
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 09 February 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.778505
 Ye Peng1†
Ye Peng1† Xiaohui Zeng2†
Xiaohui Zeng2† Liubao Peng1
Liubao Peng1 Qiao Liu1
Qiao Liu1 Lidan Yi1
Lidan Yi1 Xia Luo1
Xia Luo1 Sini Li3
Sini Li3 Liting Wang1
Liting Wang1 Shuxia Qin1
Shuxia Qin1 Xiaomin Wan1*
Xiaomin Wan1* Chongqing Tan1*
Chongqing Tan1*Objective: The ORIENT-32 clinical trial revealed that sintilimab plus bevacizumab biosimilar significantly improved the median progression-free survival and median overall survival (OS) compared with sorafenib. This analysis evaluated the cost-effectiveness of sintilimab plus bevacizumab biosimilar as a first-line treatment for unresectable hepatocellular carcinoma from the Chinese perspective of healthcare system.
Materials and methods: A Markov model with three mutual health states was constructed to evaluate the economic outcome of sintilimab plus bevacizumab biosimilar. The model cycle was 21 days, and the simulation time horizon was a lifetime. The output parameters of the model were the total cost, life-year (LY), quality-adjusted LY (QALY), and incremental cost-effectiveness ratio (ICER). Sensitivity analyses were conducted to assess the robustness of the results.
Results: The base-case results found that sintilimab plus bevacizumab biosimilar provided an improvement of 1.27 QALYs and 1.84 LYs compared with sorafenib, and the ICER was $23,352/QALY. The hazard ratio for OS had the greatest influence on the ICER. The probability of sintilimab plus bevacizumab biosimilar was 85% at willingness-to-pay thresholds of $30,552/QALY.
Conclusion: The findings of this analysis suggested that sintilimab plus bevacizumab biosimilar was a cost-effective first-line therapy for patients with unresectable hepatocellular carcinoma.
Primary liver cancer is the third leading cause of cancer-related deaths in the world, with an estimated 830,000 deaths in 2020 (Sung et al., 2021). Its main type is hepatocellular carcinoma (HCC), which accounts for 75–85% of all cases (Sung et al., 2021). The predominant causative factor of HCC is hepatitis B virus (HBV). The new cases of HBV infection in China account for about half of the global cases (Chen et al., 2016). The burden of HCC in China is high, and the prognosis of patients with HCC is poor (Ren et al., 2021).
Sorafenib, as the first-line standard care for advanced HCC, has moderate efficacy and limited survival benefits (Llovet et al., 2008; Cheng et al., 2009; Kudo et al., 2018). However, recent research progress has shown that the combination of anti–programmed cell death-1 (PD-1) antibodies and anti-angiogenic drugs may become a potential first-line therapy for HCC (Ren et al., 2021). The ORIENT-32 clinical trial evaluated the efficacy and safety of sintilimab, a selective anti-PD-1 antibody (Gao et al., 2020), in combination with bevacizumab biosimilar in the treatment of unresectable HCC (Ren et al., 2021). The results revealed that sintilimab plus bevacizumab biosimilar significantly improved the median progression-free survival (PFS) and median overall survival (OS) when compared with sorafenib. Sintilimab plus bevacizumab biosimilar also had a lower incidence of grade 3–5 adverse events (AEs) than sorafenib (29 vs. 47%). Therefore, the sintilimab plus bevacizumab biosimilar therapy seems to be an attractive option as a first-line treatment for unresectable HCC. The present analysis evaluated the cost-effectiveness of sintilimab plus bevacizumab biosimilar as a first-line treatment for unresectable HCC from the perspective of the Chinese healthcare system.
This analysis assumed that the target population was patients with unresectable HCC who had not previously received systemic therapy, which is consistent with the ORIENT-32 trial (Ren et al., 2021). A Markov model with three mutual health states was constructed to evaluate the economic outcome of sintilimab plus bevacizumab biosimilar or with sorafenib in unresectable HCC (Figure 1). The model cycle was 21 days, and the simulation time horizon was a lifetime. The output parameters of the model were total cost, life-year (LY), quality-adjusted LY (QALY), and incremental cost-effectiveness ratio (ICER). All costs were adjusted to be expressed in US dollars, and the exchange rate was 1 US dollar = 7.07 Chinese yuan (June 2020). The costs and outcomes were adjusted using an annual discount rate of 3% (Sanders et al., 2016). The willingness-to-pay (WTP) threshold was proposed to be three times that of the per-capita gross domestic product of China in 2020 (US $30,552) (Murray et al., 2000). Statistical analysis was performed using TreeAge Pro (TreeAge Software, Williamstown, MA) and R.
