- Department of Anesthesiology, Liuzhou Workers Hospital/The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, China
Background: Emergence agitation (EA) is a common problem often observed in children after sevoflurane anesthesia, which can be prevented by dexmedetomidine and alfentanil. This study aims to compare the effectiveness of dexmedetomidine alone and with different doses of alfentanil in preventing EA in children under sevoflurane anesthesia.
Materials and Methods: In a double-blind trial, 80 children (ASA I or II, 3–7 years old) undergoing tonsillectomy alone and adenotonsillectomy with sevoflurane anesthesia were randomly assigned into four groups: the control group, dexmedetomidine (DEX) group, dexmedetomidine plus 10 μg/kg alfentanil group (DEX + Alf1), and dexmedetomidine plus 20 μg/kg alfentanil group (DEX + ALf2). The incidence of EA was assessed with the Aono’s scale, and the severity of EA was evaluated with the Pediatric Anesthesia Emergence Delirium (PAED) scale. The time of tracheal extubation and time of wake were recorded. Postoperative pain and complications such as nausea and vomiting, cough, laryngospasm, and bradycardia were recorded.
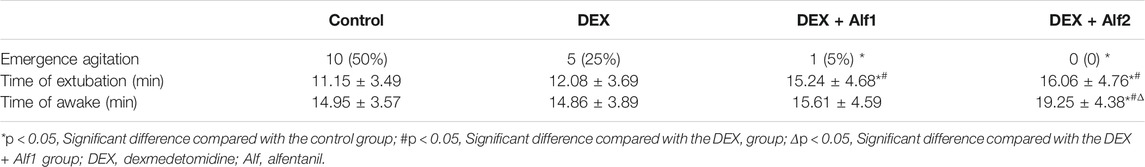
Results: The incidence of EA was 50% in the control group, 25% in the DEX group, and 5% in the DEX + Alf1 group, and it never happened in the DEX + Alf2 group. The Aono’s scale, the PAED scale, and the FLACC scale in the control group and the DEX group were significantly more than those in the DEX + Alf1 group and the DEX + Alf2 group after the tracheal extubation (p < 0.05). The time of tracheal extubation of the control group and the DEX group were significantly shorter than those in the DEX + Alf1 group and the DEX + Alf2 group (p < 0.05). The awakening time of the DEX + Alf2 group is significantly longer than those in other groups (p < 0.05). The case of postoperative nausea and vomiting in the DEX + Alf1 group was fewer than those in the other groups (p < 0.05). And, the cases of cough and laryngospasm and bronchospasm in the DEX + Alf1 group and the DEX + Alf2 group were significantly less than those in the control group and the DEX group after the tracheal extubation (p < 0.05).
Conclusion: The combined administration of alfentanil and dexmedetomidine can reduce EA in children undergoing tonsillectomy alone and adenotonsillectomy with sevoflurane anesthesia. Dexmedetomidine plus 10 μg/kg alfentanil seems to be more appropriate than other dose combinations as it reduced EA and postoperative nausea and vomiting but did not prolong the time to awake.
Introduction/Background
Sevoflurane is a popular anesthetic for children used worldwide because of its low pungency, rapid onset, and fast recovery properties. However, it is associated with a higher incidence of emergence agitation (EA) (as high as 80%) (Sato et al., 2010). EA in children is usually a short-lived phenomenon with no after-effect. And, it is potentially dangerous because it can make the children fall out of bed and remove the surgical dressings and intravenous catheters, which increased the extra medical cost (Dahmani et al., 2014; Driscoll et al., 2017; Choi et al., 2018; Abbas et al., 2019).
Pharmacological prophylactic interventions were a good method to prevent EA. Some research studies showed that propofol (Jalili et al., 2019), α2-agonist (Tsiotou et al., 2018; Tan et al., 2019), and µ-opioid agonists (Choi et al., 2011; Tan et al., 2016) were effective in preventing EA. Dexmedetomidine (DEX), a selective α2-adrenoceptor agonist, significantly reduces the incidence of EA in children after sevoflurane anesthesia (Bilgen et al., 2014; Yang et al., 2020). Sato M (Sato et al., 2010) found that intravenous 0.3 μg/kg DEX after induction of anesthesia reduced EA from 68 to 24% under sevoflurane anesthesia. Alfentanil, µ-opioid receptor agonists, can decrease the incidence of EA in children after sevoflurane anesthesia (Bilgen et al., 2014). Kim et al. (2009) showed that administration of alfentanil at the dose of 10 μg/kg and 20 μg/kg after induction of anesthesia reduced EA in children after sevoflurane anesthesia from 71 to 34%. Choi et al. (2016) revealed that 10 μg/kg alfentanil can reduce the EA from 64 to 32% in children receiving sevoflurane anesthesia. Even if both DEX and alfentanil were associated with reducing the incidence of the unsettling behavior after surgery, up to one in four children may present negative behaviors after the awakening of anesthesia.
