- 1The First Affiliated Hospital of China Medical University, Shenyang, China
- 2Liaoning Provincial Key Laboratory of Oral Diseases, School and Hospital of Stomatology, China Medical University, Shenyang, China
Background/Aims: The relationship between the efficacy of metformin and the prognosis of patients with head and neck cancer (HNC) was still unclear. This study aims to clarify the prognostic value of metformin treatment using meta-analysis.
Methods: Studies related to HNC prognosis and metformin were searched in Cochrane Library, Embase, LILACS, MEDLINE and PubMed databases. A meta-analysis was performed to evaluate the association between metformin therapy and the prognosis of HNC on overall survival (OS), disease-free survival (DFS) and disease-specific survival (DSS) and whether article quality, comorbidities, age, region or smoking had an influence on the prognosis of metformin treatment. Pooled hazard ratio (HR) and 95% confidence interval (CI) were analyzed to assess the effect.
Results: Eleven eligible studies involving 14,694 participants were included. Metformin increased the OS (HR = 0.87, 95% CI: 0.76–0.99), but failed on DFS (HR = 0.67, 95% CI: 0.40–1.09) or DSS (HR = 0.69, 95% CI: 0.41–1.14) in HNC patients. Subgroup analysis showed metformin was associated with improved OS (HR = 0.66, 95% CI: 0.49–0.88), DFS (HR = 0.49, 95% CI: 0.26–0.92) and DSS (HR = 0.38, 95% CI: 0.22–0.65) in studies with higher Newcastle-Ottawa Scale (NOS) scores. Subgroup analysis of age indicated that patients younger than 65 years (OS, HR = 0.67, 95% CI: 0.49–0.92) were more likely to benefit from metformin treatment. Subgroup analysis of comorbidities showed metformin significantly improved patient outcomes in studies without adjusted for comorbidities (OS, HR = 0.66, 95% CI: 0.51–0.85; DSS, HR = 0.38, 95% CI: 0.22–0.65), but not in studies that adjusted for comorbidities.
Conclusions: Metformin improved the prognosis of HNC patients as an adjuvant therapy, especially in those with higher NOS scores. Age and comorbidities of HNC patients influenced the therapeutic effect of metformin. Further well-conducted investigations are needed.
Introduction
Head and neck cancer (HNC) is used to describe cancers that occur in the larynx, nasal cavity, oral cavity, paranasal sinuses, throat, as well as salivary glands. Most HNCs are squamous cell carcinomas (Lutzky et al., 2008). HNC is one of the most common types of cancer, with approximately 900,000 new cases and 450,000 related deaths worldwide per year (Sung et al., 2021). The prognosis of patients with HNC remains unsatisfactory, despite advances in surgery, chemotherapy and radiotherapy. Recently, several hypoglycemic drugs have been found to be able to reduce the risk of cancer and have a positive effect on cancer treatment (Shlomai et al., 2016). Among hypoglycemic agents, metformin has attracted much attention as the most potential anti-cancer therapeutic assistant (Morales and Morris 2015; Vilaseca et al., 2020).
Metformin has shown a preventive effect on a variety of cancer types, including pancreatic cancer, colorectal cancer and hepatocellular carcinoma, reducing the incidence and mortality (Wan et al., 2018; Schulte et al., 2019; Ng et al., 2020). Metformin has been repeatedly reported to decrease the risk of HNC (Becker et al., 2014; Tseng 2014; Yen et al., 2015; Tseng 2016; Lerner et al., 2017; Tseng 2018; Veitz-Keenan et al., 2019; Mekala et al., 2020), but its role in prolonging NHC patient survival remains controversial. In 2020, there was a meta-analysis including seven retrospective cohort studies and exploring the correlations between metformin and HNC patient survival (Wang et al., 2020). The results showed that there was no significant association between the use of metformin and survival of HNC patients (HR = 0.89, 95% CI: 0.66–1.18; p = 0.413). However, this meta-analysis missed three studies (Spratt et al., 2016; Chang et al., 2017; Ogunsakin et al., 2018), which may lead to a biased analysis result. In addition, after this meta-analysis, a new study on the correlations between metformin and the survival of HNC patients was published (Hu et al., 2020). Consequently, further meta-analysis is required. To the end, we performed a meta-analysis on 11 cohort studies in order to determine the effect of metformin on HNC patient survival. Furthermore, subgroup analysis was also performed.
Methods
Systematic Literature Search
Research studies were obtained from Cochrane Library, Embase, LILACS, MEDLINE and PubMed databases, without time or language restrictions.
The search strategy for PubMed included the following terms: head and neck squamous cancer [title] OR head and neck squamous carcinoma [title]OR head and neck cancer [title] OR head and neck carcinoma [title] OR HNSCC [title] OR nasopharyngeal [title] OR oral squamous cell carcinoma [title] OR laryngeal [title] AND metformin [title] AND adjuvant [Any field]. Cited studies of the included studies was also checked. The search was performed on 3 May2021.
Eligibility Criteria
The following criteria were used to select randomized controlled trials and observational studies: participants, outcomes, and study design (PICOS). The inclusion criteria were defined as follows: 1) The population is patients with head and neck cancer; 2) Metformin is used as adjuvant therapy; 3) Metformin is associated with head and neck cancer; 4) Clinical research. The exclusion criteria were defined as follows: 1) Cell culture and animal experiments; 2) Article does not contain survival data; 3) Published not in English; 4) Data overlap.
The literature retrieval and screening were conducted independently by two authors (Jiao and Sun). If there is an unidentified document between the two authors, the third author (Chen) will make a decision based on the inclusion criteria.
