- 1College of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Neurology, Chongqing Traditional Chinese Medicine Hospital, Chongqing, China
Background: Accumulated experimental evidence suggests that resveratrol may have an effect on diabetic nephropathy by inhibiting inflammation and decreasing oxidative stress. However, the credibility of the evidence for this practice is unclear. Thus, we aimed to perform a systematic review and meta-analysis of animal studies to evaluate the antioxidant and anti-inflammatory properties of resveratrol when used in the treatment of diabetic nephropathy.
Methods: Electronic bibliographic databases including PubMed, EMBASE, and Web of Science were searched for relevant studies. The methodological quality of animal studies was assessed based on the SYstematic Review Center for Laboratory animal Experimentation Risk of Bias (SYRCLE’s RoB) tool. A meta-analysis was performed based on the Cochrane Handbook for Systematic Reviews of Interventions by using RevMan 5.4 software. This study was registered within International Prospective Register of Systematic Reviews (PROSPERO) as number CRD42021293784.
Results: Thirty-six qualified studies involving 726 animals were included. There was a significant association of resveratrol with the levels of blood glucose (BG), serum creatinine (Scr), blood urea nitrogen (BUN), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), glutathione peroxidase (GPx), and interleukin-1β (IL-1β). Nevertheless, resveratrol treatment did not effectively decrease the levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). In addition, more remarkable antioxidant and hypoglycemic effects were observed in type 2 diabetic nephropathy rather than in type 1 diabetic nephropathy based on subgroup analysis.
Conclusion: In this meta-analysis, resveratrol can exert its antioxidant activities by reducing the levels of MDA and recovering the activities of SOD, CAT, GSH, and GPx. With regard to pro-inflammatory cytokines, resveratrol had a positive effect on the reduction of IL-1β. However, the analysis indicated that resveratrol had no effect on IL-6 and TNF-α levels, probably because of the methodological quality of the studies and their heterogeneity. Current evidence supports the antioxidant and anti-inflammatory properties of resveratrol, but its relationship with the levels of some inflammatory cytokines such as IL-6 and TNF-α in animals with diabetic nephropathy needs further elucidation.
Introduction
Diabetic nephropathy (DN), one of the most common, serious, and expensive diabetic complications (Alicic et al., 2021), is a chronic progressive disorder that can result in kidney failure and is currently the primary cause of kidney replacement therapy worldwide (DeFronzo et al., 2021). Multiple factors including hyperglycemia, inflammation, and oxidative stress contribute to the pathogenesis and progression of DN (Singh et al., 2011; Markus et al., 2018; DeFronzo et al., 2021). Literature data show that oxidative stress and inflammation could directly damage renal function in streptozotocin (STZ)-induced diabetic animals leading to DN (Barman et al., 2018). Current treatments for DN, including optimized glycemic control and blood pressure control with renin-angiotensin system blockade (angiotensin-receptor blockers or angiotensin-converting enzyme inhibitors), are insufficient to delay the progression to end-stage renal disease (ESRD) in a substantial proportion of patients (Fernandez-Fernandez et al., 2014; Lytvyn et al., 2020). Therefore, it is imperative to establish novel treatment approaches for the management of DN.
To achieve this, Chinese herbal medicine (CHM) and its bioactive ingredients as natural treatment options have become the focus of DN drug research. In comparison with conventional drugs, they typically do not selectively inhibit one molecular target but exhibit a pleiotropic action profile (Meresman et al., 2021), which may simultaneously affect fundamental processes in the pathogenesis of DN and achieve higher efficacy in the treatment of DN. Polygonum cuspidatum, the dry rhizome and root of Polygonum cuspidatum Sieb. et Zucc., is well known as Hu Zhang in China and widely used as CHM to treat diseases such as jaundice, fever, and cough (Peng et al., 2013). Polygonum cuspidatum contains a variety of bioactive ingredients such as polydatin and resveratrol (RSV), and is the primary source of the extraction of RSV (Zhang et al., 2020). Modern pharmacological studies suggested that RSV has a beneficial influence on DN with its powerful anti-inflammatory and antioxidant properties. In animal models of DN, RSV treatment can significantly relieve kidney damage by increasing the activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Khamneh et al., 2012; Ramar et al., 2012). Moreover, it was suggested that RSV has renal protective effects by reducing the levels of malondialdehyde (MDA) (Zhang et al., 2019; Wang et al., 2020). In addition, RSV can improve STZ-mediated DN symptoms in animals through decreasing the inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) (Koca et al., 2016; Ma et al., 2016). Results of these animal experiments demonstrated a protective role of RSV in DN and suggested that the antioxidant and anti-inflammatory properties were implicated in the underlying mechanisms. However, these findings in the individual animal experiments are often affected by multiple factors, such as small sample sizes, DN animal models, intervention duration, and small-study effects, so it is insufficient to draw reliable conclusions about the antioxidant or anti-inflammatory properties of RSV in the treatment of DN according to this low-quality evidence. In addition, dose-response effects and time-response effects play a critical role in the treatment of DN, but it is difficult to determine the appropriate dosage and intervention duration of RSV based on the individual animal experiments. Moreover, whether RSV has adverse effects in the management of DN remains uncertain. Furthermore, methodological quality of the animal experiments and publication bias are still unclear, which may exaggerate the RSV efficacy.
Reviews based on preclinical animal data can address these aforementioned problems, assist in determining what is valuable in further research, and inform future clinical trials. In order to bridge the gap between the animal studies and clinical application, we performed a systematic review and meta-analysis of preclinical animal data to evaluate RSV in the management of DN. The purposes of this study were to 1) provide empirical evidence to assess the antioxidant and anti-inflammatory activities of RSV in the treatment of DN, 2) discuss the appropriate dosage and intervention duration of RSV on DN, 3) provide information on adverse effects of RSV in the treatment of DN, 4) provide an assessment of methodological quality of the animal studies and publication bias, and 5) provide suggestions for future animal studies and clinical trials.
Materials and Methods
This systematic review and meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions and reported based on Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Page et al., 2021). The protocol for this systematic review and meta-analysis is available on the website of PROSPERO (CRD42021293784).
Search Strategies
Electronic bibliographic databases including PubMed, EMBASE, and Web of Science were searched for relevant studies published from January 2010 to September 2021. The language was limited to English. Medical subject headings (MeSH) and free words for database searches were as follows: Subjects (diabetic nephropathies (MeSH), “diabetic renal disease,” “kidney diseases, diabetic,” “kidney disease, diabetic,” “diabetic kidney diseases,” “diabetic kidney disease,” “nephropathies, diabetic,” “diabetic nephropathy,” “nephropathy, diabetic,” “DKD,” “DN; ” Intervention [resveratrol (MeSH), “trans resveratrol,” “cis resveratrol”].
Inclusion Criteria
1) Subjects: all animal models with DN; 2) Intervention: RSV with all dosage and duration; 3) Control: same solvent (e.g., water and saline), no intervention, etc.; 4) Outcomes: blood glucose (BG), serum creatinine (Scr), and blood urea nitrogen (BUN) were the primary outcomes, IL-1β, IL-6, TNF-α, SOD, MDA, CAT, GSH, and GPx were the secondary outcomes; 5) Study design: controlled studies with a separate control group; 6) Language: English.
Exclusion Criteria
1) Subjects: animals with co-morbidity, clinical trials, and in vitro models; 2) Intervention: RSV without batch number; 3) Control: other preparation of RSV (some pharmaceutical preparation or nutritional products containing RSV); 4) Study design: case studies, cross over studies, and studies without a separate control group; 5) Not an original full research paper (e.g., review, editorials/letters, abstracts); 6) Duplicate publication; 7) Studies without full text.
Study Selection and Data Extraction
The screening of studies was performed in two phases, initial screening based on title and abstract, followed by full-text screening of the potentially eligible articles for final determination (Higgins and Green, 2021). In each phase, two reviewers independently assessed each study. Disagreements about whether a study should be included were resolved through discussion with a third reviewer.
Two reviewers extracted the following data independently from included studies: 1) Basic information: first author’s surname and year of publication; 2) Information on subjects: sample size, weight, species, and animal models of DN in the experimental group and control group; 3) Information on treatment: intervention duration and dose; 4) Outcome measures: BG, Scr, BUN, TNF-α, IL-6, IL-1β, CAT, GSH, SOD, MDA, and GPx. All the outcome measures were continuous data, so the mean and the standard deviation for each intervention group were extracted. For studies with multiple experimental groups paired with one control group, then this control group was divided up approximately evenly among the comparisons and each pair-wise comparison was involved in the meta-analysis (Higgins and Green, 2021). In case outcomes were presented at multiple time points, the data were extracted from the last time point. Any disagreements between reviewers over the data extraction were resolved through discussion with a third reviewer.
Quality Assessment
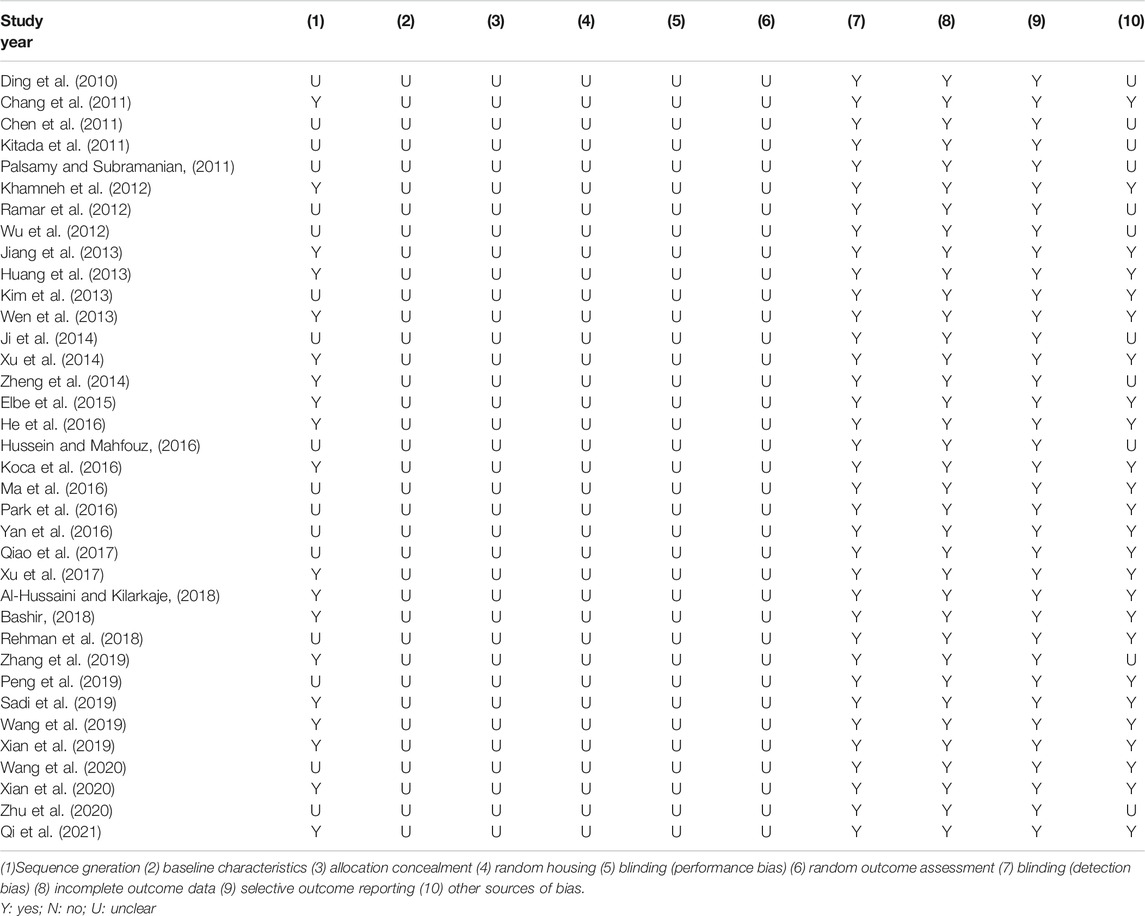
The methodological quality of the included studies was assessed according to the SYstematic Review Center for Laboratory animal Experimentation Risk of Bias (SYRCLE’s RoB) tool. The SYRCLE’s RoB tool for animal experiments contains ten entries based on six types of bias: 1) Sequence generation (selection bias); 2) Baseline characteristics (selection bias); 3) Allocation concealment (selection bias); 4) Random housing (performance bias); 5) Blinding (performance bias); 6) Random outcome assessment (detection bias); 7) Blinding (detection bias); 8) Incomplete outcome data (attrition bias); 9) Selective outcome reporting (reporting bias); (10) Other sources of bias (other). The results of assessment are “yes,” “no,” and “unclear,” representing “low risk of bias,” “high risk of bias,” and “insufficient details have been reported to assess the risk of bias properly” (Hooijmans et al., 2014).
