- 1Department of Dermatology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Clinical and Research Center for Infectious Diseases, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 3Beijing Key Laboratory of HIV/AIDS Research, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 4Infectious Disease Department, University of Chinese Academy of Sciences Shenzhen Hospital (Guangming), Shenzhen, China
Introduction: Extensive use of antiretroviral therapy has remarkably improved the survival rates of people living with HIV. Doravirine (DOR) is a newly-approved antiretroviral belonging to the class of non-nucleoside reverse transcriptase inhibitors. Here, we compared the efficacy and safety of DOR + tenofovir dipivoxil fumarate (TDF)+Lamivudine (3TC)/Emtritabine (FTC) with traditional triple therapies in treatment-naïve HIV-1-positive adults.
Methods: Randomized controlled trials involving treatment-naïve HIV-1-positive adults that met inclusion criteria were systematically retrieved and data on the following outcomes extracted: virological suppression, adverse events, severe adverse events, and drug-related adverse events. A Bayesian network meta-analysis was then performed on the data.
Results: This study included a total of 39 randomized controlled trials involving 26 antiretroviral therapies and 21,110 HIV1-positive patients. At week 48, relative to the other 25 regimens included in the network of virological suppression, DOR + TDF+3TC/FTC exhibited superiority to some efavirenz, nevirapine, atazanavir, or lopinavir-based regimens, including efavirenz + abacavir+3TC [Odd Ratio (OR) = 0.52, 95% confidence interval (CrI) = 0.35–0.77]. At week 48, the performance of DOR + TDF+3TC/FTC was relatively similar to all other analyzed regimens in terms of adverse events. The DOR + TDF+3TC/FTC regimen performed better in terms of severe adverse events and drug-related adverse events.
Conclusion: The network meta-analysis showed that DOR + TDF+3TC/FTC has good efficacy and safety at 48 weeks.
Systematic Review Registration: Open Science Framework, https://osf.io/6ybp7.
Introduction
HIV is the causative agent of AIDS. In 2020, there were 37.7 million (30.2 million–45.1 million) people living with HIV (PLWH) (UNAIDS, 2020). HIV-1, a HIV subtype is the main driver of the global AIDS pandemic. Studies have shown that antiretroviral therapy (ART) has significantly reduced the rates of HIV-1-induced, AIDS-related, morbidity and mortality, and the life expectancy of HIV-1-infected people is nearing that of the general population (Antiretroviral Therapy Cohort Collaboration, 2017; Wu, 2016; Marcus et al., 2000; GBD 2019 HIV Collaborators, 2021; Smiley et al., 2021). Regimens for initiating antiretroviral therapy in HIV-infected people are usually comprised of two nucleoside reverse transcriptase inhibitors as backbone drugs, combined with a third core drug. The core drugs may be non-nucleoside reverse transcriptase inhibitors (NNRTIs) or boosted protease inhibitors or integrase inhibitors (European AIDS Clinical Society, 2019; Panel on Antiretroviral Guidelines for Adults and Adolescents, 2021).
Doravirine (DOR), a new FDA-approved NNRTI is currently prescribed in combination with tenofovir disoproxil fumarate (TDF) and lamivudine (3TC) in the US, China, Africa and et al. (Rock et al., 2020; Panel on Antiretroviral Guidelines for Adults and Adolescents, 2021; Steegen et al., 2021). Two phase 3 clinical trials (DRIVE-AHEAD, DRIVE-FORWARD), have compared DOR + TDF+3TC/emtricitabine (FTC) with efavirenz (EFV)- or darunavir (DRV)-based regimens in treatment-naïve, HIV-1-positive patients (Molina et al., 2018; Orkin et al., 2019) and found that it is efficacious, safe and tolerable. Since the emergence of ART, the survival rate of HIV- infected people has improved significantly, turning HIV infection into a controllable chronic disease (Antiretroviral Therapy Cohort Collaboration, 2017; Wu, 2016; Marcus et al., 2000; GBD 2019 HIV Collaborators, 2021; Smiley et al., 2021). However, PLWH often need to use ART for life, and as their life expectancy increases, so does the amount of time they are on ART. Thus, ART toxicity has attracted considerable attention in clinical practice. In the case of similar efficacy, treatment regimens with less toxicity and high safety are preferred. However, due to the time-consuming and labor-intensive nature of clinical trials, DOR + TDF+3TC/FTC has not been directly compared with all first-line ARTs in randomized controlled trials (RCT). Hence, the efficacy and safety of this regimen relative to other regimens has not been directly determined. We found that network meta-analysis (NMA) is increasingly used to support the development of treatment guidelines. For example, a 2016 NMA evaluated the use of core drugs in untreated HIV-1-positive people, and found that integrase inhibitors, especially DTG, showed excellent effectiveness and safety (Kanters et al., 2016).
Here, in the absence of direct comparisons, we sought to evaluate the effectiveness and safety of various ARTs by indirect comparison. NMA combines direct and indirect evidence to simultaneously assess the relative effectiveness and safety of two or more interventions. Our objective was to compare the efficacy and safety of DOR + TDF+3TC/FTC with traditional three-drug regiments at 48 weeks in treatment-naïve HIV-1-infected adults.
Methods
Study Identification and Selection Criteria
The current NMA was conducted on the basis of the PRISMA extension statement (Hutton et al., 2015).
In November 2020 and February 2022, we systematically searched PubMed, embase, Web of Science, and Cochrane Library databases for RCTs meeting inclusion criteria. Search strategy can be found in the protocol at https://osf.io/6ybp7. Inclusion criteria were as follows: 1) phase 3 or 4 RCTs of treatment-naïve HIV-1-infected adults, 2) interventions: DOR + TDF+3TC/FTC or other ART regimens with three antiretrovirals that included 2 NRTIs (backbones) and one core agent. Some core drugs need the assistance of boosted drugs (b) like cobicistat (c) and ritonavir (r) to enhance their effect. The following cores and backbones were chosen because they were used or are still used as first-line therapies. Although some regimens may no longer be used as often, we also included these regimens to serve as comparators in order to increase the connectivity of the network, provide more indirect effects. The backbones of our interest were TDF+3TC/FTC, tenofovir alafenamide (TAF)+FTC, abacavir (ABC)+3TC, or azidothymidine (AZT)+3TC. Core drugs of our interest were raltegravir (RAL), elvitegravir (EVG), dolutegravir (DTG), bictegravir (BIC), EFV, rilpivirine (RPV), nevirapine (NVP), atazanavir (ATV), lopinavir (LPV), and DRV, 3) all included drugs were standard doses, but low-dose EFV (400mg EFV) were also included, 4) at least one of the following outcomes provided: 48-weeks virological suppression (HIV-1 RNA<50 copies/mL), adverse events, serious adverse events, drug-related adverse events, subgroup analysis (virological suppression in people with a baseline viral load of >100,000 copies/mL). Exclusion criteria were: 1) all subjects living with tuberculosis or were pregnant, 2) inability to precisely determine the backbones used. To avoid missing relevant data, we searched www.ClinicalTrials.gov and www.napnap.com, and we also read relevant systematic reviews, meta-analyses, and their references.
Past studies used third core drugs as network nodes for analysis when comparing the effectiveness and safety of ARTs in treatment-naive HIV patients (Kanters et al., 2016; Gallien et al., 2018). Considering that the backbone of the DOR + TDF+3TC/FTC we wanted to compare is defined, we used a complete treatment regimen as network node. Analyzing a comprehensive treatment regimen as a network node can also simultaneously evaluate differences between regimens of the same core drug with different backbones, thereby providing more reference for clinical treatment.
Data Extraction and Quality Evaluation
Two researchers (KZ and YZ) independently selected titles and abstracts, read full texts of articles that met the inclusion criteria, and extracted relevant data (Supplementary Table S1). Any disagreements were resolved by discussion and if no solution was reached, the opinion of the third researcher (JZ) was taken. The Cochrane Risk of Bias instrument was used to assess quality of included trials (Supplementary Table S2) (Higgins et al., 2011).
Analysis
R software (version 4.0.2) and gemtc package were used to perform NMA within a Bayesian framework. Results were calculated using Markov chain Monte Carlo method, and the convergence evaluated using a potential scale reduction factor (<1.2 is acceptable) (Shim et al., 2019). The results of fixed effect model and random effect model were calculated in this paper. By comparing deviance information criterion of the fixed effect and random effect models, the more suitable model was selected. The smaller the deviance information criterion, the more appropriate the model was. The consistency test was analysed by node splitting method, with p < 0.05 indicating inconsistency. The random-effect model was used when local comparisons were inconsistent. In case of inconsistency in many comparisons, the suitability of NMA should be discussed (Shim et al., 2019). The outcomes analyzed in this study were all binary variables, and the statistical results were expressed as odds ratio (OR) and corresponding 95% confidence interval (CrI). SUCRA values for each regimen were calculated to evaluate their ranking among various regimens. CINeMA was used to grade evidence quality (Nikolakopoulou et al., 2020).
