
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 10 March 2022
Sec. Pharmacology of Infectious Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.755745
 R. A. Akasov1,2*
R. A. Akasov1,2* E. V. Khaydukov1,2*
E. V. Khaydukov1,2* D. S. Andreyuk3,4
D. S. Andreyuk3,4 N. V. Sholina1,2
N. V. Sholina1,2 A. N. Sheremeta3
A. N. Sheremeta3 D. V. Romanov2
D. V. Romanov2 G. P. Kostyuk3
G. P. Kostyuk3 V. Ya. Panchenko1,5
V. Ya. Panchenko1,5 M. V. Kovalchuk5
M. V. Kovalchuk5Background: COVID-19 treatment remains a challenge for medicine because of the extremely short time for clinical studies of drug candidates, so the drug repurposing strategy, which implies the use of well-known and safe substances, is a promising approach.
Objective: We present the results of an observational clinical study that focused on the influence of riboflavin (vitamin B2) supplementation on the immune markers of COVID-19 severity in patients with mental health disorders.
Results: We have found that 10 mg of flavin mononucleotide (a soluble form of riboflavin) intramuscularly twice a day within 7 days correlated with the normalization of clinically relevant immune markers (neutrophils and lymphocytes counts, as well as their ratio) in COVID-19 patients. Additionally, we demonstrated that total leucocytes, neutrophils, and lymphocytes counts, as well as the neutrophils to leucocytes ratio (NLR), correlated with the severity of the disease. We also found that patients with organic disorders (F0 in ICD-10) demonstrated higher inflammation then patients with schizophrenia (F2 in ICD-10).
Conclusion: We suggest that riboflavin supplementation could be promising for decreasing inflammation in COVID-19, and further evaluation is required.
This observational clinical trial has been registered by the Sverzhevsky Research Institute of Clinical Otorhinolaryngology (Moscow, Russia), Protocol No. 4 dated 05/27/2020.
COVID-19 is a new and fast-growing challenge for medicine all over the world. Currently, there is a lack of evidence concerning the drugs with proved clinical efficacy against COVID-19 due to the limited time for laboratory and clinical evaluations. In this case, a drug repurposing strategy that involves the screening of existing compound libraries could be promising. The COVID-19 treatment used today is supportive, and the main cause of death is associated with respiratory failure due to acute respiratory distress syndrome (ARDS) (Mehta et al., 2020; Dubina, 2022). It is believed that one of the main causes of ARDS is the so-called “cytokine storm” (Giamarellos-Bourboulis et al., 2020), at which extremely high levels of inflammation markers in plasma are observed, including C-reactive protein and pro-inflammatory cytokines (IL-6, TNFα, IL-8, IL-2, etc).
Riboflavin (Rf), also called vitamin B2, is a precursor of essential coenzymes such as flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which play a vital role in cellular metabolism and have been demonstrated as promising anti-inflammatory and anti-oxidative agents (Thakur et al., 2017; Ahn and Lee, 2020; Suwannasom et al., 2020). Rf (0.2 mg/kg, i. p., single dose) protected against acute oxidant-mediated inflammatory injury in the lungs of Long-Evans rats (Seekamp et al., 1999). FAD significantly decreased inflammatory cell infiltration, reduced lung injury scores, and ameliorated lung edema in a mice model of influenza A H5N1 virus-induced lung injury (Huang et al., 2020). Rf supplementation (25 mg/kg/d, 3 days) prevents abdominal aortic aneurysm formation in a rat model through an antioxidant effect of endogenous superoxide dismutase activation (Yu et al., 2016). Diabetic mice which received Rf (10 or 20 mg/kg/day, p. o.) demonstrated the decrease of oxidative stress with an increased glucose uptake in skeletal muscles and white adipose tissue. Histological studies showed recovery in the liver and kidney tissue injury (Alam et al., 2015). More importantly, the efficacy of a riboflavin-based strategy in relieving inflammation and oxidative stress has been demonstrated in several recent clinical trials. In a double-blind, phase IIb clinical trial, patients with suspected stroke of less than 3 h of evolution received a single intravenous administration of 20 mg of Rf (da Silva-Candal et al., 2018). The decrease in glutamate concentration was significantly greater in the Rf-treated group. The percentage improvement according to the National Institutes of Health Stroke Scale score was higher in the Rf-treated group than in the placebo one (da Silva-Candal et al., 2018). Rf supplementation in patients with Crohn’s disease, a type of inflammatory bowel disease (IBD), has been evaluated in a recent prospective clinical intervention study (von Martels et al., 2020). Patients received 100 mg Rf daily for 3 weeks, which resulted in a reduction in systemic oxidative stress and anti-inflammatory effects. The concentration of free thiols significantly increased, while the concentration of IL-2 significantly decreased after 3 weeks. Serum C-reactive protein concentration also decreased after Rf supplementation, but in the subgroup with high fecal calprotectin levels only, which is usually discussed as aа marker of active inflammation. TNF-α also decreased in this group. Rf supplementation (10 mg/day, p. o) significantly decreased plasma homocysteine, a marker of inflammation and ischemic injury, in the group of elderly people with low Rf status (Tavares et al., 2009). It should be noted, that Cytoflavin (Inosine + Nicotinamide + Riboflavin + Succinic Acid) has been recently proposed for post-COVID syndrome treatment, and an anti-asthenic effect, correction of cognitive impairments, and a decrease in the severity of thrombocytopenia have been demonstrated (Putilina et al., 2021). Recently, we also discussed Rf-associated pathways as a possible target to suppress secondary infections at COVID-19 via the mucosal-associated invariant T cells activity (Akasov and Khaydukov, 2020). Several in silico studies proposed Rf/FMN/FAD as possible antiviral compounds potentially able to inhibit papain-like proteinase (PLpro) and 3C-like main protease (3CLpro) of SARS-CoV-2 (Wu et al., 2020; Anwaar et al., 2021; Hooshmand et al., 2021). The possible involvement of B vitamins in COVID-19 has also been discussed (Shakoor et al., 2021). Based on all the data discussed above, mainly on the clinically relevant efficacy in both acute (ischemic stroke) and chronic (Crohn’ disease) inflammation, we assumed the benefits of high doses of FMN (>10 mg per day intramuscularly) in COVID-19 therapy.
It is known that people with mental disorders have a higher chance of being infected with COVID-19 (De Picker et al., 2021; Taquet et al., 2021; Wang et al., 2021), and when infected they are at increased risk of a severe (Lee et al., 2020) or fatal course of illness (Nemani et al., 2021). The risk is aggravated not only by behavioral peculiarities, but also by immunological disturbances related to the nature of the mental disorder or associated medical treatment (Maes et al., 2012; Zhou et al., 2021). Moreover, patients with mental disorders often suffer from obesity, diabetes, chronic lung disease, and hypertension (De Hert et al., 2011) which can worsen the course of an illness.
