
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 25 March 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.820806
This article is part of the Research Topic MSPP 34th Scientific Meeting: Pharmacological Perspectives on Natural Products in Drug Discovery View all 10 articles
 Shivkanya Fuloria1†
Shivkanya Fuloria1† Jyoti Mehta2†
Jyoti Mehta2† Aditi Chandel2
Aditi Chandel2 Mahendran Sekar3†
Mahendran Sekar3† Nur Najihah Izzati Mat Rani4
Nur Najihah Izzati Mat Rani4 M. Yasmin Begum5
M. Yasmin Begum5 Vetriselvan Subramaniyan6
Vetriselvan Subramaniyan6 Kumarappan Chidambaram7
Kumarappan Chidambaram7 Lakshmi Thangavelu8
Lakshmi Thangavelu8 Rusli Nordin6
Rusli Nordin6 Yuan Seng Wu9
Yuan Seng Wu9 Kathiresan V. Sathasivam10
Kathiresan V. Sathasivam10 Pei Teng Lum3
Pei Teng Lum3 Dhanalekshmi Unnikrishnan Meenakshi11
Dhanalekshmi Unnikrishnan Meenakshi11 Vinoth Kumarasamy6,12
Vinoth Kumarasamy6,12 Abul Kalam Azad1*
Abul Kalam Azad1* Neeraj Kumar Fuloria1,8*†
Neeraj Kumar Fuloria1,8*†Curcuma longa Linn. (C. longa), popularly known as turmeric, belongs to the Zingiberaceae family and has a long historical background of having healing properties against many diseases. In Unani and Ayurveda medicine, C. longa has been used for liver obstruction and jaundice, and has been applied externally for ulcers and inflammation. Additionally, it is employed in several other ailments such as cough, cold, dental issues, indigestion, skin infections, blood purification, asthma, piles, bronchitis, tumor, wounds, and hepatic disorders, and is used as an antiseptic. Curcumin, a major constituent of C. longa, is well known for its therapeutic potential in numerous disorders. However, there is a lack of literature on the therapeutic potential of C. longa in contrast to curcumin. Hence, the present review aimed to provide in-depth information by highlighting knowledge gaps in traditional and scientific evidence about C. longa in relation to curcumin. The relationship to one another in terms of biological action includes their antioxidant, anti-inflammatory, neuroprotective, anticancer, hepatoprotective, cardioprotective, immunomodulatory, antifertility, antimicrobial, antiallergic, antidermatophytic, and antidepressant properties. Furthermore, in-depth discussion of C. longa on its taxonomic categorization, traditional uses, botanical description, phytochemical ingredients, pharmacology, toxicity, and safety aspects in relation to its major compound curcumin is needed to explore the trends and perspectives for future research. Considering all of the promising evidence to date, there is still a lack of supportive evidence especially from clinical trials on the adjunct use of C. longa and curcumin. This prompts further preclinical and clinical investigations on curcumin.
Herbs and natural products have long been exploited by humans to combat numerous diseases since the dawn of time. The Indian subcontinent boasts diverse flora, including both aromatic and therapeutic species. After all, contributions should be made to analyze, standardize, and confirm Unani and Ayurvedic medication for potential, safety, and effectiveness prior to actually introducing them to the market as first-line drug delivery. Plant-based therapies are being used across all civilizations. Plant-based medicines are already extensively utilized, and several countries invest 40%–50% of their total health budget to produce novel drugs. Herbal medicines are assumed to have a beneficial effect on health without any side effects.
The genus Curcuma has been employed from many years back due to its medicinal applications; it is composed of approximately 133 species worldwide. C. longa (Haridra), C. aromatica Salisb (Vana Haridra), C. angustifolia Roxb., C. zanthorrhiza Roxb., C. amada Roxb (Amaragandhi Haridra), C. caesia Roxb (Kali Haridra), and C. zedoaria Rosc (Zedoary) are common species of genus Curcuma found in several regions of the globe. Curcuma longa Linn. (C. longa) is the common tall herb that flourishes in tropical as well as in other Indian regions and is referred to as “Indian saffron or The Golden Spice of India” because of its use in a broad range of diseases in Indian homes as a spice, food preservative, and coloring source. C. longa belonging to the Zingiberaceae (ginger) family is a perennial plant commonly planted in Asian nations. It is among the oldest spices of India that have been used in Western and Southern parts for centuries, and is a significant part of Ayurvedic medicine. In Ayurveda, the therapeutic effects of C. longa have been well established and are discussed in Dashemani Lekhaniya (emaciating), Kusthagna (anti-dermatosis), and Visaghna (anti-poisonous) texts. It is known by many distinct names such as Haridra in Sanskrit, Haldi in Hindi, Jianghuang (yellow ginger in Chinese), manjal in South India, and Kyoo or Ukon in Japanese, which means an effective medication for jaundice (Sharma, 2000). C. longa is also extensively described in Indian material medica (Dravyaguna Shastra) and is used in the beauty regimen of Hindu girls where it is applied daily on their foreheads. The bride is smeared with a C. longa paste, which is a key aspect of Hindu tradition (Paranjpe and Pranjpe, 2001). It is well recognized by the Chinese, Japanese, and Korean Pharmacopoeias, and its application spans a broad range of medical conditions. In China, it is being used to relieve urticaria, dermatitis, hepatitis infection, inflammatory joints, sore throat, and wounds. It was mentioned in Hindu Mythology manuscripts as an aromatic stimulant and carminative. Turmeric powder combined with calcium hydroxide is indeed a popular home remedy for treating sprains and swelling induced by wounds or might be applied directly over the injury site. Traditional medicine has exploited dried curcumin powder to treat illnesses in history. C. longa is said to have antitoxic, anticancer, antibacterial, anti-inflammatory, and antioxidant effects (Ghotaslou et al., 2017). The tuberous rhizome from which C. longa is formed has a coarse and segmented skin. In the ground soil, the rhizomes mature underneath the foliage. The matured rhizomes have a yellowish-brown color with a dull orange from inside. Small pointed or tapered tubers sprout off the main rhizome measuring 2.5–7.0 cm (1–3 inches) in length and 2.5 cm (1 inch) in diameter (Prasad and Aggarwal, 2011). The dry rhizome is ground into a yellow powder form that has a bitter, yet sweet taste. A yellow-colored substance derived from the rhizome is curcumin (1,7-bis[4-hydroxy-3- methoxyphenyl]-1,6-heptadiene-3,5-dione), a combined form of resin and oil. Rhizome powder is supposed to flavor various cuisines and treat numerous disorders, including inflammation, flatulence, jaundice, menstrual troubles, hematuria, and hemorrhage. It is also a useful ointment to treat several skin disorders. Curcumin or diferuloylmethane and numerous volatile oils. C. longa of India is particularly popular when compared with those from other countries due to its high curcumin concentration, which is the most essential and active biological ingredient responsible for its therapeutic potential (Verma et al., 2018). Curcumin is a flavonoid having a lipophilic affinity that is practically water-insoluble (Dave et al., 2017) yet quite stable at the stomach’s acidic pH. C. longa and curcumin show antioxidant features close to vitamins C and E in both aqueous and fat-soluble extracts.
Due to shortcomings in the earlier published review articles, such as a lack of information on the therapeutic potential of C. longa in relation to its major compound curcumin, we have attempted to provide in-depth information by highlighting knowledge gaps in traditional and scientific evidence about C. longa in relation to the therapeutic potential of curcumin against numerous disorders. This review mainly focuses on the distribution, cultivation, botany, nutritional composition, phytochemistry, toxicology, traditional and medicinal properties, and safety aspects including the pharmacological activities of C. longa in relation to its major compound curcumin. This review will further discuss the current advances in C. longa and curcumin, such as the utilization of nanocarriers to increase curcumin bioavailability and overcome all the disadvantages in relation to drug delivery.
C. longa is a perennial herb with no stem and rootstock. Their leaves are 1 m long, lanceolate or oblong, dark green from the upper surface and pale green from beneath. The petiole and sheath are about the same length as the blade. Spike makes its appearance before the leaves. Flowers are sterile, pale yellow with a reddish covering, and flowering bract is green with a deep ferruginous purplish color. It has a 2-m-long, erect leafy shoot (pseudostems) (Rajkumari and Sanatombi, 2017) bearing 8–12 leaves and is commonly grown in rural backyard gardens. Its medicinally important parts are presented in Figure 1. The rhizomes have a balmy smell and bitter in taste (Puteri et al., 2020). The taxonomic classification of C. longa is shown in Figure 2.

FIGURE 1. Important parts of C. longa. (A) C. longa in natural habitat, (B) medicinally important part of C. longa (rhizome), and (C) powder of dried rhizome of C. longa (used as a coloring agent in food).
Turmeric is believed to have originated from South or Southeast Asia, more likely in Vietnam, China, or western India. It is only identified as a domesticated plant and has not been found in the wild. India is the biggest producer, consumer, and supplier, but it is also cultivated extensively in Cambodia, Bangladesh, Nepal, Indonesia, Thailand, Cambodia, Malaysia, West Bengal, Madagascar, Tamil Nadu, Maharashtra, Madras Indonesia, and Philippines (Royal Botanic Gardens Kew, 2021). The turmeric plant requires an average temperature from 20 to 30°C and a good annual rainfall to grow. Plant species can reach 1 m in height and have long, oblong leaves. Turmeric can be found in both tropical and subtropical regions. This will thrive best in the dark if not overcrowded, but somehow it also develops larger and better rhizomes when exposed to sunlight. Turmeric usually grows in a humid environment. Harvest time usually lasts from January to March–April. Early types are ready in 7–8 months, while medium types mature in 8–9 months. After the formation of yellow colored leaves, they begin to dry and the crop is ready to be harvested (Soudamini and Kuttan, 1989). Upon ripening, cutting of the leaves are done nearer to the soil surface, the ground is ploughed, and the rhizomes are collected by hand picking or carefully lifting the clusters with a spade. The turmeric plant needs a rich and friable soil with just a little sand content. It grows in irrigated and rain-fed areas on light black, ashy loam, and red soils to stiff loams. Total irrigation for turmeric will be defined by the climatic and soil conditions. Based on the soil types and rainfall, 15–25 irrigations are provided in medium heavy soils, and 35–40 irrigations are needed in light texture red soils. Rhizomes are typically piled under trees for shade or in well-ventilated shelters and finally wrapping is done with turmeric leaves. Matured rhizomes as a seed could be kept in sawdust pits (Aggarwal et al., 2004).
C. longa rhizome is also consumed as an herbal infusion with conventionally produced gin called “ogogoro” by the native individuals of Delta State, Nigeria, with the notion that it heals various ailments. It is used to preserve and flavor food and is also used as a condiment in Nigeria. It is used for wound-healing and pimples in Pakistan and is widely consumed as folk medicine (Wahono et al., 2017). In Bhutanese traditional medicine, it is named Yung-ba, and it is employed as a tonic, an antidote, an antiseptic, an anti-inflammatory agent, and a preservative (Ayati et al., 2019).
C. longa is also developed in Thailand, Philippines, Sri Lanka, and Malaysia and is considered an ethnomedicinally important plant in Indonesia and Malaysia. Its poultice, when rubbed to the perineum, ensures the healing of any birth canal lesions. C. longa is also used to relieve dental issues and digestive troubles like discomfort or pain in the upper abdomen and acidity, indigestion, gas, and ulcers, as well as mitigate the hallucinogenic effects of hashish and other psychoactive drugs. The tribes of Jhalda, Parulia District of West Bengal, apply rhizome paste to the body to relieve physical pain. Assamese tribal women apply a fresh rhizome paste for skin infection and also to improve their complexion. Rhizome in addition to other ingredients cures loose stools in cattle. It is considered as a source of various problems such as blood purification, brain and heart tonic, asthma, leucoderma, piles, bronchitis, spleen enlargement, tumor, biliary disorders, anorexia, cough, rheumatism, sinusitis, tuberculous glands in the neck, diabetic wounds, hepatic disorders, leucorrhea, and gonorrheal secretion. It helps to lower blood clotting and blood sugar level (Zhang et al., 2013). Curcumin is now regarded as a promising “new medicine” that is being utilized as a supplement in a number of countries, namely, India, Japan, Thailand, Korea, China, Malaysia, and Pakistan; it is added to curry, tea, cosmetics, and drinks, and used as a colorant, antiseptic, and anti-inflammatory agent to treat gastrointestinal discomfort. It is also used as a component in cheese, butter, mustard sauce, and chips, and as a preservative and a dyeing agent in the United States. Curcumin is commercially available in multiple forms, including capsules, energy drinks, soaps, tablets, ointments, and cosmetics. Goud et al. (1993) found out that C. longa is the best source of ω-3 fatty acid and α-linolenic acid (2.5%).
Rhizomes are being added to other plants to develop traditional remedies for a range of infections, such as tonsillitis, snake bites and stings, headaches, wounds, sprains, and fractured bones. Turmeric has been applied as a home remedy to heal wounds and also facilitates the treatment for digestive dysfunction, hepatic problems, leukemia, atherosclerosis, osteoarthritis, menstrual problems, bacterial infections, and eye problems. Turmeric has a role in preventing inflammation in the mucous membranes that line the throat, stomach, intestine, and lungs (Figure 3).
Evidence suggests the benefits of turmeric in relieving acne, inflammation, joint pain, asthma, eczema, and tonic and acute allergies; in wound healing; in maintaining a balanced mood and blood sugar levels; and in immunomodulation (Ammon and Wahl, 1991; Reddy and Rao, 2002).
C. longa contains carbohydrates, fiber, certain proteins and lipids (no cholesterol), vitamin C, pyridoxine, magnesium, phosphorus, potassium, and calcium, which makes it a nutritionally rich natural food ingredient. The nutritional profile of C. longa is shown in Figure 2 (Pradeep et al., 1993). To date, 719 constituents have been isolated and recognized from 32 Curcuma species, including terpenoids, flavonoids, phenylpropene derivatives, alkaloids, diphenylalkanoids, steroids, and other compounds (Sun et al., 2017). The rhizome was found to contain over 235 phytoconstituents, the majority of which are polyphenols and terpenoids. Curcuminoids are made up of 80% curcumin and are the most common polyphenols. There are 109 sesquiterpenes, 68 monoterpenes, 22 diarylheptanoids and diarylpentanoids, eight phenolics, five diterpenes, four sterols, three triterpenoids, two alkaloids, and 14 remaining constituents. In a standard form, C. longa consists of moisture (>9%), curcumin (5–6.6%), extraneous matter (<0.5%), mold (<3%), and volatile oils (<3.5%). Monoterpenes dominate the essential oils of flowers and leaves, while sesquiterpenes dominate the oils of roots and rhizomes. The oil constituents contain 25% tumerone, 11.5% curdine, and 8.55% ar-turmerone (Nisar et al., 2015). C. longa oil contains anti-mutagenic qualities and is also capable of preventing the development and excretion of urinary mutagens in those who smoke cigarettes. According to the latest analysis, essential oil content in the rhizome was approximately 3.97%, with ar-turmerone (40%), α-turmerone (10%), and curlone (23%) being the major components analyzed by gas chromatography (Guimarães et al., 2020). The ar-turmerone has been employed as a repellant for insects, and its mosquitocidal ability has been revealed in the leaf extract. C. longa is a rich source of polyphenolic curcuminoids like curcumin (about 80%), demethoxycurcumin (about 12%), and bisdemethoxycurcumin (Ashraf, 2018), as well as proteins, volatile oils (tumerone, atlantone, and zingiberone), sugars, and resins. Curcumin, which makes up 0.3%–5.4% of raw C. longa, is the well-studied active ingredient. Table 1 illustrates the principal C. longa products, their appearance, chemical contents, and use. The C. longa plant is known to possess acidic polysaccharides (which include ukonan A, B, C, and D), 4.2% volatile oils (which include turmerone, ar-turmerone, curcumene, germacrone, and ar-curcumene as main constituents), and 5.8% essential oils (which include 0.6% sabinene, 0.5% borneol, 1% α-phellandrene, 1% cineole, 53% sesquiterpines, 25% zingiberene, and 3%–4% curcumin) (Chattopadhyay et al., 2004). Phenolic diketone curcumin provides yellow color, and consists of curcumin I (94%), curcumin II (6%), and curcumin III (0.3%). Protein (6.3%), fat (5.1%), minerals (3.5%), carbohydrates (69.4%), and moisture (13.1%) were reported by Liao et al. (2011). The main phytocompounds are presented in Figure 4.
C. longa claims to offer a wide range of therapeutic properties. C. longa was reported to contain curcuminoids, glycosides, terpenoids, and flavonoids. Haridra rhizome has been employed by healthcare professionals for diabetes, cholesterol, inflammation, diarrhea, liver problems, asthma, and cancer with minimal cytotoxicity to normal cells, and has been used as a cosmetic ingredient (Paranjpe and Pranjpe, 2001). In human trials, curcumin is suggested to be effective and safe, and the U.S. Food and Drug Administration has certified it as “generally regarded as safe”.
C. longa has long been used to treat digestive problems, and clinical investigations have verified its therapeutic advantages. Its anti-inflammatory action has been established in preclinical research to potentially protect the gastrointestinal tract. It has also been shown to increase gastrin, secretin, and bicarbonate secretion, as well as gastric wall mucus and pancreatic enzyme (Ammon and Wahl, 1991), as well as inhibit intestinal spasms and ulcer formation caused by stress, alcohol, indomethacin, pyloric ligation, and reserpine (Rafatullah et al., 1990) and improve dyspeptic patients’ condition. The activity of curcumin against inflammation and its therapeutic effect on gastrointestinal illnesses such as dyspepsia, Helicobacter pylori infection, Crohn’s disease, gastric ulcer, acidity, and ulcerative colitis in the form of fresh juice are thought to be antihelmintic. Curcumin inhibits nuclear factor (NF)-κB and reduces gastric mucosal damage in rats suffering from NSAID-induced gastropathy, leukocyte adhesions, intercellular adhesion molecule 1, and tumor necrosis factor (TNF)-α (Thong-Ngam et al., 2012). Between baseline and 8 weeks of treatment, C. longa tablet dramatically reduced irritable bowel syndrome (IBS) prevalence and abdominal pain/discomfort score, and IBS quality of life scores showed considerable improvement (Rahimi and Abdollahi, 2012). In male mice with liver damage, curcumin protects against acetaminophen-induced hepatitis by lowering oxidative stress and liver injury, and also restores glutathione levels (Somanawat et al., 2013).
C. longa and its constituents have a relaxing impact on tracheal smooth muscles, suggesting a possible bronchodilatory influence in individuals with obstructive lung disease. They also have a protective benefit in an animal model of respiratory disorders, involving effects on inflammatory cells and mediators, lung pathological alterations, airway responsiveness, and immunomodulatory responses (Boskabady et al., 2020). Curcumin has been shown in both in vivo and in vitro investigations to have antiasthmatic properties. Curcumin therapy during OVA sensitization exhibited significant protective effects in an OVA-induced asthma paradigm in guinea pigs, attenuating bronchial constriction and hyperreactivity (Ram et al., 2003). Bronchitis is treated with fresh rhizome juice. C. longa is boiled in milk and combined with jiggery and used internally for rhinitis and cough. In cases of catarrhal cough and painful throat with infection, a rhizome decoction is gargled, and a piece of the rhizome is slightly burned and chewed. Turmerones, curcuminoids, curcumin, and tetrahydrocurcumin are chemical compounds of C. longa that have anti-asthmatic properties, and Haridradhumvarti (fumes wick) fumes are used in asthma and congestion.
