
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 12 December 2022
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1075309
This article is part of the Research Topic Clinical Trials in Drug Metabolism and Transport: 2022 View all 13 articles
 Tingting Ma1
Tingting Ma1 Bei Cao1
Bei Cao1 Lei Huang1
Lei Huang1 Yuanxun Yang1
Yuanxun Yang1 Yan Geng1
Yan Geng1 Pinhao Xie1
Pinhao Xie1 Yu Zhao1
Yu Zhao1 Hui Lin1
Hui Lin1 Kun Wang2
Kun Wang2 Chunhe Wang3*
Chunhe Wang3* Runbin Sun1*
Runbin Sun1* Juan Li1*
Juan Li1*Purpose: To evaluate the safety, tolerability, pharmacokinetics and immunogenicity of DS002 injection, an anti-nerve growth factor (anti-NGF) monoclonal antibody for treating pain conditions, in healthy Chinese subjects.
Methods: This study was a single-center, randomized, double-blind, single-dose escalation, placebo-controlled design (CTR20210155). A total of 53 healthy subjects, 27 male and 26 female, were enrolled in this study, and one subject withdrew from the study before administration. Seven dose groups were set up, which were 0.5 mg, 1.0 mg, 2.0 mg, 4.0 mg, 7.0 mg, 12.0 mg and 20.0 mg, respectively. The drug was administered by single subcutaneous injection. Four subjects were enrolled in the first dose group (0.5 mg) received DS002. Other dose groups enrolled eight subjects each, six of whom received DS002 while the other two received a placebo. Safety, tolerability, pharmacokinetic parameters and immunogenicity of DS002 were assessed.
Results: DS002 was well tolerated; all adverse events were Grade 1–2, and did not reach the termination standard of dose increment within the range of 0.5–20.0 mg. Adverse event rates were generally similar across treatments. After a single subcutaneous injection, the median Tmax in different dose groups ranged 167.77–337.38 h; mean t1/2 ranged 176.80–294.23 h, the volume of distribution (Vz) ranged 5265.42–7212.00 ml, and the clearance rate (CL) ranged 12.69–24.75 ml/h. In the dose range of 0.5–20.0 mg, Cmax ranged from 51.83 ± 22.74 ng/ml to 2048.86 ± 564.78 ng/ml, AUC0-t ranged from 20615.16 ± 5698.28 h·ng/mL to 1669608.11 ± 387246.36 h·ng/mL, and AUC0-inf ranged from 21852.45 ± 5920.21 h·ng/mL to 1673504.66 ± 389106.13 h·ng/mL. They all increased with dose escalation, and Cmax and AUC0-t did not have a significant dose-linear relationship, whilst AUC0-t was not dose-dependent at all. anti-drug antibody test results of each group of all subjects in this trial were negative.
Conclusion: DS002 showed satisfactory safety within the dose range of 0.5 mg–20.0 mg. The absorption and metabolism of DS002 were slow, it exhibited a low volume of distribution and the clearance rate was low. These data suggest that DS002, by blocking nerve growth factor, is expected to become a novel, safe and non-addictive treatment for pain conditions.
Chronic pain affects more than 30% of people in the world. The individual and societal burden of chronic pain are substantial (Cohen et al., 2021). In the state of chronic pain, the protective effect of pain becomes disordered and morbid. The pain attack leads to sleep disorder, anorexia, mental collapse, personality distortion and other consequences. Many patients even choose to commit suicide because they can’t bear the long-term pain, which jeopardized their lives and quality of life (Hooley et al., 2014). Non-steroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics are the traditional main treatment for chronic pain (Chang et al., 2016). However, long-term use of NSAIDs causes adverse effects related to gastric bleeding and cardiovascular (Schjerning et al., 2020). Opioids are effective short-term treatment but have addictive and respiratory inhibitory effects (Coussens et al., 2019; Kiyatkin 2019), which limits their clinical application. Therefore, the successful management of chronic pain remains a significant medical challenge.