The aim of the current research was to evaluate the immune patterns in COVID-19 patients with mental disorders and evaluate the possible benefits of riboflavin supplementation for COVID-19 treatment in an observational study.
We recruited 119 symptomatic adult inpatients (76 male, 43 female, mean age 59.3 ± 16.7 years) with mental disorders treated for COVID-19 at Alekseev Psychiatric Clinical Hospital no. 1 (Moscow, Russia) in June–July 2020; patients were at hospital within the treatment course. The inclusion criteria were as follows: (Mehta et al., 2020): at least 18 years of age; (Dubina, 2022); confirmed COVID-19 diagnosis (a positive test for SARS-CoV-2 RNA detected by RT-PCR collected from the upper respiratory tract; or pulmonary radiological data specific for COVID-19; or antibodies ratio specific for acute viral infection). Exclusion criteria: (Mehta et al., 2020): known or suspected active viral, bacterial, mycobacterial, or fungal infection other than COVID-19, including Epstein-Barr virus, cytomegalovirus, herpesvirus family, HIV, hepatitis C virus, etc; (Dubina, 2022); pregnancy and/or breastfeeding; (Giamarellos-Bourboulis et al., 2020); oncology diseases. The participants were assigned to experimental (50 patients) and control (69 patients) groups. All of these patients received antiviral treatment according to the national clinical guideline (Ministry of Health of Rusussian Federation, 2020), namely chloroquine (500 mg twice a day for 7 days), hydroxychloroquine (400 mg twice at the first day, then 200 mg twice at the next 6 days), lopinavir-ritonavir combination (400 mg + 100 mg p. o. every 12 h within 14 days), azithromycin (500 mg p. o, 5 days, in combination with hydroxyloroquine), and interferon preparations (IFN-β1b, 0.25 mg/ml, 8,000,000 ME, 14 days; IFN-α2b, 3,000 ME, 5 times a day, 5 days). Additionally, dexamethasone (12 mg per day p. o. or 4 mg three times a day i. v.) was used in case of CRP value growth. In addition, patients in the experimental group received the full course of riboflavin supplementation on medical advice (10 mg flavin mononucleotide intramuscularly twice a day).
Demographic, clinical, treatment, and outcome data were obtained from the electronic medical records of Alekseev Psychiatric Clinical Hospital no. 1 (Moscow, Russia). Data were anonymized by removing personally identifiable information prior to processing. Laboratory data included RBC, platelets, WBC, lymphocytes, neutrophils, and monocytes counts determined using a SYSMEX hematological analyzer (Japan); serum levels of C-reactive protein and hemoglobin; serum levels of IL-1β, IL-2, IL-6, MCP-1, TNF-α, and IFN-γ cytokines assessed using ELISA reagent kits (Vector-Best, Novosibirsk, Russia); chest computed tomography (CT) scans and their description by radiologists (unilateral and bilateral ground glass opacity, and lung involvement).
Statistical processing of the results was carried out using the GraphPad Prism software, version 6.01. The p values were estimated using the Wilcoxon Matched-Pairs Signed Ranks Test (paired, non-parametric, two-tailed) or Mann–Whitney U test (non-paired, non-parametric, two-tailed). The data are presented as median [IQR] values or box-and-whiskers plots using Tukey’s modification.
We analyzed demographic, clinical, treatment, and outcome data for 119 patients with mental disorders treated for COVID-19 at Alekseev Psychiatric Clinical Hospital no. 1 (Moscow, Russia) in June–July 2020. All of these patients received antiviral treatment according to the national clinical guideline (Ministry of Health of Rusussian Federation, 2020). We retrospectively divided this cohort to two groups: the first one additionally received 2 × 10 mg/day FMN intramuscularly (10 mg twice a day) on medical advice within 1 week; the second one did not receive FMN supplementation (Table 1).
The groups were comparable in terms of age [median (IQR) 59.00 (48.25; 68.60) for the experimental group vs 62.50 (47.00; 72.75) for the control group, p = 0.4192 in Mann–Whitney U test), CRP value on admission to the hospital [18.20 (2.570; 64.24) vs. 14.21 (3.285; 37.11), p = 0.5331], CT grade 1/CT grade 2–4 ratio (two-tailed p value of 0.0761 in Fisher’s exact test), and outcome (two-tailed p value of 0.6486 in Fisher’s exact test). The only statistically significant difference between the experimental and control groups is the male/female ratio with a predominance of men in the experimental group (two-tailed p value of 0.0214 in Fisher’s exact test).
The distribution of the study sample patients according to ICD-10 diagnoses of mental disorders is listed in Table 2. In total, most patients (n = 65; 55.1%) were diagnosed with schizophrenia spectrum disorders, F2 in ICD-10. Different types of organic mental disorders, F0 in ICD-10, accounted for 26.7% of cases. Other disorders, including affective disorders (8.8%) and intellectual disability (2.2%), were less frequent. The distribution of ICD-10 diagnoses within the FMN and control groups can also be considered comparable [F (1,3) = 9.417; p = 0.0546 in two-way ANOVA]. Based on these demographical and clinical parameters, we assume that both studied groups are similar in their demographic and clinical characteristics.
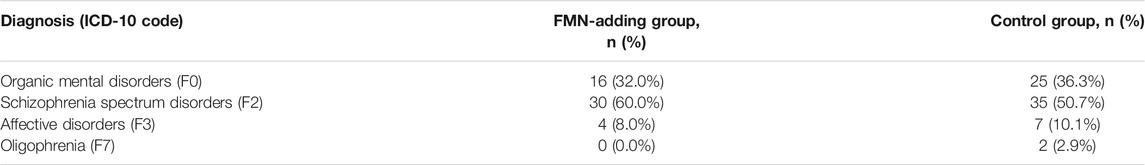
TABLE 2. Distribution of the patients (n = 119) according to ICD-10 diagnoses of mental disorders in the FMN-adding group (n = 50) and control group (n = 69).
We analyzed a number of the main blood and biochemical parameters, namely RBC, platelets, WBC, lymphocytes, neutrophils, monocytes, and hemoglobin values in both groups. The correlation of these parameters with the two most clinically relevant characteristics (C-reactive protein value and lung involvement in chest computed tomography) was evaluated. It was found that neutrophils values positively correlated with the severity of the disease (with both CRP value and lung involvement), while lymphocytes values correlated with these parameters in a negative manner (Figure 1, 2). Accordingly, the neutrophils to lymphocytes ratio also correlated positively.