The sedation caused by DEX with minimal respiratory depression made it a safer option when used in combination with opioids. We hypothesized that alfentanil and DEX have a synergistic effect, and the co-administration of DEX and alfentanil can decrease the EA to a satisfactory degree that is lower than using one drug alone in children after sevoflurane anesthesia.
In this study, we aimed to evaluate the effects of DEX alone and the co-administration of DEX and two different doses of alfentanil to prevent the unsettling behavior after sevoflurane anesthesia in children undergoing tonsillectomy and adenoidectomy.
Materials and Methods
The study was approved by the institutional ethics committee and the Chinese clinical trial registry with written informed consent (the ethics number: ChiCTR-2000040530). After signing the informed consent with the parents of all children, 89 children aged between 3 and 7 years old with an ASA physical status I or II, who were scheduled for either a tonsillectomy alone or both an adenoidectomy and tonsillectomy, were enrolled in this prospective, randomized, double-blind, controlled study. The inclusion criteria were the children with tonsils enlargement more than II degrees, repeated infection of tonsils, or snoring. The excluded criteria were the children with asthma, cardiac disease, abnormal upper airway, obstructive sleep apnea syndrome (OSAS), developmental delay, or a history of the upper respiratory tract infection in the preceding 4 weeks.
The children were randomly divided into four groups according to the random number table: The children in the control group received normal saline intravenously for 10 mins from the induction; the children in the DEX group were given intravenously 0.4 μg/kg dexmedetomidine for 10 mins from the induction; the children in the DEX + Alf1 group were administered intravenously with 0.4 μg/kg dexmedetomidine for 10 mins from the induction and alfentanil (10 μg/kg) at the induction of anesthesia; the children in the DEX + Alf2 group were administered intravenously with 0.4 μg/kg dexmedetomidine for 10 minutes from the induction and alfentanil (20 μg/kg) at the induction of anesthesia.
The primary outcome of our study is if DEX and DEX-added alfentanil can decrease the degree of EA. The second outcome of our study is which combination of drugs inhibits EA the best and has the least side effects.
In the operating room, we monitored the children with noninvasive blood pressure (NIBP), electrocardiography (ECG), pulse oximetry (SpO2), and the bispectral index (BIS). The children were inducted with 3% sevoflurane with 5 L/min oxygen, lidocaine 1 mg/kg, propofol 2–2.5 mg/kg, atracurium 0.3 mg/kg, and study drugs which were prepared with another anesthesiologist. The anesthesiologist responsible for anesthesia and observation did not know which drug it was. 3 min later, we performed the tracheal intubation. After the induction of anesthesia, we intravenously administrated 2 mg/kg tramadol and 0.1 mg/kg dexamethasone for postoperative analgesia and preventing nausea and vomiting after surgery. We maintained the anesthesia with 2–3% sevoflurane mixed in 2 L/min 50% oxygen. The concentration of sevoflurane was adjusted so that the BIS value is in the range of 40–60. During the surgery, we injected 3 ml 0.5% lidocaine and 1:200,000 epinephrine mixture into the mucosa surrounding each tonsillar fossa for local anesthesia and vasoconstriction. The mean blood pressure (MAP), heart rate (HR), and SpO2 were recorded on arrival in the operating room (baseline), intubation time (T0), and 1 min (T1), 5 min (T5), 10 min (T10), 20 min (T20), and 30 min (T30) after intubation and at tracheal extubation (E0), 5 min after tracheal extubation (E5) and 10 min after tracheal extubation (E10). Tracheal extubation was performed when the patients breathed spontaneously, moved, and coughed.
