
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pharmacol., 18 March 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.790136
This article is part of the Research TopicAction and Mechanism of Herbal GlycansView all 13 articles
 Xuemin Xie1†
Xuemin Xie1† Youliang Wu1†
Youliang Wu1† Haitao Xie1†
Haitao Xie1† Haiyan Wang1
Haiyan Wang1 Xiaojing Zhang1
Xiaojing Zhang1 Jiabin Yu1
Jiabin Yu1 Shaofang Zhu1
Shaofang Zhu1 Jing Zhao2*
Jing Zhao2* Lisen Sui1*
Lisen Sui1* Shaoping Li2*
Shaoping Li2*Epilepsy is a chronic neurological disorder. Current pharmacological therapies for epilepsy have limited efficacy that result in refractory epilepsy (RE). Owing to the limitations of conventional therapies, it is needed to develop new anti-epileptic drugs. The beneficial effects of polysaccharides from Chinese medicines, such as Lycium barbarum polysaccharides (COP) and Ganoderma lucidum polysaccharides (GLP), for treatment of epilepsy include regulation of inflammatory factors, neurotransmitters, ion channels, and antioxidant reactions. Especially, polysaccharides could be digested by intestinal microbial flora, referred as “intestinal brain organ” or “adult’s second brain”, may be the target for treatment of epilepsy. Actually, polysaccharides can effectively improve the type and quantity of intestinal flora such as bifidobacteria and lactic acid bacteria and achieve the purpose of treating epilepsy. Therefore, polysaccharides are hypothesized and discussed as potential agent for treatment of epilepsy.
Epilepsy is a chronic neurological disease that affects more than 50 million people worldwide and accounts for 0.6% of the global economic disease burden (WHO, 2016). Although WHO estimates that the seizures can be controlled by appropriate medications in 70% of epilepsy patients, only less than half of them have access to antiepileptic drugs in developing countries. In addition, there is still an estimated of 15 million patients have refractory epilepsy (RE) due to the pool response to existing anti-epileptic drugs (Lum et al., 2020). Therefore, RE has become a hot research topic of neurological treatment. In the past few decades, more than 20 kinds of anti-epileptic drugs have been developed while the incidence of RE has not been significantly reduced. The commonly used clinical antiepileptic drugs include sodium valproate (VPA), carbamazepine, phenobarbital, phenytoin sodium, ethylamine, oxcarbazepine, lamotrigine, topiramate, levetiracetam, lacoamide and so on. Each drug has its own unique physiological activity. For example, VPA is a broad-spectrum antiepileptic agent that can be used as either monotherapy or adjunctive therapy for generalized epilepsy. Common adverse reactions of VPA include gastrointestinal reaction, liver injury, tremor, increased sleep, and long-term application may include weight gain, hair loss, menstrual disorders, polycystic ovary syndrome, etc. Compared with carbamazepine, oxcarbazepine is characterized by weak liver enzyme induction, high bioavailability, better efficacy and safety. oxcarbazepine is mainly used for partial epilepsy in children. Common adverse reactions of oxcarbazepine include nausea, dizziness and diplopia. It is important to note that these drugs require long-term or even lifelong use, which makes patients more prone to hematopoietic damage, Stevens-Johnson syndrome, severe liver dysfunction, and aggravated cognitive impairment. Considering the limitation of anti-epileptics, it is need to develop new drugs with lower side effects and higher efficacy (Mehla et al., 2010). In last years, more and more studies have shown that chemicals in traditional Chinese medicines (TCMs), such as triglycerides and saponins, could be used as therapeutic agents for epilepsy by regulating inflammatory factors, neurotransmitters, ion channels and antioxidant responses (Zhang et al., 2019), which has unique advantages such as low side effects and reduced complications (Yuan et al., 2019). Polysaccharides might also be a potential agent for treatment of epilepsy. The therapeutic efficacy may be mainly derived from their prebiotic effect on gut microbiota.