Data Collection Process and Data Items
An author (YJ) selected the required information from the selected studies: author, publication year, country, sample size, study design, stage/location, the mean or median age of metformin, outcomes, definition of metformin exposure, follow-up (months), adjusted variables, results and conclusions. If there was no original data required for meta-analysis in the article, we sent an email to the corresponding author or calculated from the data extracted from Kaplan-Meier curves using the method given by Jayne F Tierney (Tierney et al., 2007). The second author (DJL) cross-checked all the retrieved information. Likewise, any disagreements were resolved through discussion and mutual agreement between the two authors. The third author (ZDC) participated in making the final decision when needed.
It is worth noting that: first, in Yung-An Tsou’s article, the number of people in the metformin treatment group and the control group in the abstract was inconsistent with the number in the results. After communicating with the author, the correct value was confirmed (Tsou et al., 2019). Second, Xin Hu’s article did not give HR values (Hu et al., 2020), and the corresponding HR and CI values were calculated from the data extracted from Kaplan-Meier curves using the method given by Jayne F Tierney (Tierney et al., 2007). Third, although the HR values were not directly provided in Amie Ogunsakin’s article (Ogunsakin et al., 2018) and Pei Hun Chang’s article (Chang et al., 2017), accurate HR and CI values can be calculated from the data in the articles.
Quality Assessment
Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of non-randomized studies (Stang 2010; Lo et al., 2014). The quality of studies was independently assessed by two authors (YJ and DJL). Any differences were discussed and resolved by the two authors or judged by the third author (YS).
Statistical Analysis
The summarized data was analyzed by Review manager 5.4 and Stata 12. HRs with 95% CIs were used to calculate the summary survival effect. The pooled analysis was performed by obtaining the corresponding HR and CI values. Heterogeneity was evaluated by the I2 statistics. I2 values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively (Higgins and Thompson 2002). By removing one study at a time, a sensitivity analysis was performed to assess the impact of each study on heterogeneity. Publication bias was intuitively assessed by examining the asymmetry of the funnel plot. In addition, Begg’s test was also used to evaluate publication bias (Hayashino et al., 2005; Peters et al., 2006). The probability value was bilateral, and p < 0.05 was considered statistically significant. Use Review manager 5.4 for data analysis.
Results
Literature Search
The selection process is shown in Figure 1. A total of 502 studies were identified from the Cochrane Library, Embase, LILACS, MEDLINE and PubMed databases initially. After screening the titles and abstracts, 84 studies were evaluated further for eligibility. Of the 84 studies, 72 did not meet the inclusion criteria: 59 studies belonged to cell culture or animal experiments; 12 studies could not contain survival data; one study was not English study; one study was data overlap. Thus, 11 cohort studies were included in this systematic review (Sandulache et al., 2014; Kwon et al., 2015; Spratt et al., 2016; Chang et al., 2017; Ogunsakin et al., 2018; Quimby et al., 2018; Stokes et al., 2018; Alcusky et al., 2019; Lee et al., 2019; Tsou et al., 2019; Hu et al., 2020).
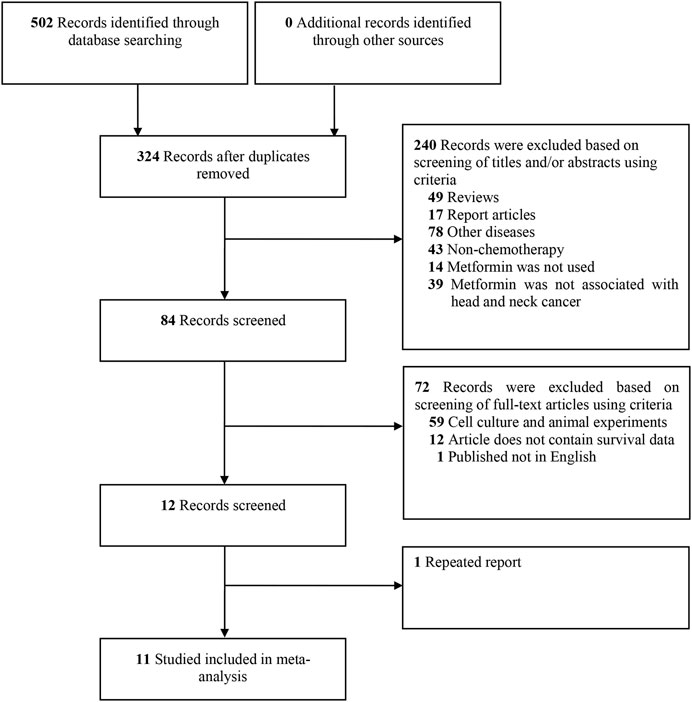
FIGURE 1. Flow diagram of studies search and selection criteria for systemic review and meta-analysis.
