- 1Drug Development Center, Institute of New Drug Development, Institute of Biomedical Sciences, China Medical University, Taichung, Taiwan
- 2Department of Family Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, South Korea
- 3Clinical Research Center of H PLUS Yangji Hospital, Seoul, South Korea
- 4Golden Biotechnology Corporation, New Taipei City, Taiwan
- 5Department of Biological Science and Technology, China Medical University, Taichung, Taiwan
Objective: It has been reported that antroquinonol extracted from Golden-Antrodia camphorate exerts protective effects on liver function both in vitro and in vivo. However, the protective effects of Golden-Antrodia camphorata on liver function have not been fully investigated in human clinical studies. Therefore, the present study aimed to evaluate the beneficial effects of Golden-Antrodia camphorata on hepatic function after alcohol consumption in human subjects.
Methods: A total of 80 participants with increased γ-glutamyl transferase levels (60–180 U/L) were enrolled in the current study and were randomly divided into two groups. Participants in the first group were orally administrated with 300 mg/day Golden-Antrodia camphorata (tablets), while those in the second group received placebo tablets for 12 weeks. Biochemical routine blood tests were performed at 6 and 12 weeks following the first administration.
Results: At 12 weeks post the first Golden-Antrodia camphorata administration, the serum levels of aspartate aminotransferase (AST; p < 0.0001), alanine aminotransferase (ALT; p = 0.0002) and triglyceride (p = 0.0158) were notably declined in the Golden-Antrodia camphorata treatment group compared with the placebo group. No clinically significant differences were observed between the Golden-Antrodia camphorata treatment and placebo groups in terms of general safety parameters.
Conclusion: A statistically significant difference was obtained in the serum levels of AST, ALT and triglycerides between the Golden-Antrodia camphorata and placebo groups. However, no clinical significance was observed in any of the safety parameters examined. Overall, these findings indicated that treatment with Golden-Antrodia camphorata exerted protective effects on liver function.
Introduction
Alcoholic hepatitis most commonly occurs in individuals with mild or chronic alcohol consumption history and may lead to liver damage, which has a high short-term mortality rate of ∼40% within 1 month of clinical presentation (Lucey et al., 2009; Singal et al., 2014; Thursz et al., 2015) demonstrated that treatment of patients with severe alcoholic hepatitis with prednisolone or pentoxifylline resulted in a 90-days mortality rate of ∼30%. Additionally, it has been reported that long-term alcohol abuse increases the risk of developing liver diseases (Younossi and Guyatt, 1998). Gutierrez-Ruiz et al showed that heavy alcohol intake could promote the production of several factors, including cytokines such as TNF-α, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and reactive oxygen species (ROS), thus resulting in the pathogenesis and progression of alcohol-induced liver diseases (Gutierrez-Ruiz et al., 1999).
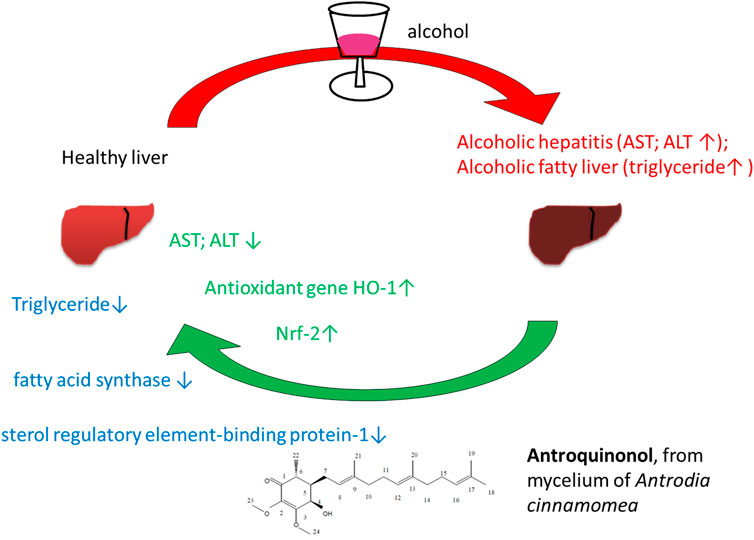
Oxidative stress, produced during alcohol metabolism, may cause liver cell injury. Liver cell injury-induced alcoholic liver diseases account for ∼20% of all alcohol-related liver diseases (Lucey et al., 2009; Serste et al., 2018). This can occur as four by-products of alcohol metabolism can mediate liver cell injury, which in turn may lead to steatohepatitis, gangrene, liver cirrhosis and/or hepatocellular carcinoma. Alcohol consumption may also cause the overproduction of free radicals, which may result in the spontaneous depletion of hepatic glutathione (Papanicolas et al., 2018). However, the overproduction of free radicals not only causes liver damage, but may also lead to vascular injury and lipid peroxidation in the blood, which in turn can promote the development of several diseases. Those who abuse alcohol long-term may develop alcoholic fatty liver disease (AFLD), which may lead to liver cancer (Osna et al., 2017). Antrodia camphorata is a well-known Chinese medicine that suppresses the generation of ROS and in vitro studies have suggested that its antioxidative properties are beneficial in reducing liver cell injury (Kumar et al., 2011; Yi et al., 2020).
Antrodia camphorata, also known as “niu-chang-chih”, is a precious and common medicinal fungus in Taiwan. Therefore, it is claimed as a “national treasure of Taiwan” (Liu et al., 2012). Taiwanese aborigines alleviate the discomfort caused by excessive alcohol consumption by chewing raw fruits or drinking fruit decoction to minimize alcohol hangover (Ao et al., 2009). Currently, the fruiting bodies of Antrodia camphorata have been widely used to treat liver diseases, hypertension, allergies and cancer (Ao et al., 2009). Geethangili et al suggested that Antrodia camphorataand its bioactive compounds exhibited numerous bioactive properties, including anticancer, hepatoprotective and neuroprotective functions (Ao et al., 2009; Geethangili and Tzeng, 2011). Antrodia camphorata contains several bioactive compounds such as flavonoids, terpinoids, polyphenolics and polysaccharides (Chiang et al., 2010). Antroquinonol, a major active compound of Golden-Antrodia camphorata, displays anticancer and anti-inflammatory properties (Chiang et al., 2010; Geethangili and Tzeng, 2011). Antirroquinonol from Antrodia camphorata protects against ethanol-induced oxidative stress in hepatic cell lines by activating Nrf-2 and HO-1 (Kumar et al., 2011).
