- 1Blokhin Cancer Research Center, Moscow, Russia
- 2Peoples’ Friendship University of Russia, Moscow, Russia
- 3Pavlov Ryazan State Medical University, Ryazan, Russia
- 4Belozersky Institute of Physicochemical Biology, Lomonosov Moscow State University, Moscow, Russia
- 5Faculty of Bioengineering and Bioinformatics, Lomonosov Moscow State University, Moscow, Russia
7-Methylguanine (7-MG) competitively inhibits the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) and RNA-modifying enzyme tRNA-guanine transglycosylase (TGT) and represents a potential anticancer drug candidate. Furthermore, as a natural compound, it could escape the serious side effects characteristic for approved synthetic PARP inhibitors. Here we present a comprehensive study of toxicological and carcinogenic properties of 7-MG. It was demonstrated that 7-MG does not induce mutations or structural chromosomal abnormalities, and has no blastomogenic activity. A treatment regimen with 7-MG has been established in mice (50 mg/kg per os, 3 times per week), exerting no adverse effects or changes in morphology. Preliminary data on the 7-MG anticancer activity obtained on transplantable tumor models support our conclusions that 7-MG can become a promising new component of chemotherapy.
1 Introduction
7-Methylguanine (7-MG) (Shapiro et al., 1968) is a degradation product of nucleic acids which is present in small amounts in human urine (Bromberg et al., 1957a; Bromberg et al., 1957b; Lothrop and Uziel, 1983; Svoboda and Kasai, 2004; Rodríguez-Gonzalo et al., 2013; Raćkowska et al., 2019) and may be considered an indicator of whole-body RNA turnover (Sander et al., 1986a; Sander et al., 1986b; Sander et al., 1986c; Topp et al., 1987). In mRNA the guanosine cap is methylated due to methyltransferase activity, that is required for maturation and translation (Shuman, 2002; Shafer et al., 2005; Topisirovic et al., 2011; Varshney et al., 2016). Furthermore, 7-MG adducts are normally present in DNA, exposed to various exogenous and endogenous methylating agents, and their number is increasing on aging (Park and Ames, 1988; Tan et al., 1990; Mustonen and Hemminki, 1992; O'Connor, 1993; Tamae et al., 2009). There is no evidence, however, for synthesis of nucleotides from free 7-MG base or for its direct incorporation into nucleic acids (Craddock et al., 1968; Kaina et al., 1983; Kerr, 1985; Kerr, 1990). A certain proportion of 7-MG is converted to 8-hydroxy-7-methylguanine by xanthine oxidase or demethylated (Weissmann and Gutman, 1957; Borowitz et al., 1965; Litwack and Weissmann, 1966; Skupp and Ayvazian, 1969).
Recently we have shown that 7-MG inhibits DNA repair enzymes poly(ADP-ribose) polymerases, PARP1 and PARP2, in a competitive manner and accelerates apoptotic death of cancer cells induced by cisplatin and doxorubicin (Nilov et al., 2016; Nilov et al., 2018a; Nilov et al., 2020a). These PARP enzymes bind to DNA breaks and synthesize a signal polymer poly (ADP-ribose) from NAD+ molecules to activate the excision repair proteins (Hassler and Ladurner, 2012; Drenichev and Mikhailov, 2015; Ray Chaudhuri and Nussenzweig, 2017; Alemasova and Lavrik, 2019; Nilov et al., 2020b). Inhibitors of PARP1/2, therefore, can exert anti-proliferative effect and be combined with DNA damaging agents (Cepeda et al., 2006; Martin et al., 2008; Ferraris, 2010; Lord et al., 2015; Nilov et al., 2018b). We have demonstrated that 7-MG forms substrate-specific interactions with the Gly863 and Tyr907 residues in the PARP1/2 active site and suppresses DNA-dependent PARP activity in three different assays (biochemical assay with radiolabeled NAD+, fluorescence anisotropy assay, and Förster resonance energy transfer microscopy assay with nucleosome particles) (Nilov et al., 2016; Nilov et al., 2020a). This results in the formation of nonproductive PARP–nucleosome complexes and likely prevents further steps in DNA repair, replication and transcription, leading to cancer cell death (Maluchenko et al., 2019; Nilov et al., 2020a). 7-MG is also known as a competitive inhibitor of RNA-modifying enzyme tRNA-guanine transglycosylase (TGT) which substitutes the guanine base with 7-deazaguanine derivative queuine (Farkas et al., 1984; Johannsson et al., 2018). In a recent paper, it was shown that TGT deficiency could significantly suppress the proliferation and migration of cancer cells (Zhang et al., 2020). From the point of view of polypharmacology, such a multitarget (PARP1/2, TGT) mechanism of a drug candidate may be promising, if adverse effects are negligible (Bolognesi, 2013; Medina-Franco et al., 2013).
FDA-approved synthetic PARP1/2 inhibitors olaparib, rucaparib, niraparib (Frampton, 2015; Mittica et al., 2018; Zimmer et al., 2018) can cause side effects likely related to the nonselective interaction with numerous NAD+-binding proteins and nonspecific effects on the organism. Myelodysplastic syndrome/acute myeloid leukemia occurred in some patients after treatment with above-mentioned synthetic inhibitors, and some cases were fatal (Malyuchenko et al., 2015; Sonnenblick et al., 2015; Wang et al., 2016; Ohmoto and Yachida, 2017; Jain and Patel, 2019). 7-MG, being a natural compound, may have a more favorable toxicity profile, which is also supported by QSAR modeling (Nilov et al., 2016; Nilov et al., 2018a). In this article, we present the results of a comprehensive experimental study of toxicological and carcinogenic properties of 7-MG that establish the basis for further testing of its anticancer activity.