Study Characteristics
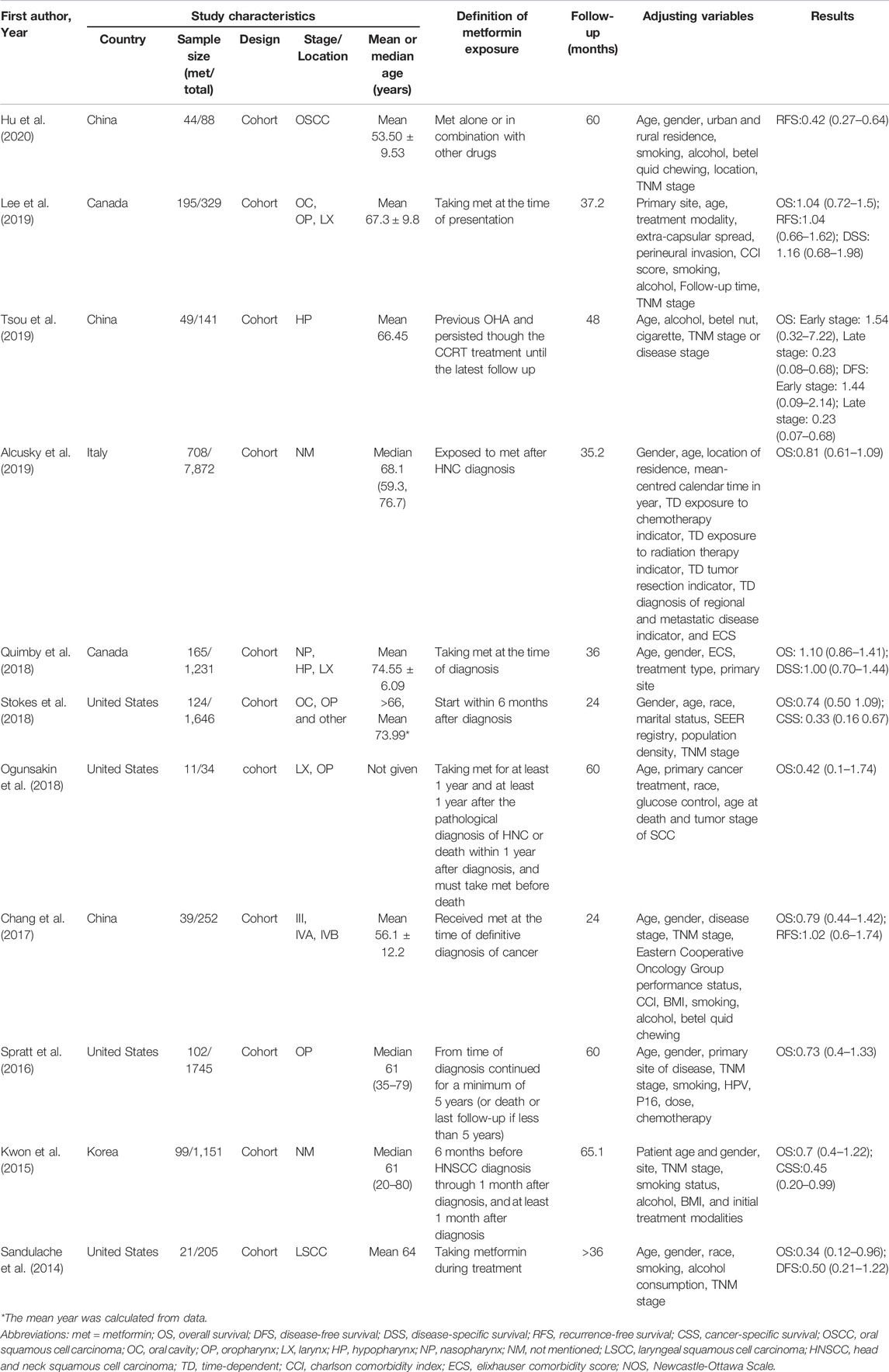
The summary characteristics of the included studies were shown in Table 1. Sample sizes ranged from 34 to 7,872 patients. All included studies were from 2014 to 2020, and all studies were published in English. Belonging to cohort studies, these studies were conducted in different countries: Canada (Quimby et al., 2018; Lee et al., 2019), China (Chang et al., 2017; Tsou et al., 2019; Hu et al., 2020), the United States (Sandulache et al., 2014; Spratt et al., 2016; Ogunsakin et al., 2018; Stokes et al., 2018), Europe (Alcusky et al., 2019) and South Korea (Kwon et al., 2015). All selected studies are clinical studies and belong to cohort studies. In these 11 studies, exposure to metformin was given different definitions.
Four studies defined metformin exposure as the use of metformin when diagnosing head and neck cancer (Chang et al., 2017; Quimby et al., 2018; Alcusky et al., 2019; Lee et al., 2019), four studies defined metformin exposure as continuous use of metformin during treatment (Sandulache et al., 2014; Spratt et al., 2016; Tsou et al., 2019; Hu et al., 2020), one study defined metformin exposure as the use of metformin within 6 months after diagnosis (Stokes et al., 2018), and one study defined metformin exposure as having to take metformin for at least one year and at least one year after the pathological diagnosis of HNC or death within one year after diagnosis, and must take metformin before death (Ogunsakin et al., 2018). In addition, another study divided the definition of metformin exposure into three categories: prediagnosis (>6 months prediagnosis), per-diagnosis (6 months before HNC diagnosis through 1 month after diagnosis), and postdiagnosis (>1 month after diagnosis) (Kwon et al., 2015). In the analysis of the research outcomes, three indicators: overall survival (OS) (Sandulache et al., 2014; Kwon et al., 2015; Spratt et al., 2016; Chang et al., 2017; Ogunsakin et al., 2018; Quimby et al., 2018; Stokes et al., 2018; Alcusky et al., 2019; Lee et al., 2019; Tsou et al., 2019), disease-free survival (DFS) (Sandulache et al., 2014; Chang et al., 2017; Lee et al., 2019; Tsou et al., 2019; Hu et al., 2020), and disease-specific survival (DSS) (Kwon et al., 2015; Quimby et al., 2018; Stokes et al., 2018; Lee et al., 2019) were selected. The impact of confounding factors on the results was considered.