Results
Studies Included
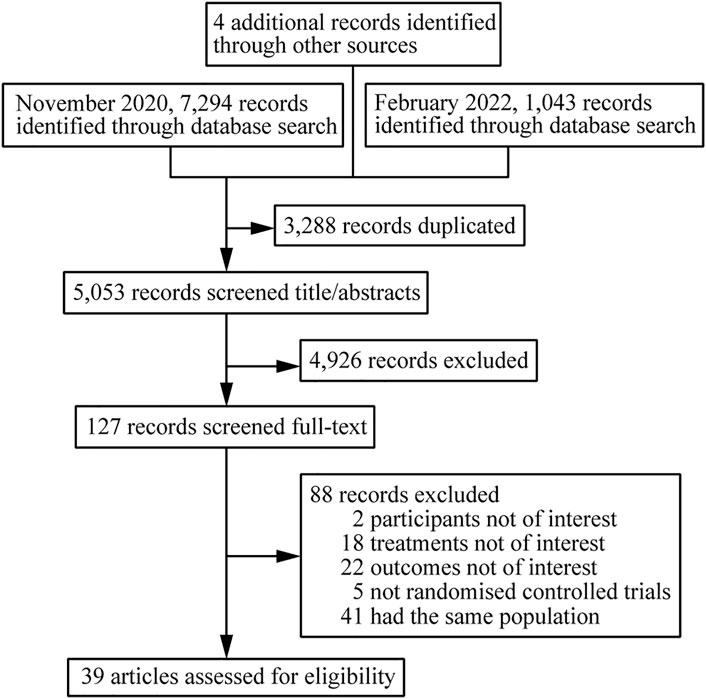
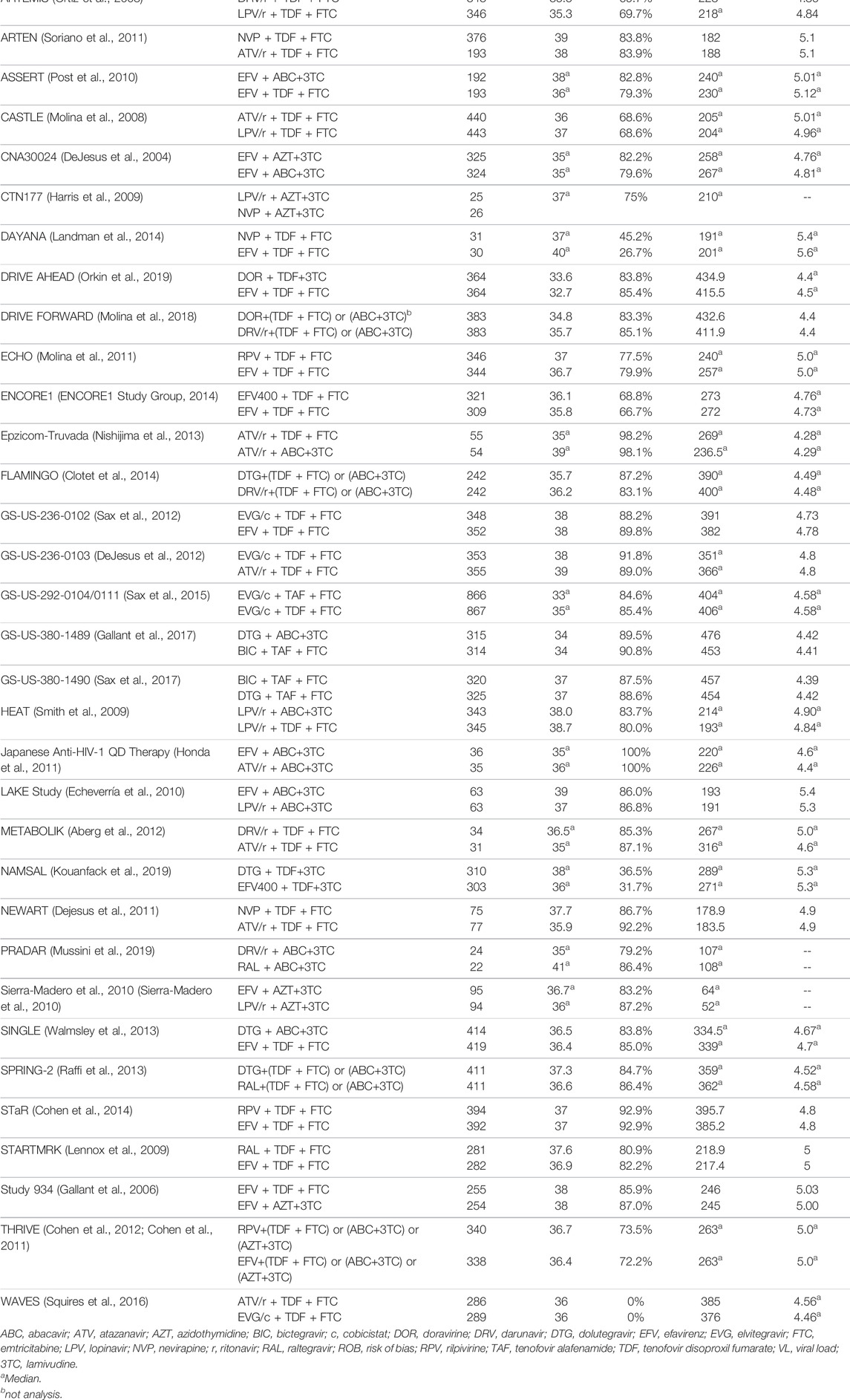
A total of 8341 citations were obtained using database searches and other methods. Of these, 3288 duplicates were excluded. After screening, 39 articles (Orkin et al., 2019; Molina et al., 2018; Lennox et al., 2014; Miro et al., 2015; Puls et al., 2010; Eron et al., 2018; Orrell et al., 2017; Ortiz et al., 2008; Soriano et al., 2011; Post et al., 2010; Molina et al., 2008; DeJesus et al., 2004; Harris et al., 2009; Landman et al., 2014; Molina et al., 2011; ENCORE1 Study Group, 2014; Nishijima et al., 2013; Clotet et al., 2014; Sax et al., 2012; DeJesus et al., 2012; Sax et al., 2015; Gallant et al., 2017; Sax et al., 2017; Smith et al., 2009; Honda et al., 2011; Echeverría et al., 2010; Aberg et al., 2012; Kouanfack et al., 2019; Dejesus et al., 2011; Mussini et al., 2019; Sierra-Madero et al., 2010; Walmsley et al., 2013; Raffi et al., 2013; Cohen et al., 2014; Lennox et al., 2009; Gallant et al., 2006; Cohen et al., 2012; Cohen et al., 2011; Squires et al., 2016), involving 39 RCTs and 21,110 participants, met the inclusion criteria (Figure 1). The number of participants in different studies ranged from 46 to 1,809, and most of the studies involved predominantly male participants. In different studies, baseline characteristics like the mean/median age, CD4 and viral load of participants in different treatment groups were 32.7–41 years, 30 to 476 cells/mm3, 4.28 to 5.6 log copies/mL respectively. Table 1 shows the basic information for each trial and the baseline characteristics of the study population. The ARTs analyzed for each outcome are plotted in network plots, with straight line connections between the different ARTs indicating the existence of a RCT that directly compared the 2 ARTs (Figure 2). Fixed-effect model was preferred for all the analysis, except analysis in adverse events.
