- 1Department of Pharmacy and Biotechnology (FABIT), University of Bologna, Bologna, Italy
- 2LAQV, Rede de Química e Tecnologia (REQUIMTE), University of Porto, Porto, Portugal
- 3Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal
Chemoprevention is a strategy aimed to not only reduce the risk but also delay the development or recurrence of cancer. An ideal chemopreventive agent is not dangerous and ought not to result in side effects or damage to human health. In this context, epigallocatechin-3-gallate (EGCG) is considered a suitable chemopreventive agent, but its clinical use is limited by many factors, namely, the difference in source, administration, individual metabolism, absorption, and distribution. Genetic and dietary differences greatly cause this variability, which has limited the rational use of EGCG in chemoprevention and, particularly, the definition of a safe and efficient concentration. In the present mini review, the main limitations to a complete understanding of the use of EGCG as a chemopreventive agent will be briefly illustrated. This review also indicates the introduction and trialing of lipid-based nanoparticles (NPs) as a proper strategy to deliver EGCG at a well-defined concentration for better investigation of the chemopreventive activity. Finally, some examples of cancers that might benefit from EGCG treatment in different stages of the disease are proposed.
Introduction
EGCG, the most abundant catechin in green tea, is considered a suitable chemopreventive agent based on epidemiologic (Yuan et al., 2011; Yi et al., 2019; Zhao et al., 2021) and animal model studies (Khan and Mukhtar, 2007; Yang et al., 2011; Yiannakopoulou, 2014). In vitro studies on human cancer cells have also provided a large set of data that indicate the cytotoxicity of EGCG on cancer cells (Singh et al., 2011; Gan et al., 2018; Shirakami and Shimizu, 2018; Aggarwal et al., 2020). On the other hand, many contradictory results have also been published, and doubts on its potential use in humans exist (Filippini et al., 2020; Kim et al., 2020). The complexity of unraveling the activity of EGCG against cancer cells is due to the very high number of variables, which are difficult to interpret correctly (Mereles and Hunstein, 2011). A first issue concerns the poor physicochemical stability and low bioavailability of EGCG (Lambert and Yang, 2003; Chow and Hakim, 2011; Sang et al., 2011). Oral consumption of EGCG bioavailability after oral intake can be reduced or increased by assuming various food and beverages (Kale et al., 2010; Peters et al., 2010; Naumovsky et al., 2015). Thus, EGCG, which is only partly degraded in the stomach at low pH, reaches the intestine, where the pH is neutral-alkaline, and is further degraded (Neilson et al., 2007). The quantity of EGCG that crosses the enterocytes is low: EGCG enters the enterocytes mainly by passive diffusion since no specific receptors carrying EGCG exist on the surface of enterocytes. Active outflow by multidrug resistance-associated protein 2 (MRP2) may occur, further lowering EGCG absorption (Vaidyanathan and Walle, 2001; Hong et al., 2003; Scholl et al., 2018). Once into the enterocytes, the EGCG is actively metabolized by phase II enzymes, conjugated with glucuronic acid and sulfate, or by methylation or methylated by catechol-Omethyltransferase (COMT). Glucuronidation and sulfation mainly occur in the intestine, whereas glucuronidation, sulfation, and methylation occur later in the liver (Singh et al., 2011). Some conjugates are further methylated. Genetic heterogeneity due to a polymorphism involving COMT results in a low-activity variant, which possesses a 40–75% less catalytic capacity than the enzyme coded with wild-type alleles and can introduce a high variability in EGCG activity (Wu et al., 2003; Miller et al., 2012; Lai et al., 2019). A large part of orally taken EGCG is effluxed from the enterocytes into the intestinal lumen or from the liver to the bile and excreted in the feces, and hence lost. Gut microbiota plays a critical role in the metabolism of EGCG. Microbiota can deconjugate and degrade EGCG (Zhang et al., 2013; Liu et al., 2020). In contrast, it has been found that metabolites of green tea, including EGCG, produced by gut microbes, have significant health benefits: small molecules derived from breakdown can show antioxidant and anti-inflammatory capacity, correct dysbiosis, and decrease harmful metabolites (Zhang et al., 2019; Xu et al., 2020). Then, ultimately, microbiota may concur to improve the health effects of EGCG. In addition to cancer cell cytotoxicity, indirect advantages of EGCG on cancer onset and development are also related to the metabolic (antidiabetic and contrasting obesity effects) (Hursel et al., 2009; Hara-Terawaki et al., 2017; Quezada-Fernández et al., 2019), antioxidative, and anti-inflammatory actions of EGCG (Chen et al., 2018), all these conditions being clearly associated with cancer development (Thielecke and Boschmann, 2009; Yang et al., 2016; Oz, 2017; Potenza et al., 2020).
Then, why should EGCG be proposed as a chemopreventive agent? The relative potency of all these variables to stability and bioavailability makes defining the health effects of green tea catechins and EGCG unpredictable. Several observational and interventional studies have been reviewed extensively over the last years (Clement, 2009; Fujiki et al., 2018; Almatroodi et al., 2020). The findings are conflicting, but a consistent number of studies on several human malignant neoplasia support the potential use of EGCG as a chemopreventive agent despite this variability: any improvement in EGCG stability and bioavailability is, therefore, supposed to improve the efficacy. When we turn to animal models, there is a general agreement that a clear chemopreventive effect independent of the experimental model used (chemical carcinogenesis, xenograft tumors, knock-out animals spontaneously developing cancer, etc.) occurs. A large majority of studies clearly demonstrated that EGCG, usually used as a beverage, delayed cancer onset and the metastatic process and reduced the size and number of neoplastic foci (Ju et al., 2007; Fujiki et al., 2018; Gan et al., 2018). In the context of animal models, the number of variables under evaluation and concurring to the final target decreases: animals are genetically related, frequently inbred, exposed to the same environmental conditions that are controlled from the beginning to the end of the experimental treatments, and consume the same food, which is very simple and not varied. In these conditions, EGCG shows chemopreventive efficacy (Ju et al., 2007; Yang et al., 2011).