Two reviewers performed quality assessment independently, and discrepancies were discussed with a third reviewer.
Statistical Analysis
All the outcome measures were continuous data (e.g., BG and Scr), so standardized mean difference (SMD) was considered to describe the effect sizes of the intervention effects. The confidence interval (CI) was established at 95%, and a p value <0.05 was considered to be statistically significant. Random-effect model was implemented to calculate the pooled results. To evaluate between-study heterogeneity, the chi-squared test and I2 statistics were used. The chi-squared test with a significance level of α = 0.1 was used as statistical measure of heterogeneity, and I2 > 50% was considered to represent substantial heterogeneity. Subgroup analysis was performed to investigate the possible sources of heterogeneity based on following variables if there were adequate studies: dosage (low ≤ 10 mg/kg/day; 10 < middle ≤ 20 mg/kg/day; high >20 mg/kg), intervention duration (<12 weeks; ≥ 12 weeks), DN models (type 1 DN; type 2 DN), and species (rats; mice). Sensitivity analysis was conducted to evaluate whether a single study affects the overall effect sizes by removing one study at each stage. Publication bias was assessed with the funnel plot as well as the Egger’s test (Egger et al., 1997) if there were at least 10 studies for each outcome. For Egger’s test, a p value less than 0.05 was considered as statistically significant (Egger et al., 1997). Meta-analysis was performed with RevMan 5.4 software.
Results
Study Inclusion
A total number of 475 animal studies were identified through searching the databases for systematic review and meta-analysis. After removing duplicates, 311 publications remained. In screening titles and abstracts, 267 publications were excluded because of the following reasons: 1) clinical trials; 2) review articles; 3) not RSV or DN; 4) in vitro studies; 5) others. Then, full-text selection of the 44 remaining animal studies revealed that eight studies were ineligible due to following reasons: 1) studies without full text (n = 3); 2) inappropriate outcome measures (n = 1); 3) conference abstracts (n = 4). Ultimately, 36 eligible studies were included in this systematic review and meta-analysis. The process of study selection is shown in Figure 1.
Study Characteristics
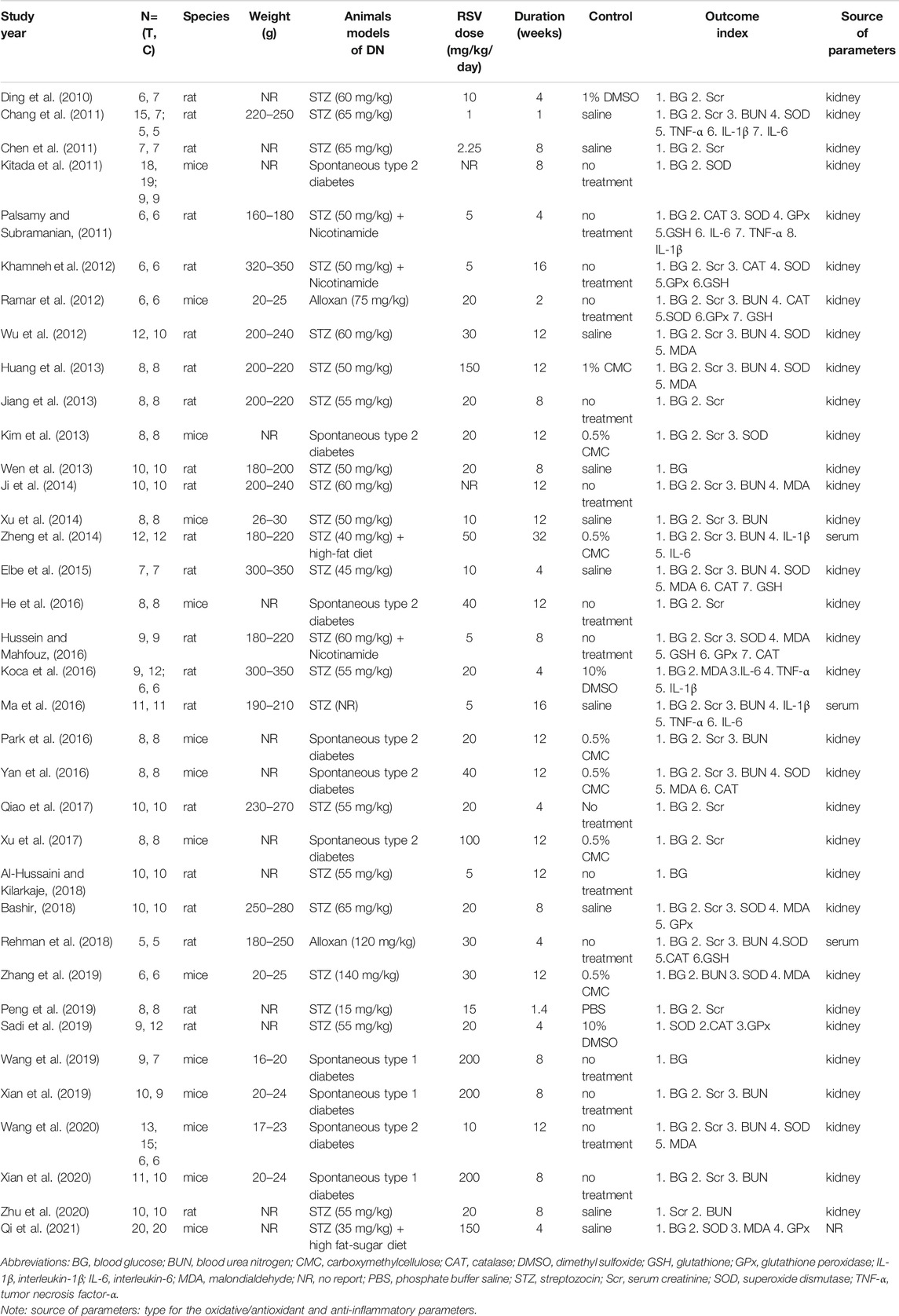
The 36 qualified studies involving 726 animals were published between 2010 and 2021. The number of all animals in the experimental group was 365 and that in the control group was 361. The animal species included mice and rats, 14 studies (38.9%) of all which used mice, and 22 studies (61.1%) used rats. The weight of rats ranged from 160 to 350 g in all studies and that of mice ranged from 16 to 30 g, and 13 studies did not report the weight of animals. There were two animal models in these studies, type 1 DN (66.7%) and type 2 DN (33.3%).
Three levels (low, medium, and high) of RSV doses were implemented in these studies and the dose ranged from 1 to 200 mg/kg/day, and two studies did not report RSV dosage. Control groups mainly included the same solvent, such as dimethyl sulfoxide (DMSO), saline, carboxymethyl cellulose (CMC), and phosphate buffer saline (PBS). Three studies (8.3%) used DMSO as the control group, ten studies (27.8%) selected saline, seven studies (19.4%) used CMC, one study (2.8%) selected phosphate buffer saline, and the remaining 15 studies (41.7%) had no treatment. The intervention duration consisted of <12 weeks and ≥12 weeks, and the duration ranged from 1 week to 32 weeks. The intervention duration were ≥12 weeks in 15 studies (41.7%) and <12 weeks in 21 studies (58.3%). The characteristics of the 36 qualified studies are outlined in Table 1. In addition, a summary table describing the RSV is shown in Supplementary Table S1.
Study Quality
Random allocation to experimental and control groups was mentioned in 19 studies (52.8%), and the remaining 17 studies did not report the methods of allocation. None of the studies reported whether the distribution of relevant baseline levels was balanced between the experimental groups and control groups. None of the studies reported the blinded allocation. Random housing, blinding (performance bias), and random outcome assessment were not mentioned in all studies. All these studies had complete outcome data and reported expected outcomes. As for other sources of bias, 25 studies (69.4%) stated that there was no conflict of interest among the authors, and the remaining 11 studies (30.6%) did not mention it. The methodological quality of included studies is displayed in Table 2.
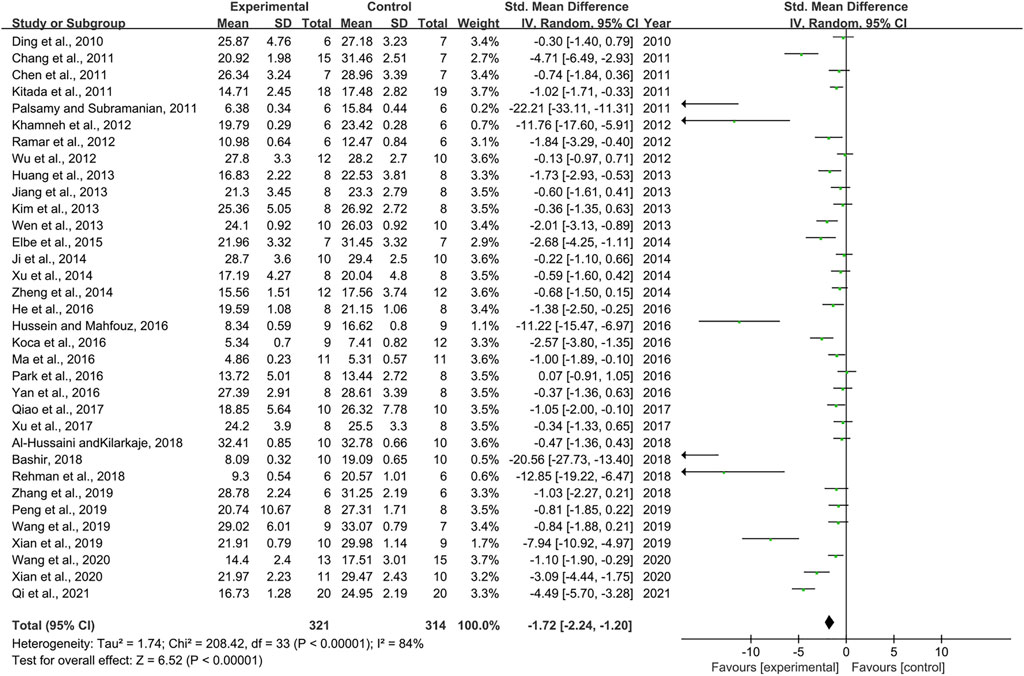
Effect of Resveratrol on Blood Glucose
Thirty-four pair-wise comparisons reported the influence of RSV on BG. The pooled results showed that RSV could significantly decrease BG levels compared with the control group [n = 635, SMD = −1.72, 95% CI (−2.24, −1.20), p < 0.00001; Heterogeneity: X2 = 208.42, p < 0.00001; I2 = 84%, Figure 2]. Subgroup analysis was conducted based on the DN models, intervention duration, dosage, and species. More beneficial effects were observed when studies employed type 2 DN models (p = 0.000), intervention duration of <12 weeks (p = 0.000), and rats (p = 0.000), as well as when they used RSV in a low-dose group (p = 0.000) (Supplementary Table S2). In addition, visual inspection of funnel plots showed asymmetry for the effects of RSV on BG (Supplementary Figure S1), while the result of Egger’s test was statistically significant [intercept: −5.37, 95% CI (−6.66, −4.09); p = 0.000].
