- 1The First Clinical Medical College, Henan University of Chinese Medicine, Zhengzhou, China
- 2Department of Spleen, Stomach, Hepatobiliary Diseases, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 3Zhengzhou Key Laboratory of Traditional Chinese Medicine for Prevention and Treatment of Hepatobiliary Diseases, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
Background: The incidence of Non-alcoholic fatty liver disease (NAFLD) is increasing year by year. Researches showed that Chinese patent medicines (CPMs) had achieved good efficacy in the treatment of Non-alcoholic fatty liver disease. However, the debate on optimum Chinese patent medicine (CPM) persists. Therefore, we conducted a network meta-analysis to objectively compare the efficacy of different Chinese patent medicines in the treatment of Non-alcoholic fatty liver disease.
Methods: PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wanfang Database, China Science and Technology Journal Database, and Chinese Biomedical Literature Database were used as databases for RCT researches retrieval. The retrieval time was from establishment of the database to July 2022. After effective data was extracted, Review Manager 5.4 and Cochrane Collaboration System Evaluator’s Manual were used to assess bias risk. STATA 16.0 based on frequency theory was used for the network meta-analysis.
Results: Totally 39 studies were included, involving 13 Chinese patent medicines, including 4049 patients, of which 42 patients were lost. In terms of improving clinical efficiency rate, Zhibitai capsule was most likely the best choice of Chinese patent medicine for Non-alcoholic fatty liver disease. Liuwei Wuling tablet had the best effect in reducing serum ALT and AST; Gandan Shukang capsule had the best effect in reducing serum GGT; Qianggan capsule had the best effect in reducing serum TG; Dangfei Liganning capsule had the best effect in reducing serum TC. None of the included studies had serious adverse reactions.
Conclusion: For patients with Non-alcoholic fatty liver disease in this NMA, Zhibitai capsule, Liuwei Wuling tablet, Gandan Shukang capsule, Qianggan capsule, Dangfei Liganning capsule might be noteworthy. Due to the uclear risk bias, better designed double-blind, multi center and large sample RCTs are needed which resolve the problems of blinding, selective reporting and allocation concealment.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022341240.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is complex chronic liver disease linked to being overweight, obesity, insulin resistance. In the past 40 years, NAFLD has become the most common chronic liver disease, with a global incidence rate of 25% and an estimated 3.6 million new cases every year (Shiha et al., 2021). NAFLD is the fastest growing cause of liver related mortality in the world. It has become an important pathogeny of end-stage liver disease, primary liver cancer and liver transplantation. A 51 years cohort study in Sweden reported that even mild fatty liver disease would increase the risk of death by 71%, and the risk was proportional to the severity of fatty liver disease (Sheka et al., 2020).
At present, NAFLD patients are mainly treated with lifestyle interventions, such as diet and exercise (Kamada et al., 2021). However, it is extremely difficult for many NAFLD patients to adhere to these therapies for a long time. In recent years, with the deepening of scholars’ understanding of the pathogenesis of NAFLD, important progress has been made in the research and development of drugs to treat NAFLD (Younossi et al., 2018). Western medicines for NAFLD can be divided into two categories (Fan et al., 2019). The first type is drugs for metabolic syndrome. Such as orlistat, liraglutide, metformin, statins, etc. However, anti-obesity drugs are easy to cause gastrointestinal adverse reactions (Anirban et al., 2018). Metformin, pioglitazone and other drugs can reduce blood sugar and improve insulin resistance, but there was no evidence that hypoglycemic drugs could improve Non-alcoholic steatohepatitis (NASH). Statins can reduce serum low density lipoprotein (LDL) cholesterol levels, but there was no evidence that they could improve NASH and liver fibrosis. The second type is drugs to improve liver injury. Such as vitamin E, silymarin, bicyclol alcohol, polyene phosphatidylcholine, diammonium glycyrrhizinate, reduced glutathione, S-adenosylmethionine, ursodeoxycholic acid, tiopronin, etc. The safety of long-term use of vitamin E is worrying (Traber and Head, 2021). The therapeutic effect of other liver protecting and anti-inflammatory drugs on NASH and liver fibrosis has been unclear, which requires evidence support from large-scale evidence-based medicine. To sum up, although there are many western medicines for NAFLD treatment, their safety and exact effect still need to be confirmed by standardized and large-scale clinical studies. To date, there is no drug approved for NAFLD (Cusi et al., 2022).
Chinese patent medicines (CPMs) such as Qianggan capsule and Danning tablet, which are made of Chinese medicinal materials, also showed good efficacy in the treatment of NAFLD (Liu et al., 2018; Ding et al., 2021). However, there are few studies directly comparing different CPMs in the treatment of NAFLD. It is difficult to evaluate the efficacy of various CPMs in the treatment of NAFLD. A network meta-analysis (NMA) can enhance evidence by combining direct evidence and indirect evidence to compare different interventions. In addition, it also carries out a comprehensive evaluation and ranking of interventions to identify the advantages and disadvantages of various interventions.
In this study, a network meta-analysis technique was used to systematically evaluate the efficacy of a variety of CPMs in the treatment of NAFLD, providing a basis for clinical treatment.
2 Methods
This study follows the systematic evaluation of the network meta analysis list and the preferred reporting items of meta analysis (Page et al., 2021). The protocol for the research has been submitted to the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022341240).
3 Eligibility criteria
Inclusion criteria were as follows: 1) Study type: randomized clinical trials (RCTs); 2) Study object: NAFLD patients, refer to the series of guidelines for non-alcoholic fatty liver disease formulated by Fatty Liver and Alcoholic Liver Disease Group of Liver Disease Branch of Chinese Medical Association (National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, 2001; National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, 2006; National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, 2010; National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, 2018); 3) Intervention measures: The experiment group was treated with Chinese patent medicine (CPM), while the control group was treated with western medicine, another CPM different from the experiment group, lifestyle modification or placebo. CPMs need to be included in the China Medical Information Platform (https://www.dayi.org.cn/); 4) Outcome indicators: clinical efficiency rate (defined by symptoms, signs, ultrasonic or computed tomography (CT) or other imaging assessment of fatty liver degree, liver function, blood lipids, and comprehensive improvement), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), triglyceride (TG), total cholesterol (TC); 5) Language: only studies in English and Chinese were analysed.
Exclusion criteria were as follows: 1) Patients with other liver diseases such as viral hepatitis; 2) Non-randomized controlled studies, case control studies, experimental studies, case reports, conference summaries, reviews, retrospective studies, meta-analysis; 3) Unable to get full-text; 4) Repeated published researches.
4 Search strategy
PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP), and Chinese Biomedical Literature Database (CBM) were used as databases for RCT researches retrieval. The retrieval time was from the establishment of the database to July 2022.
Key words include: Non-alcoholic Fatty Liver Disease, NAFLD, Non-alcoholic Fatty Liver Disease, Fatty Liver, Non-alcoholic, Fatty Livers, Non-alcoholic, Liver, Non-alcoholic Fatty, Livers, Non-alcoholic Fatty, Non-alcoholic Fatty Liver, Non-alcoholic Fatty Livers, Non-alcoholic Steatohepatitis, Non-alcoholic, Steatohepatitis, Non-alcoholic, NASH, non-alcoholic steatohepatitis, metabolic associated fatty liver disease, metabolic associated steatohepatitis, MAFLD, MASH, Proprietary Chinese medicine, Chinese patent medicine, capsule, tablet, pellet, pill, powder.
5 Data collection and quality assessment
The characteristics of the included literatures were extracted into Microsoft Excel 2016, including the main author, year of publication, diagnosis, sample size, age, intervention measures, duration, and outcome indicators.
Two authors respectively evaluated the methodological quality of the literatures. In case of disagreement, they would discuss or ask the third senior researcher to make a decision. The quality evaluation included in the studies were conducted according to “Bias Risk Assessment” tool in Handbook 5.1.0 of Cochrane Evaluation Manual: ① Random allocation method; ② Hide allocation scheme; ③ Blind method shall be adopted for subjects and test personnel; ④ The outcome evaluators were blinded; ⑤ Integrity of result data; ⑥ Selective reporting of research results; ⑦ Other sources of bias (such as sample size estimation, baseline comparability, study design, etc.). Make a judgment of “low risk”, “unclear” and “high risk” for the literature.
6 Data synthesis and analysis
We used Review Manager 5.4 to draw a Cochrane bias risk map. Stata16.0, JAGS and R (version x64 4.2.1) were used for NMA. We estimated summary odds ratios (ORs) for dichotomous outcomes and mean differences (MD) for continuous outcomes using pairwise and network meta analysis. The significance of an effect was expressed by 95% confidence interval (CI).
All results of the pairwise meta-analysis were described in the tables and forest plots (Cipriani et al., 2018). Network evidence plots were used to show the relationship between interventions. In the network evidence plots, the size of the dot represents the sample size of the treatment method. The larger the dot is, the more the sample size is. The thickness of the line between two points represents the number of studies. The thicker the line is, the more the number of studies is. The Surface Under the Cumulative Ranking (SUCRA) was used to reflect the probability order of different CPMs to be the best treatment option. A higher SUCRA score indicated a more effective or acceptable intervention.
Comparison adjusted funnel plots were used to assess the presence of publication bias. When there was a closed loop, we carried out the inconsistency test. In the inconsistency test, if p < 0.05, it was considered that the results of direct comparison and indirect comparison were inconsistent. Global I2 was used to measure the overall heterogeneity. If I2 > 75% (Page et al., 2021), it was considered that there was a large heterogeneity. When the degree of heterogeneity was high, we chose random effect network meta-analysis model. Otherwise, the fixed-effects model was selected. Sensitivity analysis was used to evaluate the stability of aggregate effect.
7 Results
7.1 Search results
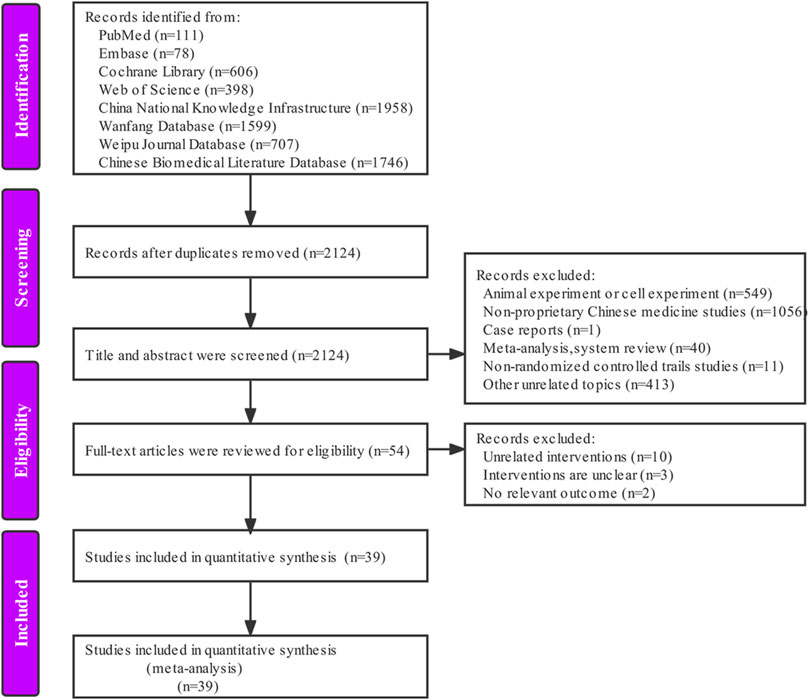
According to the pre-determined retrieval strategy, 7203 documents were retrieved, and the documents that did not meet the inclusion criteria were excluded by removing duplicates, reading the abstract and full text of the documents. Finally, we included 39 articles (Chen et al., 2006; Huang and Zhang, 2007; Ji et al., 2008; Wu et al., 2008; Yuan, 2008; Li and Jiang, 2009; Lv, 2009; Meng, 2009; Fan et al., 2010; Li et al., 2010; Xu et al., 2010; Deng et al., 2011; Jin, 2011; Liu, 2011; Zhang, 2011; Li et al., 2012; Qi et al., 2012; Lin et al., 2013; Liu and Lv, 2014; Luo and Jiang, 2014; Ma, 2014; Wang, 2014; Yang et al., 2014; Yu et al., 2014; Zhang et al., 2014; Wang et al., 2015; Wei et al., 2015; Yang et al., 2015; He et al., 2016; Ou et al., 2016; Li et al., 2017; Ning et al., 2017; Zhong and Yang, 2017; Wang et al., 2018; Wu and Peng, 2018; Xu and Tao, 2018; Peng, 2019; Zhang, 2019; Nan, 2020). The specific retrieval process is shown in Figure 1.
