- Beijing University of Chinese Medicine, Beijing, China
Objectives: Topical Chinese herbal medicine (TCHM) is widely used to prevent radiodermatitis in patients who receive radiation therapy in China. However, evidence regarding its efficacy remains limited. The purpose of the review is to evaluate the effects of TCHM in preventing radiodermatitis.
Methods: The protocol of this review was registered in PROSPERO (CRD42020220620). Relevant clinical trials were identified (from January 1, 2010, to April 24, 2022) through 11 electronic databases, including PubMed, SpringerLink, Proquest, the Cochrane Central Register of Controlled Trials, Scopus, the ProQuest Dissertation & Theses Global, PsycINFO, Applied Social Sciences Index and Abstracts, the Chinese National Knowledge Infrastructure Databases, Wangfang Data Knowledge Service Platform, and the Chongqing VIP Chinese Science and Technology Periodical Database. The quality of the included trials was assessed through a risk of bias assessment using Version 2 of the Cochrane risk-of-bias tool (RoB 2.0). We included RCTs that compared TCHM single used or as adjunctive treatment with routine drugs, conventional therapy, or placebo for cancer patients who are about to start radiation therapy and do not possess any type of dermatitis or skin lesions at that time. Primary outcomes of interest were the incidence of radiodermatitis and the grade of radiodermatitis according to the RTOG (Radiation Therapy Oncology Group). Secondary outcomes included the recovery time of skin and mucosa, the occurrence time of radiodermatitis, the radiation dose, quality of life, and adverse events. Data were summarized using risk ratio (RR) calculations and 95% confidence intervals (CI) for binary outcomes or mean difference (MD) with 95% CI for continuous outcomes. Certainty of the evidence was assessed according to the GRADE criteria.
Results: In this review, 38 randomized controlled trials (RCTs) were included. Risk of bias assessment through RoB 2.0 showed that two studies were rated as low risk, two studies were rated as high risk, and the rest were rated as having some concerns. Compared with routine drugs, TCHM may have an advantage in reducing RTOG grading (RR = 0.46, 95%CI 0.35–0.60), decreasing the recovery time of radiodermatitis (MD = −2.35, 95%CI 3.58 to −1.12 days), delaying the occurrence of radiodermatitis (MD = 2.36, 95%CI 1.74–2.98), and improving the quality of life of patients (RR = 1.46, 95%CI 1.03–2.06). Compared with conventional therapy, TCHM may also have an advantage in decreasing the grade of RTOG (RR = 0.28, 95%CI 0.21–0.38).
Conclusion: Current low evidence revealed that TCHM may have better efficacy in the prevention of radiodermatitis; however, more high-quality RCTs are still warranted to testify this conclusion.
Systematic Review Registration: (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020220620), identifier (PROSPERO 2020 CRD42020220620).
1 Introduction
Radiodermatitis (RD) is one of the most common side effects of radiation therapy for cancer (Ashack et al., 2020; Behroozian et al., 2021; Yao and Cheng, 2021). At present, about 95% of patients who receive radiation therapy will develop RD (Rosenthal et al., 2019). Patients with breast cancer, head and neck cancer, lung cancer, or sarcoma are more likely to suffer from RD (Fuzissaki et al., 2019; Tinkle et al., 2021; Yokota et al., 2021; Louie et al., 2022). Erythema is the predominant sign among RD patients, while 42 and 15% of patients developed RT-induced grade 3 and 4 skin toxicities characterized by desquamation, ulceration, and pigment changes (Hu et al., 2018; Bontempo et al., 2021). The consequences of RD can be serious, including dose reduction or discontinuation of therapy, delayed treatment, diminished esthetic appeal, and significantly impaired quality of life.
The main risk factors for RD are both treatment and patient-related, they may relate to the parts of the body that received radiation therapy (Xie et al., 2021), coexisting diseases (such as diseases related to DNA repair defects), obesity, advanced age, female, long-term sun exposure, smoking (Hymes et al., 2006; Meyer et al., 2012; Bray et al., 2016), total radiation dose, segmented metering, and exposed volume and surface area (Kole et al., 2017; Liu et al., 2020). It has been reported that traditional chemotherapy or EGFR inhibitors such as anthracyclines or taxanes will increase the risk of severe RD (Tejwani et al., 2009).
General management of RD includes daily preventive measures and topical or systemic pharmaceutical treatment. Daily preventive measures include keeping the radiation therapy area clean and dry, washing with warm water and mild soap, avoiding skin irritation, wearing loose clothing, preventing abrasions, etc. (Finkelstein et al., 2022; Yang et al., 2020). Recommendations for use of topical skin agents including glucocorticoids, Vaseline ointment, olive oil, ascorbic acid, sulfadiazine, etc. (Finkelstein et al., 2022; Lee et al., 2016; Menon et al., 2021). Recommendations for systemic drugs include protease (Gujral et al., 2001), Pentoxifylline (Aygenc et al., 2004), antioxidants (Lin et al., 2006), etc. Previous published systematic reviews (Miller et al., 2011; Chan et al., 2014; Hindley et al., 2014; Ulff et al., 2017) showed that cleaning in daily care can reduce itching, redness, and peeling, but does not reduce the overall risk of radiation dermatitis, regular topical use of corticosteroids can reduce the incidence of severe dermatitis during radiation therapy and within a few weeks after radiation therapy. However, the use of glucocorticoids during radiation therapy did not reduce the incidence of RD (Chan et al., 2014) and there is no evidence to support the standard approach for the prevention and treatment of RD.
Meanwhile, several studies have shown that topical Chinese herbal medicine (TCHM) has certain advantages in the prevention of RD, it may reduce the incidence of grade 3 and 4 RD according to RTOG (Wang et al., 2016; Wu, 2019), shorten the course of the disease, and delay the occurrence of dermatitis (Wang et al., 2014). Some traditional Chinese herbals and compound prescriptions such as aloe, moist burn ointment, and Kangfuxin liquid (dry insect extract of Periplaneta americana) have been proved to promote the proliferation and renewal of granulation tissue and improve the blood circulation of the wound and its surroundings to promote the repair and regeneration of wound skin (Wu et al., 2010; Wei, 2012; Zhu et al., 2013). Curcumin in turmeric has the effect of scavenging free radicals, increasing the activity of oxidase, anti-inflammation, and anti-infection (Huang et al., 2016). Compound ulcer oil can inhibit the production and release of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), IL-1, and IL-8, and promote the production of epidermal growth factor (EGF), thus delaying the occurrence of skin inflammation and improving the speed of recovery (Zhao et al., 2016).
RD may lead to delays in treatment, diminished cosmesis, a decline in quality of life, and functional deficits. Although it confuses clinicians and patients, there is no gold standard in the prevention and management of this condition, and many interventions are based on provider experience, anecdotal evidence, or poorly powered studies (Leventhal and Young, 2017). TCHM is widely used in China, and many clinical studies have reported that it has preventive and treatment effects on RD. To some extent, prevention is more valuable than treatment (Martin et al., 2020). Therefore, we conducted this systematic review of TCHM for RD prevention to obtain evidence.
2 Methods
2.1 Study Design
Protocol of this review was registered in PROSPERO as Huibo Yu, Baojin Han, and Huijuan Cao. Prevention of radiodermatitis with topical medication of Chinese herbal medicine: a systematic review of randomized controlled trials. PROSPERO 2020 CRD42020220620. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020220620. We conducted the systematic review in strict accordance with the predefined protocol.
The reporting of this systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Checklist.
2.2 Eligibility Criteria
In Chinese or English, studies were included when the following criteria were met: 1) Patient: Cancer patients, of any age, who is about to start radiation therapy and do not possess any type of dermatitis or skin lesions before the radiotherapy. 2) Intervention: TCHM, including herbal patent, preparation produced in hospitals, herbal lotion, etc. 3) Comparators: Controls include routine drugs (such as Recombinant Human Epidermal Growth Factor Derivative for External Use, Trolamine Cream, Hydrocortisone Butyrate Cream), conventional therapy (proper rest, routine radiodermatitis missionary, vitamin, and other energy), placebo or no treatment. 4) Outcomes: primary outcomes included incidence rate of radiodermatitis and RTOG (Radiation Therapy Oncology Group) Grading criteria. Secondary outcomes included recovery time of skin mucosa, time of incidence of radiodermatitis, exposure dose in the incidence of radiodermatitis, quality of life (e.g., Karnofsky Performance Status, KPS), and adverse events. 5) Study type: Randomized controlled trials (RCTs). Literature that is unable to obtain the full text or the analyzable data was excluded.
2.3 Exclusion Criteria
1) Insufficient data. 2) Irregular outcome evaluation criteria.
2.4 Literature Searching
PubMed, SpringerLink, Proquest, the Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, the ProQuest Dissertation & Theses Global (PQDT), PsycINFO, Applied Social Sciences Index and Abstracts (ASSIA), the Chinese National Knowledge Infrastructure Databases (CNKI), Wangfang Data Knowledge Service Platform, and the Chongqing VIP Chinese Science and Technology Periodical Database (VIP) were searched from 1 January 2010 to 24 April 2022, in Chinese and English. The searching terms of retrieval strategy were different based on each database, which included “radio dermatitis,” or “radiation reaction,” or “radioactive lesions,” or “radio epithelitis” combined with “Chinese medicine,” or “herbal medicine,” or “herbs,” or “TCM,” and combined with “random.” We searched for additional trials by reviewing the reference lists of studies related to the prevention of RD with TCHM. All studies were independently searched by two reviewers (HBY and BJH). Any disagreement was resolved through the third reviewer (HJC).
2.5 Study Screening and Data
Studies using the search strategy were retrieved and the full text of these potentially eligible studies was retrieved and independently assessed for eligibility by two reviewers (HBY and BJH). Any disagreement was resolved through discussion with the third reviewer (HJC).