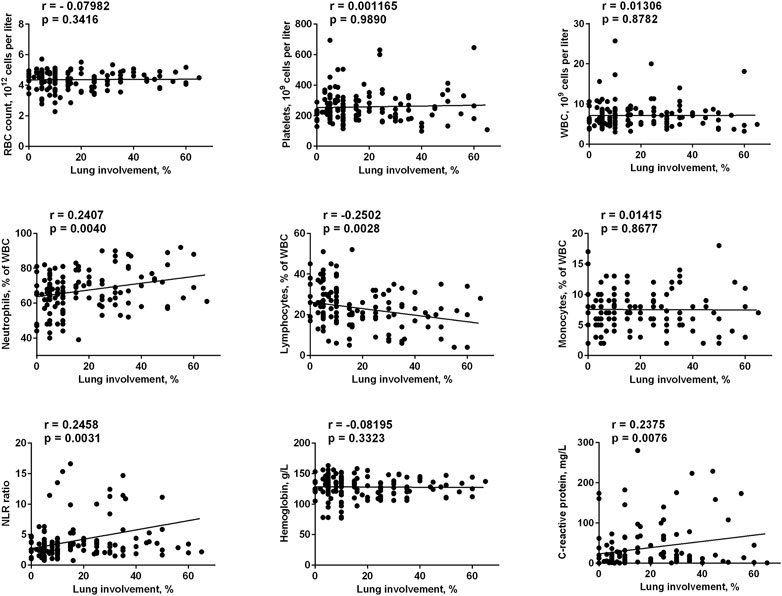
FIGURE 1. Correlation of main blood and biochemical parameters taken on admission to the hospital with the lung involvement in chest computed tomography. The data on day 1 for FMN group and days 1 and 7 for control group were used.
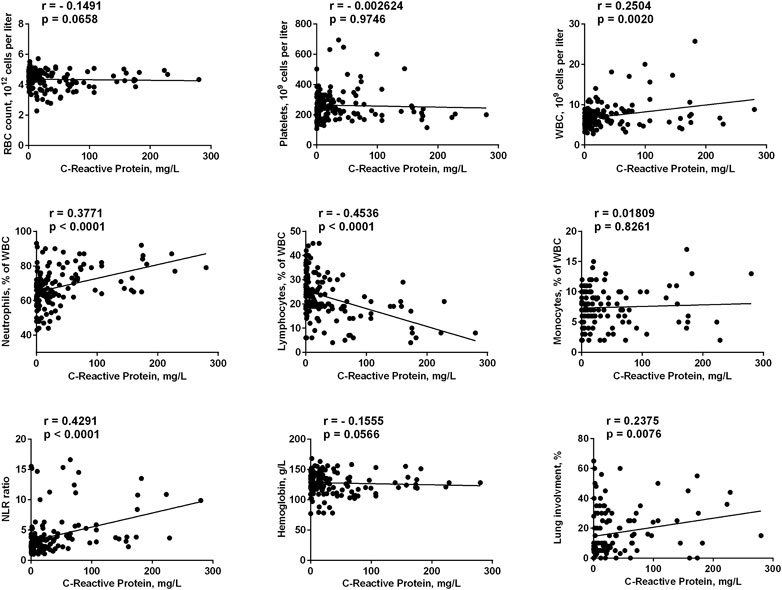
FIGURE 2. Correlation of main blood and biochemical parameters taken on admission to the hospital with C-reactive protein. The data on day 1 for FMN group and days 1 and 7 for control group were used.
The only blood test parameter that correlated with CRP values, but not with the lung involvement, was the total leucocytes count. This could be explained by a better sensitivity of the CRP value to the inflammation status. Indeed, for all studied parameters the correlation with CRP values was clearer than with the lung involvement (e.g., for neutrophils r = 0.3771, p < 0.0001 vs. r = 0.2407, p = 0.004). Finally, lung involvement positively correlated with CRP values (r = 0.2375, p = 0.0076). We can conclude that in our cohort we had four parameters that correlated with the severity of the disease (in addition to well-established clinically relevant lung involvement and CRP values), namely total leucocytes, neutrophils, lymphocytes, and NLR, while RBC, platelets, monocytes and hemoglobin did not correlate with COVID-19 progression. This is in agreement with the literature data: neutrophils and lymphocytes counts, as well as their ratio, were the parameters that predicted the severity and outcome of the COVID-19 infection (Li et al., 2020; Yang et al., 2020; Imran et al., 2021).
The median values of the main blood and biochemical parameters, namely RBC, platelets, WBC, lymphocytes, neutrophils, monocytes, hemoglobin, and CRP, were estimated and compared in each group (day 1 vs. day 7) and in each time point (experimental group vs. control group), and summarized in Table 3. Neutrophils and lymphocytes counts, as well as their ratio, provide the most promising data for the correlation between the COVID-19 course and FMN supplementation. Neutrophils decreased in both cohorts, but only in the experimental group was this decrease statistically significant in the Mann–Whitney U test [67.00 (63.00; 73.00) to 63.00 (57.00; 69.00), p = 0.0406 vs. 69.00 (58.75; 72.25) to 66.00 (58.50; 71.50), p = 0.7904]. Lymphocytes also increased in both groups, but statistical significance has been found for the FMN group only [21.00 (19.00; 27.00) to 27.00 (20.00; 33.00), p = 0.0262 vs. 21.50 (19.00; 30.25) to 24.00 (19.00; 31.50), p = 0.5651]. Finally, the NLR demonstrated a similar pattern for the experimental and control groups [3.190 (2.267; 3.789) to 2.296 (1.781; 3.190), p = 0.0152 vs. 3.117 (1.935; 3.798) to 2.680 (1.888; 3.789), p = 0.6224].