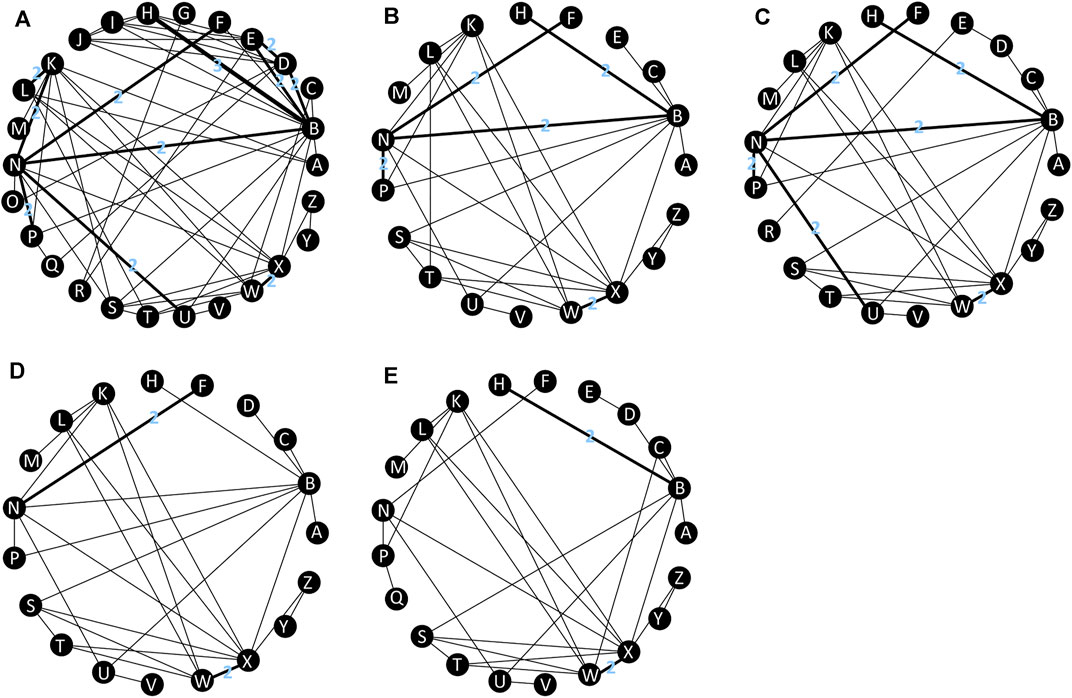
FIGURE 2. Network plot of treatment outcomes in terms of (A) virological suppression, (B) adverse events, (C) serious adverse events, (D) drug-related adverse events, (E) subgroup analysis. All connecting straight lines are based either on single studies or in case this does not hold true, then every connection with n > 1 should show the actual n. A: DOR + TDF + XTC; B: EFV + TDF + XTC; C: EFV400 + TDF + XTC; D: EFV + ABC+3TC; E: EFV + AZT+3TC; F: NVP + TDF + XTC; G: NVP + AZT+3TC; H: RPV + TDF + XTC; I: RPV + ABC+3TC; J: RPV + AZT+3TC; K: DRV/b + TDF + XTC; L: DRV/r + ABC+3TC; M: DRV/c + TAF + FTC; N: ATV/r + TDF + XTC; O: ATV/r + ABC+3TC; P: LPV/r + TDF + XTC; Q: LPV/r + ABC+3TC; R: LPV/r + AZT+3TC; S: RAL + TDF + XTC; T: RAL + ABC+3TC; U: EVG/c + TDF + XTC; V: EVG/c + TAF + FTC; W: DTG + TDF + XTC; X: DTG + ABC+3TC; Y: DTG + TAF + FTC; Z: BIC + TAF + FTC. ABC: abacavir; ATV: atazanavir; AZT: Azidothymidine; BIC: bictegravir; c: cobicistat; DOR: doravirine; DRV: darunavir; DTG: dolutegravir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NVP: nevirapine; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate; XTC: emtricitabine or lamivudine; 3TC: lamivudine.
Virologic Suppression
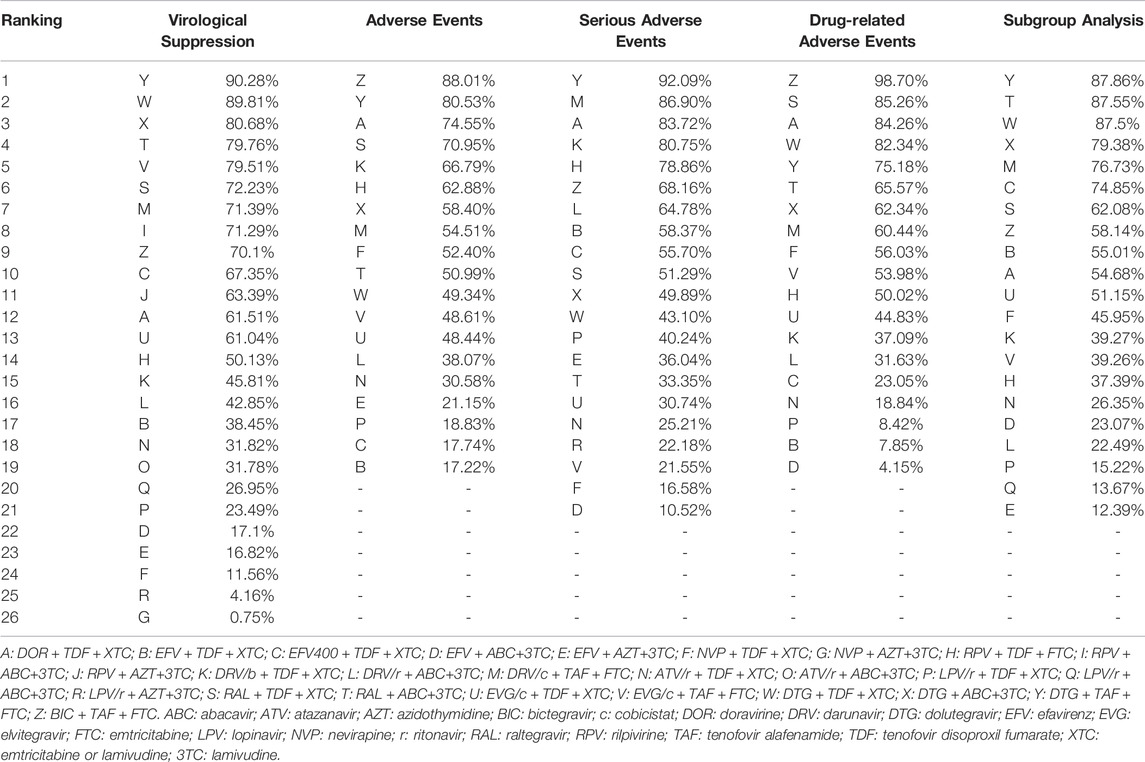
All included studies reported the number of participants with a HIV RNA load of <50 copies/mL at 48 weeks. A total of 26 treatment regimens were analyzed for this outcome. Network analysis revealed that EFV + TDF+3TC/FTC had the most connection times (Figure 2A). Fixed-effect model analysis found that DOR + TDF+3TC/FTC has a higher proportion of people achieved virological suppression relative to most NNRTI and PI-based regimens (Figure 3A), indicating good efficacy. DTG + TAF+3TC/FTC had the highest SUCRA value (SUCRA = 90.28%), indicating that it is the best regimen with regards to virological suppression, while DOR + TDF+3TC/FTC (SUCRA = 61.51%) ranked 12th, indicating relatively lower effectiveness (Table 2).
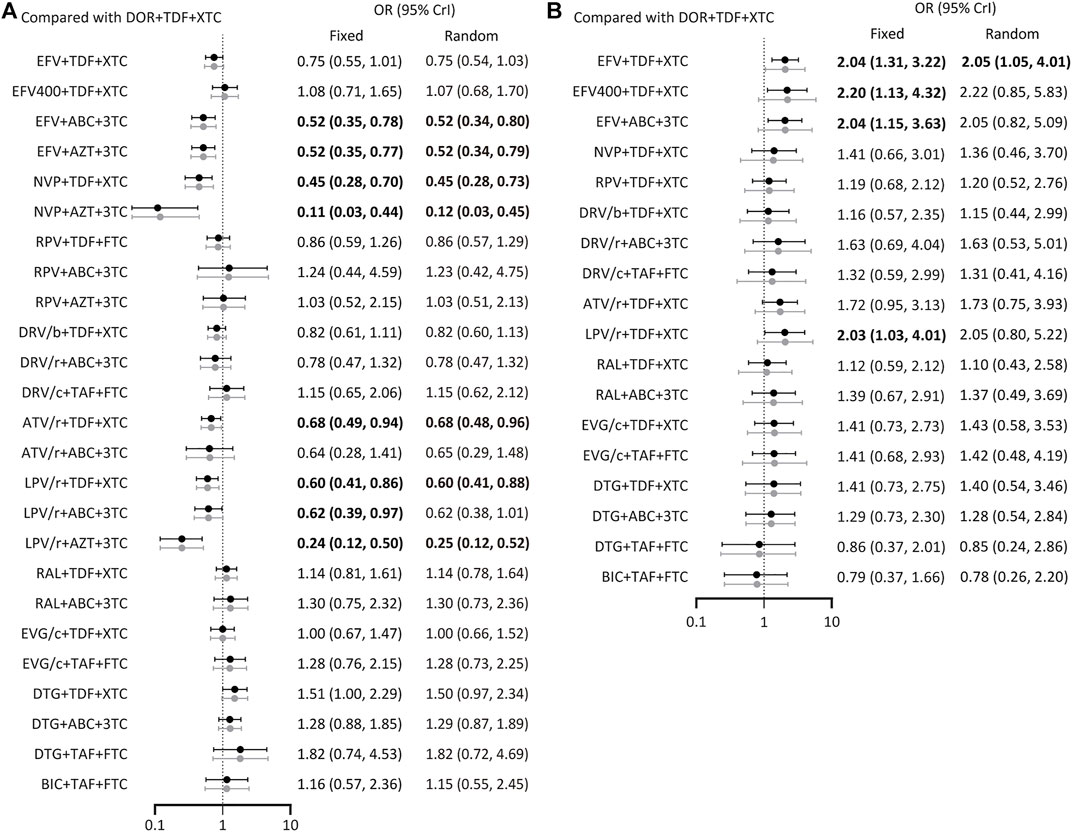
FIGURE 3. Forest plot of outcomes in terms of (A) virological suppression, (B) adverse events. Results of fixed effect model were drawn in black, and results of random effect model were drawn in grey. ABC: abacavir; ATV: atazanavir; AZT: Azidothymidine; BIC: bictegravir; c: cobicistat; DOR: doravirine; DRV: darunavir; DTG: dolutegravir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NVP: nevirapine; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate; XTC: emtricitabine or lamivudine; 3TC: lamivudine.