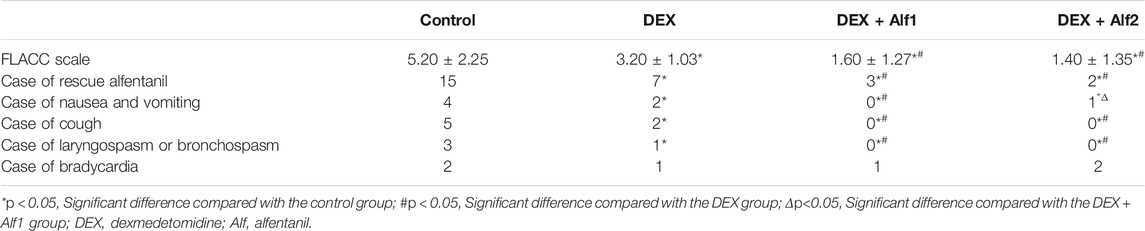
The time of anesthesia was defined from the administration of DEX or saline until the tracheal tube was extubated. The time of tracheal extubation was defined from the end of the surgery and the discontinuation of sevoflurane until the tracheal tube was extubated. The time of awakening was defined from the end of the surgery and discontinuation of sevoflurane until the children acted on command. The incidence of EA was rated on the following Aono’s (Aono et al., 1997) four-point scale: 1 = calm; 2 = not calm but could be easily consoled; 3 = moderately agitated or restless and not easily calmed; and 4 = combative, excited or disoriented, and thrashing around. Scores of one and two were considered nonproblematic behavior, and scores of three and four were considered EA. The severity of EA was evaluated with the Pediatric Anesthesia Emergence Delirium (PAED) scale (Sikich and Lerman, 2004), which consists of five items: 1) the child makes eye contact with the caregiver, 2) the child shows purposeful actions, 3) the child is aware of his or her surroundings, 4) the child is restless, and 5) the child is inconsolable. Items 1–3 are scored as follows: 4 = not at all, 3 = just a little, 2 = quite a bit, 1 = very much, and 0 = extremely. Items 4 and 5 are scored as follows: 0 = not at all, 1 = just a little, 2 = quite a bit, 3 = very much, and 4 = extremely. When the comprehensive score was greater than 15, we considered that severe agitation appeared. Postoperative pain was assessed with the Face, Legs, Activity, Cry, Consolability scale (FLACC) (Redmann et al., 2017) at 10-min intervals from admission in the PACU for 30 min. When the FLACC scale of children was more than 4, 5 μg/kg alfentanil was administered intravenously. The incidence and severity of EA and pain were measured at E0, E5, and E10. The adverse reactions after surgery such as nausea and vomiting, cough, laryngospasm and bronchospasm, and bradycardia were recorded at E0, E5, and E10.
Statistical analyses were performed with SPSS 23.0. Demographic data such as the age, gender, weight, type of surgery, and duration of anesthesia and surgery were compared with unpaired Student’s t-tests. Differences in the incidence of EA and severe EA among the groups were analyzed using a χ2 test with the Fisher’s exact test correction compared in the three timepoints. Intra and postoperative hemodynamic and respiratory variables in the same subjects were compared with the Bonferroni test after repeated measures of analysis of variance. p < 0.05 was considered to be statistically significant.
Results
A total of 89 children were enrolled in our study, and out of them, 9 children were excluded. Two children with asthma, 1 child with abnormal upper airway, two children with OSAS, and 4 children having a history of upper respiratory tract infection were excluded. In total, 80 children finished this study, with 20 children in each group. The demographic data such as the age, gender, surgery type, weight, duration of surgery, and anesthesia showed no significant differences among the four groups (p > 0.05) (Table 1).
DEX alone treatment reduced the occurrence rate of EA from 50% in the control group to 25% in the DEX group. Co-administered 10 μg/kg alfentanil further decreased the incidence to 5% in the DEX + Alf1 group, and co-administered 20 μg/kg alfentanil decreased the incidence to 0% in the DEX + Alf2 group, respectively (Table 2). There was no difference in the time of tracheal extubation between the DEX group and the control group (p > 0.05), while the time of tracheal extubation was prolonged by administered alfentanil from 12.08 ± 3.69 min in the DEX group to 15.24 ± 4.68 min in the DEX + Alf1 group and 16.06 ± 4.76 min in the DEX + Alf2 group (p < 0.05), respectively. There was no difference in the time of tracheal extubation between the DEX + Alf1 group and the DEX + Alf2 group (p > 0.05). The time of awakening in the DEX + Alf two group was significantly longer than those in the other groups (p < 0.05) (Table 2).