Golden-Antrodia camphorata has not been studied thoroughly in human clinical studies for its safety and protective effect on liver function. In this study, we sought to assess the beneficial effects of Golden-Antrodia camphorata on human hepatic function after alcohol consumption. Antrodia camphorata containing antroquinonol has been found to have clinical and pharmacological benefits in numerous cancerous studies, including breast, lung, pancreatic, colon, brain, and liver cancer (Angamuthu et al., 2019; Ganesan et al., 2019). In addition, antroquinonol has been approved by the US FDA for clinical trials (ClinicalTrials.gov Identifier: NCT02047344). Lu et al demonstrated that mycelia from Antrodia camphorata exerted protective effects against ethanol-induced liver damage in rats (Lu et al., 2007). However, the effects of Golden-Antrodia camphorata on lipid metabolism and protection of liver function have not been evaluated in human clinical studies. The results of the present study revealed that antroquinonol extracted from the ethanolic extracts of mycelium of Golden-Antrodia camphorata exhibited hepatoprotective effects.
Materials and Methods
Participants
This study was registered at the Clinical Research Information Service (CRIS; registration no. KCT0003692; research unique no. 2018AS0229; https://cris.nih.go.kr/cris/search/detailSearch.do/15180). In addition, the study protocol was approved by the Institutional Review Board of the Korea University Ansan Hospital (approval no. 2018AS0229) and H Plus Yangji Hospital (approval no. HYJ 2018-04–006-006) prior to the initiation of the study. The entire study was performed in accordance with the Declaration of Helsinki and the ethical standards provided by the Korean Good Clinical Practice guidelines. The current multicenter randomized, double blind, placebo-controlled study was conducted between 26 December 2018 and 4 November 2020.
All subjects were recruited from the Clinic Trial Center for Functional Foods at Korea University Ansan Hospital and H Plus Yangji Hospital. Written informed consent was obtained from all participants prior to enrollment in this study. The inclusion criteria were as follows: (1) Men or women, aged 20–75 years (range, 21-72) at the time of the screening test; (2) subjects with serum γ-glutamyltranspeptidase (γ-GTP) levels of 60–180 U/L; and (3) subjects who fully understood the detailed description of the study and voluntarily agreed to participate.
Treatment Regimen
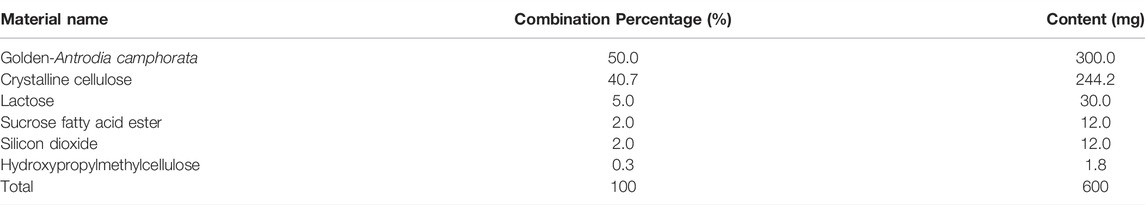
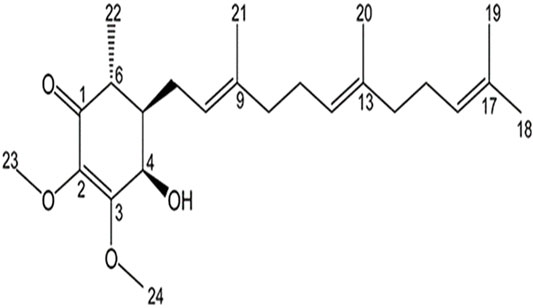
Participants in the test group consumed Golden-Antrodia camphorata, while those in the placebo group were orally administrated with tablets without Golden-Antrodia camphorata. Both tablets were indistinguishable in appearance or taste. The components of the Golden-Antrodia camphorata tablets are listed in Table 1. Antroquinonol is the main bioactive ingredient in Golden-Antrodia camphorata tablets. Figure 1 illustrates the chemical structure of antroquinonol. The molecular formula of antroquinonol is C24H38O4 and its molecular weight is 390.6.
Design, Intervention, and Study Protocol
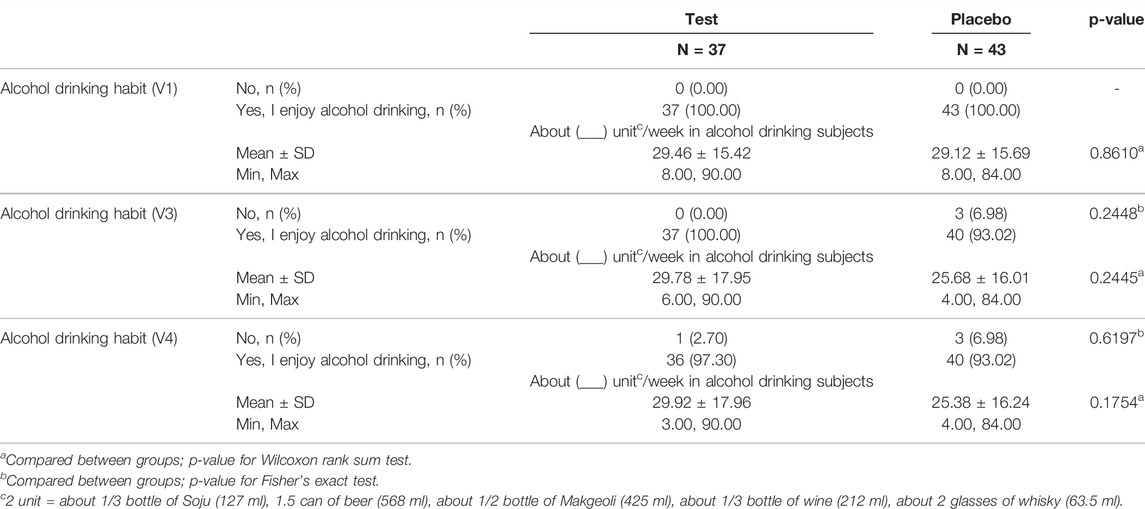
In the current multicenter randomized, double blind and placebo-controlled study, after the participants signed the consent for voluntary participation in the clinical study, their demographic features, medical and medication history, physical test results, vital signs, possibility of pregnancy (applicable only for females in their childbearing age) and clinical laboratory test results, electrocardiogram and abdominal ultrasonography findings and alcohol drinking habits were recorded before their randomization by inclusion/exclusion criteria. Participants in the test or placebo groups were treated with tablets with or without Golden-Antrodia camphorata, respectively, for a total of 12 weeks. The protocol of the trial specifies that safety checkpoints are held every 6 weeks, while efficacy checkpoints and safety inspections are held at the end of 12 weeks. Participants were randomly assigned to the test or placebo group at a ratio of 1:1 (Figure 2).
