
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 11 January 2023
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1069321
This article is part of the Research TopicDrug Metabolism and Transport: The Frontier of Personalized MedicineView all 22 articles
 Kakeru Sato1
Kakeru Sato1 Tatsuya Seki1
Tatsuya Seki1 Asuka Mizutani2
Asuka Mizutani2 Yuka Muranaka1
Yuka Muranaka1 Shiho Hirota1
Shiho Hirota1 Kodai Nishi3
Kodai Nishi3 Kana Yamazaki4
Kana Yamazaki4 Ryuichi Nishii4
Ryuichi Nishii4 Takeo Nakanishi5
Takeo Nakanishi5 Ikumi Tamai6
Ikumi Tamai6 Keiichi Kawai2,7
Keiichi Kawai2,7 Masato Kobayashi2*
Masato Kobayashi2*Chemoradiotherapy is frequently used to treat cancer. Stereotactic body radiotherapy (SBRT) is a single high-dose radiotherapy used to treat a variety of cancers. The anticancer drug methotrexate (MTX) shows affinity for solute carrier (SLC) and ATP-binding cassette (ABC) transporters. This study investigated relationships between accumulation of methotrexate and gene expression levels of solute carrier and ATP-binding cassette transporters in cancer cells after a single and high-dose X-ray irradiation. Cancer cell lines were selected from lung and cervical cancer cell line that are commonly used for stereotactic body radiotherapy and effective with methotrexate. We examined expression levels of organic anion-transporting polypeptide (OATP)1B1, OATP1B3, OATP1B7, and organic anion transporter (OAT)1 as solute carrier transporters and multidrug resistance-associated protein (MRP)1 and MRP2 as ATP-binding cassette transporters, using real-time polymerase chain reaction and accumulation of 3H-MTX in cancer cells after 10-Gy irradiation, assuming stereotactic body radiotherapy. Cells were divided into three groups: Control without irradiation; 4 h after irradiation; and 24 h after irradiation. In control, gene expression levels of OAT1 in all cells was below the limit of measurement. After irradiation, gene expression levels of OATP1B1/1B3/1B7 showed changes in each cell line. Gene expression levels of MRP1/2 tended to increase after irradiation. Gene expression levels of OATP1B1/1B3/1B7 were much lower than those of MRP1/2. Accumulation of 3H-MTX tended to decrease over time after irradiation. Irradiation of cancer cells thus alters gene expression levels of both solute carrier transporters (OATP1B1/1B3/1B7) and ABC transporters (MRP1/2) and decreases accumulation of 3H-MTX in cancer cells over time due to elevated expression of MRP1/2.
Chemoradiotherapy combines chemotherapy with anticancer drugs and radiotherapy to treat cancer. Chemoradiotherapy can be divided into three categories according to the timing of anticancer drug administration: Neoadjuvant; concurrent; and adjuvant (Baldini et al., 2018). The accumulation of anticancer drugs in cancer cells usually depends on gene expression levels of solute carrier (SLC) and ATP-binding cassette (ABC) transporters (Nakanishi, 2007; Carmichael and Day, 2022). SLC transporters mainly contribute to the uptake of anticancer drugs, while ABC transporters are involved in their excretion (Nakanishi, 2007; Carmichael and Day, 2022).
