
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 22 December 2022
Sec. Experimental Pharmacology and Drug Discovery
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1093666
This article is part of the Research Topic Modulation of Immune Function: Drug Discovery and Translational Application View all 6 articles
Immune checkpoint inhibitors have made significant progress in the treatment of various cancers. However, due to the low ICI responsive rate for the gynecologic cancer, ICI two-drug combination therapy tends to be a predominant way for clinical treatment. Antibody-drug conjugates, a promising therapeutic modality for cancer, have been approved by the FDA for breast cancer, lymphoma, multiple myeloma and gastric cancer. On September 2021, the FDA granted accelerated approval to tisotumab vedotin for patients with recurrent or metastatic cervical cancer. Currently, the role of therapy of ADCs on gynecologic tumors was also included in medication regimens. Now more than 30 ADCs targeting for 20 biomarkers are under clinical trials in the field, including monotherapy or combination with others for multiple lines of therapy. Some ADCs have been proved to enhance the antitumor immunity effect on both pre-clinical models and clinical trials. Therefore, combination of ADCs and ICIs are expected in clinical trials. In this review, we discuss current development of ADCs in gynecologic oncology and the combination effects of ICIs and ADCs.
The first antibody-drug conjugate (ADC) drug, Mylotarg, was approved by the FDA in 2009. After a decade of advancement in conjugation biochemistry, a third-generation ADC drug, DS-8201, was approved in 2019, providing new possibilities for the use of ADC in cancer treatment. To date, ADCs have been approved for breast cancer, lymphoma, multiple myeloma, and gastric cancer and hundreds are in different phases of clinical trials for other tumor types (Beck et al., 2017; Abdollahpour-Alitappeh et al., 2019). In September 2021, tisotumab vedotin was granted FDA accelerated approval for recurrent or metastatic cervical cancer, becoming the 12th ADC approved by the FDA and the first for use against gynecologic cancers. Now more than 30 ADCs targeting 20 biomarkers are being tested in clinical trials, of which IMGN853 and tisotumab vedotin for ovarian and cervical cancer, respectively, are the first to be tested in phase III clinical trials. Over the last 10 years, cancer immunotherapy has undergone revolutionary changes. Immune checkpoint inhibitors (ICI), PD-1, PD-L1 and CTLA-4 monoclonal antibodies, represent major milestones in the treatment of human cancers, especially Hodgkin’s lymphoma and melanoma (Drakes et al., 2020; Kurnit et al., 2020). On October 2021, the FDA approved pembrolizumab (Keytruda) for use in combination with chemotherapy, with or without bevacizumab, for patients with persistent, recurrent, or metastatic cervical tumors that express PD-L1. On March 2022 pembrolizumab was FDA approved as a single agent for patients with advanced endometrial carcinoma that is microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR). However, the effect of ICIs on gynecologic cancer remain less than ideal. Combination ADC-ICI therapy is thought to be a potential solution.
ADCs contain biomarker-specific antibody, cytotoxic payloads and linkers (Ritchie et al., 2013). ADCs expand the therapeutic window by specifically delivering cytotoxic drugs to tumors and reducing their retention in healthy tissues (Tarcsa et al., 2020).
Biomarkers are highly expressed in tumors and have little or no expression in normal tissues. IgG, in particular IgG1 and occasionally IgG2, IgG4, are often used in ADCs due to their long circulation half-life and high affinity. Some ADCs employ unique monoclonal antibodies such as STRO-002 with structures that are specifically designed for site-specific conjugation (Cheng et al., 2018).
Linker stability in vivo ensures that cytotoxic molecules are not launched prematurely and that drug cleavage occurs once it enters the tumor, preventing systemic toxicity (Jain et al., 2015). There is a negative correlation between linker stability and ADC toxicity. The first marketed ADC, Mylotarg® (Gemtuzumab ozogamicin), was removed from the market in 2011 because its hydrazone linker was shown to be unstable causing high toxicity (van der Velden et al., 2001; Lu et al., 2016). Other recent cleavable linkers have increased stability, though off-target effects may persist. Cleavable linkers are more likely than non-cleavable linkers to cause bystander effects that contribute to tumor death (Chari et al., 2014; Burton et al., 2019).
The two types of linkers are shown in the Table 1. In addition, some ADCs such as DMUC4064A, XMT-1536, and stro-002, are being coupled by proprietary technologies (Ohri et al., 2018; Abrahams et al., 2019; Yurkovetskiy et al., 2021).
ADCs are dependent on the inclusion of highly cytotoxic molecules because the complex mechanisms by which ADCs function tends to reduce their utility. In addition, cytotoxic molecules have stable circulation and are easy to conjugate with antibodies (Teicher and Chari, 2011). The cytotoxic molecules used in ADCs are very limited, primarily falling into two categories, microtubule-targeting and DNA-damaging agents; however, the next-generation of ADCs is making use of RNA polymerase inhibitors and other agents (Pahl et al., 2018). Microtubule-targeting agents are the most widely used payloads, targeting maytansine sites (DM1 and DM4) and vinca alkaloid sites (MMAE and MMAF) to inhibit tubulin, disrupt microtubules, and arrest the cell cycle in the G2/M phase, inducing cell death (Chen et al., 2017). Importantly, these toxic properties are only functional in proliferating cells. In contrast, DNA-damaging agents are cytotoxic in both proliferating and non-proliferating cells since their mode of cytotoxic action is not dependent on the cell cycle. The categories of cytotoxic payloads are shown in the Table 2.
The function of ADC drugs against refractory gynecologic cancers is receiving increasing attention. Clinical trials for some biomarkers are in progress. The Table 3 summarizes the ADCs of gynecological oncology in clinical trials (Figure 1).
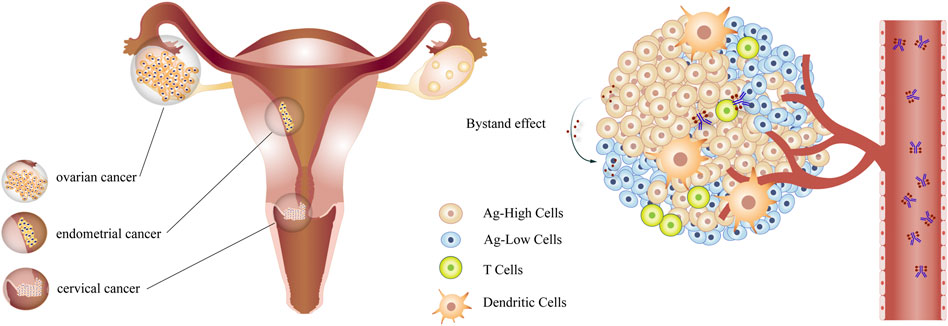
FIGURE 1. The ADCs in gynecologic cancer ADCs target antigen-high cells in gynecological tumors through the circulation and kill antigen-low cells by bystander effects.
Alpha folate receptor binding antibody (FRα) is a 38–40 kDa glycosyl-phosphatidylinositol (GPI) glycoprotein anchored to the cell surface that binds folate for intracellular transport (Elnakat and Ratnam, 2004; Kitamura et al., 2010). Normally, the expression of FRα is restricted to a small number of polarized epithelia in the choroid plexus, lung, kidney, uterus, and placenta, while it is overexpressed in the majority of ovarian, uterine, and ependymal brain tumors and in varying percentages of lung, breast, kidney and colon carcinomas (Elnakat and Ratnam, 2006; Birrer et al., 2019; Salazar and Ratnam, 2007; Scaranti et al., 2020; O'Shannessy et al., 2012). FRα is expressed in >80% of epithelial ovarian cancers (EOC) and is associated with poor prognosis of EOC (Ross et al., 1994; Kelemen, 2006; Vergote et al., 2015).
Mirvetuximab soravtansine (IMGN853), an advanced ADC in gynecological oncology, includes an FRα binding antibody, a cleavable disulfide link, and a maytansinoid DM4 payload (Ab et al., 2015). A phase I dose-escalation trial of IMGN853 has a favorable safety profile. The most common adverse events (AEs) were fatigue (25%), blurred vision (23%), diarrhea (21%), and peripheral neuropathy (21%), the majority of which were grades 1 or 2. Treatment-related serious adverse events (SAE) (9%) included grade 3 hypophosphatemia, punctate keratitis, episode, corneal opacity, and pulmonary edema. The blurred vision and keratopathy were reversible. All 44 patients who received IMGN853 were not required with expression of FRα. Of 43 assessable patients, two EOC patients had confirmed partial responses (PRs) with an the objective response rate (ORR) of 5%, 22 patients had stable disease (SD) and five patients (four with EOC and one with endometrial cancer) had a confirmed CA 125 response with an overall clinical benefit rate (ORR + SD ≥4 months + CA 125 response) of 23% (Moore et al., 2017a). The phase I expansion cohort study included 46 patients for whom ≥ 25% of their tumor cells had at least 2+ FRα staining intensity. The ORR was 26%, including one complete response (CR) and 11 PRs, the median progression-free survival (PFS) was 4.8 months, and the median duration of response (DoR) was 19.1 weeks. Remarkably, there appeared to be a relationship between the receipt of prior lines of therapy and the response to IMGN853. An ORR of 39% was observed in patients who had received one to three prior lines, compared with 13% among patients who had received at least four (Moore et al., 2017b). The overall ORR in a phase Ib study was 22%, including two CRs and four PRs. This study also revealed a correlation between FRα expression and clinical efficacy. Low-expression cases had no objective response, with a median PFS of 2.8 months, while the ORR of the medium-expression cohort was 20% (1/5), with a median PFS of 3.9 months and the ORR of the high-expression cohort was 31% (5/16, including two CRs) with a median PFS of 5.4 months (Martin et al., 2017).
These early trials provided sufficient evidence to inform the inclusion criteria of FORWARD I (NCT02631876), a phase III monotherapy trial for patients with platinum-resistant FRα positive advanced EOC, primary peritoneal cancer, and/or fallopian tube cancer. A total of 336 patients were randomized to receive IMGN853 or chemotherapy (paclitaxel, pegylated liposomal doxorubicin, or topotecan) in a 2:1 ratio. The IMGN853 and chemotherapy groups had ORRs of 22% and 12%, respectively; however, both the PFS (HR = 0.98, p = 0.897), the primary endpoint, and the overall survival (HR = 0.81, p = 0.248) were not significantly different. While not reaching the primary endpoint, IMGN853 demonstrated superior clinical activity and fewer adverse events than chemotherapy (Moore et al., 2021). Two new Phase III studies, SORAYA (NCT04296890) and MIRASOL (NCT04209855) based on FORWARD I, are in progress (Moore et al., 2021).
MORAb-202 is an ADC in which farletuzumab is bonded to eribulin by a cathepsin-B cleavable linker (Cheng et al., 2018). As a human monoclonal antibody specific for FRα, farletuzumab combined with standard chemotherapy did not meet the primary endpoint in a phase III trial for platinum-sensitive recurrent ovarian cancer (Vergote et al., 2016). This phase I study included 22 patients with FRα-positive solid tumors, including 12 patients with ovarian cancer. A total of 21 of 22 patients (95%) experienced treatment-emergent AEs, ten (45%) of whom developed leukopenia and neutropenia, the most frequent adverse events. One patient experienced a grade 3 rise in alanine aminotransferase and γ-glutamyl transferase as dose-limiting toxicities and five patients (23%) developed pneumonitis/interstitial lung disease related to MORAb-202. The overall ORR of the study was 45.45% (10/22) including one CR, nine PRs, and eight (36%) SDs. Notably, normalized serum FRα was associated with the highest level of tumor shrinkage (Shimizu et al., 2021). Phase I/II studies (NCT04300556) to further assess MORAb-202 are ongoing.
