
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 08 April 2022
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.849758
A correction has been applied to this article in:
Corrigendum: Neurological adverse events associated with esketamine: A disproportionality analysis for signal detection leveraging the FDA adverse event reporting system
Esketamine was approved for the treatment of treatment-resistant depression in 2019. After the approval of esketamine, numerous concerns have been raised regarding its long-term safety and tolerability. A previous systematic pharmacovigilance study on esketamine-related adverse events (AEs) was published in 2020; however, it has not been updated 2 years later. The primary aim of this study was to detect and characterize neurological safety signals of esketamine to partially update the knowledge in this field using the FDA pharmacovigilance database. Reporting odds ratio (ROR) was calculated for esketamine-related neurological AEs from 2019 to 2021 with a signal considered when the lower limit of the 95% confidence interval (CI) of ROR (ROR025) exceeded one. Severe and non-severe cases were compared using an independent samples t-test or chi-squared (χ2) test, and a rating scale was used to prioritize the signals. The database contained 720 cases of esketamine-associated neurological AEs, with 21 signals detected, ranging from a ROR025 of 1.05 (disturbance in attention) to 204.00 (sedation). 16 latest neurological AEs emerged in the second year of marketing approval of esketamine, with eight signals detected. The associations between esketamine and nervous system disorders persisted when stratifying by sex, age, and reporter type, whereas the spectrum of neurological AEs differed in stratification regimens. Esketamine dosage, antidepressant polypharmacy, or co-prescription with benzodiazepines affected AEs severity (t = 2.41, p = 0.017; χ2 = 6.75, p = 0.009; and χ2 = 4.10, p = 0.043; respectively), while age and sex did not (p = 0.053 and p = 0.397, respectively). Three signals were categorized as moderate clinical priority [i.e., sedation, dizziness, and dysgeusia (priority points 7, 5, and 5, respectively)], showing the same early failure type profiles. Notably, seven detected disproportionality signals were not previously detected in clinical trials. Although the majority of results were in line with those obtained in the previous study, there were discrepancies in the spectrum of neurological AEs and the effects of several risk factors on AEs severity among the two studies that should be recognized and managed early in clinical treatments.
The United States (U.S.) Food and Drug Administration (FDA) and European Medicines Agency approved esketamine plus an oral antidepressant for treatment of treatment-resistant depression (TRD) in adults in 2019 (Kim et al., 2019; Schatzberg, 2019; U.S. Food and Drug Administration, 2019; Horowitz and Moncrieff, 2020; Psychopharmacologic Drugs Advisory Committee, 2020) based primarily on two pivotal positive phase 3 trials (Daly et al., 2019; Popova et al., 2019) that demonstrated a statistically significantly larger reduction in the Montgomery–Asberg Depression Rating Scale scores and decrease in the risk of relapse. Soon after, the authorities expanded the indication of esketamine to include patients with major depressive disorder with acute suicidal behaviors or ideation (McIntyre et al., 2021). Esketamine, a non-selective, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, is the S-enantiomer of racemic ketamine (Bozymski et al., 2020; Janssen Inc, 2020). By antagonizing the NMDA receptor, esketamine transiently increases glutamate release, resulting in increased stimulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, with this stimulation augmenting neurotrophic signaling and possibly facilitating the restoration of synaptic function in brain areas responsible for the adjustment of mood and emotional behavior (Janssen Inc, 2020). However, the exact mechanisms by which esketamine exerts its antidepressant effects remains unclear.
After the approval of esketamine, numerous concerns have been raised regarding its long-term safety and tolerability. A previous systematic pharmacovigilance study of esketamine-associated AEs was published in August 2020 and included all reports recorded in the FDA Adverse Event Reporting System (FAERS) from the second quarter (Q2) of 2019–2020 Q1 (Gastaldon et al., 2020). This real-world study detected a range of new, unexpected signals and identified several risk factors related to AEs severity, such as the female, patients treated with higher doses, and those receiving multidrug therapy (Gastaldon et al., 2020). Because esketamine has been on the market for >2 years, it would be instructive for clinicians and pharmacovigilance experts to be presented what has changed 1 year after the publication of a similar article. Additionally, it remains unclear whether the risk factors identified in the previous study would be reliable predictors of the outcomes of AEs in a specific system organ class. Two recent meta-analyses reveal that, in general, neurological AEs are among the most commonly reported ones in patients following treatment with esketamine (Zheng et al., 2020; Wang et al., 2021). Also, many esketamine-associated neurological side effects emerged in the previous pharmacovigilance study (Gastaldon et al., 2020). On these grounds, the characteristics of neurological AEs associated with esketamine are the special focus in this study.
In the present study, we analyzed the safety data of esketamine from the FAERS database in order to detect and characterize relevant neurological safety signals. We then compared the main results of these two pharmacovigilance studies in order to identify new findings in the present study and partially update the current knowledge in this field. Additionally, we performed stratification analyses, clinical prioritization of signals, and time-to-onset analysis in order to further investigate the properties of esketamine-related neurological AEs.
A disproportionality analysis reflecting the case/non-case study design, was used to quantify the associations between esketamine and neurological AEs. This measures the occurrence of target AEs associated with a drug compared to all other drugs in the database. If the proportion of a target AE is larger in the suspected group of drugs (case) than in the non-exposed group (non-case), an association is hypothesized to exist between the drug and the AE and conceived as a disproportionality signal (Gastaldon et al., 2020). Because esketamine was approved for marketing by the U.S. FDA in March 2019, this study included all reports from the first quarter (Q1) of 2019–2021 Q2.
Seven data files, including patient demographics and administrative information, drug/biologic information, the Medical Dictionary for Regulatory Activities (MedDRA) terms for AEs, patient outcomes, report sources, drug therapy start/end dates, and MedDRA terms for diagnoses/indications, were downloaded from the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html#collapse2020) and processed using Golang (v1.15; http://go.dev/doc/go1.15). Because FAERS may sporadically include duplicate reports submitted by various sources, duplicates were identified and removed accordingly, as described in previous studies (Carnovale et al., 2018; Gastaldon et al., 2020; Mazhar et al., 2021). The case ID was chosen as the key filter in this study to remove duplicate records. We further reviewed the records manually according to the similarities of primary ID, patient details (e.g., age and sex), suspect drugs, and AEs when the case ID was the same. Only reports with the latest FDA received date were selected, and duplicate records were removed accordingly. All reports recorded in FAERS with esketamine considered as the primary or secondary suspect medication were eligible for inclusion in the analysis. This study included all nervous system disorders according to the MedDRA terminology (v23.0), with all of these events coded on the preferred term (PT) level to identify or select specific symptoms or signs of neurological entities. We ultimately retrieved and described detailed information, including patient characteristics (sex, age at onset, and weight), general information (reporter region, reporting year, and reporter qualification), drugs (dosage regimen), reactions (reported terms, MedDRA classification terms, and event date), seriousness (serious and non-serious), final outcome, and concomitant drugs. Means (± standard deviation) were used to characterize continuous normally distributed variables and proportions for categorical variables. A flowchart reporting the multi-step process of data extraction, processing, and analysis is shown in Figure 1.
