
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 02 March 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.768980
This article is part of the Research Topic From Clinical Trials to Real-World Data Sciences: Evidence-Based Medicine for Value in Health View all 36 articles
Objective: This study aimed to assess neoplasm risk in patients with rheumatoid arthritis (RA) treated with fostamatinib.
Methods: Studies were collected from electronic databases of OVID Medline, OVID EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science. We included studies that reported neoplasms in patients with RA treated with fostamatinib. Study selection was repeated by two reviewers based on the study selection criteria. Data were collected and methodological quality assessment was performed. Data were pooled using the Peto odds ratio (OR) with a 95% confidence interval (CI). Subgroup analyses of the fostamatinib dose, trial duration, neoplasm nature, and neoplasm-originating systems were conducted. A funnel plot was used to estimate publication bias, and sensitivity analysis was performed to test the robustness of the results.
Results: Seven trials involving 4,971 participants showing low to moderate risk of bias were included. Compared with the placebo, fostamatinib use was not associated with the risks of overall neoplasms (Peto OR = 2.62, 95%CI 0.97–7.10), malignant neoplasms (Peto OR = 3.08, 95%CI 0.96–9.91), or benign neoplasms (Peto OR = 1.71, 95%CI 0.26–11.36). Nevertheless, compared with the placebo, a longer duration of fostamatinib use had a higher risk of malignant neoplasms (Peto OR = 4.49, 95%CI 1.03–19.60) at 52 weeks. As for malignant neoplasms in the digestive system, lower doses of fostamatinib reduced the neoplasm risk (100 mg bid vs 150 mg qd: Peto OR = 0.06, 95%CI 0.01–0.59). Sensitivity analysis showed no significant differences in the effective trends, and no publication bias was found.
Conclusion: Fostamatinib is not associated with the risks of overall neoplasms as compared to placebo. Nevertheless, a longer duration of fostamatinib use may be associated with a risk of malignant neoplasms and higher doses of fostamatinib may increase malignant neoplasms in the digestive system. Further well-planned cohort studies with a larger study population are needed to elucidate these outcomes.
Systematic Review Registration: PROSPERO (CRD42020202121).
Rheumatoid arthritis (RA) is an autoimmune disease characterized by persistent synovitis formation, systematic inflammation, and autoantibodies presence, leading to bone and cartilage damage if not treated appropriately (Scott et al., 2010; Smolen et al., 2016). The risk factors for RA include susceptibility genes, environmental factors (e.g., oral microbiome, smoking, periodontitis, and microbiome), epigenetic modifications, and posttranslational modifications (e.g., methylation, acetylation, and citrullination). RA is associated with several comorbidities, such as cancer and cardiovascular diseases, which are risk factors for increased mortality (Dougados, 2016; Widdifield et al., 2018).
Compared with the general population, patients with RA are associated with increased risks of overall malignant neoplasms [standardized incidence ratio (SIR) = 1.05, 95% confidence interval (CI) (1.01–1.09)], lymphoma [2.08, 95%CI (1.80–2.39)], lung cancer [1.63, 95%CI (1.43–1.87)], and malignant melanoma [1.23, 95%CI (1.01–1.49)] (Smitten et al., 2008; Simon et al., 2015; De Cock and Hyrich, 2018). The high inflammatory activity of RA is a major risk factor for developing lymphomas (Baecklund et al., 2006). Compared with low disease activity, moderate and high inflammatory disease activity increase the risk of developing lymphomas by 8-fold (odds ratio [OR] = 7.7, 95%CI 4.8–12.3) and 70-fold (OR = 71.3, 95%CI 24.1–211.4), respectively. Therefore, achieving remission of disease activity or maintaining low disease activity is the treatment target (Smolen et al., 2016).
Spleen tyrosine kinase (Syk), a non-receptor protein tyrosine located in the cytoplasm, plays a fundamental role in the activation of the B-cell receptor, which is necessary for B-cell development, proliferation, and survival. Thus, pharmacological targeting of Syk is effective in affecting the signal transduction of B-cell receptors, leading to cell apoptosis and inhibition of the activation and migration of B-cells, which are therapeutic targets for B-cell dominant diseases, such as chronic lymphocytic leukemia, B-cell malignancies, and autoimmune disorders (Buchner et al., 2010; Hoellenriegel et al., 2012). Fostamatinib is a Syk inhibitor, and R406 is the active metabolite of fostamatinib, which has been reported to effectively treat RA (Kunwar et al., 2016; Kang et al., 2019). Nevertheless, fostamatinib is reported to have an increased risk of infection, diarrhea, hypertension, neutropenia, and hypertransaminasaemia (Salgado et al., 2014; Kunwar et al., 2016; Kang et al., 2019; Chen et al., 2021). Whether the use of fostamatinib is associated with an increased risk of malignancy remains unknown. Therefore, we performed a systematic review and meta-analysis by including all the available evidence to assess neoplasm risk in patients with RA treated with fostamatinib.
