
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 04 March 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.817265
This article is part of the Research Topic The Effect of Anti-Cancer Drug Therapies in the Treatment of Lung Cancer View all 29 articles
Currently, the predictive role of POLE mutations for immunotherapy is under intense investigation. The POLE gene encodes one of the four subunits of DNA polymerase important for DNA replication and repair. POLE mutations are related to other favorable predicative factors such as high expression of PD-L1, high TMB, and infiltration of CD8+ cells in the tumor microenvironment. No formal clinical trials studied the efficacy of immunotherapy in lung patients harboring POLE mutation, and only few cases were mentioned in the literature. Moreover, lung cancer patients are prone to brain metastasis, which is notorious for the unresponsiveness to chemotherapy. The efficacy of immunotherapy for brain metastasis is still controversial. Here, we described a case of a POLEmt non-small-cell lung cancer (NSCLC) patient with brain metastasis who was treated with immunotherapy. His brain lesions disappeared after treatment. Our report strongly supported the benefit of immune-combined therapy for advanced NSCLC patients with POLE mutation, even with brain metastasis.
A 65-year-old man was admitted because of a nodule in the upper lobe of his right lung in his annual health screen without any discomfort. He had a smoking history of 30 years. The patient underwent an enhanced CT scan of his head before surgery, and no obvious metastases were found. He underwent right upper lobectomy and lymph node dissection. Postoperative pathological examination revealed invasive adenocarcinoma (alveolar, papillary, and solid) with pleural involvement, supported by typical immunohistochemistry (IHC) staining as follows: CK7(+), TTF-1 (+), NapsinA (+), CK5/6 (−), P63 (−), P40 (−), CD56 (−), CgA (−), Syn (−), and Ki-67 (50%). PD-L1 expression of the tumor proportion score (TPS) was evaluated using the IHC 22C3 pharmDx assay, and a combined positive sore of 30 was assessed (Figure 1). All the 13 excised lymph nodes were free of tumor cells including group 2 (0/2), group 4 (0/2), group 7 (0/3), and group 11 (0/1). He was diagnosed with pT2aN0M0, IB stage. However, after surgery, multiple enhanced intracranial nodules were observed in contrasted head MRI (Figures 2C–E) without neurological symptoms. The diagnosis was corrected to cT0N0M1c, IVc stage. The next-generation sequencing of his tumor detected POLE mutation (exon 26, p. P1025fs, 47.81%) and TP53 mutation (exon5, c.376-1G>A, 5.74%), microsatellite stabilization (MSS), and TMB 16.95 mut/Mb.
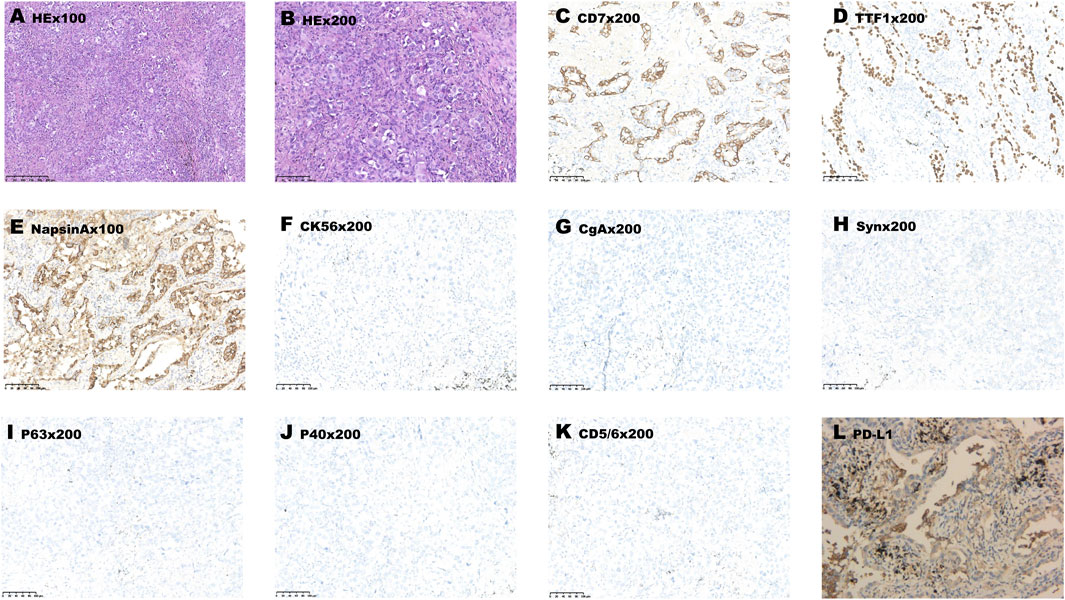
FIGURE 1. Pathological examination showed adenocarcinoma morphology (A,B). (C–L) Immunohistochemistry data: CK7 (+), TTF-1 (+), NapsinA (+), CK5/6 (−), P63 (−), P40 (−), CD56 (−), CgA (−), Syn (−) and PD-L1 (+, positive proportion about 30%), supported the diagnosis. Original magnification: (A) ×100 and (B–K) × 200.