The nerve growth factor (NGF) belongs to the neurotrophic factor family. It was originally extracted from mouse submandibular gland and snake venom, and exists in almost all vertebrates (Tong et al., 2012). NGF exists in various species as different polymers (precursors), among which the β subunit has NGF biological activity and is called β-NGF; Mature dissociation β-NGF is composed of two 118-amino acid peptides connected through non-covalent bonds (Shooter 2001). Although NGF has a critical role in early neural development, in adults, this role changes to other functions, including neuronal plasticity, hypersensitization to noxious stimuli, and pain signaling. (Enomoto et al., 2019; Wise et al., 2021). When NGF binds to the functional receptor tropomyosin receptor kinase A (TrkA) on the nociceptor, it activates cytoplasmic ERK, PLC/PKC and other signal pathways. NGF-TrkA signaling reduces the threshold of the neuronal action potential, improves neuronal excitability, and then sensitizes pain (Tong et al., 2012; Wise et al., 2021). Elevated NGF levels have been associated with acute and chronic pain conditions and injured and inflamed tissues. NGF is expected to become a potential non-addictive analgesic target. The anti-NGF antibody can bind to NGF and block its interaction with TrkA to interrupt the pain-sensing neurons to send signals.
Anti-NGF antibody drugs include Pfizer/Lilly’s Tanezumab and Regeneron/Tiva’s Fasinumab, both of which have achieved significant efficacy in the treatment of moderate to severe osteoarthritis and chronic lower back pain. However, both drugs reported severe adverse reactions in joints, including osteonecrosis and rapidly progressive osteoarthritis (RPOA), (Jayabalan and Schnitzer 2016). Therefore, it is of great social significance and clinical prospect to develop safer anti-NGF antibodies, in order to meet clinical needs and improve patients’ quality of life.
DS002 is an anti-NGF monoclonal antibody independently developed by Dartsbio Pharmaceuticals, Ltd. In vitro studies have shown that DS002 can successfully block the binding of NGF to TrkA receptors. In pre-clinical studies, DS002 treatment demostrated potent analgesic effects in several pain models including OA pain, chemotherapy-induced pain, and cancer pain. Based on the preclinical repeated toxicity test and efficacy test, as well as the clinical trial of drugs at the same target (Jonsson et al., 2016), the dose range selection of this first-in-human trial for the healthy subject was set as 0.5 mg–20.0 mg. The objective of this first-in-human study was to evaluate the safety, tolerability, pharmacokinetics and immunogenicity of a single subcutaneous injection of DS002 in healthy volunteers.
This study (CTR20210155) was approved by the ethics committee of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, and carried out according to the Declaration of Helsinki (2013 version), the Guideline For Good Clinical Practice issued by the International Council for Harmonisation (ICH), the Good Clinical Practice (GCP) issued by the National Medical Products Administration (NMPA) and relevant regulations. Written informed consent was obtained from all subjects.
The overall study was a single-center, randomized, double-blind, single-dose escalation, placebo-controlled phase I clinical trial. A total of 53 Chinese healthy subjects were enrolled in this study, including 27 males and 26 females, and 52 subjects completed this clinical trial.
The dose-escalation design was used to evaluate the safety, tolerability, pharmacokinetics and immunogenicity of DS002 after a single administration. Seven dose groups were set up, including 0.5, 1.0, 2.0, 4.0, 7.0, 12.0 and 20.0 mg, not per kg, respectively. DS002 was administered by subcutaneous injection. Four subjects were enrolled in the first dose group (0.5 mg), and each subject received a singledose of DS002 subcutaneously. In the 2nd to 7th dose groups, eight subjects were enrolled in each group, among whom 6 received DS002 and two subjects received placebo. A series of blood samples were collected before and after DS002 administration to evaluate the PK parameters and immunogenicity of DS002 in healthy subjects. Subjects were clinically followed up on the 4th, 8th, 15th, 22nd, 29th, 43rd, 57th, 85th and 113th days, biological samples were collected and safety and tolerability were assesseded. Subjects were followed up by telephone on the 120th day, which was the end of each dose group. (Figure 1). The next dose escalation group could only be started after the assessment results on the 29th day of all subjects in the previous dose group were confirmed as safe.
