
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 18 February 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.840695
 Limin Liao1,2,3*†
Limin Liao1,2,3*† Qinggang Liu1,2,3†
Qinggang Liu1,2,3† Huiling Cong1,2
Huiling Cong1,2 Zhihui Xu4
Zhihui Xu4 Enhui Li4
Enhui Li4 Zhiliang Weng5
Zhiliang Weng5 Haihong Jiang5
Haihong Jiang5 Ben Liu6
Ben Liu6 Xiao Huang6
Xiao Huang6 Shujie Xia7
Shujie Xia7 Wei Wen7
Wei Wen7 Juan Wu1,2
Juan Wu1,2 Guowei Shi8
Guowei Shi8 Yang Wang8
Yang Wang8 Peijun Li9
Peijun Li9 Yang Yu9
Yang Yu9 Zujun Fang10
Zujun Fang10 Jie Zheng10
Jie Zheng10 Ye Tian11
Ye Tian11 Donghao Shang11
Donghao Shang11 Hanzhong Li12
Hanzhong Li12 Zhongming Huang12
Zhongming Huang12 Liqun Zhou13
Liqun Zhou13 Yunxiang Xiao13
Yunxiang Xiao13 Yaoguang Zhang14
Yaoguang Zhang14 Jianlong Wang14
Jianlong Wang14 Xiaodong Zhang15
Xiaodong Zhang15 Peng Zhang15
Peng Zhang15 Dongwen Wang16
Dongwen Wang16 Xuhui Zhang16
Xuhui Zhang16 Keji Xie17
Keji Xie17 Bin Wang17
Bin Wang17 Lulin Ma18
Lulin Ma18 Xiaojun Tian18
Xiaojun Tian18 Lijun Chen19
Lijun Chen19 Jinkai Dong19
Jinkai Dong19Objective: To evaluate the efficacy and safety of Hengli® Chinese botulinum toxin type A (BTX-A; 100 U) in Chinese patients with overactive bladder.
Methods: This study was a multicenter, randomized, double-blind, placebo-controlled trial in Chinese patients who were inadequately managed with anticholinergic medications. Eligible patients were randomized 2:1 to receive intradetrusor injections of Hengli® BTX-A (n = 144) or placebo (n = 72). The primary endpoint was the change in the number of daily micturition episodes at week 6 from baseline. The secondary efficacy endpoints included the average frequency of urgency and urinary incontinence (UI) episodes per day, urgency score, average micturition volume per day, OABSS, and QoL score.
Results: In the Hengli® BTX-A group, there was a significantly greater reduction in the average number of micturition episodes per 24 h compared with the placebo group (3.28 vs. 1.43; p = 0.003). Moreover, there was a significantly greater improvement in the daily number of urgency episodes, micturition volume and OABSS score. An increased post-void residual urine volume, dysuria, and urinary tract infection represented adverse events (AEs) in the Hengli® BTX-A group. Most AEs were mild or moderate in severity. One patient in the BTX-A group initiated clean intermittent catheterization (CIC) during treatment.
Conclusion: Hengli® BTX-A treatment was well-tolerated and resulted in significant improvements in OAB symptoms among Chinese patients inadequately managed by anticholinergics.
Clinical Trial Registration: http://www.chinadrugtrials.org.cn/clinicaltrials.prosearch.dhtml, Identifier: CTR20131190.
As defined by the International Continence Society, overactive bladder (OAB) is characterized by symptoms of “urgency, with or without urge incontinence, usually accompanied by frequency and nocturia” (Abrams et al., 2002). OAB is a common disabling condition (Irwin et al., 2011). OAB has detrimental effects on daily activities and mental health, thus significantly reducing the quality of life (QoL) (Abrams et al., 2000; Irwin et al., 2006). According to an epidemiologic survey conducted in China, the prevalence of OAB is 23.9% in adults >40 years of age, and increases proportionally with advancing age (Chuang et al., 2019).
Anticholinergic agents are the mainstay of pharmacologic treatment for OAB (Allahdin and Oo, 2012; De Nunzio et al., 2021). Use of anticholinergic agents, however, is often restricted by insufficient efficacy and/or intolerable side effects, such as constipation and dry mouth (Benner et al., 2010; Sexton et al., 2011). Alternatively, minimally invasive options are justified in the case of inadequate symptom control, including minimally posterior tibial nerve stimulation (PTNS), sacral nerve stimulation (SNS), and intradetrusor botulinum toxin A (BTX-A) injections (Lightner et al., 2019). BTX-A is produced from Clostridium botulinum and inhibits the release of neurotransmitters at presynaptic nerve terminals. When administered into the detrusor muscle of the bladder, BTX-A inhibits the release of acetylcholine, thus suppressing involuntary contractions of the detrusor muscle. Additionally, it has been suggested that BTX-A suppresses afferent pathways by blocking the release of neurotransmitters, including substance P and adenosine triphosphate (ATP), and inhibiting expression of the TRPV1 and P2X3 receptors (Apostolidis et al., 2006; Leong et al., 2010).