STRO-002 is a novel FRα-targeting ADC that includes the tubulin-targeting 3-aminophenyl hemiasterlin warhead, SC209, joined to the antibody, SP8166. This drug is generated using a cell-free antibody production system (XpressCF™) and a site-specific conjugation (XpressCF+™) platform (Abrahams et al., 2019). A phase I dose-escalation study of STRO-002 (NCT03748186) contained 39 platinum-resistant patients without requirement for FRα expression. Most (86%) treatment-emergent AEs were grades 1 or 2. The most common treatment-related grade 3 and 4 AEs were reversible neutrophil reduction (36%), neutropenia (33%), arthralgia (12.8%), and neuropathy (7.7%). Significantly, no ocular toxicity signals have been observed, distinguishing STRO-002 from other ADCs. Most ADCs revealed ocular toxicities that are driven by the payload present and not by antigen expression. And the most commonly reported cases above are ADCs that contain DM4 or MMAF whose biomarkers barely express in the eye (Eaton et al., 2015). Within assessable patients, an ORR of 32% (10/31) was observed, including one CR (3%), four confirmed PRs, and five unconfirmed PRs, with a median PFS of 7.2 months and a median DoR of 5.8 weeks. There were also 18 SDs (58%) and 3 PDs (9.67%) (Naumann et al., 2021).
Mesothelin is a 40-kDa glycoprotein that is attached to the cell membrane by a glycosylphosphatidylinositol (GPI) (Hassan et al., 2004; Pastan and Hassan, 2014). Under physiological conditions, mesothelin is expressed in the upper part of the vagina, the uterus, and the fallopian tubes developed from the mesodermal Müllerian (paramesonephric) duct. Expression of mesothelin increases substantially during malignant transformation (Chang and Pastan, 1996; Jirsova et al., 2010), in particular, that associated with pancreatic cancer, mesothelioma, lung cancer, pancreatic cancer, breast cancer, ovarian cancer, endometrial cancer and cervical adenocarcinoma (Argani et al., 2001; Tchou et al., 2012; Iizuka et al., 2013; Qiao et al., 2014; Qiao et al., 2016; Stewart and Cristea, 2019; Weidemann et al., 2021). Mesothelin expression increases in 70%–85% ovarian tumors, making it a potential target for this disease (Hassan et al., 2005; Stewart and Cristea, 2019).
Anetumab Ravtansine is an ADC consisting of maytansinoid DM-4 conjugated to an anti-mesothelin-monoclonal antibody IgG1 via a disulfide-containing linker [a reducible SPDB linker (N-succinimidyl 4-(2-pyridyldithio) butanoate)]. A total of 148 patients with high-expression mesothelin cancer, including 21 with ovarian cancer, participated in phase I clinical trials (NCT01439152) for anetumab ravtansine. At the q3w maximum tolerated dose (MTD) (6.5 mg/kg), the most common drug-related AEs were fatigue, nausea, diarrhea, anorexia, vomiting, and peripheral sensory neuropathy, and the PR, SD, and DCR for ovarian cancer patients were 5%, 52%, and 57%, respectively, with a median DoR of 62 days and the median PFS of 2.8 months (Hassan et al., 2020). In a subsequent phase Ib study, anetumab ravtansine was combined with pegylated liposomal doxorubicin in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer. Within 21 assessable patients, the disease control rate (DCR) was 86% of 11 PRs (52%) and 7 SDs (33%), and a durable PR (>250 days) was observed in six patients (29%) (Santin et al., 2020).
DMOT4039A is composed of the humanized IgG1 anti-mesothelin mAb, h7D9.v3, and monomethyl auristatin E (MMAE) combined with a protease-labile valine-citrulline linker (Scales et al., 2014). In a phase I study of patients with unresectable pancreatic or platinum-resistant ovarian cancer, 31 ovarian cancer patients had strong staining for mesothelin. The main AEs were gastrointestinal or constitutional. Cumulative peripheral neuropathy (grades 1–3) resulting from microtubule inhibitor-specific toxicity, occurred in 20% of patients. At the q3w RP2D, three of ten ovarian cancer patients revealed confirmed partial responses, with durations of 2.7, 3.6, and 4.1 months and an additional three patients showed a CA125 response without a RECIST response. The median PFS for ovarian cancer patients at RP2D was 4.9 months. The trial also found that both serum mesothelin levels and tissue mesothelin immunohistochemistry (IHC) scores were not associated with the clinical activity of DMOT4039A (Weekes et al., 2016).
BMS-986148 contains tubulysin, a cytotoxic peptide with antimitotic activity that is attached to a fully human IgG1 anti-mesothelin mAb via a valine-citrulline linker (Clarke et al., 2019). A phase I/IIa trial (CA008-002, NCT02341625) assessed the safety, tolerability, and preliminary efficacy of BMS-986148 ± nivolumab against mesothelioma, ovarian cancer, pancreatic cancer, gastric cancer, and non-small cell lung cancer (NSCLC). Hepatic TRAEs occurred in all treatment cohorts, the most common (≥10%) of which were elevated aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase. On study day 130, an ovarian cancer patient died from pneumonitis related BMS-986148 monotherapy at the MTD level. The ORR and DCR were 9% and 13%, respectively, including two PRs (DOR, 19.91 and 3.02 months) and 11 SDs among 22 ovarian cancer patients, with a PFS of 2.8 months. There were also seven PDs (Rottey et al., 2022).
RC88, composed of anti-mesothelin mAb and MMAE, is being tested in a phase I clinical trial in mesothelin-positive solid tumor patients, including those with ovarian cancer (Jiang et al., 2021).
MUC16, a large type I transmembrane mucin of the MUC family (Yin and Lloyd, 2001; Thériault et al., 2011; Aithal et al., 2018), is a precursor of the most widely used biomarker for recurrent ovarian cancer, CA125. MUC16 is overexpressed in the majority (80%) of human EOCs but not in the epithelium of normal ovaries, and also plays a role in endometrial, fallopian tube, pancreatic, colon, peritoneal, nasopharyngeal, lung, breast and stomach cancers (Bast et al., 1981; Kabawat et al., 1983; Macdonald et al., 1988; Rosen et al., 2005).
DMUC5754A contains the humanized IgG1 anti-MUC16 monoclonal antibody and MMAE linked through a protease-labile linker, maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl (Chen et al., 2007). A phase I dose-escalation trial of DMUC5754A in 66 ovarian cancer and 11 pancreatic cancer patients found that fatigue, peripheral neuropathy, nausea, decreased appetite, vomiting, diarrhea, alopecia, pyrexia, anemia, neutropenia, alopecia, decreased appetite and hypomagnesemia were the most common AEs across all drug dose levels. Drug-related SAEs included small intestine obstruction, hypocalcemia, neutropenia, dehydration, diarrhea, nausea, and posterior reversible encephalopathy syndrome (PRES). The only case of PRES was reversed following drug cessation. No ocular toxicity signals were reported. The ORR was 11%, including one CR and six PRs, and there were six additional SDs lasting >6 months. Notably, the objective responses were only observed in MUC16-high patients (Liu et al., 2016).
DMUC4064A contains a humanized IgG1 anti-MUC16 monoclonal antibody (MMUC3333A) and two MMAE connected by a protease-cleavable linker. Using the new technology, “THIOMAB™ drug conjugates” (TDC) for site-directed conjugation, DMUC4064A was shown to have a more homogenous payload than other ADCs (Ohri et al., 2018). A phase I dose-escalation trial of DMUC4064A in patients with platinum-resistant ovarian cancer found that fatigue, nausea, abdominal pain, constipation, blurred vision, diarrhea, and anemia were the most common AEs. Ocular AE related to DMUC4064A, including blurred vision, dry eye, keratitis, cataract, corneal epithelial microcysts, eye pain, and photophobia, occurred in 40% of patients. A total of 27 SAEs occurred in 25% of patients including one death due to septic shock. The trial was associated with one CR and 20 PRs. An ORR of 46% was observed in the 54 patients with a high MUC16 IHC score of 2 + or 3+ and, an overall median PFS of 3.9 months. There were also 19 SDs (35%) and 12 PDs (22%). The best responses were SD (n = 4, 57%) and PD (n = 3, 43%) in seven patients with MUC16 IHC scores of 0 or 1+ (Liu et al., 2021). However, clinical development of DMUC4064A has been discontinued for non-safety-related reasons.
NaPi2b is a multitransmembrane protein involved in transcellular inorganic phosphate absorption and the maintenance of phosphate homeostasis (Xu et al., 1999). NaPi2b is also associated with cell differentiation and tumorigenesis and is broadly expressed in human lung, ovarian, and thyroid cancers (Andersson et al., 2009; Finstad et al., 1997; Lin et al., 2015; Rangel et al., 2003; Jarząb et al., 2005; Suzuki et al., 2011; Xiao, 2005). Differential expression between tumors and normal tissues, cell surface localization and endocytosis make NaPi2b a potential target for ADC design.
XMT-1536 (upifitamab rilsodotin) is a first-in-class Dolaflexin ADC, employing the Dolaflexin platform to provide ten DolaLock auristatin payload molecules per anti-NaPi2b antibody (Yurkovetskiy et al., 2021). In a phase I study (NCT03319628), fatigue, nausea, vomiting, pyrexia, decreased appetite, diarrhea, and a transient increase in AST were the most common treatment-related AEs. The ORR was 39%, including two CRs, and the DCR was 81% in 26 ovarian cancer patients with high NaPi2b expression. The two CR patients had previously received bevacizumab and PARPi treatment. More than 60% of patients had high expression of NaPi2b (Hamilton et al., 2020; Richardson et al., 2020; Concin et al., 2021; Richardson et al., 2021).
Lifastuzumab Vedotin is an ADC that includes a humanized IgG1 anti-NaPi2b monoclonal antibody (MNIB2126A) and a MMAE that are connected by a protease-labile linker, maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl. A phase Ia study (NCT01363947) of lifastuzumab vedotin in NSCLC or platinum-resistant ovarian cancer patients assessed its safety and preliminary antitumor activity. Most (89%) patients experienced AEs related to the study drug, the most common of which were fatigue (52%), nausea (38%), decreased appetite (33%), peripheral sensory neuropathy (29%), and vomiting (24%). A total of 16% of patients who received the 2.4 mg/kg dose stopped treatment due to AEs and one dose-limiting toxicity (DLT) occurred at 1.8 mg/kg. This study established the RP2D at 2.4 mg/kg, identical to the RP2D of most Genentech MMAE-ADCs with similar drug-antibody ratios. Peripheral neuropathy associated with MMAE was reported in 41% of patients and 54 patients (63%) experienced grade 1–3 pulmonary toxicity, a common risk attributed to ADCs. At active doses of ≥1.8 mg/kg, partial responses were observed in 11 of 24 (46%) patients with PROC, all of which were NaPi2b-high, with DoR ranging from 43 to 561 days (median, 342.0) (Gerber et al., 2020). In a randomized phase II study (NCT01991210), lifastuzumab vedotin (DNIB0600A) was compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. While a higher ORR was observed in patients treated with Lifastuzumab than liposomal doxorubicin (34% versus 15%, respectively), the study did not reach its end point because the median PFS (5.3 versus 3.1 months, respectively) was not statistically significant (Banerjee et al., 2018). As a result, Lifastuzumab Vedotin has not been studied further.