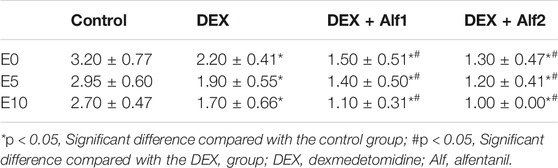
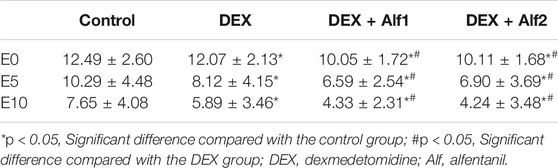
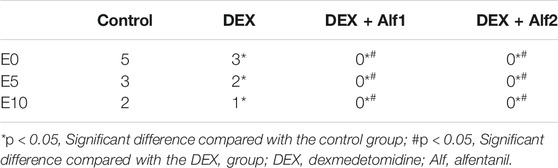
The Aono’s scores and the scale of PAED in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly less than those in the control group at E0, E5, and E10 (p < 0.05). The Aono’s scores and the scale of PAED in the DEX + Alf1 group and the DEX + Alf2 group were significantly less than those in the DEX group at E0, E5, and E10 (p < 0.05) (Table 3) (Table 4). There was no severe EA in the DEX + Alf1 group and the DEX + Alf2 group. The severe EA in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly lower than that in the control group at E0, E5, and E10 (p < 0.05). The severe EA in the DEX + Alf1 group and the DEX + Alf2 group were significantly lower than that in the DEX group at E0, E5, and E10 (p < 0.05) (Table 5).
The FLACC scale and the case of rescue alfentanil in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly less than those in the control group (p < 0.05). The FLACC scale and the case of rescue alfentanil in the DEX + Alf1 group and the DEX + Alf2 group were significantly less than those in the DEX group (p < 0.05). The cases of nausea and vomiting in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly lower than those in the control group (p < 0.05). And, the case of nausea and vomiting in the DEX + Alf1 group was significantly lower than those in the DEX group and the DEX + Alf2 group (p < 0.05) (Table 6). Furthermore, DEX plus 10 μg/kg alfentanil seems to be more appropriate than other dose combinations because it has a lower incidence of postoperative nausea and vomiting and shorter awakening time. Administration of alfentanil at the doses of 10 μg/kg and 20 μg/kg with DEX significantly reduced cases of cough and laryngospasm and bronchospasm (p < 0.05) (Table 6)
During the late stage of surgery and the period of extubation, the HR and the MAP increased gradually; DEX alone and a combination of alfentanil at the doses of 10 μg/kg and 20 μg/kg significantly inhibited the increase of the HR and the MAP (p < 0.05). The HR and the MAP in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly less than that in the control group at T10, T20, T30, E0, E5, and E10 (p < 0.05) (Figure 1 and Figure 2).
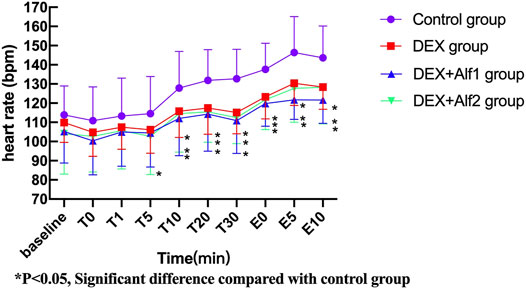
FIGURE 1. Heart rate of patients during anesthesia among groups. *p ˂ 0.05, Significant difference compared with the control group.
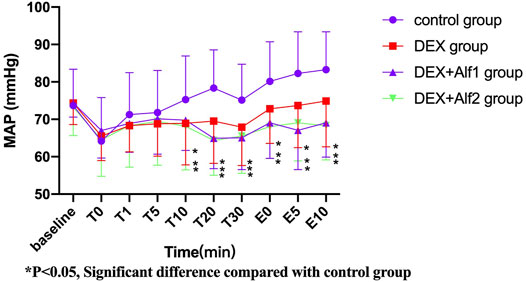
FIGURE 2. MAP of patients during anesthesia among groups. *p ˂ 0.05, Significant difference compared with the control group.
