
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 30 November 2022
Sec. Pharmacology of Infectious Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1058669
This article is part of the Research TopicStrategies to optimize the use of antimicrobials in a clinical setting to combat antimicrobial resistanceView all 5 articles
Objectives: This cross-sectional study was conducted at Jordan university hospital to evaluate the impact of microbial culture data and sensitivity results on optimizing UTI treatment.
Methods: All positive urine cultures requested for adult patients (≥18 years) admitted to Jordan University Hospital (JUH) within the period from January 2019–July 2021 were evaluated. The antibiotics prescribed before and after culture data and sensitivity results were compared to evaluate the impact of these diagnostic measures on optimizing UTI treatment.
Results: During the study period, 2400 urine cultures revealed positive results. Among those patients, 1,600 (66.7%) were discharged before the availability of culture results and excluded. Of the remaining 800 patients, 701 patients (87.6%) received empiric treatment. After culture and sensitivity results were available, overall, 84 (10.5%) patients had optimization (improvement) in their UTI management after culture results were known, while 6 (0.8%) patients had a worsening in their treatments. Based on the culture results, we found that only 12.4% of patients were appropriately treated before and after the culture results. Moreover, our results revealed that 31.9% were inappropriately treated for their UTIs before and after culture results.
Conclusion: This study revealed an alarmingly high rate of inappropriate treatment of UTIs despite the availability of urine culture and sensitivity data, and that culture results were not used to optimize treatment strategies for UTI. This practice can potentially result in poor health-related outcomes and adversely affects efforts to battle AMR. Multifaceted strategies must be implemented to help clinicians follow the best current evidence and current guidelines in their selection of antibiotics for the management of UTIs.
Optimum therapeutic antimicrobial management should target a specific pathogen with a precise dose and treatment duration in order to effectively combat causative microbes, reduce the risk of complications, minimize adverse drug reactions, and lower the risk of antimicrobial resistance (AMR) (Niederman, 2003). It has been reported that in order to efficiently optimize antimicrobial use and reduce the risk of antimicrobial resistance, healthcare providers should follow a multiple-step approach to reduce the risk of AMR (Graber et al., 2015). This approach includes different points of assessment for the appropriateness, dose, duration, and route of administration of antimicrobials. This assessment is especially important after microbial culture results become available, commonly after the initiation of empiric treatment, to evaluate the sensitivity of the identified pathogen (Kinn et al., 2018). Following these assessments has been concluded as a strategy to help better manage infections, reduce adverse drug reactions, and unnecessary exposure to antimicrobial agents, which can potentially help control AMR (Wolfe et al., 2019).
The optimal use of antimicrobial agents in hospitalized patients includes a correct selection of empiric antimicrobials agents as well as the targeted agents (Carson and Naber, 2004). Inappropriate prescribing not only results in negative health-related outcomes but also increases the risk of AMR. According to the Centers for Disease Control and Prevention (CDC), more than 2.6 million people get infected with antimicrobial-resistant microorganisms each year in the United States, resulting in about 44,000 deaths as a minimum (Dadgostar, 2019).
Irrational and excessive use of antibiotics has been documented by different studies in Jordan (Shehadeh et al., 2012; Yusef et al., 2018; Alsayed et al., 2022). Self-medication of antibiotics without a doctor’s prescription is one example of irrational usage, which can contribute to the development of bacterial resistance toward the antimicrobial agents (Al-Azzam et al., 2007). According to previous studies, the prevalence of irrational use of antibiotics in Jordan was approximately 40.7%, which is considered significant (Al-Azzam et al., 2007; Sawair et al., 2009; Yusef et al., 2018). The effectiveness of antimicrobial agents decreases with time depending on the frequency of use, this feature makes them different than other drug classes (Llor and Bjerrum, 2014; Reygaert, 2018). Thus, all healthcare professionals must use the currently available antimicrobials rationally and prescribe them properly in order to avoid going back to the era before the discovery of antimicrobials (Alanis, 2005).