Inflammatory markers include C-reactive protein (CRP), complements, and fibrinogen, all of which are induced by inflammatory cytokines in response to stimulation. According to Sandur et al. (2007), curcumin, demethoxycurcumin, and bisdemethoxycurcumin are the active compounds in C. longa that inhibit TNF-induced NF-κB activation. The methoxy groups on the phenyl ring were discovered to be responsible for their actions. C. longa extract was examined to improve serum inflammatory markers and mental health and mood disturbance in healthy participants who are overweight (Uchio et al., 2021). Researchers discovered that curcumin has anti-inflammatory properties by inhibiting the pro-inflammatory transcription factor (NF-κB) in 1995. They also discovered the molecular mechanism that underlies this inhibition (Singh and Aggarwal, 1995). TNF-α quickly activates NF-κB, which consists of the p50 and p65 subunits in human myeloid ML-1 cells, while curcumin prevented this activation. Curcumin also inhibits the binding of activator protein 1 (AP-1) binding factors, but the Sp1 binding factor remained unaffected. Curcumin inhibits the activation of NF-κB by phorbol ester and hydrogen peroxide, in addition to TNF-α. Furthermore, curcumin suppresses the NF-κB activation pathway after the convergence of multiple stimuli but before human I kappa B alpha phosphorylation. The capacity of C. longa to suppress both inflammatory prostaglandin derivative of arachidonic acid and neutrophil activity during inflammation may also indicate its anti-inflammatory activities. Curcumin is frequently used with bromelain to improve absorption and anti-inflammatory activity. Curcumin is equally efficacious as cortisone or phenylbutazone when given orally in acute inflammation. C. longa given orally to reduce inflammatory edema considerably. Curcumin’s therapeutic effect in sepsis appears to be achieved by activation of peroxisome proliferator-activated receptor gamma (PPAR-γ), which leads to inhibition of pro-inflammatory cytokine along with expression and release of TNF-α (Jacob et al., 2008). One trial evaluated 43 kidney transplant patients; 480 mg of curcumin and 20 mg of quercetin per capsule were observed to be potent during delayed graft rejection. Significant lower serum creatinine after transplant was attained in 43% of control patients and 71% of low-dose-treated participants. Induction of the hemeoxygenase enzyme, proinflammatory cytokines, and free radical scavenger associated with tissue injury possibly caused the enhanced early performance of transplanted kidneys (Figure 5) (Shoskes et al., 2006). Majority of the benefits seemed to be due to the anti-inflammatory and antioxidant properties of curcumin, while the quercetin in the molecule was negligible.
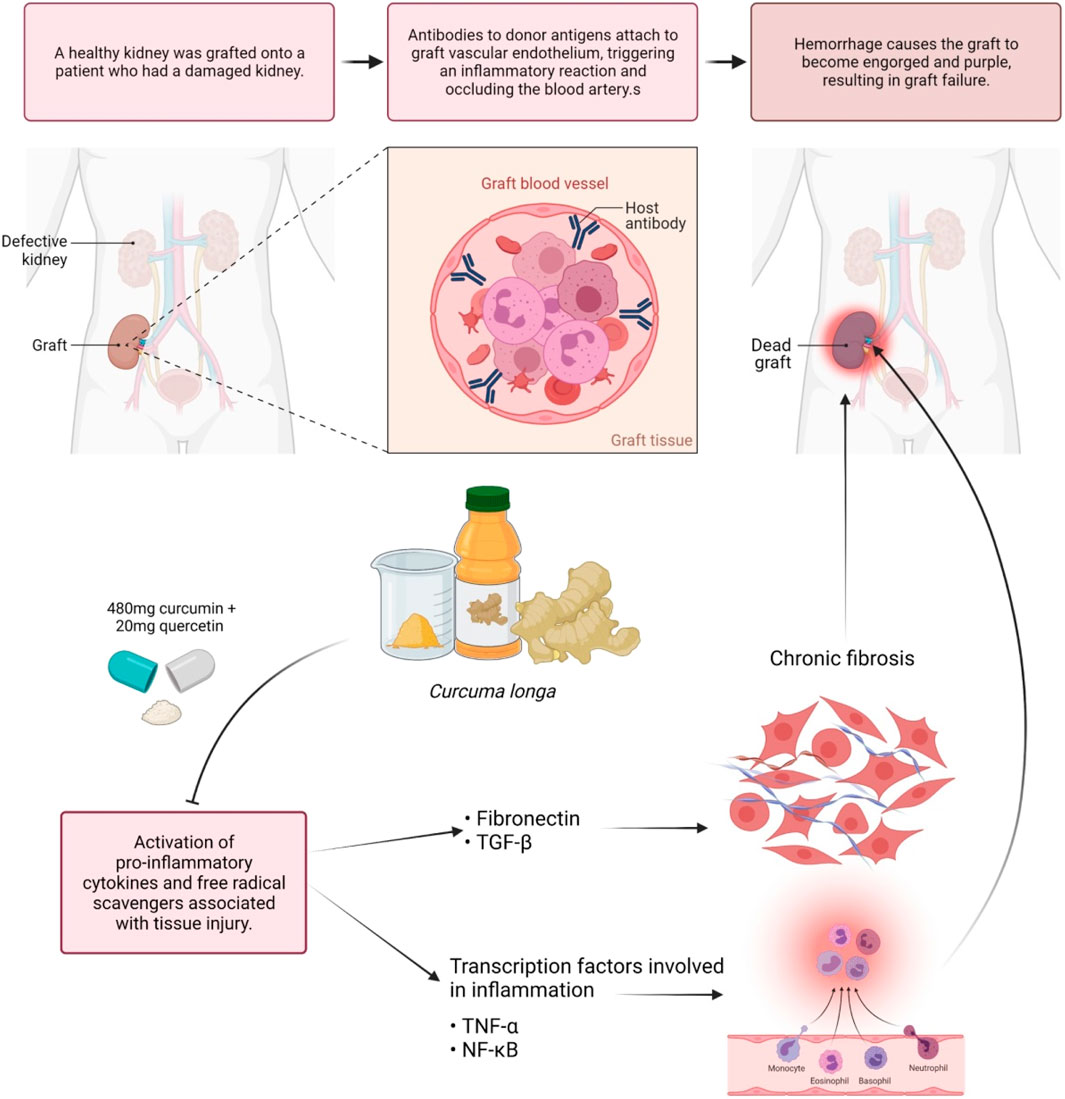
FIGURE 5. Modulation of antibodies by curcumin. Curcumin assists in the modulation of antibodies that react with endothelium and causes hyperacute graft rejection. Blocking the expression of pro-inflammatory cytokines and transcription factors linked to inflammation and fibrosis may help to prevent dead grafts.
In diabetes mellitus, C. longa rhizome powder is particularly beneficial when added to amla juice and honey. Curcuminoids, the active component in the rhizome, reduce lipid peroxidation by keeping superoxide dismutase, catalase, and glutathione peroxidase active at higher levels. Curcuminoids have been demonstrated in diabetes mellitus type 2 patients to improve insulin resistance, reduce glucose and insulin levels, enhance adiponectin secretion, and lower levels of leptin, resistin, interleukin (IL-6, IL-1β), and TNF-α (Hajavi et al., 2017). According to the findings, C. longa ethanolic extract containing both curcuminoids and sesquiterpenoids is more hypoglycemic than curcuminoids or sesquiterpenoids alone (Nishiyama et al., 2005). C. longa extracts examined under in vivo conditions towards type 2 diabetes in mice models predict that it has a hypoglycemic impact by reducing blood glucose levels (Ponnusamy et al., 2012). C. longa has a low impact on postprandial plasma glucose and insulin in healthy individuals; it was found that consumption of 6 g of C. longa had no noticeable effect on the glycemic level. The change in insulin was substantially greater 30 and 60 min after the oral glucose tolerance test (OGTT) with C. longa. Following the OGTT, the insulin area under the curve was likewise considerably greater after consuming C. longa. Curcumin and its three derivatives (dimethoxy curcumin, bisdemethoxycurcumin, and diacetyl curcumin) were reported for their antioxidant capabilities (Faizal et al., 2009). C. longa dried rhizome powder diluted in milk has antidiabetic, hypolipidemic, and hepatoprotective properties, according to the scientific and systemic investigation, and could be used as an efficient and safe antidiabetic dietary supplement with great potential (Rai et al., 2010). Both isopropanol and acetone extract of C. longa inhibited human pancreatic amylase enzyme, which reduces starch hydrolysis, resulting in lower glucose levels (Ponnusamy et al., 2010).
Cardiovascular diseases (CVDs) seem to be a global health issue that is linked to high disease and death rates. Anti-hypercholesterolemic, anti-atherosclerotic (Gao et al., 2019), and protective capabilities against cardiac ischemia and reperfusion (Wang et al., 2018) of curcumin have been proven in preclinical and clinical trials. Curcumin has anti-CVD potential by improving the lipid profile of patients, and it might be administered alone or as a dietary supplement to traditional CV medicines (Qin et al., 2017). Curcumin is also seen in many studies to protect against coronary heart disease (Li H. et al., 2020) and also possesses anticoagulant properties. CV preventive characteristics of C. longa include reduction in the level of cholesterol and triglycerides, decrease in the vulnerability of low-density lipoprotein (LDL) to lipid peroxidation, and platelet aggregation prevention, which helps to defend against atherosclerosis according to animal studies and also inhibits thromboxane formation. Curcumin increases VLDL cholesterol trans-protein plasma, causing increased levels and mobilization of α-tocopherol from adipose tissue that protects against oxidative stress that occurs during atherosclerosis. However, the fatty acids in the animals were less susceptible to oxidation in the blood vessel. It was suggested that oral intake of 500 mg/day curcumin for a week leads to a significant reduction in serum lipid peroxide (33%) and total serum cholesterol (12%) levels while increasing HDL cholesterol (29%). Curcumin may reduce chronic heart failure by boosting p38 MAPK, JNK, and ASK1, according to Cao et al. (2018). Curcumin and its components were used in recent research to determine the utility of nanotechnology-based medication delivery systems in CVD patients (Salehi et al., 2020).
In jaundice, rhizome powder added to amla juice is utilized. Jaundice is also cured by combining corriliyum (Anjana) with Haridra, Red ochre (Gairika), and Amalaki (Emblica officinalis Gaertn.) (Tripathi, 2009). C. longa’s hepatoprotective abilities have been proven in studies against several hepatotoxic ailments, including carbon tetrachloride, galactosamine, and acetaminophen (paracetamol) (Rao et al., 1995). The ethanolic crude extract of rhizomes was detected with curcumin, tumerone, atlantone, and zingiberene, which had substantial hepatoprotective ability at an oral dose of 250 and 500 mg/kg (Park et al., 2000). Karamalakova et al. (2019) investigated the decrease in the level of plasma bilirubin and gamma glutamyl transpeptidase, and the decrease in lipid peroxidation and provided significant hepatic protection against bleomycin toxicity by decreasing reactive oxygen species (ROS), which improves superoxide dismutase, catalase, and malondialdehyde levels. Curcumin is said to increase apoptosis in injured hepatocytes while also reducing inflammatory effects, hepatic fibrogenesis, and substantially liver injury. The hepatoprotective attribute of C. longa and curcumin might be due to direct free radical scavenging mechanisms, boosting glutathione levels, and assisting in liver detoxification. Aflatoxin-induced biliary hyperplasia, lipid alterations, and necrosis were likewise cured by C. longa and curcumin. Sodium curcuminate is a salt of curcumin that has choleretic effects, boosting biliary excretion of bile salts, cholesterol, bilirubin, and bile solubility, thus helping to prevent and treat cholelithiasis. This could be related to the antioxidant capacity of curcumin’s phenolic groups. Tacrine is well-known for its hepatotoxic and T-cell-destructive properties. Curcumin was over ten times more efficient than standard therapy, ascorbic acid, in research involving human hepatocytes cells that had been disrupted by tacrine (Song et al., 2001).
Curcuma oil lowers ischemia’s negative effect by decreasing nitrosative and oxidative stress. Ischemia collapses the membrane potential of the mitochondria, cytochrome c releases, Bax:Bcl-2 protein ratio changes, and caspase-activated, which leads to the apoptotic initiation in a sequential manner, which was considerably inhibited by Curcuma oil. As a result, there is evidence for the action of Curcuma oil in neuroprotection with a wide therapeutic window for the reduction in ischemic brain injury (Dohare et al., 2008).
In an Alzheimer’s disease transgenic mouse, curcumin decreased oxidative stress and repaired amyloid pathology. Antioxidant and anti-inflammatory features of curcumin helped to minimize the manifestation of Alzheimer’s disease, which is characterized by inflammation and oxidation. Parkinson’s disease (PD) is found to be the second most common neurodegenerative disease following Alzheimer’s disease, which affects dopaminergic neurons of the substantia nigra pars compacta (SNpc) and decreases dopamine (DA) in their striatal terminals. Curcumin is suggested to be an effective therapeutic and nutraceutical agent for PD treatment. Interestingly, curcumin was found to inhibit the synthesis of MOA-B enzyme (Khatri and Juvekar, 2016), which would lead to an increase in the level and availability of DA in the brain. Neuroprotective effects of curcumin in a 6-hydroxydopmine animal model of PD (El Nebrisi et al., 2020) indicated an increase in the survival of striatal TH fibers and SNpc neurons, decreased abnormal turning behavior, and exerted neuroprotective properties. These findings provide evidence that α7-nicotinic acetylcholine receptors could be a potential therapeutic target and curcumin would be the first natural source that is found to modulate nicotinic receptors in PD. Curcumin can be a future therapy for various neurological illnesses including major depression, involuntary movement, as well as diabetic neuropathy (Kulkarni and Dhir, 2010). Ethanol extract of C. longa was found to show neuroprotective effects on neuronal loss induced by dexamethasone treatment in rat hippocampus (Issuriya et al., 2014). In 2018, an in vivo study revealed that administration of C. longa extract at a dose of 200 mg/kg in trimethyltin (TMT)-treated Sprague–Dawley rats with neurotoxic damage seems to prevent the deficits in the spatial memory performance and partially inhibit the decrease in the number of CA2–CA3 region pyramidal neurons. Therefore, the anti-inflammatory as well as antioxidant effects of C. longa were observed (Yuliani and Mustofa, 2018) Furthermore, Yuliani and Mustofa (2019) examined the neuroprotective effects of ethanolic C. longa extract at 200 mg/kg in an in vivo analysis via preventing oxidative stress by decreasing the plasma and brain malondialdehyde levels and increasing the superoxide dismutase, catalase, and glutathione peroxidase enzyme activities and glutathione levels in the brain on TMT-exposed Sprague–Dawley rats. Terrestrial animals and aquatic animals are also required to be used for research purposes. An aquatic environment serves as a sink for environmental contaminants including Benzo[a]pyrene (B[a]P), and research on the fish model is also needed to understand the influence of B[a]P on oxidative stress-induced neurotoxicity and anxiety-like behavioral responses in aquatic animals (Billiard et al., 2006; Satpathy L. and Parida S. P., 2021). B[a]P is important in the mechanical aspects of oxidative stress to lipid membranes, nucleic acids, and proteins, as well as changes in antioxidant capacity. Curcumin has a potential to act as a co-supplement by reducing anti-anxiety behavioral response and altering antioxidant activity with a significant increase in pyknotic neuronal counts in the periventricular gray zone of the optic tectum that regulates anxiety against B[a]P-induced neurotoxicity in adult zebrafish (Satpathy L. and Parida S., 2021).
Banji et al. (2021) demonstrated the neuroprotective and antioxidant activity of C. longa extract in synergy with essential oil against neurotoxicity mediated by aluminum. Detection of free curcumin and its metabolites in the brain and plasma has increased bioavailability and tissue distribution, implying that it could be used in neurodegenerative illnesses.
Another neurodegenerative disease, amyotrophic lateral sclerosis (ALS), causes a selective loss of motor neurons in the spinal cord, brainstem, and motor cortex. Curcumin was studied to determine if it could help ALS patients, particularly those with bulbar involvement, survive longer (Ahmadi et al., 2018). Curcumin therapy reduced the development of ALS and oxidative damage in a double-blind therapeutic trial (Chico et al., 2018). Curcumin-based drug delivery systems are beneficial for the treatment of ALS, according to a study (Tripodo et al., 2015), although Rakotoarisoa and co-workers pointed out that curcumin has chemical instability, low oral bioavailability, and low water solubility rate in the ALS disease condition (Rakotoarisoa and Angelova, 2018).
The ability of curcumin to interact indirectly with a diverse array of transcription factors, including NF-κB, activator protein 1 (AP-1), β-catenin, and signal transducer and activator of transcription (STAT) proteins, and to act as a partial agonist of the PPAR-γ, a ligand-activated transcription factor involved in both neuroprotective and anti-inflammatory signaling pathways (Chen et al., 2015; Kunnumakkara et al., 2017). Curcumin has been demonstrated to help with a variety of diseases, including multiple sclerosis (MS) (Mohajeri et al., 2015). Curcumin-D-monoglucuronide (curcumin monoglucuronide, CMG) was developed as a prodrug form of curcumin due to its low bioavailability in the body. CMG is deemed to be safe for use and can be injected intravenously, revealing an anticancer impact on mice implanted with human colorectal cancer cells by achieving a 1,000-fold higher blood concentration of free-form curcumin than curcumin administered orally (Ozawa et al., 2017). In mouse xenograft models, CMG given intraperitoneally appears to have antitumor effects on oxaliplatin-resistant colon cancer with minimal toxicity (Ozawa-Umeta et al., 2020). After CMG delivery, the microbiota changes, which may be linked to immunopathology suppression in an autoimmune model for MS (Chearwae and Bright, 2008) and experimental autoimmune encephalomyelitis (EAE). The gut microbiota has been suggested to play a major role in the development and severity of MS. When compared to healthy controls, MS patients had an increased number of bacteria from the genera Akkermansia, Blautia, and Pseudomonas, as well as a lower number of bacteria from the genera Prevotella and Parabacteroides (Chen et al., 2016; Park et al., 2017; Tsunoda, 2017).
C. longa and its curcumin constituent have significant antioxidant activity, equivalent to both vitamin C and vitamin E, in both water- and fat-soluble extracts. Curcumin can help the body rid itself of hydroxyl radicals, singlet oxygen, superoxide radicals, nitrogen dioxide, and NO. Curcumin pretreatment was proven to reduce ischemia-induced mutations in the heart (Dikshit et al., 1995). The efficiency of curcumin on endothelial heme oxygenase-1 (inducible stress protein) employing bovine aortic endothelial cells was discovered in an in vitro investigation that resulted in increased cellular resistance to oxidative stress. Curcumin can also help Caenorhabditis elegans live longer by lowering intracellular ROS and lipofuscin levels during aging (Liao et al., 2011). Previous research into the potential of C. longa to sustain hippocampal cells of male Wistar rats from lead-induced damage and reduces lipid peroxidation caused by toxic heavy metals. Resveratrol and curcumin alleviate and synergistically repair oxidative stress to the tissues by enhancing antioxidant response through free radical scavenging (Al-Basher et al., 2020). In one of the earlier studies, the anti-inflammatory and antioxidant capability of curcumin was detected to be synergistically enhanced with quercetin, and a synergistic protective effect was also demonstrated in diazinon-induced rats (Abdel-Diam et al., 2019). The anti-inflammatory impact of berberine and curcumin may decrease oxidative stress, liver inflammation, and lipid metabolism (Feng et al., 2018), and the berberine combination also reduced inflammatory and oxidative stress responses in the cortex and hippocampus of rats (Lin et al., 2020).
