
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Pharmacol. , 01 April 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.636180
This article is part of the Research Topic Reducing the Harm of Medication - Recent Trends in Pharmacovigilance View all 17 articles
Background: Treatment of respiratory tract diseases with inhaled glucocorticoids is a form of therapy that has been used for many years. It shows lower potency of side effects; nevertheless, microbiome change, sinopulmonary dysbiosis, secondary immunodeficiency, and immunomodulatory effects are underestimated. The latest guideline recommendations introduce the use of empirical antibiotic and/or multiplying inhaled glucocorticoids in therapeutic intervention of asthma and chronic pulmonary obstructive disease.
Aims and objectives: The aim of the study was to describe a simple, universal, and cost-effective method of microbiome analysis for clinical trials. Such a general method for monitoring pharmacovigilance should be widely available and reliable.
Methods: The study material included two kinds of swabs, taken from the same mouth ulcerations of patients with asthma treated with a temporary quadruple dose of fluticasone. The microbiological investigation was performed, and identification of the isolates was carried out using the matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF-MS) Biotyper.
Results: The analysis of dry swab demonstrated the presence of typical oral bacteria (Neisseria spp. and Streptococcus spp.), alongside with the potentially pathogenic Actinomyces spp. and three different Rothia species, identified simultaneously: R. aeria, R. dentocariosa, and R. mucilaginosa. Although quadrupled dose of corticoids was discontinued and ulcer healing was observed, the patients required topical therapy for maintained xerostomia. Progressive systemic autoimmunity (seronegative Sjögren’s syndrome with major organ involvement) was observed later.
Conclusion: Topical steroids (especially in quadruple dose) require attention to safety, immunomodulation, and microbiological outcome. They showed systemic side effects: microbiome alteration, humoral (IgG) immunodeficiency, and systemic autoimmunity. Isolation of three species of Rothia from a patient with mouth ulcers after steroid therapy suggests their participation in infectious and inflammatory processes. The proposed a methodology using MALDI-TOF-MS may be a prototype approach for microbial diagnostics in clinical trials of immunomodulatory drugs.
Asthma and chronic obstructive pulmonary disease (COPD) have been treated with steroids for many years. Inhaled glucocorticoids (ICS) were introduced later, initially as a topical application of hydrocortisone (London and Alexander, 1951), with higher efficacy than antihistamines or allergen-immunotherapy (Bagratuni, 1960). ICS have become the first-line treatment for asthma and sometimes COPD, because of beneficial effects in many inflammatory respiratory system diseases. On the other hand, bacterial infection of the lower respiratory tract contributes to approximately 50% of COPD exacerbations. Lung microbiome may reflect micro-aspiration of oral microbiota, but the strict role of the lung microbiome remains unidentified (Pragman et al., 2012; Zdziarski, 2020). For example, bacteria-associated exacerbation was defined as colony-forming units greater than 107/ml sputum or a positive culture result (Ghebre et al., 2018), but collected microorganisms in the respiratory tract may be micro-aspiration-derived or through carryover (e.g., bronchoscopic) (Charlson et al., 2011). Although recently in pediatric (Cazeiro et al., 2017) and adult practice (Chen et al., 2020) there are ample data and meta-analyses showing the increased incidence of ICS-induced infections, they raise serious doubts. Many studies of ICS safety had reporting bias: infectious and inflammatory complications are insufficiently described with non-adequate terminology such as pneumonia, upper respiratory tract infection, etc. (Zdziarski et al., 2017a). Confirmation of infectious process with laboratory and microbiological testing was not carried out. Furthermore, in a meta-analysis, cases of serious pneumonia were defined as for hospitalization or as death from pneumonia (Yang et al., 2017). In clinical trials, drug-induced dysbiosis, secondary immunodeficiency, and opportunistic infection profile are not reported. Although the changes in the microbiome in asthmatic and COPD patients are well described, the effects of ICS have not been evaluated (Charlson et al., 2011; Erb-Downward et al., 2011; Ghebre et al., 2018). Only one study shows data with increasing caries and dental plaque in asthmatic adolescents using ICS but without strict microbiological analysis (Santos et al., 2012). Sample collection and microbiological analysis are crucial for further interpretation and conclusions. Only one recent study revealed an increased risk of oropharyngeal colonization by Streptococcus pneumoniae (Zhang et al., 2013), but it is not known whether such colonization should be considered as a preclinical phase of the disease or a change in the natural microbiome. However, one meta-analysis revealed a protective effect of ICS against pneumonia in patients with asthma (Bansal et al., 2015). The anti-proliferative and immunosuppressive effects of ICS, the direct effect on the respiratory epithelium, and the disruption of lung microbiome are most likely to be implicated.
The aim of the study was to describe a simple, universal, and cost-effective method of microbiome analysis for clinical trials. Such a general method for monitoring pharmacovigilance should be widely available and reliable. We were looking for the optimal method of sampling, culturing, and microbiological analysis. By trying the method in one of the patients treated with high doses of ICS, we made an unexpected finding: severe dysbiosis during induction phase of autoimmune lymphoproliferative disease, i.e., Sjögren’s Syndrome (SS).
A 32-year-old, non-smoking male patient was admitted to the Department of Clinical Immunology of Lower Silesian Oncology Center in Wroclaw, Poland, with suspicion of Sjögren’s syndrome (difficulty in swallowing dry food, xerostomia, and ocular discomfort). Previously, he has been treated with a low dose of ICS (i.e., fluticasone propionate 150–250 µg/day) and long-acting beta2 agonists (LABA, i.e., formoterol) due to atopic asthma (Figure 1). On exertion, he was treated with high doses, up to 1,000 mcg, of fluticasone daily (quadrupled dose) and was asked to rinse his mouth with water after using the inhaler, but without the use of a spacer to reduce side effects in the mouth and throat. The patient was neither on an extreme diet, disease-modifying antirheumatic drugs, retinoids, antibiotics nor had mucositis/gastroenteritis or dental intervention for at least 2 months prior to sampling.