Currently, there are several commercial brands of BTX-A available, including Botox® (Allergan, Irvine, CA, United States), Dysport® (Ipsen, Slough, United Kingdom), Xeomin® (Merz Pharma, Frankfurt am Main, Germany) and Hengli® (Lanzhou Biological Products Institute, Lanzhou, China) (Jiang et al., 2015). The efficacy and safety of Botox® BTX-A for OAB has been well-established in two large, phase III placebo-controlled trials and in a long-term extension study conducted in the United States and Europe (Chapple et al., 2013; Nitti et al., 2016; Nitti et al., 2017). Since Hengli® BTX-A was approved by the China Ministry of Health in 1993 as a national new drug, it has been produced and sold for more than 20 years. The trade names, Hengli®, Prosigne®, and Lantox®, are used in China, Brazil, and Russia, respectively. It has been concluded that Lantox® and Botox® have similar efficacy, safety, tolerability profiles, and equivalence of doses for the treatment of blepharospasm, hemifacial spasm, and cervical dystonia (Rieder et al., 2007; Quagliato et al., 2010); however, there are no large randomized trials to confirm the safety and efficacy of Hengli® BTX-A in Chinese adults with OAB. Herein, we report a multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of Hengli® BTX-A treatment in Chinese patients with OAB.
Eligible patients were 18–75 years old with a primary diagnosis of idiopathic OAB and average ≥8 micturitions per day. The patients were not adequately managed with anticholinergic drugs (insufficient efficacy and/or intolerable side effects). Anticholinergics were maintained at the same dose in patients who were treated at the time of enrollment, while patients not treated with anticholinergics were not administered anticholinergics for the duration of the study. Patients not using clean intermittent catheterization (CIC) at baseline were required to initiate CIC if needed.
The key exclusion criteria included dysuria, post-void residual (PVR), urine volume >50 ml with spontaneous micturition, long-term indwelling catheterization or CIC, previous botulinum toxin treatment for any urologic condition within 6 months before screening, bladder or prostate cancer, and any urologic abnormalities or diseases, such as urinary tract infection (UTI) and urolithiasis, which may affect bladder function. The definition of UTI in this study included a positive urine culture (bacteriuria count >105 colony forming cells/ml) and leukocyturia (>10 cells per high power field) without distinguishing between symptomatic and asymptomatic UTIs.
Throughout the period of April 2016 to December 2018, 17 sites across China were selected to participate in this multicenter, randomized, double-blind, placebo-controlled study. The study protocol was reviewed and approved by the NMPA of China (clinical trial ID: 2013L01406). All study sites received Ethics Committee approval before study initiation and all participants provided written informed consent before enrollment in the study. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guideline.
There was a screening period of 1 week before treatment in which patients presenting to this clinic completed a 3-day bladder diary, urinalysis, PVR measurement, and other laboratory tests, as well as relevant evaluations. The participants were recruited based on the results of the 1-week screening period. Following the 1-week screening period, all eligible patients were randomly assigned using the automated Interactive Web Response System (IWRS) to botulinum toxin A (100U, Hengli®; Lanzhou Biological Products, Lanzhou, China) or placebo in a 2:1 ratio. Stratification was based on whether or not anticholinergic therapy used. The study drug was injected into the detrusor layer approximately 2 mm deep with 0.5 ml per injection site and separated by 1 cm between each site. A total of 20 sites, including the bladder trigone, were injected with the special 5F injection needle. The bladder neck was avoided during injection. Patients in the placebo group received the same volume of placebo without BTX-A, and only freeze-dried adjuvant preparations, which are combinations of gelatin, sucrose, and dextran. Patients were administered local or general anesthesia. Antibiotics were administered prophylactically before injection and continued for 1–2 days afterward. The patients were evaluated 2, 6, 12, 14, 18 and 24 weeks after treatment to assess the efficacy and safety of the drug. If the frequency of micturition decreased <50% at 12 weeks compared to baseline and the patient was willing to undergo retreatment with botulinum toxin A, then there was a window for expansion. In the extended treatment phase, patients could undergo BTX-A retreatment beginning at week 12 for both groups and were followed up at weeks 14, 18, and 24 post-treatment.
A 3-day bladder diary was recorded to evaluate OAB symptoms before baseline and at each follow-up evaluation. The primary efficacy endpoint of this study was the change from baseline in the number of micturition episodes per 24 h after 6 weeks of treatment. The secondary efficacy endpoints included the change in the average number of micturition episodes per 24 h from baseline at weeks 2 and 12, as well as the change from baseline in the average frequency of urgency and urinary incontinence (UI) episodes per day, urgency score, average micturition volume per time, overactive bladder symptom score (OABSS) (Homma et al., 2006), and QoL score at weeks 2, 6, and 12 after treatment. The health-related (HR) QoL was assessed using the International Prostate Symptom Score-QoL Subscore (Chapple et al., 2009).