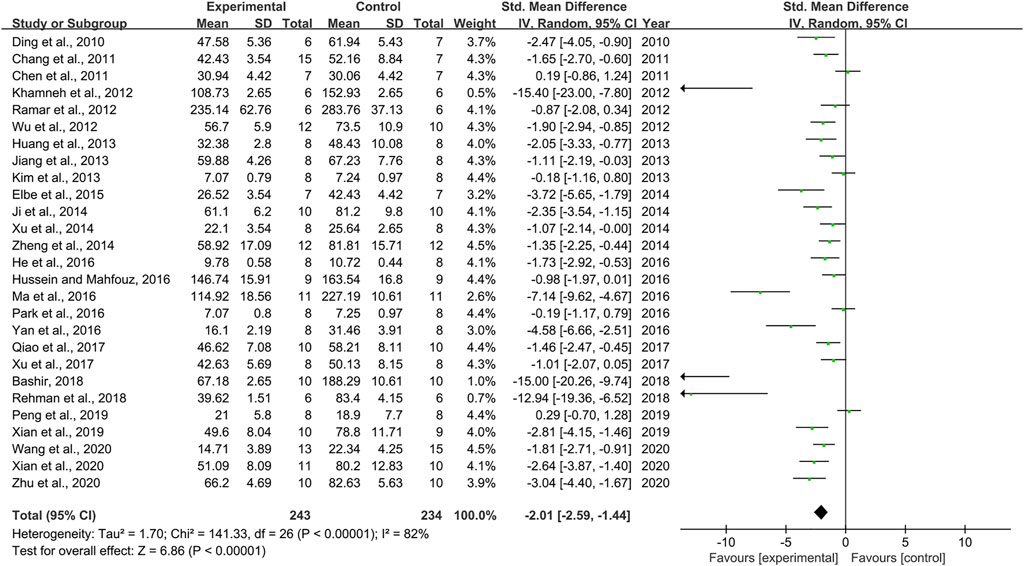
Effect of Resveratrol on Serum Creatinine
Combining effect sizes from 27 pair-wise comparisons, a significant reduction in Scr level was observed after RSV administration, compared to that in the control group [n = 477, SMD = −2.01, 95% CI (−2.59, −1.44), p < 0.00001; Heterogeneity: X2 = 141.33, p < 0.00001; I2 = 82%, Figure 3]. Subgroup analysis was performed according to DN models, intervention duration, dosage, and species. More beneficial effects were observed when studies applied type 1 DN models (p = 0.000), rats (p = 0.000), and intervention duration of <12 weeks (p = 0.000), as well as when they used RSV in a low-dose group (p = 0.000) (Supplementary Table S2). Furthermore, visual inspection of funnel plots showed asymmetry for the effects of RSV on Scr (Supplementary Figure S2), and the result of Egger’s test was statistically significant [intercept: −5.63, 95% CI (−7.15, −4.10); p = 0.000].
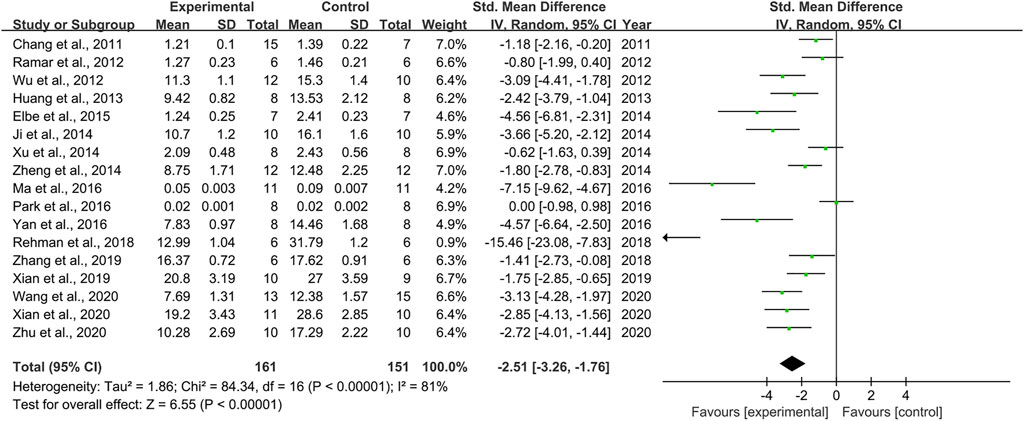
Effect of Resveratrol on Blood Urea Nitrogen
Seventeen pair-wise comparisons reported the influence of RSV on BUN. The pooled results suggested that RSV could significantly decrease BUN level compared with the control group [n = 312, SMD = −2.51, 95% CI (−3.26, −1.76), p < 0.00001; Heterogeneity: X2 = 84.34, p < 0.00001; I2 = 81%, Figure 4]. The included studies were stratified according to variables including DN models, intervention duration, dosage, and species. Better therapeutic effects were observed when studies used type 1 DN models (p = 0.000), intervention duration of ≥12 weeks (p = 0.000), and rats (p = 0.000), as well as when they used RSV in a low-dose group (p = 0.000) (Supplementary Table S2). Funnel plots showed asymmetry for the effects of RSV on BUN (Supplementary Figure S3), while the result of Egger’s test was statistically significant [intercept: −5.84, 95% CI (−8.09, −3.59); p = 0.019].
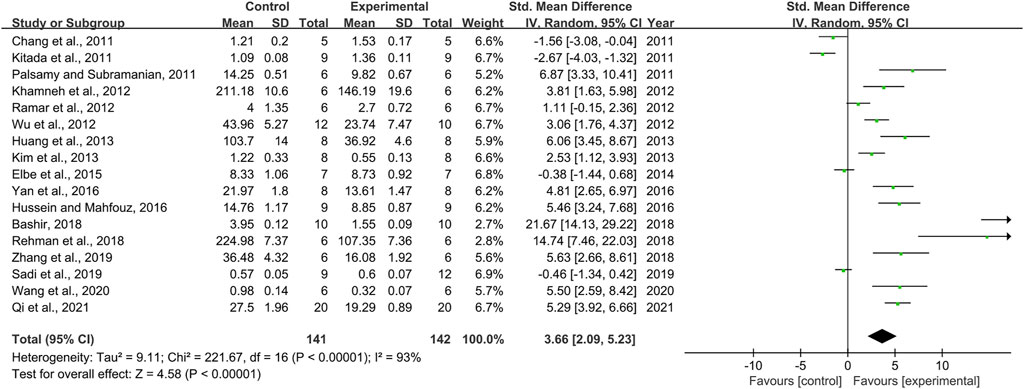
Effect of Resveratrol on Superoxide Dismutase
Seventeen pair-wise comparisons mentioned the influence of RSV on SOD. The pooled effect sizes indicated that RSV could significantly increase the level of SOD compared with a control group [n = 283, SMD = 3.66, 95% CI (2.09, 5.23), p < 0.00001; Heterogeneity: X2 = 221.67, p < 0.00001; I2 = 93%, Figure 5]. Subgroup analysis was conducted based on DN models, intervention duration, dosage, and species. More beneficial effects were demonstrated when studies used type 2 DN models (p = 0.002), intervention duration of ≥12 weeks (p = 0.000), and rats (p = 0.000), as well as studies that employed high dosage (p = 0.000) (Supplementary Table S2). Funnel plots showed asymmetry for the effects of RSV on SOD (Supplementary Figure S4), while the result of Egger’s test was statistically significant [intercept: 6.01, 95% CI (2.95, 9.08); p = 0.001].
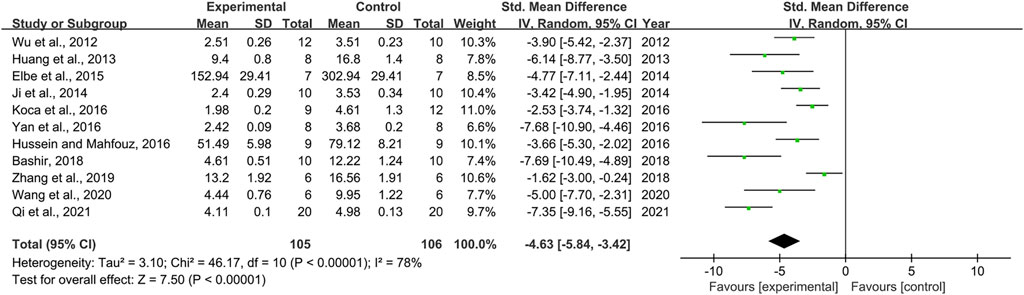
Effect of Resveratrol on Malondialdehyde
Effect sizes for MDA were pooled from a total of 11 pair-wise comparisons. There was a significant association of RSV with MDA level [n = 211, SMD = −4.63, 95% CI (−5.84, −3.42), p < 0.00001; Heterogeneity: X2 = 46.17, p < 0.00001; I2 = 78%, Figure 6]. The included studies were stratified according to variables including DN models, intervention duration, dosage, and species. More beneficial effects were found when studies used type 2 DN models (p = 0.000), intervention duration of <12 weeks (p = 0.000), and mice (p = 0.002), as well as when they used RSV in a high-dose group (p = 0.000) (Supplementary Table S2). Furthermore, funnel plots indicated asymmetry for the effects of RSV on MDA (Supplementary Figure S5), and the result of Egger’s test was statistically significant [intercept: −5.34, 95% CI (−8.63, −2.05); p = 0.005].