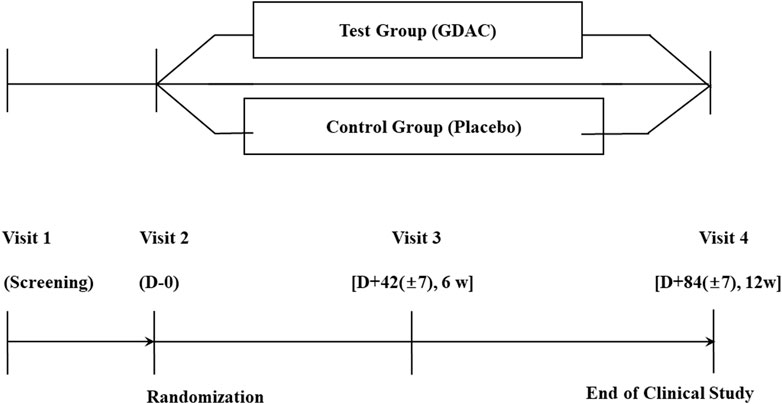
FIGURE 2. A flow diagram of the enrolment process in the human study. A subject who wishes to be enrolled in this clinical study should be given an explanation of the study and evaluated at the 1st visit (Screening visit). Upon completion of our first visit, we planned to conduct a second visit within 14 days and conduct an evaluation. Three days after the second visit, a third visit was to happen, and evaluations were to take place. The fourth evaluation was conducted 84 (±7) days after the second one.
Measurement of Dietary Intake
To record the dietary habits of participants during the study period, a food intake questionnaire was provided to each participant during visits 2 and 3 (Figure 2). The subject was asked to record their regular meals, as well as other meals, for 3 days in the week prior to their next scheduled visit (including 1 day on the weekend if possible). At visit days 3 and 4, the investigator evaluated the answers of the food intake questionnaire.
Safety Investigation
Golden-Antrodia camphorata administration was evaluated based on the classification (Table 7), frequency, and severity (Table 8) of adverse events (ΑΕs), which were recorded in each participant’s AE report, and abnormal findings in clinical laboratory tests, including blood tests (Table 9), biochemical and urine tests (Table 10), vital signs (blood pressure and pulse), weight, and electrocardiogram findings. Analyzing statistically the abnormal laboratory test results recorded within each participant’s AE report, we evaluated their clinical significance.
Statistical Analysis
Statistical analysis was carried out with SAS® software (version 9.4; SAS Institute, Cary, North Carolina, United States)., A two-tailed test was performed. In all analyses, p-values were rounded off up to 4 decimal places. p < 0.05 was considered to indicate a statistically significant difference. The significance level for the efficacy, safety and demographic and nutrition analysis data was set at p < 0.05.
Results
Participant Characteristics
Herein, 188 individuals were screened to select the suitable candidates. A total of 84 subjects were excluded from the study, while the remaining 104 participants were randomly allocated to the test (n = 53) and placebo (n = 51) groups. Among them, 17 subjects were excluded due to violation of inclusion/exclusion criteria (n = 7), consent withdrawal (n = 7), follow-up failure (n = 1), principal investigator directed termination (n = 1) and prohibited