
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 17 February 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.843110
 Ming-kun Chen1,2†
Ming-kun Chen1,2† Zhi-jian Liang1,2†
Zhi-jian Liang1,2† Dao-Sheng Luo3†
Dao-Sheng Luo3† Kang-yi Xue1,2
Kang-yi Xue1,2 De-ying Liao1,2
De-ying Liao1,2 Zheshen Li4
Zheshen Li4 Yuzhong Yu5
Yuzhong Yu5 Zhe-Sheng Chen4*
Zhe-Sheng Chen4* Shan-Chao Zhao1,2,5*
Shan-Chao Zhao1,2,5*Objective: To summarize the current therapeutic status using chemotherapeutic agent docetaxel and endocrine therapeutic agents (ARAT, abiraterone, orteronel or enzalutamide) for the treatment of metastatic castration-resistant prostate cancer (mCRPC), including sequential therapy and combined therapy, to promote the consensus on the optimal regimen for achieving superior treatment efficacy.
Methods: Through literature search in PubMed, articles with the following relevant keywords were collected and anlyzed: CRPC, abiraterone, orteronel and enzalutamide, median survival, overall survival, prostate specific antigen (PSA), PSA response rate and median radiologic progression-free survival.
Results: Fifty-eight articles were obtained and analyzed in this review. These articles included androgen axis-targeting agents after docetaxel, docetaxel after androgen axis-targeting agents, Triple sequential and combination therapy, covering four current drugs for mCRPC treatment: docetaxel, abiraterone, orteronel, and enzalutamide. It was found that there may be some cross-resistance between androgen axis-targeting agents, which will reduce the efficacy of subsequent drug treatment. Although neither of the studies of using combination therapy showed serious drug toxicity, the efficacy of sequential therapy was not as good as expected. Most adverse reactions after treatment were reported to be level 1–2.
Conclusion: Based on the results of the current studies, abiraterone followed by enzalutamide treatment is the best sequential treatment for most docetaxel-naïve patients. This treatment achieves not only good OS, but also PFS and PSA response rates. In addition, for patients who have previously failed docetaxel treatment, enzalutamide is the best choice as the subsequent treatment.
Prostate cancer is one of the most common malignancies among men in the world (Siegel et al., 2019). Since Huggins and Hodges (Huggins, 1941) discovered the effect of androgens on prostate cancer, androgen deprivation therapy (ADT) has became the main treatment for metastatic castration-sensitive prostate cancer (mCSPC) (Scher and Sawyers, 2005). However, although most patients can be relieved by ADT, most of them eventually progressed into castration-resistant prostate cancer (CRPC) (Bastos et al., 2014). The in-depth understanding of the mechanism of metastatic castration-resistant prostate cancer (mCRPC) led to the development of several new therapies, including taxanes (such as docetaxel) (Tannock et al., 2004), agents targeting androgen synthesis (such as abiraterone acetate and orteronel) (Potter et al., 1995; Yamaoka et al., 2012), and the androgen receptor inhibitor (enzalutamide) (Tran et al., 2009).
Docetaxel, one of the tubulin-binding taxanes, was the first chemotherapeutic agent demonstrated having survival benefits for mCRPC through two large randomized studies in 2004 (Table 1) (Petrylak et al., 2004; Tannock et al., 2004). Subsequently, the androgen receptor (AR) was gradually understood. AR is a nuclear hormone receptor, which depends on the activation of dihydrotestosterone and a ligand produced by the intracellular conversion of testosterone, which induces nuclear localization and target gene transcription (Lu et al., 2006). The emergence of new endocrine agents including abiraterone, orteronel and enzalutamide have also been added to the treatment options for mCRPC. Among them, abiraterone is a potent and irreversible cytochrome inhibitor that inhibits androgen synthesis and acts as an androgen receptor antagonist (Richards et al., 2012). Similarly, Orteronel inhibits androgen synthesis by selecting CYP17, 20-lyase as a reversible inhibitor (Kaku et al., 2011; Yamaoka et al., 2012). Followed by enzalutamide, which is a second-generation antiandrogen agent developed on the basis of a preclinical model of bicalutamide resistance with an androgen receptor mutation or overexpresses the androgen receptor (Hoffman-Censits and Kelly, 2013). Both endocrine therapies have been shown to improve overall survival of mCRPC either as a single medicine or combined with others.
However, there were controvercial about the sequential or combined treatment strategy. The presence of a two-compartment, potentially adaptive, feedback loop in single-agent studies of androgen blockers suggests that combination therapies may eliminate adaptive responses between the respective drugs (Efstathiou et al., 2012; Efstathiou et al., 2015). Compared to traditional sequential therapies, a new drug is changed until the prodrug fails, they believe that simultaneous combination use may help improve patient outcomes (Attard et al., 2018; Efstathiou et al., 2020). Thus, we summarize the current and ongoing clinical studies on the sequences and combination of these agents for the treatment of mCRPC in this review.
Relevant keywords involving mCRPC, docetaxel, abiraterone, orteronel, and enzalutamide were used for literuature search in Pubmed. The median survival was recognized as the primary outcome, and the median time to PSA progression, PSA response and median radiologic progression-free survival were defined as secondary outcomes. After constructing the literature pool and extracting the above results of patients with various treatment strategies that were available, the results were compared with the corresponding large randomized Phase III study (Table 1).
