
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 April 2022
Sec. Translational Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.833303
Experience in the clinical use of posaconazole (PCZ) in pediatric patients is limited, and no specific dose recommendations exist. This study aimed to investigate an appropriate dosing regimen, and assess the exposure-response relationships of PCZ in children. We reviewed the medical records of inpatients aged <18 years who subjected to PCZ concentrations monitoring. Clinical data, PCZ dosing and monitoring data were collected. A total of 375 PCZ trough concentrations (Cmin) from 105 pediatric patients were included. For children receiving PCZ for prophylaxis, the median doses required to achieve the therapeutic range at the ages of <6, 6–12 and >12 years were 14.80, 14.52 and 12.90 mg/kg/day, respectively (p = 0.001); and for those receiving PCZ for treatment, the median doses were 23.50, 20.96 and 15.38 mg/kg/day, respectively (p = 0.001). Among children taking PCZ for prophylaxis, 12% developed a proven or probable breakthrough IFIs; the median PCZ concentrations were significantly lower than those children with successful treatment response (0.43 versus 1.20 μg mL−1; p < 0.001). 79.2% patients taking PCZ for treatment had a positive clinical response, and the median PCZ concentrations were significantly higher than those children with disease progression (1.06 versus 0.53 μg mL−1; p = 0.024). No association between Cmin values and hepatotoxicity was observed. Factors such as age, CRP, ALT and co-administration with proton pump inhibitors exhibited significant effects on PCZ Cmin. It is necessary to adjust the dosing regimens based on PCZ Cmin to individualize antifungal therapy and provide guidelines for dose adjustment in children.
Invasive fungal infections (IFIs) represent a major cause of morbidity and mortality in immunocompromised children (Lehrnbecher and Groll, 2011). Posaconazole (PCZ) is an extended-spectrum triazole antifungal used as prophylaxis of and treatment for IFIs in these patients (Groll et al., 2014; Arrieta et al., 2019). Several studies have demonstrated a correlation between PCZ plasma levels and clinical efficacy (Jang et al., 2010; Moore et al., 2015). Therefore, therapeutic drug monitoring (TDM) of PCZ can improve its efficacy and safety (Ashbee et al., 2014; Chau et al., 2014; Döring et al., 2017). Our previous research which conducted in adults demonstrated that a number of factors exhibited significant effects on PCZ Cmin including gender, albumin and interactions with co-medications, leading to high inter-individual and intra-individual variability in plasma concentrations. A 25.9% lower in the exposure of the oral PCZ suspension was reported when administered with proton pump inhibitors (PPIs) in adults (Dolton et al., 2014; Jia et al., 2020). However, fewer data are available in children and most of them are based on adult experience for pediatric patients.
Individual differences in concentrations of this drug in children blood serum are greater than adults, achieving and maintaining therapeutic concentrations for the duration of therapy is difficult due to the drug interactions and erratic absorption in PCZ pharmacokinetics. thus, the monitoring of trough concentrations in children is of the utmost importance for PCZ therapeutic effectiveness and safety (Jancel et al., 2017; Boonsathorn et al., 2019). There is an agreement that children need higher and personalized doses to reach therapeutic Cmin (McMahon et al., 2017; Lai et al., 2020). It is difficult to attain the target concentration of PCZ for children and there is a significant variability in the dose required because of its unpredictable bioavailability (Gwee et al., 2015). To date, there is few published recommendations on PCZ dosing for pediatric patients.
Therefore, we performed a retrospective analysis to investigate a potential relationship between PCZ Cmin and its efficacy and safety among children, and explore an appropriate dosing regimen as well as the potential factors influencing PCZ Cmin in children.
This retrospective study was performed in The First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). Pediatric patients (age <18) taking PCZ oral suspension for treatment or prophylaxis of IFIs and performing at least one PCZ concentration determination during therapy between June, 2018 and December, 2020 were enrolled in this study. Medical records and demographic information of these children were reviewed. Details of PCZ therapy, outcomes of therapy, adverse events, concomitant medications that could potentially interfere with PCZ absorption, and biochemical parameters including CRP, albumin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), g-GT (gamma-glutamyl transferase), and bilirubin values were also collected.
The blood samples for measuring PCZ Cmin were collected for routine care. Only children having achieved steady-state were included in the analysis and PCZ was considered to have achieved a steady-state plasma concentration after at least 7 days of dosing (Lai et al., 2020). Dose adjustments were taken according to the current guidelines and the manufacturer’s recommendations (Vicenzi et al., 2018). PCZ trough plasma concentrations were determined using a previously described ultra-high-performance liquid chromatograph-tandem mass spectrometry method (UPLC-MS/MS, Waters, United States) (Jia et al., 2020). The analytical range was 0.025–5.00 μg mL−1. For the purpose of this study, plasma concentrations ≥0.7 μg mL−1 were considered therapeutic for prophylaxis and ≥1.0 μg mL−1 for treatment (Bernardo et al., 2020).