Another conflicting point concerns “in vitro” experiments on cell lines. In this case, the EGCG concentrations used to obtain significant results, including cytotoxicity and downregulation of molecular pathways involved in cancer onset and development, are far from those measured in vivo, in blood and tissues, after EGCG oral administration (Gan et al., 2018; Almatroodi et al., 2020). This discrepancy seems to be related to the fact that EGCG dissolved in culture medium is barely taken up by cells and is unstable at pH greater than 5 (Hong et al., 2002). Furthermore, oxidation processes may occur, and they are considered misleading with respect to the interpretation of the final cytotoxic effects (Lambert and Elias, 2010; Wei et al., 2016). On the other hand, currently a large number of studies are underway on human cancer cell lines: extensive meta-analysis in the last years reported coherent results concerning the molecular targets (Singh et al., 2011; Gan et al., 2018; Shirakami and Shimizu, 2018; Aggarwal et al., 2020; Farooqi et al., 2020) and stressed another crucial point: EGCG is safe for normal cells since very high concentrations can only induce cell death (Weisburg et al., 2004; Papi et al., 2013; Tyagi et al., 2015; Luo et al., 2018; Ni et al., 2018; Xie et al., 2018). So, EGCG is preferentially taken up by cancer cells rather than by normal cells, fulfilling one of the basic characteristics of a molecule suitable for chemoprevention. Can the potentiality of EGCG as a chemopreventive agent be improved in view of future applications?
Application of Nanotechnology in Epigallocatechin-3-Gallate Delivery Systems to Cancer Cells
The use of nanotechnology, namely, the development of drug delivery systems, especially biocompatible nanoparticles (NPs), constitutes a promising approach to increase the bioavailability and stability of this natural compound (Granja et al., 2017; Cai et al., 2018; Granja et al., 2019). Different types of NPs, including lipid-based NPs, polymeric NPs, and gold NPs, among others, can be used, and for more comprehensive information, the reader can consult the following studies: Min and Kwon (2014), Granja et al. (2017), and Li et al. (2020). NPs are extremely versatile and can be easily modified at their surface to selectively target cancer cells and, therefore, selectively release ECGC, especially inside tumor cells (Li et al., 2020). The main ligands that are used include antibodies that recognize tumor cells and folic acid and analogs because of the higher expression of folic acid receptors in cancer cell lines than in healthy cells (Granja et al., 2017). Among the different types of NPs, lipid-based NPs emerge as the most promising drug delivery systems because their composition is based on lipids, which also exist in the human body, making the NPs biocompatible and biodegradable (Nature Reviews Materials, 2021). One important advantage of their use is also their production simplicity, which is based on nanoemulsion oil/water production, and their high physicochemical stability, which is demanding for their scale-up (Frias et al., 2016).
Lipid-Based Nanoparticles
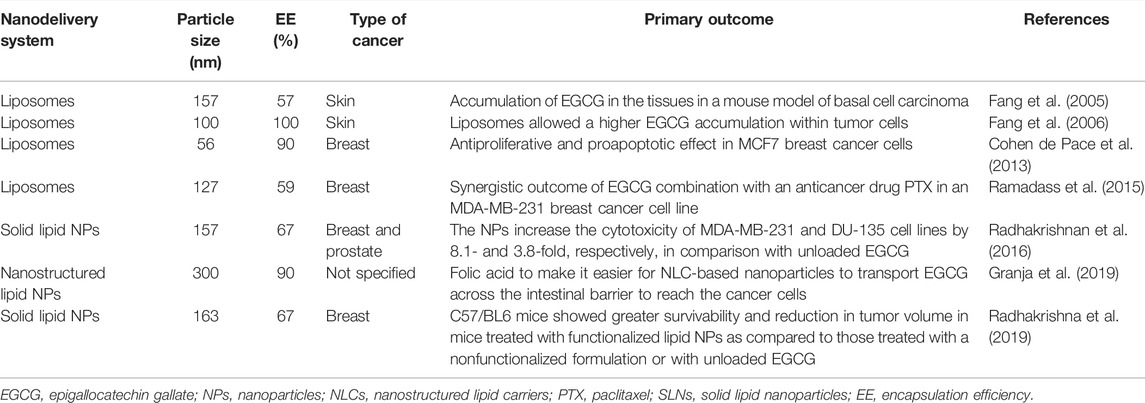
Lipid-based NPs constitute a broad and diverse group that includes liposomes and lipid NPs (Garcia-Pinel et al., 2019). Liposomes were discovered in 1965; are vesicular structures, constituted by phospholipid bilayers, enclosing an aqueous medium; and have been attracting interest as nanocarriers for many years (Bangham et al., 1965a; Bangham et al., 1965b). Thus, liposomes are already used in the clinical industry in several marketed formulations, including Doxil®, Ambisome®, and DepoDur™, among others (Bulbake et al., 2017). Solid lipid nanocarriers (SLNs) were developed in the 1990s, and next-generation nanostructured lipid carriers (NLCs) found almost 10 years later to improve the stability and capacity loading of SNLs (Naseri et al., 2015). The lipid NPs approved for clinical use were recently used in COVID-19 mRNA vaccines (Nature Reviews Materials, 2021). This mini review highlights the main contributions of EGCG lipid-based NPs developed in recent years and applied in cancer therapy and their enormous advantages and potential in clinical use. The main characteristics of lipid-based NP EGCG delivery systems in cancer therapy are summarized in Table 1. In 2005, Fang et al. developed liposomes loaded with EGCG of 157 nm diameter and with an encapsulation efficiency (EE) of 57%. The authors demonstrated that the formulation allowed the accumulation of EGCG in the tissues of Balb/c-nu, a mouse model of basal cell carcinoma. One year later, the authors developed liposomes of size 100 nm and with an EE of 100%. The authors demonstrated that in comparison with unloaded EGCG, the liposomal formulations demonstrated a 20-fold higher EGCG deposition (Fang et al., 2005; Fang et al., 2006). In 2013, Cohen de Pace et al. developed nanoliposomes loaded with EGCG of diameter 56 nm and with an EE of 90% and demonstrated that this formulation significantly enhanced EGCG stability, improved its sustained release, and increased the intracellular EGCG content in MCF7 cells, inducing apoptosis of MCF7 cells and inhibiting MCF7 cell proliferation compared to unloaded EGCG. In addition, the authors demonstrated that the developed formulation retained its antiproliferative and proapoptotic effectiveness at 10 µM or lower, at which unloaded EGCG does not have any beneficial effects (Cohen de Pace et al., 2013). In 2015, Ramadass et al. developed liposomes with EGCG of diameter 127 nm and with an EE of 59%. They demonstrated the synergistic outcome of a combination