2 Materials and Methods
2.1 Toxicology Studies
Six-week-old female CBA, BALB/c, and C57BL/6 mice were obtained from the Stolbovaya farm of the Federal Medical Biological Agency (http://www.scbmt.ru). These mouse strains are widely used in toxicology studies and in studies involving transplantable tumor models. 7-MG (Sigma-Aldrich, product No. 67073) was administered orally by gavage in experiments A and B (Figure 1). In experiment A, CBA mice were divided randomly to four treatment groups of 10 animals: control group I, drinking water (3 times per week for 4 weeks); group II, 50 mg/kg 7-MG (3 times per week for 4 weeks); group III, 200 mg/kg 7-MG (3 times per week for 1 week); group IV, 600 mg/kg 7-MG (single dose). Animals were euthanized by cervical dislocation 1 week after the last treatment. In experiment B, mice of each strain (CBA, BALB/c, C57BL/6) were divided into two groups, group I (drinking water) and group II (600 mg/kg 7-MG), and euthanized 4 weeks after single-dose administration. Lungs, heart, liver, spleen, thymus, kidneys, adrenal glands, pancreas, stomach, small and large intestines were collected from euthanized mice and inspected. The tissues were processed for light microscopy by fixing in 10% buffered formalin, dehydrating, and embedding in paraffin. Histological analysis was performed on sections stained by hematoxylin-eosin. Organ lesions were detected on the examined sections (representative microphotographs of the found abnormalities are shown in Supplementary Figure S1), and the number of lesions per animal was counted.
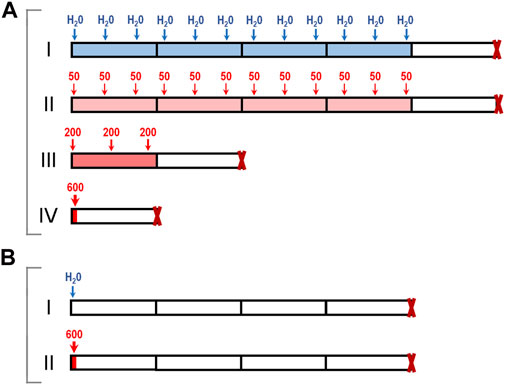
FIGURE 1. Toxicology study of 7-MG: schematic representation of experiments. (A) CBA mice were treated either with water (group I) or with single and multiple doses of 7-MG (groups II–IV, total dose for each group was 600 mg/kg); (B) CBA, BALB/c, or C57BL/6 mice were treated with water (groups I) or a single 600 mg/kg dose of 7-MG (group II), and euthanized 4 weeks later.
2.2 Carcinogenicity Studies
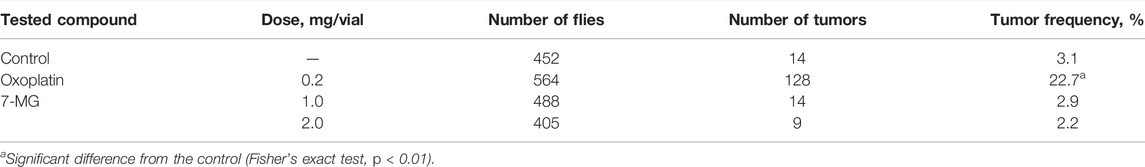
The Ames test was performed in S. typhimurium strains TA98 and TA100 as described previously (Maron and Ames, 1983). Three 7-MG doses (1.4, 7.0, and 35.0 µg/plate) were tested with and without rat liver S9 fraction. Distilled water was used as a negative control; 3,4-benzopyrene (4.4 µg/plate), 2-acetylaminofluorene (22.0 µg/plate), 4,9-diazapyrene derivative (8.8 µg/plate), and sodium azide (8.8 µg/plate) served as positive controls. Plates were incubated for 72 h and then revertant colonies were counted. The comet assay was performed as described previously (Singh et al., 1988). Immortalized human kidney epithelial cells (NKE-hTERT) were treated with different concentrations of 7-MG (0.02 and 0.2 mg/ml) for 24 h and then embedded in agarose on microscope slides. After cell lysis and electrophoresis, slides were stained with DNA dye (Vista Green) and the number of comets was counted. Distilled water was used as a negative control and cisplatin (25 µg/ml) served as a positive control. The somatic mutation and recombination test in D. melanogaster was based on previous work (Kirsanov et al., 2011). Five wild type males and 10 wtsP4/TM3 females were placed into vials for breeding. Heterozygous larvae were treated with 7-MG (1 and 2 mg/vial); distilled water and oxoplatin (0.2 mg/vial) were used as controls. Adult F1 males and females were examined for the presence of tumors using a binocular microscope.
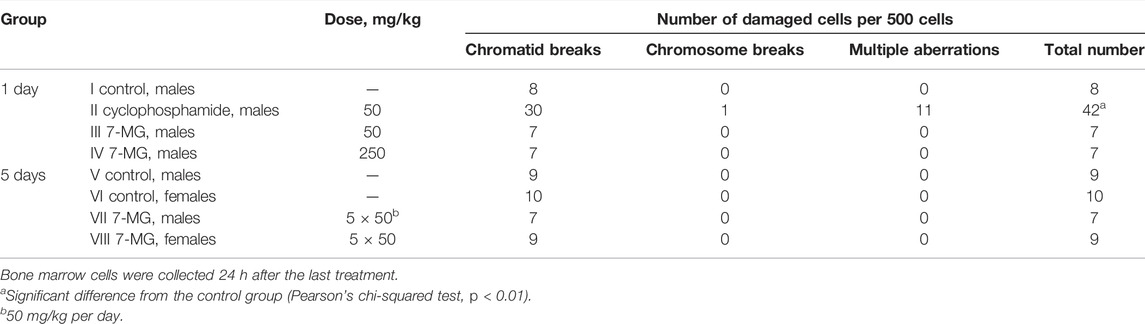
The chromosomal aberration assay was performed using standard procedure (Albertini et al., 2000). C57BL/6 mice were divided to groups of 5 animals and treated with 7-MG (50–250 mg/kg per os). Distilled water was used as a negative control and cyclophosphamide (50 mg/kg i.p.) served as a positive control. Bone marrow cells were collected 24 h after the treatment; to arrest proliferating cells at metaphase, animals received colchicine (0.004% i.p.) 3 h prior to euthanasia. Cells were obtained from the femurs, stained on slides with Giemsa, and analyzed by microscopy.