Several investigations have been conducted to assess the pattern of antimicrobial resistance in Jordan (Abdullah and Shara, 2011; Shakhatreh et al., 2019). A study conducted by Abdullah et al. showed that 90.9% of Klebsiella pneumonia isolated from different clinical specimens were resistant to imipenem (Abdullah and Shara, 2011). Furthermore, another study found that 81.9% of E. coli isolates from urine cultures exhibited resistance to at least three different types of antimicrobials (Shakhatreh et al., 2019). However, the need to implement Antimicrobial stewardship (AMS) programs in Jordan has arisen because millions of people are infected annually with antimicrobial-resistant organisms and tens of thousands of them die. AMS programs are among the most effective strategies to overcome bacterial resistance via taking various actions aimed to directly influence antibiotic use and reduce unnecessary antibacterial prescriptions (Dyar et al., 2017). Therefore, AMS is an important approach that must be applied in all hospitals, regardless of their size (Stenehjem et al., 2017).
Urinary tract infections (UTIs) are common infectious diseases at the community level that attack any region of the urinary system (Amawi et al., 2021). The prevalence of UTIs in females is 30 times higher than in males under the age of 50 (Amawi et al., 2021). Complicated UTIs (those infections in immunocompromised patients, males, and those associated with anatomical abnormalities) are often hard to treat and caused mainly by a diverse species of gram-negative and gram-positive bacteria, increasing antimicrobial resistance, and a higher prevalence of recurrent infections. The most common causative microbes are gram-negative bacteria, including Escherichia coli (E. coli), followed by Klebsiella and Proteus species (Foxman, 2010). Patients with complicated UTIs most likely require empiric broad-spectrum intravenous antimicrobial therapy (O’Grady et al., 2019). Early detection and confirmation of the causative organisms by culture and sensitivity testing is of critical importance for the management of UTIs. Urine culture remains the gold standard for UTIs investigation, and antimicrobial therapy should be tailored based on the results of the urine culture (Tan and MPJSmj, 2016). This is of special significance in order to improve therapeutic outcomes in treated patients, minimize side effects, and help combat AMR. Thus, the aim of this study is to evaluate the impact of microbial culture data and sensitivity results on optimizing UTI management in a tertiary teaching hospital in Jordan.
This is a retrospective cross-sectional study that was conducted at Jordan University Hospital (JUH), Amman-Jordan, all urine cultures requested for patients (≥18 years) admitted to JUH between January 2019–July 2021 were reviewed, and only patients with positive culture were considered.
Following patients’ identification, information regarding urine culture and sensitivity testing were obtained from JUH laboratory electronic system. Data on the prescribed empiric antimicrobials were collected from patients’ medical records among other clinical and demographic data. Any change in the selection of antimicrobials following the urine culture and sensitivity results were also documented.
Urinary tract infection was considered to be appropriately treated empirically with antimicrobial if the identified microorganism, as per the microbial culture results, was within the spectrum covered by that empiric antimicrobial, and if the organism was reported as susceptible to that antimicrobial agent (Harvey et al., 2012). UTIs were flagged as inappropriately treated if any of the following was documented; having no antimicrobial therapy prescribed “untreated”, being treated with antimicrobial that does not cover the identified microorganism “lack of coverage”, and being treated with antimicrobial that was reported as “resistant”. In some cases, it was not possible to judge the appropriateness of treatment due to the lack of sensitivity testing. In a similar way, the appropriateness of treatment following culture results was evaluated.
After that, antimicrobials prescribed before and after culture and sensitivity testing were compared to evaluate the appropriateness of the management of UTIs. The treatment of UTIs was either 1) improved (treatment was inappropriate before culture and became appropriate after culture results), 2) worsened (treatment was appropriate before culture and became inappropriate after culture results), 3) not changed since the patients were treated appropriately before and after culture and sensitivity testing, or 4) not changed since the patients were treated inappropriately before and after culture and sensitivity testing.
The World Medical Association Declaration of Helsinki guidance was followed in the study (World Medical, 2013). The study was initiated after obtaining approval from the Institutional Review Board (IRB) committee at JUH which is the teaching hospital affiliated with the University of Jordan (Reference No. 196/2021). Patient informed consent was waived by the ethics committee due to the retrospective nature of the study. All the collected information was kept on the personal computer of the principal investigator using password-protected files.