Adverse Events
A total of 19 regimens reported data on adverse events (Figure 2B). Since the comparison between DTG + ABC+3TC and RAL + TDF+3TC/FTC had a p < 0.05 using the node splitting method, the random-effects model was applied. The incidence of adverse events with DOR + TDF+3TC/FTC was lower than with EFV + TDF+3TC/FTC and was statistically significant (OR = 2.05, 95% CrI = 1.05–4.0). Comparisons between DOR + TDF+3TC/FTC and ARTs except EFV + TDF+3TC/FTC showed no statistical differences (Figure 3B). The SUCRA value for DOR + TDF+3TC/FTC was 74.55% (third rank), indicating its incidence of adverse events maybe higher than DTG + TAF + FTC and BIC + TAF + FTC, but lower than other regimens (Table 2).
Serious Adverse Events
A total of 27 studies reported the occurrence of serious adverse events involving 21 treatment regimens (Figure 2C). Forest plot showed DOR + TDF+3TC/FTC was statistically different from EFV + ABC+3TC (OR = 3.55, 95% CrI = 1.33–9.85), NVP + TDF+3TC/FTC (OR = 3.04, 95% CrI = 1.16–8.19), ATV/r + TDF+3TC/FTC (OR = 2.51, 95% CrI = 1.12–5.81), EVG/c + TDF+3TC/FTC (OR = 2.37, 95% CrI = 1.06–5.49), and EVG/c + TAF + FTC (OR = 2.72, 95% CrI = 1.12–6.75). These ORs and 95% CrIs were all >1, indicating that DOR + TDF+3TC/FTC had higher safety compared with the above regimens with regards to serious adverse events (Figure 4A). Based on SUCRA value, DOR + TDF+3TC/FTC ranks third (SUCRA = 83.72%), indicating its incidence of serious adverse events maybe higher than DTG + TAF + FTC and DRV/c + TAF + FTC, but lower than other regimens (Table 2).
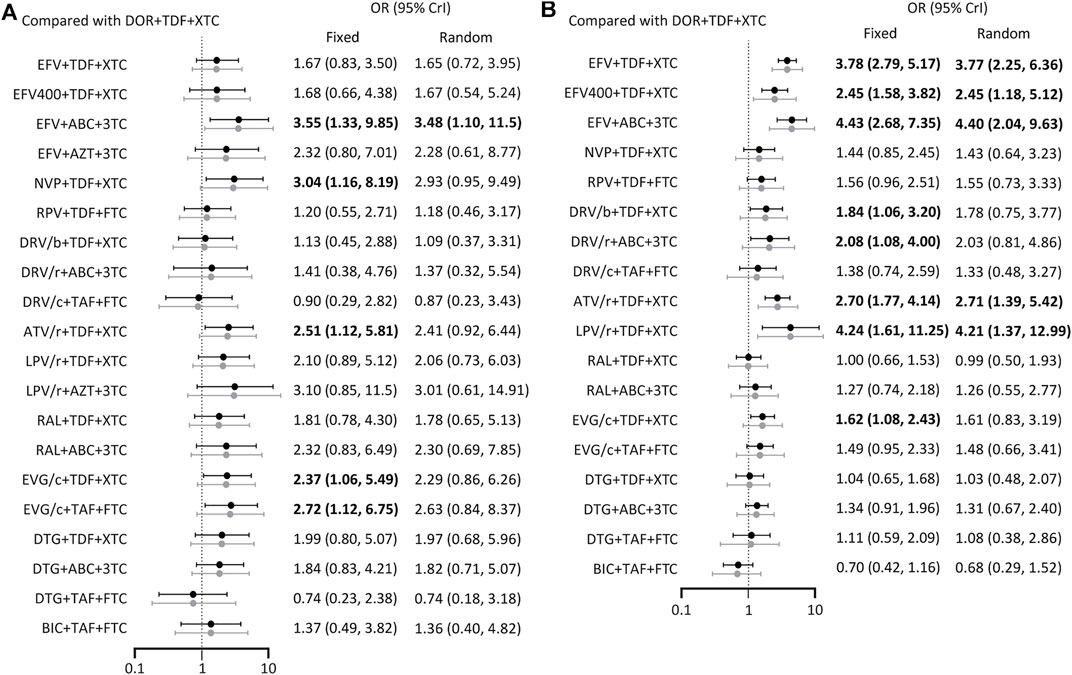
FIGURE 4. Forest plot of outcomes in terms of (A) serious adverse events (B) drug-related adverse events. Results of fixed effect model were drawn in black, and results of random effect model were drawn in grey. ABC: abacavir; ATV: atazanavir; AZT: Azidothymidine; BIC: bictegravir; c: cobicistat; DOR: doravirine; DRV: darunavir; DTG: dolutegravir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NVP: nevirapine; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate; XTC: emtricitabine or lamivudine; 3TC: lamivudine.
Drug-Related Adverse Events
The 19 ARTs compared in the 19 trials constituted a network of evidence for drug-related adverse events (Figure 2D). DOR + TDF+3TC/FTC exhibited better safety than EFV-based regimens. Comparison between DOR + TDF+3TC/FTC and DRV-based regimens revealed different results for different backbones. ABC+3TC and TDF+3TC/FTC with DRV had more drug-related adverse events, while TAF + FTC with DRV had no statistically significant difference relative to DOR + TDF+3TC/FTC. Most of the comparisons between DOR + TDF+3TC/FTC and ARTs containing INI showed no statistical differences. Only comparison with EVG/c + TDF+3TC/FTC showed relatively poor safety of the latter (Figure 4B). The SUCRA value of DOR + TDF+3TC/FTC was 84.26%, indicating its incidence of drug-related adverse events maybe higher than BIC + TAF + FTC and RAL + TDF+3TC/FTC, but lower than other regimens (Table 2).
Subgroup Analysis
With regards to virological suppression in patients with viral loads >100,000 copies/mL, all other ARTs had no statistically significant difference relative to DOR + TDF+3TC/FTC (Figure 5). SUCRA value showed that DOR + TDF+3TC/FTC ranked relatively low (Table 2). This is probably because many trials did not report results for subgroup analysis, resulting in a corresponding reduction in the number of treatment regimens (Figure 2E) and participants, thus increasing the uncertainty and CrI.