of EGCG with an anticancer drug paclitaxel (PTX) in an MDA-MB-231 breast cancer cell line and concluded on the suitability of PTX/EGCG co-loaded liposomes for the treatment of invasive breast cancer (Ramadass et al., 2015). In 2016, Radhakrishnan et al. developed EGCG solid lipid NPs of diameter 157 nm and with an EE of 67%. This formulation was tested in MDA-MB-231 and DU-135 cell lines, and an increase in cytotoxicity of 8.1- and 3.8-fold, respectively, was observed. In the same year, Frias et al. developed SLNs and NLCs loaded with EGCG of diameter 300–400 nm that demonstrated a higher EE of 80% and 90%, respectively, and a high stability during long-term storage (Radhakrishnan et al., 2016). Later, in 2019, Granja et al. developed NLCs of 300 nm diameter and with an EE of 90% and functionalized the NPs with folic acid to increase their transport across the intestinal barrier to reach the cancer cells (Granja et al., 2019). Recently, Radhakrishnan et al. developed solid lipid NPs loaded with EGCG and functionalized the NPs with a gastrin-releasing peptide receptor (GRPR)-specific peptide as GRPRs are overexpressed in breast cancer. The “in vivo” studies performed on C57/BL6 mice showed greater survivability and reduction in tumor volume in mice treated with functionalized solid lipid NPs than in mice treated with a nonfunctionalized formulation or with unloaded EGCG (Radhakrishnan et al., 2019). Table 1 summarizes examples of successful nanoformulations with EGCG. An update on this topic can be found in Yang et al. (2020), Kazi et al. (2020), and Rashidinejad et al. (2021).
Potential Applications and Key Issues for Further Research
Neoplastic disease is extremely heterogeneous, and even in the same tissue and organ, the multiplicity of cancer types having different degrees of malignancy and evolution is very high. Chemoprevention is classified as primary, secondary, or tertiary. Primary chemoprevention aims at preventing the development of premalignant lesions (often assessed by appropriate markers) and subsequent cancer in high-risk cohorts. Secondary chemoprevention prevents the evolution of premalignant markers/lesions into cancer. Finally, tertiary chemoprevention prevents the recurrence of cancer (Landis-Piwowar and Iyer, 2014).
Premalignant Lesions in Sporadic Cancer
Sporadic cancer represents the most common human cancer. In some cases, premalignant lesions precede the development of frankly malignant neoplasms with invasive growth. Here, we discuss some aspects of a few neoplasms largely found in the human population, for example, prostatic intraepithelial neoplasia (PIN) and high-grade PIN in prostatic cancer, atypical hyperplasia and lobular/ductal carcinoma in situ (LCIS, DCIS) of the breast, and colon premalignant lesions, which can be identified by screening or are detected occasionally, and some open questions that might be addressed by a chemopreventive intervention based on EGCG. In some cases, surgery is the first option, but evolution of a premalignant lesion is difficult to predict and the risk of overtreatment must be evaluated too (Curtius et al., 2017). For a critically reviewed list of premalignant diseases potentially subject to chemopreventive interventions, the reader can consult Maresso et al. (2015). Chemoprevention might be an option to be investigated in all those cases where surgery is not considered necessary or a second-line intervention after surgery to target potentially evolving situations. A successful example of a green tea catechin (GTC) extract, orally administered in patients with high-grade PIN, was reported by Naponelli and coworkers (Naponelli et al., 2017). After 1 year of treatment, only one tumor was diagnosed among 30 GTC-treated men, while nine cancers were found among 30 placebo-treated men. Despite the limitations of stability and bioavailability of GTCs, many successful trials on the use of green tea or EGCG in prostate cancer prevention have been reported and critically analyzed (Guo et al., 2017; Jacob et al., 2017; Perletti et al., 2019).
In breast atypical hyperplasia and LCIS or DCIS, tamoxifen and raloxifene have been demonstrated to reduce breast cancer risk, but they must be used in postmenopausal women, reducing the number of patients who might obtain significant risk reduction benefits without incurring serious harm (Moen and Keating, 2008; Sauter, 2018). Aromatase inhibitors, which increase osteoporosis risk, are also preferentially used in postmenopausal women, but estrogen-insensitive breast neoplasms do not respond to this kind of treatment (Trivedi et al., 2017; Thorat and Balasubramanian, 2020). Therefore, premenopausal women and women with estrogen-negative neoplasms cannot benefit from this plan of chemoprevention. Patients with premalignant lesions, especially those in premenopause, might be eligible for a chemoprevention trial based on EGCG or green tea extract. In early breast carcinoma, EGCG administration 4 weeks before the surgery resulted in a higher concentration of EGCG in the tumor than in the adjacent normal tissue, which correlated with a lower Ki-67 index with respect to untreated patients (Lazzeroni et al., 2017). Mammographic density was found to be reduced by GTE supplementation in women at high risk of breast cancer, similar to tamoxifen treatment (Samavat et al., 2017). On the basis of numerous studies indicating a protective effect of green tea catechins in breast cancer development (Yu et al., 2019), EGCG nanoformulations might have the potential to reduce the risk in patients who develop premalignant breast lesions, independent of the presence or absence of estrogen receptors and age.