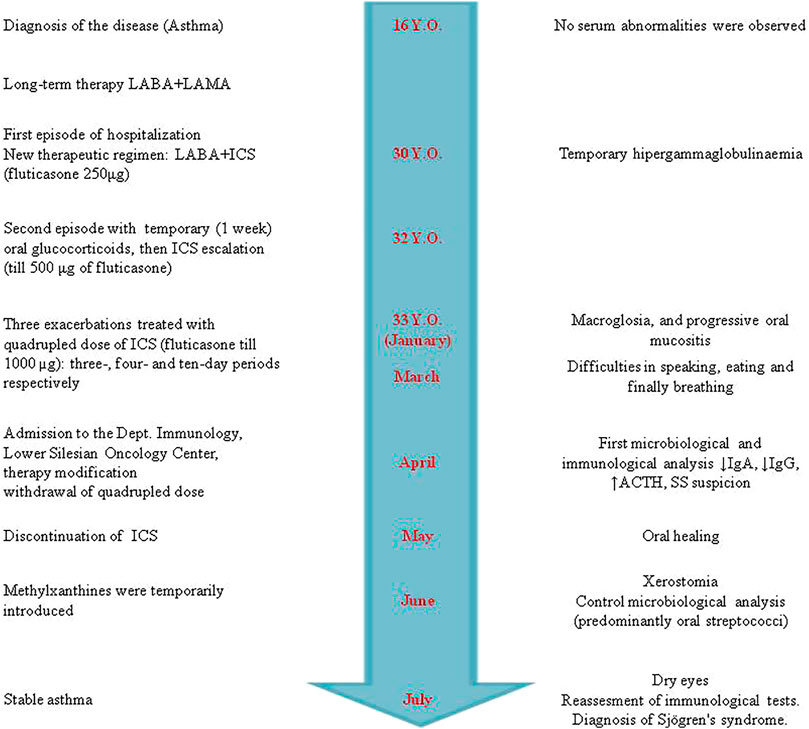
FIGURE 1. Timeline of clinical presentation, therapeutic regimen of asthma, and Sjögren’s syndrome development. The course and therapeutic interventions of asthma are presented on the left. The right side shows the sequence of symptoms and immune parameters during the development of Sjögren’s syndrome. In contrast to the similar adverse drug reactions (ADRs), i.e., drug-induced lupus (DIL), disappearance of clinical manifestation was not observed. The patient has Sjögren’s syndrome (SS) symptoms even now [more than a year and a half after the inhaled corticoid (ICS) discontinuation].
The clinic-based World Health Organization–Uppsala Monitoring Center system (WHO–UMC) or Naranjo’s algorithm was used for causality assessment in type B (unpredictable) and type A (predictable) adverse drug reactions (ADRs) (Pande, 2018). The WHO–UMC scale was used, intended mainly as a convenient tool for assessing individual case. In Naranjo’s algorithm, ADR was categorized into the following four categories: ≥9 = definite ADR, 5–8 = probable ADR, 1–4 = possible ADR, and 0 = doubtful ADR.
Oral dry swab was taken with simple Viscose Swabs Applicator (Equimed, ELATALA®, DELTALAB S.L. SP) or with transport Amies medium without charcoal (Equimed®, DB S.L. SP) in the laboratory, seeded within 20 min and 6 h, respectively. In this way, it was compared whether the transport and the use of the transport medium can positively or negatively affect the microbiological result. This pre-analytical element influences the results and reporting of clinical trials, usually with the submission of materials to a central laboratory. Both swabs were cultured on solid media: blood agar, nutrient agar, tryptic soy-thioglycolate agar (Paściak et al., 2003), and brain–heart infusion (BHI) agar in duplicate to investigate different growth conditions, i.e., aerobic and anaerobic. Aerobic culture takes from 24 to 48 h at 37°C. Anaerobic conditions were obtained with the use of GasPak EZ Anaerobe Pouch System® (Pouchoxygen Becton, Dickinson and Company) and 5–7 days of incubation at 37°C. All different colonies were selected by two experienced microbiologists and subjected to matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF-MS) analysis.
After excising the separate colony, the standard ethanol-formic acid protein extraction method was used according to the procedure recommended by the spectrometer manufacturer (Pasciak et al., 2015). Alpha-cyano-4-hydroxycinnamic acid was used as a matrix, and MALDI-TOF-MS analysis was performed on the Ultraflex mass spectrometer (Bruker Daltonics, Germany). Spectra were recorded in the positive linear mode for a mass range of 2,000–20,000 Da and were obtained by at least 2,800 laser shots acquired from four spot positions under control of Flex Control software 3.1 (Bruker Daltonics). The spectra were externally calibrated using the E. coli DH5-alpha standard (Bruker Daltonics) consisting of six ribosomal E. coli proteins, RNase A, and myoglobin. The Biotyper 3.1 software (Bruker Daltonics) with a database containing 4,613 entries was used for strain identification. Criteria used in identification, according to the manufacturer, were as follows: a score value below 1.699 meant that the identification was unreliable, 1.7–1.999 probable genus identification, 2.0–2.299 reliable genus identification, and 2.3–3.0 highly probable species identification.
The clinical material was taken by the application of dry swabs with simple Viscose Swabs Applicator and transport Amies medium without charcoal. Identification of the isolates after cultivation in appropriate conditions was carried out using the MALDI-TOF Biotyper (Table 1). Comparing the aerobic and anaerobic cultures, only two species taken from dry swab were repeated (i.e., Staphylococcus epidermidis and Streptococcus salivarius), but none from the transport Amies medium. Surprisingly, we found three different species of Rothia genus in one patient: R. aeria, R. dentocariosa, and Rothia mucilaginosa in the same niche, characterized by different MALDI-TOF mass spectra. Collection of microbiota with transport swab gave a significantly narrower microbiome repertoire because strains of S. epidermidis, Neisseria macacae, N. perflava, as well as R. mucilaginosa were not detected in these conditions. The dry swab collection method and anaerobic growth conditions allowed us to detect a significantly higher abundance of oral microbiota, but without Rothia spp. (Table 1).
Because of mucositis, dysbiosis state, and clinical manifestation of adverse ICS reaction (Figure 2), the immunoparameter analysis was performed (Table 2). Although a quadrupled dose was not continued over the 10-day period (Figure 1), insignificant adrenocortical suppression was observed (i.e., with a mild increase of ACTH) and a slight decrease of serum IgG and IgA. Iron deficiency was not observed (Table 2). Further immunological analysis excluded primary immunodeficiency, especially deficiency of IgA, which was at a normal level in blood, but a quantitative deficit of saliva was later observed. Physical examination showed no significant eye dryness (i.e., Schirmer’s test 10 mm in 5 min) nor any further abnormalities, but submandibular lymph glands were slightly swollen and oral mucosa showed WHO stage 3 of oral mucositis and 3–8 mm erosion/ulcers and a bitter taste on the back of the tongue (Figure 2). Hypertrophy of the tongue (macroglossia) with consequences and malfunction of the upper respiratory tract caused breathing and speech problems. Tongue base-induced obstructive sleep apnea was observed. After the withdrawal of ICS in quadrupled dose, the oral ulcer disappeared, but prolonged WHO stage 2/3 of oral mucositis was observed. It required topical therapy with mucoprotectants, drugs usually used for chemotherapy-induced oral mucositis. The duration of quadrupled treatment was as short as possible and was not continued after the symptoms disappeared (Figure 1). Although higher doses were not continued over a 10-day period, insignificant adrenocortical suppression was observed (i.e., with mild increase of ACTH and sodium retention) as well as secondary immunodeficiency (i.e., weak decrease of serum IgG level and destruction of mucosal barrier with epithelitis) (Table 2). After discontinuation of ICS, the oral mucositis prolonged as mild signs of seronegative primary Sjögren’s syndrome with major organ involvement (Tezcan et al., 2017). Three months later, the patient showed no serum abnormality, but prolonged lymphadenopathy, splenomegaly, saliva deficiency, and positive autoantibody such as rheumatoid factor, SS-A, were observed (Figure 1; Table 2). On the basis of the clinical presentation and formal criteria, which require the presence of immunologic abnormalities (i.e., SS-A antibody or lymphocytic infiltration in labial salivary gland), the SS diagnosis was made (Sandhya et al., 2017). After ICS discontinuation, the patient did not develop asthma exacerbation within 6 months (Figure 1). The Rothia spp. diversity was not observed later—streptococcal growth predominated (Figure 1). Contrary to the first microbial analysis (presented in Table 1), the second one revealed S. salivarius, Streptococcus oralis, S. pneumoniae, Neisseria spp., as well as Candida albicans. However, this could be due to the inflammatory response, changes in immunity, and the microbiome seen in Sjögren’s syndrome as described previously (Almståhl et al., 2003).