In radiotherapy, stereotactic radiotherapy involves the delivery of higher doses (7–18 Gy or more) than the usual single-beam dose (1.8–2 Gy) and is used to treat various cancers (Marcrom et al., 2017; Jardel et al., 2020; Sarudis et al., 2021; Ugurluer et al., 2021). Stereotactic radiotherapy was originally used to treat brain cancers, with stereotactic body radiotherapy (SBRT) representing the application of this technology to the trunk of the body, such as for lung and liver cancers (Song et al., 2004; Donovan and Swaminath, 2018; Tandberg et al., 2018; Sarudis et al., 2021; Ugurluer et al., 2021). SBRT has also been shown to be effective against cervical cancer, which is still frequently treated using intracavitary small-source radiotherapy (Ito et al., 2019; Facondo et al., 2021)
Methotrexate (MTX) is a folate antagonist used as an anticancer drug (Visentin et al., 2012). This agent stops cancer growth by preventing the uptake of folic acid, which is necessary in DNA synthesis (Yu et al., 2020). MTX is effective against lung and cervical cancers, where SBRT also appears useful (Conroy et al., 1976; Smyth and Ford, 1981). MTX has shown affinity for the SLC transporters organic anion-transporting polypeptide (OATP) and organic anion transporter (OAT), and the ABC transporters multidrug resistance-associated protein (MRP), multidrug resistance protein (MDR), and breast cancer resistance protein (BCRP) (Hagenbuch and Meier, 2004; Nakanishi, 2007; Murakami and Mori, 2012; Gao et al., 2021). While irradiation increases the expressions of MRP1 and MRP2, contributing to the efflux of MTX (Henness et al., 2002; Bartkowiak et al., 2009), the effects of irradiation on SLC transporters have not been examined. Further, correlations between the kinetics of anticancer drugs including MTX and SLC and ABC transporters after irradiation have yet to be clarified. The purpose of this study was thus to investigate the relationships between accumulation of MTX and expression levels of the genes for SLC and ABC transporters in cancer cells after irradiation. Temporal changes in MTX accumulation in cancer cells after a single and high-dose irradiations assuming SBRT were examined.
The human-derived lung adenocarcinoma cancer cell lines NCI-H441 (American Type Culture Collection, Manassas, VA, United States) and PC-14 (RIKEN Cell Bank, Tsukuba, Japan) were used. The HeLa human-derived cervical cancer line (RIKEN Cell Bank) was also used. H441, PC-14 and HeLa cell lines were cultivated using RPMI-1640 (FUJIFILM Wako Chemical, Osaka, Japan), Dulbecco’s Modified Eagle’s Medium (FUJIFILM Wako Chemicals) and Eagle’s minimum essential medium (FUJIFILM Wako Chemicals) mixed with 10% fetal bovine serum and 1% sodium pyruvate at 37°C under conditions of 5% CO2.
After achieving 70–80% confluence in a 10-cm diameter plate, each cell line was irradiated with a single 10-Gy X-ray (dose rate, 1.0 Gy/min) using X-ray irradiation equipment (MBR1520R-3; Hitachi, Tokyo, Japan). Cells were divided into three groups: Control without irradiation; 4 h after irradiation; and 24 h after irradiation.
An RNeasy Plus Mini Kit (QIAGEN, Hilden, Germany) was used to extract RNA from the cancer cells used in this study. The quality of the extracted RNA was evaluated using the RNA integrity number (RIN) as an indicator of quality. The RIN is expressed as a number from 1 to 10, with a higher number reflecting higher quality of RNA. An Affinity Script QPCR cDNA Synthesis kit (Agilent Technologies, Tokyo, Japan) was used for synthesizing cDNA.
Real-time PCR was performed using an AriaMx 5P system (Agilent Technologies). OATP1B1/1B3/1B7 as the combination of OATP1B1, OATP1B3 and OATP1B7 for SLC transporters (because the primer sequences of these transporters are quite similar) and MRP1 and MRP2 for ABC transporters were selected as the targets of PCR. The gene ACBT for β-actin was used as the internal control gene, as a housekeeping gene that is constantly expressed in all cells. Also, ACBT was used to correct for differences in the amounts of initial RNA and cDNA due to differences in sample organization and purification methods. Primer design was outsourced to Eurofins Genomics (Tokyo, Japan). Preparation of cloned plasmids used for the creation of standard curves was outsourced to GenScript (Tokyo, Japan). Primer sequences and concentrations of the genes used are shown in Table 1. A 20-µL volume of PCR reaction solution contained 10 µL of Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies), .4 µL of primer, 1 µL of template (10–50 ng of cDNA or cloned plasmid) and 8.6 µL of nuclease-free water. The thermal profile of reaction conditions was: 95°C hot start for 3 min, then 45 cycles of amplification at 95°C for 5 s and 62°C for 15 s, ending with 95°C for 1 min, 55°C for 30 s and 95°C for 30 s.
