- 1State Key Laboratory of Quality Research in Chinese Medicine, Macau Institute for Applied Research in Medicine and Health, Macau University of Science and Technology, Macao, China
- 2Dr. Neher’s Biophysics Laboratory for Innovative Drug Discovery, Macau University of Science and Technology, Macao, China
- 3Department of Urology, The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China
- 4Institute of Traditional Chinese Medicine Research, Tianjin University of Traditional Chinese Medicine, Zhuhai, China
Background: Coronavirus disease 2019 (COVID-2019), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a worldwide epidemic and claimed millions of lives. Accumulating evidence suggests that cytokines storms are closely associated to COVID-19 severity and death. Here, we aimed to explore the key factors related to COVID-19 severity and death, especially in terms of the male patients and those in western countries.
Methods: To clarify whether inflammatory cytokines have role in COVID-19 severity and death, we systematically searched PubMed, Embase, Cochrane library and Web of Science to identify related studies with the keywords “COVID-19″ and “cytokines”. The data were measured as the mean with 95% confidence interval (CI) by Review Manager 5.3 software. The risk of bias was assessed for each study using appropriate checklists.
Results: We preliminarily screened 13,468 studies from the databases. A total of 77 articles with 13,468 patients were ultimately included in our study. The serum levels of cytokines such as interleukin-6 (IL-6), IL-10, interleukin-2 receptor (IL-2R), tumor necrosis factor (TNF)-α, IL-1β, IL-4, IL-8 and IL-17 were higher in the severity or death group. Notably, we also found that the circulating levels of IL-6, IL-10, IL-2R and TNF-α were significantly different between males and females. The serum levels of IL-6, IL-10, IL-2R and TNF-α were much higher in males than in females, which implies that the increased mortality and severity in males was partly due to the higher level of these cytokines. Moreover, we found that in the severe and non-survivor groups, European patients had elevated levels of IL-6 compared with Asian patients.
Conclusion: These large-scale data demonstrated that the circulating levels of IL-6, IL-10, IL-2R, IL-1β, IL-4, IL-8 and IL-17 are potential risk factors for severity and high mortality in COVID-19. Simultaneously, the upregulation of these cytokines may be driving factors for the sex and region predisposition.
Introduction
Coronavirus disease 2019 (COVID-2019), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has raised major public health crises since 2019. Though many patients with COVID-19 present no symptoms or only mild symptoms (including fever, cough, and fatigue), some suffer severe symptoms and may progress to pneumonia, acute respiratory distress syndrome (ARDS), multi organ dysfunction and even death. The severity of COVID-19 is known to be closely correlated to cytokines storms, when the immune system is unable to counteract the virus, cytokine storms in patients may lead to macrophage hyperactivity and further systemic abnormal reactions (Lang et al., 2020; Liang et al., 2020; Makaronidis et al., 2020). However, the characteristics of the cytokine storms in COVID-19 patients have not been fully illustrated.
In death cases, patients with COVID-19 shows a higher risk of mortality in males sex (Griffith et al., 2020). According to the largest sex-disaggregated data from 47 countries, men with COVID-19 have higher morbidity than women with COVID-19 (63.8% men; 36.2% women). In addition, the overall mortality of COVID-19 is more than 2.3 times higher in men than in women (Control and Response, 2020). The discrepancy in COVID-19 outcomes between male and female patients may be attributed to several biological and social factors, especially cytokine storms (Griffith et al., 2020). Moreover, as the Covid-19-related literatures grows increasingly, the racial and ethnic disparities showed that the death and severity rate of Asians are lower than the other population (Tirupathi et al., 2020a; Mackey et al., 2021a).
To this end, we conducted a systematic review and meta-analysis to identify the key factors associated to COVID-19 severity and death, especially in terms of the sex and race bias detected in severe COVID-19 patients.
Methods
Search Strategy
We screened databases (Web of Science, Embase, the Cochrane Library, and PubMed) from December 2019 to June 2021. We also registered on the INPLASY (International Platform of Registered Systematic Review and Meta analysis Protocols platform). The number for our study is INPLASY2021120050. To search for more articles, we also screened related reference lists from relevant studies. The search terms included (“2019 novel coronavirus disease”) OR (“COVID19”) OR (“COVID-19 pandemic”) OR (“SARS-CoV-2 infection”) OR (“COVID-19 virus disease”) OR (“2019 novel coronavirus infection”) OR (“2019-nCoV infection”) OR (“coronavirus disease 2019”) OR (“coronavirus disease-19”) OR (”2019-nCoV disease”) OR (“COVID-19 virus infection”) OR (“cytokines”).
Inclusion and Exclusion Criteria
All the included studies met the following criteria: 1) the types of studies considered for inclusion were prospective or retrospective cohort studies comparing mild groups and severe groups; 2) the circulating levels of cytokines were analyzed before treatment. The exclusion criteria were reviews, studies of interventions other than cytokines, in vitro studies and in vivo animal experiments. To further reduce the accidental error of our study, each analysis of cytokines should contain more than two studies. Only English studies were screened in our study. After screening and collecting the literature, two authors removed duplicate publications by Endnote and independently evaluated each study based on their title and abstract. The symptom criteria are listed as follows.
For the mild group, patients had respiratory symptoms (fever, cough, fatigue, anorexia, headache), without evidence of viral pneumonia or hypoxia.
For the severe group, patients had one or more of the following conditions: respiratory distress, respiratory rate ≥30 times/minute, oxygen saturation (SpO2) ≤93% at rest, arterial partial pressure of oxygen (PaO2)/Fraction of inspiration O2 (FiO2) in arterial blood ≤300 mmHg, >50% lung imaging progress in the short term within 24–48 h, respiratory failure and mechanical ventilation required, shock, combined with other organ failure, and transfer to the intensive care unit (8).
Data Extraction and Quality Assessment
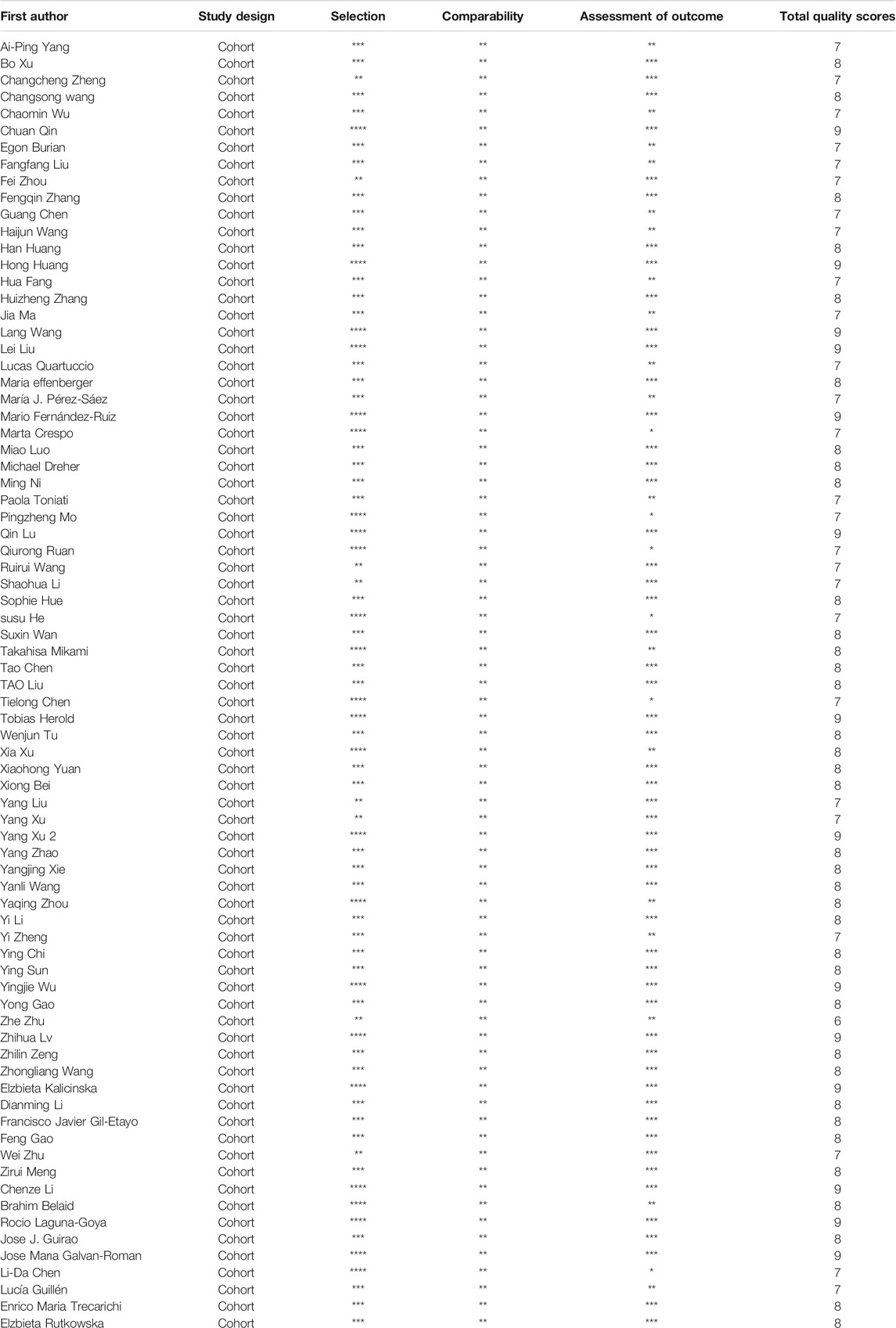
Two authors (Hu & Pan) collected data from the included studies, including the first author, study country, inclusion time, age, sex, sample sizes, mild group/severe group, survivors/non-survivors, study design, and outcomes. Another two authors assessed the quality of the studies using the Newcastle-Ottawa Scale (NOS) and scored points for each included study independently.