Extracted information included: General information (document number, title, first author, year(s), location (city, country), source, etc.), methodological related information (study design type, random scheme concealment, random allocation method, randomization blind method, statistical analyst blinded, loss of follow-up, baseline comparability, and selective reporting), participants information (diagnostic criteria, inclusion criteria, source, sample size, age, gender, types of carcinoma, and site of radiation exposure), intervention information (types of experimental group interventions, types of control group interventions, and treatment duration), and outcome relevant information (incidence rate of radiodermatitis, RTOG Grading criteria, exposure dose in the incidence of radiodermatitis, the recovery time of skin mucosa, time of incidence of radiodermatitis, and quality of life). We intended to contact the original authors of the included trials to acquire the missing information if needed; however, we found no key information that might affect our judgment of inclusion was missing.
2.6 Methodological Quality Assessment
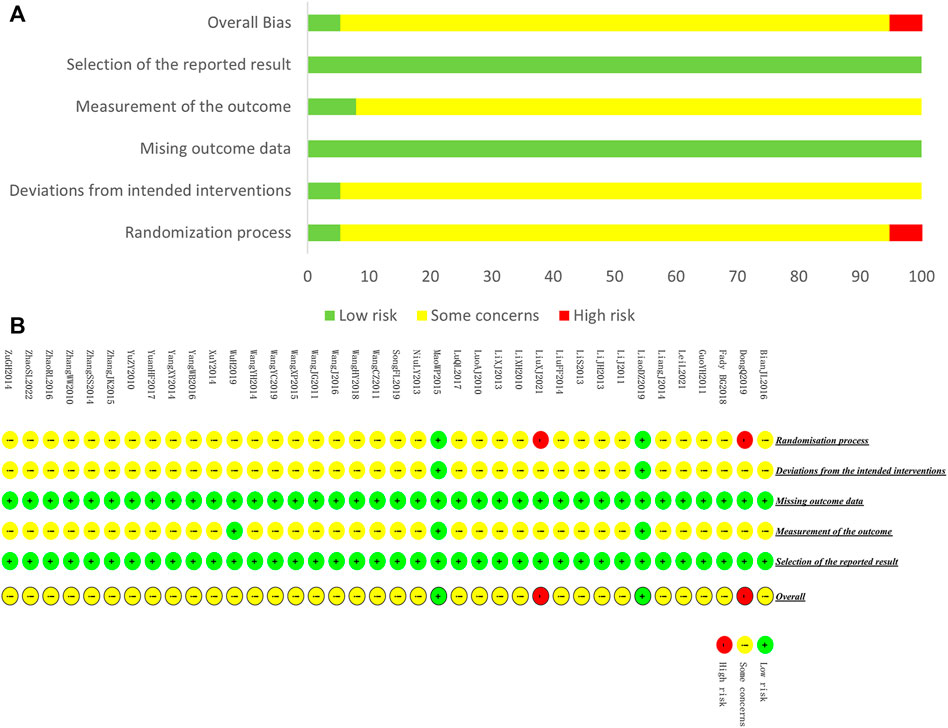
Two reviewers (HBY and BJH) independently evaluated the included studies, and any disagreement arising from this process was resolved by a third reviewer (HJC). Risk of bias assessment Version 2 of the Cochrane risk-of-bias tool (RoB 2.0) was used to assess the quality of included studies, in which five domains were assessed: randomization process, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result. Within each domain, a series of questions were designed to elicit information about trial features associated with bias risk, making the assessment more accurate. Overall bias was considered as “low-risk” if the study was classified as low-risk in all domains, “some concerns” if there was at least 1 domain rated as having some concerns, and “high-risk” if there was at least 1 domain rated as high-risk or several domains rated as having some concerns that could affect the validity of the results.
2.7 Data Analysis
All statistical analyses were performed using RevMan 5.4 (The Cochrane Collaboration) software. Data were summarized using risk ratio (RR) calculations and 95% confidence intervals (CI) for binary outcomes or mean difference (MD) with 95% CI for continuous outcomes.
Intention to treat analysis was not employed to deal with the missing data, since almost all the included trials reported no missing data during the data collection and analysis. Statistical heterogeneity among included trials was evaluated by the I2 test. Meta-analysis was conducted if there is no significant clinical and statistical heterogeneity (I2 < 75%) among included trials. Considering the potential clinical heterogeneity among trials caused by the effect of different prescriptions, we used random-effects model to pool the data to ensure the rationality of the results. Pooling analysis was not used if there is significant statistical heterogeneity (I2 ≥ 75%) among trials. The funnel plot was used to explore the potential publication bias if there are ten or more trials in the meta-analysis.
Subgroup analysis was performed when there was significant heterogeneity (I2 > 25%) in the results. The subgroups were classified by the effects of different radiation therapy dosages, different radiation dermatitis and mucositis grading, different western drugs, different types of carcinoma, or different dosage forms of the TCHM on the results. Sensitivity analysis was conducted to challenge the robustness of the primary analysis, for trials with or without a high-risk of bias, and for meta-analyses conducted using the fixed-effect model or the random-effect model.
We used the Grading of Recommendations Assessment Development and Evaluation Criteria (GRADE) to assess the certainty of the evidence for outcomes with meta-analysis. By assessing the risk of bias, inconsistency of trials, indirectness of evidence, imprecision of results, and publication bias, we determined whether to downgrade the evidence. The evidence was assessed on four levels: high, moderate, low, or very low.
3 Results
3.1 Searching of the Literature
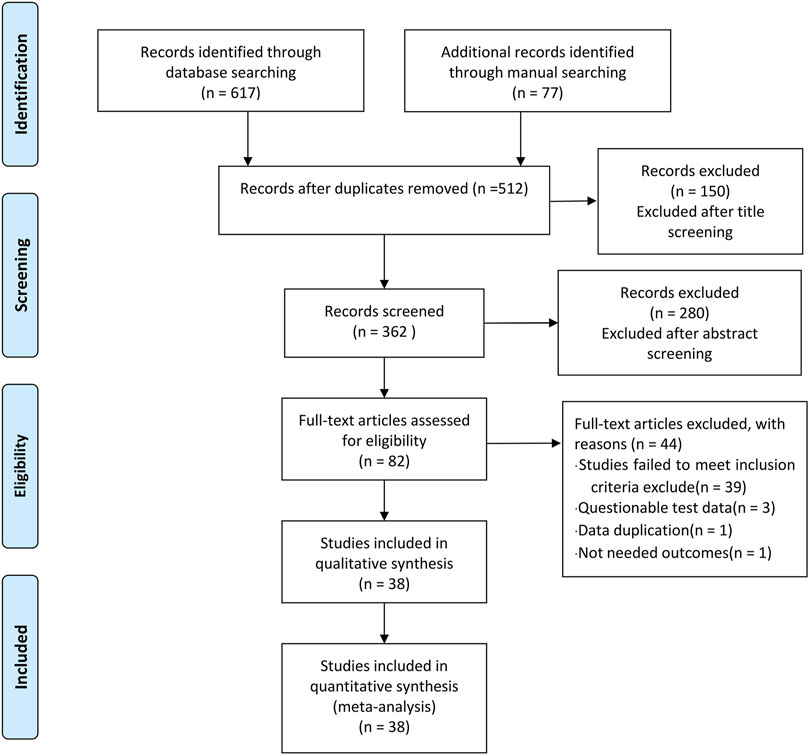
In phase 1 of study selection, 674 articles were identified across 11 electronic databases and through manual searching. After the duplicated articles were removed, 512 articles remained. A thorough screening of the title was completed, and 150 articles were excluded. Two hundred and eighty articles were further excluded through the abstracts screening. In phase 2, 82 articles remained for full-text screening. This process led to the exclusion of 44 articles. In total, 38 articles were selected for data extraction and qualitative synthesis. The specific process is shown in Figure 1.