Among the three health states, the probability of staying in the progression-free disease (PFD) state was based on the PFS curve. The transition probability from PFD state to progressed disease (PD) state was estimated by the difference between OS and PFS curves. The probability of death was obtained by subtracting the area under the OS curve from 1. The PFS and OS data of patients in the sorafenib arm were derived from the results of the ORIENT-32 trial (Ren et al., 2021), and the data beyond the clinical trial time horizon were extrapolated using the method described by Guyot et al. (2012). First, the data points in the survival curves were gathered using GetData Graph Digitizer, and then the parametric survival functions of Weibull, log-logistic, log-normal, exponential, Gompertz, and generalized gamma were fitted. The final survival functions were selected for the sorafenib arm based on the Akaike information criterion. The transition parameters and proportions are shown in Table 1 and Figure 2. The PFS and OS data for the sintilimab plus bevacizumab biosimilar arm were calculated based on the hazard ratios (HRs) of sintilimab plus bevacizumab biosimilar versus sorafenib reported in the ORIENT-32 trial. After the disease progressed, the data on patients receiving subsequent treatment were derived from the ORIENT-32 trial (Ren et al., 2021). The key clinical inputs are demonstrated in Table 1.
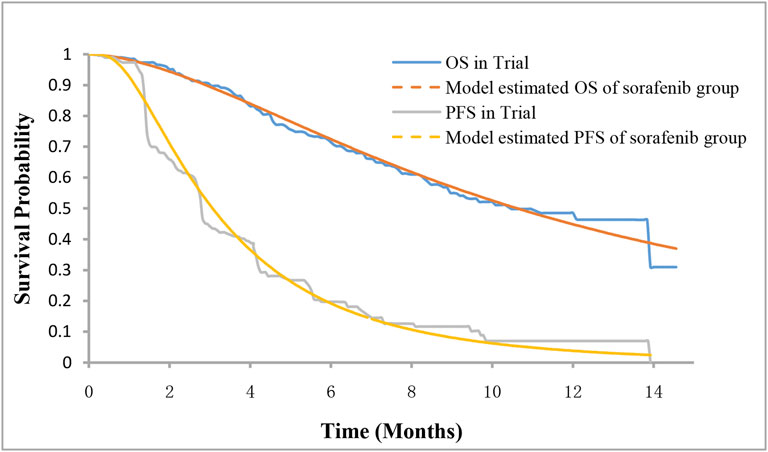
FIGURE 2. Survival curves for model and the phase III trial. PFS, progression-free survival; OS, overall survival.
Only direct medical costs such as drugs cost, testing cost, AEs management cost, and best supportive treatment were considered (Table 1). The costs were reported in 2020 US dollars and adjusted to 2020 value using the consumer price index (National Bureau Of Statistics Of China, 2021).
Based on the ORIENT-32 trial (Ren et al., 2021), patients in the sintilimab plus bevacizumab biosimilar arm received intravenous injections of 200 mg sintilimab and 15 mg/kg bevacizumab biosimilar every 3 weeks. Patients in the sorafenib arm received 400 mg of sorafenib orally twice daily. To calculate the dose of bevacizumab biosimilar, a typical Chinese patient weighing 60 kg was assumed (Wen et al., 2021). Treatment in both arms continued until disease progression or unacceptable toxicity, or the sintilimab plus bevacizumab biosimilar arm was treated for up to 24 months. After disease progression, subsequent therapy was received in 29% of patients in the sintilimab plus bevacizumab biosimilar arm and 47.0% of patients in the sorafenib arm (Ren et al., 2021). Tyrosine kinase inhibitors (regorafenib) and immune agents (pembrolizumab) were hypothesized to be the second-line options of the two arms based on the ORIENT-32 trial (Ren et al., 2021). All cost information are shown in Table 1.
The utility values of PFD and PD states related to HCC were assumed to be 0.76 and 0.68 (Su et al., 2021), respectively, based on the published cost-effectiveness analysis of patients with HCC. The disutility values related to AEs and the costs related to managing grade ≥3 AEs were also included in this analysis (Table 1) (Su et al., 2021; Wen et al., 2021).
