
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 24 January 2022
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.821944
This article is part of the Research Topic Women in Drug Metabolism and Transport: 2021 View all 6 articles
Objective: Pharmacokinetics (PK), pharmacodynamics (PD), safety and immunogenicity studies were conducted to evaluate the bioequivalence of CMAB807, a biosimilar to denosumab (Prolia®), which is the only approved RANKL inhibitor for the treatment of osteoporosis.
Methods: In this randomized, double-blind, single-dose phase I study, 132 healthy Chinese male subjects received a subcutaneous injection of 60 mg of CMAB807 or denosumab at a 1:1 ratio. The PK, PD, safety and immunogenicity results were assessed prior to and up to 126 days after administration.
Results: The PK profiles of CMAB807 and denosumab were similar. The geometric mean ratios of the maximum concentration (Cmax), AUC0-t and AUCo-∞ were 102.41, 104.15 and 103.89%, respectively, and the 90% confidence interval was observed to be within 80.00–125.00%, which indicated the bioequivalence of CMAB807 and denosumab. The PD profiles of the two groups were also comparable. The production of the C-terminal cross-linking telopeptide of type I collagen (CTX1) was inhibited by up to 85% for 10 days, and this inhibition was sustained for up to 126 days in both the CMAB807 and denosumab groups. No subjects in the CMAB807 group, three subjects in the denosumab group before administration, and two subjects in the denosumab group after administration were positive for anti-drug antibody (ADA). Adverse events (AEs) were observed in 98.5% of subjects in both groups. The most common AE recorded was increased parathyroid hormone (PTH) levels, with incidences of 92.4 and 95.5% in the CMAB807 and denosumab groups, respectively. No clinically meaningful differences were observed in safety and immunogenicity between CMAB807 and denosumab.
Conclusion: CMAB807 represents a new potential treatment option for patients with osteoporosis.
Clinical Trial Registration: https://clinicaltrials.gov (Registration No. NCT03925051), http://www.chinadrugtrial/org.cn/index.html (Registration No. CTR20190800).
Denosumab is a fully human IgG κ-type monoclonal antibody (mAB) that binds to receptor activator of nuclear factor-κB ligand (RANKL) (Tanaka et al., 2005). This binding prevents the activation of RANK and inhibits the formation, activation and survival of osteoclasts, thus reducing the number and activity of osteoclasts and subsequently resulting in a reduction in bone resorption and an increase in the bone mass and strength of cortical bone and trabecular bone (Deeks, 2018). Denosumab (Prolia®) was approved by the Food and Drug Administration (FDA) as a treatment to increase bone mass in postmenopausal women with osteoporosis and men with osteoporosis who are at high risk of fractures (Amgen, 2021). However, the high price of denosumab makes it difficult for patients in developing countries such as China to afford.
Biosimilars can improve the health of patients by increasing their accessibility to biological molecules due to decreasing healthcare-associated costs (Mulcahy et al., 2018). Denosumab biosimilars are being actively developed worldwide (Zhang et al., 2020; Khan, 2021; Singh et al., 2021; Zhang et al., 2021). However, no denosumab biosimilar has been marketed in China to date. CMAB807, a recombinant anti-RANKL human mAB injection, is a candidate biosimilar of denosumab developed according to Chinese biosimilar guidelines (NMPA, 2015) and current FDA and European Medicines Agency (EMA) guidelines for the development of biosimilar products (EMA, 2015; FDA, 2015). The similarity of CMAB807 to the reference product was established through a stepwise approach of physicochemical and biological characterization and preclinical comparability experiments (unpublished data).
Comparative pharmacokinetics (PK) studies designed to document similar PK profiles for key parameters of biosimilar and reference medicinal products are an essential component of biosimilar development programs. The primary endpoints are usually PK parameters, such as the area under the curve (AUC) and maximum concentration (Cmax) (NMPA, 2015). Serum concentration-time profiles of denosumab were best characterized by a two-compartment model with first-order absorption and parallel linear and nonlinear elimination (FDA, 2010). In the present study, the PK profile of 60 mg of denosumab was compared with that of CMAB807 to evaluate the bioequivalence of the two products. The pharmacodynamics (PD) profiles, safety and immunogenicity were also evaluated.
