
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 17 January 2023
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1068613
This article is part of the Research Topic Gut Microbiota-Mediated Natural Products Metabolism and Its Role in the Pharmacokinetics and Pharmacodynamics View all 3 articles
Dysbiosis of gut microbiota plays a fundamental role in the pathogenesis and development of chronic kidney disease (CKD) and its complications. Natural products from plants and microorganisms can achieve recognizable improvement in renal function and serve as an alternative treatment for chronic kidney disease patients with a long history, yet less is known on its beneficial effects on kidney injury by targeting the intestinal microbiota. In this review, we summarize studies on the effects of natural products from plants and microorganisms, including herbal medicines and their bioactive extracts, polysaccharides from plants and microorganisms, and phytochemicals, on the prevention and treatment of chronic kidney disease through targeting gut microflora. We describe the strategies of these anti-CKD effects in animal experiments including remodulation of gut microbiota structure, reduction of uremic toxins, enhancement of short-chain fatty acid (SCFA) production, regulation of intestinal inflammatory signaling, and improvement in intestinal integrity. Meanwhile, the clinical trials of different natural products in chronic kidney disease clinical practice were also analyzed and discussed. These provide information to enable a better understanding of the renoprotective effects of these effective natural products from plants and microorganisms in the treatment of chronic kidney disease. Finally, we propose the steps to prove the causal role of the intestinal microflora in the treatment of chronic kidney disease by natural products from plants and microorganisms. We also assess the future perspective that natural active products from plants and microorganisms can beneficially delay the onset and progression of kidney disease by targeting the gut flora and highlight the remaining challenges in this area. With the continuous deepening of studies in recent years, it has been proved that gut microbiota is a potential target of natural active products derived from plants and microorganisms for chronic kidney disease treatment. Fully understanding the functions and mechanisms of gut microbiota in these natural active products from plants and microorganisms is conducive to their application as an alternative therapeutic in the treatment of chronic kidney disease.
Chronic kidney disease (CKD) has become an increasing public health problem with a wide range of complications and high risk of mortality. The worldwide prevalence of CKD (8%–16%) brings a heavy economic burden for middle- and low-income countries in particular (Zhang et al., 2012; Lv and Zhang, 2019). Due to the concealed symptom of early CKD, clinical diagnosis and therapeutic interventions for early CKD patients are lagging (Flagg, 2018); most therapeutic methods for ESRD (end-stage renal disease) patients such as lowering blood pressure, controlling blood glucose, and reducing proteinuria have poor effect in preventing kidney failure (Chen T. K. et al., 2019). As current anti-CKD therapies have limited effectiveness and/or severe adverse effects, alternative treatments like natural products have gained much more attention, especially with current studies implying that gut microbiota is an anti-CKD target.
Gut microbiota are composed of over 1,000 genera of bacteria colonized in the human intestine, which play a crucial role in many important human physiological functions, including maintenance of energy balance, modulation of intestinal homeostasis, and regulation of the immune system (Fan and Pedersen, 2021). The features and functional effect of intestinal flora have been extensively studied in the past few decades, and lots of studies have revealed that dysbiosis of gut microflora is highly associated with the onset and progression of CKD (Jiang et al., 2017; Hu J. et al., 2020; Andrianova et al., 2020; Wang X. et al., 2020; Zhong et al., 2020; Du et al., 2021a). Gut microflora influence the gut–kidney axis, namely, the crosstalk between the intestinal microflora, CKD, and changes in the intestinal environment (Evenepoel et al., 2017), in a bidirectional way; on the one hand, kidney function failure is highly related to the reduced bacterial diversity and biased community constitutions of the gut microflora (Cigarran Guldris et al., 2017; Hobby et al., 2019). Increasing sources of evidence support that altered gut microbiota composition has been reported in patients with different kidney diseases, including those with end-stage renal disease (ESRD), acute kidney injury (AKI), IgA nephropathy, CKD, and diabetic nephropathy (Jiang et al., 2017; Hu J. et al., 2020; Andrianova et al., 2020; Zhong et al., 2020; Du et al., 2021a). CKD patients had lower bacterial diversities than healthy subjects, with an increasing abundance of harmful microbes such as Proteobacteria and Actinobacteria and a decreasing level of beneficial microbes such as Lactobacillaceae (Hu J. et al., 2020; Andrianova et al., 2020; Zhong et al., 2020). Moreover, the change in gut microflora structure and function is highly associated with the severity of CKD (Hu X. et al., 2020). Specific gut microbes producing short-chain fatty acids (SCFAs) are potentially helpful for CKD early diagnosis and prognosis monitoring (Gao B. et al., 2021; Sato et al., 2021). On the other hand, various metabolic pathways including uremic toxin, SCFAs, and bile acid pathway are affected by the altered gut microflora. Experimental and clinical evidence demonstrated that altered gut microflora in CKD patients could accelerate the biosynthesis of uremic toxins, whose concentrations would consequently increase in the blood (Wang X. et al., 2020; Rysz et al., 2021). Also, these resulted in the progression of impairment in the intestinal epithelial barrier and inflammation which intensified the cardiovascular and kidney diseases (Espi et al., 2020; Opdebeeck et al., 2020), suggesting that the gut flora could serve as a novel therapeutic target for CKD and related complications.
In order to clarify the gut microbiota and anti-CKD molecular mechanisms of these natural products, we reviewed the knowledge based on publications in English- and Chinese-language journals. Table 1 summarizes the mechanisms of different natural products from plants and microorganisms, including herbal decoction, crude extracts, polysaccharides from plants and microorganisms, and phytochemicals, in CKD progression through targeting the gut microbial based on these studies, which focus on remodulation of gut microbiota structure, reduction of uremic toxins, enhancement of SCFA production, regulation of intestinal inflammatory signaling, and improvement in intestinal integrity of natural products commonly used in kidney diseases. Meanwhile, the clinical trials of different natural products in CKD clinical practice were also analyzed and discussed (Table 2).
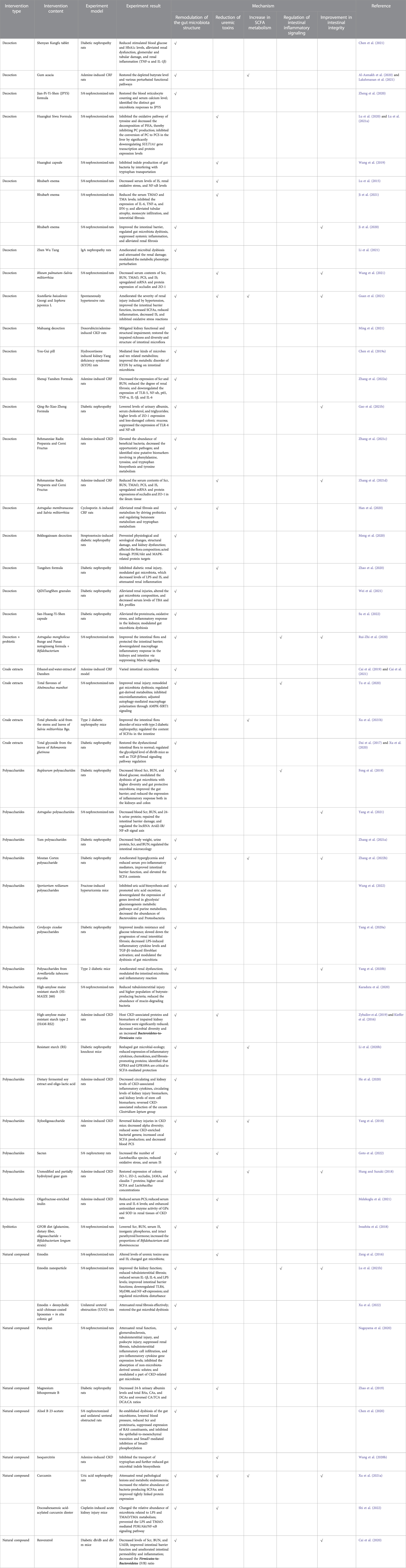
TABLE 1. Mechanisms of gut microbiota as an alternative target for natural products from plants and microorganisms for the treatment of CKD in animal experiments.
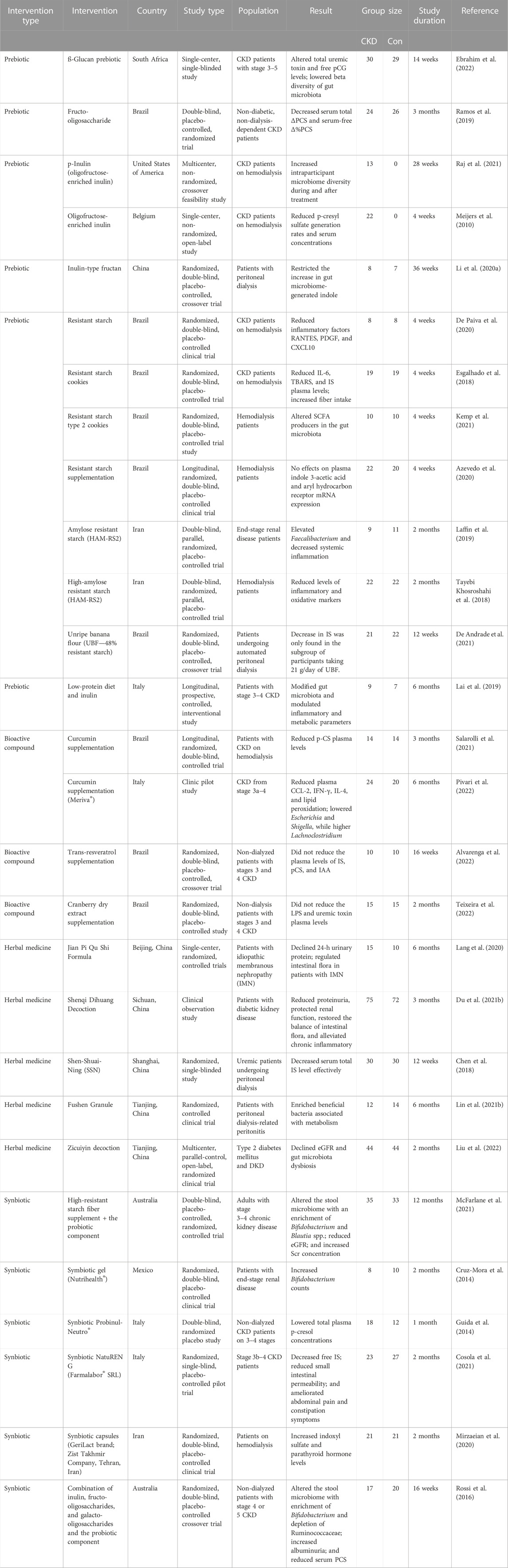
TABLE 2. Clinical trial of natural products from plants and microorganisms in the treatment of CKD patients by modulating gut microbiota.
Natural products from plants and microorganisms exhibited their prebiotic-like effects by remodulating the gut flora structure (Figure 1A).

FIGURE 1. Schematic graphic representation of strategies of gut microbiota as an alternative target for natural products from plants and microorganisms in the treatment of CKD. The strategies of these anti-CKD effects of herbal medicine include (A) remodulation of the gut microbiota structure; (B) improvement in intestinal integrity; (C) reduction of uremic toxins; (D) regulation of intestinal inflammatory signaling; and (E) enhancement of SCFA production.
At the phylum level, Firmicutes and Bacteroidetes are the representative intestinal microbiota. For diabetic nephropathy, the Firmicutes-to-Bacteroidetes ratio (F/B ratio) has an important influence on obesity (Etxeberria et al., 2015), body mass index (Koliada et al., 2017), and glucose levels (Remely et al., 2016). Some studies found that the F/B ratio was significantly increased in the diabetic nephropathy model. Phytochemical resveratrol treatment significantly reduced body and kidney weights but not the fasting blood sugar (FBS) of db/db mice, which were associated with the decrease in the F/B ratio after resveratrol treatment (Cai et al., 2020). Qing-Re-Xiao-Zheng formula (Gao Y. et al., 2021), Cordyceps cicadae polysaccharides (Yang J. et al., 2020), and San-Huang-Yi-Shen capsule (Su et al., 2022) significantly lowered serum glucose and the ratio of Firmicutes-to-Bacteroidetes in mice with diabetic kidney disease. While treatment with QiDiTangShen granules and Tangshen formula had no significant effects on the F/B ratio in db/db mice, this maybe partly explains why they do not alter body weight and glycemic levels in those mice (Zhao et al., 2020; Wei et al., 2021). For other nephropathy models, the value of F/B was decreased after the intervention of Astragalus membranaceus and Salvia miltiorrhiza in CsA-induced chronic nephrotoxicity (Han et al., 2020). The combination of Scutellaria baicalensis Georgi (SB) and Sophora japonica L. (SL) lowered the F/B ratio in spontaneously hypertensive rats (Guan et al., 2021). Resistant starch HAM-RS2 (HI-MAIZE 260) resulted in a decrease in the F/B ratio in the adenine-induced CKD model (Kieffer et al., 2016; Zybailov et al., 2019; Li Y. J. et al., 2020).