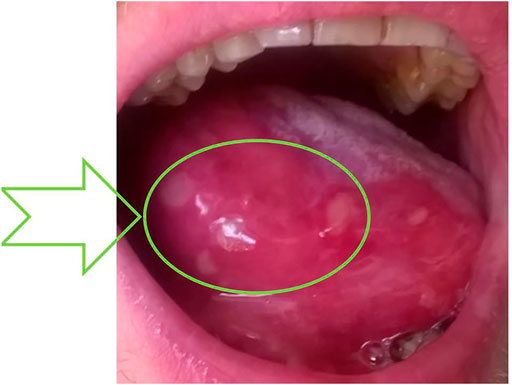
FIGURE 2. WHO stage 3 of oral mucositis caused by dysbiosis after quadrupling fluticasone therapy. Macroglossia—enlargement of the tongue with acute epithelial disruption with fibrin, called epithelitis—and many ulcers were observed as a sign of lymphocytic infiltration of the epithelia. Formation of a fibrin coating—the place of subsequent sampling (dysbiosis state) —is indicated by an arrow. The probable ADRs are reported as L453/2020 in Polish Pharmacovigilance Service, collectively with data presented in the upper part of Table 2.
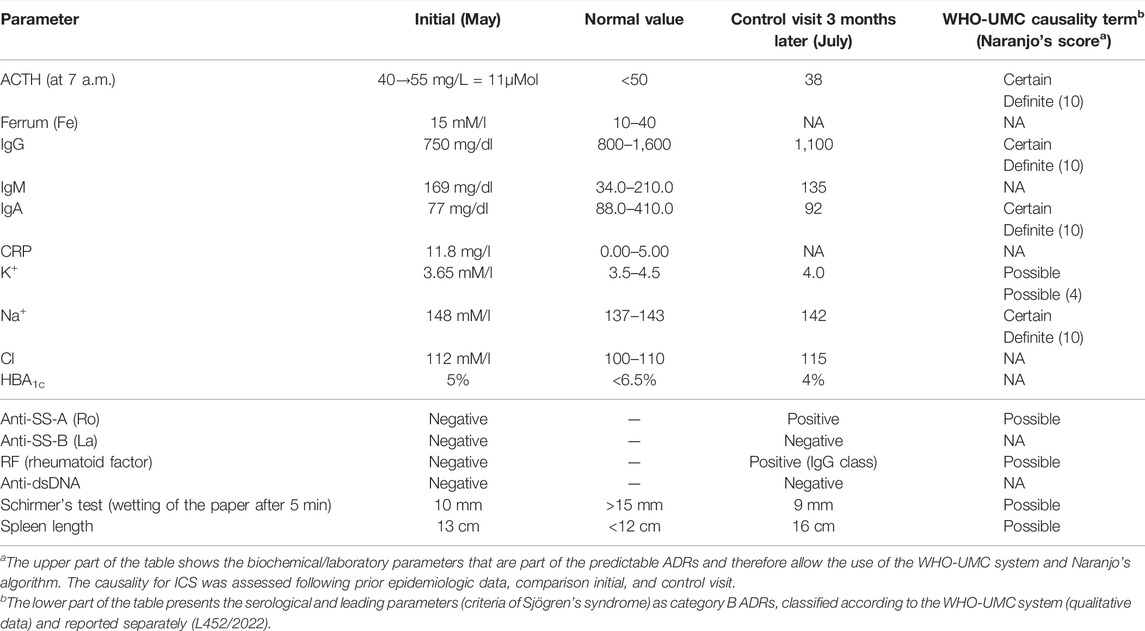
TABLE 2. Laboratory data of patients with quadrupling inhaled glucocorticoid (i.e., fluticasone 1,000 µg/day) and patient’s characteristics.
The clinical course to a limited extent allowed for unambiguous answers in individual parts of the questionnaires. Some symptoms can be attributed to the increased dose (quadrupled) and some to steroids. The change in ACTH (and other biochemical/laboratory parameters presented in Table 2) is an indirect indicator of ICS toxic concentrations. Basing on prior epidemiologic studies (prior knowledge) and information obtained from a given case (especially dechallenge), these ADRs were classified as predictable and certain according to the WHO-UMC system. Although rechallenge was difficult to perform ethically (especially in quadrupled dose of ICS), discontinuation of ICS (dechallenge) corresponded with oral reconstitution (Figure 1) without Rothia overgrowth. Furthermore, dysbiosis and macroglossia were in strict time relationship with quadrupled dose (onset in January), and the response to withdrawal was observed (May) (Figure 1), and the ADRs was classified as probable. The answer to the survey question in Naranjo’s algorithm (Was the mucositis more severe when the dose was increased?) is positive in the first period (i.e., when the patient escalated their ICS doses). However, the follow-up question (Was the mucositis less severe when the dose was decreased?) is not so clear after ICS withdrawal (SS prolonged). Therefore, oral mucositis may be classified as probable or possible according to the WHO-UMC system. Formoterol (LABA) and temporary short-acting beta-agonists (e.g., salbutamol) were still used; therefore, the ADRs were unlikely the cause of biochemical parameters Table 2.