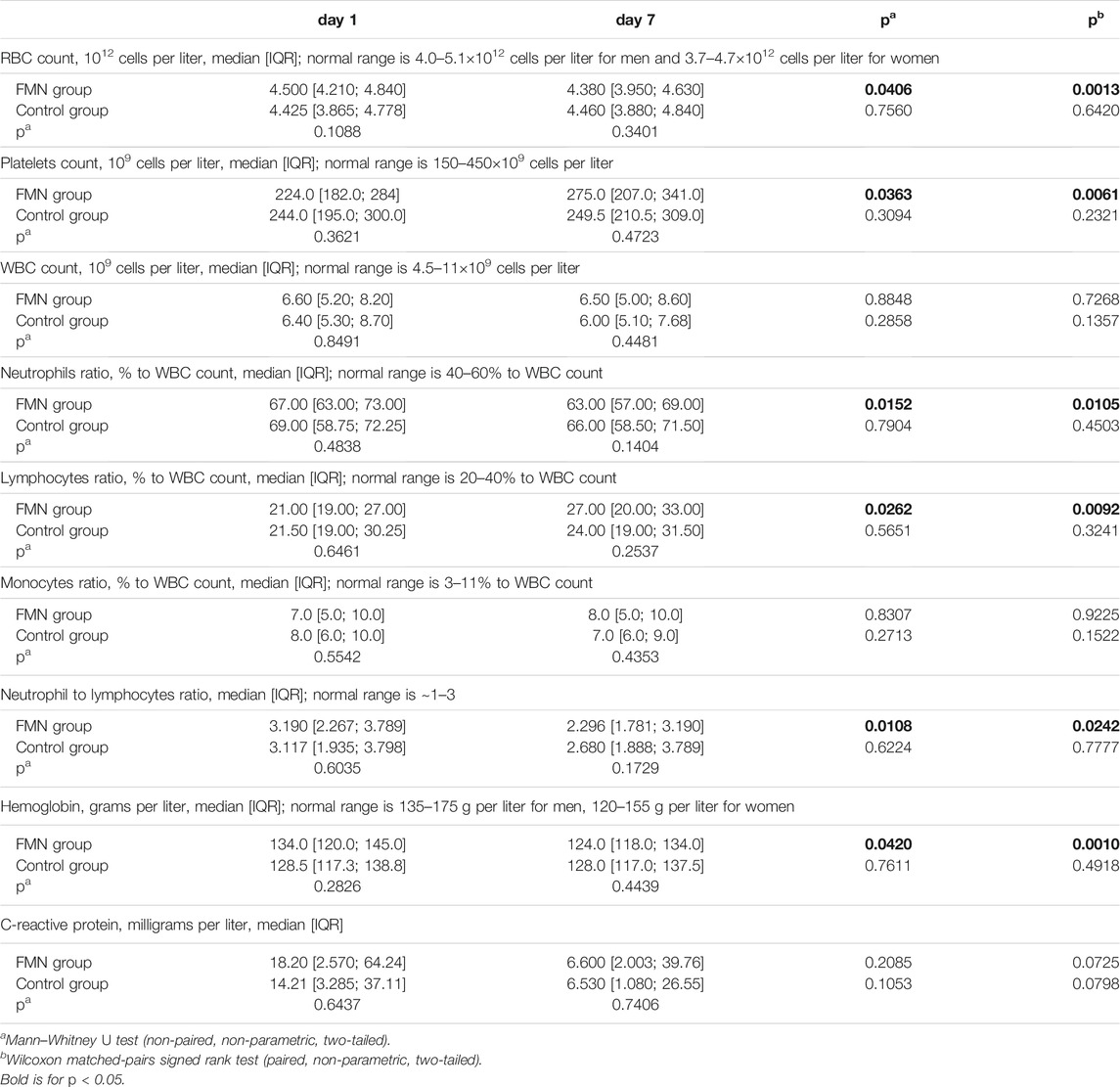
TABLE 3. Dynamics of main blood and biochemical parameters within 1 week after admission to the hospital in FMN group (n = 50) and control group (n = 69).
Surprisingly, we have found a statistically significant increase in the platelets count [224.0 (182.0; 284) to 275.0 (207.0; 341.0), p = 0.0363] and a statistically significant decrease in the RBC count [4.500 (4.210; 4.840) to 4.380 (3.950; 4.630), p = 0.0406] and hemoglobin value [134.0 (120.0; 145.0) to 124.0 (118.0; 134.0), p = 0.0420]. None of these parameters changed significantly in the control group and none of them correlated with COVID-19 severity in our cohort. Although thrombocytopenia (platelets count <150×109 cells per liter) is one of the COVID-19 symptoms (Mei et al., 2020; Zong et al., 2021), we had only seven patients that demonstrated thrombocytopenia within the COVID-19 course. Therefore, we split the normal platelets range (150–450×109 cells per liter) into two parts and evaluated groups with lower (<300×109 cells per liter) and higher (>300×109 cells per liter) platelet counts separately (Supplementary Data S1). In fact, platelets in the lower (<300×109 platelets per liter) FMN group increased from 205.5 [178.5; 240.3] to 272.0 [197.3; 327.3], p = 0.0014, while in the higher (>300×109 platelets per liter) FMN group they decreased from 336.0 [325.0; 353.0] to 291.0 [217.0; 352.0], p = 0.1657), so median values shifted to the center of normal range.
Moreover, we found a decrease in both hemoglobin and RBC count in the experimental group, but not in the control group. Since hemoglobin and RBC values did not correlate with the severity of COVID-19 (CRP values or lung involvement), we assume that this decrease could be explained with fractional eryptosis occurring via visible light irradiation of the patients’ skin. It is known that the light illumination of red blood cells incubated with riboflavin results in partial cell death (Qadri et al., 2017). Fortunately, both median RBC and hemoglobin changed slightly in absolute values and generally remained in the normal range (Supplementary Data S2). Moreover, riboflavin plays an important role in erythropoiesis, as it improves iron absorption and ferritin mobilization (Suwannasom et al., 2020), so we can assume an increase in the RBC count in the FMN group soon.
We additionally picked out patients with advanced severity of COVID-19 (CRP value >10 mg/l and/or CT grade 2–4 at any time of treatment) and evaluated the median values of all studied biochemical parameters in this sub-cohort (Table 4). The obtained data were similar to Table 3 with statistically significant changes for RBC, platelets, neutrophils, lymphocytes, NLR, and hemoglobin in the experimental group, but not in the control group. However, we additionally found statistically significant differences between the experimental and control groups on day 7 for neutrophils and NLR. On day 1 median values of neutrophils were 68.00 [63.00; 79.00] in the experimental group and 70.00 [63.75; 76.00] in the control group (p = 0.7504), while on day 7 median values were 64.00 [55.00; 71.00] and 68.00 [63.00; 77.00], respectively (p = 0.0317). Similarly, median NLR on day 1 was 3.342 [2.222; 4.139] in the experimental group and 3.600 [2.680; 4.676] in the control group (p = 0.2834), while on day 7 median NLR was 2.240 [1.610; 3.300] and 3.000 [2.207; 4.588], respectively (p = 0.0284). We assume this result is direct evidence of the anti-inflammatory efficacy of high doses of riboflavin in COVID-19 patients.