All the collected data were coded, entered, and analyzed using the Statistical Package for Social Sciences (SPSS) version 22. The descriptive analysis was conducted usingmedian/interquartile range (IQR) f continuous variables, while frequency and percentages were used for categorical variables. Checking for normality was carried out using the Shapiro-Wilk test (with p ≤ 0.05 indicating that our continuous variables were not normally distributed). McNemar’s test was carried out to evaluate differences in antimicrobials appropriateness rate before and after obtaining culture results. A p ≤ 0.05 was considered statistically significant. All tests were two-tailed.
During the study period, urine cultures were ordered for 6,950 patients, 4,550 (65.5%) patients tested negative and were excluded from the study and only those with positive culture were included in the study (n = 2,400, 34.5%). Among those patients with positive culture episodes, 1,600 patients (66.7%) were discharged too early before the availability of culture results and were also excluded which left us with a total sample of 800 patients.
The median age of participants (n = 800) was 64 years (IQR = 29), with 71.0% of the participants (n = 568) being above 50 years old, and more than two-thirds of them were females (n = 555, 69.4%). Moreover, more than half of the patients (n = 437, 54.6%) were receiving polypharmacy (defined as ≥4 medications), and they had a median length of hospital stay of 12 days (IQR = 11). For more details about the demographic and medical characteristics of the study sample, refer to Table 1.
Antimicrobials were primarily prescribed empirically before culture results for 701 patients (87.6%), while 12% of the patients (n = 99) did not receive any empiric antimicrobial. The median number of the prescribed empiric antimicrobials for all of the recruited patients was 1.0 (IQR = 0.0), with a total of 873 prescribed empiric antimicrobials. The most frequently prescribed empiric antimicrobials were ceftriaxone (n = 214, 24.5%), imipenem/cilastatin (n = 209, 23.9%), and levofloxacin (n = 128, 14.7%).
Following culture and sensitivity testing, 367 (45.9%) patients had their antimicrobials continued, 270 (33.8%) had them changed, 114 (14.4%) had additional agents to have targeted antimicrobial coverage, 48 (6.0%) patients had agents discontinued (Figure 1). Following the availability of culture results, patients received a total of 972 antimicrobials. These agents were prescribed for 743 patients (92.9%), while 57 patients (7.1%) received no antimicrobial and were flagged as “untreated”. The most frequently prescribed antimicrobial following culture results were imipenem/cilastatin (n = 276, 28.4%), levofloxacin (n = 115, 11.8%), and ceftriaxone (n = 105, 10.8%).
Most urine culture specimens revealed one microorganism (n = 559, 69.9%), with few specimens showed two or more pathogens (241, 30.1%). The most frequently reported pathogens were E. coli (n = 313, 29.5%), Enterococcus (n = 189, 17.8%), and Staphylococcus (n = 158, 14.9%). Less than half of the patients had their sensitivity testing reports performed (n = 391, 48.9%). There were 136 instances of resistance to antimicrobials out of the 873 prescribed empiric antimicrobials (15.6%). These antimicrobials reported as “resistant” were prescribed for 117 patients out of the 800 eligible patients (14.6%). Ceftriaxone (n = 48, 35.3%), levofloxacin (n = 27, 19.9%), and imipenem/cilastatin (n = 21, 15.4%) were the main empiric antimicrobials that with reported resistence.
The difference between the appropriateness of antimicrobials before and after culture and sensitivity testing is presented in Figure 2. The number of untreated patients was reduced from 99 (12.4%) to 57 (7.1%) following culture and sensitivity testing. Moreover, the “lack of coverage” was reduced from 214 (26.8%) to 161 (20.1%). Also, the incorrect treatment (the identified pathogens were reported as resistant to the prescribed antimicrobial) was reduced from 117 (14.6%) to 64 (8.0%). Finally, the number of patients with correct treatment was increased from 107 (13.4%) to 203 (25.4%), p < 0.001.