Quality of Studies
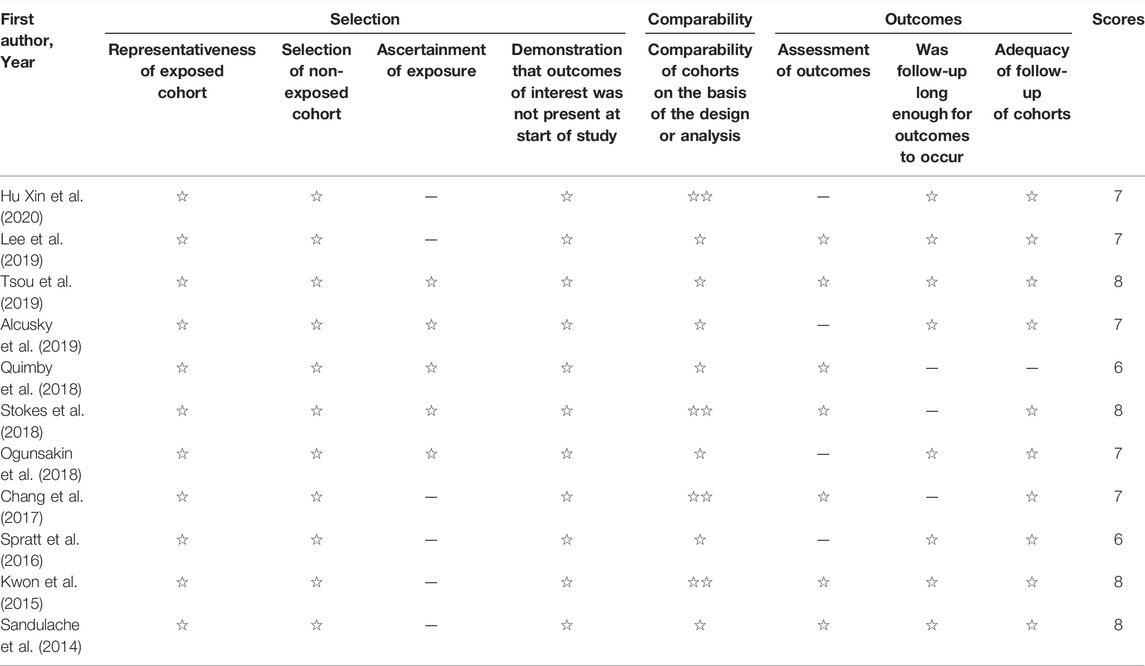
The risk of bias of the studies was accessed by NOS (Lo et al., 2014). The NOS scores of included studies were shown in Table 2. Of the 11 studies, the NOS scores of two studies were six, five studies were seven, and the remaining four studies were eight. The average score calculated for NOS is 7.18.
Results of Individual Studies
The forest map of survival data is shown in Figure 2. To evaluate the outcome, OS (13,311 patients) was used in ten studies; DFS (972 patients) was used in five studies; and DSS (4,355 patients) was used in four studies. When applying the fixed effects model, OS (HR = 0.87, 95% CI: 0.76–0.99, p = 0.04, Figure 2A) shows that metformin is beneficial to the treatment of head and neck cancer, but DFS (HR = 0.67, 95% CI: 0.40–1.09, p = 0.11, Figure 2B) and DSS (HR = 0.69, 95% CI: 0.41–1.14, p = 0.15, Figure 2C) on the random effects model do not indicate a significant effect of metformin in HNC patients.
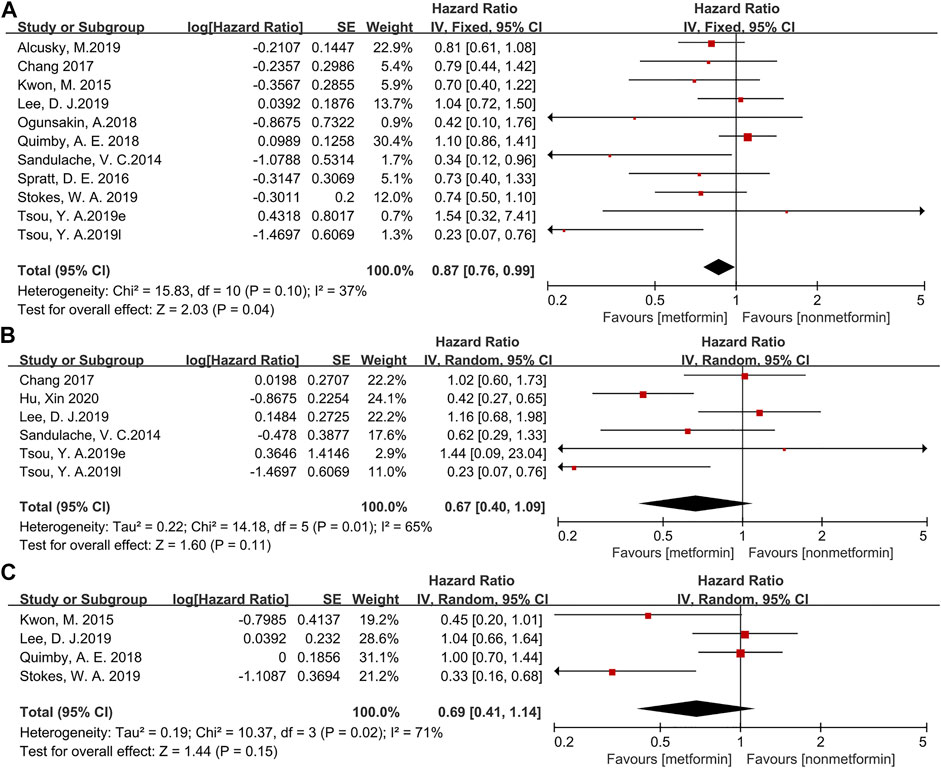
FIGURE 2. The forest plot of head and neck cancer according to metformin use for overall survival (A), disease-free survival (B), disease-specific survival (C).
Sensitivity Analysis
The heterogeneity of OS (χ2 = 15.83, df = 10, p = 0.10, I2 = 37%) was obvious. A sensitivity analysis was performed by deleting one study at a time to evaluate the impact of each study on heterogeneity. Alexandra E Quimby’s article was considered to be the main reason for the high heterogeneity. After excluding this article, the heterogeneity was reduced (χ2 = 10.61, df = 9, p = 0.30, I2 = 15%), and the results were not greatly affected (HR = 0.78, 95% CI: 0.66–0.92, p = 0.003).