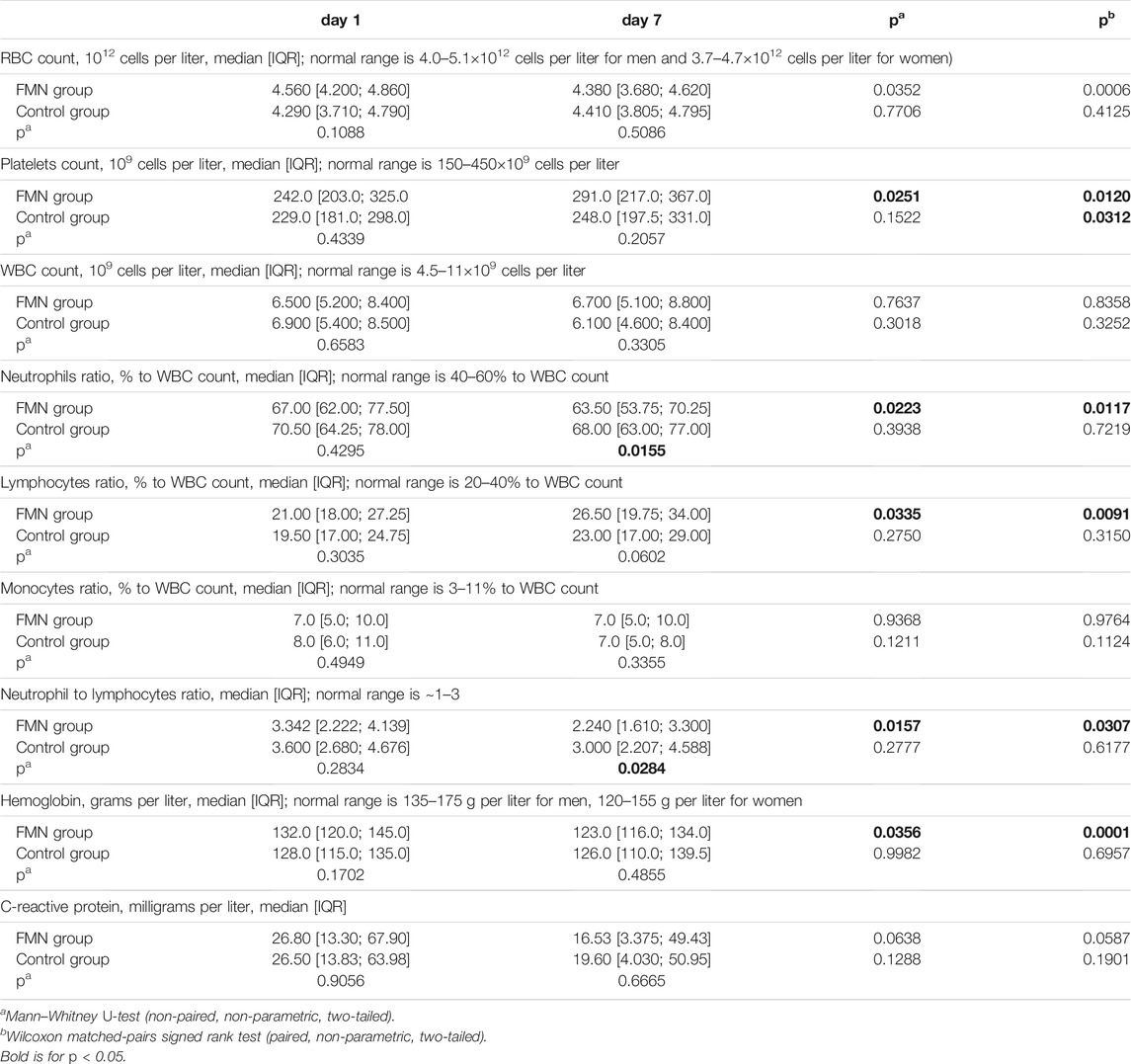
TABLE 4. Dynamics of main blood and biochemical parameters within 1 week after admission to the hospital for patients demonstrated CRP value >10 mg/l and/or CT grade 2–4 at any time of treatment in FMN group (n = 38) and control group (n = 43).
Mental disorders vary greatly in their characteristics, including behavior, pathogenesis, and even biochemical markers (García-Gutiérrez et al., 2020). Based on this knowledge, we can assume alterations in the course of the disease in patients with different psychiatric diagnoses. Our cohort included two main types of diagnosis, namely organic mental spectrum disorders (F0, n = 41) and schizophrenia spectrum disorders (F2, n = 65), while affective disorders (F3, n = 11) and oligophrenia (F7, n = 2) were minor fractions. Here we compared main blood and biochemical parameters in patients with organic mental disorders and schizophrenia. The median values are summarized in Table 5. Indeed, F0 patients demonstrated higher inflammation than F2 patients, which was expressed in higher neutrophils [72.00 (66.00; 79.25) vs. 67.00 (60.75; 72.00), p = 0.0026], lower lymphocytes [19.00 (13.50; 25.25) vs. 23.35 (19.00; 31.00), p = 0.0029], and a higher NLR ratio [3.642 (2.703; 5.923) vs. 2.867 (2.006; 3.640), 0.0020]. In addition, the median CRP value was also higher in the F0 subgroup [24.30 (5.750; 63.14) vs. 16.20 (1.900; 40.74)], although this difference was not significant (p = 0.2111). Higher inflammation in the F0 subgroup can be explained by background neuroinflammation, which is one of the central mechanisms in dementia or Alzheimer’s disease (Kinney et al., 2018; Hur et al., 2020). However, schizophrenia is also often discussed in terms of inflammatory biomarkers (Müller et al., 2015; Miller and Goldsmith, 2019), so this issue needs further evaluation. Additionally, median RBC value was lower in F0 subgroup in comparison to F2 [4.320 (3.620; 4.640) vs. 4.540 (4.218; 4.860), 0.0072]. Anemia conditions are often found in chronic psychiatry patients (Korkmaz et al., 2015), so we can suppose mild anemia in F0 subgroup. The other possible explanation is statistical bias since absolute values are rather close.
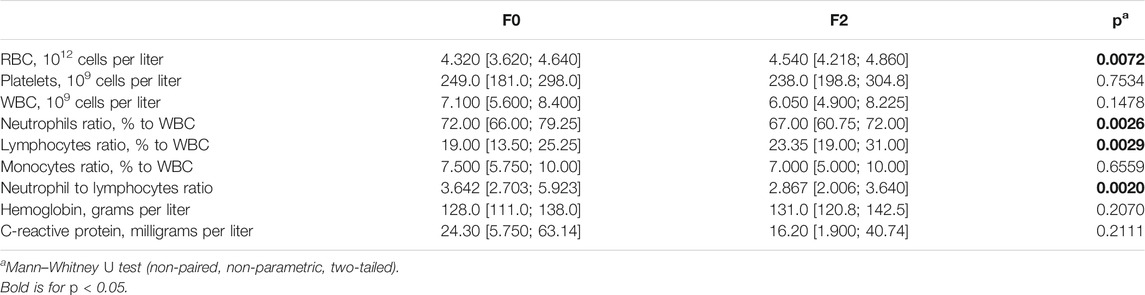
TABLE 5. Main blood and biochemical parameters in organic mental spectrum disorders (F0, n = 41) and schizophrenia spectrum disorders (F2, n = 65) subgroups, median [IQR].
