Levels with Coronary Artery Calcium Progression Among post-menopausal Women in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Cardiovasc. Comput. Tomogr 13, 41–47. doi:10.1016/j.jcct.2018.09.010
Taechakraichana, N., Limpaphayom, K., Ninlagarn, T., Panyakhamlerd, K., Chaikittisilpa, S., and Dusitsin, N. (2000). A Randomized Trial of Oral Contraceptive and Hormone Replacement Therapy on Bone mineral Density and Coronary Heart Disease Risk Factors in Postmenopausal Women. Obstet. Gynecol. 95 (1), 87–94. doi:10.1016/s0029-7844(99)00493-7
Tandon, V. R., Mahajan, A., Sharma, S., and Sharma, A. (2010). Prevalence of Cardiovascular Risk Factors in Postmenopausal Women: A Rural Study. J. Midlife Health 1 (1), 26–29. doi:10.4103/0976-7800.66993
Taskinen, M. R., Puolakka, J., Pyörälä, T., Luotola, H., Bjäörn, M., Kääriänen, J., et al. (1996). Hormone Replacement Therapy Lowers Plasma Lp(a) Concentrations. Comparison of Cyclic Transdermal and Continuous Estrogen-Progestin Regimens. Arterioscler Thromb. Vasc. Biol. 16 (10), 1215–1221. doi:10.1161/01.atv.16.10.1215
Teede, H. J., Liang, Y. L., Kotsopoulos, D., Zoungas, S., Cravent, R., and McGrath, B. P. (2001). A Placebo-Controlled Trial of Long-Term Oral Combined Continuous Hormone Replacement Therapy in Postmenopausal Women: Effects on Arterial Compliance and Endothelial Function. Clin. Endocrinol. (Oxf) 55 (5), 673–682. doi:10.1046/j.1365-2265.2001.01382.x
Terauchi, M., Honjo, H., Mizunuma, H., and Aso, T. (2012). Effects of Oral Estradiol and Levonorgestrel on Cardiovascular Risk Markers in Postmenopausal Women. Arch. Gynecol. Obstet. 285 (6), 1647–1656. doi:10.1007/s00404-012-2222-9
Thurston, R. C., Bhasin, S., Chang, Y., Barinas-Mitchell, E., Matthews, K. A., Jasuja, R., et al. (2018). Reproductive Hormones and Subclinical Cardiovascular Disease in Midlife Women. J. Clin. Endocrinol. Metab. 103, 3070–3077. doi:10.1210/jc.2018-00579
Tilly-Kiesi, M., Lappi, M., Puolakka, J., Luotola, H., Pyörälä, T., and Taskinen, M. R. (1996). Different Effects of Continuous Oestrogen-Progestin and Transdermal Oestrogen with Cyclic Progestin Regimens on Low-Density Lipoprotein Subclasses. Eur. J. Clin. Invest. 26 (12), 1125–1133. doi:10.1046/j.1365-2362.1996.450594.x
Tuck, C. H., Holleran, S., and Berglund, L. (1997). Hormonal Regulation of Lipoprotein(a) Levels: Effects of Estrogen Replacement Therapy on Lipoprotein(a) and Acute Phase Reactants in Postmenopausal Women. Arterioscler Thromb. Vasc. Biol. 17 (9), 1822–1829. doi:10.1161/01.atv.17.9.1822
Ulloa, N., Arteaga, E., Bustos, P., Durán-Sandoval, D., Schulze, K., Castro, G., et al. (2002). Sequential Estrogen-Progestin Replacement Therapy in Healthy Postmenopausal Women: Effects on Cholesterol Efflux Capacity and Key Proteins Regulating High-Density Lipoprotein Levels. Metabolism 51 (11), 1410–1417. doi:10.1053/meta.2002.35580
Vaisar, T., Gordon, J. L., Wimberger, J., Heinecke, J. W., Hinderliter, A. L., Rubinow, D. R., et al. (2021). Perimenopausal Transdermal Estradiol Replacement Reduces Serum HDL Cholesterol Efflux Capacity but Improves Cardiovascular Risk Factors. J. Clin. Lipidol. 15 (1), 151–e0.e150. doi:10.1016/j.jacl.2020.11.009
van Dam-Nolen, D. H. K., van Dijk, A. C., Crombag, G. A. J. C., Lucci, C., Kooi, M. E., Hendrikse, J., et al. (2021). Lipoprotein(a) Levels and Atherosclerotic Plaque Characteristics in the Carotid Artery: The Plaque at RISK (PARISK) Study. Atherosclerosis 329, 22–29. doi:10.1016/j.atherosclerosis.2021.06.004
Villa, P., Sagnella, F., Perri, C., Suriano, R., Costantini, B., Macrí, F., et al. (2008). Low- and Standard-Estrogen Dosage in Oral Therapy: Dose-dependent Effects on Insulin and Lipid Metabolism in Healthy Postmenopausal Women. Climacteric 11 (6), 498–508. doi:10.1080/13697130802471058
Villa, P., Suriano, R., Ricciardi, L., Tagliaferri, V., De Cicco, S., De Franciscis, P., et al. (2011). Low-dose Estrogen and Drospirenone Combination: Effects on Glycoinsulinemic Metabolism and Other Cardiovascular Risk Factors in Healthy Postmenopausal Women. Fertil. Steril 95 (1), 158–163. doi:10.1016/j.fertnstert.2010.07.001