Only patients with a PCZ treatment regimen of ≥14 days were incorporated into the study to assess clinical response. Fungal infections and the response to antifungal therapy were retrospectively classified according to the definitions of the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC-MSG) (Pauw et al., 2008). For patients receiving PCZ for prophylaxis, it would be assessed as a positive outcome if the course was completed without breakthrough fungal infection (Pauw et al., 2008; Chinese Association Hematologists; Chinese Invasive Fungal Infection Working Group, 2020). For patients receiving PCZ for treatment, of a fungal infection, a successful outcome was defined as partial improvement or improvement of clinically significant signs and symptoms, improvement or resolution of radiological signs of infection and evidence of microbiological cure; otherwise, patients were classified as clinical failure. Adverse drug reactions and their relationship with PCZ were defined according to the Common Terminology Criteria for Adverse Events (CTCAE) defined by the National Cancer Institute (National Cancer Institute, 2006).
Statistical analysis was performed with SPSS Statistics for Mac Ver. 26 (SPSS Inc., Chicago, IL). Patients were divided into three groups (<6, 6–12, and >12 years old) according to previous literature (Döring et al., 2017; Lavigne et al., 2019). Descriptive statistics included the mean, standard deviation, and median. The categorical variables were expressed as frequency and percentage. Categorical variables were compared using χ2 test or Fisher’s exact test. Continuous variables were compared using Mann-Whitney U-test or Kruskal-Wallis H test. The correlation between two continuous variables was examined using the Spearman correlation coefficient. The association of Cmin with efficacy and safety was analyzed by logistic regression. Affecting factors of PCZ trough concentrations were analyzed using multivariate analysis by linear regression. p values < 0.05 were considered statistically significant.
A total of 375 PCZ Cmin drawn in 105 pediatric patients were available in this research (Figure 1) 32 (30.5%) females and 73 (69.5%) male patients were included, with a median age of 10 years (range, 1.5–18 years). The most frequent underlying condition was acute lymphoblastic leukaemia (34.29%). Seventy-two (68.6%) received an allogeneic hematopoietic stem cell transplantation (HSCT) before using PCZ. A median of 2 trough levels (range, 1–15) were measured per patient, and the median therapy duration of all patients was 84 days (range, 7–320 days). Demographic information of these patients is summarized in Table 1.
The median PCZ plasma concentration for all 375 trough levels was 1.07 μg mL−1 (range, 0.04–4.84 μg mL−1; mean 1.16 ± 0.76 μg mL−1). Timing of the first TDM sample varied widely, with a median of 7 days (range, 7–34 days) after starting therapy. Distribution of PCZ trough concentrations is shown in Figure 2.
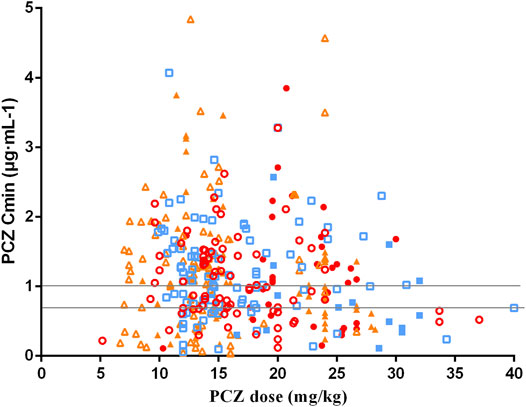
FIGURE 2. Distribution for PCZ Cmin at different weight-adjusted doses received. Solid and hollow red circles represent the data obtained of PCZ prophylaxis and treatment in children aged <6 years old, respectively. Solid and hollow blue square represent the data obtained of prophylaxis and treatment in children aged 6–12 years old, respectively. Solid and hollow orange triangles represent the data obtained of prophylaxis and treatment in children aged >12 years old, respectively. Two horizontal lines indicates the therapeutic range of PCZ prophylaxis and treatment (0.7 μg·mL-1 and 1.0 μg·mL-1).
Seventy-eight patients (74.3%) received PCZ for prophylaxis with 273 PCZ plasma concentration measured. The median PCZ plasma concentration was 1.10 μg mL−1 (range, 0.04–4.84 μg mL−1; mean 1.18 ± 0.73 μg mL−1). The median duration of therapy was 51 days (range, 8–306 days). 74.4% of these patients had therapeutic serum concentrations (≥0.7 μg mL−1). There is no significant difference in the number of PCZ Cmin reaching the target concentration among the three age groups (<6 ages, 71.43%; 6–12 ages, 79.79%; >12 ages, 71.57%; χ2 = 2.216, p = 0.333). Twenty-seven (25.7%) received PCZ for treatment with 102 PCZ plasma concentration measured. The median PCZ Cmin was 0.91 μg mL−1 (range, 0.07–3.85 μg mL−1; mean 1.11 ± 0.84 μg mL−1). 48.04% of these patients had therapeutic concentrations (≥1.0 μg mL−1). No significant difference in the number of PCZ Cmin within the therapeutic range among the three age groups were observed (<6 ages, 56.25%; 6–12 ages, 35.29%; >12 ages, 47.17%; χ2 = 1.987, p = 0.370).