The studies of the human microbiome have revealed that healthy individuals differ remarkably in the oral cavity microbiota composition. Based on the analysis of 2,589 16S rRNA clones, the bacterial diversity of the microflora from nine different sites of five clinically healthy subjects revealed the genus Rothia among many others (Aas et al., 2005). In further study of 10 healthy human individuals, Bik et al. (2010) found that the genus Rothia was abundant in the oral cavity and was present in all 10 individuals. However, Rothia spp. are not described as a typical commensal in “Structure, function and diversity of the healthy human microbiome” (Human Microbiome Project Consortium, 2012). Microbiome encompasses the microbiota and its host environment, but the latter is rarely included in the analysis (Zdziarski, 2020; Raffatellu and Bäumler, 2021; Zdziarski et al., 2017b). In other words, there is no “healthy” microbiome (Yong, 2014), and an integrated approach is crucial (Brinker et al., 2019). Interestingly, higher abundance of R. mucilaginosa was observed previously in periodontal patients (Camelo-Castillo et al., 2015), but another publication shows the three most active microbial players, e.g., Porphyromonas gingivalis, Treponema denticola, and Fusobacterium nucleatum (Deng et al., 2018). Noteworthy, in several case reports (the description of four Rothia species is the last decade’s finding), the isolation of only one species of Rothia predominates (Falcone et al., 2012; Zhou and Li, 2015). Our observation of three different Rothia species in the same sample from the patient is the first in the literature, regarding the described tissue and disease conditions. Appropriate specimen collection and storage before arrival at the molecular diagnostic laboratory are crucial (Huse et al., 2014). Furthermore, DNA/RNA false-negative results are minimized by avoiding the use of swabs with wooden shafts or cotton tips (the swab that has been validated for the amplification assay must be used). It prompts the use of a dry swab (without transport medium) or brush sampling method rather than lavage or biopsy in microbiome sampling as described elsewhere for the assessment of gut microbiota (Huse et al., 2014). The concerns raised above indicate that the pre-analytical stage of sample management is crucial to get credible results. However, the first and second elements of the diagnostic chain seem to be crucial for the final result (Table 1), which is partly the answer why the composition of the oral microbiome in various publications is so diverse (e.g., Human Microbiome Project Consortium, 2012; Zhou and Li, 2015). An identification method using MALDI-TOF-MS turned out to be very efficient in identification down to the species level. This is crucial because identification at the genus level is insufficient for proper classification (Nouioui et al., 2007). Therefore, without strict uniform diagnostic chain, it is impossible to define a healthy microbiome by itemizing microbial species or cataloguing their genes (Zdziarski 2020; Raffatellu and Bäumler, 2021).
Based on our observations, we propose a widely available and reliable diagnostic chain for monitoring pharmacovigilance that should consist of the following:
• Sample collection (near laboratory) with dry swab (transport medium is not useful—Table 1)
• Aerobic as well as anaerobic (Gas-Pack) culture of microorganisms (various media)
• Identification with MALDI-TOF-MS
• ADR reporting and terminology (dysbiosis, macroglossia, immunodeficiency, and secondary inflammatory disease)
• Modification of therapeutic regimen (e.g., microbiota transplantation, pre–pro-biotics, and ICS withdrawal), nomenclature, and guidelines, e.g., the Global Initiative for Asthma (2019).
Our data may be important for patients with long-term oxygen therapy as well as for storing and transporting of clinical material to the laboratory (i.e., with access to oxygen). In hypoxic patients, shifts and changes in the microbiome and alpha diversity may occur when oxygen therapy (or the use of oxygen for ICS nebulization) is administered, similar as presented in Table 1. Charlson et al. (2011) suggest that the lung microbiome reflects micro-aspiration of the oral flora. The risk of microbial transition is much higher after aerosol delivery, especially ICS (O’Malley, 2015). Risk factors for R. mucilaginosa bacteremia include prolonged and profound neutropenia, malignancy, and an indwelling vascular foreign body. Unfortunately, most of the literature indicates the risk factor, but the retrospective study identified no qualitative or statistically significant differences between the two groups for any of the variables collected, including recent corticosteroid use (6% versus 11%, p = 1.0) and the presence of neutropenia (88% versus 89%, p= 1.0) (Ramanan et al., 2014).
There are major challenges in specifying relevant outcomes and study designs for evaluating adverse drug reactions (Zdziarski et al., 2017a). High diversity in reporting, as well as variation in their definition, methods of ascertainment, and grading, is an important problem in clinical studies (Peryer et al., 2020). One of the crucial limiting factors in clinic-based causality assessment in clinical immunology practice is the long latency of many immunomediated ADRs (Zdziarski, 2019) as presented previously for anaphylaxis: IgE-mediated (type 1—“immediate”) allergic reactions were observed 5 or 14 days after drug administration (Zdziarski et al., 2017a). Naranjo’s algorithm was not useful (Pande, 2018). In our observation, it is difficult to unambiguously associate biochemical changes with the chronic use of ICS or a quadrupled dose, and there may have been an accumulation of them (Figure 1; Table 2). On the other hand, macroglossia and mucositis are symptoms directly and continuously observed by the patient, and the time relationship is clearer (Figure 1). Although oral mucositis is prolonged, the type of inflammation (bacterial to autoimmune) and the clinical picture (i.e., presented in Figure 2 to dry mucositis in SS syndrome) changed. The Common Terminology Criteria for Adverse Events (CTCAE) and Naranjo’s algorithm do not provide for such specific and qualitative scenario. Information taken from published reports may be incomplete or may lack specificity because of usually observed differences in coding and categorization of adverse effects between studies (Pande, 2018). Most of the studies do not differentiate between adverse event (harmful outcome that occurs, not necessarily caused by a drug) and adverse effect (causal relation between the drug and the event is at least a reasonable possibility). Following CTCAE, the infectious complications are, therefore, described usually as localized or life-threatening colitis, pneumonia, etc., which is an inflammatory rather than a strictly infectious process. For example, the last meta-analysis of 17 randomized controlled trials (20,478 patients) showed a significantly increased risk of upper respiratory tract infections in COPD patients with ICS therapy (Chen et al., 2020). These studies were dedicated to assessing the efficacy and safety of ICS treatment rather than the infectious profile. Upper respiratory tract infection (URTI) and pneumonia were not accurately defined as an infection without microbiological analysis, species identification, or at least type (opportunistic vs. pathogen-related). For example, there were eight deaths among patients from pneumonia as such in the combination therapy group, seven in the placebo group, nine in the salmeterol group, and 13 in the fluticasone group. Surprisingly, the URTI rate is higher in the combination therapy group than in the placebo group and in patients with fluticasone monotherapy (i.e., 0.11, 0.1, and 0.09 rate per year, respectively) (Calverley et al., 2007). The infection is, therefore, difficult to link with ICS (incidence rate lower than placebo), especially endogenous and opportunistic infections. Moreover, the meta-analysis corresponds with our report:
• Fluticasone was observed with an increased risk of URTI in comparison with other ICS (e.g., mometasone);
• High-dose fluticasone treatment was associated with a significantly higher risk of URTI but not low dose.