Colorectal cancer (CRC) is one of the leading causes of cancer deaths in the world (Arnold et al., 2017). Although recommendations for aspirin-based chemoprevention strategies have recently been established, hazards of the long-term use of aspirin make identification of individuals for whom the protective benefits outweigh the harms important (Katona and Weiss, 2020). Some premalignant diseases develop in the context of inflammatory bowel diseases (Nadeem et al., 2020) and require surgery to alleviate symptoms, independent of possible future cancer, such as colectomy for patients suffering from severe colitis symptoms. EGCG might be suggested as a chemoprevention strategy, particularly in patients suffering from severe colitis, due to the anti-inflammatory property of EGCG (Sokolosky and Wargovich, 2012; Wang et al., 2020). The development of a suitable nanoformulation for proper delivery to the colic mucosa might be an achievable target for these patients and an opportunity to delay and reduce cancer risk.
Disease-free intervals between chemo- and radiotherapy treatments occur in many cancer patients. In these phases, patients often do not receive any support (apart from some nutrition suggestions) to achieve remission or to prevent relapse. Compounds such as EGCG that have anti-inflammatory, antioxidant, and chemopreventive properties might also offer an opportunity of treatment for all patients experiencing undesired consequences of therapies. A special mention concerns childhood neoplasms: treatments in young cancer patients may open the way to secondary neoplasms that have time to insurge. Especially for very young cancer survivors, the option of safe intervention is mandatory (Gebauer et al., 2019).
Conclusion
This mini review summarized the main contributions of lipid-based NPs for EGCG delivery in cancer prevention and therapy and demonstrated that lipid-based NPs offer a great opportunity to increase the potential of EGCG as a chemopreventive and therapeutic agent. Adequate clinical trials to establish the safety and efficacy of nanoformulations are urgently needed to validate EGCG as a crucial component in experiencing a new innovative chemoprevention and treatment strategy for cancer.
Author Contributions
FF wrote and revised the manuscript. MP wrote and revised the manuscript and prepared Table 1.
Funding
FF was a recipient of a grant (ex-RFO) from the University of Bologna, Italy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, V., Tuli, H. S., Tania, M., Srivastava, S., Ritzer, E. E., Pandey, A., et al. (2020). Molecular Mechanisms of Action of Epigallocatechin Gallate in Cancer: Recent Trends and Advancement. Semin. Cancer Biol. 24, S1044–579X. doi:10.1016/j.semcancer.2020.05.011
Almatroodi, S. A., Almatroudi, A., Khan, A. A., Alhumaydhi, F. A., Alsahli, M. A., and Rahmani, A. H. (2020). Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and its Role in the Therapy of Various Types of Cancer. Molecules 25 (14), 3146. doi:10.3390/molecules25143146
Arnold, M., Sierra, M. S., Laversanne, M., Soerjomataram, I., Jemal, A., and Bray, F. (2017). Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 66 (4), 683–691. doi:10.1136/gutjnl-2015-310912
Bangham, A. D., Standish, M. M., and Miller, N. (1965a). Cation Permeability of Phospholipid Model Membranes: Effect of Narcotics. Nature 208, 1295–1297. doi:10.1038/2081295a0
Bangham, A. D., Standish, M. M., and Watkins, J. C. (1965b). Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 13 (1), 238–252. doi:10.1016/s0022-2836(65)80093-6
Bulbake, U., Doppalapudi, S., Kommineni, N., and Khan, W. (2017). Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 9 (2), 12. doi:10.3390/pharmaceutics9020012
Cai, Z. Y., Li, X. M., Liang, J. P., Xiang, L. P., Wang, K. R., Shi, Y. L., et al. (2018). Bioavailability of Tea Catechins and its Improvement. Molecules 23 (9), 2346. doi:10.3390/molecules23092346
Chen, C. Y., Kao, C. L., and Liu, C. M. (2018). The Cancer Prevention, Anti-inflammatory and Anti-oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 19 (9), 2729. doi:10.3390/ijms19092729
Chow, H. H., and Hakim, I. A. (2011). Pharmacokinetic and Chemoprevention Studies on tea in Humans. Pharmacol. Res. 64 (2), 105–112. doi:10.1016/j.phrs.2011.05.007
Clement, Y. (2009). Can green tea Do that? A Literature Review of the Clinical Evidence. Prev. Med. 49 (2-3), 83–87. doi:10.1016/j.ypmed.2009.05.005
Curtius, K., Wright, N. A., and Graham, T. A. (2017). Evolution of Premalignant Disease. Cold Spring Harb. Perspect. Med. 7 (12), a026542. doi:10.1101/cshperspect.a026542
de Pace, R. C. C., Liu, X., Sun, M., Nie, S., Zhang, J., Cai, Q., et al. (2013). Anticancer Activities of (−)-Epigallocatechin-3-Gallate Encapsulated Nanoliposomes in MCF7 Breast Cancer Cells. J. Liposome Res. 23 (3), 187–196. doi:10.3109/08982104.2013.788023
Fang, J. Y., Hung, C. F., Hwang, T. L., and Huang, Y. L. (2005). Physicochemical Characteristics and In Vivo Deposition of Liposome-Encapsulated tea Catechins by Topical and Intratumor Administrations. J. Drug Target. 13 (1), 19–27. doi:10.1080/10611860400015977