Statistical Analysis
Review Manager 5.3 was used to perform all statistical analyses. The mean and standard deviation (SD) were used as measurements across articles. We calculated the sample mean and SD by the sample size and interquartile range (IQR) (Wan et al., 2014; Luo et al., 2018). The circulating levels of cytokines between different groups were collected from the selected articles and analyzed using a random-effects model when I2>50%. The standard Cochran’s Q test and I2 statistics were also used to identify heterogeneity from the included articles. Significant heterogeneity was determined when I2 value > 50% and p-value <0.05.
Results
Large Scale Data From Clinical Reports
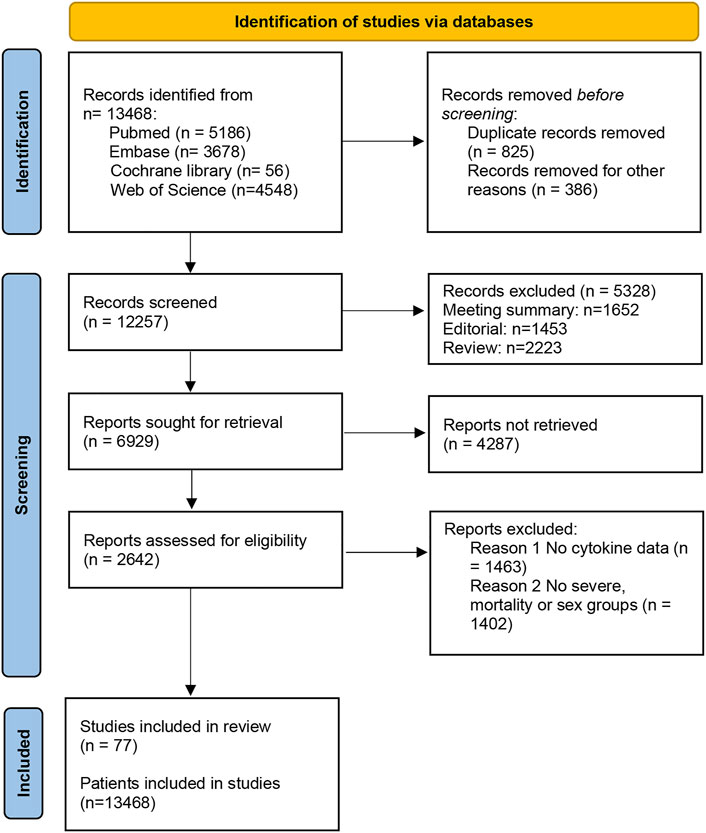
A total of 13,468 studies were screened out by the database search. After removing 826 duplicates, we excluded 8452 articles by reading the titles and abstracts of the studies. Then, we read the remaining literature and excluded studies that were not matched to the inclusion and exclusion criteria. There were 77 articles with 13,986 patients ultimately included in this study (Han et al., 2020; Yang et al., 2020; Rutkowska et al., 2021) (Figure 1). The baseline features of all included studies are presented in Table 1.
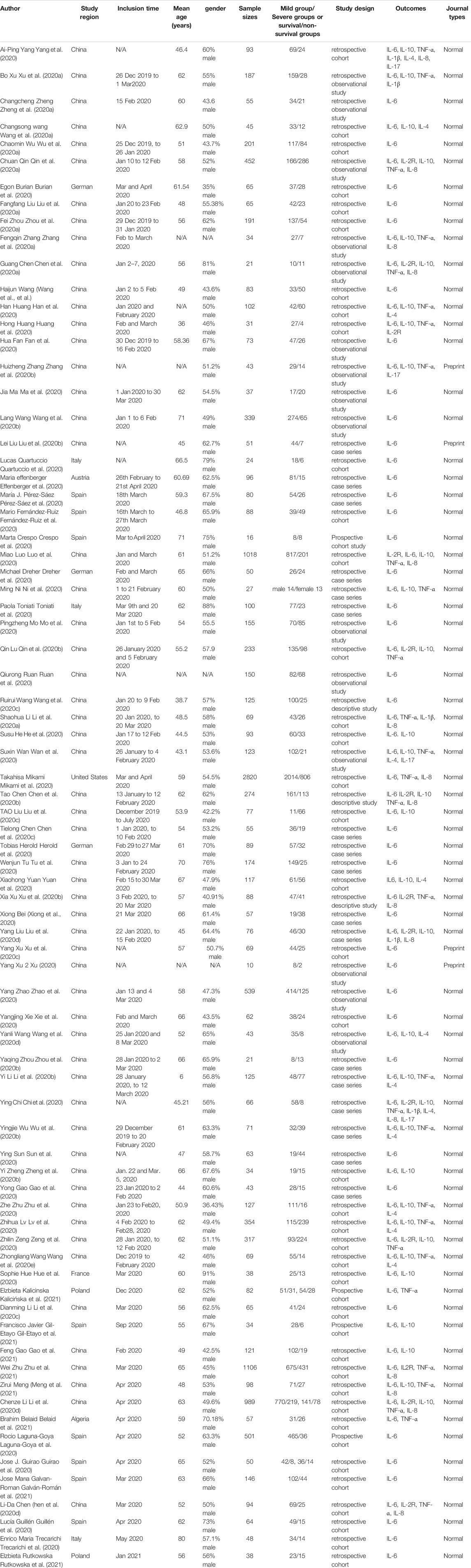
Studies were published between December 2019 and June 2021. Among the 77 studies, 57 studies were performed in China, eight in Spain, three in Germany, three in Italy, two in Poland, and one each in Austria, the USA, France and Algeria. Seventy-three studies were published in normal journals, and four were published in preprint journals.14 cytokines were reported in these 77 studies, including IL-1β, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-8, IL10, IL-15, IL-17, TNF-α, IFN-γ, MCP-1, and CXCL-10. Review Manager 5.3 was used to calculate and compare the sample mean and SD by the sample size and interquartile range. After removing the cytokines that having no statistical difference in either severe or death group, the cytokines that only contain two articles were also removed. Totally eight cytokines were included in our meta-analysis, containing IL-1β, IL-2R, IL-4, IL-6, IL-8, IL-10, IL-17, and TNF- α. Furthermore, we also screened the cytokines associated with gender or regions of COVID-19 patients. IL-2R, IL-6, IL-10 and TNF-α, which were correlated with gender or regions of COVID-19 patients, were finally presented in this study. All the included studies detected the serum levels of IL-6, while 13 studies focused on IL-2R, 31 studies analyzed IL-10 and 29 studies were related to the serum levels of TNF-α. Five studies analyzed IL-1β, 12 studies analyzed IL-4, 11 studies analyzed IL-8 and IL-17 was studies by four studies. Moreover, five studies analyzed the correlation between genders and cytokines. Fifty-seven and twenty-four studies analyzed the serum levels of cytokines in severity and mortality groups. All 77 studies had NOS quality scores greater than 6, indicating that all these studies have high levels of quality, as shown in Table 2.