Each cell was seeded at 1.0 ×105 cells/well in 12-well plastic plates. At about 24 h after seeding, cells were irradiated and divided into three groups: 4 h after irradiation; 24 h after irradiation; and control without irradiation. Each group was pre-incubated for 5 min in phosphate-buffered saline (PBS), then incubated with 3H-MTX (10 kBq/well) for 5, 10, 30, or 60 min. After incubation, cells were washed twice with 600 µL of PBS and lysed by 500 µL of .1 M NaOH. Three hundred microliters of cell lysate were mixed with 5 mL of ULTIMA GOLD (Perkin Elmer, Waltham, MA, United States) and the radioactivity of the mixture was measured using a liquid scintillation counter (LSC-5100; Hitachi Aloka Medical, Tokyo, Japan). The results are shown as the percent injected dose/number of living cells measured by an automatic cell counter (LUNA FX7™; Logo Biosystems, Gyeonggi-do, South Korea).
All cell lines showed RIN >9, indicating high-quality RNA. Measured expression levels of drug transporter genes in each cell line in the three groups by conducting Real-time PCR are shown in Table 2. For SLC transporters, the total gene expression level of OATP1B1/1B3/1B7 was higher in H441 cells than in PC-14 or HeLa cells, but OAT1 in all cells was below the limit of measurement. After cell irradiation, total gene expression levels of OATP1B1/1B3/1B7 decreased in H441 in comparison to before irradiation (control), but increased slightly in PC-14 and HeLa cells.
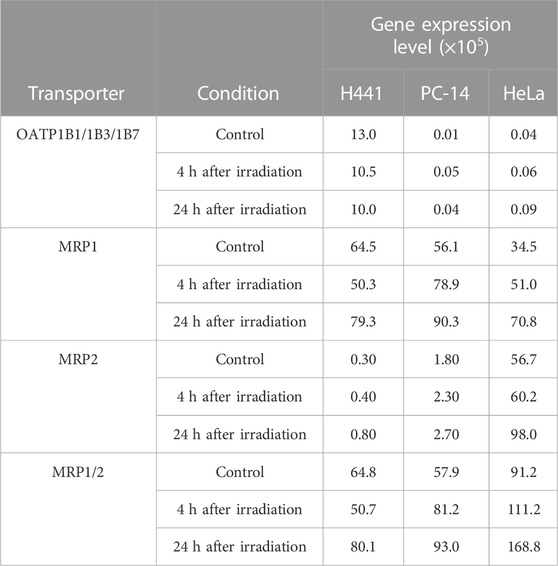
TABLE 2. Gene expression levels of measured drug transporter in each cell by conducting Real-time PCR.
For ABC transporters, expression levels of MRP1/2, as the combination of MRP1 and MRP2, were higher than levels of OATP1B1/1B3/1B7 and OAT1 in control samples of all cancer cells. In control samples, MRP1/2 showed higher expression in HeLa than in H441 or PC-14. In addition, H441 and PC-14 showed higher gene expression levels of MRP1 than MRP2, while HeLa displayed higher gene expression levels of MRP2 than MRP1. After irradiation, gene expression levels of MRP1/2 tended to increase over time in all cancer cell lines.
Figure 1 shows the accumulation of 3H-MTX in H441, PC-14 and HeLa cells in the control, 4 h after irradiation and 24 h after irradiation groups at 5, 10, 30, and 60 min after 3H-MTX injection. Accumulation of 3H-MTX was decreased at 4 and 24 h after irradiation in H441 cells and at 24 h after irradiation in PC-14 cells. In HeLa cells, accumulation of 3H-MTX was significantly decreased compared to control from 10 min after 3H-MTX injection in the 4 h after irradiation group and from 30 min after 3H-MTX injection in the 24 h after irradiation group.