Fang, J. Y., Lee, W. R., Shen, S. C., and Huang, Y. L. (2006). Effect of Liposome Encapsulation of tea Catechins on Their Accumulation in Basal Cell Carcinomas. J. Dermatol. Sci. 42 (2), 101–109. doi:10.1016/j.jdermsci.2005.12.010
Farooqi, A. A., Pinheiro, M., Granja, A., Farabegoli, F., Reis, S., Attar, R., et al. (2020). EGCG Mediated Targeting of Deregulated Signaling Pathways and Non-coding RNAs in Different Cancers: Focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL Mediated Signaling Pathways. Cancers (Basel) 12 (4), 951. doi:10.3390/cancers12040951
Filippini, T., Malavolti, M., Borrelli, F., Izzo, A. A., Fairweather-Tait, S. J., Horneber, M., et al. (2020). Green tea (Camellia Sinensis) for the Prevention of Cancer. Cochrane Database Syst. Rev. 3 (3), CD005004. doi:10.1002/14651858.CD005004.pub3
Frias, I., Neves, A. R., Pinheiro, M., and Reis, S. (2016). Design, Development, and Characterization of Lipid Nanocarriers-Based Epigallocatechin Gallate Delivery System for Preventive and Therapeutic Supplementation. Drug Des. Devel. Ther. 10, 3519–3528. doi:10.2147/DDDT.S109589
Fujiki, H., Watanabe, T., Sueoka, E., Rawangkan, A., and Suganuma, M. (2018). Cancer Prevention with Green Tea and its Principal Constituent, EGCG: from Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cell 41 (2), 73–82. doi:10.14348/molcells.2018.2227
Gan, R. Y., Li, H. B., Sui, Z. Q., and Corke, H. (2018). Absorption, Metabolism, Anti-cancer Effect and Molecular Targets of Epigallocatechin Gallate (EGCG): An Updated Review. Crit. Rev. Food Sci. Nutr. 58 (6), 924–941. doi:10.1080/10408398.2016.1231168
García-Pinel, B., Porras-Alcalá, C., Ortega-Rodríguez, A., Sarabia, F., Prados, J., Melguizo, C., et al. (2019). Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials (Basel) 9 (4), 638. doi:10.3390/nano9040638.9
Gebauer, J., Higham, C., Langer, T., Denzer, C., and Brabant, G. (2019). Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr. Rev. 40 (3), 711–767. doi:10.1210/er.2018-00092
Granja, A., Frias, I., Neves, A. R., Pinheiro, M., and Reis, S. (2017). Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. Biomed. Res. Int. 2017, 5813793. doi:10.1155/2017/5813793
Granja, A., Neves, A. R., Sousa, C. T., Pinheiro, M., and Reis, S. (2019). EGCG Intestinal Absorption and Oral Bioavailability Enhancement Using Folic Acid-Functionalized Nanostructured Lipid Carriers. Heliyon 5, e02020. doi:10.1016/j.heliyon.2019.e02020
Guo, Y., Zhi, F., Chen, P., Zhao, K., Xiang, H., Mao, Q., et al. (2017). Green tea and the Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 96 (13), e6426. doi:10.1097/MD.0000000000006426
Hara-Terawaki, A., Takagaki, A., Kobayashi, H., and Nanjo, F. (2017). Inhibitory Activity of Catechin Metabolites Produced by Intestinal Microbiota on Proliferation of HeLa Cells. Biol. Pharm. Bull. 40 (8), 1331–1335. doi:10.1248/bpb.b17-00127
Hong, J., Lambert, J. D., Lee, S. H., Sinko, P. J., and Yang, C. S. (2003). Involvement of Multidrug Resistance-Associated Proteins in Regulating Cellular Levels of (-)-Epigallocatechin-3-Gallate and its Methyl Metabolites. Biochem. Biophys. Res. Commun. 310, 222–227. doi:10.1016/j.bbrc.2003.09.007
Hong, J., Lu, H., Meng, X., Ryu, J. H., Hara, Y., and Yang, C. S. (2002). Stability, Cellular Uptake, Biotransformation, and Efflux of tea Polyphenol (-)-Epigallocatechin-3-Gallate in HT-29 Human colon Adenocarcinoma Cells. Cancer Res. 62 (24), 7241–7246.
Hursel, R., Viechtbauer, W., and Westerterp-Plantenga, M. S. (2009). The Effects of green tea on Weight Loss and Weight Maintenance: a Meta-Analysis. Int. J. Obes. (Lond) 33 (9), 956–961. doi:10.1038/ijo.2009.135
Jacob, S. A., Khan, T. M., and Lee, L. H. (2017). The Effect of Green Tea Consumption on Prostate Cancer Risk and Progression: A Systematic Review. Nutr. Cancer 69 (3), 353–364. doi:10.1080/01635581.2017.1285037
Ju, J., Lu, G., Lambert, J. D., and Yang, C. S. (2007). Inhibition of Carcinogenesis by tea Constituents. Semin. Cancer Biol. 17 (5), 395–402. doi:10.1016/j.semcancer.2007.06.013
Kale, A., Gawande, S., Kotwal, S., Netke, S., Roomi, W., Ivanov, V., et al. (2010). Studies on the Effects of Oral Administration of Nutrient Mixture, Quercetin and Red Onions on the Bioavailability of Epigallocatechin Gallate from green tea Extract. Phytother. Res. 24, S48–S55. doi:10.1002/ptr.2899.S48–S55
Katona, B. W., and Weiss, J. M. (2020). Chemoprevention of Colorectal Cancer. Gastroenterology 158 (2), 368–388. doi:10.1053/j.gastro.2019.06.047
Kazi, J., Sen, R., Ganguly, S., Jha, T., Ganguly, S., and Chatterjee Debnath, M. (2020). Folate Decorated Epigallocatechin-3-Gallate (EGCG) Loaded PLGA Nanoparticles; In-Vitro and In-Vivo Targeting Efficacy against MDA-MB-231 Tumor Xenograft. Int. J. Pharm. 585, 119449. doi:10.1016/j.ijpharm.2020.119449
Khan, N., and Mukhtar, H. (2007). Tea Polyphenols for Health Promotion. Life Sci. 81 (7), 519–533. doi:10.1016/j.lfs.2007.06.011
Kim, T. L., Jeong, G. H., Yang, J. W., Lee, K. H., Kronbichler, A., van der Vliet, H. J., et al. (2020). Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv. Nutr. 11 (6), 1437–1452. doi:10.1093/advances/nmaa077