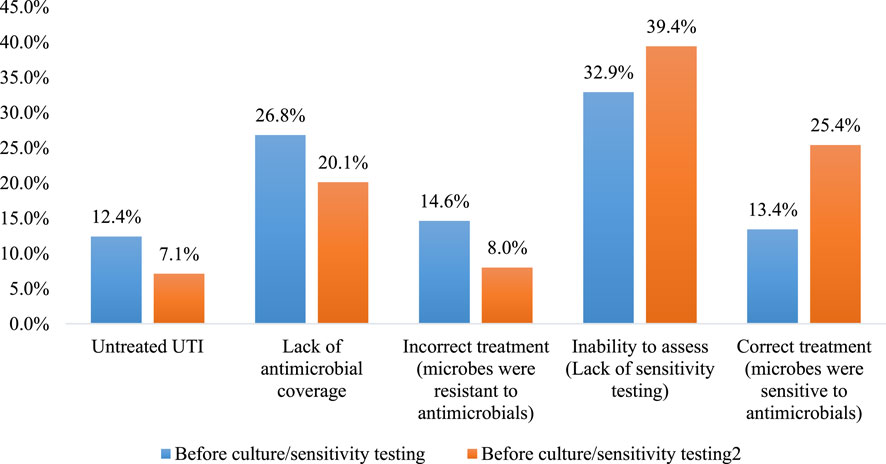
FIGURE 2. The difference between the appropriateness of antimicrobials before and after culture and sensitivity testing (n = 800). p < 0.001 using McNemar test.
The impact of urine culture and sensitivity testing on optimizing antimicrobials prescribing was evaluated and the results are presented in Figure 3. Results showed that the treatment of 10.5% of the patients (n = 84) was improved, while only 0.8% of the patients (n = 6) had less appropriate treatment (worsening). The remaining patients have no change in the appropriateness of their treatments, where 99 patients (12.4%) have received correct antimicrobials before and after culture and sensitivity testing, while 255 patients (31.9%) have received inappropriate antimicrobials before and after culture and sensitivity testing. The impact of antimicrobial changes could not be assessed for 356 patients (44.5%) since sensitivity testing was not performed to patients. Several examples of the impact of urine culture and sensitivity testing on optimizing antimicrobials prescribing were presented in Table 2.
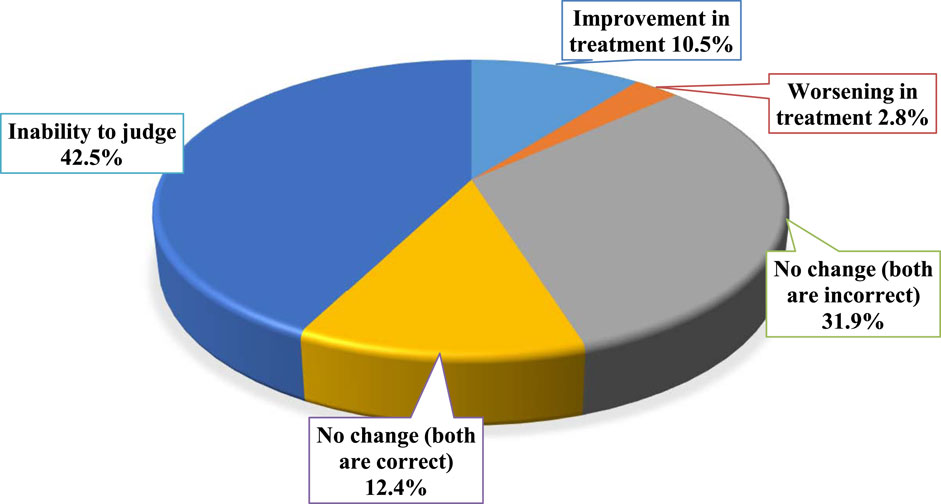
FIGURE 3. The impact of urine culture and sensitivity testing (if available) on optimizing antimicrobials prescribing (n = 800).

TABLE 2. Examples of the effect of antimicrobial adjustment following culture and sensitivity results.
This study assessed the impact of urine culture and sensitivity testing in optimizing UTI treatment for 800 hospitalized patients in Jordan. According to our observations, UTIs management was improved in 10.5% of patients when results of culture and sensitivity testing were available. Several studies in the literature have supported the importance of microbial culturing and sensitivity tests in guiding the appropriate antimicrobial therapy (Reller et al., 2009; Bayot and Bragg, 2019; Benkova et al., 2020). Sensitivity tests are of special importance for the appropriate management of infection, they help guide physicians in determining which antimicrobials are most likely to be effective in combating microbial growth (Khan et al., 2019).