Gut microbiota, which is called “intestinal brain organ” and “adult’s second brain”, is related to metabolic diseases, autoimmune diseases, and nervous system diseases (Pellegrini et al., 2018). Recent studies indicate that gut dysfunction/dysbiosis is presumably involved in the pathogenesis of and susceptibility to epilepsy. In addition, the reconstruction of the intestinal microbiome through, for example, faecal microbiota transplantation, probiotic intervention, and a ketogenic diet, has exhibited beneficial effects on drug-resistant epilepsy (Yue et al., 2021). Indeed, a few recent studies have highlighted differences in fecal microbiota profiles from selected epileptic individuals as compared to healthy controls (Lum et al., 2020). In addition, a 22-year-old Crohn’s disease patient with a 17-year history of seizures underwent a fecal microbiota transplant to treat Crohn’s disease (He et al., 2017). During the 20-month follow-up, the patient had no seizures despite discontinuation of the antiepileptic treatment with sodium valproate. Another study showed that probiotic treatment reduced the frequency of seizures by more than 50% in 28.9% of patients with drug-resistant to epilepsy (Gómez-Eguílaz et al., 2018). It was found that intestinal dysbiosis is associated with chronic stress-induced epilepsy in rats and members of the intestinal microbiota influence the anti-seizure effect of the ketogenic diet in mice. Recent studies in human cohorts suggest a dysbiosis in children with epilepsy. It may be possible that dysbiosis is more relevant in certain subtypes of epilepsy though larger studies with age-matched controls are needed to confirm (Dahlin and Prast-Nielsen, 2019). Braakman and Ingen described 6 cases of drug-resistant epilepsy, of which 5 had no seizures and 1 had a reduction in seizure frequency by more than 90% during antibiotic treatment. This effect disappeared within 2 weeks of stopping treatment, presumably due to the recovery of certain gut microbes (Braakman and Van Ingen, 2018) though other potential mechanisms might not be excluded (Sander and Perucca, 2003). Peng et al. (2018) found that the abundance of rare intestinal flora in patients with drug-resistant epilepsy increased abnormally, and the number of beneficial bacteria such as bifidobacteria and lactobacilli decreased. Olson et al. (2018) found that the ketogenic diet changes intestinal flora, which is a necessary part of the ketogenic diet, to exert its anti-epileptic effect. This information is linked to that a decreased amount of long-chain (such as arachidic, and oleic acid) and medium-chain fatty acids (sebacic acid and isocaproic acid) as well as bile acid was observed in patients with inflammatory bowel disease (Weng et al., 2019). Indeed, sebacic acid (SA) is a component of ketogenic diet and administered in pure form to inhibit the P-glycoprotein function and expression in an experimental model of refractory epilepsy (Enrique et al., 2021). Those above studies have indicated that regulating gut microbiota can achieve therapeutic effect of epilepsy. There are multiple interactions between gut microbiota and central nervous system (CNS). Gut microbiota affects the development and homeostasis of CNS through immune, circulatory and neural pathways, while CNS induces gut microbiota through stress and endocrine responses (Dinan and Cryan, 2017; Tremlett et al., 2017). The term “brain gut axis” is used to describe these two-way interactions (Bauer et al., 2016). Bagheri et al. found that there was a significant imbalance of intestinal flora in experimentally induced epileptic rats, and there was a certain proportion between the dominant flora in intestinal flora and seizures (Bagheri et al., 2019). The concentration of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) increased and the severity of epileptic seizures was significantly reduced in rats treated with probiotic supplements. This may be related to the fact that selective probiotics modulate the expression of specific GABA receptor subunits in brain regions (Liang et al., 2017). Studies have found that intestinal microflora α-diversity significantly increased in patients with refractory epilepsy, and the lower level of bifidobacteria and lactobacillus, the more frequent the seizures. Recently, some scholars have observed that intestinal flora can regulate the function of CNS in multiple ways and can affected epileptic seizures. In addition, intestinal microflora disorder may be caused by regulating immune and inflammatory responses, changing nutrient metabolism, activating and improving microglia and astrocyte functions, changing vagal nerve activity, and reducing neuroactive substances in limbic system such as hippocampus [such as brain-derived neurotrophin (neurotrophic factor)] increased the risk and susceptibility of epilepsy (Vuong et al., 2017). Therefore, it is believed that the intestinal flora may be a target for RE treatment (De Caro et al., 2019; He et al., 2021). The gut-brain bidirectional axis and the underlying mechanism of KD-based therapy targeting gut microbiome in in vivo animal models and clinical studies in neurological diseases have been reviewed (Rawat et al., 2020). Briefly, the intestinal microbiota can affect the balance of excitement/inhibition through neurotransmitters (mainly GABA, glutamate and 5-HT) or their precursors (such as tryptophan) (O'Mahony et al., 2015; Mittal et al., 2017), thereby affecting the occurrence and maintenance of epileptic seizures and the occurrence of epilepsy. Immune system-mediated pro-inflammatory effects (for example, the release of cytokines and chemokines) also increase the level of LPS due to the passage of the intestinal barrier. Increased permeability (Belkaid and Hand, 2014; Blander et al., 2017) and production of short-chain fatty acids (especially butyric acid, propionic acid and acetic acid) has an anti-inflammatory effect (Stilling et al., 2016; van de Wouw et al., 2018). In addition, neural (such as vagus nerve afferent, enteric nervous system) and neuroendocrine (such as hypothalamus-pituitary-adrenal axis) networks (Sudo et al., 2004; Cheung et al., 2019), as well as the endocannabinoid system (Rousseaux et al., 2007) and brain-derived neurotrophic factor level, may be affected by gut microbiota, and therefore further effect on the seizure mechanism. Many highly modifiable gut microbiota–brain axis pathways may be related to epilepsy.