FIGURE 2. The red arrow represents the primary lesion of the lung (A,B). The red triangle represents brain metastases (C–H). And the enhanced MRI showed complete response of brain metastases (I–K).
He was prescribed with two cycles of combination therapy of pemetrexed (500 mg/kg, Hansoh Inc.) plus carboplatin (AUC = 5, Yangtze Inc.) plus bevacizumab (7.5 mg/kg, Roche Inc.) plus tislelizumab (an anti-PD1 antibody, BeiGene Inc., 200 mg). After two cycles of therapy, the intracranial metastases became smaller in size (from 1.6 cm × 1.0 cm to 0.6 cm × 0.6 cm, Figures 2F–H). After four cycles of combined therapy, the metastases had completely disappeared (Figures 2I–K). He received tislelizumab, pemetrexed, and bevacizumab for two cycles of consolidation therapy. After six cycles of treatment, the patient felt fatigue and poor appetite. We tested the ACTH, 24 h urinary free cortisol excretion (UFC), and 8 h cortisol (PTC-8). The results showed that the 24 h UFC (4.8 ug/24h, normal: 20.3–127.6 ug/24h) and PTC-8 (19.9 nmol/L, normal: 133.0–537.0 nmol/L) decreased significantly. The patient was diagnosed with immune-related hypophysitis (grade 2) after multi-disciplinary treatment. He received glucocorticoids for a week, and the hypophysitis gradually relieved. Then, the treatment was switched to tislelizumab and bevacizumab for six cycles until now. Currently, 11 months after the initiation of the combined therapy, the patient is still on therapy and responding with no further treatment-related adverse events. The complete treatment process of the patient is shown in Table 1.
In the past decade, immune checkpoint inhibitors (ICIs) have emerged as a new treatment modality beyond chemotherapy for advanced non-small-cell lung cancer (NSCLC) without driver mutations. However, the question is that only a minority (20–30%) of patients can benefit from immunotherapy (Reck et al., 2016; Hellmann et al., 2019).
Currently, the predictive role of POLE mutations for immunotherapy is under intense investigation. The POLE gene is located in chromosome 12q24.33, encoding one of the four subunits of DNA polymerase important for DNA replication and repair (Rossi et al., 2020). POLE mutations are related to other favorable predicative factors such as high expression of PD-L1, high TMB, and infiltration of CD8+ cells in the tumor microenvironment (TME) (Wang et al., 2019; Yao et al., 2019). In one report, the density of CD8+ T cells was consistently higher in tumors harboring POLE mutation (POLEmt) than wild-type (POLEwt), either in endometrial cancer (59.4 vs. 24.7 CD8+ cells per HPF, p = 0.11) or colorectal intraepithelial neoplasia (59.4 vs. 14.8 CD8+ cells per HPF, p = 0.029) or colorectal cancer (154.9 vs. 34.0 CD8+ cells per HPF, p value undescribed) (Temko et al., 2018). The high CD8+ T-cell infiltration in POLEmt colorectal cancer was also reported by Domingo et al. (Domingo et al., 2016). In POLEmt endometrial cancer (n = 37), PD-L1 expression (>1%) was 29.6% and intratumoral T-cell infiltrates were 27.8% (Pasanen et al., 2020). In another report, Howitt et al. showed a PD-L1 expression (>10%) of 84% and a number of 32.8 CD8+TIL per HPF in POLEmt endometrial cancer (Howitt et al., 2015).