The improvement in UTI management reported in this study included treating those who were untreated prior to the availability of culture results. The number of patients with untreated UTIs decreased from 12.4% to 7.1% with the availability of culture results. These results indicate a delay in antimicrobial therapy which can potentially increase morbidity and the risk of complications of UTIs. Despite the need to control antimicrobial resistance and refrain from prescribing antimicrobial agents unless necessary, delay in antimicrobial therapy has been reported to negatively impact patients health-related outcomes and adds to the economic burden of the management of UTIs (Lodise et al., 2019). Rapid diagnostic testing has been recommended to guarantee appropriate and timely use of antimicrobial agents, this can help improve patient outcome and reduce the risk of antimicrobial resistance (Reuter et al., 2019).
Unfortunately, about one-third of patients (31.9%) still inappropriately treated even after culture results are available. Moreover, six (0.8%) patients had a worsening in their treatments after the results of the culture were available. Our results are consisted with the conclusion of a recent review where a change to targeted antimicrobial therapy occurred in 50% of patients in 22 hospitals in the Netherlands, however, only 32% of the changes were correct (Hulscher et al., 2017). Antibiotic stewardship to support appropriate antibiotic prescribing patterns is essential to provide efficient and cost effective treatment minimizing the risk of complications and antimicrobial resistance (Hulscher et al., 2017).
Furthermore, we were unable to assess the impact of urine culture and sensitivity testing on optimizing UTI treatments in 44.5% of the patients due to the lack of sensitivity reports which indicate that microbiological sensitivity testing is not being carried out as recommended for all patients. Previous studies stated that the resistance pattern of uropathogens in UTI patients changes over time, which mandates special attention and monitoring to decrease the risk of therapeutic failure and microbial resistance (Linhares et al., 2000; Akram et al., 2007). Recent studies have shown the value of culture and sensitivity testing in decreasing inappropriate antibacterial use (Tabak et al., 2018; Maugeri et al., 2019). The value of performing microbiological sensitivity testing over culture alone was assessed in Denmark by Holm et al. (2015). Because of the rising prevalence of resistance, they predicted that treating enterococci based on culture would result in 20%–30% inappropriate antibiotic therapy. In contrast, sensitivity testing should increase proper antibiotic prescriptions by more than 90%. Another study conducted in Nicaragua (Latin America) concluded that the choice of antimicrobial therapy should be based on the results of sensitivity testing to decrease the possibility of resistance and the emergence of ESBL producing species (Bours et al., 2010). These results support what was previously proposed regarding the importance of sensitivity testing in tracking antibiotic resistance levels, increasing appropriate treatment, and improving patient outcomes.
In this study, we identified a significant number of patients who were excluded from the study (66.7%) with pending lab tests, where results of culture were obtained after discharge. Unfortunately, lab results showed positive urine culture in those patients. A similar finding was observed in a study conducted by Roy et al. (2005), which has identified a considerable proportion of patients (41%) that were discharged too early before the availability of culture results, and 12.6% of them required immediate action to initiate or modify the antibiotic therapy, which could result in negative consequences in patient outcomes. They observed that this was especially relevant at tertiary care academic hospitals since primary care physicians and inpatient physicians were frequently unaware that a test had been requested (Roy et al., 2005). Another study conducted by Walz et al. (2005) reported a high number (32%) of pending lab tests at a single academic medical facility in the Unites States. These findings emphasize the importance of developing reliable interventions to improve the communication of pending lab tests at discharge between hospital laboratory facilities and inpatient and outpatient providers, and to ensure follow-up (Roy et al., 2005; Walz et al., 2011).