However, the definite association between F/B ratio and biochemical indicators of diabetes is not well established and still needs to be further studied (Mokhtari et al., 2021). On the contrary, the Firmicutes-to-Bacteroidetes ratio was found to have declined significantly in some studies of diabetic nephropathy (Dai et al., 2017; Zhao et al., 2019; Chen et al., 2020). The Shenyan Kangfu tablet (Chen et al., 2020) and Rehmannia glutinosa leaves total glycoside (Dai et al., 2017; Xu et al., 2020) reduced stimulated blood glucose and increased the F/B ratio in db/db mice. Magnesium lithospermate B did not affect FBG levels and increased the F/B ratio slightly in the streptozotocin (STZ)-induced mice (Zhao et al., 2019). In the other CKD models, the decreased F/B ratio was also observed in adenine-induced CKD rats, and the combination of Rehmanniae Radix Preparata (RR) and Corni Fructus (CF) significantly altered the ratio trend (Zhang et al., 2021c & 2021d). After supplementation with dietary docosahexaenoic acid-acylated curcumin diester and curcumin, the ratio of F/B was remarkably elevated in acute kidney injury mice (Shi et al., 2022). Sporisorium reilianum polysaccharides could notably increase the ratio of F/B in hyperuricemic mice (Wang et al., 2022).
The phylum Proteobacteria was mainly composed of opportunistic pathogens, which were found at low levels (less than 4%) in the normal group. The expansion of Proteobacteria abundance is considered a hallmark for dysbiosis (Shin et al., 2015). For CKD rats, the abundance of Enterobacteriaceae was largely increased (Chen et al., 2020; Xu X. et al., 2021; Ji et al., 2021; Ming et al., 2021), which is responsible for the production of uremic toxins, such as indoxyl sulfate (IS) and p-cresol sulfate (PCS) (Li and Young, 2013). Emodin via colonic irrigation (Zeng et al., 2016) and Astragalus polysaccharide (Yang et al., 2021) remarkably decreased Escherichia coli, which is positively correlated with both urea and IS. The relative abundance of Escherichia and Shigella from family Enterobacteriaceae was significantly increased in the injured kidney mice which were restored by the intervention of curcumin (Xu X. et al., 2021) and alisol B 23-acetate (Chen et al., 2020). Tangshen formula-treated rats exhibited decreases in the Enterobacteriaceae family (OTU167 and OTU218) in DN rats (Zhao et al., 2020). As a pathogenic genus from phyla Proteobacteria, Desulfovibrio was proved to reduce sulfate to intestinal toxin hydrogen sulfide (Kushkevych et al., 2019). The increased number of Desulfovibrio in the CKD rats was strongly decreased by You-Gui pill (Chen R. et al., 2019) and the combination of RR and CF treatment (Zhang et al., 2021c; Zhang et al., 2021d).
The phylum Actinobacteria is the most important flora that causes intestinal inflammation and stimulates multiple inflammatory reactions (Burge et al., 2019). Oral administration of Bekhogainsam decoction resulted in a significant decrease in Actinobacteria in the STZ-induced DN mice (Meng et al., 2020). The abundance of Actinobacteria was increased in CKD rats, and the prebiotic acacia gum (Lakshmanan et al., 2021) and Jian-Pi-Yi-Shen decoction (Zheng et al., 2020) treatment successfully reversed those levels.
The relative abundance of bacteria producing SCFAs, including Lactobacillus, Akkermansia, Lachnospiraceae, and Ruminococcaceae, was significantly lower in CKD animal models. Lactobacillus, as a lactic acid-producing probiotic, has been observed to decrease in CKD patients and different CKD models (Zeng et al., 2016; Nagayama et al., 2020). Its increase could protect kidney function by reducing the accumulation of urinary toxins such as IS, repairing the intestinal barrier, and improving inflammation and oxidative stress (Zhu et al., 2021; Kim et al., 2022; Tungsanga et al., 2022). Treatment with adenine reduced the Lactobacillaceae count, whereas additional supplementation with acacia gum (Al-Asmakh et al., 2020) and guar gum dietary fibers (Hung and Suzuki, 2018) reversed this effect in the intestinal tract. Compared to spontaneously hypertensive rats, the relative abundance of Lactobacillus was increased in the group of SB and SL treatments (Guan et al., 2021). Lactobacillus were relatively less abundant in STZ-induced diabetic nephrotoxicity (Su et al., 2022), which was increased by high-dose San-Huang-Yi-Shen capsule. Emodin via colonic irrigation (Zeng et al., 2016), sacran (Goto et al., 2022), and alisol B 23-acetate (Chen et al., 2020) remarkably increased Lactobacillus in 5/6 nephrectomized (Nx) mice. A. membranaceus and S. miltiorrhiza (Han et al., 2020), Moutan Cortex polysaccharide (Zhang M. et al., 2022), polysaccharides from Armillariella tabescens mycelia (Yang R. et al., 2020), and Cordyceps cicadae polysaccharides (Yang J. et al., 2020) not only recalled the content of Lactobacillus but also significantly increased the abundance of Akkermansia in chronic nephrotoxicity models. Akkermansia, a genus of the phylum Verrucomicrobia, is also known as a beneficial gut microbe because of its advantage in the maintenance of gut integrity (Taherali et al., 2018; Cani et al., 2022). A. muciniphila slows down the development and progression of diabetes, obesity, and IBD in mice (Rodrigues et al., 2022), which was enriched by the total flavones of Abelmoschus manihot treatment in CRF rat models (Tu et al., 2020).
A number of studies have illustrated that Ruminococcaceae and Lachnospiraceae families have been discovered to be related to the enhancement of immunological response (Davar et al., 2021), the improvement in renal function (Xu et al., 2017; Xu X. et al., 2021), and primary bile acid production (Zhao et al., 2019). Furthermore, the amount of Ruminococcaceae, which contains many butyrate-producing genera, such as Ruminococcaceae UCG-014 and Ruminococcus 1, was greatly enhanced by RR and CF (Zhang et al., 2021c; Zhang et al., 2021d), Bupleurum polysaccharides (Feng et al., 2019), and curcumin (Xu X. et al., 2021) in CKD rats. Resistant starch slowed the progression of renal injury by enriching the butyrate producers Ruminococcus torques and Eubacterium ruminantium in 5/6 nephrectomy CKD rats (Karaduta et al., 2020). The Lachnospiraceae family, which was reduced in the CKD models, was increased by emodin nanoparticle treatment (Lu Z. et al., 2021) and yam polysaccharide (Zhang W. et al., 2021). The acetic acid-producing genera Lachnospiraceae UCG-001 and the Lachnospiraceae NK4A136 group, which significantly decreased in CKD rats, were drastically enriched following the intervention of RR and CF (Zhang et al., 2021c and 2021d) and dietary docosahexaenoic acid-acylated curcumin diester or curcumin (Shi et al., 2022). The genus Lactonifactor was significantly increased in the total flavones of Abelmoschus manihot treatment of the potassium oxonate-induced CRF rat model (Tu et al., 2020).
For other probiotic microbes, Mahuang decoction (Ming et al., 2021) and resistant starch diet (Li Y. J. et al., 2020) could promote the expansion of SCFA-producing bacteria of the genus Prevotella, which are disrupted in CKD models. Prevotella-9 were increased by the SB and SL in spontaneously hypertensive rats. Butyric acid-producing bacteria Bacteroidales S24-7 were enriched with the administration of Mahuang decoction (Ming et al., 2021), You-Gui pill (Chen R. et al., 2019), and rhubarb enema (Ji et al., 2020) in CKD rats. Coprococcus play an important role in body health through its production of acetate acid and vitamin B (Nogal et al., 2021) and have become significantly more abundant in the group treated with paramylon (Nagayama et al., 2020) and Jian-Pi-Yi-Shen decoction (Zheng et al., 2020) than the 5/6 Nx group. Tangshen formula (Zhao et al., 2020), rhubarb enema treatment (Ji et al., 2021), fermented soybean product (Koji polysaccharides ®) (He et al., 2020), Astragalus polysaccharide (Yang et al., 2021), Coptidis Rhizoma extracts (Cui et al., 2018), and a resistant starch diet (Li Y. J. et al., 2020) enhanced the Bifidobacteriaceae genus that was decreased in CKD models. The proportions of Bifidobacterium and Ruminococcus were significantly elevated in the GFOB diet-fed 5/6 Nx rats (Iwashita et al., 2018). The abundance of Lachnoclostridium was significantly raised in the Sporisorium reilianum polysaccharide-treated hyperuricemia mice (Wang et al., 2022). Shenqi Yanshen Formula significantly modified the dysbiosis of gut microbiota by increasing the abundance of Succinivibrionaceae in the adenine-induced CKD model (Zhang L. et al., 2022).
However, some studies showed that the abundance of SCFA-producing bacteria was enhanced in the kidney injury model, which is contrary to the aforementioned examples. It may be due to the different strains even within the same family having existential discrepancies in their responses to one kind of nature products, which cannot be distinguished by 16 S rDNA sequencing. The abundance of Lactobacillus and Bacteroides enriched in the diabetic mice, which were significantly reduced by QiDiTangShen granules (Wei et al., 2021) and the Shenyan Kangfu tablet (Chen et al., 2021). The IgAN rat group had higher abundance of Lachnospiraceae, Lactobacillaceae, and Bacteroidaceae than the normal group, which was reduced upon Zhen Wu Tang intervention (Li et al., 2021). Following treatment with total flavones of Abelmoschus manihot, the abundance of Bacteroidales and Lactobacillales was decreased, which were enriched in potassium oxonate-induced CRF rat models (Tu et al., 2020). The relative abundance of Bacteroidetes and Bacteroidales S24-7 in db/db mice was significantly increased, which was significantly reduced by Rehmannia glutinosa leaves total glycoside (Dai et al., 2017; Xu et al., 2020). The Ruminococcus genus (OTU82, OTU230, and OTU51) that increased in the 5/6 Nx group was found to be associated with kidney injury (Zeng et al., 2016; Zhao et al., 2020). The water extract of Dansen (Cai et al., 2019; Cai et al., 2021) and alisol B 23-acetate (Chen et al., 2020) could downregulate the Ruminococcus induced by adenine-induced injury. Gum acacia treatment reduced the genus Akkermansia, which was found enriched in the adenine-induced CKD rats (Lakshmanan et al., 2021) and the 5/6 Nx group (Ji et al., 2020).
In a word, natural products from plants and microorganisms could stimulate the growth of beneficial bacteria and inhibit the colonization of potential or opportunistic pathogens in the intestine.
The intestinal epithelial barrier is formed by epithelial cells to prevent enteral substances from entering the rest of the body, and it plays a crucial role in resisting the colonization of exogenous microorganisms. Aberrant physical conditions induced by the gastrointestinal dysbiosis of CKD lead to disruption of the intestinal muco/sa (Figure 1B). Damaged intestinal barriers increase intestinal permeability, which permits the pathogenic bacteria or endotoxin (such as lipopolysaccharide (LPS)) to translocate across the intestinal epithelial cells into the circulation of the blood and lymphoid system (Boyapati et al., 2016). Therefore, maintaining the integrity and function of the intestinal epithelial barrier has therapeutic significance for CKD.