Annapurna et al. (2011) evaluated the ability of C. longa prophylactically and therapeutically, i.e., pre-induction treatment and post-induction treatment via oral and topical application to modulate the N-methyl-N-nitrosourea-induced mammary cancer in rats for 24 weeks. Prophylactic topical application given at 200 mg/kg of C. longa has significantly reduced the mean tumor volume compared with therapeutic topical application. This was the first report to show the anticancer activity of C. longa with topical application in a breast cancer model. In an in vivo research involving the topical application of curcumin in CD-1 mice and dietary administration of 1% C. longa, 0.05% of its ethanol extract significantly reduced tumor incidence, tumor burden, and tumor volume in dimethyl benz[a]anthracene (DMBA)-initiated and 12,O-tetradecanoylphorbal-13-acetate (TPA)-promoted skin tumors (Huang et al., 1988). Kuttan and his colleague’s work was the first to demonstrate curcumin’s anti-cancer potential in both in vitro and in vivo experimental models (Kuttan et al., 1985). Curcumin activates DNA damage response, laying the foundation for the therapeutic use of these nutraceuticals in prostate cancer chemoprevention (Horie, 2012). The general anti-carcinogenic effect of curcumin involves mechanisms like induction of apoptosis and inhibition of cell-cycle progression in rat aortic smooth muscle cells (Chen and Huang, 1998). The antiproliferative effect is regulated partly through hindrance of protein tyrosine kinase activity and c-myc mRNA expression, while the apoptotic effect may partly be mediated via preventing the functioning of protein tyrosine kinase, protein kinase C, and expressions of c-myc mRNA and bcl-2 mRNA (Chen and Huang, 1998). Curcumin inhibits the transcription factor NF-κB (Figure 6) and various downstream gene products like c-myc, Bcl-2, COX-2, nitric oxide synthase (NOS), Cyclin D1, TNF-α, ILs, and matrix metallopeptidase 9 (MMP-9) and has anti-proliferative activities in a diversity of malignancies. Curcumin could be used to avoid colorectal cancer (CRC) in diabetics with type 2 diabetes by lowering leptin blood levels and increasing adiponectin levels. Poloxamer 407 can be employed as a polymer to expand the colorectal medicine liberation mechanism for curcuminoids in CRC treatment, according to the study of Chen et al. (2012).
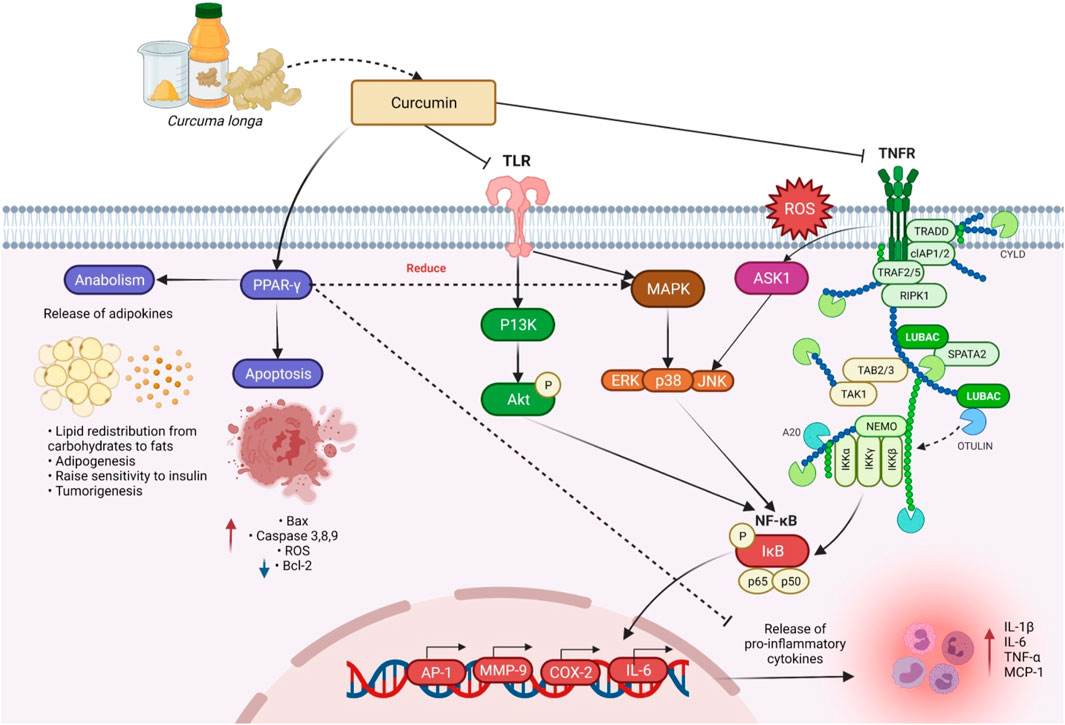
FIGURE 6. Curcumin’s mechanism of action in reducing inflammation, anabolism, and apoptosis. By inhibiting the pro-inflammatory transcription factor (NF-κB), and activation of PPAR-γ, curcumin aids in anabolism and apoptosis, suppression of pro-inflammatory cytokines, as well as the expression and release of TNF-α. Abbreviations: TLR, Toll-like receptors; TNFR, Tumor necrosis factor receptor; ROS, Reactive oxygen species; TRADD, Tumor necrosis factor receptor type 1-associated death domain protein; CYLD, CYLD lysine 63 deubiquitinase; cIAP1/2, Cellular inhibitor of apoptosis protein 1/2; TRAF 2/5, Tumor necrosis factor receptor-associated factor 2/5; RIPK1, Receptor-interacting serine/threonine-protein kinase 1; LUBAC, Linear ubiquitin chain assembly complex; SPATA2, Spermatogenesis-associated protein 2; NEMO, NF-κB essential modulator; TAB2/3, TGF-beta activated kinase 1 (MAP3K7) binding protein 2; TAK1, Transforming growth factor-β-activated kinase 1; IKKα, IKKβ, and IKKγ, Inhibitory kappa b kinase alpha, beta, and gamma; IkB, Inhibitor of nuclear factor kappa Bv; PPAR-γ, Peroxisome proliferator-activated receptor gamma; P13K, Phosphoinositide 3-kinases; Akt, Ak strain transforming; ERK, Extracellular-signal-regulated kinase; JNK, Jun N-terminal kinase; Bax, Bcl-2-associated X-protein; AP-1, Activated protein-1; MMP-9, Matrix metallopeptidase 9; COX-2, Cyclooxygenase 2; IL-6 and 1β, Interleukin 6 and 1 beta; TNF-α, Tumor necrosis factor alpha; MCP-1, Monocyte chemoattractant protein-1.
A novel approach in adjuvant treatment for osteosarcoma is by combining a synthetic counterpart of the natural chemical pancratistatin with curcumin (Ma et al., 2011). One controlled study found that employing poly-lactic-co-glycolic acid to create and characterize nano-curcumin improves the water solubility as well as anticancer activity of the nanoparticulate emulsion (Nair et al., 2012). Curcumin also has an impact on numerous growth factor receptors and adhesion molecules that are implicated in tumor growth, angiogenesis, and metastasis (Wilken et al., 2011) and exerts antitumor action in cancer cells by the suppression of NF-κB and signal transducers and activators of the transcription 3 (STAT3) pathways (Jiménez-Flores et al., 2014).
A study on in vitro and in vivo models revealed that both C. longa and curcumin exhibited the ability to lessen the impacts of numerous known causative agents of mutation and cancer in different body tissues. Curcumin (50 μM) initiates destruction in the human kidney cells and causes the colorectal HT-29 cancerous cells to grow larger, which is most probably due to cell cycle arrest (Kössler et al., 2012). Curcumin also triggers programmed cell death in colon cancerous cells and inhibits micro-inflammation in the gastrointestinal system linked to inflammatory bowel illnesses, according to laboratory research (Nita, 2003). Okanlawon et al. (2020) determine the influence of the inclusion of powdered C. longa on carcass yield and intestinal increase in rabbit production. Farombi et al. (2007) explored the combined effects of curcumin and kolaviron (a bioflavonoid extracted from Garcinia kola seeds) on DBP-induced testicular injury in rats. Curcumin treatment of mice infected with human prostate cancer cells resulted in a lowered microvessel density, cell proliferation, an improvement in apoptosis. Endothelial cells derived from bovine aorta exposed to curcumin (5–15 μM) under normoxic (oxygen tensions within 10–21%) or hypoxic (oxygen tensions within 1–5%) conditions were reported to increase heme oxygenase activity and resistance to oxidative stress. Consumption of alcohol sensitizes the pancreas to give an inflammatory response through NF-κB activation via protein kinase C epsilon. One pilot study concluded that an oral dosage of 500 mg of curcumin with 5 mg of piperine could restore lipid peroxidation in patients suffering from tropical pancreatitis (Durgaprasad et al., 2005).
EGFR-, miRNA-, autophagy-, and cancer stem cell-based treatments with curcumin could be proven as potential processes and targets for tackling lung cancer (Ye et al., 2012). Curcumin also seems to promote tumor progression, reducing the efficiency of docetaxel in lung cancer patients. Meanwhile, synchronized curcumin and docetaxel treatment causes minor toxicity in normal organs, as well as the bone marrow and liver (Yin et al., 2012). In vivo curcumin lessens the migratory and invasive capabilities of A549 cells and inhibited adiponectin expression thought to be mediated through the NF-κB/MMP pathways and has been proposed as an adjuvant in lung malignancy (Tsai et al., 2015).
Yu and his colleagues evaluated the role of curcumin in inhibiting the human hepatoma SMMC-7721 cells significantly by promoting apoptosis via modulation of Bax/bcl-2 (Yu et al., 2011). Apoptosis was associated with increases in p53 levels as well as its DNA-binding ability, along with protein expression of Bax. Phosphorylation of CDC27 (cell division cycle 27) is the main mechanism of anticancer efficacy of curcumin by obstructing cell growth and proliferation in an apoptotic pathway, leading to the death of the cells (Lee and Langhans, 2012). It has been discovered that circulating miR-21 is elevated in patients with hepatocellular carcinoma (HCC); it can be exploited as a diagnostic marker and therapeutic target for HCC, and is being linked to distant metastasis (Zhang et al., 2019). According to Li and his colleagues, in human hepatoma cell lines such as HepG2 and HCCLM3, suppression of miR-21 improved anticancer action of curcumin like cell growth suppression, apoptosis via upregulated target gene, and TIMP3 expression, and the mechanism may refer to TGF-β1/smad3 signaling pathway inhibition (Li J. et al., 2020). Curcumin inhibits cancer through modulating several signaling pathways and molecular targets, including TGF-β1/smad3, IGF, PI3K/Akt, Wnt/β-catenin, and vascular endothelial growth fact (VEGF) (Figure 7) (Mohebbati et al., 2017). Although several reasons for curcumin’s antitumor potential have been hypothesized, the exact molecular mechanism of this activity against HCC is unknown.
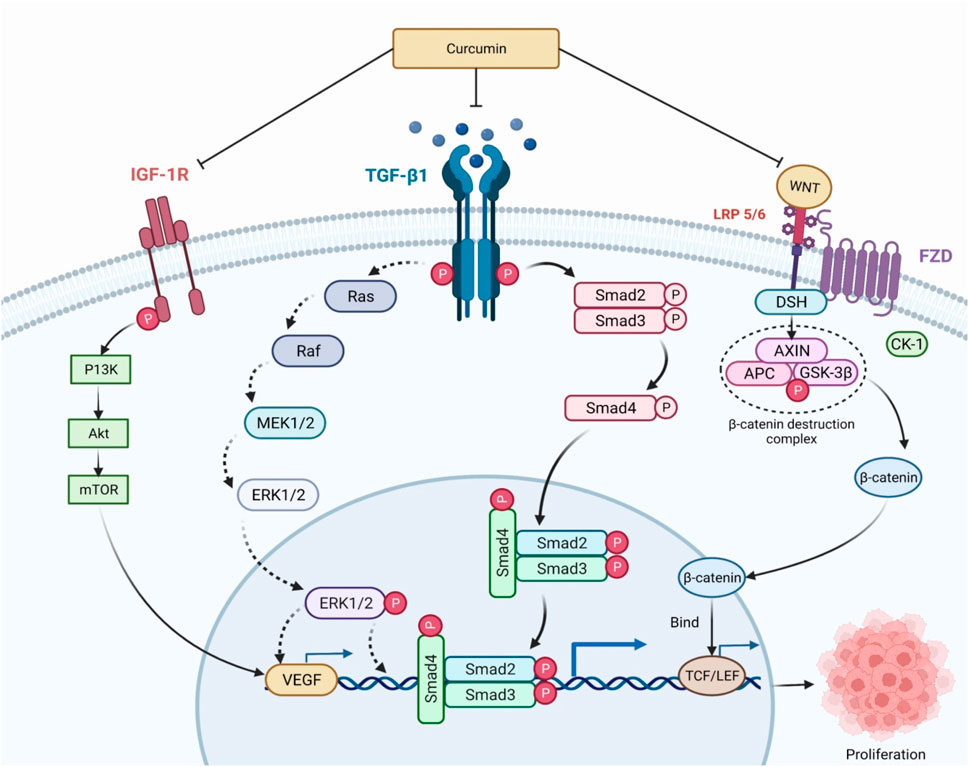
FIGURE 7. Mechanism of curcumin in regulation of cancer proliferation. TGF-β1/smad3, IGF, PI3K/Akt, Wnt/β-catenin, and vascular endothelial growth factor (VEGF) are some of the signaling pathways and molecular targets that curcumin modulates to inhibit cancer. Blocking these receptors has the potential to reduce chronic inflammation and oxidative damage. DSH and AXIN are recruited once the WNT binds to LRP 5/6, producing the β-catenin destruction complex. β-catenin that has escaped into the nucleus promotes the transcription of genes including cyclin D1 and P13k, which promote cell proliferation and growth. IGF-1R, Insulin-like growth factor 1 receptor; TGF-β1, Transforming growth factor beta 1; WNT, Wingless/integrated; LRP 5/6, Low-density lipoprotein receptor-related protein 5/6; Smad, small mothers against decapentaplegic; Ras, Rat sarcoma virus; Raf, Rapidly Accelerated Fibrosarcoma; MEK 1/2, Mitogen-activated protein kinase 1/2; ERK ½, Extracellular-signal-regulated kinase ½; P13K, Phosphoinositide 3-kinases; Akt, Ak strain transforming; mTOR, mammalian target of rapamycin; TCF/LEF, T-cell factor/lymphoid enhancer factor; FZD, Frizzled; DSH, Dishevelled; AXIN, Axis Inhibitor; APC, Adenomatous polyposis coli; GSK-3β, Glycogen synthase kinase-3 beta; CK-1, casein kinase 1.
Curcumin therapy of Burkitt’s lymphoma cell lines in combination with ionizing radiation shows that it boosts lymphoma cells’ susceptibility to ionizing radiation-induced apoptosis and improves cell cycle arrest at the G2/M phase. Curcumin and L-ASP show synergism in patients with blood and bone marrow malignancy (Jiang et al., 2015). Curcumin also hinders the cellular growth of uterine leiomyosarcoma and reduces the spread of castrate-resistant disease and human leiomyosarcoma cells via modulating the AKT-mammalian target of rapamycin pathway for inhibition (Wong et al., 2011). Curcumin or C. longa extract’s potential in decreasing tumors induced chemically was investigated. It was documented that curcumin and crude extract of C. longa is useful in reducing papilloma development throughout carcinogenesis and progression. About 0.2% and 1.0% of dietary curcumin can reduce the number of papilloma by acting on 7,12-dimethyl benz[a]anthracene (DMBA) and 12,0-tetradecanoylphorbol-13-acetate (TPA) that promoted skin tumor, which was explored by Limtrakul et al. (2001) as ras-p21 and fos-p62 oncogene expression was decreased dose-dependently by curcumin. Mohanty et al. (2006) examined the potency of C. longa on apoptosis of myocardial cells in experimentally produced myocardial ischemic–reperfusion injury because it exhibited considerable anti-apoptotic effects that lead to the preservation of cardioprotective characteristics and heart function. Aqueous C. longa extract exhibited antimutagenic activity against mutagens and also inhibited the progression of forestomach tumors induced by benzo[a]pyrene against Salmonella typhimurium strains.
Insulin-like growth factor-binding protein-3 (IGFBP-3) is a high-affinity binding protein that alters the mitogenic functions of IGFs while also having anti-proliferative and proapoptotic characteristics. Apoptosis is induced by transfection of IGFBP-3 cDNA into breast cancer cell lines expressing either mutant (T47D) or wild-type p53 (MCF-7). IGFBP-3 results in a higher ratio of pro-apoptotic to anti-apoptotic Bcl-2 family members. Curcumin induced cell apoptosis in MCF-7 via a p53-dependent pathway and could offer therapeutic promise in patients with breast cancer (Choudhuri et al., 2002). In the mouse model, the combination of curcumin and cyclophosphamide negated the efficacy of cyclophosphamide and then hindered the reduction of tumor size. Curcumin causes DNA fragmentation and base degradation in the presence of copper and cytochrome p450 isoenzymes. Furthermore, Frank et al. (2003) demonstrated that curcumin bonded to copper did not suppress spontaneous hepatic tumor growth in a rat model of liver cancer. The enhanced toxicity and oxidative stress may be explained by the excess load of copper.
Curcumin can limit the absorption and effectiveness of irinotecan, a chemotherapy medication used in colon cancer. Curcumin in combination with paclitaxel (Taxol) effectively decreased breast cancer dissemination to the lung in a xenograft mouse model of human breast cancer, relative to either curcumin or paclitaxel alone (Frank et al., 2003). Curcumin reduces T cells significantly, but a low dosage of curcumin boosts T cells retrieved from mice with the 3LL tumor. Consequently, increased CD8+ T cells demonstrated improved IFN-γ secretion and proliferation, especially against 3LL tumor cells (Han et al., 2014). C. longa extracts (containing curcuminoids, volatile oil, and water-soluble polysaccharides) could be employed as an adjuvant supplement for cancer patients who have their immune systems impaired by chemotherapy and saw an improvement in the expansion of peripheral blood mononuclear cells and the composition of cytokines. In vitro and in vivo analyses have indicated that aromatic-turmerone exhibits anti-angiogenic abilities on human endothelial cells (Yue et al., 2015).
Dietary substances such as curcumin and docosahexaenoic acid (DHA) have varying antiproliferative abilities among multiple breast cell lines and indicated synergism in SK-BR-3 (human breast cancer cell line) cells, which might be due to DHA elevation of cellular curcumin absorption and, thus, an occurrence unique to the combined action of substances (Altenburg et al., 2011). The study shows the synergistic interaction between curcumin and garcinol in pancreatic cancer cells (BxPC-3 and Panc-1) in a dose-dependent manner (Parasramka and Gupta, 2012). In vitro screening is carried out on human multiple myeloma cell lines (U266) by Ghoneum and Gollapudi (2011) to explain the synergistic apoptotic potency of arabinoxylan rice bran (MGN-3/Biobran) and curcumin (C. longa) in the United States.
Curcumin inhibited the degranulation and release of histamine from rat peritoneal mast cells caused by compound 48/80. Calcium uptake measurements and cAMP tests in mast cells were used to investigate the mechanism of action. In an animal model, curcumin dramatically reduced the mast cell-mediated passive cutaneous anaphylactoid reaction. Curcumin enhanced intracellular cAMP levels and inhibited both nonspecific and selective mast cell-mediated allergy reactions (Choi et al., 2010). Curcumin significantly reduced IgE/Ag-induced PSA (passive systemic anaphylaxis), as measured by serum-dependent leukotriene C4, dependent prostaglandin D2, and histamine levels, indicating that it might be useful to produce drugs for allergic inflammatory illnesses (Li et al., 2014). Curcumin can suppress expression of CD80, CD86, and class II antigens by dendritic cells and blocks the release of inflammatory cytokines like IL1β, IL-6, and TNF-α from LPS-stimulated dendritic cells.
