- Department of Medicine, The University of Hong Kong, Hong Kong SAR, China
Background: Previous retrospective cohorts showed that Rehmannia-6 (R-6, Liu-wei-di-huang-wan) formulations were associated with significant kidney function preservation and mortality reduction among chronic kidney disease patients with diabetes. This study aimed to investigate the potential mechanism of action of common R-6 variations in a clinical protocol for diabetic nephropathy (DN) from a system pharmacology approach.
Study Design and Methods: Disease-related genes were retrieved from GeneCards and OMIM by searching “Diabetic Nephropathy” and “Macroalbuminuria”. Variations of R-6 were identified from a published existing clinical practice guideline developed from expert consensus and pilot clinical service program. The chemical compound IDs of each herb were retrieved from TCM-Mesh and PubChem. Drug targets were subsequently revealed via PharmaMapper and UniProtKB. The disease gene interactions were assessed through STRING, and disease–drug protein–protein interaction network was integrated and visualized by Cytoscape. Clusters of disease–drug protein–protein interaction were constructed by Molecular Complex Detection (MCODE) extension. Functional annotation of clusters was analyzed by DAVID and KEGG pathway enrichment. Differences among variations of R-6 were compared. Binding was verified by molecular docking with AutoDock.
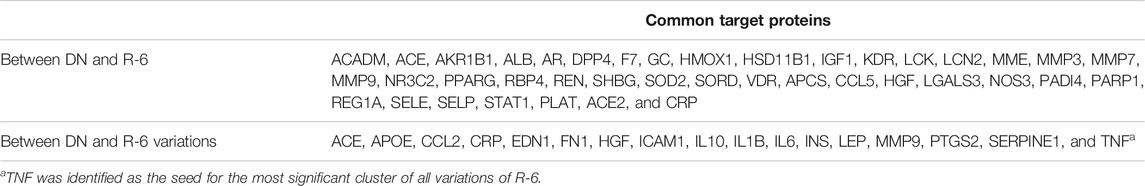
Results: Three hundred fifty-eight genes related to DN were identified, forming 11 clusters which corresponded to complement and coagulation cascades and signaling pathways of adipocytokine, TNF, HIF-1, and AMPK. Five variations of R-6 were analyzed. Common putative targets of the R-6 variations on DN included ACE, APOE, CCL2, CRP, EDN1, FN1, HGF, ICAM1, IL10, IL1B, IL6, INS, LEP, MMP9, PTGS2, SERPINE1, and TNF, which are related to regulation of nitric oxide biosynthesis, lipid storage, cellular response to lipopolysaccharide, inflammatory response, NF-kappa B transcription factor activity, smooth muscle cell proliferation, blood pressure, cellular response to interleukin-1, angiogenesis, cell proliferation, peptidyl-tyrosine phosphorylation, and protein kinase B signaling. TNF was identified as the seed for the most significant cluster of all R-6 variations. Targets specific to each formulation were identified. The key chemical compounds of R-6 have good binding ability to the putative protein targets.
Conclusion: The mechanism of action of R-6 on DN is mostly related to the TNF signaling pathway as a core mechanism, involving amelioration of angiogenesis, fibrosis, inflammation, disease susceptibility, and oxidative stress. The putative targets identified could be validated through clinical trials.
Introduction
In 2021, 10.5% of the world population were diabetic, with a rising trend (Sun et al., 2021). Of the diabetic patients, 25%–40% develop nephropathy from long-standing diabetes, characterized by glomerular basement membrane thickening, foot process effacement, mesangial expansion, glomerulosclerosis, interstitial fibrosis, and the formation of Kimmelstiel-Wilson nodules (Alsaad and Herzenberg, 2007; Alicic et al., 2017). The pathogenesis of diabetic nephropathy (DN) is multifactorial (Tang and Yiu, 2020) involving the complement cascade (Flyvbjerg, 2017; Yiu et al., 2018), metabolic (Forbes et al., 2003; Goh and Cooper, 2008), immunity (Lin et al., 2012; Pichler et al., 2017; Tang and Yiu, 2020), inflammatory, (Navarro-González et al., 2011; Pichler et al., 2017), oxidative stress (Kashihara et al., 2010; Li et al., 2014a; Sagoo and Gnudi, 2018), coagulation (Madhusudhan et al., 2016; Oe et al., 2016), and hemodynamic (Jerums et al., 2010; Premaratne et al., 2015; Alicic et al., 2017; Tonneijck et al., 2017) pathways, ending with renal fibrosis.
Diabetic kidney disease (DKD) is conventionally diagnosed by the presence of albuminuria, reduced kidney function, and clinical history excluding other etiologies (Alicic et al., 2017; Heerspink et al., 2019). Macroalbuminuria signifies established nephropathy and increases the specificity of DKD diagnosis (Furuichi et al., 2018; Persson and Rossing, 2018). The conventional renin–angiotensin–aldosterone system (RAAS) blockade offers limited effect in reducing end-stage kidney failure and mortality (Hsu et al., 2014; Palmer et al., 2015; Chan and Tang, 2018; Nistor et al., 2018). More regimens targeting other mechanisms are needed. DKD leads the cause of end-stage kidney failure in most regions among other types of chronic kidney disease (CKD). Kidney replacement therapy (dialysis or transplantation) is required once end stage is reached, and the accessibility is often limited (Thomas et al., 2016; Tang et al., 2020; Tang and Yiu, 2020).
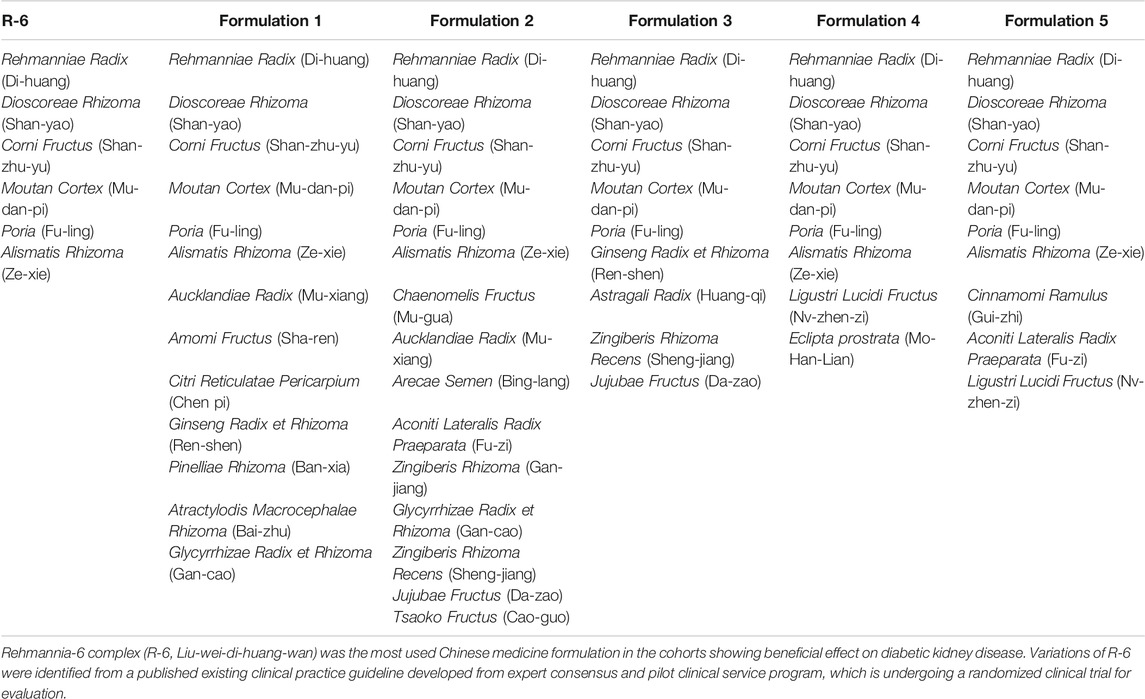
Previous registry studies showed that individualized Chinese medicine (CM) treatment was associated with slower decline of kidney function and reduced risk of end-stage kidney failure and mortality among patients with CKD and diabetes (Hsieh et al., 2014; Lin et al., 2015; Huang et al., 2018; Chan et al., 2022). CM formulations are prescribed based on symptom-based diagnosis (Chan et al., 2020a; Chan et al., 2020b; Chan et al., 2021a; Chan et al., 2021b; Chan et al., 2021c; Shu et al., 2021), which predicts renal function decline independent of blood pressure, blood glucose, lipids, and urine albumin control (Chan et al., 2021b). The CM used in these cohorts mostly contained a classical CM formulation, namely, Rehmannia-6 complex (R-6, Liu-wei-di-huang-wan) with variations according to the symptom-based diagnosis. R-6 contains Rehmanniae Radix (Di-huang) (Yokozawa et al., 2004), Corni Fructus (Shan-zhu-yu) (Gao et al., 2021), Dioscoreae Rhizoma (Shan-yao) (Luo, 2008), Poria (Fu-ling) (Sun, 2014), Moutan Cortex (Mu-dan-pi) (Wang et al., 2017b), and Alismatis Rhizoma (Ze-xie) (Zhang et al., 2017a). According to classical CM theory, R-6 replenishes the kidney and clears the waste including fluid retention.
Unlike single herbal extracts or synthesized chemical compounds, CM formulations are complex, containing multiple chemicals, and therefore exerts systemic effects through orchestrated pathways in real-world practice (Zhang et al., 2019). Traditional in vivo and in vitro studies focusing on specific mechanisms may have limitations for the assessment of interaction between CM formulations and disease with multifactorial pathophysiology (Li et al., 2014b; Zhang et al., 2019). Network pharmacology offers a whole-system approach to delineate the possible mechanisms underpinning observed clinical effect based on existing in vivo and in vitro evidence (Hopkins, 2008; Zhang et al., 2019). This systematic and systemic approach could identify the mechanisms and target proteins of each formulation for subsequent validation in clinical trials.