Tissue factor (TF), also known as thromboplastine, is the main physiological initiator of the extrinsic coagulation pathway (Bogdanov et al., 2003; Yu and Rak, 2004). In adults, TF is constitutively expressed in cells of the subendothelial vessel wall, including smooth muscle cells, pericytes, and fibroblasts (Förster et al., 2006; Pan et al., 2019). However, most cancer patients show hypercoagulability, and TF is involved in cancer cell proliferation, survival, angiogenesis, and the epithelial-to-mesenchymal that promotes tumor development (Magnus et al., 2014; Alley et al., 2019). TF is more highly expressed in the malignant tissue of the ovaries, cervix uteri, and uterus than in healthy tissue and is also expressed in solid cancers of the pancreas, lung, prostate, bladder, breast, and colon (Cocco et al., 2010; Cocco et al., 2011; Zhao et al., 2018).
Tisotumab vedotin (TV) is an ADC that was granted accelerated approval in the US for the treatment of recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. Tisotumab vedotin is comprised of an anti-TF-monoclonal antibody IgG1 and MMAE that are combined with a protease-cleavable linker (Alley et al., 2019). The first-in-human, multicenter, phase I–II (InnovaTV 201, NCT02001623) trial of tisotumab vedotin in patients with advanced or metastatic solid tumors that included relapsed, advanced, or metastatic cancers of the ovary, cervix, endometrium, bladder, prostate, or oesophagus, and squamous cell carcinoma of the head and neck or NSCLC. A total of 39 (27%) of 147 patients experienced a treatment-emergent serious AE associated with the study drug and one patient with pneumonia was considered possibly treatment-related. AEs of interest included bleeding, neuropathy, and ocular events (conjunctivitis, ulceration, keratitis, and symblepharon), which are characteristic of ADCs. Grade 1 epistaxis was most common, occurring in 98% of patients. Many (63/147; 43%) patients developed neuropathy, 51 (81%) of whom had received prior taxane chemotherapy. A total of 88 (60%) patients had an ocular event and 63 patients experienced conjunctivitis, some of which was relieved using ocular mitigation strategies. The ORR was 26.5% (9/34) for cervical cancer, 7.1% (1/14) for endometrial cancer, and 13.9% (5/36) for ovarian cancer (de Bono et al., 2019).
InnovaTV 204 (NCT03438396), a phase II study of tisotumab vedotin in patients with previously treated recurrent or metastatic cervical cancer revealed serious treatment-related AEs in 13 (13%) patients, including one death due to septic shock. The stable disease rate was 49% and the ORR was 24%, including seven (7%) CRs and 17 (17%) PRs. The DOR was 8.3 months, the median PFS was 4.2 months and the median OS was 12.1 months, with a 6-month PFS rate of 30% and a 6-month overall survival rate of 79% (Coleman et al., 2021). A randomized, phase III open-label study of this drug for cervical cancer patients and clinical studies of its use against other solid tumors are ongoing.
Protein tyrosine kinase 7 (PTK7) is a highly conserved transmembrane PTK involved in Wnt signaling during haematopoietic and somatic progenitor cell development (Puppo et al., 2011; Peradziryi et al., 2012; Hayes et al., 2013; Hashmi et al., 2016; Huang et al., 2020). PTK7 is overexpressed in multiple tumor types, including advanced NSCLC, ovarian cancer, triple-negative breast cancer (TNBC), colon, gastric, and esophageal cancers (Shin et al., 2013; Chen et al., 2014; Gärtner et al., 2014; Haiyan et al., 2014; Lhoumeau et al., 2015; Jiang et al., 2019). PTK7 has no catalytic activity so cannot be used as an inhibitor but could serve as a target for ADCs (Damelin et al., 2017).
PF-06647020 (Cofetuzumab Pelidotin) is an ADC comprised of a humanized anti-PTK7 mAb joined to the microtubule targeting drug, auristatin-0101 (Aur0101), by a cleavable valine-citrulline based linker (Sachdev et al., 2018). The first phase I trial (NCT02222922) involved 63 women with platinum-resistant ovarian cancer. The most common TRAEs were nausea, alopecia, fatigue, headache, neutropenia, and vomiting, 8% of patients developed grade 3 peripheral sensory neuropathy across dose levels, and two patients experienced grade 3 abdominal pain (DLT) at the highest dose. Grade 2–3 abdominal pain of unknown etiology has also been previously reported in the trial of the auristatin-based ADC, PF-06650808 (Rosen et al., 2020). The ORR was 27% in ovarian cancer patients (n = 63), 19% in NSCLC patients (n = 31), and 21% in TNBC patients (n = 29). The clinical effect of PF-06647020 correlated with PTK7 expression in tumor tissues (Maitland et al., 2021).
Trop2, a tumor-associated calcium signal transducer of a human trophoblast cell, is overexpressed in a variety of human epithelial cancers (Basu et al., 1995). Trop2 ADCs have been primarily researched for use against breast cancer. However, overexpression has also been observed in patients with ovarian, endometrial, and cervical cancers (Bignotti et al., 2010; Varughese et al., 2011a; Varughese et al., 2011b; Raji et al., 2011). Preliminary findings suggest that Trop2 may be a promising target in gynecological oncology (Syed, 2020).
Sacituzumab govitecan was approved by the FDA in 2021 as an ADC for mTNBC patients. This drug consists of a humanized anti-Trop2 monoclonal antibody and an SN-38 combined with a cleavable linker (Cardillo et al., 2015; Goldenberg et al., 2015). In a phase I/II basket trial (NCT01631552) of Sacituzumab govitecan, 483 pts (97.6%) experienced a treatment-related AE. The most common TRAEs included nausea (62.6%), diarrhea (56.2%), fatigue (48.3%), alopecia (40.4%), and neutropenia (57.8%), while the most prevalent treatment-related SAEs included febrile neutropenia (4.0%), diarrhea (2.8%), vomiting (1.4%), neutropenia (1.4%), and nausea (1.2%). Interestingly, treatment-related grade >2 neuropathy, serious cardiotoxicity, and ocular toxicity have not been reported. In this trial, 18 patients with platinum-resistant endometrial cancer responded more favorably to Sacituzumab govitecan than single-agent chemotherapy. The ORR of the endometrial cancer cohort was 22.2%, including four PRs. There are six SDs with a median OS of 11.9 months and a PFS of 3.2 months. The ORR was zero among eight EOC and one cervical cancer patients (Bardia et al., 2021).
PF-06664178 is an ADC that combines a humanized anti-Trop2 IgG1 antibody (PF-06478924, RN926) with an AcLys-VCAur0101 (PF-06380101), a potent inhibitor of tubulin polymerization, via an enzymatic process (Strop et al., 2013; Strop et al., 2016). A phase I dose-escalation study of PF-06664178 involved 31 patients with solid tumors, including six with ovarian and one with cervical cancer. However, skin and mucosal cells were shown to have a particular sensitivity to the Aur0101 payload, causing intolerable skin rashes and inflammation of the mucous membranes along with neutropenia at a dose associated with minimal anti-tumor activity. As a result, the development of this drug has been discontinued (King et al., 2018).
HER2, a transmembrane protein with tyrosine kinase activity, is overexpressed in many tumor types, especially breast cancer, where expression is linked to poor prognosis, resistance to chemotherapy, hormone therapy, and radiotherapy, and an elevated risk of metastasis and recurrence (Bookman et al., 2003; Liu et al., 2015; Zhou et al., 2015; Connell and Doherty, 2017; Yang et al., 2021). Recent whole-exome sequencing and confirmatory IHC studies show high HER2/neu expression in ∼35% of patients with uterine serous carcinoma (USC) (Zhao et al., 2013; Rottmann et al., 2020).
Ado-trastuzumab emtansine is FDA approved for use against HER2-positive breast cancer. This agent is composed of trastuzumab and 3–4 DM1 combined by a non-cleavable thioether linker (Verma et al., 2012). A phase II clinical trial (NCT02465060), NCI-MATCH (EAY131), involved 38 patients who had tumors with an ERRB2 copy number >7. Of the 36 evaluable patients, 8/10 (80%) patients with ovarian and endometrial cancer had stable disease with a median disease duration of 4.6 months. NGS measurements associated increased levels of the CN gene with a greater antitumor effect (Jhaveri et al., 2019). In an additional multi-histology basket trial (NCT02675829) of 58 patients with advanced lung, endometrial, salivary gland, biliary tract, ovarian, bladder, colorectal and other cancers, the overall ORR was 26% (14/53), with a lung cancer ORR of 50% (3/6), an endometrial cancer ORR of 22% (4/18, 2 CR), an ovarian cancer ORR of 17% (1/6), a salivary cancer ORR of 100% (5/5, 3 CR) and a biliary tract cancer ORR of 17% (1/6). There are also two clinical trials of T-DM1 in multi-drug combination for recurrent ovarian cancer and recurrent endometrial cancer (Li et al., 2018).
BDC-1001 is an immune-stimulating antibody conjugate (ISAC) drug that targets HER2. This drug contains an anti-HER2 IgG1 (trastuzumab bioanalogue) as the targeting component and the toxin present in conventional ADCs has been replaced with a toll-like receptor (TLR) 7/8 dual agonist that is coupled by a non-cleavable linker. TLR7/8 is important in innate immunity, helping to bridge non-specific and specific immune responses. TLR7/8 agonists attached to BDC-1001 are phagocytosed into lysosomes on HER2-positive tumor cells, activating myeloid APCs and presenting tumor-associated antigens to T cells (Ackerman et al., 2019). Phase 1/2 clinical data has revealed that fatigue, infusion-related reactions, nausea, abdominal pain, fever, arthralgia, constipation, anemia, diarrhea, dyspnea, and vomiting are the most common AEs associated with BDC-1001 (NCT04278144). DLTs have not been observed, and the q3w MTD was not achieved at 20 mg/kg. Many patients (42.1%) experienced AEs greater than grade 3, and 19 (33.3%) patients experienced SAEs, two of which were considered treatment-related. Of the 40 evaluable patients, the overall ORR was only 2.5% (one PR), with a disease control rate (DCR) of 32.5%. Twelve patients had SDs, including one with endometrial cancer for 24 months, one with cervical cancer for 23 + months, and one with ovarian cancer for 6 months (Dumbrava et al., 2021; Sharma et al., 2021).