Proinflammatory Cytokines as the Driving Factor for Severity and High Mortality in COVID-19 Patients
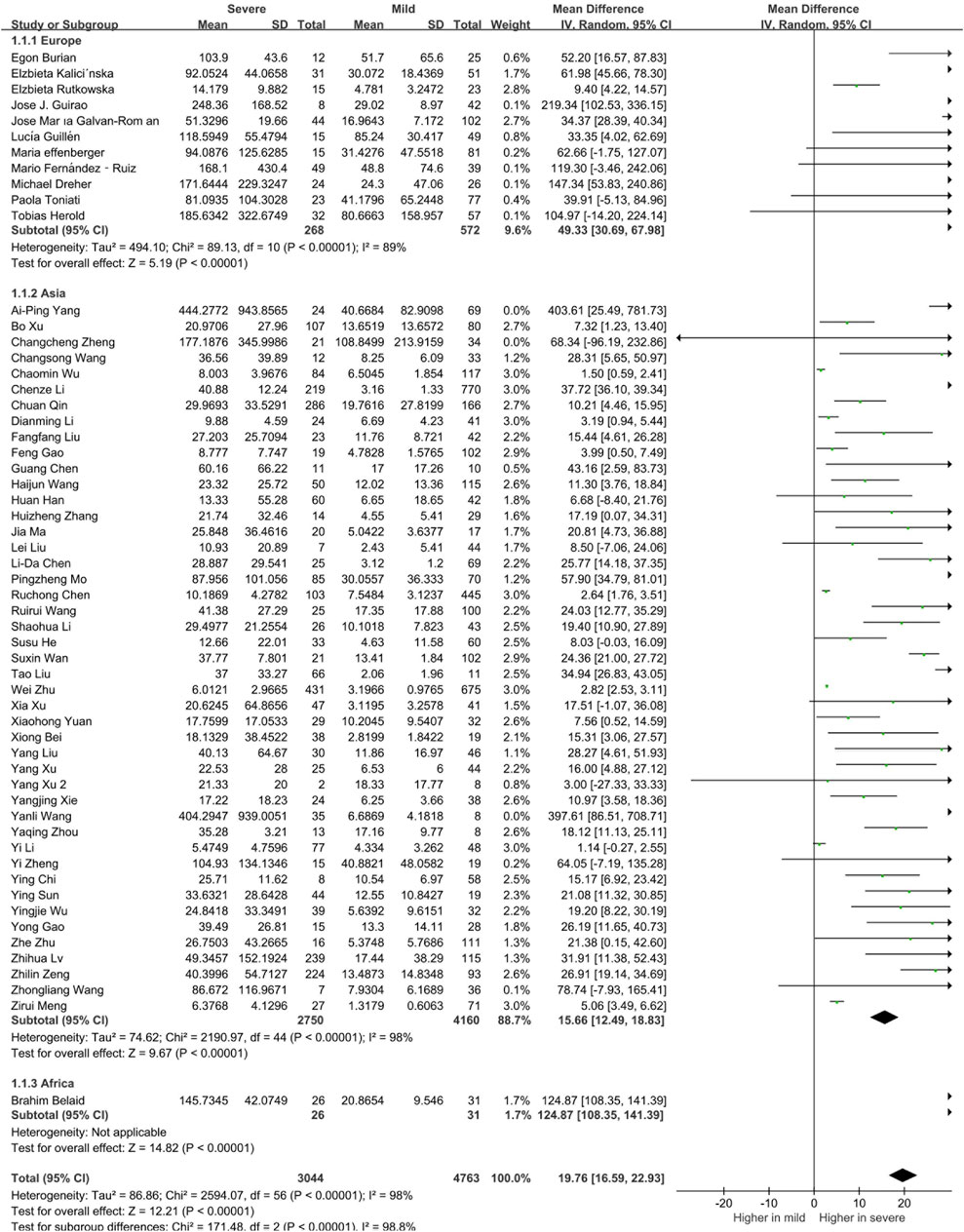
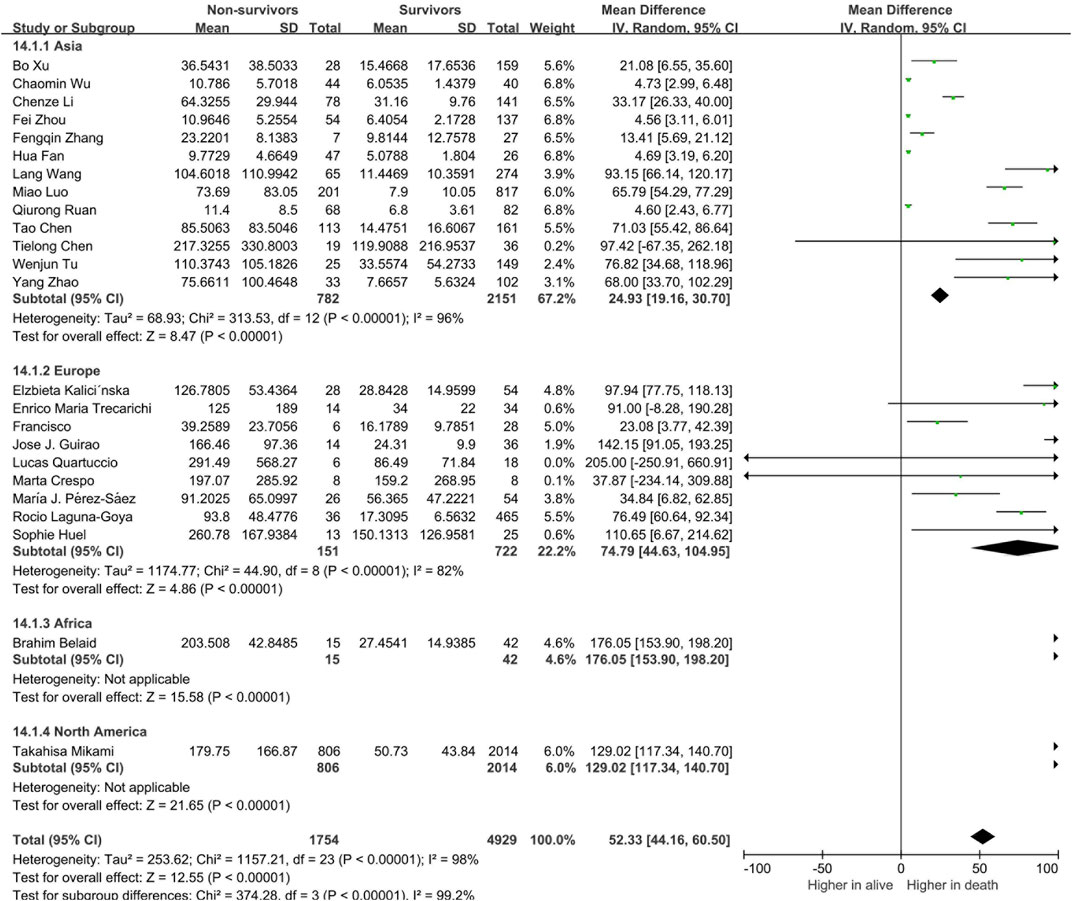
To determine whether the circulating levels of inflammatory cytokines are risk factors for severity and mortality of COVID-19 patients, we classified the patients into mild and severe groups. There were 57 studies and 7,807 patients included in this meta-analysis. Compared to patients in the mild group, circulating levels of IL-6 was found to be significantly increased in patients in the severe group (19.76 [16.59, 22.93], p < 0.00001, Supplementary Figure S1). The serum level of IL-6 in the non-surviving group was also significantly elevated compared with that in the surviving group (52.33 [44.16, 60.50], p < 0.00001, Supplementary Figure S2). In addition to IL-6, the serum levels of IL-2R, IL-10, IL-1β, IL-4, IL-8, IL-17 and TNF-α were also elevated in both severe and non-surviving COVID-19 patients (Supplementary Figures S3–S6). Suggesting that the upregulation of these cytokines were correlated with the prognosis of COVID-19 patients.
Alterations of the Distinctive Cytokines Are Related to Sex Bias in COVID-19 Patients
In this meta-analysis, four cytokines were found to be correlated with severity of male COVID-19 patients. Five studies reporting circulating interleukin-6 (IL-6) levels in male (n = 488) and female (n = 509) COVID-19 patients were included. In addition, interleukin-2 receptor (IL-2R), interleukin-10 (IL-10) and tumor necrosis factor α (TNF-α) were also different between male and female patients. Compared to female patients, the expression levels of circulating IL-6 (11.76 [7.56, 15.96], p < 0.000001), IL-2R (85.75 [3.91, 167.59], p = 0.04), IL-10 (1.54 [0.99, 2.08], p < 0.00001) and TNF-α (1.39 [0.81, 1.97], p < 0.00001) were found to be significantly elevated in male patients (Figure 2). Additionally, we conducted a sensitivity analysis to confirm the robustness of the model, and a significant sex gap was detected in circulating levels of IL-6, IL-2R, IL-10 and TNF-α.