7.2 Characteristics of included studies
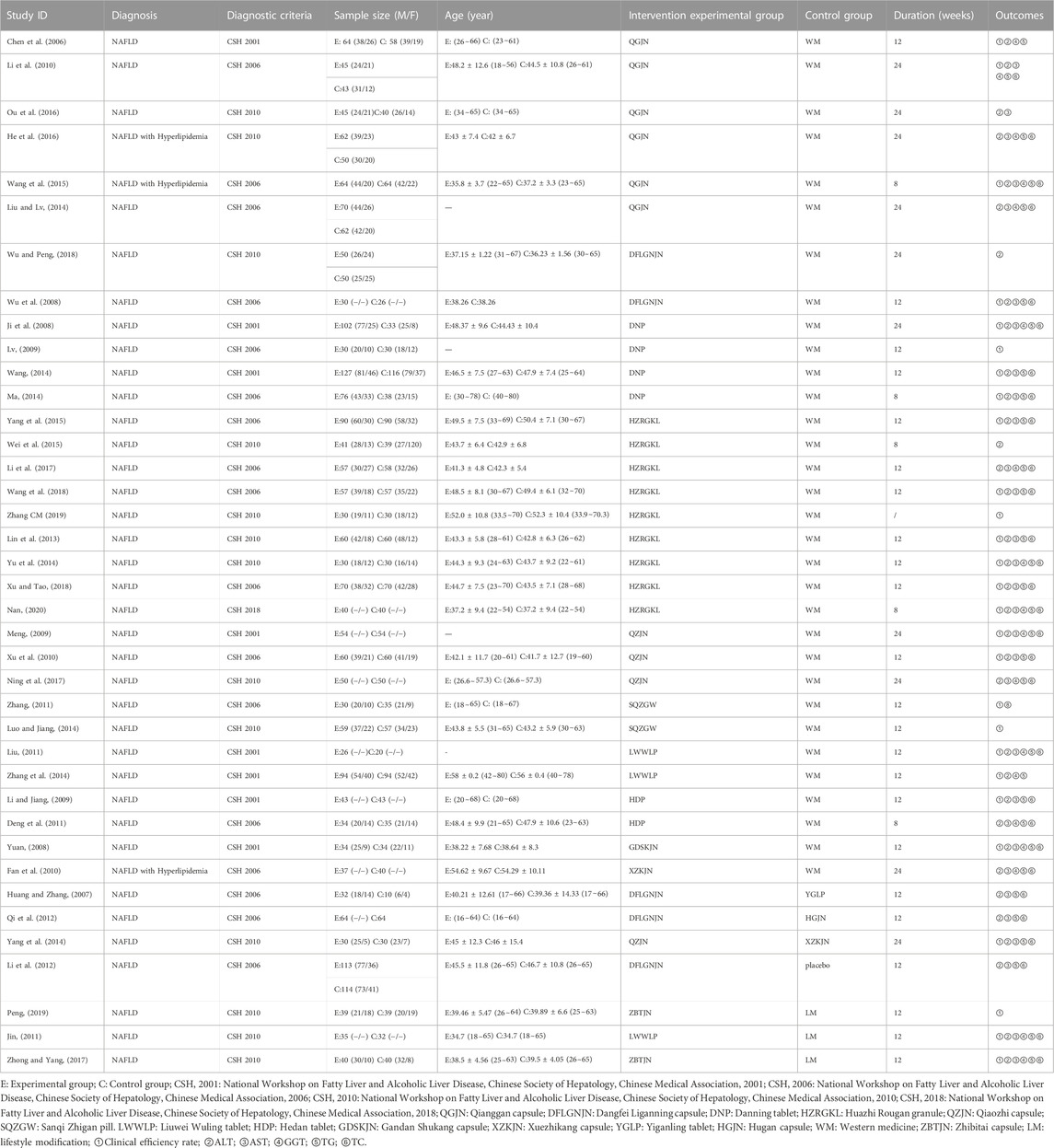
The studies included three English studies and 36 Chinese studies, involving 4049 patients, including 42 patients who were not interviewed. The articles were published from 2006 to 2020, and all of them were two arm studies. 13 CPMs were included: Qianggan capsule (6 RCTs), Dangfei Liganning capsule (5 RCTs), Danning tablet (4 RCTs), Huazhi Rougan granule (9 RCTs), Qiaozhi capsule (4 RCTs), Sanqi Zhigan pill (2 RCTs), Liuwei Wuling tablet (3 RCTs), Hedan tablet (2 RCTs), Gandan Shukang capsule (1 RCTs), Xuezhikang capsule (2 RCTs), Yiganling tablet (1 RCTs), Hugan capsule (1 RCTs), Zhibitai capsule (2RCTs). Details characterizations of 13 CPMs are show in Supplementary Table S10. Among them, 27 articles reported clinical efficiency rate (2968 cases), 34 articles reported ALT (3670 cases), 30 articles reported AST (3180 cases), 17 articles reported GGT (1695 cases), 31 articles reported TG (3405 cases), and 29 articles reported TC (3095 cases). The basic characteristics of included studies are shown in Table 1.
7.3 Risk of bias
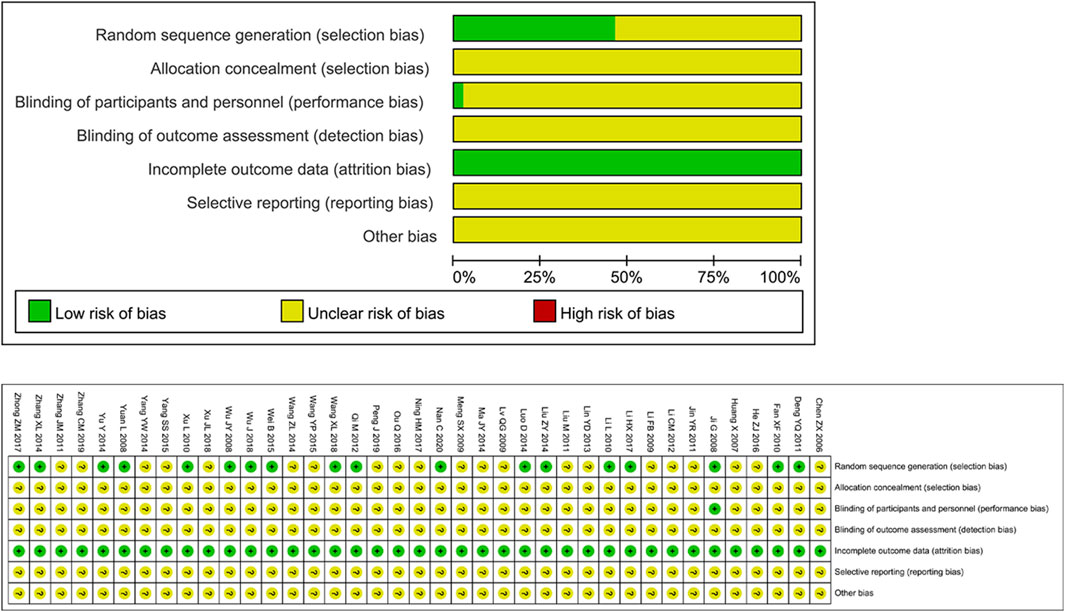
The risk assessment of 39 RCTs is shown in Figure 2. Unclear Risk bias were common due to insufficient method reporting. 19 studies used appropriate randomization generation methods, such as the method of generating random numbers or random number tables using computers. All literatures did not indicate the implementation of allocation concealment. One study (Ji et al., 2008) implemented a double-blind method. The data integrity evaluation results were “low risk”, and the selective reporting results and other biases were “unclear".
7.4 Network meta-analysis
7.4.1 Primary outcomes
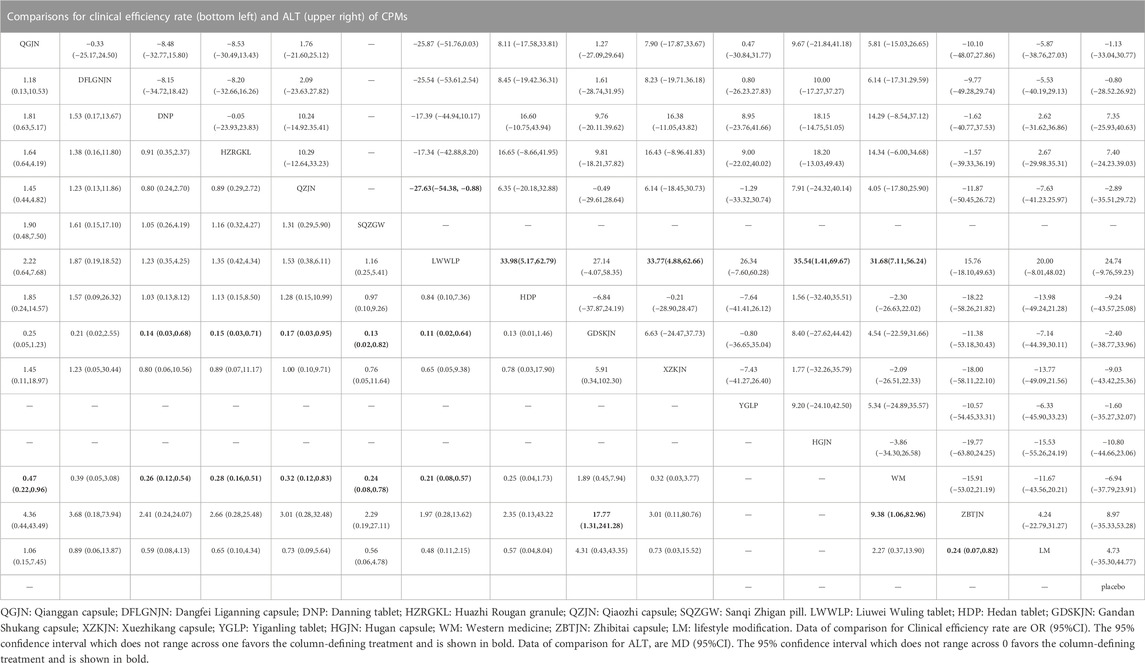
27 studies reported clinical efficiency rate, involving 11 CPMs (Qianggan capsule, Dangfei Liganning capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Sanqi Zhigan pill, Liuwei Wuling tablet, Hedan tablet, Gandan Shukang capsule, Xuezhikang capsule, Zhibitai capsule). The network relationship between the interventions is shown in Figure 3. In terms of the clinical efficiency rate, according to OR and 95%CI between all the pairwise interventions, Zhibitai capsule (OR: 0.24; 95%CI: 0.07, 0.82) was better than lifestyle modification. Qianggan capsule (OR: 0.47; 95%CI: 0.22, 0.96), Danning tablet (OR: 0.26; 95%CI: 0.12, 0.54), Huazhi Rougan granule (OR: 0.28; 95%CI: 0.16, 0.51), Qiaozhi capsule (OR: 0.32; 95%CI: 0.12, 0.83), Sanqi Zhigan pill (OR: 0.24; 95%CI: 0.08, 0.78), Liuwei Wuling tablet (OR: 0.21; 95%CI: 0.08, 0.57), Zhibitai capsule (OR: 9.38; 95%CI: 1.06, 82.96) were better than western medicine. Danning tablet (OR: 0.14; 95%CI: 0.03, 0.68), Huazhi Rougan granule (OR: 0.15; 95%CI: 0.03, 0.71), Qiaozhi capsule (OR: 0.17; 95%CI: 0.03, 0.95), Sanqi Zhigan pill (OR: 0.13; 95%CI: 0.02, 0.82), Liuwei Wuling tablet (OR: 0.11; 95%CI: 0.02, 0.64), Zhibitai capsule (OR: 17.77; 95%CI: 1.31, 241.28) were better than Gandan Shukang capsule. As shown in Table 2 and Supplementary Figure S1. Moreover, Zhibitai capsule with the highest-ranking probability of SUCRA (83.2%), had the best effectiveness in improving clinical efficiency rate, followed by Liuwei Wuling tablet (70.5%) and Sanqi Zhigan pill (63.6%). More details about the rank probability of SUCRA are shown in Figure 4.