Moreover, there was no significant correlation between PCZ Cmin and weight-adjusted dosage for all children <6, 6–12 and >12 years of age, regardless of the indication of treatment (p > 0.05 for all 6 groups). Large intra- and inter- patient variability for patients receiving 12 mg/kg/day of PCZ suspension who had five or more sample measurements was observed (Figure 3), and the median intra-patient variability of these children was 28.75% (range, 12.89–119.83%).
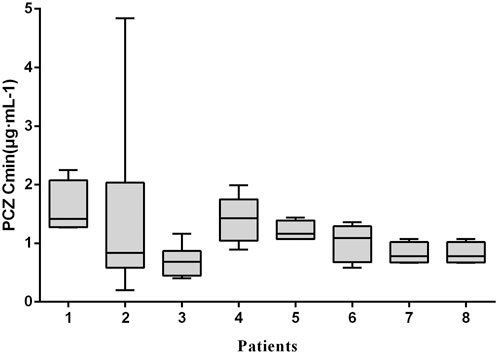
FIGURE 3. Intra-and inter-patient variability of PCZ Cmin. Each boxplot represents PCZ levels (median, minimum, maximum and interquartile range) of a single child for a total of 8 children receiving 12 mg/kg/day of PCZ suspension who had five or more sample measurements.
The median maintenance PZC doses required to achieve therapeutic Cmin of prophylaxis and treatment were 14.17 mg/kg/day (range, 7.02–30.89 mg/kg/day) and 19.56 mg/kg/day (range, 8.57–32.00 mg/kg/day), respectively (p < 0.001). The median maintenance doses required to achieve therapeutic Cmin for different age and the indication of treatment are shown in Figure 4.
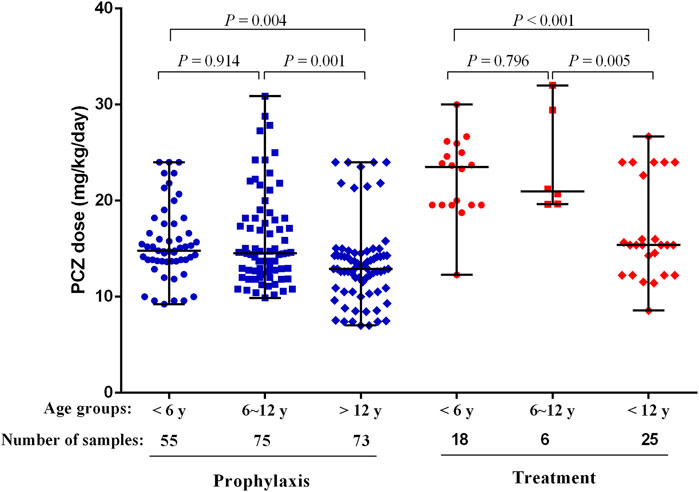
FIGURE 4. PCZ maintenance dose required to achieve therapeutic Cmin for different age and the indication of treatment. Horizontal bars represent the median dose values for each group. y, years.
Patients received PCZ for prophylaxis of <6, 6–12 and >12 years required median doses of 14.80 mg/kg/day (range, 9.23–24.00 mg/kg/day), 14.52 mg/kg/day (range, 9.88–30.89 mg/kg/day), and 12.90 mg/kg/day (range, 7.02–24.00 mg/kg/day), respectively (p = 0.001), to achieve therapeutic Cmin. Additional analysis among the three age groups showed that younger patients (<6 and 6–12 years) needed a significantly higher median PCZ dose to maintain the therapeutic trough levels compared to the older children (>12 years) (14.80 versus 12.90 mg/kg/day, 14.52 versus 12.90 mg/kg/day, respectively; p < 0.05). In contrast, the median maintenance dose between patients <6 and 6–12 years old did not differ significantly (14.80 versus 14.52 mg/kg/day, p = 0.914).
For the children taken PCZ for treatment, the median doses needed by patients <6, 6–12 and >12 years were 23.50 mg/kg/day (range, 12.28–30.00 mg/kg/day), 20.96 mg/kg/day (range, 19.64–32.00 mg/kg/day), and 15.38 mg/kg/day (range, 8.57–26.67 mg/kg/day), respectively (p < 0.001), to achieve therapeutic Cmin. Additional analysis among the three age groups showed that younger patients (<6 and 6–12 years) needed a significantly higher median PCZ dose to maintain the therapeutic trough levels compared to older children (23.50 versus 15.38 mg/kg/day, 20.96 versus 15.38 mg/kg/day, respectively; p < 0.05). In contrast, the median maintenance dose between patients <6 years and 6–12 years did not differ significantly (p = 0.796).
There was no significant difference in the dosages of PCZ required to achieve therapeutic Cmin and sub-therapeutic for all children, regardless of the indication of treatment (p > 0.05 for all 6 groups). (Table 2).
Clinical response was analyzed in patients with administration of PCZ more than 14 days. Among the 78 patients receiving PCZ for prophylaxis of IFIs, 75 patients were identified in the analysis of efficacy of PCZ prophylaxis. 9 of the 75 (12.0%) patients with a median PCZ Cmin of 0.43 μg mL−1 (range, 0.13–0.70 μg mL−1) developed proven or probable breakthrough IFIs during the study period. These trough levels were significantly lower than the other 66 patients without IFIs (median 1.20 μg mL−1, range, 0.12–2.10 μg mL−1, p < 0.001) (Table 3).
