
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 09 March 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.843469
This article is part of the Research Topic Edible and Medicinal Plants: From Ethnopharmacological Practices to Interdisciplinary Approaches and Regulations View all 18 articles
 Hyung Mook Kim1,2†
Hyung Mook Kim1,2† Yong Moon Lee2
Yong Moon Lee2 Ee Hwa Kim1
Ee Hwa Kim1 Sang Won Eun3
Sang Won Eun3 Hyun Kyung Sung4
Hyun Kyung Sung4 Heung Ko5
Heung Ko5 Sang Jun Youn6
Sang Jun Youn6 Yong Choi6
Yong Choi6 Wakana Yamada7
Wakana Yamada7 Seon Mi Shin5*†
Seon Mi Shin5*†This study aimed to evaluate skin health’s functional improvement, such as wrinkles, elasticity, moisture, and whitening, and safety following the consumption of “edible bird’s nest extract” for 12 weeks by women. This single-center, double-blinded, parallel-group, placebo-controlled study included women aged 40–60 years. Our primary purpose was to assess improvement in skin wrinkles, elasticity, and moisture after 12 weeks using an SV700, cutometer, and corneometer, respectively, compared to baseline measurements. Our secondary purpose was to evaluate skin wrinkle, elasticity, and moisture changes at 4 and 8 weeks from baseline using the aforementioned equipment, and measure transdermal water loss and melanin and erythema indexes using a tewameter and mexameter, respectively. Experts performed the visual evaluation of skin wrinkles at 4, 8, and 12 weeks from baseline. The participants were randomly allocated in a 1:1 ratio into the edible bird’s nest extract or the placebo group with 43 participants each, where they consumed 100 mg of the extract or placebo, respectively, daily for 12 weeks. The outcomes were measured at every visit. In this study, upon comparing changes in the skin elasticity value between the two intake groups at 12 weeks of ingestion, skin elasticity in the edible bird’s nest extract group decreased significantly compared with that in the placebo group. Adverse reactions were absent in both groups. In the case of laboratory test results, changes before and after the ingestion of the extract were within the normal range, thus indicating no clinically significant difference. The edible bird’s nest extract was effective in improving skin wrinkles. Moreover, it is beneficial for skin health and can be used as a skin nutritional supplement. Compared with the placebo, the edible bird’s nest extract was identified as safe.
Clinical Trial Registration: https://cris.nih.go.kr/cris/search/detailSearch.do?search_lang=E&search_page=M&pageSize=10&page=undefined&seq=21007&status=5&seq_group=20330, identifier KCT0006558.
Edible bird’s nest (EBN) is the swift’s nest that is made from its saliva and contains sialylglycoconjugates. The composition of the swift’s saliva resembles that of salivary mucin. Many studies have been carried out on the tonic effects of EBN, and it has been shown that EBN stimulates mitosis hormones and the epidermal growth factor, resulting in the repair of cells and stimulation of the immune system (Guo et al., 2017). In China, it is termed “Yan Woo (燕窩)” and was used to maintain the beauty and youth of court and upper-class ladies. In addition, according to the ancient Chinese literature, “Bencao gangmu shiyi (本草綱目拾遺) and Bencao qiu zhen (本草求眞),” it is effective in curing tuberculosis, chronic diarrhea, and lung infections (Dictionary of Chinese Traditional Drugs, 1985). According to Chinese medical books, it brightens the skin, nourishes the stomach and lungs, and improves complexion. Although previous studies showed that EBN contains sialic acid, is effective in brain development, and is used for the treatment of various chronic inflammatory diseases, studies on its effects on skin health are still lacking (Kim et al., 2021). Sialic acid is a generic term for acyl derivatives of neuraminic acid, and there are more than 30 kinds of this derivative in nature (Kiefel and von Itzstein, 2002). N-acetylneuraminic acid is present in the EBN extract, which improves learning ability (Morgan and Winick, 1980), strengthens immunity (Bagriaçik and Miller, 1999), prevents influenza infection (Biddle and Belyavin, 1963), and acts as an important ingredient at the ends of cell membrane glycoproteins and glycolipids, in addition to improving skin elasticity and moisturizing effect. Antioxidants and skin health are areas of interest to all ages, and the functionality of health functional food materials has been consistently prioritized. As the skin ages, the number of elastic fibers and the skin regeneration rate decrease and wrinkles increase. In addition, active oxygen generated as a by-product of metabolic activity, harmful external substances, and exposure to ultraviolet rays may damage the elastic fibers, advancing skin aging. Food intake can improve skin health; however, daily meals are often deficient in nutrients, and eating only certain foods can be detrimental to health. Epidermal growth factor (EGF) has been found in EBN, which has been proposed to correspond with the proliferative effect of EBN in epidermal tissues (Kong et al., 1987). In addition, N-acetylneuraminic acid, which is present in EBN, possesses a skin-whitening function (Chan et al., 2015), and additionally, EBN was shown to reduce water loss, wrinkle area, and dermal thickness of skin (Terazawa and Shimoda, 2020). Herein, we have provided a different evidence to reveal the signaling pathway of EBN extract and its regulation of the expressions of filaggrin and filaggrin-2, two important skin barrier proteins of the SFTP family playing roles in water balance of the skin surface. The EBN-mediated regulation of filaggrin and filaggrin-2 is demonstrated to be triggered by the p38-MAPK signaling pathway and various transcriptional factors, for example, GATA3, PPARα, PPARβ, and PPARγ (Lai et al., 2021).Therefore, we conducted this clinical trial to explore the effect of EBN on improving skin wrinkles, elasticity, moisture, and whitening to obtain evidence of the extract as a functional material for skin health.