The ICER was the incremental cost for each additional QALY between the sintilimab plus bevacizumab biosimilar arm and the sorafenib arm. It was assumed to be cost-effective when the ICER was below the prespecified WTP threshold ($30,552/QALY) (Neumann et al., 2014). Sensitivity analyses were conducted to assess the robustness of the results. One-way sensitivity analysis was performed on all key parameters. The range of each parameter was set based on the published literature or assumed to be a 20% change in the base-case value (Table 1). Monte Carlo simulations with 10,000 iterations were performed in the probabilistic sensitivity analysis. Pre-specified distributions for all key parameters are shown in Table 1. The cost-effectiveness acceptability curve was used to indicate the possibility of being considered cost-effective at different WTP levels.
The base-case results of the cost-effectiveness analysis are shown in Table 2. The sintilimab plus bevacizumab biosimilar treatment provided 2.30 QALYs and 3.30 overall LYs, with an accompanying cost of $59,018. The sorafenib treatment provided 1.03 QALYs and 1.47 LYs at a cost of $29,351. The ICER for sintilimab plus bevacizumab biosimilar vs sorafenib was estimated to be $23,352/QALY.
The results of the one-way sensitivity analysis showed that the HR for OS had the greatest influence on the ICER, followed by the utility of PD, the proportion receiving subsequent treatment, and the cost of bevacizumab biosimilar (Figure 3). However, these parameters varied within a range, and the ICER has always remained below the threshold of $30,552/QALY. The results of the probabilistic sensitivity analysis were displayed by the cost-effectiveness acceptability curve. The probability of sintilimab plus bevacizumab biosimilar being cost-effective increased as the WTP thresholds increased (Figure 4). Sintilimab plus bevacizumab biosimilar has an 85% probability of being cost-effective compared with sorafenib at the WTP thresholds of $30,552/QALY.
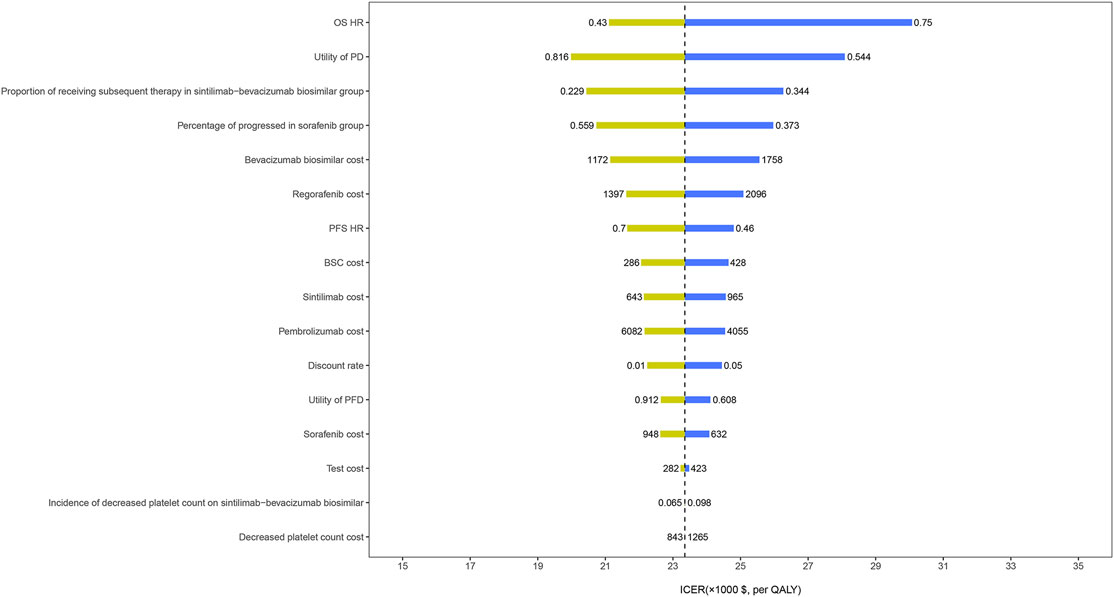
FIGURE 3. One-way sensitivity analyses. OS, overall survival; HR, hazard ratio; PD, progressed disease; PFS, progression-free survival; BSC, best supportive care; PFD, progression-free disease; QALYs, quality-adjusted life-years; ICER, incremental cost-effectiveness ratio.
We conducted a cost-effectiveness analysis of sintilimab plus bevacizumab biosimilar versus sorafenib in the first-line treatment of unresectable HCC. The ICER for sintilimab plus bevacizumab biosimilar was estimated as $23,352/QALY versus sorafenib. The probabilistic sensitivity analysis indicated that the probability of sintilimab plus bevacizumab biosimilar was 85% at the WTP threshold of $30,552/QALY.