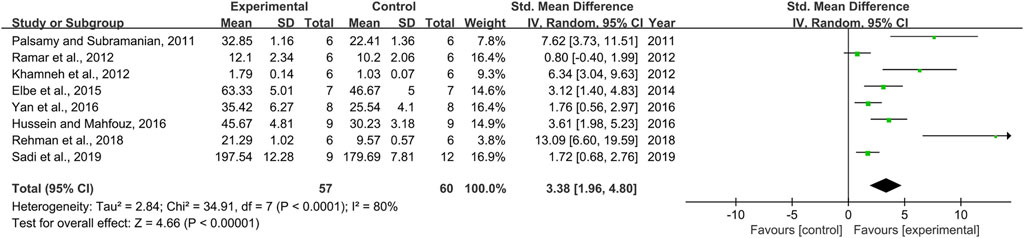
Effect of Resveratrol on Catalase
Effect sizes for CAT were pooled from a total of eight pair-wise comparisons. There was a significant association of RSV with CAT level [n = 117, SMD = 3.38, 95% CI (1.96, 4.80), p < 0.00001; Heterogeneity: X2 = 34.91, p < 0.0001; I2 = 80%, Figure 7]. Subgroup analysis was performed according to DN models, intervention duration, dosage, and species. More beneficial effects were observed when studies applied type 2 DN models (p = 0.000), intervention duration of <12 weeks (p = 0.000), and rats (p = 0.000), as well as studies that employed low dosage (p = 0.000) (Supplementary Table S2). However, publication bias was not conducted on CAT as less than 10 studies were included.
Effect of Resveratrol on Glutathione
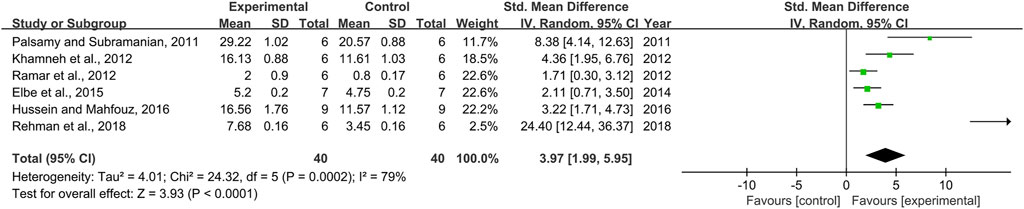
Six pair-wise comparisons mentioned the impact of RSV on GSH. The pooled effect sizes showed that RSV could significantly increase the GSH level compared with the control group [n = 80, SMD = 3.97, 95% CI (1.99, 5.95), p < 0.0001; Heterogeneity: X2 = 24.32, p = 0.0002; I2 = 79%, Figure 8]. Subgroup analysis was performed according to DN models, intervention duration, dosage, and species. More beneficial effects were observed when studies applied type 2 DN models (p = 0.000), intervention duration of ≥12 weeks (p = 0.000), and rats (p = 0.000), as well as when they used RSV in a low-dose group (p = 0.000) (Supplementary Table S2). Nevertheless, publication bias was not conducted on GSH as less than 10 studies were included.
Effect of Resveratrol on Glutathione Peroxidase
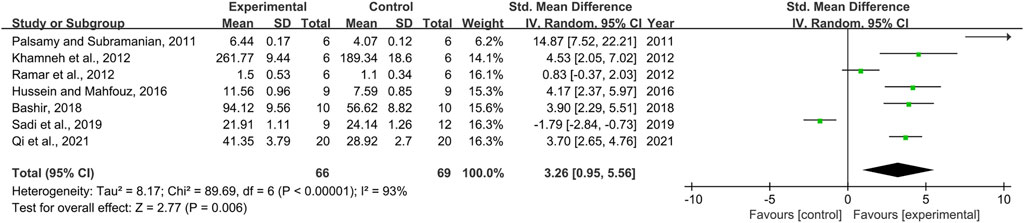
Seven pair-wise comparisons reported the impact of RSV on GPx. The pooled effect sizes showed that RSV could significantly increase the GPx level compared with control group [n = 135, SMD = 3.26, 95% CI (0.95, 5.56), p = 0.006; Heterogeneity: X2 = 89.69, p < 0.00001; I2 = 93%, Figure 9]. The included studies were stratified according to variables including DN models, intervention duration, dosage, and species. Better therapeutic effects were observed when studies used type 2 DN models (p = 0.000), intervention duration of ≥12 weeks (p = 0.000), and rats (p = 0.028), as well as when they used RSV in a low-dose group (p = 0.001) (Supplementary Table S2). However, publication bias was not conducted on GPx as less than 10 studies were included.
Effect of Resveratrol on TNF-α
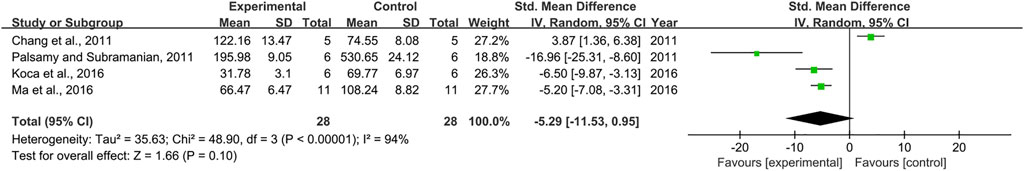
Combining effect sizes from four pair-wise comparisons, no significant decrease in the level of TNF-α was observed after RSV administration, compared to that in the control group [n = 56, SMD = −5.29, 95% CI (−11.53, 0.95), p = 0.1; Heterogeneity: X2 = 48.90, p < 0.00001; I2 = 94%, Figure 10]. Because meta-analysis indicated that there was no significant association of RSV with the level of TNF-α, subgroup analysis was not performed on TNF-α. In addition, publication bias was not conducted on TNF-α as less than 10 studies were included.
Effect of Resveratrol on IL-6
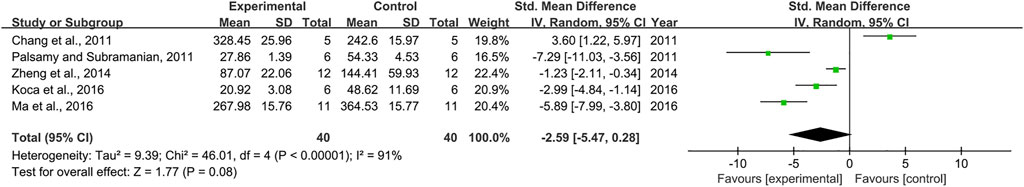
As for the effect on IL-6, 5 pair-wise comparisons mentioned the influence of RSV on this outcome. The pooled effect sizes showed that RSV did not significantly decrease IL-6 level compared with the control group [n = 80, SMD = −2.59, 95% CI (−5.47, 0.28), p = 0.08; Heterogeneity: X2 = 46.01, p < 0.00001; I2 = 91%, Figure 11]. Because meta-analysis indicated that there was no significant association of RSV with the level of IL-6, subgroup analysis was not performed on IL-6. Furthermore, publication bias was not conducted on IL-6 as less than 10 studies were included.
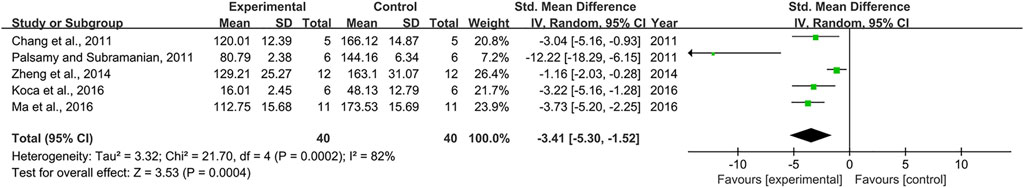
Effect of Resveratrol on IL-1β
Effect sizes for IL-1β were pooled from a total of 5 pair-wise comparisons. There was a significant association of RSV with IL-1β level (n = 80, SMD = −3.41, 95% CI (−5.30, −1.52), p = 0.0004; Heterogeneity: X2 = 21.70, p = 0.0002; I2 = 82%, Figure 12]. Subgroup analysis was conducted according to DN models, intervention duration, and dosage. More beneficial effects were observed when studies employed type 1 DN models (p = 0.000), intervention duration of <12 weeks (p = 0.004), as well as when they used RSV in a low-dose group (p = 0.001) (Supplementary Table S2). Subgroup analysis was not conducted according to species because all included studies applied rat models of DN. Publication bias was not performed on IL-6 as less than 10 studies were included.
Sensitivity Analysis
For BG, Scr, BUN, SOD, MDA, CAT, GSH, GPx, and IL-1β, the sensitivity analysis was conducted by removing one study at each stage, and the results indicated that no individual study significantly affected the pooled effect sizes.
Nevertheless, TNF-α and IL-6 were influenced in one study (Chang et al., 2011), and there was a significant association of RSV with TNF-α and IL-6 levels after removing this study [Before sensitivity analysis: TNF-α: SMD = −5.29, 95% CI (−11.53, 0.95), p = 0.1; IL-6: SMD = -2.59, 95% CI (−5.47, 0.28), p = 0.08. After sensitivity analysis: TNF-α: SMD = −7.64, 95% CI (−11.80, −3.48), p = 0.0003; IL-6: SMD = −4.03, 95% CI (−6.67, −1.38), p = 0.003].
Discussion
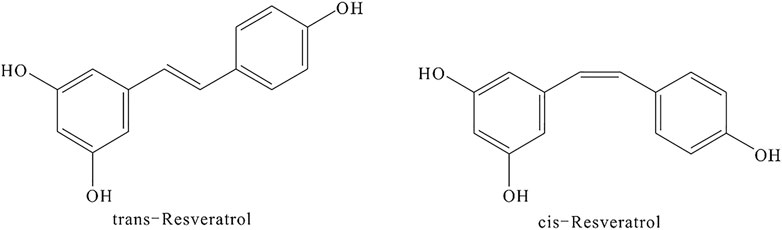
RSV is a phytoalexin phenolic metabolite produced in response to environmental stress (Gowd et al., 2020). Its chemical name is 3,4′,5-trihydroxy-trans-stilbene or 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol. RSV has a CAS registry number of 501-36-0, a molecular weight of 228.24, and an empirical formula of C14H12O3. The appearance of RSV is that of an off-white powder with a melting point of 254–257°C. It is soluble in water at 25°C and easily soluble in organic solvents such as ethyl acetate, ether, chloroform, methanol, ethanol, acetone, and ethyl acetate. RSV exists in two isoforms cis- and trans-resveratrol, and its chemical structure is shown in Figure 13.
The present systematic review and meta-analysis mainly intended to evaluate the anti- oxidant and anti-inflammatory properties of RSV when used in the treatment of DN. The results showed that RSV was significantly associated with a lower level of MDA and higher levels of SOD, CAT, GSH, and GPx. With regard to pro-inflammatory cytokines, RSV can significantly reduce the IL-1β level, but it did not effectively decrease the levels of TNF-α and IL-6. Current evidence supports the antioxidant and anti-inflammatory properties of RSV, but its relationship with some inflammatory mediators such as IL-6 and TNF-α in animals with diabetic nephropathy needs further elucidation.