We conducted a phase I, double-blind, randomized, parallel-group, single-dose study. One hundred thirty-two 132) healthy Chinese male subjects were planned to be enrolled. This study was performed at the Department of Clinical Pharmacology, Zhongshan Hospital, Fudan University, Shanghai, China, from June 2019 to March 2020. The protocol, informed consent and amendments were approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University. All subjects signed written informed consent forms before participating. The study was registered at ClinicalTrials.gov (Registration No. NCT03925051) and the Chinese Clinical Trial Registry (Registration No. CTR20190800). The study was conducted according to the guidelines for Good Clinical Practice and the ethical standards for human experimentation established in the Declaration of Helsinki.
Eligible subjects were healthy Chinese male subjects aged 18–65 years with a body weight greater than 50 kg (body mass index (BMI) of 19–26 kg/m2). They were deemed healthy after assessments of physical examinations, vital signs, medical history, laboratory safety tests, and 12-lead electrocardiogram (ECG) measurements (QTc<450 ms). In addition, their blood calcium levels were between 2.15 and 2.55 nmol/L (including the borders).
Exclusion criteria included a history of clinically significant allergies, osteomyelitis or osteonecrosis of the jaw, odontopathy or maxillary disease in the active stage or in remission, hyperthyroidism or hypothyroidism, rheumatoid arthritis, osteomalacia, Paget’s disease, lumbar disc herniation, or mental disease. Subjects were excluded if they were positive for hepatitis B surface antigen, human immunodeficiency virus, or hepatitis C antibodies; consumed more than eight cups of caffeine-containing drink per day; smoked more than five cigarettes per day; consumed over 14 servings of alcohol per day; were vaccinated with a live attenuated virus vaccine; lost more than 400 ml of blood; or who had taken any drugs, vitamins or herbal formulations within 30 days.
Subjects were screened 2–30 days prior to the administration of the study drug. Eligible subjects were admitted to the hospital the day before administration. A random table was generated by unblinded statisticians using random blocks with SAS 9.4 statistical analysis software (Cary, NC, United States). Eligible subjects were sorted according to their weights and assigned to the denosumab or CMAB807 group in a 1:1 ratio to minimize interindividual differences in each group. A single dose of CMAB807 (60 mg/1 ml, batch number: 201903001, Shanghai Biomabs Pharmaceutical Co., Ltd., protein concentration: 62.24 mg/ml) was administered to the CMAB807 group, and denosumab (60 mg/1 ml, batch number: 1093571, Amgen, Thousand Oaks, California, United States, protein concentration: 60.6 mg/ml) was administered to the denosumab group subcutaneously within 10 cm of the periumbilical region. Subjects remained in the hospital for 3 days after administration and were followed up on days 4–126.
Blood samples were collected for PK analysis within 30 min prior to dosing (predose) and at 6 h, 2, 3, 4, 6, 8, 10, 12, 14, 18, 21, 28, 35, 42, 56, 70, 84, 112, and 126 days after dosing. Tubes containing whole blood (3 ml) were placed at room temperature for at least 30 min to induce coagulation and centrifuged promptly at 1921 × g at 4°C for 10 min. Shortly after the separation of the whole blood, serum was carefully transferred to another tube and stored at −70°C until shipment to United-Power Pharma Tech Co., Ltd. for analysis using an electrochemiluminescence assay (ECLA). The overall assay accuracy (% deviation from nominal) was -13.9–13.1%, and the precision (% coefficient of variation) was 7.1–16.2%. Both CMAB807 and Prolia® were confirmed to be stable at -90∼-60 °C for 6 months, at -30∼-10 °C for 3 months, at room temperature for 1 h and 10 min, and after five freeze-thaw cycles. Linear calibration curves were obtained in the range of 25.0–6,400 ng/ml, with a lower limit of quantitation (LLOQ) of 25.0 ng/ml and an upper limit of quantitation (ULOQ) of 6,400 ng/ml. Values below the LLOQ were treated as 0 when observed before Cmax and were excluded from the PK analysis when observed after Cmax.
The primary PK endpoints were Cmax, AUC0-t and AUC0-∞. Other parameters assessed for denosumab included the time to reach the maximum concentration (Tmax), apparent terminal t1/2, apparent total plasma clearance (CL/F) and volume of distribution (Vz/F), which were measured as secondary PK parameters. All PK parameters were calculated with a noncompartmental analysis (NCA) using Phoenix® WinNonlin® software (version 8.0, Certara L.P., Princeton, NJ, United States). The actual sample collection times were considered for the PK analysis. Cmax and Tmax were obtained visually from the concentration-time curve. The AUC0-∞ was determined by adding AUC0–t to the extrapolated area, which was calculated using the linear trapezoidal method.