Recently, the immune checkpoint inhibitor (ICI) combination with PD-1 antibody and cytotoxic T-lymphocyte–associated protein-4 (CTLA-4) antibody has been proved to be effective for advanced HCC. The Phase 1/2 clinical trial (CheckMate 040) from 31 centers in 10 countries showed that nivolumab plus ipilimumab had manageable safety, promising objective response rate, and durable responses for patients with advanced HCC previously treated with sorafenib (Yau et al., 2020). In 2020, the U.S. Food and Drug Administration approved nivolumab in combination with ipilimumab for the treatment of patients with HCC previously treated with sorafenib (Saung et al., 2021). However, there is currently no relevant and sufficient large-scale phase III clinical trial data. Once the data is available, we will conduct a pharmacoeconomic evaluation on it.
The choice of the comparator in the model is an important issue in cost-effectiveness analysis. According to the treatment guidelines for HCC, there are several first-line therapy options for unresectable HCC (Bureau of Medical Administration National Harmon Health Commission of the People’s Republic of China, 2020). This makes the choice of comparator a challenge. Lenvatinib, another first-line targeted therapy approved for unresectable HCC has not been included in this study. But we speculate that the ICER of this group might be lower than the estimate in our study due to similar clinical efficacy and higher cost when compared with sorafenib (Kudo et al., 2018). Due to the lack of head-to-head data, other immune checkpoint inhibitors have not been included in the evaluation, such as atezolizumab plus bevacizumab, which also have favorable survival benefits as a first-line treatment therapy for unresectable HCC (Finn et al., 2020). Two economic evaluations in China comparing sorafenib with atezolizumab plus bevacizumab have found sorafenib to be cost-effective (Wen et al., 2021; Hou and Wu 2020).
In our study, sintilimab plus bevacizumab biosimilar was superior to sorafenib, which is different from a previous study on atezolizumab and bevacizumab. The present study suggests that the cost-effectiveness of the ICI combination therapy might not be due to the therapeutic effect but to biosimilar itself. Bevacizumab biosimilars are similar to the reference bevacizumab in terms of efficacy and safety (Yang et al., 2019; Zhang et al., 2019), but the price of bevacizumab biosimilars is much lower than the price of the original drug. Therefore, this will increase the probability that sintilimab combined with bevacizumab is cost-effective. In recent years, value-based cancer treatment has become a hot topic in oncology. The development of biosimilar products provides patients with affordable alternative therapy.
Some studies have evaluated the cost-effectiveness of immunotherapy in HCC. Almost all studies have shown that immunotherapy is unlikely to be a cost-effective option compared to sorafenib, although the ICERs reported by these studies vary (Hou and Wu 2020; Chiang et al., 2021a; Su et al., 2021; Wen et al., 2021; Zhang et al., 2021). Four of these studies that used clinical data from the IMbrave150 trial evaluated the economics of atezolizumab plus bevacizumab in the first-line treatment of HCC from the perspective of the United States (Chiang et al., 2021a; Su et al., 2021; Wen et al., 2021; Zhang et al., 2021). The study by Wen et al. (2021) also evaluated the economics of atezolizumab plus bevacizumab from the perspective of China, with an ICER of $145,546.21/QALY (Wen et al., 2021). A China-based study showed that atezolizumab plus bevacizumab was unlikely to be cost-effective compared with sorafenib as a frontline treatment for patients with unresectable HCC, with an ICER was $64,613/QALY (Hou and Wu 2020). Based on the clinical data of the KEYNOTE-240 trial, the study by Chiang et al. (2021b) conducted a cost-effectiveness analysis of pembrolizumab as a second-line treatment for HCC in the United States. The results indicated that pembrolizumab was unlikely to be cost-effective. To our knowledge, our study was the first to analyze the cost-effectiveness of sintilimab plus bevacizumab biosimilar in the first-line treatment of HCC. There is some strength to this analysis that is worth highlighting. First, this analysis evaluated the economic outcomes of sintilimab plus bevacizumab biosimilar in the treatment of unresectable HCC through economic modeling methods and the synthesis of the latest evidence. Monotherapy blockade of PD-1 alone or combined with other regimens is becoming an attractive option for the treatment of unresectable HCC (Mahipal et al., 2019). Furthermore, this study addressed the unmet needs of the economic evaluation related to advanced HCC.