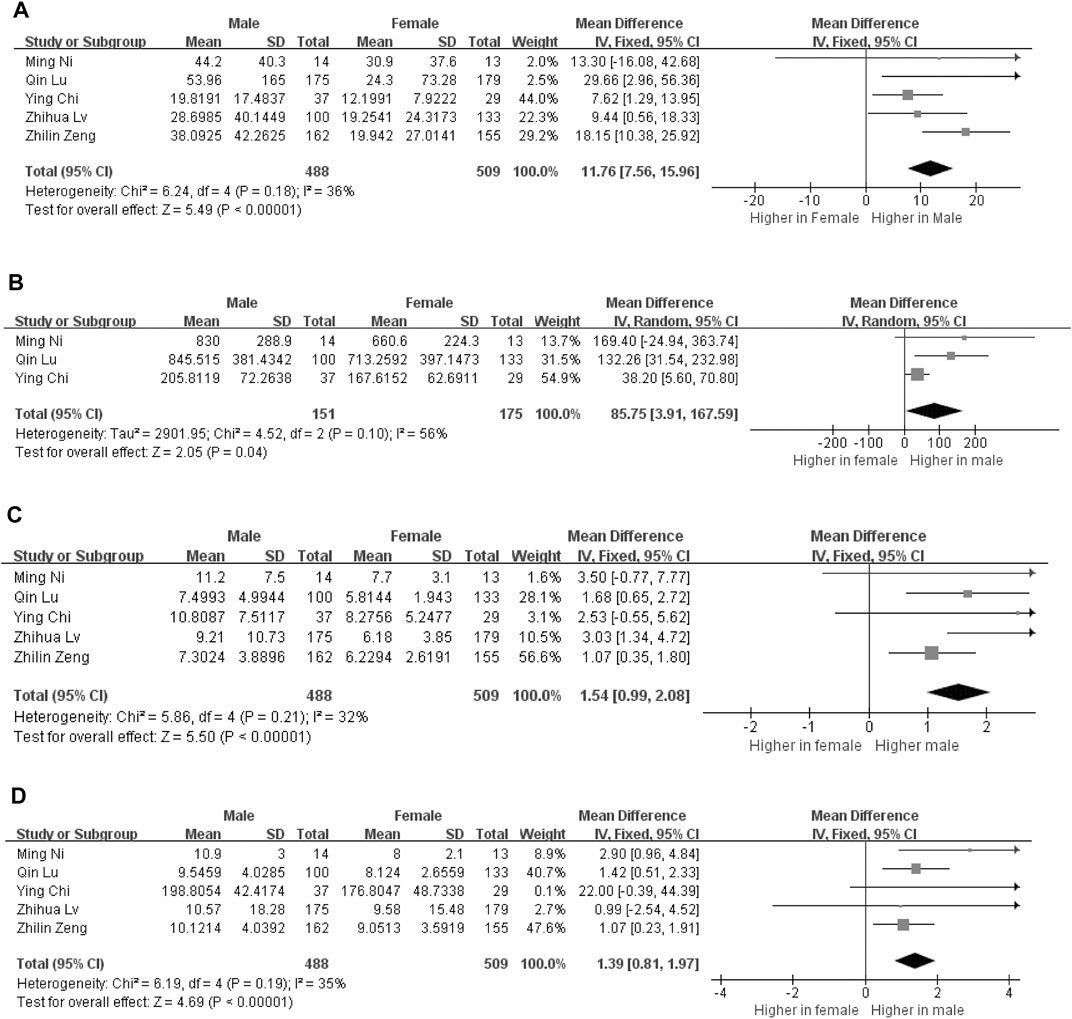
FIGURE 2. Forest plot for the male and female groups. The serum levels of IL-6 levels in the groups of male and female (A). The serum levels of IL-2R levels in the groups of male and female (B). The serum levels of IL-10 levels in the groups of male and female (C). The serum levels of TNF-α levels in the groups of male and female (D).
The Levels of IL-6 Related to Severity and High Mortality in COVID-19 Patients From Different Continents
We further analyzed the correlation between cytokines and continents. We classified the articles into Asia, Europe, Africa and North America groups, and there were 840 European patients, 6,910 Asian patients and 57 African patients in the selected studies. To better interpret the differences between countries, we compared the ages, sex distributions and the severe rate of the included patients in the two territories. Results showed that ages and the proportions of severe or dead patients were comparable, while the male patients in the severe COVID-19 patients in Europe was significantly higher than that in Asia (Supplementary Tables 1, 2). The results of our meta-analysis showed that Asian, European, and African patients with severe COVID-19 had elevated circulating IL-6 levels and the circulating IL-6 levels of European and African was higher than the Asian patients (Figure 3). Notably, we found that there were 997 Asian, 223 European, 19 African and 1007 North American in the analysis of mortality. Among them, all the death patients with COVID-19 had higher IL-6 levels than the survive patients. Moreover, Asian death patients still the have the lowest circulating IL-6 levels than the other continents’ patients (Figure 4). Unlike IL-6, the serum level of IL-10 had the potential to predict the risk of mortality in Asian patients, but it showed no correlation with mortality in European patients (Supplementary Figure S7).
Discussion
The SARS-CoV-2 S protein engages with the host ACE2 receptor and is subsequently cleaved at S1/S2 and S2′ sites by TMPRSS2 protease, which leads to activation of the S2 domain and drives fusion of the viral and host membranes. The secretion of interferon is the first step to start the antiviral program. Alveolar cells are an important part of the epithelial endothelial barrier. After respiratory epithelial cells were first infected by virus, virus infection activates pattern recognition receptors in these cells, triggering the production and release of type I and type III interferons (IFNs) and other proinflammatory mediators (such as cytokines, chemokines and antimicrobial peptides), so as to start the host’s innate and acquired immune response, which further activated the secondary cytokines (such as IL-10, IFN-γ, MCP-1, IL-4, and IL-17) and lead to cytokines storm (Vabret et al., 2020). In the mild patients, immune cells have the ability of eliminating viruses completely and inhibit the them from invading alveoli, which lead to low cytokines in serum (Figure 5). In this study, we identified that the serum levels of IL-6, IL-2R, IL-10, TNF-α, IL-1β, IL-4, IL-8 and IL-17 were significantly elevated in the severe or death cases and probably play crucial roles in the progression of COVID-19. Male sex was identified as a hazard for more severe disease and higher mortality in COVID-19 (Takahashi et al., 2020; Zeng et al., 2020). The recognition of how sex influences COVID-19 outcomes have important significance for clinical management and remission tactics. In this large-scale worldwide meta-analysis, the related cytokines affecting the development of severe disease in male patients were identified and the serum of IL-6, as well as IL-10, IL-2R and TNF-α, in males was obviously higher than that in females.
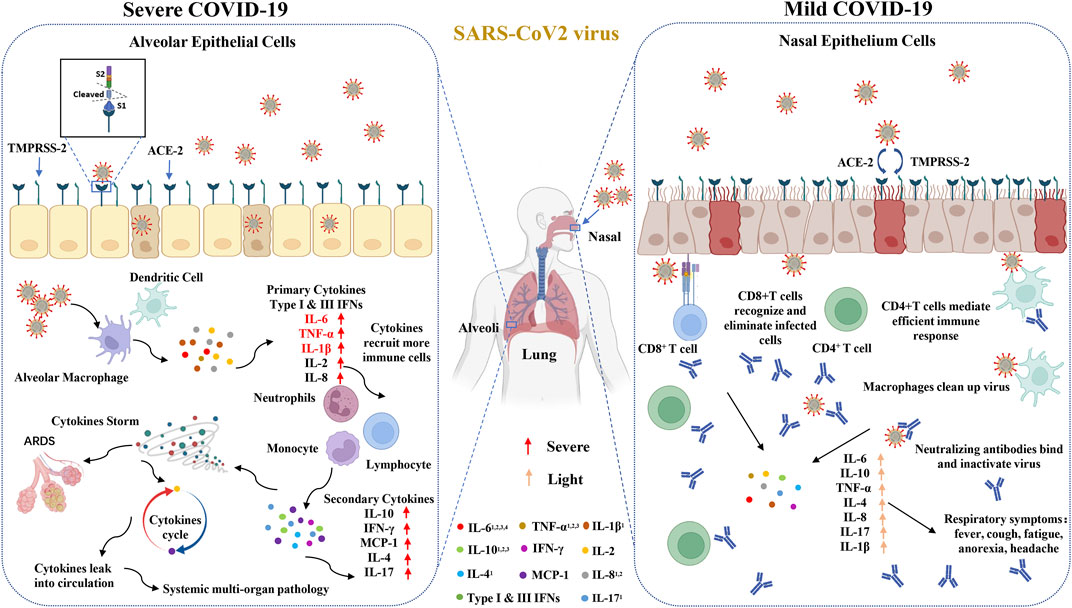
FIGURE 5. Increased circulating cytokines affect the development of COVID-19. The SARS-CoV-2 S protein engages with the host ACE2 receptor and is subsequently cleaved at S1/S2 and S2’ sites by TMPRSS2 protease. In the severe patients, COVID-19 invades the alveoli and activates innate immune responses to primary cytokines, such as type I and III IFNs, IL-6 and TNF-α, which further evokes the secondary cytokines and leads to cytokines storm. In the mild patients, immune cells have the ability of eliminating viruses and inhibiting them from invading alveoli, which leads to down-regulate cytokines in serum. 1The cytokines have significant differences between mild and severe groups. 2The cytokines between alive and death groups have significant differences. 3This cytokines have significant differences between male and female groups. 4This cytokines have significant differences between different regions.