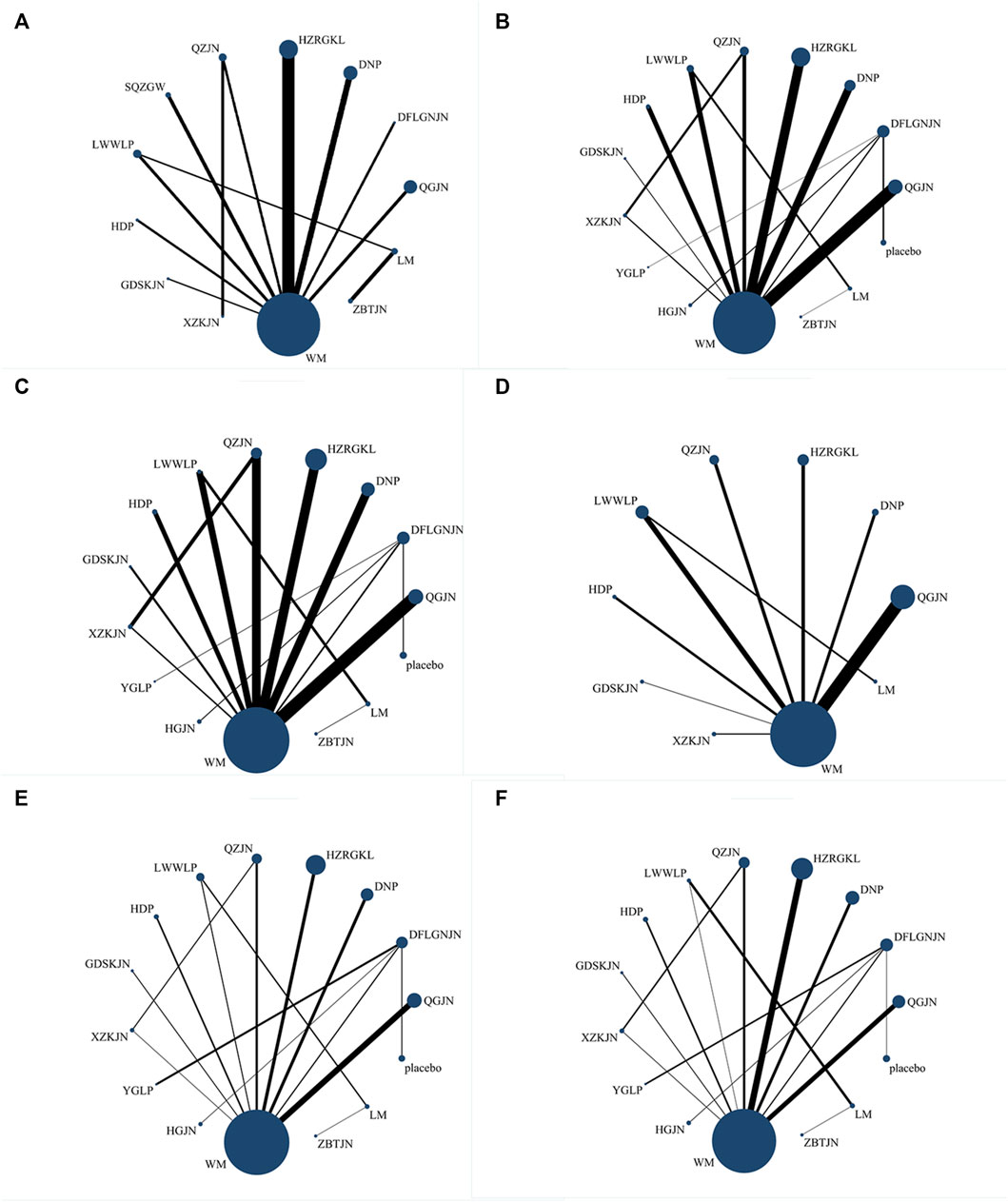
FIGURE 3. Network evidence plots (A) Clinical efficiency rate (B) ALT (C) AST (D) GGT (E) TG (F) TC; WM, Western medicine; QGJN, Qianggan capsule; DFLGNJN, Dangfei Liganning capsule; DNP, Danning tablet; HZRGKL, Huazhi Rougan granule; QZJN, Qiaozhi capsule; SQZGW, Sanqi Zhigan pill; LWWLP, Liuwei Wuling tablets; HDP, Hedan tablet; GDSKJN, Gandan Shukang capsule; XZKJN, Xuezhikang capsule; YGLP, Yiganling tablet; HGJN, Hugan capsule; ZBTJN, Zhibitai capsule; LM, lifestyle modification.

FIGURE 4. Summary of results from SUCRA (A) Clinical efficiency rate (B) ALT (C) AST (D) GGT (E) TG (F) TC; WM, Western medicine; QGJN, Qianggan capsule; DFLGNJN, Dangfei Liganning capsule; DNP, Danning tablet; HZRGKL, Huazhi Rougan granule; QZJN, Qiaozhi capsule; SQZGW, Sanqi Zhigan pill; LWWLP, Liuwei Wuling tablet; HDP, Hedan tablet; GDSKJN, Gandan Shukang capsule; XZKJN, Xuezhikang capsule; YGLP, Yiganling tablet; HGJN, Hugan capsule; ZBTJN, Zhibitai capsule; LM, lifestyle modification.
34 studies reported ALT, involving 12 CPMs (Qianggan capsule, Dangfei Liganning capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Liuwei Wuling tablet, Hedan tablet, Gandan Shukang capsule, Xuezhikang capsule, Yiganling tablet, Hugan capsule, Zhibitai capsule). The network relationship between the interventions is shown in Figure 3. In terms of ALT improvement, according to MD and 95%CI between all the pairwise interventions, Liuwei Wuling tablet (MD: 31.68; 95%CI: 7.11, 56.24) was superior to western medicine. In addition, Liuwei Wuling tablet was superior to Qiaozhi capsule (MD: −27.63; 95%CI: −54.38, −0.88), Hedan tablet (MD: 33.98; 95%CI: 5.17, 62.79), Xuezhikang capsule (MD: 33.77; 95%CI: 4.88, 62.66), Hugan capsule (MD: 35.54; 95%CI: 1.41, 69.67), as shown in Table 2 and Supplementary Figure S2. Moreover, Liuwei Wuling tablet, with the highest-ranking probability of SUCRA (98.0%), had the best effectiveness in reducing ALT, followed by Huazhi Rougan granule (75.8%) and Danning tablet (73.6%). More details about the rank probability of SUCRA are shown in Figure 4.
7.4.2 Secondary outcomes
30 studies reported AST, involving 12 CPMs (Qianggan capsule, Dangfei Liganning capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Liuwei Wuling tablet, Hedan tablet, Gandan Shukang capsule, Xuezhikang capsule, Yiganling tablet, Hugan capsule, Zhibitai capsule). The network relationship between the interventions is shown in Figure 3. According to MD and 95%CI between all the pairwise interventions, in terms of AST improvement, there was no significant difference between 12 CPMs and western medicine. In addition, there was no significant difference between 12 CPMs, as shown in Table 3 and Supplementary Figure S3. However, Liuwei Wuling tablet, with the highest-ranking probability of SUCRA (86.7%), had the best effectiveness in reducing AST, followed by Danning tablet (74.8%). More details about the rank probability of SUCRA are shown in Figure 4.