Discussion
This study demonstrated that intravenous administration of DEX 0.4 μg/kg alone or combined with intravenous different doses of alfentanil from induction of anesthesia could reduce EA in children with sevoflurane anesthesia undergoing adenotonsillectomy surgery. In our study, the Aono’s score and the PAED scale of the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly less than that of the control group. The Aono’s score and the PAED scale of the DEX + Alf1 group and the DEX + Alf2 group were significantly less than that of the DEX group; therefore, co-administration of DEX and alfentanil has a better effect to prevent EA than DEX alone. And, the time of awakening in the DEX + Alf2 group was longer than that in the DEX + Alf1 group, and the case of nausea and vomiting was more in the DEX + Alf2 group than that in the DEX + Alf1 group significantly. So intravenous administration of 0.4 μg/kg DEX with 10 μg/kg alfentanil was the appropriate dose because it can prevent EA after sevoflurane anesthesia, do not prolong the awakening time, and do not increase postoperative nausea and vomiting.
EA is a common phenomenon with children after sevoflurane anesthesia. A lot of risk factors may be considered during the development of EA, for example, pain, age, different types of surgery and inhaled anesthetics with fast emergence, and anesthetic techniques such as sevoflurane (Choi et al., 2019). In some research studies, the prophylactic use of analgesics successfully reduced EA after sevoflurane anesthesia, showed that pain may be one of the reasons for EA (Lynch et al., 1998; Kosar et al., 2014). On the other hand, post-anesthetic EA has been observed when the pain was effectively controlled (Weldon et al., 2004) or in the absence of pain (Cravero et al., 2000). In our study, The FLACC scale and the cases of rescue alfentanil in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group were significantly less than those in the control group. The differences of the FLACC scale and the Anno’s scale and the PAED scale between the groups were similar. Then, in our study, the result showed that pain is one of the major reasons for post-anesthetic EA. Children aged less than or equal to 7 years old (preschool children) are more likely to undergo EA (Przybylo et al., 2003). The patients have emergence delirium and not pain, maybe related to the inhaled anesthetics with fast emergence and anesthetic techniques as sevoflurane, the age, and the type of the surgery. In this study, we enrolled 3–7-year-old children who were more likely to suffer from EA, and in our study, the incidence of EA is 50% which is similar to the 44% in Przybylo’s research (Przybylo et al., 2003) at roughly the same age children. There is some relationship between EA and the type of surgery such as head and neck surgery, tonsils, thyroid, and middle ear surgery (Przybylo et al., 2003; Sato et al., 2010). In our study, the children we enrolled underwent tonsillectomy and adenoidectomy surgery. The age and the type of surgery subject the children at a high risk of EA.
The mechanism of DEX prevention of EA for children undergoing tonsillectomy and adenoidectomy may have sedative, analgesic, and anxiolytic properties (Olutoye et al., 2010; Zhang et al., 2019). Meanwhile, both a single dose administered and continuous intravenous infusion of DEX showed that can reduce EA after sevoflurane anesthesia in children (Yang et al., 2020). In our study, the continuous infusion of DEX for 10 minutes during induction decreased EA from 50 to 25% without prolonging awakening time. Kim found that the 50% effective dose and 95% effective dose of DEX to prevent EA in children undergoing tonsillectomy or adenoidectomy after desflurane anesthesia is 0.25 μg/kg or 0.38 μg/kg (Kim et al., 2015). And, the 0.3–0.5 μg/kg bolus dose of DEX has no hemodynamic effects in children (Guler et al., 2005; Ibacache et al., 2015). Although many research studies showed that DEX could be safely used in children, a recent study found that DEX caused some hemodynamic changes in children (Jooste et al., 2010). In our study, we choose a 0.4 μg/kg dose of DEX; it has a good effect of preventing EA and does not show any side effects.
Several research studies demonstrated that intravenous alfentanil 10 μg/kg and 20 μg/kg could decrease the incidence of EA (Aono et al., 1997; Kim et al., 2009; Choi et al., 2016). In our study, we selected these two doses combined with DEX. The mechanism of alfentanil to prevent EA after sevoflurane anesthesia may be related to its analgesic and slightly sedative effect (Choi et al., 2016). In our study, the difference of the FLACC scale and Anno’s scale and PAED scale between the groups was similar. This supported the analgesic effect of alfentanil to prevent EA. One of the side effects of opioids is postoperative nausea and vomiting. In our study, although we use the prophylactic dexamethasone, the case of nausea and vomiting in the DEX + Alf2 group was more than that in the DEX + Alf1 group. Dexamethasone can decrease postoperative edema and improve subsequent oral take after tonsillectomy because it has anti-inflammatory effects (Yiu et al., 2017).