Polysaccharides are biological macromolecules formed by the polymerization of more than 10 monosaccharides through glycosidic bonds. They are widely found in animals, plants and microorganisms. Indeed, TCMs are usually administered as decoction which contains larger proportion of polysaccharides. In recent years, with the development of “glycobiology”, studies have found that polysaccharides not only participate in various physiological activities, but also have a wide range of biological effects (Chen et al., 2016). Since lack of polysaccharides hydrolase, most polysaccharides cannot be directly digested and absorbed by human body. Intestinal flora play a mediating role in the process of interaction between polysaccharides and human body. Most of the beneficial health effects of polysaccharides have been associated with its reversal impacts on gut microbiota dysbiosis (Wang et al., 2021b). Mental illnesses, such as depression, Parkinson’s disease, Alzheimer’s disease and autism have been linked to gut microbiota. Actually, Flammulina velutipes polysaccharides contributed to significant improvements in mice learning and memory behavior through its gut microbiota regulation (Ma et al., 2021). Polysaccharides may also mainly contribute to their treatment of epilepsy through gut microbiota. The potential effects of polysaccharides from edible mushroom Grifola frondosa (GFP) on gut microbiota dysbiosis were investigated (Li et al., 2019). Metagenomic analysis revealed that GFP supplementation (400 mg/kg/day) resulted in significant structure changes on gut microbiota in high-fat diet (HFD)-fed rats, in particular modulating the relative abundance of functionally relevant microbial phylotypes compared with the HFD group. SP2-1, one homogeneous polysaccharide isolated from Scutellaria baicalensis Georgi can repair the intestinal barrier through up-regulated expressions of ZO-1, Occludin and Claudin-5. Furthermore, as compared with model group, the abundance of Firmicutes, Bifidobacterium, Lactobacillus, and Roseburia were significantly increased and the levels of Bacteroides, Proteobacteria and Staphylococcus were significantly inhibited with SP2-1 treatment. The modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides (ZJP) on intestinal microbiota were investigated and the gut flora structure was then analyzed using high-throughput sequencing. After ZJP treatment, there was a significant decrease in Firmicutes/Bacteroidetes, which suggested that ZJP showed prebiotic-like activities by positively modulating intestinal microbiota (Ji et al., 2020). Lycium barbarum polysaccharides (LBPS) treatment also could modulate the composition of the gut microbiota, increasing the relative abundances of Bacteroidaceae, Lactobacillaceae, Prevotellaceae and Verrucomicrobiaceae, which were positively associated with immune traits. The present results indicated that LBPS might regulate the immune response depending on the modulation of the gut microbiota, suggesting that LBPS could be developed as special ingredients for immunoregulation in association with the modulation of the gut microbiota (Ding et al., 2019).
A few studies provided evidence that intestinal inflammation was also a contributing factor to epileptic events for susceptible patients and a possible reason for the reduced efficacy of antiepileptic drugs, which made intestinal inflammation a promising antiepileptic drug target (Yue et al., 2021). Many Chinese herbal polysaccharides have immune regulation functions such as protecting the body’s immune organs, activating immune cells, activating the complement system, and releasing cytokines (Hong et al., 2019; Zeng et al., 2019; Xi et al., 2020; Wang et al., 2021a), these are beneficial to the treatment of epilepsy. In the early stage of epilepsy, excessive reactive oxygen species (ROS) free radical is produced in the body, causing inflammation. Polysaccharides are beneficial for the treatment of epilepsy through antiinflammation, regulating excitatory neurotransmitters and receptors, sodium/potassium ion channels and antioxidant activities (Yuan et al., 2019). Dendrobium officinale polysaccharides have anti-inflammatory, antioxidative and immunity-enhancement effects, which attribute to the treatment of epilepsy due to their strong anti-inflammatory and antioxidative effects (Zhang et al., 2019). Cornus officinalis fruit polysaccharides reduce the activation of ROS and Mitogen-activated protein kinaseMAPK cascade pathways in hippocampus after epilepsy, the change of mitochondrial membrane potential, the leakage of cytochrome C, and the activation of cleaved caspase-3, thereby reducing neuronal apoptosis and having neuroprotective effects on epilepsy (Sun et al., 2018). Glycyrrhiza uralensis polysaccharides (GUP) may inhibit the oxidative stress and inflammation in epileptic rats ignited by pentylenetetrazol by down-regulating the expression of hippocampal P2X7 receptor and NF-κB protein, and reduce neuropathological damage (Xiao et al., 2021). Additionally, after the intervention of LBPS in epilepsy model rats, the number of BrdU-positive cells in the granular layer of the hippocampus dentate gyrus, the expression of MAP-2 and NeuN-positive neurons were improved to a certain extent, and it has a good neuroprotective effect (Feng et al., 2017). LBPS also can improve the learning and memory ability of epileptic rats, and its mechanism may be related to the protection of hippocampal neurons by enhancing the anti-oxidative stress effect (Chen et al., 2020). GLP may increase the expression of GLAST, GLT-1 and EAAC1 to reduce neuronal excitability and reduce or inhibit epileptic seizures (Zhu et al., 2015). It may reduce the influx of calcium ions in nerve cells, thereby indirectly inhibiting the activation of NF-κB induced by pentylenetetrazol, reducing the excitability of nerve cells, and achieving anti-epileptic effects (Zhang et al., 2010). Table 1 summarized some TCMs polysaccharides and their effects on cell culture or animal model, and the mechanism.