Rhizome juice is utilized as an antiparasitic in the therapeutics of numerous skin problems, and rhizome powder is added to cow’s urine to relieve internal itching and dermatitis (Paranjpe and Pranjpe, 2001). Leaves hold great potential against human pathogenic fungi on account of their various in vitro and in vivo antifungal activities, for instance, strong fungicidal ability, long shelf life, high inoculum density tolerability, thermostability, a large number of antidermatophytic effects, and lack of any side effects. Curcumin has been proven to have antimutagenic, antioxidant, free radical scavenging, anti-inflammatory, and anti-carcinogenic abilities, allowing it to protect the skin from detrimental UV-induced impacts (Binic et al., 2013).
C. longa and curcumin caused a considerable increase in hepatic glutathione S-transferase (GST) and sulfhydryl (SH), Cytochrome b5, and cytochrome P450 levels, implying that C. longa and/or curcumin metabolites can be passed down through the milk supply. C. longa and curcumin are nontoxic and non-mutagenic during pregnancy in animals, although additional research in humans is needed (Soleimani et al., 2018).
IBS patients have far more inflammatory cells in the mucosa of the colon and ileum part of the intestine. Ng et al. (2018) looked into the possibility that curcumin can aid in IBS manifestation. Crohn’s disease (CD) and ulcerative colitis (UC) are the two primary forms of inflammatory bowel disease (IBD), characterized by abdominal pain, bloating, altered bowel habits, and increased stool frequency. Holt et al. (2005) carried out a pilot study to see how curcumin therapy affected IBD patients who had earlier received standard UC or CD therapy. Curcumin with standard treatment exerts more beneficial effects than placebo plus conventional UC treatment in maintaining recovery, according to Hanai et al. (2006). Bundy et al. (2004) examined that abdominal pain or discomfort score was lowered significantly by 22% and 25% in the one- and two-tablet group volunteers, respectively, and revealed the role of C. longa on IBS pathology.
Six hundred milligrams of curcumin five times a day for 12 weeks to individuals with peptic ulcers could prevent ulcer development but also cause symptomatic erosions, dyspepsia, and gastritis in some patients. Abdominal pain along with other symptoms has greatly decreased with curcumin within 1–2 weeks. Kim et al. (2005) found that orally administered ethanolic C. longa extract decreased stomach acid, gastric juice secretion, and ulcer initiation in male rats by inhibiting H2 histamine receptors, which is similar to the effects of ranitidine. Similarly, the antiulcer action of C. longa ethanolic extract was seen as it lowers ulcer index in addition to stomach acidity significantly. C. longa extract also suppressed hypothermic-restraint stress depletion of stomach wall mucus and diminished the severity of necrotizing agent-induced lesions.
Rats suffering from chronic mild stress (CMS) lead to much less sucrose intake; increased IL-6, TNF-α, CRF, and cortisol levels; smaller medulla oblongata; and reduced splenic NK cellular activity. The condition created in CMS was cured with ethanolic extract, which even caused the medulla oblongata to be lower than normal. C. longa has antidepressant potential because it tends to hinder monoamine oxidase accumulation in the central nervous system (Yu et al., 2002). Curcumin has a wide range of characteristics that are important to depression pathogenesis. The ethanolic C. longa extract prevented the decrease in serotonin, noradrenalin, and dopamine concentrations while increasing serotonin turnover, cortisol levels, and serum corticotrophin-releasing factor levels (Xia et al., 2007). The consequences of orally administered curcumin seem on behavior under chronic stress or depression condition in the rat model. Curcumin administration showed a similar impact to imipramine, a known antidepressant drug, and it has been indicated by various authors to be a feasible alternative source in depression condition (Mohammed et al., 2019; Qi et al., 2020).
Curcumin possesses a powerful anti-drug resistance agent. It has a novel capacity to suppress adriamycin-induced elevation of P-glycoprotein and its mRNA, and this ability is linked to increased intracellular drug accumulation, thereby increasing ADM lethality (Xu et al., 2011). Curcumin blocks NF-κB activation, which results in chemosensitivity in drug-resistant cancer cells. Furthermore, curcumin and tamoxifen co-therapy has also been illustrated to expose tamoxifen-resistant breast cancer cells, suggesting that it could be a viable method for either minimizing tamoxifen resistance or re-sensitizing refractory illness to tamoxifen therapy (Mimeault and Batra, 2011).
The essential oil as well as extracts of C. longa can suppress a diverse range of bacteria, infectious fungus, and parasites.
Some of the researchers assessed the antimicrobial activity of curcumin against different bacterial strains such as Salmonella paratyphi, Trichophytongypseum, Staphylococcus aureus, Streptococci mutans, and Mycobacterium tuberculosis (Tajbakhsh et al., 2008; Maghsoudi et al., 2017). The extract showed antimicrobial activity towards Trichophyton longifusus, Microsporum canis, and S. aureus while toxicity was indicated against Lemna minor. The C. longa-treated rabbit group had a significantly greater mean value for wound contraction, and therefore, wounds revealed decreased inflammation and a rising tendency in collagen formation. The extract of C. longa in ethanol was active for Shigella flexneri, Staphylococcus epidermis, Klebsiella pneumoniae, Lactobacillus, Pseudomonas aeruginosa, Vibrio cholerae, and Salmonella typhi (Oghenejobo and Bethel, 2017). Drug combinations can cause powerful or reductive pharmacokinetic effects, which may enhance or diminish the clinical efficiency of one another via regulation of absorption, distribution, metabolism, and excretion (Chou, 2006). Another study revealed the synergistic combinatorial impact of copper metal ions with aqueous extracts of C. longa against Paenibacillus popilliae, a known food spoilage bacterium, and detected the phytoconstituents including alkaloid, flavonoid, anthocyanin, steroids, and coumarin in C. longa extracts (Jassal et al., 2015). C. longa aqueous extract and chitosan possess significant synergism and antibacterial potency at 512 μg/ml and 1,024 μg/ml against MDR pathogens such as methicillin-resistant S. aureus, carbapenem-resistant Pseudomonas, carbapenem-resistant Enterobacteriaceae and AmpC-producing Enterobacteriaceae, and antibiofilm producers (Etemadi et al., 2021). The aqueous extract, curcumin component, and oil fraction of C. longa revealed antibacterial activity and suppresses H. pylori, Streptococcus, Staphylococcus, and Lactobacillus species (Mahady et al., 2002).
Curcumin has been identified to have synergistic effects but not antagonistic effects when combined with antibiotics such as oxacillin, ampicillin, ciprofloxacin, gentamicin, amikacin, polymyxin B, and norfloxacin, and anti-inflammatory effects when combined with a certain cytotoxic agent, with chemotherapy (Lin et al., 2007), or with a polyphenol derivative-containing diet (Strimpakos and Sharma, 2008). Various researchers have employed different extracts prepared from medicinal plants to treat MDR bacteria (Mehta and Jandaik, 2016; Urmila Jandaik et al., 2016; Mehta et al., 2021; Mehta et al., 2022a), which has been recognized as a global concern. A mixture of C. longa, galanga powder, and essential oil of lemongrass slowed the deterioration of raw white hard clam muscle, which improved the seafood quality during preservation (Nguyen, 2020).
Curcumin was shown to improve the activity of common azole and polyene anti-fungal (Sharma et al., 2010). Another study indicated that C. longa oil suppressed dermatophytes and pathogenic fungus when applied externally on guinea pigs infected with dermatophytes, molds, and yeast. The guinea pigs with dermatophytes and fungal infected lesions improved, and the lesions become invisible after 7 days of C. longa administration. The antifungal, antibacterial, phytotoxic, cytotoxic, and insecticidal activities of a C. longa ethanolic extract were explored by Khattak et al. (2005). Ether, chloroform, and ethanol extracts of C. longa along with its oil possess antifungal effect against Aspergillus flavus, Aspergillus parasiticus, Fusarium moniliforme, and Penicillium digitatum, as suggested by several authors (Wuthi-Udomlert et al., 2000; Jayaprakasha et al., 2001). C. longa methanolic extract exhibited antifungal action for Cryptococcus neoformans and Candida albicans, which indicates minimum inhibitory concentration (MIC) values of 128 and 256 g/ml, respectively. Curcumin has antifungal action over all Candida test strains, at MICs varying from 250 to 2,000 g/L, but is less efficacious than fluconazole, according to a recent analysis. It could be attributable to changes in membrane-associated ATPase activity, ergosterol production, or proteinase secretion (Neelofar et al., 2011).
Curcumin has antiviral potential (von Rhein et al., 2016) even for HIV; inhibiting HIV-1 LTR promoter directed gene expression with no effect on cell viability (Ashraf, 2018). Curcumin had moderate effectiveness towards Plasmodium falciparum and Leishmania organisms. The ethanol extracts exhibit anti-Entamoeba histolytica activity while curcumin has anti-P. falciparum and anti-Leishmania effect in vitro. Curcumin seems to have its antiviral activity for Epstein–Barr virus and HIV (Taher et al., 2003). An extract of C. longa in both aqueous and ethanol is used in aquaculture as a treatment for bacterial infections (Sahu et al., 2005). Curcumin exerts anti-parasitic action against African trypanosomes, has schistosomicidal activities against Schistosoma mansoni adult worms, and has anti-malarial in addition to nematocidal effects. Diets supplemented with C. longa reduced small intestine lesion scores and boosted weight gain in chicks infected with the cecal protozoan, Eimeria maxima. Curcumin fits well to the active site of the protease, according to in silico modeling studies (Vajragupta et al., 2005) and proved to be a powerful inhibitor of HIV integrase, as it can bind acidic residues in the integrase’s catalytic site, limiting it from interacting with its substrates. Molecular docking analysis revealed that particularly the keto-enol and terminal o-hydroxyl group of curcumin are tightly linked to the integrase’s binding region formed by residues such as Glu92, Thr93, Asp116, Ser119, Asp64, His67, Thr66, Asn120, and Lys159 (Vajragupta et al., 2005). Recent analysis has also shown the therapeutic potential of C. longa against coronavirus disease 2019 (COVID-19) (Emirik, 2020), and its ability to modulate cytokine storm in COVID-19 patients (Valizadeh et al., 2020; Mehta et al., 2022b) has produced formidable renewed interest in C. longa.
Traditional medicine has been recommended by the World Health Organization as a cost-effective substitute to manufactured antifertility medicines. Parkes mouse strain was given aqueous rhizome extract of C. longa via the oral route (600 mg/kg body weight/day for 8 and 12 weeks), which causes reversible spermatogenesis, decreased seminiferous tubules diameter, and loosening of germinal epithelium, thus indicating its potential in male fertility. Hembrom et al. (2015) also examined the influence of an aqueous C. longa rhizome extract in sperm count, spermatozoa motility, and seminal pH in Swiss Albino male mice leading to infertility. The combined action of curcumin and andrographolide significantly suppressed the number of implants and litter size in female Sprague–Dawley rats, changed the duration of phases involved in the estrus cycle, and lowered the number of ovarian follicles (Shinde et al., 2015). Petroleum ether in addition to aqueous extract of rhizome shows antifertility impact on rats via oral administration and results in complete inhibition of implantation. Curcumin also reduces human sperm motility, suggesting its usage as intravaginal contraceptive and its antispermatogenic activity.
In the management and therapy of diseases caused by immune system malfunctioning, immune response modulation is necessary. The most commonly used immunosuppressant in transplant rejection is cyclosporin A, a microbial peptide (Elgert, 2009); however, cyclosporin A exhibited toxicities and adverse effects like nephrotoxic activity and gingival hypertrophy. Unfortunately, the majority of commercially available medications include adverse effects. The main important side effects of NSAIDs include injury to the stomach and intestinal mucosa. Corticosteroids, an immunosuppressive medicine, have several negative health risks, including decreased bone marrow and skin fragility. Natural products continue to be a valuable source of innovative and safe anti-inflammatory compounds (Elgert, 2009). Due to the existence of bioactive metabolites, many Curcuma species, including C. longa, C. zanthorrhiza Roxb., C. amada Roxb., C. manga Valeton & Zijp, C. aeruginosa Roxb., and C. zedoaria Rosc., have been shown to have a variety of immunomodulatory effects. Several recent analyses on the phytochemistry, biological, and pharmacological action of the Curcuma genus have been published (Rajkumari and Sanatombi, 2017; Sun et al., 2017; Mahadevi and Kavitha, 2020; USDA, 2021). Curcumin can inhibit the expansion of T cells triggered by plant lectin concanavalin A (Con A), according to a report on the role of the genus Curcuma and its bioactive metabolites to control the immunological response. Curcumin inhibits lymphoma B-cell proliferation by lowering the potency of c-MYC, BCL-XL, and NF-κB. Curcumin has also been demonstrated to suppress the production of ROS in macrophages. Curcumin also stimulates NK cell apoptosis by modulating the NF-κB pathway and inhibiting BCL-XL and Cyclin D. Curcumin inhibits IL-1 and IL-6 inflammatory cytokines such as from LPS-stimulated dendritic cells and suppresses the expressions of CD80, CD86, and MHC class II by dendritic cells. Curcumin also causes reduced LPS-induced MAPK activation and NF-κB p65 translocation in dendritic cells (Nair et al., 2017) along with impaired activation of Th1 responses. Curcumin significantly suppressed the formation of IL-6, IL-8, TNF-α, and MCP-1 from higher glucose-cultured monocytes, according to Jain et al. (2009). Curcumin decreased NOS activity and macrophages’ ability to secrete nitric oxide (NO) at low doses. Curcumin has been revealed to be linked to the viral S1 protein, which is required for SARS-CoV-2 entry in an in silico approach; thus, it may inhibit cytokine storm in the severe stage of COVID-19 (Pawitan, 2020). Table 2 shows the immunomodulating activity of curcumin with its mechanisms of action.
In the last few years, the use of nanoformulation antibiotics has become increasingly popular in improving pharmacological therapeutic benefits. Nanomedicine is a relatively new field that is rapidly expanding in conjunction with nanotechnology and pharmaceutical disciplines (Caster et al., 2017). Nanopharmaceuticals, on the other hand, confront numerous hurdles, including improved characterization, toxicity issues, regulatory standards, high expenses, and healthcare warnings. Curcumin’s practical applications are frequently limited by factors such as poor water solubility and physicochemical instability, poor bioactive absorption, rapid metabolization, low pharmacokinetics, bioavailability, penetration and targeting efficacy, sensitivity to metal ions, alkaline conditions, heat, and light, among others (Flora et al., 2013). These barriers are being overcome by encapsulating curcumin in nanoformulations (nano curcumin) (Yallapu et al., 2012). Integrating curcumin into nanocarriers is an effective and efficient way to improve curcumin’s biological features, such as bioavailability and solubility, long-term circulation, and long-term retention in the body, as well as overcome curcumin’s physiological challenges (Das et al., 2010; Li et al., 2013; Fonseca-Santos et al., 2016). It can also limit unintentional toxicity to neighboring normal cells/tissues by dispersing the intended tissues. Curcumin encapsulated in poly(lactic-co-glycolic acid) nanoparticles showed a ninefold increase in nano curcumin when compared to natural curcumin (Shaikh et al., 2009). Experiments demonstrate that nanoforms of curcumin are effective in the treatment of liver and heart problems (Shimatsu et al., 2012), cancer (Mohanty and Sahoo, 2010), and brain tumors (Mohanty and Sahoo, 2010; Lim et al., 2011). Moreover, curcumin nanoformulation exhibited threefold higher anti-HIV activity in comparison with its free form; obstructed the HIV-1-triggered expression of interleukin-1β (IL-1β), Topo II α, and cyclooxygenase 2 (COX-2); and blocked the synthesis of viral complementary DNA (cDNA) (Gandapu et al., 2011).
Moreover, metal-based green synthesis is gaining importance due to its chemical, optical, photochemical, and electronic properties (Mohanpuria et al., 2008). Among several metals, silver has gained huge attention for the green synthesis of NPs because of its numerous applications in various industries, particularly because of its nonlinear optical, biolabeling, and antibacterial capacity. Silver nanoparticles (AgNPs) are widely used in various fields like in drug delivery (Basu et al., 2018), nanomedicine (Carabineiro, 2017), agriculture, and cosmetics, and most importantly, they are used as an antimicrobial agent (Zhang et al., 2017). However, many scientists report that AgNPs also cause toxicity (McGillicuddy et al., 2017), but still, they play a major role as a disinfectant and as a antimicrobial agent. An emergence of nanotechnology that helps in the production of AgNPs has served as a new therapeutic modality. Because of their characteristic broad-spectrum antimicrobial ability, AgNPs have gained increasing attention in biomedical applications including wound management (Parveen et al., 2018; Ahsan and Farooq, 2019; Ravindran et al., 2019), but the hydrophobic nature of curcumin limits its biomedical applications. Hydrogels are in natural or synthetic forms including bacterial cellulose as one of the promising synthetic candidates used in wound dressings because of its ability to maintain a moist microclimate at the wound site, which has been proven to facilitate healing (Tao et al., 2019; Xue et al., 2019). The hydrophobicity of curcumin was overcome by its microencapsulation in cyclodextrins. A combination of AgNP with curcumin in the biosynthetic bacterial cellulose is used for the preparation of hydrogel dressings that exhibit antimicrobial activity against wound-infecting pathogenic microbes like S. aureus, P. aeruginosa, and Candida auris (Gupta et al., 2020). Among several different approaches to synthesizing AgNP, the use of extracts from natural plant sources has received wide research consideration because of the safe and eco-friendly procedure (Alsammarraie et al., 2018; Hemmati et al., 2019; Ravindran et al., 2019; Keshari et al., 2020). The antibacterial action of nano formed curcumin against a wide range of microorganisms, including fungi, bacteria, and viruses, has been carried out by researchers. Curcumin-modified AgNPs, for example, are utilized to inhibit respiratory syncytial virus (RSV) infection cells and reduce viral loads while having no deleterious side effects (Yang et al., 2016). Naseri et al. then investigated the antiviral effects of curcumin nanomicelles on the attachment and entrance of hepatitis C virus (HCV) infection, finding out that the viral load in HCV cells treated with curcumin nanomicelles was reduced (Naseri et al., 2017). Nanocurcumin was shown to exhibit improved antibacterial activity compared to curcumin because of its enhanced aqueous-phase solubility and simple dispersibility. An efficient antibacterial potential was found against Bacillus subtilis, H. pylori, S. aureus, and P. aeruginosa (Basniwal et al., 2011). An aqueous extract from C. longa-coated cotton fabrics, as well as formulated metallic AgNPs, also exhibited noticeable antimicrobial potency against S. aureus, P. aeruginosa, S. pyogenes, and C. albicans, and even AgNP-loaded cotton fabrics displayed potent wound healing activity in fibroblast (L929) cells (Maghimaa and Alharbi, 2020). Moreover, recently, it has been found that the combination of AgNP with the rhizome extract of C. longa showed antimicrobial effect towards plant affecting bacteria such as Xanthomonas axonopodis and Erwinia amylovora, which could be useful for nano-drug delivery applications (Gaurav, 2021). Curcumin’s bioavailability was also discussed in Figure 8, as well as a method for dealing with it.