This analysis also has limitations. First, the efficacy and safety parameters of the model are essentially based on the results of the ORIENT-32 trial. Any bias in this trial may inevitably affect the cost and effectiveness. Second, based on the Kaplan–Meier PFS and OS data reported in the ORIENT-32 trial, the long-term survival data beyond the observation time was extrapolated by the fitting of the parametric distribution. This may lead to uncertainty in the output of the model, although the model and observation data have been validated. Third, data availability and assumptions have also led to the limitation of our analysis. The use of ±20% variation in range in the sensitivity analysis to explain the uncertainty may not reflect the true situation. However, it had also been used as an acceptable boundary in similar studies (Kohn et al., 2017). Fourth, the management cost of grade 1–2 AEs has not been considered in this analysis. However, the sorafenib arm had a higher incidence of grade 1–2 AEs than the sintilimab plus bevacizumab biosimilar arm, which may cause ICER to be lower than the estimated value of our study. Moreover, the results of the one-way sensitivity analysis showed that the costs associated with AEs were minor. This study showed that sintilimab plus bevacizumab biosimilar was a cost-effective first-line therapy for patients with unresectable HCC. These findings may help clinicians make optimal decisions for the treatment of HCC.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Study design and supervision: XW and CT; data analysis and interpretation: SL, LY, and XL; data collection: LP, LW, SQ, and QL; manuscript writing: YP and XZ; final approved of manuscript: All authors.
The work was supported by grants from the National Natural Science Foundation of China (grant numbers: 82073818 and 71874209); and the Key Science-Technology Research and Development Program of Hunan Province (grant number: 2020JJ8046); and the Hunan Provincial Natural Science Foundation of China (grant number: 2019JJ40411).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bureau of Medical AdministionNational Harmon Health Commission of the People’s Republic of China (2020). Guidellines for Diagonosis and Treatment of Prime Minister Cancer in China (2019 Edition). J. Clin. Hepatol. 36 (2), 277–292. doi:10.3969/j.issn.001-526.2020.007
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer Statistics in China, 2015. CA Cancer J. Clin. 66 (2), 115–132. doi:10.3322/caac.21338
Cheng, A. L., Kang, Y. K., Chen, Z., Tsao, C. J., Qin, S., Kim, J. S., et al. (2009). Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: a Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 10 (1), 25–34. doi:10.1016/S1470-2045(08)70285-7
Chiang, C.-L., Chan, S.-K., Lee, S.-F., and Choi, H. C.-W. (2021). First-Line Atezolizumab Plus Bevacizumab versus Sorafenib in Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Cancers 13 (5), 931. doi:10.3390/cancers13050931
Chiang, C. L., Chan, S. K., Lee, S. F., Wong, I. O., and Choi, H. C. (2021). Cost-effectiveness of Pembrolizumab as a Second-Line Therapy for Hepatocellular Carcinoma. JAMA Netw. Open 4 (1), e2033761. doi:10.1001/jamanetworkopen.2020.33761
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2020). Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 382 (20), 1894–1905. doi:10.1056/NEJMoa1915745
Gao, S., Li, N., Gao, S., Xue, Q., Ying, J., Wang, S., et al. (2020). Neoadjuvant PD-1 Inhibitor (Sintilimab) in NSCLC. J. Thorac. Oncol. 15 (5), 816–826. doi:10.1016/j.jtho.2020.01.017
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Hou, Y., and Wu, B. (2020). Atezolizumab Plus Bevacizumab versus Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: a Cost-Effectiveness Analysis. Cancer Commun. (Lond) 40 (12), 743–745. doi:10.1002/cac2.12110
Kohn, C. G., Zeichner, S. B., Chen, Q., Montero, A. J., Goldstein, D. A., and Flowers, C. R. (2017). Cost-Effectiveness of Immune Checkpoint Inhibition in BRAF Wild-type Advanced Melanoma. J. Clin. Oncol. 35 (11), 1194–1202. doi:10.1200/JCO.2016.69.6336
Kudo, M., Finn, R. S., Qin, S., Han, K. H., Ikeda, K., Piscaglia, F., et al. (2018). Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: a Randomised Phase 3 Non-inferiority Trial. Lancet 391 (10126), 1163–1173. doi:10.1016/S0140-6736(18)30207-1
Li, M., Lin, S., Wilson, L., Huang, P., Wang, H., Lai, S., et al. (2021). Cost-Effectiveness Analysis of Hepatic Arterial Infusion of FOLFOX Combined Sorafenib for Advanced Hepatocellular Carcinoma with Portal Vein Invasion. Front. Oncol. 11, 562135. doi:10.3389/fonc.2021.562135
Llovet, J. M., Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J. F., et al. (2008). Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 359 (4), 378–390. doi:10.1056/NEJMoa0708857
Mahipal, A., Tella, S. H., Kommalapati, A., Lim, A., and Kim, R. (2019). Immunotherapy in Hepatocellular Carcinoma: Is There a Light at the End of the Tunnel. Cancers (Basel) 11 (8). doi:10.3390/cancers11081078
Murray, C. J., Evans, D. B., Acharya, A., and Baltussen, R. M. (2000). Development of WHO Guidelines on Generalized Cost-Effectiveness Analysis. Health Econ. 9 (3), 235–251. doi:10.1002/(sici)1099-1050(200004)9:3<235:aid-hec502>3.0.co;2-o
National Bureau Of Statistics Of China.(2021) National Bureau of Statistics of China. Available at: http://data.stats.gov.cn/english/index.htm, (accessed 24 July 2021).