Hyperglycemia is a predominant cause underlying the development and progression of DN (DeFronzo et al., 2021), and improving BG levels plays an important role in renal protection. However, whether RSV can decrease BG levels remains controversial. Recent studies indicated that RSV could reduce BG levels in animal models of DN (Xian et al., 2020; Qi et al., 2021). Other studies suggested that RSV had no significant hypoglycemic effect (Park et al., 2016; Yan et al., 2016). In this meta-analysis, compared to the control group, the level of BG was significantly decreased in RSV-treated groups. In addition, DN models might influence the hypoglycemic effect of RSV according to subgroup analysis. The efficacy of RSV on reducing BG was more significant in type 2 DN animal models, but the hypoglycemic effect of RSV in type 1 DN animal models was relatively diminished. Type 1 DN most commonly results from an autoimmune disorder in which pancreatic beta cells are destroyed, while type 2 DN is characterized by an insufficient response to circulatory insulin by peripheral tissues because of insulin resistance (Li et al., 2021). Hence, the insulin resistance is the primary difference between type 1 DN and type 2 DN. In a double-blind clinical trial, the insulin resistance and the level of insulin in type 2 diabetic patients were significantly improved by RSV treatment (Zare Javid et al., 2017). Another study also showed that RSV treatment can improve insulin sensitivity in type 2 diabetic patients (Brasnyó et al., 2011). Thus, the ability of RSV to improve insulin resistance may be the reason it has a more powerful hypoglycemic effect in type 2 DN.
Scr and BUN are the most important markers that reflect renal function status. Early reports found that RSV treatment could significantly reduce the levels of Scr and BUN in animal models of DN. However, previous studies also indicated that there was no significant difference in Scr between treatment and control groups (Peng et al., 2019). With regard to BUN, one study showed that RSV was not associated with a lower level of BUN (Xu et al., 2014). Therefore, conflicting actions of RSV in animal models of DN still exist in the current researches. Our meta-analysis suggested that RSV treatment was significantly associated with lower levels of Scr and BUN. This result indicated that RSV has a beneficial effect on DN animal models.
Dose-response effects and time-response effects play an important role in clinical medication. However, no animal studies reported the dose-response effects and time-response effects of RSV when used in the treatment of DN. In the meta-analysis reported here, with regard to BG, Scr, and BUN, the greatest effects were recorded in low-dose groups rather than in high-dose groups. Moreover, in a randomized, double-blind, placebo-controlled clinical trial, high-dose RSV had no significant influence on BG and insulin sensitivity in obese human subjects (Poulsen et al., 2013). According to these findings, variability in dosage of RSV may influence the efficacy, and low-dose RSV may be more suitable for the treatment of DN. Thus, it is necessary to notice that the dosage of RSV should be increased slowly starting from a low dosage in clinical use. Nevertheless, whether an excessive dose of RSV will suppress its therapeutic effects in the treatment of DN should be further investigated. For time-response effects, subgroup analysis indicated that intervention duration of RSV can influence the hypoglycemic effect, and more beneficial effects were observed when studies had a drug administration time of <12 weeks. However, there was no significant difference in the influence of RSV on Scr and BUN between intervention duration <12 weeks and intervention duration >12 weeks. Therefore, it is difficult to determine the appropriate intervention duration based on current evidence. According to the above findings, attention should be paid to the following two aspects in the future studies: first, both patients with type 1 DN and type 2 DN need to be included in clinical trials. During clinical trials, the levels of insulin and BG must be strictly monitored in order to determine which type of DN is more sensitive to RSV treatment. Second, the particular multi-arm clinical trials (two or more experimental intervention groups with a common control group) need to be conducted. In multi-arm clinical trials, multiple levels of dosage or intervention duration are required to evaluate the dose-response effects or time-response effects and determine the optimal dosage or intervention duration.
Oxidative stress is considered to be an etiologic factor in the pathogenesis of DN, and an imbalance between excess generation of pro-oxidants and antioxidants including SOD, CAT, GPx, and GSH is believed to play an important role in modulating kidney tissue damage such as renal tubular cell death, tubulointerstitial fibrosis, and glomerular mesangial expansion (Singh et al., 2011). SOD is a major antioxidant enzyme found in mitochondria and cytoplasm, and can change super-oxide into molecular oxygen and hydrogen peroxide. GPx utilizes GSH to detoxify hydrogen peroxide to lipid peroxides and water, along with serving as a peroxynitrite reductase (Yamaguchi et al., 1998). The increased generation of CAT in the proximal tubules of transgenic diabetic animals can mitigate tubular apoptosis and interstitial fibrosis (Brezniceanu et al., 2008). MDA is an unsaturated aldehyde produced by the oxidation of polyunsaturated fatty acids (Mandlik et al., 2021). SOD, CAT, GPx, GSH, and MDA are used in vivo as markers of oxidative stress. Previous studies found that RSV treatment could effectively mitigate renal dysfunction by recovering SOD, CAT, GPx, and GSH activity (Khamneh et al., 2012; Ramar et al., 2012). Consistent with the previous findings, in our meta-analysis the increased levels of SOD, CAT, GPx, and GSH were observed following RSV treatment. Recent work suggested that the MDA content in control group is significantly higher than that in RSV-treated group (Zhang et al., 2019; Wang et al., 2020). This result was also supported by our study. Interestingly enough, our subgroup analysis indicated that DN models may affect the antioxidant effects, the increased levels of SOD, CAT, GPx, and GSH and decreased MDA content were all observed when studies used models for type 2 DN. One point that should be considered in relation to this result is the better hypoglycemic effect of RSV in type 2 DN. Chronic hyperglycemia plays a major part in the occurrence of oxidative stress and impairment of the antioxidant response (Nishikawa et al., 2000), so the better hypoglycemic effect in type 2 DN might contribute to a higher antioxidant capacity of RSV in type 2 DN. These findings raise a question regarding the potential value of RSV as a promising candidate in the treatment of type 2 DN instead of type 1 DN. With regard to dose-response effects, the increased levels of SOD and GSH and decreased MDA level were recorded in high-dose groups. However, a higher level of CAT and GPx was observed in studies with low-dose groups. As for time-response effects, the increased SOD, GSH, and GPx levels were found when studies had intervention duration ≥12 weeks, but the decreased MDA content and enhanced CAT levels were observed in intervention duration <12 weeks. These contradictory results might be due to the small sample size in subgroup analysis. For example, only one study of GSH and GPx was included in the high-dose and intervention duration ≥12 weeks groups. Thus, the dose-response effects and time-response effects of RSV in antioxidant capacity in the treatment of DN should be further clarified through increasing sample size.
Pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 are critical mediators in the development and progression of DN. In animal models of DN, the renal levels of TNF-α, IL-1β, and IL-6 are significantly upregulated when compared with those in healthy animals (Koca et al., 2016; Ma et al., 2016). TNF-α is cytotoxic to glomerular mesangial and epithelial cells, and can lead directly to kidney tissue damage (Palsamy and Subramanian, 2011). The IL-1β level is related to increased permeability of vascular endothelial cells (Pérez-Morales et al., 2019). IL-6 can induce fibronectin expression, promote mesangial cell proliferation, and increase the permeability of endothelial cells (Navarro-González and Mora-Fernández, 2008). A previous study indicated that RSV obviously decreased the level of IL-1β but enhanced that of IL-6 and TNF-α in the DN animal models (Chang et al., 2011). Other studies suggested that RSV was significantly associated with a lower level of TNF-α, IL-1β, and IL-6 (Koca et al., 2016; Ma et al., 2016). Based on the above results, there is no consensus about the anti-inflammatory property of RSV in the treatment of DN. Our meta-analysis found that RSV did not decrease the levels of IL-6 and TNF-α but was significantly associated with a low level of IL-1β. Therefore, the anti-inflammatory effects of RSV still need to be confirmed in more studies.
The molecular mechanisms of antioxidant and anti-inflammatory properties of RSV in the treatment of DN may be related to multiple signaling pathways. Recent studies demonstrated that the mechanisms of RSV against oxidative stress were mainly through activating the silent information regulator T1 (SIRT1) signaling pathway to inhibit the production of reactive oxygen species (ROS), reduce the expression of the receptor for advanced glycation end products (RAGE), activate adenosine monophosphate-activated protein kinase (AMPK), inhibit the activation of nuclear factor kappa B (NF-κB), regulate NF-E2-related-factor 2-Kelch-like ECH associated protein 1 (Nrf2-Keap1) signaling pathway, and decrease the expression of glutathione S-transferase (Deng et al., 2018). In addition, one study found that RSV can upregulate the expression of 3-hydroxy-3-methylglutaryl coenzyme in the db/db mice kidney, which promotes the degradation of insulin-like growth factor 1 receptor protein (IGF-1R), thereby enhancing the activity of antioxidant enzymes (Yan et al., 2016). Forkhead box O3a (FoxO3a) plays a critical role in the regulation of oxidative stress. Recent studies indicated that RSV treatment can increase SIRT1 deacetylase activity, subsequently decreasing the expression of acetylated-FoxO3a and inhibiting the oxidative stress caused by hyperglycemia both in vitro and in vivo (Wang et al., 2017). Furthermore, several studies confirmed that Forkhead box O1 (FoxO1) is also associated with oxidative stress. With an increase in MDA levels and a decrease in SOD activity, the expression of FoxO1 was reduced. Nevertheless, FoxO1 expression was increased and SIRT1 deacetylase activity was enhanced after RSV treatment. These results suggest that RSV can reduce renal oxidative stress injury by regulating SIRT1/FoxO1 signaling pathway (Deng et al., 2018). Moreover, in the pathological state of hyperglycemia and hemodynamic disorder, a large number of renal macrophages are infiltrated, and multiple inflammatory cytokines such as IL-1β, TNF-α, and IL-6 are released. Among them, the activation of the NF-κB signal transduction pathway is considered to be an important intermediate link for many signal pathways. Several studies suggested that RSV can significantly reduce the expression of IL-6, IL-1β, intercellular cell adhesion molecule-1 (ICAM-1), and other inflammatory cytokines in the kidney tissue through modulating AMPK/NF-κB, Akt/NF-κB, phosphoinositide 3-kinases/protein kinase B (PI3K/Akt) signaling pathway, and the pancreatic insulin signal transduction pathway (Chang et al., 2011; Ojima et al., 2013; Xu et al., 2014; Sadi et al., 2015). In addition, RSV could inhibit the expression of inflammatory cytokines such as TNF-α and IL-6 by inhibiting the phosphorylation of extracellular signal-regulated kinase 1/2 (Chen and Wei, 2015).