The relationship among polysaccharides, intestinal flora and human health have been well reviewed and summarized (Liu et al., 2019; Song et al., 2021; Liang et al., 2021; Ma et al., 2021; Yin et al., 2020; Su et al., 2021). Polysaccharides could improve intestinal microecology by repairing intestinal barrier function, regulating the composition of intestinal flora, and regulating intestinal cytokine levels. Under normal circumstances, polysaccharides from TCMs can increase the number of beneficial bacteria such as Bacteroides, Firmicutes, and lactic acid bacteria, and reduce the number of harmful bacteria such as Enterococcus and Fusobacterium. It has also been detected that Chinese medicine polysaccharides can affect TNF-α, slgA, and NF⁃κB and other disease-related changes in biochemical indicators (Zhou et al., 2019). The effects of gut microbiota on epilepsy and potential anti-epileptic mechanism of polysaccharides were shown in Figure 1.
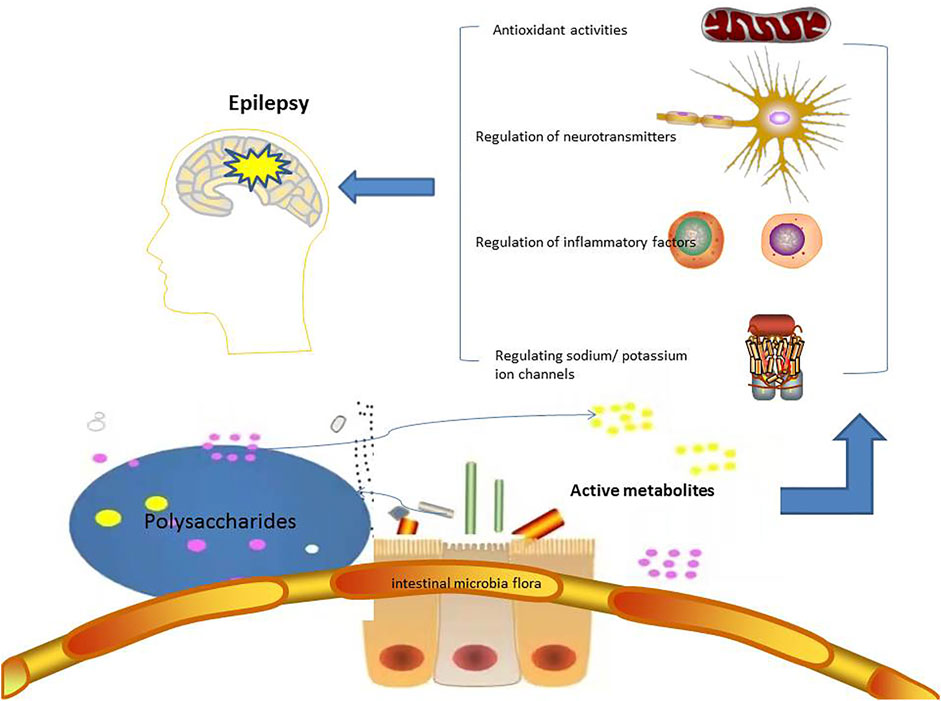
FIGURE 1. The potential anti-epileptic mechanism of polysaccharides targeting on gut microbiota. Polysaccharides are degraded into active metabolites by gut microbiota, and then to treat epilepsy in the ways of regulating inflammatory factors, neurotransmitters, ion channels, and antioxidant activites.
Current studies support the hypothesis that polysaccharides could be beneficial to the treatment of epilepsy. The evidence includes: 1) polysaccharides have the abilities to regulate inflammatory factors, neurotransmitters, ion channels, enhance immune function, promote the growth of intestinal flora and antioxidant responses. 2) polysaccharide can improve the gastrointestinal health function of the body, regulate the composition of intestinal flora, reshape the intestinal flora ecology and finally to produce anti-epileptic effects. Studies have shown that microbiota intervention could control seizures in animal models. However, in patients with epilepsy, polysaccharides as the next treatment of epilepsy drugs need to be comprehensively investigated with or without combination of anti-epilepsy drugs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
SL, JZ, and LS designed the topic and reviewed the manuscript. HW, XZ, JY, and SZ collected the references and did the statistical analysis. XX, YW, and HX wrote the manuscript. All authors contributed to the article and approved the submitted version.