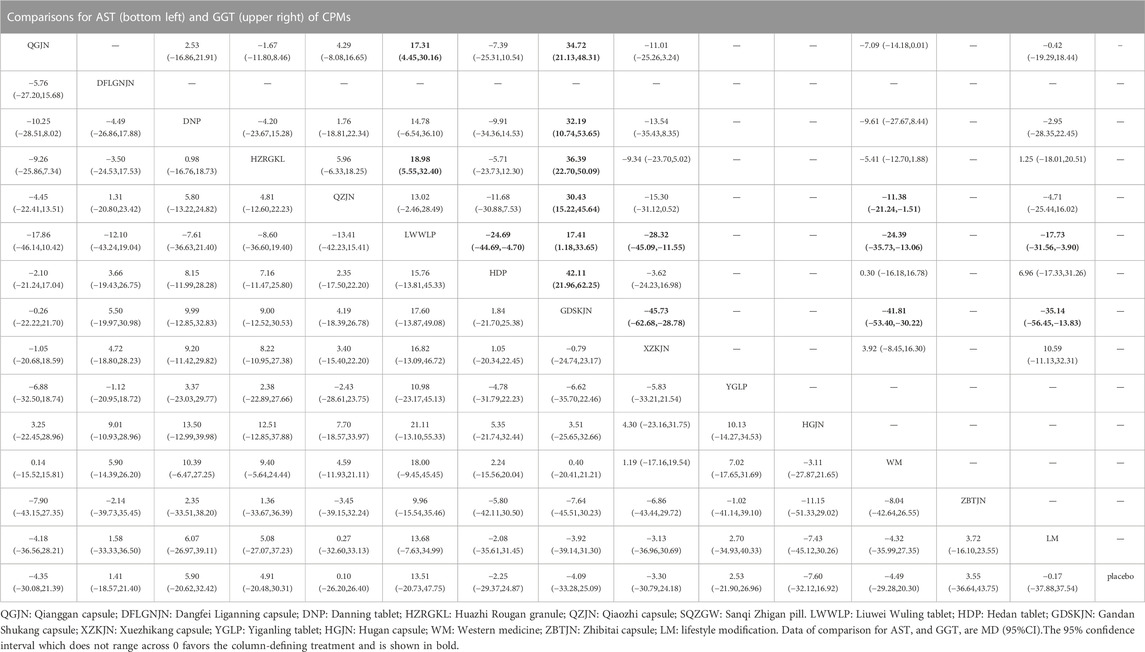
17 studies reported GGT, involving 8 CPMs (Qianggan capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Liuwei Wuling tablet, Hedan tablet, Gandan Shukang capsule, Xuezhikang capsule). The network relationship between the interventions is shown in Figure 3. In terms of GGT improvement, according to MD and 95%CI between all the pairwise interventions, Liuwei Wuling tablet (MD: −17.73; 95%CI: −31.56, −3.90) and Gandan Shukang capsule (MD: −35.14; 95%CI: −56.45, −13.83) were superior to lifestyle modification. Qiaozhi capsule (MD: −11.38; 95%CI: −21.24, −1.51), Liuwei Wuling tablet (MD: −24.39; 95%CI: −35.73, −13.06) and Gandan Shukang capsule (MD: −41.81; 95%CI: −53.40, −30.22) were superior to western medicine. In addition, Gandan Shukang capsule was superior to Qianggan capsule (MD: 34.72; 95%CI: 21.13, 48.31), Danning tablet (MD: 32.19; 95%CI: 10.74, 53.65), Huazhi Rougan granule (MD: 36.39; 95%CI: 22.70, 50.09), Qiaozhi capsule (MD: 30.43; 95%CI: 15.22, 45.64), Liuwei Wuling tablet (MD: 17.41; 95%CI: 1.18, 33.65), Hedan tablet (MD: 42.11; 95%CI: 21.96, 62.25), Xuezhikang capsule (MD: −45.73; 95%CI: −62.68, −28.78). Liuwei Wuling tablet was superior to Qianggan capsule (MD: 17.31; 95%CI: 4.45, 30.16), Huazhi Rougan granule (MD: 18.98; 95%CI: 5.55, 32.40), Hedan tablet (MD: −24.69; 95%CI: −44.69, −4.70), Xuezhikang capsule (MD: −28.32; 95%CI: −45.09, −11.55). The detailed results of pairwise comparison are shown in Table 3 and Supplementary Figure S4. Moreover, Gandan Shukang capsule, with the highest-ranking probability of SUCRA (99.8%), had the best effectiveness in reducing GGT, followed by Liuwei Wuling tablet (87.3%) and Qiaozhi capsule (63.7%). More details about the rank probability of SUCRA are shown in Figure 4.
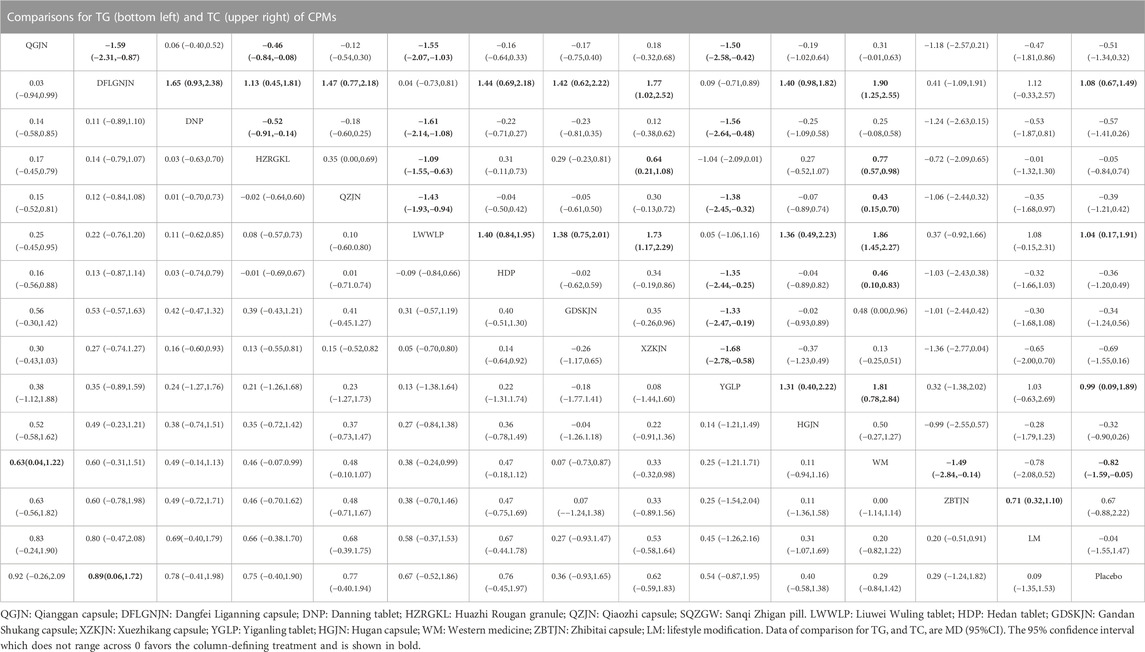
31 studies reported TG, involving 12 CPMs (Qianggan capsule, Dangfei Liganning capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Liuwei Wuling tablet, Hedan tablet, Gandan Shukang capsule, Xuezhikang capsule, Yiganling tablet, Hugan capsule, Zhibitai capsule). The network relationship between the interventions is shown in Figure 3. According to MD and 95%CI between all the pairwise interventions, in terms of TG improvement, Qianggan capsule (MD: 0.63; 95%CI: 0.04, 1.22)) was superior to western medicine, as shown in Table 4 and Supplementary Figure S5. Moreover, Qianggan capsule, with the highest-ranking probability of SUCRA (83.3%), had the best effectiveness in reducing TG, followed by Dangfei Liganning capsule (76.2%) and Danning tablet (69.2%). More details about the rank probability of SUCRA are shown in Figure 4.
29 studies reported TC, involving 12 CPMs (Qianggan capsule, Dangfei Liganning capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Liuwei Wuling tablet, Hedan tablet, Gandan Shukang capsule, Xuezhikang capsule, Yiganling tablet, Hugan capsule, Zhibitai capsule). The network relationship between the interventions is shown in Figure 3. In terms of TC improvement, according to MD and 95%CI between all the pairwise interventions, Dangfei Liganning capsule (MD: 1.08; 95%CI: 0.67, 1.49), Liuwei Wuling tablet (MD: 1.04; 95%CI: 0.17, 1.91), Yiganling tablet (MD: 0.99; 95%CI: 0.09, 1.89) were superior to placebo. Zhibitai capsule (MD: 0.71; 95%CI: 0.32, 1.10) was superior to lifestyle modification. Dangfei Liganning capsule (MD: 1.90; 95%CI: 1.25, 2.55), Huazhi Rougan granule (MD: 0.77; 95%CI: 0.57, 0.98), Qiaozhi capsule (MD: 0.43; 95%CI: 0.15, 0.70), Liuwei Wuling tablet (MD: 1.86; 95%CI: 1.45, 2.27), Hedan tablet (MD: 0.46; 95%CI: 0.10, 0.83) and Yiganling tablet (MD: 1.81; 95%CI: 0.78, 2.84) were superior to western medicine. In addition, Dangfei Liganning capsule was superior to Qianggan capsule (MD: −1.59; 95%CI: −2.31, −0.87), Danning tablet (MD: 1.65; 95%CI: 0.93, 2.38), Huazhi Rougan granule (MD: 1.13; 95%CI: 0.45, 1.81), Qiaozhi capsule (MD: 1.47; 95%CI: 0.77, 2.18), Hedan tablet (MD: 1.44; 95%CI: 0.69, 2.18), Gandan Shukang capsule (MD: 1.42; 95%CI: 0.62, 2.22), Xuezhikang capsule (MD: 1.77; 95%CI: 1.02, 2.52), Hugan capsule (MD: 1.40; 95%CI: 0.98, 1.82). Liuwei Wuling tablet was superior to Qianggan capsule (MD: −1.55; 95%CI: −2.07, −1.03), Danning tablet (MD: −1.61; 95%CI: −2.14, −1.08), Huazhi Rougan granule (MD: −1.09; 95%CI: −1.55, −0.63), Qiaozhi capsule (MD: −1.43; 95%CI: −1.93, −0.94), Hedan tablet (MD: 1.40; 95%CI: 0.84, 1.95), Gandan Shukang capsule (MD: 1.38; 95%CI: 0.75, 2.01), Xuezhikang capsule (MD: 1.73; 95%CI: 1.17, 2.29), Hugan capsule (MD: 1.36; 95%CI: 0.49, 2.23). Yiganling tablet was superior to Qianggan capsule (MD: −1.50; 95%CI: −2.58, −0.42), Danning tablet (MD: −1.56; 95%CI: −2.64, −0.48), Qiaozhi capsule (MD: −1.38; 95%CI: −2.45, −0.32), Hedan tablet (MD: −1.35; 95%CI: −2.44, −0.25), Gandan Shukang capsule (MD: −1.33; 95%CI: −2.47, −0.19), Xuezhikang capsule (MD: −1.68; 95%CI: −2.78, −0.58), Hugan capsule (MD: 1.31; 95%CI: 0.40, 2.22). Huazhi Rougan granule was superior to Qianggan capsule (MD: −0.46; 95%CI: −0.84, −0.08), Danning tablet (MD: −0.52; 95%CI: −0.91, −0.14), Xuezhikang capsule (MD: 0.64; 95%CI: 0.21, 1.08). The detailed results of pairwise comparison are shown in Table 4 and Supplementary Figure S6. Moreover, Dangfei Liganning capsule, with the highest-ranking probability of SUCRA (91.1%), had the best effectiveness in reducing TC, followed by Liuwei Wuling tablet (90.5%) and Yiganling tablet (87.9%). More details about the rank probability of SUCRA are shown in Figure 4.
7.5 Adverse reactions
22 studies mentioned adverse reactions. 12 studies reported that neither group had adverse reactions. The other 10 studies reported specific adverse reactions, including Liuwei Wuling tablet and Gandan Shukang capsule. Adverse reactions mainly occurred in Qianggan capsule, Danning tablet, Huazhi Rougan granule, Qiaozhi capsule, Hedan tablet and Xuezhikang capsule, but most of them were mild gastrointestinal reactions. Specific adverse reactions are shown in Supplementary Table 10.
7.6 Publication bias
The comparison adjusted funnel plot for the six outcomes is shown in Figure 5. The comparison adjusted funnel chart for the outcome indicator of clinical efficiency rate showed that its symmetry was poor, and there might be publication bias. The reason may be related to the small number of included studies and the small total sample size. The comparison adjusted funnel chart for the five outcome indicators ALT, AST, GGT, TG and TC showed that all studies were symmetrically distributed in the upper middle part and clustered towards the middle line, suggesting that the risk of publication bias were low.