A protocol with five variations of R-6 was formulated based on previous expert consensus and a pilot service program and is undergoing clinical trial (Chan et al., 2016). R-6 was modified based on the phenotypes of patients according to the traditional CM theory. There are limited system pharmacology data regarding the putative mechanism of these R-6 variations on DN. This study aims to investigate the potential mechanism of the therapeutic action of common R-6 variations for DN. We retrieved the genes and potential targets of DN, the chemical compounds of CMs, and the corresponding targets of R-6 through data mining. A drug–disease network was then constructed with pathway analysis to delineate the interaction between R-6 and DN. Through comparing the pathways expected to act on by different R-6 variations, we identified the putative targets of R-6 on DN and the potential differences among different variations.
Materials and Methods
Genes and Potential Targets Related to DN
The computational workflow is summarized in Figure 1. First, disease-related genes were retrieved from GeneCards (https://www.genecards.org/) (Stelzer et al., 2016) and the Online Mendelian Inheritance in Man (OMIM) (https://www.omim.org/) (Hamosh et al., 2000). GeneCards, operated by The Weizmann Institute of Science and LifeMap Sciences, provides richly annotated disease genetics integrated from over 150 web sources. OMIM provides genotypic and phenotypic information on molecular contributions in human genetic disorders. We searched the databases with keywords “Diabetic Nephropathy” and “Macroalbuminuria” as macroalbuminuria signifies the development of nephropathy and increases the specificity of DN (Tang and Yiu, 2020) and is commonly included as a recruitment criterion of diabetic kidney disease-related clinical trials (Heerspink et al., 2019; Perkovic et al., 2019). The genetic datasets from GeneCards and OMIM were integrated for a more comprehensive analysis (Supplementary Table S1).
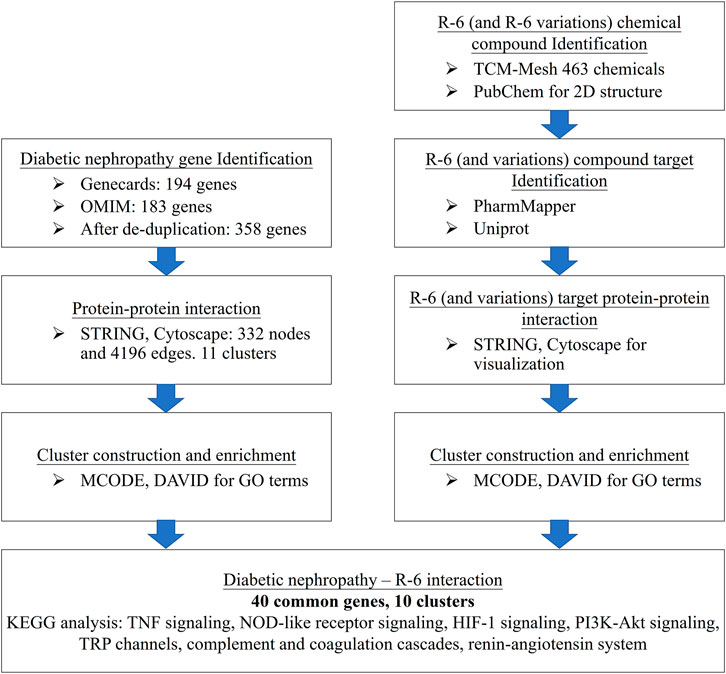
FIGURE 1. Computational workflow of disease–drug network analysis. Disease-related genes were retrieved from GeneCards and OMIM. The chemical compound IDs of each herb were retrieved from TCM-Mesh and PubChem. Drug targets were revealed via PharmaMapper and UniProtKB. The disease gene interactions were assessed through STRING, and the disease–drug protein–protein interaction network was integrated and visualized by Cytoscape. Functional annotation of clusters was analyzed by DAVID and KEGG pathway enrichment.
Compounds of R-6 and Their Potential Targets
The variations of R-6 were identified from an existing clinical practice guideline developed based on expert consensus in mainland China (Zhang, 2010; Chan, 2018), which is undergoing a randomized clinical trial for evaluation in Hong Kong (Chan et al., 2016; Chan et al., 2019). The clinical guideline is compatible with the prescription pattern of previously reported big-data studies from Taiwan (Hsieh et al., 2014; Lin et al., 2015; Huang et al., 2018; Guo et al., 2021b) and a pilot service program in Hong Kong (Chan, 2018; Chan et al., 2022).
TCM-Mesh (http://mesh.tcm.microbioinformatics.org/) (Zhang et al., 2019; Zhang et al., 2017b), with data on chemicals, genes, and disease association of more than 6,000 herbs, was used to collect the compound IDs of chemicals in each herb. A total of 463 chemicals were identified including flavonoids, phenolic acids, and alkaloids (Supplementary Table S2). Compound IDs were identified by PubChem (https://pubchem.ncbi.nlm.nih.gov/) (Kim et al., 2019), a freely accessible chemistry database maintained by the National Institutes of Health (NIH), where we acquired the 2D structures of each chemical in sdf format files. sdf format files were then imported into an open web server, PharmaMapper (http://www.lilab-ecust.cn/pharmmapper/) (Wang et al., 2017a), to identify human drug targets of chemicals. UniProtKB (https://www.uniprot.org/) (The UniProt Consortium, 2019), a central hub that integrates the functional annotations of proteins, was applied to convert the unstandardized protein naming into the official symbol by selecting “Homo sapiens”.
Disease–Drug Network Construction
Both the integrated disease and drug targets were imported into the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) (https://string-db.org/, version 11.0) (Szklarczyk et al., 2019) to assess and visualize the protein–protein interactions between disease and drug mechanisms at the molecular level to identify potential targets of R-6 on DN. The returned interaction data were expressed with a network visualization software, namely, Cytoscape (http://cytoscape.org/, version 3.7.2) (Shannon et al., 2003), to integrate the complex network of molecular interactions. To explore relevant clusters with extensive protein interactions, we used the Molecular Complex Detection (MCODE), a plug-in of Cytoscape, to identify clusters (densely interconnected regions) in the network. Each cluster represents an independent network of biological mechanisms. The disease, drug, and disease–drug protein–protein interaction networks and clusters were constructed for DN, R-6, and variations of R-6.
Gene Ontology and Pathway Enrichment Analysis
Each cluster returned by MCODE was imported into the Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/summary.jsp, version 6.8) (Huang et al., 2009), a web server which integrates the functional annotations of a large set of genes with reference to disease association, biological pathways from various databases for a more profound understanding of disease pathogenesis, and identification of potential targets for treatment. All functional annotation data were corrected by the Bonferroni method to minimize type 1 error arising from multiple comparisons. All targets of DN and R-6 variations and the top cluster of the disease–gene interaction were further analyzed and compared by the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment.
Validation by Molecular Docking
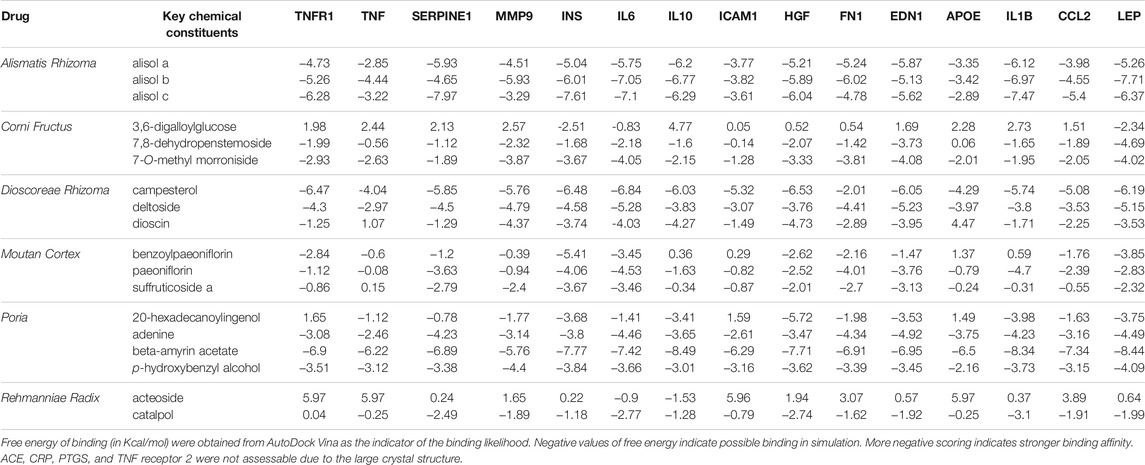
The chemical structure of key compounds of the CMs of R-6 was extracted from PubChem as mol2 files and processed in pdbqt format in AutoDockTools 1.5.6 for binding analysis. The crystal structures of the putative gene/protein targets obtained from the network and pathway analyses were extracted from the RCSB Protein Data Bank (https://www.rcsb.org/). Water molecules and inactive ligands were removed before the proteins were hydrogenated and charged (Guo et al., 2021a). A blind docking strategy was used to screen through each protein for all possible binding sites with a grid size of 126 × 126× 126 points. Free energy of binding (in kcal/mol) were obtained from AutoDock Vina as the indicator of the binding likelihood (Lee et al., 2012; Cournia et al., 2017; Heinzelmann and Gilson, 2021). Negative values of free energy indicate possible binding in simulation. More negative scoring indicates stronger binding affinity.