This study has several limitations. In this study, the lack of data on patient’s medical history can influence the physician’s choice of antibiotic, dosage, and duration of therapy. In addition, patients’ data were obtained from a single Jordanian tertiary center’s (JUH) database, which limits the generalizability of the conclusion. Multicenter studies covering different regions of Jordan should be conducted to confirm these findings. Also, the evaluation of the appropriateness of the UTI empiric treatment was judged based on the empirical treatment and the diagnosis only, without knowing the history of the patient or what drove the physician to prescribe a particular drug. Moreover, without knowing a patient medical history, it was not possible to evaluate the quality of the prescription according to the clinical practice guidelines. However, this is the first study to evaluate the impact of urine culture and sensitivity testing on optimizing UTI treatment in Jordan and taking into consideration the observational design of the study, it is considered satisfactory to provide background data at this stage.
This study revealed an alarmingly high rate of inappropriate treatment of UTI even after the availability of urine culture and sensitivity data, and that culture results were not used to optimize treatment strategies for UTI. This may increase the risk of therapeutic failure and microbial resistance. We also report a high rate of lack of sensitivity testing which is equally important and can potentially lead to increased risk of complications and antimicrobial resistance. Multifaceted strategies including antibiotic stewardship programs must be implemented to enhance clinicians’ appropriate management of UTIs starting from ordering the right diagnostic tests ending with the appropriate selection of antibiotics for treatment of UTI which should be guided by culture and sensitivity tests results.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The study was approved by the Institutional Review Board (IRB) committee at JUH (Reference No. 196/2021).
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdullah, M., and Shara, A. (2011). Emerging antimicrobial resistance of Klebsiella pneumoniae strains isolated from pediatric patients in Jordan. New Iraqi J. Med. 7 (2), 81–87.
Akram, M., Shahid, M., and Khan, A. (2007)., 6. India, 1–7.Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in JNMC Hospital Aligarh1
Al-Azzam, S. I., Al-Husein, B. A., Alzoubi, F., Masadeh, M. M., Al-Horani Sjijoom, , and health, e. (2007). Self-medication with antibiotics in Jordanian population. Int. J. Occup. Med. Environ. Health 20 (4), 373–380. doi:10.2478/v10001-007-0038-9
Alanis, A. J. (2005). Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 36 (6), 697–705. doi:10.1016/j.arcmed.2005.06.009
Alsayed, A. R., El Hajji, F. D., Al-Najjar, M. A., Abazid, H., and Al-Dulaimi, A. J. S. P. J. (2022). Patterns of antibiotic use, knowledge, and perceptions among different population categories: A comprehensive study based in Arabic countries. Saudi Pharm. J. 30 (3), 317–328. doi:10.1016/j.jsps.2022.01.013
Amawi, H. A., U’wais, H. T., Nusair, M. B., Al-okour, R., Amawi, S., Al-Shatnawi, S., et al. (2021). Management of urinary tract infections and antibiotic susceptibility patterns of bacterial isolates. Int. J. Clin. Pract. 75 (10), e14475. doi:10.1111/ijcp.14475
Bayot, M. L., and Bragg, B. N. (2019). Antimicrobial susceptibility testing, Clin. Infect. Dis., 49(11), 1749–1755.
Benkova, M., Soukup, O., and Joam, M. (2020). Antimicrobial susceptibility testing: Currently used methods and devices and the near future in clinical practice. J. Appl. Microbiol. 129 (4), 806–822. doi:10.1111/jam.14704
Bours, P., Polak, R., Hoepelman, A., Delgado, E., Jarquin, A., and Matute, A. (2010). Increasing resistance in community-acquired urinary tract infections in Latin America, five years after the implementation of national therapeutic guidelines. Int. J. Infect. Dis. 14 (9), e770–e774. doi:10.1016/j.ijid.2010.02.2264
Carson, C., and Naber, K. G. J. D. (2004). Role of fluoroquinolones in the treatment of serious bacterial urinary tract infections. Drugs 64 (12), 1359–1373. doi:10.2165/00003495-200464120-00007
Dadgostar, P. (2019). Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 12, 3903–3910. doi:10.2147/IDR.S234610
Dyar, O., Huttner, B., Schouten, J., and Pulcini, C. (2017). What is antimicrobial stewardship? Clin. Microbiol. Infect. 23 (11), 793–798. doi:10.1016/j.cmi.2017.08.026
T. P. Lodise, A. Berger, A. Altincatal, R. Wang, T. Bhagnani, P. Gillardet al. (2019). Antimicrobial resistance or delayed appropriate therapy—does one influence outcomes more than the other among patients with serious infections due to carbapenem-resistant versus carbapenem-susceptible enterobacteriaceae? Open forum infectious diseases (Oxford University Press US).