3.2 Characteristics of the Included Studies
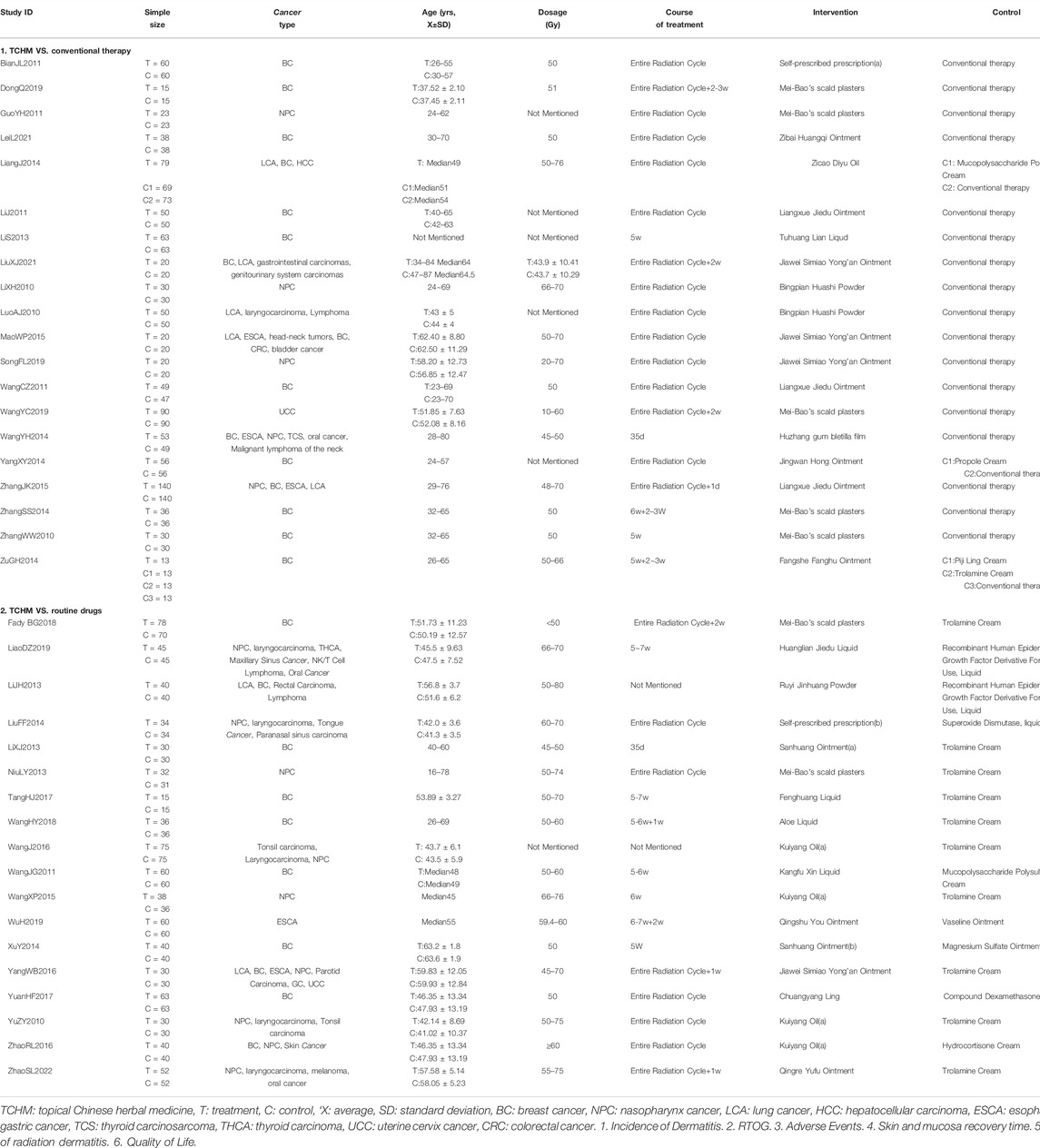
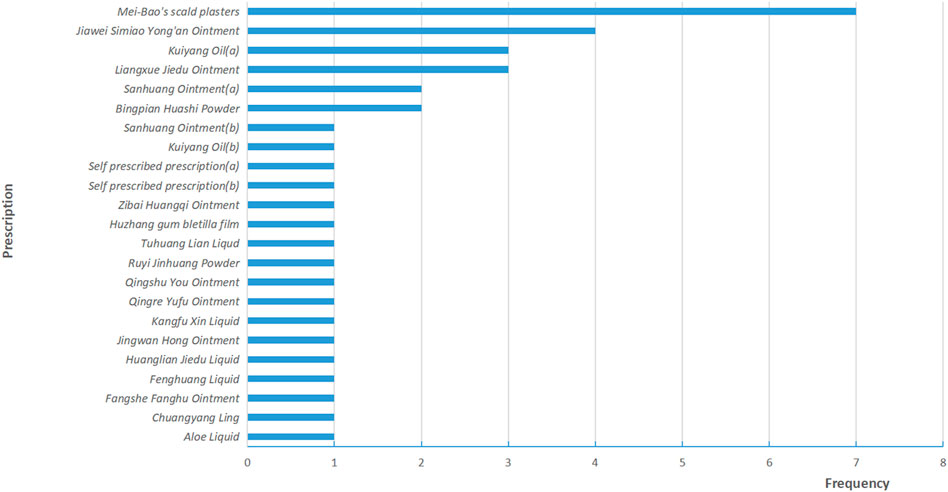
A total of 38 RCTs (Li et al., 2010; Luo and Lan, 2010; Yu et al., 2010; Zhang and Xue, 2010; Bian et al., 2011; Guo and Shi, 2011; Li et al., 2011; Wang C. et al., 2011; Wang J. et al., 2011; Li et al., 2013; Li and Liu, 2013; Lin et al., 2013; Niu, 2013; Liang and Huang, 2014; Liu et al., 2014; Wang et al., 2014; Xu et al., 2014; Yang and Guan, 2014; Zhang, 2014; Zu et al., 2014; Mao, 2015; Wang et al., 2015; Zhang, 2015; Wang et al., 2016; Yang, 2016; Zhao et al., 2016; Tang et al., 2017; Yuan et al., 2017; Geara et al., 2018; Wang et al., 2018; Dong, 2019; Liao et al., 2019; Wang et al., 2019; Wu, 2019; Song et al., 2020; Lei et al., 2021; Liu, 2021; Zhao et al., 2022) and 3,439 patients were included in this review, 1724 patients were treated by TCHM. In TCHM group, ointment was used in 21 studies (Zhang and Xue, 2010; Guo and Shi, 2011; Li et al., 2011; Wang C. et al., 2011; Li et al., 2013; Niu, 2013; Xu et al., 2014; Yang and Guan, 2014; Zhang, 2014; Zu et al., 2014; Mao, 2015; Zhang, 2015; Yuan et al., 2017; Geara et al., 2018; Dong, 2019; Wang et al., 2019; Wu, 2019; Song et al., 2020; Lei et al., 2021; Liu, 2021; Zhao et al., 2022), oil was used in 8 studies (Yu et al., 2010; Bian et al., 2011; Liang and Huang, 2014; Liu et al., 2014; Wang et al., 2015; Wang et al., 2016; Yang, 2016; Zhao et al., 2016), solution was used in 5 studies (Wang J. et al., 2011; Lin et al., 2013; Tang et al., 2017; Wang et al., 2018; Liao et al., 2019), powder was used in 3 studies (Li et al., 2010; Luo and Lan, 2010; Li and Liu, 2013), and TCM drug films were used in 1 study. The general principle of treatment included clearing heat-toxin, supplemented by replenishing qi and nourishing yin, activating blood, resolving stasis, and relieving pain. A total of 23 prescriptions were used in all experiments, involving 53 kinds of herbal medicine. The herbal medicine repetition rate in all prescriptions was 49.1%. All the prescriptions have the effect of clearing the heat-toxin. The specific prescription, herbal and dosage, treatment principle, and general medicine are shown in Table 1. The frequency of prescriptions is shown in Figure 2, and the frequency of the top 20 herbs is shown in Figure 3. The controls included routine drugs and conventional therapy. There were 18 RCTs, including 794 patients who employed routine drugs as control. Triethanolamine was the most commonly used drug. Other 20 RCTs, including 921 patients, used conventional therapy in the control group. The main cancer types were breast cancer and head and neck cancer, and the radiation dose levels ranged from 45 to 80 Gy. All the studies reported the incidence of RD, 37 studies reported the RTOG grading of RD, 6 studies reported the occurrence time of RD, 7 studies reported the recovery time of RD, none of the trials reported the exposure dose for RD, 6 studies reported the patients’ change in the quality of life, and adverse events were reported in 13 studies. The basic characteristics of the included literature are shown in Table 1.
3.3 Study Quality Assessment
We assessed the risk of bias for 38 included studies. For “Randomization process,” only one study used envelopes to perform allocation concealment (Mao, 2015), and 1 study mentioned double-blind (Liao et al., 2019). These 2 studies were rated as low-risk. Two studies (Dong, 2019; Liu, 2021) were rated high-risk because they generated the random sequence based on the admission order. The rest of the studies were rated with some concerns. For “Deviations from the intended interventions,” only 2 studies were rated as low-risk because the randomization of these studies was reasonable, while the rest of the studies were rated as having some concerns since the absence of blinding may induce the detection bias. For “Missing outcome data,” all the included studies were evaluated as low-risk of bias because of the potential completeness of the data. For “Measurement of the outcome,” only two studies that applied blind method were rated as low-risk. For “Selection of the reported result,” all studies were assessed as having a low-risk of bias because their experimental analysis methods were consistent with the present methods. A summary of the results of the risk of bias assessment for included studies is shown in Figure 4.
3.4 Estimate Effects (See Table 2)
3.4.1 Topical Chinese Herbal Medicine vs. Routine Drugs
A total of 18 studies contributed data to this comparison.
3.4.1.1 The Incidence of Radiation Dermatitis/Radiation Therapy Oncology Group Grading
A total of 1,584 patients in 18 studies reported the incidence of RD (Yu et al., 2010; Wang C. et al., 2011; Li et al., 2013; Li and Liu, 2013; Niu, 2013; Liu et al., 2014; Xu et al., 2014; Wang et al., 2015; Wang et al., 2016; Yang, 2016; Zhao et al., 2016; Tang et al., 2017; Yuan et al., 2017; Geara et al., 2018; Wang et al., 2018; Liao et al., 2019; Wu, 2019; Zhao et al., 2022) (shown in Figure 5). Meta-analysis showed that there was no significant difference in the incidence of RD between TCHM and routine drugs (REM, RR = 0.99, 95%CI 0.97 to 1.01, 1,584 participants, I2 = 54%, p = 0.43). The subgroups classified by types of cancer, radiation dose, and dosage form of the TCHM could not explain the source of heterogeneity. We found that heterogeneity was mainly derived from TangHJ 2017. After excluding this study, the heterogeneity dropped to zero. The false-positive result may be caused by the too small sample size of this study (T/C = 15/15). But the meta-analysis also showed that compared with TCHM, routine drugs had an advantage in reducing RTOG grading (REM, RR = 0.46, 95%CI 0.35 to 0.60, 1,584 participants, I2 = 27%, p < 0.00001, see Figure 6). Subgroups classified by types of cancer showed some differences between subgroup (p = 0.10, I2 = 51.6%).