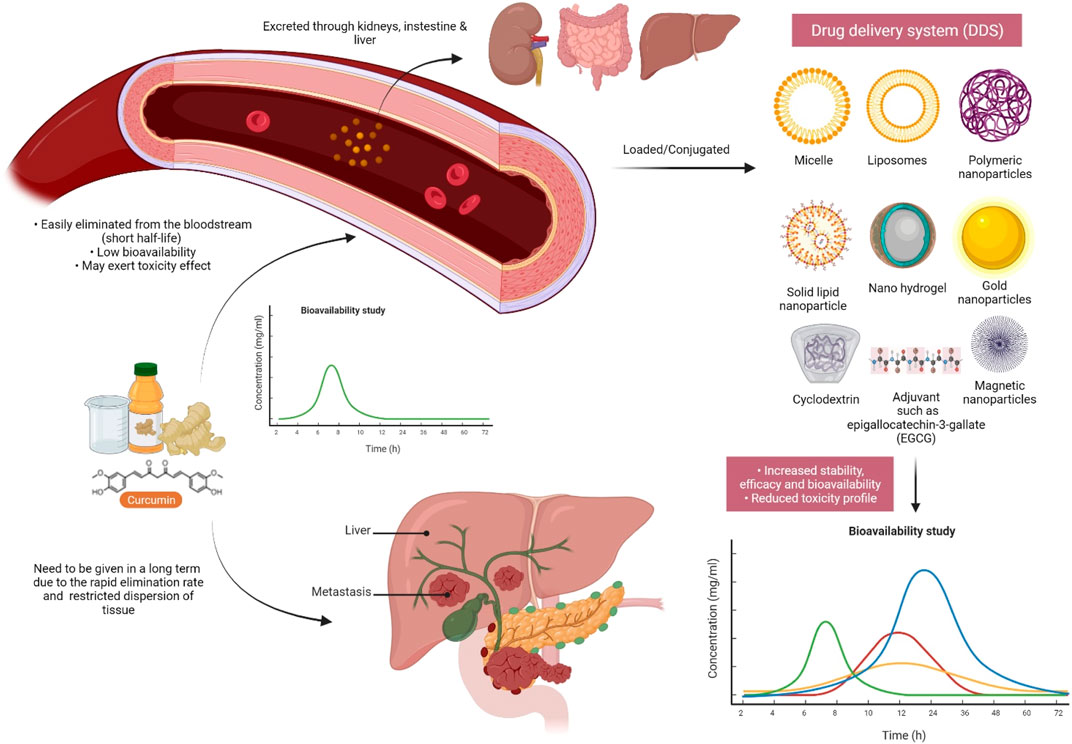
FIGURE 8. Curcumin must be modified/improved prior to future research due to its extremely low bioavailability. Due to the quick elimination of curcumin in the systemic circulation as a result of enzyme metabolism, conjugating curcumin to adjuvants or other delivery systems may be beneficial in order to increase its half-life and bioavailability.
Facts suggest that excessive turmeric consumption may trigger uterine contraction in pregnancy, and may hinder iron absorption (and so must be used with caution in iron-deficient individuals). Turmeric has been reported to reduce testosterone levels and sperm movement in men (when administered orally) and delay blood clotting (and so its use must be terminated at least 2 weeks before a scheduled surgery). According to some reports, turmeric should not be consumed if one has gallbladder and bleeding problems (Deshpande et al., 1998; Park et al., 2000). Figure 9 depicts curcumin’s safety and toxicity profiles, and a way to manage them (Cheng et al., 2001; Perkins et al., 2002; Rithaporn et al., 2003; Liddle et al., 2006; Sharma et al., 2007; Vareed et al., 2008; Dance-Barnes et al., 2009). In addition, Table 3 summarizes the clinical uses of C. longa and its major component curcumin.
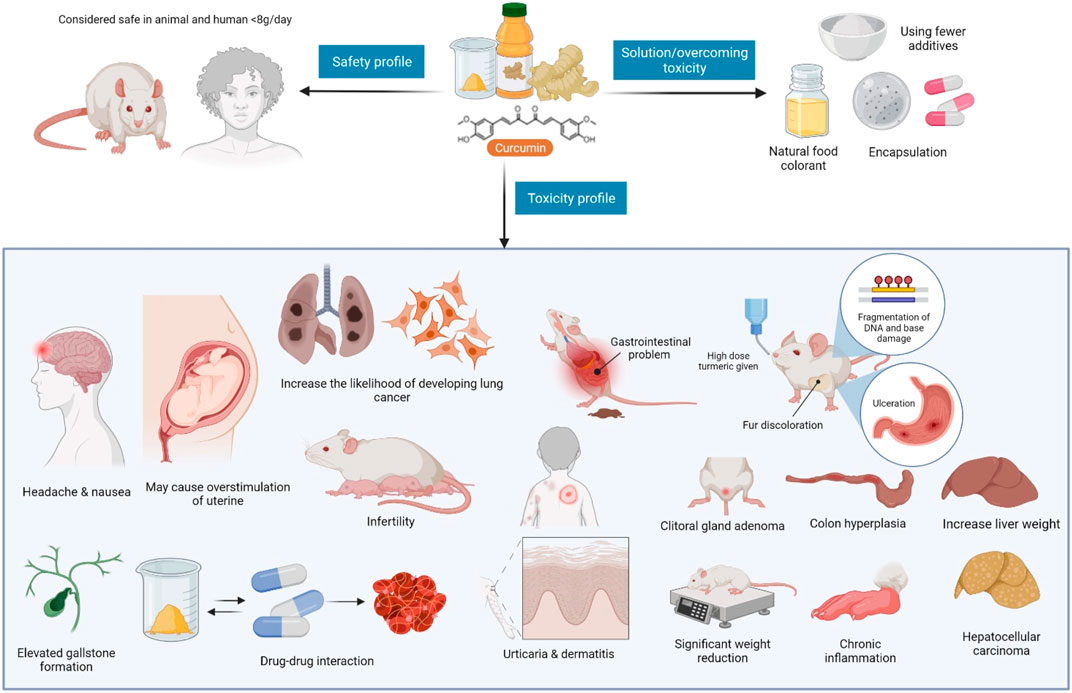
FIGURE 9. Curcumin safety, toxicity profiles, and solution to overcome it. Curcumin safety, toxicity profiles, and a way to circumvent it. Despite its recognized safety, certain investigations have highlighted unprecedented side effects of curcumin, including gastrointestinal problems, chronic inflammations, and others. To address this issue, fewer additives should be utilized, and curcumin microencapsulation should be tailored.
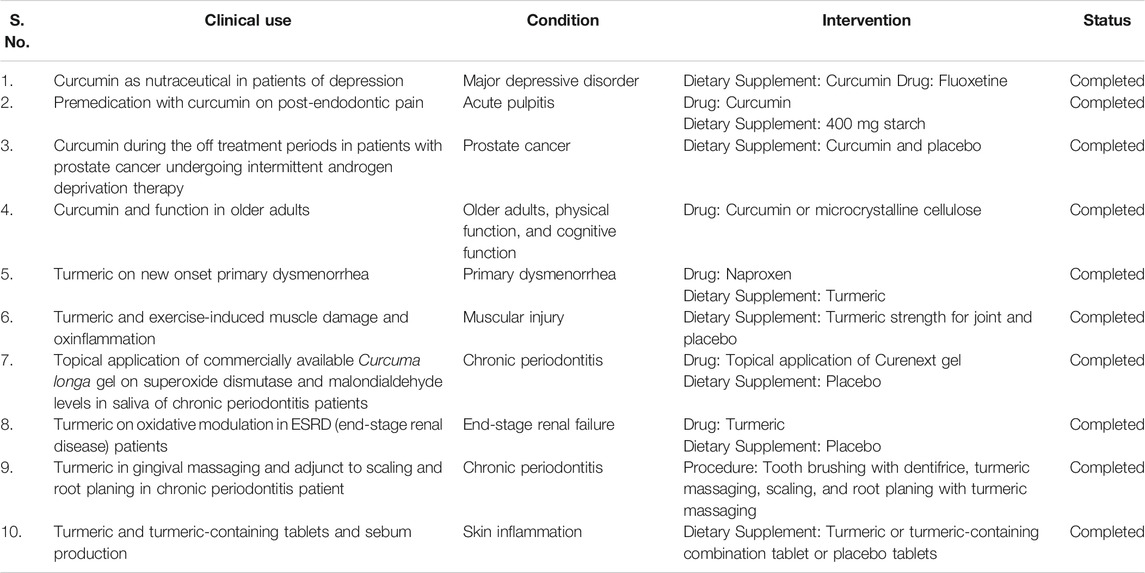
TABLE 3. Clinical uses of C. longa and its major compound curcumin (https://clinicaltrials.gov/).
C. longa with its various pharmacological features has been characterized as a universal panacea among herbal remedies, as per the literature survey. This plant is regarded as a potent medicinal plant with a wide range of potent pharmacological properties due to the presence of numerous chemical components including starch, essential elements, proteins, vitamins, volatile oils, curcumin, and curcuminoids. Curcumin has a long history of use as a culinary spice and food color, as well as a component of Ayurvedic and Chinese medicine. Curcumin has a variety of beneficial effects on humans, according to science. Curcumin is still utilized as a cooking ingredient today, but modern technology has made it possible to use it in a range of food- and health-related applications. Curcumin’s efficacy, safety, and pharmacokinetics have all been examined extensively in clinical studies over the last 50 years (Gupta et al., 2013; Subramani et al., 2018). The development of innovative nanomedicine formulations to increase curcumin targeting, pharmacokinetics, efficacy, and cellular uptake has been prompted by a significant therapeutic limitation (Salehi et al., 2020a; Salehi et al., 2020b). Cancer, CVD, arthritis, atherosclerosis, diabetes, gastric illness, IBD, psoriasis, acquired immunodeficiency syndrome, and other inflammatory disorders are all examples of pleiotropic activities. Several studies in this review discovered the anti-inflammatory effects of C. longa and curcumin, including decreased white blood cell, neutrophil, and eosinophil numbers, as well as protective effects on serum levels of inflammatory mediators like phospholipase A2 and total protein in various inflammatory disorders. Curcumin has anticancer properties by interfering with many cellular systems and inhibiting/inducing the production of multiple cytokines, enzymes, or IκKβ, TNF-α, STAT3, COX-2, PKD1, NF-κB, epidermal growth factor, and MAPK, among others. Under oxidative stress conditions, C. longa extracts and curcumin decreased MDA and NO levels while increasing thiol, SOD, and catalase levels. Curcumin also influenced the lifespan of organisms by regulating important signaling pathways such as the mTOR, PKA, and FOXO signaling pathways. In conditions where the immune system was disturbed, treatment with C. longa and curcumin enhanced IgE, IL-4, TGF-β, IL-17, IFN-γ, and the Th1/Th2 ratio. The pharmacological effects of C. longa extracts and curcumin on respiratory, allergy, and immunologic problems suggest that C. longa and curcumin may have a possible therapeutic effect on these illnesses. C. longa extracts and curcumin delay the onset of diabetes, improve β-cell functioning, prevent β-cell death, and reduce insulin resistance in animal models. Curcumin’s use has been limited due to its low water solubility, which can result in poor chemical stability, oral bioavailability, and cellular uptake. Other strategies that have been aggressively studied include delivering medications at a controlled rate, slow delivery, and targeted delivery. Curcumin nanoformulations have been produced to improve the solubility and bioavailability of the compound. Curcumin’s medicinal applications and clinical efficacy could be expanded if biotechnology and nanotechnology were used to address the current limitations.
This review adds to the growing body of evidence supporting the use of turmeric as a preventative and therapeutic strategy. We believe that more progress in the development of strategies incorporating natural products can be exploited to be used against COVID-19 in the upcoming years. Hence, this paper also suggests the use of gold nanoparticles in combination with neutralizing antibody Ty1, which may assist selectively in the receptor binding domain of the SARS-CoV-2 spike, directly hindering angiotensin-converting enzyme 2 interaction. The inclusion of curcumin and bevacizumab further enhanced the efficacy of the proposed strategy as both may target and potently neutralize VEGF, thereby decreasing and slowing tumor growth (Figure 10).
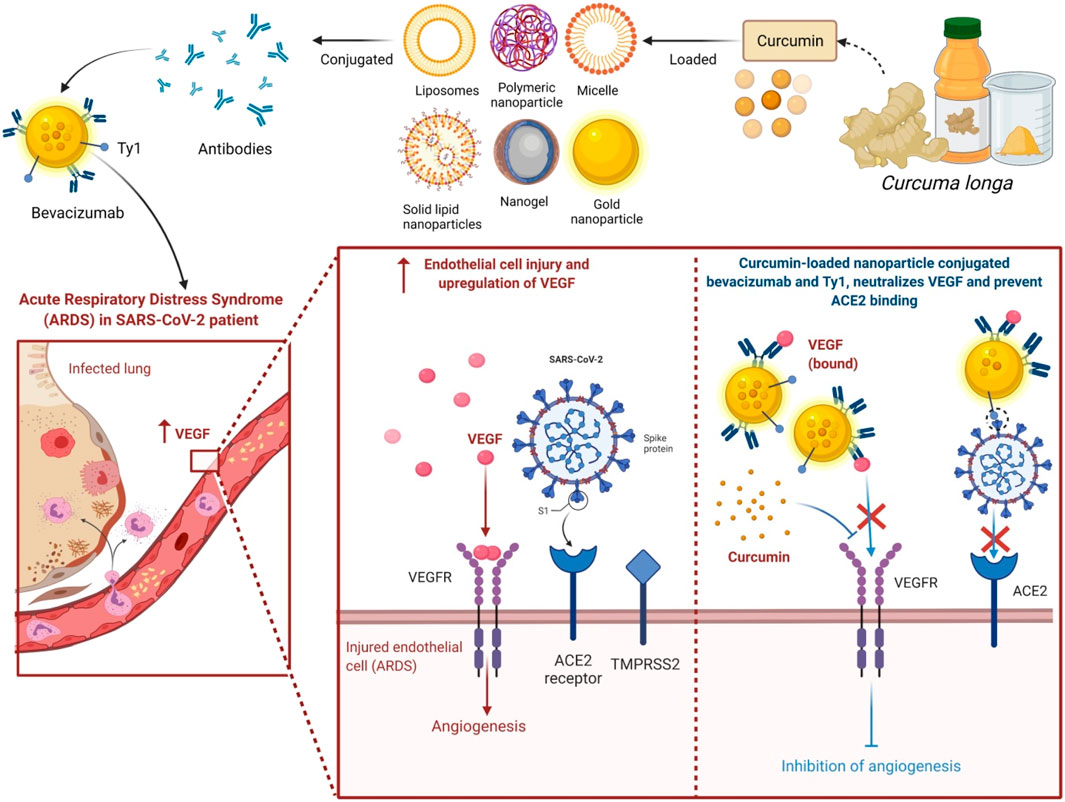
FIGURE 10. Future perspective of curcumin-loaded gold nanoparticles functionalized with Ty1 and bevacizumab in a patient infected with SARS-CoV-2 (COVID-19).
C. longa entailed extensive research and development work to fully exploit its medicinal value, and efforts should be made to investigate the possibilities of practical clinical applications as well as the details of hidden and untold areas in order to maximize its utility for the benefit of humanity. It is suggested to inhibit cytokine storm in the severe stage of COVID-19. So, we can further work on it to get out of this pandemic situation of COVID-19 mutations. For the rational usage of turmeric and curcumin in the therapies of human diseases especially COVID-19, an accurate knowledge of effective dose, safety, and mode of action is necessary.
Writing—original draft: JM, AC, SF, MS, and NF; Conceptualization: JM and AC; Resources: SF, JM, AC, MS, NR, MB, VS, KC, LT, RN, YW, KS, PL, DM, VK, AA, and NF; Data curation: SF, JM, AC, MS, NR, MB, VS, KC, LT, RN, YW, KS, PL, DM, VK, AA, and NF; Writing—review and editing: SF, JM, AC, MS, NR, MB, VS, KC, LT, RN, YW, KS, PL, DM, VK, AA, and NF. All authors have read and agreed to the published version of the manuscript.
This study was funded by the Deanship of Scientific Research through Research Group (Small) (project number RGP.1/330/42) of the King Khalid University, Abha 61421, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group (Small) (project number RGP.1/330/42). All the authors of this manuscript are thankful to their respective Departments/Universities for successful completion of this study. The figures in this manuscript were created with the support of https://biorender.com under a paid subscription (Ref: C08A1A0B-0002; 3 November 2021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors MS.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Diam, M. M., Samak, D. H., El-Sayed, Y. S., Aleya, L., Alarifi, S., and Alkahtani, S. (2019). Curcumin and Quercetin Synergistically Attenuate Subacute Diazinon-Induced Inflammation and Oxidative Neurohepatic Damage, and Acetylcholinesterase Inhibition in Albino Rats. Environ. Sci. Pollut. Res. Int. 26 (4), 3659–3665. doi:10.1007/s11356-018-3907-9
Afolayan, F. I. D., Erinwusi, B., and Oyeyemi, O. T. (2018). Immunomodulatory Activity of Curcumin-Entrapped Poly D,l-Lactic-Co-Glycolic Acid Nanoparticles in Mice. Integr. Med. Res. 7 (2), 168–175. doi:10.1016/j.imr.2018.02.004
Aggarwal, B. B., Takada, Y., and Oommen, O. V. (2004). From Chemoprevention to Chemotherapy: Common Targets and Common Goals. Expert Opin. Investig. Drugs 13 (10), 1327–1338. doi:10.1517/13543784.13.10.1327
Ahmadi, M., Agah, E., Nafissi, S., Jaafari, M. R., Harirchian, M. H., Sarraf, P., et al. (2018). Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients with Amyotrophic Lateral Sclerosis: a Pilot Randomized Clinical Trial. Neurotherapeutics 15 (2), 430–438. doi:10.1007/s13311-018-0606-7
Ahsan, A., and Farooq, M. A. (2019). Therapeutic Potential of green Synthesized Silver Nanoparticles Loaded PVA Hydrogel Patches for Wound Healing. J. Drug Deliv. Sci. Tech. 54, 101308. doi:10.1016/j.jddst.2019.101308
Alambra, J. R., Alenton, R. R. R., Gulpeo, P. C. R., Mecenas, C. L., Miranda, A. P., Thomas, R. C., et al. (2012). Immunomodulatory Effects of Turmeric, Curcuma Longa (Magnoliophyta, Zingiberaceae) on macrobrachium Rosenbergii (Crustacea, Palaemonidae) against Vibrio Alginolyticus (Proteobacteria, Vibrionaceae). Aquac. Aquarium, Conservation Legis. 5 (1), 13–17.
AlBasher, G., Abdel-Daim, M. M., Almeer, R., Ibrahim, K. A., Hamza, R. Z., Bungau, S., et al. (2020). Synergistic Antioxidant Effects of Resveratrol and Curcumin against Fipronil-Triggered Oxidative Damage in Male Albino Rats. Environ. Sci. Pollut. Res. Int. 27 (6), 6505–6514. doi:10.1007/s11356-019-07344-8
Alsammarraie, F. K., Wang, W., Zhou, P., Mustapha, A., and Lin, M. (2018). Green Synthesis of Silver Nanoparticles Using Turmeric Extracts and Investigation of Their Antibacterial Activities. Colloids Surf. B Biointerfaces 171, 398–405. doi:10.1016/j.colsurfb.2018.07.059
Altenburg, J. D., Bieberich, A. A., Terry, C., Harvey, K. A., VanHorn, J. F., Xu, Z., et al. (2011). A Synergistic Antiproliferation Effect of Curcumin and Docosahexaenoic Acid in SK-BR-3 Breast Cancer Cells: Unique Signaling Not Explained by the Effects of Either Compound Alone. BMC cancer 11 (1), 149. doi:10.1186/1471-2407-11-149
Ammon, H. P., and Wahl, M. A. (1991). Pharmacology of Curcuma Longa. Planta Med. 57 (01), 1–7. doi:10.1055/s-2006-960004
Annapurna, A., Suhasin, G., Raju, B., Jaya, G., and Siva, C. (2011). Anti-cancer Activity of Curcuma Longa linn.(Turmeric). J. Pharm. Res. 4 (4), 1274–1276.
Ashraf, K. (2018). A Comprehensive Review on Curcuma Longa Linn.: Phytochemical, Pharmacological, and Molecular Study. Int. J. Green Pharm. (Ijgp) 11 (04).