We calculated the reporting odds ratio (ROR) for all selected AEs with at least five reports in order to reduce the likelihood of false positives (Raschi et al., 2017). ROR is a disproportionality approach widely applied based on the principles of calculations using a 2 × 2 table (Supplementary Table S1) (Salem et al., 2018). The 95% confidence interval (CI) was estimated for the ROR, and a signal was considered when the lower limit of the 95% CI of the ROR (ROR025) exceeded one.
Previous findings report that several factors, such as patient physical and mental status, concomitant medicines, pharmaceutical formulation, route of drug administration, and disease severity, may impact the occurrence and severity of AEs associated with esketamine or ketamine (Ceban et al., 2021; McIntyre et al., 2021). However, no biomarkers or phenomenological features have been proven to be reliable predictors of outcome with ketamine in patients with TRD (McIntyre et al., 2021). We then compared the severe and non-severe reports to clarify the severity of the detected safety signals and identify risk factors in patients. AEs were classified as serious or non-serious, with serious cases defined as death, a life-threatening adverse drug experience, inpatient hospitalization or prolongation of existing hospitalization, a persistent or severe disability, a congenital anomaly/birth defect, as well as other serious medical events (Johnson et al., 2019). We compared age, weight, sex, esketamine dose, AE types, and concomitant drugs between serious and non-serious reports. Because reports from countries outside the U.S. are also been collected in the FAERS database, we further performed a subgroup analysis by including U.S. cases only in order to reduce the impact of geographic variation in AE reporting. Proportions were compared using a Pearson’s chi-squared (χ2) or Fisher’s exact test, and an independent samples t test was applied for continuous data, such as age and dose. Data were analyzed using SPSS (v22.0; IBM Corp., Armonk, NY, United States), and statistical significance was set at a two-tailed p < 0.05.
Finally, we conducted stratification analysis by sex (female and male), age (18–64 and ≥65 years), and reporter type (healthcare professionals and consumers) separately to further explore the impact of different stratification regimens on the associations between esketamine and nervous system disorders.
AEs with disproportionality signals are generally classified into three types according to the level of clinical importance: 1) weak clinical priority, 2) moderate clinical priority, and 3) strong clinical priority (Gatti et al., 2020). We applied a scale to prioritize signals in five aspects; 1) number of target AEs, 2) ROR025 values, 3) mortality proportion, 4) assessment as important medical events (IMEs) or designated medical events (DMEs), and 5) results of current evidence evaluation (Gatti et al., 2020). The detailed information is shown in Supplementary Table S2.
Time-to-onset (TTO) of a given event was calculated as the difference between the start of treatment and the date the event occurred. Analyses of TTO data were performed based on median duration, quartiles, and the Weibull shape parameter (WSP) test (Mazhar et al., 2021), which is used to determine the varying rate of incidence of AEs (Mazhar et al., 2021). The scale parameter α and shape parameter β are used to describe the Weibull distribution (Kinoshita et al., 2020; Mazhar et al., 2021). We calculated the median TTO and WSP of signals with moderate or strong clinical priority after the beginning of the esketamine therapy in order to predict the hazard of the occurrence of these AEs over time. The selection of parameters and criteria for evaluation were described in previous studies (Kinoshita et al., 2020; Mazhar et al., 2021). All WSP tests were conducted using Minitab statistical software (v19.1.0; Minitab LLC, State College, PA, United States).
A total of 5,592,554 individual cases of AEs have been submitted to the FAERS database since 2019 and containing 993 esketamine-related neurological AEs in 720 patients. The detailed clinical characteristics of patients with esketamine-induced AEs are listed in Table 1. Serious cases of neurological and overall AEs, including six (1.28%) and 87 (6.06%) deaths, were recorded in 420 and 1,282 patients, respectively. Other serious events and hospitalization were the most frequently reported severe outcomes and occurred in 443 (94.25%) and 1,265 (88.15%) cases of neurological and overall events, respectively. There were 214 (29.72%), 329 (45.69%), and 177 (24.58%) neurological reports received in 2019, 2020, and the first half of 2021, respectively. The detailed quarterly numbers of submitted cases for neurological and overall AEs are shown in Figure 2A. Age data were available for 519 patients (mean age: 46.59 ± 15.32 years); notably, no patient was <18 years of age. Neurological AEs predominantly affected females (64.01%), with a female to male sex ratio of 1.78:1. Nervous system AEs reports were submitted by healthcare professionals and consumers in 616 (85.67%) and 103 (14.33%) cases, respectively.
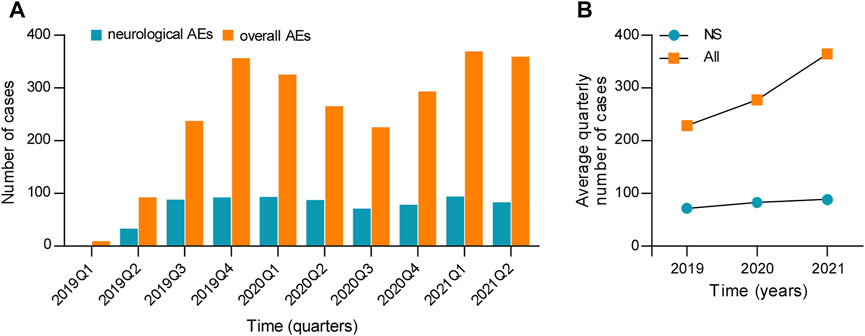
FIGURE 2. (A) Number of cases and (B) the average quarterly number of cases for neurological and overall AEs associated with esketamine. NS, nervous system; AEs, adverse events.
From 2019 to 2021, 90 different PTs related to neurological AEs after receiving esketamine were reported in the FAERS database, 30 of which were mentioned in at least five reports (Figure 3). The three most commonly reported neurological events were sedation (n = 361, 36.35%), dizziness (n = 130, 13.09%), and headache (n = 70, 7.05%).