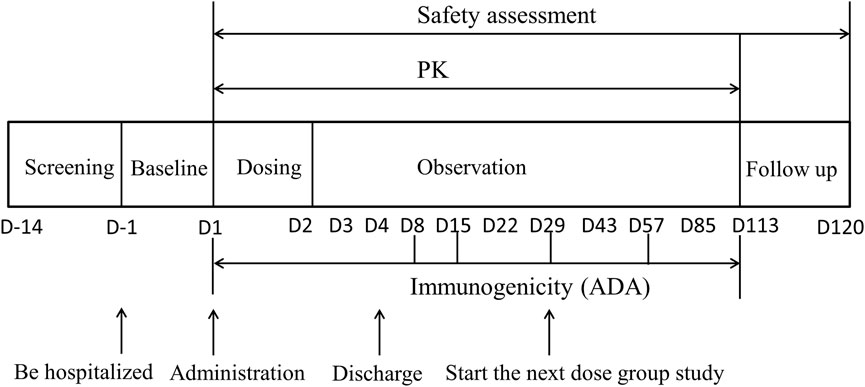
FIGURE 1. The flow chart of DS002 single-dose administration. PK, pharmacokinetics; ADA, anti-drug antibody.
During hospitalization in the research center, the subjects were managed with a unified diet and rest protocol. On the day before DS002 administration, the subjects stayed in the Phase I trials unit, took a unified dinner, and then fasted overnight for at least 10 h. The next morning, they ate a small amount of standard breakfast and then received DS002 injection subcutaneously. After injection, they ate the standard meals. Within 48 h prior to enrollment and during the whole trial period, it was forbidden to take any food and drink rich in caffeine or xanthine (such as tea, coffee, chocolate, cola, etc.), smoking and drinking alcohol-containing products were also not allowed. Strenuous exercise (exceeding normal daily activities) were avoided during the whole trial period. Subjects were told to take proper contraception from the start of the study period to 6 months after DS002 administration.
This study was a double-blind trial design (except for the first dose group). Blindness was maintained for the generation of random numbers, the coding of drugs used in the trial, the drug matched to each subject, the recording and evaluation of test results, the monitoring of the test process and data management. On the day of DS002 administration, the investigator checked the information of all subjects to ensure that they met the inclusion criteria of the trial, and then administered according to the drug number matched with the random number of the subject. All relevant personnel remained blind during the clinical study.
PK blood sampling time points: for determination of the concentration of DS002, blood samples were collected at 17 time points in each dose group: within 1 h before DS002 administration, and 6 h, 8 h, 12 h, D2 (24 h), D2 (36 h), D3 (48 h), D3 (60 h), D4 (72 h), D8 (168 h), D15 (336 h), D22 (504 h), D29 (672 h), D43 (1,008 h), D57 (1,344 h), D85 (2016 h), and D113 (2688 h) after administration. About 3 ml of venous blood was collected at each time point (Figure 1).
Immunogenicity (ADA) blood sampling time points: blood samples were collected at 6 time points in each dose group, including within 1 h before administration, D8 (168 h), D15 (336 h), D29 (672 h), D57 (1,344 h) and D113 (2688 h) after administration, and about 3 ml of venous blood was collected at each time point (Figure 1).
Subjects were healthy volunteers aged 18–45 years, including both males and females (not pregnant or breastfeeding). The body mass index (BMI) of the subjects was 19.0–26.0 kg/m2, the male body weight was ≥50 kg and female ≥45 kg. Effective contraception should be used during the trial and continued for 6 months after administration. Subjects fully understood the content, objectives and characteristics of the study and could complete the study as planned, voluntarily participated in the study and signed written informed consent.
Subjects were excluded if they met one of the following: Those who had an allergic history or allergic constitution in the past; Those who had a history of bone or joint diseases, peripheral or autonomic neuropathy, spontaneous bleeding of unknown causes and (or) disease or medical history of continuous bleeding after trauma, abnormal thyroid function or thyroid hormone, or malignant tumor; Smokers, alcoholics or drug abusers. Subjects were not allowed to take any prescription drugs, over-the-counter drugs and natural health products within 14 days before screening.
The primary objective of this study was to evaluate the safety and tolerability of DS002 as a single administration in healthy subjects. The secondary objective was to evaluate the pharmacokinetic parameters and immunogenicity of DS002 in healthy subjects after a single administration.
The safety indicators of this study included the incidence of adverse event (AE, Graded according to CTCAE v5.0) and serious adverse event (SAE); Laboratory examinations; Neurological examination; Injection site reaction; 12-lead electrocardiogram (ECG); Abdominal B ultrasound; Vital signs; Physical examination.