Results
DN
A total of 358 DN-related genes were obtained from GeneCards and OMIM (Figure 2A). The network contains 332 nodes and 4,196 edges. Eleven clusters (Figures 2B–F) were identified from the analysis by MCODE. Gene ontology analysis showed that these genes corresponded to 71 ontology terms. The top 20 most significant terms included the response to hypoxia (GO: 0001666), inflammatory response (GO: 0006954), glucose homeostasis (GO: 0042593), response to drug (GO: 0042493), positive regulation of angiogenesis (GO: 0045766), regulation of blood pressure (GO: 0008217), leukocyte migration (GO: 0050900), positive regulation of smooth muscle cell proliferation (GO: 0048661), response to activity (GO: 0014823), positive regulation of cytosolic calcium ion concentration (GO: 0007204), acute-phase response (GO: 0006953), platelet degranulation (GO: 0002576), positive regulation of gene expression (GO: 0010628), positive regulation of nitric oxide biosynthetic process (GO: 0045429), aging (GO: 0007568), response to glucocorticoid (GO: 0051384), positive regulation of transcription from RNA polymerase II promoter (GO: 0045944), cellular response to lipopolysaccharide (GO: 0071222), negative regulation of apoptotic process (GO: 0043066), and response to mechanical stimulus (GO: 0009612) (Supplementary Datasheet S3).
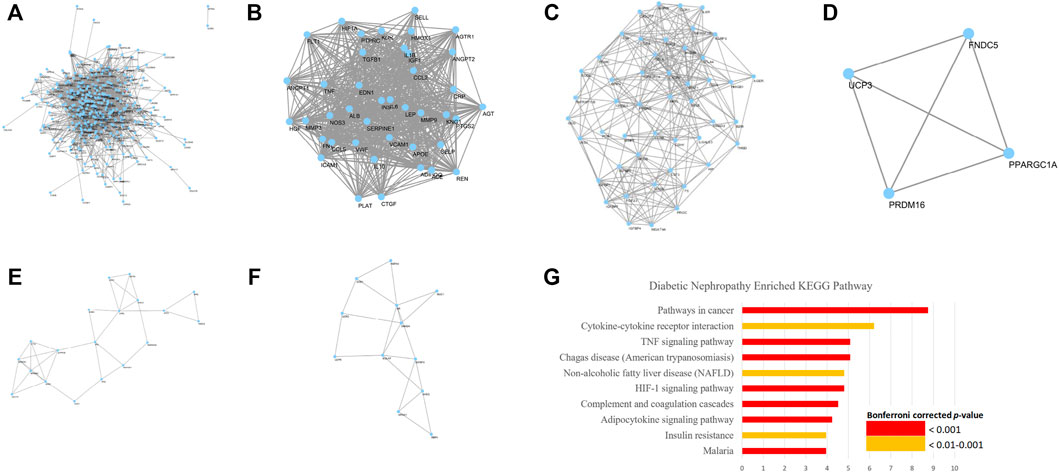
FIGURE 2. Network and representative clusters of DN. Key pathogenesis of DN includes the complement and coagulation cascades, adipocytokine signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, and AMPK signaling pathway. (A) A total of 9,621 pairs of genes were identified from GeneCards and OMIM. (B–F) Major Cluster of Genes Related to Diabetic Nephropathy and (G) KEGG enrichment analysis on the DN pathogenesis
Further KEGG pathway analysis showed that the DN-related pathways were closely related to the complement and coagulation cascades, adipocytokine signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, and AMPK signaling pathway, which share similar mechanisms with insulin resistance, rheumatoid arthritis, non-alcoholic fatty liver disease, and various infectious diseases and cancers (Figure 2G; Supplementary Datasheet S4).
Putative Targets of R-6
R-6 is a formulation containing Rehmanniae Radix, Corni Fructus, Dioscoreae Rhizoma, Poria, Moutan Cortex, and Alismatis Rhizoma (Table 1) and contains 64 identifiable chemical compounds (Supplementary Table S2) (out of the 463 chemicals from all R-6 variations). Four hundred forty-nine potential gene targets of R-6 were identified (Figure 3A). The top 20 most significant terms included steroid hormone-mediated signaling pathway (GO: 0043401), peptidyl-tyrosine autophosphorylation (GO: 0038083), transcription initiation from RNA polymerase II promoter (GO: 0006367), negative regulation of apoptotic process (GO: 0043066), proteolysis (GO: 0006508), protein autophosphorylation (GO: 0046777), extracellular matrix disassembly (GO: 0022617), peptidyl-tyrosine phosphorylation (GO: 0018108), response to hypoxia (GO: 0001666), oxidation-reduction process (GO: 0055114), response to drug (GO: 0042493), positive regulation of phosphatidylinositol 3-kinase signaling (GO: 0014068), protein phosphorylation (GO: 0006468), cellular response to insulin stimulus (GO: 0032869), glutathione metabolic process (GO: 0006749), phosphatidylinositol-mediated signaling (GO: 0048015), collagen catabolic process (GO: 0030574), response to estrogen (GO: 0043627), leukocyte migration (GO: 0050900), and purine-containing compound salvage (GO: 0043101) (Supplementary Data S5).
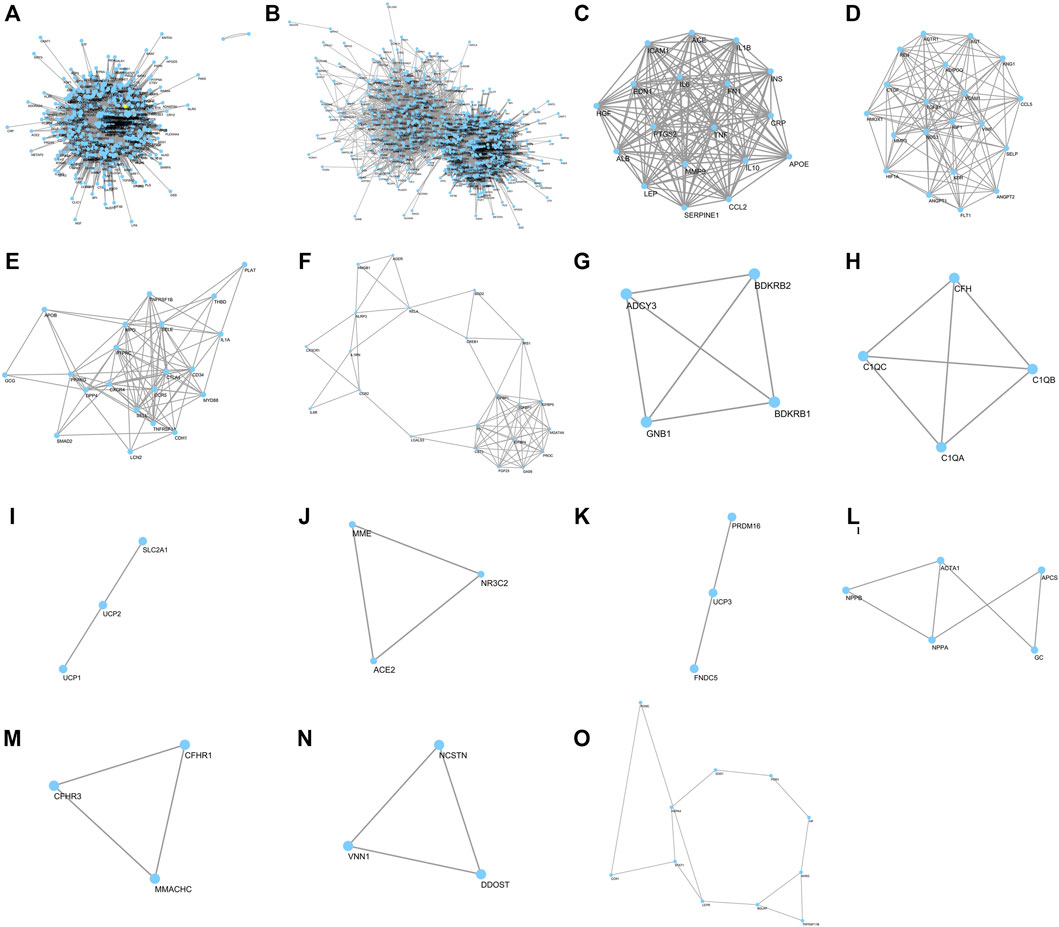
FIGURE 3. Network of R-6 putative targets and clusters of DN–R-6 interaction network. Forty DN-related genes were overlapped with and R-6; (A) 449 potential gene targets of R-6; (B) putative targets of R-6 on diabetic DN; (C–O) Major clusters of putative targets of R-6 on DN
KEGG pathway analysis showed that the R-6-related pathways involve complement and coagulation cascades, VEGF signaling pathway, HIF-1 signaling pathway, T-cell and B-cell receptor signaling pathway, Fc epsilon RI signaling pathway, PI3K-Akt signaling pathway, MAPK signaling pathway, Ras signaling pathway, ErbB signaling pathway, FoxO signaling pathway, PPAR signaling pathway, sphingolipid signaling pathway, Rap1 signaling pathway, neurotrophin signaling pathway, prolactin, estrogen and thyroid hormone signaling pathway, metabolic pathways, insulin signaling pathway, and purine metabolism, sharing similar mechanisms with various infectious diseases and cancers (Supplementary Data S6).