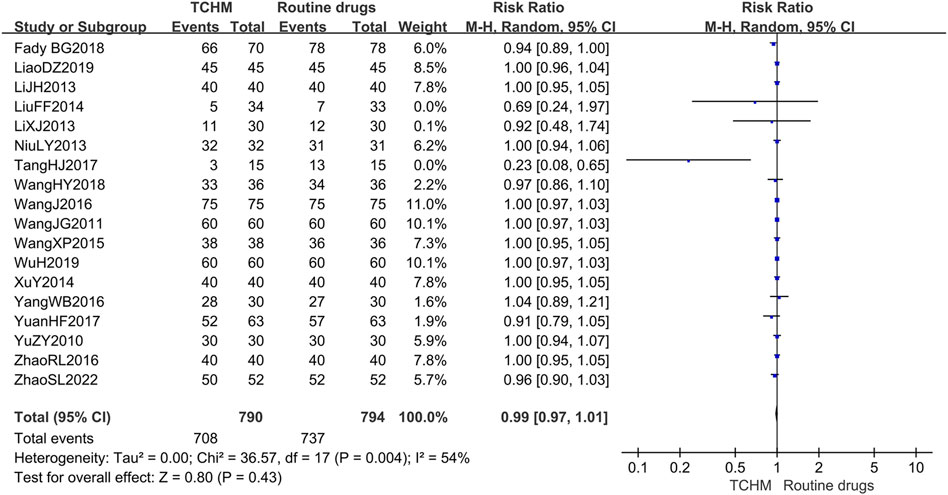
FIGURE 5. Forest plot and pooled risk ratios for association of incidence rate of radiodermatitis with TCHM and routine drugs.
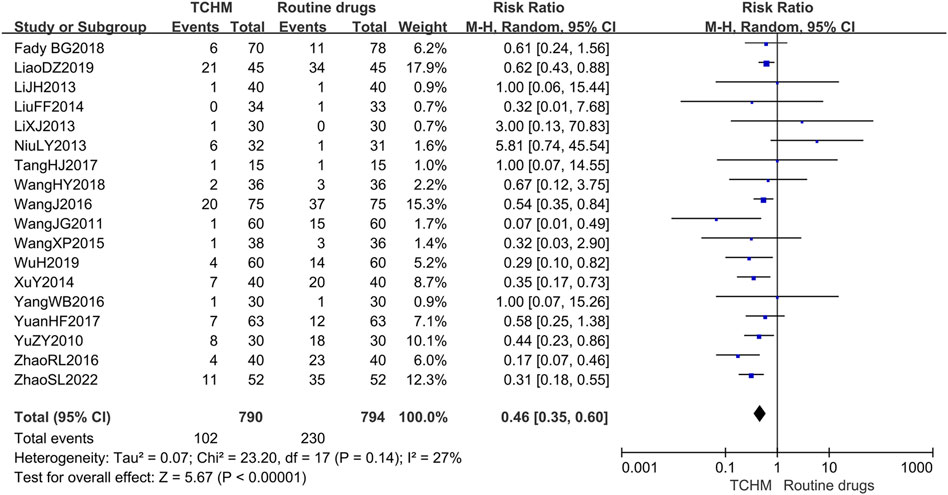
FIGURE 6. Forest plot and pooled risk ratios for association of RTOG grading criteria with TCHM and routine drugs.
3.4.1.2 Skin and Mucosa Recovery Time
Four studies including 301 patients reported skin and mucosa recovery time (Li et al., 2013; Wang et al., 2016; Zhao et al., 2016; Zhao et al., 2022). Meta-analysis showed that compared with routine drugs, TCHM may reduce the recovery time of RD (REM, MD = −2.35, 95%CI −3.58 to −1.12 days, 301 participants, I2 = 68%, p = 0.0002) (shown in Supplementary Figure S1).
3.4.1.3 The Occurrence Time of Radiation Dermatitis
Four studies including 334 patients reported the occurrence time of RD (Li et al., 2013; Zhao et al., 2016; Liao et al., 2019; Zhao et al., 2022). Meta-analysis showed that TCHM may delay the occurrence of RD compared to routine drugs (REM, MD = 2.36, 95%CI 1.74 to 2.98, 334 participants, I2 = 0%, p < 0.00001) (shown in Supplementary Figure S2).
3.4.1.4 The Radiation Dose
No study mentioned this outcome.
3.4.1.5 Quality of Life
Four studies including 366 patients reported quality of life, of which 180 cases in two studies reported changes in the quality of life before and after treatment (Yang, 2016; Wu, 2019). Due to huge heterogeneity, we were unable to conduct a meta-analysis; however, results from each trial showed no difference in this outcome between groups.
Meta-analysis of another two studies (Yu et al., 2010; Yuan et al., 2017) showed that TCHM had an advantage in improving the quality of life in patients with RD compared with routine drugs (REM, RR = 1.46, 95%CI 1.03 to 2.06, 186 participants, I2 = 0%, p = 0.03) (shown in Supplementary Figure S3).
3.4.1.6 Adverse Events
Seven studies mentioned the adverse events (Yu et al., 2010; Xu et al., 2014; Wang et al., 2015; Yang, 2016; Liao et al., 2019; Wu, 2019; Zhao et al., 2022), and six of them reported no adverse events. The remaining study reported that one patient in the treatment group had a rash in the smeared area and two patients in the control group had pruritus in the smeared area (Wang et al., 2015).
3.4.2 Topical Chinese Herbal Medicine vs. Conventional Therapy
3.4.2.1 The Incidence of Radiation Dermatitis
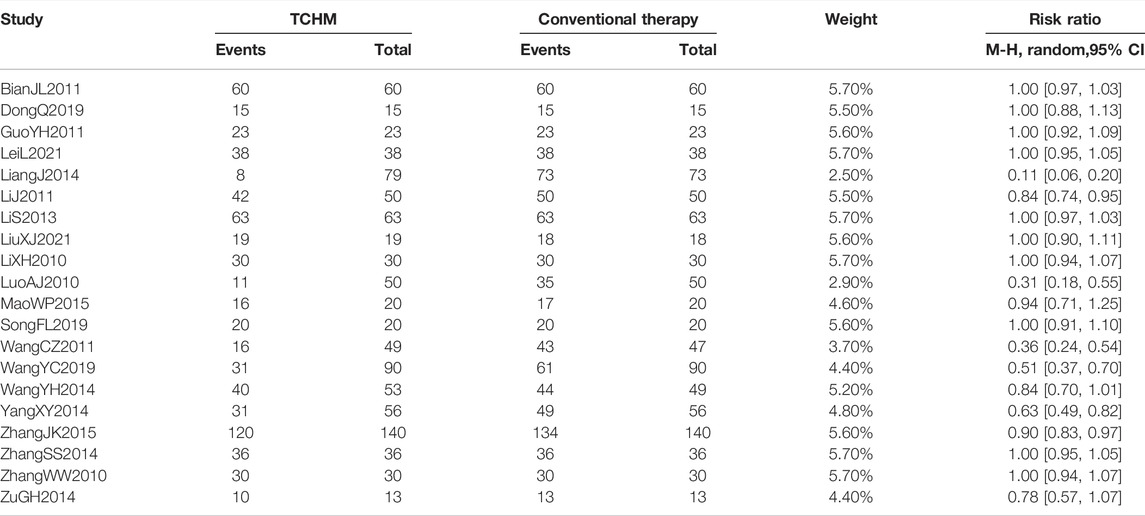
A total of 1855 patients in 20 studies reported the incidence of RD (Li et al., 2010; Luo and Lan, 2010; Zhang and Xue, 2010; Bian et al., 2011; Guo and Shi, 2011; Li et al., 2011; Wang C. et al., 2011; Lin et al., 2013; Liang and Huang, 2014; Wang et al., 2014; Yang and Guan, 2014; Zhang, 2014; Zu et al., 2014; Mao, 2015; Zhang, 2015; Dong, 2019; Wang et al., 2019; Song et al., 2020; Lei et al., 2021; Liu, 2021). Due to the huge heterogeneity, we were unable to conduct a meta-analysis. Fourteen of the 20 studies showed that there was no statistical difference in the incidence of RD between TCHM and conventional therapy. Among the 14 trials, 10 studies showed that the incidence of RD was 100% in both groups (Li et al., 2010; Zhang and Xue, 2010; Bian et al., 2011; Guo and Shi, 2011; Lin et al., 2013; Zhang, 2014; Dong, 2019; Song et al., 2020; Lei et al., 2021; Liu, 2021), the remaining 4 studies showed that the incidence rate was about 80% in both groups (Wang et al., 2014; Yang and Guan, 2014; Zu et al., 2014; Mao, 2015). Six of the 20 studies have showed a difference between the two groups (Luo and Lan, 2010; Li et al., 2011; Wang J. et al., 2011; Liang and Huang, 2014; Zhang, 2015; Wang et al., 2019). Compared to conventional therapy, the incidence of RD is reduced by 10–90% in TCHM group. The specific results of each study are shown in Table 2. Subgroups classified by types of cancer, radiation dose, and dosage form of the TCHM could not explain the source of heterogeneity.
3.4.2.2 Radiation Therapy Oncology Group Grading
A total of 1703 patients in 19 studies reported RTOG grading (Li et al., 2010; Luo and Lan, 2010; Zhang and Xue, 2010; Bian et al., 2011; Guo and Shi, 2011; Li et al., 2011; Wang C. et al., 2011; Lin et al., 2013; Wang et al., 2014; Yang and Guan, 2014; Zhang, 2014; Zu et al., 2014; Mao, 2015; Zhang, 2015; Dong, 2019; Wang et al., 2019; Song et al., 2020; Lei et al., 2021; Liu, 2021). Meta-analysis showed that compared with conventional therapy, TCHM had an advantage in reducing RTOG grading (REM, RR = 0.28, 95%CI 0.21 to 0.38, 1703 participants, I2 = 6%, p < 0.00001) (shown in Figure 7).