2.3 Anticancer Activity
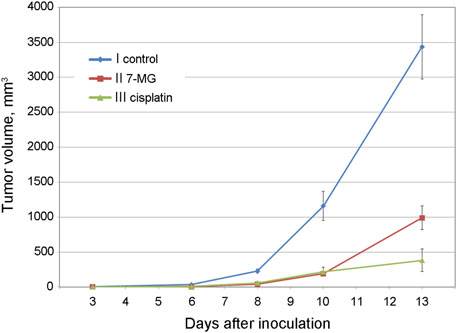
Six-week-old female CBA mice were obtained from the Stolbovaya farm. Uterine sarcoma US-322 and cervical squamous cell carcinoma RShM-5 (originally derived from CBA mice exposed to 1,2-dimethylhydrazine and 3-methylcholanthrene, respectively) (Treshalina et al., 2000; Turusov et al., 2005; Bunyatyan et al., 2019) were inoculated subcutaneously by injecting 0.5 ml of tumor cell suspension (0.1 g/ml in PBS) into the right axillary cavity. Mice with US-322 were divided into three treatment groups of 10 animals: control group I, PBS (s.c., 3 times per week); group II, 7-MG (50 mg/kg per os, 3 times per week); group III, cisplatin (2.5 mg/kg s.c., 2 times within a week after inoculation). Mice with RShM-5 were divided into five groups of 10 animals: group I, PBS (s.c., 3 times per week); group II, 7-MG (50 mg/kg per os, 3 times per week); group III, 7-MG for 1 week (50 mg/kg per os, 3 times within a week after inoculation); group IV, cisplatin (1.5 mg/kg s.c., 3 times within a week after inoculation); group V, cisplatin + 7-MG (1.5 mg/kg s.c. + 50 mg/kg per os, 3 times within a week after inoculation). For the combined treatment, 7-MG was administered 3 h prior to cisplatin. The length and width of a subcutaneous tumor were measured with a digital caliper, and the tumor volume was calculated as 1/2 (length×width2).
The animal protocols were approved by the Local Committee for Ethics of Animal Experimentation (Blokhin Cancer Research Center, decision 2019-5 dated 11 February 2019), experiments were conducted in accordance with resolution 81 of the Eurasian Economic Commission and directive 2010/63/EU (on the protection of animals used for scientific purposes).
2.4 Statistical Analysis
Statistical significance of the difference between animal groups was assessed with the Pearson’s chi-squared test (study of anticancer activity, study of chromosomal abnormalities in mice), Student’s t-test (analysis of organ weights), and Fisher’s exact test (study of blastomogenic activity in flies). Significant differences between cells in the comet assay were assessed with the Fisher’s exact test. Data processing was carried out using the Statistica software (StatSoft Inc.).
3 Results
3.1 Toxicology Studies
The adverse effects of 7-MG that can result either from a single or multiple exposures were assessed using four groups of female CBA mice, as presented in Figure 1A. A maximum dose of 600 mg/kg was chosen based on mouse/rat oral LD50 values (40–500 mg/kg) predicted for 7-MG with QSAR software ACD/Percepta (www.acdlabs.com). Oral administration of 7-MG to group II (50 mg/kg, 3 times per week for 4 weeks), group III (200 mg/kg, 3 times per week for 1 week), and group IV (600 mg/kg, single dose) was not lethal to any of the animals. Visual observation revealed no apparent lesions or abnormalities of internal organs in treated mice. However, a significant elevation in spleen weight was produced in group IV (Supplementary Table S1). Histological analysis of the heart, thymus, kidney, adrenal gland, pancreas and stomach showed no abnormalities in all treatment groups. Lung, liver, and spleen tissues were affected only in group IV (Table 1). In this group, small lymphoid infiltrates developed in the lungs and liver (Supplementary Figures S1A,B). The splenic white pulp had poorly defined follicles and lacked germinal centers, and the red pulp was infiltrated with lymphoid cells (Supplementary Figure S1C). In addition, focal lymphoid hyperplasia of the small and large intestines was found in groups III and IV (Supplementary Figure S1D).
The stimulating effect on lymphoid tissue caused by a single 600 mg/kg dose of 7-MG was then investigated in more detail using different mouse strains: CBA, BALB/c and C57BL/6. Animals were administered with either water or 7-MG (600 mg/kg), and samples of lung, liver, spleen, and intestine were collected after 4 weeks (Figure 1B). Microscopic examination revealed the persistence of lymphoid lesions produced by a high dose of 7-MG in all strains. Lung, liver and intestine abnormalities were found in nearly all animals (Supplementary Table S2), and the splenic microarchitecture was affected in 33% of CBA mice, 30% of BALB/c, and 60% of C57BL/6. However, 7-MG treatment was not lethal to any of the animals and had no significant effect on body weight (Supplementary Figure S2). The median lethal dose (LD50) is therefore expected to be substantially greater than 600 mg/kg, which allows us to classify 7-MG as only slightly toxic inhibitor.
These results lead to a conclusion that 50 mg/kg administration 3 times per week may be an optimal regimen, which is devoid of adverse effects and can be readily applied in further testing for anticancer activity of 7-MG in mice (see the Section 3.3).
3.2 Carcinogenicity Studies
The mutagenic and carcinogenic properties of 7-MG have been studied using various short term tests: the Ames test (uses bacterial strains to assess the mutagenic potential) (Maron and Ames, 1983), comet assay (detects DNA strand breaks at the level of the individual cell) (Collins, 2004), chromosomal aberration assay (detects structural chromosomal abnormalities in mice) (Albertini et al., 2000), and somatic mutation and recombination test (uses Drosophila melanogaster to assess the mutagenic, recombinogenic and blastomogenic potential) (Sidorov et al., 2001). The mutagenicity of 7-MG was tested in Salmonella typhimurium strains, TA98 and TA100, both with and without metabolic activation by rat liver S9 fraction. It was demonstrated that 7-MG does not induce frameshift mutations or base-pair substitutions (Table 2). The comet assay showed that 7-MG does not produce DNA damage in immortalized human kidney epithelial cells (Figure 2; Supplementary Figure S3), and the chromosomal aberration assay showed that it does not induce chromatid or chromosome breaks in bone marrow cells of C57BL/6 mice (Table 3; Supplementary Figure S4). The somatic mutation and recombination test detected no blastomogenic activity of 7-MG in wts/+ heterozygotes of D. melanogaster (Table 4).