The positive relationship between POLEmt and immunotherapy was studied in a pancancer research study (Wang et al., 2019). Patients harboring POLEmt were divided into the MSS group and MSI group, and the prognosis of the MSI group was better. However, in another phase II multicenter study where metastatic or unresectable colorectal cancer patients (n = 33) with dMMR/MSI-H or POLEmt were enrolled, salvage (≥2 line) avelumab was prescribed. Unfortunately, all patients with POLEmt (n = 3) had progressive disease in 2 months (Kim et al., 2020). Therefore, the role of POLEmt is still controversial, and more studies are needed.
POLE mutations are common in endometrial cancer and colorectal cancer, but account for only about 3% of NSCLC (Wang et al., 2019; Song et al., 2018). It was reported that POLEmt was a favorable prognostic factor in lung cancer (Liu et al., 2018). A study found a mutation rate of 2.8% (9/319) in NSCLC patients, and all were adenocarcinomas. The TMB of these patients was 12.2/Mb, higher than 7.8/Mb for the rest. None had MSI tumors. Seven patients were positive for CD8+ T cells, and five patients had a PD-L1 expression more than 25% (Song et al., 2018). No formal clinical trials studied the efficacy of immunotherapy in these patients, and only few cases were mentioned in the literature (Table 2) (Rizvi et al., 2015; Johanns et al., 2016; Gong et al., 2017; Song et al., 2018; Lee et al., 2019; Veneris et al., 2019; Zhu et al., 2020).
Moreover, lung cancer patients are prone to brain metastasis, which is notorious for unresponsiveness to chemotherapy. The efficacy of immunotherapy for brain metastasis is still controversial. In the brain metastasis subgroup of OAK research, atezolizumab outperformed docetaxel in the survival of these patients (16.0 vs. 11.9 months, p = 0.163, HR = 0.74) (Gadgeel et al., 2019). Recently, pembrolizumab was tested in a phase II trial for NSCLC with brain metastases, and 42 patients were divided into cohort 1 (PD-L1≥1%, n = 37) or 2 (PD-L1<1%, n = 5). The ORR of cohort 1 was 29.7% with four patients having CR, while there were no objective responses in cohort 2 (Goldberg et al., 2020). Another phase II trial evaluated the safety and efficacy of pembrolizumab on melanoma and NSCLC with brain metastases. All 18 patients in the NSCLC cohort had PD-L1 ≥ 1%. The ORR in this cohort was 33% (CR: n = 4, PR: n = 2), and the median survival was 7.7 months (Goldberg et al., 2016). In the RATIONALE 304 study, tislelizumab plus chemotherapy had a significantly longer median PFS than chemotherapy (9.7 vs. 7.6 m, p = 0.004). In addition, 18 NSCLC patients with brain metastases were enrolled in this study; however, it did not give exact data for the subgroup (Lu et al., 2021). Bevacizumab had encouraging efficacy against NSCLC with brain metastases. The combination of bevacizumab plus carboplatin and paclitaxel in the treatment of advanced non-squamous NSCLC was tested in phase II trial BRAIN (NCT00800202). The results showed that the ORR of intracranial lesions, median PFS, and median OS were 61.2%, 6.7 months, and 16.0 months, respectively (Besse et al., 2015). So, we chose four-drug combination therapy for this patient.