The flow-chart for the retrieval process is shown in Figure 1. A total of 5,481 publications were identified by PubMed by search keywords, which were then filtered according to the content of the articles, resulting in a total of 58 compliant articles.
For those who had previously treated with abiraterone followed by docetaxel (A-D), Schweizer et al. described up to 100% of patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1 (Schweizer et al., 2014). By comparison, the PSA decline of ≥50% (38% vs 63%; p = 0.02), median progression-free survival (PFS) (4.4 months vs 7.6 months; p = 0.003), and median prostate-specific antigen progression-free survival (PSA-PFS) (4.1 month vs 6.7 months; p = 0.002) in the A-D group were lower than those in the D group (only experienced docetaxel) (Supplementary Table S1, Supplementary Figure S1). This indicated that the progression risk for the A-D group may be higher than that of Group D and is unlikely to receive a PSA response (Figure 2). In contrast, the median OS and median PFS of patients in the studies with a relatively low proportion of ECOG PS 0–1 were not significantly affected, suggesting that cross-resistance may be absent between the two agents (Azad et al., 2014; Miyake et al., 2017). Combined with different levels of PSA baselines in different retrospective studies, A-D sequential therapy may be more suitable for patients with advanced disease and large tumor burden.
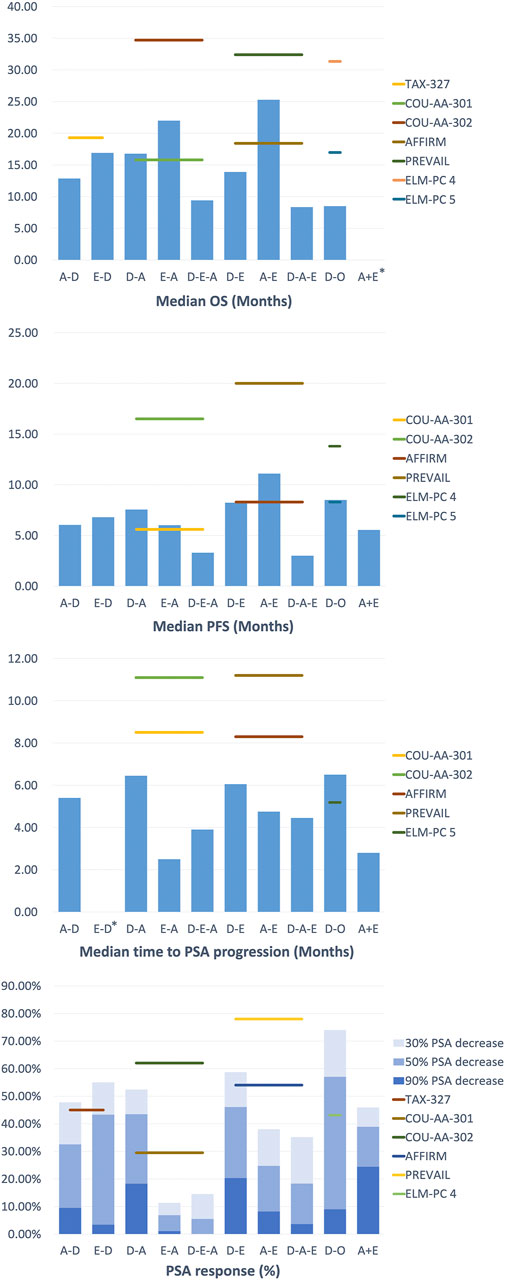
FIGURE 2. Clinical Outcomes of treatment strategy in Metastatic Castration-Resistant Prostate Cancer.
For patients who were treated with enzalutamide followed by docetaxel (E-D), Miyake et al. reported similar results to the TAX-327 experimental group, suggesting that there may be no cross-resistance in enzalutamide and docetaxel. At the same time, they performed multivariate analyses to determine the independent prognostic indicator: PS for PSA PFS and PS and visceral metastasis for OS. The findings indicated that as mCRPC patients progress with enzalutamide, the introduction of docetaxel was best in patients with favorable performance status (PS) to maximize prognostic benefits. Finally, docetaxel was well tolerated, and no unintended toxic side effects are reported in A-D, while specific side effects of this sequential therapy have not been reported in E-D.
Treatment with docetaxel before abiraterone (D-A) generally shows satisfactory effect and an acceptable safety profile, but the subsequent improvement in treatment results is lacking.
As in a multi-center study by Satoh et al., the 50% PSA reduction rate for mCRPC patients at 3 months was 28.3%, and the median OS at 6 months was 89.1%. Additionally, the ≥50% PSA decline rate was 48.4%, the median OS and PFS were 30.2 and 7.3 months in the study of Chang et al. (Chang et al., 2019), which is similar to the outcomes (≥50% PSA decrease in 51.6%, median OS in 24 months and PFS in 6.6 months) of the study of Li et al. (Li et al., 2017). These results were better than that in the D-A sequential treatment in COU-AA-301 (29.5%, 15.8 months, 5.6 months, respectively), yet they were inferior to COU-AA-302 (62%, not reported, 16.5 months, respectively). This may be due to the basic characteristics of the patients in the above study (100% ECOG PS 0–1) and the longer follow-up time, which leads to a more extended OS shift. Furthermore, in the study of Lin et al. (Lin et al., 2019), D-A sequence also appeared to be inferior compared to those who have not previously received D treatment (median OS in 18 vs 27 months, p = 0.016, median rPFS in 12.5 vs 17 months, p = 0.003), revealing the possible cross-resistance between docetaxel and abiraterone.