Adverse events (AEs), PVR, CIC were monitored throughout the trial. In patients who do not use CIC, PVR measurements were also recorded. Retention of urine was defined as a PVR ≥200 ml that required CIC. As was the case in patients with urinary retention, the decision to initiate CIC post-treatment rested primarily with the investigator’s clinical judgment. Specifically, CIC was initiated if the PVR was >200 and <350 ml and if the patient had symptoms that warranted CIC, or if the PVR was >350 ml with or without symptoms.
The full analysis set (FAS) population was analyzed (all randomized patients except three who did not use experimental drugs as prescribed) to determine efficacy. The safety population (all patients who received treatment, analyzed by treatment received) was analyzed to determine safety. The planned enrollment was 216 patients, including a 20% attrition rate. The study design provided 80% power to detect a between-group difference as reported in a previous study in change from baseline of 1.43 micturition times per 24 h at week 6, assuming a common standard deviation of 3 micturition times (Chapple et al., 2013).
Comparisons of numerical variables between groups were done using t-tests and Wilcoxon’s test. An analysis of categorical variables between two groups was done using a chi-squared test and Fisher’s exact test. Analysis of continuous variables and categorical variables within the treatment groups was performed using paired t-tests and Wilcoxon signed-rank tests. A significance level of 0.05 was used for all statistical tests. For data with a normal or non-normal distribution, the mean ± standard deviation or median (interquartile range) was used. Statistical calculations were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC, United States).
The ITT (intention-to-treat) populations consisted of 216 randomized patients (144 patients in the BTX-A group and 72 patients in the placebo control group), of whom 189 completed the 12-week study and 27 terminated early (Figure 1). In addition, 34 and 47 patients initially received BTX-A and placebo received retreatment, respectively. The FAS populations included 213 patients, 142 in the BTX-A group and 71 in the placebo group. AEs caused discontinuation in three patients. There was a balanced distribution of baseline characteristics between the treatment groups (Table 1). The duration of OAB was 0.57 (0.12, 1.99) years, and 1.00 (1.00, 2.00) treatment measures were administered in the past 6 months before entry into the study. Inadequate efficacy was the primary cause of failed OAB management. Patients had 14.00 (11.15, 18.85) micturitions per day at baseline.
After 6 weeks, all treatment groups demonstrated significant reductions in the average number of micturition episodes per 24 h from baseline, and the difference between the two groups was statistically significant (p < 0.001 and p = 0.008 respectively). Compared with baseline, the number of micturition episodes per 24 h decreased by 2.40 (0.70, 4.60) times for the BTX-A group and 0.70 (−1.00, 3.00) times for the placebo control group, and the difference between the two groups was statistically significant (p = 0.003; Figure 2A). Furthermore, the change rates of average micturition episodes per 24 h from baseline at week 6 in the BTX-A group was significantly greater compared with placebo (16 vs. 8%, p = 0.014; Table 2).
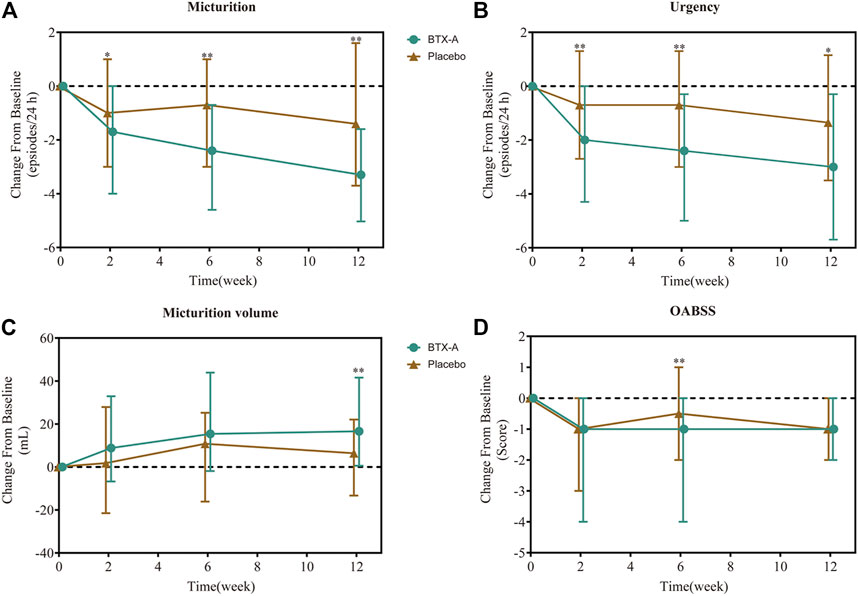
FIGURE 2. Change from baseline in daily average number of micturition episodes (A), daily average number of urgency episodes (B), average micturition volume per time (C), and OABSS score (D). Values are represented as median (interquartile range). *p < 0.05; **p < 0.01; ***p < 0.001 vs. placebo.