Tight junction proteins, such as zonula occludens-1 (ZO-1), claudin-1, and occludin, play an indispensable role in maintaining the permeability of the intestinal epithelial barrier. Once the expression of tight junction proteins in intestinal mucosa decreases, the paracellular permeability would increase and the tight junctions would be destroyed, resulting in intestinal barrier damage (Paone and Cani, 2020). Decreased expression of tight junction proteins was detected in different nephropathy models. Couplet medicine of Rheum palmatum–S. miltiorrhiza can effectively improve intestinal barrier function in CRF rats by upregulating the expression of occludin and ZO-1 in the ileum tissue of the 5/6 nephrectomy model (Wang et al., 2021). Higher levels of ZO-1 expression and less-damaged colonic mucosa with a lower serum level of FITC-dextra, a marker of intestinal permeability, implied the beneficial role of Qing-Re-Xiao-Zheng formula for gut barrier integrity in the diabetic nephrectomy model (Gao Y. et al., 2021). Fermentable dairy fiber unmodified guar gum (GG) restored colonic barrier integrity by higher expression of colonic ZO-1, occludin, and claudin-1 proteins in adenine-induced CKD mice (Hung and Suzuki, 2018). Astragalus polysaccharide (Yang et al., 2021), rhubarb enema decoction (Ji et al., 2020), the combination of Astragalus mongholicus Bunge and Panax notoginseng formula with Bifidobacterium (Rui-Zhi T, et al., 2020) and alisol B 23-acetate (Chen et al., 2020) treatment restored intestinal epithelial tight junctions and reduced intestinal permeability with upregulated expression of ZO-1, occludin, and claudin-1 proteins in 5/6 Nx rat models. Polysaccharides from Armillariella tabescens mycelia (Yang R. et al., 2020), Bupleurum (Feng et al., 2019; Liu et al., 2019), and Moutan Cortex (Zhang M. et al., 2022) improved the gut barrier by increasing the protein expression of occludin and claudin-1 in the colonic tissues of the diabetic nephropathy rats. Resveratrol (Cai et al., 2020) and curcumin supplementation (Xu X. et al., 2021) and the combination of SB and SL (Guan et al., 2021) protected the intestine epithelial barrier by substantially recovering ZO-1 and claudin-1 protein expression in CKD models. A. membranaceus and S. miltiorrhiza recovered intestinal permeability by enhancing the expression of ZO-1 in CsA-induced chronic nephrotoxicity (Han et al., 2020). Thus, improving the intestinal barrier with natural products from plants and microorganisms can effectively alleviate intestinal inflammation and renal fibrosis.
Intake of choline, tyrosine, and tryptophan increases the amounts of trimethylamine (TMA), IS, hydrogen sulfide, and indole produced by intestinal bacteria. After absorption, these compounds are further metabolized in the liver to generate the typical uremic toxin trimethylamine N-oxide (TMAO), PCS, and p-indoxyl sulfate. These toxins, which cannot be removed efficiently even by hemodialysis and would accumulate in advanced CKD patients (Wu et al., 2020), are highly related to the progression and mortality of multiple cardiovascular diseases (CVDs) and CKDs (Lin et al., 2015; Cheng et al., 2020; Zhang Y. et al., 2021; Lim et al., 2021) (Figure 1C).
Obvious studies showed that natural products could reduce the production of uremic toxin. Orally administered Tangshen formula significantly inhibited diabetic renal injury by decreasing the amount of bacteria producing the precursor of IS and then the serum levels of LPS and IS (Zhao et al., 2020). Rhubarb enema granule treatment ameliorated tubulointerstitial fibrosis in the kidneys of CKD rats, most likely by alleviating circulating TMAO and IS levels (Lu et al., 2015; Ji et al., 2021). The small molecule compound piceatannol (PIC) could inhibit the synthesis of uremic toxin precursors in Bacillus, thereby reducing the accumulation of IS and PCS in CKD mice (Li et al., 2022). The DHA-acylated curcumin diester treatment remarkably lowered the LPS and TMAO/TMA of AKI mice by decreasing the relative abundance of intestinal microflora with their metabolism (Shi et al., 2022). Emodin, an abundant anthraquinone in the roots and bark of the traditional Chinese medicine rhubarb (Da Huang), has been demonstrated to reduce uremic toxins and is used in China for the treatment of CKD (Li et al., 2015; Sun et al., 2019). Emodin via colonic irrigation (ECI) remodeled gut microflora and decreased the levels of urea and IS in CKD rats (Zeng et al., 2016). Deoxycholic acid–chitosan-coated liposomes could enhance the renoprotective effect of emodin (Xu et al., 2022).
Supplementation with amylose resistant starch HAM-RS2 (Kieffer et al., 2016) could reduce urine IS and p-cresol (PC) in adenine-induced CKD rats. Sulfated polysaccharide sacran (Goto et al., 2022) and GFOB diet (containing prebiotics such as glutamine, dietary fiber, and oligosaccharide and probiotic strain Bifidobacterium longum) (Iwashita et al., 2018) completely diminished serum levels of IS in 5/6 Nx rats, whereas xylooligosaccharide supplementation could decrease serum levels of IS and PCS by altering microbial tyrosine metabolism (Yang et al., 2018). The combination of SB and SL attenuated higher serum levels of IS and severe oxidative stress in the kidneys of spontaneously hypertensive rats (Guan et al., 2021). Oligofructose-enriched inulin significantly reduced serum PCS and urea and enhanced antioxidant enzyme activity in renal tissues of CKD rats (Melekoglu et al., 2021). Further studies revealed that transporting tryptophan during indole production may be an important inhibition target for natural products. Huangkui capsule (water extract of Abelmoschus moschatus) inhibited the tryptophan transport in the main indole-synthesizing bacteria Enterobacteriaceae, resulting in the decease of uremic toxin IS production in CKD rats (Wang et al., 2019). Huangkui Siwu Formula (HKSWF), containing Abelmoschus moschatus, Astragalus mongholicus, Polygonum cuspidatum, and Curcuma longa L., could inhibit the conversion of p-cresol into urotoxin PCS in the liver and directly inhibit the oxidative pathway of tyrosine and decrease the PC production in CKD rats (Lu et al., 2020; Lu J. et al., 2021). As a natural flavonoid isolated from Bidens bipinnata L., isoquercitrin (quercetin-3-O-d-glucopyranoside) could disturb microbiota-mediated indole production by inhibiting the transport of exogenous tryptophan into indole-synthesizing bacteria and further reducing indole biosynthesis (Wang Y. et al., 2020). To sum up, modulation of uremic toxin production by gut microbiota is one of the main strategies of the mechanisms of natural products from plants and microorganisms to delay CKD progression.
The dysbiosis of gut microbiota induced by kidney injury can affect the intestinal microenvironment through modulation of the inflammatory process in the gastrointestinal tract (Chi et al., 2021) (Figure 1D). For example, the abundance of Proteobacteria and the Gram-negative family, including pathogenic Enterobacteriaceae, Vibrionaceae, and Pseudomonadaceae, was found to increase significantly in the CKD patients (Jiang et al., 2017). They can secrete pro-inflammatory elements, such as the endotoxin LPS, which would stimulate both local intestinal and systematic chronic inflammation when accumulating in the blood (Ciesielska et al., 2021). These trigger TLR-4 signaling activation and the subsequent release of inflammatory cytokines (Wang et al., 2014). A&P combined with Bifidobacterium could protect nephridial tissue against CKD by downregulating Mincle/NF-κB inflammatory signaling transduction in the intestine (Rui-Zhi et al., 2020). Emodin-NP via colonic irrigation (Lu Z. et al., 2021) and rhubarb enema treatment (Ji et al., 2020) remarkably alleviated microbiota disturbance in CKD rats and inhibited the expression of TLR-4, MyD88, and NF-κB of the TLR-4 signaling pathway in the intestinal tract of the 5/6 Nx model. Bupleurum polysaccharides could significantly reduce the expression of HMGB1/TLR-4/NF-κB/IL-6 inflammatory factors in the colon tissue (Feng et al., 2019). In addition, resveratrol treatment significantly decreased IFN-γ and TNF-α levels in the intestine of db/db mice (Cai et al., 2020). Treatment with polysaccharides from Armillariella tabescens mycelia decreased the concentrations of colonic pro-inflammatory cytokines TNF-α and IL-1β (Yang R. et al., 2020). Therefore, reducing the intestinal inflammatory reaction with natural products from plants and microorganisms could contribute to attenuating the systematic chronic inflammation in CKD.
SCFAs (acetate, propionate, butyrate, etc.) are generated from the fermentation of various types of cellulose by SCFA-producing gut microbiota such as Prevotella, Faecalibacterium, Bacteroides, and Akkermansia under anaerobic conditions (Koh et al., 2016) (Figure 1E). The easily absorbed SCFAs exert beneficial physiological effects on the host via epigenetic modification or the G protein-coupled receptors, including providing energy for intestinal epithelial cells, promoting their proliferation, maintaining intestinal barrier function, maintaining intestinal homeostasis, and improving immune tolerance (Koh et al., 2016; Parada Venegas et al., 2019). For example, gut γδ T cells play indispensable roles in host defense and regulation of intestinal chronic inflammation. Propionate could inhibit γδ T cells producing interleukin-17A (IL-17) in a histone deacetylase-dependent manner (Dupraz et al., 2021). Compared to the non-CKD controls, SCFAs were significantly decreased in patients with severe CKD (Wu et al., 2020).
Dietary administration of gum acacia water extracts in CKD rats improved their renal function by modulating the microbiome composition and plasma levels of ethanoic acid, propionic acid, butanoic acid, and pentanoic acid (Al-Asmakh et al., 2020; Lakshmanan et al., 2021). It has been demonstrated that the combination of SB and SJ is effective in improving kidney injury caused by hypertensive in clinic. The combination of SB and SJ treatments increased SCFA production, upregulated the expression of receptor GPR41, and downregulated the expression of Olfr78 in male spontaneously hypertensive rats (Guan et al., 2021). A. membranaceus and S. miltiorrhiza increased serum SCFA content by enhancing the growth of butyric acid- and lactic acid-producing probiotics, especially Lactobacillus and Akkermansia (Han et al., 2020). Total phenolic acid from the stems and leaves of S. miltiorrhiza could ameliorate the intestinal microflora disorder of mice with diabetic nephropathy and regulate the content of SCFAs via adjusting the amount of some SCFA-producing bacteria in the intestine (Xu Z. et al., 2021). Furthermore, resistant starch (Li Y. J. et al., 2020), guar gum (Hung and Suzuki, 2018), xylooligosaccharide (Yang et al., 2018), and Moutan Cortex polysaccharide (Zhang M. et al., 2022) could also increase cecal SCFA production. Altering SCFAs with natural products from plants and microorganisms has proved to be another potential therapeutic strategy to mitigate kidney injury and slow the progression of renal decline.
In summary, natural products from plants and microorganisms were effective in the treatment of different kinds of CKD by modulating the gut microbiota in a multi-channel and multi-target way (Figure 2). Some ancient classic formula, crude extracts from herbal medicine, and dietary fiber could change the imbalance within different intestinal floras of CKD by increasing the number of probiotics and reducing the amount of deleterious microbes. Natural polysaccharides, which led to an increase in SCFAs, decreased the intestinal mucosal barrier permeability by enhancing the tight junction expression of the intestinal epithelium. Also, these prevented pathogenic bacterial growth and large amount of endotoxin LPS produced by Gram-negative bacilli over-proliferated in the intestine, translocating into the blood to enhance systematic inflammatory responses. Some crude extracts and natural polyphenol compounds could disrupt immune responses by inhibiting the inflammatory signal transduction produced by pathogenic bacteria-stimulating dendritic cells in the colon; some herbal medicines, flavonoid and anthrone compounds, and polysaccharides from plants and microorganisms could inhibit the production of IS, PCS, and TMAO fermented by intestinal pathogenic bacteria. Moreover, the restoration of dysbiosis of gut microbiota was not independent from attenuating metabolic endotoxins and inhibiting the inflammatory signaling pathway.