Alfentanil can depress the respiratory depression; DEX also has minimal effect on depressing the respiratory depression. In our study, the children had not experienced respiratory depression during extubation and in the PACU. And, the children did not show hypoxemia when they came back to the ward. The MAP was stable during extubation and in the PACU in the DEX group, the DEX + Alf1 group, and the DEX + Alf2 group. The sedative effects of DEX and alfentanil make the patients’ heart rate to not increase very high. The combination of DEX and alfentanil reduced the case of nausea and vomiting, cough, laryngospasm, and bronchospasm and did not increase the case of bradycardia after surgery. Thus, the combination of DEX and alfentanil has the synergistic effect to prevent EA after sevoflurane anesthesia and has no severe side effects.
Conclusion
Co-administration of alfentanil and DEX can reduce EA in children after sevoflurane anesthesia. DEX plus 10 μg/kg alfentanil seems to be more appropriate than other dose combinations with 20 μg/kg alfentanil as it reduced EA and postoperative nausea and vomiting but did not prolong the time to awake.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Liuzhou workers hospital/Chinese clinical trial registry. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
Y-zZ, M-yY, and X-lW designed experiments and carried out the experiment; BT, Y-yQ, and MO collected the data; X-hJ and Y-fT analyzed experimental results; Y-zZ and M-yY wrote the manuscript.
Funding
This work was supported by the Health Commission of Guangxi Zhuang Autonomous Region Self-Funded Scientific Research Project (Grant No. Z20210009).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Qin Huan-hua and Rao Zhen-xing for their great support in tonsillectomy and adenoidectomy surgery.
References
Abbas, M. S., El-Hakeem, E. E. A., and Kamel, H. E. (2019). Three Minutes Propofol after Sevoflurane Anesthesia to Prevent Emergence Agitation Following Inguinal Hernia Repair in Children: a Randomized Controlled Trial. Korean J. Anesthesiol 72 (3), 253–259. doi:10.4097/kja.d.18.00345
Aono, J., Ueda, W., Mamiya, K., takimoto, e., and Manabe, M. (1997). Greater Incidence of Delirium during Recovery from Sevoflurane Anesthesia in Preschool Boys. Anesthesiology 87, 1298–1300. doi:10.1097/00000542-199712000-00006
Bilgen, S., Köner, Ö., Karacay, S., Sancar, N. K., Kaspar, E. C., and Sözübir, S. (2014). Effect of Ketamine versus Alfentanil Following Midazolam in Preventing Emergence Agitation in Children after Sevoflurane Anaesthesia: a Prospective Randomized Clinical Trial. J. Int. Med. Res. 42 (6), 1262–1271. doi:10.1177/0300060514543039
Choi, E. K., Lee, S., Kim, W. J., and Park, S. J. (2018). Effects of Remifentanil Maintenance during Recovery on Emergence Delirium in Children with Sevoflurane Anesthesia. Paediatr. Anaesth. 28 (8), 739–744. doi:10.1111/pan.13446
Choi, E. K., Park, S., Park, K. B., Kwak, K. H., and Park, S. (2019). Postoperative Emergence Agitation and Intraoperative Sevoflurane Sedation under Caudal Block in Children: a Randomized Comparison of Two Sevoflurane Doses. Anesth. Pain Med. (Seoul) 14 (4), 434–440. doi:10.17085/apm.2019.14.4.434
Choi, H. R., Cho, J. K., Lee, S., Yoo, B. H., Yon, J. H., and Kim, K. M. (2011). The Effect of Remifentanil versus N(2)O on Postoperative Pain and Emergence Agitation after Pediatric Tonsillectomy/adenoidectomy. Korean J. Anesthesiol 61 (2), 148–153. doi:10.4097/kjae.2011.61.2.148
