
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 10 November 2022
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1063625
This article is part of the Research Topic Pharmacovigilance and Drug Repositioning Research using Pharmacoepidemiology View all 7 articles
Objectives: Intraocular administration of vascular endothelial growth factor (VEGF) inhibitors may be associated with pregnancy loss. However, little is known about intraocular anti-VEGF therapy during pregnancy. Here, we conducted a pharmacovigilance study using a spontaneous reporting database to evaluate the relationship between intraocular VEGF inhibitors and pregnancy loss.
Methods: We used the JAPIC AERS database which is composed of the Food and Drug Administration Adverse Event Reporting System (FAERS) dataset preprocessed by the Japan Pharmaceutical Information Center (JAPIC) to investigate the VEGF inhibitors ranibizumab, aflibercept, and bevacizumab. Disproportionality analyses were conducted for VEGF inhibitors and pregnancy loss. The lower limit of the 95% confidence interval (CI) of the reporting odds ratio (ROR) > 1 and a minimum of three reported cases of pregnancy loss were the detection criteria used in the current study.
Results: In the FAERS, 19 pregnancy loss cases were reported for ranibizumab with an ROR of 4.44 (95% CI: 2.42–8.16), 6 for intraocular bevacizumab with an ROR of 32.25 (95% CI: 3.88–267.9), and 4 for intraocular aflibercept with an ROR of 5.37 (95% CI: 1.34–21.49). All these drugs met the detection criteria.
Conclusion: Potential safety signals of pregnancy loss were obtained from intraocular administration of VEGF inhibitors during pregnancy. These signals should be validated using a causal design study.
Intraocular anti-vascular endothelial growth factor (VEGF) therapy is commonly used because of its efficacy in various diseases such as choroidal neovascularization, retinal vascular occlusion, and diabetic retinopathy/macular edema. All these diseases are seen in not only older adult patients but also women of childbearing age, meaning that pregnant patients could be exposed to these drugs without awareness of their pregnancy (Kianersi et al., 2016; Naderan et al., 2021). Intraocular administration of VEGF inhibitors such as ranibizumab, aflibercept, and bevacizumab has been shown to enter systemic circulation and reduce VEGF levels in the blood (Avery et al., 2014). VEGF contributes to vascular shape and function (Bautch, 2012), and both VEGF-A polymorphisms and reduced VEGF expression have been associated with spontaneous miscarriage, likely owing to defective fetal and placental angiogenesis (Almawi et al., 2013). Because human immunoglobin G is known to cross the placental barrier, anti-VEGF antibodies may also cross the placenta. Therefore, intraocular administration of VEGF inhibitors may be associated with pregnancy loss. However, only a few studies based on a small number of case reports have examined the association between VEGF inhibitors and adverse pregnancy outcomes, and the results are controversial (Polizzi and Mahajan, 2015; Naderan et al., 2021). Further evidence is required, but the number of cases at individual medical institutions is limited.
Spontaneous reporting is a fundamental source of information in pharmacovigilance. Usually, the risk of adverse events from drug exposure during pregnancy is examined in study designs involving a control group, such as in cohort studies. However, these studies require a sufficient number of cases and may require significant time and effort to conduct. To obtain safety signal data for small study populations earlier, some pharmacovigilance researchers have begun using the spontaneous reporting database (Deepak and Stobaugh, 2014; Sakai et al., 2017; Sessa et al., 2019; van De Ven et al., 2020). An international survey of pharmacovigilance centers has also reported intentions to implement or improve spontaneous reporting for drug exposure during pregnancy (Kant et al., 2019). Lareb, a pharmacovigilance center in the Netherlands, has discussed the use of a spontaneous reporting database to develop a toolkit for drug safety surveillance in pregnant women (Lareb, 2018).
Here, we conducted a pharmacovigilance study using a large spontaneous reporting database to evaluate the relationship between intraocular VEGF inhibitors and pregnancy loss.