In conclusion, in this report, we described a case of a POLEmt NSCLC patient with brain metastasis who was treated with immunotherapy plus chemotherapy and bevacizumab. His brain lesions disappeared after treatment. Our report strongly supported the benefit of immune-combined therapy for advanced NSCLC patients with POLE mutation, even with brain metastasis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Z-YD and YF contributed to conception and drafted the manuscript. Z-YD reviewed the manuscript. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Besse, B., Le Moulec, S., Mazières, J., Senellart, H., Barlesi, F., Chouaid, C., et al. (2015). Bevacizumab in Patients with Nonsquamous Non-small Cell Lung Cancer and Asymptomatic, Untreated Brain Metastases (BRAIN): A Nonrandomized, Phase II Study. Clin. Cancer Res. 21, 1896–1903. doi:10.1158/1078-0432.CCR-14-2082
Domingo, E., Freeman-Mills, L., Rayner, E., Glaire, M., Briggs, S., Vermeulen, L., et al. (2016). Somatic POLE Proofreading Domain Mutation, Immune Response, and Prognosis in Colorectal Cancer: a Retrospective, Pooled Biomarker Study. Lancet Gastroenterol. Hepatol. 1, 207–216. doi:10.1016/S2468-1253(16)30014-0
Gadgeel, S. M., Lukas, R. V., Goldschmidt, J., Conkling, P., Park, K., Cortinovis, D., et al. (2019). Atezolizumab in Patients with Advanced Non-small Cell Lung Cancer and History of Asymptomatic, Treated Brain Metastases: Exploratory Analyses of the Phase III OAK Study. Lung Cancer 128, 105–112. doi:10.1016/j.lungcan.2018.12.017
Goldberg, S. B., Gettinger, S. N., Mahajan, A., Chiang, A. C., Herbst, R. S., Sznol, M., et al. (2016). Pembrolizumab for Patients with Melanoma or Non-small-cell Lung Cancer and Untreated Brain Metastases: Early Analysis of a Non-randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 17, 976–983. doi:10.1016/S1470-2045(16)30053-5
Goldberg, S. B., Schalper, K. A., Gettinger, S. N., Mahajan, A., Herbst, R. S., Chiang, A. C., et al. (2020). Pembrolizumab for Management of Patients with NSCLC and Brain Metastases: Long-Term Results and Biomarker Analysis from a Non-randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 21, 655–663. doi:10.1016/S1470-2045(20)30111-X
Gong, J., Wang, C., Lee, P. P., Chu, P., and Fakih, M. (2017). Response to PD-1 Blockade in Microsatellite Stable Metastatic Colorectal Cancer Harboring a POLE Mutation. J. Natl. Compr. Canc Netw. 15, 142–147. doi:10.6004/jnccn.2017.0016
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S. W., Carcereny Costa, E., et al. (2019). Nivolumab Plus Ipilimumab in Advanced Non-small-cell Lung Cancer. N. Engl. J. Med. 381, 2020–2031. doi:10.1056/NEJMoa1910231
Howitt, B. E., Shukla, S. A., Sholl, L. M., Ritterhouse, L. L., Watkins, J. C., Rodig, S., et al. (2015). Association of Polymerase E-Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 1, 1319–1323. doi:10.1001/jamaoncol.2015.2151
Johanns, T. M., Miller, C. A., Dorward, I. G., Tsien, C., Chang, E., Perry, A., et al. (2016). Immunogenomics of Hypermutated Glioblastoma: A Patient with Germline POLE Deficiency Treated with Checkpoint Blockade Immunotherapy. Cancer Discov. 6, 1230–1236. doi:10.1158/2159-8290.CD-16-0575
Kim, J. H., Kim, S. Y., Baek, J. Y., Cha, Y. J., Ahn, J. B., Kim, H. S., et al. (2020). A Phase II Study of Avelumab Monotherapy in Patients with Mismatch Repair-Deficient/Microsatellite Instability-High or POLE-Mutated Metastatic or Unresectable Colorectal Cancer. Cancer Res. Treat. 52, 1135–1144. doi:10.4143/crt.2020.218