Our observation indicates the need for the implementation of microbiological testing and species identification in the coding and reporting of adverse effects in clinical trials. Identification using MALDI-TOF-MS as a relatively inexpensive and increasingly accessible method should be the standard. The species diversity of the cultured bacteria (Table 1) indicates that the dysbiotic state precedes the subsequent general symptoms and should, therefore, be described as a separate category or at least a separate grade in infectious complications in CTCAE. Regretfully, clinical trials of inhalation drugs, as well as all therapeutic regimens, do not implement microbiome analysis.
Although Rothia were described as health-associated genera, these bacteria can cause disease in severe immunodeficiency. Rothia spp. are increasingly being recognized as emerging opportunistic pathogens (Abidi et al., 2016). Our observation shows the clinical repercussion of inhalators, especially steroid overuse. Unfortunately, routine checks for microbial colonization and surveillance cultures from patients are not recommended (O’Malley, 2015), but asthma exacerbation is frightening for a patient, and self-management of ICS prompts overdosing. The concept and strict plan for patients, which included a temporary quadrupling of the ICS dose, were described previously (McKeever et al., 2018). The finding showed five events of severe pneumonia (0.5%) with one death in the quadrupling group of 957 patients. Our findings (Table 2) indicate that inhaled corticoids are absorbed with systemic side effects. Furthermore, the post-ICS epithelitis and macroglossia (Figure 2) caused breathing perturbation. The oral cavity can act as the site of origin of dissemination of pathogenic organisms to distant body sites in immunocompromised hosts, especially those suffering from malignancies, diabetes, and rheumatoid arthritis, or SS immunosuppressive treatment. Only one study shows lung microbiome alteration in patients treated with ICS (Pragman et al., 2012). Acquired causes of macroglossia may include endocrine disorders and inflammatory or infectious diseases. However, this rare symptom has not been described in more detail, especially in the light of ADRs and the microbiome as well as endocrine and immune system disorders (Genetic and Rare Diseases Information Center, 2022).
Innate immune response is disturbed in our patient (Table 2) (Bishop and Gleeson, 2009). Our observation corresponds with the data that only high-dose ICSs are associated with a significantly increased risk of infection of the upper respiratory tract but preferentially endogenous with dysbiosis (Figure 3). Furthermore, invasive or recurrent pneumococcal diseases were observed only in immunodeficient child with antibody deficiency (Butters et al., 2019). The ICS-induced transient humoral immunodeficiency observed here requires reflection. Until now, ICS has not been described as a risk factor for Rothia opportunistic complication. This is the first description and, what is noteworthy, of three different species simultaneously in the same niche. The genome-based taxonomic classification of the phylum Actinobacteria as well as Rothia spp. is still an open issue (Nouioui et al., 2007). We hypothesize that alterations in the oral mucosa microbiome and/or its interactions with the host immune system (e.g., low IgG, lymphadenopathy, and splenomegaly (Table 2; Figure 3) may lead to disordered immune tolerance and the development of an inflammatory state that accelerates the progression of asthma as well as induces autoimmune-lymphoproliferative disorder, i.e., Sjögren’s syndrome (Figure 3). Unfortunately, strong alloantigenic stimulation by microbiome and narrow lymphocyte repertoire prompt lymphoproliferative disease (Zdziarski et al., 2017c). T cell receptor (TCR) threshold activity leading to such drastically opposing signaling outcomes (life or death) is modulated in part by glucocorticoids (Figure 3) (Wiegers et al., 2011). Dysbiosis and the role of microbiota in Sjögren’s syndrome clinical presentation are the area for further research. Endogenous glucocorticoids, to some extent blocked by ICS with the increase of ACTH (Table 2), are required for a robust adaptive immune response because of their promotion of the selection of T cells, and the absence of thymocyte glucocorticoid signaling results in an immunocompromised state with alterations in the TCR repertoire of polyclonal T cells (Mittelstadt et al., 2012). Only through an integrated approach that considers influences of multiple interacting factors we will be able to gain a better understanding of host–microbe associations (Brinker et al., 2019; Zdziarski, 2020).
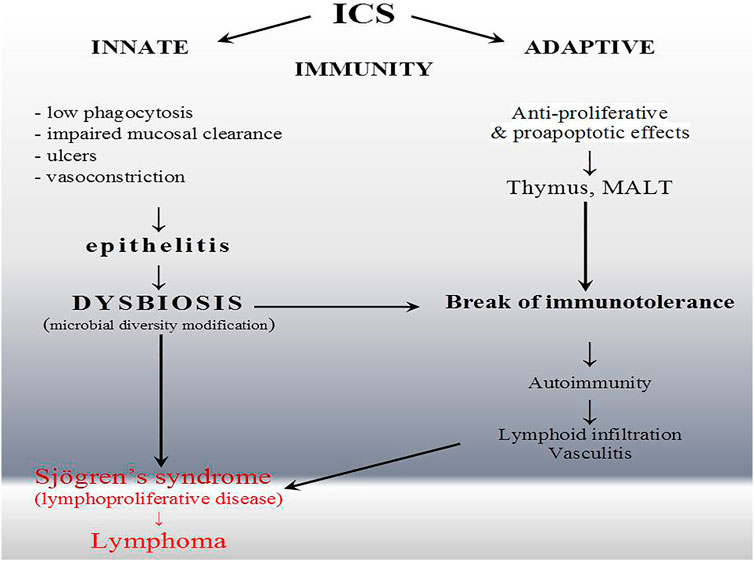
FIGURE 3. Inflammatory complication of inhaled corticoid (ICS) overuse. The wide spectrum of immunomodulatory effects of ICS and influence on epithelial barrier prompt lymphocyte selection in thymus (T cell) or mucosa-associated lymphatic tissue (MALT) (B and T cells) intensified by non-specific perturbation and dysbiosis. ICS, unlike drugs typically associated with drug-induced lupus, have a direct influence on the immune system. Nevertheless, they also induced systemic rather than organ-specific autoimmunity.