We recruited healthy participants through a written notice posted on the hospital’s homepage and bulletin board of the Chungbuk Cosmetics Clinical Research Support Center (Cheongju, Chungcheongbuk-do, the Republic of Korea) until the target sample size was reached. Women willing to participate in the study voluntarily visited the Chungbuk Cosmetics Clinical Research Support Center. After obtaining informed consent, we enrolled women who met the inclusion criteria. The first participant was enrolled in January 2021. The duration of the recruitment period was 8 months. The total sample size was 105. This clinical trial protocol (IRB No. SMCJH 2021-06) was approved by the Institutional Review Board of the Chungju Semyung University Korean Medicine Hospital and registered at the Korean Clinical Research Information Service (KCT0006558). Details of the eligibility and exclusion criteria are provided in Table 1.
EBN extract, the primary ingredient of the test food, was obtained from Japan’s Oryza Oil & Fat Chemical Co., Ltd. and is similar to the product commercially sold by EBN extract-PK, which contains 20% of the hydrolyzed swiftlet nest extract. Therefore, one capsule of this test food contains 20 mg of the hydrolyzed swiftlet nest extract (Supplementary Table S2). The HPLC analysis for sialic acid was included in the test food and is presented in Supplementary Table S1 and Figures 1B,C. The active and placebo capsules were identical in shape, size, and color (Figure 1). The standardization of this test food capsule was guaranteed by strict process controls during manufacture and analysis of the hydrolyzed bird’s nest extract. EBN extract is a powder that is dried by adding maltodextrin after enzymatic treatment of edible bird’s nest with protease; the difference is that it has been subjected to enzyme treatment to increase absorption efficiency in the body and has been standardized with sialic acid, an indicator and functional ingredient (Kim et al., 2021). The participants were orally administered either an active or placebo tablet per dose once a day for 12 weeks. They received a 1-month supply of the investigational product at baseline and during weeks 4 and 8, following which they were encouraged to continue the prescribed dosage regimen. At weeks 4, 8, and 12, the unused capsules were returned and counted to evaluate participant compliance. We advised the participants to maintain their usual diet and exercise levels during the study. They were prohibited from consuming medicines or foods that could affect their skin during the study, and during the screening visit (visit 1), the subjects taking such medicines or foods were excluded so as not to affect the results of the outcome. Medicines, foods, exercise therapy, and diets maintained prior to their enrollment were allowed at the discretion of the principal investigator. Information regarding all concomitant medications, including the product or ingredient name, dosage, and duration, was recorded at every visit. We discontinued the intervention under the following conditions: a severe adverse event; a participant had used a drug or underwent a physical procedure that could affect the skin; a participant wished to discontinue study participation; difficulties in assessment for administrative reasons, such as violations of the dosage method or the visit schedule; and difficulties in follow-up owing to personal reasons.
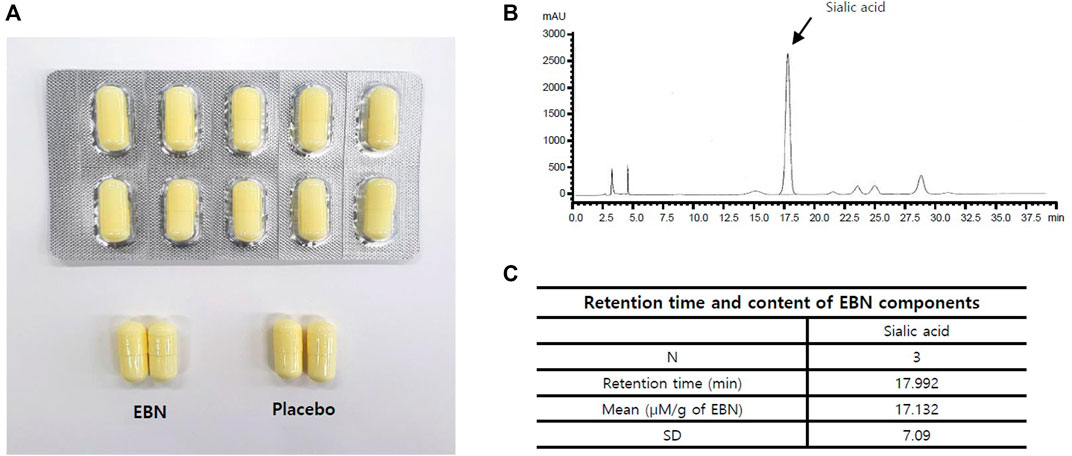
FIGURE 1. (A) EBN tablet and placebo. (B,C) Fingerprinting analysis of EBN. (B) Chromatogram of sialic acid HPLC specifications (x: time, y: sialic acid concentration). (C) The retention time of sialic acid in EBN components.