DS-8201 consists of trastuzumab and an exatecan derivative topoisomerase I inhibitor, combined by a cleavable tetrapeptide linker that is linked to the antibody via a cysteine residue. This drug was approved by the FDA in 2019 to treat HER2-positive breast cancer. The phase II clinical trial, STATICE (UMIN00002956, NCCH1615), included 34 patients with HER2-positive uterine cancer sarcoma, of whom 22 had 2 + or 3 + HER2 expression and ten had 1 + expression. Of those with 2 + or 3 + expression, the objective remission rate was 55% (12/22), ten had stable disease, none had progression, and the disease control rate was 100%. Of those with 1 + HER2 expression, the ORR was 70% (7/10), three had stable disease, none had progression, and the DCR was 100% (Hasegawa et al., 2021). Another multicenter, multicohort, phase II study (NCT04482309) is ongoing and includes seven groups of patients (∼40 patients/group) with urothelial bladder, biliary tract, cervical, endometrial, ovarian, pancreatic, or rare tumors (Meric-Bernstam et al., 2022).
Trastuzumab duocarmazine consists of Trastuzumab and duocarmazine connected by a cleavable linker (Black et al., 2016). A phase I/II trial (NCT02277717) assessed the use of Trastuzumab duocarmazine against multiple HER2-positive advanced solid tumors. Treatment-related SAEs were reported in 11% of patients during the dose-expansion phase, the most frequent of which were infusion-related reactions and dyspnea. The most common AEs were fatigue, conjunctivitis, and dry eye. Most patients (104/146; 71%) had at least one ocular AE, and one dose-limiting pneumonia death was associated with the highest treatment dose. In the non-breast cancer extension cohort, 25 of 45 evaluable patients (57%) had a reduction in target lesions. The ORR was 6% and 25% among patients with gastric cancer and uroepithelial cancer, respectively. A total of 5 of 13 patients (39%) with endometrial cancer had an ORR and a median PFS of 4–3 months (2.4–9.9) (Banerji et al., 2019). A phase II trial of SYD985 for the treatment of HER2-positive endometrial cancer (NCT04205630) and a phase I trial of this drug in combination with niraparib (NCT04235101) for the treatment of HER2-positive solid tumors are currently ongoing.
A166 is a monoclonal antibody specific for HER2 that is coupled with distortion derived from MMAF-5. A phase I–II clinical study (NCT03602079) is underway among patients with HER2-positive solid tumors, including those with cervical cancer. Preliminary findings suggest that the drug is well tolerated; however, eye toxicity, including dry eye syndrome and blurred vision, was common. SD and PR were observed in almost 60% of patients (Liu et al., 2020).
In gynecological oncology, new targets are continuously identified, prompting the design of novel treatments. After clinical trials, however, several ADCs in Table 4 have been discontinued due to a lack of efficacy at tolerable doses, issues with safety, or other reasons (Figure 2).
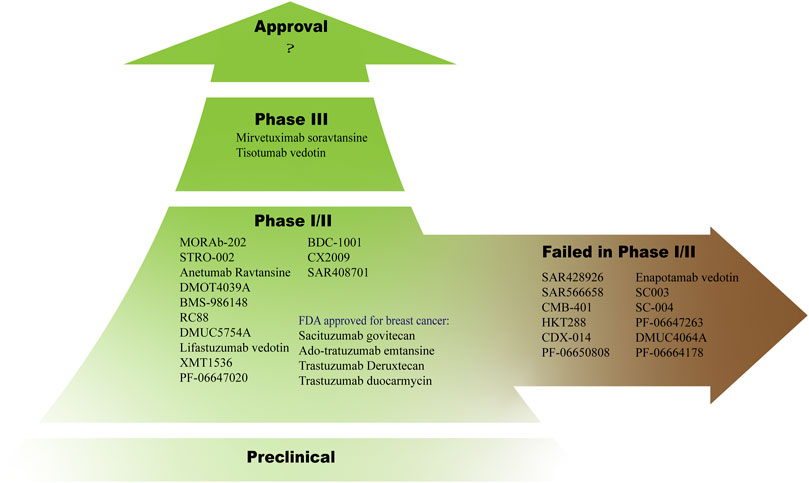
FIGURE 2. Dozens of ADCs are in clinical trials, most of them are in phase I or II and some of them have failed in this phase.
ICIs induce long-lasting responses and even cure in some patient. These drugs enhance existing anti-tumor immune responses by preventing tumors from inhibiting T cell activation and anti-tumor immunological memory. ICIs have shown some success against endometrial cancer and an ORR of 53.0%–57.1% was observed among patients with MSI-H/dMMR (Marabelle et al., 2020). PD-1 inhibitors are recommended for those with recurrent/metastatic PD-L1-positive cervical cancer. However, ICIs are not effective against ovarian cancer. T cell-specific inflammation of the tumor microenvironment may explain why melanoma and lung cancer demonstrate high response rates to ICIs while most other cancer patients respond poorly. “T cell-inflamed” (hot) tumors that already have an ongoing anti-tumor T-cell response are more responsive to ICIs while “immune excluded” (cold) tumors are less responsive (Maleki Vareki, 2018). Thus, ICI in combination with other drugs may represent a direction for the development of future tumor immunotherapies. ADCs can theoretically turn tumors from immunologically cold to hot, providing the T cell-inflamed microenvironment needed for ICI treatment (Torres and Emens, 2022). In the WSG-ADAPT trial, T-DM1 increased the number and density of tumor-infiltrating T cells in breast cancer patients (Müller et al., 2015).
One of the mechanisms by which cytotoxic compounds, such as metantrine and dorastatin, induce antitumor immunity is by directly activating and inducing the maturation of dendritic cells (DCs) and the production of proinflammatory cytokines (Martin et al., 2014; Müller et al., 2014). There is also evidence that topoisomerase I inhibitors can act as immunomodulators to activate DCs (Gavrilescu et al., 2020).
Cytotoxic compounds also induce antitumor immunity by promoting immunogenic cell death (ICD), a functionally unique response pattern that occurs after tumor cells have been treated with certain chemotherapeutic drugs, oncolytic viruses, physicochemical therapies, photodynamic therapy, and radiotherapy. The death of tumor cells promotes the exposure, active secretion, and passive release of various signaling molecules. These include damage-associated molecular patterns (DAMPs), such as calreticulin, high mobility group protein 1 (HMGB1), ATP molecules, and heat shock proteins (HSP70, HSP90). DAMPs released during ICD are recognized by pattern recognition receptors (PRRs) on DCs, causing activation, homing, and/or maturation, that results in the cross-presentation of tumor antigens to CD8+ cytotoxic T lymphocytes (CTLs) and ultimately activates both the innate and adaptive immune responses (Fucikova et al., 2020). Only a few chemotherapeutics, including anthracyclines and oxaliplatin, are ICD-inducing and are thus of particular interest for ICI combination treatments (Figure 3).
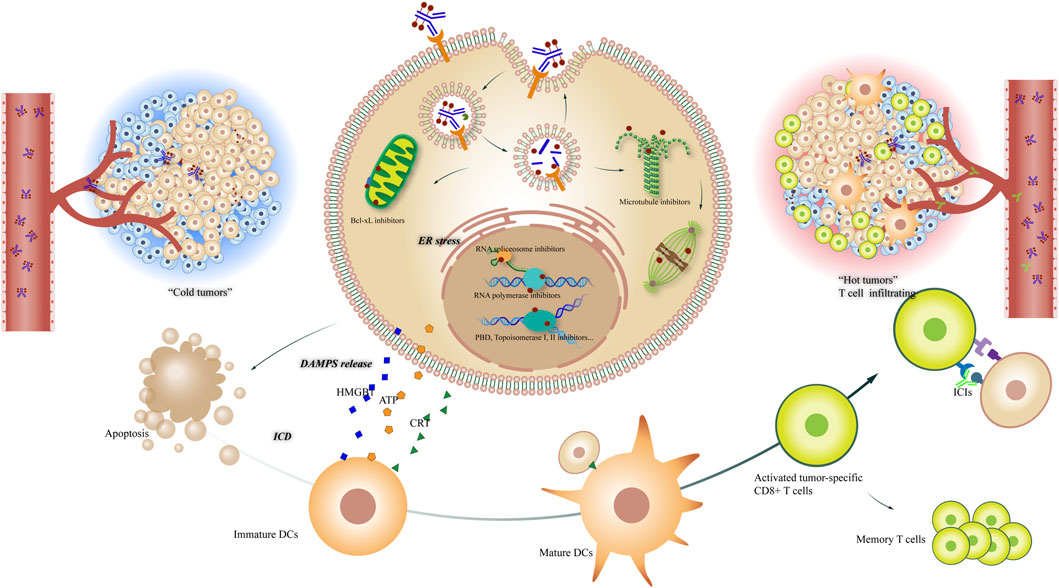
FIGURE 3. Potential mechanisms of ADCs in combination with tumor immunology therapy. Left: “cold tumor” without T cell infiltrating; right: “hot tumor” with active tumor-specific CD8+ T cells, offering a tumor microenvironment to ICIs; center: The different mechanisms of ADCs killing tumor cells, including Bcl-xL inhibiting, RNA spliceosome inhibiting, RNA polymerase inhibiting, DNA damaging and microtubule inhibiting, etc; down: The process that ICDs induce DCs maturing and T cell activating, leading to the convert of tumor microenvironment.
Some ADC payloads, such as maytansinoids and auristatins, and DNA alkylating agents such as pyrrolobenzodiazepines, can stimulate immune cells and enhance the anti-tumor efficacy of ICIs in preclinical models (Rios-Doria et al., 2017), which are shown to have a synergistic rather than an additive effect. For gynecologic cancer, mirvetuximab soravtansine induces ICD in vivo, as shown by the upregulation of ICD markers. Combined mirvetuximab soravtansine and pembrolizumab treatment have a synergistic antitumor effect that is dependent on CD8+ cells in a murine EOC model. These findings support the development of clinical trials to test the use of this drug combination in humans (Gavrilescu et al., 2020). Tumor cells treated with tisotumab vedotin are shown to release DAMPs and co-culture with allogeneic human PBMCs leads to innate immune cell activation and T cell proliferation in vitro. The combined use of tisotumab vedotin and pembrolizumab further enhances T cell proliferation and cytokine production. In vivo studies have shown that tisotumab vedotin treatment recruits F4/80+ and CD11+ innate immune cells to xenograft tumors. These data provide evidence for the immunomodulatory effects of tisotumab vedotin (Gray et al., 2020).
Table 5 summarizes ongoing clinical trials which are evaluating the combined use of ADCs and ICIs for the treatment of gynecological tumors. One study assessed 14 patients with platinum-resistant ovarian cancer who were treated with both the PD-1 inhibitor, pembrolizumab (Keytruda), and mirvetuximab soravtansine (NCT02606305). The safety profile was consistent with the known profiles of each agent. The confirmed ORR was 43% (6/14 patients) and the overall median PFS of the trial was 5.2 months, with a median DOR of 30.1 weeks. The group with high FRα expression had a PFS of 8.6 months with a median DOR of 36.1 weeks. In contrast, the ORR of pembrolizumab monotherapy in PD-L1-positive ovarian cancer was only 11.5%, with a median PFS of 1.9 months (Matulonis et al., 2018). Combined use of tisotumab vedotin and pembrolizumab was tested among 33 patients with cervical cancer. The observed safety was generally consistent with a single agent. The confirmed ORR among 32 evaluable patients was 41%, with three (9%) CRs and ten (31%) PRs. The median time to response was 1.4 months and the median PFS was 5.3 months (Lorusso et al., 2022). A study assessing the combined use of trastuzumab emtansine and atezolizumab among patients with recurrent or persistent endometrial cancer is ongoing.