A panel of cytokines, including IL-1β, IL-2, IL-6, TNF-α, МРС-1, and IFN-γ, was also monitored over the hospital stay for a limited number of patients that were recruited to the trial at a late stage (July 2020). All of these cytokines have been earlier studied for clinical relevance in COVID-19. E.g., IL-6 has been previously shown as a predictor for respiratory failure (Jøntvedt Jørgensen et al., 2020) and disease outcome (Del Valle et al., 2020). TNF-α serum levels have been described as an independent and significant predictor of disease severity and death (Del Valle et al., 2020). Studies involving a larger cohort of severe COVID-19 patients showed the importance of IL-2 levels (Shi et al., 2020), while IL-1β has been demonstrated as a low predictive value marker due to its minor expression in patients (Del Valle et al., 2020). In the current research, IL-6 measurements showed the most promising results, decreasing in the experimental group and growing in control group (Table 6). The limited number of patients with cytokine data resulted in a lack of statistical significance, but in line with absolute values changes, we assume IL-6 as the most interesting marker for further research; TNF-α, МСP-1, and МСP-1 as of moderate interest; and IL-2 and IL1β as of low interest.
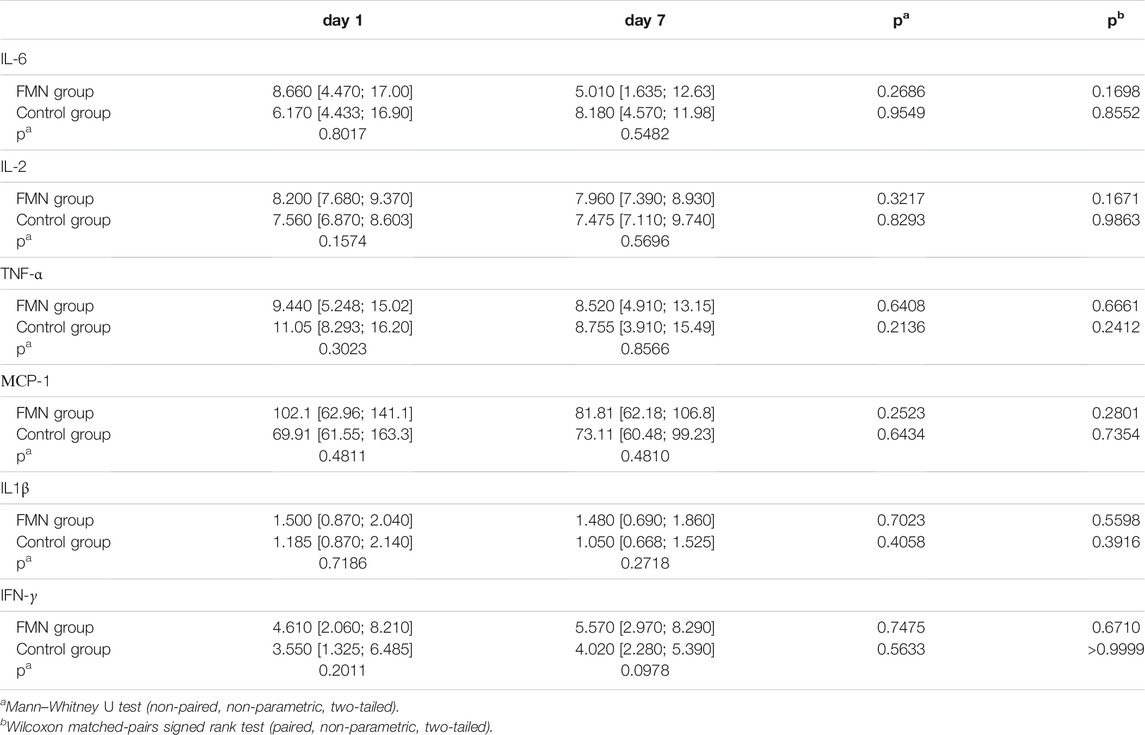
TABLE 6. Dynamics of cytokines within 1 week after admission to the hospital in FMN group (n = 30) and control group (n = 14).
An observational trial of riboflavin (vitamin B2) impact on the immune status of COVID-19 patients with mental disorders has been performed. We demonstrated that a full course of riboflavin supplementation (10 mg of flavin mononucleotide intramuscularly twice a day within 7 days) correlated with a normalization of clinically relevant immune markers (neutrophils and lymphocytes counts, as well as their ratio) in COVID-19 patients. We also found that patients with organic disorders (F0 in ICD-10) demonstrated higher inflammation than patients with schizophrenia patients (F2 in ICD-10). We suppose that riboflavin supplementation could be promising for decreasing inflammation in COVID-19, and further evaluation is required.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Commission of the Sverzhevsky Research Institute of Clinical Otorhinolaryngology (Moscow, Russia), Protocol No. 4 dated 05/27/2020. The patients/participants provided their written informed consent to participate in this study.
RA, EK, DA, and GK designed the research project. NS and DR obtained the data from electronic medical records, while RA, EK, DA, NS, and DR performed the statistical analysis. AS, GK, PV, and MK were involved in the discussion of the obtained results and preparation of the manuscript in the present form. All the authors have contributed and approved the manuscript.