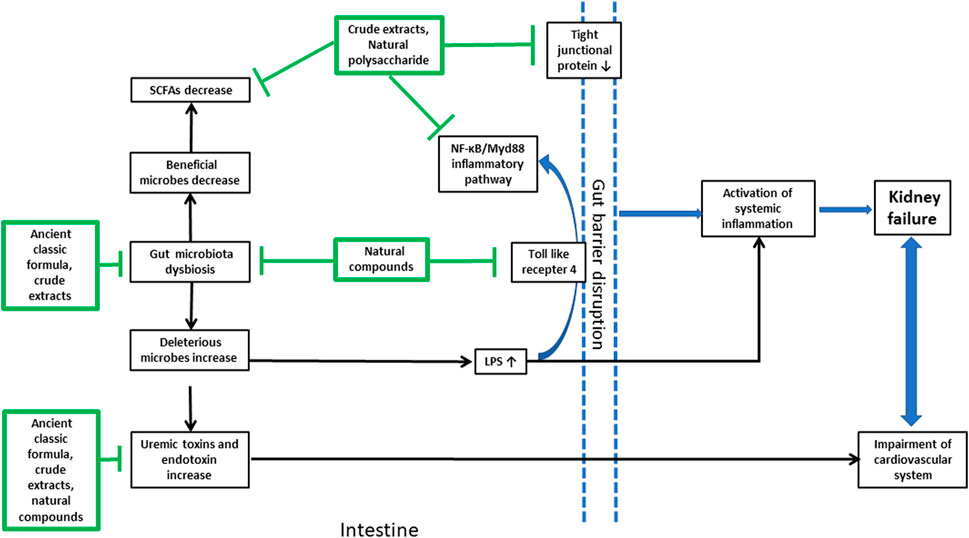
FIGURE 2. Brief summary of the natural products from plants and microorganisms used in the treatment of CKD and their multi-targets in the gut microenvironment. The arrow line with a pointed tail means promoting effects and that with a flat tail means inhibitory effects.
Besides animal experiment, accumulating clinical trials have shown that natural products from plant and microorganism have an effect on the reduction of proteinuria and improvement of renal function by modifying the gut microbiota (Table 2). The first benefit of administering natural products during CKD is recovery of the dysbiosis of gut microflora. The Jian Pi Qu Shi Formula, made of Astragalus membranaceus Bge., Codonopsis pilosula, and other eight herbal medicines, was shown to regulate intestinal flora in the pilot trial of patients with idiopathic membranous nephropathy (Lang et al., 2020). A cohort study of 160 diabetic kidney patients suggested that Modified Shenqi Dihuang decoction has good curative effect on reducing proteinuria, protecting renal function, restoring the balance of intestinal flora by increasing the numbers of Bacteroides, Bifidobacteria, and Lactobacillus, but decreasing the numbers of Enterobacter, Enterococcus, and yeast (Du et al., 2021b). Zicuiyin decoction, which had better efficacy in improving and protecting kidney function in diabetic kidney disease (DKD) patients, could decline eGFR and ameliorate gut microbiota dysbiosis in a multicenter, parallel-control, open-label, randomized clinical trial (Liu et al., 2022). Fushen Granule (FSG) ameliorated BUN and Scr and improved albumin (ALB) by enriching beneficial bacteria associated with metabolism in patients with peritoneal dialysis-related peritonitis (PDRP) in a randomized controlled trial (Lin W. et al., 2021). Supplementation of amylose resistant starch HAM-RS2 (20 g/d resistant starch) led to an improvement of constipation severity and renal function, elevation in Faecalibacterium, and a decrease in serum urea, IL-6, TNF-α, and Malondialdehyde (MDA) of systemic inflammation in hemodialysis (HD) patients (Tayebi Khosroshahi et al., 2018; Laffin et al., 2019; Jia et al., 2021). Long-term synbiotic supplementation for 12 months, consisted of high-resistant starch fiber supplement HI-MAIZE 260 (20 g/d, 50% resistant starch) and the probiotic components of nine strains from Bifidobacteria, Lactobacillus, and Streptococcus genera, could reduce eGFR and increase Scr concentration by altering the gut microbiome with an enrichment of Bifidobacterium and Blautia spp. in stage 3–4 CKD patients (McFarlane et al., 2021). While short-term treatment of synbiotic (Nutrihealth®), containing a mix of probiotics and a prebiotic fiber, omega-3 fatty acids, and vitamins, could increase Bifidobacterium counts and maintain the intestinal microbial balance in Mexican patients with ESRD(Cruz-Mora et al., 2014).
The second benefit of supplementation with natural products is uremic toxin removal in the clinical application of CKD treatment. The curcumin supplementation showed a significant decrease in PCS plasma levels in HD patients (Salarolli et al., 2021) and reduced the inflammatory response by modifying the gut microbiota structure of CKD patients after 3-month administration (Pivari et al., 2022). In a clinical trial of 60 peritoneal dialysis patients, Shen-Shuai-Ning granules could decrease IS serum concentration after 12 weeks of treatment (Chen et al., 2018). Supplementation with the functional food HI-MAIZE® 260 cookies (16 g/d resistant starch) could increase fiber intake, reduce IL-6 and IS plasma levels (Esgalhado et al., 2018), decrease different growth factors (De Paiva et al., 2020), and alter the SCFA-producing microbiota in HD patients (Kemp et al., 2021), but no indole-3-acetic acid (IAA) levels or aryl hydrocarbon receptor (AhR) expression in patients with end-stage CKD (Azevedo et al., 2020). However, the unripe banana flour (21 g/d, 48% resistant starch) only decreased IS in the subgroup of participants undergoing peritoneal dialysis (PD) (De Andrade et al., 2021). The prebiotic fructo-oligosaccharide (FOS) decreased the serum total ΔPCS and serum-free Δ%PCS, but not IS and IAA in non-diabetic- and non-dialysis-dependent CKD patients (Ramos et al., 2019). The prebiotic inulin-type fructans restricted the increase in gut-derived indole in PD patients (Li L. et al., 2020). The ß-glucan prebiotic (50% ß-glucan) significantly altered the levels of total and free p-cresyl glucuronide (pCG) and lowered the beta diversity of the gut microbiome in stage 3–5 CKD predialysis participants (Ebrahim et al., 2022). The prebiotic oligofructose-inulin significantly increased intraparticipant microbiome diversity and reduced serum PCS concentrations in HD patients (Meijers et al., 2010; Raj et al., 2021). Inulin with low-protein diet modified gut microbiota and reduced inflammatory factors (TNF-α and NOX2) and metabolic parameters (serum uric acid (SUA) and C-reactive protein (CRP)) in the plasma of CKD patients (Lai et al., 2019). A synbiotic Probinul-Neutro lowered total plasma PC concentrations in non-dialyzed CKD patients with stage 3–4 CKD (Guida et al., 2014). Two-month administration of the synbiotic NatuREN G® resulted in the amelioration of the abdominal pain and constipation symptoms and a decrease in free IS in the stage 3b–4 CKD group (Cosola et al., 2021). The synbiotic capsules (GeriLact brand, Iran) supplementation containing a mix of probiotics and FOS as prebiotic might increase IS and parathyroid hormone levels in HD patients (Mirzaeian et al., 2020). A low-protein diet plus a new formulation of probiotics (Bifidobacterium longum and Lactobacillus reuteri) was effective in reducing IS in patients affected by advanced CKD (De Mauri et al., 2022). Synbiotic therapy, which consisted of a combination of high-molecular weight inulin, FOS, and galacto-oligosaccharides (GOSs) and the probiotic component including nine strains from the Lactobacillus, Bifidobacteria, and Streptococcus genera, decreased serum PCS of CKD patients with enrichment of Bifidobacterium and depletion of Ruminococcaceae after 6 weeks (4-week washout) in a randomized, double-blind, placebo-controlled, crossover trial (Rossi et al., 2016). However, supplementation with trans-resveratrol (Alvarenga et al., 2022) and cranberry dry extract (Teixeira et al., 2022) did not reduce the plasma levels of uremic toxins IS, PCS, and IAA produced by the intestinal microflora in patients with stage 3–4 CKD. In brief, natural products from plants and microorganisms may be a promising treatment for CKD patients in clinical practices.
The studies summarized in this review provide correlative insights into how host–gut microbiota–natural products from plant and microorganism interactions can contribute to CKD management. Advances in constantly updated sequencing technologies let investigators determine the change in microorganisms in experiments with CKD animals treated with different traditional Chinese formulas, crude extracts, natural polymers, and phytochemicals (Table 1). Abnormal changes in the gut microbiota of CKD animals make the pathogenic bacteria-producing urinary toxin accumulate in the blood and fail to be eliminated by the impaired kidney. Simultaneously, the damage to the intestinal epithelial barrier increases the permeability of the intestinal mucosa, which allows pathogenic bacteria and enterogenous endotoxins to translocate into the blood circulation and activates the immune system of the intestinal mucosa. All of these contribute to the imbalance in intestinal microecology, the inducement of systemic inflammatory response, and the impairment of kidney tissue and function. The aforementioned intestinal events are not mutually exclusive. Natural products from plants and microorganisms may act on the dysbiosis of intestinal microecology in CKD through a variety of channels; for example, a natural polysaccharide may modulate the structure of gut microflora, fortify the intestinal mucosa barrier, and reduce the inflammatory reaction in the colon tissue. Even components from the same herb may have different targets. For instance, ethanol extract from S. miltiorrhiza could increase the diversity of intestinal microflora and regulate the amount of SCFAs, which display the multi-target characteristics of natural products. Interestingly, herbal medicine plus probiotics or synbiotics appeared to strengthen their effectiveness on the dysbiosis of gut microbiota.
Previous studies on the human microbiome have identified correlative microorganisms associated with CKD patients. Transferring the CKD patients’ microbiome into germ-free mice aggravated CKD-associated phenotypes, which proved the causality between gut microflora and the pathogenesis of CKD (Wang X. et al., 2020). Gut microflora is thought to be the most potential therapeutic target for CKD treatment. Accumulating studies have demonstrated that an ancient classic herbal formula, natural polysaccharide, or bioactive compound could alter the diversity of the intestinal microbiota and the production of uremic toxins in CKD patients (Table 2). However, different kinds of natural active products from plants and microorganisms have different mechanisms and limits that need to be distinguished, though they are similar adjuvant treatments via targeting gut microbiota. Fermentable dietary fibers, such as inulin, FOS, galacto-oligosaccharides (GOSs), and resistant starch, are utilized as prebiotics that are selectively digested by host microorganisms, which could stimulate the growth of one or a limited number of beneficial bacteria and reduce the uremic toxin released in the colon. However, common side effects including mild diarrhea and gassiness were often accompanied with administration of these fibers (Sanctuary et al., 2019); Moreover, FOS and GOS were reported to have harmful effects on the glucose metabolism by reducing butyrate-producing microbes (Liu et al., 2017). Therefore, whether they have potential adverse effects and became intolerant, especially in long-term interventions, need to be further researched. The administration of probiotic supplementation only failed to reduce uremic toxins and inflammatory markers in CKD patients (Borges et al., 2018), and the combination of probiotics with healthy components like prebiotics and bioactive compounds, called synbiotics, could benefit the gut ecosystem and host life. Many cohort studies of synbiotics listed in the review are short-term RCTs with small-scale participants, and we are unable to rule out the bioactive components or a specific bacterium that mainly contribute to the efficacy, not to mention the best dosage and proportion of prebiotics, route of administration, and duration of intervention. The variation of a single element in the study may result in different outcomes.
Herbal medicine has been one of the most widely used alternative methods for the prevention and treatment of CKD in China and other Asian countries for hundreds of years. Our review demonstrated that different proportions of traditional herbal formulas or different herbal ingredients have different focuses on the intestinal dysbacteriosis and abnormal immune system of CKD, which presented the great advantage of herbal medicine in CKD treatment. The synergistic effects of multiple ingredients of herbal medicine on the gut microbiota of patients with CKD are reflected in two aspects: on the one hand, the bioactive ingredients have multi-target characteristics. The discrepancy in the regulatory objectives of gut microbiota makes them supplement each other. Taking Astragali Radix as an example, where Astragalus polysaccharide and astragaloside IV are the most important bioactive ingredients purified from Astragali Radix, both of which have to be biotransformed by gut microbiota to get better potency (Wang and Chen, 2017; Hong et al., 2021). Astragalus polysaccharide can modify the gut flora structure and fortify the intestinal mucosa barrier of the colon tissue, while astragaloside IV can protect against kidney injury by alleviating oxidative stress, attenuating mitochondrial dysfunction, and reducing inflammatory reactions in the kidney tissue (Zhang et al., 2020). On the other hand, the different proportions or bioactive ingredients cooperate with each other to improve the therapeutic effects by regulating the structure and metabolites of gut microbiota. Moreover, the synergistic effect can also reduce the possible toxic and side effects during the treatment process. Taking RR and CF as examples, they have been used together to treat CKD for thousands of years. The relative abundance of probiotic genera Ruminococcaceae and Lachnospiraceae was decreased in CKD rats, which could not be significantly recovered by the treatment of RR or CF, respectively, but they are notably increased in the RR + CF treatment groups (Zhang et al., 2021d). However, there are still some problems that need to be solved. First of all, even though many bioactive components from herbal medicine were identified through modern technology including standardized phytochemistry, pharmacology, pharmacokinetics, pharmacodynamics, and toxicology research procedures (Lin T. L. et al., 2021), the bottleneck barrier of herbal medicine development i.e., investigating how the complex ingredients in herbal medicines work together in a synergistic pattern is still not fully elucidated. Second, herbal medicine can avoid the adverse therapeutic effect caused by Western medicine treatment in the clinical practices of CKD treatment (Zhao et al., 2021), but most studies of these herbal medicines are focused on animal experiments. There are few clinical trials about herbal medicine plus first-line Western medicine for CKD treatment except for the multicenter clinical trial of Abelmoschus manihot and irbesartan (Zhao et al., 2022). High-quality interventional trials of well-studied, high-quality RCTs investigating herbal medicine treatment in CKD are still lacking. Third, though a variety of active substances identified from the herbal medicines, such as alkaloids, polysaccharides, glycosides, lipids, and vitamins, provide material basics for the multi-level and multi-target characteristic of herbal medicine, side effects and safety validations of herbal medicine on CKD patients need further large size and long duration clinical trials.