Lai, C. Y., Kerr, C. L., Huang, C. C., Chen, C. C., Tsai, C. H., Tang, Y. M., et al. (2019). Genetic Polymorphism of Catechol-O-Methyltransferase Modulates the Association of green tea Consumption and Lung Cancer. Eur. J. Cancer Prev. 28, 316–322. doi:10.1097/CEJ.0000000000000464
Lambert, J. D., and Elias, R. J. (2010). The Antioxidant and Pro-oxidant Activities of green tea Polyphenols: a Role in Cancer Prevention. Arch. Biochem. Biophys. 501, 65–72. doi:10.1016/j.abb.2010.06.013
Lambert, J. D., and Yang, C. S. (2003). Cancer Chemopreventive Activity and Bioavailability of tea and tea Polyphenols. Mutat. Res. 523-524, 201–208. doi:10.1016/s0027-5107(02)00336-6
Landis-Piwowar, K. R., and Iyer, N. R. (2014). Cancer Chemoprevention: Current State of the Art. Cancer Growth Metastasis 7, 19–25. doi:10.4137/CGM.S11288
Lazzeroni, M., Guerrieri-Gonzaga, A., Gandini, S., Johansson, H., Serrano, D., Cazzaniga, M., et al. (2017). A Presurgical Study of Lecithin Formulation of Green Tea Extract in Women with Early Breast Cancer. Cancer Prev. Res. (Phila) 10 (6), 363–370. doi:10.1158/1940-6207.CAPR-16-0298
Li, K., Teng, C., and Min, Q. (2020). Advanced Nanovehicles-Enabled Delivery Systems of Epigallocatechin Gallate for Cancer Therapy. Front. Chem. 8, 573297. doi:10.3389/fchem.2020.573297
Liu, Z., de Bruijn, W. J. C., Bruins, M. E., and Vincken, J. P. (2020). Reciprocal Interactions between Epigallocatechin-3-Gallate (EGCG) and Human Gut Microbiota In Vitro. J. Agric. Food Chem. 68 (36), 9804–9815. doi:10.1021/acs.jafc.0c03587
Luo, K. W., Lung, W. Y., Chun, X, Luo, X. L., and Huang, W. R. (2018). EGCG Inhibited Bladder Cancer T24 and 5637 Cell Proliferation and Migration via PI3K/AKT Pathway. Oncotarget 9 (15), 12261–12272. doi:10.18632/oncotarget.24301
Maresso, K. C., Tsai, K. Y., Brown, P. H., Szabo, E., Lippman, S., and Hawk, E. T. (2015). Molecular Cancer Prevention: Current Status and Future Directions. CA Cancer J. Clin. 65 (5), 345–383. doi:10.3322/caac.21287
Mereles, D., and Hunstein, W. (2011). Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls Than Promises? Int. J. Mol. Sci. 12 (9), 5592–5603. doi:10.3390/ijms12095592
Miller, R. J., Jackson, K. G., Dadd, T., Nicol, B., Dick, J. L., Mayes, A. E., et al. (2012). A Preliminary Investigation of the Impact of Catechol-O-Methyltransferase Genotype on the Absorption and Metabolism of green tea Catechins. Eur. J. Nutr. 51 (1), 47–55. doi:10.1007/s00394-011-0189-0
Min, K. J., and Kwon, T. K. (2014). Anticancer Effects and Molecular Mechanisms of Epigallocatechin-3-Gallate. Integr. Med. Res. 3, 16–24. doi:10.1016/j.imr.2013.12.001
Moen, M. D., and Keating, G. M. (2008). Raloxifene: a Review of its Use in the Prevention of Invasive Breast Cancer. Drugs 68 (14), 2059–2083. doi:10.2165/00003495-200868140-00008
Nadeem, M. S., Kumar, V., Al-Abbasi, F. A., Kamal, M. A., and Anwar, F. (2020). Risk of Colorectal Cancer in Inflammatory Bowel Diseases. Semin. Cancer Biol. 64, 51–60. doi:10.1016/j.semcancer.2019.05.001
Naponelli, V., Ramazzina, I., Lenzi, C., Bettuzzi, S., and Rizzi, F. (2017). Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges. Antioxidants (Basel) 6 (2), 26. doi:10.3390/antiox6020026
Naseri, N., Valizadeh, H., and Zakeri-Milani, P. (2015). Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 5 (3), 305–313. doi:10.15171/apb.2015.043
Naumovski, N., Blades, B., and Roach, P. (2015). Food Inhibits the Oral Bioavailability of the Major green tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 4, 373–393. doi:10.3390/antiox4020373
Neilson, A. P., Hopf, A. S., Cooper, B. R., Pereira, M. A., Bomser, J. A., and Ferruzzi, M. G. (2007). Catechin Degradation with Concurrent Formation of Homo- and Heterocatechin Dimers during In Vitro Digestion. J. Agric. Food Chem. 55 (22), 8941–8949. doi:10.1021/jf071645m
Ni, J., Guo, X., Wang, H., Zhou, T., and Wang, X. (2018). Differences in the Effects of EGCG on Chromosomal Stability and Cell Growth between Normal and Colon Cancer Cells. Molecules 23 (4), 788. doi:10.3390/molecules23040788
Oz, H. S. (2017). Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients 9 (6), 561. doi:10.3390/nu9060561
Papi, A., Farabegoli, F., Iori, R., Orlandi, M., De Nicola, G. R., Bagatta, M., et al. (2013). Vitexin-2-O-xyloside, Raphasatin and (-)-Epigallocatechin-3-Gallate Synergistically Affect Cell Growth and Apoptosis of colon Cancer Cells. Food Chem. 138 (2-3), 1521–1530. doi:10.1016/j.foodchem.2012.11.112
Perletti, G., Magri, V., Vral, A., Stamatiou, K., and Trinchieri, A. (2019). Green tea Catechins for Chemoprevention of Prostate Cancer in Patients with Histologically-Proven HG-PIN or ASAP. Concise Review and Meta-Analysis. Arch. Ital. Urol. Androl. 91 (3), 153–156. doi:10.4081/aiua.2019.3.153
Peters, C. M., Green, R. J., Janle, E. M., and Ferruzzi, M. G. (2010). Formulation with Ascorbic Acid and Sucrose Modulates Catechin Bioavailability from green tea. Food Res. Int. 43, 95–102. doi:10.1016/j.foodres.2009.08.016
Potenza, M. A., Iacobazzi, D., Sgarra, L., and Montagnani, M. (2020). The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules 25 (13), 3061. doi:10.3390/molecules25133061