This systematic review and meta-analysis was conducted to investigate the neoplasm risk in patients with RA treated with fostamatinib and reported based on the preferred reporting items for systematic reviews and meta-analyses guidelines (Moher et al., 2009). This study was registered in PROSPERO with registration number CRD42020202121.
The inclusion criteria were set based on the PICO principle: P (patients) were RA patients; I (intervention) was fostamatinib, regardless of the dose and usage; C (comparison) was placebo, other treatment, or different doses of fostamatinib; O (outcomes) were neoplasms regardless of the neoplasm nature (malignant or benign); in addition, we also included s (study designs) for randomized controlled trials (RCTs), cohort studies, or case-control studies.
A study was excluded if it was a duplicate, commentary, conference abstract, and or did not have relevant outcomes.
Electronic databases of OVID Medline, OVID EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials were searched on 3 July 2020, using both MeSH terms and key words without language limitations. Search terms included “rheumatoid arthritis” and “fostamatinib”. Detailed search strategy can be found in the supplemental file or the published study (Chen et al., 2021). Manual searches of reference lists of included studies and clnicaltrials.gov was also performed to identify potentially eligible studies.
Study selection was performed independently and repeated by a pair of reviewers (CYH, LH, and TYR), which was managed by Microsoft Office Access 2013. After preliminary screening by titles and abstracts, full texts were read based on the study selection criteria. Reference lists of included studies and published reviews and the clinicaltrials.gov website were manually checked. Any disagreement was resolved via discussion or judged by a third reviewer, if necessary.
Data was collected independently and repeated by two authors (CYH, TYR, and LZL) on trial registration number, publication date or release date, trial duration-from the trial beginning to the time assessing neoplasm incidence, treatment information, number of neoplasms, and number of participants. Any disagreement was resolved via discussion or judged by a third reviewer, if necessary.
The Cochrane Collaboration tool was used to assess the risk of bias of included RCTs (JPT and So, 2011). Which focused on the items of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. For each item, if the answer was yes and correctly described, the assessment was low risk; if the answer was yes but lacked detailed description, the assessment was unclear; if the answer was yes but with the inappropriate method or if the method was not performed, the assessment was high risk. The risk of bias of the included studies was judged based on overall evidence. Methodological quality assessment was performed by two reviewers (CYH and LH), and any disagreement was resolved via discussion or judged by a third reviewer, if necessary. Quality assessment for cohort and case-control studies was not described here, as no such study design was included.
RevMan software (version 5.1.3) was used to analyze the data. The effect size of the meta-analysis was estimated using the Peto OR with 95% CIs, considering the very low event. I2 and heterogeneity p-value at the level of 0.1 was used to assess the clinical diversity, and I2 had values of 25, 50, and 75% indicating low, moderate, and high heterogeneity, respectively, as recommended by the Cochrane Handbook (Higgins et al., 2003). Pre-set subgroup analyses by fostamatinib dose, trial duration, neoplasm nature, and neoplasm-originating systems were conducted. Sensitivity analysis using the Mantel-Haenszel random effect model was performed to test the robustness of the results. Publication bias was assessed using funnel plots.
Classification of neoplasm-originating sites and systems was based on the 10th version of the International Classification of Diseases (ICD10) (WHO), including malignant neoplasms (C00-C97), benign neoplasms (D10-D36), bone and articular cartilage (C40-C41), ill-defined, secondary, and unspecified sites (C76-C80), digestive organs (C15-C26), and urinary tract (C64-C68).
As this study is a systematic review and meta-analysis, no ethical concerns or patients were involved.
A total of 558 citations were identified from the OVID Medline (n = 118), OVID EMBASE (n = 269), Web of Science (n = 127), and Cochrane Library (n = 44). After excluding duplicates (n = 159), irrelevant studies (n = 358) screened by titles and abstracts, and studies without relevant outcomes (n = 34) by reading the full texts, finally, seven trials (Evaluation of Effectivene, 1197a; Evaluation of Effectivene, 1197b; Evaluation of Effectivene, 1197c; Evaluation of Efficacy an, 1264; Treatment of Arthritis Wi, 2633; Efficacy and Safety Study; Evaluation of Long-term S, 1242) that enrolled a total of 4,971 RA patients were included (Figure 1). No additional studies were included by the manual checking. No cohort or case-control studies were included.