Restrictive off-target toxicity is the main safety issue currently faced by ADCs, with fatigue, nausea, diarrhea, anorexia, vomiting, and peripheral sensory neuropathy being the primary AEs. Notably, ocular toxicity and pulmonary damage appear to be ADC-specific. In resistance, ADCs have resistance mechanisms that are like those of their individual components. Endocytosis, lysosomal function, and medication efflux pumps are also linked to resistance. The development of novel cytotoxic agents and the innovation of linkers and conjugation technology will help to break through the competition of ADCs.
Identifying additional agents that can generate “immune-synergy” has been a major focus since the initial successes of ICIs. Some ADCs payloads can induce ICDs. However, lymphopenia and neutropenia, known adverse effects of microtubule-targeting agents, are a concern of combined ADC and ICI treatment, which can influence anti-tumor immunity. Clinical trials are required to show whether combination therapies can benefit patient populations that do not respond well to monotherapy.
A-JW is the main writer of the review, who completes the collection and analysis of relevant literature and the writing of the first draft of the paper; YG and Y-YS participate in the analysis and arrangement of the literature; M-YD and H-BC are the designer and person in charge of the project, and guide the writing of the paper. All authors read and agree to the final text.
The work in the lab was supported by the National Natural Science Foundation of China (81972447, 81272866), National Natural Science Foundation of China Youth Project (82002770). The Key Research and Development Program of Hubei Province (No. 2022BCA004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ab, O., Whiteman, K. R., Bartle, L. M., Sun, X., Singh, R., Tavares, D., et al. (2015). IMGN853, a folate receptor-α (FRα)-Targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors. Mol. Cancer Ther. 14 (7), 1605–1613. doi:10.1158/1535-7163.MCT-14-1095
Abdollahpour-Alitappeh, M., Lotfinia, M., Gharibi, T., Mardaneh, J., Farhadihosseinabadi, B., Larki, P., et al. (2019). Antibody–drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J. Cell. Physiology 234 (5), 5628–5642. doi:10.1002/jcp.27419
Abrahams, C., Krimm, S., Li, X., Zhou, S., Hanson, J., Masikat, M. R., et al. (2019). Abstract NT-090: Preclinical activity and safety of stro-002, A novel adc targeting folate receptor alpha for ovarian and endometrial cancer. Clin. Cancer Res. 25, NT-090. NT-090-NT-090. doi:10.1158/1557-3265.ovcasymp18-nt-090
Ackerman, S. E., Gonzalez, J. C., Gregorio, J. D., Paik, J. C., Hartmann, F. J., Kenkel, J. A., et al. (2019). Abstract 1559: TLR7/8 immune-stimulating antibody conjugates elicit robust myeloid activation leading to enhanced effector function and anti-tumor immunity in pre-clinical models. Cancer Res. 79, 1559. doi:10.1158/1538-7445.am2019-1559
Aithal, A., Rauth, S., Kshirsagar, P., Shah, A., Lakshmanan, I., Junker, W. M., et al. (2018). MUC16 as a novel target for cancer therapy. Expert Opin. Ther. Targets 22 (8), 675–686. doi:10.1080/14728222.2018.1498845
Alley, S. C., Harris, J. R., Cao, A., Heuvel, E. G. v. d., Velayudhan, J., Satijn, D., et al. (2019). Abstract 221: Tisotumab vedotin induces anti-tumor activity through MMAE-mediated, Fc-mediated, and Fab-mediated effector functions in vitro. Cancer Res. 79, 221. doi:10.1158/1538-7445.am2019-221
Andersson, H., Cederkrantz, E., Back, T., Divgi, C., Elgqvist, J., Himmelman, J., et al. (2009). Intraperitoneal α-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F (ab′) 2—a phase I study. J. Nucl. Med. 50 (7), 1153–1160. doi:10.2967/jnumed.109.062604
Argani, P., IaCobuzio-Donahue, C., Ryu, B., Rosty, C., GogginsM., , Wilentz, R. E., et al. (2001). Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin. Cancer Res. 7 (12), 3862–3868.
Banerjee, S., Oza, A. M., Birrer, M. J., Hamilton, E. P., Hasan, J., LeAry, A., et al. (2018). Anti-NaPi2b antibody–drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann. Oncol. 29, 917–923. doi:10.1093/annonc/mdy023
Banerji, U., van Herpen, C. M. L., Saura, C., Thistlethwaite, F., Lord, S., Moreno, V., et al. (2019). Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20 (8), 1124–1135. doi:10.1016/S1470-2045(19)30328-6
Bardia, A., Messersmith, W. A., Kio, E. A., Berlin, J. D., Vahdat, L., Masters, G. A., et al. (2021). Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 32 (6), 746–756. doi:10.1016/j.annonc.2021.03.005
Bast, R. C., FeeneyM., , Lazarus, H., Nadler, L. M., Colvin, R. B., and Knapp, R. C. (1981). Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investigation 68 (5), 1331–1337. doi:10.1172/jci110380
Basu, A., Goldenberg, D. M., and Stein, R. (1995). The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7–3G11, is phosphorylated on serine 303. Int. J. Cancer 62 (4), 472–479. doi:10.1002/ijc.2910620419
Beck, A., Goetsch, L., Dumontet, C., and Corvaia, N. (2017). Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 16 (5), 315–337. doi:10.1038/nrd.2016.268
Bignotti, E., Todeschini, P., Calza, S., Falchetti, M., Ravanini, M., Tassi, R. A., et al. (2010). Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur. J. Cancer 46 (5), 944–953. doi:10.1016/j.ejca.2009.12.019
Birrer, M. J., Betella, I., Martin, L. P., and Moore, K. N. (2019). Is targeting the folate receptor in ovarian cancer coming of age? Oncol. 24 (4), 425–429. doi:10.1634/theoncologist.2018-0459
Black, J., Menderes, G., Bellone, S., Schwab, C. L., Bonazzoli, E., Ferrari, F., et al. (2016). SYD985, a novel duocarmycin-based HER2-targeting antibody–drug conjugate, shows antitumor activity in uterine serous carcinoma with HER2/neu expression. Mol. Cancer Ther. 15 (8), 1900–1909. doi:10.1158/1535-7163.MCT-16-0163
Bogdanov, V. Y., Balasubramanian, V., Hathcock, J., Vele, O., Lieb, M., and Nemerson, Y. (2003). Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat. Med. 9 (4), 458–462. doi:10.1038/nm841
Bookman, M. A., Darcy, K. M., Clarke-Pearson, D., Boothby, R. A., and Horowitz, I. R. (2003). Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the gynecologic oncology group. J. Clin. Oncol. 21 (2), 283–290. doi:10.1200/JCO.2003.10.104
Burton, J. K., Bottino, D., and Secomb, T. W. (2019). A systems Pharmacology model for drug delivery to solid tumors by antibody-drug conjugates: Implications for bystander effects. AAPS J. 22 (1), 12. doi:10.1208/s12248-019-0390-2
Cardillo, T. M., Govindan, S. V., Sharkey, R. M., Trisal, P., Arrojo, R., Liu, D., et al. (2015). Sacituzumab govitecan (IMMU-132), an anti-trop-2/SN-38 antibody–drug conjugate: Characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjugate Chem. 26 (5), 919–931. doi:10.1021/acs.bioconjchem.5b00223
Chang, K., and Pastan, I. (1996). Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc. Natl. Acad. Sci. 93 (1), 136–140. doi:10.1073/pnas.93.1.136
Chari, R. V. J., Miller, M. L., and Widdison, W. C. (2014). Antibody–drug conjugates: An emerging concept in cancer therapy. Angew. Chem. Int. Ed. 53 (15), 3796–3827. doi:10.1002/anie.201307628
Chen, Y., Clark, S., Wong, T., Chen, Y., Dennis, M. S., Ross, S., et al. (2007). Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res. 67 (10), 4924–4932. doi:10.1158/0008-5472.CAN-06-4512
Chen, R., Khatri, P., Mazur, P. K., Polin, M., Zheng, Y., Vaka, D., et al. (2014). A meta-analysis of lung cancer gene expression identifies PTK7 as a survival gene in lung adenocarcinoma. Cancer Res. 74 (10), 2892–2902. doi:10.1158/0008-5472.CAN-13-2775
Chen, H., Lin, Z., Arnst, K. E., Miller, D. D., and Li, W. (2017). Tubulin inhibitor-based antibody-drug conjugates for cancer therapy. Molecules 22 (8), 1281. doi:10.3390/molecules22081281
Cheng, X., Li, J., Tanaka, K., Majumder, U., Milinichik, A. Z., Verdi, A. C., et al. (2018). MORAb-202, an antibody-drug conjugate utilizing humanized anti-human FRα farletuzumab and the microtubule-targeting agent eribulin, has potent antitumor activity. Mol. Cancer Ther. 17 (12), 2665–2675. doi:10.1158/1535-7163.MCT-17-1215
Clarke, J., Chu, S. C., Siu, L. L., Machiels, J. P., Markman, B., Heinhuis, K., et al. (2019). Abstract B057: BMS-986148, an anti-mesothelin antibody-drug conjugate (ADC), alone or in combination with nivolumab demonstrates clinical activity in patients with select advanced solid tumors. Mol. Cancer Ther. 18, B057. doi:10.1158/1535-7163.targ-19-b057
Cocco, E., Hu, Z., Richter, C. E., Bellone, S., CasagrandeF., , BelloneM., , et al. (2010). hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor for immunotherapy of uterine serous papillary carcinoma. Br. J. Cancer 103 (6), 812–819. doi:10.1038/sj.bjc.6605760
Cocco, E., Varughese, J., Buza, N., Bellone, S., Glasgow, M., Bellone, M., et al. (2011). Expression of tissue factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix: Implications for immunotherapy with hI-con1, a factor VII-IgGFcchimeric protein targeting tissue factor. BMC Cancer 11 (1), 263. doi:10.1186/1471-2407-11-263
Coleman, R. L., Lorusso, D., Gennigens, C., Gonzalez-Martin, A., Randall, L., Cibula, D., et al. (2021). Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 22 (5), 609–619. doi:10.1016/S1470-2045(21)00056-5
Concin, N., Burger, R., Mosher, R., Hamilton, E., Randall, L., Monk, B., et al. (2021). 184 UPLIFT (ENGOT-ov67/GOG-3048) a pivotal cohort of upifitamab rilsodotin, a NaPi2b-directed ADC in platinum-resistant ovarian cancer. Int. J. Gynecol. Cancer 31, A205. doi:10.1136/ijgc-2021-ESGO.351
Connell, C. M., and Doherty, G. J. (2017). Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2 (5), e000279. doi:10.1136/esmoopen-2017-000279
Damelin, M., Bankovich, A., Bernstein, J., Lucas, J., Chen, L., Williams, S., et al. (2017). A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci. Transl. Med. 9 (372), eaag2611. doi:10.1126/scitranslmed.aag2611
de Bono, J. S., Concin, N., Hong, D. S., Thistlethwaite, F. C., Machiels, J. P., Arkenau, H. T., et al. (2019). Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 20, 383–393. doi:10.1016/S1470-2045(18)30859-3
Drakes, M. L., Czerlanis, C. M., and Stiff, P. J. (2020). Immune checkpoint blockade in gynecologic cancers: State of affairs. Cancers 12 (11), 3301. doi:10.3390/cancers12113301
Dumbrava, E. I., Sharma, M. R., Carvajal, R. D., Catenacci, D., Emens, L. A., Gadgeel, S. M., et al. (2021). Abstract OT-03-02: Phase 1/2 study of a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), BDC-1001, as a single agent and in combination with an immune checkpoint inhibitor in patients with advanced HER2-expressing solid tumors. Cancer Res. 81. doi:10.1158/1538-7445.sabcs20-ot-03-02
Eaton, J. S., Miller, P. E., Mannis, M. J., and Murphy, C. J. (2015). Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J. Ocul. Pharmacol. Ther. 31 (10), 589–604. doi:10.1089/jop.2015.0064
Elnakat, H., and Ratnam, M. (2004). Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv. drug Deliv. Rev. 56 (8), 1067–1084. doi:10.1016/j.addr.2004.01.001
Elnakat, H., and Ratnam, M. (2006). Role of folate receptor genes in reproduction and related cancers. Front. Biosci. 11, 506–519. doi:10.2741/1815
Finstad, C. L., Lloyd, K. O., Federici, M. G., Divgi, C., Venkatraman, E., Barakat, R. R., et al. (1997). Distribution of radiolabeled monoclonal antibody MX35 F (ab') 2 in tissue samples by storage phosphor screen image analysis: evaluation of antibody localization to micrometastatic disease in epithelial ovarian cancer. Clin. cancer Res. 3 (8), 1433–1442.