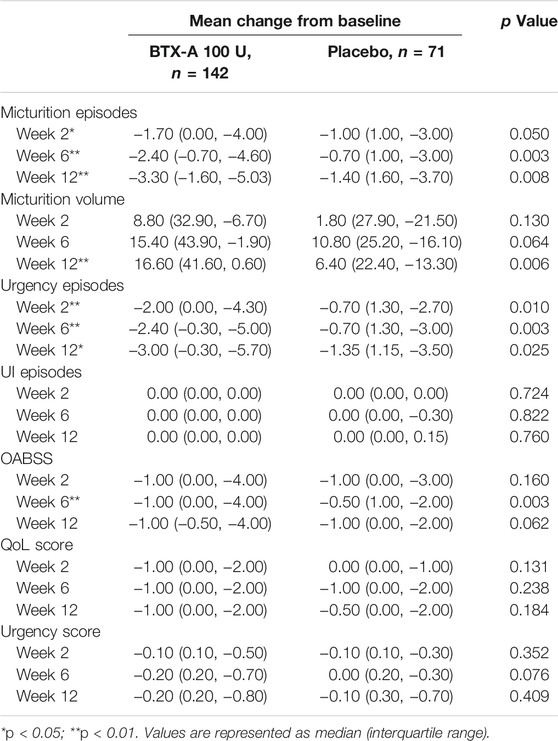
TABLE 2. Change from baseline in daily average episodes up to post-treatment week 12 in FAS population.
In the first visit at week 2 post-treatment, significant differences in the average number of micturitions as well as urgency episodes were already observed compared with the placebo. The average number of micturitions per 24 h at weeks 2 and 12 decreased by 1.70 (0.00, 4.00) and 3.30 (1.60, 5.03) times for the BTX-A group, 1.00 (−1.00, 3.00) and 1.40 (−1.60, 3.70) times for the placebo control group compared to baseline; the difference between the two groups was statistically significant (p = 0.008 and p = 0.014, respectively; Figure 2A). Efficacy was also observed in patients retreated with BTX-A. Moreover, BTX-A significantly decreased the number of urgency episodes compared with placebo 2, 6 and 12 weeks after treatment (p = 0.010, p = 0.003, and p = 0.025, respectively). The decreased number of urgency episodes from baseline at week 6 were 2.40 (0.30, 5.00) and 0.70 (−1.30, 3.00) for the BTX-A and placebo groups, respectively (Figure 2B). At week 12, the average micturition volume per time in the BTX-A group was significantly higher than the placebo group (p = 0.01; Figure 2C). Significant reductions in the OABSS from baseline were also observed during the primary phase following treatment with BTX-A (p = 0.003), with a decreased OABSS score by 1.00 (0.00, 4.00) and 0.50 (−1.00, 2.00) from baseline for the BTX-A and placebo groups at week 6 (Figure 2D). Nevertheless, no significant changes were observed at other follow-up periods. There was, however, no statistically significant difference between the groups in terms of the mean daily urgency score, the QoL score, and the number of UI episodes compared to baseline at each follow-up evaluation (Table 2).
AEs were mainly localized in the lower urinary tract. The most common AEs in the BTX-A group, occurred within the first 12 weeks of treatment, as well as over the entire treatment cycle, as follows: increased PVR (19.01%); dysuria (14.79%); and UTI (13.38%; Table 3). UTIs were all uncomplicated with no involvement of the upper urinary tract.
There were no statistically significant differences in the incidence of AEs between groups during the primary treatment phase, with the exception of PVR (p = 0.003). There was one patient (0.7%) who had a PVR >300 ml among the 27 patients (19.01%) in the BTX-A group during the primary treatment phase. The PVR in the placebo group for all 3 patients (4.23%) did not exceed 100 ml during the primary treatment phase. Of the 11 patients (13.58%) with an increased PVR in the extended treatment phase, 7 patients (8.64%) had a urine volume <100 ml. No patients had a PVR >200 ml during the extended treatment phase (Figure 3). There was one patient who required CIC for urinary retention during treatment (Table 3). In other cases, the patients managed their PVR with oral medications or did not require treatment, and the PVR decreased spontaneously over time.
The BTX-A and placebo groups had low discontinuation rates due to AEs. Specifically, 1.41 and 0.70% of patients withdrew during the primary treatment phase, respectively. No deaths occurred in this study.