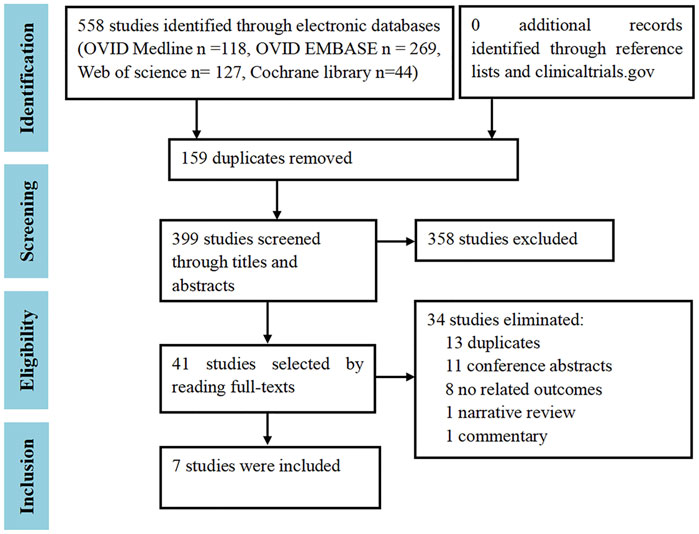
FIGURE 1. Study selection flowchart. Adapted from Chen et al. (2021).
The trial duration was a median (range) of 26 (12–109) weeks. The number of participants varied from 189 to 1912, with a median of 457. About 82.7% (4,113/4,971) of the participants were women. The age was around 50 years, ranging from 50 to 53 years. The common dosage of fostamatinib was 100 mg twice daily or 150 mg once daily, which were taken orally (Table 1).
Only one study correctly reported methods of random sequence generation, three studies correctly reported allocation concealment, and all trials performed blinding of participants, personnel, and outcome assessment, reported complete outcome data, and did not selectively report outcome data (Figures 2, 3). Overall, the risk of bias in the included trials was low to moderate.
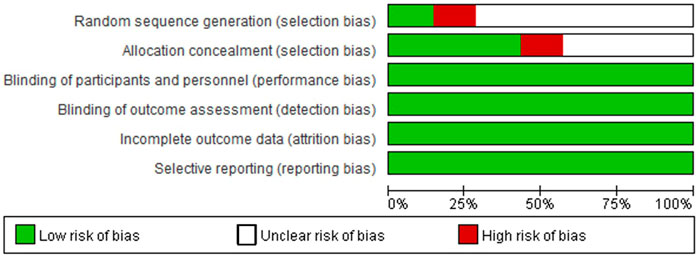
FIGURE 2. Risk of bias graph. Adapted from Chen et al. (2021).
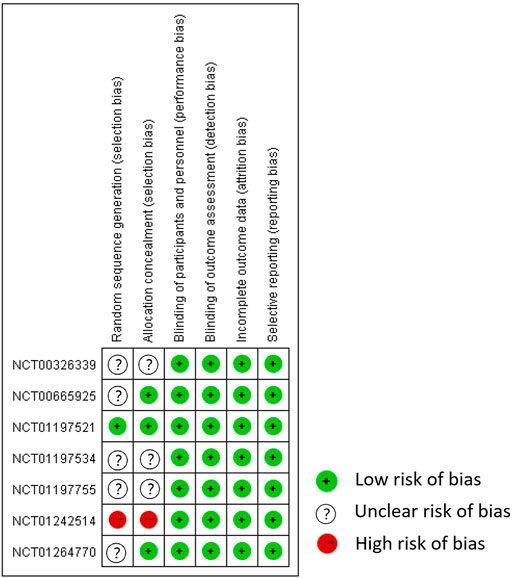
FIGURE 3. Risk of bias summary. Adapted from Chen et al. (2021).
Different statistical methods were used, the Mantel-Haenszel random effect model vs. Peto fixed-effect model, to conduct sensitivity analysis to test the robustness of the results, which did not change the effect direction (data not shown). Publication bias, taking the data from overall neoplasms in patients treated with fostamatinib vs. placebo for an example, was assessed using a funnel plot, and the results showed that the funnel plot was symmetrical; thus, publication bias was not likely to occur (Figure 4).
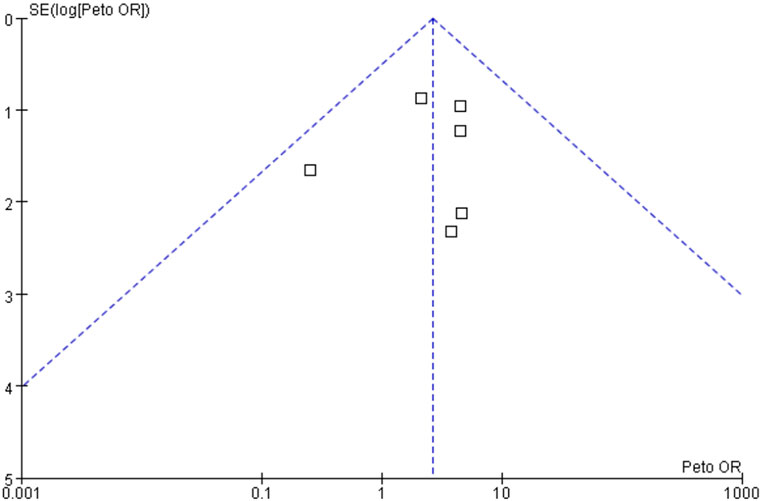
FIGURE 4. Publication bias based on overall neoplasms in patients treated with fostamatinib versus placebo.