In the treatment of docetaxel followed by enzalutamide (D-E), Noonan et al. and Chang et al. found that the ≥30% PSA reduction rates were as high as 70 and 76.9%, the ≥50% PSA reduction rates were 60 and 69.2%, and the median PFS was 11.9 and 9.5 months respectively (Noonan et al., 2013; Chang et al., 2019). There was no significant difference between the results of the two studies. However, compared with the other group of D-A sequential therapy in their respective studies, the D-E therapy seemed to be slightly better in maintaining PSA response rate and PSA decline, except for PFS and OS (Cheng et al., 2015). Furthermore, in the docetaxel followed by orteronel (D-O), Cathomas et al. concluded that orteronel significantly lengthens EFS in patients achieving disease stabilization (median EFS in 8.5 vs 2.9 months, p = 0.001), but other aspects are slightly inferior to other sequential treatments (Cathomas et al., 2016).
The above results indicated that there was no significant difference in efficacy in sequential therapy. Still, D-A sequential therapy can achieve better results in PSA response rate and PFS for patients with mCRPC. The most common adverse events were fatigue, pain, and anemia.
In the study of the therapeutic effect relationship between androgen axis-targeting agents, two sequential treatment groups were established: abiraterone followed by enzalutamide treatment (A-E) and the opposite order (E-A). This study design provided a better understanding of two AR-axis targeted (ARAT) drugs, with the opposite order for therapeutic effects and the possibility of cross-resistance. Interestingly, in the current research reports, the conclusions of each study are strikingly consistent.
Studies by Terada et al. and Komura et al. showed that enzalutamide remained effective when used as a second-line drug following abiraterone treatment (Terada et al., 2017; Komura et al., 2019). However, in the sequential treatment of E-A, the PSA response of abiraterone as a second-line treatment was significantly lower than the experimental group in COU-AA-301 (≥50% PSA decline rate in 29.5%) and COU-AA-302 (≥50% PSA decline rate in 62%). On the other hand, the PSA response of A-E sequence with better healing effect also decreased to a certain extent compared with AFFIRM (≥50% PSA decline rate in 54%) and PREVAL (≥50% PSA decline rate in 78%) (Azad et al., 2015; Attard et al., 2018; Khalaf et al., 2019). These results revealed the possibility of cross-resistance between abiraterone and enzalutamide.
Overall, these studies revealed the cross-resistance between abiraterone and enzalutamide, and their efficacy was similar in the first-line treatments. Still, the A-E sequence was more effective in second-line PFS and combined PFS, even if there was no difference observed in OS. The most common level 3–4 adverse event in the treatment is hypertension, but no treatment-related deaths have occurred.
In the third-line sequential therapy, only a few studies evaluated the efficacy of androgen blockers as third-line therapy in mCRPC patients. After undergoing docetaxel and enzalutamide sequential therapy plus abiraterone (D-E-A), Noonan et al. found that although 70% of patients had a ≥30% PSA decrease in enzalutamide, only 11 and 3% of patients had at least a ≥30% and ≥50% PSA decrease in subsequent treatment with abiraterone acetate (Noonan et al., 2013). The median PFS and duration of abiraterone acetate treatment were short, at 3.9 months (95% CI 2.7–5.1) and 3 months (95% CI 0.3–12.8), respectively. Similarly, the results from the study of Loriot et al. were generally not satisfactory (Loriot et al., 2013). In contrast, when enzalutamide was lastly used (D-A-E), the results of studies such as Thomsen et al. and Badrising et al. were broadly consistent (Badrising et al., 2014; Bianchini et al., 2014; Thomsen et al., 2014). They showed that the outcomes were slightly less than in the D-E group in AFFIRM (50% PSA decrease: 54%, the median PFS: 8.3 months), revealing the heterogeneity of prostate cancer in terms of AR signaling addiction and drug resistance.
Interestingly, the above study suggested that some patients who were sensitive to the primary AR axis inhibitors may maintained a degree of response to subsequent treatment. Therefore, although there may be cross-resistance between abiraterone and enzalutamide as a whole, for some patients who progress after enzalutamide treatment, certain sensitivity to abiraterone may remain. Further prospective studies of prostate cancer heterogeneity are particularly important to assess the sequencial use of abiraterone and enzalutamide to help determine the best sequencing of using these drugs.
Additionally, in a prospective study by Schmid et al., only 10% of patients achieved a ≥50% reduction in PSA response, with a median OS of 7.5 months and a median PFS of 3.1 months (Schmid et al., 2014). This prospective study suggested that in the sequential treatment of D-A-E, the clinical effect of enzalutamide was more modest than its role in early tumor stage. They concluded that the preclinical results did not support the continuous use of abiraterone and enzalutamide. They proposed that a reasonable treatment strategy may be the alternating use of chemotherapy and antihormonal drugs, such as A-D-E.