Serum samples used to measure denosumab pharmacodynamics were obtained predose, 6 h, and 3, 10, 28, 56, 84, 112, and 126 days postdose in the fasted state in the morning. Two milliliters of whole blood were collected, centrifuged and stored until PK sample procedures were performed. The serum C-terminal cross-linking telopeptide of type I collagen (CTX1) concentrations were analyzed by United-Power Pharma Tech Co., Ltd. using an electrochemiluminescence immunoassay (ECLIA). Concentrations ranging from 0.010 to 6.00 ng/ml can be accurately detected using the validated method. Serum CTX1 concentrations below the LLOQ (0.010 ng/ml) were set to 0.010 ng/ml before data analysis. The actual sample collection times were also considered for the PD analysis. The concentration-time data included the minimum observable serum CTX1 concentration (Imin), the observed time of the lowest serum CTX1 concentration (Tmin), the observed highest inhibition percentage of CTX1 (Imax), the AUC from zero to 126 days (AUEC0-126d), and the final quantifiable CTX1 concentration (AUEC0-t). Additionally, the percent change in the CTX1 concentration from baseline between the two groups was compared visually.
Blood samples (4 ml) were collected for the immunogenicity evaluation at predose and 28, 56, 84, 112, and 126 days after dosing. The anti-drug antibody (ADA) samples were analyzed at United-Power Pharma Tech Co., Ltd. using the bridging electrochemiluminescence immunoassay (Bridging-ECLIA) and the MSD Discovery Workbench (v 4.0.12).
Clinical safety evaluations were conducted by performing physical examinations and assessing vital signs, 12-lead ECGs, and immunogenicity. Laboratory safety evaluations were conducted and included routine hematology (including hematocrit, hemoglobin, platelet count and white blood cell count), serum chemistry (including alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ-glutamyltranspeptidase, total protein, albumin, total bilirubin, direct bilirubin, creatinine, urea, uric acid, glucose, triglyceride, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), potassium, sodium, calcium, magnesium, phosphorus and chloride), coagulation functions (including the prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), and thrombin time (TT)), thyroid functions (including thyroid stimulating hormone (TSH), free triiodothyronine, free thyroxine, triiodothyronine, thyroxine and parathyroid hormone (PTH)) and urinalysis (including protein, glucose, ketones, white blood cells, blood, pH and gravity). Both clinical and laboratory safety evaluations were performed before and at certain time points after administration. Adverse events (AEs) were recorded and graded according to the Common Terminology Criteria for Adverse Reactions, V. 5.0 (CTCAE 5.0) of the National Cancer Institute and were evaluated for intensity, duration, outcome, and relationship to the study drug by the investigators. Investigators monitored AEs throughout the study.
Sample size calculations were performed using PASS (version 16.0.2, NCSS, Kaysville, UT, United States), assuming that Cmax and AUC were the primary endpoints and AUC0-∞, Tmax, and t1/2 were the secondary endpoints. The coefficient of variation (CV) of the PK parameters was predicted to be 32% based on data obtained from healthy volunteers (Bekker et al., 2004). One hundred sixteen subjects (58 per group) needed to complete the study to achieve 90% power (1-β) at the 5% nominal level (α = 0.05, two one-sided tests) such that the 90% confidence intervals (CIs) for the geometric mean ratio (GMR) fell within the acceptance criterion of 80.00–125.00%. Allowing for 10% potential drop-outs, the sample size was defined to be 132 (66 per group).
All subjects who were treated with the study drug were included in the safety analysis set. Subjects who were administered the study drug and had at least one PK or PD parameter collected were included in the PK or PD set populations, respectively. In addition, subjects whose drug concentration before administration was greater than 5% Cmax were eliminated from the PK analysis set.
All statistical tests were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, United States). A descriptive statistical analysis was conducted for the PK and PD parameters and the demographic data. The comparisons were performed using the chi-square test for categorical variables, Student’s t-test for normally distributed data, or the Wilcoxon signed-rank test for data with an unknown distribution. p < 0.05 was considered statistically significant.
For PK parameters, analysis of variance (ANOVA) was used to analyze the difference in the least-square means between denosumab and CMAB807 after the natural logarithmic transformation of Cmax, AUC0-t, and AUC0-∞. The GMRs and 90% CIs were obtained after conversion to an inverse natural logarithm. PK values were concluded to be bioequivalent between denosumab and CMAB807 if the 90% CIs for Cmax, AUC0-t, and AUC0-∞ were recorded to be between 80.00 and 125.00%. Notably, t1/2, CL/F and Vz/F were not included if AUC_%Extrap >20%. In addition, the Wilcoxon-signed rank test was used to compare the Tmax of denosumab and its biosimilar.