Sjögren’s syndrome is a chronic autoimmune disease that classically affects the lacrimal and salivary glands and can affect almost any organ system in the body including the lungs (Sandhya et al., 2017). Lung involvement in primary SS is mainly related to the small airway disease (Papiris et al., 1999). Intriguingly, even in SS, the gut microbiome is more likely to be studied than the respiratory one, as reviewed elsewhere (Tsigalou et al., 2018). It revealed significant compositional differences compared to controls, while Firmicutes/Bacteroidetes ratio and Actinobacteria decreased (Moon et al., 2020). A similar observation was presented in our case. As SS developed, a wide variety of microbes (Table 1) became dominated by streptococci. Whether it is an effect or a cause, most studies are not conclusive, and indirect pieces of evidence are the only elements in this puzzle thus far (Tsigalou et al., 2018). MALT activation in preclinical phase of SS overlaps with inflammatory symptoms, and clinical presentation of inflammatory respiratory diseases usually treated with ICS (Zdziarski and Gamian, 2019), bronchial hyper responsiveness, cough, and bronchiolitis or bronchiectasis, are reported in SS with a prevalence between 7% and 61% (Flament et al., 2016). There are currently no SS treatments available that address the underlying disease etiology, and systemic or topical steroids are not effective. Initial autoimmune inflammation and epithelitis—a deregulated immune response—are the first phase of SS development (Tapinos et al., 1999) (Figure 2). The overuse of ICS and quadrupling of therapeutic regimen in asthma may be paradoxically the source of microbial dysbiosis, systemic inflammatory complication, and systemic autoimmunity (relatively rare seronegative SS with major organ involvement). The lack of hyper-gammaglobulinemia and low IgG observed here show that patients receiving ICS may develop secondary immunodeficiency as well as atypical SS presentation (Table 2). In our observation, dysbiosis and Rothia spp. overgrowth were not observed after ICS withdrawal (patient recovers well after ICS stopping), but contrary to early immunoabnormalities (top of Table 2), late immunoabnormalities and SS are difficult for causality assessment (qualified as possible as presented in the bottom part of Table 2). ICS show extraordinary pleiotropic effects, and SS is an unknown-etiology disease (Figure 3).
Our research had several limitations. Firstly, the baseline samples were not collected prior to treatment initiation. The study was not prospective, and the method and diagnostic chain were presented in one, which is the most transparent case. It would be very difficult to obtain patients with a newly diagnosed disease, then to observe them with waiting for a similar situation and a quadrupling ICS dose. Besides, it would never be certain whether the starting sample is a native microbiome or disease altered (e.g., asthma). Secondly, the limitation of the presented diagnostic chain is transport and culture (Table 1). These do not allow the detection and identification of organisms that have not been cultured in the laboratory, e.g., Archaea, which are involved in periodontal disease (Vianna et al., 2006). Thirdly, there is no simple and direct evidence that the observed therapy and microbiome are the direct cause of the development of Sjögren’s syndrome, a relatively rare autoimmune disease. However, apart from classical rheumatic fever and Group A streptococcal infection, the early stages of autoimmune diseases (the primary immune response) and the induction phase of the disease are not known. This accidental finding in the later observation of the patient, however, may be a sufficient example of the undiscovered role of the respiratory microbiome. Most of the studies, including in Sjögren’s syndrome, are of the gut microbiome (Tsigalou et al., 2018).
Our findings shed a new light on an adverse effect of ICS and the initial phase and possible pathogenesis of Sjögren’s syndrome. ICS and their overuse or quadrupling prompt oral and respiratory tract microbiome discrepancy (dysbiosis) and secondary immunodeficiency; therefore, opportunistic infections with microorganisms such as from the genus Rothia are erroneously omitted. Such dysbiosis should be considered a preclinical phase of the disease, and an area for further research should be provided. Identification with MALDI-TOF-MS as a cheap and increasingly accessible method may be a prototype approach.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: All data generated or analyzed during this study are included in this article. The clinical isolate was deposited in a publicly accessible culture collection—Polish Collection of Microorganisms.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
PZ and MP conceived and designed the experiments. PZ was responsible for the clinical diagnosis and patient management. MP performed the microbiological and MALDI-TOF analysis. PZ, MP, and AG analyzed the data. AG contributed reagents/materials/analysis tools. PZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the statutory activity of Hirszfeld Institute and from private funds of the first author. The work is part of the following project: Analysis of the structural properties of a microbial biofilm matrix.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACRH, adrenocorticotropic hormone; ADRs, adverse drug reactions; AE, adverse event; COPD, chronic obstructive pulmonary disease; CTCAE, Common Terminology Criteria for Adverse Events; ICS, inhaled glucocorticoids; LABA, long-acting beta2-agosts; MALDI-TOF-MS, matrix-assisted laser desorption ionization–time of flight mass spectrometry; MALT, mucosa-associated lymphoid tissue; SS, Sjögren’s syndrome; TCR, T cell receptor; URTI, upper respiratory tract infection.
Aas, J. A., Paster, B. J., Stokes, L. N., Olsen, I., and Dewhirst, F. E. (2005). Defining the normal Bacterial flora of the Oral Cavity. J. Clin. Microbiol. 43 (11), 5721–5732. doi:10.1128/JCM.43.11.5721-5732.2005
Abidi, M. Z., Ledeboer, N., Banerjee, A., and Hari, P. (2016). Morbidity and Mortality Attributable to Rothia Bacteremia in Neutropenic and Nonneutropenic Patients. Diagn. Microbiol. Infect. Dis. 85, 116–120. doi:10.1016/j.diagmicrobio.2016.01.005
AlmståhI, A., Wikström, M., Stenberg, I., Jakobsson, A., and Fagerberg-Mohlin, B. (2003). Oral Microbiota Associated with Hyposalivation of Different Origins. Oral Microbiol. Immunol. 18, 1–8. doi:10.1034/j.1399-302X.2003.180101.x
Bagratuni, L. (1960). A Comparative Study of Topical Steroids, Antihistamines and Pollen Vaccine in the Treatment of hay Fever and hay Asthma. Ann. Allergy 18, 859–865.