In this clinical trial, we performed block randomization. During screening, participants who met the eligibility criteria and were judged suitable for the clinical trial were assigned to the EBN and placebo groups. The randomization table is a sequence of random number permutations generated by the randomization program of the SAS 9.4 (SAS Institute, Cary, NY, the United States), beginning from number 1 for human subjects. The sponsor attached the food label for the human application test according to the randomization table during food packaging and supplied it to the testing institution before the commencement of this clinical trial.
The randomization codes were not disclosed until the end of the trial, except when it was unavoidably necessary to read the code. The clinical trial tester supplied the intervention that matched the registration number assigned to the selected participant.
The primary endpoint comprised changes in skin wrinkles (in R1: skin roughness, R2: maximum roughness, R3: average roughness, R4: smoothness depth, and R5: arithmetic roughness average), skin elasticity (in R2: total elasticity, R5: real elasticity, and R7: the ratio of elasticity to the entire curve), and skin moisture, each after 12 weeks from the baseline.
The secondary endpoint comprised changes in skin wrinkles (in R1, R2, R3, R4, and R5), skin elasticity (in R2, R5, and R7), and skin moisture, each at 4 and 8 weeks from the baseline. Moreover, it comprised changes in the amount of transdermal water loss, melanin and erythema index, and visual evaluation of skin wrinkles measured by experts, each at 4, 8, and 12 weeks from the baseline. The visual evaluation was analyzed as one result by discussion among two experts.
Experts performed a visual evaluation of skin wrinkles at visits 1 (screening visit), 2 (week 0), 3 (week 4), 4 (week 8), and 5 (week 12), and after visits 1 and 2. The results of visit 1 could be recorded for visit 2 conducted within a week. At visit 1, the selection criteria were confirmed by identifying the presence of wrinkles around the eyes and the Global Photo Damage Score (approximately 2–6 points), visually or by photographs. The SV700 evaluated skin wrinkles, the Cutometer MPA 5809 evaluated skin elasticity values, the Corneometer CM 825 evaluated skin moisture values, the Tewameter TM 300 evaluated transdermal moisture loss, and the Mexameter MX 18 evaluated skin whitening. The evaluation time comprised four visits, including baseline, visit 3 (week 4), visit 4 (week 8), and visit 5 (week 12). Skin wrinkle evaluation using the SV700 measured the area 2–3 cm away from the left eye, and the remaining techniques measured the right-angle intersection of the corner of the eye and the tip of the nose. The test site was kept clean and dry to ensure similar measurement conditions in all participants. In addition, the skin was stabilized in a place with constant temperature and humidity (22 ± 2°C, R.H. 40−60%) for at least 30 min before proceeding. Water intake was restricted 1 h before the measurement.
Safety was evaluated through adverse reactions, laboratory tests (hematology, blood chemistry, and urine), and vital signs (systolic blood pressure, diastolic blood pressure, pulse, and body temperature).
We referred to the article by Hwang et al. (2015) and used the amount of change in the average depth of skin wrinkles (R4 smoothness depth) using a micro-wrinkle analyzer. More than 104 people who met the inclusion and exclusion criteria were recruited to be allocated to the investigational product for human clinical research. Furthermore, it was planned to analyze more than 41 people per group with the number of subjects who completed the study as per the protocol and have no major protocol deviations that may affect the interpretation of the primary endpoint.
The formula for calculating the number of subjects in the human application test was as follows:
A statistical hypothesis test was conducted at a significance level of 0.05 (two-sided). The number of subjects, mean, and standard deviation were presented for primary and secondary endpoints. In addition, depending on whether the data normality assumption was satisfied, analysis of covariate (ANCOVA) used baselines as a covariate, or the Wilcoxon rank-sum test was performed to compare the difference between the groups. For demographic characteristics and laboratory test results, mean and standard deviation were presented, and a two-sample t-test or the Wilcoxon rank-sum test was used for inter-group comparison for continuous data. However, for categorical data, frequency and percentage were calculated, and Pearson’s chi-square test or Fisher’s exact test was used for inter-group comparison. Intra-group analysis was performed using the paired t-test or the Wilcoxon signed-rank test. Per-protocol set was used as the analysis for primary and secondary endpoints. Per-protocol set included all subjects who completed the study per the protocol and had no major protocol deviations. Moreover, a safety set that included all subjects who received at least one capsule of the investigational product and had at least one safety assessment was used as the safety analysis.