Twenty-four patients were identified in the analysis of efficacy of PCZ treatment. The median PCZ concentration of 1.06 μg mL−1 (range, 0.55–3.08 μg mL−1) in 19 patients (79.2%) with a positive clinical response was significantly higher than that in the remaining 5 patients (20.8%) with disease progression (median 0.53 μg mL−1, range, 0.30–0.66 μg mL−1, p < 0.024) (Table 3).
Logistic regression analysis showed that PCZ steady-state average plasma concentrations correlated well with clinical responsiveness. The predicted probability of successful clinical response of prophylaxis and treatment with increasing PCZ concentration from the logistic regression analysis is displayed in Figure 5. Out of 105 patients, 3 (4.76%) died during the observation period, due to relapse of the underlying disease. None of the patients included in the present analysis died from IFIs during the observation period.
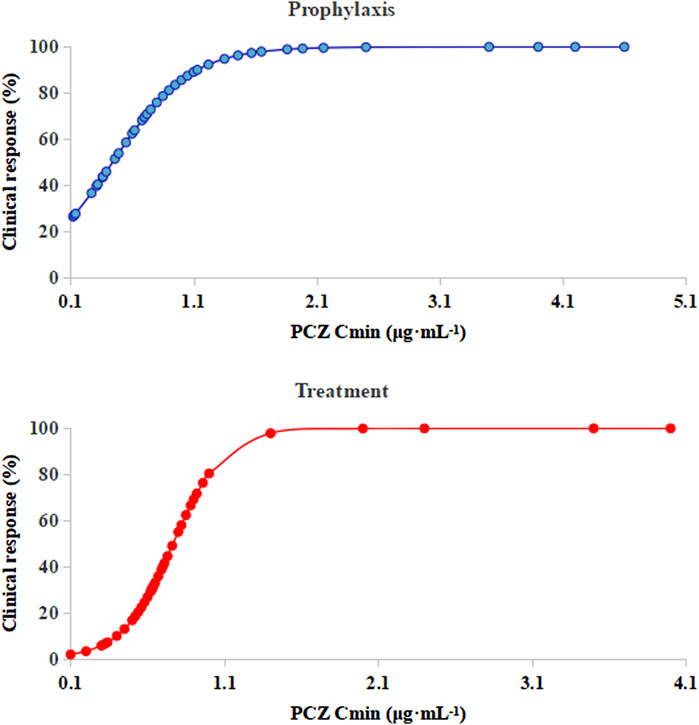
FIGURE 5. Logistic regression model showing PCZ Cmin predicting the probability of successful clinical response of prophylaxis and treatment. The solid lines represent the regression fit; Cmin, posaconazole average steady-state plasma concentrations.
Twenty-four experienced hepatotoxicity (24/105, 22.8%). The treatment-related hepatotoxicity emerged by day 12 (range, 3–30) after starting PCZ therapy. Univariate analysis did not reveal any statistically significant association between PCZ trough level and the occurrence of hepatotoxicity (0.95 ± 0.67 μg mL−1 versus 1.13 ± 0.58 μg mL−1, p = 0.369). Three patients discontinued PCZ treatment due to adverse events. These adverse events were considered likely to be related to PCZ treatment for two patients and possibly related (hepatotoxicity and hypokalemia) for one of them. These side effects were always controlled by discontinuing PCZ without any consequences.
Multiple linear regression analysis identified a number of drug interactions and clinical factors associated with a significant change in dose-normalized plasma PCZ concentration (Table 4). Concomitant administration of PPIs had a 64.4% lower blood plasma concentration (median trough concentration, 1.26 versus 3.54 μg mL−1, p = 0.002) (Figure 6). In addition, patients’ age (p < 0.001), CRP (p = 0.042), and ALT (p = 0.022) were also associated with significantly reduced PCZ concentrations.