Foxman, B. J. N. R. U. (2010). The epidemiology of urinary tract infection. Nat. Rev. Urol. 7 (12), 653–660. doi:10.1038/nrurol.2010.190
Graber, C. J., Jones, M. M., Glassman, P. A., Weir, C., Butler, J., Nechodom, K., et al. (2015). Taking an antibiotic time-out: Utilization and usability of a self-stewardship time-out program for renewal of vancomycin and piperacillin-tazobactam. Hosp. Pharm. 50 (11), 1011–1024. doi:10.1310/hpj5011-1011
Harvey, R. A., Clark, M., Finkel, R., Rey, J., and Whalen, K. (2012). Lippincott’s illustrated reviews. Philadelphia: Pharmacology.
Holm, A., Cordoba, G., Sørensen, T. M., Jessen, L. R., Siersma, V., and Ljbfp, B. (2015). Point of care susceptibility testing in primary care-does it lead to a more appropriate prescription of antibiotics in patients with uncomplicated urinary tract infections? BMC Fam. Pract. 16 (1), 106–108. doi:10.1186/s12875-015-0322-x
Hulscher, M., Prins, J. J. C. M., and Infection, (2017). Antibiotic stewardship: Does it work in hospital practice? Clin. Microbiol. Infect. 23 (11), 799–805. doi:10.1016/j.cmi.2017.07.017
Khan, Z. A., Siddiqui, M. F., and Park, S. J. D. (2019). Current and emerging methods of antibiotic susceptibility testing. Diagn. (Basel). 9 (2), 49. doi:10.3390/diagnostics9020049
Kinn, P., Postelnick, M., Schroeder, S., and Schulz, L. T. (2018). A timeout on the antimicrobial timeout: Where does it stand and what is its future? Curr. Treat. Options Infect. Dis. 10 (2), 281–290. doi:10.1007/s40506-018-0146-z
Linhares, I., Raposo, T., Rodrigues, A., and Almeida, A. (2000). Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: A ten-year surveillance study. BMC Infect. Dis. 13 (1), 19–14. doi:10.1186/1471-2334-13-19
Llor, C., and Bjerrum, L. (2014). Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 5 (6), 229–241. doi:10.1177/2042098614554919
Maugeri, G., Lychko, I., Sobral, R., and Roque, A. (2019). Identification and antibiotic-susceptibility profiling of infectious bacterial agents: A review of current and future trends. Biotechnol. J. 14 (1), 1700750. doi:10.1002/biot.201700750
Niederman, M. S. (2003). Appropriate use of antimicrobial agents: Challenges and strategies for improvement. Crit. Care Med. 31 (2), 608–616. doi:10.1097/01.CCM.0000050464.70382.D6
O’Grady, M. C., Barry, L., Corcoran, G. D., Hooton, C., Sleator, R., and BjjoAC, L. (2019). Empirical treatment of urinary tract infections: How rational are our guidelines? J. Antimicrob. Chemother. 74 (1), 214–217. doi:10.1093/jac/dky405
Reller, L. B., Weinstein, M., Jorgensen, J. H., and Mjjcid, F. (2009). Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 49 (11), 1749–1755. doi:10.1086/647952
Reuter, C. H., Palac, H. L., Kociolek, L. K., Zheng, X. T., Chao, Y. Y., Patel, R. M., et al. (2019). Ideal and actual impact of rapid diagnostic testing and antibiotic stewardship on antibiotic prescribing and clinical outcomes in children with positive blood cultures. Pediatr. Infect. Dis. J. 38 (2), 131–137. doi:10.1097/INF.0000000000002102
Reygaert, W. C. (2018). An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 4 (3), 482–501. doi:10.3934/microbiol.2018.3.482
Roy, C. L., Poon, E. G., Karson, A. S., Ladak-Merchant, Z., Johnson, R. E., Maviglia, S. M., et al. (2005). Patient safety concerns arising from test results that return after hospital discharge. Ann. Intern. Med. 143 (2), 121–128. doi:10.7326/0003-4819-143-2-200507190-00011