Furthermore, to verify the impact of the treatment of previous docetaxel on subsequent treatment, Azad et al. set up two groups of treatment using D-A-E and A-E (Azad et al., 2015). They found that the efficacy of enzalutamide was comparable between the two groups, with similar PSA response rates (22 vs 26%, p = 0.8), median radiology/clinical time to progression (4.6 vs 6.6 months, p = 0.6) and median OS (10.6 vs 8.6 months, p = 0.2). The efficacy of enzalutamide was similar regardless of previous docetaxel use, suggesting that the cross-resistance between abiraterone and enzalutamide was independent to docetaxel. Although the activity of enzalutamide was limited, 21% of patients in the study remained enzalutamide-sensitive for at least 6 months, demonstrating that the D-A-E sequential treatment regimen could provide long-term benefits for patients. In general, there is a certain degree of cross-resistance between abiraterone and enzalutamide. Regardless of whether or not docetaxel has been used, the overall activity of abiraterone and subsequent enzalutamide treatment is limited, but some patients can still get long-term benefits. The need to develop reliable predictive biomarkers to identify these patients is critical. Patients treated with triple sequences were well tolerated, had no accidental toxicity, and most adverse reactions were grades 1–2.
The studies of combined treatment that can be retrieved in the present study is between androgen blockers (A + E), and the outcomes of the search was quite different. For example, in the study by Attard et al., the median PFS was only 7.5 months, the ≥30% and ≥50% PSA decline rates were 4.8 and 0.8%, respectively (Attard et al., 2018) (Supplementary Table S2). In contrast, Efstathiou et al. reported the 50% PSA decline rate was as high as 77% and the median PFS was 8.4 months, better than the results of Attard et al. (Efstathiou et al., 2020). However, this might be related to the characteristics and previous medications of the patients. The proportion of patients with a Gleason score of ≥8 in the study of Attard et al. was as high as 92.5%, and the median age was 72 years, which was higher than that of the survey by Efstathiou et al. Moreover, the patients in the study by Attard et al. had previously received the treatment of enzalutamide and were treated with combination therapy until the disease progressed.
In addition, COU-AA-302 and PREVAIL seem to take longer than the time reported above in terms of time to progress and 50% PSA decline (11.1 months, 24%; 11.2 months, 78%). Both studies suggested that the results of the efficacy study do not support the treatment plan of abiraterone combined with enzalutamide. Although the combination therapy was not as good as expected, some patients in the study by Efstathiou et al. achieved excellent results. This may be because inhibition of AR and androgen biosynthesis may further prolong the survival of some mCRPC patients by delaying the emergence of drug resistance. Therefore, finding and verifying biomolecular markers is necessary to identify patients who will benefit from the combination therapy.
No severe drug toxicity occurred in either study, reflecting the safety of the combined use of the two drugs.
Over the past 10 years, tremendous changes have taken place in the treatment strategy of mCRPC, especially after the emergence of many new drugs and their clinical application. The traditional docetaxel chemotherapy has obvious limitations, such as requiring patients with high PS, moderate survival benefits, and relatively serious side effects. But docetaxel has its own unique advantages: the course of treatment is shorter than that of abiraterone and enzalutamide, the total cost is low, and glucocorticoids are not required (Caffo et al., 2011; Al-Batran et al., 2015). On the other hand, the new ARAT agents, abiraterone, orteronel and enzalutamide, have been proven to have considerable survival benefits, with low requirements for the patient’s PS condition. At the same time, the side effects caused by the agents are not obvious compared to docetaxel (de Bono et al., 2011; Scher et al., 2012; Ryan et al., 2013; Beer et al., 2014). Multiple studies have shown that subsequent ARAT agents are somewhat less effective during sequential D-ARAT treatment, suggesting that previous docetaxel chemotherapy may produce resistance to AR (Esch et al., 2014; Lorente et al., 2015). Since there was no study on the median OS reported in the A + E combination therapy, it is not possible to compare with sequential treatments at the median OS level. However, Attard et al. found that there was no significant difference in PFS by comparing A + E and E-A (5.7 vs 5.6 months, p = 0.22), and the frequency of grade 3 hypertension (10 vs 2%) and increased ALT (6 vs 2%) or AST (2 vs 0%) was more frequent in the A + E group. Current studies on combination therapies have been unable to support substantial efficacy benefits. For the time being, different combination therapy trials for mCRPC are ongoing (Table 2), which is expected to continue to supplement the vacuum in this field. Therefore, ARAT agents are recommended as a first-line treatment for mCRPC, rather than docetaxel or combination therapy.
The effect of the sequence of A and E is relatively better than that of the ARAT-D sequence, especially in OS. Also, when ARAT agents were used as the first-line treatment, komura et al. showed that there was no significant difference in efficacy between abiraterone and enzalutamide (Komura et al., 2019). However, in the second-line treatment, A-E is superior to E-A in PFS (15 vs 7 months, p = 0.04), time to PSA progression (TTPP; 6 vs 3 months, p = 0.008) and PSA response (p = 0.01), except that OS did not reach significance (14 vs 23 months, p = 0.35).
A meta-analysis to compare oncologic outcomes between the treatment sequences of A-E and E-A by Chung et al. also came to similar conclusions (Chung et al., 2019). They reported that the former could obtain better outcomes in PFS than the latter (p < 0.0001), especially in docetaxel-naïve CRPC patients, except for OS (p = 0.10). The adverse event rate of grade 3 or worse was roughly similar between A-E and E-A. Therefore, according to the current research results, A-E sequential therapy is the best choice for patients with mCRPC.
The selection of the best treatment strategy for mCRPC patients, in addition to focusing on the best efficacy, other factors should also be considered, such as economic conditions and comorbidities et al. Fortunately, the total cost of A-E is lower in sequential treatment of ARAT on the premise of maintaining relatively superior efficacy. Because the use of drug treatment as the first-line treatment may have the longest duration until the disease progresses, and it costs the most, abiraterone might be more cost-effective than enzalutamide and docetaxel. Abiraterone is not applicable if the patient has a contraindication to the use of glucocorticoids.