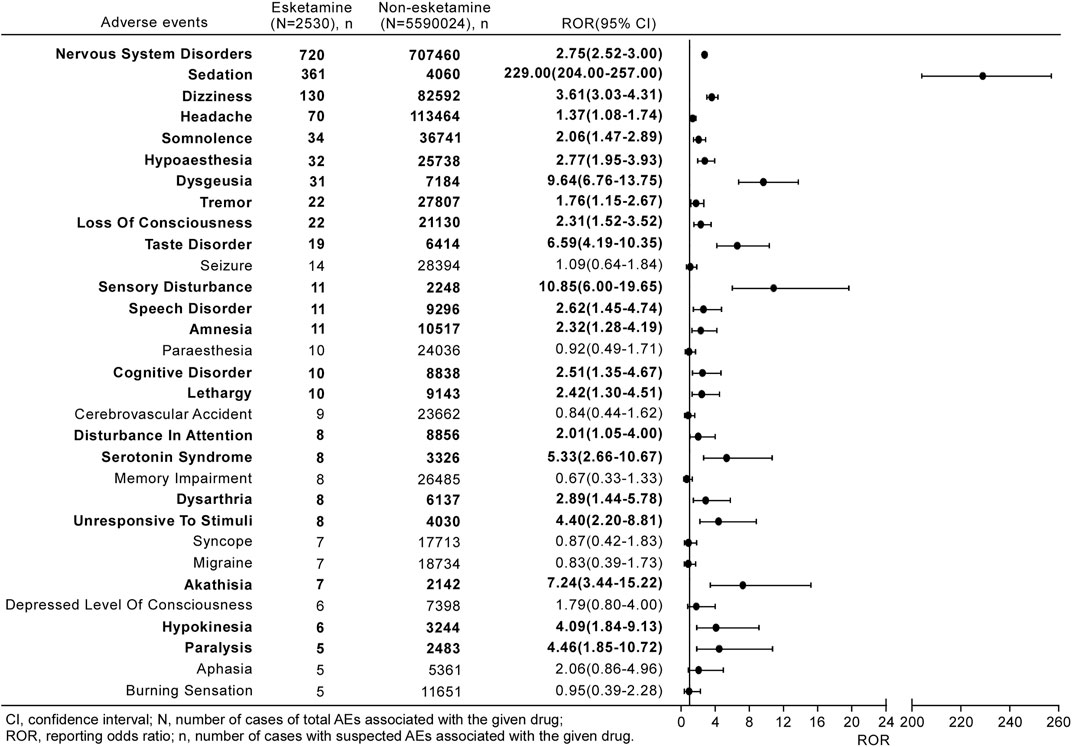
FIGURE 3. Reporting odds ratios (ROR) with 95% CI for all esketamine-related neurological AEs with at least five counts. Results that are statistically significant are in bold.
Figure 3 shows a full list of disproportionality results for the esketamine-associated neurological AEs occurring in at least five reports. The frequency of the reported nervous system disorders with esketamine was strikingly higher than that for non-esketamine in the entire database, with an ROR025 of 2.52. Further investigation of the subgroups revealed 21 neurological signals after esketamine treatment, with values of signals ranging from a ROR025 of 1.05 (disturbance in attention) to 204.00 (sedation). Other events, including paresthesia, memory impairment, and cerebrovascular accidents, were not over-reported in this population.
Esketamine dose differed statistically significantly between severe and non-severe cases of neurological AEs (69.88 ± 15.46 mg vs. 63.93 ± 15.54 mg, respectively; p = 0.017) (Table 2). By contrast, weight and age did not differ between the two groups (p = 0.521 and p = 0.053, respectively). Additionally, a higher proportion of males exhibited serious AEs than females; however, the difference was not statistically significant (χ2 = 0.72, p = 0.397). Sedation (χ2 = 31.15, p < 0.001), loss of consciousness (χ2 = 14.49, p < 0.001), and serotonin syndrome (p = 0.024) were more likely to be reported as serious AEs, whereas dizziness (χ2 = 4.12, p = 0.042), dysgeusia (χ2 = 7.98, p = 0.005), taste disorders (χ2 = 9.64, p = 0.002), and cognitive disorders (p = 0.002) were more likely to be reported as non-serious AEs. Patients with severe AEs were more likely to receive combination therapy with antidepressant polypharmacy (χ2 = 6.75, p = 0.009), benzodiazepines (χ2 = 4.10, p = 0.043), or somatic medications (χ2 = 5.05, p = 0.025) than patients with non-serious AEs. After assessing U.S. cases only, risk factors related to the severity of neurological AEs persisted. Detailed results are shown in Supplementary Table S3.
We used three different stratification strategies to increase the robustness of the findings. After separately assessing nervous system disorders stratifying by sex, age, and reporter type (Figure 4), the ROR025 values of all stratification subgroups were greater than one and the associations between esketamine and nervous system disorders persisted. However, the spectrum of neurological AEs differed in stratification regimens, with the detailed stratification analysis results shown in Figure 5.
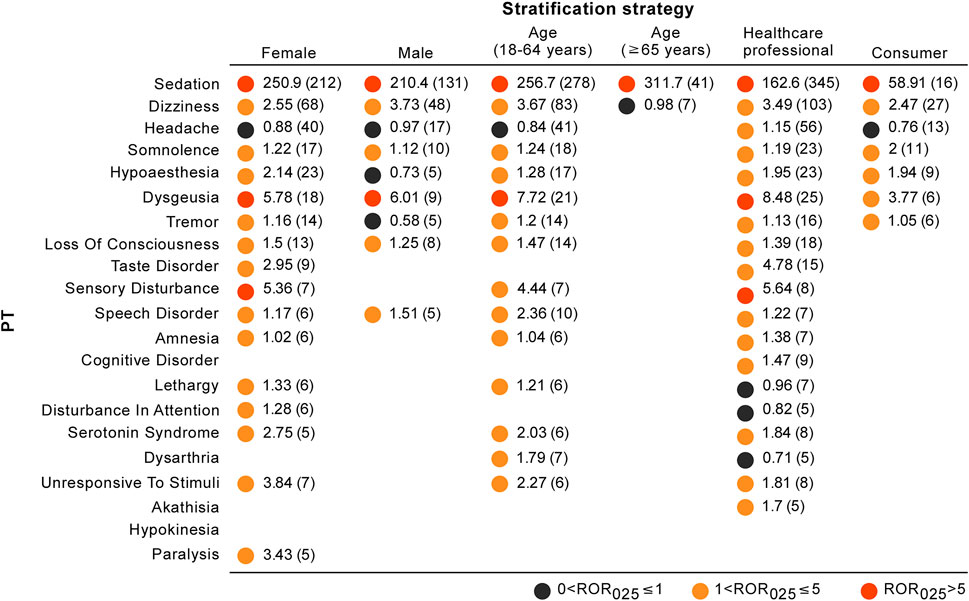
FIGURE 5. Neurological toxicity spectrums for different stratification strategies. The results are expressed in the form of ROR025 (n). Blank spaces represent not eligible for disproportionality analysis (AEs with at least 5 reports were eligible for analysis).
In total, 10 of the 21 PTs (47.62%) with disproportionality signals were categorized as IMEs, whereas none represented DMEs (Table 3). According to the clinical priority assessment results, 18 (85.71%), three (14.29%), and zero PTs were graded as weak, moderate, and strong clinical priority, respectively. Sedation (n = 361; ROR025 = 204.00; and priority score = 7), dizziness (n = 130; ROR025 = 3.03; and priority score = 5), and dysgeusia (n = 31; ROR025 = 6.76; and priority score = 5) were considered moderate clinical priorities. In the assessment of the relevant evidence, 10 PTs showed a strong level of evidence, and seven detected disproportionality signals were not previously detected in clinical trials (i.e., sensory disturbance, amnesia, cognitive disorder, serotonin syndrome, akathisia, hypokinesia, and paralysis).