We collected data from the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), a spontaneous reporting database containing records from pharmaceutical companies, medical institutions, and consumers. Data collected were split into seven tables reporting these essential categories: patient demographic information (DEMO), drug information (DRUG), indications for the use of reported drugs (INDI), therapy start and end dates (THER), adverse events (REAC), adverse event outcomes (OUTC), and report sources (RPSR). The cases included in this study were reported from the fourth quarter of 1997 to the fourth quarter of 2021. The FAERS dataset version used was processed by the Japan Pharmaceutical Information Center (JAPIC). They removed duplicate cases, such as those with the same “CASEID,” leaving the non-duplicated cases. In the dataset, drug names were mapped to generic names using their respective drug name dictionaries; reported adverse events and indication of use were assigned to their preferred term (PT) codes and the age was converted to years. The drug name dictionary is based on the World Health Organization drug dictionary and Drugs@FDA (https://www.accessdata.fda.gov). For drug names not included in these dictionaries, dictionaries were created based on the Summary of Product Characteristics for each product, as well as on drug information databases such as Martindale and MIMS (https://www.mims.com/). This preprocessed FAERS dataset (JAPIC AERS) was coded in the Medical Dictionary for Regulatory Activities (MedDRA) ver 25.0, which we used for analysis in the current study.
Subgroup disproportionality analyses are used to control for possible bias when analyzing the relationship between drugs and pregnancy outcomes in datasets in which most reports are from non-pregnant women (Beyer-Westendorf et al., 2020; Huybrechts et al., 2021). However, there is no established algorithm for identifying reports of pregnant women from spontaneous reporting databases (Deepak and Stobaugh, 2014; Sessa et al., 2019; Sandberg et al., 2020; Sakai et al., 2022). To our knowledge, no regulatory authority has provided any specific guidance for such procedure. Because the spontaneous reporting databases do not usually contain a dedicated field to identify reports of pregnant women, measures are being taken to identify such reports using the standard MedDRA query (SMQ) (Sessa et al., 2019; Sakai et al., 2022). Free text and author-specific keyword searches other than dictionaries have also been conducted, but their reliability is unknown (Deepak and Stobaugh, 2014). Therefore, in the current study, we used our previously described method to identify reports of pregnant women in the FAERS (Sakai et al., 2022).
All sub-SMQs of the SMQ “pregnancy and neonatal topics,” except for “lactation related topics (including neonatal exposure through breast milk)” were utilized to identify candidate cases for pregnancy-related reports. Based on these reports, cases that contained pregnancy-related exposure PTs in Figure 1 or where the route of administration was transplacental were defined as definitive pregnancy-related reports. Except for definitive pregnancy-related reports, we excluded cases with patients of ineligible sex and age, along with those of paternal exposure (Figure 1). The reports obtained through these procedures were considered as pregnancy-related reports.
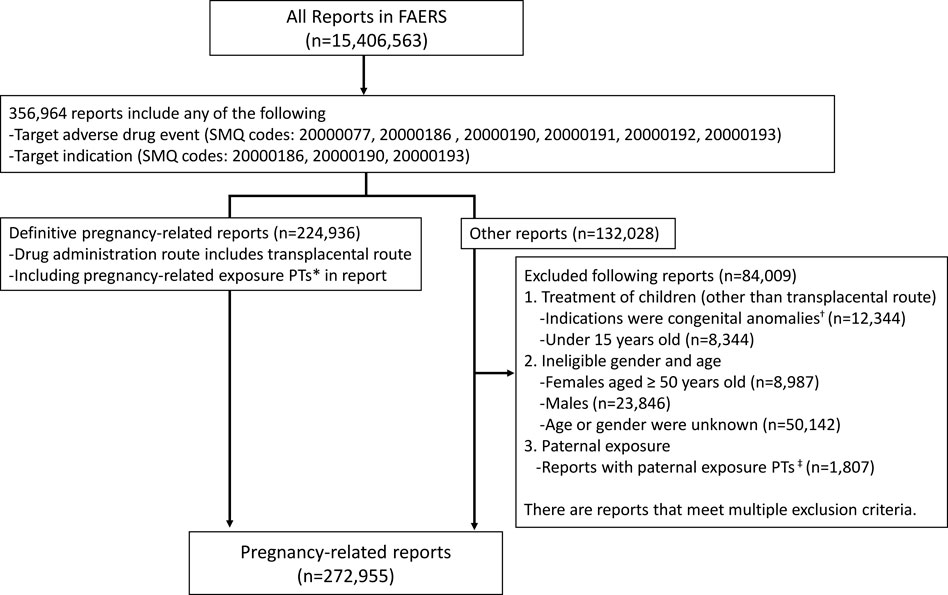