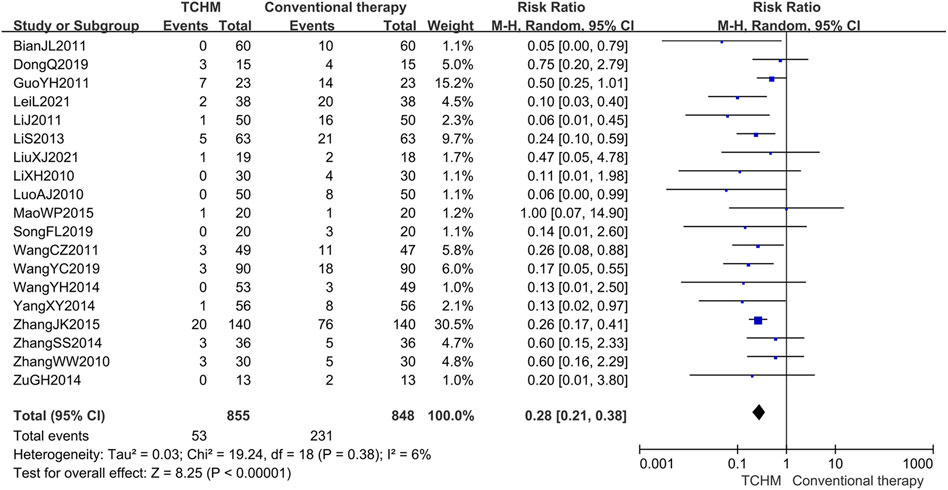
FIGURE 7. Forest plot and pooled risk ratios for association of RTOG grading criteria with TCHM and conventional therapy.
3.4.2.3 Skin and Mucosa Recovery Time
Three studies including 358 patients reported skin and mucosa recovery time (Wang et al., 2014; Wang et al., 2019; Lei et al., 2021). Due to the huge heterogeneity, we were unable to conduct a meta-analysis. These studies showed that TCM topical medicine could shorten the recovery time of skin and mucosa compared with conventional education, and the MD values (95%CI) were respectively −5.53 (−6.63, −4.43) days; −4.64 (−5.18, −4.10) days; −19.40 (−21.79, −17.01) days.
3.4.2.4 The Occurrence Time of Radiodermatitis
Two studies including 178 patients reported this outcome (Wang et al., 2014; Lei et al., 2021). Due to huge heterogeneity, we were unable to conduct a meta-analysis. One study showed that TCHM could delay the occurrence of radiation dermatitis for 4 days compared to conventional therapy. Another study showed that there was no difference in this outcome between groups.
3.4.2.5 The Radiation Dose
No study mentioned this outcome.
3.4.2.6 Quality of Life
Two studies including 77 patients reported quality of life (Mao, 2015; Lei et al., 2021). Due to the huge heterogeneity, we were unable to conduct a meta-analysis. One study showed that TCHM could improve patients’ quality of life compared with conventional therapy, while another study showed that there was no difference in this outcome between groups.
3.4.2.7 Adverse Events
Six studies mentioned the adverse events (Wang et al., 2014; Mao, 2015; Zhang, 2015; Dong, 2019; Lei et al., 2021; Liu, 2021), all of them reported no adverse events.
3.5 Funnel Plot
The funnel plot within data from 18 studies concerning TCHM compared to routine drugs with the incidence of radiation dermatitis/RTOG grading reduction showed potential asymmetry (Figures 8A,B). The funnel plot within data from 19 studies concerning TCHM compared to conventional therapy with RTOG Grading reduction showed potential asymmetry (Figure 8C). These may be caused by both of small study effect and publication bias.
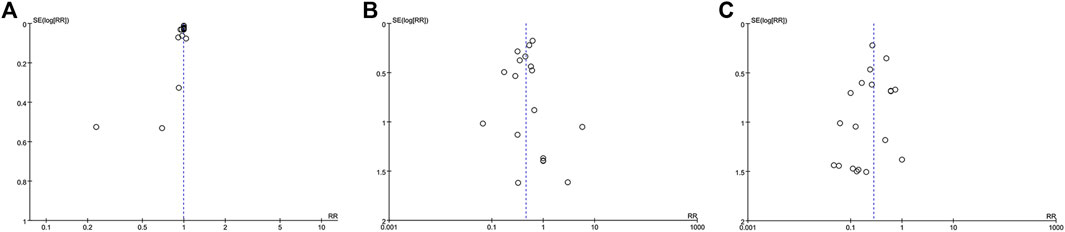
FIGURE 8. Funnel plot (A) TCHM compared to routine drugs with the incidence of radiation dermatitis reported in 22 trails (B) TCHM compared to routine drugs with RTOG grading criteria reported in 20 trails (C) TCHM compared to conventional therapy with RTOG grading criteria reported in 19 trails.
3.6 Sensitivity Analysis
According to the protocol, sensitivity analysis was used to evaluate the influence of removing the high-risk study and switching the random/fixed-effect model on the results. There was only two high-risk trials in our study, the results showed that there was no difference after removing the high-risk trial from the meta-analysis (the result from original Figure 7 to RR = 0.27, 95%CI 0.20 to 0.36, p < 0.00001). After switching random/fixed-effect model, only one meta-analysis was changed (the result from original Figure 5 to RR = 0.97, 95%CI 0.94 to 0.99, p = 0.005). Since RR value is close to 1 and the huge heterogeneity in these trials, we still adopt the result from random-effect model.
3.7 Quality of Evidence
As shown in Table 3, when comparing TCHM to routine drugs, the quality of evidence was low for RTOG grading criteria, time of incidence of radiodermatitis, and very low for the incidence rate of radiodermatitis, recovery time of skin mucosa. As for the outcome of recovery time of skin mucosa and time of incidence of radiodermatitis, the publication bias of these endpoints was not used for GRADE evaluation since only 4 studies were included. As shown in Table 4, when comparing TCHM to conventional therapy, the quality of evidence was low for RTOG grading criteria.
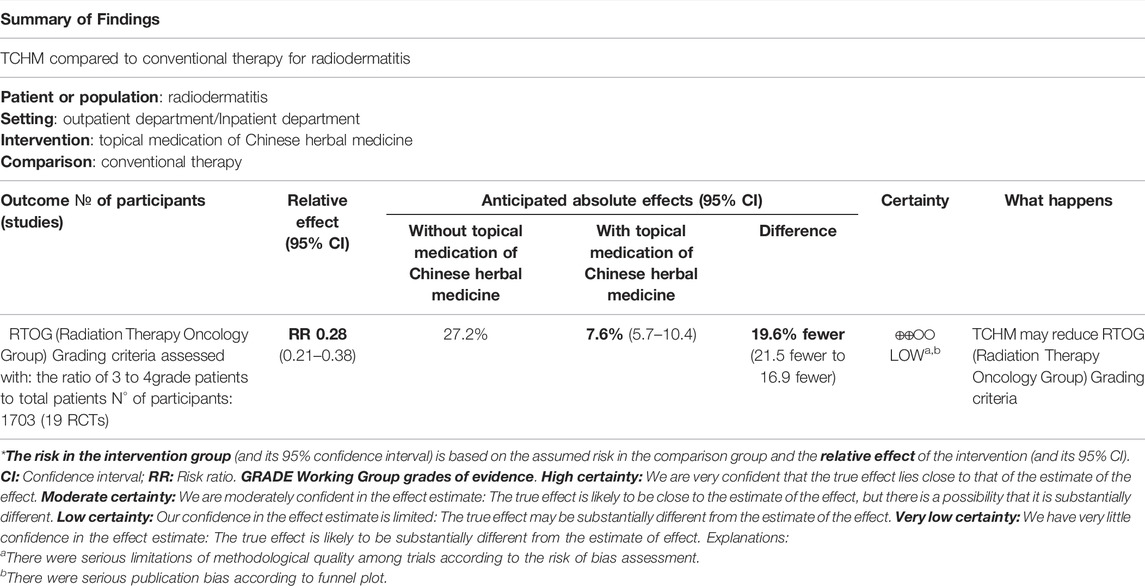
TABLE 4. Summary of finding table of topical medication of Chinese herbal medicine compared to conventional therapy for radiodermatitis.
4 Discussion
4.1 The Main Findings of the Review
A total of 38 studies were included in this study. Most of them were having some concerns about the risk of bias according to ROB 2.0 assessment. Very low-quality evidence was found that TCHM had no better effect on reducing the incidence rate of RD compared to routine drugs. However, low-quality evidence was found that TCHM may lower the RTOG grade of patients with RD (15.6% fewer than routine drugs) and delay the incidence of RD for about 2 days. Very low-quality evidence showed that TCHM may also cure the patients’ skin mucosa 2 days faster compared to routine drugs. When compared to conventional therapy, we got low-quality evidence that TCHM may reduce the RTOG grade (average 19.6% fewer than control). Insufficient evidence leads to inconclusive results on the safety of TCHM in preventing RD.
4.2 Advantages and the Limitations of the Review
Only the Chinese and English databases are searched, but TCHM is also used in some other countries, so the literature retrieval may be incomplete. As all included studies in this review reported acute RD, no studies concerning chronic RD as found, which may affect the reliability of the conclusions. Two studies reported missing data, and the missing data ratio was below 10%. We consider the impact of the missing data on the current conclusion may not be significant, so we did not use intention-to-treat analysis during the data synthesis.
Although several previously published systematic reviews are similar to our research topics, they are all different from this review. Ding et al. (2021) evaluated the therapeutic effect of TCHM for patients with RD, they focused on the cure rate and the cure time for RD patients treated by TCHM, but our review was aimed to evaluate the preventive effect of TCHM for patients with cancers who were about to accept radiation therapy. Hu and Hu (2019) included only six studies to evaluate the preventive effect of aloe gel for RD, which were concerned with all kinds of TCHM. Zheng (2021) did not target herbal medicine for preventing RD. Thus, this may be the first systematic review to assess the preventive effect of TCHM for RD.
Although we included 23 different prescriptions, the herbal medicine repetition rate in the prescriptions was 49.1% See Table 5 for specific patented formulations, botanical or chemical cited in the article. Meanwhile, the principle of all prescriptions is consistent, which is to clear heat-toxin. With the appropriate statistical analysis methods and the pre-designed subgroup analysis, we minimized the impact of clinical heterogeneity on the results and objectively evaluated the quality of evidence according to GRADE criteria. Although the quality of the evidence is low, it seems that TCHM can reduce the severity of RD, delay its occurrence, and accelerate the healing of skin lesions.