This study is the first multicenter, randomized, double-blind, placebo-controlled study in Chinese patients with OAB who were inadequately managed (insufficient efficacy or intolerable side effects) with anticholinergic therapy. The results at 6 weeks showed that treatment with Hengli® BTX-A was associated with statistically significant improvement in the primary efficacy end point (number of daily micturition episodes). Patients perceived an overall improvement in their condition, which was clearly supported by the results of the micturition episodes, urgency episodes and OABSS. Results from the present study are comparable to the two phase III trials carried out in the U.S. and Europe, where the mean difference in daily micturition episodes from baseline at week 6 after Botox treatment was −1.96 and −2.40, respectively (Chapple et al., 2013; Nitti et al., 2013), which was in agreement with the mean decrease of −3.28 observed in the present study. OABSS improvements with BTX-A treatment were also similar to the results in a study conducted in Japan, with a mean decrease of −3.4 in the previous trial compared with −1.94 in this trial (Yokoyama et al., 2020). Finally, the results of Hengli® BTX-A in this trial were comparable to other trials that showed Botox treatment had a positive effect on OAB symptoms.
Hengli® BTX-A was well-tolerated in this study. In the BTX-A group there were more AEs and treatment-related AEs that were mainly related to the urinary tract. These factors included an increase in PVR, dysuria, UTI, and urinary retention. A great number of patients in the BTX-A group had a PVR >100 ml that was either managed with medication or ignored; the PVR decreased as a result. CIC was initiated by only one patient which was less than previous studies (Chapple et al., 2013; Nitti et al., 2013; Yokoyama et al., 2020). Most adverse events were mild or moderate in severity. The number of study discontinuations in the BTX-A group was quite low (1.41%). The safety of Hengli® BTX-A in the current study was comparable to Botox in trials conducted in the United States and Europe.
The active pharmaceutical ingredient (API) of Hengli® is crystalline neurotoxin. Like Botox®, Xeomin®, and Dysport®, the neurotoxin in Hengli® is derived from the Hall strain of C. botulinum type A and has an identical amino acid sequence. The high-performance liquid chromatography (HPLC) analysis of neurotoxin determined that its molecular mass was 150 kDa, with a heavy chain of 100 kDa and a light chain of 50 kDa. Its chemical purity reached 99.5% (Zhao et al., 2010). Hengli®, like other products, is a complex mixture of compounds containing botulinum neurotoxin, complexing proteins, and excipients. In contrast to Xeomin®, which only contains the 150 kD neurotoxin, Hengli®, Botox®, and Dysport® contain the 150 kD neurotoxin as part of a complex with additional proteins which do not participate in the mechanism of action. The SDS-PAGE of Hengli® complex exhibited the following protein bands: neurotoxin (152 kDa), nontoxic non-HA (136 kDa, 120 kDa), hemagglutinin (HA) 70 component (57 kDa, 17 kDa, 15 kDa), HA 33 component (30 and 28 kDa). These basic components are common to all products with BTX-A complex. Besides, Hengli® uses gelatin as the stabilizing protein rather than human serum albumin. Other adjuvant preparations include dextran and sucrose. Comparison of the potency of Hengli®, Botox®, and Xeomin® shows no difference in their potency labeling (Liang et al., 2017; Dressler, 2020). Hengli® contains about 5 ng of API per vial (100U), so a small amount of protein confers clinical benefit while posing a very low risk of stimulating the immune system. No neutralizing antibody was detected against active BTX-A after four repeated-dose animal studies with Hengli® (Liang et al., 2017).
The clinical efficacy of Hengli® BTX-A may result from its dual action involving both efferent and afferent processes. In the efferent nerves, BTX-A injections temporarily inhibit the contraction of detrusor tissues by blocking the release of acetylcholine through cleavage of SNAP-25 in both the pre- and post-ganglionic nerves (Cruz, 2014). Additionally, BTX-A blocks the release of ATP from purinergic efferent nerves in the detrusor muscle (Lawrence et al., 2010). A BTX-A injection inhibits the conduction of the afferent nerves in the bladder detrusor by decreasing urothelial ATP release and increasing urothelial NO release (Khera et al., 2004; Collins et al., 2013). Moreover, reduction in TRPV1 and P2X3 immunoreactivity in biopsies of patients with OAB after a BTX-A injection is associated with better clinical outcomes (Apostolidis et al., 2005). Overall, BTX-A injections may ameliorate OAB symptoms at multiple levels. Further research could shed light on the comprehensive mechanism underlying BTX-A action.