PK indexes: PK parameters of DS002 after a single administration, including but not limited to Cmax, Tmax, AUC0-t, AUC0-inf, t1/2, MRT, λz; Immunogenicity index: ADA after single administration of DS002.
Electrochemiluminescence Immunoassay (ECLIA) methods for the analysis of the pharmacokinetics (PK) and anti-drug antibody (ADA) of DS002 in serum were established.
For PK samples, the MSD plate was pre-coated with huNGF-his, and after adding the sample, it was incubated to form an antigen-antibody complex. After incubation, the unbound DS002 was washed away with a washing solution. Detection antibody Sulfo-Anti-DS002 antibody was then added and incubated; after incubation, unbound antibody was washed off with washing solution; finally the reading buffer was added, and MSD (MESO QuickPlexSQ120, acquisition software MSD Workbench V4.0.12) was used to collect the signal. The data were analyzed and processed with Watson LIMS (V7.4.1 and V7.6.1). The calibration ranges of DS002 were 2.50 ng/ml∼500.00 ng/ml. The quality control samples (7.5, 50 and 400 ng/ml) were determined with an accuracy of 89.0%–114.3%, the precision of 17.6% (CV) for inter-assay and accuracy of 104.0%–106.1%, precision of 19.8% (CV) for intra-assay. There was no matrix effect for human serum. No hook effect was observed for samples up to 5000.00 ng/ml. The sample can be diluted up to 1,250 fold with a total CV(%) of 5.5%.
The ADA samples were measured by screening assay, confirmatory assay, and titer assay. The method is based on the multivalency of antibodies and the specific binding of antigen-antibody. First, each control sample and the test sample were diluted by 1:20 times of acidification. Then each diluted sample solution was added to TrkA-hFc working solution for purification to remove interferences. The purified solution was then added with Biotin-DS002 and Suflo-DS002 Master Mixture (with or without drug), incubating on a dilution plate to allow a bridging reaction between the drug and the anti-drug antibody to form a bridging complex. After the reaction was completed, it was transferred to the MSD plate for incubation. After incubation, the plate was washed and then the reading buffer was added, and the signal was read on the MSD. For the screening assay, the HPC samples (5000 ng/ml) were determined with a precision of 4.4% (CV) for inter-assay and 11.9% (CV) for intra-assay. The LPC samples (16 ng/ml) were determined with a precision of 14% (CV) for inter-assay and 10.8% (CV) for intra-assay. For the confirmatory assay, the HPC samples (5000 ng/ml) were determined with a precision of 0.1% (CV) for inter-assay and 0.1% (CV) for intra-assay. The LPC samples (16 ng/ml) were determined with a precision of 25.9% (CV) for inter-assay and 18.3% (CV) for intra-assay. There was no matrix effect for human serum. No hook effect was observed for samples up to 41000.00 ng/ml.
Unless otherwise specified, SAS (version 9.4 and above) and Phoenix™ WinNonlin ® (Version 8.0 and above) were used for statistical analysis. The classified variables were summarized by frequency and percentage. Continuous variables were summarized by sample size, mean, standard deviation, median, minimum and maximum. For some continuous variables, the coefficient of variation or the geometric coefficient of variation, geometric mean, lower quartile and upper quartile were also counted. The number of subjects enrolled in each dose group and the situation of dropped cases were summarized. A descriptive statistical analysis on baseline characteristics of the enrolled cases were made. Safety and PK data were summarized using appropriate statistical tables and diagrams.
Full analysis set (FAS): all randomized subjects. Safety analysis set (SS): all subjects who have received the study drug at least once and have at least one safety evaluation index. PK concentration and parameter analysis set (PKCS and PKPS): all subjects who have used the study drug and have at least one PK concentration or parameter data. Immunogenicity analysis set (IS): all subjects who have received the study drug at least once and have at least one post-baseline immunogenicity evaluation data.
Based on the PKCS, the concentration data were tabulated and described by dose groups. The drug concentration-time curves (linear and semi-logarithmic) were drawn according to the planned blood collection time.
Based on PKPS, a Non-Compartmental Analysis by Phoenix™ WinNonlin® (version 8.0 or above) was adopted. PK parameters were calculated according to the actual blood collection time, and PK parameters were listed and described statistically. A power model was used to analyze the linear relationship between main PK parameters (Cmax, AUC) and dose.