combination of drug intake (n = 1). Finally, 80 subjects (37 in the test and 43 in the placebo groups) completed the clinical study. In all, 104 participants drank. The general characteristics of the study population and alcohol drinking habit are listed in Table 2 and 3. No statistically significant differences were observed in sex, age and high fat food intake between the two groups.
Efficacy Evaluation
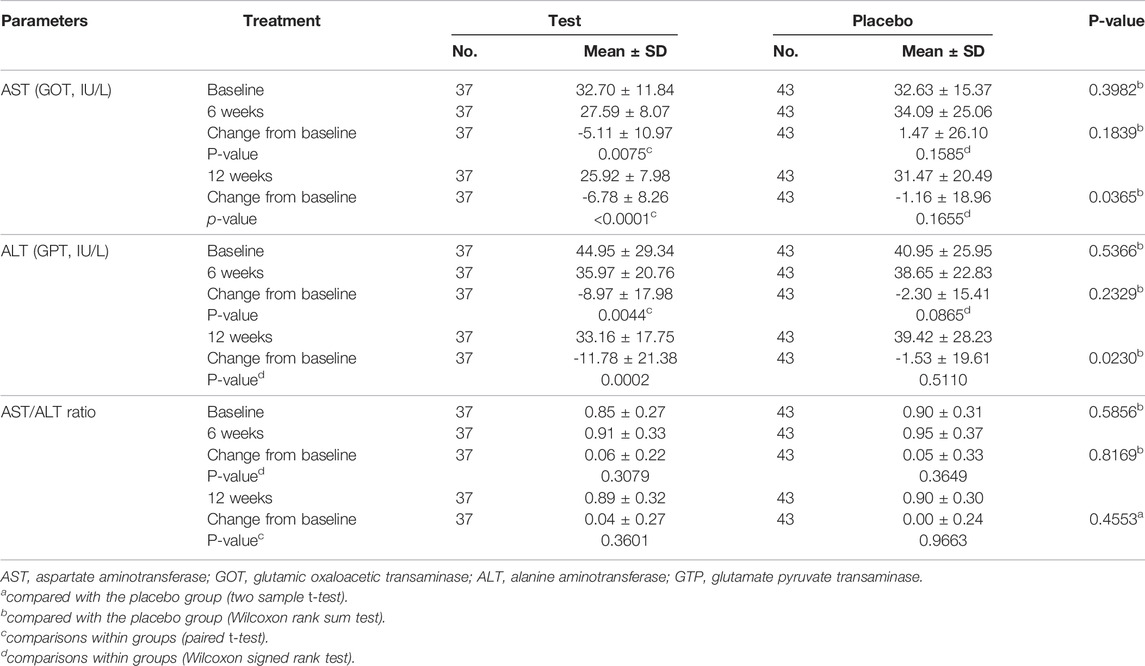
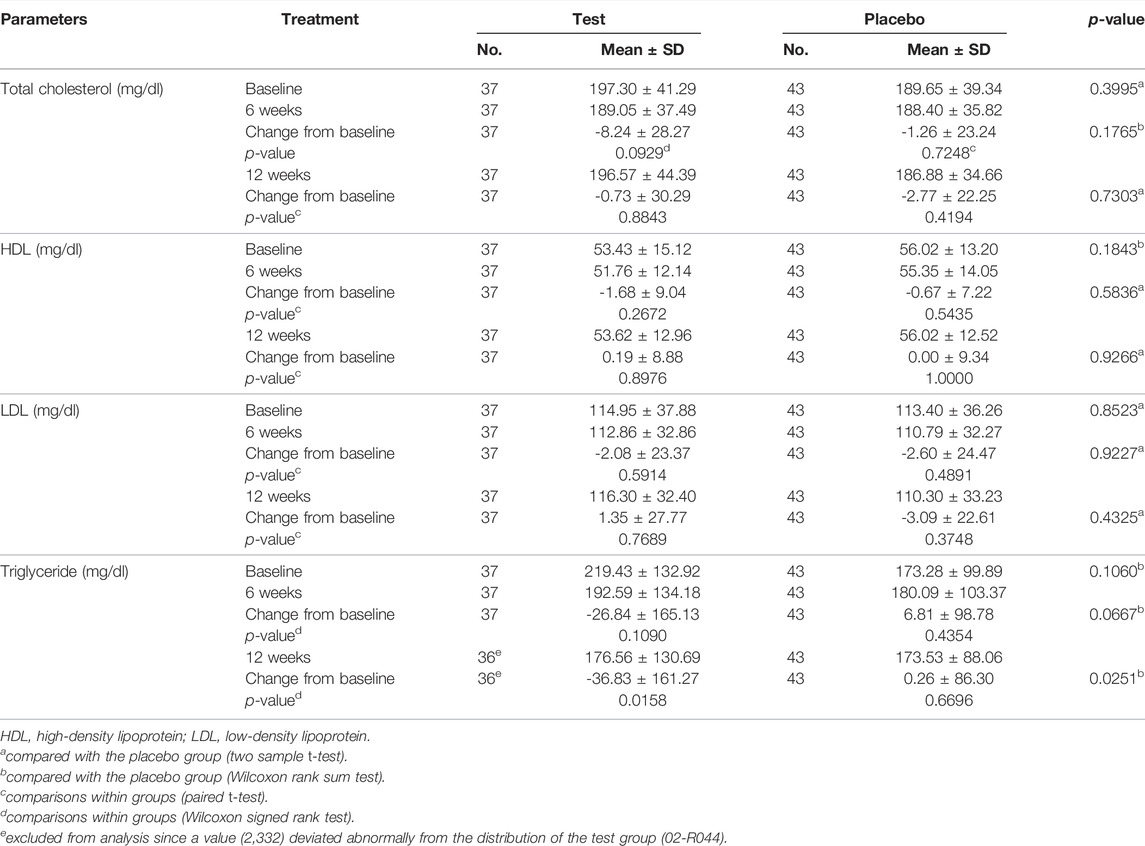
Tables 4 and 5 demonstrate the liver function markers and Table 6 shows the lipid levels prior to and 12 weeks after the study began. At 12 weeks following Golden-Antrodia camphorata intake, the levels of ALT, also known as glutamate pyruvate transaminase (GPT) were reduced from the baseline levels by 11.78 ± 21.38 IU/L in the test group (p = 0.0002) and by 1.53 ± 19.61 IU/L in the placebo group (p = 0.5110), resulting in a statistically significant difference between the two groups (p = 0.0230). In addition, although AST/glutamic oxaloacetic transaminase (GOT) levels were decreased by 5.11 ± 10.97 IU/L in the test group (p = 0.0075) and increased by 1.47 ± 26.10 IU/L in the placebo group (p = 0.1585) at 6 weeks following tablet administration, there was no statistically significant difference identified between the groups. However, at 12 weeks after first administration, AST levels were notably reduced by 6.78 ± 8.26 IU/L in the test group (p < 0.0001) and 1.16 ± 18.96 IU/L in the placebo group (p = 0.1655) compared with the baseline values. Therefore, a statistically significant difference was observed in AST levels between the test and placebo groups (p = 0.0365). In addition, ALT levels were significantly reduced in the test food group at 12 weeks after ingestion (p < 0.05) compared to the placebo food group in specifically selected 31–51 IU/L ALT. With regards to the changes from baseline (PP set) triglyceride levels, triglyceride content was notably decreased by 36.83 ± 161.27 mg/dl in the test group (p = 0.0158) and elevated by 0.26 ± 86.30 mg/dl in the placebo group (p = 0.6696) at 12 weeks after the first intake, and a statistically significant difference was identified between the two groups (p = 0.0251). In males, no statistically significant differences were observed in γ-GTP levels across the groups.
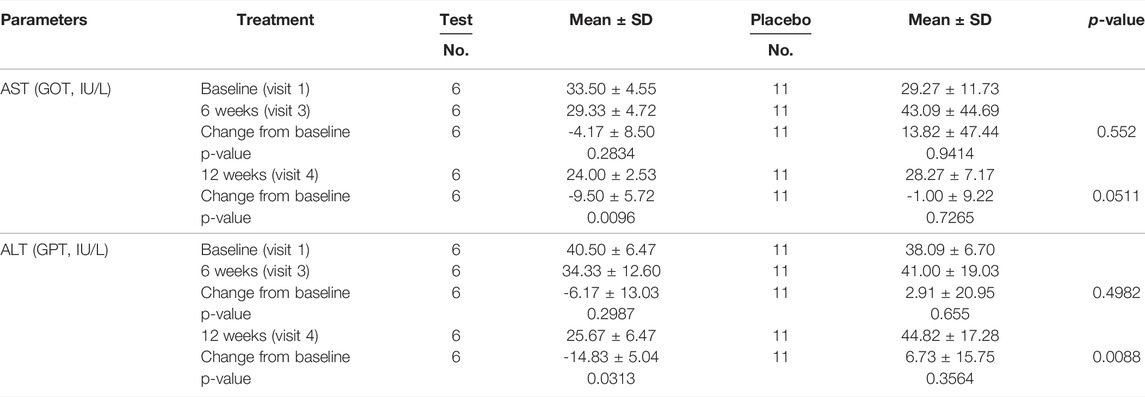
TABLE 5. The variances of AST and ALT levels in subgroups (ALT between 31 and 51 IU/L and gamma-GTP between 51 and 100 IU/L) by visit (PP Set).