Lee, E. K., Lindeman, N. I., Matulonis, U. A., and Konstantinopoulos, P. A. (2019). POLE-mutated clear Cell Cervical Cancer Associated with In-Utero Diethylstilbestrol Exposure. Gynecol. Oncol. Rep. 28, 15–17. doi:10.1016/j.gore.2019.01.012
Liu, L., Ruiz, J., O'Neill, S. S., Grant, S. C., Petty, W. J., Yang, M., et al. (2018). Favorable Outcome of Patients with Lung Adenocarcinoma Harboring POLE Mutations and Expressing High PD-L1. Mol. Cancer 17, 81. doi:10.1186/s12943-018-0832-y
Lu, S., Wang, J., Yu, Y., Yu, X., Hu, Y., Ai, X., et al. (2021). Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J. Thorac. Oncol. 16, 1512–1522. doi:10.1016/j.jtho.2021.05.005
Pasanen, A., Ahvenainen, T., Pellinen, T., Vahteristo, P., Loukovaara, M., and Bützow, R. (2020). PD-L1 Expression in Endometrial Carcinoma Cells and Intratumoral Immune Cells: Differences across Histologic and TCGA-Based Molecular Subgroups. Am. J. Surg. Pathol. 44, 174–181. doi:10.1097/PAS.0000000000001395
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-small-cell Lung Cancer. N. Engl. J. Med. 375, 1823–1833. doi:10.1056/NEJMoa1606774
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J., et al. (2015). Cancer Immunology. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-small Cell Lung Cancer. Science 348, 124–128. doi:10.1126/science.aaa1348
Rossi, G., Russo, A., Tagliamento, M., Tuzi, A., Nigro, O., Vallome, G., et al. (2020). Precision Medicine for NSCLC in the Era of Immunotherapy: New Biomarkers to Select the Most Suitable Treatment or the Most Suitable Patient. Cancers (Basel) 12, 1125. doi:10.3390/cancers12051125
Song, Z., Cheng, G., Xu, C., Wang, W., Shao, Y., and Zhang, Y. (2018). Clinicopathological Characteristics of POLE Mutation in Patients with Non-small-cell Lung Cancer. Lung Cancer 118, 57–61. doi:10.1016/j.lungcan.2018.02.004
Temko, D., Van Gool, I. C., Rayner, E., Glaire, M., Makino, S., Brown, M., et al. (2018). Somatic POLE Exonuclease Domain Mutations Are Early Events in Sporadic Endometrial and Colorectal Carcinogenesis, Determining Driver Mutational Landscape, Clonal Neoantigen burden and Immune Response. J. Pathol. 245, 283–296. doi:10.1002/path.5081
Veneris, J. T., Lee, E. K., Goebel, E. A., Nucci, M. R., Lindeman, N., Horowitz, N. S., et al. (2019). Diagnosis and Management of a Recurrent Polymerase-Epsilon (POLE)-mutated Endometrial Cancer. Gynecol. Oncol. 153, 471–478. doi:10.1016/j.ygyno.2019.03.247
Wang, F., Zhao, Q., Wang, Y.-N., Jin, Y., He, M.-M., Liu, Z.-X., et al. (2019). Evaluation of POLE and POLD1 Mutations as Biomarkers for Immunotherapy Outcomes across Multiple Cancer Types. JAMA Oncol. 5, 1504. doi:10.1001/jamaoncol.2019.2963
Yao, J., Gong, Y., Zhao, W., Han, Z., Guo, S., Liu, H., et al. (2019). Comprehensive Analysis of POLE and POLD1 Gene Variations Identifies Cancer Patients Potentially Benefit from Immunotherapy in Chinese Population. Sci. Rep. 9, 15767. doi:10.1038/s41598-019-52414-z
Keywords: POLE mutation, lung adenocarcinoma, PD-L1, brain metastases, CR
Citation: Fu Y, Zheng Y, Wang P-P, Chen Y-Y and Ding Z-Y (2022) Immunotherapy for a POLE Mutation Advanced Non-Small-Cell Lung Cancer Patient. Front. Pharmacol. 13:817265. doi: 10.3389/fphar.2022.817265
Received: 17 November 2021; Accepted: 10 February 2022;
Published: 04 March 2022.
Edited by:
Zhi Li, The First Affiliated Hospital of China Medical University, ChinaReviewed by:
Kailun Fei, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2022 Fu, Zheng, Wang, Chen and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Yu Ding, ZGluZ3poZW55dUBzY3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.