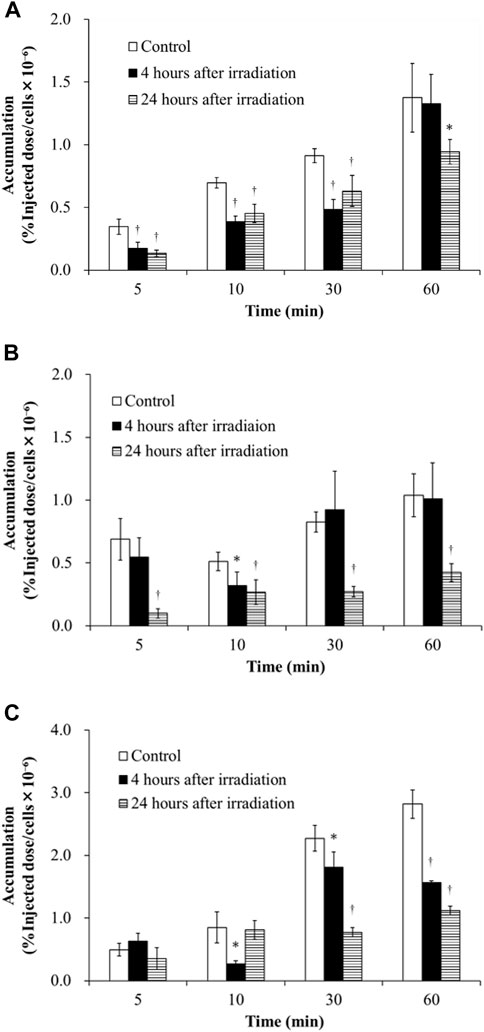
FIGURE 1. Accumulation of 3H-MTX in H441 (A), PC-14 (B) and HeLa (C) cells in the three groups including control, 4 h after irradiation and 24 h after irradiation at 5, 10, 30, and 60 min after adding 3H-MTX. Accumulation of 3H-MTX tended to decrease after irradiation in all three cancer cells. †p < .01 and *p < .05 vs. control.
Consideration of the effects of irradiation on the kinetics of anticancer drugs in chemoradiotherapy is important. This study examined how the accumulation of 3H-MTX in cancer cells was impacted by the effects of gene expression levels for SLC and ABC transporters after X-ray irradiation. Since the degree to which gene expression levels of SLC and ABC transporters are changed under the influence of irradiation was unknown, we examined these gene expressions after irradiation by conducting Real-time PCR (Table 2). Total gene expression levels of OATP1B1/1B3/1B7 decreased after irradiation in H441 cells compared to control, but increased slightly in PC-14 and HeLa cells. For ABC transporters, total gene expression levels of MRP1/2 were higher than OATP1B1/1B3/1B7 and OAT1 in all cancer cell lines under the control conditions. Accumulation of 3H-MTX tended to decrease over time after irradiation in all cancer cell lines (Figure 1), and accumulation was significantly decreased at 24 h.
The correlation between accumulation of 3H-MTX and gene expression levels of drug transporters is discussed for each cell line. In H441 cells (Table 2), gene expression levels of OATP1B1/1B3/1B7 decreased over time, and MRP1/2 was slightly decreased at 4 h after irradiation in comparison with control and increased further at 24 h after irradiation. Accumulation of 3H-MTX in H441 cells was significantly decreased compared to control at 5, 10, and 30 min after adding 3H-MTX (Figure 1A). Although we selected MRP1/2 as representative ABC transporters for MTX, changes in MDR and BCRP gene expressions might also influence the accumulation of 3H-MTX in H441 (Ji et al., 2013). Henness et al. (2002) reported that expressions of MRP1 and MRP2 were increased after fractionated irradiation, but these expression levels might change over time after a single high-dose irradiation. In PC-14 and HeLa cells (Table 2), gene expression levels of MRP1/2 were much greater than those of OATP1B1/1B3/1B7. After irradiation, the difference between OATP1B1/1B3/1B7 and MRP1/2 became greater over time.