Another important aspect that has to be considered is that the ingredients of natural products may be metabolized by gut microflora and affect their structures and efficacy. Accumulating evidence showed that the bioactive compounds from herbal medicines like Astragali Radix and S. miltiorrhiza were effective in ameliorating kidney injury via gut microbiota biotransformation (Wang and Chen, 2017; Cai et al., 2019). It was reported that APS could be fermented by intestinal microorganisms Desulfovibrio vulgaris to produce acetic acids (Hong et al., 2021). Astragaloside IV (ASIV) is the most abundant saponin purified from Astragali Radix. It is gradually deglycosylated by human intestinal flora and transformed into cycloastragenol (Wang and Chen, 2017), which is the effective form with better permeability and absorptivity to improve the bioavailability in vivo (Zhai et al., 2016). Tanshinones and salvianolic acid from S. miltiorrhiza would exert effect after being metabolized through methylation, demethylation, dehydrogenation, hydrogenation, and hydroxylation by intestinal bacteria (Cai et al., 2019).
Identifying gut microbiota as the alternative target of natural products from plants and microorganisms in the treatment of CKD still face more challenges. These associative studies should spur further causal investigations by using modern microbial technology, such as genomic sequencing, germ-free (GF) animals, and antibiotic treatments. Additionally, to understand how natural products from plants and microorganisms affect the gut microbiota in CKD patients and to develop new alternative therapies for CKD treatment, the strain- and molecular-level connections between the gut microbiome, natural products from plants and microorganisms, and host CKD phenotype should be established in future research based on the hypotheses developed from these correlative studies (Chaudhari et al., 2021). The systematic research on functional compounds from natural products metabolized by gut microbiota might seek out a new approach to uncover these problems. Identification and functional testing of gut microbiota metabolites related to natural product components would deepen the understanding of how the natural products modulate CKD patients’ physiology via targeting gut microbiota by using cutting-edge omics platforms including next-generation sequencing (NGS), proteomics, metabolomics, and cultureomics. These strategies have pushed the development of novel disease-related probiotics, prebiotics, and functional proteins for the treatment of CKD by targeting the dysbiosis of the gut microbiota in recent years (Lobel et al., 2020; Zhu et al., 2021). The synergistic effect of multiple methods such as herbal medicine and these disease-related probiotics or prebiotics would provide a novel therapeutic method for CKD patients.
In summary, the literature analyzed in this review suggests a great advantage in the adoption of natural products from plants and microorganisms to treat CKD via targeting gut microbiota. These natural products from plants and microorganisms have an impact on the biogenesis and progression of CKD and its relative metabolic complications through alteration of the diversity and composition of the gut microbiota. More research should be carried out to prove the causal role of intestinal microflora in the treatment of CKD by natural products from plants and microorganisms based on these associative studies.
JC, LZ, HZ, and ML contributed to the conception and design of the study. LZ collected the papers. LZ wrote the first draft of the manuscript. JC, HZ, and ML revised the draft of the manuscript. All authors contributed to the manuscript and approved the submitted version.
This research was supported by the Sanming Project of Medicine in Shenzhen (SZZYSM202111002), Natural Science Foundation of China (81804052 and 82004248), Shenzhen Science and Technology Program (JCYJ20220531091809022, JSGG20191129102216637, JSGG20210802093208023, and ZDSYS201606081515458), and Traditional Chinese Medicine Bureau of Guangdong Province (20231286).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Asmakh, M., Sohail, M. U., Al-Jamal, O., Shoair, B. M., Al-Baniali, A. Y., Bouabidi, S., et al. (2020). The effects of gum acacia on the composition of the gut microbiome and plasma levels of short-chain fatty acids in a rat model of chronic kidney disease. Front. Pharmacol. 11, 569402. doi:10.3389/fphar.2020.569402
Alvarenga, L., Cardozo, L. F. M. F., Leal, V. O., Kemp, J. A., Saldanha, J. F., Ribeiro-Alves, M., et al. (2022). Can resveratrol supplementation reduce uremic toxin plasma levels from the gut microbiota in nondialyzed patients with chronic kidney disease? J. Ren. Nutr. 3 (22), 68500010–68506913. doi:10.1053/j.jrn.2022.01.010
Andrianova, N. V., Popkov, V. A., Klimenko, N. S., Tyakht, A. V., Baydakova, G. V., Frolova, O. Y., et al. (2020). Microbiome-metabolome signature of acute kidney injury. Metabolites 10 (4), 142. doi:10.3390/metabo10040142
Azevedo, R., Esgalhado, M., Kemp, J. A., Regis, B., Cardozo, L. F., Nakao, L. S., et al. (2020). Resistant starch supplementation effects on plasma indole 3-acetic acid and aryl hydrocarbon receptor mRNA expression in hemodialysis patients: Randomized, double blind and controlled clinical trial. J. Bras. Nefrol. 42 (3), 273–279. doi:10.1590/2175-8239-JBN-2020-0003
Borges, N. A., Carmo, F. L., Stockler-Pinto, M. B., de Brito, J. S., Dolenga, C. J., Ferreira, D. C., et al. (2018). Probiotic supplementation in chronic kidney disease: A double-blind, randomized, placebo-controlled trial. J. Ren. Nutr. 28 (1), 28–36. doi:10.1053/j.jrn.2017.06.010
Boyapati, R. K., Rossi, A. G., Satsangi, J., and Ho, G. T. (2016). Gut mucosal DAMPs in IBD: From mechanisms to therapeutic implications. Mucosal Immunol. 9, 567–582. doi:10.1038/mi.2016.14
Burge, K., Gunasekaran, A., Eckert, J., and Chaaban, H. (2019). Curcumin and intestinal inflammatory diseases: Molecular mechanisms of protection. Int. J. Mol. Sci. 20 (8), 1912. doi:10.3390/ijms20081912
Cai, H., Su, S., Guo, J., and Duan, J. (2021). Effect of Salviae Miltiorrhizae Radix et Rhizoma on diversity of intestinal flora in diabetic nephropathy rats. China J. Chin. Mater Med. 46 (2), 426–435. doi:10.19540/j.cnki.cjcmm.20200723.402
Cai, H., Su, S., Li, Y., Zhu, Z., Guo, J., Zhu, Y., et al. (2019). Danshen can interact with intestinal bacteria from normal and chronic renal failure rats. Biomed. Pharmacother. 109, 1758–1771. doi:10.1016/j.biopha.2018.11.047
Cai, T. T., Ye, X. L., Li, R. R., Chen, H., Wang, Y. Y., Yong, H. J., et al. (2020). Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front. Pharmacol. 11, 1249. doi:10.3389/fphar.2020.01249
Cani, P. D., Depommier, C., Derrien, M., Everard, A., and de Vos, W. M. (2022). Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 19 (10), 625–637. doi:10.1038/s41575-022-00631-9
Chaudhari, S. N., McCurry, M. D., and Devlin, A. S. (2021). Chains of evidence from correlations to causal molecules in microbiome-linked diseases. Nat. Chem. Biol. 17 (10), 1046–1056. doi:10.1038/s41589-021-00861-z
Chen, H., Wang, M. C., Chen, Y. Y., Chen, L., Wang, Y-N., Vaziri, N. D., et al. (2020). Alisol b 23-acetate attenuates CKD progression by regulating the renin–angiotensin system and gut–kidney axis. Ther. Adv. Chronic Dis. 11, 2040622320920025–21. doi:10.1177/2040622320920025
Chen, Q., Ren, D., Wu, J., Yu, H., Chen, X., Wang, J., et al. (2021). Shenyan Kangfu tablet alleviates diabetic kidney disease through attenuating inflammation and modulating the gut microbiota. J. Nat. Med. 75 (1), 84–98. doi:10.1007/s11418-020-01452-3
Chen, R., Wang, J., Zhan, R., Zhang, L., and Wang, X. (2019a). Fecal metabonomics combined with 16s rRNA gene sequencing to analyze the changes of gut microbiota in rats with kidney-yang deficiency syndrome and the intervention effect of you-gui pill. J. Ethnopharmacol. 244, 112139. doi:10.1016/j.jep.2019.112139
Chen, T. K., Knicely, D. H., and Grams, M. E. (2019b). Chronic kidney disease diagnosis and management: A review. JAMA 322 (13), 1294–1304. doi:10.1001/jama.2019.14745
Chen, X., Gao, S., Ruan, M., Chen, S., Xu, J., Xing, X., et al. (2018). Shen-Shuai-Ning granule decreased serum concentrations of indoxyl sulphate in uremic patients undergoing peritoneal dialysis. Biosci. Rep. 38 (5), BSR20171694. doi:10.1042/BSR20171694
Cheng, T. H., Ma, M. C., Liao, M. T., Zheng, C. M., Lu, K. C., Liao, C. H., et al. (2020). Indoxyl sulfate, a tubular toxin, contributes to the development of chronic kidney disease. Toxins 12 (11), 684. doi:10.3390/toxins12110684
Chi, M., Ma, K., Wang, J., Ding, Z., Li, Y., Zhu, S., et al. (2021). The immunomodulatory effect of the gut microbiota in kidney disease. J. Immunol. Res. 2021 (A7), 5516035–16. doi:10.1155/2021/5516035
Ciesielska, A., Matyjek, M., and Kwiatkowska, K. (2021). TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 78 (4), 1233–1261. doi:10.1007/s00018-020-03656-y
Cigarran Guldris, S., González Parra, E., and Cases Amenós, A. (2017). Gut microbiota in chronic kidney disease. Nefrologia 37 (1), 9–19. doi:10.1016/j.nefro.2016.05.008
Cosola, C., Rocchetti, M. T., di Bari, I., Acquaviva, P. M., Maranzano, V., Corciulo, S., et al. (2021). Efficacy of divinylbenzenic resin in removing indoxyl sulfate and P-cresol sulfate in hemodialysis patients: Results from an in vitro study and an in vivo pilot trial (xuanro4-Nature 3.2). Toxins (Basel). 13 (5), 170. doi:10.3390/toxins12030170
Cruz-Mora, J., Martínez-Hernández, N. E., Martín del Campo-López, F., Viramontes-Hörner, D., Vizmanos-Lamotte, B., Muñoz-Valle, J. F., et al. (2014). Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Ren. Nutr. 24 (5), 330–335. doi:10.1053/j.jrn.2014.05.006
Cui, X., Tao, J., Jiang, S., Wei, X., Xu, J., Qian, D., et al. (2018). Research on interaction between Coptidis Rhizoma extracts and intestinal bacteria. Chin. Tradit. Herb. Drug 49 (9), 2103–2107.
Dai, X., Cai, H., Su, S., Zheng, T., Wei, D., Yan, H., et al. (2017). Regulatory effect of the leaves of Rehmannia glutinosa Libosch on intestinal microflora in diabetic nephropathy rats. Acta Pharm. Sin. 52 (11), 1683–1691.