One hundred thirty-two subjects were enrolled in this study after screening 408 potential subjects. Sixty-six subjects were allocated to the CMAB807 group, and the other 66 subjects were allocated to the denosumab group. All 132 subjects were administered drugs, and at least one value for the PD dataset was collected; therefore, all subjects were included in the safety analysis and PD analysis sets. One subject in each group withdrew from the study for personal reasons. In addition, one subject (No. 1128) in the CMAB807 group was not included in the PK analysis set because his predose drug concentration was greater than 5% of Cmax. Therefore, 65 and 66 subjects were included in the PK analysis set in the CMAB807 and denosumab groups, respectively (Figure 1).
Demographic data are presented in Table 1. The mean age, height, body weight, and BMI were 27.6 years, 170.8 cm, 65.2 kg, and 22.3 kg/m2, respectively. The demographic parameters were not significantly different between the CMAB807 and denosumab groups (p > 0.05).
As shown in Figure 2, the entire PK profiles of CMAB807 and denosumab were similar. The PK parameters of denosumab and its biosimilar and the bioequivalence assessment of the PK parameters in this study are shown in Table 2. Both CMAB807 and denosumab were absorbed after a single-dose SC injection, with a median Tmax of 9 days. Following Tmax, serum concentrations decreased biexponentially with an average apparent terminal t1/2 values of 17.2 and 18.0 days in the CMAB807 and denosumab groups, respectively.
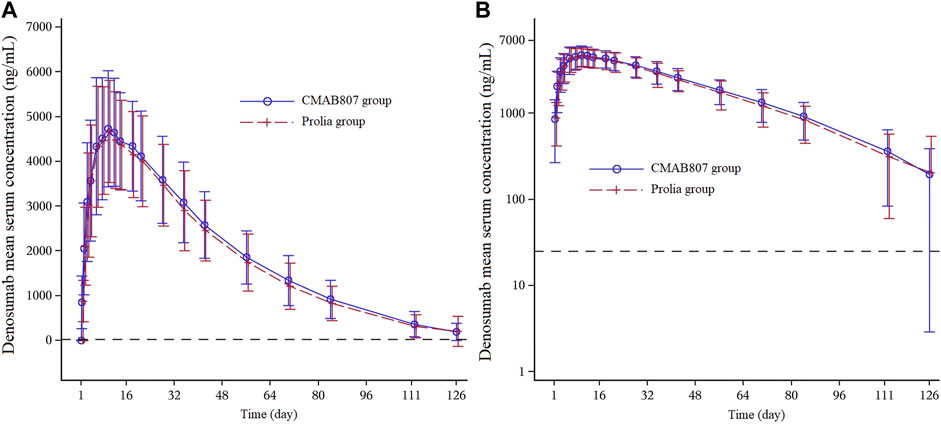
FIGURE 2. Mean serum concentration-time profiles following the SC injection of 60 mg of CMAB807 or denosumab in healthy Chinese male subjects (A): Linear scale (B): semilog scale. Note: the error bars indicate the standard deviations (SD); the dashed horizontal line refers to the LLOQ (25.0 ng/ml).
Cmax, AUC0-t and AUC0-∞ are the primary bioequivalence evaluation parameters in this study. As shown in Table 2, following a single SC administration of CMAB807 or denosumab, the 90% CIs for the GMRs of Cmax, AUC0-t and AUC0-∞ were fully contained within 80.00–125.00%, confirming the bioequivalence between them. In addition, all the primary and secondary PK parameters were comparable in the two groups (p > 0.05).
The median percent change in CTX levels from baseline versus time profiles after the administration of CMAB807 and denosumab are shown in Figure 3. The PD parameters (including Imin, Tmin, Imax, AUEC0-t, and AUEC0–126 d) and the PD profiles of the CMAB807 group and the denosumab group were similar (Table 3; Figure 3). CTX1 production was inhibited by up to approximately 85% (Imax) after the administration of a single dose of denosumab or CMAB807 for 10 days. The median value of Imax was 648 h in both groups. The inhibition status was sustained for up to 126 days. The CTX1 reduction rate seemed to be attenuated at the end of the study. On day 126, the median CTX1 inhibition rates of the CMAB807 and denosumab groups were 82.8 and 82.4%, respectively.