For this clinical trial, the first participant for the human application trial was screened on 11th January 2021, whereas the last participant was screened on 23rd April 2021.
A total of 105 participants were enrolled for the clinical trial, and the per-protocol set comprised 86 participants (Table 2), 43 each in the EBN and placebo groups (Figure 2 and Supplementary Table S3).

FIGURE 2. Study flowchart. Evaluation*: Adverse reactions and vital signs check, laboratory test (safety evaluation), pregnancy test, drug history/concomitant drug examination, efficacy evaluation, and skin functional test.
The EBN group showed a significant decrease in the skin wrinkle value compared to the placebo group at baseline and week 12. Elasticity decreased in both the groups; however, there was no statistical significance. Regarding the amount of skin moisture change before and after intake, there was no statistically significant difference between the EBN and the placebo groups (Figure 3 and Supplementary Table S4).

FIGURE 3. Effect of EBN on skin wrinkle parameters at baseline and week 12. Data are presented as the means ± SD. *Indicates that the p-value between the two groups is <0.05. **Indicates that the p-value between the two groups is <0.01. †Indicates that the p-value compared within groups is <0.05. † indicates that the p-value compared within groups is <0.01. N.S. is an abbreviation for not significant.
The skin elasticity value (R2: total elasticity, R5: real elasticity, and R7: ratio of elasticity to the entire curve) had decreased in both the EBN and placebo groups at baseline and week 12 (Supplementary Table S4).
Compared to the previous reading, there was no tendency for decrease or increase in the skin wrinkle value, skin elasticity value, and skin moisture value at week 4 and week 8 (Supplementary Table S5).
The change in transdermal water loss displayed a tendency to increase, and the melanin index and erythema index decreased in both groups at baseline and week 12 (Supplementary Table S6). However, there was no significant difference in secondary outcomes between the two groups.
No adverse reactions occurred during this study. There was no minimally detectable change or minimal clinically significant change among the vital sign variables. Laboratory test results (hematology, blood chemistry, and urine) showed minimal clinically significant changes (Supplementary Table S7).
EBN is a swiftlets’ (Aerodramus fuciphagus) residence made of their salivary gland secretions mixed with feathers or grass during breeding (Kew et al., 2014; Dai et al., 2021). The largest number of EBN was often found in caves or houses in Southeast Asia and the South Pacific, depending on the habitat distribution (Kew et al., 2014; Daud et al., 2021). EBN has been regarded as a high-grade healthy food that only privileged individuals can eat, with functions of moistening the lung, nourishing the stomach, relieving the liver, clearing the eyes, and tonifying the heart (Hwang et al., 2015). It is composed of protein, carbohydrate, ash, fat, and dietary elements (Quek et al., 2018), and has various effects, including antiviral effects and inhibitory hemagglutination (Guo et al., 2017); immunological enhancement (Zhao et al., 2016); improving intelligence and memory (Oliveros et al., 2018); improvement of neurodegenerative disease (Yew et al., 2014; Careena et al., 2018) and cardiovascular disease (Hun Lee et al., 2020); promoting cell division (Roh et al., 2012); antioxidant, anti-inflammatory, and anti-aging effects (Yida et al., 2015; Hun Lee et al., 2020) on epidermal growth factor-like activity (Kong et al., 1987); and stimulating collagen production and epithelial tissue proliferation (Park et al., 2017). EBN is expected to have various effects on skin health through these effects. Therefore, we tried to investigate the effect of EBN extract on skin wrinkles and whitening. We conducted this randomized, double-blind study to determine the effect of EBN extract on improving skin wrinkles, elasticity, moisture, and whitening. In a previous experimental study, the authors confirmed the safety of the extract (Haghani et al., 2016).
We observed a causal relationship between EBN and skin wrinkle improvement. We compared the amount of change in skin wrinkle (R1, R2, R3, R4, and R5) before (0 weeks) and after (12 weeks) the intake of EBN extract with the R1 value measured before the intake. Afterward, it was confirmed that the EBN group had significantly decreased the skin wrinkle value compared to the placebo group at baseline and week 12. In addition, R2, R3, R4, and R5 decreased significantly in the EBN group compared with those in the placebo group. In addition, the EBN and placebo groups demonstrated a statistically significant decrease in the skin wrinkle value before and after 12 weeks of the intervention. As a result, it was demonstrated that consumption of EBN extract positively affects skin wrinkle improvement.