IL-6, the core factor of “cytokine storm”, plays a pivotal role in the severity and high mortality of COVID-19. It enhances the production of TNF-α and IL-8 by stimulating the differentiation of T follicular helper cells, inhibits antiviral helper T cell 1 (Th1) cell commitment and improves the differentiation of helper T cell 2 (Th2) cells by regulating the circulating of IL-4 and interferon γ (IFN-γ) (Ahmadpoor and Rostaing, 2020; Wu and Yang, 2020). Moreover, elevated levels of IL-6 lead to acute lung injury and suppress the functions of T lymphocytes, macrophages and dendritic cells, which impair the immune system (Zhang et al., 2004). Tocilizumab, an IL-6 antagonist, revealed good capacity in inhibiting inflammation and cytokine storms in COVID-19 and various clinical studies have verified the beneficial effect of IL-6 and its receptor antagonists in treating severe and critical COVID-19 patients (Xu et al., 2020d; Potere et al., 2021). Besides IL-6, TNF-α inhibitor can also reduce lung exudation and inflammatory reactions, it has been used in the treatment of patients with covid-19 patients (Tirupathi et al., 2020b; Mackey et al., 2021b). However, blocking IL-6 and TNF-α inhibitor may not be used to all patients due to its potential adverse events and expensive price (Wang et al., 2020f; Keewan et al., 2021). The identification of which COVID-19 patients are suitable for treatment with IL-6 antagonists and TNF-α inhibitor are meaningful in the clinic. In our study, the cytokines IL-6, IL-10, and TNF-α were significantly upregulated in severe COVID-19 patients, especially in male patients, indicating that IL-6 antagonists and TNF-α inhibitors are more appropriate used in male patients to reduce both severity and mortality rate of COVID-19.
An increasing number of studies have pointed out that there are ethnicity-related differences in cytokines in systemic lupus erythematosus, chronic rhinosinusitis and other autoimmunity diseases (Niewold et al., 2012; Wang et al., 2016; Slight-Webb et al., 2020). We also focused on ethnicity-related differences in cytokines in COVID-19 patients and the results showed that there were lower circulating levels of IL-6 in Asian patients than in European and African patients, suggesting that IL-6 antagonists are recommended to use earlier in western countries.
This study had some limitations. Firstly, the articles that described the differential serum levels of cytokines in males and females were all from China. More clinical experiments should focus on the sex bias of cytokines in COVID-19. Secondly, our meta-analysis mainly investigated studies written in English, which might lead to language bias.
Conclusion
These large-scale data revealed that the serum levels of IL-6, IL-10, IL-2R, TNF-α, IL-1β, IL-4, IL-8, and IL-17 are potential risk factors for severity and high mortality in COVID-19. The IL-6 antagonist and TNF-α inhibitor are likely to be a proper therapeutic strategy to reduce mortality in males with COVID-19 and in Western countries.
Author Contributions
HH wrote the manuscript, HP conceived and designed the study, HH and HP reviewed and revised the manuscript. HH and KH searched the database and extracted the data, HH and RL carried out the Meta-analysis and made figures, HP and HZ revised and sorted out the data, LL designed and performed the final review of the manuscript, all authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Key Research and Development Project of China (2020YFA0708003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.802228/full#supplementary-material
References
Ahmadpoor, P., and Rostaing, L. (2020). Why the Immune System Fails to Mount an Adaptive Immune Response to a COVID-19 Infection. Transpl. Int. 33 (7), 824–825. doi:10.1111/tri.13611
Belaid, B., Lamara Mahammad, L., Mihi, B., Rahali, S., Djidjeli, A., Larab, Z., et al. (2021). T Cell Counts and IL-6 Concentration in Blood of North African COVID-19 Patients Are Two Independent Prognostic Factors for Severe Disease and Death. J. Leukoc. Biol.
Burian, E., Jungmann, F., Kaissis, G. A., Lohöfer, F. K., Spinner, C. D., Lahmer, T., et al. (2020). Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort. J. Clin. Med. 9 (5). doi:10.3390/jcm9051514
Chen, G., Wu, D., Guo, W., Cao, Y., Huang, D., Wang, H., et al. (2020). Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Invest. 130 (5), 2620–2629. doi:10.1172/JCI137244
Chen, L. D., Zhang, Z. Y., Wei, X. J., Cai, Y. Q., Yao, W. Z., Wang, M. H., et al. (2020). Association between Cytokine Profiles and Lung Injury in COVID-19 Pneumonia. Respir. Res. 21 (1), 201. doi:10.1186/s12931-020-01465-2
Chen, T., Dai, Z., Mo, P., Li, X., Ma, Z., Song, S., et al. (2020). Clinical Characteristics and Outcomes of Older Patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: A Single-Centered, Retrospective Study. J. Gerontol. A. Biol. Sci. Med. Sci. 75 (9), 1788–1795. doi:10.1093/gerona/glaa089
Chen, T., Wu, D., Chen, H., Yan, W., Yang, D., Chen, G., et al. (2020). Clinical Characteristics of 113 Deceased Patients with Coronavirus Disease 2019: Retrospective Study. BMJ 368, m1091. doi:10.1136/bmj.m1091
Chi, Y., Ge, Y., Wu, B., Zhang, W., Wu, T., Wen, T., et al. (2020). Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 222 (5), 746–754. doi:10.1093/infdis/jiaa363
Control, C. C. D., and Response, P. E. W. G. N. E. (2020). The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 41 (2), 145–151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003
Crespo, M., Pérez-Sáez, M. J., Redondo-Pachón, D., Llinàs-Mallol, L., Montero, M. M., Villar-García, J., et al. (2020). COVID-19 in Elderly Kidney Transplant Recipients. Am. J. Transpl. 20 (10), 2883–2889. doi:10.1111/ajt.16096
Dreher, M., Kersten, A., Bickenbach, J., Balfanz, P., Hartmann, B., Cornelissen, C., et al. (2020). The Characteristics of 50 Hospitalized COVID-19 Patients with and without ARDS. Dtsch Arztebl Int. 117 (16), 271–278. doi:10.3238/arztebl.2020.0271
Effenberger, M., Grander, C., Grabherr, F., Griesmacher, A., Ploner, T., Hartig, F., et al. (2020). Systemic Inflammation as Fuel for Acute Liver Injury in COVID-19. Digestive and Liver Disease : Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver.
Fan, H., Zhang, L., Huang, B., Zhu, M., Zhou, Y., Zhang, H., et al. (2020). Cardiac Injuries in Patients with Coronavirus Disease 2019: Not to Be Ignored. Int. J. Infect. Dis. 96, 294–297. official publication of the International Society for Infectious Diseases. doi:10.1016/j.ijid.2020.05.024
Fernández-Ruiz, M., López-Medrano, F., Pérez-Jacoiste Asín, M., Maestro de la Calle, G., Bueno, H., Caro-Teller, J., et al. (2020). Tocilizumab for the Treatment of Adult Patients with Severe COVID-19 Pneumonia: A Single-center Cohort Study. J. Med. Virol. 93 (2), 831–842. doi:10.1002/jmv.26308
Galván-Román, J., Rodríguez-García, S., Roy-Vallejo, E., Marcos-Jiménez, A., Sánchez-Alonso, S., Fernández-Díaz, C., et al. (2021). IL-6 Serum Levels Predict Severity and Response to Tocilizumab in COVID-19: An Observational Study. J. Allergy Clin. Immunol. 147 (1), 72–80. e8.