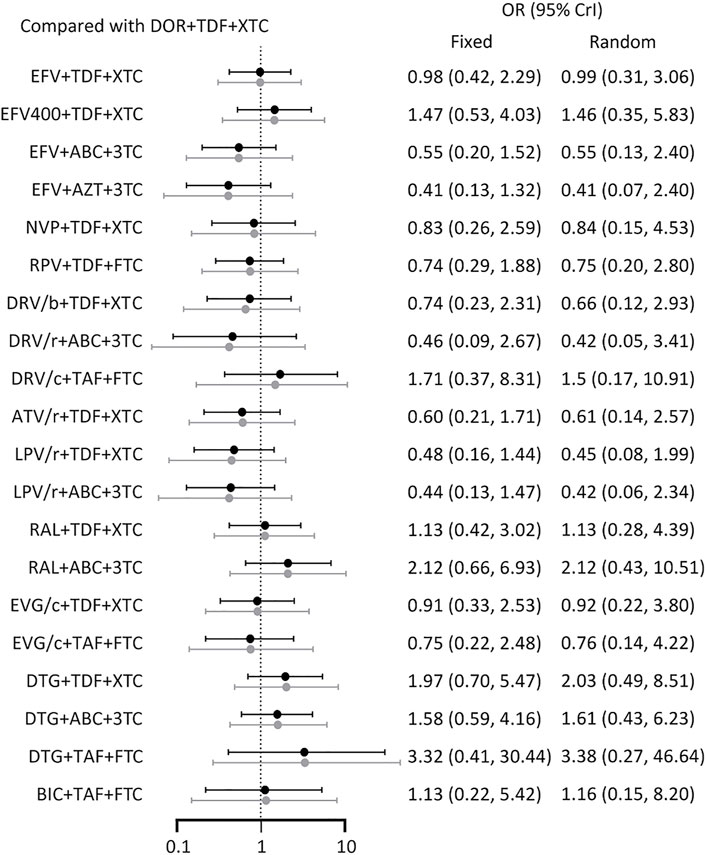
FIGURE 5. Forest plot of outcomes in terms of subgroup analysis. Results of fixed effect model were drawn in black, and results of random effect model were drawn in grey. ABC: abacavir; ATV: atazanavir; AZT: Azidothymidine; BIC: bictegravir; c: cobicistat; DOR: doravirine; DRV: darunavir; DTG: dolutegravir; EFV: efavirenz; EVG: elvitegravir; FTC: emtricitabine; LPV: lopinavir; NVP: nevirapine; r: ritonavir; RAL: raltegravir; RPV: rilpivirine; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate; XTC: emtricitabine or lamivudine; 3TC: lamivudine.
Evidence Grading
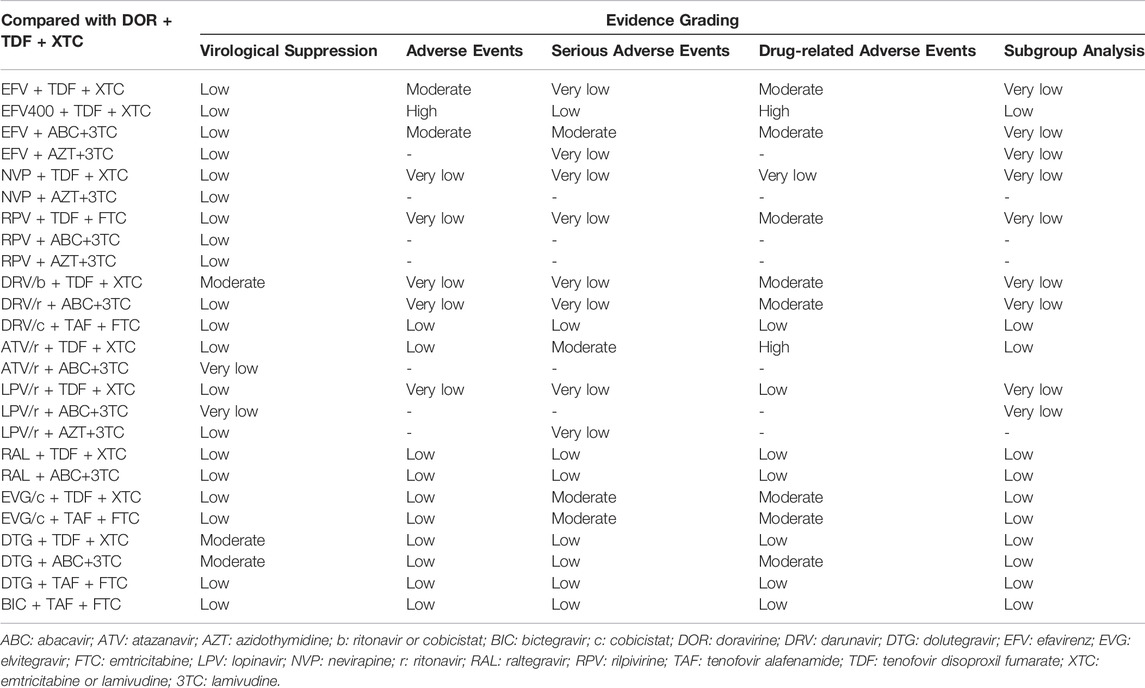
Based on evidence grading by CINeMA, most results were rated as “moderate” and “low” (Table 3). There are two main reasons for downgrading. First, many trials were not blinded, resulting in a trial rating of “low quality,” which may affect the results of the comparison between relevant regimens, leading to the downgrade. Additionally, where CrI included 1, results were not statistically significant, and were downgraded.
Discussion
This study compared the efficacy and safety of DOR + TDF+3TC/FTC with traditional three-drug regiments at 48 weeks in treatment-naïve HIV-1-infected adults. Compared with cohort studies and case-control studies, RCTs are of higher grade, so this study chose to include RCTs for NMA. A total of 39 RCTs involving more than 20,000 patients were included according to the inclusion criteria, and a maximum of 26 treatments were compared simultaneously (Orkin et al., 2019; Molina et al., 2018; Lennox et al., 2014; Miro et al., 2015; Puls et al., 2010; Eron et al., 2018; Orrell et al., 2017; Ortiz et al., 2008; Soriano et al., 2011; Post et al., 2010; Molina et al., 2008; DeJesus et al., 2004; Harris et al., 2009; Landman et al., 2014; Molina et al., 2011; ENCORE1 Study Group, 2014; Nishijima et al., 2013; Clotet et al., 2014; Sax et al., 2012; DeJesus et al., 2012; Sax et al., 2015; Gallant et al., 2017; Sax et al., 2017; Smith et al., 2009; Honda et al., 2011; Echeverría et al., 2010; Aberg et al., 2012; Kouanfack et al., 2019; Dejesus et al., 2011; Mussini et al., 2019; Sierra-Madero et al., 2010; Walmsley et al., 2013; Raffi et al., 2013; Cohen et al., 2014; Lennox et al., 2009; Gallant et al., 2006; Cohen et al., 2012; Cohen et al., 2011; Squires et al., 2016). Baseline characteristics of the participants were similar. The results of this study are consistent with those of DRIVE-AHEAD and DRIVE-FORWORD (Molina et al., 2018; Orkin et al., 2019). Two phase three RCTs (DRIVE-AHEAD, DRIVE-FORWORD) have proven that DOR has good efficacy and safety in treatment-naïve HIV-1 adults (Molina et al., 2018; Orkin et al., 2019). DRIVE-FORWORD showed that compared to DRV/r (TDF + FTC or ABC+3TC backbone), DOR was not less effective in virological suppression and had similar safety (Molina et al., 2018). DRIVE-AHEAD compared DOR + TDF+3TC with EFV + TDF + FTC and uncovered similar virologic efficacy, but the safety of the former was better (Orkin et al., 2019).
In this study, in terms of virological suppression, DOR++TDF+3TC/FTC was not inferior to EFV + TDF + FTC/3TC and DRV/r-based regimens. But ORs between DOR++TDF+3TC/FTC and INI-based regimens were ≥1, indicating INI-based regimens had a higher proportion of patients achieving virological suppression. In subgroup analysis, DOR performed similarly in the high viral load population as in the full population, except that the comparison with EVG had an OR more than 1. For safety analysis, adverse events, serious adverse events, and drug-related adverse events which were commonly reported in studies were selected for analysis. In the above three outcome events, DOR performed well and SUCRA value ranked the third. It needs to be mentioned that local inconsistency was found in the analysis of adverse events (p < 0.05 for node splitting analysis between DTG + ABC+3TC and RAL + TDF+3TC/FTC), in other words, direct and indirect comparisons between DTG + ABC+3TC and RAL + TDF+3TC/FTC were inconsistent. We found that I2 was lower than 50% by anohe analysis, considering the heterogeneity between the data is acceptable, and the inconsistency may be due to some differences in the baseline characteristics of the studies included in this outcome event.
With the continuous development of ART, HIV infection has changed from a deadly disease to a chronic long-term disease. Although AIDS cannot be cured at present, ART can significantly reduce the viral loads, maintain patients’ immune function, and prolong life expectancy (Antiretroviral Therapy Cohort Collaboration, 2017; Wu, 2016; Marcus et al., 2000; GBD 2019 HIV Collaborators, 2021; Smiley et al., 2021). In recent years, the role of NNRTIs in ART has been weakened to some extent with the emergence of more and more tolerated antiretroviral drugs and regimens. DOR is a new NNRTI, which can be used as a single pill with other anti-retroviral drugs, or as a fixed-dose combination with 3TC and TDF. This study showed that DOR + TDF+3TC/FTC has good efficacy and safety at 48 weeks. Additionally, the marketing of DOR + TDF+3TC as a fixed-dose combination may improve patient compliance as fewer drugs need to be taken using this regimen (Godman et al., 2020). These findings support the use of DOR + TDF+3TC/FTC in treatment-naive HIV-positive adults.