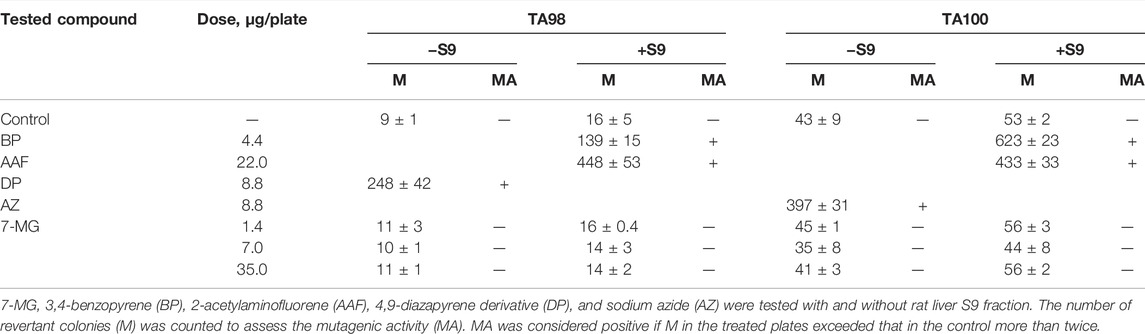
TABLE 2. Study of the mutagenicity of 7-MG in S. typhimurium strains TA98 (detects frameshift mutagens) and TA100 (detects mutagens that cause base-pair substitutions).
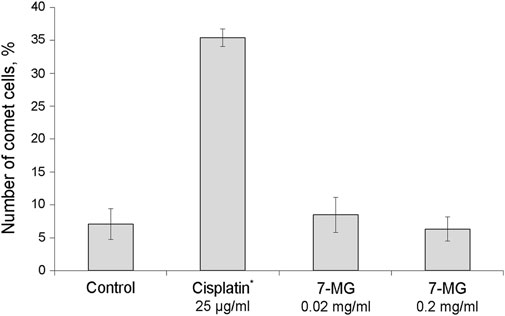
FIGURE 2. Study of the ability of 7-MG to produce DNA strand breaks in immortalized human kidney epithelial cells using the comet assay (500 cells were analyzed per slide). *Significant difference from the control cells (Fisher’s exact test, p < 0.05).
3.3 Anticancer Activity
In previous sections, the following findings were made: 1) 7-MG is not carcinogenic and 2) it can be safely administered in an appropriate dosage. As molecular mechanisms of 7-MG are known (PARP1/2 and TGT inhibition), a thorough investigation of its anticancer properties could be initiated in vivo, involving various transplantable tumor models and a set of existing drugs as active controls. Below are two illustrative examples demonstrating the utility of 7-MG as a component of chemotherapy.
Preliminary studies of 7-MG anticancer activity at a safe dose were carried out using mouse transplantable tumor models of uterine sarcoma US-322 (Figure 3, Supplementary Table S3) and cervical cancer RShM-5 (Figure 4, Supplementary Table S4). Tumor nodes appeared in all control group animals within 3 and 7 days after inoculation, respectively. The US-322 model demonstrated a statistically significant inhibition of tumor growth by the treatment with 7-MG, 50 mg/kg 3 times per week (Figure 3, group II), and the effect of 7-MG was comparable to cisplatin (group III). In the case of RShM-5 model, an ineffective dose of cisplatin was used to test the ability of 7-MG to sensitize the tumor to DNA-damaging agents. It was revealed that the combined treatment with cisplatin and 7-MG within a week after inoculation inhibits tumor growth, whilst 7-MG or cisplatin administration alone exerted no significant effect (Figure 4, groups III–V). It is noteworthy that single-agent 7-MG treatment extended to more than 1 week (group II) had an even more pronounced antitumor effect compared to 1 week of combined treatment. Furthermore, group III (7-MG given for 1 week) clearly demonstrates that interruption of the 7-MG treatment results in accelerated tumor growth starting from the 10th day.
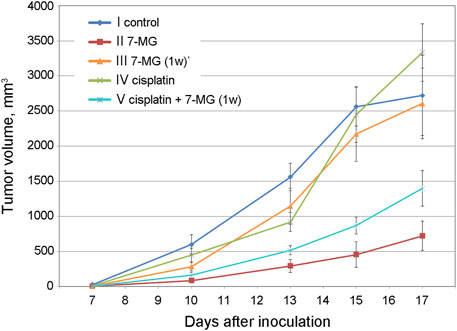
FIGURE 4. Dynamics of RShM-5 tumor growth in female CBA mice at different treatment regimens. *7-MG was given for 1 week only.
4 Discussion
PARP1/2 inhibitors represent a novel class of anticancer agents. Although initially proposed for treatment of BRCA-deficient tumors in women (breast or ovarian cancer), these inhibitors have also demonstrated efficacy in other models such as prostate and gastric cancers (Underhill et al., 2011; Sachdev et al., 2019). Soft tissue sarcomas were also shown to be sensitive to PARP inhibition combined with genotoxic chemotherapy (Ordóñez et al., 2015). The strong PARP1/2 suppression seems to be inherently toxic due to an important role played by these proteins in the organism, but attempts are continuing to find the proper balance between efficacy and toxicity of inhibitors. Fatigue, anemia, nausea and neutropenia together with a risk of myelodysplastic syndrome/acute myeloid leukemia accompany the use of synthetic PARP1/2 inhibitors (Walsh, 2018). The recently described inhibitor 7-MG is a natural nitrogenous base that could escape the serious side effects and become a promising new component of chemotherapy. Additionally, 7-MG inhibits the RNA-modifying enzyme TGT, which may enhance its anticancer activity.