Gao, F., Zheng, K. I., Yan, H. D., Sun, Q. F., Pan, K. H., Wang, T. Y., et al. (2021). Association and Interaction between Serum Interleukin-6 Levels and Metabolic Dysfunction-Associated Fatty Liver Disease in Patients with Severe Coronavirus Disease 2019. Front. Endocrinol. (Lausanne) 12, 604100. doi:10.3389/fendo.2021.604100
Gao, Y., Li, T., Han, M., Li, X., Wu, D., Xu, Y., et al. (2020). Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID-19. J. Med. Virol. 92 (7), 791–796. doi:10.1002/jmv.25770
Gil-Etayo, F. J., Suàrez-Fernández, P., Cabrera-Marante, O., Arroyo, D., Garcinuño, S., Naranjo, L., et al. (2021). T-helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front Cel Infect Microbiol 11, 624483. doi:10.3389/fcimb.2021.624483
Griffith, D. M., Sharma, G., Holliday, C. S., Enyia, O. K., Valliere, M., Semlow, A. R., et al. (2020). Men and COVID-19: A Biopsychosocial Approach to Understanding Sex Differences in Mortality and Recommendations for Practice and Policy Interventions. Prev. Chronic Dis. 17, E63. doi:10.5888/pcd17.200247
Guillén, L., Padilla, S., Fernández, M., Agulló, V., García, J., Telenti, G., et al. (2020). Preemptive Interleukin-6 Blockade in Patients with COVID-19. Sci. Rep. 10 (1), 16826.
Guirao, J. J., Cabrera, C. M., Jiménez, N., Rincón, L., and Urra, J. M. (2020). High Serum IL-6 Values Increase the Risk of Mortality and the Severity of Pneumonia in Patients Diagnosed with COVID-19. Mol. Immunol. 128, 64–68. doi:10.1016/j.molimm.2020.10.006
Han, H., Ma, Q., Li, C., Liu, R., Zhao, L., Wang, W., et al. (2020). Profiling Serum Cytokines in COVID-19 Patients Reveals IL-6 and IL-10 Are Disease Severity Predictors. Emerg. Microbes Infect. 9 (1), 1123–1130. doi:10.1080/22221751.2020.1770129
He, S., Zhou, C., Lu, D., Yang, H., Xu, H., Wu, G., et al. (2020). Relationship between Chest CT Manifestations and Immune Response in COVID-19 Patients. Int. J. Infect. Dis. 98, 125–129. doi:10.1016/j.ijid.2020.06.059
Herold, T., Jurinovic, V., Arnreich, C., Lipworth, B. J., Hellmuth, J. C., von Bergwelt-Baildon, M., et al. (2020). Elevated Levels of IL-6 and CRP Predict the Need for Mechanical Ventilation in COVID-19. J. Allergy Clin. Immunol. 146 (1), 128–e4. e4. doi:10.1016/j.jaci.2020.05.008
Huang, H., Zhang, M., Chen, C., Zhang, H., Wei, Y., Tian, J., et al. (2020). Clinical Characteristics of COVID-19 in Patients with Preexisting ILD: A Retrospective Study in a Single center in Wuhan, China. J. Med. Virol. 92 (11), 2742–2750. doi:10.1002/jmv.26174
Hue, S., Beldi-Ferchiou, A., Bendib, I., Surenaud, M., Fourati, S., Frapard, T., et al. (2020). Uncontrolled Innate and Impaired Adaptive Immune Responses in Patients with COVID-19 Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 202 (11), 1509–1519. doi:10.1164/rccm.202005-1885OC
Kalicińska, E., Szymczak, D., Zińczuk, A., Adamik, B., Smiechowicz, J., Skalec, T., et al. (2021). Immunosuppression as a Hallmark of Critical COVID-19: Prospective Study. Cells 10 (6). doi:10.3390/cells10061293
Keewan, E., Beg, S., and Naser, S. A. (2021). Anti-TNF-α Agents Modulate SARS-CoV-2 Receptors and Increase the Risk of Infection through Notch-1 Signaling. Front. Immunol. 12, 641295. doi:10.3389/fimmu.2021.641295
Laguna-Goya, R., Utrero-Rico, A., Talayero, P., Lasa-Lazaro, M., Ramirez-Fernandez, A., Naranjo, L., et al. (2020). IL-6-based Mortality Risk Model for Hospitalized Patients with COVID-19. J. Allergy Clin. Immunol. 146 (4), 799–e9. e9. doi:10.1016/j.jaci.2020.07.009
Lang, F. M., Lee, K. M., Teijaro, J. R., Becher, B., and Hamilton, J. A. (2020). GM-CSF-based Treatments in COVID-19: Reconciling Opposing Therapeutic Approaches. Nat. Rev. Immunol. 20 (8), 507–514. doi:10.1038/s41577-020-0357-7
Li, C., Jiang, J., Wang, F., Zhou, N., Veronese, G., Moslehi, J. J., et al. (2020). Longitudinal Correlation of Biomarkers of Cardiac Injury, Inflammation, and Coagulation to Outcome in Hospitalized COVID-19 Patients. J. Mol. Cel Cardiol 147, 74–87. doi:10.1016/j.yjmcc.2020.08.008
Li, D., Liu, C., Liu, J., Hu, J., Yang, Y., and Zhou, Y. (2020). Analysis of Risk Factors for 24 Patients with COVID-19 Developing from Moderate to Severe Condition. Front. Cel Infect Microbiol 10, 548582. doi:10.3389/fcimb.2020.548582
Li, S., Jiang, L., Li, X., Lin, F., Wang, Y., Li, B., et al. (2020). Clinical and Pathological Investigation of Patients with Severe COVID-19. JCI insight 5 (12). doi:10.1172/jci.insight.138070
Li, Y., Deng, W., Xiong, H., Li, H., Chen, Z., Nie, Y., et al. (2020). Immune-related Factors Associated with Pneumonia in 127 Children with Coronavirus Disease 2019 in Wuhan. Pediatr. Pulmonol 55 (9), 2354–2360. doi:10.1002/ppul.24907
Liang, W. H., Guan, W. J., Li, C. C., Li, Y. M., Liang, H. R., Zhao, Y., et al. (2020). Clinical Characteristics and Outcomes of Hospitalised Patients with COVID-19 Treated in Hubei (Epicentre) and outside Hubei (Non-epicentre): a Nationwide Analysis of China. Eur. Respir. J. 55 (6). doi:10.1183/13993003.00562-2020
Liu, F., Ji, C., Luo, J., Wu, W., Zhang, J., Zhong, Z., et al. (2020). Clinical Characteristics and Corticosteroids Application of Different Clinical Types in Patients with corona Virus Disease 2019. Sci. Rep. 10 (1), 13689. doi:10.1038/s41598-020-70387-2
Liu, L., Gao, J-Y., Hu, W-m., Zhang, X-x., Guo, L., Liu, C-q., et al. (2020). Clinical Characteristics of 51 Patients Discharged from Hospital with COVID-19 in Chongqing, China. medRxiv 2020. 02.20.20025536.
Liu, T., Zhang, J., Yang, Y., Ma, H., Li, Z., Zhang, J., et al. (2020). The Role of Interleukin-6 in Monitoring Severe Case of Coronavirus Disease 2019. EMBO Mol. Med. 12 (7), e12421. doi:10.15252/emmm.202012421
Liu, Y., Liao, W., Wan, L., Xiang, T., and Zhang, W. (2020). Correlation between Relative Nasopharyngeal Virus RNA Load and Lymphocyte Count Disease Severity in Patients with COVID-19. Viral Immunol.
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-range, And/or Mid-quartile Range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Luo, M., Liu, J., Jiang, W., Yue, S., and Wei, S. (2020). IL-6 Combined with CD8+ T Cell Count Early Predict In-Hospital Mortality for Patients with COVID-19. JCI Insight 5 (13). doi:10.1172/jci.insight.139024
Lv, Z., Cheng, S., Le, J., Huang, J., Feng, L., Zhang, B., et al. (2020). Clinical Characteristics and Co-infections of 354 Hospitalized Patients with COVID-19 in Wuhan, China: a Retrospective Cohort Study. Microbes Infect. 22, 195–199. doi:10.1016/j.micinf.2020.05.007
Ma, J., Yin, J., Qian, Y., and Wu, Y. (2020). Clinical Characteristics and Prognosis in Cancer Patients with COVID-19: A Single center's Retrospective Study. J. Infect. 81 (2), 318–356. doi:10.1016/j.jinf.2020.04.006
Mackey, K., Ayers, C. K., Kondo, K. K., Saha, S., Advani, S. M., Young, S., et al. (2021). Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths : A Systematic Review. Ann. Intern. Med. 174 (3), 362–373. doi:10.7326/M20-6306
Mackey, K., Ayers, C. K., Kondo, K. K., Saha, S., Advani, S. M., Young, S., et al. (2021). Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths. Ann. Intern. Med. 174 (3), 362–373. doi:10.7326/m20-6306
Makaronidis, J., Mok, J., Balogun, N., Magee, C. G., Omar, R. Z., Carnemolla, A., et al. (2020). Seroprevalence of SARS-CoV-2 Antibodies in People with an Acute Loss in Their Sense of Smell And/or Taste in a Community-Based Population in London, UK: An Observational Cohort Study. Plos Med. 17 (10), e1003358. doi:10.1371/journal.pmed.1003358
Meng, Z., Wang, M., Zhao, Z., Zhou, Y., Wu, Y., Guo, S., et al. (2021). Development and Validation of a Predictive Model for Severe COVID-19: A Case-Control Study in China. Front. Med. (Lausanne) 8, 663145. doi:10.3389/fmed.2021.663145
Mikami, T., Miyashita, H., Yamada, T., Harrington, M., and Siau, E. (2020). Risk Factors for Mortality in Patients with COVID-19 in New York City. J. Gen. Intern. Med. 36 (1), 17–26. doi:10.1007/s11606-020-05983-z
Mo, P., Xing, Y., Xiao, Y., Deng, L., Zhao, Q., Wang, H., et al. (2020). Clinical Characteristics of Refractory COVID-19 Pneumonia in Wuhan, China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America.