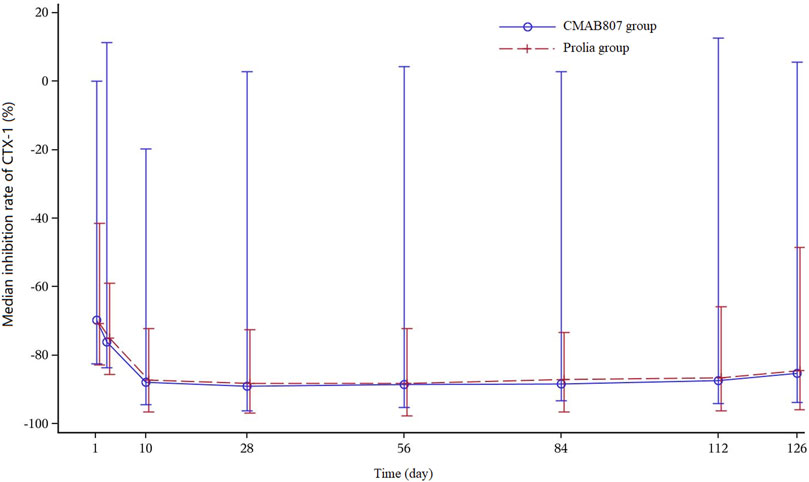
FIGURE 3. Median percent change in the CTX1-time profiles from baseline. Note: the error bars indicate the SD.
The PK and PD profiles indicate that the concentration-effect relationship for the two treatments is not different and that equivalent exposure results in an equivalent response, thus supporting their biosimilarity.
All subjects in the CMAB807 group were negative for ADA before dosing and throughout the study period. In the denosumab group, three subjects (No. 1004, 1011, and 1123) were positive for ADA before administration, and two subjects (No. 1011 and 1033) were ADA-positive after administration (on day 28 and day 84, respectively) but were negative at the end of the study. Therefore, the ADA characteristics between CMAB807 and denosumab were similar in this study.
Treatment-emergent adverse events (TEAEs) among 65 subjects (199 events, 98.5% [65/66]) in the CMAB807 group and 65 subjects (191 events, 98.5% [65/66]) in the denosumab group were recorded. No AEs led to death or discontinuation. No abnormal reactions at the injection site were observed. Most TEAEs were grade 1 or 2. Drug-related TEAEs (greater than grade 3 based on the CTCAE 5.0 criterion) were observed in three subjects in the CMAB807 group (increased ALT, AST, and triglyceride levels) and in one subject in the denosumab group (increased AST level). One serious adverse event (SAE) was reported in the CMAB807 group (open injury of the left hand because of an accident) and was estimated as grade 3, but not drug-related by the investigating physician. No drug-related SAE was reported (Table 4).
All of the common TEAEs (≥5% of subjects in any of the treatment groups) were comparable in the two groups (p ≥ 0.05). The most common AEs in this study were increased parathyroid hormone (PTH) levels (92.4 vs 95.5%, CMAB807 vs Denosumab) (Table 4). Increased PTH levels were observed 8 days after administration and generally decreased after the 21st day. Most of the subjects returned to the baseline level at the end of the study without any treatment (Figure 4). The PTH profile was generally consistent with the PK characteristics of denosumab (median Tmax = 9 days, range from 3 to 21 days). Among the subjects with elevated serum PTH levels, some also had hypophosphatemia, but very few had hypocalcemia. No clinically significant changes corresponding to the increased PTH levels were found during thyroid function detection. Thus, after the administration of 60 mg of CMAB807 or denosumab, the incidence of AEs that appeared in the two groups was similar, and both of the study drugs were considered generally safe and well tolerated.
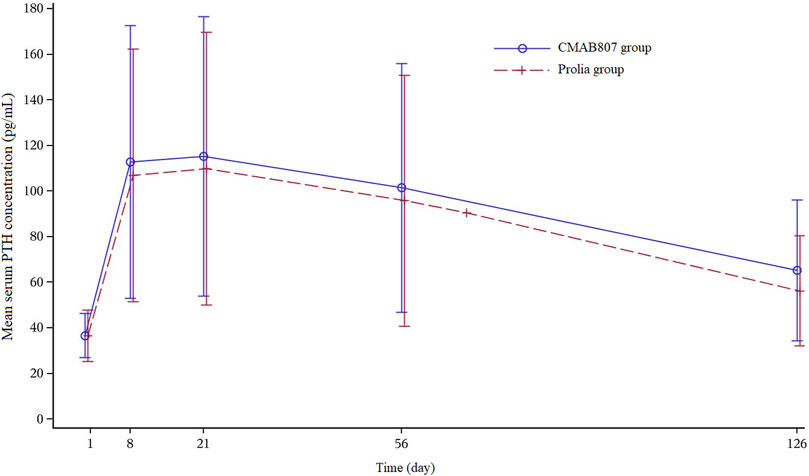
FIGURE 4. Mean serum PTH concentration versus time profiles after the SC injection of 60 mg of CMAB807 or denosumab in healthy Chinese male subjects. Note: the error bars indicate the SD.