There was no significant difference in secondary outcomes between the two groups. The skin elasticity value (R2: total elasticity, R5: real elasticity, and R7: ratio of elasticity to the entire curve) was decreased in both the EBN and placebo groups at baseline and week 12. The amount of change in transdermal water loss displayed a tendency to increase in both groups at baseline and week 12, and the melanin index and erythema index decreased in both groups at baseline and week 12.
There are studies that have documented that EBN containing EGF promotes and activates the synthesis of DNA, and thus promotes cell proliferation (Kong. et al., 1987). A further experiment was conducted to study the effects of EBN extract, with rich EGF, on human skin cell (epidermal keratinocytes and dermal fibroblasts) proliferation. The results showed that EBN extract promoted the proliferation of normal keratinocytes and fibroblasts dose-dependently (Kong et al., 1987). Keratinocytes play an important role in healthy skin barrier function, improved proliferation of keratinocytes directly improves skin barrier function, thus promoting skin suppleness and improving the overall skin texture. Meanwhile, fibroblasts synthesize extracellular matrix and collagen, and play a critical role in wound healing, influence skin elasticity, and physically apparent aging.
EGF plays an important role in the process of wound healing. Studies showed that wound healing is delayed in mice whose submandibular gland is removed as the submandibular gland produces EGF (Niall et al., 1982). Proliferation and migration of epithelial cells play a critical role in wound healing, and EGF is a known factor in promoting fibroblast proliferation and epithelial cells migration, thus promoting wound healing. The effect of EBN extract on wound healing was examined by a scratch test. Normal human keratinocytes were cultured in a petri dish. A damage model was induced by scratching a line in the center of the dish. Wound healing at the scratched area was observed after 24-h. Accelerated wound healing effect was observed in the dish treated with EBN extract, and the effect was dose-dependent. Thus, it is suggestive that EBN extract promotes wound healing (Niall et al., 1982).
Tight junctions (TJs) refer to a closely associated area of two adjacent cells whose membrane join together forming a virtually impermeable barrier to fluid. In the epidermis, TJs play a crucial role in the formation and maintenance of epithelial barriers and prevent invasion of foreign particles. In the stratum corneum, ceramide acts as a first line of skin barrier. TJs in the stratum granulosum act as the second line of skin barrier. Occludin and claudin are integral plasma membrane proteins located at the TJs (Niessen, 2007). They form important barrier that protect the skin from external organism, prevent excessive water loss, and selectively transport small solutes through the skin (Yamamoto et al., 2008). This has prompted further understanding into the effect of EBN on skin barrier function where an experiment was conducted to evaluate the effect of EBN on skin TJs. Among the TJ proteins, claudin-1 (Cldn-1) and claudin-4 (Cldn-4) have been demonstrated to have a role in skin barrier function (Furuse et al., 2002; Yuki et al., 2011). The effect of EBN on the expression of TJ proteins was examined. Both genetic expression and protein expression of Cldn-1 and Cldn-4 was upregulated by EBN-P (PC) 0.1% in vitro. In addition, the effect of EBN on the protein expression of Cldn-4 of normal keratinocytes was examined and observed using fluorescence microscopy. The results showed that the protein expression of Cldn-4 was upregulated by EBN-P (PC) 0.1%.
Sialic acid is the major carbohydrate found in EBN and in several tissues and fluids in humans (Kim et al., 2021). A study demonstrated the antioxidant role of sialic acid as a hydroxyl radical scavenger (Ogasawara et al., 2007), and another study found that sialic acid from EBN has the effects of scavenging DPPH radicals and hydroxyl radicals (Wang et al., 2019). Several studies have demonstrated that sialic acid is essential for brain function, immune function, and cell proliferation and repair (Wang, 2009; Varki and Gagneux, 2012; Rashed et al., 2021); however, there was no statistically significant difference between the two groups in the amount of skin moisture change. Considering that the present trial was conducted during the COVID-19 pandemic, all participants routinely used medical masks. Therefore, the increase in skin moisture content and transdermal moisture loss was a possible outcome of using masks for a prolonged period. Furthermore, this is a single-center study, not a cross-over study; therefore, it is highly likely that only subjects from a specific region had participated, which may lead to consequential bias. It can be seen that there was a slightly negative effect as the mask dries the skin, which can lead to wrinkles and loss of moisture in the skin. In contrast, we evaluated skin wrinkles at a position 2–3 cm away from the left eye, which was not affected by long-term mask-wearing; therefore, it is considered an objective functional evaluation index and can be evaluated by excluding other external factors (wearing a mask). There were no adverse reactions or clinically meaningful changes in the laboratory test values following the ingestion of EBN extract. The consumption of EBN extract for 12 weeks objectively confirmed skin wrinkle improvement and a tendency to increase skin moisture and decrease the melanin and erythema index. In addition, there were no safety-associated problems. Therefore, EBN extract was effective in improving skin wrinkles. Moreover, it is beneficial for skin health and can be used as a skin nutritional supplement.