The research was partially funded by grants from the Science and Technology Development Fund, Macau SAR (File no. 034/2017/A1 and 0017/2019/AKP), the Key-Area Research and Development Program of Guangdong Province (File no. 2020B1111110006) and the University of Macau (File no. MYRG2018-00083-ICMS/MYRG2019-00128-ICMS/CPG2022-00014-ICMS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bagheri, S., Heydari, A., Alinaghipour, A., and Salami, M. (2019). Effect of Probiotic Supplementation on Seizure Activity and Cognitive Performance in PTZ-Induced Chemical Kindling. Epilepsy Behav. 95, 43–50. doi:10.1016/j.yebeh.2019.03.038
Bauer, K. C., Huus, K. E., and Finlay, B. B. (2016). Microbes and the Mind: Emerging Hallmarks of the Gut Microbiota-Brain axis. Cell. Microbiol. 18, 632–644. doi:10.1111/cmi.12585
Belkaid, Y., and Hand, T. W. (2014). Role of the Microbiota in Immunity and Inflammation. Cell 157, 121–141. doi:10.1016/j.cell.2014.03.011
Blander, J. M., Longman, R. S., Iliev, I. D., Sonnenberg, G. F., and Artis, D. (2017). Regulation of Inflammation by Microbiota Interactions with the Host. Nat. Immunol. 18, 851–860. doi:10.1038/ni.3780
Braakman, H. M. H., and van Ingen, J. (2018). Can Epilepsy Be Treated by Antibiotics. J. Neurol. 265, 1934–1936. doi:10.1007/s00415-018-8943-3
Chen, B., Song, X., Song, Y., and Li, Y. (2020). Effects Of Lycium barbarum Polysaccharides On Learning And Memory Ability And Antioxidant Stress In Epileptic Rats. Chin. J. Tradit. Med. Sci. Technol. 27, 204–207.
Chen, Y., Yao, F., Ming, K., Wang, D., Hu, Y., and Liu, J. (2016). Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 21, 1705. doi:10.3390/molecules21121705
Cheung, S. G., Goldenthal, A. R., Uhlemann, A. C., Mann, J. J., Miller, J. M., and Sublette, M. E. (2019). Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 10, 34. doi:10.3389/fpsyt.2019.00034
Dahlin, M., and Prast-Nielsen, S. (2019). The Gut Microbiome and Epilepsy. EBioMedicine 44, 741–746. doi:10.1016/j.ebiom.2019.05.024
De Caro, C., Iannone, L. F., Citraro, R., Striano, P., De Sarro, G., Constanti, A., et al. (2019). Can We 'seize' the Gut Microbiota to Treat Epilepsy. Neurosci. Biobehav. Rev. 107, 750–764. doi:10.1016/j.neubiorev.2019.10.002
Dinan, T. G., and Cryan, J. F. (2017). Gut Instincts: Microbiota as a Key Regulator of Brain Development, Ageing and Neurodegeneration. J. Physiol. 595, 489–503. doi:10.1113/JP273106
Ding, Y., Yan, Y., Peng, Y., Chen, D., Mi, J., Lu, L., et al. (2019). In Vitro digestion under Simulated Saliva, Gastric and Small Intestinal Conditions and Fermentation by Human Gut Microbiota of Polysaccharides from the Fruits of Lycium Barbarum. Int. J. Biol. Macromol. 125, 751–760. doi:10.1016/j.ijbiomac.2018.12.081
Enrique, A. V., Di Ianni, M. E., Goicoechea, S., Lazarowski, A., Valle-Dorado, M. G., Costa, J. J. L., et al. (2021). New Anticonvulsant Candidates Prevent P-Glycoprotein (P-Gp) Overexpression in a Pharmacoresistant Seizure Model in Mice. Epilepsy Behav. 121, 106451. doi:10.1016/j.yebeh.2019.106451
Feng, Y., Liu, J., Tang, H. D., Huang, X. H., Chen, H. Y., and Su, L. (2017). Nervous Protection And Mechanism Of Lycium Barbarum Polysaccharides On Epileptic Rats. Chin. J. Gerontol. 37, 6036–6038.