Results of TTO and WSP analyses for sedation, dizziness, and dysgeusia are summarized in Table 4. The median TTO of sedation, dizziness, and dysgeusia associated with esketamine was 1.00 (range: 0–621), 0.00 (range: 0–433), and 0.00 (range: 0–17) days, respectively. In the WSP test for sedation, both the shape parameter β and the upper limit of its 95% CI were <1, suggesting an early failure type, with the same failure type identified for dizziness and dysgeusia.
This report provides the most updated findings linking esketamine with the neurological safety profiles based on the real-world population. Although the majority of results were in line with those obtained in the previous study, there were discrepancies in the spectrum of neurological AEs and the effects of several risk factors on AEs severity among the two studies. Overall, several major findings emerged that deserve further discussion.
Because esketamine was approved in March 2019, the number of neurological and overall AEs was low in 2019 Q1, with only one (0.14%) and nine (0.36%) cases, respectively. Therefore, we calculated the average number of quarterly reports by excluding data from 2019 Q1 in order to more precisely compare the annual growth trend of esketamine safety reports (Figure 2B). Figure 2B shows that there has been a marked increase in the mean number of esketamine reports per quarter from 2019 to 2020, with the annual total counts in 2020 almost 1.6-fold higher than that in 2019. This growth trend of esketamine reports across this time window is similar to the results reported in the preceding study, which indicated that the increasing trend was noteworthy, and that the number of monthly esketamine-associated AEs had nearly doubled in 2020 relative to 2019 (Gastaldon et al., 2020). Additionally, the authors predicted that the number of esketamine reports might expand further in the next year (Gastaldon et al., 2020). The present findings confirm this prediction, with a higher increase in the average number of quarterly reports in 2021 compared to 2020 (Figure 2B). Assuming that the Weber effect is correct [i.e., the reporting peak happened 2 years after approval of the drug (Hoffman et al., 2014)], we propose the hypothesis that esketamine reports may gradually stabilize from 2021 Q3 onwards.
We then evaluated the increasing trend of esketamine-related neurological AEs. The counts of neurological safety reports increased in the first 9 months of 2019 and have exhibited a relatively stable trend thereafter (Figure 2A). In summary, although we argue that notoriety bias [e.g., substantially increased AEs reporting stimulated by media attention (Khouri et al., 2021)] has no marked impact on the reporting pattern of esketamine-related neurological AEs, an enhanced post-marketing safety surveillance remains necessary.
Generally, disproportionality analysis cannot be used as an independent method to assess drug-related risks in real-world populations or replace formal clinical trials owing to its inability to quantify the incidence rates of adverse events. However, recent findings suggest that there is a significant correlation with the strength of association between disproportionality analyses and risk estimates in clinical studies (e.g., relative risks) (Van Puijenbroek et al., 2002; Maciá-Martínez et al., 2016; Khouri et al., 2021). Therefore, in the absence of data from clinical trials or epidemiological studies, disproportionality analysis provides at least an important indication of the prioritization of AEs and helps in the design of future studies (Maciá-Martínez et al., 2016; Khouri et al., 2021). Although the results for a majority of signals were in line with those obtained in the previous study, there were discrepancies in the spectrum of esketamine-associated neurological AEs between the two studies. One year after esketamine approval, 18 esketamine-related neurological AEs with at least four reports were registered in the FAERS database (Gastaldon et al., 2020), whereas in the present study, the total number increased to 34, representing 16 newly recorded AEs emerged in the following year. The full list of all new esketamine-relateded neurological AEs is provided in Supplementary Table S4. Further analysis of these new AEs detected eight signals, including amnesia (ROR025 = 1.28), loss of consciousness (ROR025 = 1.52), paralysis (ROR025 = 1.85), serotonin syndrome (ROR025 = 2.66), disturbance in attention (ROR025 = 1.05), hypertonia (ROR025 = 4.63), nystagmus (ROR025 = 3.97), and unresponsive to stimuli (ROR025 = 2.20). Interestingly, three events, i.e., cognitive disorder, headache, and tremor were less frequently reported in the previous study (Gastaldon et al., 2020) than in our study (ROR025 were respectively 0.89 vs. 1.35, 0.75 vs. 1.08, and 0.86 vs. 1.15 in the previous study vs. our analysis). In summary, the spectrum of safety signals may change over time as more reports are submitted in the future. Healthcare professionals should continuously monitor medication safety and ensure timely reporting of any AEs to spontaneous reporting systems.
The present study showed the three most commonly reported neurological events were sedation, dizziness, and headache. In particular, sedation was the only signal related to all stratification regimens (Figure 5) and more likely to be reported as a severe AE. Similarly, sedation was the signal with the highest ROR025 values among all neurological AEs in both previous pharmacovigilance study (Gastaldon et al., 2020) and the present analysis, stimulating our interest to further explore its essential features. We extracted a total of 361 (50.14%) sedation cases, with 248 (68.70%) eventually reported as serious cases, including six deaths. These results are consistent with those reported in a series of randomized controlled studies, where sedation was substantially more frequent in the esketamine groups (49–61%) than in the placebo-treated groups (10–19%), with a relative risk of up to 4.75 (Fedgchin et al., 2019; Popova et al., 2019; Ochs-Ross et al., 2020; Zheng et al., 2020). Moreover, other clinical characteristics of sedation warrant further attention. In the TTO analysis, the median TTO of sedation was 1 day, implying that at least half of the patients developed a rapid (within 24 h) onset of sedation. This also accords with previous clinical trials, which demonstrated that the latest onset and resolution time of any degree of sedation among all participants were 90 and 210 min after esketamine use, respectively (Fedgchin et al., 2019; Popova et al., 2019; Ochs-Ross et al., 2020; Psychopharmacologic Drugs Advisory Committee, 2020). Furthermore, several subjects experienced marked fluctuations in the way sedation occurred, indicating that the post-dose times of onset, peak, resolution, and severity varied among follow-up visits and suggesting that previous experience cannot accurately predict the future onset pattern (Fedgchin et al., 2019; Popova et al., 2019; Ochs-Ross et al., 2020; Psychopharmacologic Drugs Advisory Committee, 2020). Therefore, due to the potential severity and fluctuating mode of sedation, clinicians should monitor patients for at least 2-h post-dose to alleviate excessive sedation risk (e.g., motor vehicle accidents, falls) associated with esketamine.