In this study, upon comparing changes in skin wrinkle values between the two intake groups at 12 weeks of ingestion, those in the EBN extract group decreased significantly compared with the placebo group. However, the skin elasticity value decreased and skin moisture value increased, but there was no statistical significance between the two groups. The change in transdermal water loss displayed a tendency to increase, and the melanin index and erythema index decreased in both groups at baseline and week 12. However, there was no significant difference in the secondary outcomes between the two groups.
No adverse reactions were recorded during the study. In the case of laboratory test results, changes before and after ingestion of the test food were within the normal range, and there was no minimally detectable change or minimal clinically significant change.
This study has several limitations, including that this is a single-center study, not a cross-over study. Moreover, there is no verum group in the study. However, if multicenter and cross-over studies are conducted in the future, it is expected that clearer and more reliable research results will be obtained. A verum group should be included in the next study, where a follow-up phase should be performed to assess skin wrinkle values of the EBN group after a period of no drug treatment. Furthermore, in future studies, it is thought that research and statistical analysis on the skin improvement effect by age should be performed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to SE: ZGFlaGFuY2hlbXRlY2hAZGhjaGVtdGVjaC5jb20= / SY: c2p5b3VuQHJuYnMuY28ua3I= / YC: Y2hvaXlAcm5icy5jby5rcg==.
All patients voluntarily participated, and were informed prior so that they could withdraw from the study without penalty. This study was approved by the Institutional Review Boards of Chungju Semyung University Korean Medicine Hospital (IRB reference No.: SMCJH-2021-06, Protocol version 1.1) and registered at CRIS (KCT0006558). The patients/participants provided their written informed consent to participate in this study.
HMK: clinical trial progress and result report preparation; EK, YL, and HK: clinical trial supervision and editing; HS: editing, writing—reviewing, and editing; SE: clinical trial support; SY: statistical processing, figure, and table creation; YC: statistical processing, figure, and table creation; WY: reviewing and raw material information advice; and SS: writing—original draft, editing, and reviewing.
This study received funding from Daehan Chemtech and Oryza Oil & Fat Chemical CO.,Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Author SE was employed by Daehan Chemtech Co., Ltd. Authors SY and YC were employed by RnBS Corp. Author WY was employed by Oryza Oil & Fat Chemical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.843469/full#supplementary-material
Bagriaçik, E. U., and Miller, K. S. (1999). Cell Surface Sialic Acid and the Regulation of Immune Cell Interactions: The Neuraminidase Effect Reconsidered. Glycobiology9, 267–275. doi:10.1093/glycob/9.3.267
Biddle, F., and Belyavin, G. (1963). The Haemagglutination Inhibitor in Edible Bird-Nest: Its Biological and Physical Properties. J. Gen. Microbiol.31, 31–44. doi:10.1099/00221287-31-1-31
Careena, S., Sani, D., Tan, S. N., Lim, C. W., Hassan, S., Norhafizah, M., et al. (2018). Effect of Edible Bird's Nest Extract on Lipopolysaccharide-Induced Impairment of Learning and Memory in Wistar Rats. Evid. Based Complement. Alternat Med.2018, 9318789. doi:10.1155/2018/9318789
Chan, G. K. L., Wong, Z. C. F., Lam, K. Y. C., Cheng, L. K. W., Zhang, L. M., Lin, H., et al. (2015). Edible Bird's Nest, an Asian Health Food Supplement, Possesses Skin Lightening Activities: Identification of N-Acetylneuraminic Acid as Active Ingredient. Jcdsa05, 262–274. doi:10.4236/jcdsa.2015.54032
Dai, Y., Cao, J., Wang, Y., Chen, Y., and Jiang, L. (2021). A Comprehensive Review of Edible Bird's Nest. Food Res. Int.140, 109875. doi:10.1016/j.foodres.2020.109875
Daud, N. A., Mohamad Yusop, S., Babji, A. S., Lim, S. J., Sarbini, S. R., and Hui Yan, T. (2021). Edible Bird's Nest: Physicochemical Properties, Production, and Application of Bioactive Extracts and Glycopeptides. Food Rev. Int.37, 177–196. doi:10.1080/87559129.2019.1696359
Dictionary of Chinese Traditional Drugs. (1985)(Shanghai Science and Technology Publisher, Shogakukan Inc.).