Gómez-Eguílaz, M., Ramón-Trapero, J. L., Pérez-Martínez, L., and Blanco, J. R. (2018). The Beneficial Effect of Probiotics as a Supplementary Treatment in Drug-Resistant Epilepsy: a Pilot Study. Benef. Microbes 9, 875–881. doi:10.3920/BM2018.0018
He, L. Y., Hu, M. B., Li, R. L., Zhao, R., Fan, L. H., He, L., et al. (2021). Natural Medicines for the Treatment of Epilepsy: Bioactive Components, Pharmacology and Mechanism. Front. Pharmacol. 12, 604040. doi:10.3389/fphar.2021.604040
He, Z., Cui, B. T., Zhang, T., Li, P., Long, C. Y., Ji, G. Z., et al. (2017). Fecal Microbiota Transplantation Cured Epilepsy in a Case with Crohn's Disease: The First Report. World J. Gastroenterol. 23, 3565–3568. doi:10.3748/wjg.v23.i19.3565
Hong, C. Y., Zhang, H. D., Liu, X. Y., and Xu, Y. (2019). Attenuation of Hyperoxic Acute Lung Injury by Lycium Barbarum Polysaccharide via Inhibiting NLRP3 Inflammasome. Arch. Pharm. Res. 42, 902–908. doi:10.1007/s12272-019-01175-4
Ji, X., Hou, C., Gao, Y., Xue, Y., Yan, Y., and Guo, X. (2020). Metagenomic Analysis of Gut Microbiota Modulatory Effects of Jujube (Ziziphus Jujuba Mill.) Polysaccharides in a Colorectal Cancer Mouse Model. Food Funct. 11, 163–173. doi:10.1039/c9fo02171j
Jia, D., Rao, C., Xue, S., and Lei, J. (2015). Purification, Characterization and Neuroprotective Effects of a Polysaccharide from Gynostemma Pentaphyllum. Carbohydr. Polym. 122, 93–100. doi:10.1016/j.carbpol.2014.12.032
Li, L., Guo, W. L., Zhang, W., Xu, J. X., Qian, M., Bai, W. D., et al. (2019). Grifola Frondosa Polysaccharides Ameliorate Lipid Metabolic Disorders and Gut Microbiota Dysbiosis in High-Fat Diet Fed Rats. Food Funct. 10, 2560–2572. doi:10.1039/c9fo00075e
Liang, J., Wu, Y., Yuan, H., Yang, Y., Xiong, Q., Liang, C., et al. (2019). Dendrobium Officinale Polysaccharides Attenuate Learning and Memory Disabilities via Anti-oxidant and Anti-inflammatory Actions. Int. J. Biol. Macromol. 126, 414–426. doi:10.1016/j.ijbiomac.2018.12.230
Liang, J., Zhang, M., Wang, X., Ren, Y., Yue, T., Wang, Z., et al. (2021). Edible Fungal Polysaccharides, the Gut Microbiota, and Host Health. Carbohydr. Polym. 273, 118558. doi:10.1016/j.carbpol.2021.118558
Liang, L., Zhou, H., Zhang, S., Yuan, J., and Wu, H. (2017). Effects of Gut Microbiota Disturbance Induced in Early Life on the Expression of Extrasynaptic GABA-A Receptor α5 and δ Subunits in the hippocampus of Adult Rats. Brain Res. Bull. 135, 113–119. doi:10.1016/j.brainresbull.2017.09.014
Liu, L., Li, M., Yu, M., Shen, M., Wang, Q., Yu, Y., et al. (2019). Natural Polysaccharides Exhibit Anti-tumor Activity by Targeting Gut Microbiota. Int. J. Biol. Macromol. 121, 743–751. doi:10.1016/j.ijbiomac.2018.10.083
Lum, G. R., Olson, C. A., and Hsiao, E. Y. (2020). Emerging Roles for the Intestinal Microbiome in Epilepsy. Neurobiol. Dis. 135, 104576. doi:10.1016/j.nbd.2019.104576
Ma, G., Du, H., Hu, Q., Yang, W., Pei, F., and Xiao, H. (2021). Health Benefits of Edible Mushroom Polysaccharides and Associated Gut Microbiota Regulation. Crit. Rev. Food Sci. Nutr., 1–18. doi:10.1080/10408398.2021.1903385
Mehla, J., Reeta, K. H., Gupta, P., and Gupta, Y. K. (2010). Protective Effect of Curcumin against Seizures and Cognitive Impairment in a Pentylenetetrazole-Kindled Epileptic Rat Model. Life Sci. 87, 596–603. doi:10.1016/j.lfs.2010.09.006
Mittal, R., Debs, L. H., Patel, A. P., Nguyen, D., Patel, K., O'Connor, G., et al. (2017). Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cel. Physiol. 232, 2359–2372. doi:10.1002/jcp.25518
O'Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome axis. Behav. Brain Res. 277, 32–48. doi:10.1016/j.bbr.2014.07.027