Analysis of Adverse Events
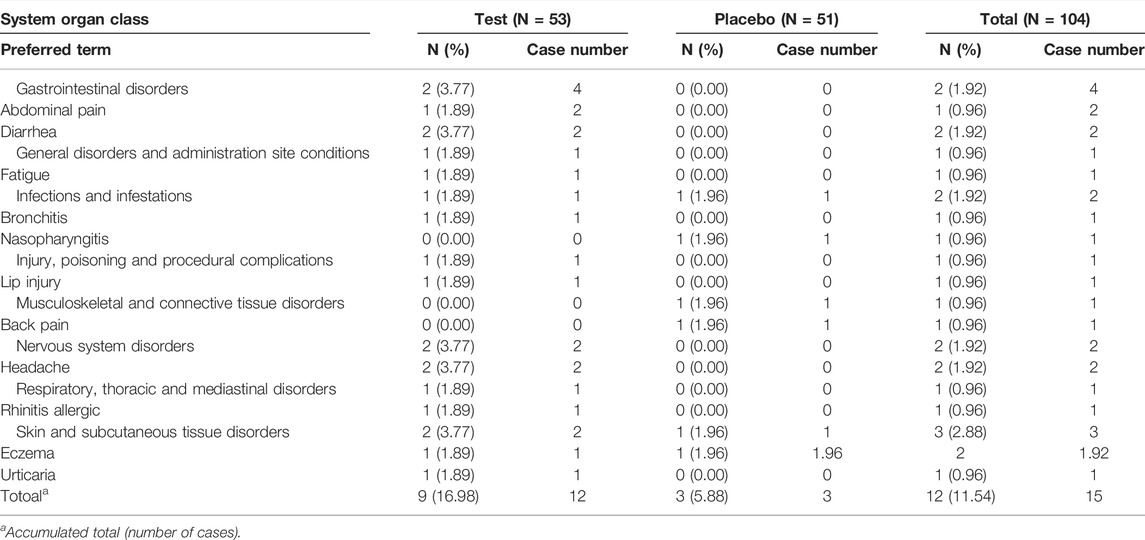
The classification and severity level of adverse events and their association with Golden- Antrodia camphorata treatment are listed in Tables 7, 8. The survey on the severity of adverse events revealed 7 and 5 cases of mild and moderate adverse events, respectively, in the test group. In the placebo group, 3 cases of mild adverse events were recorded. No severe adverse reactions were reported in the Golden-Antrodia camphorata treatment group. Furthermore, clinical indicators, such as heart function, blood pressure and pulse, and hematological parameters, including white blood cell, platelet and red blood cell counts, total bilirubin, albumin, blood urea nitrogen, creatinine, uric acid, total protein and glucose levels, and hematocrit and pH were also determined. However, no statistically significant changes were observed before and after treatment in both groups, since all clinical parameters were within normal ranges (data not shown). Incidence of any AEs was slightly higher in the Golden-Antrodia camphorata compared with placebo group, but the difference was non-significant. No serious AEs occurred in this clinical study.
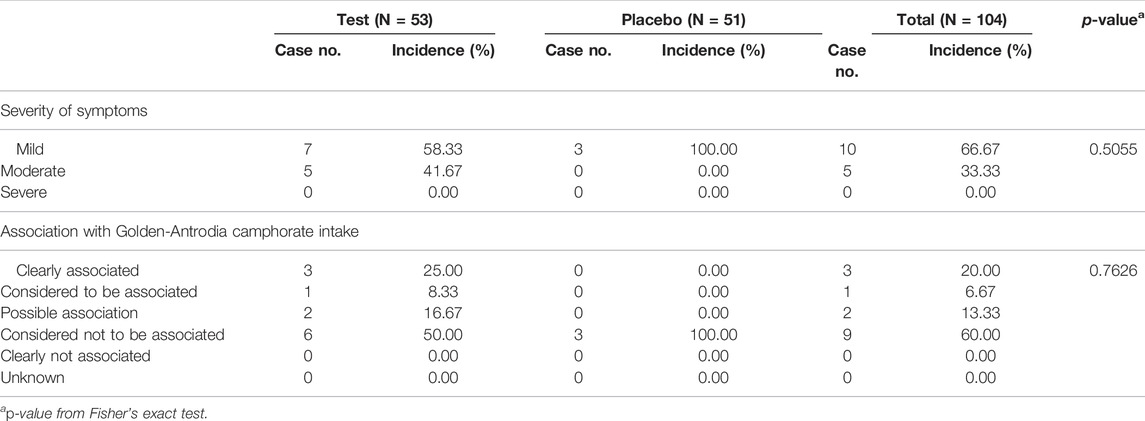
TABLE 8. The severity of adverse events and their association with consumption of Golden-Antrodia Camphorata (Safety set).
Biochemical Blood Test in Safety Set
Tables 9, 10 summarizes the biochemical blood test results indicating the severity of safety set. No statistically significant differences were observed in the values of biochemical parameters between both groups at 12 weeks after the initiation of the study.
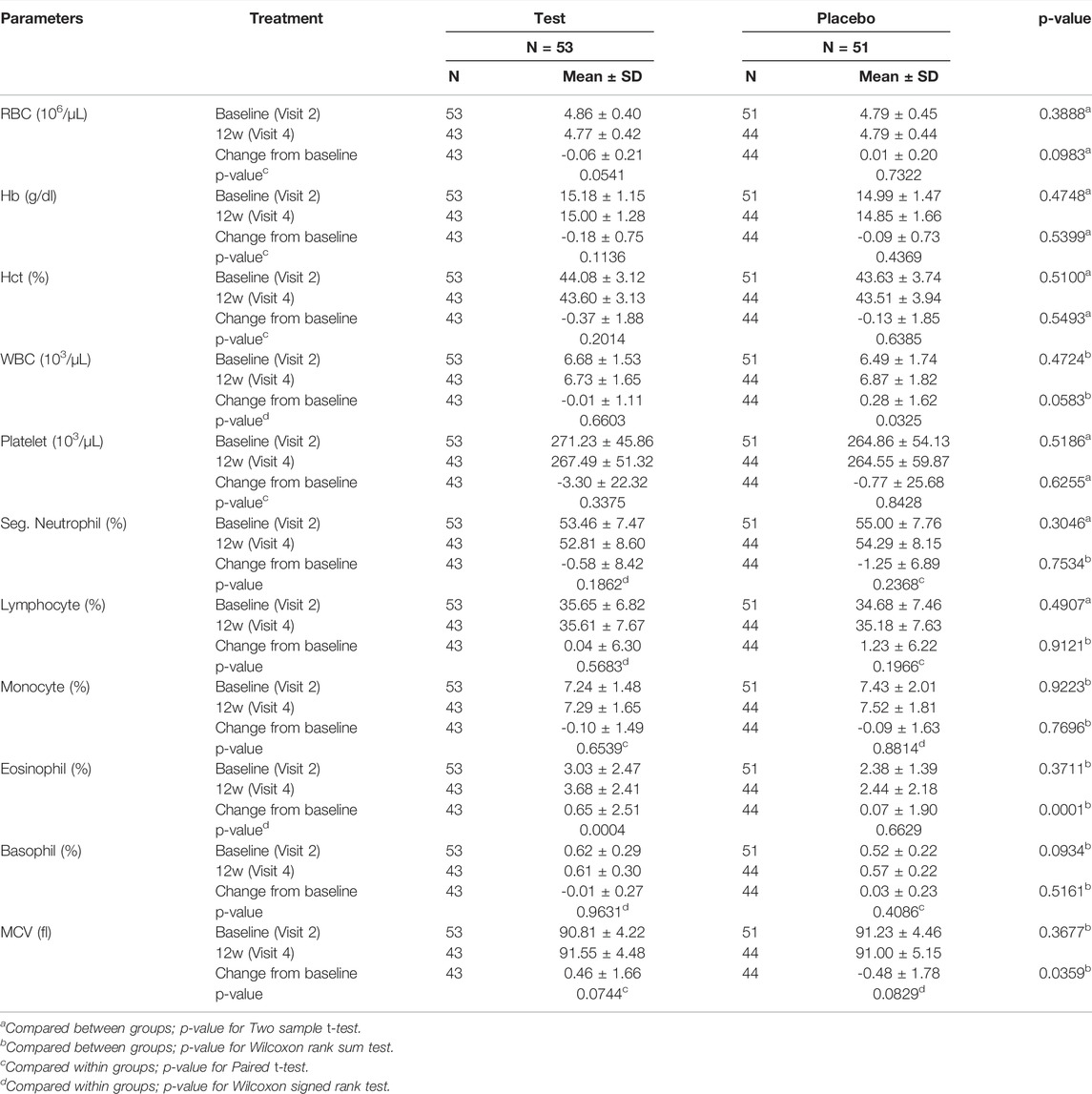
TABLE 9. The association between the blood parameters and Golden-Antrodia Camphorata consumption (Safety set).