Of particular interest to clinicians and patients is whether they would be more or less likely to be safe to benefit from ketamine or esketamine management from an a priori perspective (McIntyre et al., 2021). The present analysis indicated that esketamine dosage, antidepressant polypharmacy, or co-prescription with benzodiazepines but not age or sex may correlate with an increased risk of AEs severity. The descriptive analysis in Table 1 showed that females (64.01%) were more inclined to report neurological AEs, and further comparison of severe and non-severe cases revealed that although the proportion of serious AEs was numerically higher in males than in females (66.52 vs. 62.84%), the two groups did not differ statistically. This result is slightly different from the finding of a previous pharmacovigilance study that suggested women were at a higher risk of reporting severe AEs after receiving esketamine (Gastaldon et al., 2020). Few studies have evaluated the effect of sex on esketamine-related side effects; however, some previous observations provided evidence of the differential effects of sex on AE occurrence in patients receiving the esketamine parent compound ketamine (Winstock et al., 2012; Zhang et al., 2013; Chen et al., 2014). An observational study found that male patients receiving ketamine were more likely to undergo psychotic disorders (Zhang et al., 2013), and other studies revealed that cognitive impairment mainly affected female ketamine users (Zhang et al., 2013; Chen et al., 2014). By contrast, Winstock et al. (2012) noted an absence of any association with sex in urinary symptoms among ketamine patients. Additionally, the present stratification analysis showed that the spectrum of neurological AEs differed between females and males, with females associated with more neurological event categories than males (Figure 5). The probable mechanisms of distinct sex effects on ketamine-related toxicities remain unclear, although several explanations have been proposed, such as sex hormones and pharmacokinetic properties (Lee et al., 2000; Zarate et al., 2012; Gastaldon et al., 2020). Although no general conclusions have been reached on the exact effects of sex on esketamine-induced toxicity, sex should be viewed as a key factor in clinical treatment and future research. In the previous study, Gastaldon et al. (2020) compared age between reports with serious and non-serious outcomes and found that there was no statistical difference in age among two groups (p = 0.807), which is consistent with the finding in our current study. Additionally, we performed stratification analysis by age (18–64 and ≥65 years) and found that the ROR025 values of nervous system disorders were similar among these two subgroups (2.89 and 2.50, respectively) and two disproportionality signals were present (Figure 4). Therefore, the associations between esketamine and nervous system disorders persisted when stratifying by age. However, this study observed the spectrum of neurological AEs differed markedly between stratification regimens by age, and patients aged ≥65 years reported fewer neurological event categories than those <65 years (Figure 5). This is consistent with recent studies indicating that the common AEs were reported less frequently in patients ≥65 years of age than in those <65 years after esketamine use (Bozymski et al., 2020; Ochs-Ross et al., 2020; Psychopharmacologic Drugs Advisory Committee, 2020). However, our results should be interpreted with caution and require further investigations for verification based on the low reporting cases among geriatric patients.
The present study revealed that patients taking a higher dose of esketamine were more prone to develop serious neurological toxicity. A possible explanation for this might be that the increases in Cmax and area under the receiver operating characteristic curve were nearly dose-proportional when the dose of esketamine was increased from 56 to 84 mg, with a subsequent increase in the risk of dose-dependent adverse reactions (U.S. Food and Drug Administration, 2019; McIntyre et al., 2021). Other possible high-risk factors were the intake of combination therapy with antidepressant polypharmacy, somatic medications, or benzodiazepines, potentially due to channeling bias (e.g., selectively co-prescribing other drugs with esketamine to patients with more severe conditions) and potential drug–drug interactions affecting treatment outcomes (Gastaldon et al., 2020; Raschi et al., 2018; Carmona-Huerta et al., 2019). The above findings support the conclusions of another study in this field, with the exception of patients receiving co-medication with antipsychotics or mood stabilizers (Gastaldon et al., 2020). Moreover, we noticed that benzodiazepines were more tend to be administered concomitantly with esketamine than other hypnotics in the reported cases (Table 2). However, the concomitant use of benzodiazepines increased the risk of developing severe neurological AEs (χ2 = 4.10, p = 0.043). This finding is consistent with those of previous studies, which suggested that concomitant esketamine use with central nervous system depressants (e.g., benzodiazepines, opioids, alcohol) may worsen sedation (U.S. Food and Drug Administration, 2019; Diekamp et al., 2021). Given the frequent need for co-prescriptions of hypnotics with esketamine, we recommend that non-benzodiazepines (e.g., zolpidem, zopiclone, zaleplon and eszopiclone) should be administered to mitigate the risk of severe neurological events.
In recent years, there has been an exponential increase in research on disproportionality analysis, particularly concerning the rapid detection of safety signals for recently approved drugs (Raschi et al., 2018). In the present study, we sought to advance the application of our disproportionality analysis by utilizing a rating scale in order to prioritize safety signals and avoid unnecessary warnings. This approach may also be beneficial in helping clinicians and pharmacovigilance researchers improve the reliability of disproportionality signals by evaluating the current evidence. The disproportionality signals with moderate clinical priority other than sedation were dizziness and dysgeusia. Dizziness is among the most commonly observed adverse reactions in patients being treated with esketamine and usually manifests as exertion dizziness, postural dizziness, and procedural dizziness. A pooled analysis of completed phase 3 studies found that dizziness was less tolerable in the esketamine group than in the placebo group (27.6–33.0% vs. 6.4–9.7%) (Fedgchin et al., 2019; Popova et al., 2019; Ochs-Ross et al., 2020), with an odds ratio (OR) of 4.47 (95% CI: 3.27–6.11, p < 0.0001) (Wang et al., 2021). Additionally, in the TTO analysis, the median TTO of dizziness was 0 days, with an early failure type profile, suggesting that most patients would report dizziness within the same day of esketamine treatment, and then the risk of dizziness occurrence gradually decreased over time. However, the data on time-to-onset for dizziness slightly contrasted what is known from the previous pharmacovigilance study, showing dizziness with a mean time-to-onset of 15.7 days (Gastaldon et al., 2020). This discrepancy mainly resulted from the different computation methods of TTO data. According to a recent meta-analysis of controlled trials, dysgeusia was statistically significantly more commonly reported in the intranasal esketamine arm than in the placebo arm (OR = 1.67, 95% CI: 1.21–2.31; p = 0.022) (Wang et al., 2021). Furthermore, the frequency of dysgeusia in various age ranges differs, and patients aged <65 years appear to be associated with relatively higher dysgeusia frequency than patients aged ≥65 years (18.8 vs. 5.6%) (Fedgchin et al., 2019; Popova et al., 2019; Ochs-Ross et al., 2020), which further supports the findings of our stratification analysis (number of dysgeusia reports: 21 vs. 2, respectively) (Figure 5).