The study was conducted from 22 February 2021 to 24 May 2022. In this trial, a total of 53 healthy Chinese subjects in seven dose groups were included in the FAS, and 52 were included in the SS (one subject withdrew from the study before administration), including 40 subjects receiving DS002, four in the 0.5 mg dose group, and six in other dose groups; There were 12 subjects receiving placebo. All 52 subjects completed the study (Figure 2). The demographics and baseline characteristics analysis of the subjects are shown in Table 1.
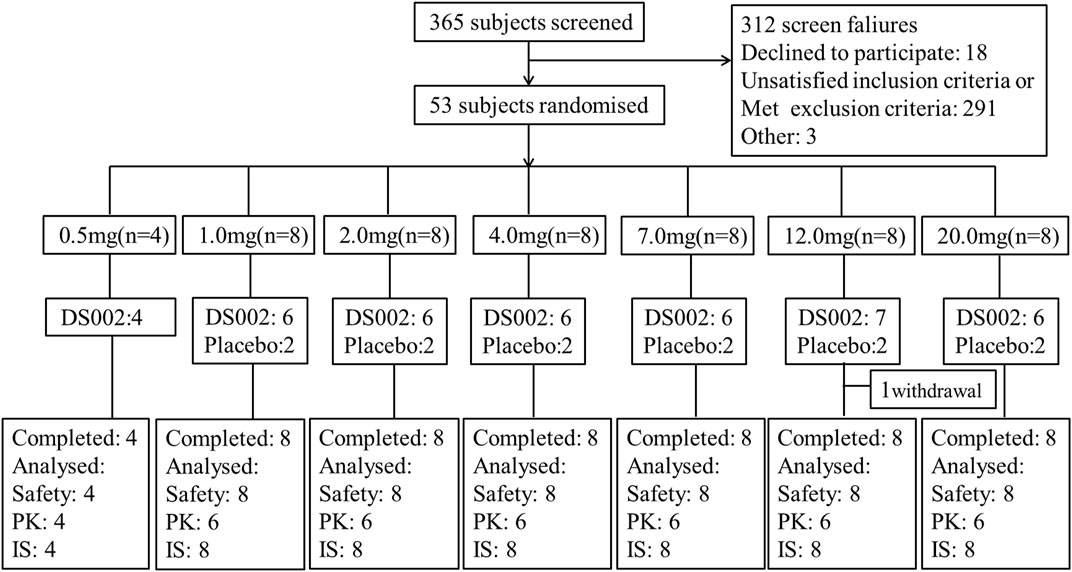
FIGURE 2. Subject disposition of participants in the study. PK, pharmacokinetics; IS, Immunogenicity analysis set.
At least one adverse event (AE) occurred in 36 of 40 (90%) healthy subjects who received DS002 and 12 of 12 (100%) subjects who received placebo. Forty-five subjects (86.54%) had adverse reactions (ARs). 12 (25.00%) subjects of DS002 had AEs of grade 2 (vs. placebo, 1/12, 8.33%). No grade 3 or grade 4 AEs were reported. The TEAEs of subjects are shown in Table 2.
Among the 40 subjects injected DS002, the AEs with an incidence rate of more than 5% include the following: upper respiratory tract infection (25.00%, 10/40); decreased triiodothyronine, and elevated serum uric acid (22.50%, 9/40); elevated amylase (20.00%, 8/40); elevated serum thyrotropin (15.00%, 6/40); decreased white blood cell count (12.50%, 5/40); arthralgia (10.00%, 4/40); positive urine red blood cell, positive urine occult blood, elevated triglycerides, elevated alanine aminotransferase, prolonged QTc interval of ECG, hypokalemia, and decreased free thyroxine (7.50%, 3/40); increased neutrophil count, abnormal ECG T wave, increased white blood cell count, elevated free thyroxine, elevated γ-glutamyltransferase, elevated aspartate aminotransferase, elevated serum calcium and elevated free triiodothyronine (5.00%, 2/40).
In the placebo group, 12 subjects (100.00%) had AEs. The incidence rates from high to low were elevated amylase (33.33%, 4/12); elevated serum thyrotropin, decreased leukocyte count, and increased neutrophil count (25.00%, 3/12); decreased triiodothyronine, abnormal ECG T wave, and positive urine protein (16.67%, 2/12); all (100.00%) had ARs. Of which one subject (8.33%) had 2 times Grade 2 AE.