Olson, C. A., Vuong, H. E., Yano, J. M., Liang, Q. Y., Nusbaum, D. J., and Hsiao, E. Y. (2018). The Gut Microbiota Mediates the Anti-seizure Effects of the Ketogenic Diet. Cell 173, 1728–e13. doi:10.1016/j.cell.2018.04.027
Pellegrini, C., Antonioli, L., Colucci, R., Blandizzi, C., and Fornai, M. (2018). Interplay Among Gut Microbiota, Intestinal Mucosal Barrier and Enteric Neuro-Immune System: a Common Path to Neurodegenerative Diseases. Acta Neuropathol. 136, 345–361. doi:10.1007/s00401-018-1856-5
Peng, A., Qiu, X., Lai, W., Li, W., Zhang, L., Zhu, X., et al. (2018). Altered Composition of the Gut Microbiome in Patients with Drug-Resistant Epilepsy. Epilepsy Res. 147, 102–107. doi:10.1016/j.eplepsyres.2018.09.013
Qin, X., Hua, J., Lin, S. J., Zheng, H. T., Wang, J. J., Li, W., et al. (2020). Astragalus Polysaccharide Alleviates Cognitive Impairment and β-amyloid Accumulation in APP/PS1 Mice via Nrf2 Pathway. Biochem. Biophys. Res. Commun. 531, 431–437. doi:10.1016/j.bbrc.2020.07.122
Rawat, K., Singh, N., Kumari, P., and Saha, L. (2020). A Review on Preventive Role of Ketogenic Diet (KD) in CNS Disorders from the Gut Microbiota Perspective. Rev. Neurosci. 32, 143–157. doi:10.1515/revneuro-2020-0078
Rousseaux, C., Thuru, X., Gelot, A., Barnich, N., Neut, C., Dubuquoy, L., et al. (2007). Lactobacillus Acidophilus Modulates Intestinal Pain and Induces Opioid and Cannabinoid Receptors. Nat. Med. 13, 35–37. doi:10.1038/nm1521
Sander, J. W., and Perucca, E. (2003). Epilepsy and Comorbidity: Infections and Antimicrobials Usage in Relation to Epilepsy Management. Acta Neurol. Scand. Suppl. 180, 16–22. doi:10.1034/j.1600-0404.108.s180.3.x
Song, Q., Wang, Y., Huang, L., Shen, M., Yu, Y., Yu, Q., et al. (2021). Review of the Relationships Among Polysaccharides, Gut Microbiota, and Human Health. Food Res. Int. 140, 109858. doi:10.1016/j.foodres.2020.109858
Stilling, R. M., van de Wouw, M., Clarke, G., Stanton, C., Dinan, T. G., and Cryan, J. F. (2016). The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain axis. Neurochem. Int. 99, 110–132. doi:10.1016/j.neuint.2016.06.011
Su, D., Li, S., Zhang, W., Wang, J., Wang, J., and Lv, M. (2017). Structural Elucidation of a Polysaccharide from Lonicera japonica Flowers, and its Neuroprotective Effect on Cerebral Ischemia-Reperfusion Injury in Rat. Int. J. Biol. Macromolecules 99, 350–357. doi:10.1016/j.ijbiomac.2017.02.096
Su, Y., Li, J., Wu, L., and Kuang, H. (2021). Polysaccharides from TCM Herbs Exhibit Potent Anti-obesity Effect by Mediating the Community Structure of Gut Microbiota. Pharmazie 76, 473–479. doi:10.1691/ph.2021.1463
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., et al. (2004). Postnatal Microbial Colonization Programs the Hypothalamic-Pituitary-Adrenal System for Stress Response in Mice. J. Physiol. 558, 263–275. doi:10.1113/jphysiol.2004.063388
Sun, X., Kong, L., and Zhou, L. (2018). Protective Effect of Fructus Corni Polysaccharide on Hippocampal Tissues and its Relevant Mechanism in Epileptic Rats Induced by Lithium Chloride-Pilocarpine. Exp. Ther. Med. 16, 445–451. doi:10.3892/etm.2018.6142
Tremlett, H., Bauer, K. C., Appel-Cresswell, S., Finlay, B. B., and Waubant, E. (2017). The Gut Microbiome in Human Neurological Disease: A Review. Ann. Neurol. 81, 369–382. doi:10.1002/ana.24901
van de Wouw, M., Boehme, M., Lyte, J. M., Wiley, N., Strain, C., O'Sullivan, O., et al. (2018). Short-chain Fatty Acids: Microbial Metabolites that Alleviate Stress-Induced Brain-Gut axis Alterations. J. Physiol. 596, 4923–4944. doi:10.1113/JP276431
Vuong, H. E., Yano, J. M., Fung, T. C., and Hsiao, E. Y. (2017). The Microbiome and Host Behavior. Annu. Rev. Neurosci. 40, 21–49. doi:10.1146/annurev-neuro-072116-031347