Subgroup Analysis
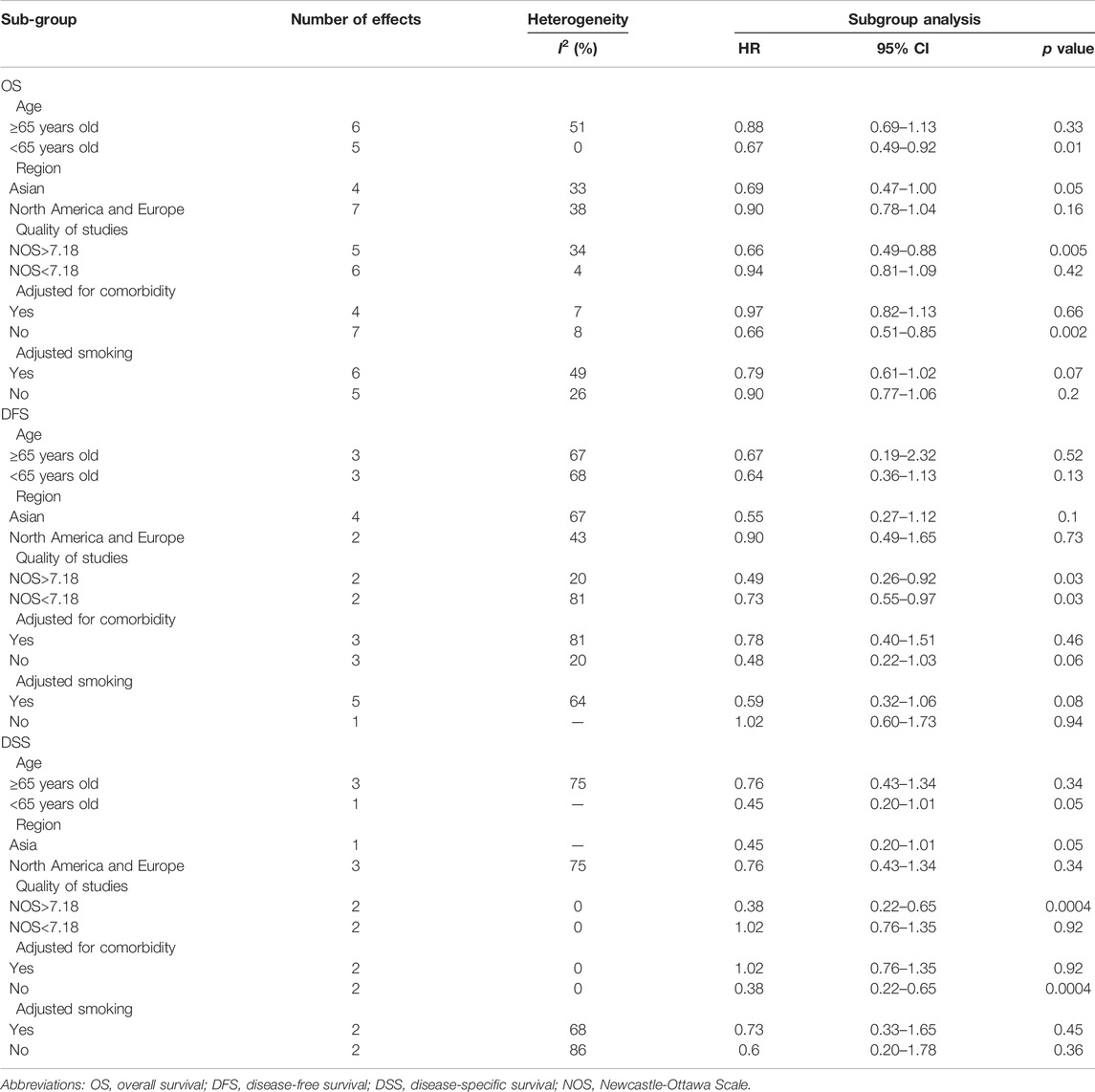
The results of the subgroup analysis are demonstrated in Table 3.
The average score of NOS was 7.18. Studies with NOS > 7.18 were considered to belong to the high-quality group, and studies with NOS < 7.18 were classified as low-quality group. Four of the 11 studies were classified into the high-quality group (Sandulache et al., 2014; Kwon et al., 2015; Stokes et al., 2018; Tsou et al., 2019). In the subgroup of high-quality, significantly better survival was observed in metformin group compared to the non-metformin group on OS (HR = 0.66, 95% CI: 0.49–0.88, p = 0.005), DFS (HR = 0.49, 95% CI: 0.26–0.92, p = 0.03) and DSS (HR = 0.38, 95% CI: 0.22–0.65, p = 0.0004).
Subgroup analysis based on comorbidity showed that metformin significantly improved patient outcomes in studies without adjusted for comorbidities (OS, HR = 0.66, 95% CI: 0.51–0.85, p = 0.002; DSS, HR = 0.38, 95% CI: 0.22–0.65, p = 0.0004), but not in studies that adjusted for comorbidities.
When analyzing populations in different regions, OS (HR = 0.69, 95% CI: 0.47–1.00, p = 0.05), DFS (HR = 0.55, 95% CI: 0.27–1.12, p = 0.1), and DSS (HR = 0.45, 95% CI: 0.20–1.01, p = 0.05) showed that although it was not statistically significant, metformin was associated with better prognosis only in Asian HNC patients.
Subgroup analysis with the 65-year-old showed a better prognosis in the metformin group in the population under 65-years-old (OS, HR = 0.67, 95% CI: 0.49–0.92, p = 0.01).
In addition, subgroup analysis of adjusted smoking indicated that although there was no statistically significant difference between the use and non-use of metformin on OS (HR = 0.79, 95% CI: 0.61–1.02, p = 0.07), DFS (HR = 0.59, 95% CI: 0.32–1.06, p = 0.08), DSS (HR = 0.73, 95% CI: 0.33–1.65, p = 0.45).