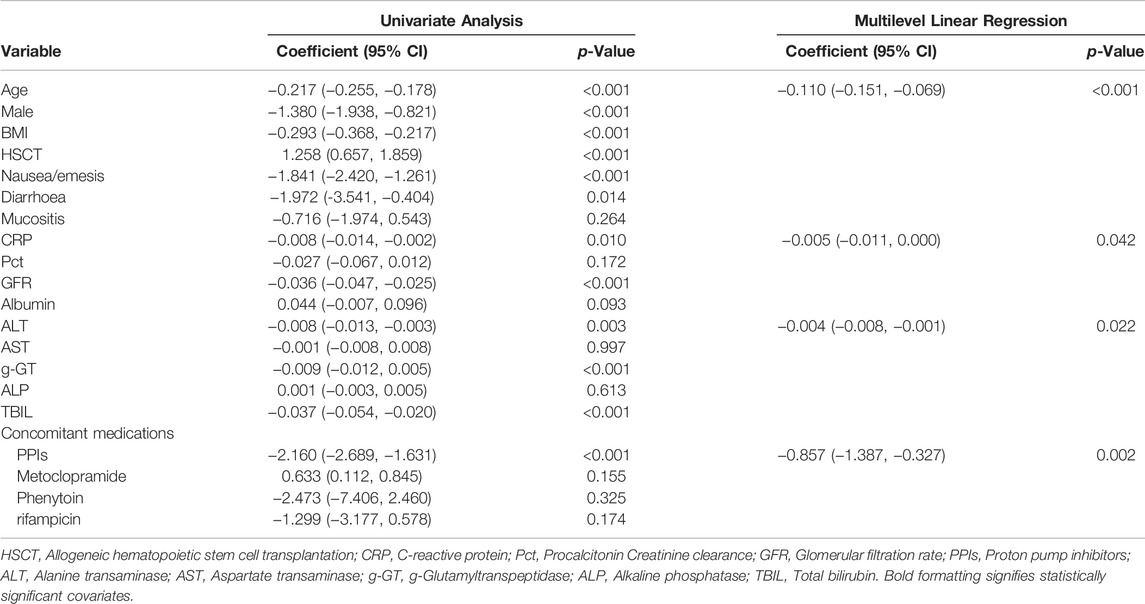
TABLE 4. Univariate and multilevel linear regression to examine factors influencing dose normalized plasma PCZ concentration.
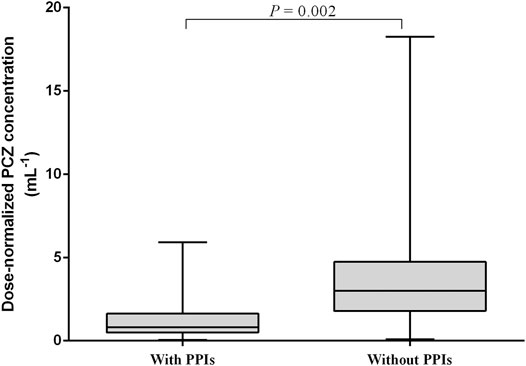
FIGURE 6. Effect of proton pump inhibitors (PPIs) on dose-normalized PCZ Cmin. The graph shows boxplots with whiskers (min to max) of dose-normalized Cmin.
We reported on the relationship between the PCZ therapeutic drug monitoring and dose in immunocompromised children. It is difficult to attain target concentrations in children, especially in those <13 years old and there is a significant variability in the dose required as a result of unpredictable bioavailability. A large interindividual variability in PCZ Cmin was observed which cannot be explained by the dose-concentration relationship. This study demonstrated that dose-normalized serum concentrations of PCZ were affected by age, CRP, ALT, and coadministration of PPIs (Table 4).
The pharmacokinetics of PCZ in children has not been fully studied to date, and limited information is available on optimal dosing regimen for oral suspension for pediatric patients. Mathew et al. examined thirty-two immunocompromised children (aged 3–18 years) receiving PCZ for both prophylaxis and treatment (Mathew et al., 2017). Sixteen of thirty-two patients (50%) achieved target PCZ (0.7 μg mL−1) with a median initial dose of 22.8 mg/kg/day (range, 9.2–44 mg/kg/day). The remaining sixteen patients did not achieve PCZ target with a median initial dose of 15.8 mg/kg/day (range, 9.3–50 mg/kg/day). Vicenzi et al. assessed the correlation between PCZ daily dose and trough plasma level in hematology-oncology pediatric patients (Vicenzi et al., 2018). For the prophylaxis group, a median dose of 12 mg/kg/day (range, 4–35 mg/kg/day) of PCZ oral solution was associated with a PCZ Cmin > 0.7 μg mL−1 in 63% of PCZ assessments at day ≥7 after starting PCZ. In the group of patients which used PCZ as salvage therapy for IFI, a median dosage of 15 mg/kg/day (range, 5–30 mg/kg/day) was associated with the achievement of a threshold above 1 μg mL−1 in 77% of measurements performed in the first week of treatment.
In our study, the median PCZ dose for prophylaxis was 14.80 mg/kg/day for patients <6 years, 14.52 mg/kg/day for patients 6–12 years and 12.9 mg/kg/day for patients >12 years, respectively. The median dose for treatment was 23.50 mg/kg/day for patients <6 years, 20.96 mg/kg/day for patients 6–12 years and 15.38 mg/kg/day for patients >12 years, respectively (Table 2). Higher PCZ doses were required to achieve the target Cmin among younger children. However, the median maintenance dose did not differ significantly between patients aged <6 and 6–12 years old in this study, although a higher trend is shown in patients <6 years old (Figure 4). One possible explanation for the higher doses in patients <13 years is that patients in this group were dosed based on current available data versus patients 13 years and older who were dosed based on package insert recommendations (Bernardo et al., 2020).