In comparing fostamatinib vs. placebo, a total of six trials reported 16 neoplasms in 2,038 RA patients treated with fostamatinib, which did not increase the neoplasm risk compared with the placebo of two neoplasms in 967 RA patients (Peto OR = 2.62, 95%CI 0.97–7.10, I2 = 0, 16/2038 vs. 2/967) (Table 2; Figure 5). Longer durations of fostamatinib use also did not increase the neoplasm risk without clinical diversity across the studies (Peto OR = 3.78, 95%CI 0.04–352.61, 1/142 vs. 0/47 for 12 weeks; Peto OR = 0.75, 95%CI 0.06–9.58, 2/372 vs. 1/161 for 24 weeks; Peto OR = 4.53, 95%CI 0.41–50.05, 3/304 vs. 0/153 for 26 weeks; Peto OR = 2.98, 95%CI 0.85–10.49, 10/1220 vs. 1/606 for 52 weeks) (Table 2; Figure 5).
For fostamatinib dosages of 100 mg bid vs. 150 mg qd, seven trials reported 25 neoplasms in 2,954 RA patients treated with 100 mg of fostamatinib twice daily; 100 mg fostamatinib twice daily did not increase the neoplasm risk compared with 150 mg fostamatinib use once daily for 12 neoplasms in 1,339 RA patients (Peto OR = 0.81, 95%CI 0.39–1.69, and I2 = 35). Similarly, there was no difference in the neoplasm risk between fostamatinib dosages of 100 mg twice daily and 150 mg once daily, with longer durations of fostamatinib use (Table 2; Supplementary Figure S1).
For fostamatinib dosages of 100 mg bid vs. 100 mg qd, two studies were concerned with the neoplasm risk in RA patients treated with these different usage times of fostamatinib. A dosage timing of twice daily did not have a higher neoplasm risk compared with 100 mg of fostamatinib once daily (Peto OR = 3.22, 95%CI 0.77–13.52, 16/1424 vs. 0/269) (Table 2; Supplementary Figure S2).
When comparing fostamatinib and placebo, six trials reported 12 malignant neoplasms in 2038 RA patients treated with fostamatinib, which did not increase the neoplasm risk compared with the placebo in one malignant neoplasm in 967 RA patients (Peto OR = 3.08, 95%CI 0.96–9.91, and I2 = 6) (Table 2; Supplementary Figure S3). Nevertheless, the longer use of fostamatinib had a higher malignant neoplasm risk than the placebo (Peto OR = 4.49, 95%CI 1.03–19.60, 8/1220 vs. 0/606 for 52 weeks, I2 = 0) (Table 2, Supplementary Figure S3).
When comparing different doses of fostamatinib, compared with 150 mg of fostamatinib once daily, 100 mg of fostamatinib twice daily did not have a higher malignant neoplasm risk (Peto OR = 1.09, 95%CI 0.45–2.60, I2 = 39, 19/2954 vs. 7/1339, n = 7) (Table 2, Supplementary Figure S4). In comparison with 100 mg of fostamatinib once daily, 100 mg of fostamatinib twice daily did not increase the malignant neoplasm risk (Peto OR = 3.21, 95%CI 0.57–18.05, and 11/1424 vs. 0/269, and n = 2) (Table 2, Supplementary Figure S5).
Regarding fostamatinib vs. Adamumab, one trial examined the differences in the malignant neoplasm risk, and the use of fostamatinib did not have a higher malignant neoplasm risk compared to adamumab (Peto OR = 1.03, 95%CI 0.04–25.70, and 1/159 vs. 0/54) (Table 2, Supplementary Figure S6).
For the bone and articular cartilage system, one malignant neoplasm was reported in 765 RA patients treated with fostamatinib, which was comparable to that in RA patients treated with placebo (Peto OR = 0.36, 95%CI 0.02–7.67, 1/765 vs. 1/354, I2 = 68, and n = 2) (Table 2; Supplementary Figure S7).
For ill-defined, secondary, and unspecified sites, four studies reported malignant neoplasms. Compared to placebo, fostamatinib did not have a higher malignant neoplasm risk than placebo (Peto OR = 1.58, 95%CI 0.16–15.90, 2/1222 vs. 1/759, I2 = 74, and n = 3) (Table 2; Supplementary Figure S8). A dosage of 100 mg of fostamatinib twice daily did not increase the risk of malignant neoplasms compared to 150 mg of fostamatinib once daily (Peto OR = 4.64, 95%CI 0.80–26.88, 6/2719 vs. 0/1111, I2 = 0, and n = 4) (Table 2; Supplementary Figure S9).
For the digestive organs, two trials reported malignant neoplasms. RA patients who used fostamatinib 100 mg twice daily had a lower risk of malignant neoplasms than those who used 150 mg of fostamatinib once daily (Peto OR = 0.06, 95%CI 0.01–0.59, 1/1957 vs. 3/661, I2 = 0) (Table 2; Supplementary Figure S10).