Denosumab and its biosimilar (CMAB807) were generally well tolerated. The common TEAEs that occurred in this study were generally consistent with those listed on the label of denosumab, such as hypocalcemia, hypophosphatemia, increased PTH levels, and arthralgia (Amgen, 2021). The only SAE in the study was an “open injury of the hand” by accident in the CMAB807 group, which was judged as a nondrug-related AE. Furthermore, the number of drug-related TEAEs over CTCAE grade 3 in the CMAB807 group (3 events, which were increased ALT, increased AST and hypertriglyceridemia, respectively) was greater than the number in the Prolia® group (1 event, which was increased AST). However, there were also subjects with increased ALT and hypertriglyceridemia in the Prolia® group, just not to the extent of grade 3. The difference between the two groups was not statistically significant. The most common TEAE in this study was an increased PTH level (92.4% in the CMAB807 group and 95.5% in the denosumab group). The PTH level was temporarily increased by denosumab treatment. PTH is a critical regulator of skeletal development that promotes both bone formation and bone resorption (Li et al., 2020) and regulates calcium and phosphorus homeostasis (Civitelli and Ziambaras, 2011). The calcium status is strictly regulated by intestinal calcium absorption, bone resorption, and renal reabsorption (Nakamura et al., 2015). Therefore, denosumab strongly inhibits bone resorption, resulting in an increase in calcium release from the bones, causing hypocalcemia, followed by effects on the parathyroid gland, which is the reason for the increased serum PTH level and decreased serum phosphate level.
As the distribution and elimination of mABs are often proportional to body weight (Wang et al., 2008; Grant, 2009; Glassman and Balthasar, 2016), subjects were sorted according to their weights to reduce PK errors. However, a population PK meta-analysis of 22,944 serum-free denosumab concentrations in 495 healthy subjects and 1069 patients showed that the denosumab PK parameters do not depend on body weight (Sutjandra et al., 2011). Additionally, differences in exposure do not affect the response to denosumab (Chen et al., 2018). Therefore, a fixed dose (60 mg) for all patients is supported.
Overall, six subjects in the CMAB807 group and two subjects in the denosumab group received treatment for various adverse reactions. As denosumab and its biosimilar are recombinant mABs whose metabolic pathway usually involves the phagocytic function of mesenchymal cells rather than cytochrome P450 metabolism (Zhang et al., 2021), concomitant medications were considered to have little effect on the PK/PD characteristics of denosumab and its biosimilar.
Serum denosumab concentrations decreased at a faster rate when the serum denosumab concentration was less than approximately 1 μg/ml. The mechanism underlying this change in elimination rate is likely related to denosumab binding to RANKL (i.e., target-mediated disposition). This mechanism predominates at low serum denosumab concentrations (i.e., <1 μg/ml in this case) and becomes saturated as the serum denosumab concentration increases (An, 2020). A bioequivalence study confirmed that CMAB807 showed PK profiles similar to those of denosumab evaluated in healthy Chinese male subjects. The 90% CIs of the test-to-reference ratios for the primary PK endpoints, namely, Cmax, AUC0-∞ and AUC0-last, were observed to be within the predefined bioequivalence approved range of 80.00–125.00%, according to Chinese biosimilar guidelines (NMPA, 2015). The PK profiles in this study were also generally comparable to the profiles observed in Caucasian and Chinese populations in previous reports (Chen et al., 2018; Amgen, 2020; Zhang et al., 2021).