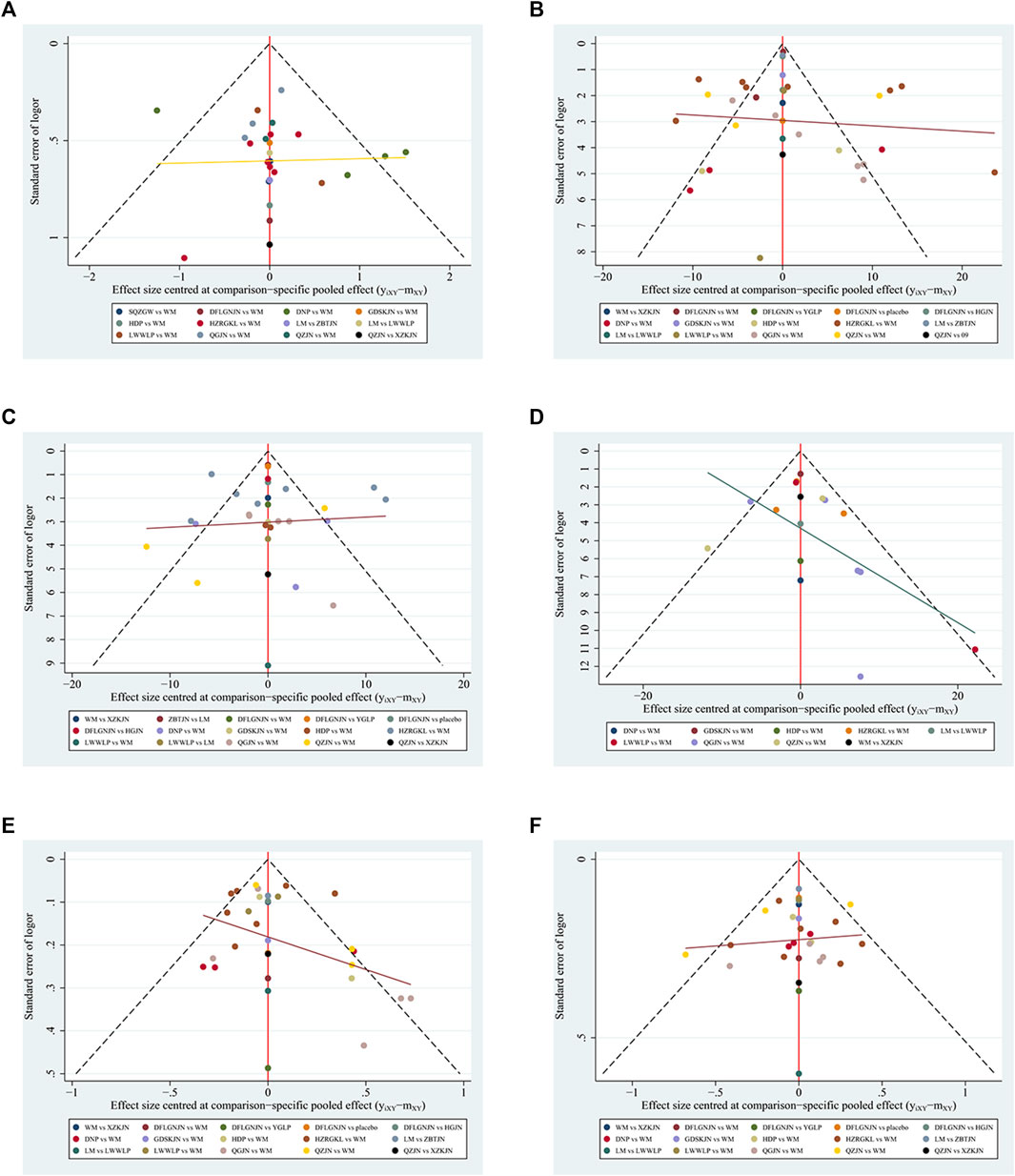
FIGURE 5. Funnel plots (A) Clinical efficiency rate (B) ALT (C) AST (D) GGT (E) TG (F) TC; WM, Western medicine; QGJN, Qianggan capsule; DFLGNJN, Dangfei Liganning capsule; DNP, Danning tablets; HZRGKL, Huazhi Rougan granule; QZJN, Qiaozhi capsule; SQZGW, Sanqi Zhigan pill; LWWLP, Liuwei Wuling tablet; HDP, Hedan tablet; GDSKJN, Gandan Shukang capsule; XZKJN, Xuezhikang capsule; YGLP, Yiganling tablet; HGJN, Hugan capsule; ZBTJN, Zhibitai capsule; LM, lifestyle modification.
7.7 Network inconsistency and heterogeneity
The network evidence diagrams of ALT, AST, TG and TC formed a closed loop respectively. Node splitting analysis was used to evaluate inconsistency. The NMA did not show any inconsistency in ALT, AST, TG and TC. The details are shown in Supplementary Figures S7–S10. As shown in Supplementary Table 11, the global I2 of clinical efficiency rate and ALT were54.2%, 94.2% respectively. The global I2 of AST, GGT, TG, TC were 91.1%, 73.6%, 80.3%, 44.2% respectively. Sensitivity analysis showed that excluding any one study would not change the expected confidence interval, and the pooled results were stable. The results of sensitivity analysis are shown in Supplementary Figures S11–S13.
8 Discussion
Traditional Chinese medicine (TCM) believes that the causes of NAFLD patients are eating much fat and sweet, lying more and moving less, and emotional disorders. These causes lead to liver loss, spleen loss, endogenous dampness, phlegm accumulation, kidney essence loss, and phlegm retention. Finally, it will cause dysfunction of the liver, spleen and kidney. Phlegm, dampness and stasis will block the liver collaterals (Zhang and Li, 2017). In clinical practice, the main treatment principle should be “resolving phlegm, removing dampness and activating blood circulation”. All CPMs included in these studies are Chinese herbal prescriptions established on the basis of the above treatment principles.
Based on 39 related research materials and six main results, we systematically evaluated the efficacy of 13 commonly used CPMs in the treatment of NAFLD by using a network meta-analysis technique. According to the results of NMA, most CPMs were superior to placebo or lifestyle modification or western medicine in all results. The difference between groups was statistically significant. According to the results of NMA, except for AST, most CPMs were better than placebo or lifestyle modification or western medicine in the outcomes, with statistically significant differences between groups. The reason why there was no significant difference in serum AST may be that the patients with NAFLD often showed an increase in serum ALT and GGT, while the increase in serum AST was not significant. Therefore, there was no significant difference between the two comparisons in this NMA.
As far as the results of this NMA were concerned, Zhibitai capsule had the best efficacy in improving clinical efficiency rate. It is composed of hawthorn, rhizoma alismatis, atractylodes macrocephala and monascus, which has the effect of eliminating phlegm, dampness and blood stasis. It directly treats the phlegm, dampness and blood stasis of NAFLD core pathogenesis. Therefore, it can significantly improve the clinical efficacy. In addition, modern research showed that maslinic acid in hawthorn could reduce the content of fat in the liver cells of NAFLD mice and inflammation injury (Li et al., 2022). Alisol B in alisma orientalis could attenuate hepatic steatosis, inflammation, and fibrosis in high-fat diet plus carbon tetrachloride (DIO + CCl4)-induced and choline-deficient and amino acid-defined (CDA)-diet-induced NASH mice (Zhao Z et al., 2022). Monascus had the effects of lowering blood lipid and anti-inflammation (Hsu et al., 2014). Liuwei Wuling tablet had the best effect in reducing serum ALT and AST level for NAFLD patients. It is composed of Schisandra chinensis, Ligustrum lucidum, Forsythia suspensa, Curcuma zedoary, Sonchus chicory, Ganoderma lucidum spore powder, which has the effect of promoting blood circulation and nourishing liver. Long term stagnation of blood stasis may lead to liver inflammation. Liuwei Wuling tablet has the effect of promoting blood circulation and nourishing liver. Therefore, it is more effective in improving the inflammatory damage of NAFLD. Modern pharmacological studies showed that the extract of Schisandra chinensis could reduce liver damage and serum ALT, AST levels (Zhao J et al., 2022). Liqustri lucidi Fructus could regulate AMPK signaling pathway to protect hepatocytes from oxidative damage (Seo et al., 2017). Phillygenin, an extract of Forsythia suspensa, could reduce liver lipid deposition and serum ALT, AST levels in NAFLD mice induced by high-fat diet (Zhou W et al., 2022). Ganoderma lucidum spore powder could protect mice against developing obesity caused by increased fat intake by regulating inflammatory factors and lipid metabolism (Zhong et al., 2022). Gandan Shukang capsule had the best effect in reducing serum GGT level. It is composed of white peony, herba artemisiae, bupleurum, turmeric, salvia miltiorrhiza, turtle shell (made of) and jujube. It has the effect of clearing the liver, regulating the spleen, promoting qi and removing blood stasis. The effect of Gandan Shukang capsule on removing blood stasis might be the reason for its improvement of NAFLD inflammation. Paeoniflorin, the extract of paeony, could improve the liver inflammation and reduce serum GGT level in rats with non-alcoholic steatohepatitis (Ma et al., 2016; Ma et al., 2020). Saikosaponin-d, the extract of Bupleurum chinense, could inhibit the inflammatory reaction in NAFLD mice and achieve the liver protective effect (Chang et al., 2021). Salvianolic acid B, Tanshinone IIA and Salvianolic acid A in Salvia miltiorrhiza extract could improve the inflammatory damage in NAFLD mice (Li et al., 2020; Meng et al., 2022; Xu et al., 2022). Qianggan capsule had the best effect in reducing serum TG level. It is composed of Herba Artemisiae, Radix Isatidis, Angelica, Radix Paeoniae Alba, Radix Salviae Miltiorrhizae, Radix Curcumae, Radix Astragali, Codonopsis, Rhizoma Alisma, Rhizoma Polygonati, Rhizoma Rehmanniae, Chinese Yam, Hawthorn, Six God Qu, Gentiana macrophylla, and Glycyrrhiza. It has the effects of clearing away heat and dampness, nourishing the spleen and blood, and supplementing qi and relieving depression. Its diuretic effect may be the reason why it reduces blood lipids. Diosgenin is abundant in yam, Study confirmed that the abundant diosgenin in yam could suppress excessive weight gain, reduce serum levels of total cholesterol and triglycerides, and decrease liver fat accumulation in high-fat diet-induced NAFLD rats (Zhou Y et al., 2022). Hawthorn could reduce blood lipid, especially serum TG level (Al Humayed, 2016; Han et al., 2016; Kim et al., 2022). Curcumin in turmeric could reduce blood lipid (Jarhahzadeh et al., 2021). Dangfei Liganning capsule had the best effect in reducing serum TC level. It is composed of local medicine and silymarin, which has the effect of clearing damp heat, benefiting liver and removing jaundice. Its diuretic effect could also reduce blood lipids. Modern pharmacological research showed that sweroside may ameliorate obesity with fatty liver via the regulation of lipid metabolism and inflammatory responses (Yang et al., 2020). Silymarin is a mixture of flavonoid obtained from Silybum marianum (L.) Gaertn. [Asteraceae] with good clincial evidence for liver protecting effect and a wide use in phytotherapy especially in Europe. It can also reduce blood lipids. Many studies had shown that silymarin, an extract of silymarin, could regulate lipid metabolism, reduce blood lipids, and achieve the goal of treating NAFLD (Ni and Wang, 2016; Jiang et al., 2022). Some scholars also pointed out that silymarin could be used for NAFLD without elevated transaminase (Liu et al., 2022). None of the included studies had serious adverse reactions. Most of the studies only showed mild gastrointestinal reactions, which could be eliminated after relevant treatment.