Förster, Y., Meye, A., Albrecht, S., and Schwenzer, B. (2006). Tissue factor and tumor: Clinical and laboratory aspects. Clin. Chim. Acta 364 (1), 12–21. doi:10.1016/j.cca.2005.05.018
Fucikova, J., Kepp, O., Kasikova, L., Petroni, G., Yamazaki, T., Liu, P., et al. (2020). Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 11 (11), 1013. doi:10.1038/s41419-020-03221-2
Gärtner, S., Gunesch, A., Knyazeva, T., Wolf, P., Hogel, B., Eiermann, W., et al. (2014). PTK 7 is a transforming gene and prognostic marker for breast cancer and nodal metastasis involvement. PloS one 9 (1), e84472. doi:10.1371/journal.pone.0084472
Gavrilescu, L. C., Setiady, Y., Dong, L., Chicklas, S., Harris, L., Pinkas, J., et al. (2020). Abstract B21: Synergistic antitumor immunity observed with combination FRα-targeting antibody-drug conjugate plus anti-PD-1 therapy is CD8+ cell dependent. Cancer Immunol. Res. 8, B21. doi:10.1158/2326-6074.tumimm18-b21
Gerber, D. E., Infante, J. R., Gordon, M. S., Goldberg, S. B., Martin, M., Felip, E., et al. (2020). Phase Ia study of anti-NaPi2b antibody–drug conjugate lifastuzumab vedotin DNIB0600A in patients with non–small cell lung cancer and platinum-resistant ovarian cancer. Clin. Cancer Res. 26 (2), 364–372. doi:10.1158/1078-0432.CCR-18-3965
Goldenberg, D. M., Cardillo, T. M., Govindan, S. V., Rossi, E. A., and Sharkey, R. M. (2015). Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 6 (26), 22496–22512. doi:10.18632/oncotarget.4318
Gray, E., Gow, J., Brady, L., Bieda, M., Smith, K., Hensley, K., et al. (2020). 617 Tisotumab vedotin shows immunomodulatory activity through induction of immunogenic cell death. J. Immunother. Cancer 8, A371. doi:10.1136/jitc-2020-SITC2020.0617
Haiyan, W., Yin, Y., Guo, Q., Wei, W., Wen, J., Peng, L., et al. (2014). Expression and clinical significance of PTK7 in ovarian serous tumors. China Oncol. 24 (7), 481–486. doi:10.3969/j.issn.1007-3969.2014.07.001
Hamilton, E. P., Barve, M., Tolcher, A., Buscema, J., Papadopoulos, K., Zarwan, C., et al. (2020). 836P safety and efficacy of XMT-1536 in ovarian cancer: A subgroup analysis from the phase I expansion study of XMT-1536, a NaPi2b antibody-drug conjugate. Ann. Oncol. 31, S627–S628. doi:10.1016/j.annonc.2020.08.975
Hasegawa, K., Nishikawa, T., Hirakawa, A., Mori, M., Kato, T., Hamada, A., et al. (2021). 813P - efficacy and safety of trastuzumab deruxtecan in HER2-expressing uterine carcinosarcoma (STATICE trial, NCCH1615): A multicenter, phase II clinical trial. J. Oncol. 32, S737. doi:10.1016/j.annonc.2021.08.1255
Hashmi, F., Liu, M., Shen, S., and Qiao, L. Y. (2016). EXPRESS: Phospholipase C gamma mediates endogenous brain-derived neurotrophic factor - regulated calcitonin gene-related peptide expression in colitis - induced visceral pain. Mol. Pain 12, 1744806916657088. doi:10.1177/1744806916657088
Hassan, R., Bera, T., and Pastan, I. (2004). Mesothelin: a new target for immunotherapy. Clin. Cancer Res. 10, 3937–3942. doi:10.1158/1078-0432.CCR-03-0801
Hassan, R., Kreitman, R. J., Pastan, I., and Willingham, M. C. (2005). Localization of mesothelin in epithelial ovarian cancer. Appl. Immunohistochem. Mol. Morphol. 13 (3), 243–247. doi:10.1097/01.pai.00000141545.36485.d6
Hassan, R., Blumenschein, G. R., Moore, K. N., Santin, A. D., Kindler, H. L., Nemunaitis, J. J., et al. (2020). First-in-Human, multicenter, phase I dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J. Clin. Oncol. 38 (16), 1824–1835. doi:10.1200/JCO.19.02085
Hayes, M., Naito, M., Daulat, A., Angers, S., and Ciruna, B. (2013). Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/β-catenin-dependent cell fate decisions during vertebrate development. Development 140 (8), 1807–1818. doi:10.1242/dev.090183
Huang, W. J., Ruan, S., Wen, F., Lu, X. N., Gu, S. P., Chen, X. X., et al. (2020). Multidrug resistance of gastric cancer: The mechanisms and Chinese medicine reversal agents. Cancer Manag. Res. 12, 12385–12394. doi:10.2147/CMAR.S274599
Iizuka, T., Sawabe, M., Takubo, K., Liu, M., Homma, Y., Suzuki, M., et al. (2013). hTERT promoter polymorphism, -1327C>T, is associated with the risk of epithelial cancer. SpringerPlus 2 (1), 249. doi:10.1186/2193-1801-2-249
Jain, N., Smith, S. W., Ghone, S., and Tomczuk, B. (2015). Current ADC linker chemistry. Pharm. Res. 32 (11), 3526–3540. doi:10.1007/s11095-015-1657-7
Jarząb, B., Wiench, M., Fujarewicz, K., Simek, K., Jarzab, M., Oczko-Wojciechowska, M., et al. (2005). Gene expression profile of papillary thyroid cancer: Sources of variability and diagnostic implications. Cancer Res. 65 (4), 1587–1597. doi:10.1158/0008-5472.CAN-04-3078
Jhaveri, K. L., Wang, X. V., Makker, V., Luoh, S. W., Mitchell, E. P., Zwiebel, J. A., et al. (2019). Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann. Oncol. 30 (11), 1821–1830. doi:10.1093/annonc/mdz291
Jiang, W., He, J., Lv, B., Xi, X., He, G., and He, J. (2019). PTK7 expression is associated with lymph node metastasis, ALK and EGFR mutations in lung adenocarcinomas. Histology Histopathol. 35 (5), 489–495. doi:10.14670/HH-18-183
Jiang, J., Li, S., Tang, N., Wang, L., Xin, W., and Li, S. (2021). Preclinical safety profile of RC88-ADC:a novel mesothelin-targeted antibody conjugated with Monomethyl auristatin E. Drug Chem. Toxicol., 1–11. doi:10.1080/01480545.2021.2005085
Jirsova, K., Neuwirth, A., Kalasova, S., Vesela, V., and Merjava, S. (2010). Mesothelial proteins are expressed in the human cornea. Exp. Eye Res. 91 (5), 623–629. doi:10.1016/j.exer.2010.08.002
Kabawat, S. E., Bast, R. C., Welch, W. R., Knapp, R. C., and Colvin, R. B. (1983). Immunopathologic characterization of a monoclonal antibody that recognizes common surface antigens of human ovarian tumors of serous, endometrioid, and clear cell types. Am. J. Clin. Pathology 79 (1), 98–104. doi:10.1093/ajcp/79.1.98
Kelemen, L. E. (2006). The role of folate receptor α in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 119 (2), 243–250. doi:10.1002/ijc.21712
King, G. T., Eaton, K. D., Beagle, B. R., Zopf, C. J., Wong, G. Y., Krupka, H. I., et al. (2018). A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody-drug conjugate in patients with advanced or metastatic solid tumors. Invest New Drugs 36 (5), 836–847. doi:10.1007/s10637-018-0560-6
Kitamura, T., SuzukiM., , NisHimatsu, H., Kurosaki, T., Enomoto, Y., FukuHara, H., et al. (2010). Final report on low-dose estramustine phosphate (EMP) monotherapy and very low-dose EMP therapy combined with LH-RH agonist for previously untreated advanced prostate cancer. Aktuelle Urol. 41 (1), S34–S40. doi:10.1055/s-0029-1224657
Kurnit, K. C., Reid, P., Moroney, J. W., and Fleming, G. F. (2020). Immune checkpoint inhibitors in women with gynecologic cancers: Practical considerations. Gynecol. Oncol. 158 (3), 531–537. doi:10.1016/j.ygyno.2020.06.499
Lhoumeau, A.-C., Martinez, S., Boher, J. M., Monges, G., Castellano, R., Goubard, A., et al. (2015). Overexpression of the promigratory and prometastatic PTK7 receptor is associated with an adverse clinical outcome in colorectal cancer. PloS one 10 (5), e0123768. doi:10.1371/journal.pone.0123768
Li, B. T., Makker, V., Shen, R., Kris, M. G., Berger, M. F., Ulaner, G., et al. (2018). A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. Am. Soc. Clin. Oncol. 36, 2502. doi:10.1200/JCO.2018.36.15_suppl.2502
Lin, K., Rubinfeld, B., Zhang, C., Firestein, R., Harstad, E., Roth, L., et al. (2015). Preclinical development of an anti-NaPi2b (SLC34A2) antibody–drug conjugate as a therapeutic for non–small cell lung and ovarian cancers. Clin. Cancer Res. 21 (22), 5139–5150. doi:10.1158/1078-0432.CCR-14-3383
Liu, M., Shen, S., Kendig, D. M., Mahavadi, S., Murthy, K. S., Grider, J. R., et al. (2015). Inhibition of NMDAR reduces bladder hypertrophy and improves bladder function in cyclophosphamide induced cystitis. J. Urology 193 (5), 1676–1683. doi:10.1016/j.juro.2014.12.092
Liu, J. F., Moore, K. N., Birrer, M. J., Berlin, S., Matulonis, U. A., Infante, J. R., et al. (2016). Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody–drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann. Oncol. 27 (11), 2124–2130. doi:10.1093/annonc/mdw401
Liu, Y., Lian, W., Zhao, X., Qi, W., Xu, J., Xiao, L., et al. (2020). A first in-human study of A166 in patients with locally advanced/metastatic solid tumors which are HER2-positive or HER2-amplified who did not respond or stopped responding to approved therapies. J. Clin. Oncol. 38, 1049. doi:10.1200/jco.2020.38.15_suppl.1049
Liu, J., Burris, H., Wang, J. S., Barroilhet, L., Gutierrez, M., Wang, Y., et al. (2021). An open-label phase I dose-escalation study of the safety and pharmacokinetics of DMUC4064A in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 163 (3), 473–480. doi:10.1016/j.ygyno.2021.09.023