Publication Bias
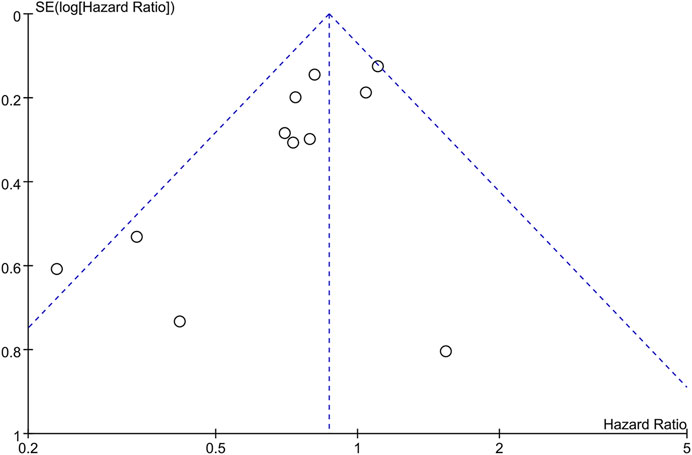
The funnel plots (Figure 3) and Begg’s test (p = 0.119) suggested that there was no publication bias.
Discussion
This systematic review and meta-analysis investigated the effect of metformin on HNC patients concerning survival. Our study demonstrated that metformin significantly prolonged the survival of HNC patients for the study of OS analysis. Especially, the survival benefit showed more obscure in studies with high-quality and among patients without comorbidity. In addition, it was worth noting that the efficacy of metformin therapy seems to be better for Asian HNC patients, although there was no statistical significance, there was a corresponding trend (p = 0.05).
The effect of metformin on prevention and treatment of HNC has been strengthened both by epidemiological data and laboratory studies. However, whether metformin is beneficial to the survival of HNC patients remains controversial. Wang et al. reported a meta-analysis of seven cohort studies of metformin treatment and HNC patients with unfavorable prognoses (Wang et al., 2020). Our results indicated that metformin significantly benefited the survival of HNC patients, which was different from the previous analysis. The difference in results may be related to the included participants. Our study included 11 studies with 14,694 participants, which is about five times the previous sample size. And the insignificance of DFS and DSS analysis of metformin on the survival of patients may also be connected with the small sample size.
Subgroup analysis based on quality showed that the results of OS, DFS and DSS all indicated that metformin significantly prolonged the survival of HNC patients in high-quality group. This result suggested that higher quality studies were more inclined to conclude that metformin was beneficial to patient survival. However, further high-quality studies with large sample sizes are needed.
Age-based subgroup analysis suggested that metformin significantly prolonged survival in HNC patients in people younger than 65 years. The results suggested that age might influence the effect of metformin, but more samples are needed to investigate.
Many HNC patients used metformin because of type 2 diabetes mellitus. In a subgroup analysis of comorbidities, mainly diabetes, metformin significantly improved patient outcomes in studies without adjusted for comorbidities (OS, HR = 0.66, 95% CI: 0.51–0.85, p = 0.002; DSS, HR = 0.38, 95% CI: 0.22–0.65, p = 0.0004), but not in studies that adjusted for comorbidities. This suggested that metformin use was associated with diabetes, and that HNC patients with previously diagnosed diabetes may have a better prognosis. However, the result made the impact of metformin on the prognosis of HNC patients confusing. Of the 11 studies, three were performed only in patients with type 2 diabetes (Ogunsakin et al., 2018; Tsou et al., 2019; Hu et al., 2020), and all three showed that metformin was associated with improved outcomes in HNC patients. However, there was no study performed only in patients without diabetes. In order to minimize the influence of diabetes on the prognosis of HNC patients with metformin, it is necessary to study the effect of metformin on the prognosis of patients without diabetes in the future.
Many reviews showed that metformin adjuvant treatment significantly associated with a survival benefit for Asian cancer patients (Tian et al., 2016; Wan et al., 2016; Wan et al., 2018). Subgroup analysis by region showed Subgroup analysis by region showed metformin was connected with longevity of Asian HNC patients despite no statistical significance. In addition, it was worth mentioning that there was an article that also showed that metformin can promote the prognosis of oral cancer patients (Huang et al., 2021). Because it was published in Chinese, this article was not included in this meta-analysis. Therefore, Asian HNC patients seemed more likely to benefit from metformin treatment. However, further epidemiological and laboratory studies are needed to understand these associations better.
Smoking is an important factor affecting the prognosis of patients with HNC. Subgroup analysis by adjusted smoking suggested that there was a trend that those who accepted metformin therapy lived longer than those who did not.
Several limitations of this study should be considered in this meta-analysis. First, only 11 cohort studies were included. Although we included more studies than that in the meta-analysis performed by Wang, it was still insufficient. Second, the influence of confounding factors on the results was beyond control. Third, measurement errors were unavoidable. Fourth, the dose and exposure time of metformin and the survival rate of HNC patients were unable to determine. The last, the effect of the interaction between metformin and other drugs or treatments on the outcome was unable to evaluate.
Conclusion
This systematic review and meta-analysis showed that metformin had a significant improvement in overall survival of HNC patients, thus supporting metformin as an adjunct to the treatment of head and neck cancer. Especially, the efficacy of metformin in the high-quality group was more significant than that in the low-quality group. In a subgroup analysis of comorbidities, mainly diabetes, metformin significantly improved patient outcomes in studies without adjusted for comorbidities, but not in studies that adjusted for comorbidities. It is necessary to study the effect of metformin on the prognosis of HNC patients in the population without diabetes in the future. In addition, Asian HNC patients seemed to be more likely to benefit from the metformin therapy. However, the quantitative limitation of studies undermined the power of the analysis. There is a need for further studies with large sample sizes to investigate the relationship between metformin and the survival of HNC patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YJ: Methodology, Formal analysis, Investigation, Data Curation, Writing—Original Draft. DL: Investigation, Data Curation, Writing—Original Draft. YS: Investigation, Visualization. ZC: Validation. SL: Conceptualization, Writing—Reviewing and Editing.