For the urinary tract, there were five trials focused on malignant neoplasms. The use of fostamatinib did not have a higher risk of malignant neoplasms than the placebo (Peto OR = 4.33, 95%CI 0.52–36.07, 4/1273 vs. 0/613, I2 = 0, and n = 4) (Table 2; Supplementary Figure S11). RA patients who used fostamatinib 100 mg twice daily did not have an increased risk of malignant neoplasms compared to those who used 150 mg of fostamatinib once daily (Peto OR = 0.60, 95%CI 0.08–4.81, 2/2263 vs. 2/968, I2 = 50, and n = 5) (Table 2; Supplementary Figure S12).
Compared to placebo, the use of fostamatinib did not have a higher benign neoplasm risk (Peto OR = 1.71, 95%CI 0.26–11.36, I2 = 0, 4/2038 vs. 1/967, and n = 6) (Table 2, Supplementary Figure S13). A longer duration of fostamatinib use did not increase the risk of benign neoplasms (Table 2; Supplementary Figure S13). Compared to 150 mg of fostamatinib once daily, 100 mg of fostamatinib twice daily did not elevate the risk of benign neoplasms (Peto OR = 0.41, 95%CI 0.10–1.57, I2 = 0, 6/2954 vs. 5/1339, and n = 7) (Table 2; Supplementary Figure S14). Similarly, long-term use of 100 mg of fostamatinib twice daily did not increase the risk of benign neoplasms (Table 2; Supplementary Figure S14).
In total, two studies reported a benign neoplasm risk in RA patients treated with different dosage times of fostamatinib. For 100 mg of fostamatinib, a dosage time of twice daily did not increase benign neoplasm risk compared to once daily (Peto OR = 3.19, 95%CI 0.25–41.20, 5/1424 vs. 0/269) (Table 2; Supplementary Figure S15).
Our results indicate that fostamatinib was not associated with the risks of overall neoplasms as compared to placebo (16 cases in 2038 participants vs 2 cases in 967 participants), whereas, use of fostamatinib might be related to increased malignant neoplasm risk: longer duration of fostamatinib use might be correlated with an increased risk of malignant neoplasms, 8 cases in 1220 participants vs 0 cases in 606 participants at 52 weeks, and higher dose of fostamatinib may increase the malignant neoplasm risk in the digestive system, 3 cases in 661 participants who used 150 mg once a day vs 1 case in 1957 participants taking 100 mg twice a day.
Askling et al. reported that the highest crude incidence rates of all malignancies excluding non-melanoma skin cancer, solid malignancies, all skin cancers, and malignant lymphomas in RA patients treated with fostamatinib were 1.36, 1.47, 0.74, and 0.10 per 100 person-years, respectively (Askling et al., 2016), which were similar in RA patients with other treatments. The incidence of solid cancer in RA patients treated with tumor necrosis factor α inhibitors (TNFi) and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) was 0.81 and 1.17 per 100 person-years, respectively, and no difference was found between the two groups after adjusting for baseline characteristics (hazard ratio [HR] = 0.83, 95%CI 0.64–1.07) (Mercer et al., 2015).
Although the high inflammatory activity of RA has been reported to be associated with increased lymphoma (Baecklund et al., 2006), Simon et al. suggested that some types of malignant tumors are related to treatment rather than the underlying disease (Simon et al., 2015). However, compared with no treatment, treatments such as those involving TNFi (incidence rate ratios [IRR] = 1.1, 95%CI 0.8–1.6) and tocilizumab (IRR = 1.2, 95%CI 0.5–2.9) were not associated with increased melanoma risk (Mercer et al., 2017). Further, compared with csDMARD, TNFi did not increase the risk of lymphomas, solid cancers excluding non-melanoma skin cancer, non-melanoma skin cancer, and melanoma skin cancer in patients with RA (Chen et al., 2016; De Cock and Hyrich, 2018).
By targeting Syk, fostamatinib has been used to treat a wide range of diseases, such as graft-versus-host disease (Flynn et al., 2015), follicular lymphoma (Fruchon et al., 2012), chronic lymphocytic leukemia (Quiroga et al., 2009), Waldenström macroglobulinemia (Kuiatse et al., 2015), ulcerative colitis (Can et al., 2015), and idiopathic thrombocytopenic purpura (Bajpai, 2009), in addition to treating RA. The mechanisms underlying the treatment of such diseases include targeting Syk signaling in B-cells and promoting their apoptosis for graft-versus-host disease (Flynn et al., 2015); suppressing the expression of matrix metalloproteinase 9 and angiogenesis through Syk-mTOR pathway for follicular lymphoma (Fruchon et al., 2012); inhibiting the phosphorylation of B-cell downstream signaling molecules, Syk, ERK, and AKT to reduce the production of CXCL12 and CXCL13 chemokines for chronic lymphocytic leukemia (Quiroga et al., 2009); inhibiting the activation of Syk and Bruton’s tyrosine kinase and suppressing downstream signaling through MAPK kinase (MEK), p44/42 MAPK, and protein kinase B/Akt to prolong the onset of tumor growth and reduce viability of primary Waldenström macroglobulinemia cells (Kuiatse et al., 2015); inhibiting tissue myeloperoxidase activity and suppressing the molecular expressions of TNFα, CD3, Syk, and phospho-Syk in tissues (Can et al., 2015); reducing inflammation through decreased major inflammatory cytokines such as TNFα, IL-1, and IL-6 and inhibiting bone degradation for the autoimmune diseases idiopathic thrombocytopenic purpura and RA (Bajpai, 2009; Boccia et al., 2020).