The complete mechanism of CPMs in treating NAFLD is still unclear. Some potential mechanisms have been clarified. Animal experiments showed that Qianggan capsule had a good therapeutic effect on liver lipid and inflammation in NAFLD model of SD male rats prepared with high-fat diet. The possible mechanism was to improve leptin resistance and increased the expression of leptin receptor mRNA and phosphorylated protein tyrosine kinase JAK-2 (P-JAK2), signal transducer and activator of transcription three phosphorylation (P-STAT3) protein in the liver (Zheng et al., 2009). Another study showed that Qianggan capsule might improve the NAFLD model of Wistar rats prepared with high-fat diet by inhibiting the expression of interleukin-8 (IL-8) mediated by early growth response protein 1 (EGR-1) (Hao and Liu, 2018). The mechanism of Dangfei Liganning capsule in treating NAFLD might be to reduce subcellular localization of nuclear factor E2-related factor 2 (Nrf2) mediated liver oxidative stress, reduce the expression of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory body related genes and nuclear factor kappa-B (NF-ΚB) protein (Xu et al., 2015; Zhao et al., 2021). In addition, the regulation of Dangfei Liganning capsule on adiponectin, tumor necrosis factor-α (TNF-α) and insulin resistance was also an important mechanism for its treatment of NAFLD (Song et al., 2012). The mechanism of Danning tablet in the treatment of NAFLD was to reduce the content of TNF-α, transforming growth factor-β1 (TGF-β1), malondialdehyde (MDA) in liver tissue and increase the content of succinate dehydrogenase (SDH) (Zhang et al., 2016; Jiang et al., 2017). Huazhi Rougan granule could prevent NAFLD by reducing the levels of cytokines interleukin18 (IL-18) and interleukin-1β (IL-1β) (Shi et al., 2020). Qiaozhi capsule could regulate the secretion of GLUT-4, and significantly reduce the secretion of TNF-α and interleukin6 (IL-6). It could also improve IR and regulate fat metabolism by up regulating the protein and gene expression of peroxisome proliferator-activated receptor γ (PPAR-γ) and IR in liver tissue. In addition, it could reduce the expression of heme oxygenase 1 (HO-1) and cytochrome P450 family member 2E1 (CYP2E1) in liver tissue, reduce oxidative stress and lipid peroxidation, and prevent the occurrence and development of NAFLD (Wang et al., 2011; Zhao et al., 2014; Zhao et al., 2015).
This study still has the following limitations: ① The quality of the included studies was low. In the 39 studies, only 19 studies reported random methods, and one study used a double-blind method. Most of the literatures quality was evaluated as “unclear”, which led to a certain risk of publication bias; ② The included studies were all small sample studies, which would reduce the statistical reliability of this study; ③ Not all the CPMs included in these studies could follow the principle of “syndrome differentiation and treatment”. There were unified interventions for NAFLD of different syndrome types, which might affect the results; ④ The length of treatment varied. Most treatment cycles were 12 weeks and 24 weeks, but a few studies had treatment cycles of 8 weeks, which might cause clinical heterogeneity. ⑤ The number of RCTs involved in Zhibitai Capsule (2RCTs), Liuwei Wuling Tablet (3RCTs), Gandan Shukang Capsule (1RCT), Dangfei Liganning Capsule (5RCTs), Qianggan Capsule (6RCTs) is limited, and the results of NMA merger may not be convincing enough. ⑥ All these studies were conducted in China, which has certain restrictions on the promotion and application of Chinese patent medicines. In view of the above limitations, it is suggested that the following four points should be noted during the implementation of future studies: ① Apply the correct randomization method. For example, a random number table or a computer is used to generate random numbers. At the same time, attention should be paid to hiding the random serial number, such as using orderly numbered, opaque and sealed envelopes to hide the random number. These can avoid selectivity bias. ② The study design shall be at least double blind. The blind method shall be adopted for both the subjects and the main researchers, and the blind method shall not be easily damaged to avoid implementation bias. ③ It is recommended that all RCT studies should be registered for clinical trials before implementation, so as to avoid publication bias due to only reporting positive outcomes.④ It is suggested to carry out multi center, large sample studies of international cooperation to clarify the exact efficacy of these Chinese patent medicines in the treatment of NAFLD.
9 Conclusion
For patients with NAFLD in this NMA, Zhibitai capsule might have best efficacy in improving clinical efficiency rate, Liuwei Wuling tablet might have best effect in reducing serum ALT and AST level, Gandan Shukang capsule might have best effect in reducing serum GGT level, Qianggan capsule might have best effect in reducing serum TG level, Dangfei Liganning capsule might have best effect in reducing serum TC level. The results of this NMA are limited to the data analysis of literatures, and cannot fully explain the clinical efficacy of CPMs in treating NAFLD, so there are some limitations. Better designed double-blind, multi center and large sample RCTs are needed which resolve the problems of blinding, selective reporting and allocation concealment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
LZ and SL done conception, designed of the study, performed the network meta-analysis. YG and SL conducted literature retrieval, screening and extraction. ML and WZ reviewed and edited the manuscript.
Funding
This work was sponsored by Special Research Project of Traditional Chinese Medicine in Henan Province (No. 2022JDZX098, and No. 2022JDZX006), National Natural Science Foundation of China (No. 81904154, and No. 82205086), and Key R&D and Promotion Projects in Henan Province (No. 192102310425, and No. 202102310168).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1077180/full#supplementary-material
References
Al Humayed, S. (2017). Protective and therapeutic effects of Crataegus aronia in non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 123 (1), 23–30. doi:10.1080/13813455.2016.1205097
Anirban, M., Soumyabrata, R. C., Debmalya, S., and Bhattacharjee, K. (2018). Liraglutide-Indian experience. Indian J. Endocrinol. Metab. 22, 818–826. doi:10.4103/ijem.IJEM_187_18
Chang, G. R., Lin, W. L., Lin, T. C., Liao, H. J., and Lu, Y. W. (2021). The ameliorative effects of saikosaponin in thioacetamide-induced liver injury and non-alcoholic fatty liver disease in mice. Int. J. Mol. Sci. 22 (21), 11383. doi:10.3390/ijms222111383
Chen, Z. X., Zhang, S. J., and Yun, L. R. (2006). Clinical study of treatment nonalcoholic fatty liver with qianggan capsule. Zhongguo Zhong Yao Za Zhi 31 (20), 1739–1741.
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 391 (10128), 1357–1366. doi:10.1016/s0140-6736(17)32802-7
Cusi, K., Isaacs, S., Barb, D., Basu, R., Caprio, S., Garvey, W. T., et al. (2022). American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: Co-sponsored by the American association for the study of liver diseases (AASLD). Endocr. Pract. 28, 528–562. doi:10.1016/j.eprac.2022.03.010
Deng, Y. Q., Fan, X. F., and Li, J. P. (2011). Clinical observation of Hedan tablet in the treatment of non-alcoholic fatty liver disease. Zhejiang J. Inte Tradit. Chin. West Med. 21 (10), 697–699.
Ding, C. M., Wang, Z. Y., Bai, H. H., Hou, L. X., Gou, X. J., and Xu, M. L. (2021). Systematic review of clinical efficacy of danning tablet in the treatment of non-alcoholic fatty liver disease. Eval. Anal. Drug-Use Hosp. Chin. 21 (04), 459–463. doi:10.14009/j.issn.1672-2124.2021.04.018
Fan, J. G., Wei, L., and Zhuang, H. (2019). Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J. Dig. Dis. 20 (4), 163–173. doi:10.1111/1751-2980.12685
Fan, X. F., Deng, Y. Q., Ye, L., Li, Y. D., Chen, J., Lu, W. W., et al. (2010). Effect of Xuezhikang Capsule on serum tumor necrosis factor-alpha and interleukin-6 in patients with nonalcoholic fatty liver disease and hyperlipidemia. Chin. J. Integr. Med. 16 (2), 119–123. doi:10.1007/s11655-010-0119-7
Han, X., Li, W., Huang, D., and Yang, X. (2016). Polyphenols from hawthorn peels and fleshes differently mitigate dyslipidemia, inflammation and oxidative stress in association with modulation of liver injury in high fructose diet-fed mice. Chem. Biol. Interact. 257, 132–140. doi:10.1016/j.cbi.2016.08.002
Hao, L. L., and Liu, X. X. (2018). Improvement of Qianggan Capsules on non-alcoholic fatty liver in rats based on regulation of Egr-1 on IL-8 expression. Drugs & Clin. 33 (02), 214–219. doi:10.7501/j.issn.1674-5515.2018.02.002
He, Z. J., Wang, M. X., and Ning, M. M. (2016). Clinical efficacy of Qianggan capsule on non-alcoholic fatty liver disease complicated with hyperlipidemia. J. Hainan Med. Coll. 22 (14), 1518–1520. doi:10.13210/j.cnki.jhmu.20160301.026
Hsu, W. H., Chen, T. H., Lee, B. H., Hsu, Y. W., and Pan, T. M. (2014). Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 64, 94–103. doi:10.1016/j.fct.2013.11.015
Huang, X., and Zhang, Z. Y. (2007). A clinical observation on Dangfei liganning capsule for 32 cases of nonalcoholic fatty liver disease. J. Tradit. Chin. Med. 48 (06), 524–525. doi:10.13288/j.11-2166/r.2007.06.018
Jarhahzadeh, M., Alavinejad, P., Farsi, F., Husain, D., and Rezazadeh, A. (2021). The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: A randomized double blind clinical trial. Diabetol. Metab. Syndr. 13 (1), 112. doi:10.1186/s13098-021-00731-7
Ji, G., Fan, J. G., Chen, J. J., Lu, L. G., Xing, L. J., Zheng, P. Y., et al. (2008). Effectiveness of danning tablet in patients with non-alcoholic fatty liver of damp-heat syndrome type: A multicenter randomized controlled trial. Zhong Xi Yi Jie He Xue Bao 6 (2), 128–133. doi:10.3736/jcim20080205
Jiang, G., Sun, C., Wang, X., Mei, J., Li, C., Zhan, H., et al. (2022). Hepatoprotective mechanism of Silybum marianum on nonalcoholic fatty liver disease based on network pharmacology and experimental verification. Bioengineered 13 (3), 5216–5235. doi:10.1080/21655979.2022.2037374
Jiang, N., Zhang, J. H., Wang, X. N., Xu, Y., and Liu, Z. P. (2017). Experimental study on the protective effect of Danning Pian on liver in rats with non-alcoholic fatty liver disease. J. Gansu Univ. Chin. Med. 34 (06), 12–16. doi:10.16841/j.issn1003-8450.2017.06.03
Jin, Y. R. (2011). Therapeutic effect of Liuweiwuling tablets on nonalcoholic steatohepatitis. Chin. J. Liver Dis. 4 (03), 29–31.