In PC-14 (Figure 1B), accumulation of 3H-MTX showed little change between control and 4 h after irradiation, but was significantly decreased at all time points after adding 3H-MTX in cells at 24 h after irradiation. Since gene expression levels of OATP1B1/1B3/1B7 were slightly greater in PC-14 cells, the effects on gene expression levels of OATP1B1/1B3/1B7 may be greater than the effects on accumulation of 3H-MTX at 4 h after irradiation. At 24 h after irradiation, a correlation was noted between decrease in accumulation of 3H-MTX and much higher gene expression levels of MRP1/2.
In HeLa cells (Figure 1C), accumulation of 3H-MTX was significantly decreased compared to control at 4 h after irradiation from 10 min after adding 3H-MTX, and at 24 h after irradiation from 30 min after adding 3H-MTX. Although the effects of drug transporters are usually seen at 5 min after adding 3H-MTX, no significant differences at this time points were seen between control and groups at 4 and 24 h after irradiation. An equilibrium state appears to exist between functions of OATP1B1/1B3/1B7 and MRP1/2 at around 5 min after adding 3H-MTX, but gene expression levels of MRP1/2 were higher than those of OATP1B1/1B3/1B7 (Table 2). With greater expression of MRP1/2 over time, accumulation of 3H-MTX decreased significantly compared to control (Figure 1C). From 10 min after adding 3H-MTX, accumulation of 3H-MTX was higher in HeLa than in H441 and PC-14. Gene expression levels of MRP2 were also higher in HeLa than in H441 or PC-14, and expression of MRP2 was also higher than that of MRP1. These results may suggest that 3H-MTX has higher affinity for MRP1 than for MRP2.
This study was performed assuming SBRT, in which a single exposure provides a higher dose than conventional radiotherapy (Marcrom et al., 2017; Jardel et al., 2020; Sarudis et al., 2021; Ugurluer et al., 2021). Since Lei et al. (2021) reported that the survival rate of HeLa was less than 50% after a single 10-Gy irradiation, we selected a single high-dose of 10-Gy irradiation. In our experiments, cancer cells after a single high-dose irradiation have shown a tendency to excrete anticancer drugs as a foreign body. Neoadjuvant chemotherapy, which administers anticancer drugs prior to radiation, may therefore prove effective in the combination of SBRT and chemotherapy. However, these results only reflect temporal changes following a single irradiation. Future experiments will need to consider fractional irradiation at high dose. In addition, in vivo experiments will be required to confirm our in vitro results for the accumulation of 3H-MTX and expression of drug transporters.
For a more detailed examination, next-generation sequencers might be useful in the future because this method is capable of comprehensively quantifying multitude of various genes (Slatko et al., 2018). However, we intentionally selected MTX which has affinity primarily for OATP and MRP transporters as an anti-cancer drug. Therefore, Real-time PCR, which can accurately quantify gene expression levels of specific transporters, would be appropriate in this study. The Real-time PCR was also used in the study of (Sutherland et al., 2020). They examined the relationship between accumulation of anti-cancer drugs in cancer cells and gene expression levels of specific SLC transporters by Real-time PCR.
X-ray irradiation with a single, high dose to cancer cells alters gene expression levels of both SLC transporters (OATP1B1/1B3/1B7) and ABC transporters (MRP1/2). In particular, changes in MRP1/2 were much greater than those in OATP1B1/1B3/1B7. Irradiation decreased accumulation of 3H-MTX in cancer cells over time due to higher expression of MRP1/2.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Conceptualization, MK; methodology, KS, TS, and SH; investigation, KS, TS, AM, SH, YM, KN, and MK; resources, KY and RN; writing—original draft preparation, KS; writing—review and editing, TN, IT, KK, and MK; supervision, TN, IT, KK, and MK. All authors have read and agreed to the published version of the manuscript.