Sawair, F., Baqain, Z., Karaky, A., and Rajmp, E. (2009). Assessment of self-medication of antibiotics in a Jordanian population. Med. Princ. Pract. 18 (1), 21–25. doi:10.1159/000163041
Shakhatreh, M. A. K., Swedan, S. F., Ma'en, A., and Khabour OfjjoKSU-, S. (2019). Uropathogenic Escherichia coli (UPEC) in Jordan: Prevalence of urovirulence genes and antibiotic resistance. J. King Saud Univ. - Sci. 31 (4), 648–652. doi:10.1016/j.jksus.2018.03.009
Shehadeh, M., Suaifan, G., Darwish, R. M., Wazaify, M., Zaru, L., and Alja’fari Sjspj, (2012). Knowledge, attitudes and behavior regarding antibiotics use and misuse among adults in the community of Jordan. A pilot study. Saudi Pharm. J. 20 (2), 125–133. doi:10.1016/j.jsps.2011.11.005
Stenehjem, E., Hyun, D. Y., Septimus, E., Yu, K. C., Meyer, M., Raj, D., et al. (2017). Antibiotic stewardship in small hospitals: Barriers and potential solutions. Clin. Infect. Dis. 65 (4), 691–696. doi:10.1093/cid/cix407
Tabak, Y. P., Vankeepuram, L., Ye, G., Jeffers, K., Gupta, V., and Murray, P. (2018). Blood culture turnaround time in US acute care hospitals and implications for laboratory process optimization. J. Clin. Microbiol. 56 (12), e00500–e00518. doi:10.1128/JCM.00500-18
Tebano, G., Mouelhi, Y., Zanichelli, V., Charmillon, A., Fougnot, S., Lozniewski, A., et al. (2020). Selective reporting of antibiotic susceptibility testing results: A promising antibiotic stewardship tool. Expert Rev. anti. Infect. Ther. 18 (3), 251–262. doi:10.1080/14787210.2020.1715795
Walz, S. E., Smith, M., Cox, E., Sattin, J., and Kind, A. (2011). Pending laboratory tests and the hospital discharge summary in patients discharged to sub-acute care. J. Gen. Intern. Med. 26 (4), 393–398. doi:10.1007/s11606-010-1583-7
Wolfe, J. R., Bryant, A. M., and Khoury, J. A. (2019). Impact of an automated antibiotic time-out alert on the de-escalation of broad-spectrum antibiotics at a large community teaching hospital. Infect. Control Hosp. Epidemiol. 40 (11), 1287–1289. doi:10.1017/ice.2019.197
World Medical, A. (2013). World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 310 (20), 2191–2194.
Yusef, D., Babaa, A. I., Bashaireh, A. Z., Al-Bawayeh, H. H., Al-Rijjal, K., Nedal, M., et al. (2018). Knowledge, practices & attitude toward antibiotics use and bacterial resistance in Jordan: A cross-sectional study. Infect. Dis. Health 23 (1), 33–40. doi:10.1016/j.idh.2017.11.001
Yusef, D., Hayajneh, W. A., Bani Issa, A., Haddad, R., Al-Azzam, S., Lattyak, E. A., et al. (2021). Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J. Antimicrob. Chemother. 76 (2), 516–523. doi:10.1093/jac/dkaa464
Keywords: urinary tract infection, urine culture, antimicrobial, Jordan, sensitivity tests
Citation: Alkhawaldeh R, Abu Farha R, Abu Hammour K and Alefishat E (2022) Optimizing antimicrobial therapy in urinary tract infections: A focus on urine culture and sensitivity testing. Front. Pharmacol. 13:1058669. doi: 10.3389/fphar.2022.1058669
Received: 30 September 2022; Accepted: 15 November 2022;
Published: 30 November 2022.
Edited by:
Israel Abebrese Sefah, University of Health and Allied Sciences, GhanaReviewed by:
Mainul Haque, National Defence University of Malaysia, MalaysiaCopyright © 2022 Alkhawaldeh, Abu Farha, Abu Hammour and Alefishat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eman Alefishat, ZW1hbi5hbGVmaXNoYXRAa3UuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.