Putative Targets of R-6 on DN
Forty DN-related genes were overlapped with R-6 (Figure 3B–O; Table 2). Gene ontology analysis on the most significant cluster showed that the putative targets of R-6 on DN are strongly related to 44 ontology terms in 10 clusters, which included positive regulation of nitric oxide biosynthetic process (GO: 0045429), negative regulation of lipid storage (GO: 0010888), cellular response to lipopolysaccharide (GO: 0071222), inflammatory response (GO: 0006954), positive regulation of NF-kappa B transcription factor activity (GO: 0051092), positive regulation of smooth muscle cell proliferation (GO: 0048661), regulation of blood pressure (GO: 0008217), response to glucocorticoid (GO: 0051384), cellular response to interleukin-1 (GO: 0071347), angiogenesis (GO: 0001525), and negative regulation of blood coagulation (GO: 0030195) in cluster 1; positive regulation of phosphatidylinositol 3-kinase signaling (GO: 0014068), angiogenesis (GO: 0001525), positive regulation of cellular protein metabolic process (GO: 0032270), response to hypoxia (GO: 0001666), positive regulation of peptidyl-tyrosine phosphorylation (GO: 0050731), platelet degranulation (GO: 0002576), renin–angiotensin regulation of aldosterone production (GO: 0002018), positive regulation of ERK1 and ERK2 cascade (GO: 0070374), positive regulation of cell migration (GO: 0030335), regulation of blood pressure (GO: 0008217), and response to glucose (GO: 0009749) in cluster 2; inflammatory response (GO: 0006954), leukocyte migration (GO: 0050900), and response to lipopolysaccharide (GO: 0032496) in cluster 3; positive regulation of dendritic cell differentiation (GO: 2001200) and inflammatory response (GO: 0006954) in cluster 4; positive regulation of cytosolic calcium ion concentration (GO: 0007204) in cluster 5; complement activation (GO: 0006956), innate immune response (GO: 0045087), and proteolysis (GO: 0006508) in cluster 6; mitochondrial transport (GO: 0006839) in cluster 7; angiotensin maturation (GO: 0002003) in cluster 8; positive regulation of brown fat cell differentiation (GO: 0090336) in cluster 9; and response to hydrogen peroxide (GO: 0042542) in cluster 13 (Supplementary Data S7).
KEGG pathway analysis showed that the possible mechanisms of R-6 on DN are related to the TNF signaling pathway, NOD-like receptor signaling pathway, HIF-1 signaling pathway, PI3K-Akt signaling pathway, inflammatory mediator regulation of TRP channels, complement and coagulation cascades, and renin–angiotensin system, which share similar mechanisms with non-alcoholic fatty liver disease, inflammatory bowel disease, rheumatoid arthritis, and various types of infectious diseases and cancers (Figure 4; Supplementary Data S8).
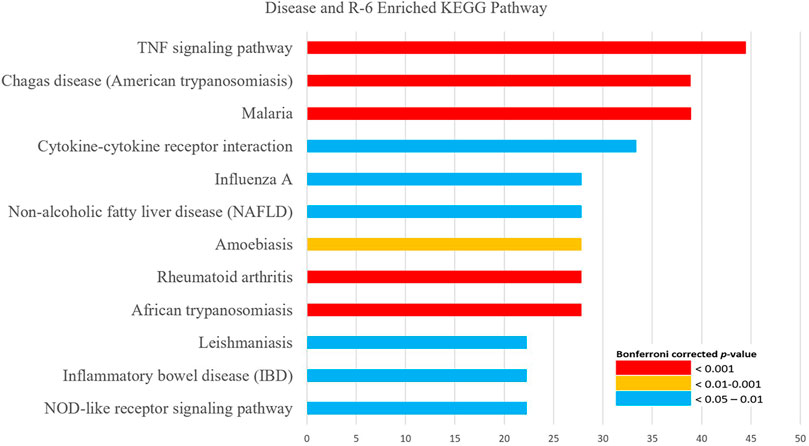
FIGURE 4. KEGG pathway enrichment analysis of the interaction between DN and R-6. KEGG pathway analysis showed that the putative targets of R-6 on diabetic nephropathy are related to the TNF signaling pathway, NOD-like receptor signaling pathway, HIF-1 signaling pathway, PI3K-Akt signaling pathway, inflammatory mediator regulation of TRP channels, complement and coagulation cascades, and renin–angiotensin system.
Comparative Analysis on the Putative Targets of R-6 Variations on DN
Five variations of R-6 were identified from an existing clinical practice guideline and were analyzed (Table 1). The common putative targets of the R-6 variations on DN included ACE, APOE, CCL2, CRP, EDN1, FN1, HGF, ICAM1, IL10, IL1B, IL6, INS, LEP, MMP9, PTGS2, SERPINE1, and TNF. TNF was identified as the seed for the most significant cluster of all R-6 variations. These common targets were related to the TNF signaling pathway, NOD-like receptor signaling pathway, HIF-1 signaling pathway, PI3K-Akt signaling pathway, inflammatory mediator regulation of TRP channels, complement and coagulation cascades, and renin–angiotensin system, which share similar mechanisms with rheumatoid arthritis, systemic lupus erythematosus, non-alcoholic fatty liver disease, inflammatory bowel disease, and various types of infectious diseases and cancers.
Formulation 1, 3, and 4 had 9, 10, and 7 clusters with significant interactions with DN, respectively. These clusters involved positive regulation of the nitric oxide biosynthetic process (GO: 0045429), negative regulation of lipid storage (GO: 0010888), cellular response to lipopolysaccharide (GO: 0071222), and inflammatory response (GO: 0006954) and positive regulation of NF-kappa B transcription factor activity (GO: 0051092) in cluster 1; positive regulation of phosphatidylinositol 3-kinase signaling (GO: 0014068), angiogenesis (GO: 0001525), positive regulation of cellular protein metabolic process (GO: 0032270), response to hypoxia (GO: 0001666), positive regulation of peptidyl-tyrosine phosphorylation (GO: 0050731), platelet degranulation (GO: 0002576), and renin–angiotensin regulation of aldosterone production (GO: 0002018) in cluster 2; and inflammatory response (GO: 0006954) and leukocyte migration (GO: 0050900) in cluster 3, which was highly similar to R-6. Both formulations 1 and 4 also had a cluster with significant interaction on complement activation (GO: 0006956).
There were six clusters with a significant function between formulation 2 and DN. The major cluster of interaction involved regulation of blood pressure (GO: 0008217), positive regulation of ERK1 and ERK2 cascade (GO: 0070374), positive regulation of cell proliferation (GO: 0008284), positive regulation of cell migration (GO: 0030335), positive regulation of T-cell proliferation (GO: 0042102), negative regulation of the endothelial cell apoptotic process (GO: 2000352), cellular response to interleukin-1 (GO: 0071347), negative regulation of the extrinsic apoptotic signaling pathway via death domain receptors (GO: 1902042), positive regulation of MAPK cascade (GO: 0043410), positive regulation of protein kinase B signaling (GO: 0051897), aging (GO: 0007568), cellular response to tumor necrosis factor (GO: 0071356), and extracellular matrix organization (GO: 0030198) in addition to that of R-6 and DN alone.
Formulation 5 formed eight clusters with significant interactions with DN. The two clusters with the most significant interactions included positive regulation of phosphatidylinositol 3-kinase signaling (GO: 0014068), angiogenesis (GO: 0001525), positive regulation of cellular protein metabolic process (GO: 0032270), response to hypoxia (GO: 0001666), positive regulation of peptidyl-tyrosine phosphorylation (GO: 0050731), platelet degranulation (GO: 0002576), and renin–angiotensin regulation of aldosterone production (GO: 0002018) in cluster 2; inflammatory response (GO: 0006954) and leukocyte migration (GO: 0050900) in cluster 3; and complement activation (GO: 0006956) in cluster 6 (Supplementary Data S9).
KEGG analysis showed that formulations 1, 3, and 5 involved the TNF signaling pathway, NOD-like receptor signaling pathway, non-alcoholic fatty liver disease, and inflammatory bowel disease, and formulations 2 and 4 involved inflammatory mediator regulation of TRP channels in addition to the shared pathways (Supplementary Data S10).
Validation of Binding
From the molecular docking, the key chemical constituents from R-6 have good binding affinity to APOE, CCL2, EDN1, FN1, HGF, ICAM1, IL10, IL1B, IL6, INS, LEP, MMP9, PTGS2, SERPINE1, TNF, and TNF receptor 1, which supported the mechanistic involvement of these related pathways in the R-6’s effect on DN. ACE, CRP, PTGS, and TNF receptor 2 were not assessable due to the large crystal structure (Table 3).
Discussion
DN, R-6, and the Interaction From a Whole-System Perspective
Eleven clusters of pathophysiology were identified from DN. From a systemic perspective, the complement and coagulation cascades, adipocytokine signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, and AMPK signaling pathway were the key pathways mediating the pathogenesis of DN based on the current evidence from in vivo and in vitro studies. These pathways are also shared by insulin resistance, rheumatoid arthritis, non-alcoholic fatty liver disease, and various types of infectious diseases and cancers, indicating a possibility in repurposing established therapeutics of these diseases for the DN management.
R-6’s mechanistic action involves modulation of complement, coagulation, angiogenesis (VEGF), oxidative stress (HIF-1), innate and adaptive immunity (T cell, B cell receptor and Fc epsilon RI), cell proliferation and survival (PI3K-Akt, MAPK, Ras, ErbB, neurotrophin, and FoxO), cell adhesion (Rap1 and MAPK), and hormonal (prolactin, estrogen, and thyroid) and metabolism (PPAR, sphingolipid, insulin, and purine) pathways.
From the network inferences, R-6 likely acts on DN via the TNF, NOD-like receptor, HIF-1 and PI3K-Akt signaling pathway, complement and coagulation cascades, and renin–angiotensin system. These share similar mechanisms with non-alcoholic fatty liver disease, inflammatory bowel disease, and rheumatoid arthritis, which could support the use of R-6 in these conditions.