Moreover, fostamatinib can reduce inflammatory cell adhesion and migration, diminish macrophage survival, and normalize upregulated monocytosis and inflammatory gene expression induced by a high-cholesterol diet (Hilgendorf et al., 2011). Thus, fostamatinib can be used to treat RA. Theoretically, fostamatinib can reduce the disease activity of RA and be used to treat lymphoma; thus, the finding of this study that longer-time use of fostamatinib could increase malignant neoplasm risk should be explained with caution. Fostamatinib is only effective in approximately 50% (680/1419) of RA patients assessed by ACR20, (Kunwar et al., 2016); therefore, uncontrolled disease activity would contribute to the risk of malignant tumors. In contrast, owing to the limited data, subgroup analyses were only performed for the nature of the neoplasm, follow-up periods, and the neoplasm-originating system, but analysis of subtypes of malignant neoplasms was not conducted. Thus, we could not focus on a specific neoplasm type, such as B-cell lymphoma, as Syk is reported to be necessary for B-cell development, proliferation, and survival.
Several limitations of this meta-analysis and systematic review should be considered. First, only RCTs were eligible for inclusion, whereas no cohort and case-control studies were included. Although RCTs can balance the baseline measurements, having the least potential bias and less likely to be affected by possible confounders, the sample size and follow-up duration were relatively small, and short. For the small smaple size, the minimal reported sample size in the subgroup in our review was only based on 96 participants. Especially for rare cases, such as neoplasm, a small sample size may be underpowered to detect the outcomes. Moreover, the number of included studies was small and only seven RCTs were included. When performing subgroup analysis, several outcomes were reported only by one study; thus, the confidence intervals were relatively wide, affecting the reliability of the results. Therefore, well-planned observational studies with large study populations, such as cohort studies, are needed to determine whether fostamatinib is associated with increased or decreased malignant neoplasm risk in RA patients.
Our findings suggest that a longer duration of fostamatinib use in RA patients increases the risk of malignant neoplasms and a higher dose of fostamatinib may increase malignant neoplasms in the digestive system. However, owing to the small sample size and short follow-up duration, further studies such as cohort studies with large study populations and longer follow-up times are required to rule out the results.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
QX, GY, and YC conceived the study, YC and HL designed the study forms, GY and QX guided this study. YC searched the literature; YC, HL, YT, and ZL screened the studies for inclusion, extracted data, and assessed methodological quality; YC, GY, and QX organized and analyzed data. All authors drafted and revised the manuscript.
This study is supported by Post-doctoral Research and Development Fund of West China Hospital of Sichuan University (2019HXBH090), Clinical Research Incubation Project, West China Hospital, Sichuan University (2019HXFH038 and 2021HXFH018), and Sichuan Science and Technology Program (2021JDRC0045, 2021YFS0164, 2021YJ0472, and 2021JDRC0169).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.768980/full#supplementary-material
Askling, J., Berglind, N., Franzen, S., Frisell, T., Garwood, C., Greenberg, J. D., et al. (2016). How Comparable Are Rates of Malignancies in Patients with Rheumatoid Arthritis across the World? A Comparison of Cancer Rates, and Means to Optimise Their Comparability, in Five RA Registries. Ann. Rheum. Dis. 75 (10), 1789–1796. doi:10.1136/annrheumdis-2015-208105
Baecklund, E., Iliadou, A., Askling, J., Ekbom, A., Backlin, C., Granath, F., et al. (2006). Association of Chronic Inflammation, Not its Treatment, with Increased Lymphoma Risk in Rheumatoid Arthritis. Arthritis Rheum. 54 (3), 692–701. doi:10.1002/art.21675
Bajpai, M., (2009). Fostamatinib, a Syk Inhibitor Prodrug for the Treatment of Inflammatory Diseases. IDrugs 12 (3), 174–185.