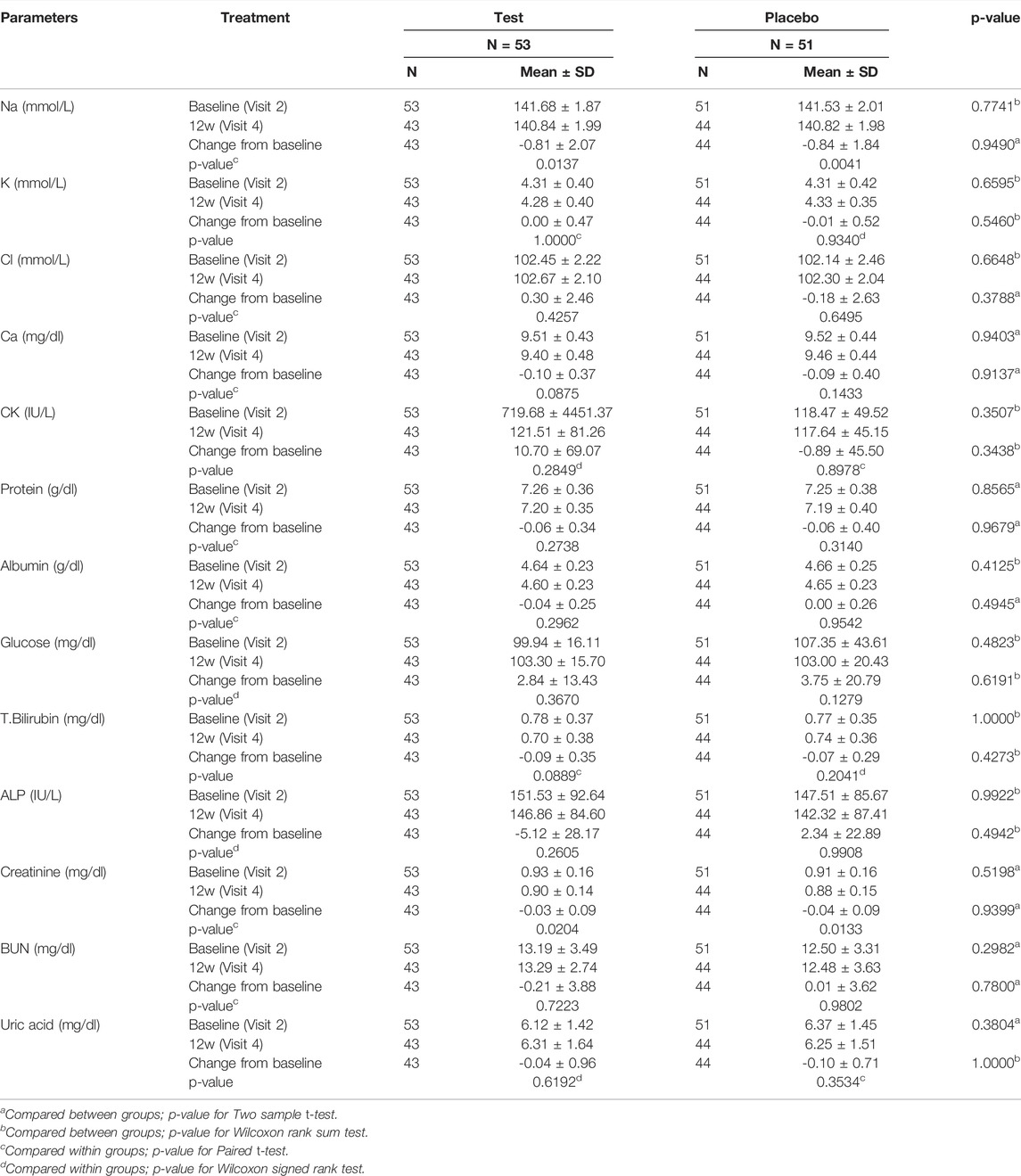
TABLE 10. The association between the blood chemistry parameters and Golden-Antrodia Camphorata (Safety set).
Discussion
The liver function index of aspartate aminotransferase (AST; p < 0.0001), alanine aminotransferase AST; p < 0.0001), and triglyceride (p = 0.0158) were markedly reduced in the Golden-Antrodia camphorata treatment group compared with the placebo group per day for 12 weeks. According to a previous study, Golden-Antrodia camphorata pretreatment inhibited ethanol-induced AST, ALT, ROS, NO, MDA production, and GSH depletion via increased activation of Nrf-2 and the downstream antioxidant gene HO-1 through the MAPK pathway (Kumar et al., 2011). An animal model of hepatic injury (caused by a high-fat diet) showed that Golden-Antrodia camphorata inhibited lipid accumulation (Chen et al., 2019). By upregulating hepatic ADP-activated protein kinase and downregulating both sterol regulatory element-binding protein-1 protein expression and fatty acid synthase expression, Golden-Antrodia camphorata reduced hepatic lipids and inflammatory cytokines.