4.3 Implications for Clinical Practice
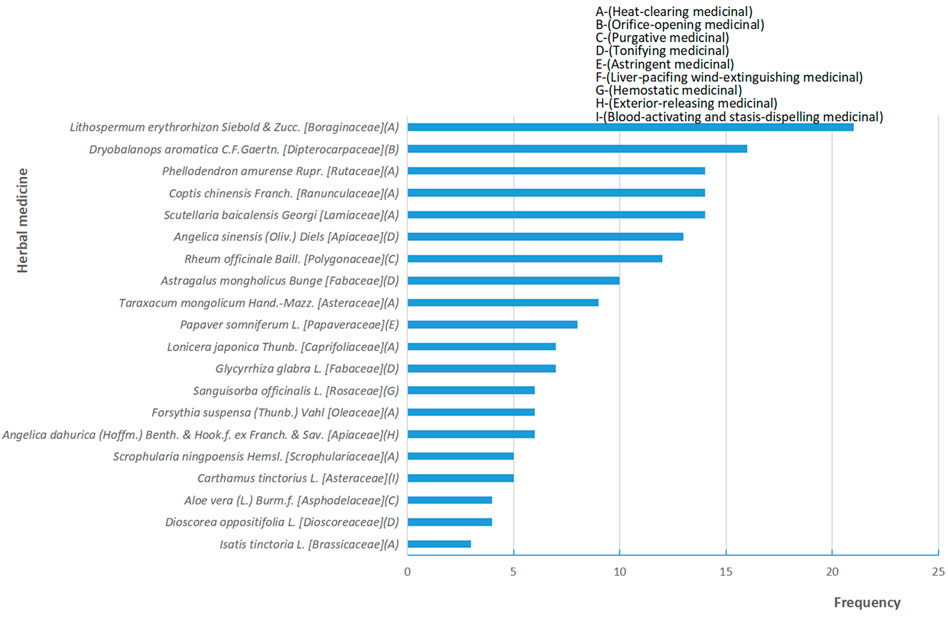
This review suggests that topical herbal medicine can be used for the cancer patients who are about to accept radiotherapy to prevent severe RD and to accelerate the healing of skin lesions. The main treatment principle of the included trials is heat-clearing since, in TCM theory, radiation therapy is kind of an exogenous dry-heat toxin. Thus, Lithospermum erythrorhizon Siebold & Zucc [Boraginaceae], Coptis chinensis Franch [Ranunculaceae], Phellodendron amurense Rupr [Rutaceae], Scutellaria baicalensis Georgi [Lamiaceae], Dryobalanops aromatica C.F.Gaertn [Dipterocarpaceae] and other herbs with an effect on clearing heat-toxin can be used in self-made prescriptions during the treatment. The most frequently used herbal plant in this review is Mei-Bao’s scald plasters, and the most frequently used herb is Lithospermum erythrorhizon Siebold & Zucc. [Boraginaceae]. The possible optimal time for application of topical herbal medicine is 24–30 h before radiotherapy. In addition, the radiation dose included in this review was between 45 and 80 Gy.
4.4 Instructions for Future Research
Most parts of the studies included in this review may not be reported strictly in accordance with CONSORT reporting standards, so all results are rated as low or very low at the time of GRADE rating. In the future, researchers should conduct well-designed, high-quality clinical studies in accordance with CONSORT guidelines to verify the efficacy and safety of local use of TCHM in the prevention of radiation dermatitis. In future studies, we can consider the type of cancer as a variable to explore the dominant diseases. In addition, some studies in this review do not specify the specific dose of traditional Chinese medicine or the ratio between drugs, and the relationship between dose and efficacy is not clear, which may lead to differences in the results of different studies, and the composition of TCM compound prescription is often not clear. It limits the extrapolation of clinical research results. In future research, we should pay attention to the relationship between the dose and the efficacy of TCM to determine the best drug dose.
5 Conclusion
This study has shown that TCHM can reduce the severity of RD, delay its occurrence, and accelerate the healing of skin lesions. However, the evidence is low or very low due to the low quality of the included studies. Furthermore, the efficacy of topical medication of Chinese herbal medicine in the prevention of radiodermatitis still needs to be verified in future well-designed, high-quality clinical trials that adhere to CONSORT guidelines.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
H-JC designed the research. H-BY and B-JH performed literature search and article selection, assessed methodological bias risk, performed data extraction, conducted a meta-analysis, assessed study quality, and finished the manuscript draft. H-JC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
H-JC was supported by the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.819733/full#supplementary-material
Abbreviations
TCHM, topical Chinese herbal medicine; RTOG, Radiation Therapy Oncology Group; RR, risk ratio; CI, confidence intervals; MD, mean difference; RCT, randomized controlled trials; RD, radiodermatitis; TCM, traditional Chinese medicine; CENTRAL, Cochrane Central Register of Controlled Trials; CNKI, the Chinese National Knowledge Infrastructure Databases; VIP, the Chongqing VIP Chinese Science and Technology Periodical Database; GRADE, Grading of Recommendations Assessment Development and Evaluation criteria; CONSORT, Consolidated Standards Of Reporting Trials; T, treatment; C, control; X, average; SD, standard deviation; BC, breast cancer; NPC, nasopharynx cancer; LCA, lung cancer; HCC, hepatocellular carcinoma; ESCA, esophageal carcinoma; GC, gastric cancer; TCS, thyroid carcinosarcoma; THCA, thyroid carcinoma; UCC, uterine cervix cancer; CRC, colorectal cancer.
References
Ashack, K. A., Kuritza, V., Visconti, M. J., and Ashack, L. (2020). Dermatologic Sequelae Associated with Radiation Therapy. Am. J. Clin. Dermatol 21 (4), 541–555. doi:10.1007/s40257-020-00519-x
Aygenc, E., Celikkanat, S., Kaymakci, M., Aksaray, F., and Ozdem, C. (2004). Prophylactic Effect of Pentoxifylline on Radiotherapy Complications: a Clinical Study. Otolaryngol. Head. Neck Surg. 130 (3), 351–356. doi:10.1016/j.otohns.2003.08.015
Behroozian, T., Milton, L., Zhang, L., Lou, J., Karam, I., Lam, E., et al. (2021). How Do Patient-Reported Outcomes Compare with Clinician Assessments? A Prospective Study of Radiation Dermatitis in Breast Cancer. Radiotherapy Oncol. 159, 98–105. doi:10.1016/j.radonc.2021.03.020
Bian, J., Luo, Z., and Zhang, S. (2011). Clinical Observation of Chinese Herb Mixtureon the Prevention of Acute Radiation-Induced Skin Injury with Post-Surgical Breast Cancer Patient. Hebei J. Traditional Chin. Med. 33 (12), 1797–1798. CNKI:SUN:HBZY.0.2011-12-017.
Bontempo, P. S. M., Ciol, M. A., Menêses, A. G., Simino, G. P. R., Ferreira, E. B., and Reis, P. E. D. D. (2021). Acute Radiodermatitis in Cancer Patients: Incidence and Severity Estimates. Rev. Esc. Enferm. Usp. 55, e03676. doi:10.1590/S1980-220X2019021703676
Bray, F. N., Simmons, B. J., Wolfson, A. H., and Nouri, K. (2016). Acute and Chronic Cutaneous Reactions to Ionizing Radiation Therapy. Dermatol Ther. (Heidelb) 6 (2), 185–206. doi:10.1007/s13555-016-0120-y
Chan, R. J., Webster, J., Chung, B., Marquart, L., Ahmed, M., and Garantziotis, S. (2014). Prevention and Treatment of Acute Radiation-Induced Skin Reactions: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC cancer 14, 53. doi:10.1186/1471-2407-14-53
Ding, T., Nian, J., Zhang, Q., and Wang, X. (2021). Meta-analysis of Curative Effect of External Treatment of Traditional Chinese Medicine on Acute Radiation Dermatitis. Beijing Tradit. Chin. Med. 40 (01), 90–95. doi:10.16025/j.1674-1307.2021.01.025
Dong, Q. (2019). Nursing Effect of MEBO on Preventing Radiation Skin Injury after Modified Radical Mastectomy for Breast Cancer. Drug Eval. 16 (05), 37–39. CNKI:SUN:YPPJ.0.2019-05-009.