Davar, D., Dzutsev, A. K., McCulloch, J. A., Rodrigues, R. R., Chauvin, J. M., Morrison, R. M., et al. (2021). Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Sci. (New York, NY) 371 (6529), 595–602. doi:10.1126/science.abf3363
De Andrade, L. S., Sardá, F. A. H., Pereira, N. B. F., Teixeira, R. R., Rodrigues, S. D., de Lima, J. D., et al. (2021). Effect of unripe banana flour on gut-derived uremic toxins in individuals undergoing peritoneal dialysis: A randomized, double-blind, placebo-controlled, crossover trial. Nutrients 13 (2), 646. doi:10.3390/nu13020646
De Mauri, A., Carrera, D., Bagnati, M., Rolla, R., Vidali, M., Chiarinotti, D., et al. (2022). Probiotics-supplemented low-protein diet for microbiota modulation in patients with advanced chronic kidney disease (ProLowCKD): Results from a placebo-controlled randomized trial. Nutrients 14 (8), 1637. doi:10.3390/nu14081637
De Paiva, B. R., Esgalhado, M., Borges, N. A., Kemp, J. A., Alves, G., Leite, P. E. C., et al. (2020). Resistant starch supplementation attenuates inflammation in hemodialysis patients: A pilot study. Int. Urol. Nephrol. 52 (3), 549–555. doi:10.1007/s11255-020-02392-3
Du, X., Liu, J., Xue, Y., Kong, X., Lv, C., Li, Z., et al. (2021a). Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine 73 (1), 71–84. doi:10.1007/s12020-021-02721-1
Du, X., Pan, W., Liang, Y., and Zhang, Q. (2021b). Observation on the curative effect of modified Shenqi Dihuang decoction in treating diabetic kidney disease with deficiency of both qi and yin and its effects on intestinal flora and inflammation factors. Tradit. Chin. Drug Res. Clin. Pharmacol. 32 (4), 566–572.
Dupraz, L., Magniez, A., Rolhion, N., Richard, M. L., Da Costa, G., Touch, S., et al. (2021). Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 36 (1), 109332. doi:10.1016/j.celrep.2021.109332
Ebrahim, Z., Proost, S., Tito, R. Y., Raes, J., Glorieux, G., Moosa, M. R., et al. (2022). The effect of ß-glucan prebiotic on kidney function, uremic toxins and gut microbiome in stage 3 to 5 chronic kidney disease (CKD) predialysis participants: A randomized controlled trial. Nutrients 14 (4), 805. doi:10.3390/nu14040805
Esgalhado, M., Kemp, J. A., Azevedo, R., Paiva, B. R., Stockler-Pinto, M. B., Dolenga, C. J., et al. (2018). Could resistant starch supplementation improve inflammatory and oxidative stress biomarkers and uremic toxins levels in hemodialysis patients? A pilot randomized controlled trial. Food Funct. 9 (12), 6508–6516. doi:10.1039/c8fo01876f
Espi, M., Koppe, L., Fouque, D., and Thaunat, O. (2020). Chronic kidney disease-associated immune dysfunctions: Impact of protein-bound uremic retention solutes on immune cells. Toxins (Basel) 12 (5), 300. doi:10.3390/toxins12050300
Etxeberria, U., Arias, N., Boqué, N., Macarulla, M. T., Portillo, M. P., Martínez, J. A., et al. (2015). Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 26 (6), 651–60. doi:10.1016/j.jnutbio.2015.01.002
Evenepoel, P., Poesen, R., and Meijers, B. (2017). The gut-kidney axis. Pediatr. Nephrol. 32 (11), 2005–2014. doi:10.1007/s00467-016-3527-x
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 (1), 55–71. doi:10.1038/s41579-020-0433-9
Feng, Y., Weng, H., Ling, L., Zeng, T., Zhang, Y., Chen, D., et al. (2019). Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int. J. Biol. Macromol. 132, 1001–1011. doi:10.1016/j.ijbiomac.2019.03.242
Flagg, A. J. (2018). Chronic renal therapy. Nurs. Clin. North Am. 53 (4), 511–519. doi:10.1016/j.cnur.2018.07.002
Gao, B., Jose, A., Alonzo-Palma, N., Malik, T., Shankaranarayanan, D., Regunathan-Shenk, R., et al. (2021a). Butyrate producing microbiota are reduced in chronic kidney diseases. Sci. Rep. 11 (1), 23530. doi:10.1038/s41598-021-02865-0
Gao, Y., Yang, R., Guo, L., Wang, Y., Liu, W. J., Ai, S., et al. (2021b). Qing-Re-Xiao-Zheng formula modulates gut microbiota and inhibits inflammation in mice with diabetic kidney disease. Front. Med. (Lausanne). 8, 719950. doi:10.3389/fmed.2021.719950
Goto, M., Kobira, Y., Kaneko, S., Arima, H., Michihara, A., Azuma, K., et al. (2022). The effects of sacran, a sulfated polysaccharide, on gut microbiota using chronic kidney disease model rats. Biol. Pharm. Bull. 45 (5), 576–582. doi:10.1248/bpb.b21-00897
Guan, Y., Chen, K., Quan, D., Kang, L., Yang, D., Wu, H., et al. (2021). The combination of Scutellaria baicalensis Georgi and Sophora japonica L. ameliorate renal function by regulating gut microbiota in spontaneously hypertensive rats. Front. Pharmacol. 11, 575294. doi:10.3389/fphar.2020.575294
Guida, B., Germanò, R., Trio, R., Russo, D., Memoli, B., Grumetto, L., et al. (2014). Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: A randomized clinical trial. Nutr. Metab. Cardiovasc Dis. 24 (9), 1043–1049. doi:10.1016/j.numecd.2014.04.007
Hu, X., Ouyang, S., Xie, Y., Gong, Z., and Du, J. (2020b). Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad. Med. 132 (6), 495–505. doi:10.1080/00325481.2020.1744335
Han, C., Jiang, Y. H., Li, W., and Liu, Y. (2020). Astragalus membranaceus and Salvia miltiorrhiza ameliorates cyclosporin a-induced chronic nephrotoxicity through the "gut-kidney axis. J. Ethnopharmacol. 269 (5), 113768. doi:10.1016/j.jep.2020.113768
He, L. X., Abdolmaleky, H. M., Yin, S., Wang, Y., and Zhou, J. R. (2020). Dietary fermented soy extract and oligo-lactic acid alleviate chronic kidney disease in mice via inhibition of inflammation and modulation of gut microbiota. Nutrients 12 (8), 2376. doi:10.3390/nu12082376
Hobby, G. P., Karaduta, O., Dusio, G. F., Singh, M., Zybailov, B. L., and Arthur, J. M. (2019). Chronic kidney disease and the gut microbiome. Am. J. Physiol. Ren. Physiol. 316 (6), F1211–F1217. doi:10.1152/ajprenal.00298.2018
Hong, Y., Sheng, L., Zhong, J., Tao, X., Zhu, W., Ma, J., et al. (2021). Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 13 (1), 1–20. doi:10.1080/19490976.2021.1930874
Hu, J., Zhong, X., Yan, J., Zhou, D., Qin, D., Xiao, X., et al. (2020a). High-throughput sequencing analysis of intestinal flora changes in ESRD and CKD patients. BMC Nephrol. 21 (1), 12. doi:10.1186/s12882-019-1668-4
Hung, T. V., and Suzuki, T. (2018). Dietary fermentable fibers attenuate chronic kidney disease in mice by protecting the intestinal barrier. J. Nutr. 148 (4), 552–561. doi:10.1093/jn/nxy008
Iwashita, Y., Ohya, M., Yashiro, M., Sonou, T., Kawakami, K., Nakashima, Y., et al. (2018). Dietary changes involving Bifidobacterium longum and other nutrients delays chronic kidney disease progression. Am. J. Nephrol. 47 (5), 325–332. doi:10.1159/000488947
Ji, C., Deng, Y., Yang, A., Lu, Z., Chen, Y., Liu, X., et al. (2020). Rhubarb Enema improved colon mucosal barrier injury in 5/6 nephrectomy rats may associate with gut microbiota modification. Front. Pharmacol. 11, 1092. doi:10.3389/fphar.2020.01092
Ji, C., Li, Y., Mo, Y., Lu, Z., Lu, F., Lin, Q., et al. (2021). Rhubarb Enema decreases circulating trimethylamine N-oxide level and improves renal fibrosis accompanied with gut microbiota change in chronic kidney disease rats. Front. Pharmacol. 12, 780924. doi:10.3389/fphar.2021.780924
Jia, L., Dong, X., Li, X., Jia, R., and Zhang, H. L. (2021). Benefits of resistant starch type 2 for patients with end-stage renal disease under maintenance hemodialysis: A systematic review and meta-analysis. Int. J. Med. Sci. 18 (3), 811–820. doi:10.7150/ijms.51484
Jiang, S., Xie, S., Lv, D., Wang, P., He, H., Zhang, T., et al. (2017). Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci. Rep. 7 (1), 2870. doi:10.1038/s41598-017-02989-2
Karaduta, O., Glazko, G., Dvanajscak, Z., Arthur, J., Mackintosh, S., Orr, L., et al. (2020). Resistant starch slows the progression of CKD in the 5/6 nephrectomy mouse model. Physiol. Rep. 8 (19), e14610. doi:10.14814/phy2.14610
Kemp, J. A., Regis de Paiva, B., Fragoso Dos Santos, H., Emiliano de Jesus, H., Craven, H., Z Ijaz, U., et al. (2021). The impact of enriched resistant starch type-2 cookies on the gut microbiome in hemodialysis patients: A randomized controlled trial. Mol. Nutr. Food Res. 65 (19), e2100374. doi:10.1002/mnfr.202100374
Kieffer, D. A., Piccolo, B. D., Vaziri, N. D., Liu, S., Lau, W. L., Khazaeli, M., et al. (2016). Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am. J. Physiol. Ren. Physiol. 310 (9), F857–871. doi:10.1152/ajprenal.00513.2015
Kim, H., Nam, B. Y., Park, J., Song, S., Kim, W. K., Lee, K., et al. (2022). Lactobacillus acidophilus KBL409 reduces kidney fibrosis via immune modulatory effects in mice with chronic kidney disease. Mol. Nutr. Food Res. 66 (22), e2101105. doi:10.1002/mnfr.202101105
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165 (6), 1332–1345. doi:10.1016/j.cell.2016.05.041
Koliada, A., Syzenko, G., Moseiko, V., Budovska, L., Puchkov, K., Perederiy, V., et al. (2017). Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 17 (1), 120. doi:10.1186/s12866-017-1027-1
Kushkevych, I., Dordević, D., Kollar, P., Vítězová, M., and Drago, L. (2019). Hydrogen sulfide as a toxic product in the small-large intestine axis and its role in IBD development. J. Clin. Med. 8 (7), 1054. doi:10.3390/jcm8071054
Laffin, M. R., Tayebi Khosroshahi, H., Park, H., Laffin, L. J., Madsen, K., Kafil, H. S., et al. (2019). Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: Microbial analysis from a randomized placebo-controlled trial. Hemodial. Int. 23 (3), 343–347. doi:10.1111/hdi.12753
Lai, S., Molfino, A., Testorio, M., Perrotta, A. M., Currado, A., Pintus, G., et al. (2019). Effect of low-protein diet and inulin on microbiota and clinical parameters in patients with chronic kidney disease. Nutrients 11 (12), 3006. doi:10.3390/nu11123006
Lakshmanan, A. P., Al Za'abi, M., Ali, B. H., and Terranegra, A. (2021). The influence of the prebiotic gum acacia on the intestinal microbiome composition in rats with experimental chronic kidney disease. Biomed. Pharmacother. 133, 110992. doi:10.1016/j.biopha.2020.110992
Lang, R., Wang, X. H., Li, A. F., Liang, Y., Yu, R. H., Shi, B., et al. (2020). Effects of jian Pi Qu Shi formula on intestinal bacterial flora in patients with idiopathic membranous nephropathy: A prospective randomized controlled trial. Chronic Dis. Transl. Med. 6, 124–133. doi:10.1016/j.cdtm.2020.04.004
Li, C., Wang, Y., Wang, Y., Yin, J., Yang, S., Liu, Y., et al. (2022). Piceatannol alleviates host inflammation in chronic kidney disease model mice through regulating gut microbiota. Acta Pharm. Sin. 57 (02), 364–374. doi:10.16438/j.0513-4870.2021-0990
Li, G., and Young, K. D. (2013). Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiol. Read. 159 (2), 402–410. doi:10.1099/mic.0.064139-0
Li, J., Cao, Y., Lu, R., Li, H., Pang, Y., Fu, H., et al. (2021). Zhen-Wu-Tang protects IgA nephropathy in rats by regulating exosomes to inhibit NF-κB/NLRP3 pathway. Front. Pharmacol. 11, 1080. doi:10.3389/fphar.2020.01080
Li, L., Xiong, Q., Zhao, J., Lin, X., He, S., Wu, N., et al. (2020a). Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: A randomized crossover study. Am. J. Clin. Nutr. 111 (5), 1087–1099. doi:10.1093/ajcn/nqz337
Li, Y. J., Chen, X., Kwan, T. K., Loh, Y. W., Singer, J., Liu, Y., et al. (2020b). Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 31 (6), 1267–1281. doi:10.1681/ASN.2019101029
Li, Y., Xiong, W., Yang, J., Zhong, J., Zhang, L., Zheng, J., et al. (2015). Attenuation of inflammation by emodin in lipopolysaccharide-induced acute kidney injury via inhibition of Toll-like receptor 2 signal pathway. Iran. J. Kidney Dis. 9 (3), 202–208.