This study was funded in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Nos 21H02865, 22H03016, and 22K19504) and the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science.
The authors would like to thank Mikie Ohtake and other staff of the Faculty of Health Sciences, Kanazawa University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baldini, E. H., Le Cesne, A., and Trent, J. C. (2018). Neoadjuvant chemotherapy, concurrent chemoradiation, and adjuvant chemotherapy for high-risk extremity soft tissue sarcoma. Am. Soc. Clin. Oncol. Educ. Book 23 (38), 910–915. doi:10.1200/EDBK_201421
Bartkowiak, D., Stempfhuber, M., Wiegel, T., and Bottke, D. (2009). Radiation- and chemoinduced multidrug resistance in colon carcinoma cells. Strahlenther Onkol. 185 (12), 815–820. doi:10.1007/s00066-009-1993-9
Carmichael, Natasha, and Day, Philip J. R. (2022). Cell surface transporters and novel drug developments. Front. Pharmacol. 13, 852938. doi:10.3389/fphar.2022.852938
Conroy, J. F., Lewis, G. C., Brady, L. W., Brodsky, I., Kahn, S. B., Ross, D., et al. (1976). Low dose bleomycin and methotrexate in cervical cancer. Cancer 37 (2), 660–664. doi:10.1002/1097-0142(197602)37:2<660::aid-cncr2820370208>3.0.co;2-v
Donovan, E. K., and Swaminath, A. (2018). Stereotactic body radiation therapy (SBRT) in the management of non-small-cell lung cancer: Clinical impact and patient perspectives. Cancer 9, 13–23. doi:10.2147/LCTT.S129833
Facondo, G., Vullo, G., De Sanctis, V., Valeriani, M., Ascolese, A. M., Massaro, M., et al. (2021). Stereotactic body radiation therapy boost in patients with cervical cancer ineligible for brachytherapy. Cancer Diagn Progn. 1 (2), 53–60. doi:10.21873/cdp.10008
Gao, J., Wang, C., and Wei, W. (2021). The effects of drug transporters on the efficacy of methotrexate in the treatment of rheumatoid arthritis. Life Sci. 268, 118907. doi:10.1016/j.lfs.2020.118907
Hagenbuch, B., and Meier, P. J. (2004). Organic anion transporting polypeptides of the OATP/SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 447 (5), 653–665. doi:10.1007/s00424-003-1168-y
Henness, S., Davey, M. W., Harvie, R. M., and Davey, R. A. (2002). Fractionated irradiation of H69 small-cell lung cancer cells causes stable radiation and drug resistance with increased MRP1, MRP2, and topoisomerase IIalpha expression. Int. J. Radiat. Oncol. Biol. Phys. 54 (3), 895–902. doi:10.1016/s0360-3016(02)03037-7
Ito, K., Kito, S., Nakajima, Y., Shimizuguchi, T., Ogawa, H., Nihei, K., et al. (2019). Determining the recommended dose of stereotactic body radiotherapy boost in patients with cervical cancer who are unsuitable for intracavitary brachytherapy: a phase I dose-escalation study. Jpn. J. Clin. Oncol. 49 (9), 856–861. doi:10.1093/jjco/hyz074
Jardel, P., Kammerer, E., Villeneuve, H., and Thariat, J. (2020). Stereotactic radiation therapy for breast cancer in the elderly. Transl. Cancer Res. 9 (1), 86–96. doi:10.21037/tcr.2019.07.18
Ji, X. N., Yang, F., Sui, X. M., Wang, F. G., Ge, R. G., Quan, X. L., et al. (2013). Effect of fractionated irradiation on the expression of multidrug resistance genes in the CNE1 human nasopharyngeal carcinoma cell line. Mol. Med. Rep. 7 (1), 187–194. doi:10.3892/mmr.2012.1148
Lei, H., Shi, J., Teng, Y., Song, C., Zou, L., Ye, F., et al. (2021). Baicalein modulates the radiosensitivity of cervical cancer cells in vitro via miR-183 and the JAK2/STAT3 signaling pathway. Adv. Clin. Exp. 30 (7), 727–736. doi:10.17219/acem/135478
Marcrom, S. R., McDonald, A. M., Thompson, J. W., Popple, R. A., Riley, K. O., Markert, J. M., et al. (2017). Fractionated stereotactic radiation therapy for intact brain metastases. Adv. Radiat. Oncol. metastases 2 (4), 564–571. doi:10.1016/j.adro.2017.07.006
Murakami, T., and Mori, N. (2012). Involvement of multiple transporters-mediated transports in mizoribine and methotrexate pharmacokinetics. Pharmaceuticals 5 (8), 802–836. doi:10.3390/ph5080802
Nakanishi, T. (2007). Drug transporters as targets for cancer chemotherapy. Cancer Genomics Proteomics 4 (3), 241–254.