The primary aim of this research was to outline the safety profile of 7-MG in vivo. We have established an oral regimen for 7-MG treatment in CBA mice (50 mg/kg, 3 times per week for up to 4 weeks) that exerts no adverse effects or changes in morphology. Adverse events were detected only at a maximum single dose of 600 mg/kg, in the form of small lymphoid infiltrates of non-inflammatory origin in the lungs and liver. These lesions may be resulted from the excessive inhibition of PARP (Beneke and Möröy, 2001; Ricks et al., 2015; Dasa et al., 2018) at concentrations much higher than therapeutic levels. The safety of 7-MG was also confirmed by the examination in four short-term carcinogenicity assays where it showed no mutagenic or blastomogenic effects.
Preliminary data obtained on mouse transplantable tumor models (uterine sarcoma, cervical carcinoma) demonstrated that 7-MG significantly reduces tumor growth at a safe dose and can also potentiate the activity of cisplatin. The interruption of the 7-MG treatment results in accelerated tumor growth, which highlights the advantages of a multiple-dose regimen. The molecular mechanism of 7-MG is fundamentally different from that of drugs like cisplatin. It acts by modulating the enzyme activity instead of causing DNA damage, and the multiple exposure to 7-MG is needed for the effective modulation. The present study of the natural 7-MG compound has confirmed its safety and potential tumor suppressing activity in mice. For further development, it would be important to identify the most sensitive tumors for the 7-MG treatment as well as to select DNA-damaging agents for the combination treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by The Local Committee for Ethics of Animal Experimentation (Blokhin Cancer Research Center, Moscow, Russia).
Author Contributions
Conceptualization and funding acquisition, DN; investigation, KK, TF, EA, LT, TG, OV, IK, EL, NK, and TS; writing—original draft, KK, EL, and DN; supervision and writing—review and editing, GB, MY, and VŠ.
Funding
This research was funded by the Russian Science Foundation, grant number 19-74-10072.
Conflict of Interest
DN and VŠ are named on a patent for using 7-methylguanine to suppress PARP activity.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.842316/full#supplementary-material
Abbreviations
7-MG, 7-methylguanine; PARP, poly(ADP-ribose) polymerase; TGT, tRNA-guanine transglycosylase.
References
Albertini, R. J., Anderson, D., Douglas, G. R., Hagmar, L., Hemminki, K., Merlo, F., et al. (2000). IPCS Guidelines for the Monitoring of Genotoxic Effects of Carcinogens in Humans. International Programme on Chemical Safety. Mutat. Res. 463, 111–172. doi:10.1016/s1383-5742(00)00049-1
Alemasova, E. E., and Lavrik, O. I. (2019). Poly(ADP-ribosyl)ation by PARP1: Reaction Mechanism and Regulatory Proteins. Nucleic Acids Res. 47, 3811–3827. doi:10.1093/nar/gkz120
Beneke, R., and Möröy, T. (2001). Inhibition of poly(ADP-Ribose) Polymerase Activity Accelerates T-Cell Lymphomagenesis in P53 Deficient Mice. Oncogene 20, 8136–8141. doi:10.1038/sj.onc.1205056
Bolognesi, M. L. (2013). Polypharmacology in a Single Drug: Multitarget Drugs. Curr. Med. Chem. 20, 1639–1645. doi:10.2174/0929867311320130004
Borowitz, I. J., Bloom, S. M., Rothschild, J., and Sprinson, D. B. (1965). Methylated Purines and Pyrimidines. I. Syntheses of 8-Hydroxy-7-Methylguanine and Methylation of a 5-(benzylideneamino) Pyrimidine. Biochemistry 4, 650–655. doi:10.1021/bi00880a006
Bromberg, P. A., Gutman, A. B., and Weissmann, B. (1957). The Purine Bases of Human Urine. II. Semiquantitative Estimation and Isotope Incorporation. J. Biol. Chem. 224, 423–434. doi:10.1016/s0021-9258(18)65041-0
Bromberg, P. A., Gutman, A. B., and Weissmann, B. (1957). The Purine Bases of Human Urine. I. Separation and Identification. J. Biol. Chem. 224, 407–422. doi:10.1016/s0021-9258(18)65040-9
Bunyatyan, N. D., Oborotova, N. A., Nikolaeva, L. L., Saprykina, N. S., Borisova, L. M., Kiseleva, M. P., et al. (2019). Comparative Analysis of Bioactivity of the Russian-made Antitumor Substances of the Nitrosourea Group. Bull. Exp. Biol. Med. 166, 456–460. doi:10.1007/s10517-019-04371-w
Cepeda, V., Fuertes, M. A., Castilla, J., Alonso, C., Quevedo, C., Soto, M., et al. (2006). Poly(ADP-ribose) Polymerase-1 (PARP-1) Inhibitors in Cancer Chemotherapy. Recent Pat. Anticancer Drug Discov. 1, 39–53. doi:10.2174/157489206775246430
Collins, A. R. (2004). The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 26, 249–261. doi:10.1385/MB:26:3:249
Craddock, V. M., Mattocks, A. R., and Magee, P. N. (1968). The Fate of 7[14C]-Methylguanine after Administration to the Rat. Biochem. J. 109, 75–78. doi:10.1042/bj1090075