The incidences of AEs and ARs in each dose group showed an overall upward trend with dose escalation, and the incidence of AEs and ARs between DS002 and placebo subjects was similar. One case of Grade 2 AE (elevated amylase without symptom) was reported in the 12.0 mg dose group, whilst the rest of elevated amylase AEs were all Grade 1. No serious adverse event (SAE) was reported in subjects who received DS002 injection. None of the subjects died or dropped out to TEAE.
In brief, after a single dose of DS002, none of the dose groups reached the termination standard of dose escalation, and the safety and tolerability profiles of DS002 were satisfied.
All 40 subjects receiving DS002 were included in the PK analysis. The PK parameters of the DS002 and placebo groups are shown in Table 3.
In this study, AUC_% Extrap of DS002 in the dose range of 0.5 mg–20 mg was less than 5.81% (Table 3), which indicated that the design of the blood sampling time points for PK test was reasonable, and the calculated terminal clearance rate constant and related parameters were reliable.
After a single subcutaneous injection of DS002 in healthy subjects, the median Tmax was 167.77–337.38 h; The mean t1/2 was 176.80–294.23 h. Except for the 0.5 mg dose group, of which t1/2 was 176.80 h, t1/2 of the remaining dose groups ranged 205.46–294.23 h (Table 3). In the dose range of 0.5–20.0 mg, Cmax, AUC0-t and AUC0-inf of prototype drug DS002 increased with the increase of dose (Table 3; Figures 3A,B).
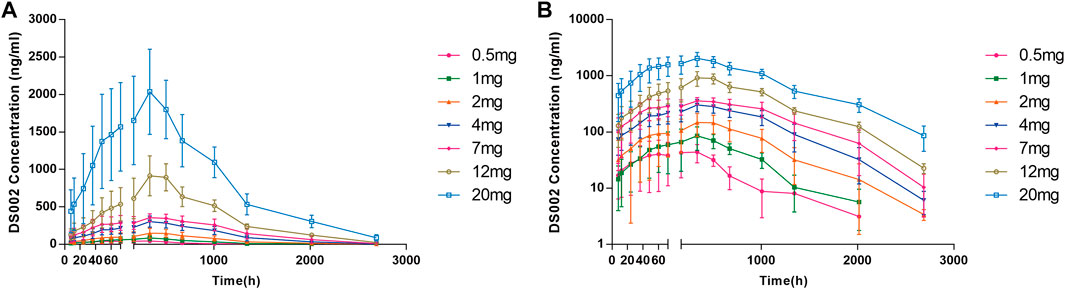
FIGURE 3. Plasma concentration-time profile (PKCS) of DS002. (A) DS002 concentrations time curves of subjects; (B) Semi-logarithmic curves of DS002 concentrations time of subjects. PKCS, pharmacokinetics concentration set. Data are presented as mean ± SD.
After a single DS002 administration to healthy subjects, Cmax, AUC0-t, AUC0-inf and dose were simultaneously logarithmically converted to perform linear PK analysis. The 90% confidence interval of Cmax slope was 90.61%–106.92%, the 90% confidence interval of AUC0-t slope was 107.41%–121.13%, and the 90% confidence interval of AUC0-inf slope was 106.05%–119.33%. The results indicated that Cmax and AUC0-inf increased with dose, but did not have a significant dose-linear relationship, whilst AUC0-t was not dose dependent at all. (Table 4).
The ADA test results of every group of subjects in this trial were negative at all follow-up visits, indicating that the immunogenicity of the DS002 injection was low.
Pain can be treated more effectively with analgesics that operate on different mechanisms. Traditional NSAIDs and opioids are the most widely used analgesics, but their long-term use causes side effects and addiction, which is unable to meet clinical needs (Chang et al., 2016; Coussens et al., 2019; Kiyatkin 2019; Schjerning et al., 2020). DS002, an anti-NGF monoclonal antibody, is developed as a potential analgesic. Preclinical studies have shown that it could effectively supress NGF/TrkA signalingand reset the pain threshold without obvious adverse reactions and addiction problems. Here in this first-in-human study, we evaluated the safety, tolerability and PK parameters of DS002 as a single subcutaneous injection in healthy subjects. DS002 was well tolerated and safe within the dose range of 0.5–20.0 mg. PK results showed that the absorption and metabolism of DS002 was slow, and had a long effect time. Immunogenicity results suggested that ADA was negative throughout all follow-up visits.