Neumann, P. J., Cohen, J. T., and Weinstein, M. C. (2014). Updating Cost-Effectiveness-Tthe Curious Resilience of the $50,000-Per-QALY Threshold. N. Engl. J. Med. 371 (9), 796–797. doi:10.1056/NEJMp1405158
Ren, Z., Xu, J., Bai, Y., Xu, A., Cang, S., Du, C., et al. (2021). Sintilimab Plus a Bevacizumab Biosimilar (IBI305) versus Sorafenib in Unresectable Hepatocellular Carcinoma (ORIENT-32): a Randomised, Open-Label, Phase 2-3 Study. Lancet Oncol. 22 (7), 977–990. doi:10.1016/S1470-2045(21)00252-7
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama 316 (10), 1093–1103. doi:10.1001/jama.2016.12195
Saung, M. T., Pelosof, L., Casak, S., Donoghue, M., Lemery, S., Yuan, M., et al. (2021). FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist 26 (9), 797–806. doi:10.1002/onco.13819
Su, D., Wu, B., and Shi, L. (2021). Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma. JAMA Netw. Open 4 (2), e210037. doi:10.1001/jamanetworkopen.2021.0037
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Wen, F., Zheng, H., Zhang, P., Liao, W., Zhou, K., and Li, Q. (2021). Atezolizumab and Bevacizumab Combination Compared with Sorafenib as the First-Line Systemic Treatment for Patients with Unresectable Hepatocellular Carcinoma: A Cost-Effectiveness Analysis in China and the United states. Liver Int. 41 (5), 1097–1104. doi:10.1111/liv.14795
Yang, Y., Wu, B., Huang, L., Shi, M., Liu, Y., Zhao, Y., et al. (2019). Biosimilar Candidate IBI305 Plus Paclitaxel/carboplatin for the Treatment of Non-squamous Non-small Cell Lung Cancer. Transl Lung Cancer Res. 8 (6), 989–999. doi:10.21037/tlcr.2019.12.23
Yau, T., Kang, Y. K., Kim, T. Y., El-Khoueiry, A. B., Santoro, A., Sangro, B., et al. (2020). Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 6 (11), e204564. doi:10.1001/jamaoncol.2020.4564
Zhang, H., Zhu, X., Wei, H., Li, C., Chen, H., Li, X., et al. (2019). A phase I, randomized, double-blinded, single-dose study evaluating the pharmacokinetic equivalence of the biosimilar IBI305 and bevacizumab in healthy male subjects;. Int. J. Clin. Pharmacol. Ther. 57 (3), 167–174. doi:10.5414/CP203349
Keywords: sintilimab, bevacizumab biosimilar, cost-effectiveness, hepatocellular carcinoma, markov model
Citation: Peng Y, Zeng X, Peng L, Liu Q, Yi L, Luo X, Li S, Wang L, Qin S, Wan X and Tan C (2022) Sintilimab Plus Bevacizumab Biosimilar Versus Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma: A Cost-Effectiveness Analysis. Front. Pharmacol. 13:778505. doi: 10.3389/fphar.2022.778505
Received: 17 September 2021; Accepted: 14 January 2022;
Published: 09 February 2022.
Edited by:
Francis Kalemeera, University of Namibia, NamibiaReviewed by:
Kaiyan Liu, University of Maryland, Baltimore, United StatesCopyright © 2022 Peng, Zeng, Peng, Liu, Yi, Luo, Li, Wang, Qin, Wan and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomin Wan, d2FueGlhb21pbkBjc3UuZWR1LmNu; Chongqing Tan, dGFuY2hvbmdxaW5nQGNzdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.