For patients who have previously failed docetaxel, whether ARAT drugs could be used as the next line of treatment is another critical issue. In the D-ARAT sequential therapy, Chang et al. reported that D-E group is slightly better than D-A group on PSA response rate (≥50% PSA decrease: 69.2 vs 48.4%, p = 0.171; ≥90% PSA decrease: 38.5 vs 25%, p = 0.320) and median PFS (9.5 vs 7.3 months, p = 0.734). In Fang et al., a trial-level meta-analysis, also showed no significant statistical difference in OS, although D-E was 2.2 months more than D-A (Fang et al., 2017). In another assessment analysis of the efficacy in the study of Chopra et al., the efficacy of abiraterone and enzalutamide was indirectly compared in both the pre-docetaxel and post-docetaxel settings, based on published phase III randomized trials (Chopra et al., 2017). It is concluded that enzalutamide outperforms abiraterone in terms of PSA response rate, median PFS and TTPP. D-O is currently known to have fewer studies, only reported significantly prolonged EFS in patients with stable disease, the rest of the results were not significantly prominent. The adverse event rate of grade 3 or worse was roughly similar in androgen axis-targeting agents. Therefore, enzalutamide as the next treatment agent i.e. D-E, may be a more reasonable and effective choice in patients who fail after treatment with docetaxel.
Additionally, in a prospective study by Schmid et al., only 10% of patients achieved a ≥50% reduction in PSA response, with a median OS of 7.5 months and a median PFS of 3.1 months. This prospective study demonstrated that the clinical effect of enzalutamide is modest using the sequential treatment of D-A-E. They concluded that the preclinical results do not support the continuous use of abiraterone and enzalutamide. They proposed that a reasonable treatment strategy may be the alternating use of chemotherapy and antihormonal drugs, such as A-D-E. Although there is no other prospective study to confirm this result, and this trial has limitations such as small sample size and no random design, an appropriate sequencial treatment may include A-D-E. In addition to AR signaling conduction in mCRPC, poly (ADP-ribose) polymerase inhibitors (PARPi) for the treatment of defective tumors is of great interest. Among them, the new drugs niraparib and olaparib both showed anti-tumor activity against metastatic deaggressive prostate cancer with abnormal DNA damage response (DDR) gene(Smith et al., 2019; Mateo et al., 2020). In the future, PARPi may become the first choice of targeted therapy for mCRPC.
Most of the current studies are limited to retrospective features, and there might be some biases compared to the real world. To date, there are no high-level research data to prove and support any scheme related to mCRPC treatment. Therefore, to select the most suitable and effective treatment strategy based on the essential characteristics of each patient, further high-quality clinical research is urgently needed.
The research in the treatment of mCRPC in the past 10 years has played an essential role in promoting the choice of the most effective treatment method according to individual patients. Based on the results of the current studies, A-E may be the best sequential treatment for most docetaxel-naïve patients. This treatment has good feedback not only on OS, but also on PFS and PSA response rates. In addition, for patients who have previously failed docetaxel treatment, enzalutamide is the best choice for the next stage of treatment.
The main conceptual ideas and outline: M-kC, Z-jL, Z-SC, and S-CZ. Collected and analysis data: MC, Z-jL, D-SL, K-yX, D-yL, YY, and ZL. Wrote and Edited the paper: MC, Z-jL, D-SL, K-yX, D-yL, YY, ZL, Z-SC, and S-CZ. All authors have read and agreed to the published version of the manuscript.
This study was supported by the National Natural Science Foundation of China (No. 81972394), the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515010066) and the President Foundation of the Third Affiliated Hospital of Southern Medical University (Nos. YM2021010, YM2021011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.843110/full#supplementary-material
Al-Batran, S. E., Hozaeel, W., Tauchert, F. K., Hofheinz, R. D., Hinke, A., Windemuth-Kieselbach, C., et al. (2015). The Impact of Docetaxel-Related Toxicities on Health-Related Quality of Life in Patients with Metastatic Cancer (QoliTax). Ann. Oncol. 26 (6), 1244–1248. doi:10.1093/annonc/mdv129