In contrast to randomized controlled trials that evaluate Botox® or other BTX-A products in OAB patients, micturition episodes were chosen as the primary endpoint to evaluate efficacy in this study. This setting was based on the results of an epidemiological study of OAB in China, in which 70% of Chinese OAB patients present without UI (OAB-dry) (Wang et al., 2011). In accordance with this conclusion, our data also showed that most Chinese OAB patients rarely suffer from urinary incontinence (Table2). Furthermore, our study showed Hengli® is comparable to Botox® when it comes to relieving frequency in patients with OAB. Additionally, due to the double-blinded nature of this study, researchers and patients were both blinded to treatment, so an ethically sound method was used. Therefore, patients were not required to stop taking anticholinergic medications throughout the study, but rather to maintain the same dosage as before enrollment.
It is worth investigating whether gender factors affect the responses to BTX-A treatment in overactive bladder patients, as there is a gender factor in OAB pathophysiology. In this study, 82.39% of patients in BTX-A group and 85.92% of patients in placebo group were females. However, when comparing the safety and efficacy of botulinum toxin after using sex grouping, there was no significant difference. Our results may be influenced by the large difference in the number of patients between males and females, as we did not restrict recruitment to certain genders. Another male-female cohort study in a larger patient population would provide insights into differences in responses to botulinum toxin between genders.
One limitation of the study was that comparisons with placebo were not feasible after week 12, because patients were able to request and receive retreatment beyond that point. In an extended study phase, repeated treatment with Hengli® BTX-A are being evaluated to determine the efficacy over the long term. Secondly, no statistically significant differences were found between the groups in terms of the number of UI episodes compared to baseline at each follow-up evaluation, which was the primary efficacy endpoint of Botox trials conducted in the U.S. and Europe. Consistent with prior epidemiologic studies, there were fewer UI episodes among the 2 groups at baseline (Wang et al., 2011). As a result, fewer UI episodes may result in an insignificant difference between groups. Moreover, no significant difference was observed in QoL scores between the Hengli® and the placebo group. As the International Prostate Symptom Score-QoL Subscore questionnaire was used to evaluate QoL, which consists of one question with a score between 0 and 6. In this questionnaire one highly subjective question was asked, and the OAB population tends to have extremely high expectations of efficacy, thus a statistically significant score may not be obtained.
Hengli® BTX-A treatment resulted in significant improvement of OAB symptoms compared with placebo in Chinese OAB patients who were poorly managed by anticholinergic therapy. The participants in this study reported improvements in their condition. In Chinese OAB patients, Hengli® BTX-A was well-tolerated, with a low incidence of PVR elevation requiring CIC. In this study we showed that Hengli® BTX-A is an effective treatment option for Chinese patients with OAB in whom prior anticholinergic therapy failed to manage their condition adequately.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of China Rehabilitation Research Center. The patients/participants provided their written informed consent to participate in this study.
Principal investigator (PI), Project development, data collection and management, data analysis, manuscript writing and revising: LL. Data management, data analysis, and manuscript writing: QL. Project development, data collection and management: HC, ZX, EL, ZW, HJ, BL, XH, SX, WW, JW, GS, YW, PL, YY, ZF, JZ, YT, HS, HL, ZH, LZ, YX, YZ, JW, XZ, PZ, DW, XZ, KX, BW, LM, XT, LC, JD. All authors approved the final manuscript.
This study was funded by the National Natural Science Foundation of China, Grant/Award Number: No. 81870523.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the patients/participants in this study.
Abrams, P., Cardozo, L., Fall, M., Griffiths, D., Rosier, P., Ulmsten, U., et al. (2002). The Standardisation of Terminology of Lower Urinary Tract Function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21, 167–178. doi:10.1002/nau.10052
Abrams, P., Kelleher, C. J., Kerr, L. A., and Rogers, R. G. (2000). Overactive Bladder Significantly Affects Quality of Life. Am. J. Manag. Care 6, S580–S590.