Boccia, R., Cooper, N., Ghanima, W., Boxer, M. A., Hill, Q. A., Sholzberg, M., et al. (2020). Fostamatinib Is an Effective Second-Line Therapy in Patients with Immune Thrombocytopenia. Br. J. Haematol. 190 (6), 933–938. doi:10.1111/bjh.16959
Buchner, M., Baer, C., Prinz, G., Dierks, C., Burger, M., Zenz, T., et al. (2010). Spleen Tyrosine Kinase Inhibition Prevents Chemokine- and Integrin-Mediated Stromal Protective Effects in Chronic Lymphocytic Leukemia. Blood 115 (22), 4497–4506. doi:10.1182/blood-2009-07-233692
Can, G., Ayvaz, S., Can, H., Demirtas, S., Aksit, H., Yilmaz, B., et al. (2015). The Syk Inhibitor Fostamatinib Decreases the Severity of Colonic Mucosal Damage in a Rodent Model of Colitis. J. Crohns Colitis 9 (10), 907–917. doi:10.1093/ecco-jcc/jjv114
Chen, Y., Liu, H., Huang, Y., Lin, S., Yin, G., and Xie, Q. (2021). The Cardiovascular Risks of Fostamatinib in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12, 632551. doi:10.3389/fphar.2021.632551
Chen, Y., Sun, J., Yang, Y., Huang, Y., and Liu, G. (2016). Malignancy Risk of Anti-tumor Necrosis Factor Alpha Blockers: an Overview of Systematic Reviews and Meta-Analyses. Clin. Rheumatol. 35 (1), 1–18. doi:10.1007/s10067-015-3115-7
De Cock, D., and Hyrich, K. (2018). Malignancy and Rheumatoid Arthritis: Epidemiology, Risk Factors and Management. Best Pract. Res. Clin. Rheumatol. 32 (6), 869–886. doi:10.1016/j.berh.2019.03.011
Dougados, M. (2016). Comorbidities in Rheumatoid Arthritis. Curr. Opin. Rheumatol. 28 (3), 282–288. doi:10.1097/BOR.0000000000000267
Efficacy and Safety Study of R935788 Tablets to Treat Rheumatoid Arthritis (Taski-2). Available at: https://www.clinicaltrials.gov/ct2/show/NCT00665925?term=NCT00665925&draw=2&rank=1.
Evaluation of Effectiveness of Two Dosing Regimens of Fostamatinib Compared to Placebo in Patients with Rheumatoid Arthritis (RA) Who Are Taking Disease Modifying Anti-rheumatic Drug (DMARD) but Not Responding. (OSKIRA - 2). Available at: https://www.clinicaltrials.gov/ct2/show/NCT01197534?term=NCT01197534&draw=1&rank=1.
Evaluation of Effectiveness of Two Dosing Regimens of Fostamatinib Compared to Placebo in Patients with Rheumatoid Arthritis (RA) Who Are Taking Methotrexate and Have Had Inadequate Response to Single TNF-Alpha Antagonist (OSKIRA - 3). Available at: https://www.clinicaltrials.gov/ct2/show/NCT01197755?term=NCT01197755&draw=2&rank=1.
Evaluation of Effectiveness of Two Dosing Regimens of Fostamatinib Compared to Placebo in Patients with Rheumatoid Arthritis (RA) Who Are Taking Methotrexate but Not Responding. (OSKIRA - 1). Available at: https://www.clinicaltrials.gov/ct2/show/NCT01197521?term=NCT01197521&draw=2&rank=1.
Evaluation of Efficacy and Safety of Fostamatinib Monotherapy Compared with Adalimumab Monotherapy in Patients with Rheumatoid Arthritis (RA) (OSKIRA -4). https://www.clinicaltrials.gov/ct2/show/NCT01264770?term=NCT01264770&draw=1&rank=1.
Evaluation of Long-Term Safety and Effectiveness of Fostamatinib in the Treatment of Rheumatoid Arthritis (RA) (OSKIRA-X). Available at: https://www.clinicaltrials.gov/ct2/show/NCT01242514?term=NCT01242514&draw=2&rank=1.