Ni, M., Tian, F. B., Xiang, D. D., and Yu, B. (2020). Characteristics of Inflammatory Factors and Lymphocyte Subsets in Patients with Severe COVID-19. J. Med. Virol. 92 (11), 2600–2606. doi:10.1002/jmv.26070
Niewold, T. B., Kelly, J. A., Kariuki, S. N., Franek, B. S., Kumar, A. A., Kaufman, K. M., et al. (2012). IRF5 Haplotypes Demonstrate Diverse Serological Associations Which Predict Serum Interferon Alpha Activity and Explain the Majority of the Genetic Association with Systemic Lupus Erythematosus. Ann. Rheum. Dis. 71 (3), 463–468. doi:10.1136/annrheumdis-2011-200463
Pérez-Sáez, M., Blasco, M., Redondo-Pachón, D., Ventura-Aguiar, P., Bada-Bosch, T., Pérez-Flores, I., et al. (2020). Use of Tocilizumab in Kidney Transplant Recipients with COVID-19. Am. J. Transplant. : official J. Am. Soc. Transplant. Am. Soc. Transpl. Surgeons. 20 (11), 3182–3190.
Potere, N., Di Nisio, M., Cibelli, D., Scurti, R., Frattari, A., Porreca, E., et al. (2021). Interleukin-6 Receptor Blockade with Subcutaneous Tocilizumab in Severe COVID-19 Pneumonia and Hyperinflammation: a Case-Control Study. Ann. Rheum. Dis. 80 (2), 1–2. doi:10.1136/annrheumdis-2020-218243
Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., et al. (2020). Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71 (15), 762–768. doi:10.1093/cid/ciaa248
Qin, L., Li, X., Shi, J., Yu, M., Wang, K., Tao, Y., et al. (2020). Gendered Effects on Inflammation Reaction and Outcome of COVID-19 Patients in Wuhan. J. Med. Virol. 92 (11), 2684–2692. doi:10.1002/jmv.26137
Quartuccio, L., Sonaglia, A., Pecori, D., Peghin, M., Fabris, M., Tascini, C., et al. (2020). Higher Levels of IL-6 Early after Tocilizumab Distinguish Survivors from Nonsurvivors in COVID-19 Pneumonia: A Possible Indication for Deeper Targeting of IL-6. J. Med. Virol. 92 (11), 2852–2856. doi:10.1002/jmv.26149
Ruan, Q., Yang, K., Wang, W., Jiang, L., and Song, J. (2020). Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China. Intensive Care Med. 46 (5), 846–848. doi:10.1007/s00134-020-05991-x
Rutkowska, E., Kwiecień, I., Żabicka, M., Maliborski, A., Raniszewska, A., Kłos, K., et al. (2021). Cytokines and Leukocytes Subpopulations Profile in SARS-CoV-2 Patients Depending on the CT Score Severity. Viruses 13 (5). doi:10.3390/v13050880
Slight-Webb, S., Smith, M., Bylinska, A., Macwana, S., Guthridge, C., Lu, R., et al. (2020). Autoantibody-positive Healthy Individuals with Lower Lupus Risk Display a Unique Immune Endotype. J. Allergy Clin. Immunol. 146 (6), 1419–1433. doi:10.1016/j.jaci.2020.04.047
Sun, Y., Dong, Y., Wang, L., Xie, H., Li, B., Chang, C., et al. (2020). Characteristics and Prognostic Factors of Disease Severity in Patients with COVID-19: The Beijing Experience. J. Autoimmun. 112, 102473. doi:10.1016/j.jaut.2020.102473
Takahashi, T., Ellingson, M. K., Wong, P., Israelow, B., Lucas, C., Klein, J., et al. (2020). Sex Differences in Immune Responses that Underlie COVID-19 Disease Outcomes. Nature 588 (7837), 315–320. doi:10.1038/s41586-020-2700-3
Tirupathi, R., Muradova, V., Shekhar, R., Salim, S. A., Al-Tawfiq, J. A., and Palabindala, V. (2020). COVID-19 Disparity Among Racial and Ethnic Minorities in the US: A Cross Sectional Analysis. Trav. Med Infect Dis 38, 101904. doi:10.1016/j.tmaid.2020.101904
Tirupathi, R., Muradova, V., Shekhar, R., Salim, S. A., Al-Tawfiq, J. A., Palabindala, V., et al. (2020). COVID-19 Disparity Among Racial and Ethnic Minorities in the US: A Cross Sectional Analysis. Trav. Med Infect Dis 38, 101904. doi:10.1016/j.tmaid.2020.101904
Toniati, P., Piva, S., Cattalini, M., Garrafa, E., Regola, F., Castelli, F., et al. (2020). Tocilizumab for the Treatment of Severe COVID-19 Pneumonia with Hyperinflammatory Syndrome and Acute Respiratory Failure: A Single center Study of 100 Patients in Brescia, Italy. Autoimmun. Rev. 19 (7), 102568. doi:10.1016/j.autrev.2020.102568
Trecarichi, E. M., Mazzitelli, M., Serapide, F., Pelle, M. C., Tassone, B., Arrighi, E., et al. (2020). Clinical Characteristics and Predictors of Mortality Associated with COVID-19 in Elderly Patients from a Long-Term Care Facility. Sci. Rep. 10 (1), 20834. doi:10.1038/s41598-020-77641-7
Tu, W. J., Cao, J., Yu, L., Hu, X., and Liu, Q. (2020). Clinicolaboratory Study of 25 Fatal Cases of COVID-19 in Wuhan. Intensive Care Med. 46 (6), 1117–1120. doi:10.1007/s00134-020-06023-4
Vabret, N., Britton, G. J., Gruber, C., Hegde, S., Kim, J., Kuksin, M., et al. (2020). Immunology of COVID-19: Current State of the Science. Immunity 52 (6), 910–941. doi:10.1016/j.immuni.2020.05.002
Wan, S., Yi, Q., Fan, S., Lv, J., Zhang, X., Guo, L., et al. (2020). Relationships Among Lymphocyte Subsets, Cytokines, and the Pulmonary Inflammation index in Coronavirus (COVID-19) Infected Patients. Br. J. Haematol. 189 (3), 428–437. doi:10.1111/bjh.16659
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range And/or Interquartile Range. BMC Med. Res. Methodol. 14, 135. doi:10.1186/1471-2288-14-135
Wang, C., Fei, D., Li, X., Zhao, M., and Yu, K. (2020). IL-6 May Be a Good Biomarker for Earlier Detection of COVID-19 Progression. Intensive Care Med. 46 (7), 1475–1476. doi:10.1007/s00134-020-06065-8
Wang, H., Luo, S., Shen, Y., Li, M., Zhang, Z., Dong, Y., et al. Multiple Enzyme Release, Inflammation Storm and Hypercoagulability AreProminent IndicatorsFor Disease ProgressionIn COVID-19: A Multi-Centered, CorrelationStudy with CT Imaging Score. Social Science Electronic Publishing.