Attard, G., Borre, M., Gurney, H., Loriot, Y., Andresen-Daniil, C., Kalleda, R., et al. (2018). Abiraterone Alone or in Combination with Enzalutamide in Metastatic Castration-Resistant Prostate Cancer with Rising Prostate-specific Antigen during Enzalutamide Treatment. J. Clin. Oncol. 36 (25), 2639–2646. doi:10.1200/jco.2018.77.9827
Azad, A. A., Eigl, B. J., Murray, R. N., Kollmannsberger, C., and Chi, K. N. (2015). Efficacy of Enzalutamide Following Abiraterone Acetate in Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer Patients. Eur. Urol. 67 (1), 23–29. doi:10.1016/j.eururo.2014.06.045
Azad, A. A., Leibowitz-Amit, R., Eigl, B. J., Lester, R., Wells, J. C., Murray, R. N., et al. (2014). A Retrospective, Canadian Multi-center Study Examining the Impact of Prior Response to Abiraterone Acetate on Efficacy of Docetaxel in Metastatic Castration-Resistant Prostate Cancer. Prostate 74 (15), 1544–1550. doi:10.1002/pros.22872
Badrising, S., van der Noort, V., van Oort, I. M., van den Berg, H. P., Los, M., Hamberg, P., et al. (2014). Clinical Activity and Tolerability of Enzalutamide (MDV3100) in Patients with Metastatic, Castration-Resistant Prostate Cancer Who Progress after Docetaxel and Abiraterone Treatment. Cancer 120 (7), 968–975. doi:10.1002/cncr.28518
Bastos, D. A., Dzik, C., Rathkopf, D., and Scher, H. I. (2014). Expanding Androgen- and Androgen Receptor Signaling-Directed Therapies for Castration-Resistant Prostate Cancer. Oncology (Williston Park) 28 (8), 693–699. doi:10.1158/1541-7786.MCR-14-0036
Beer, T. M., Armstrong, A. J., Rathkopf, D. E., Loriot, Y., Sternberg, C. N., Higano, C. S., et al. (2014). Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 371 (5), 424–433. doi:10.1056/NEJMoa1405095
Bianchini, D., Lorente, D., Rodriguez-Vida, A., Omlin, A., Pezaro, C., Ferraldeschi, R., et al. (2014). Antitumour Activity of Enzalutamide (MDV3100) in Patients with Metastatic Castration-Resistant Prostate Cancer (CRPC) Pre-treated with Docetaxel and Abiraterone. Eur. J. Cancer 50 (1), 78–84. doi:10.1016/j.ejca.2013.08.020
Caffo, O., Sava, T., Comploj, E., Fariello, A., Zustovich, F., Segati, R., et al. (2011). Impact of Docetaxel-Based Chemotherapy on Quality of Life of Patients with Castration-Resistant Prostate Cancer: Results from a Prospective Phase II Randomized Trial. BJU Int. 108 (11), 1825–1832. doi:10.1111/j.1464-410X.2011.10277.x
Cathomas, R., Crabb, S. J., Mark, M., Winterhalder, R., Rothermundt, C., Elliott, T., et al. (2016). Orteronel Switch Maintenance Therapy in Metastatic Castration Resistant Prostate Cancer after First-Line Docetaxel: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial (SAKK 08/11). Prostate 76 (16), 1519–1527. doi:10.1002/pros.23236
Chang, L. W., Hung, S. C., Wang, S. S., Li, J. R., Yang, C. K., Chen, C. S., et al. (2019). Abiraterone Acetate and Enzalutamide: Similar Efficacy in Treating Post Docetaxel Metastatic Castration-Resistant Prostate Cancer: Single Center Experience. Anticancer Res. 39 (7), 3901–3908. doi:10.21873/anticanres.13541
Cheng, H. H., Gulati, R., Azad, A., Nadal, R., Twardowski, P., Vaishampayan, U. N., et al. (2015). Activity of Enzalutamide in Men with Metastatic Castration-Resistant Prostate Cancer Is Affected by Prior Treatment with Abiraterone And/or Docetaxel. Prostate Cancer Prostatic Dis. 18 (2), 122–127. doi:10.1038/pcan.2014.53
Chopra, A., Georgieva, M., Lopes, G., Yeo, C. M., and Haaland, B. (2017). Abiraterone or Enzalutamide in Advanced Castration-Resistant Prostate Cancer: An Indirect Comparison. Prostate 77 (6), 639–646. doi:10.1002/pros.23309
Chung, D. Y., Kang, D. H., Kim, J. W., Kim, D. K., Lee, J. Y., Hong, C. H., et al. (2019). Comparison of Oncologic Outcomes between Two Alternative Sequences with Abiraterone Acetate and Enzalutamide in Patients with Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 12 (1). doi:10.3390/cancers12010008
de Bono, J. S., Logothetis, C. J., Molina, A., Fizazi, K., North, S., Chu, L., et al. (2011). Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 364 (21), 1995–2005. doi:10.1056/NEJMoa1014618
Efstathiou, E., Titus, M., Tsavachidou, D., Tzelepi, V., Wen, S., Hoang, A., et al. (2012). Effects of Abiraterone Acetate on Androgen Signaling in Castrate-Resistant Prostate Cancer in Bone. J. Clin. Oncol. 30 (6), 637–643. doi:10.1200/jco.2010.33.7675
Efstathiou, E., Titus, M., Wen, S., Hoang, A., Karlou, M., Ashe, R., et al. (2015). Molecular Characterization of Enzalutamide-Treated Bone Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 67 (1), 53–60. doi:10.1016/j.eururo.2014.05.005