Lorusso, D., Vergote, I., O'Cearbhaill, R. E., Westermann, A. M., Banerjee, S. N., Van Nieuwenhuysen, E., et al. (2022). Tisotumab vedotin (TV) + pembrolizumab (pembro) in first-line (1L) recurrent or metastatic cervical cancer (r/mCC): Interim results of ENGOT Cx8/GOG 3024/innovaTV 205. J. Clin. Oncol. 40, 5507. doi:10.1200/jco.2022.40.16_suppl.5507
Lu, J., Jiang, F., Lu, A., and Zhang, G. (2016). Linkers having a crucial role in antibody-drug conjugates. Int. J. Mol. Sci. 17 (4), 561. doi:10.3390/ijms17040561
Macdonald, F., Downing, R., and Allum, W. H. (1988). Expression of CA125 in pancreatic carcinoma and chronic pancreatitis. Br. J. cancer 58 (4), 505–506. doi:10.1038/bjc.1988.251
Magnus, N., Garnier, D., Meehan, B., McGraw, S., Lee, T. H., Caron, M., et al. (2014). Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc. Natl. Acad. Sci. U. S. A. 111 (9), 3544–3549. doi:10.1073/pnas.1314118111
Maitland, M. L., Sachdev, J. C., Sharma, M. R., Moreno, V., Boni, V., Kummar, S., et al. (2021). First-in-Human study of PF-06647020 (cofetuzumab Pelidotin), an antibody–drug conjugate targeting protein tyrosine kinase 7, in advanced solid tumors. Clin. Cancer Res. 27 (16), 4511–4520. doi:10.1158/1078-0432.CCR-20-3757
Maleki Vareki, S. (2018). High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 6 (1), 157. doi:10.1186/s40425-018-0479-7
Marabelle, A., Le, D. T., Ascierto, P. A., Di Giacomo, A. M., De Jesus-Acosta, A., Delord, J. P., et al. (2020). Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38 (1), 1–10. doi:10.1200/JCO.19.02105
Martin, K., Muller, P., Schreiner, J., Prince, S. S., Lardinois, D., Heinzelmann-Schwarz, V. A., et al. (2014). The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol. Immunother. 63 (9), 925–938. doi:10.1007/s00262-014-1565-4
Martin, L. P., Konner, J. A., Moore, K. N., Seward, S. M., Matulonis, U. A., Perez, R. P., et al. (2017). Characterization of folate receptor alpha (FRα) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: A phase I expansion study of the FRα-targeting antibody-drug conjugate mirvetuximab soravtansine. Gynecol. Oncol. 147 (2), 402–407. doi:10.1016/j.ygyno.2017.08.015
Matulonis, U. A., Moore, K., Martin, L., Vergote, I., Castro, C., Gilbert, L., et al. (2018). Mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), with pembrolizumab in platinum-resistant ovarian cancer (PROC): Initial results of an expansion cohort from FORWARD II, a phase Ib study. Ann. Oncol. 29, viii339. doi:10.1093/annonc/mdy285.157
Meric-Bernstam, F., Anoka, C., Chaudry, A., Puvvada, S., Rowbottom, J., Gustavson, M., et al. (2022). A phase 2, multicenter, open-label study evaluating trastuzumab deruxtecan (T-DXd) for the treatment of select human epidermal growth factor receptor 2 (HER2)-expressing solid tumors (DESTINY-PanTumor02). J. Clin. Oncol. 32, S1253. doi:10.1016/j.annonc.2021.08.756
Moore, K. N., Borghaei, H., O'Malley, D. M., Jeong, W., Seward, S. M., Bauer, T. M., et al. (2017). Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer 123 (16), 3080–3087. doi:10.1002/cncr.30736
Moore, K. N., Martin, L. P., O'Malley, D. M., Matulonis, U. A., Konner, J. A., Perez, R. P., et al. (2017). Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A phase I expansion study. J. Clin. Oncol. 35 (10), 1112–1118. doi:10.1200/JCO.2016.69.9538
Moore, K. N., Oza, A. M., Colombo, N., Oaknin, A., Scambia, G., Lorusso, D., et al. (2021). Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann. Oncol. 32 (6), 757–765. doi:10.1016/j.annonc.2021.02.017
Müller, P., Martin, K., Theurich, S., Schreiner, J., Savic, S., Terszowski, G., et al. (2014). Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol. Res. 2 (8), 741–755. doi:10.1158/2326-6066.CIR-13-0198
Müller, P., Kreuzaler, M., Khan, T., Thommen, D. S., Martin, K., Glatz, K., et al. (2015). Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 7 (315), 315ra188. doi:10.1126/scitranslmed.aac4925
Naumann, R. W., Braiteh, F. S., Martin, L. P., Hamilton, E. P., Diaz, J. P., Diab, S., et al. (2021). Phase 1 dose-escalation study of STRO-002, an antifolate receptor alpha (FRα) antibody drug conjugate (ADC), in patients with advanced, progressive platinum-resistant/refractory epithelial ovarian cancer (EOC). J. Clin. Oncol. 39, 5550. doi:10.1200/jco.2021.39.15_suppl.5550
Ohri, R., Bhakta, S., Fourie-O'Donohue, A., Dela Cruz-Chuh, J., Tsai, S. P., Cook, R., et al. (2018). High-throughput cysteine scanning to identify stable antibody conjugation sites for maleimide- and disulfide-based linkers. Bioconjugate Chem. 29 (2), 473–485. doi:10.1021/acs.bioconjchem.7b00791
O'Shannessy, D. J., Yu, G., Smale, R., Fu, Y. S., Singhal, S., Thiel, R. P., et al. (2012). Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget 3 (4), 414–425. doi:10.18632/oncotarget.489
Pahl, A., Lutz, C., and Hechler, T. (2018). Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov. Today Technol. 30, 85–89. doi:10.1016/j.ddtec.2018.08.005
Pan, L., Yu, Y., Yu, M., Yao, S., Mu, Q., Luo, G., et al. (2019). Expression of flTF and asTF splice variants in various cell strains and tissues. Mol. Med. Rep. 19 (3), 2077–2086. doi:10.3892/mmr.2019.9843
Pastan, I., and Hassan, R. (2014). Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 74 (11), 2907–2912. doi:10.1158/0008-5472.CAN-14-0337
Peradziryi, H., Tolwinski, N. S., and Borchers, A. (2012). The many roles of PTK7: a versatile regulator of cell–cell communication. Archives Biochem. biophysics 524 (1), 71–76. doi:10.1016/j.abb.2011.12.019
Puppo, F., Thome, V., Lhoumeau, A. C., Cibois, M., Gangar, A., Lembo, F., et al. (2011). Protein tyrosine kinase 7 has a conserved role in Wnt/β-catenin canonical signalling. EMBO Rep. 12 (1), 43–49. doi:10.1038/embor.2010.185
Qiao, Z., Xia, C., Shen, S., Corwin, F. D., Liu, M., Guan, R., et al. (2014). Suppression of the PI3K pathway in vivo reduces cystitis-induced bladder hypertrophy and restores bladder capacity examined by magnetic resonance imaging. PloS one 9 (12), e114536. doi:10.1371/journal.pone.0114536
Qiao, L. Y., Shen, S., LiuM.,, , Xia, C., Kay, J. C., and Zhang, Q. L. (2016). Inflammation and activity augment brain-derived neurotrophic factor peripheral release. Neuroscience 318, 114–121. doi:10.1016/j.neuroscience.2016.01.018
Raji, R., Guzzo, F., Carrara, L., Varughese, J., Cocco, E., Bellone, S., et al. (2011). Uterine and ovarian carcinosarcomas overexpressing Trop-2 are sensitive to hRS7, a humanized anti-Trop-2 antibody. J. Exp. Clin. Cancer Res. 30 (1), 106. doi:10.1186/1756-9966-30-106
Rangel, L. B. A., Sherman-Baust, C. A., Wernyj, R. P., Schwartz, D. R., Cho, K. R., and Morin, P. J. (2003). Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene 22 (46), 7225–7232. doi:10.1038/sj.onc.1207008
Richardson, D. L., Hamilton, E., Tolcher, A., Burns, T., Edenfield, W., Papadopoulos, K., et al. (2020). A phase 1 study of XMT-1536 in patients with solid tumors likely to express NaPi2b: A summary of dose escalation. Gynecol. Oncol. 159, 52. doi:10.1016/j.ygyno.2020.06.109
Richardson, D. L., Hamilton, E. P., Oaknin, A., Randall, L. M., Banerjee, S. N., Taylor, S. K., et al. (2021). Uplift (ENGOT-ov67): A pivotal cohort to evaluate XMT-1536 (upifitamab rilsodotin), a NaPi2b-directed antibody drug conjugate for platinum-resistant ovarian cancer. J. Clin. Oncol. 39, TPS5607. doi:10.1200/jco.2021.39.15_suppl.tps5607
Rios-Doria, J., Harper, J., Rothstein, R., Wetzel, L., Chesebrough, J., Marrero, A., et al. (2017). Antibody-drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 77 (10), 2686–2698. doi:10.1158/0008-5472.CAN-16-2854
Ritchie, M., Tchistiakova, L., and Scott, N. (2013). Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs 5 (1), 13–21. doi:10.4161/mabs.22854
Rosen, D. G., Wang, L., Atkinson, J. N., Yu, Y., Lu, K. H., Diamandis, E. P., et al. (2005). Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol. Oncol. 99 (2), 267–277. doi:10.1016/j.ygyno.2005.06.040
Rosen, L. S., Wesolowski, R., Baffa, R., Liao, K. H., Hua, S. Y., Gibson, B. L., et al. (2020). A phase I, dose-escalation study of PF-06650808, an anti-Notch3 antibody–drug conjugate, in patients with breast cancer and other advanced solid tumors. Investig. New Drugs 38 (1), 120–130. doi:10.1007/s10637-019-00754-y
Ross, J. F., Chaudhuri, P. K., and Ratnam, M. (1994). Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 73 (9), 2432–2443. doi:10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s
Rottey, S., Clarke, J., Aung, K., Machiels, J. P., Markman, B., Heinhuis, K. M., et al. (2022). Phase I/IIa trial of BMS-986148, an anti-mesothelin antibody–drug conjugate, alone or in combination with nivolumab in patients with advanced solid tumors. Clin. Cancer Res. 28 (1), 95–105. doi:10.1158/1078-0432.CCR-21-1181
Rottmann, D., Snir, O. L., Wu, X., Wong, S., Hui, P., Santin, A. D., et al. (2020). HER2 testing of gynecologic carcinosarcomas: tumor stratification for potential targeted therapy. Mod. Pathol. 33 (1), 118–127. doi:10.1038/s41379-019-0358-x