FIGURE 1. Data extraction of pregnancy-related reports from the Food and Drug Administration Adverse Event Reporting System. *PTs: maternal exposure during delivery (10071407), foetal exposure during delivery (10071409), maternal exposure before pregnancy (10071406), maternal exposure during pregnancy (10071408), fetal exposure during pregnancy (10071404), exposure during pregnancy (10073513), maternal exposure timing unspecified (10071415), foetal exposure timing unspecified (10071405), maternal drugs affecting foetus (10026923), drug exposure before pregnancy (10064998). †SMQs: congenital, familial, and genetic disorders (20000077). ‡PTs: paternal drugs affecting the fetus (10050425), exposure via father (10071403), paternal exposure during pregnancy (10080091), paternal exposure timing unspecified (10080092), paternal exposure before pregnancy (10080093), and maternal exposure via partner during pregnancy (10084938). PT, Preferred term; SMQ, standard MedDRA query.
Drugs under investigation included the VEGF inhibitors ranibizumab, aflibercept, and bevacizumab. Because aflibercept and bevacizumab can be administered both intraocularly and intravenously, we included only reports of intraocular administration. Cases with an unknown route of administration were excluded. Drugs in the FAERS database were categorized into four drug roles: “primary suspect drug,” “secondary suspect drug,” “concomitant,” and “interacting,” depending on their level of involvement in the expected adverse events. Only reports of “primary suspect drug” or “secondary suspect drug” were included in this analysis.
Regarding adverse events, we initially analyzed the number of reports of the PT included in the SMQ “termination of pregnancy and risk of abortion,” which includes adverse events that are unlikely to have been caused directly by the drug, such as induced abortion and infectious miscarriage. As a result, a definition of pregnancy loss was created that excluded these adverse events, and the remaining terms were used as target adverse events (Table 1).
We created two by two contingency tables from pregnancy-related reports and calculated reporting odds ratios (RORs) (Figure 2). The detection criteria were defined as the lower limit of the 95% confidence interval of the ROR > 1 under the condition that a minimum of three cases of the target adverse event were reported, as per the criteria of the European Medicines Agency (Wisniewski et al., 2016; Sessa et al., 2019). We also conducted two sensitivity analyses, one using the PT “abortion spontaneous” as the only target adverse event, and one using definitive pregnancy-related reports. Statistical analyses were performed using the open-source R software (version 4.2.1).
For the drugs where signals were detected, individual safety reports were reviewed to further assess the relationship. The survey items included age, drug, indication, adverse events, and reporting period. Drugs were checked to see if any have been shown to be harmful during pregnancy with the Australian Therapeutic Goods Administration classification. We also checked for duplicate reports that could not be excluded by preprocessing.
A total of 272,955 reports of pregnant women were extracted from the FAERS. Pregnancy loss was reported in 42,821 cases. Among pregnancy-related reports, 42 involved ranibizumab, 8 involved intraocular aflibercept, and 7 included intraocular bevacizumab. The reported adverse events for the SMQ “termination of pregnancy and risk of abortion” for each drug are shown in Table 2. Among these events, the PT “abortion spontaneous” was the most reported, and fewer than three “stillbirth” PTs were reported for any of the investigated drugs.
Pregnancy loss was reported in 19 cases with ranibizumab, 6 cases with intraocular bevacizumab, and 4 cases with intraocular aflibercept (Table 3). Ranibizumab met the detection criteria with an ROR of 4.44 (95% CI: 2.42–8.16), as did the sensitivity analysis results. Intraocular bevacizumab also met the detection criteria with an ROR of 32.25 (95% CI: 3.88–267.9), as did the sensitivity analysis results. Intraocular aflibercept also met the detection criteria with an ROR of 5.37 (95% CI: 1.34–21.49), but the sensitivity analysis results did not meet the criteria.