Ayati, Z., Ramezani, M., Amiri, M. S., Moghadam, A. T., Rahimi, H., Abdollahzade, A., et al. (2019). Ethnobotany, Phytochemistry and Traditional Uses of Curcuma Spp. And Pharmacological Profile of Two Important Species (C. Longa and C. Zedoaria): a Review. Curr. Pharm. Des. 25 (8), 871–935. doi:10.2174/1381612825666190402163940
Banji, D., Banji, O. J., and Srinivas, K. (20212021). Neuroprotective Effect of Turmeric Extract in Combination with its Essential Oil and Enhanced Brain Bioavailability in an Animal Model. Biomed. Res. Int. doi:10.1155/2021/6645720
Basu, S., Samanta, H. S., and Ganguly, J. (2018). Green Synthesis and Swelling Behavior of Ag-Nanocomposite Semi-IPN Hydrogels and Their Drug Delivery Using Dolichos Biflorus Linn. Soft Mater. 16 (1), 7–19. doi:10.1080/1539445x.2017.1368559
Bhawana, R. K., Basniwal, R. K., Buttar, H. S., Jain, V. K., and Jain, N. (2011). Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 59 (5), 2056–2061. doi:10.1021/jf104402t
Billiard, S. M., Timme-Laragy, A. R., Wassenberg, D. M., Cockman, C., and Di Giulio, R. T. (2006). The Role of the Aryl Hydrocarbon Receptor Pathway in Mediating Synergistic Developmental Toxicity of Polycyclic Aromatic Hydrocarbons to Zebrafish. Toxicol. Sci. 92 (2), 526–536. doi:10.1093/toxsci/kfl011
Binic, I., Lazarevic, V., Ljubenovic, M., Mojsa, J., and Sokolovic, D. (20132013). Skin Ageing: Natural Weapons and Strategies.Evidence-Based Complement. Altern. Med.
Boskabady, M. H., Shakeri, F., and Naghdi, F. (2020). “The Effects of Curcuma Longa L. And its Constituents in Respiratory Disorders and Molecular Mechanisms of Their Action,” in Studies in Natural Products Chemistry (Elsevier), 239–269. doi:10.1016/b978-0-12-817905-5.00007-x
Bundy, R., Walker, A. F., Middleton, R. W., and Booth, J. (2004). Turmeric Extract May Improve Irritable Bowel Syndrome Symptomology in Otherwise Healthy Adults: a Pilot Study. J. Altern. Complement. Med. 10 (6), 1015–1018. doi:10.1089/acm.2004.10.1015
Cao, Q., Zhang, J., Gao, L., Zhang, Y., Dai, M., and Bao, M. (2018). Dickkopf-3 U-pregulation M-ediates the C-ardioprotective E-ffects of C-urcumin on C-hronic H-eart F-ailure. Mol. Med. Rep. 17 (5), 7249–7257. doi:10.3892/mmr.2018.8783
Carabineiro, S. (2017). Applications of Gold Nanoparticles in Nanomedicine: Recent Advances in Vaccines. Molecules 22 (5), 857. doi:10.3390/molecules22050857
Caster, J. M., Patel, A. N., Zhang, T., and Wang, A. (2017). Investigational Nanomedicines in 2016: a Review of Nanotherapeutics Currently Undergoing Clinical Trials. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol 9 (1), e1416. doi:10.1002/wnan.1416
Chattopadhyay, I., Biswas, K., Bandyopadhyay, U., and Banerjee, R. K. (2004). Turmeric and Curcumin: Biological Actions and Medicinal Applications. Curr. Sci., 44–53.
Chearwae, W., and Bright, J. J. (2008). 15-deoxy-Delta(12,14)-prostaglandin J(2) and Curcumin Modulate the Expression of Toll-like Receptors 4 and 9 in Autoimmune T Lymphocyte. J. Clin. Immunol. 28 (5), 558–570. doi:10.1007/s10875-008-9202-7
Chen, H. W., and Huang, H. C. (1998). Effect of Curcumin on Cell Cycle Progression and Apoptosis in Vascular Smooth Muscle Cells. Br. J. Pharmacol. 124 (6), 1029–1040. doi:10.1038/sj.bjp.0701914
Chen, J., Chia, N., Kalari, K. R., Yao, J. Z., Novotna, M., Paz Soldan, M. M., et al. (2016). Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci. Rep. 6 (1), 28484. doi:10.1038/srep28484
Chen, M. J., Cheng, Y. M., Lai, P. H., Wu, J. F., and Hsu, Y. C. (2012). In Vitro biocompatibility of Thermally Gelling Liquid Mucoadhesive Loaded Curcuminoids in Colorectal Cancer Chemoprevention. Int. J. Colorectal Dis. 27 (7), 869–878. doi:10.1007/s00384-011-1393-3
Chen, R., Peng, X., Du, W., Wu, Y., Huang, B., Xue, L., et al. (2015). Curcumin Attenuates Cardiomyocyte Hypertrophy Induced by High Glucose and Insulin via the PPARγ/Akt/NO Signaling Pathway. Diabetes Res. Clin. Pract. 108 (2), 235–242. doi:10.1016/j.diabres.2015.02.012
Cheng, A. L., Hsu, C. H., Lin, J. K., Hsu, M. M., Ho, Y. F., Shen, T. S., et al. (2001). Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-malignant Lesions. Anticancer Res. 21 (4B), 2895–2900.
Chico, L., Ienco, E. C., Bisordi, C., Lo Gerfo, A., Petrozzi, L., Petrucci, A., et al. (2018). Amyotrophic Lateral Sclerosis and Oxidative Stress: a Double-Blind Therapeutic Trial after Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 17 (10), 767–779. doi:10.2174/1871527317666180720162029
Choi, Y. H., Yan, G. H., Chai, O. H., and Song, C. H. (2010). Inhibitory Effects of Curcumin on Passive Cutaneous Anaphylactoid Response and Compound 48/80-induced Mast Cell Activation. Anat. Cel Biol 43 (1), 36–43. doi:10.5115/acb.2010.43.1.36
Chou, T. C. (2006). Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 58 (3), 621–681. doi:10.1124/pr.58.3.10
Choudhuri, T., Pal, S., Agwarwal, M. L., Das, T., and Sa, G. (2002). Curcumin Induces Apoptosis in Human Breast Cancer Cells through P53-dependent Bax Induction. FEBS Lett. 512 (1-3), 334–340. doi:10.1016/s0014-5793(02)02292-5
Dance-Barnes, S. T., Kock, N. D., Moore, J. E., Lin, E. Y., Mosley, L. J., D'Agostino, R. B., et al. (2009). Lung Tumor Promotion by Curcumin. Carcinogenesis 30 (6), 1016–1023. doi:10.1093/carcin/bgp082
Das, R. K., Kasoju, N., and Bora, U. (2010). Encapsulation of Curcumin in Alginate-Chitosan-Pluronic Composite Nanoparticles for Delivery to Cancer Cells. Nanomedicine 6 (1), 153–160. doi:10.1016/j.nano.2009.05.009
Dave, S., Vijay, S., Keswani, H., and Sharma, S. (2017). Curcumin-a Magical Medicine: a Comprehensive Review. Int. ayurvedic Med. J. 5 (2), 458–467.
Deshpande, U. R., Gadre, S. G., Raste, A. S., Pillai, D., Bhide, S. V., and Samuel, A. M. (1998). Protective Effect of Turmeric (Curcuma Longa L.) Extract on Carbon Tetrachloride-Induced Liver Damage in Rats. Indian J. Exp. Biol. 36 (6), 573–577.
Dikshit, M., Rastogi, L., Shukla, R., and Srimal, R. C. (1995). Prevention of Ischaemia-Induced Biochemical Changes by Curcumin & Quinidine in the Cat Heart. Indian J. Med. Res. 101, 31–35.
Dohare, P., Garg, P., Sharma, U., Jagannathan, N. R., and Ray, M. (2008). Neuroprotective Efficacy and Therapeutic Window of Curcuma Oil: in Rat Embolic Stroke Model. BMC Complement. Altern. Med. 8 (1), 55–20. doi:10.1186/1472-6882-8-55
Durgaprasad, S., Pai, C. G., Vasanthkumar, J. F., Alvres, J. F., and Namitha, S. (2005). A Pilot Study of the Antioxidant Effect of Curcumin in Tropical Pancreatitis. Indian J. Med. Res. 122 (4), 315–318.
El Nebrisi, E., Javed, H., Ojha, S. K., Oz, M., and Shehab, S. (2020). Neuroprotective Effect of Curcumin on the Nigrostriatal Pathway in a 6-Hydroxydopmine-Induced Rat Model of Parkinson's Disease Is Mediated by α7-Nicotinic Receptors. Int. J. Mol. Sci. 21 (19), 7329. doi:10.3390/ijms21197329
Emirik, M. (2020). Potential Therapeutic Effect of Turmeric Contents against SARS-CoV-2 Compared with Experimental COVID-19 Therapies: In Silico Study. J. Biomol. Struct. Dyn., 1–14. doi:10.1080/07391102.2020.1835719
Etemadi, S., Barhaghi, M. H. S., Leylabadlo, H. E., Memar, M. Y., Mohammadi, A. B., and Ghotaslou, R. (2021). The Synergistic Effect of Turmeric Aqueous Extract and Chitosan against Multidrug-Resistant Bacteria. New Microbes New Infect. 41, 100861. doi:10.1016/j.nmni.2021.100861
Faizal, P., Suresh, S., Satheesh Kumar, R., and Augusti, K. T. (2009). A Study on the Hypoglycemic and Hypolipidemic Effects of an Ayurvedic Drug Rajanyamalakadi in Diabetic Patients. Indian J. Clin. Biochem. 24 (1), 82–87. doi:10.1007/s12291-009-0014-1
Farombi, E. O., Abarikwu, S. O., Adedara, I. A., and Oyeyemi, M. O. (2007). Curcumin and Kolaviron Ameliorate Di-n-butylphthalate-induced Testicular Damage in Rats. Basic Clin. Pharmacol. Toxicol. 100 (1), 43–48. doi:10.1111/j.1742-7843.2007.00005.x
Feng, W. W., Kuang, S. Y., Tu, C., Ma, Z. J., Pang, J. Y., Wang, Y. H., et al. (2018). Natural Products Berberine and Curcumin Exhibited Better Ameliorative Effects on Rats with Non-alcohol Fatty Liver Disease Than Lovastatin. Biomed. Pharmacother. 99, 325–333. doi:10.1016/j.biopha.2018.01.071
Flora, G., Gupta, D., and Tiwari, A. (2013). Nanocurcumin: a Promising Therapeutic Advancement over Native Curcumin. Crit. Rev. Ther. Drug Carrier Syst. 30 (4), 331–368. doi:10.1615/critrevtherdrugcarriersyst.2013007236
Fonseca-Santos, B., Dos Santos, A. M., Rodero, C. F., Gremião, M. P., and Chorilli, M. (2016). Design, Characterization, and Biological Evaluation of Curcumin-Loaded Surfactant-Based Systems for Topical Drug Delivery. Int. J. Nanomedicine 11, 4553–4562. doi:10.2147/IJN.S108675
Frank, N., Knauft, J., Amelung, F., Nair, J., Wesch, H., and Bartsch, H. (2003). No Prevention of Liver and Kidney Tumors in Long-Evans Cinnamon Rats by Dietary Curcumin, but Inhibition at Other Sites and of Metastases. Mutat. Res. 523-524, 127–135. doi:10.1016/s0027-5107(02)00328-7
Gandapu, U., Chaitanya, R. K., Kishore, G., Reddy, R. C., and Kondapi, A. K. (2011). Curcumin-loaded Apotransferrin Nanoparticles Provide Efficient Cellular Uptake and Effectively Inhibit HIV-1 Replication In Vitro. PloS one 6 (8), e23388. doi:10.1371/journal.pone.0023388
Gao, S., Zhang, W., Zhao, Q., Zhou, J., Wu, Y., Liu, Y., et al. (2019). Curcumin Ameliorates Atherosclerosis in Apolipoprotein E Deficient Asthmatic Mice by Regulating the Balance of Th2/Treg Cells. Phytomedicine 52, 129–135. doi:10.1016/j.phymed.2018.09.194
Gaurav, I., and Tanuja, fnm. (2021). Green Synthesis and Characterization of Silver Nanoparticles with Rhizome Extract of Curcuma Longa (AgNPs-RECL) for Antimicrobial Activity towards Xanthomonas and Erwinia Species. Res. J. Pharm. Tech. 14 (1), 325–330. doi:10.5958/0974-360x.2021.00060.3
Ghoneum, M., and Gollapudi, S. (2011). Synergistic Apoptotic Effect of Arabinoxylan rice Bran (MGN-3/Biobran) and Curcumin (Turmeric) on Human Multiple Myeloma Cell Line U266 In Vitro. Neoplasma 58 (2), 118–123. doi:10.4149/neo_2011_02_118
Ghotaslou, R., Leylabadlo, H. E., Akhi, M. T., Sadeghi, J., Yousefi, L., bialvaei, A. Z., et al. (2017). The Importance of Helicobacter pylori tnpA, tnpB, and cagA Genes in Various Gastrointestinal Diseases. Mol. Genet. Microbiol. Virol. 32 (1), 62–65. doi:10.3103/s0891416817010049
Goud, V. K., Polasa, K., and Krishnaswamy, K. (1993). Effect of Turmeric on Xenobiotic Metabolising Enzymes. Plant Foods Hum. Nutr. 44 (1), 87–92. doi:10.1007/BF01088486
Guimarães, A. F., Vinhas, A. C. A., Gomes, A. F., Souza, L. H., and Krepsky, P. B. (2020). Essential Oil of Curcuma Longa L. Rhizomes Chemical Composition, Yield Variation and Stability. Química Nova 43, 909–913. doi:10.21577/0100-4042.20170547
Gupta, A., Briffa, S. M., Swingler, S., Gibson, H., Kannappan, V., Adamus, G., et al. (2020). Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 21 (5), 1802–1811. doi:10.1021/acs.biomac.9b01724
Gupta, S. C., Patchva, S., and Aggarwal, B. B. (2013). Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 15 (1), 195–218. doi:10.1208/s12248-012-9432-8
Hajavi, J., Momtazi, A. A., Johnston, T. P., Banach, M., Majeed, M., and Sahebkar, A. (2017). Curcumin: a Naturally Occurring Modulator of Adipokines in Diabetes. J. Cel Biochem 118 (12), 4170–4182. doi:10.1002/jcb.26121
Han, F., Luo, B., Shi, R., Han, C., Zhang, Z., Xiong, J., et al. (2014). Curcumin Ameliorates Rat Experimental Autoimmune Neuritis. J. Neurosci. Res. 92 (6), 743–750. doi:10.1002/jnr.23357
Hanai, H., Iida, T., Takeuchi, K., Watanabe, F., Maruyama, Y., Andoh, A., et al. (2006). Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 4 (12), 1502–1506. doi:10.1016/j.cgh.2006.08.008
Hembrom, A. R., Verma, A., and Singh, V. N. (2015). Antifertility Effects of Rhizome ofCurcuma Longaon Seminal Parameters of Swiss Albino Male Mice. Rese. Jour. Pharm. Technol. 8 (4), 404–406. doi:10.5958/0974-360x.2015.00068.2
Hemmati, S., Rashtiani, A., Zangeneh, M. M., Mohammadi, P., Zangeneh, A., and Veisi, H. (2019). Green Synthesis and Characterization of Silver Nanoparticles Using Fritillaria Flower Extract and Their Antibacterial Activity against Some Human Pathogens. Polyhedron 158, 8–14. doi:10.1016/j.poly.2018.10.049
Holt, P. R., Katz, S., and Kirshoff, R. (2005). Curcumin Therapy in Inflammatory Bowel Disease: a Pilot Study. Dig. Dis. Sci. 50 (11), 2191–2193. doi:10.1007/s10620-005-3032-8
Horie, S. (2012). Chemoprevention of Prostate Cancer: Soy Isoflavones and Curcumin. Korean J. Urol. 53 (10), 665–672. doi:10.4111/kju.2012.53.10.665
Huang, M. T., Smart, R. C., Wong, C. Q., and Conney, A. H. (1988). Inhibitory Effect of Curcumin, Chlorogenic Acid, Caffeic Acid, and Ferulic Acid on Tumor Promotion in Mouse Skin by 12-O-Tetradecanoylphorbol-13-Acetate. Cancer Res. 48 (21), 5941–5946.
Inzaugarat, M. E., De Matteo, E., Baz, P., Lucero, D., García, C. C., Gonzalez Ballerga, E., et al. (2017). New Evidence for the Therapeutic Potential of Curcumin to Treat Nonalcoholic Fatty Liver Disease in Humans. PLoS One 12 (3), e0172900. doi:10.1371/journal.pone.0172900
Issuriya, A., Kumarnsit, E., Wattanapiromsakul, C., and Vongvatcharanon, U. (2014). Histological Studies of Neuroprotective Effects of Curcuma Longa Linn. On Neuronal Loss Induced by Dexamethasone Treatment in the Rat hippocampus. Acta Histochem. 116 (8), 1443–1453. doi:10.1016/j.acthis.2014.09.009
Jacob, A., Wu, R., Zhou, M., and Wang, P. (2007). Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-gamma Activation. PPAR Res. 2007, 89369. doi:10.1155/2007/89369
Jain, S. K., Rains, J., Croad, J., Larson, B., and Jones, K. (2009). Curcumin Supplementation Lowers TNF-Alpha, IL-6, IL-8, and MCP-1 Secretion in High Glucose-Treated Cultured Monocytes and Blood Levels of TNF-Alpha, IL-6, MCP-1, Glucose, and Glycosylated Hemoglobin in Diabetic Rats. Antioxid. Redox Signal. 11 (2), 241–249. doi:10.1089/ars.2008.2140
Jassal, P., Kaur, G., and Kaur, L. (2015). Synergistic Effect of Curcuma Longa and Glycyrrhiza Glabra Extracts with Copper Ions on Food Spoilage Bacteria. Int. J. Pharm. Sci. 7, 371–375.