Bansal, V., Mangi, M. A., Johnson, M. M., and Festic, E. (2015). Inhaled Corticosteroids and Incident Pneumonia in Patients with Asthma: Systematic Review and Meta-Analysis. Acta Med. Acad. 44, 135–158. doi:10.5644/ama2006-124.141
Bik, E. M., Long, C. D., Armitage, G. C., Loomer, P., Emerson, J., Mongodin, E. F., et al. (2010). Bacterial Diversity in the Oral Cavity of 10 Healthy Individuals. ISME J. 4 (8), 962–974. doi:10.1038/ismej.2010.30
Bishop, N. C., and Gleeson, M. (2009). Acute and Chronic Effects of Exercise on Markers of Mucosal Immunity. Front. Biosci. (Landmark Ed. 14, 4444–4456. doi:10.2741/3540
Brinker, P., Fontaine, M. C., Beukeboom, L. W., and Falcao Salles, J. (2019). Host, Symbionts, and the Microbiome: The Missing Tripartite Interaction. Trends Microbiol. 27, 480–488. doi:10.1016/j.tim.2019.02.002
Butters, C., Phuong, L. K., Cole, T., and Gwee, A. (2019). Prevalence of Immunodeficiency in Children with Invasive Pneumococcal Disease in the Pneumococcal Vaccine Era: A Systematic Review. JAMA Pediatr. 173 (11), 1084–1094. doi:10.1001/jamapediatrics.2019.3203
Calverley, P. M., Anderson, J. A., Celli, B., Ferguson, G. T., Jenkins, C., Jones, P. W., et al. (2007). Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 356, 775–789. doi:10.1056/NEJMoa063070
Camelo-Castillo, A. J., Mira, A., Pico, A., Nibali, L., Henderson, B., Donos, N., et al. (2015). Subgingival Microbiota in Health Compared to Periodontitis and the Influence of Smoking. Front. Microbiol. 6, 119. doi:10.3389/fmicb.2015.00119
Cazeiro, C., Silva, C., Mayer, S., Mariany, V., Wainwright, C. E., and Zhang, L. (2017). Inhaled Corticosteroids and Respiratory Infections in Children with Asthma: A Meta-Analysis. Pediatrics 139, 139. doi:10.1542/peds.2016-3271
Charlson, E. S., Bittinger, K., Haas, A. R., Fitzgerald, A. S., Frank, I., Yadav, A., et al. (2011). Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract. Am. J. Respir. Crit. Care Med. 184, 957–963. doi:10.1164/rccm.201104-0655OC
Chen, H., Feng, Y., Wang, K., Yang, J., and Du, Y. (2020). Association between Inhaled Corticosteroids and Upper Respiratory Tract Infection in Patients with Chronic Obstructive Pulmonary Disease: a Meta-Analysis of Randomized Controlled Trials. BMC Pulm. Med. 20, 282. doi:10.1186/s12890-020-01315-3
Deng, Z. L., Sztajer, H., Jarek, M., Bhuju, S., and Wagner-Döbler, I. (2018). Worlds Apart - Transcriptome Profiles of Key Oral Microbes in the Periodontal Pocket Compared to Single Laboratory Culture Reflect Synergistic Interactions. Front. Microbiol. 9, 124. doi:10.3389/fmicb.2018.00124
Erb-Downward, J. R., Thompson, D. L., Han, M. K., Freeman, C. M., McCloskey, L., Schmidt, L. A., et al. (2011). Analysis of the Lung Microbiome in the “Healthy” Smoker and in COPD. PLoS One 6, e16384. doi:10.1371/journal.pone.0016384
Falcone, E. L., Zelazny, A. M., and Holland, S. M. (2012). Rothia Aeria Neck Abscess in a Patient with Chronic Granulomatous Disease: Case Report and Brief Review of the Literature. J. Clin. Immunol. 32, 1400–1403. doi:10.1007/s10875-012-9726-8
Flament, T., Bigot, A., Chaigne, B., Henique, H., Diot, E., and Marchand-Adam, S. (2016). Pulmonary Manifestations of Sjögren's Syndrome. Eur. Respir. Rev. 25, 110–123. doi:10.1183/16000617.0011-2016
Genetic and Rare Diseases Information Center (GARD) (2022). Available at: https://rarediseases.info.nih.gov/diseases/3342/macroglossia (Accessed Jan 23, 2022).
Ghebre, M. A., Pang, P. H., Diver, S., Desai, D., Bafadhel, M., Haldar, K., et al. (2018). Biological Exacerbation Clusters Demonstrate Asthma and Chronic Obstructive Pulmonary Disease Overlap with Distinct Mediator and Microbiome Profiles. J. Allergy Clin. Immunol. 141, 2027–e12. doi:10.1016/j.jaci.2018.04.013
Huse, S. M., Young, V. B., Morrison, H. G., Antonopoulos, D. A., Kwon, J., Dalal, S., et al. (2014). Comparison of brush and Biopsy Sampling Methods of the Ileal Pouch for Assessment of Mucosa-Associated Microbiota of Human Subjects. Microbiome 2, 5. doi:10.1186/2049-2618-2-5
London, M., and Alexander, F. W. (1951). Topical Application of Cortisone in Intractable Bronchial Asthma. J. Allergy 22, 518–523. doi:10.1016/0021-8707(51)90108-6
McKeever, T., Mortimer, K., Wilson, A., Walker, S., Brightling, C., Skeggs, A., et al. (2018). Quadrupling Inhaled Glucocorticoid Dose to Abort Asthma Exacerbations. N. Engl. J. Med. 378, 902–910. doi:10.1056/NEJMoa1714257
Mittelstadt, P. R., Monteiro, J. P., and Ashwell, J. D. (2012). Thymocyte Responsiveness to Endogenous Glucocorticoids Is Required for Immunological Fitness. J. Clin. Invest. 122, 2384–2394. doi:10.1172/JCI63067
Moon, J., Choi, S. H., Yoon, C. H., and Kim, M. K. (2020). Gut Dysbiosis Is Prevailing in Sjögren's Syndrome and Is Related to Dry Eye Severity. PLoS One 15, e0229029. doi:10.1371/journal.pone.0229029
Nouioui, I., Carro, L., García-López, M., Meier-Kolthoff, J. P., Woyke, T., Kyrpides, N. C., et al. (2007). Genome-Based Taxonomic Classification of the Phylum Actinobacteria. Front. Microbiol. 9, 2007. doi:10.3389/fmicb.2018.02007
O'Malley, C. A. (2015). Device Cleaning and Infection Control in Aerosol Therapy. Respir. Care 60, 917–930. doi:10.4187/respcare.03513
Pande, S. (2018). Causality or Relatedness Assessment in Adverse Drug Reaction and its Relevance in Dermatology. Indian J. Dermatol. 63, 18–21. doi:10.4103/ijd.IJD_579_17
Papiris, S. A., Maniati, M., Constantopoulos, S. H., Roussos, C., Moutsopoulos, H. M., and Skopouli, F. N. (1999). Lung Involvement in Primary Sjögren's Syndrome Is Mainly Related to the Small Airway Disease. Ann. Rheum. Dis. 58, 61–64. doi:10.1136/ard.58.1.61
Paściak, M., Dacko, W., Sikora, J., Gurlaga, D., Pawlik, K., Miękisiak, G., et al. (2015). Creation of an In-House Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry Corynebacterineae Database Overcomes Difficulties in Identification of Nocardia Farcinica Clinical Isolates. J. Clin. Microbiol. 53, 2611–2621. doi:10.1128/JCM.00268-15
Paściak, M., Holst, O., Lindner, B., Mordarska, H., and Gamian, A. (2003). Novel Bacterial Polar Lipids Containing Ether-Linked Alkyl Chains, the Structures and Biological Properties of the Four Major Glycolipids from Propionibacterium Propionicum PCM 2431 (ATCC 14157T). J. Biol. Chem. 278, 3948–3956. doi:10.1074/jbc.M206013200
Peryer, G., Golder, S., Junqueira, D., Vohra, S., and Loke, Y. K. (2020). “Chapter 19: Adverse Effects,” in Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. Editors J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Pageet al. (Cochrane).