Wang, L., He, W., Yu, X., Hu, D., Bao, M., Liu, H., et al. (2020). Coronavirus Disease 2019 in Elderly Patients: Characteristics and Prognostic Factors Based on 4-week Follow-Up. J. Infect. 80 (6), 639–645. doi:10.1016/j.jinf.2020.03.019
Wang, R., Pan, M., Zhang, X., Han, M., Fan, X., Zhao, F., et al. (2020). Epidemiological and Clinical Features of 125 Hospitalized Patients with COVID-19 in Fuyang, Anhui, China. Int. J. Infect. Dis. 95, 421–428. official publication of the International Society for Infectious Diseases. doi:10.1016/j.ijid.2020.03.070
Wang, X., Zhang, N., Bo, M., Holtappels, G., Zheng, M., Lou, H., et al. (2016). Diversity of TH Cytokine Profiles in Patients with Chronic Rhinosinusitis: A multicenter Study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 138 (5), 1344–1353. doi:10.1016/j.jaci.2016.05.041
Wang, Y., Mao, Q., and Zhou, X. (2020). Does Tocilizumab Have a Magical Therapeutic Effect on COVID-19 Patients without Obvious Adverse Reactions. Proc. Natl. Acad. Sci. U S A. 117 (49), 30896–30897. doi:10.1073/pnas.2009961117
Wang, Y., Zhu, F., Wang, C., Wu, J., Liu, J., Chen, X., et al. (2020). Children Hospitalized with Severe COVID-19 in Wuhan. Pediatr. Infect. Dis. J. 39 (7), e91–e4. doi:10.1097/INF.0000000000002739
Wang, Z., Yang, B., Li, Q., Wen, L., and Zhang, R. (2020). Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 71 (15), 769–777. doi:10.1093/cid/ciaa272
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., et al. (2020). Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 180 (7), 934–943. doi:10.1001/jamainternmed.2020.0994
Wu, D., and Yang, X. O. (2020). TH17 Responses in Cytokine Storm of COVID-19: An Emerging Target of JAK2 Inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 53 (3), 368–370. doi:10.1016/j.jmii.2020.03.005
Wu, Y., Huang, X., Sun, J., Xie, T., Lei, Y., Muhammad, J., et al. (2020). Clinical Characteristics and Immune Injury Mechanisms in 71 Patients with COVID-19. mSphere 5 (4). doi:10.1128/mSphere.00362-20
Xie, Y., You, Q., Wu, C., Cao, S., Qu, G., Yan, X., et al. (2020). Impact of Cardiovascular Disease on Clinical Characteristics and Outcomes of Coronavirus Disease 2019 (COVID-19). Circ. J. 84 (8), 1277–1283. doi:10.1253/circj.CJ-20-0348
Xiong, B., Liu, T., Luo, P., Wei, Y., Zhou, Y., Liu, M., et al. (2020). Prominent Hypercoagulability Associated with Inflammatory State Among Cancer Patients with SARS-CoV-2 Infection. Front. Oncol. 10, 1345. doi:10.3389/fonc.2020.01345
Xu, B., Fan, C. Y., Wang, A. L., Zou, Y. L., Yu, Y. H., He, C., et al. (2020). Suppressed T Cell-Mediated Immunity in Patients with COVID-19: A Clinical Retrospective Study in Wuhan, China. J. Infect. 81 (1), e51–e60. doi:10.1016/j.jinf.2020.04.012
Xu, X., Han, M., Li, T., Sun, W., Wang, D., Fu, B., et al. (2020). Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. U S A. 117 (20), 10970–10975. doi:10.1073/pnas.2005615117
Xu, X., Yu, M. Q., Shen, Q., Wang, L. Z., Yan, R. D., Zhang, M. Y., et al. (2020). Analysis of Inflammatory Parameters and Disease Severity for 88 Hospitalized COVID-19 Patients in Wuhan, China. Int. J. Med. Sci. 17 (13), 2052–2062. doi:10.7150/ijms.47935
Xu, Y., Li, Y-r., Zeng, Q., Lu, Z-b., Li, Y-z., Wu, W., et al. (2020). Clinical Characteristics of SARS-CoV-2 Pneumonia Compared to Controls in Chinese Han Population. medRxiv 2020. 03.08.20031658.
Yang, A. P., Li, H. M., Tao, W. Q., Yang, X. J., Wang, M., Yang, W. J., et al. (2020). Infection with SARS-CoV-2 Causes Abnormal Laboratory Results of Multiple Organs in Patients. Aging (Albany NY) 12 (11), 10059–10069. doi:10.18632/aging.103255
Yuan, X., Huang, W., Ye, B., Chen, C., Huang, R., Wu, F., et al. (2020). Changes of Hematological and Immunological Parameters in COVID-19 Patients. Int. J. Hematol. 112 (4), 553–559. doi:10.1007/s12185-020-02930-w
Zeng, Z., Yu, H., Chen, H., Qi, W., Chen, L., Chen, G., et al. (2020). Longitudinal Changes of Inflammatory Parameters and Their Correlation with Disease Severity and Outcomes in Patients with COVID-19 from Wuhan, China. Crit. Care 24 (1), 525. doi:10.1186/s13054-020-03255-0
Zhang, F., Xiong, Y., Wei, Y., Hu, Y., Wang, F., Li, G., et al. (2020). Obesity Predisposes to the Risk of Higher Mortality in Young COVID-19 Patients. J. Med. Virol. 92 (11), 2536–2542. doi:10.1002/jmv.26039
Zhang, H., Wang, X., Fu, Z., Luo, M., Zhang, Z., Zhang, K., et al. (2020). Potential Factors for Prediction of Disease Severity of COVID-19 Patients. medRxiv. 03.20.20039818.
Zhang, Y., Li, J., Zhan, Y., Wu, L., Yu, X., Zhang, W., et al. (2004). Analysis of Serum Cytokines in Patients with Severe Acute Respiratory Syndrome. Infect. Immun. 72 (8), 4410–4415. doi:10.1128/IAI.72.8.4410-4415.2004
Zhao, Y., Nie, H. X., Hu, K., Wu, X. J., Zhang, Y. T., Wang, M. M., et al. (2020). Abnormal Immunity of Non-survivors with COVID-19: Predictors for Mortality. Infect. Dis. Poverty 9 (1), 108. doi:10.1186/s40249-020-00723-1
Zheng, C., Wang, J., Guo, H., Lu, Z., Ma, Y., Zhu, Y., et al. (2020). Risk-adapted Treatment Strategy for COVID-19 Patients. Int. J. Infect. Dis. 94, 74–77. official publication of the International Society for Infectious Diseases. doi:10.1016/j.ijid.2020.03.047
Zheng, Y., Sun, L. J., Xu, M., Pan, J., Zhang, Y. T., Fang, X. L., et al. (2020). Clinical Characteristics of 34 COVID-19 Patients Admitted to Intensive Care Unit in Hangzhou, China. J. Zhejiang Univ. Sci. B 21 (5), 378–387. doi:10.1631/jzus.B2000174
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., et al. (2020). Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: a Retrospective Cohort Study. Lancet 395 (10229), 1054–1062. doi:10.1016/S0140-6736(20)30566-3
Zhou, Y., Han, T., Chen, J., Hou, C., Hua, L., He, S., et al. (2020). Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin. Transl Sci. 13 (6), 1077–1086. doi:10.1111/cts.12805
Zhu, W., Zhang, H., Li, Y., Ding, Z., Liu, Z., Ruan, Y., et al. (2021). Optimizing Management to Reduce the Mortality of COVID-19: Experience from a Designated Hospital for Severely and Critically Ill Patients in China. Front. Med. (Lausanne) 8, 582764. doi:10.3389/fmed.2021.582764
Zhu, Z., Cai, T., Fan, L., Lou, K., Hua, X., Huang, Z., et al. (2020). Clinical Value of Immune-Inflammatory Parameters to Assess the Severity of Coronavirus Disease 2019. Int. J. Infect. Dis. 95, 332–339. official publication of the International Society for Infectious Diseases. doi:10.1016/j.ijid.2020.04.041
Keywords: COVID-19, cytokines, sex bias, mortality, meta-analysis
Citation: Hu H, Pan H, Li R, He K, Zhang H and Liu L (2022) Increased Circulating Cytokines Have a Role in COVID-19 Severity and Death With a More Pronounced Effect in Males: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:802228. doi: 10.3389/fphar.2022.802228
Received: 26 October 2021; Accepted: 13 January 2022;
Published: 14 February 2022.
Edited by:
Andrzej Lange, Hirszfeld Institute of Immunology and Experimental Therapy (PAN), PolandReviewed by:
Khalid Muhammad, United Arab Emirates University, United Arab EmiratesAna Cristina Simões E. Silva, Federal University of Minas Gerais, Brazil
Soraya Mezouar, Aix-Marseille university, France
Copyright © 2022 Hu, Pan, Li, He, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Liu, bGxpdUBtdXN0LmVkdS5tbw==
†These authors have contributed equally to this work
 Huating Hu
Huating Hu Hudan Pan1,2†
Hudan Pan1,2† Runze Li
Runze Li Kancheng He
Kancheng He