Few trials have described PCZ Cmin and efficacy relationgship in pediatric patients and no guidelines are available regarding the target PCZ plasma concentration for pediatric patients currently. Dolton et al. reported that among 72 patients taking PCZ for prophylaxis against IFIs, and 12 patients (17%) developed a breakthrough fungal infection (Dolton et al., 2012); the median PCZ concentrations were significantly lower than those who did not (0.289 versus 0.485 μg mL−1; p < 0.01). In the present study, a significant exposure-response relationship was observed. In the prophylaxis group, all breakthrough fungal infections were observed at the median PCZ concentrations below 0.50 μg mL−1, suggesting that this cutoff value may be a useful concentration target for PCZ prophylaxis against IFIs in children, which is considerably lower than described in our previous study in adult patients with hematologic disorders (0.76 μg mL−1) (9). In that analysis, a cut-off Cmin was identified for patients as the concentration where successful clinical response increased >80% probability (Wang et al., 2014).
Similar to a retrospective study of PCZ prophylaxis of IFIs in pediatric patients with hematology oncology [the success rate was (80/84) 95.2%], and a randomized clinical trial for prophylaxis against IFIs [the success rate was (50/70) 71.4%], the current study found that 88.0% of patients (66/75) successfully responded to PCZ prophylaxis (Vicenzi et al., 2018; Sienkiewicz-Oleszkiewicz et al., 2019). Among patients receiving PCZ for the treatment of IFI, PCZ concentrations among patients who failed therapy (0.53 μg mL−1) were lower than those who treated successfully (1.06 μg mL−1) (p = 0.024). In our previous study, PCZ Cmin values of 1.0 μg mL−1 was associated with a >80% probability of successful treatment response (Jia et al., 2020). Similar to a multicenter study of PCZ treatment of IFIs in juvenile (<18 years) with IFIs [the success rate was (5/8) 62.5%] (Krishna et al., 2007), the current study found that 79.2% of patients (19/24) successfully responded to PCZ treatment. Taken together, the relationship between PCZ Cmin and efficacy may follow a different profile among pediatric patients. Therefore, a lower limit of the target Cmin should be considered for Asian children compared with adults. This retrospective study is the first to assess the relationship between PCZ Cmin and efficacy in children. Future studies of larger patient cohorts are needed to accurately define concentration targets for PCZ in the prophylaxis and treatment of IFIs.
In our study, PCZ was well tolerated in children, and the rate of hepatotoxicity possibly related to PCZ was 22.8% and all reverted with the discontinuation of the drug, comparable to previously reported rates (3.0–16.7%) (Döring et al., 2017; Vicenzi and Cesaro, 2018). Similar to previous reports, we found no consistent correlation between PCZ trough concentrations and hepatotoxicity (Krishna et al., 2007). Because PCZ concentrations are relatively constant at steady state, the average of all plasma concentrations for each patient was calculated to provide a single steady-state plasma PCZ concentration, which was used to analyze the association of Cmin with efficacy and safety.
Concomitant PPI therapy has been shown to be significantly associated with decreased concentrations in adult patients (Jia et al., 2020; Krishna et al., 2009). In a population-pharmacokinetic study in children, Boonsathorn et al. found a 42% reduction in relative bioavailability in patients taking PPIs (Boonsathorn et al., 2019). Co-administration of PPIs was associated with a 64.4% reduction in PCZ plasma concentration in our study (Figure 6). It is unlikely that this interaction is cytochrome P450 mediated because PCZ undergoes limited metabolism primarily by UDP-glucuronosyltransferase UGT1A4 (Lipp, 2010). The later study also confirmed that the pH-dependent solubility of PCZ through monitoring of intraluminal PCZ concentrations (Walravens et al., 2011). We did not observe phase II glucuronide enzyme inducers such as phenytoin or rifampicin to be significantly associated with dose-normalized PCZ concentration (Table 4), probably due to the low samples taken concurrently with these drugs (0.5 and 2%, respectively). The univariate and multiple linear regression analysis both showed that lower PCZ concentrations were observed with increasing CRP concentrations. However, the effect is less distinct compared to adult patients (Jia et al., 2020). Therefore, therapeutic drug monitoring of PCZ should be warranted in pediatric patients with elevated CRP level.
There are limitations in our study. This was a single-institution retrospective study, and polymorphisms that could influence plasma PCZ concentration were not checked. In addition, TDM of PCZ was not routinely performed during the study period (especially low numbers of cases in children with IFI). Further pediatric studies are needed to confirm the results of the present study, and especially to standardize TDM and dose adjustment of PCZ to improve attainment of target concentrations.
This study confirmed that age, CRP, ALT and co-administration of PPIs were independent factors affecting PCZ exposure in children. In prophylaxis group, therapeutic concentrations of PCZ can be obtained in the majority of children with a dose of 14.17 mg/kg/day. In the treatment groups, the dose was 19.56 mg/kg/day. Younger patients might need a higher dosage of PCZ to achieve target concentrations compared to older children.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Human Research Ethics Committee of The First Affiliated Hospital of Zhengzhou University (2021-KY- 0365-002).
XZ and JY designed the clinical trial. MJ, ZQ and QZ performed the drug concentration analyses. PL and DW performed the clinical data collection. MJ wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China (No. 2020YFC2008304), Henan Province medical science and technology tackling key problems provincial and ministerial joint construction Youth Project (No. SBGJ202103086), Henan science and technology research plan project (No. 222102310538), and the China International Medical Foundation (No. Z-2018-31-2102).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Yang Wang and Han Xing for their help with statistical analysis and the manuscript.