Denosumab administration resulted in significant inhibition of bone resorption, as assessed by CTX1 levels, which is a specific biomarker of bone reabsorption, whose serum concentration is suggestive of osteoclast activity (Williams and Sapra, 2021). In previous clinical studies of Caucasians, treatment with 60 mg of denosumab reduced CTX1 levels by approximately 70% in 6 h and 85% by 3 days (10 days in this study), with maximal reductions occurring by 1 month (Amgen, 2021). In the present study, the CTX1 level was reduced by 85% by 10 days, with a Tmin of 27 days, which is comparable to previous results obtained from the Chinese population (Chen et al., 2018; Zhang et al., 2020). The administration of denosumab to Chinese patients appears to result in a less rapid reduction in CTX1 levels than in Caucasians. Reduced CTX1 levels were partially attenuated from a maximal reduction of ≥87% to ≥45% (range: 45–80%) at 180 days after administration, when serum denosumab levels were diminished, reflecting the reversibility of the effects of denosumab on bone remodeling (Amgen, 2021). However, in the present study, the recovery of the CTX1 inhibition rate was not fully observed because of the limited follow-up duration (126 days). The PK and PD similarities of CMAB807 and denosumab support further study of this biosimilar in phase III studies.
The immunogenicity and safety were analyzed. The overall ADA-positive rates were as low as 0% (0/66) in the CMAB807 group and 4.5% (3/66) and 3.0% (2/66) in the denosumab group before and after administration, respectively, which were similar to the positive rates reported in previous studies in both healthy subjects and patients (Amgen, 2021). The detection of ADAs does not significantly affect the PK parameters of denosumab, CTX1 inhibition rate (Zhang et al., 2020; Zhang et al., 2021), and clinically significant adverse reactions (FDA, 2010).
In this phase I clinical study, the PK, PD and ADA profiles of the denosumab biosimilar (CMAB807) were similar to those of denosumab. The safety data were also comparable between the two groups. These results support the efficacy of CMAB807 as a denosumab biosimilar.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Zhangshan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
XL, QG and XZ designed the experiment, HC, WC and FY performed the clinic trials. HC, QG and XZ analyzed the data. HC wrote the manuscript. XL revised the manuscript.
This study received funding from Shanghai Biomabs Pharmaceutical Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Authors QG, XZ and CW were employed by Taizhou Mabtech Pharmaceuticals Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank for the help of United-Power Pharma Tech Co. in serum sample bioanalysis. Also, we want to thank all the subjects who participating this study.
Amgen (2021). Prolia (Denosumab) Highlights of Prescribing Information. Available at: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/prolia/prolia_pi.pdf. (Accessed Aug 12, 2021).
Amgen (2020). Prolia (Denosumab Injection) Product Monograph. Available at: https://www.amgen.ca/products/∼/media/1e79aee7d94340df88c3d97f5bb897c3.ashx. (Accessed Aug 12, 2021).
An, G. (2020). Concept of Pharmacologic Target-Mediated Drug Disposition in Large-Molecule and Small-Molecule Compounds. J. Clin. Pharmacol. 60 (2), 149–163. doi:10.1002/jcph.1545
Bekker, P. J., Holloway, D. L., Rasmussen, A. S., Murphy, R., Martin, S. W., Leese, P. T., et al. (2004). A Single-Dose Placebo-Controlled Study of AMG 162, a Fully Human Monoclonal Antibody to RANKL, in Postmenopausal Women. J. Bone Miner. Res. 19 (7), 1059–1066. doi:10.1359/JBMR.040305
Chen, Q., Hu, C., Liu, Y., Song, R., Zhu, W., Zhao, H., et al. (2018). Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Single-Dose Denosumab in Healthy Chinese Volunteers: A Randomized, Single-Blind, Placebo-Controlled Study. PLoS One 13 (6), e0197984. doi:10.1371/journal.pone.0197984
Civitelli, R., and Ziambaras, K. (2011). Calcium and Phosphate Homeostasis: Concerted Interplay of New Regulators. J. Endocrinol. Invest. 34 (7 Suppl), 3–7. doi:10.1080/07853890701689645
Deeks, E. D. (2018). Author Correction to: Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging 35 (3), 261. doi:10.1007/s40266-018-0535-5
EMA (2015). Guideline on Similar Biological Medicinal Products. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf. (Accessed Jan 11, 2022).
FDA (2010). Clinical Pharmacology Biopharmaceutics Reviews. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/125320s000ClinPharmR.pdf. (Accessed Aug 12, 2021).
FDA (2015). Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product. (Accessed Jan 11, 2022).