Allahdin, S., and Oo, N. (2012). An Overview of Treatment of Overactive Bladder Syndrome in Women. J. Obstet. Gynaecol. 32, 217–221. doi:10.3109/01443615.2011.649317
Apostolidis, A., Dasgupta, P., and Fowler, C. J. (2006). Proposed Mechanism for the Efficacy of Injected Botulinum Toxin in the Treatment of Human Detrusor Overactivity. Eur. Urol. 49, 644–650. doi:10.1016/j.eururo.2005.12.010
Apostolidis, A., Popat, R., Yiangou, Y., Cockayne, D., Ford, A. P., Davis, J. B., et al. (2005). Decreased Sensory Receptors P2X3 and TRPV1 in Suburothelial Nerve Fibers Following Intradetrusor Injections of Botulinum Toxin for Human Detrusor Overactivity. J. Urol. 174, 977–983. doi:10.1097/01.ju.0000169481.42259.54
Benner, J. S., Nichol, M. B., Rovner, E. S., Jumadilova, Z., Alvir, J., Hussein, M., et al. (2010). Patient-reported Reasons for Discontinuing Overactive Bladder Medication. BJU Int. 105, 1276–1282. doi:10.1111/j.1464-410X.2009.09036.x
Chapple, C., Herschorn, S., Abrams, P., Sun, F., Brodsky, M., and Guan, Z. (2009). Tolterodine Treatment Improves Storage Symptoms Suggestive of Overactive Bladder in Men Treated with Alpha-Blockers. Eur. Urol. 56, 534–541. doi:10.1016/j.eururo.2008.11.026
Chapple, C., Sievert, K. D., MacDiarmid, S., Khullar, V., Radziszewski, P., Nardo, C., et al. (2013). OnabotulinumtoxinA 100 U Significantly Improves All Idiopathic Overactive Bladder Symptoms and Quality of Life in Patients with Overactive Bladder and Urinary Incontinence: a Randomised, Double-Blind, Placebo-Controlled Trial. Eur. Urol. 64, 249–256. doi:10.1016/j.eururo.2013.04.001
Chuang, Y. C., Liu, S. P., Lee, K. S., Liao, L., Wang, J., Yoo, T. K., et al. (2019). Prevalence of Overactive Bladder in China, Taiwan and South Korea: Results from a Cross-Sectional, Population-Based Study. Low Urin Tract Symptoms 11, 48–55. doi:10.1111/luts.12193
Collins, V. M., Daly, D. M., Liaskos, M., McKay, N. G., Sellers, D., Chapple, C., et al. (2013). OnabotulinumtoxinA Significantly Attenuates Bladder Afferent Nerve Firing and Inhibits ATP Release from the Urothelium. BJU Int. 112, 1018–1026. doi:10.1111/bju.12266
Cruz, F. (2014). Targets for Botulinum Toxin in the Lower Urinary Tract. Neurourol Urodyn 33, 31–38. doi:10.1002/nau.22445
De Nunzio, C., Brucker, B., Bschleipfer, T., Cornu, J. N., Drake, M. J., Fusco, F., et al. (2021). Reply to Satoshi Funada, Takashi Yoshioka, and Yan Luo's Letter to the Editor re: Cosimo De Nunzio, Benjamin Brucker, Thomas Bschleipfer, et al. Beyond Antimuscarinics: A Review of Pharmacological and Interventional Options for Overactive Bladder Management in Men. Eur Urol 2021;79:492-504. Eur. Urol. 79, e147–504. doi:10.1016/j.eururo.2020.12.03210.1016/j.eururo.2021.02.033
Dressler, D. (2020). Therapeutically Relevant Features of Botulinum Toxin Drugs. Toxicon 175, 64–68. doi:10.1016/j.toxicon.2019.12.005
Homma, Y., Yoshida, M., Seki, N., Yokoyama, O., Kakizaki, H., Gotoh, M., et al. (2006). Symptom Assessment Tool for Overactive Bladder Syndrome-Ooveractive Bladder Symptom Score. Urology 68, 318–323. doi:10.1016/j.urology.2006.02.042
Irwin, D. E., Kopp, Z. S., Agatep, B., Milsom, I., and Abrams, P. (2011). Worldwide Prevalence Estimates of Lower Urinary Tract Symptoms, Overactive Bladder, Urinary Incontinence and Bladder Outlet Obstruction. BJU Int. 108, 1132–1138. doi:10.1111/j.1464-410X.2010.09993.x
Irwin, D. E., Milsom, I., Kopp, Z., Abrams, P., and Cardozo, L. (2006). Impact of Overactive Bladder Symptoms on Employment, Social Interactions and Emotional Well-Being in Six European Countries. BJU Int. 97, 96–100. doi:10.1111/j.1464-410X.2005.05889.x
Jiang, Y. H., Liao, C. H., and Kuo, H. C. (2015). Current and Potential Urological Applications of Botulinum Toxin A. Nat. Rev. Urol. 12, 519–533. doi:10.1038/nrurol.2015.193
Khera, M., Somogyi, G. T., Kiss, S., Boone, T. B., and Smith, C. P. (2004). Botulinum Toxin A Inhibits ATP Release from Bladder Urothelium after Chronic Spinal Cord Injury. Neurochem. Int. 45, 987–993. doi:10.1016/j.neuint.2004.06.001
Lawrence, G. W., Aoki, K. R., and Dolly, J. O. (2010). Excitatory Cholinergic and Purinergic Signaling in Bladder Are Equally Susceptible to Botulinum Neurotoxin a Consistent with Co-release of Transmitters from Efferent Fibers. J. Pharmacol. Exp. Ther. 334, 1080–1086. doi:10.1124/jpet.110.169342