From the in silico binding affinity assessment, alisol (Alismatis Rhizoma), 7-o-methyl morroniside (Corni Fructus), campesterol (Dioscoreae Rhizoma), paeoniflorin (Moutan Cortex), beta-amyrin acetate (Poria), and catalpol (Rehmanniae Radix) have the highest likelihood of interacting with the proteins involved in these pathways.
TNF Signaling as the Key Mechanism of R-6’s Action on DN
From KEGG enrichment analysis, the TNF signaling pathway was demonstrated as the key signaling involved in the action of R-6 on DN with the highest number of involved pathways. TNF signaling activates multiple pathways (e.g., NF-κB, JNK, MAPK, and PI3K-Akt) via receptors including TNF receptor type I (TNFR1) and TNF receptor type II (TNFR2), leading to apoptosis, necroptosis, inflammatory response, and vascular response. The TNF signaling pathway has been suggested as a therapeutic target for general CKD (Al-Lamki and Mayadas, 2015; Breyer and Susztak, 2016), autoimmune diseases, and cancers (Shaikh et al., 2018; Steeland et al., 2018). Elevated sera TNFR1 and TNFR2 were associated with reduced total filtration surface per glomerulus, podocyte number per glomerulus, filtration slit frequency, fenestrated endothelium, and increased glomerular basement membrane width, foot process width, mesangial fractional volume, and global glomerular sclerosis in diabetic patients with early nephropathy (Pavkov et al., 2016). Previous longitudinal cohorts also demonstrated that higher baseline serum TNFR1 and TNFR2 levels increased the risk of end-stage kidney disease among type 2 diabetes patients (Niewczas et al., 2012; Pavkov et al., 2015).
TNF-α suppression by inhibitor or antibody alleviated albuminuria, macrophage infiltration, and glomerular and tubular injury in STZ-induced diabetic rats and Ins2Akita mice (Awad et al., 2015; Cheng et al., 2021). Nevertheless, clinical pan-TNF intervention leads to concerns of the off-target effect (e.g., fever, hypotension, tuberculosis, and malignancy) as the TNF signaling pathway is a master regulator of innate immunity (Steeland et al., 2018), and TNFR1 is expressed widely in most cell types. Unlike TNFR1, which is pro-inflammatory and apoptotic, TNFR2 is more restricted to endothelial, immune, neuronal, and tumor cells and associated with immunomodulatory and anti-inflammatory effects (Steeland et al., 2018; Murakoshi et al., 2020). Targeted TNFR2 inhibition that modulates the TNF signaling homeostasis could alleviate the off-target effect in TNF signaling pathway intervention (Shaikh et al., 2018; Steeland et al., 2018; Murakoshi et al., 2020). The clinical efficacy, specificity to TNFR2, and the associated adverse events of R-6 on DN requires further clinical studies.
Differences Between R-6—DN Interaction Among R-6 Variations
From the comparative analysis of the interaction between R-6 variations and DN, formulations 1, 3, and 5 involved the NOD-like receptor signaling pathway, and formulations 2 and 4 involved regulation of TRP channels on top of the common targeting mechanisms, respectively. Similar to and synergistic with the toll-like receptor, NOD-like receptors are highly conserved pattern recognition receptors that mediate innate immunity through the activation of NF-κB, MAPK, inflammasome, and the production of pro-inflammatory cytokines and chemokines (Geddes et al., 2009). Polymorphisms of TLR and NLR were also associated with dyslipidemia, higher glucose level (Geddes et al., 2009; Gomes Torres et al., 2019), and insulin resistance (Zhou et al., 2017). NLRP3 has been shown to induce epithelial–mesenchymal transition of the tubular epithelial cells via TGF-beta (Wang et al., 2013). A previous study showed that NOD2 was upregulated in diabetic patient biopsies, and NOD2 knockout alleviated the hyperglycemia-induced nephrin expression reduction in diabetic mice (Du et al., 2013).
TRP channels are extensively expressed in the kidney and pancreas that modulate the transporting and signaling mechanisms underpinning glomerular filtration, reabsorption, and secretion of water and solutes in the kidney, and insulin secretion in the pancreas (Tomilin et al., 2016). TRP channels have six subfamilies, namely, TRPC, TRPV, TRPM, TRPP, TRPA, TRPML, and TRPN (Tomilin et al., 2016). TRP channels respond to mechanical stimuli and mediate vascular remodeling in various disease models (Smani et al., 2015; Kanthakumar and Adebiyi, 2021). Under a diabetic environment, the TRPC6 expression increases via the renin–angiotensin system and ATP signaling, leading to calcium influx and subsequent damage of the podocyte structure and detachment (Roshanravan and Dryer, 2014; Sonneveld et al., 2014; Wang et al., 2020). A late clinical trial TRACTION-2 using GFB-887, a podocyte targeted small molecule TRPC5 inhibitor, to treat diabetic kidney disease was designed based on previous in vitro and in vivo studies showing a protective effect of TRPC5 inhibition via the Rac1 signaling pathway (Walsh et al., 2021). Goshajinkigan, a Japanese herbal formulation similar to R-6, was shown to suppress the oxaliplatin-induced increase of TRPA1 and TRPM8 mRNA expression in dorsal root ganglia (Mizuno et al., 2014). Nevertheless, the involvement of the NOD-like receptor pathway and TRP channels was revealed through network inferences. Current research on the direct effect of R-6 on these two mechanisms in diabetes is limited.
Strengths and Limitations
A network pharmacological approach was used to delineate the systemic interaction between DN and R-6 with multifactorial pathophysiology and multiple chemical constituents. The whole-system effect of R-6 specific to DN was revealed for further clinical validation. Nevertheless, we limited the analysis to DN with macroalbuminuria for a more specific DN diagnosis (Persson and Rossing, 2018), and we did not perform HPLC or UPLC analysis on the chemical constituents of the formulations as the CMs involved are well studied and available in the existing databases. Network pharmacology analysis focuses on protein–protein interaction. The direction of effect (e.g., positive, negative, or cyclic effect) and the associated epigenetic regulations require further investigations.
Besides, we did not perform in vivo or in vitro validation as (1) the study medication involves multiple drugs and chemicals, (2) network pharmacology analysis is already a systematic integration of all existing in vivo and in vitro data (Hopkins, 2008), and (3) further validation data from a single study with experimental models are unlikely to add much evidence to the result from network analysis. We used in silico docking analysis as a validation from the chemistry perspective. Free energy was used to estimate the strength of bonding for better estimating the binding affinity instead of using root-mean-square deviation from geometric assessment (Cournia et al., 2017; Mobley and Gilson, 2017). Subsequent validation from clinical samples screening the biomarkers of key mechanisms would serve better to evaluate the whole-system effect and the relative involvement of different mechanisms of the formulations in real clinical settings (Chan et al., 2021c).
Conclusion
The complement and coagulation cascades, adipocytokine signaling pathway, TNF signaling pathway, HIF-1 signaling pathway, and AMPK signaling pathway orchestrated the pathogenesis of DN from a systemic perspective. The therapeutic effect of R-6 on DN likely involves the TNF, NOD-like receptor, HIF-1, and PI3K-Akt signaling pathways; the complement and coagulation cascades; and the renin–angiotensin system. The analysis of R-6 and the comparative analysis of R-6 variations converged to suggest that the TNF signaling pathway is the key mechanism involved in the action of R-6 on DN among patients with DN presenting with different clinical phenotypes. Variations of R-6 used in clinical protocols may also involve the NOD-like receptor signaling pathway and TRP channels on top of the common mechanisms from indirect inferences. These putative targets could be validated through further trials with clinical samples.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
KC and ST conceived the study. KY and KC collected the data and performed the pathway analysis. KC, WY, RX, SW-YL, HY, YZ, JM, and KL interpreted the pathway-related pathophysiology. KC, KY, and ST drafted the manuscript. All authors involved in the interpretation of data and manuscript revision.
Funding
This project was made possible in part through the support of Health and Medical Research Fund (Ref: 12133341, 14151731), and the philanthropic donations from Mrs. Louise Mon (Perennial International Ltd.), Ting Lai Ling, Chan Wing Kwan, Patrick, and Sa Sa Making Life Beautiful Charity Fund. In addition, ST was supported by the HKU Outstanding Researcher Award, Croucher Senior Medical Research Fellowship Award, and the Yu endowed professorship at HKU. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.794139/full#supplementary-material
References
Al-Lamki, R. S., and Mayadas, T. N. (2015). TNF Receptors: Signaling Pathways and Contribution to Renal Dysfunction. Kidney Int. 87 (2), 281–296. doi:10.1038/ki.2014.285
Alicic, R. Z., Rooney, M. T., and Tuttle, K. R. (2017). Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 12 (12), 2032–2045. doi:10.2215/CJN.11491116
Alsaad, K. O., and Herzenberg, A. M. (2007). Distinguishing Diabetic Nephropathy from Other Causes of Glomerulosclerosis: an Update. J. Clin. Pathol. 60 (1), 18–26. doi:10.1136/jcp.2005.035592
Awad, A. S., You, H., Gao, T., Cooper, T. K., Nedospasov, S. A., Vacher, J., et al. (2015). Macrophage-derived Tumor Necrosis Factor-α Mediates Diabetic Renal Injury. Kidney Int. 88 (4), 722–733. doi:10.1038/ki.2015.162
Breyer, M. D., and Susztak, K. (2016). The Next Generation of Therapeutics for Chronic Kidney Disease. Nat. Rev. Drug Discov. 15 (8), 568–588. doi:10.1038/nrd.2016.67
Chan, G. C. W., and Tang, S. C. W. (2018). Proteinuria Reaffirmed as a Risk Modifier in Diabetic Chronic Kidney Disease. Nephrol. Dial. Transpl. 33 (11), 1873–1874. doi:10.1093/ndt/gfy208
Chan, K. (2018). Integrative Management for Diabetic Kidney Disease – Patients’ and Clinicians’ Perspectives, Clinical Effectiveness and Possible Mechanisms [PhD Thesis]. Hong Kong: The University of Hong Kong.