Pragman, A. A., Kim, H. B., Reilly, C. S., Wendt, C., and Isaacson, R. E. (2012). The Lung Microbiome in Moderate and Severe Chronic Obstructive Pulmonary Disease. PLoS One 7, e47305. doi:10.1371/journal.pone.0047305
Raffatellu, M., and Bäumler, A. J. (2021). Special Collection on the Microbiome and Infection. Infect. Immun. 89, e0035621. doi:10.1128/IAI.00356-21
Ramanan, P., Barreto, J. N., Osmon, D. R., and Tosh, P. K. (2014). Rothia Bacteremia: a 10-Year Experience at Mayo Clinic, Rochester, Minnesota. J. Clin. Microbiol. 52, 3184–3189. doi:10.1128/JCM.01270-14
Sandhya, P., Kurien, B. T., Danda, D., and Scofield, R. H. (2017). Update on Pathogenesis of Sjogren's Syndrome. Curr. Rheumatol. Rev. 13, 5–22. doi:10.2174/1573397112666160714164149
Santos, N. C., Jamelli, S., Costa, L., Baracho Filho, C., Medeiros, D., Rizzo, J. A., et al. (2012). Assessing Caries, Dental Plaque and Salivary Flow in Asthmatic Adolescents Using Inhaled Corticosteroids. Allergol. Immunopathol (Madr) 40, 220–224. doi:10.1016/j.aller.2011.04.005
Tapinos, N. I., Polihronis, M., Tzioufas, A. G., and Moutsopoulos, H. M. (1999). Sjögren's Syndrome. Autoimmune Epithelitis. Adv. Exp. Med. Biol. 455, 127–134. doi:10.1007/978-1-4615-4857-7_18
Tezcan, M. E., Kucuk, H., and Goker, B. (2017). American College of Rheumatology/European League Against Rheumatism Sjögren's Syndrome Classification Criteria May Not Be Adequate for Extraglandular Disease and Necessitate Defining "Seronegative Sjögren's Syndrome": Comment on the Article by Shiboski et al. Arthritis Rheumatol. 69, 1341–1342. doi:10.1002/art.40083
The Global Initiative for Asthma, (2019). Available at: https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/(Accessed Apr 22, 2019).
The Human Microbiome Project Consortium (2012). Structure, Function and Diversity of the Healthy Human Microbiome. Nature 486, 207–214. doi:10.1038/nature11234
Tsigalou, C., Stavropoulou, E., and Bezirtzoglou, E. (2018). Current Insights in Microbiome Shifts in Sjogren's Syndrome and Possible Therapeutic Interventions. Front. Immunol. 9, 1106. doi:10.3389/fimmu.2018.01106
Vianna, M. E., Conrads, G., Gomes, B. P., and Horz, H. P. (2006). Identification and Quantification of Archaea Involved in Primary Endodontic Infections. J. Clin. Microbiol. 44, 1274–1282. doi:10.1128/JCM.44.4.1274-1282.2006
Wiegers, G. J., Kaufmann, M., Tischner, D., and Villunger, A. (2011). Shaping the T-Cell Repertoire: a Matter of Life and Death. Immunol. Cel Biol 89, 33–39. doi:10.1038/icb.2010.127
Yang, M., Chen, H., Zhang, Y., Du, Y., Xu, Y., Jiang, P., et al. (2017). Long-term Use of Inhaled Corticosteroids and Risk of Upper Respiratory Tract Infection in Chronic Obstructive Pulmonary Disease: a Meta-Analysis. Inhal Toxicol. 29, 219–226. doi:10.1080/08958378.2017.1346006
Yong, E. (2014). There Is No ‘healthy’ Microbiome, 2 November. New York: The New York Times, 4. Available at: https://www.nytimes.com/2014/11/02/opinion/sunday/there-is-no-healthy-microbiome.html (Accessed Dec 17, 2021).
Zdziarski, P. (2019). CMV-specific Immune Response-New Patients, New Insight: Central Role of Specific IgG during Infancy and Long-Lasting Immune Deficiency after Allogenic Stem Cell Transplantation. Int. J. Mol. Sci. 20, 271. doi:10.3390/ijms20020271
Zdziarski, P., Gamian, A., and Dworacki, G. (2017c). A Case Report of Lymphoid Interstitial Pneumonia in Common Variable Immunodeficiency: Oligoclonal Expansion of Effector Lymphocytes with Preferential Cytomegalovirus-specific Immune Response and Lymphoproliferative Disease Promotion. Medicine (Baltimore) 96, e7031. doi:10.1097/MD.0000000000007031
Zdziarski, P., and Gamian, A. (2019). Lymphoid Interstitial Pneumonia in Common Variable Immune Deficiency - Case Report with Disease Monitoring in Various Therapeutic Options: Pleiotropic Effects of Rituximab Regimens. Front. Pharmacol. 9, 1559. doi:10.3389/fphar.2018.01559
Zdziarski, P., Gamian, A., Majda, J., and Korzeniowska-Kowal, A. (2017a). Passive Blood Anaphylaxis: Subcutaneous Immunoglobulins Are a Cause of Ongoing Passive Anaphylactic Reaction. Allergy Asthma Clin. Immunol. 13, 41. doi:10.1186/s13223-017-0213-x
Zdziarski, P., Paściak, M., Rogala, K., Korzeniowska-Kowal, A., and Gamian, A. (2017b). Elizabethkingia Miricola as an Opportunistic Oral Pathogen Associated with Superinfectious Complications in Humoral Immunodeficiency: a Case Report. BMC Infect. Dis. 17 (1), 763. doi:10.1186/s12879-017-2886-7
Zdziarski, P. (2020). Veillonella Atypica in Tumor as a Tripartite Interaction: Commensal – Tumor – Patient. (In Polish). Pol. Med. J. 48, 35–39.
Zhang, L., Prietsch, S. O., Mendes, A. P., Von Groll, A., Rocha, G. P., Carrion, L., et al. (2013). Inhaled Corticosteroids Increase the Risk of Oropharyngeal Colonization by Streptococcus Pneumoniae in Children with Asthma. Respirology 18, 272–277. doi:10.1111/j.1440-1843.2012.02280.x
Keywords: inhaled glucocorticoids, Rothia, adverse drug reaction, microbiome and dysbiosis, Sjögren’s syndrome, macroglossia, epithelitis, autoimmunity
Citation: Zdziarski P, Paściak M and Gamian A (2022) Microbiome Analysis and Pharmacovigilance After Inhaled Glucocorticoid: Oral Dysbiosis With the Isolation of Three Rothia Species and Subsequent Sjögren’s Syndrome. Front. Pharmacol. 13:636180. doi: 10.3389/fphar.2022.636180
Received: 21 April 2021; Accepted: 24 January 2022;
Published: 01 April 2022.
Edited by:
Elena Ramírez, University Hospital La Paz, SpainReviewed by:
Hanna Evelina Sidjabat, Griffith University, AustraliaCopyright © 2022 Zdziarski, Paściak and Gamian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Przemysław Zdziarski, emR6aWFyc2tpQG9pbC5vcmcucGw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.