Dasa, S. S. K., Diakova, G., Suzuki, R., Mills, A. M., Gutknecht, M. F., Klibanov, A. L., et al. (2018). Plectin-targeted Liposomes Enhance the Therapeutic Efficacy of a PARP Inhibitor in the Treatment of Ovarian Cancer. Theranostics 8, 2782–2798. doi:10.7150/thno.23050
Drenichev, M. S., and Mikhailov, S. N. (2015). Poly(ADP-ribose)--a Unique Natural Polymer Structural Features, Biological Role and Approaches to the Chemical Synthesis. Nucleosides Nucleotides Nucleic Acids 34, 258–276. doi:10.1080/15257770.2014.984073
Farkas, W. R., Jacobson, K. B., and Katze, J. R. (1984). Substrate and Inhibitor Specificity of tRNA-Guanine Ribosyltransferase. Biochim. Biophys. Acta 781, 64–75. doi:10.1016/0167-4781(84)90124-6
Ferraris, D. V. (2010). Evolution of poly(ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors. From Concept to Clinic. J. Med. Chem. 53, 4561–4584. doi:10.1021/jm100012m
Frampton, J. E. (2015). Olaparib: A Review of its Use as Maintenance Therapy in Patients with Ovarian Cancer. BioDrugs 29, 143–150. doi:10.1007/s40259-015-0125-6
Hassler, M., and Ladurner, A. G. (2012). Towards a Structural Understanding of PARP1 Activation and Related Signalling ADP-Ribosyl-Transferases. Curr. Opin. Struct. Biol. 22, 721–729. doi:10.1016/j.sbi.2012.08.005
Jain, P. G., and Patel, B. D. (2019). Medicinal Chemistry Approaches of Poly ADP-Ribose Polymerase 1 (PARP1) Inhibitors as Anticancer Agents - A Recent Update. Eur. J. Med. Chem. 165, 198–215. doi:10.1016/j.ejmech.2019.01.024
Johannsson, S., Neumann, P., and Ficner, R. (2018). Crystal Structure of the Human tRNA Guanine Transglycosylase Catalytic Subunit QTRT1. Biomolecules 8, 81. doi:10.3390/biom8030081
Kaina, B., Heindorff, K., and Aurich, O. (1983). O6-methylguanine, but Not N7-Methylguanine or N3-Methyladenine, Induces Gene Mutations, Sister-Chromatid Exchanges and Chromosomal Aberrations in Chinese Hamster Cells. Mutat. Res. 108, 279–292. doi:10.1016/0027-5107(83)90126-4
Kerr, S. J. (1985). Induction of Adipocyte Formation in 10T1/2 Cells by 1-methylguanine and 7-methylguanine. Tumour Biol. 6, 115–121.
Kerr, S. J. (1990). Methylated Oxypurines and Induction of Differentiation of Murine Erythroleukemia Cells. Mol. Cell. Biochem. 92, 37–44. doi:10.1007/BF00220717
Kirsanov, K. I., Lesovaya, E. A., Sidorov, R. A., Belitsky, G. A., and Yakubovskaya, M. G. (2011). Analysis of Blastomogenic Activity of Mammal Carcinogens in Drosophila Using the Wts P4 Allele and RNA Interference-Induced P53 Silencing. Russ. J. Genet. 47, 409–416. doi:10.1134/s1022795411040065
Litwack, M. D., and Weissmann, B. (1966). Source of Urinary 8-Hydroxy-7-Methylguanine in Man. Biochemistry 5, 3007–3012. doi:10.1021/bi00873a033
Lord, C. J., Tutt, A. N., and Ashworth, A. (2015). Synthetic Lethality and Cancer Therapy: Lessons Learned from the Development of PARP Inhibitors. Annu. Rev. Med. 66, 455–470. doi:10.1146/annurev-med-050913-022545
Lothrop, C. D., and Uziel, M. (1983). RNA Turnover in Cultured Hamster Embryo Cells: Identification of Modified Nucleoside End Products. J. Cell. Physiol. 114, 111–116. doi:10.1002/jcp.1041140118
Maluchenko, N., Nilov, D., Feofanov, A., Lys, A., Kutuzov, M., Gerasimova, N., et al. (2019). 7-Methylguanine Traps PARP-1 on Nucleosomes: spFRET Microscopy Study. Microsc. Microanal. 25, 1282–1283. doi:10.1017/S1431927619007141
Malyuchenko, N. V., Kotova, E. Y., Kulaeva, O. I., Kirpichnikov, M. P., and Studitskiy, V. M. (2015). PARP1 Inhibitors: Antitumor Drug Design. Acta Naturae 7, 27–37. doi:10.32607/20758251-2015-7-3-27-37
Maron, D. M., and Ames, B. N. (1983). Revised Methods for the Salmonella Mutagenicity Test. Mutat. Res. 113, 173–215. doi:10.1016/0165-1161(83)90010-9
Martin, S. A., Lord, C. J., and Ashworth, A. (2008). DNA Repair Deficiency as a Therapeutic Target in Cancer. Curr. Opin. Genet. Dev. 18, 80–86. doi:10.1016/j.gde.2008.01.016
Medina-Franco, J. L., Giulianotti, M. A., Welmaker, G. S., and Houghten, R. A. (2013). Shifting from the Single to the Multitarget Paradigm in Drug Discovery. Drug Discov. TodayToday 18, 495–501. doi:10.1016/j.drudis.2013.01.008
Mittica, G., Ghisoni, E., Giannone, G., Genta, S., Aglietta, M., Sapino, A., et al. (2018). PARP Inhibitors in Ovarian Cancer. Recent Pat. Anticancer Drug Discov. 13, 392–410. doi:10.2174/1574892813666180305165256
Mustonen, R., and Hemminki, K. (1992). 7-Methylguanine Levels in DNA of Smokers' and Non-smokers' Total White Blood Cells, Granulocytes and Lymphocytes. Carcinogenesis 13, 1951–1955. doi:10.1093/carcin/13.11.1951
Nilov, D., Maluchenko, N., Kurgina, T., Pushkarev, S., Lys, A., Kutuzov, M., et al. (2020a). Molecular Mechanisms of PARP-1 Inhibitor 7-methylguanine. Int. J. Mol. Sci. 21, 2159. doi:10.3390/ijms21062159
Nilov, D. K., Pushkarev, S. V., Gushchina, I. V., Manasaryan, G. A., Kirsanov, K. I., and Švedas, V. K. (2020b). Modeling of the Enzyme-Substrate Complexes of Human poly(ADP-Ribose) Polymerase 1. Biochem. (Mosc) 85, 99–107. doi:10.1134/S0006297920010095