Seven unexpected AEs with a detected signal require further attention in the future, including sensory disturbance, amnesia, cognitive disorder, serotonin syndrome, akathisia, hypokinesia, and paralysis. These events were not previously detected in pre-marketing pivotal clinical trials and only reported in the pharmacovigilance database. Surprisingly, an unexpected finding was the absence of convincing clinical evidence for the association between esketamine and cognitive disorder. In a phase I study that recruited healthy volunteers (Morrison et al., 2018), a single dose of 84 mg esketamine caused statistically significant cognitive performance impairment at 40-min post-dose versus placebo-treated participants (all five tests, p < 0.005). By contrast, cognitive performance on these tests was comparable between the esketamine- and placebo-treated groups at 2-, 4-, or 6-h post-dose (Morrison et al., 2018). Additionally, the CogState Computerized Test Battery used to assess cognition performance in phase 3 studies revealed no statistically significant differences between the placebo and esketamine groups, except for findings in the SUSTAIN-2 trial (this trial provided evidence of slowing of the reaction time in elderly participants) (Daly et al., 2019; Fedgchin et al., 2019; Popova et al., 2019; Ochs-Ross et al., 2020; Wajs et al., 2020). Nevertheless, it remains difficult to distinguish the drug effect from other factors because of the high degree of intra-individual variability in the SUSTAIN-2 trial (Wajs et al., 2020). Although no universally convincing conclusions have been drawn on the exact impact of esketamine on cognitive function, cognitive impairment is still listed in the warning and precaution section of the instruction manual (U.S. Food and Drug Administration, 2019), likely owing to the fact that cognitive and memory impairments have been reported with long-term ketamine use or abuse (Na and Kim, 2021). Consequently, the exact effects of esketamine on cognition and the mechanisms of this potential association require further investigation.
Clinical trials and several systematic reviews report that neurological disorders occur in ∼20–60% of patients following administration (Caddy et al., 2015; Kishimoto et al., 2016; Fedgchin et al., 2019; Janssen Inc, 2020; Xiong et al., 2021). Therefore, there is a strong need for effective preventive and management measures for these side effects in order to ensure greater acceptability and effectiveness of the treatments. Preventive interventions (e.g., establishing a comfortable therapeutic environment without over-stimulation, playing soothing music, and instruction to perform breathing and mindfulness exercises) and constant patient surveillance are the primary methods for alleviating neurological side effects (Ceban et al., 2021). Additionally, patient evaluation (e.g., prescribing esketamine to patients already at high risk should be done with extreme caution), patient education and instruction, and skillful care operations are crucial steps in mitigating adverse reactions (Ceban et al., 2021; McIntyre et al., 2021). Furthermore, clinicians need to fully consider the necessity of adjunctive medications [e.g., moderate-to-severe headaches can be addressed using analgesics, such as acetaminophen (Ceban et al., 2021); and benzodiazepines may worsen sedation and are not recommended], dosage reduction [e.g., in cases of dose-dependent neurological AEs, such as sedation, the dose of esketamine should be decreased to promote tolerability, although the efficacy may be compromised (Psychopharmacologic Drugs Advisory Committee, 2020; McIntyre et al., 2021)], and suspension or discontinuation of therapy owing to serious adverse reactions.
Notably, the findings of this study based on the FAERS database need to be interpreted with caution, in consideration of several limitations shared by all pharmacovigilance databases, including the possibility of submitting incomplete, inaccurate, untimely, and unverified information (Hauben et al., 2007; Salem et al., 2018; Caldito et al., 2021). Additionally, the incidence rates of AEs cannot be calculated by disproportionality analysis due to the lack of the total size of the population using esketamine and could also be affected by over- or under-reporting (Gastaldon et al., 2020). Furthermore, the establishment of causality between a drug and AE is also restricted in pharmacovigilance studies (Salem et al., 2018). Another limitation of the present study is that we focused on the AEs in one reaction group alone, so the generalization between our findings and other system organ classes is unknown. Notwithstanding such limitations, the new findings in the present study could potentially prompt improved awareness of esketamine-related toxicities and provide some support evidence to confirm the conclusions found by Gastaldon et al. (2020). Finally, spontaneous individual safety reports provide a valuable source of drug safety information and remain the cornerstone for post-marketing safety assessments.
To the best of our knowledge, this report provides the most updated analysis linking esketamine with nervous system safety profile based on a larger number of individual safety reports. One of the main findings is that 16 latest neurological AEs emerged in the second year of marketing approval of esketamine, with eight signals detected. Moreover, we observed a continuously increasing trend of esketamine overall reports in the period from 2019 to 2021 and proposed a hypothesis that esketamine reports may gradually stabilize from the second half of 2021 onwards. However, the growth trend in neurological AEs is somewhat different, exhibiting a relatively stable trend since 2019 Q4. A higher dose of esketamine, antidepressant polypharmacy, and combination treatment with benzodiazepines or somatic medications are more likely to be risk factors related to AEs severity, whereas age and sex are not. Three AEs with moderate signal prioritization exhibit the same early failure type profiles, suggesting that the risk of these AEs occurrence associated with esketamine increases at an earlier stage of the treatment. From a prospective viewpoint, given the growing use of esketamine, pharmacovigilance studies will likely play a central role in facilitating risk–benefit assessment through vigorous long-term monitoring, particularly for unanticipated AEs. In summary, our findings and management recommendations could potentially prompt improved awareness of esketamine-related toxicities and help clinicians/researchers mitigate the risk of neurological events. In the meantime, more intensive studies on other systemic organ classes are warranted in the future to comprehensively examine the safety profiles of esketamine.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
HG, BW, JL, MH and JW contributed to the conception and design of the work; HG, SW and SY collected and analyzed the data; BW generated the tables and figures; HG wrote the first draft of the manuscript; BW and JL critically revised the paper and provided technical and material support; and all authors reviewed and approved the publication of the final version of the manuscript.
This study does not represent the opinion of the U.S. FDA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to Hai-hang Li (South China Normal University, Guangzhou, China) for his excellent contribution to language editing during the preparation of this manuscript. Permission from Oxford University Press for the use of a rating scale to prioritize the signals is gratefully acknowledged [Gatti, M., Antonazzo, I.C., Diemberger, I., De Ponti, F., and Raschi, E. (2020). Adverse events with sacubitril/valsartan in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur. J. Prev. Cardiol. 28 (9), 983–989. doi: 10.1177/2047487320915663].