Jayaprakasha, G. K., Negi, P. S., Anandharamakrishnan, C., and Sakariah, K. K. (2001). Chemical Composition of Turmeric Oil-Aa Byproduct from Turmeric Oleoresin Industry and its Inhibitory Activity against Different Fungi. Z. Naturforsch C J. Biosci. 56 (1-2), 40–44. doi:10.1515/znc-2001-1-207
Jiang, A. J., Jiang, G., Li, L. T., and Zheng, J. N. (2015). Curcumin Induces Apoptosis through Mitochondrial Pathway and Caspases Activation in Human Melanoma Cells. Mol. Biol. Rep. 42 (1), 267–275. doi:10.1007/s11033-014-3769-2
Jiménez-Flores, L. M., López-Briones, S., Macías-Cervantes, M. H., Ramírez-Emiliano, J., and Pérez-Vázquez, V. (2014). A PPARγ, NF-Κb and AMPK-dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic Db/db Mice Liver. Molecules 19 (6), 8289–8302. doi:10.3390/molecules19068289
Kalim, H., Handono, K., Khalasha, T., Pratama, M., Dantara, T. I., Wulandari, A., et al. (2017). Immune Modulation Effects of Curcumin in Pristane-Induced Lupus Mice. Indian J. Rheumatol. 12 (2), 86. doi:10.4103/injr.injr_95_16
Karamalakova, Y. D., Nikolova, G. D., Georgiev, T. K., Gadjeva, V. G., and Tolekova, A. N. (2019). Hepatoprotective Properties of Curcuma Longa L. Extract in Bleomycin-Induced Chronic Hepatotoxicity. Drug Discov. Ther. 13 (1), 9–16. doi:10.5582/ddt.2018.01081
Keshari, A. K., Srivastava, R., Singh, P., Yadav, V. B., and Nath, G. (2020). Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum Nocturnum. J. Ayurveda Integr. Med. 11 (1), 37–44. doi:10.1016/j.jaim.2017.11.003
Khatri, D. K., and Juvekar, A. R. (2016). Kinetics of Inhibition of Monoamine Oxidase Using Curcumin and Ellagic Acid. Pharmacogn Mag. 12 (Suppl. 2), S116–S120. doi:10.4103/0973-1296.182168
Khattak, S., Saeed-ur-Rehman, H. U., Ullah Shah, H., Ahmad, W., and Ahmad, M. (2005). Biological Effects of Indigenous Medicinal Plants Curcuma Longa and Alpinia Galanga. Fitoterapia 76 (2), 254–257. doi:10.1016/j.fitote.2004.12.012
Kim, D. C., Kim, S. H., Choi, B. H., Baek, N. I., Kim, D., Kim, M. J., et al. (2005). Curcuma Longa Extract Protects against Gastric Ulcers by Blocking H2 Histamine Receptors. Biol. Pharm. Bull. 28 (12), 2220–2224. doi:10.1248/bpb.28.2220
Kössler, S., Nofziger, C., Jakab, M., Dossena, S., and Paulmichl, M. (2012). Curcumin Affects Cell Survival and Cell Volume Regulation in Human Renal and Intestinal Cells. Toxicology 292 (2-3), 123–135. doi:10.1016/j.tox.2011.12.002
Kulkarni, S. K., and Dhir, A. (2010). An Overview of Curcumin in Neurological Disorders. Indian J. Pharm. Sci. 72 (2), 149–154. doi:10.4103/0250-474X.65012
Kunnumakkara, A. B., Bordoloi, D., Padmavathi, G., Monisha, J., Roy, N. K., Prasad, S., et al. (2017). Curcumin, the golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 174 (11), 1325–1348. doi:10.1111/bph.13621
Kuttan, R., Bhanumathy, P., Nirmala, K., and George, M. C. (1985). Potential Anticancer Activity of Turmeric (Curcuma Longa). Cancer Lett. 29 (2), 197–202. doi:10.1016/0304-3835(85)90159-4
Lee, S. J., and Langhans, S. A. (2012). Anaphase-promoting Complex/cyclosome Protein Cdc27 Is a Target for Curcumin-Induced Cell Cycle Arrest and Apoptosis. BMC cancer 12 (1), 44–12. doi:10.1186/1471-2407-12-44
Li, B., Takeda, T., Tsuiji, K., Wong, T. F., Tadakawa, M., Kondo, A., et al. (2013). Curcumin Induces Cross-Regulation between Autophagy and Apoptosis in Uterine Leiomyosarcoma Cells. Int. J. Gynecol. Cancer 23 (5), 803–808. doi:10.1097/IGC.0b013e31828c9581
Li, H., Sureda, A., Devkota, H. P., Pittalà, V., Barreca, D., Silva, A. S., et al. (2020a). Curcumin, the golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 38, 107343. doi:10.1016/j.biotechadv.2019.01.010
Li, J., Wei, H., Liu, Y., Li, Q., Guo, H., Guo, Y., et al. (2020b). Evidence-Based Complementary And Alternative Medicine 2020. doi:10.1155/2020/2892917Curcumin Inhibits Hepatocellular Carcinoma via Regulating miR-21/TIMP3 axis
Li, X., Lu, Y., Jin, Y., Son, J. K., Lee, S. H., and Chang, H. W. (2014). Curcumin Inhibits the Activation of Immunoglobulin E-Mediated Mast Cells and Passive Systemic Anaphylaxis in Mice by Reducing Serum Eicosanoid and Histamine Levels. Biomol. Ther. (Seoul) 22 (1), 27–34. doi:10.4062/biomolther.2013.092
Liao, V. H., Yu, C. W., Chu, Y. J., Li, W. H., Hsieh, Y. C., and Wang, T. T. (2011). Curcumin-mediated Lifespan Extension in Caenorhabditis elegans. Mech. Ageing Dev. 132 (10), 480–487. doi:10.1016/j.mad.2011.07.008
Liddle, M., Hull, C., Liu, C., and Powell, D. (2006). Contact Urticaria from Curcumin. Dermatitis 17 (4), 196–197. doi:10.2310/6620.2006.06004
Lim, K. J., Bisht, S., Bar, E. E., Maitra, A., and Eberhart, C. G. (2011). A Polymeric Nanoparticle Formulation of Curcumin Inhibits Growth, Clonogenicity and Stem-like Fraction in Malignant Brain Tumors. Cancer Biol. Ther. 11 (5), 464–473. doi:10.4161/cbt.11.5.14410
Limtrakul, P., Anuchapreeda, S., Lipigorngoson, S., and Dunn, F. W. (2001). Inhibition of Carcinogen Induced C-Ha-Ras and C-Fos Proto-Oncogenes Expression by Dietary Curcumin. BMC cancer 1 (1), 1–7. doi:10.1186/1471-2407-1-1
Lin, L., Li, C., Zhang, D., Yuan, M., Chen, C. H., and Li, M. (2020). Synergic Effects of Berberine and Curcumin on Improving Cognitive Function in an Alzheimer's Disease Mouse Model. Neurochem. Res. 45 (5), 1130–1141. doi:10.1007/s11064-020-02992-6
Lin, Y. G., Kunnumakkara, A. B., Nair, A., Merritt, W. M., Han, L. Y., Armaiz-Pena, G. N., et al. (2007). Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor-kappaB Pathway. Clin. Cancer Res. 13 (11), 3423–3430. doi:10.1158/1078-0432.CCR-06-3072
Ma, D., Tremblay, P., Mahngar, K., Collins, J., Hudlicky, T., and Pandey, S. (2011). Selective Cytotoxicity against Human Osteosarcoma Cells by a Novel Synthetic C-1 Analogue of 7-deoxypancratistatin Is Potentiated by Curcumin. PLoS One 6 (12), e28780. doi:10.1371/journal.pone.0028780
Maghimaa, M., and Alharbi, S. A. (2020). Green Synthesis of Silver Nanoparticles from Curcuma Longa L. And Coating on the Cotton Fabrics for Antimicrobial Applications and Wound Healing Activity. J. Photochem. Photobiol. B 204, 111806. doi:10.1016/j.jphotobiol.2020.111806
Maghsoudi, A., Yazdian, F., Shahmoradi, S., Ghaderi, L., Hemati, M., and Amoabediny, G. (2017). Curcumin-loaded Polysaccharide Nanoparticles: Optimization and Anticariogenic Activity against Streptococcus Mutans. Mater. Sci. Eng. C Mater. Biol. Appl. 75, 1259–1267. doi:10.1016/j.msec.2017.03.032
Mahadevi R, R., and Kavitha R, R. (2020). Phytochemical and Pharmacological Properties of Curcuma Amada: A Review. ijrps 11 (3), 3546–3555. doi:10.26452/ijrps.v11i3.2510
Mahady, G. B., Pendland, S. L., Yun, G., and Lu, Z. Z. (2002). Turmeric (Curcuma Longa) and Curcumin Inhibit the Growth of Helicobacter pylori, a Group 1 Carcinogen. Anticancer Res. 22 (6C), 4179–4181.
McGillicuddy, E., Murray, I., Kavanagh, S., Morrison, L., Fogarty, A., Cormican, M., et al. (2017). Silver Nanoparticles in the Environment: Sources, Detection and Ecotoxicology. Sci. Total Environ. 575, 231–246. doi:10.1016/j.scitotenv.2016.10.041
Mehta, J., Rolta, R., and Dev, K. (2022a). Role of Medicinal Plants from North Western Himalayas as an Efflux Pump Inhibitor against MDR AcrAB-TolC Salmonella enterica Serovar Typhimurium: In Vitro and In Silico Studies. J. Ethnopharmacol 282, 114589. doi:10.1016/j.jep.2021.114589
Mehta, J., Rolta, R., Salaria, D., Awofisayo, O., Fadare, O. A., Sharma, P. P., et al. (2021). Phytocompounds from Himalayan Medicinal Plants as Potential Drugs to Treat Multidrug-Resistant Salmonella Typhimurium: An In Silico Approach. Biomedicines 9 (10), 1402. doi:10.3390/biomedicines9101402
Mehta, J., Jandaik, S., and ., U. (2016). Evaluation of Phytochemicals and Synergistic Interaction between Plant Extracts and Antibiotics for Efflux Pump Inhibitory Activity against Salmonella Enterica Serovar Typhimurium Strains. Int. J. Pharm. Pharm. Sci. 8 (10), 217–223. doi:10.22159/ijpps.2016v8i10.14062
Mehta, J., Rolta, R., Mehta, B. B., Kaushik, N., Choi, E. H., and Kaushik, N. K. (2022b). Role of Dexamethasone and Methylprednisolone Corticosteroids in COVID-19 Hospitalized Patients: A Review. Front. Microbiol.
Mimeault, M., and Batra, S. K. (2011). Potential Applications of Curcumin and its Novel Synthetic Analogs and Nanotechnology-Based Formulations in Cancer Prevention and Therapy. Chin. Med. 6 (1), 31–19. doi:10.1186/1749-8546-6-31
Mohajeri, M., Sadeghizadeh, M., Najafi, F., and Javan, M. (2015). Polymerized Nano-Curcumin Attenuates Neurological Symptoms in EAE Model of Multiple Sclerosis through Down Regulation of Inflammatory and Oxidative Processes and Enhancing Neuroprotection and Myelin Repair. Neuropharmacology 99, 156–167. doi:10.1016/j.neuropharm.2015.07.013
Mohammed, H. S., Khadrawy, Y. A., El-Sherbini, T. M., and Amer, H. M. (2019). Electrocortical and Biochemical Evaluation of Antidepressant Efficacy of Formulated Nanocurcumin. Appl. Biochem. Biotechnol. 187 (3), 1096–1112. doi:10.1007/s12010-018-2866-4
Mohanpuria, P., Rana, N. K., and Yadav, S. K. (2008). Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanopart Res. 10 (3), 507–517. doi:10.1007/s11051-007-9275-x
Mohanty, C., and Sahoo, S. K. (2010). The In Vitro Stability and In Vivo Pharmacokinetics of Curcumin Prepared as an Aqueous Nanoparticulate Formulation. Biomaterials 31 (25), 6597–6611. doi:10.1016/j.biomaterials.2010.04.062
Mohanty, I., Arya, D. S., and Gupta, S. K. (2006). Effect of Curcuma Longa and Ocimum Sanctum on Myocardial Apoptosis in Experimentally Induced Myocardial Ischemic-Reperfusion Injury. BMC Complement. Altern. Med. 6 (1), 3–12. doi:10.1186/1472-6882-6-3
Mohebbati, R., Anaeigoudari, A., and Khazdair, M. R. (2017). The Effects of Curcuma Longa and Curcumin on Reproductive Systems. Endocr. Regul. 51 (4), 220–228. doi:10.1515/enr-2017-0024
Nair, D. S., Krishnakumar, K., and Krishnan, B. (2017). Pharmacological Profile of Curcumin: A Review. J. Bio Innovation 6 (4), 533–541.
Nair, K. L., Thulasidasan, A. K., Deepa, G., Anto, R. J., and Kumar, G. S. (2012). Purely Aqueous PLGA Nanoparticulate Formulations of Curcumin Exhibit Enhanced Anticancer Activity with Dependence on the Combination of the Carrier. Int. J. Pharm. 425 (1-2), 44–52. doi:10.1016/j.ijpharm.2012.01.003
Naseri, S., Darroudi, M., Aryan, E., Gholoobi, A., Rahimi, H. R., Ketabi, K., et al. (2017). The Antiviral Effects of Curcumin Nanomicelles on the Attachment and Entry of Hepatitis C Virus 11 (2), 29–35.
Neelofar, K., Shreaz, S., Rimple, B., Muralidhar, S., Nikhat, M., and Khan, L. A. (2011). Curcumin as a Promising Anticandidal of Clinical Interest. Can. J. Microbiol. 57 (3), 204–210. doi:10.1139/W10-117
Ng, Q., Soh, A., Loke, W., Venkatanarayanan, N., Lim, D., and Yeo, W.-S. (2018). A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS). Jcm 7 (10), 298. doi:10.3390/jcm7100298
Nisar, T., Iqbal, M., Raza, A., Safdar, M., Iftikhar, F., and Waheed, M. (2015). Estimation of Total Phenolics and Free Radical Scavenging of Turmeric (Curcuma longa). Environ. Sci. 15 (7), 1272–1277.
Nishiyama, T., Mae, T., Kishida, H., Tsukagawa, M., Mimaki, Y., Kuroda, M., et al. (2005). Curcuminoids and Sesquiterpenoids in Turmeric (Curcuma Longa L.) Suppress an Increase in Blood Glucose Level in Type 2 Diabetic KK-Ay Mice. J. Agric. Food Chem. 53 (4), 959–963. doi:10.1021/jf0483873
Nita, C.-W. (2003). Safety and Anti-inflammatory Activity of Curcumin: a Component of Tumeric (Curcuma Longa). J. Altern. Complement. Med. 9 (1), 161–168.
Oghenejobo, M., and Bethel, O. (2017). Antibacterial Evaluation, Phytochemical Screening and Ascorbic Acid Assay of Turmeric (Curcuma Longa). MOJ Bioequiv Availab 4 (2), 00063. doi:10.15406/mojbb.2017.04.00063
Okanlawon, E. O., Okanlawon, E. O., Bello, K. O., Akinola, O. S., Oluwatosin, O. O., Irekhore, O. T., et al. (2020). Carcass Yield and Intestinal Morphology of Male Rabbits Fed Diets Supplemented with Turmeric (Curcuma Longa) Powder. Ghana J. Agric. Sci. 55 (2), 97–106. doi:10.4314/gjas.v55i2.8
Ozawa, H., Imaizumi, A., Sumi, Y., Hashimoto, T., Kanai, M., Makino, Y., et al. (2017). Curcumin β-D-Glucuronide Plays an Important Role to Keep High Levels of Free-form Curcumin in the Blood. Biol. Pharm. Bull. 40 (9), 1515–1524. doi:10.1248/bpb.b17-00339
Ozawa-Umeta, H., Kishimoto, A., Imaizumi, A., Hashimoto, T., Asakura, T., Kakeya, H., et al. (2020). Curcumin β-D-glucuronide Exhibits Anti-tumor Effects on Oxaliplatin-Resistant colon Cancer with Less Toxicity In Vivo. Cancer Sci. 111 (5), 1785–1793. doi:10.1111/cas.14383
Parasramka, M. A., and Gupta, S. V. (20122012). Synergistic Effect of Garcinol and Curcumin on Antiproliferative and Apoptotic Activity in Pancreatic Cancer Cells. J. Oncol. doi:10.1155/2012/709739
Park, A. M., Omura, S., Fujita, M., Sato, F., and Tsunoda, I. (2017). Helicobacter pylori and Gut Microbiota in Multiple Sclerosis versus Alzheimer's Disease: 10 Pitfalls of Microbiome Studies. Clin. Exp. Neuroimmunol 8 (3), 215–232. doi:10.1111/cen3.12401
Park, E. J., Jeon, C. H., Ko, G., Kim, J., and Sohn, D. H. (2000). Protective Effect of Curcumin in Rat Liver Injury Induced by Carbon Tetrachloride. J. Pharm. Pharmacol. 52 (4), 437–440. doi:10.1211/0022357001774048
Parveen, A., Kulkarni, N., Yalagatti, M., Abbaraju, V., and Deshpande, R. (2018). In Vivo efficacy of Biocompatible Silver Nanoparticles Cream for Empirical Wound Healing. J. Tissue Viability 27 (4), 257–261. doi:10.1016/j.jtv.2018.08.007
Pawitan, J. A. (2020). Curcumin as Adjuvant Therapy in COVID-19: Friend or Foe? J. Int. Dental Med. Res. 13 (2), 824–829.
Perkins, S., Verschoyle, R. D., Hill, K., Parveen, I., Threadgill, M. D., Sharma, R. A., et al. (2002). Chemopreventive Efficacy and Pharmacokinetics of Curcumin in the Min/+ Mouse, a Model of Familial Adenomatous Polyposis. Cancer Epidemiol. Biomarkers Prev. 11 (6), 535–540.
Phuoc Nguyen, M. (2020). Synergistic Effect of Turmeric (Curcuma Longa), Galanga (Alpinia Galanga) Powder and Lemongrass (Cymbopogon Citratus)) Essential Oil as Natural Preservative in Chilled Storage of White Hard Clam (Meretrix Lyrata). Orient. J. Chem. 36, 195–200. doi:10.13005/ojc/360126
Ponnusamy, S., Ravindran, R., Zinjarde, S., Bhargava, S., and Ravi Kumar, A. (2010). Evaluation of Traditional Indian Antidiabetic Medicinal Plants for Human Pancreatic Amylase Inhibitory Effect In Vitro. Evidence-Based Complement. Altern. Med. 2011, 515647. doi:10.1155/2011/515647
Ponnusamy, S., Zinjarde, S., Bhargava, S., and Kumara, A. R. (2012). Role of Curcuma Longa, a Traditional Ayurvedic Medicinal Plant, in Diabetes. CELLMED 2 (4), 3131–3137. doi:10.5667/tang.2012.0032
Prasad, S., and Aggarwal, B. (2011). Chapter 13, Turmeric, the Golden Spice. Herbal Medicine: Biomolecular and Clinical Aspects.
Puteri, A. I. S., Sandhika, W., and Hasanatuludhhiyah, N. (2020). Effect of Javanese Turmeric (Curcuma Xanthorrhiza) Extract on Hepatitis Model of Alcohol-Induced Mice. Jkb 31 (1), 39–42. doi:10.21776/ub.jkb.2020.031.01.8
Qi, X. J., Liu, X. Y., Tang, L. M., Li, P. F., Qiu, F., and Yang, A. H. (2020). Anti-depressant Effect of Curcumin-Loaded Guanidine-Chitosan Thermo-Sensitive Hydrogel by Nasal Delivery. Pharm. Dev. Technol. 25 (3), 316–325. doi:10.1080/10837450.2019.1686524
Qin, S., Huang, L., Gong, J., Shen, S., Huang, J., Ren, H., et al. (2017). Efficacy and Safety of Turmeric and Curcumin in Lowering Blood Lipid Levels in Patients with Cardiovascular Risk Factors: a Meta-Analysis of Randomized Controlled Trials. Nutr. J. 16 (1), 68–10. doi:10.1186/s12937-017-0293-y
Rafatullah, S., Tariq, M., Al-Yahya, M. A., Mossa, J. S., and Ageel, A. M. (1990). Evaluation of Turmeric (Curcuma Longa) for Gastric and Duodenal Antiulcer Activity in Rats. J. Ethnopharmacol 29 (1), 25–34. doi:10.1016/0378-8741(90)90094-a
Rahimi, R., and Abdollahi, M. (2012). Herbal Medicines for the Management of Irritable Bowel Syndrome: a Comprehensive Review. World J. Gastroenterol. 18 (7), 589–600. doi:10.3748/wjg.v18.i7.589
Rai, P. K., Jaiswal, D., Mehta, S., Rai, D. K., Sharma, B., and Watal, G. (2010). Effect of Curcuma Longa Freeze Dried Rhizome Powder with Milk in STZ Induced Diabetic Rats. Indian J. Clin. Biochem. 25 (2), 175–181. doi:10.1007/s12291-010-0032-z
Rajkumari, S., and Sanatombi, K. (2017). Nutritional Value, Phytochemical Composition, and Biological Activities of Edible Curcuma Species: A Review. Int. J. Food properties 20 (Suppl. 3), S2668–S2687. doi:10.1080/10942912.2017.1387556
Rakotoarisoa, M., and Angelova, A. (2018). Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines (Basel) 5 (4), 126. doi:10.3390/medicines5040126
Ram, A., Das, M., and Ghosh, B. (2003). Curcumin Attenuates Allergen-Induced Airway Hyperresponsiveness in Sensitized guinea Pigs. Biol. Pharm. Bull. 26 (7), 1021–1024. doi:10.1248/bpb.26.1021
Rao, C. V., Desai, D., Rivenson, A., Simi, B., Amin, S., and Reddy, B. S. (1995). Chemoprevention of colon Carcinogenesis by Phenylethyl-3-Methylcaffeate. Cancer Res. 55 (11), 2310–2315.