Chan, K. W., Chow, T. Y., Yu, K. Y., Feng, Y., Lao, L., Bian, Z., et al. (2022). Effectiveness of Integrative Chinese-Western Medicine for Chronic Kidney Disease and Diabetes: A Retrospective Cohort Study. Am. J. Chin. Med. 2022, 1–18. doi:10.1142/s0192415x2250015x
Chan, K. W., Ip, T. P., Kwong, A. S., Lui, S. L., Chan, G. C., Cowling, B. J., et al. (2016). Semi-individualised Chinese Medicine Treatment as an Adjuvant Management for Diabetic Nephropathy: a Pilot Add-On, Randomised, Controlled, Multicentre, Open-Label Pragmatic Clinical Trial. BMJ Open 6 (8), e010741. doi:10.1136/bmjopen-2015-010741
Chan, K. W., Kwong, A. S. K., Tsui, P. N., Cheung, S. C. Y., Chan, G. C. W., Choi, W. F., et al. (2021a). Efficacy, Safety and Response Predictors of Adjuvant astragalus for Diabetic Kidney Disease (READY): Study Protocol of an Add-On, Assessor-Blind, Parallel, Pragmatic Randomised Controlled Trial. BMJ Open 11 (1), e042686. doi:10.1136/bmjopen-2020-042686
Chan, K. W., Wong, V. T., and Tang, S. C. W. (2020a). COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-western Medicine for the Management of 2019 Novel Coronavirus Disease. Am. J. Chin. Med. 48 (3), 737–762. doi:10.1142/S0192415X20500378
Chan, K. W., Lee, P. W., Leung, C. P. S., Chan, G. C. W., Yiu, W. H., Cheung, H. M., et al. (2020b). Patients’ and clinicians’ Expectations on Integrative Medicine Services for Diabetes: A Focus Group Study. BMC Complement. Med. Ther. 20 (1), 205. doi:10.1186/s12906-020-02994-5
Chan, K. W., Chow, T. Y., Yu, K. Y., Xu, Y., Zhang, N. L., Wong, V. T., et al. (2021b). SYmptom-Based STratification of DiabEtes Mellitus by Renal Function Decline (SYSTEM): A Retrospective Cohort Study and Modeling Assessment. Front. Med. 8, e682090. doi:10.3389/fmed.2021.682090
Chan, K. W., Kwong, A. S. K., Chan, G. C. W., Leung, C. P. S., Yiu, W. H., Lui, S. L., et al. (2019). Semi-individualised Chinese Medicine Treatment for Diabetic Kidney Disease - from Users' Perspectives to SCHEMATIC Trial Interim Result and Potential Mechanisms. Adv. Integr. Med. 6, S12. doi:10.1016/j.aimed.2019.03.033
Chan, K. W., Lee, P. W., Leung, C. P.-s., Law, Y. K., Gao, L., Chan, G. C.-w., et al. (2021c). PRAgmatic Clinical Trial Design of Integrative MediCinE (PRACTICE): A Focus Group Series and Systematic Review on Trials of Diabetes and Kidney Disease. Front. Med. 8, 668913. doi:10.3389/fmed.2021.668913
Cheng, D., Liang, R., Huang, B., Hou, J., Yin, J., Zhao, T., et al. (2021). Tumor Necrosis Factor-α Blockade Ameliorates Diabetic Nephropathy in Rats. Clin. Kidney J. 14 (1), 301–308. doi:10.1093/ckj/sfz137
Cournia, Z., Allen, B., and Sherman, W. (2017). Relative Binding Free Energy Calculations in Drug Discovery: Recent Advances and Practical Considerations. J. Chem. Inf. Model. 57 (12), 2911–2937. doi:10.1021/acs.jcim.7b00564
Du, P., Fan, B., Han, H., Zhen, J., Shang, J., Wang, X., et al. (2013). NOD2 Promotes Renal Injury by Exacerbating Inflammation and Podocyte Insulin Resistance in Diabetic Nephropathy. Kidney Int. 84 (2), 265–276. doi:10.1038/ki.2013.113
Flyvbjerg, A. (2017). The Role of the Complement System in Diabetic Nephropathy. Nat. Rev. Nephrol. 13 (5), 311–318. doi:10.1038/nrneph.2017.31
Forbes, J. M., Cooper, M. E., Oldfield, M. D., and Thomas, M. C. (2003). Role of Advanced Glycation End Products in Diabetic Nephropathy. J. Am. Soc. Nephrol. 14 (8 Suppl. 3), S254–S258. doi:10.1097/01.asn.0000077413.41276.17
Furuichi, K., Shimizu, M., Okada, H., Narita, I., and Wada, T. (2018). Clinico-pathological Features of Kidney Disease in Diabetic Cases. Clin. Exp. Nephrol. 22 (10), 1046–1051. doi:10.1007/s10157-018-1556-4
Gao, X., Liu, Y., An, Z., and Ni, J. (2021). Active Components and Pharmacological Effects of Cornus Officinalis: Literature Review. Front. Pharmacol. 12 (513), e633447. doi:10.3389/fphar.2021.633447
Geddes, K., Magalhães, J. G., and Girardin, S. E. (2009). Unleashing the Therapeutic Potential of NOD-like Receptors. Nat. Rev. Drug Discov. 8 (6), 465–479. doi:10.1038/nrd2783
Goh, S. Y., and Cooper, M. E. (2008). Clinical Review: The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 93 (4), 1143–1152. doi:10.1210/jc.2007-1817
Gomes Torres, A. C. M. B., Leite, N., Tureck, L. V., de Souza, R. L. R., Titski, A. C. K., Milano-Gai, G. E., et al. (2019). Association between Toll-like Receptors (TLR) and NOD-like Receptor (NLR) Polymorphisms and Lipid and Glucose Metabolism. Gene 685, 211–221. doi:10.1016/j.gene.2018.11.065
Guo, C., Kang, X., Cao, F., Yang, J., Xu, Y., Liu, X., et al. (2021a). Network Pharmacology and Molecular Docking on the Molecular Mechanism of Luo-hua-zi-zhu (LHZZ) Granule in the Prevention and Treatment of Bowel Precancerous Lesions. Front. Pharmacol. 12 (144). doi:10.3389/fphar.2021.629021
Guo, J. C., Pan, H. C., Yeh, B. Y., Lu, Y. C., Chen, J. L., Yang, C. W., et al. (2021b). Associations between Using Chinese Herbal Medicine and Long-Term Outcome Among Pre-dialysis Diabetic Nephropathy Patients: A Retrospective Population-Based Cohort Study. Front. Pharmacol. 12, 616522. doi:10.3389/fphar.2021.616522
Hamosh, A., Scott, A. F., Amberger, J., Valle, D., and McKusick, V. A. (2000). Online Mendelian Inheritance in Man (OMIM). Hum. Mutat. 15 (1), 57–61. doi:10.1002/(SICI)1098-1004(200001)15:1<57::AID-HUMU12>3.0.CO;2-G
Heerspink, H. J. L., Parving, H. H., Andress, D. L., Bakris, G., Correa-Rotter, R., Hou, F. F., et al. (2019). Atrasentan and Renal Events in Patients with Type 2 Diabetes and Chronic Kidney Disease (SONAR): a Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 393 (10184), 1937–1947. doi:10.1016/S0140-6736(19)30772-X
Heinzelmann, G., and Gilson, M. K. (2021). Automation of Absolute Protein-Ligand Binding Free Energy Calculations for Docking Refinement and Compound Evaluation. Sci. Rep. 11 (1), 1116. doi:10.1038/s41598-020-80769-1
Hopkins, A. L. (2008). Network Pharmacology: the Next Paradigm in Drug Discovery. Nat. Chem. Biol. 4 (11), 682–690. doi:10.1038/nchembio.118
Hsieh, C. F., Huang, S. L., Chen, C. L., Chen, W. T., Chang, H. C., and Yang, C. C. (2014). Non-aristolochic Acid Prescribed Chinese Herbal Medicines and the Risk of Mortality in Patients with Chronic Kidney Disease: Results from a Population-Based Follow-Up Study. BMJ Open 4 (2), e004033–004033. doi:10.1136/bmjopen-2013-004033
Hsu, T. W., Liu, J. S., Hung, S. C., Kuo, K. L., Chang, Y. K., Chen, Y. C., et al. (2014). Renoprotective Effect of Renin-Angiotensin-Aldosterone System Blockade in Patients with Predialysis Advanced Chronic Kidney Disease, Hypertension, and Anemia. JAMA Intern. Med. 174 (3), 347–354. doi:10.1001/jamainternmed.2013.12700
Huang, da. W., Sherman, B. T., and Lempicki, R. A. (2009). Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 4 (1), 44–57. doi:10.1038/nprot.2008.211
Huang, K. C., Su, Y. C., Sun, M. F., and Huang, S. T. (2018). Chinese Herbal Medicine Improves the Long-Term Survival Rate of Patients with Chronic Kidney Disease in Taiwan: a Nationwide Retrospective Population-Based Cohort Study. Front. Pharmacol. 9, 1117. doi:10.3389/fphar.2018.01117
Jerums, G., Premaratne, E., Panagiotopoulos, S., and MacIsaac, R. J. (2010). The Clinical Significance of Hyperfiltration in Diabetes. Diabetologia 53 (10), 2093–2104. doi:10.1007/s00125-010-1794-9
Kanthakumar, P., and Adebiyi, A. (2021). Renal Vascular TRP Channels. Curr. Res. Physiol. 4, 17–23. doi:10.1016/j.crphys.2021.02.001