Efstathiou, E., Titus, M., Wen, S., Troncoso, P., Hoang, A., Corn, P., et al. (2020). Enzalutamide in Combination with Abiraterone Acetate in Bone Metastatic Castration-Resistant Prostate Cancer Patients. Eur. Urol. Oncol. 3 (1), 119–127. doi:10.1016/j.euo.2019.01.008
Esch, L., Schulz, W. A., and Albers, P. (2014). Sequential Treatment with Taxanes and Novel Anti-androgenic Compounds in Castration-Resistant Prostate Cancer. Oncol. Res. Treat. 37 (9), 492–498. doi:10.1159/000365530
Fang, M., Nakazawa, M., Antonarakis, E. S., and Li, C. (2017). Efficacy of Abiraterone and Enzalutamide in Pre- and Postdocetaxel Castration-Resistant Prostate Cancer: A Trial-Level Meta-Analysis. Prostate Cancer 2017, 8560827. doi:10.1155/2017/8560827
Hoffman-Censits, J., and Kelly, W. K. (2013). Enzalutamide: a Novel Antiandrogen for Patients with Castrate-Resistant Prostate Cancer. Clin. Cancer Res. 19 (6), 1335–1339. doi:10.1158/1078-0432.Ccr-12-2910
Huggins, M. L. (1941). A New Society for X-Ray and Electron Diffraction Research Workers. Science 93 (2421), 489–490. doi:10.1126/science.93.2421.489
Kaku, T., Hitaka, T., Ojida, A., Matsunaga, N., Adachi, M., Tanaka, T., et al. (2011). Discovery of Orteronel (TAK-700), a Naphthylmethylimidazole Derivative, as a Highly Selective 17,20-lyase Inhibitor with Potential Utility in the Treatment of Prostate Cancer. Bioorg. Med. Chem. 19 (21), 6383–6399. doi:10.1016/j.bmc.2011.08.066
Khalaf, D. J., Annala, M., Taavitsainen, S., Finch, D. L., Oja, C., Vergidis, J., et al. (2019). Optimal Sequencing of Enzalutamide and Abiraterone Acetate Plus Prednisone in Metastatic Castration-Resistant Prostate Cancer: a Multicentre, Randomised, Open-Label, Phase 2, Crossover Trial. Lancet Oncol. 20 (12), 1730–1739. doi:10.1016/s1470-2045(19)30688-6
Komura, K., Fujiwara, Y., Uchimoto, T., Saito, K., Tanda, N., Matsunaga, T., et al. (2019). Comparison of Radiographic Progression-free Survival and PSA Response on Sequential Treatment Using Abiraterone and Enzalutamide for Newly Diagnosed Castration-Resistant Prostate Cancer: A Propensity Score Matched Analysis from Multicenter Cohort. J. Clin. Med. 8 (8), 1251. doi:10.3390/jcm8081251
Li, J. R., Wang, S. S., Yang, C. K., Chen, C. S., Ho, H. C., Chiu, K. Y., et al. (2017). First Line Androgen Deprivation Therapy Duration Is Associated with the Efficacy of Abiraterone Acetate Treated Metastatic Castration-Resistant Prostate Cancer after Docetaxel. Front. Pharmacol. 8, 55. doi:10.3389/fphar.2017.00055
Lin, G. W., Li, G. X., Dai, B., Ye, D. W., Kong, Y. Y., Wang, Y., et al. (2019). Clinical Activity of Abiraterone Plus Prednisone in Docetaxel-Naοve and Docetaxel-Resistant Chinese Patients with Metastatic Castration-Resistant Prostate Cancer. Asian J. Androl. 21 (2), 131–136. doi:10.4103/aja.aja_85_18
Lorente, D., Mateo, J., Perez-Lopez, R., de Bono, J. S., and Attard, G. (2015). Sequencing of Agents in Castration-Resistant Prostate Cancer. Lancet Oncol. 16 (6), e279–92. doi:10.1016/s1470-2045(15)70033-1
Loriot, Y., Bianchini, D., Ileana, E., Sandhu, S., Patrikidou, A., Pezaro, C., et al. (2013). Antitumour Activity of Abiraterone Acetate against Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel and Enzalutamide (MDV3100). Ann. Oncol. 24 (7), 1807–1812. doi:10.1093/annonc/mdt136
Lu, N. Z., Wardell, S. E., Burnstein, K. L., Defranco, D., Fuller, P. J., Giguere, V., et al. (2006). International Union of Pharmacology. LXV. The Pharmacology and Classification of the Nuclear Receptor Superfamily: Glucocorticoid, Mineralocorticoid, Progesterone, and Androgen Receptors. Pharmacol. Rev. 58 (4), 782–797. doi:10.1124/pr.58.4.9
Mateo, J., Porta, N., Bianchini, D., McGovern, U., Elliott, T., Jones, R., et al. (2020). Olaparib in Patients with Metastatic Castration-Resistant Prostate Cancer with DNA Repair Gene Aberrations (TOPARP-B): a Multicentre, Open-Label, Randomised, Phase 2 Trial. Lancet Oncol. 21 (1), 162–174. doi:10.1016/s1470-2045(19)30684-9
Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., et al. (2019). Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 69 (1), 363–385. doi:10.3322/caac.2155110.3322/caac.21565
Miyake, H., Matsushita, Y., Tamura, K., Motoyama, D., Ito, T., Sugiyama, T., et al. (2017). Impact of Prior Androgen receptor-axis-targeted Agents on the Clinical Activity of Subsequent Docetaxel in Patients with Metastatic Castration-Resistant Prostate Cancer: Comparative Assessment between Abiraterone Acetate and Enzalutamide. Med. Oncol. 34 (12), 200. doi:10.1007/s12032-017-1060-9
Noonan, K. L., North, S., Bitting, R. L., Armstrong, A. J., Ellard, S. L., and Chi, K. N. (2013). Clinical Activity of Abiraterone Acetate in Patients with Metastatic Castration-Resistant Prostate Cancer Progressing after Enzalutamide. Ann. Oncol. 24 (7), 1802–1807. doi:10.1093/annonc/mdt138