It has been reported that Antrodia camphorata, a medicinal mushroom species, exerts possible hepatoprotective effects against liver injury and alcohol-induced liver diseases (Kumar et al., 2011). In addition, in vivo and in vitro experiments revealed that antroquinonol, a bioactive component of Antrodia camphorata, exerted significant anti-inflammatory and antioxidant activities via regulating nuclear factor erythroid 2-related factor 2 (Tsai et al., 2011). In addition, Golden-Antrodia camphorata displays antiviral, anti-inflammatory and antifibrotic activities (Jiang et al., 2021). To the best of our knowledge, the present study was the first to evaluate the safety and efficacy of Golden-Antrodia camphorata, derived from the ethanolic extract of the mycelium of Antrodia camphorata, in terms of a human clinical study.
GOT, also known as AST, and GPT, also known as ALT, are common enzymes associated with the metabolism of amino acids and proteins in the body. The normal AST value is between 5 and 40 units per liter. In patients with chronic renal failure due to vitamin B6 deficiency, AST and ALT levels are reported to be lower (Yasuda et al., 1995). Hepatocyte injury promotes the release of their contents, including ALT and AST, into the bloodstream. Chronic liver inflammation and alcoholic liver diseases are characterized by increased levels of both ALT and AST. Notably, a previous study demonstrated that Golden-Antrodia camphorata treatment could reduce ALT and AST levels in bloodstream or liver tissues (Thiyagarajan et al., 2015). Herein, our baseline (PP group) test result was 32.70 ± 11.84, which was within the normal range. After 12 weeks of consuming Golden-Antrodia camphorata, ALT levels decreased by 11.78 × 21.38 IU/L in the test group (p = 0.0002), and by 1.53 × 19.61 IU/L in the placebo group (p = 0.5110), resulting in a statistically significant difference (p = 0.0230). Treatment of patients with alcoholic liver diseases with Golden-Antrodia camphorata for 12 weeks declined the serum ALT and AST levels. This finding was consistent with that observed in a previous study, where treatment with Golden-A. Camphorata could protect liver from alcohol-induced liver damage both in vitro and in vivo (Kumar et al., 2011). The results of the present study provided a scientific basis for the hepatoprotective effects of Antrodia camphorata. Data indicated that Golden-Antrodia camphorata exerted the hepatoprotective effects of Antrodia camphorata. To the best of our knowledge, the current study strongly suggested that Golden-Antrodia camphorata could be considered as a potential compound for treating alcoholic liver diseases.
Triglycerides are the main components of low-density lipoproteins (LDLs) and chylomicrons. Elevated levels of triglycerides in the blood reduces the content of high-density lipoproteins, while LDLs are increased and oxidized, eventually leading to atherosclerosis (Talayero and Sacks, 2011). Herein, Golden-Antrodia camphorata treatment reduced the levels of triglycerides. Similar results were observed in high-fat diet-fed mice (Chang et al., 2018). However, further studies are required to clarify the effects of Golden-Antrodia camphorata and antroquinonol on metabolic disorders.
Overall, we found that taking Golden-Antrodia camphorata for 12 weeks improved liver function in patients with liver disease and was safe. During a 12-weeks clinical trial, no adverse effects such as dizziness or other physical discomforts were reported. Golden-Antrodia camphorata at a dose level of 600 mg daily for 12 weeks was found to be safe both for healthy subjects and people with alcoholic liver diseases. Therefore, Golden-Antrodia camphorata may be a promising health food for people who suffer from alcoholic liver diseases. Further studies are needed to clarify the effects of Golden-Antrodia camphorata on metabolic disorders. The results may provide support for the use of Golden-Antrodia camphorata in the treatment of chronic diseases.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by This study protocol was approved by the Institutional Review Board of the Korea University Ansan Hospital (approval no. 2018AS0229) and H Plus Yangji Hospital (approval no. HYJ 2018-04-006-006) prior to the initiation of the study. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-TY, J-HP, S-HK, TS, HC, W-CW, S-SL, and Y-LT performed experiments and collected and interpreted data. Y-TY, P-NC, and S-CT designed the study, interpreted data, and provided funding. Y-TY, P-NC, and S-CT wrote the manuscript. All authors had access to the primary data and approved the manuscript.
Funding
This study received funding from Golden Biotechnology Corporation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. All authors declare no other competing interests.
Conflict of Interest
Authors TS, HC, W-CW, S-SL, Y-LT, P-NC were employed by the company Golden Biotechnology Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Angamuthu, V., Shanmugavadivu, M., Nagarajan, G., and Velmurugan, B. K. (2019). Pharmacological Activities of Antroquinonol- Mini Review. Chem. Biol. Interact. 297, 8–15. doi:10.1016/j.cbi.2018.10.009
Ao, Z. H., Xu, Z. H., Lu, Z. M., Xu, H. Y., Zhang, X. M., and Dou, W. F. (2009). Niuchangchih (Antrodia Camphorata) and its Potential in Treating Liver Diseases. J. Ethnopharmacol. 121 (2), 194–212. doi:10.1016/j.jep.2008.10.039
Chang, C. J., Lu, C. C., Lin, C. S., Martel, J., Ko, Y. F., Ojcius, D. M., et al. (2018). Antrodia Cinnamomea Reduces Obesity and Modulates the Gut Microbiota in High-Fat Diet-Fed Mice. Int. J. Obes. (Lond) 42 (2), 231–243. doi:10.1038/ijo.2017.149