Angiogenesis is the formation of new blood vessels from the pre-existing vascular system (Zent and Pozzi, 2007). The process of angiogenesis mainly consists of four events including detachment from basement membranes, proliferation and migration of endothelial cells, formation of endothelial tubes, and maturation of new vessels (Pandya et al., 2006). Under physiological conditions, these processes are regulated by pro-angiogenic and anti-angiogenic factors. However, increased expression of pro-angiogenic factors and decreased expression of anti-angiogenic factors within the glomerulus were observed during the occurrence and progression of DN. This leads to increased proliferation and migration of endothelial cells, resulting in the formation of immature and leaky vessels (Zent and Pozzi, 2007). One study reported that increased density in glomerular capillaries resulting from glomerular neovascularization and an increasing number of efferent arterioles at the glomerular vascular pole were seen in biopsy specimens of patients with type 1 diabetes and animal models of DN (Osterby et al., 1999; Guo et al., 2005). Among the pro-angiogenic factors, vascular endothelial growth factor (VEGF) is probably the most effective upregulated permeability factor in DN. VEGF is produced primarily by podocytes in the glomerulus, and high glucose can increase the synthesis of VEGF in cultured podocytes and tubules (Iglesias-de la Cruz et al., 2002; Kim et al., 2005). At the early stage of DN, VEGF was found to be higher than in normal mice, and VEGF mRNA was also significantly upregulated, and was positively correlated with the urinary microprotein excretion rate. Moreover, one previous study observed that several pathological changes similar to those of DN, such as proteinuria, glomerular injury, mesangial hyperplasia, and basal membrane thickening in mice with overexpression of VEGF, could be reversed when drug-induced overexpression of VEGF was inhibited (Jiang and Ding, 2017). It has been confirmed that administration of VEGF antibodies can ameliorate renal injury in diabetic mice (Flyvbjerg et al., 2002). Furthermore, a study of renal biopsy specimens from diabetic patients showed increased expression of VEGF and increased VEGF-receptor activation in mildly impaired glomerulus. This was accompanied by increased endothelial cell proliferation, suggesting that VEGF activation in mildly affected diabetic kidney might lead to increased glomerular angiogenesis (Hohenstein et al., 2006). Angiopoietins (Angs) are a family of vascular growth factors that regulate vascular remodeling, maturation, and stability. The Angs family includes Ang 1, Ang 2, Ang 3, and Ang 4, and they interact with tyrosine kinase receptors (Tie 1 and Tie 2) (Tao et al., 2021). Ang 1 and Ang 2 have opposite effects on endothelial cell function. Ang 1 can stabilize the adhesion of endothelial cells and promote the maturation of new capillaries by binding to the Tie 2 receptor. Ang 2 blocks Ang 1/Tie 2 signaling, which leads to increased angiogenesis, vascular instability, and subsequent leakage. In the kidneys, Ang 1 counteracts the effects of VEGF and increases the trans-endothelial resistance of cultured glomerular endothelial cells. In contrast, upregulation of Ang 2 was found in the mouse model of type 1 DN and mesangial cells exposed to high glucose levels (Yamamoto et al., 2004; Ichinose et al., 2005). According to the above findings, altering the activity of these mediators such as VEGF, Ang 1, and Ang 2 might be beneficial in preventing the progression of DN. One study showed that RSV blocked diabetes-induced increase of VEGF expression (Kim et al., 2012). However, another study did not find significant changes in the level of VEGF mRNA associated with vascular remodeling after RSV treatment (Yar et al., 2012). Therefore, results on anti-angiogenic effects of RSV are contradictory (Toro et al., 2019; Pietras-Baczewska et al., 2021). Recent work found that RSV could decrease the expression of VEGF and Ang 2, and enhance the expression of Tie 2 in the rat model of DN. However, no change in Ang 1 expression was detected (Wen et al., 2013). Based on these results, whether RSV can achieve anti-angiogenic effects by regulating anti-angiogenic factors and pro-angiogenic factors remains to be further studied.
Every medical intervention comes with the risk, great or small, of adverse effects. All clinicians should comprehensively consider the adverse aspects of interventions. A study of the immunotoxicity of RSV in male B6C3F1/N mice found that after 28 days of RSV treatment (0, 156, 312, 625, 1,250, and 2,500 mg/kg/day), the humoral, cell-mediated, and innate immune function were not changed (Huang et al., 2020). However, some studies reported that RSV treatment could result in DNA damage and reduce several DNA repair pathways, which can activate cytotoxic and apoptotic pathways (Shaito et al., 2020). A human trial indicated that RSV at a dosage of 1,000 mg/day or above could suppress cytochrome P450 isoenzymes including CYP2D6, CYP3A4, and CYP2C9 (Detampel et al., 2012). It has been reported that 450 mg/day of RSV is a safe dose for a 60-kg person (Wahab et al., 2017). In this meta-analysis, no study reported the occurrence of adverse effects. The reasons for this could be that the dosage and intervention duration of RSV were within a reasonable range, which may not be sufficient to produce adverse effects. Moreover, researchers did not report any adverse effects that occurred in the experiment. On the basis of the above findings, it is necessary to pay attention to the following two points in future research: first, the association between dose and intervention duration of RSV and adverse effects needs to be further studied. Second, some researchers did not report adverse effects, which would make people mistakenly believe that there were no adverse effects from this intervention. Hence, researchers should report in detail whether there are adverse effects in the experiments.
Several limitations should be considered in this systematic review and meta-analysis. First, a number of studies did not report baseline characteristics between experimental groups and control groups. Second, Egger’s test and asymmetry of funnel plots showed that publication bias existed, which could exaggerate the therapeutic effects of RSV. Therefore, the positive findings on RSV should be interpreted with caution. Third, some studies did not report a randomization process.
Conclusion
In this meta-analysis, RSV can exert its antioxidant effect by reducing the levels of MDA and restoring the activities of SOD, CAT, GSH, and GPx. With regard to pro-inflammatory cytokines, RSV had a positive effect on the reduction of IL-1β. However, the analysis indicated that RSV had no effect on IL-6 and TNF-α levels, probably because of the methodological quality of the studies and their heterogeneity. Current evidence supports the antioxidant and anti-inflammatory properties of RSV, but its relationship with the levels of some inflammatory mediators such as IL-6 and TNF-α in animals with DN needs further elucidation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
H-CH and X-QL designed the study. Y-HL searched databases. W-HZ and Y-HL collected the data. W-HZ and Y-HL assessed the quality of the studies. H-CH and X-QL performed all analysis. H-CH and X-QL wrote the manuscript. All authors contributed to this systematic review and meta-analysis.
Funding
This work was supported by Chongqing Natural Science Foundation (No. cstc2020jcyj-msxmX0985).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.841818/full#supplementary-material
References
Al-Hussaini, H., and Kilarkaje, N. (2018). Trans-resveratrol Mitigates Type 1 Diabetes-Induced Oxidative DNA Damage and Accumulation of Advanced Glycation End Products in Glomeruli and Tubules of Rat Kidneys. Toxicol. Appl. Pharmacol. 339, 97–109. doi:10.1016/j.taap.2017.11.025
Alicic, R. Z., Cox, E. J., Neumiller, J. J., and Tuttle, K. R. (2021). Incretin Drugs in Diabetic Kidney Disease: Biological Mechanisms and Clinical Evidence. Nat. Rev. Nephrol. 17, 227–244. doi:10.1038/s41581-020-00367-2
Barman, S., Pradeep, S. R., and Srinivasan, K. (2018). Zinc Supplementation Alleviates the Progression of Diabetic Nephropathy by Inhibiting the Overexpression of Oxidative-Stress-Mediated Molecular Markers in Streptozotocin-Induced Experimental Rats. J. Nutr. Biochem. 54, 113–129. doi:10.1016/j.jnutbio.2017.11.008
Bashir, S. O. (2019). Concomitant Administration of Resveratrol and Insulin Protects against Diabetes Mellitus Type-1-Induced Renal Damage and Impaired Function via an Antioxidant-Mediated Mechanism and Up-Regulation of Na+/K+-ATPase. Arch. Physiol. Biochem. 125, 104–113. doi:10.1080/13813455.2018.1437752
Brasnyó, P., Molnár, G. A., Mohás, M., Markó, L., Laczy, B., Cseh, J., et al. (2011). Resveratrol Improves Insulin Sensitivity, Reduces Oxidative Stress and Activates the Akt Pathway in Type 2 Diabetic Patients. Br. J. Nutr. 106, 383–389. doi:10.1017/S0007114511000316
Brezniceanu, M. L., Liu, F., Wei, C. C., Chénier, I., Godin, N., Zhang, S. L., et al. (2008). Attenuation of Interstitial Fibrosis and Tubular Apoptosis in Db/db Transgenic Mice Overexpressing Catalase in Renal Proximal Tubular Cells. Diabetes 57, 451–459. doi:10.2337/db07-0013
Chang, C. C., Chang, C. Y., Wu, Y. T., Huang, J. P., Yen, T. H., and Hung, L. M. (2011). Resveratrol Retards Progression of Diabetic Nephropathy through Modulations of Oxidative Stress, Proinflammatory Cytokines, and AMP-Activated Protein Kinase. J. Biomed. Sci. 18, 47. doi:10.1186/1423-0127-18-47
Chen, K. H., Hung, C. C., Hsu, H. H., Jing, Y. H., Yang, C. W., and Chen, J. K. (2011). Resveratrol Ameliorates Early Diabetic Nephropathy Associated with Suppression of Augmented TGF-Β/smad and ERK1/2 Signaling in Streptozotocin-Induced Diabetic Rats. Chem. Biol. Interact 190, 45–53. doi:10.1016/j.cbi.2011.01.033
Chen, Y., and Wei, J. P. (2015). Research Progress of Resveratrol in Treatment of Diabetic Nephropathy. Med. Recapitulate 21, 3545–3547. doi:10.3969/j.issn.100.6-2084.2015.19.033
DeFronzo, R. A., Reeves, W. B., and Awad, A. S. (2021). Pathophysiology of Diabetic Kidney Disease: Impact of SGLT2 Inhibitors. Nat. Rev. Nephrol. 17, 319–334. doi:10.1038/s41581-021-00393-8
Deng, Y. H., Wu, F. Z., Li, Q., and Chen, W. Y. (2018). Research Progress on Molecular Mechanism of Resveratrol against Diabetic Nephropathy. Pharmacol. Clin. Chin. Materia Med. 34, 186–190. doi:10.13412/j.cnki.zyyl.2018.01.047
Detampel, P., Beck, M., Krähenbühl, S., and Huwyler, J. (2012). Drug Interaction Potential of Resveratrol. Drug Metab. Rev. 44, 253–265. doi:10.3109/03602532.2012.700715
Ding, D. F., You, N., Wu, X. M., Xu, J. R., Hu, A. P., Ye, X. L. L., et al. (2010). Resveratrol Attenuates Renal Hypertrophy in Early-Stage Diabetes by Activating AMPK. Am. J. Nephrol. 31, 363–374. doi:10.1159/000300388
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 315, 629–634. doi:10.1136/bmj,bmj.315.7109.62910.1136/bmj.315.7109.629
Elbe, H., Vardiİ, N., Ates, B., Yologlu, S., Taskapan, C., and Taskapan, C. (2015). Amelioration of Streptozotocin-Induced Diabetic Nephropathy by Melatonin, Quercetin, and Resveratrol in Rats. Hum. Exp. Toxicol. 34, 100–113. doi:10.1177/0960327114531995
Fernandez-Fernandez, B., Ortiz, A., Gomez-Guerrero, C., and Egido, J. (2014). Therapeutic Approaches to Diabetic Nephropathy-Bbeyond the RAS. Nat. Rev. Nephrol. 10, 325–346. doi:10.1038/nrneph.2014.74
Flyvbjerg, A., Dagnaes-Hansen, F., De Vriese, A. S., Tilton, R. G., Rasch, R., and Rasch, R. (2002). Amelioration of Long-Term Renal Changes in Obese Type 2 Diabetic Mice by a Neutralizing Vascular Endothelial Growth Factor Antibody. Diabetes 51, 3090–3094. doi:10.2337/diabetes.51.10.3090
Gowd, V., Kang, Q., Wang, Q., Wang, Q., Chen, F., and Cheng, K. W. (2020). Resveratrol: Evidence for its Nephroprotective Effect in Diabetic Nephropathy. Adv. Nutr. 11, 1555–1568. doi:10.1093/advances/nmaa075
Guo, M., Ricardo, S. D., Deane, J. A., Shi, M., Cullen-McEwen, L., and Bertram, J. F. (2005). A Stereological Study of the Renal Glomerular Vasculature in the Db/db Mouse Model of Diabetic Nephropathy. J. Anat. 207, 813–821. doi:10.1111/j.1469-7580.2005.00492.x
He, T., Xiong, J., Nie, L., Yu, Y., Guan, X., Xu, X., et al. (2016). Resveratrol Inhibits Renal Interstitial Fibrosis in Diabetic Nephropathy by Regulating AMPK/NOX4/ROS Pathway. J. Mol. Med. (Berl) 94, 1359–1371. doi:10.1007/s00109-016-1451-y
Higgins, J. P. T., and Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). Cochrane Collaboration website. Available: http://training.cochrane.org/handbook (Accessed November 20, 2021).