Lim, Y. J., Sidor, N. A., Tonial, N. C., Che, A., and Urquhart, B. L. (2021). Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: Mechanisms and therapeutic targets. Toxins 13 (2), 142. doi:10.3390/toxins13020142
Lin, C. J., Wu, V., Wu, P. C., and Wu, C. J. (2015). Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PloS one 10 (7), e0132589. doi:10.1371/journal.pone.0132589
Lin, T. L., Lu, C. C., Lai, W. F., Wu, T. S., Lu, J. J., Chen, Y. M., et al. (2021a). Role of gut microbiota in identification of novel TCM-derived active metabolites. Protein Cell 12 (5), 394–410. doi:10.1007/s13238-020-00784-w
Lin, W., Jiang, C., Yu, H., Wang, L., Li, J., Liu, X., et al. (2021b). The effects of Fushen Granule on the composition and function of the gut microbiota during Peritoneal Dialysis-Related Peritonitis. Phytomedicine 86, 153561. doi:10.1016/j.phymed.2021.153561
Liu, F., Li, P., Chen, M., Luo, Y., Prabhakar, M., Zheng, H., et al. (2017). Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 7 (1), 11789. doi:10.1038/s41598-017-10722-2
Liu, J., Gao, L. D., Fu, B., Yang, H. T., Zhang, L., Che, S. Q., et al. (2022). Efficacy and safety of zicuiyin decoction on diabetic kidney disease: A multicenter, randomized controlled trial. Phytomedicine 100, 154079. doi:10.1016/j.phymed.2022.154079
Liu, Z. Z., Weng, H. B., Zhang, L. J., Pan, L. Y., Sun, W., Chen, H. X., et al. (2019). Bupleurum polysaccharides ameliorated renal injury in diabetic mice associated with suppression of HMGB1-TLR4 signaling. Chi J. Nat. Med. 17 (9), 641–649. doi:10.1016/S1875-5364(19)30078-0
Lobel, L., Cao, Y. G., Fenn, K., Glickman, J. N., and Garrett, W. S. (2020). Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 369 (6510), 1518–1524. doi:10.1126/science.abb3763
Lu, J., Wang, Y., Peng, Y., Xu, X., Chen, C., Duan, J., et al. (2021a). Effect of Huangkui Siwu Formula on metabolism and transportation pathway of urotoxin p-cresyl sulfate in vivo. Chin. Tradit. Herb. Drug 52 (1), 176–185.
Lu, J., Wang, Y., Zhang, S., Li, J., Li, C., Xu, X., et al. (2020). Huang-Kui-Si-Wu Formula decreases uremic toxin production by modulating intestinal microbial metabolic pathways. Acta Pharm. Sin. 55 (6), 1229–1236.
Lu, Z., Ji, C., Luo, X., Lan, Y., Han, L., Chen, Y., et al. (2021b). Nanoparticle-mediated delivery of emodin via colonic irrigation attenuates renal injury in 5/6 nephrectomized rats. Front. Pharmacol. 11, 606227. doi:10.3389/fphar.2020.606227
Lu, Z., Zeng, Y., Lu, F., Liu, X., and Zou, C. (2015). Rhubarb Enema attenuates renal tubulointerstitial fibrosis in 5/6 nephrectomized rats by alleviating indoxyl sulfate overload. PLoS one 10 (12), e0144726. doi:10.1371/journal.pone.0144726
Lv, J. C., and Zhang, L. X. (2019). Prevalence and disease burden of chronic kidney disease. Adv. Exp. Med. Biol. 1165, 3–15. doi:10.1007/978-981-13-8871-2_1
McFarlane, C., Krishnasamy, R., Stanton, T., Savill, E., Snelson, M., Mihala, G., et al. (2021). Synbiotics easing renal failure by improving gut microbiology II (SYNERGY II): A feasibility randomized controlled trial. Nutrients 13 (12), 4481. doi:10.3390/nu13124481
Meijers, B. K., De Preter, V., Verbeke, K., Vanrenterghem, Y., and Evenepoel, P. (2010). p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transpl. 25 (1), 219–24. doi:10.1093/ndt/gfp414
Melekoglu, E., Cetinkaya, M. A., Kepekci-Tekkeli, S. E., Kul, O., and Samur, G. (2021). Effects of prebiotic oligofructose-enriched inulin on gut-derived uremic toxins and disease progression in rats with adenine-induced chronic kidney disease. PLoS One 16 (10), e0258145. doi:10.1371/journal.pone.0258145
Meng, X., Ma, J., Kang, A. N., Kang, S. Y., Jung, H. W., and Park, Y-K. (2020). A novel approach based on metabolomics coupled with intestinal flora analysis and network pharmacology to explain the mechanisms of action of Bekhogainsam decoction in the improvement of symptoms of streptozotocin-induced diabetic nephropathy in mice. Front. Pharmacol. 11, 633. doi:10.3389/fphar.2020.00633
Ming, Y., Cheng, S., Long, W., Wang, H. L., Shen, H., Liu, X., et al. (2021). The herbal formula granule prescription Mahuang decoction ameliorated chronic kidney disease which was associated with restoration of dysbiosis of intestinal microbiota in rats. Evid. Based Complement. Altern. Med. 2021 (9888), 4602612–12. doi:10.1155/2021/4602612
Mirzaeian, S., Saraf-Bank, S., Entezari, M. H., Hekmatdoost, A., Feizi, A., and Atapour, A. (2020). Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: A double-blind, placebo-controlled, randomized clinical trial. Nutrition 73, 110713. doi:10.1016/j.nut.2019.110713
Mokhtari, P., Metos, J., and Anandh Babu, P. V. (2021). Impact of type 1 diabetes on the composition and functional potential of gut microbiome in children and adolescents: Possible mechanisms, current knowledge, and challenges. Gut Microbes 13 (1), 1–18. doi:10.1080/19490976.2021.1926841
Nagayama, Y., Isoo, N., Nakashima, A., Suzuki, K., Yamano, M., Nariyama, T., et al. (2020). Renoprotective effects of paramylon, a β-1, 3-D Glucan isolated from Euglena gracilis Z in a rodent model of chronic kidney disease. PLoS one 15 (8), e0237086. doi:10.1371/journal.pone.0237086
Nogal, A., Louca, P., Zhang, X., Wells, P. M., Steves, C. J., Spector, T. D., et al. (2021). Circulating levels of the short-chain fatty acid acetate mediate the effect of the gut microbiome on visceral fat. Front. Microbiol. 12, 711359. doi:10.3389/fmicb.2021.711359
Opdebeeck, B., D'Haese, P. C., and Verhulst, A. (2020). Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and p-cresyl sulfate. Toxins (Basel). 12 (1), 58. doi:10.3390/toxins12010058
Paone, P., and Cani, P. D. (2020). Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 69 (12), 2232–2243. doi:10.1136/gutjnl-2020-322260
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277. doi:10.3389/fimmu.2019.00277
Pivari, F., Mingione, A., Piazzini, G., Ceccarani, C., Ottaviano, E., Brasacchio, C., et al. (2022). Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 14 (1), 1837. doi:10.3390/nu11081837
Raj, D. S., Sohn, M. B., Charytan, D. M., Himmelfarb, J., Ikizler, T. A., Mehrotra, R., et al. (2021). The microbiome and p-inulin in hemodialysis: A feasibility study. Kidney360. 2 (3), 445–455. doi:10.34067/KID.0006132020
Ramos, C. I., Armani, R. G., Canziani, M. E. F., Dalboni, M. A., Dolenga, C. J. R., Nakao, L. S., et al. (2019). Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: A randomized controlled trial. Nephrol. Dial. Transpl. 34 (11), 1876–1884. doi:10.1093/ndt/gfy171
Remely, M., Hippe, B., Zanner, J., Aumueller, E., Brath, H., and Haslberger, A. G. (2016). Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr. Metab. Immune Disord. Drug Targets 16 (2), 99–106. doi:10.2174/1871530316666160831093813
Rodrigues, V. F., Elias-Oliveira, J., Pereira, Í. S., Pereira, J. A., Barbosa, S. C., Machado, M. S. G., et al. (2022). Akkermansia muciniphila and gut immune system: A good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front. Immunol. 13, 934695. doi:10.3389/fimmu.2022.934695
Rossi, M., Johnson, D. W., Morrison, M., Pascoe, E. M., Coombes, J. S., Forbes, J. M., et al. (2016). Synbiotics easing renal failure by improving gut microbiology (SYNERGY): A randomized trial. Clin. J. Am. Soc. Nephrol. 11 (2), 223–231. doi:10.2215/CJN.05240515
Rui-Zhi, T., Hui, D., Jian-Chun, L., Xia, Z., Xiao-Jia, W., Dan, W., et al. (2020). Astragalus mongholicus Bunge and Panax Notoginseng formula (A&P) combined with Bifidobacterium contribute a renoprotective effect in chronic kidney disease through inhibiting macrophage inflammatory response in kidney and intestine. Front. Physiol. 11, 583668. doi:10.3389/fphys.2020.583668
Rysz, J., Franczyk, B., Ławi´ nski, J., Olszewski, R., Ciałkowska-Rysz, A., and Gluba-Brzózka, A. (2021). The impact of CKD on uremic toxins and gut microbiota. Toxins 13 (4), 252. doi:10.3390/toxins13040252
Salarolli, R. T., Alvarenga, L., Cardozo, L. F. M. F., Teixeira, K. T. R., de S G Moreira, L., Lima, J. D., et al. (2021). Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int. Urol. Nephrol. 53 (6), 1231–1238. doi:10.1007/s11255-020-02760-z
Sanctuary, M. R., Kain, J. N., Chen, S. Y., Kalanetra, K., Lemay, D. G., Rose, D. R., et al. (2019). Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One 14 (1), e0210064. doi:10.1371/journal.pone.0210064
Sato, N., Kakuta, M., Hasegawa, T., Yamaguchi, R., Uchino, E., Murashita, K., et al. (2021). Metagenomic profiling of gut microbiome in early chronic kidney disease. Nephrol. Dial. Transpl. 36 (9), 1675–1684. doi:10.1093/ndt/gfaa122
Shi, H. H., Chen, L. P., Wang, C. C., Zhao, Y. C., Wang, Y. M., Xue, C. H., et al. (2022). Docosahexaenoic acid-acylated curcumin diester alleviates cisplatin-induced acute kidney injury by regulating the effect of gut microbiota on the lipopolysaccharide- and trimethylamine-N-oxide-mediated PI3K/Akt/NF-κB signaling pathway in mice. Food Funct. 13 (11), 6103–6117. doi:10.1039/d1fo04178a
Shin, N. R., Whon, T. W., and Bae, J. W. (2015). Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33 (9), 496–503. doi:10.1016/j.tibtech.2015.06.011
Su, X., Yu, W., Liu, A., Wang, C., Li, X., Gao, J., et al. (2022). San-Huang-Yi-Shen capsule ameliorates diabetic nephropathy in rats through modulating the gut microbiota and overall metabolism. Front. Pharmacol. 12, 808867. doi:10.3389/fphar.2021.808867
Sun, J., Luo, J. W., Yao, W. J., Luo, X. T., Su, C. L., and Wei, Y. H. (2019). Effect of emodin on gut microbiota of rats with acute kidney failure. China J. Chin. Mater Med. 44 (4), 758–764. doi:10.19540/j.cnki.cjcmm.20181105.002
Taherali, F., Varum, F., and Basit, A. W. (2018). A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 124, 16–33. doi:10.1016/j.addr.2017.10.014
Tayebi Khosroshahi, H., Vaziri, N. D., Abedi, B., Asl, B. H., Ghojazadeh, M., Jing, W., et al. (2018). Effect of high amylose resistant starch (HAM-RS2) supplementation on biomarkers of inflammation and oxidative stress in hemodialysis patients: A randomized clinical trial. Hemodial. Int. 22 (4), 492–500. doi:10.1111/hdi.12653
Teixeira, K. T. R., Moreira, L. S. G., Borges, N. A., Brum, I., de Paiva, B. R., Alvarenga, L., et al. (2022). Effect of cranberry supplementation on toxins produced by the gut microbiota in chronic kidney disease patients: A pilot randomized placebo-controlled trial. Clin. Nutr. ESPEN 47, 63–69. doi:10.1016/j.clnesp.2021.11.012
Tu, Y., Fang, Q. J., Sun, W., Liu, B. H., Liu, Y. L., Wu, W., et al. (2020). Total flavones of Abelmoschus manihot remodels gut microbiota and inhibits microinflammation in chronic renal failure progression by targeting autophagy-mediated macrophage polarization. Front. Pharmacol. 11, 566611. doi:10.3389/fphar.2020.566611
Tungsanga, S., Katavetin, P., Panpetch, W., Udompornpitak, K., Saisorn, W., Praditpornsilpa, K., et al. (2022). Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules. Nephrol. Dial. Transpl. 37 (8), 1429–1442. doi:10.1093/ndt/gfac032
Wang, H., Gou, S. J., Zhao, M. H., and Chen, M. (2014). The expression of Toll-like receptors 2, 4 and 9 in kidneys of patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin. Exp. Immunol. 177 (3), 603–610. doi:10.1111/cei.12365
Wang, H., Xu, J., Jiang, Q., Kang, L., Liu, W., Yuan, L., et al. (2021). Effects of couplet medicine of Rheum palmatum-Salvia miltiorrhiza on enterogenous urotoxins contents and intestinal barrier function in rats with chronic renal failure. China Pharm. 32 (7), 825–831.