Arrieta, A. C., Sung, L., Bradley, J. S., Zwaan, C. M., Gates, D., Waskin, H., et al. (2019). A Non-randomized Trial to Assess the Safety, Tolerability, and Pharmacokinetics of Posaconazole Oral Suspension in Immunocompromised Children with Neutropenia. PLoS One 14, e0212837. doi:10.1371/journal.pone.0212837
Ashbee, H. R., Barnes, R. A., Johnson, E. M., Richardson, M. D., Gorton, R., and Hope, W. W. (2014). Therapeutic Drug Monitoring (TDM) of Antifungal Agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 69, 1162–1176. doi:10.1093/jac/dkt508
Bernardo, V., Miles, A., Fernandez, A. J., Liverman, R., Tippett, A., and Yildirim, I. (2020). Initial Posaconazole Dosing to Achieve Therapeutic Serum Posaconazole Concentrations Among Children, Adolescents, and Young Adults Receiving Delayed-Release Tablet and Intravenous Posaconazole. Pediatr. Transpl. 24, e13777. doi:10.1111/petr.13777
Boonsathorn, S., Cheng, I., Kloprogge, F., Alonso, C., Lee, C., Doncheva, B., et al. (2019). Clinical Pharmacokinetics and Dose Recommendations for Posaconazole in Infants and Children. Clin. Pharmacokinet. 58, 53–61. doi:10.1007/s40262-018-0658-1
Chau, M. M., Kong, D. C., van Hal, S. J., Urbancic, K., Trubiano, J. A., Cassumbhoy, M., et al. (2014). Consensus Guidelines for Optimising Antifungal Drug Delivery and Monitoring to Avoid Toxicity and Improve Outcomes in Patients with Haematological Malignancy, 2014. Intern. Med. J. 44, 1364–1388. doi:10.1111/imj.12600
Chinese Invasive Fungal Infection Working Group (2020). The Chinese Guidelines for the Diagnosis and Treatment of Invasive Fungal Disease in Patients with Hematological Disorders and Cancers (The 6th Revision). Zhonghua Nei Ke Za Zhi 59, 754–763. doi:10.3760/cma.j.cn112138-20200627-00624
Pauw, B. D., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., et al. (2008). Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821. doi:10.1086/588660
Dolton, M. J., Brüggemann, R. J., Burger, D. M., and McLachlan, A. J. (2014). Understanding Variability in Posaconazole Exposure Using an Integrated Population Pharmacokinetic Analysis. Antimicrob. Agents Chemother. 58, 6879–6885. doi:10.1128/AAC.03777-14
Dolton, M. J., Ray, J. E., Chen, S. C., Ng, K., Pont, L., and McLachlan, A. J. (2012). Multicenter Study of Posaconazole Therapeutic Drug Monitoring: Exposure-Response Relationship and Factors Affecting Concentration. Antimicrob. Agents Chemother. 56, 5503–5510. doi:10.1128/AAC.00802-12
Döring, M., Cabanillas Stanchi, K. M., Klinker, H., Eikemeier, M., Feucht, J., Blaeschke, F., et al. (2017). Posaconazole Plasma Concentrations in Pediatric Patients Receiving Antifungal Prophylaxis during Neutropenia. Med. Myco. 55, myw091–384. doi:10.1093/mmy/myw091
Groll, A. H., Castagnola, E., Cesaro, S., Dalle, J. H., Engelhard, D., Hope, W., et al. (2014). Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for Diagnosis, Prevention, and Treatment of Invasive Fungal Diseases in Paediatric Patients with Cancer or Allogeneic Haemopoietic Stem-Cell Transplantation. Lancet Oncol. 15, e327–40. doi:10.1016/S1470-2045(14)70017-8
Gwee, A., Cranswick, N., and Curtis, N. (2015). Posaconazole: Promising but Problematic in Practice in Pediatric Patients. Pediatr. Infect. Dis. J. 34, 604–606. doi:10.1097/INF.0000000000000635
Jancel, T., Shaw, P. A., Hallahan, C. W., Kim, T., Freeman, A. F., Holland, S. M., et al. (2017). Therapeutic Drug Monitoring of Posaconazole Oral Suspension in Paediatric Patients Younger Than 13 Years of Age: a Retrospective Analysis and Literature Review. J. Clin. Pharm. Ther. 42, 75–79. doi:10.1111/jcpt.12483
Jang, S. H., Colangelo, P. M., and Gobburu, J. V. (2010). Exposure-response of Posaconazole Used for Prophylaxis against Invasive Fungal Infections: Evaluating the Need to Adjust Doses Based on Drug Concentrations in Plasma. Clin. Pharmacol. Ther. 88, 115–119. doi:10.1038/clpt.2010.64
Jia, M. M., Zhang, Q. W., Qin, Z. F., Lu, R. Q., Tian, X. K., Yang, J., et al. (2020). Deciphering the Relationship between the Trough Concentration of Posaconazole and its Efficacy and Safety in Chinese Patients with Hematological Disorders. Front. Pharmacol. 11, 575463. doi:10.3389/fphar.2020.575463