Kashihara, N., Haruna, Y., Kondeti, V. K., and Kanwar, Y. S. (2010). Oxidative Stress in Diabetic Nephropathy. Curr. Med. Chem. 17 (34), 4256–4269. doi:10.2174/092986710793348581
Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., et al. (2019). PubChem 2019 Update: Improved Access to Chemical Data. Nucleic Acids Res. 47 (D1), D1102–d9. doi:10.1093/nar/gky1033
Lee, H. S., Jo, S., Lim, H. S., and Im, W. (2012). Application of Binding Free Energy Calculations to Prediction of Binding Modes and Affinities of MDM2 and MDMX Inhibitors. J. Chem. Inf. Model. 52 (7), 1821–1832. doi:10.1021/ci3000997
Li, R. X., Yiu, W. H., Wu, H. J., Wong, D. W., Chan, L. Y., Lin, M., et al. (2014). BMP7 Reduces Inflammation and Oxidative Stress in Diabetic Tubulopathy. Clin. Sci. 9, 9. doi:10.1042/cs20140401
Li, S., Fan, T. P., Jia, W., Lu, A., and Zhang, W. (2014). Network Pharmacology in Traditional Chinese Medicine. Evid. Based Complement. Alternat Med. 2014, 138460. doi:10.1155/2014/138460
Lin, M., Yiu, W. H., Wu, H. J., Chan, L. Y., Leung, J. C., Au, W. S., et al. (2012). Toll-like Receptor 4 Promotes Tubular Inflammation in Diabetic Nephropathy. J. Am. Soc. Nephrol. 23 (1), 86–102. doi:10.1681/ASN.2010111210
Lin, M. Y., Chiu, Y. W., Chang, J. S., Lin, H. L., Lee, C. T., Chiu, G. F., et al. (2015). Association of Prescribed Chinese Herbal Medicine Use with Risk of End-Stage Renal Disease in Patients with Chronic Kidney Disease. Kidney Int. 88 (10), 1365–1373. doi:10.1038/ki.2015.226
Luo, D. (2008). Identification of Structure and Antioxidant Activity of a Fraction of Polysaccharide Purified from Dioscorea Nipponica Makino. Carbohydr. Polym. 71 (4), 544–549. doi:10.1016/j.carbpol.2007.06.023
Madhusudhan, T., Kerlin, B. A., and Isermann, B. (2016). The Emerging Role of Coagulation Proteases in Kidney Disease. Nat. Rev. Nephrol. 12 (2), 94–109. doi:10.1038/nrneph.2015.177
Mizuno, K., Kono, T., Suzuki, Y., Miyagi, C., Omiya, Y., Miyano, K., et al. (2014). Goshajinkigan, a Traditional Japanese Medicine, Prevents Oxaliplatin-Induced Acute Peripheral Neuropathy by Suppressing Functional Alteration of TRP Channels in Rat. J. Pharmacol. Sci. 125 (1), 91–98. doi:10.1254/jphs.13244fp
Mobley, D. L., and Gilson, M. K. (2017). Predicting Binding Free Energies: Frontiers and Benchmarks. Annu. Rev. Biophys. 46, 531–558. doi:10.1146/annurev-biophys-070816-033654
Murakoshi, M., Gohda, T., and Suzuki, Y. (2020). Circulating Tumor Necrosis Factor Receptors: a Potential Biomarker for the Progression of Diabetic Kidney Disease. Ijms 21 (6), 1957. doi:10.3390/ijms21061957
Navarro-González, J. F., Mora-Fernández, C., Muros de Fuentes, M., and García-Pérez, J. (2011). Inflammatory Molecules and Pathways in the Pathogenesis of Diabetic Nephropathy. Nat. Rev. Nephrol. 7 (6), 327–340. doi:10.1038/nrneph.2011.51
Niewczas, M. A., Gohda, T., Skupien, J., Smiles, A. M., Walker, W. H., Rosetti, F., et al. (2012). Circulating TNF Receptors 1 and 2 Predict ESRD in Type 2 Diabetes. J. Am. Soc. Nephrol. 23 (3), 507–515. doi:10.1681/ASN.2011060627
Nistor, I., De Sutter, J., Drechsler, C., Goldsmith, D., Soler, M. J., Tomson, C., et al. (2018). Effect of Renin-Angiotensin-Aldosterone System Blockade in Adults with Diabetes Mellitus and Advanced Chronic Kidney Disease Not on Dialysis: a Systematic Review and Meta-Analysis. Nephrol. Dial. Transpl. 33 (1), 12–22. doi:10.1093/ndt/gfx072
Oe, Y., Hayashi, S., Fushima, T., Sato, E., Kisu, K., Sato, H., et al. (2016). Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler Thromb. Vasc. Biol. 36 (8), 1525–1533. doi:10.1161/ATVBAHA.116.307883
Palmer, S. C., Mavridis, D., Navarese, E., Craig, J. C., Tonelli, M., Salanti, G., et al. (2015). Comparative Efficacy and Safety of Blood Pressure-Lowering Agents in Adults with Diabetes and Kidney Disease: a Network Meta-Analysis. Lancet 385 (9982), 2047–2056. doi:10.1016/S0140-6736(14)62459-4
Pavkov, M. E., Nelson, R. G., Knowler, W. C., Cheng, Y., Krolewski, A. S., and Niewczas, M. A. (2015). Elevation of Circulating TNF Receptors 1 and 2 Increases the Risk of End-Stage Renal Disease in American Indians with Type 2 Diabetes. Kidney Int. 87 (4), 812–819. doi:10.1038/ki.2014.330
Pavkov, M. E., Weil, E. J., Fufaa, G. D., Nelson, R. G., Lemley, K. V., Knowler, W. C., et al. (2016). Tumor Necrosis Factor Receptors 1 and 2 Are Associated with Early Glomerular Lesions in Type 2 Diabetes. Kidney Int. 89 (1), 226–234. doi:10.1038/ki.2015.278
Perkovic, V., Jardine, M. J., Neal, B., Bompoint, S., Heerspink, H. J. L., Charytan, D. M., et al. (2019). Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 380 (24), 2295–2306. doi:10.1056/NEJMoa1811744
Persson, F., and Rossing, P. (2018). Diagnosis of Diabetic Kidney Disease: State of the Art and Future Perspective. Kidney Int. Suppl. 8 (1), 2–7. doi:10.1016/j.kisu.2017.10.003
Pichler, R., Afkarian, M., Dieter, B. P., and Tuttle, K. R. (2017). Immunity and Inflammation in Diabetic Kidney Disease: Translating Mechanisms to Biomarkers and Treatment Targets. Am. J. Physiol. Ren. Physiol 312 (4), F716–f31. doi:10.1152/ajprenal.00314.2016
Premaratne, E., Verma, S., Ekinci, E. I., Theverkalam, G., Jerums, G., and MacIsaac, R. J. (2015). The Impact of Hyperfiltration on the Diabetic Kidney. Diabetes Metab. 41 (1), 5–17. doi:10.1016/j.diabet.2014.10.003
Roshanravan, H., and Dryer, S. E. (2014). ATP Acting through P2Y Receptors Causes Activation of Podocyte TRPC6 Channels: Role of Podocin and Reactive Oxygen Species. Am. J. Physiol. Ren. Physiol 306 (9), F1088–F1097. doi:10.1152/ajprenal.00661.2013
Sagoo, M. K., and Gnudi, L. (2018). Diabetic Nephropathy: Is There a Role for Oxidative Stress? Free Radic. Biol. Med. 116, 50–63. doi:10.1016/j.freeradbiomed.2017.12.040
Shaikh, F., He, J., Bhadra, P., Chen, X., and Siu, S. W. I. (2018). TNF Receptor Type II as an Emerging Drug Target for the Treatment of Cancer, Autoimmune Diseases, and Graft-Versus-Host Disease: Current Perspectives and In Silico Search for Small Molecule Binders. Front. Immunol. 9 (1382), 1382. doi:10.3389/fimmu.2018.01382
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 13 (11), 2498–2504. doi:10.1101/gr.1239303
Shu, Z., Chang, K., Zhou, Y., Peng, C., Li, X., Cai, W., et al. (2021). Add-On Semi-individualized Chinese Medicine for Coronavirus Disease 2019 (ACCORD): a Retrospective Cohort Study of Hospital Registries. Am. J. Chin. Med. 49 (3), 1–33. doi:10.1142/s0192415x21500257
Smani, T., Shapovalov, G., Skryma, R., Prevarskaya, N., and Rosado, J. A. (2015). Functional and Physiopathological Implications of TRP Channels. Biochim. Biophys. Acta 1853 (8), 1772–1782. doi:10.1016/j.bbamcr.2015.04.016
Sonneveld, R., van der Vlag, J., Baltissen, M. P., Verkaart, S. A., Wetzels, J. F., Berden, J. H., et al. (2014). Glucose Specifically Regulates TRPC6 Expression in the Podocyte in an AngII-dependent Manner. Am. J. Pathol. 184 (6), 1715–1726. doi:10.1016/j.ajpath.2014.02.008
Steeland, S., Libert, C., and Vandenbroucke, R. E. (2018). A New Venue of TNF Targeting. Int. J. Mol. Sci. 19 (5), 1442. doi:10.3390/ijms19051442
Stelzer, G., Rosen, N., Plaschkes, I., Zimmerman, S., Twik, M., Fishilevich, S., et al. (2016). The GeneCards Suite: from Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinformatics 54, 1. doi:10.1002/cpbi.5
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2021). IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Sun, Y. (2014). Biological Activities and Potential Health Benefits of Polysaccharides from Poria Cocos and Their Derivatives. Int. J. Biol. Macromol 68, 131–134. doi:10.1016/j.ijbiomac.2014.04.010