In terms of safety, all subjects receivied DS002 injection completed the trial. No dose-limiting toxicity was observed, and the dose increment termination criteria were not reached within the dose range of 0.5–20 mg. Five subjects suffered from arthralgia, including four subjects receiving DS002 (one in 4.0 mg, one in 12.0 mg and two in 20.0 mg group) and one subject in the placebo group. The symptoms were mild without any discomfort such as activity disorder, and were all resolved without intervention. It is worth noting that no severe bone and joint events or peripheral neuropathy/paresthesia were found. No rapid progression of osteoarthritis (RPOA) was observed even at high doses, which was the most prominent concern when the US Food and Drug Administration shelved the development plan of similar anti-NGF drugs. Collectively, the safety data of DS002 indicate that it may serve as provide a new therapeutic strategy for the target of analgesic treatment.
In adults, NGF and its interaction with TrkA have been found to play a vital role in the nociception and plasticity of the nervous system under pain conditions. Therefore, various monoclonal antibody therapies targeting this pathway have been studied to treat chronic pain. Although the Food and Drug Administration once shelved the development plan for an anti-NGF monoclonal antibody due to safety concerns, this target still has great clinical value and application prospect. Currently, no anti-NGF monoclonal antibody has been approved for clinical use, but three anti-NGF drugs are under development: tanezumab, fulranumab and fasinumab (Enomoto et al., 2019; Zhao et al., 2022). Tanezumab was designated as a fast track (Hochberg et al., 2016). The monoclonal antibody has unique pharmacokinetic parameters such as non-linear pharmacokinetics metabolism, time dependence and long half-life; Target-mediated distribution (TMD) and clearance (TMC) are important pathways for macromolecular drug metabolism. The occurrence of TMD and TMC are the important sources of non-linear PK characteristics. The t1/2 of tanezumab is about 21 day (Jonsson et al., 2016), while that of DS002 was around 7–12 days. In the dose range of 0.5–20.0 mg, Cmax, AUC0-t and AUC0-inf of prototype drug DS002 increased with the increase in dosage. Cmax and AUC0-inf did not show a significant dose linear relationship, whilst AUC0-t was not dose dependent at all. The PK parameters of DS002 conform to the metabolic characteristics of monoclonal antibodies. Moreover, the immunogenicity of DS002 injection was low (ADA was negative).
In conclusion, the absorption and metabolism of a single subcutaneous injection of DS002 in healthy subjects were slow, it exhibited a low volume of distribution, and the clearance rate was low. Single subcutaneous injection of DS002 was well tolerated and non-immunogenic within the dose range of 0.5 mg–20.0 mg. The anti-NFG antibody represented by DS002 shows promise as a novel, safe and non-addictive approach for pain treatment. This first-in-human study supports further development of DS002 as a potential analgesic treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the ethics committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School. The participants provided their written informed consent to participate in this study.
CW and JL designed the research. All authors performed the research. TM and CW analyzed the data. TM and RS contributed to the writing of the manuscript.
This study was funded by the National Natural Science Foundation of China (No. 31371399), Major Scientific and Technological Special Project of Zhongshan City (210205143867019/2021A1012) and Dartsbio Pharmaceuticals, Ltd.
The authors would like to thank the following people for their contributions: All volunteers who participated in this study, all the investigators, clinical research associates and clinical research coordinators who participated in supporting the trial.