Furuse, M., Hata, M., Furuse, K., Yoshida, Y., Haratake, A., Sugitani, Y., et al. (2002). Claudin-based Tight Junctions Are Crucial for the Mammalian Epidermal Barrier: A Lesson from Claudin-1-Deficient Mice. J.Cel Biol156 (156), 1099–1111. doi:10.1083/jcb.200110122
Guo, C. T., Cao, Y., Hu, Z. P., Wo, E. K., Chen, S. S., You, J. B., et al. (2017). Effect of Edible Bird’s Nest on Hemogram and Secondary Influenza Virus Infection of Mice with Aplastic Anemia. Int. J. Epidemiol. Infect. Dis.44, 228–232. doi:10.1016/j.foodres.2020.109875
Haghani, A., Mehrbod, P., Safi, N., Aminuddin, N. A., Bahadoran, A., Omar, A. R., et al. (2016). In Vitro and In VivoMechanism of Immunomodulatory and Antiviral Activity of Edible Bird's Nest (EBN) against Influenza A Virus (IAV) Infection. J. Ethnopharmacol185, 327–340. doi:10.1016/j.jep.2016.03.020
Hun Lee, T., Hau Lee, C., Alia Azmi, N., Kavita, S., Wong, S., Znati, M., et al. (2020). Characterization of Polar and Non-polar Compounds of House Edible Bird's Nest (EBN) from Johor, Malaysia. Chem. Biodivers17, e1900419. doi:10.1002/cbdv.201900419
Hwang, E., Park, S. Y., Jo, H., Lee, D. G., Kim, H. T., Kim, Y. M., et al. (2015). Efficacy and Safety of Enzyme-Modified Panax Ginseng for Anti-wrinkle Therapy in Healthy Skin: A single-center, Randomized, Double-Blind, Placebo-Controlled Study. Rejuvenation Res.18, 449–457. doi:10.1089/rej.2015.1660
Kew, P. E., Wong, S. F., Lim, P. K., and Mak, J. W. (2014). Structural Analysis of Raw and Commercial Farm Edible Bird Nests. Trop. Biomed.31, 63–76.
Kiefel, M. J., and von Itzstein, M. (2002). Recent Advances in the Synthesis of Sialic Acid Derivatives and Sialylmimetics as Biological Probes. Chem. Rev.102, 471–490. doi:10.1021/cr000414a
Kim, O. K., Kim, D., Lee, M., Park, S. H., Yamada, W., Eun, S., et al. (2021). Standardized Edible Bird's Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin. Antioxidants (Basel)10, 1452. doi:10.3390/antiox10091452
Kong, Y. C., Keung, W. M., Yip, T. T., Ko, K. M., Tsao, S. W., and Ng, M. H. (1987). Evidence that Epidermal Growth Factor Is Present in Swiftlet's (Collocalia) Nest. Comp. Biochem. Physiol. B87, 221–226. doi:10.1016/030510.1016/0305-0491(87)90133-7
Lai, Q. W. S., Guo, M. S. S., Wu, K. Q., Liao, Z., Guan, D., Dong, T. T., et al. (2021). Edible Bird's Nest, an Asian Health Food Supplement, Possesses Moisturizing Effect by Regulating Expression of Filaggrin in Skin Keratinocyte. Front. Pharmacol.12, 685982. doi:10.3389/fphar.2021.685982
Morgan, B. L., and Winick, M. (1980). Effects of Administration of N-Acetylneuraminic Acid (NANA) on Brain NANA Content and Behavior. J. Nutr.110, 416–424. doi:10.1093/jn/110.3.416
Niall, M., Ryan, G. B., and O'Brien, B. M. (1982). The Effect of Epidermal Growth Factor on Wound Healing in Mice. J. Surg. Res.33 (2), 164–169. doi:10.1016/0022-4804(82)90024-5
Niessen, C. M. (2007). Tight Junctions/adherens Junctions: Basic Structure and Function. J.Invest. Dermatol.127 (11), 2525–2532. doi:10.1038/sj.jid.5700865
Ogasawara, Y., Namai, T., Yoshino, F., Lee, M. C., and Ishii, K. (2007). Sialic Acid Is an Essential Moiety of Mucin as a Hydroxyl Radical Scavenger. FEBS Lett.581, 2473–2477. doi:10.1016/j.febslet.2007.04.062
Oliveros, E., Vázquez, E., Barranco, A., Ramírez, M., Gruart, A., Delgado-García, J., et al. (2018). Sialic Acid and Sialylated Oligosaccharide Supplementation during Lactation Improves Learning and Memory in Rats. Nutrients10, 1519. doi:10.3390/nu10101519
Park, J. W., Hwang, S. R., and Yoon, I. S. (2017). Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules22, 1259. doi:10.3390/molecules22081259
Quek, M. C., Chin, N. L., Yusof, Y. A., Law, C. L., and Tan, S. W. (2018). Pattern Recognition Analysis on Nutritional Profile and Chemical Composition of Edible Bird's Nest for its Origin and Authentication. Int. J. Food Properties21, 1680–1696. doi:10.1080/10942912.2018.1503303
Rashed, A. A., Ahmad, H., Abdul Khalid, S. K., and Rathi, D.-N. G. (2021). The Potential Use of Sialic Acid from Edible Bird's Nest to Attenuate Mitochondrial Dysfunction by In Vitro Study. Front. Pharmacol.12, 633303. doi:10.3389/fphar.2021.633303
Roh, K. B., Lee, J., Kim, Y. S., Park, J., Kim, J. H., Lee, J., et al. (2012). Https:. Mechanisms of Edible Bird’s Nest Extract-Induced Proliferation of Human Adipose-Derived Stem Cells. Evid. Based Complement. Alternat. Med2012, 797520. doi:10.1155/2012/797520
Terazawa, S., and Shimoda, H. (2020). Keratinocyte Proliferative and Wound Healing Effects of Edible Bird’s Nest Extract on Human Skin. Int. J. Biomed. Sci.16, 43–51. Available at: http://ijbs.org/User/ContentFullText.aspx?VolumeNO=16&StartPage=43&Type=pdf (AccessedMar1, , 2021).