This study has some limitations. Some of the regimens included in NMA to analyze certain outcome events lacked the data required for analysis and were not included in the study. Additionally, immune-reconstitution (recovery of CD4+ T-cells) is also an important indicator for evaluating the efficacy of treatment regimens in HIV-infected patients after initiating ART. However, due to inconsistent reported data units, the comprehensive analysis could not be conducted. Thus, we did not compare DOR + TDF+3TC/FTC with other ARTs in immune-reconstitution. Finally, CINeMA evidence grading uses R and the netmeta package, which adopts the frequency method for statistical analysis and may differ from the Bayesian approach. Despite its limitations, NMA provided valuable evidence to support the use of DOR + TDF+3TC/FTC in untreated HIV-1-infected adults.
Conclusion
HIV/AIDS, a human immune system disorder caused by HIV infection, is mainly treated with ART. DOR is a newly-approved antiretroviral drug belonging to the class of non-nucleoside reverse transcriptase inhibitors. Two phase 3 RCTs have proven that DOR has good efficacy and safety in HIV-1 patients. In this Bayesian network meta-analysis, we compared the efficacy and safety of DOR + TDF+3TC/FTC with traditional triple therapies in treatment-naïve HIV-1-positive patients and found that DOR + TDF+3TC/FTC has good efficacy and safety at 48 weeks.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
All authors could access all data and approved the final manuscript for submission. XH and GC contributed to study conception and design. KZ, YZ, JZ, LX, and CZ were involved in acquisition, statistical analysis, or interpretation of data. KZ drafted the manuscript. All authors read and edited the manuscript.
Funding
This work was supported by the Fund for Capital Health Development Research (2022-2-2185 to XH; 2022-1G-3011 to XH), the national science and technology major project of China during the 13th Five-year plan period (2017ZX10201101 to XH), the Beijing Excellent Talent Plan (2018000021223ZK04 to XH), the Beijing Talent Project in the New Millennium (2020A35 to XH), the National Natural Science Foundation of China (81901089 to YZ), the China Postdoctoral Science Foundation (2019M660718 to YZ), the Beijing Natural Science Foundation (7222091 to YZ), and the Beijing International Postdoctoral Exchange Fellowship Programme (2019PC-11 to YZ) and the Open Project funded by Beijing Key Laboratory of HIV/AIDS Research (BJYAHKF2019001 to YZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.676831/full#supplementary-material
References
Aberg, J. A., Tebas, P., Overton, E. T., Gupta, S. K., Sax, P. E., Landay, A., et al. (2012). Metabolic Effects of Darunavir/ritonavir versus Atazanavir/ritonavir in Treatment-Naive, HIV Type 1-infected Subjects over 48 Weeks. AIDS Res. Hum. Retroviruses 28 (10), 1184–1195. doi:10.1089/aid.2011.0327
AIDS and Hepatitis C Professional Group, Society of Infectious Diseases (2021). Chinese Medical Association;Chinese Center for Disease Control and Prevention. Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS (2021 Edition). Chin. J. Intern. Med. 14 (05), 321–343. doi:10.3760/cma.j.cn112138-20211006-00676
Antiretroviral Therapy Cohort Collaboration (2017). Survival of HIV-Positive Patients Starting Antiretroviral Therapy between 1996 and 2013: a Collaborative Analysis of Cohort Studies. Lancet HIV 4 (8), e349–e56. doi:10.1016/s2352-3018(17)30066-8
Clotet, B., Feinberg, J., van Lunzen, J., Khuong-Josses, M. A., Antinori, A., Dumitru, I., et al. (2014). Once-daily Dolutegravir versus Darunavir Plus Ritonavir in Antiretroviral-Naive Adults with HIV-1 Infection (FLAMINGO): 48 Week Results from the Randomised Open-Label Phase 3b Study. Lancet 383 (9936), 2222–2231. doi:10.1016/s0140-6736(14)60084-2
Cohen, C., Wohl, D., Arribas, J. R., Henry, K., Van Lunzen, J., Bloch, M., et al. (2014). Week 48 Results from a Randomized Clinical Trial of Rilpivirine/emtricitabine/tenofovir Disoproxil Fumarate vs. Efavirenz/emtricitabine/tenofovir Disoproxil Fumarate in Treatment-Naive HIV-1-Infected Adults. Aids 28 (7), 989–997. doi:10.1097/qad.0000000000000169
Cohen, C. J., Andrade-Villanueva, J., Clotet, B., Fourie, J., Johnson, M. A., Ruxrungtham, K., et al. (2011). Rilpivirine versus Efavirenz with Two Background Nucleoside or Nucleotide Reverse Transcriptase Inhibitors in Treatment-Naive Adults Infected with HIV-1 (THRIVE): a Phase 3, Randomised, Non-inferiority Trial. Lancet 378 (9787), 229–237. doi:10.1016/s0140-6736(11)60983-5
Cohen, C. J., Molina, J. M., Cahn, P., Clotet, B., Fourie, J., Grinsztejn, B., et al. (2012). Efficacy and Safety of Rilpivirine (TMC278) versus Efavirenz at 48 Weeks in Treatment-Naive HIV-1-Infected Patients: Pooled Results from the Phase 3 Double-Blind Randomized ECHO and THRIVE Trials. J. Acquir Immune Defic Syndr. 60 (1), 33–42. doi:10.1097/QAI.0b013e31824d006e
DeJesus, E., Herrera, G., Teofilo, E., Gerstoft, J., Buendia, C. B., Brand, J. D., et al. (2004). Abacavir versus Zidovudine Combined with Lamivudine and Efavirenz, for the Treatment of Antiretroviral-Naive HIV-Infected Adults. Clin. Infect. Dis. 39 (7), 1038–1046. doi:10.1086/424009
Dejesus, E., Mills, A., Bhatti, L., Conner, C., and Storfer, S. (2011). A Randomised Comparison of Safety and Efficacy of Nevirapine vs. Atazanavir/ritonavir Combined with Tenofovir/emtricitabine in Treatment-Naïve Patients. Int. J. Clin. Pract. 65 (12), 1240–1249. doi:10.1111/j.1742-1241.2011.02807.x
DeJesus, E., Rockstroh, J. K., Henry, K., Molina, J. M., Gathe, J., Ramanathan, S., et al. (2012). Co-formulated Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Disoproxil Fumarate versus Ritonavir-Boosted Atazanavir Plus Co-formulated Emtricitabine and Tenofovir Disoproxil Fumarate for Initial Treatment of HIV-1 Infection: a Randomised, Double-Blind, Phase 3, Non-inferiority Trial. Lancet 379 (9835), 2429–2438. doi:10.1016/s0140-6736(12)60918-0
Echeverría, P., Negredo, E., Carosi, G., Gálvez, J., Gómez, J. L., Ocampo, A., et al. (2010). Similar Antiviral Efficacy and Tolerability between Efavirenz and Lopinavir/ritonavir, Administered with Abacavir/lamivudine (Kivexa), in Antiretroviral-Naïve Patients: a 48-week, Multicentre, Randomized Study (Lake Study). Antivir. Res 85 (2), 403–408. doi:10.1016/j.antiviral.2009.11.008
ENCORE1 Study Group (2014). Efficacy of 400 Mg Efavirenz versus Standard 600 Mg Dose in HIV-Infected, Antiretroviral-Naive Adults (ENCORE1): a Randomised, Double-Blind, Placebo-Controlled, Non-inferiority Trial. Lancet 383 (9927), 1474–1482. doi:10.1016/S0140-6736(13)62187-X
Eron, J. J., Orkin, C., Gallant, J., Molina, J. M., Negredo, E., Antinori, A., et al. (2018). A Week-48 Randomized Phase-3 Trial of Darunavir/cobicistat/emtricitabine/tenofovir Alafenamide in Treatment-Naive HIV-1 Patients. Aids 32 (11), 1431–1442. doi:10.1097/qad.0000000000001817
European AIDS Clinical Society (2019). EACS Guidelines Version 10.0. Available at: https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf.