Wang, L., and Chen, Y. (2017). Efficient biotransformation of Astragaloside IV to cycloastragenol by Bacillus sp. LG-502. Appl. Biochem. Biotechnol. 183, 1488–1502. doi:10.1007/s12010-017-2517-1
Wang, X., Yang, S., Li, S., Zhao, L., Hao, Y., Qin, J., et al. (2020a). Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 69, 2131–2142. doi:10.1136/gutjnl-2019-319766
Wang, Y., Li, J., Chen, C., Lu, J., Yu, J., Xu, X., et al. (2020b). Targeting the gut microbial metabolic pathway with small molecules decreases uremic toxin production. Gut Microbes 12 (1), 1–19. doi:10.1080/19490976.2020.1823800
Wang, Y., Li, J., Lu, J., Li, C., Yu, J., Zhang, S., et al. (2019). Effect and mechanism of Huangkui capsule on reduction of uremic toxin accumulation in an animal model of chronic kidney disease. Acta Pharm. Sin. 54 (12), 2267–2276.
Wang, Z., Zhang, Z., Lu, C., Zhou, J., Wang, Z., Han, J., et al. (2022). Effects of Sporisorium reiliana polysaccharides and Phoenix dactylifera monosaccharides on the gut microbiota and serum metabolism in mice with fructose-induced hyperuricemia. Arch. Microbiol. 204 (7), 436. doi:10.1007/s00203-022-03053-y
Wei, H., Wang, L., An, Z., Xie, H., Liu, W., Du, Q., et al. (2021). QiDiTangShen granules modulated the gut microbiome composition and improved bile acid profiles in a mouse model of diabetic nephropathy.files. in a mouse model of diabetic nephropathy. Biomed. Pharmacother. 133, 111061. doi:10.1016/j.biopha.2020.111061
Wu, I. W., Gao, S. S., Chou, H. C., Yang, H. Y., Chang, L. C., Kuo, Y. L., et al. (2020). Integrative metagenomic and metabolomic analyses reveal severity-specific signatures of gut microbiota in chronic kidney disease. Theranostics 10 (12), 5398–5411. doi:10.7150/thno.41725
Xu, K. Y., Xia, G. H., Lu, J. Q., Chen, M. X., Zhen, X., Wang, S., et al. (2017). Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 7 (1), 1445. doi:10.1038/s41598-017-01387-y
Xu, X., Wang, H., Guo, D., Man, X., Liu, J., Li, J., et al. (2021a). Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail 43 (1), 1063–1075. doi:10.1080/0886022X.2021.1944875
Xu, Z., Dai, X. X., Zhang, Q. Y., Su, S. L., Yan, H., Zhu, Y., et al. (2020). Protective effects and mechanisms of Rehmannia glutinosa leaves total glycoside on early kidney injury in db/db mice. Biomed. Pharmacother. 125, 109926. doi:10.1016/j.biopha.2020.109926
Xu, Z., Hou, Y., Sun, J., Zhu, L., Zhang, Q., Yao, W., et al. (2022). Deoxycholic acid-chitosan coated liposomes combined with in situ colonic gel enhances renal fibrosis therapy of emodin. Phytomedicine 101, 154110. doi:10.1016/j.phymed.2022.154110
Xu, Z., Xiang, X., Shang, E., Su, S., Guo, J., Qian, D., et al. (2021b). Regulatory effect of total phenolic acid from the stems and leaves of Salvia miltiorrhiza Bge. on intestinal microflora and short-chain fatty acids in type 2 diabetic nephropathy mice. Acta Pharm. Sin. 56 (4), 1035–1048.
Yang, J., Dong, H., Wang, Y., Jiang, Y., Zhang, W., Lu, Y., et al. (2020a). Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 163, 442–456. doi:10.1016/j.ijbiomac.2020.06.153
Yang, J., Li, Q., Henning, S. M., Zhong, J., Hsu, M., Lee, R., et al. (2018). Effects of prebiotic fiber Xylooligosaccharide in adenine-induced nephropathy in mice. Mol. Nutr. Food Res. 62, e1800014. doi:10.1002/mnfr.201800014
Yang, J., Wang, L., Yu, Q., Diao, H., and Fan, J. (2021). The protective effect of Astragalus polysaccharides on chronic renal failure in mice. Tianjin Med. J. 49 (7), 713–720.
Yang, R., Li, Y., Mehmood, S., Yan, C., Huang, Y., Cai, J., et al. (2020b). Polysaccharides from Armillariella tabescens mycelia ameliorate renal damage in type 2 diabetic mice. Int. J. Biol. Macromol. 162, 1682–1691. doi:10.1016/j.ijbiomac.2020.08.006
Zeng, Y. Q., Dai, Z., Lu, F., Lu, Z., Liu, X., Chen, C., et al. (2016). Emodin via colonic irrigation modulates gut microbiota and reduces uremic toxins in rats with chronic kidney disease. Oncotarget 7 (14), 17468–17478. doi:10.18632/oncotarget.8160
Zhai, Y., Li, P., Wang, M., Gong, M., and Qiu, F. (2016). Determination of Astragaloside III in rat plasma by liquid chromatography-tandem mass spectrometry and its application to a rat pharmacokinetic study. Biomed. Chromatogr. 30 (2), 105–110. doi:10.1002/bmc.3521
Zhang, J., Wu, C., Gao, L., Du, G., and Qin, X. (2020). Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 87, 89–112. doi:10.1016/bs.apha.2019.08.002
Zhang, L., Wang, F., Wang, L., Wang, W., Liu, B., Liu, J., et al. (2012). Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379 (9818), 815–822. doi:10.1016/S0140-6736(12)60033-6
Zhang, L., Zhang, T-J., Li, Y., and Xiong, W-J. (2022a). Shenqi Yanshen Formula (SQYSF) protects against chronic kidney disease by modulating gut microbiota. Bioengineered 13 (3), 5625–5637. doi:10.1080/21655979.2021.2023789
Zhang, M., Yang, L., Zhu, M., Yang, B., Yang, Y., Jia, X., et al. (2022b). Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 206, 849–860. doi:10.1016/j.ijbiomac.2022.03.077
Zhang, W., Lai, X., and Chen, J. (2021a). Effect of yam polysaccharides in the treatment of obese diabetic nephropathy rats and its effect on renal function and intestinal microecology. Chin. J. Microecol. 33 (1), 37–42.
Zhang, Y., Wang, Y., Ke, B., and Du, J. (2021b). TMAO: How gut microbiota contributes to heart failure. Transl. Res. 228, 109–125. doi:10.1016/j.trsl.2020.08.007
Zhang, Z. M., Yang, L., Wan, Y., Jiang, S., Shang, E. X., Qian, D. W., et al. (2021c). The synergic renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced chronic kidney disease rats based on integrated plasma metabolomics and network pharmacology approach. Life Sci. 278, 119545. doi:10.1016/j.lfs.2021.119545
Zhang, Z. M., Yang, L., Wan, Y., Liu, C., Jiang, S., Shang, E. X., et al. (2021d). Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of Rehmanniae Radix Preparata and Corni Fructus on adenine-induced CKD rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1174, 122728. doi:10.1016/j.jchromb.2021.122728
Zhao, J., Tostivint, I., Xu, L., Huang, J., Gambotti, L., Boffa, J. J., et al. (2022). Efficacy of combined Abelmoschus manihot and irbesartan for reduction of albuminuria in patients with type 2 diabetes and diabetic kidney disease: A multicenter randomized double-blind parallel controlled clinical trial. Diabetes Care 45 (7), e113–e115. doi:10.2337/dc22-0607
Zhao, J., Zhang, Q. L., Shen, J. H., Kai, W., and Jia, L. (2019). Magnesium lithospermate B improves the gut microbiome and bile acid metabolic profiles in a mouse model of diabetic nephropathy. Acta Pharmacol. Sin. 40, 507–513. doi:10.1038/s41401-018-0029-3
Zhao, M., Yu, Y., Wang, R., Chang, M., Ma, S., Qu, H., et al. (2021). Mechanisms and efficacy of Chinese herbal medicines in chronic kidney disease. Front. Pharmacol. 11, 619201. doi:10.3389/fphar.2020.619201
Zhao, T., Zhang, H., Yin, X., Zhao, H., Ma, L., Yan, M., et al. (2020). Tangshen formula modulates gut microbiota and reduces gut-derived toxins in diabetic nephropathy rats. Biomed. Pharmacother. 129, 110325. doi:10.1016/j.biopha.2020.110325
Zheng, L., Chen, S., Wang, F., Huang, S., Liu, X., Yang, X., et al. (2020). Distinct responses of gut microbiota to Jian-Pi-Yi-Shen decoction are associated with improved clinical outcomes in 5/6 nephrectomized rats. Front. Pharmacol. 11, 604. doi:10.3389/fphar.2020.00604
Zhong, Z. X., Tan, J. X., Tan, L., Tang, Y., Qin, Z. C., Pei, G. Q., et al. (2020). Modifications of gut microbiota are associated with the severity of IgA nephropathy in the Chinese population. Int. Immunopharmacol. 89 (2), 107085. doi:10.1016/j.intimp.2020.107085
Zhu, H., Cao, C., Wu, Z., Zhang, H., Sun, Z., Wang, M., et al. (2021). The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. S1550-4131 (21), 1926–1942. doi:10.1016/j.cmet.2021.06.014
Zybailov, B. L., Glazko, G. V., Rahmatallah, Y., Andreyev, D. S., McElroy, T., Karaduta, O., et al. (2019). Metaproteomics reveals potential mechanisms by which dietary resistant starch supplementation attenuates chronic kidney disease progression in rats. PLoS One 14 (1), e0199274. doi:10.1371/journal.pone.0199274
Keywords: chronic kidney disease, gut microbiota, polysaccharide, herbal medicines, phytochemicals, gut–kidney axis, natural products
Citation: Zheng L, Luo M, Zhou H and Chen J (2023) Natural products from plants and microorganisms: Novel therapeutics for chronic kidney disease via gut microbiota regulation. Front. Pharmacol. 13:1068613. doi: 10.3389/fphar.2022.1068613
Received: 13 October 2022; Accepted: 23 December 2022;
Published: 17 January 2023.
Edited by:
Xuan Zeng, Sun Yat-sen University, ChinaReviewed by:
Shengyuan Xiao, Jilin Agricultural University, ChinaCopyright © 2023 Zheng, Luo, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Chen, bHljanBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.