Krishna, G., Moton, A., Ma, L., Medlock, M. M., and McLeod, J. (2009). Pharmacokinetics and Absorption of Posaconazole Oral Suspension under Various Gastric Conditions in Healthy Volunteers. Antimicrob. Agents Chemother. 53, 958–966. doi:10.1128/AAC.01034-08
Krishna, G., Sansone-Parsons, A., Martinho, M., Kantesaria, B., and Pedicone, L. (2007). Posaconazole Plasma Concentrations in Juvenile Patients with Invasive Fungal Infection. Antimicrob. Agents Chemother. 51, 812–818. doi:10.1128/AAC.00454-06
Lai, T., Alffenaar, J. W., Kesson, A., Bandodkar, S., and Roberts, J. A. (2020). Evaluation of Target Attainment of Oral Posaconazole Suspension in Immunocompromised Children. J. Antimicrob. Chemother. 75, 726–729. doi:10.1093/jac/dkz481
Lavigne, S., Fisher, B. T., Ellis, D., Zaoutis, T. E., and Downes, K. J. (2019). Posaconazole Administration in Hospitalized Children in the United States. J. Pediatr. Infect Dis Soc 8, 481–484. doi:10.1093/jpids/piy119
Lehrnbecher, T., and Groll, A. H. (2011). Invasive Fungal Infections in the Pediatric Population. Expert Rev. Anti Infect. Ther. 9, 275–278. doi:10.1586/eri.11.1
Lipp, H. P. (2010). Clinical Pharmacodynamics and Pharmacokinetics of the Antifungal Extended-Spectrum Triazole Posaconazole: an Overview. Br. J. Clin. Pharmacol. 70, 471–480. doi:10.1111/j.1365-2125.2010.03680.x
Mathew, S., Kussin, M. L., Liu, D., Pozotrigo, M., Seyboth, B., Thackray, J., et al. (2017). Retrospective Analysis of Posaconazole Suspension Dosing Strategies in a Pediatric Oncology Population: Single-center Experience. J. Pediatr. Infect Dis Soc 6, e149–e151. doi:10.1093/jpids/pix058
McMahon, J., Théorêt, Y., Autmizguine, J., Bittencourt, H., Tapiéro, B., and Ovetchkine, P. (2017). Posaconazole Plasma Monitoring in Immunocompromised Children. J. Pediatr. Infect Dis Soc 6, 389–392. doi:10.1093/jpids/piw087
Moore, J. N., Healy, J. R., and Kraft, W. K. (2015). Pharmacologic and Clinical Evaluation of Posaconazole. Expert Rev. Clin. Pharmacol. 8, 321–334. doi:10.1586/17512433.2015.1034689
National Cancer Institute (2006). Common Terminology Criteria for Adverse Events: National Cancer Institute Online Guidelines. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Sienkiewicz-Oleszkiewicz, B., Urbańczyk, K., Stachowiak, M., Rodziewicz, A., Zięba, A., Kałwak, K., et al. (2019). Factors Influencing the Safety and Efficiency of Antifungal Prophylaxis with Posaconazole in Children with Hematological Diseases: From Genetics to Polypharmacotherapy. Indian J. Hematol. Blood Transfus. 35, 699–706. doi:10.1007/s12288-019-01134-5
Vicenzi, E. B., Calore, E., Decembrino, N., Berger, M., Perruccio, K., Carraro, F., et al. (2018). Posaconazole Oral Dose and Plasma Levels in Pediatric Hematology-Oncology Patients. Eur. J. Haematol. 100, 315–322. doi:10.1111/ejh.13017
Vicenzi, E. B., and Cesaro, S. (2018). Posaconazole in Immunocompromised Pediatric Patients. Expert Rev. Anti Infect. Ther. 16, 543–553. doi:10.1080/14787210.2018.1490177
Walravens, J., Brouwers, J., Spriet, I., Tack, J., Annaert, P., and Augustijns, P. (2011). Effect of pH and Comedication on Gastrointestinal Absorption of Posaconazole: Monitoring of Intraluminal and Plasma Drug Concentrations. Clin. Pharmacokinet. 50, 725–734. doi:10.2165/11592630-000000000-00000
Keywords: posaconazole, pediatric patients, invasive fungal infections, therapeutic drug monitoring, pharmacokinetics
Citation: Jia M, Zhang Q, Qin Z, Wang D, Liu P, Yang J and Zhang X (2022) Dose Optimisation of Posaconazole and Therapeutic Drug Monitoring in Pediatric Patients. Front. Pharmacol. 13:833303. doi: 10.3389/fphar.2022.833303
Received: 13 December 2021; Accepted: 04 April 2022;
Published: 19 April 2022.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Fabianne Carlesse, University of São Paulo, BrazilCopyright © 2022 Jia, Zhang, Qin, Wang, Liu, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yang, amluZ3lhbmdfMDEwMUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.