The data from individual cases of pregnancy loss with ranibizumab, intraocular bevacizumab, or intraocular aflibercept treatment are shown in Tables 4, 5, and 6, respectively. In each case, no suspected drugs were classified as X by the Australian Therapeutic Goods Administration (TGA) classification. Some cases reported concomitant use of ranibizumab and azathioprine, which holds an Australian TGA D classification.
Our findings present safety signals of pregnancy loss after administration of some VEGF inhibitors. Among pregnancy loss cases, the PT “abortion spontaneous” was the most reported adverse event. Pregnancy loss in this study refers primarily to spontaneous abortion, not stillbirth. Because PT level signal detection is also a common method for analyzing safety signals (Wisniewski et al., 2016), we conducted a disproportionality analysis using only the PT “abortion spontaneous” as a sensitivity analysis. The number of PT “stillbirth” events reported was fewer than three for all VEGF inhibitors, meaning that this PT did not meet the detection criteria. Therefore, we did not conduct a disproportionality analysis using only the PT “stillbirth” as a sensitivity analysis.
VEGF plays an essential role in both fetal and placental angiogenesis. Some reports suggest a link between VEGF expression level and recurrent miscarriages (Vuorela et al., 2000). Therefore, the use of a VEGF inhibitor treatment could theoretically cause pregnancy loss, owing to the reduction of the plasma level of free VEGF (Avery et al., 2014).
Ranibizumab is a protein encoding a Fab fragment of the VEGF-A antibody; the effect of the absence of the Fc site on placental transfer is unknown, and no information on placental transfer in humans is available (Briggs et al., 2021). The effect of ranibizumab on plasma VEGF concentrations is weaker than other VEGF inhibitors and is eliminated earlier (Zehetner et al., 2013; Avery et al., 2014). Although it appears to be safer for use during pregnancy than other VEGF inhibitors, experience with its use in pregnant women is limited. Ranibizumab pertains to the Australian TGA “D” classification, indicating an association with adverse effects. Three case reports showed no adverse outcomes observed after intraocular ranibizumab administration in the third trimester (Sarhianaki et al., 2012; Jouve et al., 2015). For exposure during early pregnancy, one report indicated that miscarriage occurred 6 d after exposure (Akkaya, 2019), whereas other reports indicated no adverse outcomes (Fossum et al., 2018). In this study, we performed disproportionality analyses on a larger number of cases than in previous studies using the spontaneous reporting database, allowing for consistent signal detection and enabling sensitivity analysis. We believe that our results support the hypothesis that ranibizumab is associated with pregnancy loss. Detailed analysis of individual cases showed that the patients’ age ranged from 26 to 46 years, and pregnancy loss was not necessarily associated with older age. Based on reports No. 14–17 in Table 4, the possibility that the same case was reported in duplicate cannot be ruled out, although the FAERS, PRIMARYID (a unique number for identifying a FAERS report), and CASEID (a number for identifying a FAERS case) identifiers were different. In addition, these suspected duplicate reports may have been influenced by the concomitant use of azathioprine, which is categorized as “D” by the Australian TGA. However, the safety signal was still detected when these four cases were excluded; therefore, the presence of these reports does not significantly affect the results of this study.
Bevacizumab is a recombinant humanized monoclonal IgG antibody of VEGF that was approved for the treatment of metastatic colorectal cancer and is now also being used as an off-label therapeutic for eye diseases (Polizzi and Mahajan, 2015). Bevacizumab has been reported to reduce plasma VEGF concentrations for at least 1 month (Zehetner et al., 2013; Avery et al., 2014). However, this drug was associated with the most case reports of exposure during pregnancy, and Naderan et al. (2021) argued that it is used more for pregnant women than other VEGF inhibitors. In this study, only a limited number of cases was obtained specifically for intraocular administration. However, six of the seven cases were associated with pregnancy loss, and the signal was consistently detected, including in sensitivity analyses. Cases of miscarriage have been reported following intraocular bevacizumab administration during early pregnancy (Petrou et al., 2010; Gómez Ledesma et al., 2012). Our results support the conclusions of these previous studies that intraocular bevacizumab administration is associated with pregnancy loss. On the contrary, a study reported using single injections during early pregnancy without adverse outcomes (Sullivan et al., 2014). However, it remains unclear whether bevacizumab causes spontaneous abortion. Counseling is recommended to disclose the off-label nature of the treatment and to explain both its efficacy and the potential risks to pregnant patients.