This research work was supported by the Ministry of Science and Higher Education within the State assignment FSRC “Crystallography and Photonics” RAS and by RFBR, project number № 20-04-60357 (in part of cytokines measurement).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.755745/full#supplementary-material
Ahn, H., and Lee, G. S. (2020). Riboflavin, Vitamin B2, Attenuates NLRP3, NLRC4, AIM2, and Non-canonical Inflammasomes by the Inhibition of Caspase-1 Activity. Sci. Rep. 10, 19091. doi:10.1038/s41598-020-76251-7
Akasov, R. A., and Khaydukov, E. V. (2020). Mucosal-Associated Invariant T Cells as a Possible Target to Suppress Secondary Infections at COVID-19. Front. Immunol. 11, 1896. doi:10.3389/fimmu.2020.01896
Alam, M. M., Iqbal, S., and Naseem, I. (2015). Ameliorative Effect of Riboflavin on Hyperglycemia, Oxidative Stress and DNA Damage in Type-2 Diabetic Mice: Mechanistic and Therapeutic Strategies. Arch. Biochem. Biophys. 584, 10–19. doi:10.1016/j.abb.2015.08.013
Anwaar, M. U., Adnan, F., Abro, A., Khan, R. A., Rehman, A. U., Osama, M., et al. (2021). Combined Deep Learning and Molecular Docking Simulations Approach Identifies Potentially Effective FDA Approved Drugs for Repurposing against SARS-CoV-2. Comput. Biol. Med. 141, 105049. doi:10.1016/j.compbiomed.2021.105049
da Silva-Candal, A., Pérez-Díaz, A., Santamaría, M., Correa-Paz, C., Rodríguez-Yáñez, M., Ardá, A., et al. (2018). Clinical Validation of Blood/brain Glutamate Grabbing in Acute Ischemic Stroke. Ann. Neurol. 84, 260–273. doi:10.1002/ana.25286
De Hert, M., Cohen, D., Bobes, J., Cetkovich-Bakmas, M., Leucht, S., Ndetei, D. M., et al. (2011). Physical Illness in Patients with Severe Mental Disorders. II. Barriers to Care, Monitoring and Treatment Guidelines, Plus Recommendations at the System and Individual Level. World Psychiatry 10, 138–151. doi:10.1002/j.2051-5545.2011.tb00036.x
De Picker, L. J., Yolken, R., Benedetti, F., Borsini, A., Branchi, I., Fusar-Poli, P., et al. (2021). Viewpoint | European COVID-19 Exit Strategy for People with Severe Mental Disorders: Too Little, but Not yet Too Late. Brain Behav. Immun. 94, 15–17. doi:10.1016/j.bbi.2021.01.008
Del Valle, D. M., Kim-Schulze, S., Huang, H. H., Beckmann, N. D., Nirenberg, S., Wang, B., et al. (2020). An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 26, 1636–1643. doi:10.1038/s41591-020-1051-9
Dubina, M. V. (2022). Non-immune Prophylaxis against COVID-19 by Targeting Tolerance for Angiotensin II-Triggered SARS-CoV-2 Pathogenesis. Front. Med. 8, 2982. doi:10.3389/fmed.2021.776903
García-Gutiérrez, M. S., Navarrete, F., Sala, F., Gasparyan, A., Austrich-Olivares, A., and Manzanares, J. (2020). Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 11, 432. doi:10.3389/fpsyt.2020.00432
Giamarellos-Bourboulis, E. J., Netea, M. G., Rovina, N., Akinosoglou, K., Antoniadou, A., Antonakos, N., et al. (2020). Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 27, 992–e3. e3. doi:10.1016/j.chom.2020.04.009
Hooshmand, S. A., Zarei Ghobadi, M., Hooshmand, S. E., Azimzadeh Jamalkandi, S., Alavi, S. M., and Masoudi-Nejad, A. (2021). A Multimodal Deep Learning-Based Drug Repurposing Approach for Treatment of COVID-19. Mol. Divers. 25, 1717–1730. doi:10.1007/s11030-020-10144-9
Huang, F., Zhang, C., Liu, Q., Zhao, Y., Zhang, Y., Qin, Y., et al. (2020). Identification of Amitriptyline HCl, Flavin Adenine Dinucleotide, Azacitidine and Calcitriol as Repurposing Drugs for Influenza A H5N1 Virus-Induced Lung Injury. PLOS Pathog. 16, e1008341. doi:10.1371/journal.ppat.1008341
Hur, J. Y., Frost, G. R., Wu, X., Crump, C., Pan, S. J., Wong, E., et al. (2020). The Innate Immunity Protein IFITM3 Modulates γ-secretase in Alzheimer's Disease. Nature 586, 735–740. doi:10.1038/s41586-020-2681-2
Imran, M. M., Ahmad, U., Usman, U., Ali, M., Shaukat, A., and Gul, N. (2021). Retracted: Neutrophil/lymphocyte Ratio-A Marker of COVID‐19 Pneumonia Severity. Int. J. Clin. Pract. 75, 75. doi:10.1111/ijcp.13698
Jøntvedt Jørgensen, M., Holter, J. C., Christensen, E. E., Schjalm, C., Tonby, K., Pischke, S. E., et al. (2020). Increased Interleukin-6 and Macrophage Chemoattractant Protein-1 Are Associated with Respiratory Failure in COVID-19. Sci. Rep. 10, 21697. doi:10.1038/s41598-020-78710-7
Kinney, J. W., Bemiller, S. M., Murtishaw, A. S., Leisgang, A. M., Salazar, A. M., and Lamb, B. T. (2018). Inflammation as a central Mechanism in Alzheimer's Disease. Alzheimers Dement (N Y) 4, 575–590. doi:10.1016/j.trci.2018.06.014
Korkmaz, S., Yıldız, S., Korucu, T., Gundogan, B., Sunbul, Z. E., Korkmaz, H., et al. (2015). Frequency of Anemia in Chronic Psychiatry Patients. Neuropsychiatr. Dis. Treat. 11, 2737–2741. doi:10.2147/NDT.S91581
Lee, S. W., Yang, J. M., Moon, S. Y., Yoo, I. K., Ha, E. K., Kim, S. Y., et al. (2020). Association between Mental Illness and COVID-19 Susceptibility and Clinical Outcomes in South Korea: a Nationwide Cohort Study. Lancet Psychiatry 7, 1025–1031. doi:10.1016/S2215-0366(20)30421-1
Li, X., Liu, C., Mao, Z., Xiao, M., Wang, L., Qi, S., et al. (2020). Predictive Values of Neutrophil-To-Lymphocyte Ratio on Disease Severity and Mortality in COVID-19 Patients: a Systematic Review and Meta-Analysis. Crit. Care 24, 647. doi:10.1186/s13054-020-03374-8
Maes, M., Berk, M., Goehler, L., Song, C., Anderson, G., Gałecki, P., et al. (2012). Depression and Sickness Behavior Are Janus-Faced Responses to Shared Inflammatory Pathways. BMC Med. 10, 66. doi:10.1186/1741-7015-10-66