Flynn, R., Allen, J. L., Luznik, L., MacDonald, K. P., Paz, K., Alexander, K. A., et al. (2015). Targeting Syk-Activated B Cells in Murine and Human Chronic Graft-Versus-Host Disease. Blood 125 (26), 4085–4094. doi:10.1182/blood-2014-08-595470
Fruchon, S., Kheirallah, S., Al Saati, T., Ysebaert, L., Laurent, C., Leseux, L., et al. (2012). Involvement of the Syk-mTOR Pathway in Follicular Lymphoma Cell Invasion and Angiogenesis. Leukemia 26 (4), 795–805. doi:10.1038/leu.2011.248
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hilgendorf, I., Eisele, S., Remer, I., Schmitz, J., Zeschky, K., Colberg, C., et al. (2011). The Oral Spleen Tyrosine Kinase Inhibitor Fostamatinib Attenuates Inflammation and Atherogenesis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler Thromb. Vasc. Biol. 31 (9), 1991–1999. doi:10.1161/ATVBAHA.111.230847
Hoellenriegel, J., Coffey, G. P., Sinha, U., Pandey, A., Sivina, M., Ferrajoli, A., et al. (2012). Selective, Novel Spleen Tyrosine Kinase (Syk) Inhibitors Suppress Chronic Lymphocytic Leukemia B-Cell Activation and Migration. Leukemia 26 (7), 1576–1583. doi:10.1038/leu.2012.24
JPT, H., and So, G. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updatedAssessing Risk of Bias in Included Studies. Www. cochrane-handbook. Org. The Cochrane Collaboration, Chapter 82011
Kang, Y., Jiang, X., Qin, D., Wang, L., Yang, J., Wu, A., et al. (2019). Efficacy and Safety of Multiple Dosages of Fostamatinib in Adult Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 10, 897. doi:10.3389/fphar.2019.00897
Kuiatse, I., Baladandayuthapani, V., Lin, H. Y., Thomas, S. K., Bjorklund, C. C., Weber, D. M., et al. (2015). Targeting the Spleen Tyrosine Kinase with Fostamatinib as a Strategy against Waldenström Macroglobulinemia. Clin. Cancer Res. 21 (11), 2538–2545. doi:10.1158/1078-0432.CCR-14-1462
Kunwar, S., Devkota, A. R., and Ghimire, D. K. (2016). Fostamatinib, an Oral Spleen Tyrosine Kinase Inhibitor, in the Treatment of Rheumatoid Arthritis: a Meta-Analysis of Randomized Controlled Trials. Rheumatol. Int. 36 (8), 1077–1087. doi:10.1007/s00296-016-3482-7
Mercer, L. K., Askling, J., Raaschou, P., Dixon, W. G., Dreyer, L., Hetland, M. L., et al. (2017). Risk of Invasive Melanoma in Patients with Rheumatoid Arthritis Treated with Biologics: Results from a Collaborative Project of 11 European Biologic Registers. Ann. Rheum. Dis. 76 (2), 386–391. doi:10.1136/annrheumdis-2016-209285
Mercer, L. K., Lunt, M., Low, A. L., Dixon, W. G., Watson, K. D., Symmons, D. P., et al. (2015). Risk of Solid Cancer in Patients Exposed to Anti-tumour Necrosis Factor Therapy: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 74 (6), 1087–1093. doi:10.1136/annrheumdis-2013-204851
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Quiroga, M. P., Balakrishnan, K., Kurtova, A. V., Sivina, M., Keating, M. J., Wierda, W. G., et al. (2009). B-cell Antigen Receptor Signaling Enhances Chronic Lymphocytic Leukemia Cell Migration and Survival: Specific Targeting with a Novel Spleen Tyrosine Kinase Inhibitor, R406. Blood 114 (5), 1029–1037. doi:10.1182/blood-2009-03-212837
Salgado, E., Maneiro, J. R., Carmona, L., and Gomez-Reino, J. J. (2014). Safety Profile of Protein Kinase Inhibitors in Rheumatoid Arthritis: Systematic Review and Meta-Analysis. Ann. Rheum. Dis. 73 (5), 871–882. doi:10.1136/annrheumdis-2012-203116
Scott, D. L., Wolfe, F., and Huizinga, T. W. (2010). Rheumatoid Arthritis. Lancet 376 (9746), 1094–1108. doi:10.1016/S0140-6736(10)60826-4
Simon, T. A., Thompson, A., Gandhi, K. K., Hochberg, M. C., and Suissa, S. (2015). Incidence of Malignancy in Adult Patients with Rheumatoid Arthritis: a Meta-Analysis. Arthritis Res. Ther. 17 (1), 212. doi:10.1186/s13075-015-0728-9
Smitten, A. L., Simon, T. A., Hochberg, M. C., and Suissa, S. (2008). A Meta-Analysis of the Incidence of Malignancy in Adult Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 10 (2), R45. doi:10.1186/ar2404
Smolen, J. S., Aletaha, D., and McInnes, I. B. (2016). Rheumatoid Arthritis. Lancet 388 (10055), 2023–2038. doi:10.1016/S0140-6736(16)30173-8
Treatment of Arthritis with Syk Kinase Inhibition (TASKI-1). Available at: https://www.clinicaltrials.gov/ct2/show/NCT00326339?term=NCT00326339&draw=1&rank=1.
WHO The Tenth Revision of the International Classification of Diseases (ICD-10). Available at: http://www.cdc.gov/nchs/icd/.
Keywords: fostamatinib, rheumatoid arthritis, neoplasm, meta-analysis, systematic review
Citation: Chen Y, Liu H, Tian Y, Luo Z, Yin G and Xie Q (2022) Neoplasm Risk in Patients With Rheumatoid Arthritis Treated With Fostamatinib: A Systematic Review and Meta-analysis. Front. Pharmacol. 13:768980. doi: 10.3389/fphar.2022.768980
Received: 01 September 2021; Accepted: 07 February 2022;
Published: 02 March 2022.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Ettore Silvagni, University of Ferrara, ItalyCopyright © 2022 Chen, Liu, Tian, Luo, Yin and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geng Yin, eWluZ2VuZzE5NzVAMTYzLmNvbQ==; Qibing Xie, eGllcWliaW5nMTk3MUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.