Choi, Y. H., Kim, K. M., Lee, S. K., Kim, Y. S., Kim, S. J., Hwang, W. S., et al. (2016). Effects of Remifentanil and Remifentanil-Alfentanil Administration on Emergence Agitation after Brief Ophthalmic Surgery in Children. BMC Anesthesiol 16 (1), 50. doi:10.1186/s12871-016-0213-2
Cravero, J., Surgenor, S., and Whalen, K. (2000). Emergence Agitation in Paediatric Patients after Sevoflurane Anaesthesia and No Surgery: a Comparison with Halothane. Paediatr. Anaesth. 10, 419–424. doi:10.1046/j.1460-9592.2000.00560.x
Dahmani, S., Delivet, H., and Hilly, J. (2014). Emergence Delirium in Children: an Update. Curr. Opin. Anaesthesiol 27 (3), 309–315. doi:10.1097/ACO.0000000000000076
Driscoll, J. N., Bender, B. M., Archilla, C. A., Klim, C. M., Hossain, M. J., Mychaskiw, G., et al. (2017). Comparing Incidence of Emergence Delirium between Sevoflurane and Desflurane in Children Following Routine Otolaryngology Procedures. Minerva Anestesiol 83 (4), 383–391. doi:10.23736/S0375-9393.16.11362-8
Guler, G., Akin, A., Tosun, Z., Ors, S., Esmaoglu, A., and Boyaci, A. (2005). Single-dose Dexmedetomidine Reduces Agitation and Provides Smooth Extubation after Pediatric Adenotonsillectomy. Paediatr. Anaesth. 15 (9), 762–766. doi:10.1111/j.1460-9592.2004.01541.x
Ibacache, M. E., Muñoz, H. R., Fuentes, R., and Cortínez, L. I. (2015). Dexmedetomidine-ketamine Combination and Caudal Block for Superficial Lower Abdominal and Genital Surgery in Children. Paediatr. Anaesth. 25 (5), 499–505. doi:10.1111/pan.12642
Jalili, S., Esmaeeili, A., Kamali, K., and Rashtchi, V. (2019). Comparison of Effects of Propofol and Ketofol (Ketamine-Propofol Mixture) on Emergence Agitation in Children Undergoing Tonsillectomy. Afr. Health Sci. 19 (1), 1736–1744. doi:10.4314/ahs.v19i1.50
Jooste, E. H., Muhly, W. T., Ibinson, J. W., Suresh, T., Damian, D., Phadke, A., et al. (2010). Acute Hemodynamic Changes after Rapid Intravenous Bolus Dosing of Dexmedetomidine in Pediatric Heart Transplant Patients Undergoing Routine Cardiac Catheterization. Anesth. Analg 111, 1490–1496. doi:10.1213/ANE.0b013e3181f7e2ab
Kim, H. S., Byon, H. J., Kim, J. E., Park, Y. H., Lee, J. H., and Kim, J. T. (2015). Appropriate Dose of Dexmedetomidine for the Prevention of Emergence Agitation after Desflurane Anesthesia for Tonsillectomy or Adenoidectomy in Children: up and Down Sequential Allocation. BMC Anesthesiol 15 (1), 79. doi:10.1186/s12871-015-0059-z
Kim, J. Y., Chang, Y. J., Lee, J. Y., Park, H. Y., and Kwak, H. J. (2009). Post-induction Alfentanil Reduces Sevoflurane-Associated Emergence Agitation in Children Undergoing an Adenotonsillectomy. Acta Anaesthesiol Scand. 53 (5), 678–681. doi:10.1111/j.1399-6576.2009.01943.x
Kosar, C. M., Tabloski, P. A., Travison, T. G., Jones, R. N., Schmitt, E. M., Puelle, M. R., et al. (2014). Effect of Preoperative Pain and Depressive Symptoms on the Development of Postoperative Delirium. Lancet Psychiatry 1 (6), 431–436. doi:10.1016/S2215-0366(14)00006-6
Lynch, E. P., Lazor, M. A., Gellis, J. E., Orav, J., Goldman, L., and Marcantonio, E. R. (1998). The Impact of Postoperative Pain on the Development of Postoperative Delirium. Anesth. Analg 86, 781–785. doi:10.1097/00000539-199804000-00019
Olutoye, O. A., Glover, C. D., Diefenderfer, J. W., McGilberry, M., Wyatt, M. M., Larrier, D. R., et al. (2010). The Effect of Intraoperative Dexmedetomidine on Postoperative Analgesia and Sedation in Pediatric Patients Undergoing Tonsillectomy and Adenoidectomy. Anesth. Analg 111, 490–495. doi:10.1213/ANE.0b013e3181e33429