Sarudis, S., Karlsson, A., and Bäck, A. (2021). Surface guided frameless positioning for lung stereotactic body radiation therapy. J. Appl. Clin. Med. Phys. 22 (9), 215–226. doi:10.1002/acm2.13370
Slatko, B. E., Gardner, A. F., and Ausubel, F. M. (2018). Overview of next generation sequencing Technologies. Curr. Protoc. Mol. Biol. 122 (1), e59. doi:10.1002/cpmb.59
Smyth, J. F., and Ford, H. T. (1981). Methotrexate in the chemotherapy of lung cancer. Cancer Treat. Rep. 65 (1), 161–163.
Song, D. Y., Kavanagh, B. D., Benedict, S. H., and Schefter, T. (2004). Stereotactic body radiation therapy. Rationale, techniques, applications, and optimization. Oncology 18 (11), 1419–1430. discussion 1430, 1432, 1435-6.
Sutherland, R., Meeson, A., and Lowes, S. (2020). Solute transporters and malignancy: Establishing the role of uptake transporters in breast cancer and breast cancer metastasis. Cancer Metastasis Rev. 39 (3), 919–932. doi:10.1007/s10555-020-09879-6
Tandberg, D. J., Tong, B. C., Ackerson, B. G., and Kelsey, C. R. (2018). Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: A comprehensive review. Cancer 124 (4), 667–678. doi:10.1002/cncr.31196
Ugurluer, G., Mustafayev, T. Z., Gungor, G., Atalar, B., Abacioglu, U., Sengoz, M., et al. (2021). Stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of liver metastases in oligometastatic patients: Initial clinical experience. Radiat. Oncol. J. 39 (1), 33–40. doi:10.3857/roj.2020.00976
Visentin, M., Zhao, R., and Goldman, I. D. (2012). The antifolates. Hematol. Oncol. Clin. North Am. 26 (3), 629–648. doi:10.1016/j.hoc.2012.02.002
Keywords: chemoradiotherapy, methotrexate, SLC transporter, ABC transporters, stereotactic body radiotherapy, x-ray, irradiation
Citation: Sato K, Seki T, Mizutani A, Muranaka Y, Hirota S, Nishi K, Yamazaki K, Nishii R, Nakanishi T, Tamai I, Kawai K and Kobayashi M (2023) A single high-dose irradiation changes accumulation of methotrexate and gene expression levels of SLC and ABC transporters in cancer cells. Front. Pharmacol. 13:1069321. doi: 10.3389/fphar.2022.1069321
Received: 13 October 2022; Accepted: 29 December 2022;
Published: 11 January 2023.
Edited by:
Rong Wang, People’s Liberation Army Joint Logistics Support Force 940th Hospital, ChinaReviewed by:
Li Zhiling, Shanghai Jiao Tong University, ChinaCopyright © 2023 Sato, Seki, Mizutani, Muranaka, Hirota, Nishi, Yamazaki, Nishii, Nakanishi, Tamai, Kawai and Kobayashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masato Kobayashi, a29iYXlhc2lAbWhzLm1wLmthbmF6YXdhLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.