Leong, R. K., De Wachter, S. G., and van Kerrebroeck, P. E. (2010). Current Information on Sacral Neuromodulation and Botulinum Toxin Treatment for Refractory Idiopathic Overactive Bladder Syndrome: a Review. Urol. Int. 84, 245–253. doi:10.1159/000288223
Liang, Q., Chen, S., Zhang, R., Wei, Z., Su, J., Zhou, Y., et al. (2017). Nonclinical Safety Evaluation of Botulinum Toxin Type A and Botulinum Neurotoxin Type A. (In Chinese). Chin. J. Biologicals 30, 710–717. doi:10.13200/j.cnki.cjb.001797
Lightner, D. J., Gomelsky, A., Souter, L., and Vasavada, S. P. (2019). Diagnosis and Treatment of Overactive Bladder (Non-neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 202, 558–563. doi:10.1097/JU.0000000000000309
Nitti, V. W., Dmochowski, R., Herschorn, S., Sand, P., Thompson, C., Nardo, C., et al. (2013). OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J. Urol. 189, 2186–2193. doi:10.1016/j.juro.2012.12.022
Nitti, V. W., Dmochowski, R., Herschorn, S., Sand, P., Thompson, C., Nardo, C., et al. (2017). OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J. Urol. 197, S216–S23. doi:10.1016/j.juro.2016.10.109
Nitti, V. W., Ginsberg, D., Sievert, K. D., Sussman, D., Radomski, S., Sand, P., et al. (2016). Durable Efficacy and Safety of Long-Term OnabotulinumtoxinA Treatment in Patients with Overactive Bladder Syndrome: Final Results of a 3.5-Year Study. J. Urol. 196, 791–800. doi:10.1016/j.juro.2016.03.146
Quagliato, E. M., Carelli, E. F., and Viana, M. A. (2010). Prospective, Randomized, Double-Blind Study, Comparing Botulinum Toxins Type a Botox and Prosigne for Blepharospasm and Hemifacial Spasm Treatment. Clin. Neuropharmacol 33, 27–31. doi:10.1097/WNF.0b013e3181c46f61
Rieder, C. R., Schestatsky, P., Socal, M. P., Monte, T. L., Fricke, D., Costa, J., et al. (2007). A Double-Blind, Randomized, Crossover Study of Prosigne versus Botox in Patients with Blepharospasm and Hemifacial Spasm. Clin. Neuropharmacol 30, 39–42. doi:10.1097/01.WNF.0000236771.77021.3C
Sexton, C. C., Notte, S. M., Maroulis, C., Dmochowski, R. R., Cardozo, L., Subramanian, D., et al. (2011). Persistence and Adherence in the Treatment of Overactive Bladder Syndrome with Anticholinergic Therapy: a Systematic Review of the Literature. Int. J. Clin. Pract. 65, 567–585. doi:10.1111/j.1742-1241.2010.02626.x
Wang, Y., Xu, K., Hu, H., Zhang, X., Wang, X., Na, Y., et al. (2011). Prevalence, Risk Factors, and Impact on Health Related Quality of Life of Overactive Bladder in China. Neurourol Urodyn 30, 1448–1455. doi:10.1002/nau.21072
Yokoyama, O., Honda, M., Yamanishi, T., Sekiguchi, Y., Fujii, K., Nakayama, T., et al. (2020). OnabotulinumtoxinA (Botulinum Toxin Type A) for the Treatment of Japanese Patients with Overactive Bladder and Urinary Incontinence: Results of Single-Dose Treatment from a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial (Interim Analysis). Int. J. Urol. 27, 227–234. doi:10.1111/iju.14176
Keywords: botulinum toxin type A, Hengli®, overactive bladder, randomized controlled trial, China
Citation: Liao L, Liu Q, Cong H, Xu Z, Li E, Weng Z, Jiang H, Liu B, Huang X, Xia S, Wen W, Wu J, Shi G, Wang Y, Li P, Yu Y, Fang Z, Zheng J, Tian Y, Shang D, Li H, Huang Z, Zhou L, Xiao Y, Zhang Y, Wang J, Zhang X, Zhang P, Wang D, Zhang X, Xie K, Wang B, Ma L, Tian X, Chen L and Dong J (2022) Hengli® Chinese Botulinum Toxin Type A for Treatment of Patients With Overactive Bladder: A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 13:840695. doi: 10.3389/fphar.2022.840695
Received: 22 December 2021; Accepted: 01 February 2022;
Published: 18 February 2022.
Edited by:
Martin Hennenberg, University Hospital, LMU Munich, GermanyReviewed by:
Markus Grabbert, University of Freiburg Medical Center, GermanyCopyright © 2022 Liao, Liu, Cong, Xu, Li, Weng, Jiang, Liu, Huang, Xia, Wen, Wu, Shi, Wang, Li, Yu, Fang, Zheng, Tian, Shang, Li, Huang, Zhou, Xiao, Zhang, Wang, Zhang, Zhang, Wang, Zhang, Xie, Wang, Ma, Tian, Chen and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Liao, bG1saWFvQDI2My5uZXQ=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.