Przybylo, H. J., Martini, D. R., Mazurek, A. J., Bracey, E., Johnsen, L., and Coté, C. J. (2003). Assessing Behaviour in Children Emerging from Anaesthesia: Can We Apply Psychiatric Diagnostic Techniques? Paediatr. Anaesth. 13, 609–616. doi:10.1046/j.1460-9592.2003.01099.x
Redmann, A. J., Wang, Y., Furstein, J., Myer, C. M., and de Alarcón, A. (2017). The Use of the FLACC Pain Scale in Pediatric Patients Undergoing Adenotonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 92, 115–118. doi:10.1016/j.ijporl.2016.11.016
Sato, M., Shirakami, G., Tazuke-Nishimura, M., Matsuura, S., Tanimoto, K., and Fukuda, K. (2010). Effect of Single-Dose Dexmedetomidine on Emergence Agitation and Recovery Profiles after Sevoflurane Anesthesia in Pediatric Ambulatory Surgery. J. Anesth. 24 (5), 675–682. doi:10.1007/s00540-010-0976-4
Sikich, N., and Lerman, J. (2004). Development and Psychometric Evaluation of the Pediatric Anesthesia Emergence Delirium Scale. Anesthesiology 100, 1138–1145. doi:10.1097/00000542-200405000-00015
Tan, D., Xia, H., Sun, S., and Wang, F. (2019). Effect of Ancillary Drugs on Sevoflurane Related Emergence Agitation in Children Undergoing Ophthalmic Surgery: a Bayesian Network Meta-Analysis. BMC Anesthesiol 19 (1), 138. doi:10.1186/s12871-019-0810-y
Tan, Y., Shi, Y., Ding, H., Kong, X., Zhou, H., and Tian, J. (2016). μ-Opioid Agonists for Preventing Emergence Agitation under Sevoflurane Anesthesia in Children: a Meta-Analysis of Randomized Controlled Trials. Paediatr. Anaesth. 26 (2), 139–150. doi:10.1111/pan.12815
Tsiotou, A. G., Malisiova, A., Kouptsova, E., Mavri, M., Anagnostopoulou, M., and Kalliardou, E. (2018). Dexmedetomidine for the Reduction of Emergence Delirium in Children Undergoing Tonsillectomy with Propofol Anesthesia: A Double-Blind, Randomized Study. Paediatr. Anaesth. 28 (7), 632–638. doi:10.1111/pan.13397
Weldon, B. C., Bell, M., and Craddock, T. (2004). The Effect of Caudal Analgesia on Emergence Agitation in Children after Sevoflurane versus Halothane Anesthesia. Anesth. Analg 98, 321–326. doi:10.1213/01.ane.0000096004.96603.08
Yang, X., Hu, Z., Peng, F., Chen, G., Zhou, Y., Yang, Q., et al. (2020). Effects of Dexmedetomidine on Emergence Agitation and Recovery Quality Among Children Undergoing Surgery under General Anesthesia: A Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 8, 580226. doi:10.3389/fped.2020.580226
Yiu, Y., Mahida, J. B., Cooper, J. N., Elsey, N. M., Deans, K. J., Minneci, P. C., et al. (2017). The Effect of Perioperative Dexamethasone Dosing on post-tonsillectomy Hemorrhage Risk. Int. J. Pediatr. Otorhinolaryngol. 98, 19–24. doi:10.1016/j.ijporl.2017.04.033
Keywords: emergence agitation, tonsillectomy, adenoidectomy, alfentanil, dexmedetomidine
Citation: Zhang Y-, Wei X-, Tang B, Qin Y-, Ou M, Jiang X-, Tan Y- and Ye M- (2022) The Effects of Different Doses of Alfentanil and Dexmedetomidine on Prevention of Emergence Agitation in Pediatric Tonsillectomy and Adenoidectomy Surgery. Front. Pharmacol. 13:648802. doi: 10.3389/fphar.2022.648802
Received: 02 January 2021; Accepted: 10 January 2022;
Published: 02 February 2022.
Edited by:
Judith Ann Smith, University of Texas Health Science Center at Houston, United StatesReviewed by:
Kaining Zhi, University of Tennessee Health Science Center (UTHSC), United StatesAshwin Karanam, Pfizer, United States
Copyright © 2022 Zhang, Wei, Tang, Qin, Ou, Jiang, Tan and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mao-ying Ye, eWVtYW95aW5nNjI1QDE2My5jb20=
 Yan-zhuo Zhang
Yan-zhuo Zhang Xiong-li Wei
Xiong-li Wei Mao-ying Ye
Mao-ying Ye