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., and Manson, J. J. (2020). COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 395, 1033–1034. doi:10.1016/S0140-6736(20)30628-0
Mei, H., Luo, L., and Hu, Y. (2020). Thrombocytopenia and Thrombosis in Hospitalized Patients with COVID-19. J. Hematol. Oncol. 13, 161. doi:10.1186/s13045-020-01003-z
Miller, B. J., and Goldsmith, D. R. (2019). Inflammatory Biomarkers in Schizophrenia: Implications for Heterogeneity and Neurobiology. Biomarkers in Neuropsychiatry 1, 100006. doi:10.1016/j.bionps.2019.100006
Ministry of Health of Russian Federation. [RECOMMENDATIONS for PREVENTION, DIAGNOSTICS and TREATMENT of NEW CORONAVIRUS DESEASE (COVID-19)], Ver 6. (2020) 1–165. Available at: https://www.minzdrav-irkutsk.ru/upload/iblock/32f/32f64f5dcfdeebca4c6e2f3e895f7d43.pdf [Accessed February 3, 2021]
Müller, N., Weidinger, E., Leitner, B., and Schwarz, M. J. (2015). The Role of Inflammation in Schizophrenia. Front. Neurosci. 9, 372. doi:10.3389/fnins.2015.00372
Nemani, K., Li, C., Olfson, M., Blessing, E. M., Razavian, N., Chen, J., et al. (2021). Association of Psychiatric Disorders with Mortality Among Patients with COVID-19. JAMA Psychiatry 78, 380–386. doi:10.1001/jamapsychiatry.2020.4442
Putilina, M. V., Teplova, N. V., Bairova, K. I., Petrikeeva, A. E., and Shabalina, N. I. (2021). The Result of Prospective Randomized Study CITADEL - the Efficacy and Safety of Drug Cytoflavin in Postcovid Rehabilitation. Z. Nevrol. Psikhiatr. Im. S.S. Korsakova 121, 45. doi:10.17116/jnevro202112110145
Qadri, S. M., Chen, D., Schubert, P., Perruzza, D. L., Bhakta, V., Devine, D. V., et al. (2017). Pathogen Inactivation by Riboflavin and Ultraviolet Light Illumination Accelerates the Red Blood Cell Storage Lesion and Promotes Eryptosis. Transfusion 57, 661–673. doi:10.1111/trf.13959
Seekamp, A., Hultquist, D. E., and Till, G. O. (1999). Protection by Vitamin B2 against Oxidant-Mediated Acute Lung Injury. Inflammation 23, 449–460. doi:10.1023/A:1021965026580
Shakoor, H., Feehan, J., Mikkelsen, K., Al Dhaheri, A. S., Ali, H. I., Platat, C., et al. (2021). Be Well: A Potential Role for Vitamin B in COVID-19. Maturitas 144, 108–111. doi:10.1016/J.MATURITAS.2020.08.007
Shi, H., Wang, W., Yin, J., Ouyang, Y., Pang, L., Feng, Y., et al. (2020). The Inhibition of IL-2/IL-2R Gives Rise to CD8+ T Cell and Lymphocyte Decrease through JAK1-STAT5 in Critical Patients with COVID-19 Pneumonia. Cell Death Dis 11, 429. doi:10.1038/s41419-020-2636-4
Suwannasom, N., Kao, I., Pruß, A., Georgieva, R., and Bäumler, H. (2020). Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 21, 950. doi:10.3390/ijms21030950
Taquet, M., Luciano, S., Geddes, J. R., and Harrison, P. J. (2021). Bidirectional Associations between COVID-19 and Psychiatric Disorder: Retrospective Cohort Studies of 62 354 COVID-19 Cases in the USA. Lancet Psychiatry 8, 130–140. doi:10.1016/S2215-0366(20)30462-4
Tavares, N. R., Moreira, P. A., and Amaral, T. F. (2009). Riboflavin Supplementation and Biomarkers of Cardiovascular Disease in the Elderly. J. Nutr. Health Aging 13, 441–446. doi:10.1007/s12603-009-0081-2
Thakur, K., Tomar, S. K., Singh, A. K., Mandal, S., and Arora, S. (2017). Riboflavin and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 57, 3650–3660. doi:10.1080/10408398.2016.1145104
von Martels, J. Z. H., Bourgonje, A. R., Klaassen, M. A. Y., Alkhalifah, H. A. A., Sadaghian Sadabad, M., Vich Vila, A., et al. (2020). Riboflavin Supplementation in Patients with Crohn's Disease [the RISE-UP Study]. J. Crohns Colitis 14, 595–607. doi:10.1093/ecco-jcc/jjz208
Wang, Q., Xu, R., and Volkow, N. D. (2021). Increased Risk of COVID ‐19 Infection and Mortality in People with Mental Disorders: Analysis from Electronic Health Records in the United States. World Psychiatry 20, 124–130. doi:10.1002/wps.20806
Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., et al. (2020). Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm. Sin B 10, 766–788. doi:10.1016/j.apsb.2020.02.008
Yang, A. P., Liu, J. P., Tao, W. Q., and Li, H. M. (2020). The Diagnostic and Predictive Role of NLR, D-NLR and PLR in COVID-19 Patients. Int. Immunopharmacol 84, 106504. doi:10.1016/j.intimp.2020.106504
Yu, Z., Morimoto, K., Yu, J., Bao, W., Okita, Y., and Okada, K. (2016). Endogenous Superoxide Dismutase Activation by Oral Administration of Riboflavin Reduces Abdominal Aortic Aneurysm Formation in Rats. J. Vasc. Surg. 64, 737–745. doi:10.1016/j.jvs.2015.03.045
Zhou, X., Tian, B., and Han, H. B. (2021). Serum Interleukin-6 in Schizophrenia: A System Review and Meta-Analysis. Cytokine 141, 155441. doi:10.1016/j.cyto.2021.155441
Keywords: SARS-CoV-2, COVID-19, riboflavin, flavin mononucleotide, inflammation, cytokines, schizophrenia, organic mental disorders
Citation: Akasov RA, Khaydukov EV, Andreyuk DS, Sholina NV, Sheremeta AN, Romanov DV, Kostyuk GP, Panchenko VY and Kovalchuk MV (2022) Riboflavin for COVID-19 Adjuvant Treatment in Patients With Mental Health Disorders: Observational Study. Front. Pharmacol. 13:755745. doi: 10.3389/fphar.2022.755745
Received: 16 September 2021; Accepted: 31 January 2022;
Published: 10 March 2022.
Edited by:
Kamaldeep Paul, Thapar Institute of Engineering and Technology, IndiaReviewed by:
Ouliana Ziouzenkova, The Ohio State University, United StatesCopyright © 2022 Akasov, Khaydukov, Andreyuk, Sholina, Sheremeta, Romanov, Kostyuk, Panchenko and Kovalchuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. A. Akasov, cm9tYW4uYWthc292QGdtYWlsLmNvbQ==; E. V. Khaydukov, a2hheWR1a292QG1haWwucnU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.