Kamada, Y., Takahashi, H., Shimizu, M., Kawaguchi, T., Sumida, Y., Fujii, H., et al. (2021). Clinical practice advice on lifestyle modification in the management of nonalcoholic fatty liver disease in Japan: An expert review. J. Gastroenterol. 56 (12), 1045–1061. doi:10.1007/s00535-021-01833-9
Kim, E., Jang, E., and Lee, J. H. (2022). Potential roles and key mechanisms of hawthorn extract against various liver diseases. Nutrients 14 (4), 867. doi:10.3390/nu14040867
Li, C. M., Gong, M., Li, M. Q., Xia, Q., and Chen, Y. P. (2012). Clinical study on Dangfei liganning capsule for nonalcoholic simple fatty liver disease:a report on 113 cases. J. Traditional Chin. Med. 53 (01), 38–41. doi:10.13288/j.11-2166/r.2012.01.024
Li, F. B., and Jiang, B. T. (2009). Clinical study of Hedan Tablet in the treatment of non-alcoholic fatty liver disease. J. Clin. Res. 26 (04), 722–723. doi:10.3969/j.issn.1671-7171.2009.04.070
Li, H. X., Zhou, Q., Wang, L., and Liu, R. B. (2017). Effect of Huazhi Rougan Granules on insulin resistance in patients with non-alcoholic steatohepatitis. Chin. Tradit. Pat. Med. 39 (08), 1586–1590. doi:10.3969/j.issn.1001-1528.2017.08.008
Li, L., Zhang, X. J., Lan, Y., Xu, L., Zhang, X. Z., and Wang, H. H. (2010). Treatment of non-alcoholic fatty liver disease by Qianggan Capsule. Chin. J. Integr. Med. 16 (1), 23–27. doi:10.1007/s11655-010-0023-1
Li, S., Qian, Q., Ying, N., Lai, J., Feng, L., Zheng, S., et al. (2020). Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front. Pharmacol. 11, 560905. doi:10.3389/fphar.2020.560905
Li, T., Wang, H., Dong, S., Liang, M., Ma, J., Jiang, X., et al. (2022). Protective effects of maslinic acid on high fat diet-induced liver injury in mice. Life Sci. 301, 120634. doi:10.1016/j.lfs.2022.120634
Lin, Y. D., Xu, F. G., Wu, D. Z., and Zhi, B. B. (2013). Clinical research of Huazhi Rougan Granule in the treatment of non-alcoholic fatty liver disease. Pract. Clin. Med. 17 (03), 75–77. doi:10.7619/jcmp.201303024
Liu, L., Li, Y., and Qi, X. (2022). May silymarin be selectively considered for nonalcoholic fatty liver disease without elevated transaminases? Comment re. "Impact of silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition 102, 111648. doi:10.1016/j.nut.2022.111648
Liu, L. L., Mao, D. W., Lv, J. L., and Huang, R. (2018). Meta analysis of efficacy and safety of Qianggan Capsules on patients with nonalcoholic fatty liver disease. Chin. Tradit. Pat. Med. 40 (08), 1715–1720.
Liu, M. (2011). Treatment of 26 cases of non-alcoholic fatty liver disease with Liuwei Wuling Tablet. Chin. J. Integr. Tradit. West Med. Liver Dis. 21 (05), 303–304. doi:10.3969/j.issn.1005-0264.2011.05.018
Liu, Z. Y., and Lv, J. X. (2014). Therapeutic effect of Qianggan capsule on non-alcoholic fatty liver disease. Mod. J. Integr. Tradit. Chin. West Med. 23 (06), 600–601. doi:10.3969/j.issn.1008-8849.2014.06.011
Luo, D., and Jiang, Y. (2014). Sanqi zhigan pill treat non-alcoholic fatty liver disease of abnormal liver function patients 59 cases. Chin. J. Exp. Tradit. Med. Formulae 20 (05), 202–205. doi:10.11653/syfj2014050202
Lv, Q. G. (2009). Clinical study on safety and efficacy of danning tablets in the treatment of non-alcoholic fatty liver disease (study on liver qi stagnation syndrome and damp-heat accumulation syndrome). Changchun, China: Changchun University of Chinese Medicine. [dissertation/master’s thesis]. [Changchun China].
Ma, J. Y. (2014). Clinical observation of Dan Ning tablets in the treatment of 76 cases with nonalcoholic fatty liver disease. Chi C Dr. 30 (17), 77–78. doi:10.3969/j.issn.1007-614x.2014.17.47
Ma, X., Zhang, W., Jiang, Y., Wen, J., Wei, S., and Zhao, Y. (2020). Paeoniflorin, a natural product with multiple targets in liver diseases-A mini review. Front. Pharmacol. 11, 531. doi:10.3389/fphar.2020.00531
Ma, Z., Chu, L., Liu, H., Li, J., Zhang, Y., Liu, W., et al. (2016). Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: Involvement with the ROCK/NF-κB pathway. Int. Immunopharmacol. 38, 377–384. doi:10.1016/j.intimp.2016.06.023
Meng, L. C., Zheng, J. Y., Qiu, Y. H., Zheng, L., Zheng, J. Y., Liu, Y. Q., et al. (2022). Salvianolic acid B ameliorates non-alcoholic fatty liver disease by inhibiting hepatic lipid accumulation and NLRP3 inflammasome in ob/ob mice. Int. Immunopharmacol. 111, 109099. doi:10.1016/j.intimp.2022.109099
Meng, S. X. (2009). Clinical study on treatment of non-alcoholic fatty liver disease by Qiaozhi capsule. Chin. J. Inf. Tradit. Chin. Med. 16 (03), 56–57.
Nan, C. (2020). Clinical observation on Huazhi rougan granule in treating dampness-heat accumulation type nonalcoholic fatty liver disease. GuangMing J. Chin. Med. 35 (13), 1998–2000. doi:10.3969/j.issn.1003-8914.2020.13.019
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, (2001). Diagnostic criteria for nonalcoholic fatty liver disease (draft). Chin. J. Hepatol. 9 (06), 325.
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, (2006). Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases. Chin. J. Hepatol. 14 (03), 161–163.
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, (2010). Guidelines for management of nonalcoholic fatty liver diseases: An update and revised edition. Chin. J. Hepatol. 18 (03), 163–166.
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, (2018). Guidelines of prevention and treatment for nonalcoholic fatty liver diseases: A 2018 update. Chin. J. Hepatol. 26 (03), 195–203.
Ni, X., and Wang, H. (2016). Silymarin attenuated hepatic steatosis through regulation of lipid metabolism and oxidative stress in a mouse model of nonalcoholic fatty liver disease (NAFLD). Am. J. Transl. Res. 8 (2), 1073–1081.
Ning, H. M., Ou, Q., Huang, L., and Tang, Q. (2017). Clinical effect of Qiaozhi capsules in treatment of nonalcoholic steatohepatitis. Chin. J. Clin. Hepatol. 33 (01), 137–140. doi:10.3969/j.issn.1001-5256.2017.01.030
Ou, Q., Xu, Y. H., Qu, L. Q., and Huang, L. (2016). Effect of Qianggan capsules on insulin resistance index and liver fibrosis score in patients with nonalcoholic fatty liver disease. Chin. J. Clin. Hepatol. 32 (10), 1951–1954. doi:10.3969/j.issn.1001-5256.2016.10.027
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Peng, J. (2019). To observe the effect of Zhibitai capsule on nonalcoholic simple fatty liver. Orient. Medicat. Diet. 23, 80.
Qi, M., Zhang, G. Q., Wang, L. J., Zang, K., and Li, G. T. (2012). Clinical observation of Dangfeiliganning capsule effect on 64 cases with nonalcoholic fatty liver disease. Chin. J. Gastroenter Hepatol. 21 (09), 855–857. doi:10.3969/j.issn.1006-5709.2012.09.021
Seo, H. L., Baek, S. Y., Lee, E. H., Lee, J. H., Lee, S. G., Kim, K. Y., et al. (2017). Liqustri lucidi Fructus inhibits hepatic injury and functions as an antioxidant by activation of AMP-activated protein kinase in vivo and in vitro. Chem. Biol. Interact. 262, 57–68. doi:10.1016/j.cbi.2016.11.031
Sheka, A. C., Adeyi, O., Thompson, J., Hameed, B., Crawford, P. A., and Ikramuddin, S. (2020). Nonalcoholic steatohepatitis: A review. Jama 323 (12), 1175–1183. doi:10.1001/jama.2020.2298
Shi, H. L., Fang, N. Y., Chen, Q. L., Fang, J., and Qiao, F. (2020). The observation of the therapeutic effect of modified Huazhi rougan granuleas on hyperlipidemic nonalcoholic steatohepatitis mice model. World Chin. Med. 15 (22), 3396–3400. doi:10.3969/j.issn.1673-7202.2020.22.006
Shiha, G., Alswat, K., Al Khatry, M., Sharara, A. I., Örmeci, N., Waked, I., et al. (2021). Nomenclature and definition of metabolic-associated fatty liver disease: A consensus from the Middle East and north africa. Lancet Gastroenterol. Hepatol. 6 (1), 57–64. doi:10.1016/s2468-1253(20)30213-2
Song, H. Y., Mao, Z. M., Yang, L. L., Zhang, L., Ge, Y. L., Liu, T., et al. (2012). Mechanism of Dangfei Liganning Capsule attenuating nonalcoholic steatotic hepatitis induced by high-fat diet combined with carbon tetrachloride in rats. Chin. J. Clin. Hepatol. 28 (03), 196–200.
Traber, M. G., and Head, B. (2021). Vitamin E: How much is enough, too much and why. FREE Radic. BIO Med. 177, 212–225. doi:10.1016/j.freeradbiomed.2021.10.028
Wang, R. Q., Nan, Y. M., Zhao, S. X., Mi, H. M., Kong, L. B., and Jia, Y. H. (2011). Effects of Kezhi capsule on oxidative stress in mice with non-alcoholic steatohepatitis. Chin. Hepatol. 16 (03), 216–219. doi:10.14000/j.cnki.issn.1008-1704.2011.03.019
Wang, X. L., Zhao, L. S., and Wang, X. Y. (2018). Curative effect evaluation of Huazhi rougan granule on nonalcoholic fatty liver disease with damp heat accumulation syndrome. World Chin. Med. 13 (07), 1669–1672.
Wang, Y. P., Yao, T. L., Zhang, Y., and Yu, B. (2015). Clinical study of Qianggan capsule in the treatment of non-alcoholic fatty liver disease complicated with hyperlipidemia. J. MuDanJiang Med. Univ. 36 (06), 38–40. doi:10.13799/j.cnki.mdjyxyxb.2015.06.013
Wang, Z. L. (2014). Effect of Danning tablets on non-alcoholic fatty liver disease. Guide Chin. Med. 12 (23), 158–159. doi:10.15912/j.cnki.gocm.2014.23.161
Wei, B., Chen, X. H., Luo, D. Y., Zhang, Z. H., and Zhang, X. W. (2015). Effect of Huazhusuogan granules on intestinal barrier function in patients with non-alcoholic fatty liver disease. Mod. J. Integr. Tradit. Chin. West Med. 24 (18), 2007–2009. doi:10.3969/j.issn.1008-8849.2015.18.027
Wu, J., and Peng, Y. Z. (2018). Efficacy of dangfeiliganning capsule in nonalcoholic fatty liver disease. Pract. Clin. Med. 19 (06), 4–6. doi:10.13764/j.cnki.lcsy.2018.06.002
Wu, J. Y., Jiang, N., and Huang, R. G. (2008). Studying the treatment of nonalcoholic fatty liver disease with Dangfei liganning capsule. Si Chuan Cont. Edu Colg MS 27, 192–193.