Wakatsuki, A., Okatani, Y., Ikenoue, N., and Fukaya, T. (2002). Different Effects of Oral Conjugated Equine Estrogen and Transdermal Estrogen Replacement Therapy on Size and Oxidative Susceptibility of Low-Density Lipoprotein Particles in Postmenopausal Women. Circulation 106 (14), 1771–1776. doi:10.1161/01.cir.0000032261.12632.d7
Wakatsuki, A., Okatani, Y., Ikenoue, N., Shinohara, K., Watanabe, K., and Fukaya, T. (2003). Effect of Lower Dose of Oral Conjugated Equine Estrogen on Size and Oxidative Susceptibility of Low-Density Lipoprotein Particles in Postmenopausal Women. Circulation 108 (7), 808–813. doi:10.1161/01.CIR.0000084552.54277.64
Wakatsuki, A., and Sagara, Y. (1996). Effects of Continuous Medroxyprogesterone Acetate on Lipoprotein Metabolism in Postmenopausal Women Receiving Estrogen. Maturitas 25 (1), 35–44. doi:10.1016/0378-5122(96)01044-4
Xue, W., Deng, Y., Wang, Y. F., and Sun, A. J. (2016). Effect of Half-Dose and Standard-Dose Conjugated Equine Estrogens Combined with Natural Progesterone or Dydrogesterone on Components of Metabolic Syndrome in Healthy Postmenopausal Women: A Randomized Controlled Trial. Chin. Med. J. (Engl) 129 (23), 2773–2779. doi:10.4103/0366-6999.194646
Yang, F., Li, N., Gaman, M. A., and Wang, N. (2021). Raloxifene Has Favorable Effects on the Lipid Profile in Women Explaining its Beneficial Effect on Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 166 (1), 105512. doi:10.1016/j.phrs.2021.105512
Yang, T. S., Liang, W. H., Chang, S. P., and Yuan, C. C. (2002). Effects of Period-free Hormone Replacement Therapy in Postmenopausal Women in Taiwan. Zhonghua Yi Xue Za Zhi (Taipei) 65 (1), 23–28.
Yang, T. S., Tsan, S. H., Chen, C. R., Chang, S. P., and Yuan, C. C. (1999). Evaluation of Conjugated Estrogen Plus Medroxyprogesterone Acetate versus Tibolone in Early Postmenopausal Chinese Women. Zhonghua Yi Xue Za Zhi (Taipei) 62 (5), 308–315.
Zegura, B., Guzic-Salobir, B., Sebestjen, M., and Keber, I. (2006). The Effect of Various Menopausal Hormone Therapies on Markers of Inflammation, Coagulation, Fibrinolysis, Lipids, and Lipoproteins in Healthy Postmenopausal Women. Menopause 13 (4), 643–650. doi:10.1097/01.gme.0000198485.70703.7a
Ziaei, S., Vakilinia, T., and Faghihzadeh, S. (2010). The Effects of Tibolone on Risk Factors of Cardiovascular Disease in Menopausal Women. Iranian J. Med. Sci. 35 (4), 281–286.
2019 Surveillance of Menopause (2019). Diagnosis and Management (NICE Guideline NG23) [Internet]. London: National Institute for Health and Care Excellence (UK).Copyright © NICE. Available at: https://www.ncbi.nlm.nih.gov/books/NBK563593/.
Keywords: menopause hormone therapy, lipid profile, meta-analysis, postmenopausal women, system review
Citation: Nie G, Yang X, Wang Y, Liang W, Li X, Luo Q, Yang H, Liu J, Wang J, Guo Q, Yu Q and Liang X (2022) The Effects of Menopause Hormone Therapy on Lipid Profile in Postmenopausal Women: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:850815. doi: 10.3389/fphar.2022.850815
Received: 08 January 2022; Accepted: 28 February 2022;
Published: 12 April 2022.
Edited by:
Changting Xiao, University of Saskatchewan, CanadaReviewed by:
Željko Reiner, University Hospital Centre Zagreb, CroatiaMihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2022 Nie, Yang, Wang, Liang, Li, Luo, Yang, Liu, Wang, Guo, Yu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangning Nie, Z3VhbmduaW5nbmllQGd6dWNtLmVkdS5jbg==; Qi Yu, eXVxaW1kQDE2My5jb20=; Xuefang Liang, bGlhbmd4dWVmYW5nQGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Guangning Nie
Guangning Nie Xiaofei Yang
Xiaofei Yang Yangyang Wang
Yangyang Wang Wanshi Liang
Wanshi Liang Xuewen Li
Xuewen Li Qiyuan Luo
Qiyuan Luo Hongyan Yang
Hongyan Yang Jian Liu
Jian Liu Jiajing Wang
Jiajing Wang Qinghua Guo
Qinghua Guo Qi Yu
Qi Yu Xuefang Liang
Xuefang Liang