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al. (2019). STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-wide Experimental Datasets. Nucleic Acids Res. 47 (D1), D607–d13. doi:10.1093/nar/gky1131
Tang, S. C. W., and Yiu, W. H. (2020). Innate Immunity in Diabetic Kidney Disease. Nat. Rev. Nephrol. 16 (4), 206–222. doi:10.1038/s41581-019-0234-4
Tang, S. C. W., Yu, X., Chen, H. C., Kashihara, N., Park, H. C., Liew, A., et al. (2020). Dialysis Care and Dialysis Funding in Asia. Am. J. Kidney Dis. 75 (5), 772–781. doi:10.1053/j.ajkd.2019.08.005
Thomas, M. C., Cooper, M. E., and Zimmet, P. (2016). Changing Epidemiology of Type 2 Diabetes Mellitus and Associated Chronic Kidney Disease. Nat. Rev. Nephrol. 12 (2), 73–81. doi:10.1038/nrneph.2015.173
Tomilin, V., Mamenko, M., Zaika, O., and Pochynyuk, O. (2016). Role of Renal TRP Channels in Physiology and Pathology. Semin. Immunopathol 38 (3), 371–383. doi:10.1007/s00281-015-0527-z
Tonneijck, L., Muskiet, M. H., Smits, M. M., van Bommel, E. J., Heerspink, H. J., van Raalte, D. H., et al. (2017). Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 28 (4), 1023–1039. doi:10.1681/ASN.2016060666
The UniProt Consortium (2019). UniProt: a Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 47 (D1), D506–d15. doi:10.1093/nar/gky1049
Walsh, L., Reilly, J. F., Cornwall, C., Gaich, G. A., Gipson, D. S., Heerspink, H. J. L., et al. (2021). Safety and Efficacy of GFB-887, a TRPC5 Channel Inhibitor, in Patients with Focal Segmental Glomerulosclerosis, Treatment-Resistant Minimal Change Disease, or Diabetic Nephropathy: TRACTION-2 Trial Design. Kidney Int. Rep. 6 (10), 2575–2584. doi:10.1016/j.ekir.2021.07.006
Wang, Q., Tian, X., Wang, Y., Wang, Y., Li, J., Zhao, T., et al. (2020). Role of Transient Receptor Potential Canonical Channel 6 (TRPC6) in Diabetic Kidney Disease by Regulating Podocyte Actin Cytoskeleton Rearrangement. J. Diabetes Res. 2020, 6897390. doi:10.1155/2020/6897390
Wang, W., Wang, X., Chun, J., Vilaysane, A., Clark, S., French, G., et al. (2013). Inflammasome-independent NLRP3 Augments TGF-β Signaling in Kidney Epithelium. J. Immunol. 190 (3), 1239–1249. doi:10.4049/jimmunol.1201959
Wang, X., Shen, Y., Wang, S., Li, S., Zhang, W., Liu, X., et al. (2017a). PharmMapper 2017 Update: a Web Server for Potential Drug Target Identification with a Comprehensive Target Pharmacophore Database. Nucleic Acids Res. 45 (W1), W356–w60. doi:10.1093/nar/gkx374
Wang, Z., He, C., Peng, Y., Chen, F., and Xiao, P. (2017b). Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia Suffruticosa Andrew): a Systematic Review. Molecules 22 (6), 946. doi:10.3390/molecules22060946
Yiu, W. H., Li, R. X., Wong, D. W. L., Wu, H. J., Chan, K. W., Chan, L. Y. Y., et al. (2018). Complement C5a Inhibition Moderates Lipid Metabolism and Reduces Tubulointerstitial Fibrosis in Diabetic Nephropathy. Nephrol. Dial. Transpl. 33 (8), 1323–1332. doi:10.1093/ndt/gfx336
Yokozawa, T., Kim, H. Y., and Yamabe, N. (2004). Amelioration of Diabetic Nephropathy by Dried Rehmanniae Radix (Di Huang) Extract. Am. J. Chin. Med. 32 (6), 829–839. doi:10.1142/S0192415X04002442
Zhang, L. (2010). The Optimization Study of Clinical Pathway Formulation Based on Chronic Renal Failure Diagnosis Scheme [MD Thesis]. Guangzhou: Guangzhou University of Chinese Medicine.
Zhang, L. L., Xu, W., Xu, Y. L., Chen, X., Huang, M., and Lu, J. J. (2017). Therapeutic Potential of Rhizoma Alismatis: a Review on Ethnomedicinal Application, Phytochemistry, Pharmacology, and Toxicology. Ann. N. Y Acad. Sci. 1401 (1), 90–101. doi:10.1111/nyas.13381
Zhang, R., Zhu, X., Bai, H., and Ning, K. (2019). Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 10, 123. doi:10.3389/fphar.2019.00123
Zhang, R. Z., Yu, S. J., Bai, H., and Ning, K. (2017). TCM-mesh: The Database and Analytical System for Network Pharmacology Analysis for TCM Preparations. Sci. Rep. 7 (1), 2821. doi:10.1038/s41598-017-03039-7
Zhou, H., Feng, L., Xu, F., Sun, Y., Ma, Y., Zhang, X., et al. (2017). Berberine Inhibits Palmitate-Induced NLRP3 Inflammasome Activation by Triggering Autophagy in Macrophages: a New Mechanism Linking Berberine to Insulin Resistance Improvement. Biomed. Pharmacother. 89, 864–874. doi:10.1016/j.biopha.2017.03.003
Glossary
ACE Angiotensin-converting enzyme
AMPK Adenosine monophosphate-activated protein kinase
APOE Apolipoprotein E
CCL2 chemokine (C-C motif) ligand 2
CKD Chronic kidney disease
CM Chinese medicine
CRP C-reactive protein
DAVID Database for Annotation, Visualization and Integrated Discovery
DKD Diabetic kidney disease
DN Diabetic nephropathy
EDN1 Endothelin 1
ErbB Erythroblastic leukemia viral oncogene homolog
ERK1/2 Extracellular signal-regulated protein kinase 1/2
Fc epsilon RI Fc epsilon receptor I
RI Fc epsilon receptor I
FN1 Fibronectin 1
FoxO Forkhead Box O
HGF Hepatocyte growth factor
HIF Hypoxia-inducible factor
HPLC High-performance liquid chromatography
ICAM1 Intercellular adhesion molecule 1
IL10 interleukin 10
IL1B Interleukin 1 beta
IL6 Interleukin 6
INS Insulin
Ins2Akita Heterozygous Akita spontaneous mutation
JNK c-Jun N-terminal kinase
KEGG Kyoto Encyclopedia of Genes and Genomes
LEP Leptin
MAPK Mitogen-activated protein kinase
MCODE Molecular complex detection
MMP9 Matrix metallopeptidase 9
NF-kappa B Nuclear factor-kappa B
NLR NOD-like receptor
NLRP3 NOD-like receptor protein 3
OMIM Online Mendelian Inheritance in Man
PI3K Phosphoinositide 3-kinases
AKT Protein kinase B
PPAR Peroxisome proliferator-activated receptor
PTGS2 Prostaglandin-endoperoxide synthase 2
R-6 Rehmannia-6
Rap1 Ras-related protein 1
Ras Rat sarcoma
RCSB Research Collaboratory for Structural Bioinformatics
SERPINE1 Serpin family E member 1
STRING Search Tool for the Retrieval of Interacting Genes/Proteins
STZ Streptozotocin
TLR Toll-like receptor
TNF Tumor necrosis factor
TNFR1/2 Tumor necrosis factor receptor 1 or 2
TRP Transient receptor potential
TRPA Transient receptor potential ankyrin
TRPC Transient receptor potential canonical
TRPM Transient receptor potential melastatin
TRPML Transient receptor potential mucolipin
TRPN Transient receptor potential no mechanoreceptor potential C
TRPP Transient receptor potential polycystin
TRPV Transient receptor potential vanilloi
UPLC Ultra performance liquid chromatography
Keywords: integrative medicine, traditional Chinese medicine, diabetic nephropathy, chronic kidney disease, Rehmannia, mechanism, network pharmacology, TNF
Citation: Chan KW, Yu KY, Yiu WH, Xue R, Lok SW-y, Li H, Zou Y, Ma J, Lai KN and Tang SC-w (2022) Potential Therapeutic Targets of Rehmannia Formulations on Diabetic Nephropathy: A Comparative Network Pharmacology Analysis. Front. Pharmacol. 13:794139. doi: 10.3389/fphar.2022.794139
Received: 01 November 2021; Accepted: 10 February 2022;
Published: 21 March 2022.
Edited by:
Dan-Qian Chen, Northwest University, ChinaReviewed by:
Nicolas Roberto Robles, University of Salamanca, SpainShao-Yu Yang, National Taiwan University Hospital, Taiwan
Copyright © 2022 Chan, Yu, Yiu, Xue, Lok, Li, Zou, Ma, Lai and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sydney Chi-wai Tang, c2N3dGFuZ0Boa3UuaGs=
†ORCID: Kam Wa Chan, orcid.org/0000-0002-3175-1574; Sydney Chi-wai Tang, orcid.org/0000-0002-6862-1941
 Kam Wa Chan
Kam Wa Chan Kam Yan Yu
Kam Yan Yu Wai Han Yiu
Wai Han Yiu Rui Xue
Rui Xue Sydney Chi-wai Tang
Sydney Chi-wai Tang