Wang, Y., Sun, M., Jin, H., Yang, J., Kang, S., Liu, Y., et al. (2021b). Effects of Lycium Barbarum Polysaccharides on Immunity and the Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Microbiol. 12, 701566. doi:10.3389/fmicb.2021.701566
Wang, Y., Zhang, X., Wang, Y., Zhao, W., Li, H., Zhang, L., et al. (2021a). Application of Immune Checkpoint Targets in the Anti-tumor Novel Drugs and Traditional Chinese Medicine Development. Acta Pharm. Sin. B 11, 2957–2972. doi:10.1016/j.apsb.2021.03.004
Weng, Y. J., Gan, H. Y., Li, X., Huang, Y., Li, Z. C., Deng, H. M., et al. (2019). Correlation of Diet, Microbiota and Metabolite Networks in Inflammatory Bowel Disease. J. Dig. Dis. 20, 447–459. doi:10.1111/1751-2980.12795
Xi, S., Li, Y., Yue, L., Gong, Y., Qian, L., Liang, T., et al. (2020). Role of Traditional Chinese Medicine in the Management of Viral Pneumonia. Front. Pharmacol. 11, 582322. doi:10.3389/fphar.2020.582322
Xiao, Z., Deng, Q., Zhou, W., and Zhang, Y. (2021). Immune Activities of Polysaccharides Isolated from Lycium Barbarum L. What Do We Know So Far. Pharmacol. Ther. 229, 107921. doi:10.1016/j.pharmthera.2021.107921
Yang, Y., Liu, P., Chen, L., Liu, Z., Zhang, H., Wang, J., et al. (2013). Therapeutic Effect of Ginkgo Biloba Polysaccharide in Rats with Focal Cerebral Ischemia/reperfusion (I/R) Injury. Carbohydr. Polym. 98, 1383–1388. doi:10.1016/j.carbpol.2013.07.045
Yin, C., Noratto, G. D., Fan, X., Chen, Z., Yao, F., Shi, D., et al. (2020). The Impact of Mushroom Polysaccharides on Gut Microbiota and its Beneficial Effects to Host: A Review. Carbohydr. Polym. 250, 116942. doi:10.1016/j.carbpol.2020.116942
Yuan, X., Li, Z., Wang, X. T., Li, X. Y., Hua, H., Li, X. C., et al. (2019). China J. Chin. Materia Med. 44, 9–18. doi:10.19540/j.cnki.cjcmm.20181012.006
Yue, Q., Cai, M., Xiao, B., Zhan, Q., and Zeng, C. (2021). The Microbiota-Gut-Brain axis and Epilepsy. Cell. Mol. Neurobiol.. doi:10.1007/s10571-021-01130-210.1007/s10571-021-01130-2
Zeng, P., Li, J., Chen, Y., and Zhang, L. (2019). The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl. Sci. 163, 423–444. doi:10.1016/bs.pmbts.2019.03.003
Zhang, L., Peng, H., Xu, J., Xu, Y., Yin, Y., He, B., et al. (2019). Effects of Dendrobium Officinale Polysaccharides on Brain Inflammation of Epileptic Rats. Int. J. Polym. Sci. 2019, 1–6. doi:10.1155/2019/9058161
Zhang, T., Yang, Y., Liang, Y., Jiao, X., and Zhao, C. (2018). Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 10, 1055. doi:10.3390/nu10081055
Zhang, X. Y., Guo, Z. Y., Chen, Y., Wang, D., and Chen, Y. (2010). Recent advance in Antiepileptic Chinese Medicine Monomer. J. Liaoning Univ. Tradit. Chin. Med. 22, 129–132. doi:10.1016/S1875-5364(20)60031-0
Zhou, Y., Guo, X., Chen, W., and Liu, J. (2019). Angelica Polysaccharide Mitigates Lipopolysaccharide-Evoked Inflammatory Injury by Regulating microRNA-10a in Neuronal Cell Line HT22. Artif. Cell Nanomed. Biotechnol. 47, 3194–3201. doi:10.1080/21691401.2019.1614595
Keywords: polysaccharides, epilepsy, traditional Chinese medicines, gut microbiome, treatment
Citation: Xie X, Wu Y, Xie H, Wang H, Zhang X, Yu J, Zhu S, Zhao J, Sui L and Li S (2022) Polysaccharides, Next Potential Agent for the Treatment of Epilepsy?. Front. Pharmacol. 13:790136. doi: 10.3389/fphar.2022.790136
Received: 06 October 2021; Accepted: 20 January 2022;
Published: 18 March 2022.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Alberto Lazarowski, University of Buenos Aires, ArgentinaCopyright © 2022 Xie, Wu, Xie, Wang, Zhang, Yu, Zhu, Zhao, Sui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhao, amluZ3poYW9AdW0uZWR1Lm1v; Lisen Sui, MTM3MTE1ODA4OTFAMTYzLmNvbQ==; Shaoping Li, c3BsaUB1bS5lZHUubW8=, bGlzaGFvcGluZ0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.