Ravikumar, N., and Kavitha, C. N. (2020). Therapeutic Potential of Curcumin on Immune Dysregulation in Comorbid Diabetic Asthma in Mice. Biomed. Pharmacol. J. 13 (2), 821–831. doi:10.13005/bpj/1948
Ravindran, J., Arumugasamy, V., and Baskaran, A. (2019). Wound Healing Effect of Silver Nanoparticles from Tridax Procumbens Leaf Extracts on Pangasius Hypophthalmus. Wound Med. 27 (1), 100170. doi:10.1016/j.wndm.2019.100170
Reddy, B. S., and Rao, C. V. (2002). Novel Approaches for colon Cancer Prevention by Cyclooxygenase-2 Inhibitors. J. Environ. Pathol. Toxicol. Oncol. 21 (2), 155–164. doi:10.1615/jenvironpatholtoxicoloncol.v21.i2.90
Rithaporn, T., Monga, M., and Rajasekaran, M. (2003). Curcumin: a Potential Vaginal Contraceptive. Contraception 68 (3), 219–223. doi:10.1016/s0010-7824(03)00163-x
Royal Botanic Gardens Kew (2021). Curcuma Longa L. [Online]. Available: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:796451-1 (Accessed January 20, 2021).
Sahu, S., Mishra, B., Pradhan, J., and Das, B. (2005). Antibacterial Activity of Curcuma Longa on Fish Microbial Pathogens.
Salehi, B., Del Prado-Audelo, M. L., Cortés, H., Leyva-Gómez, G., Stojanović-Radić, Z., Singh, Y. D., et al. (2020). Therapeutic Applications of Curcumin Nanomedicine Formulations in Cardiovascular Diseases. J. Clin. Med. 9 (3), 746. doi:10.3390/jcm9030746
Sandur, S. K., Pandey, M. K., Sung, B., Ahn, K. S., Murakami, A., Sethi, G., et al. (2007). Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin, Tetrahydrocurcumin and Turmerones Differentially Regulate Anti-inflammatory and Anti-proliferative Responses through a ROS-independent Mechanism. Carcinogenesis 28 (8), 1765–1773. doi:10.1093/carcin/bgm123
Satpathy, L., and Parida, S. (2021a). Neuroprotective Role of Curcumin against Benzo[a]pyrene-Induced Neurodegeneration in Zebrafish. Biointerface Res. Appl. Chem. 12, 7311–7320. doi:10.33263/BRIAC126.73117320
Satpathy, L., and Parida, S. P. (2021b). Study on the Effects of Kandhamal Haladi in Benzo [a]Pyrene-Induced Behavioral Changes in Adult Zebrafish (Danio rerio). Polycyclic Aromatic Comp., 1–8. doi:10.1080/10406638.2021.1886124
Shaikh, J., Ankola, D. D., Beniwal, V., Singh, D., and Kumar, M. N. (2009). Nanoparticle Encapsulation Improves Oral Bioavailability of Curcumin by at Least 9-fold when Compared to Curcumin Administered with Piperine as Absorption Enhancer. Eur. J. Pharm. Sci. 37 (3-4), 223–230. doi:10.1016/j.ejps.2009.02.019
Sharma, M., Manoharlal, R., Negi, A. S., and Prasad, R. (2010). Synergistic Anticandidal Activity of Pure Polyphenol Curcumin I in Combination with Azoles and Polyenes Generates Reactive Oxygen Species Leading to Apoptosis. FEMS Yeast Res. 10 (5), 570–578. doi:10.1111/j.1567-1364.2010.00637.x
Sharma, R. A., Steward, W. P., and Gescher, A. J. (2007). Pharmacokinetics and Pharmacodynamics of curcuminThe Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, 453–470.
Shimatsu, A., Kakeya, H., Imaizumi, A., Morimoto, T., Kanai, M., and Maeda, S. (2012). Clinical Application of “Curcumin”. A Multi-functional Substance. Anti-aging Med. 9 (2), 75–83.
Shinde, N., Chauhan, A. S., Gupta, S. K., Bodakhe, S. H., and Pandey, D. P. (2015). Antifertility Studies of Curcumin and Andrographolide Combination in Female Rats. Asian Pac. J. Reprod. 4 (3), 188–194. doi:10.1016/j.apjr.2015.06.012
Shoskes, D., Lapierre, C., Cruz-Correa, M., Muruve, N., Rosario, R., Fromkin, B., et al. (2006). Erratum: Beneficial Effects of the Bioflavonoids Curcumin and Quercetin on Early Function in Cadaveric Renal Transplantation: A Randomized Placebo Controlled Trial (Transplantation (December 15, 2005). Transplantation 80 (11), 1556–1559. 82(5), 715. doi:10.1097/01.tp.0000183290.64309.21
Singh, S., and Aggarwal, B. B. (1995). Activation of Transcription Factor NF-Kappa B Is Suppressed by Curcumin (Diferuloylmethane) [corrected]. J. Biol. Chem. 270 (42), 24995–25000. doi:10.1074/jbc.270.42.24995
Soleimani, V., Sahebkar, A., and Hosseinzadeh, H. (2018). Turmeric (Curcuma Longa) and its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytother Res. 32 (6), 985–995. doi:10.1002/ptr.6054
Somanawat, K., Thong-Ngam, D., and Klaikeaw, N. (2013). Curcumin Attenuated Paracetamol Overdose Induced Hepatitis. World J. Gastroenterol. 19 (12), 1962–1967. doi:10.3748/wjg.v19.i12.1962
Song, E. K., Cho, H., Kim, J. S., Kim, N. Y., An, N. H., Kim, J. A., et al. (2001). Diarylheptanoids with Free Radical Scavenging and Hepatoprotective Activity In Vitro from Curcuma Longa. Planta Med. 67 (09), 876–877. doi:10.1055/s-2001-18860
Soudamini, K. K., and Kuttan, R. (1989). Inhibition of Chemical Carcinogenesis by Curcumin. J. Ethnopharmacol 27 (1-2), 227–233. doi:10.1016/0378-8741(89)90094-9
Strimpakos, A. S., and Sharma, R. A. (2008). Curcumin: Preventive and Therapeutic Properties in Laboratory Studies and Clinical Trials. Antioxid. Redox Signal. 10 (3), 511–545. doi:10.1089/ars.2007.1769
Subramani, P. A., Cheeran, V., Munuswamy-Ramanujam, G., and Narala, V. R. (2018). Clinical Trials of Curcumin, Camptothecin, Astaxanthin and Biochanin. Nat. Prod. Clin. Trials 1, 79–113. doi:10.2174/9781681082134118010005
Sun, W., Wang, S., Zhao, W., Wu, C., Guo, S., Gao, H., et al. (2017). Chemical Constituents and Biological Research on Plants in the Genus Curcuma. Crit. Rev. Food Sci. Nutr. 57 (7), 1451–1523. doi:10.1080/10408398.2016.1176554
Taher, M. M., Lammering, G., Hershey, C., and Valerie, K. (2003). Curcumin Inhibits Ultraviolet Light Induced Human Immunodeficiency Virus Gene Expression. Mol. Cel Biochem 254 (1), 289–297. doi:10.1023/a:1027393719610
Tajbakhsh, S., Mohammadi, K., Deilami, I., Zandi, K., Fouladvand, M., Ramedani, E., et al. (2008). Antibacterial Activity of Indium Curcumin and Indium Diacetylcurcumin. Afr. J. Biotechnol. 7 (21), 3832–3835.
Tao, G., Wang, Y., Cai, R., Chang, H., Song, K., Zuo, H., et al. (2019). Design and Performance of Sericin/poly(vinyl Alcohol) Hydrogel as a Drug Delivery Carrier for Potential Wound Dressing Application. Mater. Sci. Eng. C Mater. Biol. Appl. 101, 341–351. doi:10.1016/j.msec.2019.03.111
Thong-Ngam, D., Choochuai, S., Patumraj, S., Chayanupatkul, M., and Klaikeaw, N. (2012). Curcumin Prevents Indomethacin-Induced Gastropathy in Rats. World J. Gastroenterol. 18 (13), 1479–1484. doi:10.3748/wjg.v18.i13.1479
Tripathi, B. (2009). AshtangaHrdayam of Srimadvagbhata. (1stedn). Delhi: Chaukambha Sanskrit Pratishthan. India.
Tripodo, G., Chlapanidas, T., Perteghella, S., Vigani, B., Mandracchia, D., Trapani, A., et al. (2015). Mesenchymal Stromal Cells Loading Curcumin-INVITE-Micelles: A Drug Delivery System for Neurodegenerative Diseases. Colloids Surf. B Biointerfaces 125, 300–308. doi:10.1016/j.colsurfb.2014.11.034
Tsai, J. R., Liu, P. L., Chen, Y. H., Chou, S. H., Cheng, Y. J., Hwang, J. J., et al. (2015). Curcumin Inhibits Non-small Cell Lung Cancer Cells Metastasis through the Adiponectin/NF-κb/MMPs Signaling Pathway. PLoS One 10 (12), e0144462. doi:10.1371/journal.pone.0144462
Tsunoda, I. (2017). Lymphatic System and Gut Microbiota Affect Immunopathology of Neuroinflammatory Diseases, Including Multiple Sclerosis, Neuromyelitis Optica and Alzheimer's Disease. Wiley Online Library.
Uchio, R., Kawasaki, K., Okuda-Hanafusa, C., Saji, R., Muroyama, K., Murosaki, S., et al. (2021). Curcuma Longa Extract Improves Serum Inflammatory Markers and Mental Health in Healthy Participants Who Are Overweight: a Randomized, Double-Blind, Placebo-Controlled Trial. Nutr. J. 20 (1), 91–14. doi:10.1186/s12937-021-00748-8
Uma Pradeep, K., Geervani, P., and Eggum, B. O. (1993). Common Indian Spices: Nutrient Composition, Consumption and Contribution to Dietary Value. Plant Foods Hum. Nutr. 44 (2), 137–148. doi:10.1007/BF01088378
Urmila, Jandaik, S., Mehta, J., and Mohan, M. (2016). A Synergistic and Efflux Pump Inhibitory Activity of Plant Extracts and Antibiotics on staphylococcus Aureus Strains. Asian J. Pharm. Clin. Res. 9, 277–282.
USDA (2021). Natural Resources Conservation Services. Plants Database [Online]. Available at: https://plants.usda.gov/java/ClassificationServlet?source=display&classid=CURCU (Accessed January 25, 2021).
Vajragupta, O., Boonchoong, P., Morris, G. M., and Olson, A. J. (2005). Active Site Binding Modes of Curcumin in HIV-1 Protease and Integrase. Bioorg. Med. Chem. Lett. 15 (14), 3364–3368. doi:10.1016/j.bmcl.2005.05.032
Valizadeh, H., Abdolmohammadi-Vahid, S., Danshina, S., Ziya Gencer, M., Ammari, A., Sadeghi, A., et al. (2020). Nano-curcumin Therapy, a Promising Method in Modulating Inflammatory Cytokines in COVID-19 Patients. Int. Immunopharmacol 89, 107088. doi:10.1016/j.intimp.2020.107088
Vareed, S. K., Kakarala, M., Ruffin, M. T., Crowell, J. A., Normolle, D. P., Djuric, Z., et al. (2008). Pharmacokinetics of Curcumin Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomarkers Prev. 17 (6), 1411–1417. doi:10.1158/1055-9965.EPI-07-2693
Verma, R. K., Kumari, P., Maurya, R. K., Kumar, V., Verma, R., and Singh, R. K. (2018). Medicinal Properties of Turmeric (Curcuma Longa L.): A Review. Int. J. Chem. Stud. 6 (4), 1354–1357.
von Rhein, C., Weidner, T., Henß, L., Martin, J., Weber, C., Sliva, K., et al. (2016). Curcumin and Boswellia Serrata Gum Resin Extract Inhibit Chikungunya and Vesicular Stomatitis Virus Infections In Vitro. Antivir. Res 125, 51–57. doi:10.1016/j.antiviral.2015.11.007
Wahono, C. S., Wahyuni, Z. D., and Kalim, H. (2017). Effect of Curcuma Xanthorrhiza Suplementation in Vitamin D3 Administration towards Proteinuria, Serum Anti-dsDNA and IL-17 Levels on Systemic Lupus Erythematosus (Sle) Patients with Hypovitamin. D Int. J. Clin. Rheumatol. 12, 121–129.
Wang, R., Zhang, J. Y., Zhang, M., Zhai, M. G., Di, S. Y., Han, Q. H., et al. (2018). Curcumin Attenuates IR-Induced Myocardial Injury by Activating SIRT3. Eur. Rev. Med. Pharmacol. Sci. 22 (4), 1150–1160. doi:10.26355/eurrev_201802_14404
Wilken, R., Veena, M. S., Wang, M. B., and Srivatsan, E. S. (2011). Curcumin: A Review of Anti-cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 10 (1), 12–19. doi:10.1186/1476-4598-10-12
Wong, T. F., Takeda, T., Li, B., Tsuiji, K., Kitamura, M., Kondo, A., et al. (2011). Curcumin Disrupts Uterine Leiomyosarcoma Cells through AKT-mTOR Pathway Inhibition. Gynecol. Oncol. 122 (1), 141–148. doi:10.1016/j.ygyno.2011.03.001
Wuthi-Udomlert, M., Grisanapan, W., Luanratana, O., and Caichompoo, W. (2000). Antifungal Activity of Curcuma Longa Grown in Thailand. Southeast. Asian J. Trop. Med. Public Health 31 Suppl 1, 178–182.
Xia, X., Cheng, G., Pan, Y., Xia, Z. H., and Kong, L. D. (2007). Behavioral, Neurochemical and Neuroendocrine Effects of the Ethanolic Extract from Curcuma Longa L. In the Mouse Forced Swimming Test. J. Ethnopharmacol 110 (2), 356–363. doi:10.1016/j.jep.2006.09.042
Xu, D., Tian, W., and Shen, H. (2011). Curcumin Prevents Induced Drug Resistance: A Novel Function? Chin. J. Cancer Res. 23 (3), 218–223. doi:10.1007/s11670-011-0218-9
Xue, H., Hu, L., Xiong, Y., Zhu, X., Wei, C., Cao, F., et al. (2019). Quaternized Chitosan-Matrigel-Polyacrylamide Hydrogels as Wound Dressing for Wound Repair and Regeneration. Carbohydr. Polym. 226, 115302. doi:10.1016/j.carbpol.2019.115302
Yadav, V. S., Mishra, K. P., Singh, D. P., Mehrotra, S., and Singh, V. K. (2005). Immunomodulatory Effects of Curcumin. Immunopharmacol Immunotoxicol 27 (3), 485–497. doi:10.1080/08923970500242244
Yallapu, M. M., Jaggi, M., and Chauhan, S. C. (2012). Curcumin Nanoformulations: a Future Nanomedicine for Cancer. Drug Discov. Today 17 (1-2), 71–80. doi:10.1016/j.drudis.2011.09.009
Yang, X. X., Li, C. M., and Huang, C. Z. (2016). Curcumin Modified Silver Nanoparticles for Highly Efficient Inhibition of Respiratory Syncytial Virus Infection. Nanoscale 8 (5), 3040–3048. doi:10.1039/c5nr07918g
Ye, M. X., Li, Y., Yin, H., and Zhang, J. (2012). Curcumin: Updated Molecular Mechanisms and Intervention Targets in Human Lung Cancer. Int. J. Mol. Sci. 13 (3), 3959–3978. doi:10.3390/ijms13033959
Yin, H., Guo, R., Xu, Y., Zheng, Y., Hou, Z., Dai, X., et al. (2012). Synergistic Antitumor Efficiency of Docetaxel and Curcumin against Lung Cancer. Acta Biochim. Biophys. Sin (Shanghai) 44 (2), 147–153. doi:10.1093/abbs/gmr106
Yu, J., Zhou, X., He, X., Dai, M., and Zhang, Q. (2011). Curcumin Induces Apoptosis Involving Bax/bcl-2 in Human Hepatoma SMMC-7721 Cells. Asian Pac. J. Cancer Prev. 12 (8), 1925–1929.
Yu, Z. F., Kong, L. D., and Chen, Y. (2002). Antidepressant Activity of Aqueous Extracts of Curcuma Longa in Mice. J. Ethnopharmacol 83 (1-2), 161–165. doi:10.1016/s0378-8741(02)00211-8
Yue, G. G.-L., Kwok, H.-F., Lee, J. K.-M., Jiang, L., Chan, K.-M., Cheng, L., et al. (2015). Novel Anti-angiogenic Effects of Aromatic-Turmerone, Essential Oil Isolated from Spice Turmeric. J. Funct. Foods 15, 243–253. doi:10.1016/j.jff.2015.03.030
Yuliani, S., and Mustofa, P. G. (2019). The Neuroprotective Effects of an Ethanolic Turmeric (Curcuma Longa L.) Extract against Trimethyltin-Induced Oxidative Stress in Rats. Nutr. Neurosci. 22 (11), 797–804. doi:10.1080/1028415X.2018.1447267
Yuliani, S., and Mustofa, P. G. (2018). Turmeric (Curcuma Longa L.) Extract May Prevent the Deterioration of Spatial Memory and the Deficit of Estimated Total Number of Hippocampal Pyramidal Cells of Trimethyltin-Exposed Rats. Drug Chem. Toxicol. 41 (1), 62–71. doi:10.1080/01480545.2017.1293087
Zhang, J., Si, G., Zou, J., Fan, R., Guo, A., and Wei, X. (2017). Antimicrobial Effects of Silver Nanoparticles Synthesized by Fatsia Japonica Leaf Extracts for Preservation of Citrus Fruits. J. Food Sci. 82 (8), 1861–1866. doi:10.1111/1750-3841.13811
Zhang, N., Hu, Z., Qiang, Y., and Zhu, X. (2019). Circulating miR-130b- and miR-21-Based Diagnostic Markers and Therapeutic Targets for Hepatocellular Carcinoma. Mol. Genet. Genomic Med. 7 (12), e1012. doi:10.1002/mgg3.1012
Keywords: Curcuma longa, curcumin, phytochemical, pharmacology, toxicology
Citation: Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Subramaniyan V, Chidambaram K, Thangavelu L, Nordin R, Wu YS, Sathasivam KV, Lum PT, Meenakshi DU, Kumarasamy V, Azad AK and Fuloria NK (2022) A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 13:820806. doi: 10.3389/fphar.2022.820806
Received: 23 November 2021; Accepted: 27 January 2022;
Published: 25 March 2022.
Edited by:
Mohd Farooq Shaikh, Monash University, MalaysiaReviewed by:
Sadiq Umar, University of Illinois at Chicago, United StatesCopyright © 2022 Fuloria, Mehta, Chandel, Sekar, Rani, Begum, Subramaniyan, Chidambaram, Thangavelu, Nordin, Wu, Sathasivam, Lum, Meenakshi, Kumarasamy, Azad and Fuloria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neeraj Kumar Fuloria, bmVlcmFqa3VtYXJAYWltc3QuZWR1Lm15; Abul Kalam Azad, YXphZDIwMTFpaXVtQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.