Aflibercept is a recombinant fusion protein comprising the extracellular domains of VEGF receptors 1 and 2 fused to the Fc domain of human IgG and binds to VEGF and placental growth factor. Because of its higher affinity, aflibercept is reported to reduce plasma VEGF concentrations the most among the three VEGF inhibitors investigated in this study (Avery et al., 2014; Polizzi and Mahajan, 2015). Studies using animal models have exhibited external, skeletal, and visceral malformations after intravenous aflibercept administration during pregnancy. It is categorized as D in the Australian TGA classification. Intraocular aflibercept (EYLEA™) is contraindicated for pregnant women in Japan, and embryo-fetotoxicity is listed as a potential risk in the European risk management plan (European Medicines Agency, 2022). In this study, a safety signal of pregnancy loss was detected for intraocular aflibercept. However, the number of cases was limited, and the sensitivity analysis did not meet the signal detection criteria. To our knowledge, no published case reports currently describe human aflibercept exposure during pregnancy; therefore, we consider this finding to be an important signal.
The current study had several limitations derived from using the spontaneous reporting database (Sakai et al., 2020; Noguchi et al., 2021). First, it is well known that signals from disproportionality analysis frequently show false positive results owing to the effects of reporting biases (Wisniewski et al., 2016). Second, the lack of denominator information in the spontaneous reporting database, make us impossible to calculate the incidence rate of pregnancy loss. The ROR results do not necessarily reflect the risk intensity. Owing to the limited number of cases, statistical analysis was unstable, showing a wide range of ROR values. Third, limited information is available on individual case safety reports (Tsuchiya et al., 2020). Age, history of miscarriage (Magnus et al., 2019), alcohol consumption, smoking, and obesity (Magnus et al., 2022) are risk factors for miscarriage. Age was obtained in some reports, but data for other factors were not available. However, the findings provided evidence of important safety signals regarding the association between VEGF inhibitors and miscarriage, even though limited case reports were available until now. Given that signal detection is a hypothesis-generating design study, future efforts to collect cases and confirm/disprove hypotheses with well-designed comparative safety studies will greatly contribute to this investigation.
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
TS and CM were involved in the conception and design of the study; TS carried out the data extraction and analyses. All authors contributed to data interpretation and review. TS and FO mainly edited the manuscript. All authors have read and approved of the final manuscript.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant No. JP 19K16457.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akkaya, S. (2019). Early miscarriage occurring six days after intravitreal ranibizumab injection. Med. Hypothesis Discov. Innov. Ophthalmol. 8, 69–72. https://pubmed.ncbi.nlm.nih.gov/31263715/
Almawi, W. Y., Saldanha, F. L., Mahmood, N. A., Al-Zaman, I., Sater, M. S., and Mustafa, F. E. (2013). Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum. Reprod. 28, 2628–2635. doi:10.1093/humrep/det308
Avery, R. L., Castellarin, A. A., Steinle, N. C., Dhoot, D. S., Pieramici, D. J., See, R., et al. (2014). Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 98, 1636–1641. doi:10.1136/bjophthalmol-2014-305252
Bautch, V. L. (2012). VEGF-directed blood vessel patterning: From cells to organism. Cold Spring Harb. Perspect. Med. 2, a006452. doi:10.1101/cshperspect.a006452
Beyer-Westendorf, J., Tittl, L., Bistervels, I., Middeldorp, S., Schaefer, C., Paulus, W., et al. (2020). Safety of direct oral anticoagulant exposure during pregnancy: A retrospective cohort study. Lancet. Haematol. 7, e884–e891. doi:10.1016/S2352-3026(20)30327-6
Briggs, G. G., Towers, C. V., and Forinash, A. B. (2021). Drugs in pregnancy and lactation: A reference guide to fetal and neonatal risk. Philadelphia: Wolters Kluwer.