Funding
The study was supported by the National Natural Science Foundation of China (grant nos. 81902772 and 81800975) and Shenyang Young and Middle-aged Science and Technology Innovation Talent Support Program (grant nos. RC210038 and RC210041).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alcusky, M., Keith, S. W., Karagiannis, T., Rabinowitz, C., Louis, D. Z., and Maio, V. (2019). Metformin Exposure and Survival in Head and Neck Cancer: A Large Population-Based Cohort Study. J. Clin. Pharm. Ther. 44, 588–594. doi:10.1111/jcpt.12820
Becker, C., Jick, S. S., Meier, C. R., and Bodmer, M. (2014). Metformin and the Risk of Head and Neck Cancer: a Case-Control Analysis. Diabetes Obes. Metab. 16, 1148–1154. doi:10.1111/dom.12351
Chang, P. H., Yeh, K. Y., Wang, C. H., Chen, E. Y., Yang, S. W., Chou, W. C., et al. (2017). Impact of Metformin on Patients with Advanced Head and Neck Cancer Undergoing Concurrent Chemoradiotherapy. Head. Neck 39, 1573–1577. doi:10.1002/hed.24793
Hayashino, Y., Noguchi, Y., and Fukui, T. (2005). Systematic Evaluation and Comparison of Statistical Tests for Publication Bias. J. Epidemiol. 15, 235–243. doi:10.2188/jea.15.235
Higgins, J. P., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 21, 1539–1558. doi:10.1002/sim.1186
Hu, X., Xiong, H., Chen, W., Huang, L., Mao, T., Yang, L., et al. (2020). Metformin Reduces the Increased Risk of Oral Squamous Cell Carcinoma Recurrence in Patients with Type 2 Diabetes Mellitus: A Cohort Study with Propensity Score Analyses. Surg. Oncol. 35, 453–459. doi:10.1016/j.suronc.2020.09.023
Huang, D. N., Chen, W. X., Xiong, H. F., Hu, X., Mao, T., and Su, T. (2021). Preliminary Clinical Study on the Effect of Metformin on Prognosis of Patients with Oral Squamous Cell Carcinoma after Surgical Treatment]. Shanghai Kou Qiang Yi Xue 30, 61–65.
Kwon, M., Roh, J. L., Song, J., Lee, S. W., Kim, S. B., Choi, S. H., et al. (2015). Effect of Metformin on Progression of Head and Neck Cancers, Occurrence of Second Primary Cancers, and Cause-specific Survival. Oncologist 20, 546–553. doi:10.1634/theoncologist.2014-0426
Lee, D. J., McMullen, C. P., Foreman, A., Huang, S. H., Lu, L., Xu, W., et al. (2019). Impact of Metformin on Disease Control and Survival in Patients with Head and Neck Cancer: a Retrospective Cohort Study. J. Otolaryngol. Head. Neck Surg. 48, 34. doi:10.1186/s40463-019-0348-5
Lerner, M. Z., Mor, N., Paek, H., Blitzer, A., and Strome, M. (2017). Metformin Prevents the Progression of Dysplastic Mucosa of the Head and Neck to Carcinoma in Nondiabetic Patients. Ann. Otol. Rhinol. Laryngol. 126, 340–343. doi:10.1177/0003489416688478
Lo, C. K., Mertz, D., and Loeb, M. (2014). Newcastle-Ottawa Scale: Comparing Reviewers' to Authors' Assessments. BMC Med. Res. Methodol. 14, 45. doi:10.1186/1471-2288-14-45
Lutzky, V. P., Moss, D. J., Chin, D., Coman, W. B., Parsons, P. G., and Boyle, G. M. (2008). Biomarkers for Cancers of the Head and Neck. Clin. Med. Ear, nose throat 1, CMENT.S1051. doi:10.4137/cment.S1051
Mekala, M. R., Bangi, B. B., N, J., Lebaka, R. R., Nadendla, L. K., and Ginjupally, U. (2020). Association of Diabetes with Oral Cancer- an Enigmatic Correlation. Asian Pac J. Cancer Prev. 21, 809–814. doi:10.31557/apjcp.2020.21.3.809
Morales, D. R., and Morris, A. D. (2015). Metformin in Cancer Treatment and Prevention. Annu. Rev. Med. 66, 17–29. doi:10.1146/annurev-med-062613-093128
Ng, C. W., Jiang, A. A., Toh, E. M. S., Ng, C. H., Ong, Z. H., Peng, S., et al. (2020). Metformin and Colorectal Cancer: a Systematic Review, Meta-Analysis and Meta-Regression. Int. J. Colorectal Dis. 35, 1501–1512. doi:10.1007/s00384-020-03676-x
Ogunsakin, A., Infield, J., Zuber, J., and Solomon, S. S. (2018). Metformin Associated with Improved Outcomes in Diabetic Patients with Laryngeal and Oropharyngeal Carcinoma. Am. J. Med. Sci. 356, 574–575. doi:10.1016/j.amjms.2018.09.002
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2006). Comparison of Two Methods to Detect Publication Bias in Meta-Analysis. Jama 295, 676–680. doi:10.1001/jama.295.6.676
Quimby, A. E., Lebo, N. L., Griffiths, R., Hall, S., Dimitroulakos, J., and Johnson-Obaseki, S. (2018). Does Metformin Usage Improve Survival in Head and Neck Squamous Cell Carcinoma? A Population-Based Study. J. Otolaryngol. Head. Neck Surg. 47, 74. doi:10.1186/s40463-018-0322-7
Sandulache, V. C., Hamblin, J. S., Skinner, H. D., Kubik, M. W., Myers, J. N., and Zevallos, J. P. (2014). Association between Metformin Use and Improved Survival in Patients with Laryngeal Squamous Cell Carcinoma. Head. Neck 36, 1039–1043. doi:10.1002/hed.23409