Gallant, J., Lazzarin, A., Mills, A., Orkin, C., Podzamczer, D., Tebas, P., et al. (2017). Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Dolutegravir, Abacavir, and Lamivudine for Initial Treatment of HIV-1 Infection (GS-US-380-1489): a Double-Blind, Multicentre, Phase 3, Randomised Controlled Non-inferiority Trial. Lancet 390 (10107), 2063–2072. doi:10.1016/s0140-6736(17)32299-7
Gallant, J. E., DeJesus, E., Arribas, J. R., Pozniak, A. L., Gazzard, B., Campo, R. E., et al. (2006). Tenofovir DF, Emtricitabine, and Efavirenz vs. Zidovudine, Lamivudine, and Efavirenz for HIV. N. Engl. J. Med. 354 (3), 251–260. doi:10.1056/NEJMoa051871
Gallien, S., Massetti, M., Flandre, P., Leleu, H., Descamps, D., and Lazaro, E. (2018). Comparison of 48-week Efficacies of Elvitegravir/cobicistat/emtricitabine/tenofovir Alafenamide and Nucleoside/nucleotide Reverse Transcriptase Inhibitor-Sparing Regimens: a Systematic Review and Network Meta-Analysis. HIV Med 19, 559–571. doi:10.1111/hiv.12643
GBD 2019 HIV Collaborators (2021). Global, Regional, and National Sex-specific burden and Control of the HIV Epidemic, 1990-2019, for 204 Countries and Territories: the Global Burden of Diseases Study 2019. Lancet HIV 8 (10), e633–51. doi:10.1016/S2352-3018(21)00152-1
Godman, B., McCabe, H., and D Leong, T. (2020). Fixed Dose Drug Combinations - Are They Pharmacoeconomically Sound? Findings and Implications Especially for Lower- and Middle-Income Countries. Expert Rev. Pharmacoecon Outcomes Res. 20 (1), 1–26. doi:10.1080/14737167.2020.1734456
Harris, M., Côté, H., Ochoa, C., Allavena, C., Negredo, E., Thorne, A., et al. (2009). A Randomized, Open-Label Study of a Nucleoside Analogue Reverse Transcriptase Inhibitor-Sparing Regimen in Antiretroviral-Naive HIV-Infected Patients. J. Acquir Immune Defic Syndr. 50 (3), 335–337. doi:10.1097/QAI.0b013e3181938fc9
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Honda, M., Ishisaka, M., Ishizuka, N., Kimura, S., and Oka, S. (2011). Open-label Randomized Multicenter Selection Study of once Daily Antiretroviral Treatment Regimen Comparing Ritonavir-Boosted Atazanavir to Efavirenz with Fixed-Dose Abacavir and Lamivudine. Intern. Med. 50 (7), 699–705. doi:10.2169/internalmedicine.50.4572
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Kanters, S., Vitoria, M., Doherty, M., Socias, M. E., Ford, N., Forrest, J. I., et al. (2016). Comparative Efficacy and Safety of First-Line Antiretroviral Therapy for the Treatment of HIV Infection: a Systematic Review and Network Meta-Analysis. Lancet HIV 3 (11), e510–e20. doi:10.1016/s2352-3018(16)30091-1
Kouanfack, C., Kouanfack, C., Mpoudi-Etame, M., Omgba Bassega, P., Eymard-Duvernay, S., Leroy, S., et al. (2019). Dolutegravir-Based or Low-Dose Efavirenz-Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 381 (9), 816–826. doi:10.1056/NEJMoa1904340
Landman, R., Koulla-Shiro, S., Sow, P. S., Ngolle, M., Diallo, M. B., Guèye, N. F., et al. (2014). Evaluation of Four Tenofovir-Containing Regimens as First-Line Treatments in Cameroon and Senegal: the ANRS 12115 DAYANA Trial. Antivir. Ther. 19 (1), 51–59. doi:10.3851/imp2675
Lennox, J. L., DeJesus, E., Lazzarin, A., Pollard, R. B., Madruga, J. V., Berger, D. S., et al. (2009). Safety and Efficacy of Raltegravir-Based versus Efavirenz-Based Combination Therapy in Treatment-Naive Patients with HIV-1 Infection: a Multicentre, Double-Blind Randomised Controlled Trial. Lancet 374 (9692), 796–806. doi:10.1016/s0140-6736(09)60918-1
Lennox, J. L., Landovitz, R. J., Ribaudo, H. J., Ofotokun, I., Na, L. H., Godfrey, C., et al. (2014). Efficacy and Tolerability of 3 Nonnucleoside Reverse Transcriptase Inhibitor-Sparing Antiretroviral Regimens for Treatment-Naive Volunteers Infected with HIV-1: a Randomized, Controlled Equivalence Trial. Ann. Intern. Med. 161 (7), 461–471. doi:10.7326/m14-1084
Marcus, J. L., Leyden, W. A., Alexeeff, S. E., Anderson, A. N., Hechter, R. C., Hu, H., et al. (2000). Comparison of Overall and Comorbidity-free Life Expectancy between Insured Adults with and without HIV Infection, 2000-2016. JAMA Netw. Open 3 (6), e207954. doi:10.1001/jamanetworkopen.2020.7954
Miro, J. M., Manzardo, C., Ferrer, E., Loncà, M., Guardo, A. C., Podzamczer, D., et al. (2015). Immune Reconstitution in Severely Immunosuppressed Antiretroviral-Naive HIV-1-Infected Patients Starting Efavirenz, Lopinavir-Ritonavir, or Atazanavir-Ritonavir Plus Tenofovir/Emtricitabine: Final 48-Week Results (The Advanz-3 Trial). J. Acquir Immune Defic Syndr. 69 (2), 206–215. doi:10.1097/qai.0000000000000567
Molina, J. M., Andrade-Villanueva, J., Echevarria, J., Chetchotisakd, P., Corral, J., David, N., et al. (2008). Once-daily Atazanavir/ritonavir versus Twice-Daily Lopinavir/ritonavir, Each in Combination with Tenofovir and Emtricitabine, for Management of Antiretroviral-Naive HIV-1-Infected Patients: 48 Week Efficacy and Safety Results of the CASTLE Study. Lancet 372 (9639), 646–655. doi:10.1016/S0140-6736(08)61081-8
Molina, J. M., Cahn, P., Grinsztejn, B., Lazzarin, A., Mills, A., Saag, M., et al. (2011). Rilpivirine versus Efavirenz with Tenofovir and Emtricitabine in Treatment-Naive Adults Infected with HIV-1 (ECHO): a Phase 3 Randomised Double-Blind Active-Controlled Trial. Lancet 378 (9787), 238–246. doi:10.1016/s0140-6736(11)60936-7
Molina, J. M., Squires, K., Sax, P. E., Cahn, P., Lombaard, J., DeJesus, E., et al. (2018). Doravirine versus Ritonavir-Boosted Darunavir in Antiretroviral-Naive Adults with HIV-1 (DRIVE-FORWARD): 48-week Results of a Randomised, Double-Blind, Phase 3, Non-inferiority Trial. Lancet HIV 5 (5), e211–e20. doi:10.1016/s2352-3018(18)30021-3
Mussini, C., Roncaglia, E., Borghi, V., Rusconi, S., Nozza, S., Cattelan, A. M., et al. (2019). A Prospective Randomized Trial on Abacavir/lamivudine Plus Darunavir/ritonavir or Raltegravir in HIV-Positive Drug-Naïve Patients with CD4. PLoS One 14 (9), e0222650. doi:10.1371/journal.pone.0222650
Nikolakopoulou, A., Higgins, J. P. T., Papakonstantinou, T., Chaimani, A., Del Giovane, C., Egger, M., et al. (2020). CINeMA: An Approach for Assessing Confidence in the Results of a Network Meta-Analysis. Plos Med. 17 (4), e1003082. doi:10.1371/journal.pmed.1003082
Nishijima, T., Takano, M., Ishisaka, M., Komatsu, H., Gatanaga, H., Kikuchi, Y., et al. (2013). Abacavir/lamivudine versus Tenofovir/emtricitabine with Atazanavir/ritonavir for Treatment-Naive Japanese Patients with HIV-1 Infection: a Randomized Multicenter Trial. Intern. Med. 52 (7), 735–744. doi:10.2169/internalmedicine.52.9155
Orkin, C., Squires, K. E., Molina, J. M., Sax, P. E., Wong, W. W., Sussmann, O., et al. (2019). Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate Is Non-inferior to Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate in Treatment-Naive Adults with Human Immunodeficiency Virus-1 Infection: Week 48 Results of the DRIVE-AHEAD Trial. Clin. Infect. Dis. 68 (4), 535–544. doi:10.1093/cid/ciy540