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.849758/full#supplementary-material
Bozymski, K. M., Crouse, E. L., Titus-Lay, E. N., Ott, C. A., Nofziger, J. L., and Kirkwood, C. K. (2020). Esketamine: A Novel Option for Treatment-Resistant Depression. Ann. Pharmacother. 54 (6), 567–576. doi:10.1177/1060028019892644
Caddy, C., Amit, B. H., Mccloud, T. L., Rendell, J. M., Furukawa, T. A., Mcshane, R., et al. (2015). Ketamine and Other Glutamate Receptor Modulators for Depression in Adults. Cochrane. Database. Syst. Rev. 23 (9), Cd011612. doi:10.1002/14651858.CD011612.pub2
Caldito, N. G., Shirani, A., Salter, A., and Stuve, O. (2021). Adverse Event Profile Differences between Rituximab and Ocrelizumab: Findings from the FDA Adverse Event Reporting Database. Mult. Scler. 27 (7), 1066–1076. doi:10.1177/1352458520949986
Carmona-Huerta, J., Castiello-De Obeso, S., Ramírez-Palomino, J., Duran-Gutiérrez, R., Cardona-Muller, D., Grover-Paez, F., et al. (2019). Polypharmacy in a Hospitalized Psychiatric Population: Risk Estimation and Damage Quantification. BMC Psychiatry 19 (1), 78. doi:10.1186/s12888-019-2056-0
Carnovale, C., Mazhar, F., Pozzi, M., Gentili, M., Clementi, E., and Radice, S. (2018). A Characterization and Disproportionality Analysis of Medication Error Related Adverse Events Reported to the FAERS Database. Expert Opin. Drug Saf. 17 (12), 1161–1169. doi:10.1080/14740338.2018.1550069
Ceban, F., Rosenblat, J. D., Kratiuk, K., Lee, Y., Rodrigues, N. B., Gill, H., et al. (2021). Prevention and Management of Common Adverse Effects of Ketamine and Esketamine in Patients with Mood Disorders. CNS Drugs 35 (9), 925–934. doi:10.1007/s40263-021-00846-5
Chen, W. Y., Huang, M. C., and Lin, S. K. (2014). Gender Differences in Subjective Discontinuation Symptoms Associated with Ketamine Use. Subst. Abuse. Treat. Prev. Pol. 9, 39. doi:10.1186/1747-597X-9-39
Daly, E. J., Trivedi, M. H., Janik, A., Li, H., Zhang, Y., Li, X., et al. (2019). Efficacy of Esketamine Nasal Spray Plus Oral Antidepressant Treatment for Relapse Prevention in Patients with Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 76 (9), 893–903. doi:10.1001/jamapsychiatry.2019.1189
Diekamp, B., Borentain, S., Fu, D. J., Murray, R., Heerlein, K., Zhang, Q., et al. (2021). Effect of Concomitant Benzodiazepine Use on Efficacy and Safety of Esketamine Nasal Spray in Patients with Major Depressive Disorder and Acute Suicidal Ideation or Behavior: Pooled Randomized, Controlled Trials. Neuropsychiatr. Dis. Treat. 17, 2347–2357. doi:10.2147/NDT.S314874
Fedgchin, M., Trivedi, M., Daly, E. J., Melkote, R., Lane, R., Lim, P., et al. (2019). Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined with a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 22 (10), 616–630. doi:10.1093/ijnp/pyz039
Gastaldon, C., Raschi, E., Kane, J. M., Barbui, C., and Schoretsanitis, G. (2020). Post-Marketing Safety Concerns with Esketamine: A Disproportionality Analysis of Spontaneous Reports Submitted to the FDA Adverse Event Reporting System. Psychother. Psychosom. 90 (1), 1–8. doi:10.1159/000510703
Gatti, M., Antonazzo, I. C., Diemberger, I., De Ponti, F., and Raschi, E. (2020). Adverse Events with Sacubitril/valsartan in the Real World: Emerging Signals to Target Preventive Strategies from the FDA Adverse Event Reporting System. Eur. J. Pre. Cardiol. 28, 983–989. doi:10.1177/2047487320915663
Hauben, M., Reich, L., DeMicco, J., and Kim, K. (2007). 'Extreme Duplication' in the US FDA Adverse Events Reporting System Database. Drug Saf. 30 (6), 551–554. doi:10.2165/00002018-200730060-00009
Hoffman, K. B., Dimbil, M., Erdman, C. B., Tatonetti, N. P., and Overstreet, B. M. (2014). The Weber Effect and the United States Food and Drug Administration's Adverse Event Reporting System (FAERS): Analysis of Sixty-Two Drugs Approved from 2006 to 2010. Drug Saf. 37 (4), 283–294. doi:10.1007/s40264-014-0150-2
Horowitz, M. A., and Moncrieff, J. (2020). Are We Repeating Mistakes of the Past? A Review of the Evidence for Esketamine. Br. J. Psychiatry 219, 614–617. doi:10.1192/bjp.2020.89
Janssen Inc (2020). Spravato® Esketamine Nasal spray, Product Monography Including Patient Medication Information. Available at: https://pdf.hres.ca/dpd_pm/00055812.PDF (Accessed Jul 21, 2021).
Johnson, D. B., Manouchehri, A., Haugh, A. M., Quach, H. T., Balko, J. M., Lebrun-Vignes, B., et al. (2019). Neurologic Toxicity Associated with Immune Checkpoint Inhibitors: a Pharmacovigilance Study. J. Immunother. Cancer 7 (1), 134. doi:10.1186/s40425-019-0617-x
Khouri, C., Petit, C., Tod, M., Lepelley, M., Revol, B., Roustit, M., et al. (2021). Adverse Drug Reaction Risks Obtained from Meta-Analyses and Pharmacovigilance Disproportionality Analyses Are Correlated in Most Cases. J. Clin. Epidemiol. 134 (2021), 14–21. Epub 2021 Jan 26. PMID: 33508405. doi:10.1016/j.jclinepi.2021.01.015
Kim, J., Farchione, T., Potter, A., Chen, Q., and Temple, R. (2019). Esketamine for Treatment-Resistant Depression - First FDA-Approved Antidepressant in a New Class. N. Engl. J. Med. 381 (1), 1–4. doi:10.1056/NEJMp1903305
Kinoshita, S., Hosomi, K., Yokoyama, S., and Takada, M. (2020). Time-to-onset Analysis of Amiodarone-Associated Thyroid Dysfunction. J. Clin. Pharm. Ther. 45 (1), 65–71. doi:10.1111/jcpt.13024
Kishimoto, T., Chawla, J. M., Hagi, K., Zarate, C. A., Kane, J. M., Bauer, M., et al. (2016). Single-dose Infusion Ketamine and Non-ketamine N-Methyl-D-Aspartate Receptor Antagonists for Unipolar and Bipolar Depression: a Meta-Analysis of Efficacy, Safety and Time Trajectories. Psychol. Med. 46 (7), 1459–1472. doi:10.1017/S0033291716000064
Lee, C. J., Do, B. R., Kim, J. K., and Yoon, Y. D. (2000). Pentobarbital and Ketamine Suppress Serum Concentrations of Sex Hormones in the Female Rat. J. Anesth. 14 (4), 187–190. doi:10.1007/s005400070003
Maciá-Martínez, M. A., De Abajo, F. J., Roberts, G., Slattery, J., Thakrar, B., and Wisniewski, A. F. (2016). An Empirical Approach to Explore the Relationship between Measures of Disproportionate Reporting and Relative Risks from Analytical Studies. Drug Saf. 39 (1), 29–43. doi:10.1007/s40264-015-0351-3
Mazhar, F., Battini, V., Gringeri, M., Pozzi, M., Mosini, G., Marran, A. M. N., et al. (2021). The Impact of Anti-TNFα Agents on Weight-Related Changes: New Insights from a Real-World Pharmacovigilance Study Using the FDA Adverse Event Reporting System (FAERS) Database. Expert Opin. Biol. Ther. 21 (9), 1281–1290. doi:10.1080/14712598.2021.1948529
McIntyre, R. S., Rosenblat, J. D., Nemeroff, C. B., Sanacora, G., Murrough, J. W., Berk, M., et al. (2021). Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am. J. Psychiatry 178 (5), 383–399. doi:10.1176/appi.ajp.2020.20081251
Morrison, R. L., Fedgchin, M., Singh, J., Van Gerven, J., Zuiker, R., Lim, K. S., et al. (2018). Effect of Intranasal Esketamine on Cognitive Functioning in Healthy Participants: a Randomized, Double-Blind, Placebo-Controlled Study. Psychopharmacology (Berl) 235 (4), 1107–1119. doi:10.1007/s00213-018-4828-5
Na, K. S., and Kim, Y. K. (2021). Increased Use of Ketamine for the Treatment of Depression: Benefits and Concerns. Prog. Neuropsychopharmacol. Biol. Psychiatry 104, 110060. doi:10.1016/j.pnpbp.2020.110060
Ochs-Ross, R., Daly, E. J., Zhang, Y., Lane, R., Lim, P., Morrison, R. L., et al. (2020). Efficacy and Safety of Esketamine Nasal Spray Plus an Oral Antidepressant in Elderly Patients with Treatment-Resistant Depression-TRANSFORM-3. Am. J. Geriatr. Psychiatry 28 (2), 121–141. doi:10.1016/j.jagp.2019.10.008
Popova, V., Daly, E. J., Trivedi, M., Cooper, K., Lane, R., Lim, P., et al. (2019). Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined with a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 176 (6), 428–438. doi:10.1176/appi.ajp.2019.19020172
Psychopharmacologic Drugs Advisory Committee (2020). Drug Safety and Risk Management (DSaRM). FDA Briefing Document. Available at: https://www.fda.gov/media/121376/download (Accessed Apr 1, 2020).