Sachdev, J. C., Sharma, M., Moreno, V., Boni, V., Calvo, E., Powell, E. C., et al. (2018). PF-06647020 (PF-7020), an antibody-drug conjugate (ADC) targeting protein tyrosine kinase 7 (PTK7), in patients (pts) with advanced solid tumors: Results of a phase I dose escalation and expansion study. Am. Soc. Clin. Oncol. 36, 5565. doi:10.1200/JCO.2018.36.15_suppl.5565
Salazar, M. D., and Ratnam, M. (2007). The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 26 (1), 141–152. doi:10.1007/s10555-007-9048-0
Santin, A., Martin, A., Moore, K., Walter, A., Romero, I., Diab, S., et al. (2020). 372 Safety and activity of the anti-mesothelin antibody–drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer. Int. J. Gynecol. Cancer 30 (3), A154. doi:10.1136/ijgc-2020-IGCS.322
Scales, S. J., Gupta, N., Pacheco, G., Firestein, R., French, D. M., Koeppen, H., et al. (2014). An antimesothelin-monomethyl auristatin E conjugate with potent antitumor activity in ovarian, pancreatic, and mesothelioma models. Mol. Cancer Ther. 13 (11), 2630–2640. doi:10.1158/1535-7163.MCT-14-0487-T
Scaranti, M., Cojocaru, E., Banerjee, S., and Banerji, U. (2020). Exploiting the folate receptor alpha in oncology. Nat. Rev. Clin. Oncol. 17 (6), 349–359. doi:10.1038/s41571-020-0339-5
Sharma, M., Carvajal, R. D., Hanna, G. J., Li, B. T., Moore, K. N., Pegram, M. D., et al. (2021). Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J. Clin. Oncol. 39, 2549. doi:10.1200/jco.2021.39.15_suppl.2549
Shimizu, T., Fujiwara, Y., Yonemori, K., Koyama, T., Sato, J., Tamura, K., et al. (2021). First-in-Human phase 1 study of MORAb-202, an antibody-drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-α-positive advanced solid tumors. Clin. Cancer Res. 27 (14), 3905–3915. doi:10.1158/1078-0432.CCR-20-4740
Shin, W.-S., Kwon, J., Lee, H. W., Kang, M. C., Na, H. W., Lee, S. T., et al. (2013). Oncogenic role of protein tyrosine kinase 7 in esophageal squamous cell carcinoma. Cancer Sci. 104 (8), 1120–1126. doi:10.1111/cas.12194
Stewart, D., and Cristea, M. (2019). Antibody-drug conjugates for ovarian cancer: current clinical development. Curr. Opin. Obstet. Gynecol. 31 (1), 18–23. doi:10.1097/GCO.0000000000000515
Strop, P., Liu, S. H., Dorywalska, M., Delaria, K., Dushin, R. G., Tran, T. T., et al. (2013). Location matters: Site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 20 (2), 161–167. doi:10.1016/j.chembiol.2013.01.010
Strop, P., Tran, T. T., Dorywalska, M., Delaria, K., Dushin, R., Wong, O. K., et al. (2016). RN927C, a site-specific trop-2 antibody–drug conjugate (ADC) with enhanced stability, is highly efficacious in preclinical solid tumor models. Mol. Cancer Ther. 15 (11), 2698–2708. doi:10.1158/1535-7163.MCT-16-0431
Suzuki, M., Liu, M., Kurosaki, T., Arai, T., Sawabe, M., Homma, Y., et al. (2011). Association of rs6983561 polymorphism at 8q24 with prostate cancer mortality in a Japanese population. Clin. Genitourin. Cancer 9 (1), 46–52. doi:10.1016/j.clgc.2011.04.004
Syed, Y. Y. (2020). Sacituzumab govitecan: first approval. Drugs 80 (10), 1019–1025. doi:10.1007/s40265-020-01337-5
Tarcsa, E., Guffroy, M. R., Falahatpisheh, H., Phipps, C., and Kalvass, J. C. (2020). Antibody-drug conjugates as targeted therapies: Are we there yet? A critical review of the current clinical landscape. Drug Discov. Today Technol. 37, 13–22. doi:10.1016/j.ddtec.2020.07.002
Tchou, J., Wang, L. C., Selven, B., Zhang, H., Conejo-Garcia, J., Borghaei, H., et al. (2012). Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res. Treat. 133 (2), 799–804. doi:10.1007/s10549-012-2018-4
Teicher, B. A., and Chari, R. V. (2011). Antibody conjugate therapeutics: challenges and potential. Clin. Cancer Res. 17 (20), 6389–6397. doi:10.1158/1078-0432.CCR-11-1417
Thériault, C., Pinard, M., Comamala, M., Migneault, M., Beaudin, J., Matte, I., et al. (2011). MUC16 (CA125) regulates epithelial ovarian cancer cell growth, tumorigenesis and metastasis. Gynecol. Oncol. 121 (3), 434–443. doi:10.1016/j.ygyno.2011.02.020
Torres, E. T. R., and Emens, L. A. (2022). Emerging combination immunotherapy strategies for breast cancer: dual immune checkpoint modulation, antibody–drug conjugates and bispecific antibodies. Breast Cancer Res. Treat. 191 (2), 291–302. doi:10.1007/s10549-021-06423-0
van der Velden, V. H. J., te Marvelde, J. G., Hoogeveen, P. G., Bernstein, I. D., Houtsmuller, A. B., Berger, M. S., et al. (2001). Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 97 (10), 3197–3204. doi:10.1182/blood.v97.10.3197
Varughese, J., Cocco, E., Bellone, S., Ratner, E., Silasi, D. A., Azodi, M., et al. (2011). Cervical carcinomas overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Am. J. Obstetrics Gynecol. 205 (6), 567. doi:10.1016/j.ajog.2011.06.093
Varughese, J., Cocco, E., Bellone, S., Bellone, M., Todeschini, P., Carrara, L., et al. (2011). High-grade, chemotherapy-resistant primary ovarian carcinoma cell lines overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody. Gynecol. Oncol. 122 (1), 171–177. doi:10.1016/j.ygyno.2011.03.002
Vergote, I. B., Marth, C., and Coleman, R. L. (2015). Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 34 (1), 41–52. doi:10.1007/s10555-014-9539-8
Vergote, I., Armstrong, D., Scambia, G., Teneriello, M., Sehouli, J., Schweizer, C., et al. (2016). A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J. Clin. Oncol. 34 (19), 2271–2278. doi:10.1200/JCO.2015.63.2596
Verma, S., Miles, D., Gianni, L., Krop, I. E., Welslau, M., Baselga, J., et al. (2012). Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367 (19), 1783–1791. doi:10.1056/NEJMoa1209124
Weekes, C. D., Lamberts, L. E., Borad, M. J., Voortman, J., McWilliams, R. R., Diamond, J. R., et al. (2016). Phase I study of DMOT4039A, an antibody-drug conjugate targeting mesothelin, in patients with unresectable pancreatic or platinum-resistant ovarian cancer. Mol. Cancer Ther. 15 (3), 439–447. doi:10.1158/1535-7163.MCT-15-0693
Weidemann, S., Perez, D., Izbicki, J. R., Neipp, M., Mofid, H., Daniels, T., et al. (2021). Mesothelin is commonly expressed in pancreatic adenocarcinoma but unrelated to cancer aggressiveness. Cancer Invest 39 (9), 711–720. doi:10.1080/07357907.2021.1943747
Xiao, S. (2005). Spantide inhibits up - regulation of NOS in the pericentral canal region of the spinal cord in the rat formalin test. Chin. J. Pathophysiol.
Xu, H., Bai, L., Collins, J. F., and Ghishan, F. K. (1999). Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium–phosphate (Na+–Pi) transporter (SLC34A2). Genomics 62 (2), 281–284. doi:10.1006/geno.1999.6009
Yang, J., Gao, C., Liu, M., Liu, Y. C., Kwon, J., Qi, J., et al. (2021). Targeting an inducible SALL4-mediated cancer vulnerability with sequential therapy. Cancer Res. 81 (23), 6018–6028. doi:10.1158/0008-5472.CAN-21-0030
Yin, B. W. T., and Lloyd, K. O. (2001). Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16 *. J. Biol. Chem. 276 (29), 27371–27375. doi:10.1074/jbc.M103554200
Yu, J. L., and Rak, J. W. (2004). Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J. Thrombosis Haemostasis 2 (11), 2065–2067. doi:10.1111/j.1538-7836.2004.00972.x
Yurkovetskiy, A. V., Bodyak, N. D., Yin, M., Thomas, J. D., Clardy, S. M., Conlon, P. R., et al. (2021). Dolaflexin: A novel antibody–drug conjugate platform featuring high drug loading and a controlled bystander effect. Mol. Cancer Ther. 20 (5), 885–895. doi:10.1158/1535-7163.MCT-20-0166
Zhao, S., Choi, M., Overton, J. D., Bellone, S., Roque, D. M., Cocco, E., et al. (2013). Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. U. S. A. 110 (8), 2916–2921. doi:10.1073/pnas.1222577110
Zhao, X., Cheng, C., Gou, J., Yi, T., Qian, Y., Du, X., et al. (2018). Expression of tissue factor in human cervical carcinoma tissue. Exp. Ther. Med. 16 (5), 4075–4081. doi:10.3892/etm.2018.6723
Keywords: gynecologic oncology, immune checkpoint inhibitors (ICI), antibody-drug conjugates (ADC)s, mirvetuximab soravtansine (IMGN853), tisotumab vedotin
Citation: Wang A-J, Gao Y, Shi Y-Y, Dai M-Y and Cai H-B (2022) A review of recent advances on single use of antibody-drug conjugates or combination with tumor immunology therapy for gynecologic cancer. Front. Pharmacol. 13:1093666. doi: 10.3389/fphar.2022.1093666
Received: 09 November 2022; Accepted: 12 December 2022;
Published: 22 December 2022.
Edited by:
Jun-Ning Zhao, Sichuan Academy of Chinese Medicine Sciences, ChinaReviewed by:
Adam Yongxin Ye, Boston Children’s Hospital and Harvard Medical School, United StatesCopyright © 2022 Wang, Gao, Shi, Dai and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng-Yuan Dai, bWVuZ3l1YW5kYWlAd2h1LmVkdS5jbg==; Hong-Bing Cai, Y2hiMjEwNTFAMTYzLmNvbQ==
†Present address: Hong-Bing Cai, Zhongnan Hospital of Wuhan University, Wuhan, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.