Orrell, C., Hagins, D. P., Belonosova, E., Porteiro, N., Walmsley, S., Falcó, V., et al. (2017). Fixed-dose Combination Dolutegravir, Abacavir, and Lamivudine versus Ritonavir-Boosted Atazanavir Plus Tenofovir Disoproxil Fumarate and Emtricitabine in Previously Untreated Women with HIV-1 Infection (ARIA): Week 48 Results from a Randomised, Open-Label, Non-inferiority, Phase 3b Study. Lancet HIV 4 (12), e536–e46. doi:10.1016/s2352-3018(17)30095-4
Ortiz, R., Dejesus, E., Khanlou, H., Voronin, E., van Lunzen, J., Andrade-Villanueva, J., et al. (2008). Efficacy and Safety of Once-Daily Darunavir/ritonavir versus Lopinavir/ritonavir in Treatment-Naive HIV-1-Infected Patients at Week 48. Aids 22 (12), 1389–1397. doi:10.1097/QAD.0b013e32830285fb
Panel on Antiretroviral Guidelines for Adults and Adolescents (2021). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Rockville: Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
Post, F. A., Moyle, G. J., Stellbrink, H. J., Domingo, P., Podzamczer, D., Fisher, M., et al. (2010). Randomized Comparison of Renal Effects, Efficacy, and Safety with Once-Daily Abacavir/lamivudine versus Tenofovir/emtricitabine, Administered with Efavirenz, in Antiretroviral-Naive, HIV-1-Infected Adults: 48-week Results from the ASSERT Study. J. Acquir Immune Defic Syndr. 55 (1), 49–57. doi:10.1097/QAI.0b013e3181dd911e
Puls, R. L., Srasuebkul, P., Petoumenos, K., Boesecke, C., Duncombe, C., Belloso, W. H., et al. (2010). Efavirenz versus Boosted Atazanavir or Zidovudine and Abacavir in Antiretroviral Treatment-Naive, HIV-Infected Subjects: Week 48 Data from the Altair Study. Clin. Infect. Dis. 51 (7), 855–864. doi:10.1086/656363
Raffi, F., Rachlis, A., Stellbrink, H. J., Hardy, W. D., Torti, C., Orkin, C., et al. (2013). Once-daily Dolutegravir versus Raltegravir in Antiretroviral-Naive Adults with HIV-1 Infection: 48 Week Results from the Randomised, Double-Blind, Non-inferiority SPRING-2 Study. Lancet 381 (9868), 735–743. doi:10.1016/s0140-6736(12)61853-4
Rock, A. E., Lerner, J., and Badowski, M. E. (2020). Doravirine and its Potential in the Treatment of HIV: An Evidence-Based Review of the Emerging Data. HIV AIDS (Auckl) 12, 201–210. doi:10.2147/hiv.S184018
Sax, P. E., DeJesus, E., Mills, A., Zolopa, A., Cohen, C., Wohl, D., et al. (2012). Co-formulated Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir versus Co-formulated Efavirenz, Emtricitabine, and Tenofovir for Initial Treatment of HIV-1 Infection: a Randomised, Double-Blind, Phase 3 Trial, Analysis of Results after 48 Weeks. Lancet 379 (9835), 2439–2448. doi:10.1016/s0140-6736(12)60917-9
Sax, P. E., Pozniak, A., Montes, M. L., Koenig, E., DeJesus, E., Stellbrink, H. J., et al. (2017). Coformulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide versus Dolutegravir with Emtricitabine and Tenofovir Alafenamide, for Initial Treatment of HIV-1 Infection (GS-US-380-1490): a Randomised, Double-Blind, Multicentre, Phase 3, Non-inferiority Trial. Lancet 390 (10107), 2073–2082. doi:10.1016/s0140-6736(17)32340-1
Sax, P. E., Wohl, D., Yin, M. T., Post, F., DeJesus, E., Saag, M., et al. (2015). Tenofovir Alafenamide versus Tenofovir Disoproxil Fumarate, Coformulated with Elvitegravir, Cobicistat, and Emtricitabine, for Initial Treatment of HIV-1 Infection: Two Randomised, Double-Blind, Phase 3, Non-inferiority Trials. Lancet 385 (9987), 2606–2615. doi:10.1016/s0140-6736(15)60616-x
Shim, S. R., Kim, S. J., Lee, J., and Rücker, G. (2019). Network Meta-Analysis: Application and Practice Using R Software. Epidemiol. Health 41, e2019013. doi:10.4178/epih.e2019013
Sierra-Madero, J., Villasis-Keever, A., Méndez, P., Mosqueda-Gómez, J. L., Torres-Escobar, I., Gutiérrez-Escolano, F., et al. (2010). Prospective, Randomized, Open Label Trial of Efavirenz vs Lopinavir/Ritonavir in HIV+ Treatment-Naive Subjects with CD4+. J. Acquir Immune Defic Syndr. 53 (5), 582–588. doi:10.1097/QAI.0b013e3181cae4a1
Smiley, C. L., Rebeiro, P. F., Cesar, C., Belaunzaran-Zamudio, P. F., Crabtree-Ramirez, B., Padgett, D., et al. (2021). Estimated Life Expectancy Gains with Antiretroviral Therapy Among Adults with HIV in Latin America and the Caribbean: a Multisite Retrospective Cohort Study. Lancet HIV 8 (5), e266–73. doi:10.1016/S2352-3018(20)30358-1
Smith, K. Y., Patel, P., Fine, D., Bellos, N., Sloan, L., Lackey, P., et al. (2009). Randomized, Double-Blind, Placebo-Matched, Multicenter Trial of Abacavir/lamivudine or Tenofovir/emtricitabine with Lopinavir/ritonavir for Initial HIV Treatment. AIDS 23 (12), 1547–1556. doi:10.1097/QAD.0b013e32832cbcc2
Soriano, V., Arastéh, K., Migrone, H., Lutz, T., Opravil, M., Andrade-Villanueva, J., et al. (2011). Nevirapine versus Atazanavir/ritonavir, Each Combined with Tenofovir Disoproxil Fumarate/emtricitabine, in Antiretroviral-Naive HIV-1 Patients: the ARTEN Trial. Antivir. Ther. 16 (3), 339–348. doi:10.3851/IMP1745
Squires, K., Kityo, C., Hodder, S., Johnson, M., Voronin, E., Hagins, D., et al. (2016). Integrase Inhibitor versus Protease Inhibitor Based Regimen for HIV-1 Infected Women (WAVES): a Randomised, Controlled, Double-Blind, Phase 3 Study. Lancet HIV 3 (9), e410–e20. doi:10.1016/s2352-3018(16)30016-9
Steegen, K., Moorhouse, M., Wensing, A. M., Venter, W. D., and Hans, L. (2021). Is There a Role for Doravirine in African HIV Treatment Programmes? A Large Observational Resistance Study in South Africa. J. Int. AIDS Soc. 24 (5), e25706. doi:10.1002/jia2.25706
UNAIDS (2020). Global HIV & AIDS Statistics — Fact Sheet. Available at: https://www.unaids.org/en/resources/fact-sheet.
Walmsley, S. L., Antela, A., Clumeck, N., Duiculescu, D., Eberhard, A., Gutiérrez, F., et al. (2013). Dolutegravir Plus Abacavir-Lamivudine for the Treatment of HIV-1 Infection. N. Engl. J. Med. 369 (19), 1807–1818. doi:10.1056/NEJMoa1215541
Keywords: HIV, antiretroviral therapy, randomized controlled trials, doravirine, network meta-analysis
Citation: Zhang K, Zhang Y, Zhou J, Xu L, Zhou C, Chen G and Huang X (2022) Comparison of the Efficacy and Safety of a Doravirine-Based, Three-Drug Regimen in Treatment-Naïve HIV-1 Positive Adults: A Bayesian Network Meta-Analysis. Front. Pharmacol. 13:676831. doi: 10.3389/fphar.2022.676831
Received: 09 March 2021; Accepted: 14 March 2022;
Published: 20 April 2022.
Edited by:
Vicente Estrada, San Carlos University Clinical Hospital, SpainReviewed by:
Brian Godman, University of Strathclyde, United KingdomKurt Neumann, Independent researcher, Kerékteleki, Hungary
Copyright © 2022 Zhang, Zhang, Zhou, Xu, Zhou, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanzhi Chen, Y2hlbmd1YW56aGlxZEBob3RtYWlsLmNvbQ==; Xiaojie Huang, aHVhbmd4aWFvamllNzhAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Ke Zhang
Ke Zhang Yang Zhang
Yang Zhang Jing Zhou4
Jing Zhou4 Lulu Xu
Lulu Xu Chi Zhou
Chi Zhou Guanzhi Chen
Guanzhi Chen Xiaojie Huang
Xiaojie Huang