Hohenstein, B., Hausknecht, B., Boehmer, K., Riess, R., Brekken, R. A., and Hugo, C. P. (2006). Local VEGF Activity but Not VEGF Expression Is Tightly Regulated during Diabetic Nephropathy in Man. Kidney Int. 69, 1654–1661. doi:10.1038/sj.ki.5000294
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Ritskes-Hoitinga, M., Langendam, M. W., and Langendam, M. W. (2014). SYRCLE's Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 14, 43. doi:10.1186/1471-2288-14-43
Huang, K., Huang, J., Xie, X., Wang, S., Chen, C., Shen, X., et al. (2013). Sirt1 Resists Advanced Glycation End Products-Induced Expressions of Fibronectin and TGF-Β1 by Activating the Nrf2/ARE Pathway in Glomerular Mesangial Cells. Free Radic. Biol. Med. 65, 528–540. doi:10.1016/j.freeradbiomed.2013.07.029
Huang, M. C., White, K. L., Elmore, S. A., Guo, T. L., and Germolec, D. (2020). Immunotoxicity Studies of Trans-resveratrol in Male B6C3F1/N Mice. J. Immunotoxicol 17, 194–201. doi:10.1080/1547691X.2020.1833113
Hussein, M. M., and Mahfouz, M. K. (2016). Effect of Resveratrol and Rosuvastatin on Experimental Diabetic Nephropathy in Rats. Biomed. Pharmacother. 82, 685–692. doi:10.1016/j.biopha.2016.06.004
Ichinose, K., Maeshima, Y., Yamamoto, Y., Kitayama, H., Takazawa, Y., Hirokoshi, K., et al. (2005). Antiangiogenic Endostatin Peptide Ameliorates Renal Alterations in the Early Stage of a Type 1 Diabetic Nephropathy Model. Diabetes 54, 2891–2903. doi:10.2337/diabetes.54.10.2891
Iglesias-de la Cruz, M. C., Ziyadeh, F. N., Isono, M., Kouahou, M., Han, D. C., Kalluri, R., et al. (2002). Effects of High Glucose and TGF-Beta1 on the Expression of Collagen IV and Vascular Endothelial Growth Factor in Mouse Podocytes. Kidney Int. 62, 901–913. doi:10.1046/j.1523-1755.2002.00528.x
Ji, H., Wu, L., Ma, X., Ma, X., and Qin, G. (2014). The Effect of Resveratrol on the Expression of AdipoR1 in Kidneys of Diabetic Nephropathy. Mol. Biol. Rep. 41, 2151–2159. doi:10.1007/s11033-014-3064-2
Jiang, B., Guo, L., Li, B. Y., Zhen, J. H., Song, J., Peng, T., et al. (2013). Resveratrol Attenuates Early Diabetic Nephropathy by Down-Regulating Glutathione S-Transferases Mu in Diabetic Rats. J. Med. Food 16, 481–486. doi:10.1089/jmf.2012.2686
Jiang, H., and Ding, G. H. (2017). Research Progress in the Regulation of VEGF in Kidney. J. Med. Res. 46, 182–185. doi:10.11969/j.issn.1673-548X.2017.11.044
Khamneh, S., Ghadiri Soufi, F., and Afshar, F. (2012). Long-term Resveratrol Administration Reduces Renal Oxidative Stress and Apoptosis Rate in Experimental Model of Type 2 Diabetes. Life Sci. J. 9, 2997–3001.
Kim, M. Y., Lim, J. H., Youn, H. H., Hong, Y. A., Yang, K. S., Park, H. S., et al. (2013). Resveratrol Prevents Renal Lipotoxicity and Inhibits Mesangial Cell Glucotoxicity in a Manner Dependent on the AMPK-SIRT1-Pgc1α axis in Db/db Mice. Diabetologia 56, 204–217. doi:10.1007/s00125-012-2747-2
Kim, N. H., Oh, J. H., Seo, J. A., Lee, K. W., Kim, S. G., Choi, K. M., et al. (2005). Vascular Endothelial Growth Factor (VEGF) and Soluble VEGF Receptor FLT-1 in Diabetic Nephropathy. Kidney Int. 67, 167–177. doi:10.1111/j.1523-1755.2005.00067.x
Kim, Y. H., Kim, Y. S., Roh, G. S., Choi, W. S., and Cho, G. J. (2012). Resveratrol Blocks Diabetes-Induced Early Vascular Lesions and Vascular Endothelial Growth Factor Induction in Mouse Retinas. Acta Ophthalmol. 90, e31–7. doi:10.1111/j.1755-3768.2011.02243.x
Kitada, M., Kume, S., Imaizumi, N., and Koya, D. (2011). Resveratrol Improves Oxidative Stress and Protects against Diabetic Nephropathy through Normalization of Mn-SOD Dysfunction in AMPK/SIRT1-independent Pathway. Diabetes 60, 634–643. doi:10.2337/db10-0386
Koca, H., Pektas, M., Koca, S., Pektas, G., and Sadi, G. (2016). Diabetes-induced Renal Failure Is Associated with Tissue Inflammation and Neutrophil Gelatinase-Associated Lipocalin: Effects of Resveratrol. Arch. Biol. Sci. Belgra 68, 747–752. doi:10.2298/ABS151105031K
Li, K. X., Ji, M. J., and Sun, H. J. (2021). An Updated Pharmacological Insight of Resveratrol in the Treatment of Diabetic Nephropathy. Gene 780, 145532. doi:10.1016/j.gene.2021.145532
Lytvyn, Y., Bjornstad, P., van Raalte, D. H., Cherney, D. Z. I., and Cherney, D. Z. I. (2020). The New Biology of Diabetic Kidney Disease-Mechanisms and Therapeutic Implications. Endocr. Rev. 41. doi:10.1210/endrev/bnz010
Ma, L., Fu, R., Duan, Z., Lu, J., Gao, J., Tian, L., et al. (2016). Sirt1 Is Essential for Resveratrol Enhancement of Hypoxia-Induced Autophagy in the Type 2 Diabetic Nephropathy Rat. Pathol. Res. Pract. 212, 310–318. doi:10.1016/j.prp.2016.02.001
Mandlik, D. S., Mandlik, S. K., and Patel, S. (2021). Protective Effect of Sarsasapogenin in TNBS Induced Ulcerative Colitis in Rats Associated with Downregulation of Pro-inflammatory Mediators and Oxidative Stress. Immunopharmacology and Immunotoxicology 43, 571–583. doi:10.1080/08923973.2021.1955919
Markus, M. R. P., Ittermann, T., Baumeister, S. E., Huth, C., Thorand, B., Herder, C., et al. (2018). Prediabetes Is Associated with Microalbuminuria, Reduced Kidney Function and Chronic Kidney Disease in the General Population: The KORA (Cooperative Health Research in the Augsburg Region) F4-Study. Nutr. Metab. Cardiovasc. Dis. 28, 234–242. doi:10.1016/j.numecd.2017.12.005
Meresman, G. F., Götte, M., and Laschke, M. W. (2021). Plants as Source of New Therapies for Endometriosis: a Review of Preclinical and Clinical Studies. Hum. Reprod. Update 27, 367–392. doi:10.1093/humupd/dmaa039
Navarro-González, J. F., and Mora-Fernández, C. (2008). The Role of Inflammatory Cytokines in Diabetic Nephropathy. Jasn 19, 433–442. doi:10.1681/ASN.2007091048
Nishikawa, T., Edelstein, D., Du, X. L., Yamagishi, S., Matsumura, T., Kaneda, Y., et al. (2000). Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature 404, 787–790. doi:10.1038/35008121
Ojima, A., Ishibashi, Y., Matsui, T., Maeda, S., Nishino, Y., Takeuchi, M., et al. (2013). Glucagon-like Peptide-1 Receptor Agonist Inhibits Asymmetric Dimethylarginine Generation in the Kidney of Streptozotocin-Induced Diabetic Rats by Blocking Advanced Glycation End Product-Induced Protein Arginine Methyltranferase-1 Expression. Am. J. Pathol. 182, 132–141. doi:10.1016/j.ajpath.2012.09.016
Osterby, R., Asplund, J., Bangstad, H. J., Nyberg, G., Rudberg, S., Viberti, G. C., et al. (1999). Neovascularization at the Vascular Pole Region in Diabetic Glomerulopathy. Nephrol. Dial. Transpl. 14, 348–352. doi:10.1093/ndt/14.2.348
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 372, n160. doi:10.1136/bmj.n160
Palsamy, P., and Subramanian, S. (2011). Resveratrol Protects Diabetic Kidney by Attenuating Hyperglycemia-Mediated Oxidative Stress and Renal Inflammatory Cytokines via Nrf2-Keap1 Signaling. Biochim. Biophys. Acta 1812, 719–731. doi:10.1016/j.bbadis.2011.03.008
Pandya, N. M., Dhalla, N. S., and Santani, D. D. (2006). Angiogenesis--a New Target for Future Therapy. Vascul Pharmacol. 44, 265–274. doi:10.1016/j.vph.2006.01.005
Park, H. S., Lim, J. H., Kim, M. Y., Kim, Y., Hong, Y. A., Choi, S. R., et al. (2016). Resveratrol Increases AdipoR1 and AdipoR2 Expression in Type 2 Diabetic Nephropathy. J. Transl Med. 14, 176. doi:10.1186/s12967-016-0922-9
Peng, W., Qin, R., Li, X., and Zhou, H. (2013). Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review. J. Ethnopharmacol 148, 729–745. doi:10.1016/j.jep.2013.05.007
Peng, X., Su, H., Liang, D., Li, J., Ting, W. J., Liao, S. C., et al. (2019). Ramipril and Resveratrol Co-treatment Attenuates RhoA/ROCK Pathway-Regulated Early-Stage Diabetic Nephropathy-Associated Glomerulosclerosis in Streptozotocin-Induced Diabetic Rats. Environ. Toxicol. 34, 861–868. doi:10.1002/tox.22758
Pérez-Morales, R. E., del Pino, M. D., Ortiz, A., Mora-Fernández, C., Navarro-González, J. F., and Navarro-González, J. F. (2019). Inflammation in Diabetic Kidney Disease. Nephron 143, 12–16. doi:10.1159/000493278
Pietras-Baczewska, A., Nowomiejska, K., Brzozowska, A., Toro, M. D., Załuska, W., Sztanke, M., et al. (2021). Antioxidant Status in the Vitreous of Eyes with Rhegmatogenous Retinal Detachment with and without Proliferative Vitreoretinopathy, Macular Hole and Epiretinal Membrane. Life (Basel) 11, 453. doi:10.3390/life11050453
Poulsen, M. M., Vestergaard, P. F., Clasen, B. F., Radko, Y., Christensen, L. P., Stødkilde-Jørgensen, H., et al. (2013). High-dose Resveratrol Supplementation in Obese Men: an Investigator-Initiated, Randomized, Placebo-Controlled Clinical Trial of Substrate Metabolism, Insulin Sensitivity, and Body Composition. Diabetes 62, 1186–1195. doi:10.2337/db12-0975
Qi, M., Liang, X., Lu, J., Zhao, H., and Jin, M. (2021). Effect of Resveratrol Intervention on Renal Pathological Injury and Spermatogenesis in Type 2 Diabetic Mice. Am. J. Transl Res. 13, 4719–4725.