Quezada-Fernández, P., Trujillo-Quiros, J., Pascoe-González, S., Trujillo-Rangel, W. A., Cardona-Müller, D., Ramos-Becerra, C. G., et al. (2019). Effect of green tea Extract on Arterial Stiffness, Lipid Profile and sRAGE in Patients with Type 2 Diabetes Mellitus: a Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 70 (8), 977–985. doi:10.1080/09637486.2019.1589430
Radhakrishnan, R., Kulhari, H., Pooja, D., Gudem, S., Bhargava, S., Shukla, R., et al. (2016). Encapsulation of Biophenolic Phytochemical EGCG within Lipid Nanoparticles Enhances its Stability and Cytotoxicity against Cancer. Chem. Phys. Lipids 198, 51–60. doi:10.1016/j.chemphyslip.2016.05.006
Radhakrishnan, R., Pooja, D., Kulhari, H., Gudem, S., Ravuri, H. G., Bhargava, S., et al. (2019). Bombesin Conjugated Solid Lipid Nanoparticles for Improved Delivery of Epigallocatechin Gallate for Breast Cancer Treatment. Chem. Phys. Lipids 224, 104770. doi:10.1016/j.chemphyslip.2019.04.005
Ramadass, S. K., Anantharaman, N. V., Subramanian, S., Sivasubramanian, S., and Madhan, B. (2015). Paclitaxel/epigallocatechin Gallate Coloaded Liposome: a Synergistic Delivery to Control the Invasiveness of MDA-MB-231 Breast Cancer Cells. Colloids Surf. B Biointerfaces 125, 65–72. doi:10.1016/j.colsurfb.2014.11.005
Rashidinejad, A., Boostani, S., Babazadeh, A., Rehman, A., Rezaei, A., Akbari-Alavijeh, S., et al. (2021). Opportunities and Challenges for the Nanodelivery of green tea Catechins in Functional Foods. Food Res. Int. 142, 110186. doi:10.1016/j.foodres.2021.110186
Samavat, H., Ursin, G., Emory, T. H., Lee, E., Wang, R., Torkelson, C. J., et al. (2017). A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev. Res. (Phila) 10 (12), 710–718. doi:10.1158/1940-6207.CAPR-17-0187
Sang, S., Lambert, J. D., Ho, C. T., and Yang, C. S. (2011). The Chemistry and Biotransformation of tea Constituents. Pharmacol. Res. 64 (2), 87–99. doi:10.1016/j.phrs.2011.02.007
Sauter, E. R. (2018). Breast Cancer Prevention: Current Approaches and Future Directions. Eur. J. Breast Health 14 (2), 64–71. doi:10.5152/ejbh.2018.3978
Scholl, C., Lepper, A., Lehr, T., Hanke, N., Schneider, K. L., Brockmöller, J., et al. (2018). Population Nutrikinetics of green tea Extract. PLoS One 13 (2), e0193074. doi:10.1371/journal.pone.0193074
Shirakami, Y., and Shimizu, M. (2018). Possible Mechanisms of Green Tea and its Constituents against Cancer. Molecules 23 (9), 2284. doi:10.3390/molecules23092284
Singh, B. N., Shankar, S., and Srivastava, R. K. (2011). Green tea Catechin, Epigallocatechin-3-Gallate (EGCG): Mechanisms, Perspectives and Clinical Applications. Biochem. Pharmacol. 82 (12), 1807–1821. doi:10.1016/j.bcp.2011.07.093
Sokolosky, M. L., and Wargovich, M. J. (2012). Homeostatic Imbalance and colon Cancer: the Dynamic Epigenetic Interplay of Inflammation, Environmental Toxins, and Chemopreventive Plant Compounds. Front. Oncol. 2, 57. doi:10.3389/fonc.2012.00057
Thielecke, F., and Boschmann, M. (2009). The Potential Role of green tea Catechins in the Prevention of the Metabolic Syndrome - a Review. Phytochemistry 70, 11–24. doi:10.1016/j.phytochem.2008.11.011
Thorat, M. A., and Balasubramanian, R. (2020). Breast Cancer Prevention in High-Risk Women. Best Pract. Res. Clin. Obstet. Gynaecol. 65, 18–31. doi:10.1016/j.bpobgyn.2019.11.006
Trivedi, M. S., Coe, A. M., Vanegas, A., Kukafka, R., and Crew, K. D. (2017). Chemoprevention Uptake Among Women with Atypical Hyperplasia and Lobular and Ductal Carcinoma In Situ. Cancer Prev. Res. (Phila) 10 (8), 434–441. doi:10.1158/1940-6207.CAPR-17-0100
Tyagi, T., Treas, J. N., Mahalingaiah, P. K., and Singh, K. P. (2015). Potentiation of Growth Inhibition and Epigenetic Modulation by Combination of green tea Polyphenol and 5-Aza-2'-Deoxycytidine in Human Breast Cancer Cells. Breast Cancer Res. Treat. 149 (3), 655–668. doi:10.1007/s10549-015-3295-3510.1007/s10549-015-3295-5
Vaidyanathan, J. B., and Walle, T. (2001). Transport and Metabolism of the tea Flavonoid (-)-epicatechin by the Human Intestinal Cell Line Caco-2. Pharm. Res. 18, 1420–1425. doi:10.1023/a:1012200805593
Wang, S. T., Cui, W. Q., Pan, D., Jiang, M., Chang, B., and Sang, L. X. (2020). Tea Polyphenols and Their Chemopreventive and Therapeutic Effects on Colorectal Cancer. World J. Gastroenterol. 26 (6), 562–597. doi:10.3748/wjg.v26.i6.562
Wei, Y., Chen, P., Ling, T., Wang, Y., Dong, R., Zhang, C., et al. (2016). Certain (-)-Epigallocatechin-3-Gallate (EGCG) Auto-Oxidation Products (EAOPs) Retain the Cytotoxic Activities of EGCG. Food Chem. 204, 218–226. doi:10.1016/j.foodchem.2016.02.134
Weisburg, J. H., Weissman, D. B., Sedaghat, T., and Babich, H. (2004). In Vitro cytotoxicity of Epigallocatechin Gallate and tea Extracts to Cancerous and normal Cells from the Human Oral Cavity. Basic Clin. Pharmacol. Toxicol. 95 (4), 191–200. doi:10.1111/j.1742-7843.2004.pto_950407.x
Wu, A. H., Tseng, C. C., Van Den Berg, D., and Yu, M. C. (2003). Tea Intake, COMT Genotype, and Breast Cancer in Asian-American Women. Cancer Res. 63 (21), 7526–7529.