Nilov, D. K., Tararov, V. I., Kulikov, A. V., Zakharenko, A. L., Gushchina, I. V., Mikhailov, S. N., et al. (2016). Inhibition of poly(ADP-Ribose) Polymerase by Nucleic Acid Metabolite 7-methylguanine. Acta Naturae 8, 108–115. doi:10.32607/20758251-2016-8-2-108-115
Nilov, D., Kirsanov, K., Antoshina, E., Maluchenko, N., Feofanov, A., Kurgina, T., et al. (2018a). 7-Methylguanine: A Natural DNA Repair Inhibitor and a Promising Anticancer Compound. FEBS Open Bio 8. doi:10.1002/2211-5463.12453
Nilov, D. K., Yashina, K. I., Gushchina, I. V., Zakharenko, A. L., Sukhanova, M. V., Lavrik, O. I., et al. (2018b). 2,5-Diketopiperazines: A New Class of poly(ADP-Ribose)polymerase Inhibitors. Biochem. (Mosc) 83, 152–158. doi:10.1134/S0006297918020074
O'Connor, T. R. (1993). Purification and Characterization of Human 3-Methyladenine-DNA Glycosylase. Nucleic Acids Res. 21, 5561–5569. doi:10.1093/nar/21.24.5561
Ohmoto, A., and Yachida, S. (2017). Current Status of poly(ADP-Ribose) Polymerase Inhibitors and Future Directions. Onco Targets Ther. 10, 5195–5208. doi:10.2147/OTT.S139336
Ordóñez, J. L., Amaral, A. T., Carcaboso, A. M., Herrero-Martín, D., del Carmen García-Macías, M., Sevillano, V., et al. (2015). The PARP Inhibitor Olaparib Enhances the Sensitivity of Ewing Sarcoma to Trabectedin. Oncotarget 6, 18875–18890. doi:10.18632/oncotarget.4303
Park, J. W., and Ames, B. N. (1988). 7-Methylguanine Adducts in DNA Are Normally Present at High Levels and Increase on Aging: Analysis by HPLC with Electrochemical Detection. Proc. Natl. Acad. Sci. U. S. A. 85, 7467–7470. doi:10.1073/pnas.85.20.7467
Raćkowska, E., Bobrowska-Korczak, B., and Giebułtowicz, J. (2019). Development and Validation of a Rapid LC-MS/MS Method for Determination of Methylated Nucleosides and Nucleobases in Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1128, 121775. doi:10.1016/j.jchromb.2019.121775
Ray Chaudhuri, A., and Nussenzweig, A. (2017). The Multifaceted Roles of PARP1 in DNA Repair and Chromatin Remodelling. Nat. Rev. Mol. Cell Biol. 18, 610–621. doi:10.1038/nrm.2017.53
Ricks, T. K., Chiu, H. J., Ison, G., Kim, G., McKee, A. E., Kluetz, P., et al. (2015). Successes and Challenges of PARP Inhibitors in Cancer Therapy. Front. Oncol. 5, 222. doi:10.3389/fonc.2015.00222
Rodríguez-Gonzalo, E., Hernández-Prieto, R., García-Gómez, D., and Carabias-Martínez, R. (2013). Capillary Electrophoresis-Mass Spectrometry for Direct Determination of Urinary Modified Nucleosides. Evaluation of Synthetic Urine as a Surrogate Matrix for Quantitative Analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 942–943, 21–30. doi:10.1016/j.jchromb.2013.10.022
Sachdev, E., Tabatabai, R., Roy, V., Rimel, B. J., and Mita, M. M. (2019). PARP Inhibition in Cancer: An Update on Clinical Development. Target. Oncol. 14, 657–679. doi:10.1007/s11523-019-00680-2
Sander, G., Hülsemann, J., Topp, H., Heller-Schöch, G., and Schöch, G. (1986). Protein and RNA Turnover in Preterm Infants and Adults: A Comparison Based on Urinary Excretion of 3-methylhistidine and of Modified One-Way RNA Catabolites. Ann. Nutr. Metab. 30, 137–142. doi:10.1159/000177186
Sander, G., Topp, H., Heller-Schöch, G., Wieland, J., and Schöch, G. (1986). Ribonucleic Acid Turnover in Man:RNA Catabolites in Urine as Measure for the Metabolism of Each of the Three Major Species of RNA. Clin. Sci. 71, 367–374. doi:10.1042/cs0710367
Sander, G., Topp, H., Wieland, J., Heller-Schöch, G., and Schöch, G. (1986). Possible Use of Urinary Modified RNA Metabolites in the Measurement of RNA Turnover in the Human Body. Hum. Nutr. Clin. Nutr. 40, 103–118.
Shafer, B., Chu, C., and Shatkin, A. J. (2005). Human mRNA Cap Methyltransferase: Alternative Nuclear Localization Signal Motifs Ensure Nuclear Localization Required for Viability. Mol. Cell. Biol. 25, 2644–2649. doi:10.1128/MCB.25.7.2644-2649.2005
Shapiro, R. (1968). “Chemistry of Guanine and its Biologically Significant Derivatives,”. Editors J. N. Davidson, and W. E. Cohn (New York, USA: Academic Press).Prog. Nucleic Acid Res. Mol. Biol., Vol. 8, 73–112. doi:10.1016/s0079-6603(08)60544-9
Shuman, S. (2002). What Messenger RNA Capping Tells Us about Eukaryotic Evolution. Nat. Rev. Mol. Cell Biol. 3, 619–625. doi:10.1038/nrm880
Sidorov, R. A., Ugnivenko, E. G., Khovanova, E. M., and Belitsky, G. A. (2001). Induction of Tumor Clones in D. melanogaster Wts/+ Heterozygotes with Chemical Carcinogens. Mutat. Res. 498, 181–191. doi:10.1016/s1383-5718(01)00277-7
Singh, N. P., McCoy, M. T., Tice, R. R., and Schneider, E. L. (1988). A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 175, 184–191. doi:10.1016/0014-4827(88)90265-0
Skupp, S., and Ayvazian, J. H. (1969). Oxidation of 7-methylguanine by Human Xanthine Oxidase. J. Lab. Clin. Med. 73, 909–916.