Qiao, Y., Gao, K., Wang, Y., Wang, X., and Cui, B. (2017). Resveratrol Ameliorates Diabetic Nephropathy in Rats through Negative Regulation of the P38 MAPK/TGF-β1 Pathway. Exp. Ther. Med. 13, 3223–3230. doi:10.3892/etm.2017.4420
Ramar, M., Manikandan, B., Raman, T., Priyadarsini, A., Palanisamy, S., Velayudam, M., et al. (2012). Protective Effect of Ferulic Acid and Resveratrol against Alloxan-Induced Diabetes in Mice. Eur. J. Pharmacol. 690, 226–235. doi:10.1016/j.ejphar.2012.05.019
Rehman, K., Saeed, K., Munawar, S. M., and Akash, M. S. H. (2018). Resveratrol Regulates Hyperglycemia-Induced Modulations in Experimental Diabetic Animal Model. Biomed. Pharmacother. 102, 140–146. doi:10.1016/j.biopha.2018.03.050
Sadi, G., Pektaş, M. B., Koca, H. B., Tosun, M., and Koca, T. (2015). Resveratrol Improves Hepatic Insulin Signaling and Reduces the Inflammatory Response in Streptozotocin-Induced Diabetes. Gene 570, 213–220. doi:10.1016/j.gene.2015.06.010.1016/j.gene.2015.06.019 19
Sadi, G., Şahin, G., and Bostanci, A. (2019). Modulation of Renal Insulin Signaling Pathway and Antioxidant Enzymes with Streptozotocin-Induced Diabetes: Effects of Resveratrol. Medicina 55, 3. doi:10.3390/medicina55010003
Shaito, A., Posadino, A. M., Younes, N., Hasan, H., Halabi, S., Alhababi, D., et al. (2020). Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 21, 2084. doi:10.3390/ijms21062084
Singh, D. K., Winocour, P., and Farrington, K. (2011). Oxidative Stress in Early Diabetic Nephropathy: Fueling the Fire. Nat. Rev. Endocrinol. 7, 176–184. doi:10.1038/nrendo.2010.212
Tao, Q. R., Chu, Y. M., Wei, L., Tu, C., and Han, Y. Y. (2021). Antiangiogenic Therapy in Diabetic Nephropathy: A Double-edged S-word (Review). Mol. Med. Rep. 23, 260. doi:10.3892/mmr.2021.11899
Toro, M. D., Nowomiejska, K., Avitabile, T., Rejdak, R., Tripodi, S., Porta, A., et al. (2019). Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: a Systematic Review. Int. J. Mol. Sci. 20, 3503. doi:10.3390/ijms20143503
Wahab, A., Gao, K., Jia, C., Zhang, F., Tian, G., Murtaza, G., et al. (2017). Significance of Resveratrol in Clinical Management of Chronic Diseases. Molecules 22, 1329. doi:10.3390/molecules22081329
Wang, C., Chi, J., Che, K., Ma, X., Qiu, M., Wang, Z., et al. (2019). The Combined Effect of Mesenchymal Stem Cells and Resveratrol on Type 1 Diabetic Neuropathy. Exp. Ther. Med. 17, 3555–3563. doi:10.3892/etm.2019.73810.3892/etm.2019.7383
Wang, F., Li, R., Zhao, L., Ma, S., and Qin, G. (2020). Resveratrol Ameliorates Renal Damage by Inhibiting Oxidative Stress-Mediated Apoptosis of Podocytes in Diabetic Nephropathy. Eur. J. Pharmacol. 885, 173387. doi:10.1016/j.ejphar.2020.173387
Wang, X., Meng, L., Zhao, L., Wang, Z., Liu, H., Liu, G., et al. (2017). Resveratrol Ameliorates Hyperglycemia-Induced Renal Tubular Oxidative Stress Damage via Modulating the SIRT1/FOXO3a Pathway. Diabetes Res. Clin. Pract. 126, 172–181. doi:10.1016/j.diabres.2016.12.005
Wen, D., Huang, X., Zhang, M., Zhang, L., Chen, J., Gu, Y., et al. (2013). Resveratrol Attenuates Diabetic Nephropathy via Modulating Angiogenesis. PloS one 8, e82336. doi:10.1371/journal.pone.0082336
Wu, L., Zhang, Y., Ma, X., Zhang, N., and Qin, G. (2012). The Effect of Resveratrol on FoxO1 Expression in Kidneys of Diabetic Nephropathy Rats. Mol. Biol. Rep. 39, 9085–9093. doi:10.1007/s11033-012-1780-z
Xian, Y., Gao, Y., Lv, W., Ma, X., Hu, J., Chi, J., et al. (2020). Resveratrol Prevents Diabetic Nephropathy by Reducing Chronic Inflammation and Improving the Blood Glucose Memory Effect in Non-obese Diabetic Mice. Naunyn Schmiedebergs Arch. Pharmacol. 393, 2009–2017. doi:10.1007/s00210-019-01777-1
Xian, Y., Lin, Y., Cao, C., Li, L., Wang, J., Niu, J., et al. (2019). Protective Effect of Umbilical Cord Mesenchymal Stem Cells Combined with Resveratrol against Renal Podocyte Damage in NOD Mice. Diabetes Res. Clin. Pract. 156, 107755. doi:10.1016/j.diabres.2019.05.034
Xu, F., Wang, Y., Cui, W., Yuan, H., Sun, J., Wu, M., et al. (2014). Resveratrol Prevention of Diabetic Nephropathy Is Associated with the Suppression of Renal Inflammation and Mesangial Cell Proliferation: Possible Roles of Akt/NF-Κb Pathway. Int. J. Endocrinol. 2014, 289327. doi:10.1155/2014/10.1155/2014/289327 289327
Xu, X. H., Ding, D. F., Yong, H. J., Dong, C. L., You, N., Ye, X. L., et al. (2017). Resveratrol Transcriptionally Regulates miRNA-18a-5p Expression Ameliorating Diabetic Nephropathy via Increasing Autophagy. Eur. Rev. Med. Pharmacol. Sci. 21, 4952–4965.
Yamaguchi, T., Sano, K., Takakura, K., Saito, I., Shinohara, Y., Asano, T., et al. (1998). Ebselen in Acute Ischemic Stroke: a Placebo-Controlled, Double-Blind Clinical Trial. Ebselen Study Group. Stroke 29, 12–17. doi:10.1161/01.str.29.1.12
Yamamoto, Y., Maeshima, Y., Kitayama, H., Kitamura, S., Takazawa, Y., Sugiyama, H., et al. (2004). Tumstatin Peptide, an Inhibitor of Angiogenesis, Prevents Glomerular Hypertrophy in the Early Stage of Diabetic Nephropathy. Diabetes 53, 1831–1840. doi:10.2337/diabetes.53.7.1831
Yan, C., Xu, W., Huang, Y., Li, M., Shen, Y., You, H., et al. (2016). HRD1-mediated IGF-1R Ubiquitination Contributes to Renal protection of Resveratrol in Db/db Mice. Mol. Endocrinol. 30, 600–613. doi:10.1210/me.2015-1277
Yar, A. S., Menevse, S., Dogan, I., Alp, E., Ergin, V., Cumaoglu, A., et al. (2012). Investigation of Ocular Neovascularization-Related Genes and Oxidative Stress in Diabetic Rat Eye Tissues after Resveratrol Treatment. J. Med. Food 15, 391–398. doi:10.1089/jmf.2011.0135
Zare Javid, A., Hormoznejad, R., Yousefimanesh, H. A., Zakerkish, M., Haghighi-Zadeh, M. H., Dehghan, P., et al. (2017). The Impact of Resveratrol Supplementation on Blood Glucose, Insulin, Insulin Resistance, Triglyceride, and Periodontal Markers in Type 2 Diabetic Patients with Chronic Periodontitis. Phytother Res. 31, 108–114. doi:10.1002/ptr.5737
Zent, R., and Pozzi, A. (2007). Angiogenesis in Diabetic Nephropathy. Semin. Nephrol. 27, 161–171. doi:10.1016/j.semnephrol.2007.01.007
Zhang, T., Chi, Y., Kang, Y., Lu, H., Niu, H., Liu, W., et al. (2019). Resveratrol Ameliorates Podocyte Damage in Diabetic Mice via SIRT1/PGC-1α Mediated Attenuation of Mitochondrial Oxidative Stress. J. Cel Physiol 234, 5033–5043. doi:10.1002/jcp.27306
Zhang, Y. T., Huang, X., Chen, Y. Z., Li, J. D., and Yu, K. (2020). Chemical Constituents and Their Biosynthesis Mechanisms of. Polygonum Cuspidatum. China J. Chin. Materia Med. 45, 4364–4372.
Zheng, X. Y., Zhu, S. Y., Zhou, Y. D., Cao, Y. N., Dong, J., and Li, J. (2014). Amelioration of Diabetic Nephropathy in Rats by Resveratrol: Involvement of Anti -inflammatory Effect. Chin. Pharm. J. 49, 919–924. doi:10.11669/cpj.2014.11.003
Keywords: resveratrol, diabetic nephropathy, plant-derived agents, anti-inflammatory, antioxidant, meta-analysis
Citation: Hu H-C, Lei Y-H, Zhang W-H and Luo X-Q (2022) Antioxidant and Anti-inflammatory Properties of Resveratrol in Diabetic Nephropathy: A Systematic Review and Meta-analysis of Animal Studies. Front. Pharmacol. 13:841818. doi: 10.3389/fphar.2022.841818
Received: 22 December 2021; Accepted: 18 February 2022;
Published: 09 March 2022.
Edited by:
Thomas Brendler, PlantaPhile®, United StatesReviewed by:
Mario Damiano Toro, Medical University of Lublin, PolandVíctor Manuel Mendoza-Núñez, Universidad Nacional Autónoma de México, Mexico
Copyright © 2022 Hu, Lei, Zhang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Qiong Luo, bHhxY3F0Y21AMTYzLmNvbQ==
 Heng-Chang Hu
Heng-Chang Hu Yuan-Hong Lei
Yuan-Hong Lei Wei-Hua Zhang
Wei-Hua Zhang Xiao-Qiong Luo
Xiao-Qiong Luo