Deepak, P., and Stobaugh, D. J. (2014). Maternal and foetal adverse events with tumour necrosis factor-alpha inhibitors in inflammatory bowel disease. Aliment. Pharmacol. Ther. 40, 1035–1043. doi:10.1111/apt.12936
European Medicines Agency (2022). Eylea: EPAR – product information. Available at: https://www.ema.europa.eu/documents/product-information/eylea-epar-product-information_en.pdf (Accessed September 19, 2022).
Fossum, P., Couret, C., Briend, B., Weber, M., and Lagarce, L. (2018). Safety of intravitreal injection of ranibizumab in early pregnancy: A series of three cases. Eye 32, 830–832. doi:10.1038/eye.2017.305
Gómez Ledesma, I., de Santiago Rodríguez, M. Á., Follana Neira, I., and León Garrigosa, F. (2012). Neovascular membrane and pregnancy. Treatment with bevacizumab. Arch. Soc. Esp. Oftalmol. 87, 297–300. doi:10.1016/j.oftal.2011.09.011
Huybrechts, K. F., Hernández-Díaz, S., and Bateman, B. T. (2021). Antiemetic drugs during pregnancy: What can we learn from spontaneous reporting system database analyses?-Reply. JAMA Pediatr. 175, 327–328. doi:10.1001/jamapediatrics.2020.5177
Jouve, L., Akesbi, J., and Nordmann, J. P. (2015). Safety and efficacy of ranibizumab for pregnant women in idiopathic choroidal neovascularization. Acta Ophthalmol. 93, e597–e598. doi:10.1111/aos.12611
Kant, A., de Vries, L., and Rolfes, L. (2019). Surveillance of drug safety during pregnancy: Insight in current international activities, future intentions and need for support of national pharmacovigilance centres. Drug Saf. 42, 35–43. doi:10.1007/s40264-018-0729-0
Kianersi, F., Ghanbari, H., Naderi Beni, Z., and Naderi Beni, A. (2016). Intravitreal vascular endothelial growth factor (VEGF) inhibitor injection in unrecognised early pregnancy. Invest. New Drugs 34, 650–653. doi:10.1007/s10637-016-0361-8
Lareb (2018). Pregnancy PV toolkit. Available at: https://www.lareb.nl/media/5s3jqdid/lareb-toolkit-pregnancy-okt18-02_final.pdf (Accessed September 19, 2022).
Magnus, M. C., Wilcox, A. J., Morken, N. H., Weinberg, C. R., and Håberg, S. E. (2019). Role of maternal age and pregnancy history in risk of miscarriage: Prospective register based study. BMJ 364, l869. doi:10.1136/bmj.l869
Magnus, M. C., Hockey, R. L., Håberg, S. E., and Mishra, G. D. (2022). Pre-pregnancy lifestyle characteristics and risk of miscarriage: The Australian Longitudinal Study on Women’s Health. BMC Pregnancy Childbirth 22, 169. doi:10.1186/s12884-022-04482-9
Naderan, M., Sabzevary, M., Rezaii, K., Banafshehafshan, A., and Hantoushzadeh, S. (2021). Intravitreal anti-vascular endothelial growth factor medications during pregnancy: Current perspective. Int. Ophthalmol. 41, 743–751. doi:10.1007/s10792-020-01610-2
Noguchi, Y., Tachi, T., and Teramachi, H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 22, bbab347. doi:10.1093/bib/bbab347
Petrou, P., Georgalas, I., Giavaras, G., Anastasiou, E., Ntana, Z., and Petrou, C. (2010). Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 88, e136. doi:10.1111/j.1755-3768.2009.01572.x
Polizzi, S., and Mahajan, V. B. (2015). Intravitreal anti-VEGF injections in pregnancy: Case series and review of literature. J. Ocul. Pharmacol. Ther. 31, 605–610. doi:10.1089/jop.2015.0056
Sakai, T., Ohtsu, F., Mori, C., Tanabe, K., and Goto, N. (2017). Signal of miscarriage with aripiprazole: A disproportionality analysis of the Japanese adverse drug event report database. Drug Saf. 40, 1141–1146. doi:10.1007/s40264-017-0560-z