Sonnenblick, A., de Azambuja, E., Azim, H. A., and Piccart, M. (2015). An Update on PARP Inhibitors-Mmoving to the Adjuvant Setting. Nat. Rev. Clin. Oncol. 12, 27–41. doi:10.1038/nrclinonc.2014.163
Svoboda, P., and Kasai, H. (2004). Simultaneous HPLC Analysis of 8-hydroxydeoxyguanosine and 7-methylguanine in Urine from Humans and Rodents. Anal. Biochem. 334, 239–250. doi:10.1016/j.ab.2004.08.021
Tamae, K., Kawai, K., Yamasaki, S., Kawanami, K., Ikeda, M., Takahashi, K., et al. (2009). Effect of Age, Smoking and Other Lifestyle Factors on Urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci. 100, 715–721. doi:10.1111/j.1349-7006.2009.01088.x
Tan, B. H., Bencsath, F. A., and Gaubatz, J. W. (1990). Steady-state Levels of 7-methylguanine Increase in Nuclear DNA of Postmitotic Mouse Tissues during Aging. Mutat. Res. 237, 229–238. doi:10.1016/0921-8734(90)90004-b
Topisirovic, I., Svitkin, Y. V., Sonenberg, N., and Shatkin, A. J. (2011). Cap and Cap-Binding Proteins in the Control of Gene Expression. Wiley Interdiscip. Rev. RNA. 2, 277–298. doi:10.1002/wrna.52
Topp, H., Sander, G., Heller-Schöch, G., and Schöch, G. (1987). Determination of 7-methylguanine, N2,N2-Dimethylguanosine, and Pseudouridine in Ultrafiltrated Serum of Healthy Adults by High-Performance Liquid Chromatography. Anal. Biochem. 161, 49–56. doi:10.1016/0003-2697(87)90650-6
Treshalina, H. M., Lukasheva, E. V., Sedakova, L. A., Firsova, G. A., Guerassimova, G. K., Gogichaeva, N. V., et al. (2000). Anticancer Enzyme L-Lysine α-Oxidase: Properties and Application Perspectives. Abab 88, 267–274. doi:10.1385/abab:88:1-3:267
Turusov, V. S., Siniak, IuE., Grigor'ev, A. I., Zaridze, D. G., Antoshina, E. E., Trukhanova, L. S., et al. (2005). Low-deuterium Water Effect on Transplantable Tumors. Vopr. Onkol. 51, 99–102.
Underhill, C., Toulmonde, M., and Bonnefoi, H. (2011). A Review of PARP Inhibitors: from Bench to Bedside. Ann. Oncol. 22, 268–279. doi:10.1093/annonc/mdq322
Varshney, D., Petit, A. P., Bueren-Calabuig, J. A., Jansen, C., Fletcher, D. A., Peggie, M., et al. (2016). Molecular Basis of RNA Guanine-7 Methyltransferase (RNMT) Activation by RAM. Nucleic Acids Res. 44, 10423–10436. doi:10.1093/nar/gkw637
Walsh, C. (2018). Targeted Therapy for Ovarian Cancer: the Rapidly Evolving Landscape of PARP Inhibitor Use. Minerva Ginecol. 70, 150–170. doi:10.23736/S0026-4784.17.04152-1
Wang, Y. Q., Wang, P. Y., Wang, Y. T., Yang, G. F., Zhang, A., and Miao, Z. H. (2016). An Update on poly(ADP-Ribose)polymerase-1 (PARP-1) Inhibitors: Opportunities and Challenges in Cancer Therapy. J. Med. Chem. 59, 9575–9598. doi:10.1021/acs.jmedchem.6b00055
Weissmann, B., and Gutman, A. B. (1957). The Identification of 6-succinoaminopurine and of 8-Hydroxy-7-Methylguanine as Normal Human Urinary Constituents. J. Biol. Chem. 229, 239–250. doi:10.1016/s0021-9258(18)70612-1
Zhang, J., Lu, R., Zhang, Y., Matuszek, Ż., Zhang, W., Xia, Y., et al. (2020). tRNA Queuosine Modification Enzyme Modulates the Growth and Microbiome Recruitment to Breast Tumors. Cancers (Basel) 12, 628. doi:10.3390/cancers12030628
Keywords: 7-Methylguanine, inhibitor, cancer, carcinogenicity, toxicity
Citation: Kirsanov K, Fetisov T, Antoshina E, Trukhanova L, Gor’kova T, Vlasova O, Khitrovo I, Lesovaya E, Kulbachevskaya N, Shcherbakova T, Belitsky G, Yakubovskaya M, Švedas V and Nilov D (2022) Toxicological Properties of 7-Methylguanine, and Preliminary Data on its Anticancer Activity. Front. Pharmacol. 13:842316. doi: 10.3389/fphar.2022.842316
Received: 23 December 2021; Accepted: 13 June 2022;
Published: 06 July 2022.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesReviewed by:
Katia Mangano, University of Catania, ItalyHarald Krenzlin, Johannes Gutenberg University Mainz, Germany
Copyright © 2022 Kirsanov, Fetisov, Antoshina, Trukhanova, Gor’kova, Vlasova, Khitrovo, Lesovaya, Kulbachevskaya, Shcherbakova, Belitsky, Yakubovskaya, Švedas and Nilov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dmitry Nilov, bmlsb3ZkbUBnbWFpbC5jb20=
 Kirill Kirsanov1,2
Kirill Kirsanov1,2 Ekaterina Lesovaya
Ekaterina Lesovaya Vytas Švedas
Vytas Švedas Dmitry Nilov
Dmitry Nilov