Xu, J. L., and Tao, Y. (2018). Observe on curative effect of Huazhi Rougan granule in treating Non-alcoholic Fatty liver Disease with damp-heat accumulation. J. C Med. Lit. 5 (26), 79–81. doi:10.16281/j.cnki.jocml.2018.26.043
Xu, J. Y., Xiao, T. G., Shu, X. B., Li, Z. P., Song, H. Y., Zhang, L., et al. (2015). Dangfei Liganning Capsule ameliorate susceptibility of liver injury in NAFLD rats through regulating inflammasome. China J. Tradit. Chin. Med. Pharm. 30 (05), 1580–1584.
Xu, L., Liu, X., Jia, T., Sun, Y., Du, Y., Wei, S., et al. (2022). Tanshinone IIA ameliorates nonalcoholic steatohepatitis in mice by modulating neutrophil extracellular traps and hepatocyte apoptosis. Evid. Based Complement. Altern. Med. 2022, 5769350. doi:10.1155/2022/5769350
Xu, L., Yan, Y., and Mi, Y. Q. (2010). Clinical study on effect of Qiaozhi capsule in treating nonalcoholic fatty liver diseases. Chin. J. Integr. Tradit. West Med. Liver Dis. 20 (03), 146–148. doi:10.3969/j.issn.1005-0264.2010.03.007
Yang, Q., Shu, F., Gong, J., Ding, P., Cheng, R., Li, J., et al. (2020). Sweroside ameliorates NAFLD in high-fat diet induced obese mice through the regulation of lipid metabolism and inflammatory response. J. Ethnopharmacol. 255, 112556. doi:10.1016/j.jep.2020.112556
Yang, S. S., Guo, Y., Li, T., and Su, Z. W. (2015). Huazhi rougan granule in treating damp-heat accumulation type non-alcoholic fatty liver disease. Chin. J. Exp. Tradit. Med. Formulae 21 (24), 157–160. doi:10.13422/j.cnki.syfjx.2015240157
Yang, Y. W., Xiao, G. M., Zhao, B. M., Yang, H. Z., Dai, M., and Teng, L. C. (2014). Randomized study of Qiaozhi capsules in treating nonalconolic fatty liver. J. Pract. Med. 30 (04). doi:10.3969/j.issn.1006-5725.2014.04.048
Younossi, Z. M., Loomba, R., Rinella, M. E., Bugianesi, E., Marchesini, G., Neuschwander-Tetri, B. A., et al. (2018). Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 68 (1), 361–371. doi:10.1002/hep.29724
Yu, Y., Qian, L. Q., Hou, P., Wu, B., Li, S. N., and Ren, J. T. (2014). Observation of Huazhi Rougan granule in the treatment of nonalcoholic aftty liver disease. Mod. J. Integr. Tradit. Chin. West Med. 23 (21), 2302–2304.
Yuan, L. (2008). The Clinical observation of the Treatment of Nonalcoholic Fatty Liver(hepatochlic hygropyrexia amphiblood stasis)for using the Capsule of GANDANSHUKANG. College of Traditional Chinese Medicine. [dissertation/master’s thesis]. [Henan, China]: Henan.
Zhang, C. M. (2019). Effect of dehysteresis and ruohe granules on the treatment of humid and hot deflated non-alcoholic liver and its effect on clinical symptoms. J. C Med. Lit. 6 (89), 20–21. doi:10.16281/j.cnki.jocml.2019.89.010
Zhang, J. H., Wang, X. N., Jiang, N., Zhou, S. L., Feng, Y. X., Liu, Z. P., et al. (2016). Therapeutic eff ect of Danning tablets on nonalcoholic fatty liver disease in rats. World Chin. J. Digestol. 24 (18), 2875–2880. doi:10.11569/wcjd.v24.i18.2875
Zhang, J. M. (2011). Clinical study on 30 cases of non-alcoholic fatty liver disease treated with Sanqi Zhigan pill. Med. Foru 15 (08), 234–235.
Zhang, S. S., and Li, J. X. (2017). Expert consensus on TCM diagnosis and treatment of nonalcoholic fatty liver disease (2017). Chin. J. Clin. Hepatol. 33 (12), 2270–2274. doi:10.3969/j.issn.1001-5256.2017.12.002
Zhang, X. L., Zhou, L. J., Chen, H. J., and Zhou, R. H. (2014). Clinical observation of Liuwei Wuling tablets in the treatment of non-alcoholic steatohepatitis. Chin. J. Integr. Tradit. West Med. Liver Dis. 24 (03), 167–168. doi:10.3969/j.issn.1005-0264.2014.03.015
Zhao, J., Ding, K., Hou, M., Li, Y., Hou, X., Dai, W., et al. (2022). Schisandra chinensis essential oil attenuates acetaminophen-induced liver injury through alleviating oxidative stress and activating autophagy. Pharm. Biol. 60 (1), 958–967. doi:10.1080/13880209.2022.2067569
Zhao, W. H., Yu, Y. Q., Liu, L. J., Wang, Y. L., Mao, T. Y., and Li, J. X. (2014). Experimental study of Qiaozhi capsule on PPAR-γ and IR expression in non-alcoholic steatohepatitis rats. Chin. J. Integr. Tradit. West Med. Dig. 22 (09), 501–505. doi:10.3969./j.issn.1671-038X.2014.09.04
Zhao, W. H., Yu, Y. Q., Ye, Y., Cheng, J. W., Gao, K. L., Wang, Y. L., et al. (2015). Impact of Qiaozhi capsule on TNF-αIL-6 and GLUT-4 expression in non-alcoholic steatohepatitis rat. Chin. J. Integr. Tradit. West Med. Dig. 23 (04), 231–234. doi:10.3969/j.issn.1671-038X.2015.04.02
Zhao, Y. T., Shu, X. B., Yang, Z. X., and Li, G. P. (2021). Mechanism of Dangfei Liganning Capsule on non-alcoholic fatty liver disease via inhibiting liver oxidative stress in mice. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai 35 (05), 37–43. doi:10.16306/j.1008-861x.2021.05.007
Zhao, Z., Deng, Z. T., Huang, S., Ning, M., Feng, Y., Shen, Y., et al. (2022). Alisol B alleviates hepatocyte lipid accumulation and lipotoxicity via regulating rarα-pparγ-CD36 cascade and attenuates non-alcoholic steatohepatitis in mice. Nutrients 14 (12), 2411. doi:10.3390/nu14122411
Zheng, P. Y., Wang, L., Zhang, L., Liu, T., Xing, L. J., and Ji, G. (2009). Therapeutic effect of Qiangganjiaonang on nonalcoholic rat fatty liver by increasing the expression of liver leptin receptor and P-JAK2/P-STAT3. Chin. J. Integr. Tradit. West Med. Dig. 17 (03), 141–145.
Zhong, B., Li, F. L., Zhao, J. Y., Fu, Y., and Peng, C. (2022). Sporoderm-broken spore powder of Ganoderma lucidum ameliorate obesity and inflammation process in high-fat diet-induced obese mice. Food Nutr. Res. 66, 10–29219. doi:10.29219/fnr.v66.8745
Zhong, Z. M., and Yang, J. (2017). Clinical observation of Zhibitai capsules combined with diet and exercise intervention for nonalcoholic fatty liver disease of phlegm and blood stasis type. New J. Tradit. Chin. Med. 49 (11), 44–47. doi:10.13457/j.cnki.jncm.2017.11.014
Zhou, W., Yan, X., Zhai, Y., Liu, H., Guan, L., Qiao, Y., et al. (2022). Phillygenin ameliorates nonalcoholic fatty liver disease via TFEB-mediated lysosome biogenesis and lipophagy. Phytomedicine 103, 154235. doi:10.1016/j.phymed.2022.154235
Zhou, Y., Li, R., Zheng, Y., Song, M., Zhang, S., Sun, Y., et al. (2022). Diosgenin ameliorates non-alcoholic fatty liver disease by modulating the gut microbiota and related lipid/amino acid metabolism in high fat diet-fed rats. Front. Pharmacol. 13, 854790. doi:10.3389/fphar.2022.854790
Glossary
ALT Alanine aminotransferase
AST Aspartate aminotransferase
CPMs Chinese patent medicines
CPM Chinese patent medicine
CT Computed tomography
CNKI China National Knowledge Infrastructure
CYP2E1 Cytochrome P450 family member 2E1
CI Confidence interval
CBM Chinese Biomedical Literature Database
DFLGNJN Dangfei Liganning capsule
DNP Danning tablet
GDSKJN Gandan Shukang capsule
GGT Gamma glutamyl transferase
HZRGKL Huazhi Rougan granule
HDP Hedan tablet
HO-1 Heme oxygenase 1
IL-18 Interleukin18
IL-1β Interleukin-1β
IR Insulin resistance
LDL Low density lipoprotein
LM lifestyle modification
LWWLP Liuwei Wuling tablet
MAFLD Metabolic associated fatty liver disease
MASH Metabolic associated steatohepatitis
MD Mean differences
MDA Malondialdehyde
NMA Network meta-analysis
NAFLD Non-alcoholic fatty liver disease
NASH Non-alcoholic steatohepatitis
Nrf2 Nuclear factor E2-related factor 2
NLRP3 NOD-like receptor thermal protein domain associated protein 3
NF-ΚB Nuclear factor kappa-B
ORs Odds ratios
PPAR-γ peroxisome proliferator-activated receptor γ
QGJN Qianggan capsule
QZJN Qiaozhi capsule
RCTs Randomized clinical trials
SDH Succinate dehydrogenase
SQZGW Sanqi Zhigan pill
SUCRA Surface Under the Cumulative Ranking
TG Triglyceride
TC Total cholesterol
TNF-α Tumor necrosis factor-α
TGF-β1 Transforming growth factor-β1
VIP China Science and Technology Journal Database
WM Western medicine
XZKJN Xuezhikang capsule
YGLP Yiganling tablet
HGJN Hugan capsule
ZBTJN Zhibitai capsule
Keywords: non-alcoholic fatty liver disease, Chinese patent medicine (CPM), network meta-analysis, western medicine, efficacy
Citation: Zhang L, Liu S, Gu Y, Li S, Liu M and Zhao W (2023) Comparative efficacy of Chinese patent medicines for non-alcoholic fatty liver disease: A network meta-analysis. Front. Pharmacol. 13:1077180. doi: 10.3389/fphar.2022.1077180
Received: 22 October 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Juei-Tang Cheng, Chang Jung Christian University, TaiwanReviewed by:
Junzhao Ye, The First Affiliated Hospital of Sun Yat-sen University, ChinaHuichun Xing, Beijing Ditan Hospital, Capital Medical University, China
Copyright © 2023 Zhang, Liu, Gu, Li, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghao Liu, bGl1bWgwMTVAMTYzLmNvbQ==; Wenxia Zhao, emhhby13ZW54aWFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Lihui Zhang
Lihui Zhang Sutong Liu
Sutong Liu Yajiao Gu2
Yajiao Gu2 Minghao Liu
Minghao Liu Wenxia Zhao
Wenxia Zhao