Petrylak, D. P., Tangen, C. M., Hussain, M. H., Lara, P. N., Jones, J. A., Taplin, M. E., et al. (2004). Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 351 (15), 1513–1520. doi:10.1056/NEJMoa041318
Potter, G. A., Barrie, S. E., Jarman, M., and Rowlands, M. G. (1995). Novel Steroidal Inhibitors of Human Cytochrome P45017 Alpha (17 Alpha-Hydroxylase-C17,20-Lyase): Potential Agents for the Treatment of Prostatic Cancer. J. Med. Chem. 38 (13), 2463–2471. doi:10.1021/jm00013a022
Richards, J., Lim, A. C., Hay, C. W., Taylor, A. E., Wingate, A., Nowakowska, K., et al. (2012). Interactions of Abiraterone, Eplerenone, and Prednisolone with Wild-type and Mutant Androgen Receptor: a Rationale for Increasing Abiraterone Exposure or Combining with MDV3100. Cancer Res. 72 (9), 2176–2182. doi:10.1158/0008-5472.Can-11-3980
Ryan, C. J., Smith, M. R., de Bono, J. S., Molina, A., Logothetis, C. J., de Souza, P., et al. (2013). Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 368 (2), 138–148. doi:10.1056/NEJMoa1209096
Scher, H. I., Fizazi, K., Saad, F., Taplin, M. E., Sternberg, C. N., Miller, K., et al. (2012). Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 367 (13), 1187–1197. doi:10.1056/NEJMoa1207506
Scher, H. I., and Sawyers, C. L. (2005). Biology of Progressive, Castration-Resistant Prostate Cancer: Directed Therapies Targeting the Androgen-Receptor Signaling axis. J. Clin. Oncol. 23 (32), 8253–8261. doi:10.1200/jco.2005.03.4777
Schmid, S. C., Geith, A., Böker, A., Tauber, R., Seitz, A. K., Kuczyk, M., et al. (2014). Enzalutamide after Docetaxel and Abiraterone Therapy in Metastatic Castration-Resistant Prostate Cancer. Adv. Ther. 31 (2), 234–241. doi:10.1007/s12325-014-0092-1
Schweizer, M. T., Zhou, X. C., Wang, H., Bassi, S., Carducci, M. A., Eisenberger, M. A., et al. (2014). The Influence of Prior Abiraterone Treatment on the Clinical Activity of Docetaxel in Men with Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 66 (4), 646–652. doi:10.1016/j.eururo.2014.01.018
Smith, M. R., Sandhu, S. K., Kelly, W. K., Scher, H. I., Efstathiou, E., Lara, P., et al. (2019). Phase II Study of Niraparib in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and Biallelic DNA-Repair Gene Defects (DRD): Preliminary Results of GALAHAD. J. Clin. Oncol. 37 (7_Suppl. l), 202. doi:10.1200/jco.2019.37.7_suppl.202
Tannock, I. F., de Wit, R., Berry, W. R., Horti, J., Pluzanska, A., Chi, K. N., et al. (2004). Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 351 (15), 1502–1512. doi:10.1056/NEJMoa040720
Terada, N., Maughan, B. L., Akamatsu, S., Kobayashi, T., Yamasaki, T., Inoue, T., et al. (2017). Exploring the Optimal Sequence of Abiraterone and Enzalutamide in Patients with Chemotherapy-Naïve Castration-Resistant Prostate Cancer: The Kyoto-Baltimore Collaboration. Int. J. Urol. 24 (6), 441–448. doi:10.1111/iju.13346
Thomsen, F. B., Røder, M. A., Rathenborg, P., Brasso, K., Borre, M., and Iversen, P. (2014). Enzalutamide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer Progressing after Chemotherapy and Abiraterone Acetate. Scand. J. Urol. 48 (3), 268–275. doi:10.3109/21681805.2013.860189
Tran, C., Ouk, S., Clegg, N. J., Chen, Y., Watson, P. A., Arora, V., et al. (2009). Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science 324 (5928), 787–790. doi:10.1126/science.1168175
Yamaoka, M., Hara, T., Hitaka, T., Kaku, T., Takeuchi, T., Takahashi, J., et al. (2012). Orteronel (TAK-700), a Novel Non-steroidal 17,20-lyase Inhibitor: Effects on Steroid Synthesis in Human and Monkey Adrenal Cells and Serum Steroid Levels in Cynomolgus Monkeys. J. Steroid Biochem. Mol. Biol. 129 (3-5), 115–128. doi:10.1016/j.jsbmb.2012.01.001
Keywords: metastatic castration-resistant prostate cancer, sequential therapy, combined therapy, docetaxel, abiraterone, orteronel, enzalutamide
Citation: Chen M-k, Liang Z-j, Luo D-S, Xue K-y, Liao D-y, Li Z, Yu Y, Chen Z-S and Zhao S-C (2022) Abiraterone, Orteronel, Enzalutamide and Docetaxel: Sequential or Combined Therapy?. Front. Pharmacol. 13:843110. doi: 10.3389/fphar.2022.843110
Received: 24 December 2021; Accepted: 12 January 2022;
Published: 17 February 2022.
Edited by:
Benyi Li, University of Kansas Medical Center, United StatesReviewed by:
Zhi-hang Zhou, Chongqing Medical University, ChinaCopyright © 2022 Chen, Liang, Luo, Xue, Liao, Li, Yu, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe-Sheng Chen, Y2hlbnpAc3Rqb2hucy5lZHU=; Shan-Chao Zhao, bHVsdWx1QHNtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.