Glassman, P. M., and Balthasar, J. P. (2016). Physiologically-based Pharmacokinetic Modeling to Predict the Clinical Pharmacokinetics of Monoclonal Antibodies. J. Pharmacokinet. Pharmacodyn 43 (4), 427–446. doi:10.1007/s10928-016-9482-0
Grant, S. K. (2009). Therapeutic Protein Kinase Inhibitors. Cell. Mol. Life Sci. 66 (7), 1163–1177. doi:10.1007/s00018-008-8539-7
Jose, V., Singh, I., Patel, R., and Arora, S. (2021). Denosumab Biosimilar in Postmenopausal Osteoporotic Women: A Randomized, Assessor-Blind, Active-Controlled Clinical Trial. Indian J. Pharmacol. 53 (1), 6–12. doi:10.4103/ijp.IJP_346_19
Khan, T. H. (2021). Received Acceptance Notification for Clinical Trial Application from NMPA for its Denosumab Biosimilar HLX14. Available at: https://pharmashots.com/press-releases/henlius-received-acceptance-notification-for-clinical-trial-application-from-nmpa-for-its-denosumab-biosimilar-hlx14/. (Accessed Jan 11, 2022).
Li, J. Y., Yu, M., Pal, S., Tyagi, A. M., Dar, H., Adams, J., et al. (2020). Parathyroid Hormone-dependent Bone Formation Requires Butyrate Production by Intestinal Microbiota. J. Clin. Invest. 130 (4), 1767–1781. doi:10.1172/JCI133473
Mulcahy, A. W., Hlavka, J. P., and Case, S. R. (2018). Biosimilar Cost Savings in the United States: Initial Experience and Future Potential. Available at: https://www.rand.org/pubs/periodicals/health-quarterly/issues/v7/n4/03.html. (Accessed January 11, 2022).
Nakamura, Y., Kamimura, M., Ikegami, S., Mukaiyama, K., Uchiyama, S., Taguchi, A., et al. (2015). Changes in Serum Vitamin D and PTH Values Using Denosumab with or without Bisphosphonate Pre-treatment in Osteoporotic Patients: a Short-Term Study. BMC Endocr. Disord. 15 (1), 81. doi:10.1186/s12902-015-0077-3
NMPA, (2015). Guidelines on Development and Evaluation of Biosimilars. Available at: http://www.cde.org.cn/zdyz.do?method=largePage&id=2f41e8f3c64fedad. (Accessed Jan 11, 2022).
Sutjandra, L., Rodriguez, R. D., Doshi, S., Ma, M., Peterson, M. C., Jang, G. R., et al. (2011). Population Pharmacokinetic Meta-Analysis of Denosumab in Healthy Subjects and Postmenopausal Women with Osteopenia or Osteoporosis. Clin. Pharmacokinet. 50 (12), 793–807. doi:10.2165/11594240-000000000-00000
Tanaka, S., Nakamura, K., Takahasi, N., and Suda, T. (2005). Role of RANKL in Physiological and Pathological Bone Resorption and Therapeutics Targeting the RANKL-RANK Signaling System. Immunol. Rev. 208, 30–49. doi:10.1111/j.0105-2896.2005.00327.x
Wang, W., Wang, E. Q., and Balthasar, J. P. (2008). Monoclonal Antibody Pharmacokinetics and Pharmacodynamics. Clin. Pharmacol. Ther. 84 (5), 548–558. doi:10.1038/clpt.2008.170
Williams, C., and Sapra, A. (2021). Osteoporosis Markers. Available at: https://www.ncbi.nlm.nih.gov/books/NBK559306/ (Accessed Aug 12, 2021).
Zhang, H., Wu, M., Zhu, X., Li, C., Li, X., Sun, J., et al. (2020). A Phase I, Randomized, Single-Dose Study to Evaluate the Biosimilarity of QL1206 to Denosumab Among Chinese Healthy Subjects. Front. Pharmacol. 11, 01329. doi:10.3389/fphar.2020.01329
Keywords: denosumab, biosimilar, pharmacokinetics, pharmacodynamics, immunogenicity, osteoporosis, RANKL
Citation: Chen H, Chen W, Yuan F, Guo Q, Zhang X, Wang C and Li X (2022) Pharmacokinetics, Pharmacodynamics, Safety and Immunogenicity of CMAB807, a New Denosumab Biosimilar, in Healthy Chinese Subjects. Front. Pharmacol. 13:821944. doi: 10.3389/fphar.2022.821944
Received: 25 November 2021; Accepted: 04 January 2022;
Published: 24 January 2022.
Edited by:
Sabina Passamonti, University of Trieste, ItalyCopyright © 2022 Chen, Chen, Yuan, Guo, Zhang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuening Li, bGkueHVlbmluZ0B6cy1ob3NwaXRhbC5zaC5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.