Finkelstein, S., Kanee, L., Behroozian, T., Wolf, J. R., van den Hurk, C., Chow, E., et al. (2022). Comparison of Clinical Practice Guidelines on Radiation Dermatitis: a Narrative Review. Support Care Cancer 30 (6), 4663–4674. doi:10.1007/s00520-022-06829-6
Fuzissaki, M. A., Paiva, C. E., Oliveira, M. A., Lajolo Canto, P. P., and Paiva Maia, Y. C. (2019). The Impact of Radiodermatitis on Breast Cancer Patients' Quality of Life during Radiotherapy: A Prospective Cohort Study. J. Pain Symptom Manage 58 (1), 92. doi:10.1016/j.jpainsymman.2019.03.017
Geara, F. B., Eid, T., Zouain, N., Thebian, R., Andraos, T., Chehab, C., et al. (2018). Randomized, Prospective, Open-Label Phase III Trial Comparing Mebo Ointment with Biafine Cream for the Management of Acute Dermatitis during Radiotherapy for Breast Cancer. Am. J. Clin. Oncol. 41 (12), 1257–1262. doi:10.1097/COC.0000000000000460
Gujral, M. S., Patnaik, P. M., Kaul, R., Parikh, H. K., Conradt, C., Tamhankar, C. P., et al. (2001). Efficacy of Hydrolytic Enzymes in Preventing Radiation Therapy-Induced Side Effects in Patients with Head and Neck Cancers. Cancer Chemother. Pharmacol. 47 (Suppl. l), S23–S28. doi:10.1007/s002800170005
Guo, Y., and Shi, L. (2011). Clinical Observation of Meibao Burn Ointment in Prevention and Treatment of Radiodermatitis in Patients with Nasopharyngeal Carcinoma. China Pract. Med. 6 (03), 143–144. doi:10.14163/j.cnki.11-5547/r.2011.03.203
Hindley, A., Zain, Z., Wood, L., Whitehead, A., Sanneh, A., Barber, D., et al. (2014). Mometasone Furoate Cream Reduces Acute Radiation Dermatitis in Patients Receiving Breast Radiation Therapy: Results of a Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 90 (4), 748–755. doi:10.1016/j.ijrobp.2014.06.033
Hu, J. J., Urbanic, J. J., Case, L. D., Takita, C., Wright, J. L., Brown, D. R., et al. (2018). Association between Inflammatory Biomarker C-Reactive Protein and Radiotherapy-Induced Early Adverse Skin Reactions in a Multiracial/Ethnic Breast Cancer Population. J. Clin. Oncol. 36 (24), 2473–2482. doi:10.1200/JCO.2017.77.1790
Hu, M., and Hu, H. (2019). Effect of Aloe Gel on Prevention of Radioactive Dermatitis: a Meta-Analysis. Nurs. Res. 33 (10), 1670–1674. doi:10.12102/j.issn.1009-6493.2019.10.007
Huang, Z., Zeng, Y., Jia, Z., and Zhang, L. (2016). Research Progress of Antioxygenation in Curcumin and its Derivants. J. Prev. Med. Inf. 32 (11), 1237–1240. CNKI:SUN:YFYX.0.2016-11-028.
Hymes, S. R., Strom, E. A., and Fife, C. (2006). Radiation Dermatitis: Clinical Presentation, Pathophysiology, and Treatment 2006. J. Am. Acad. Dermatol 54 (1), 28–46. doi:10.1016/j.jaad.2005.08.054
Kole, A. J., Kole, L., and Moran, M. S. (2017). Acute Radiation Dermatitis in Breast Cancer Patients: Challenges and Solutions. Breast Cancer (Dove Med. Press) 9, 313–323. doi:10.2147/BCTT.S109763
Lee, J., Lee, S. W., Hong, J. P., Shon, M. W., Ryu, S. H., and Ahn, S. D. (2016). Foam Dressing with Epidermal Growth Factor for Severe Radiation Dermatitis in Head and Neck Cancer Patients. Int. Wound J. 13 (3), 390–393. doi:10.1111/iwj.12317
Lei, L., Wang, Y., and Yin, Y. (2021). Clinical Observation of Zibai Huangqi Ointment in Preventing and Treating Acute Radiation Dermatitis. Guangming J. Chin. Med. 36 (03), 386–388. CNKI:SUN:GMZY.0.2021-03-023.
Leventhal, J., and Young, M. R. (2017). Radiation Dermatitis: Recognition, Prevention, and Management. Oncol. Willist. Park) 31 (12), 885-9–894-9.
Li, J., and Liu, Y. (2013). Clinical Study on Ruyi Jinhuang Powder in Preventing and Treating Radiation Dermatitis. Glob. Tradit. Chin. Med. 6 (01), 49–50. CNKI:SUN:HQZY.0.2013-01-022.
Li, J., Zhang, Y., Gao, J., Wang, C., Han, Y., and Jia, J. (2011). Clinical Study on Prevention and Cure of Radioactive Cutaneous Injury with Liangxue Jiedu Gao. J. Hebei Traditional Chin. Med. Pharmacol. 26 (04), 13–14+12. doi:10.16370/j.cnki.13-1214/r.2011.04.030
Li, X., Li, K., and Lin, H. (2013). Clinical Study of Sanhuang Ointment in Preventing and Treating Acute Radiation Dermatitis. Shandong Med. J. 53 (17), 85–86. doi:10.1524/ncrs.2013.0056
Li, X., Liu, Y., Wen, M., and Yang, Z. (2010). Clinical Observation of Bingpian Huashi Powder in Preventing Radiodermatitis in Patient with Nasopharyngeal Carcinoma. Chin. Youjiang Med. J. 38 (03), 286–287. CNKI:SUN:YJYX.0.2010-03-022.
Liang, J., and Huang, L. (2014). Clinical Observation of Zicao Diyu Compound in Preventing Radiodermatitis. Intern. Med. 9 (03), 306–307. doi:10.1111/ijs.12096
Liao, D., Li, J., Liu, C., Zhao, M., Pu, Q., and Tang, Q. (2019). Clinical Effect of Huanglian -Jiedu Mistura on Prevention and Treatment of Radiation Dermatitis in Head and Neck Neoplasms. J. Chengdu Univ. Traditional Chin. Med. 42 (02), 60–63. doi:10.13593/j.cnki.51-1501/r.2019.02.060
Lin, L. C., Que, J., Lin, L. K., and Lin, F. C. (2006). Zinc Supplementation to Improve Mucositis and Dermatitis in Patients after Radiotherapy for Head-And-Neck Cancers: a Double-Blind, Randomized Study. Int. J. Radiat. Oncol. Biol. Phys. 65 (3), 745–750. doi:10.1016/j.ijrobp.2006.01.015
Lin, S., Huang, Y., and Zhuo, R. (2013). Clinical Observation of Spraying with Tuhuanglian Solution to Prevent Radiodermatitis in Breast Cancer Patient. all Health 7 (16), 138. CNKI:SUN:JKXS.0.2013-16-154.
Liu, F., Li, L., and Jia, R. (2014). The Effect of External Application of Chinese Oil on the Prevention of Radiation-Induced Damage. Chin. J. Nurs. Educ. 11 (08), 576–578. CNKI:SUN:ZHHU.0.2014-08-006.
Liu, L., Yang, Y., Guo, Q., Ren, B., Peng, Q., Zou, L., et al. (2020). Comparing Hypofractionated to Conventional Fractionated Radiotherapy in Postmastectomy Breast Cancer: a Meta-Analysis and Systematic Review. Radiat. Oncol. 15 (1), 17. doi:10.1186/s13014-020-1463-1
Liu, X. (2021). Clinical Study of Jiawei Simiao Yongan Oil on Preventing Acute Radiation Dermatitis. Beijing, China: Beijing University of Chinese Medicine.
Louie, A. V., Granton, P. V., Fairchild, A., Bezjak, A., Gopaul, D., Mulroy, L., et al. (2022). Palliative Radiation for Advanced Central Lung Tumors with Intentional Avoidance of the Esophagus (PROACTIVE): A Phase 3 Randomized Clinical Trial. JAMA Oncol. 8 (4), 1–7. doi:10.1001/jamaoncol.2021.7664
Luo, A., and Lan, Y. (2010). Clinical Observation of Bingpian Huashi Powder in Preventing Radiodermatitis in Patient with Head and Neck Cancer. Chin. General Pract. Nurs. 8 (16), 1446–1447. CNKI:SUN:JTHS.0.2010-16-030.
Mao, W. (2015). Clinical Study of Jiawei Simiao Yongan Oil on Preventing Acute Radiation Dermatitis. Beijing, China: Beijing University of Chinese Medicine.
Martin, S., Lomas, J., and Claxton, K. (2020). Is an Ounce of Prevention Worth a Pound of Cure? A Cross-Sectional Study of the Impact of English Public Health Grant on Mortality and Morbidity. BMJ Open 10 (10), e036411. doi:10.1136/bmjopen-2019-036411
Menon, A., Prem, S. S., and Kumari, R. (2021). Topical Betamethasone Valerate as a Prophylactic Agent to Prevent Acute Radiation Dermatitis in Head and Neck Malignancies: A Randomized, Open-Label, Phase 3 Trial. Int. J. Radiat. Oncol. Biol. Phys. 109 (1), 151–160. doi:10.1016/j.ijrobp.2020.08.040
Meyer, F., Fortin, A., Wang, C. S., Liu, G., and Bairati, I. (2012). Predictors of Severe Acute and Late Toxicities in Patients with Localized Head-And-Neck Cancer Treated with Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 82 (4), 1454–1462. doi:10.1016/j.ijrobp.2011.04.022
Miller, R. C., Schwartz, D. J., Sloan, J. A., Griffin, P. C., Deming, R. L., Anders, J. C., et al. (2011). Mometasone Furoate Effect on Acute Skin Toxicity in Breast Cancer Patients Receiving Radiotherapy: a Phase III Double-Blind, Randomized Trial from the North Central Cancer Treatment Group N06C4. Int. J. Radiat. Oncol. Biol. Phys. 79 (5), 1460–1466. doi:10.1016/j.ijrobp.2010.01.031
Niu, L. (2013). Efficacy Comparison between Trolamine Cream and Meibao Burn Ointment in Prevention and Treatment of Radiodermatitis in Patients with Nasopharyngeal Carcinoma. Med. J. West China 25 (11), 1626–1627+1631. CNKI:SUN:XIBU.0.2013-11-009.
Rosenthal, A., Israilevich, R., and Moy, R. (2019). Management of Acute Radiation Dermatitis: A Review of the Literature and Proposal for Treatment Algorithm. J. Am. Acad. Dermatol 81 (2), 558–567. doi:10.1016/j.jaad.2019.02.047
Song, F., Liao, Z., Li, T., Kang, N., Li, Z., Fan, S., et al. (2020). Topical Use of Jiawei Simiao Yongan Gao to Prevent Radiodermatitis in Patients with Head and Neck Cancer: A Retrospective Cohort Study. Med. Baltim. 99 (48), e23318. doi:10.1097/md.0000000000023318
Tang, H., Jiang, Y., and Zhao, L. (2017). Clinical Observation of Fenghuang Liquid as Early Stage Intervention for Post-Surgical Breast Cancer with Radiation Dermatitis. Electron. J. Pract. Clin. Nurs. Sci. 2 (02), 123–126. CNKI:SUN:SLHL.0.2017-02-090.