Raschi, E., Parisotto, M., Forcesi, E., La Placa, M., Marchesini, G., De Ponti, F., et al. (2017). Adverse Events with Sodium-Glucose Co-transporter-2 Inhibitors: A Global Analysis of International Spontaneous Reporting Systems. Nutr. Metab. Cardiovasc. Dis. 27 (12), 1098–1107. doi:10.1016/j.numecd.2017.10.008
Raschi, E., Poluzzi, E., Salvo, F., Pariente, A., De Ponti, F., Marchesini, G., et al. (2018). Pharmacovigilance of Sodium-Glucose Co-transporter-2 Inhibitors: What a Clinician Should Know on Disproportionality Analysis of Spontaneous Reporting Systems. Nutr. Metab. Cardiovasc. Dis. 28 (6), 533–542. doi:10.1016/j.numecd.2018.02.014
Salem, J. E., Manouchehri, A., Moey, M., Lebrun-Vignes, B., Bastarache, L., Pariente, A., et al. (2018). Cardiovascular Toxicities Associated with Immune Checkpoint Inhibitors: an Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol. 19 (12), 1579–1589. doi:10.1016/S1470-2045(18)30608-9
Schatzberg, A. F. (2019). A Word to the wise about Intranasal Esketamine. Am. J. Psychiatry 176 (6), 422–424. doi:10.1176/appi.ajp.2019.19040423
U.S. Food and Drug Administration (2019). Full Prescribing Information of Esketamine. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (Accessed Mar 5, 2019).
Van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A Comparison of Measures of Disproportionality for Signal Detection in Spontaneous Reporting Systems for Adverse Drug Reactions. Pharmacoepidemiol. Drug Saf. 11 (1), 3–10. doi:10.1002/pds.668
Wajs, E., Aluisio, L., Holder, R., Daly, E. J., Lane, R., Lim, P., et al. (2020). Esketamine Nasal Spray Plus Oral Antidepressant in Patients with Treatment-Resistant Depression: Assessment of Long-Term Safety in a Phase 3, Open-Label Study (SUSTAIN-2). J. Clin. Psychiatry 81 (3), e1–10. doi:10.4088/JCP.19m12891
Wang, S.-M., Kim, N.-Y., Na, H.-R., Lim, H. K., Woo, Y. S., Pae, C.-U., et al. (2021). Rapid Onset of Intranasal Esketamine in Patients with Treatment Resistant Depression and Major Depression with Suicide Ideation: A Meta-Analysis. Clin. Psychopharmacol. Neurosci. 19 (2), 341–354. doi:10.9758/cpn.2021.19.2.341
Winstock, A. R., Mitcheson, L., Gillatt, D. A., and Cottrell, A. M. (2012). The Prevalence and Natural History of Urinary Symptoms Among Recreational Ketamine Users. BJU. Int. 110 (11), 1762–1766. doi:10.1111/j.1464-410X.2012.11028.x
Xiong, J., Lipsitz, O., Chen-Li, D., Rosenblat, J. D., Rodrigues, N. B., Carvalho, I., et al. (2021). The Acute Antisuicidal Effects of Single-Dose Intravenous Ketamine and Intranasal Esketamine in Individuals with Major Depression and Bipolar Disorders: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 134, 57–68. doi:10.1016/j.jpsychires.2020.12.038
Zarate, C. A., Brutsche, N., Laje, G., Luckenbaugh, D. A., Venkata, S. L., Ramamoorthy, A., et al. (2012). Relationship of Ketamine's Plasma Metabolites with Response, Diagnosis, and Side Effects in Major Depression. Biol. Psychiatry 72 (4), 331–338. doi:10.1016/j.biopsych.2012.03.004
Zhang, Y., Lu, C., Zhang, J., Hu, L., Song, H., Li, J., et al. (2013). Gender Differences in Abusers of Amphetamine-type Stimulants and Ketamine in Southwestern China. Addict. Behavbehav 38 (1), 1424–1430. doi:10.1016/j.addbeh.2012.06.024
Keywords: esketamine, pharmacovigilance, neurological adverse events, signal, FAERS, disproportionality analysis
Citation: Guo H, Wang B, Yuan S, Wu S, Liu J, He M and Wang J (2022) Neurological Adverse Events Associated With Esketamine: A Disproportionality Analysis for Signal Detection Leveraging the FDA Adverse Event Reporting System. Front. Pharmacol. 13:849758. doi: 10.3389/fphar.2022.849758
Received: 06 January 2022; Accepted: 21 March 2022;
Published: 08 April 2022.
Edited by:
Emanuel Raschi, University of Bologna, ItalyReviewed by:
Chiara Gastaldon, Integrated University Hospital Verona, ItalyCopyright © 2022 Guo, Wang, Yuan, Wu, Liu, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, bGppbmdfc3l5QDE2My5jb20=; Miaoquan He, aG1pYW9xdWFuX3N5eUAxNjMuY29t; Jisheng Wang, d2ppc2hlbmdfc3l5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.