Chen, J. R., Yeh, W. J., Tan, H. Y., and Yang, H. Y. (2019). Antroquinonol Attenuated Abdominal and Hepatic Fat Accumulation in Rats Fed an Obesogenic Diet. J. Food Sci. 84 (9), 2682–2687. doi:10.1111/1750-3841.14746
Chiang, P. C., Lin, S. C., Pan, S. L., Kuo, C. H., Tsai, I. L., Kuo, M. T., et al. (2010). Antroquinonol Displays Anticancer Potential against Human Hepatocellular Carcinoma Cells: a Crucial Role of AMPK and mTOR Pathways. Biochem. Pharmacol. 79 (2), 162–171. doi:10.1016/j.bcp.2009.08.022
Ganesan, N., Baskaran, R., Velmurugan, B. K., and Thanh, N. C. (2019). Antrodia Cinnamomea-An Updated Minireview of its Bioactive Components and Biological Activity. J. Food Biochem. 43 (8), e12936. doi:10.1111/jfbc.12936
Geethangili, M., and Tzeng, Y. M. (2011). Review of Pharmacological Effects of Antrodia Camphorata and Its Bioactive Compounds. Evid. Based Complement. Altern. Med. 2011, 212641. doi:10.1093/ecam/nep108
Gutiérrez-Ruiz, M. C., Quiroz, S. C., Souza, V., Bucio, L., Hernández, E., Olivares, I. P., et al. (1999). Cytokines, Growth Factors, and Oxidative Stress in HepG2 Cells Treated with Ethanol, Acetaldehyde, and LPS. Toxicology 134 (2-3), 197–207. doi:10.1016/s0300-483x(99)00044-x
Jiang, M., Wu, Z., Liu, L., and Chen, S. (2021). The Chemistry and Biology of Fungal Meroterpenoids (2009-2019). Org. Biomol. Chem. 19 (8), 1644–1704. doi:10.1039/d0ob02162h
Kumar, K. J., Chu, F. H., Hsieh, H. W., Liao, J. W., Li, W. H., Lin, J. C., et al. (2011). Antroquinonol from Ethanolic Extract of Mycelium of Antrodia Cinnamomea Protects Hepatic Cells from Ethanol-Induced Oxidative Stress through Nrf-2 Activation. J. Ethnopharmacol. 136 (1), 168–177. doi:10.1016/j.jep.2011.04.030
Liu, Y. W., Lu, K. H., Ho, C. T., and Sheen, L. Y. (2012). Protective Effects of Antrodia Cinnamomea against Liver Injury. J. Tradit. Complement. Med. 2 (4), 284–294. doi:10.1016/s2225-4110(16)30114-6
Lu, Z. M., Tao, W. Y., Zou, X. L., Fu, H. Z., and Ao, Z. H. (2007). Protective Effects of Mycelia of Antrodia Camphorata and Armillariella Tabescens in Submerged Culture against Ethanol-Induced Hepatic Toxicity in Rats. J. Ethnopharmacol. 110 (1), 160–164. doi:10.1016/j.jep.2006.09.029
Lucey, M. R., Mathurin, P., and Morgan, T. R. (2009). Alcoholic Hepatitis. N. Engl. J. Med. 360 (26), 2758–2769. doi:10.1056/NEJMra0805786
Osna, N. A., Donohue, T. M., and Kharbanda, K. K. (2017). Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 38 (2), 147–161.
Papanicolas, I., Woskie, L. R., and Jha, A. K. (2018). Health Care Spending in the United States and Other High-Income Countries. JAMA 319 (10), 1024–1039. doi:10.1001/jama.2018.1150
Sersté, T., Cornillie, A., Njimi, H., Pavesi, M., Arroyo, V., Putignano, A., et al. (2018). The Prognostic Value of Acute-On-Chronic Liver Failure during the Course of Severe Alcoholic Hepatitis. J. Hepatol. 69 (2), 318–324. doi:10.1016/j.jhep.2018.02.022
Singal, A. K., Kamath, P. S., Gores, G. J., and Shah, V. H. (2014). Alcoholic Hepatitis: Current Challenges and Future Directions. Clin. Gastroenterol. Hepatol. 12 (4), 555–564. doi:10.1016/j.cgh.2013.06.013
Talayero, B. G., and Sacks, F. M. (2011). The Role of Triglycerides in Atherosclerosis. Curr. Cardiol. Rep. 13 (6), 544–552. doi:10.1007/s11886-011-0220-3
Thiyagarajan, V., Tsai, M. J., and Weng, C. F. (2015). Antroquinonol Targets FAK-Signaling Pathway Suppressed Cell Migration, Invasion, and Tumor Growth of C6 Glioma. PLoS One 10 (10), e0141285. doi:10.1371/journal.pone.0141285
Thursz, M. R., Forrest, E. H., Ryder, S., and Investigators, S. (2015). Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N. Engl. J. Med. 373 (3), 282–283. doi:10.1056/NEJMc1506342
Tsai, P. Y., Ka, S. M., Chao, T. K., Chang, J. M., Lin, S. H., Li, C. Y., et al. (2011). Antroquinonol Reduces Oxidative Stress by Enhancing the Nrf2 Signaling Pathway and Inhibits Inflammation and Sclerosis in Focal Segmental Glomerulosclerosis Mice. Free Radic. Biol. Med. 50 (11), 1503–1516. doi:10.1016/j.freeradbiomed.2011.02.029
Yasuda, K., Okuda, K., Endo, N., Ishiwatari, Y., Ikeda, R., Hayashi, H., et al. (1995). Hypoaminotransferasemia in Patients Undergoing Long-Term Hemodialysis: Clinical and Biochemical Appraisal. Gastroenterology 109 (4), 1295–1300. doi:10.1016/0016-5085(95)90591-x
Yi, Z. W., Xia, Y. J., Liu, X. F., Wang, G. Q., Xiong, Z. Q., and Ai, L. Z. (2020). Antrodin A from Mycelium of Antrodia Camphorata Alleviates Acute Alcoholic Liver Injury and Modulates Intestinal Flora Dysbiosis in Mice. J. Ethnopharmacol. 254, 112681. doi:10.1016/j.jep.2020.112681
Keywords: antroquinonol, Antrodia camphorata, liver function, aspartate aminotransferase/alanine aminotransferase ratio, anti-lipid function
Citation: Yen Y-T, Park J-H, Kang S-H, Su T, Cheng H, Wen W-C, Lin S-S, Tai Y-L, Chen P-N and Tsai S-C (2022) Clinical Benefits of Golden-Antrodia Camphorata Containing Antroquinonol in Liver Protection and Liver Fat Reduction After Alcoholic Hepatitis. Front. Pharmacol. 13:757494. doi: 10.3389/fphar.2022.757494
Received: 12 August 2021; Accepted: 30 May 2022;
Published: 21 June 2022.
Edited by:
Kusum K. Kharbanda, University of Nebraska Medical Center, United StatesReviewed by:
Maria José García Barrado, University of Salamanca, SpainYi Tao, Zhejiang University of Technology, China
Nagarajan Ganesan, King Faisal University, Saudi Arabia
Copyright © 2022 Yen, Park, Kang, Su, Cheng, Wen, Lin, Tai, Chen and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei-Ni Chen, cG5jaGVuNzlAZ29sZGVuYmlvdGVjaC5jb20=; Shih-Chang Tsai, c2N0c2FpQG1haWwuY211LmVkdS50dw==
 Yu-Ting Yen
Yu-Ting Yen Joo-Hyun Park2
Joo-Hyun Park2 Shih-Chang Tsai
Shih-Chang Tsai