Tejwani, A., Wu, S., Jia, Y., Agulnik, M., Millender, L., and Lacouture, M. E. (2009). Increased Risk of High-Grade Dermatologic Toxicities with Radiation Plus Epidermal Growth Factor Receptor Inhibitor Therapy. Cancer 115 (6), 1286–1299. doi:10.1002/cncr.24120
Tinkle, C. L., Singh, C., Lloyd, S., Guo, Y., Li, Y., Pappo, A. S., et al. (2021). Stereotactic Body Radiation Therapy for Metastatic and Recurrent Solid Tumors in Children and Young Adults. Int. J. Radiat. Oncol. Biol. Phys. 109 (5), 1396–1405. doi:10.1016/j.ijrobp.2020.11.054
Ulff, E., Maroti, M., Serup, J., Nilsson, M., and Falkmer, U. (2017). Prophylactic Treatment with a Potent Corticosteroid Cream Ameliorates Radiodermatitis, Independent of Radiation Schedule: A Randomized Double Blinded Study. Radiother. Oncol. 122 (1), 50–53. doi:10.1016/j.radonc.2016.11.013
Wang, C., Ma, L., Li, J., Zhang, Y., and Hao, L. (2011). Liangxue Jiedu Ointment and Nursing Care on Preventing Radiodermatitis in Patients with Post-Surgical Breast Cancer. Hebei Med. J. 33 (23), 3674–3675. CNKI:SUN:HBYZ.0.2011-23-104.
Wang, H., Li, X., Liang, W., Chen, L., Ling, Y., Tang, Z., et al. (2018). Clinical Observation of Fresh Aloe Liquid in Preventing Radiodermatitis in Breast Cancer Patient. World Latest Med. Inf. 18 (88), 202–206. doi:10.19613/j.cnki.1671-3141.2018.88.094
Wang, J., Yin, L., and Ding, T. (2016). Chinese Medicine Kuiyang Oil in the Prevention of Skin Damage Caused by Radiotherapy in Head and Neck Cancer for 75 Cases. Chin. Med. Mod. Distance Educ. China 14 (24), 75–77. CNKI:SUN:ZZYY.0.2016-24-037.
Wang, J., Yuan, B., Xiong, J., Chen, Y., Xie, L., Xu, X., et al. (2011). Clinical Observation of Kangfuxin Liquid in Preventing Radiodermatitis. Mod. J. Integr. Tradit. Chin. West Med. 20 (27), 3426–3427. CNKI:SUN:XDJH.0.2011-27-030.
Wang, X., Li, X., and Wang, Z. (2015). Clinical Research of Kuiyangyou Preventing and Treating 38 Cases of Nasopharyngeal Carcinoma Acute Radiodermatitis. J. Traditional Chin. Med. 56 (23), 2030–2032. doi:10.13288/j.11-2166/r.2015.23.014
Wang, Y., Li, L., Wang, C., Xu, Y., Yin, Z., and Lou, H. (2019). Clinical Study of MEBO in Preventing Acute Radiation Dermatitis of Cervical Cancer. China Mod. Dr. 57 (35), 31–34. CNKI:SUN:ZDYS.0.2019-35-009.
Wang, Y., Lin, Y., Xu, L., and Yang, B. (2014). Evaluation of Fufang Huzhang Baiji Film in the Prevention and Treatment of Radiation Dermatitis. China Pharm. 23 (11), 101–102. CNKI:SUN:YYGZ.0.2014-11-053.
Wei, Y. (2012). Clinical Observation of Meibao Burn Ointment Topical Use in Preventing Childen with Radiodermatitis. Shandong Med. J. 52 (26), 81–82. CNKI:SUN:SDYY.0.2012-26-043.
Wu, H. (2019). Evaluation of Therapeutic Efficiency and Exploration on the Potential Mechanism of Qingshu Ointment in Preventing and Treating Radiation-Induced Skin Toxicity. Nanjing, China: Nanjing University of Chinese Medicine.
Wu, S., Bai, H., Chen, J., Zhang, C., and Bai, S. (2010). Efficacy of Kangfuxin Liquid in the Treatment of 60 Cases with Esophagitis. Jilin Med. J. 31 (32), 5730–5731. CNKI:SUN:JLYX.0.2010-32-020.
Xie, Y., Wang, Q., Hu, T., Chen, R., Wang, J., Chang, H., et al. (2021). Risk Factors Related to Acute Radiation Dermatitis in Breast Cancer Patients after Radiotherapy: A Systematic Review and Meta-Analysis. Front. Oncol. 11, 738851. doi:10.3389/fonc.2021.738851
Xu, Y., Yang, W., and Zhao, S. (2014). Efficacy Observation of Sanhuang Ointment Combined with Honey in the Treatment of Radio-Dermatitis of Breast Cancer. China Pharm. 25 (19), 1789–1791. CNKI:SUN:ZGYA.0.2014-19-025.
Yang, W. (2016). Clinical Observation of Jiawei Simiao Yongan Oil Coated on Preventing Acute Radiation Dermatitis. Beijing, China: Beijing University of Chinese Medicine.
Yang, X., Ren, H., Guo, X., Hu, C., and Fu, J. (2020). Radiation-induced Skin Injury: Pathogenesis, Treatment, and Management. Aging (Albany NY) 12 (22), 23379–23393. doi:10.18632/aging.103932
Yang, X., and Guan, L. (2014). Curative Effect of Jingwanhong Ointment in the Treatment of Radioactive Skin Injury for Breast Cancer Patients. Pract. Pharm. Clin. Rem. 17 (03), 373–375. doi:10.14053/j.cnki.ppcr.2014.03.017
Yao, Z., and Cheng, B. (2021). Morbidity in Patients with Nasopharyngeal Carcinoma and Radiation-Induced Skin Lesions: Cause, Risk Factors, and Dermatitis Evolution and Severity. Adv. Skin. Wound Care 34 (12), 1–8. doi:10.1097/01.ASW.0000797952.41753.f4
Yokota, T., Zenda, S., Ota, I., Yamazaki, T., Yamaguchi, T., Ogawa, T., et al. (2021). Phase 3 Randomized Trial of Topical Steroid versus Placebo for Prevention of Radiation Dermatitis in Patients with Head and Neck Cancer Receiving Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 111 (3), 794–803. doi:10.1016/j.ijrobp.2021.05.133
Yu, Z., Li, W., and Yasuka, M. (2010). Study of Ulcer Oil on Prevention and Treatment for Radiotherapy-Induced Cutaneous Injury in Patients with Head and Neck Tumors. J. Guangzhou Univ. Tradit. Chin. Med. 27 (05), 474–477+481. doi:10.13359/j.cnki.gzxbtcm.2010.05.029
Yuan, H., Wang, Y., Cheng, C., Chang, F., and Di, L. (2017). Clinical Research on Chuangyangling Treatment of Radioactive Dermatitis Caused by Breast Cancer Radiotherapy. China J. Chin. Med. 32 (08), 1385–1387. doi:10.16368/j.issn.1674-8999.2017.08.364
Zhang, J. (2015). Clinical Study of Liangxue Jiedu Ointment in Preventing Radiodermatitis. Shijiazhuang, China: Heibei Medical University.
Zhang, S. (2014). Clinical Observation of Meibao Burn Ointment in Prevention and Treatment of Radiodermatitis in Patients with Post-surgical Breast Cancer. China Health Care & Nutr. 24 (4), 2247–2248.
Zhang, W., and Xue, M. (2010). Clinical Observation of Meibao Burn Ointment in Preventing Radiodermatitis with Breast Cancer Patients. China Pract. Med. 5 (30), 161–162. doi:10.14163/j.cnki.11-5547/r.2010.30.221
Zhao, R., Shen, H., Zhang, M., Zhou, Y., and Ruan, Y. (2016). Effect of Compound Ulcer Oil on Blood Cytokines in Patients with Radiation Dermatitis. Chin. J. Exp. Tradit. Med. Formulae 22 (09), 153–157. doi:10.13422/j.cnki.syfjx.2016090153
Zhao, S., Liu, F., and Xue, L. (2022). Observation on the Effect and Safety of Qingre Yufu Prescription in Prevention and Treatment of Acute Radiodermatitis of Malignant Tumor of Head and Neck. J. Emerg. Tradit. Chin. Med. 31 (01), 145–147. doi:10.3969/j.issn.1004-745X.2022.01.038
Zheng, Y. (2021). Meta-analysis and Clinical Application Study of Dressing Drugs on Radiation Dermatitis Prevention. Hengyang, China: University of South China.
Zhu, X., Liao, H., Chen, L., Chen, B., and Qin, Q. (2013). Clinal Observation on the Effect of Self-Made Compound Aloe Liquid on Preventing Radiation Dermatitis in Patients with Nasopharyngeal Carcinoma. J. Nursing(China) 20 (16), 67–68. doi:10.16460/j.issn1008-9969.2013.16.026
Keywords: radiodermatitis, topical Chinese herbal medicine, systematic review, meta-analysis, radiotherapy
Citation: Yu H-B, Han B-J and Cao H-J (2022) Prevention of Radiodermatitis With Topical Chinese Herbal Medicine: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:819733. doi: 10.3389/fphar.2022.819733
Received: 22 November 2021; Accepted: 09 May 2022;
Published: 22 June 2022.
Edited by:
Esra Küpeli Akkol, Gazi University, TurkeyReviewed by:
Walbert Vieira, State University of Campinas, BrazilYonggang Zhang, Sichuan University, China
Copyright © 2022 Yu, Han and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Juan Cao, aHVpanVhbmNhbzMyN0Bob3RtYWlsLmNvbQ==
†These authors share first authorship
 Hui-Bo Yu
Hui-Bo Yu Bao-Jin Han†
Bao-Jin Han† Hui-Juan Cao
Hui-Juan Cao