Varki, A., and Gagneux, P. (2012). Multifarious Roles of Sialic Acids in Immunity. Ann. N. Y. Acad. Sci.1253, 16–36. doi:10.1111/j.1749-6632.2012.06517.x
Wang, B. (2009). Sialic Acid Is an Essential Nutrient for Brain Development and Cognition. Annu. Rev. Nutr.29, 177–222. doi:10.1146/annurev.nutr.28.061807.155515
Wang, C.Y., Cheng, L.J., Shen, B., Yuan, Z.L., Feng, Y.Q., and Lu, S.H. (2019). Antihypertensive and Antioxidant Properties of Sialic Acid, the Major Component of Edible Bird’s Nests. Curr. Top. Nutraceutical Res.17, 376–380. doi:10.37290/ctnr2641-452X.17:376-379
Yamamoto, T., Saeki, Y., Kurasawa, M., Kuroda, S., Arase, S., Sasaki, H., et al. (2008). Effect of RNA Interference of Tight junction-related Molecules on Intercellular Barrier Function in Cultured Human Keratinocytes. Arch. Dermatol. Res.300, 517–524. doi:10.1007/s00403-008-0868-8
Yew, M. Y., Koh, R. Y., Chye, S. M., Othman, I., and Ng, K. Y. (2014). Edible Bird's Nest Ameliorates Oxidative Stress-Induced Apoptosis in SH-Sy5yHuman Neuroblastoma Cells. BMC Complement. Altern. Med.14, 391. doi:10.1186/1472-6882-14-391
Yida, Z., Imam, M. U., Ismail, M., Hou, Z., Abdullah, M. A., Ideris, A., et al. (2015). Edible Bird's Nest Attenuates High Fat Diet-Induced Oxidative Stress and Inflammation via Regulation of Hepatic Antioxidant and Inflammatory Genes. BMC Complement. Altern. Med.15, 310. doi:10.1186/s12906-015-0843-9
Yuki, T., Hachiya, A., Kusaka, A., Sriwiriyanont, P., Visscher, M. O., Morita, K., et al. (2011). Characterization of Tight Junctions and Their Disruption by UVB in Human Epidermis and Cultured Keratinocytes. J.Invest. Dermatol.131, 744–752. doi:10.1038/jid.2010.385
Zhao, R., Li, G., Kong, X. J., Huang, X. Y., Li, W., Zeng, Y. Y., et al. (2016). The improvement effects of edible bird's nest on proliferation and activation of B lymphocyte and its antagonistic effects on immunosuppression induced by cyclophosphamide. Drug Des Devel Ther. 21(10), 371–381. doi:10.2147/DDDT.S88193
Keywords: bird nest extract, placebo-controlled study, randomized trial, skin health, nutritional supplements
Citation: Kim HM, Lee YM, Kim EH, Eun SW, Sung HK, Ko H, Youn SJ, Choi Y, Yamada W and Shin SM (2022) Anti-Wrinkle Efficacy of Edible Bird’s Nest Extract: A Randomized, Double-Blind, Placebo-Controlled, Comparative Study. Front. Pharmacol. 13:843469. doi: 10.3389/fphar.2022.843469
Received: 26 December 2021; Accepted: 14 February 2022;
Published: 09 March 2022.
Edited by:
X. Y. Zhang,University of Minho, PortugalReviewed by:
Wei Hsum Yap, Taylor’s University, MalaysiaCopyright © 2022 Kim, Lee, Kim, Eun, Sung, Ko, Youn, Choi, Yamada and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seon Mi Shin, YnVuZ2d1ankyMUBoYW5tYWlsLm5ldA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.