Xie, C. R., You, C. G., Zhang, N., Sheng, H. S., and Zheng, X. S. (2018). Epigallocatechin Gallate Preferentially Inhibits O6-Methylguanine DNA-Methyltransferase Expression in Glioblastoma Cells rather Than in Nontumor Glial Cells. Nutr. Cancer 70 (8), 1339–1347. doi:10.1080/01635581.2018.1539189
Xu, M., Yang, K., and Zhu, J. (2020). Monitoring the Diversity and Metabolic Shift of Gut Microbes during Green Tea Feeding in an In Vitro Human Colonic Model. Molecules 25 (21), 5101. doi:10.3390/molecules25215101
Yang, C. S., Wang, H., Li, G. X., Yang, Z., Guan, F., and Jin, H. (2011). Cancer Prevention by tea: Evidence from Laboratory Studies. Pharmacol. Res. 64 (2), 113–122. doi:10.1016/j.phrs.2011.03.001
Yang, C. S., Zhang, J., Zhang, L., Huang, J., and Wang, Y. (2016). Mechanisms of Body Weight Reduction and Metabolic Syndrome Alleviation by tea. Mol. Nutr. Food Res. 60 (1), 160–174. doi:10.1002/mnfr.201500428
Yang, Q. Q., Wei, X. L., Fang, Y. P., Gan, R. Y., Wang, M., Ge, Y. Y., et al. (2020). Nanochemoprevention with Therapeutic Benefits: An Updated Review Focused on Epigallocatechin Gallate Delivery. Crit. Rev. Food Sci. Nutr. 60 (8), 1243–1264. doi:10.1080/10408398.2019.1565490
Yi, M., Wu, X., Zhuang, W., Xia, L., Chen, Y., Zhao, R., et al. (2019). Tea Consumption and Health Outcomes: Umbrella Review of Meta-Analyses of Observational Studies in Humans. Mol. Nutr. Food Res. 63 (16), e1900389. doi:10.1002/mnfr.201900389
Yiannakopoulou, E. Ch. (2014). Effect of green tea Catechins on Breast Carcinogenesis: a Systematic Review of In-Vitro and In-Vivo Experimental Studies. Eur. J. Cancer Prev. 23 (2), 84–89. doi:10.1097/CEJ.0b013e328364f23e
Yu, S., Zhu, L., Wang, K., Yan, Y., He, J., and Ren, Y. (2019). Green tea Consumption and Risk of Breast Cancer: A Systematic Review and Updated Meta-Analysis of Case-Control Studies. Medicine (Baltimore) 98 (27), e16147. doi:10.1097/MD.0000000000016147
Yuan, J. M., Sun, C., and Butler, L. M. (2011). Tea and Cancer Prevention: Epidemiological Studies. Pharmacol. Res. 64, 123–135. doi:10.1016/j.phrs.2011.03.002
Zhang, S., Zhao, Y., Ohland, C., Jobin, C., and Sang, S. (2019). Microbiota Facilitates the Formation of the Aminated Metabolite of green tea Polyphenol (-)-Epigallocatechin-3-Gallate Which Trap Deleterious Reactive Endogenous Metabolites. Free Radic. Biol. Med. 131, 332–344. doi:10.1016/j.freeradbiomed.2018.12.023
Zhang, X., Zhu, X., Sun, Y., Hu, B., Sun, Y., Jabbar, S., et al. (2013). Fermentation In Vitro of EGCG, GCG and EGCG3"Me Isolated from Oolong tea by Human Intestinal Microbiota. Food Res. Int. 54 (2), 1589–1595. doi:10.1016/j.foodres.2013.10.005
Keywords: cancer, catechins, medicinal chemistry, nanomedicine, nutraceutics, EGCG
Citation: Farabegoli F and Pinheiro M (2022) Epigallocatechin-3-Gallate Delivery in Lipid-Based Nanoparticles: Potentiality and Perspectives for Future Applications in Cancer Chemoprevention and Therapy. Front. Pharmacol. 13:809706. doi: 10.3389/fphar.2022.809706
Received: 05 November 2021; Accepted: 15 March 2022;
Published: 14 April 2022.
Edited by:
Mukerrem Betul Yerer Aycan, Erciyes University, TurkeyReviewed by:
Song Zhu, Jiangnan University, ChinaCopyright © 2022 Farabegoli and Pinheiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fulvia Farabegoli, ZnVsdmlhLmZhcmFiZWdvbGlAdW5pYm8uaXQ=
 Fulvia Farabegoli
Fulvia Farabegoli Marina Pinheiro2,3
Marina Pinheiro2,3