Sakai, T., Wada, Y., Kokan, A., Tanabe, K., Goto, N., and Ohtsu, F. (2020). Creating a checklist and a survey on research that used the Japanese Adverse Drug Event Report database. Jpn. J. Drug Inf. 22, 7–16. doi:10.11256/jjdi.22.7
Sakai, T., Mori, C., Koshiba, H., Yuminaga, R., Tanabe, K., and Ohtsu, F. (2022). Pregnancy loss signal from prostaglandin eye drop use in pregnancy: A disproportionality analysis using Japanese and US spontaneous reporting databases. Drugs Real World Outcomes 9, 43–51. doi:10.1007/s40801-021-00287-y
Sandberg, L., Taavola, H., Aoki, Y., Chandler, R., and Norén, G. N. (2020). Risk factor considerations in statistical signal detection: using subgroup disproportionality to uncover risk groups for adverse drug reactions in VigiBase. Drug Saf. 43, 999–1009. doi:10.1007/s40264-020-00957-w
Sarhianaki, A., Katsimpris, A., Petropoulos, I. K., Livieratou, A., Theoulakis, P. E., and Katsimpris, J. M. (2012). Intravitreal administration of ranibizumab for idiopathic choroidal neovascularization in a pregnant woman. Klin. Monbl. Augenheilkd. 229, 451–453. doi:10.1055/s-0031-1299207
Sessa, M., Mascolo, A., Callréus, T., Capuano, A., Rossi, F., and Andersen, M. (2019). Direct-acting oral anticoagulants (DOACs) in pregnancy: New insight from VigiBase®. Sci. Rep. 9, 7236–7236. doi: doi:10.1038/s41598-019-43715-4
Sullivan, L., Kelly, S. P., Glenn, A., Williams, C. P., and McKibbin, M. (2014). Intravitreal bevacizumab injection in unrecognised early pregnancy. Eye 28, 492–494. doi:10.1038/eye.2013.311
Tsuchiya, M., Obara, T., Sakai, T., Nomura, K., Takamura, C., and Mano, N. (2020). Quality evaluation of the Japanese adverse drug event report database (JADER). Pharmacoepidemiol. Drug Saf. 29, 173–181. doi:10.1002/pds.4944
van De Ven, N. S., Pozniak, A. L., Levi, J. A., Clayden, P., Garratt, A., Redd, C., et al. (2020). Analysis of pharmacovigilance databases for dolutegravir safety in pregnancy. Clin. Infect. Dis. 70, 2599–2606. doi:10.1093/cid/ciz684
Vuorela, P., Carpén, O., Tulppala, M., and Halmesmäki, E. (2000). VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol. Hum. Reprod. 6, 276–282. doi:10.1093/molehr/6.3.276
Wisniewski, A. F., Bate, A., Bousquet, C., Brueckner, A., Candore, G., Juhlin, K., et al. (2016). Good signal detection practices: Evidence from IMI PROTECT. Drug Saf. 39, 469–490. doi:10.1007/s40264-016-0405-1
Zehetner, C., Kirchmair, R., Huber, S., Kralinger, M. T., and Kieselbach, G. F. (2013). Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br. J. Ophthalmol. 97, 454–459. doi:10.1136/bjophthalmol-2012-302451
Keywords: pregnancy loss, pharmacovigilance, signal detection, spontaneous reporting databases, VEGF inhibitors
Citation: Sakai T, Mori C and Ohtsu F (2022) Potential safety signal of pregnancy loss with vascular endothelial growth factor inhibitor intraocular injection: A disproportionality analysis using the Food and Drug Administration Adverse Event Reporting System. Front. Pharmacol. 13:1063625. doi: 10.3389/fphar.2022.1063625
Received: 07 October 2022; Accepted: 27 October 2022;
Published: 10 November 2022.
Edited by:
Yoshihiro Noguchi, Gifu Pharmaceutical University, JapanCopyright © 2022 Sakai, Mori and Ohtsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takamasa Sakai, dGtzYWthaUBtZWlqby11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.