Schulte, L., Scheiner, B., Voigtländer, T., Koch, S., Schweitzer, N., Marhenke, S., et al. (2019). Treatment with Metformin Is Associated with a Prolonged Survival in Patients with Hepatocellular Carcinoma. Liver Int. 39, 714–726. doi:10.1111/liv.14048
Shlomai, G., Neel, B., LeRoith, D., and Gallagher, E. J. (2016). Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol. 34, 4261–4269. doi:10.1200/jco.2016.67.4044
Spratt, D. E., Beadle, B. M., Zumsteg, Z. S., Rivera, A., Skinner, H. D., Osborne, J. R., et al. (2016). The Influence of Diabetes Mellitus and Metformin on Distant Metastases in Oropharyngeal Cancer: A Multicenter Study. Int. J. Radiat. Oncol. Biol. Phys. 94, 523–531. doi:10.1016/j.ijrobp.2015.11.007
Stang, A. (2010). Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 25, 603–605. doi:10.1007/s10654-010-9491-z
Stokes, W. A., Eguchi, M., Amini, A., Hararah, M. K., Ding, D., McDermott, J. D., et al. (2018). Survival Impact and Toxicity of Metformin in Head and Neck Cancer: An Analysis of the SEER-Medicare Dataset. Oral Oncol. 84, 12–19. doi:10.1016/j.oraloncology.2018.06.022
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tian, R. H., Zhang, Y. G., Wu, Z., Liu, X., Yang, J. W., and Ji, H. L. (2016). Effects of Metformin on Survival Outcomes of Lung Cancer Patients with Type 2 Diabetes Mellitus: a Meta-Analysis. Clin. Transl. Oncol. 18, 641–649. doi:10.1007/s12094-015-1412-x
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S., and Sydes, M. R. (2007). Practical Methods for Incorporating Summary Time-To-Event Data into Meta-Analysis. Trials 8, 16. doi:10.1186/1745-6215-8-16
Tseng, C. H. (2018). Metformin and Risk of Developing Nasopharyngeal Cancer in Patients with Type 2 Diabetes Mellitus. Metabolism 85, 223–226. doi:10.1016/j.metabol.2018.04.009
Tseng, C. H. (2016). Metformin May Reduce Oral Cancer Risk in Patients with Type 2 Diabetes. Oncotarget 7, 2000–2008. doi:10.18632/oncotarget.6626
Tseng, C. H. (2014). Metformin Reduces Thyroid Cancer Risk in Taiwanese Patients with Type 2 Diabetes. PLoS One 9, e109852. doi:10.1371/journal.pone.0109852
Tsou, Y. A., Chang, W. D., Lu, J. J., Wu, T. F., Chen, H. L., Chen, C. M., et al. (2019). The Effect of Metformin Use on Hypopharyngeal Squamous Cell Carcinoma in Diabetes Mellitus Patients. BMC Cancer 19, 862. doi:10.1186/s12885-019-6083-5
Veitz-Keenan, A., Silvestre Calle, T. D., and Bergamini, M. (2019). Limited Evidence Suggests Metformin Might Be Beneficial to Reduce Head and Neck Cancer Risk and Increase Overall Survival, while Any Benefit with Antiinflammatory Drugs Is Inconsistent. J. Evid. Based Dent. Pract. 19, 298–300. doi:10.1016/j.jebdp.2019.101340
Vilaseca, I., Fuster, G., and Avilés-Jurado, F. X. (2020). The Impact of Diabetes in Head and Neck Cancer. Curr. Opin. Otolaryngol. Head. Neck Surg. 28, 107–111. doi:10.1097/moo.0000000000000606
Wan, G., Sun, X., Li, F., Wang, X., Li, C., Li, H., et al. (2018). Survival Benefit of Metformin Adjuvant Treatment for Pancreatic Cancer Patients: a Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 49, 837–847. doi:10.1159/000493214
Wan, G., Yu, X., Chen, P., Wang, X., Pan, D., Wang, X., et al. (2016). Metformin Therapy Associated with Survival Benefit in Lung Cancer Patients with Diabetes. Oncotarget 7, 35437–35445. doi:10.18632/oncotarget.8881
Wang, Y., Fu, T., Liu, Y., Yang, G., Yu, C., and Zhang, Z. J. (2020). The Association between Metformin and Survival of Head and Neck Cancer: A Systematic Review and Meta-Analysis of 7 Retrospective Cohort Studies. Curr. Pharm. Des. 26, 3161–3170. doi:10.2174/1381612826666200218095310
Keywords: metformin, head and neck cancer, meta-analysis, therapy, survival
Citation: Jiao Y, Liu D, Sun Y, Chen Z and Liu S (2022) Survival Benefit of Metformin as an Adjuvant Treatment for Head and Neck Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:850750. doi: 10.3389/fphar.2022.850750
Received: 08 January 2022; Accepted: 28 April 2022;
Published: 13 May 2022.
Edited by:
Xuanbin Wang, Hubei University of Medicine, ChinaReviewed by:
Guo-qing Zheng, Zhejiang Chinese Medical University, ChinaXinmao Song, Fudan University, China
Copyright © 2022 Jiao, Liu, Sun, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sai Liu, bGl1c2FpQGNtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yu Jiao
Yu Jiao Dongjuan Liu
Dongjuan Liu Yi Sun1
Yi Sun1 Sai Liu
Sai Liu