CW was employed by the Company Dartsbio Pharmaceuticals, Ltd. KW was employed by the Company Beijing Highthink Pharmaceutical Technology Service Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chang, D. S., Hsu, E., Hottinger, D. G., and Cohen, S. P. (2016). Anti-nerve growth factor in pain management: Current evidence. J. Pain Res. 9, 373–383. doi:10.2147/JPR.S89061
Cohen, S. P., Vase, L., and Hooten, W. M. (2021). Chronic pain: An update on burden, best practices, and new advances. Lancet 397 (10289), 2082–2097. doi:10.1016/S0140-6736(21)00393-7
Coussens, N. P., Sittampalam, G. S., Jonson, S. G., Hall, M. D., Gorby, H. E., Tamiz, A. P., et al. (2019). The opioid crisis and the future of addiction and pain therapeutics. J. Pharmacol. Exp. Ther. 371 (2), 396–408. doi:10.1124/jpet.119.259408
Enomoto, M., Mantyh, P. W., Murrell, J., Innes, J. F., and Lascelles, B. D. X. (2019). Anti-nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet. Rec. 184 (1), 23. doi:10.1136/vr.104590
Hochberg, M. C., Tive, L. A., Abramson, S. B., Vignon, E., Verburg, K. M., West, C. R., et al. (2016). When is osteonecrosis not osteonecrosis?: Adjudication of reported serious adverse joint events in the tanezumab clinical development program. Arthritis Rheumatol. 68 (2), 382–391. doi:10.1002/art.39492
Hooley, J. M., Franklin, J. C., and Nock, M. K. (2014). Chronic pain and suicide: Understanding the association. Curr. Pain Headache Rep. 18 (8), 435. doi:10.1007/s11916-014-0435-2
Jayabalan, P., and Schnitzer, T. J. (2016). Tanezumab in the treatment of chronic musculoskeletal conditions. Expert Opin. Biol. Ther. 17 (2), 245–254. doi:10.1080/14712598.2017.1271873
Jonsson, E. N., Xie, R., Marshall, S. F., and Arends, R. H. (2016). Population pharmacokinetics of tanezumab in phase 3 clinical trials for osteoarthritis pain. Br. J. Clin. Pharmacol. 81 (4), 688–699. doi:10.1111/bcp.12850
Kiyatkin, E. A. (2019). Respiratory depression and brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin, and fentanyl. Neuropharmacology 151, 219–226. doi:10.1016/j.neuropharm.2019.02.008
Schjerning, A.-M., McGettigan, P., and Gislason, G. (2020). Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 17 (9), 574–584. doi:10.1038/s41569-020-0366-z
Shooter, E. M. (2001). Early days of the nerve growth factor proteins. Annu. Rev. Neurosci. 24 (1), 601–629. doi:10.1146/annurev.neuro.24.1.601
Tong, Q., Wang, F., Zhou, H.-Z., Sun, H.-L., Song, H., Shu, Y.-Y., et al. (2012). Structural and functional insights into lipid-bound nerve growth factors. FASEB J. 26 (9), 3811–3821. doi:10.1096/fj.12-207316
Wise, B. L., Seidel, M. F., and Lane, N. E. (2021). The evolution of nerve growth factor inhibition in clinical medicine. Nat. Rev. Rheumatol. 17 (1), 34–46. doi:10.1038/s41584-020-00528-4
Zhao, D., Luo, M.-h., Pan, J.-k., Zeng, L.-f., Liang, G.-h., Han, Y.-h., et al. (2022). Based on minimal clinically important difference values, a moderate dose of tanezumab may be a better option for treating hip or knee osteoarthritis: A meta-analysis of randomized controlled trials. Ther. Adv. Musculoskelet. Dis. 14, 1759720X2110676. doi:10.1177/1759720x211067639
Keywords: DS002, anti-nerve growth factor antibody, first-in-human trial, pharmacodynamics, novel analgesic
Citation: Ma T, Cao B, Huang L, Yang Y, Geng Y, Xie P, Zhao Y, Lin H, Wang K, Wang C, Sun R and Li J (2022) First-in-human study to assess the safety, tolerability, pharmacokinetics and immunogenicity of DS002, an anti-nerve growth factor monoclonal antibody. Front. Pharmacol. 13:1075309. doi: 10.3389/fphar.2022.1075309
Received: 20 October 2022; Accepted: 29 November 2022;
Published: 12 December 2022.
Edited by:
Zhihao Liu, Dalian Medical University, ChinaReviewed by:
Jiaqi Mi, Boehringer Ingelheim, GermanyCopyright © 2022 Ma, Cao, Huang, Yang, Geng, Xie, Zhao, Lin, Wang, Wang, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, anVhbmxpMjAwM0BuamdseXkuY29t; Runbin Sun, cnVuYmluc3VuQGdtYWlsLmNvbQ==; Chunhe Wang, d2FuZ2NAZGFydHNiaW8uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.