- 1College of Pharmacy, Pusan National University, Busan, South Korea
- 2Department of Pharmacy, Pusan National University Hospital, Busan, South Korea
- 3College of Pharmacy, Kyung Hee University, Seoul, South Korea
- 4Department of Regulatory Science, Graduate School, Kyung Hee University, Seoul, South Korea
- 5Institute of Regulatory Innovation Through Science, Kyung Hee University, Seoul, South Korea
Background: Adverse drug events (ADEs) in the elderly frequently occur because of their multiple chronic diseases and complexity of drug therapy. To better understand adverse drug events, the prevalence and characteristics of adverse drug events in elderly South Korean patients were assessed.
Methods: The National Health Insurance databases for 2015 and 2016 were used for the analysis. We included patients aged ≥65 years that had at least one claim with the diagnosis codes ‘drug-induced,’ ‘poisoning by drug,’ and ‘vaccine-associated’ each year for the base-case analysis. To minimize the underestimation of adverse drug event prevalence, we also used an extended definition analysis by adding the ‘adverse drug event very likely’ codes. We estimated the prevalence of adverse drug events by sex, age group, and type of insurance and examined the frequent types of adverse drug events in 2015 and 2016.
Results: In the base-case analysis, adverse drug event prevalence in individuals aged 65 years and older was 2.75% in 2015 and 2.77% in 2016. With advanced age, the prevalence of adverse drug event tended to increase, peaking in the age group of 75–79 years. In addition, the adverse drug event prevalence was higher in females and Medical Aid enrollees. The most frequently occurring adverse drug event was ‘allergy, unspecified,’ followed by ‘other drug-induced secondary parkinsonism,’ and ‘generalized skin eruption due to drugs and medicaments.’ When we examined the extended definition analysis, the prevalence of adverse drug events was 4.47% in 2015 and 4.52% in 2016, which significantly increased from those estimated in the base-case analysis.
Conclusion: Among the older adults, the prevalence of adverse drug event was higher in advanced age, females, and Medical Aid enrollees. In particular, allergy and drug-induced secondary parkinsonism frequently occurred. This study provides evidence that health policies addressing the prevention and management of adverse drug events should be a priority for the most vulnerable elderly patients.
Introduction
Adverse drug events (ADE) are untoward complications that may occur during drug therapy (Nebeker et al., 2004). Bates et al. defined an ADE as any injury resulting from drug-related medical interventions, including medication errors (Bates et al., 1997). ADE is a broad spectrum of definitions compared with an adverse drug reaction (ADR), which is harmful and unintended consequences, occurring at appropriate use of drugs (Nebeker et al., 2004). Because nearly half of ADEs come from medication errors and can be prevented, only considering the effect of medications normally used underestimates the problem (Bates et al., 1995; Lghoul-Oulad Saïd et al., 2020).
ADEs are an essential public health issue that contribute to morbidity and a considerable economic burden on healthcare resources (Bates et al., 1995; Bates et al., 1997; Classen et al., 1997). According to a review of forty-seven European studies, hospital admissions due to ADRs, a subset of ADEs, were 3.6%, and the occurrence of ADRs during the hospital stay was 10.1% (Bouvy et al., 2015). The costs associated with ADEs in two tertiary care hospitals were estimated at $5.6 million annually, even in the late 1990s (Bates et al., 1997).
In particular, elderly patients are at high risk of ADEs because they have altered drug metabolism, have more chronic diseases, and take several medications (Field et al., 2004; Pedrós et al., 2014). For example, in South Korea, 86.4% of those aged 65 years or above had polypharmacy, defined as the concurrent use of six or more medications per person (Kim H. et al., 2014). Furthermore, a large meta-analysis reported that hospital admission related to ADR in the elderly was four times higher than in younger adults (Beijer and de Blaey, 2002). Therefore, efforts to improve patient safety in the elderly by reducing ADEs are a public health priority (Bates et al., 2009).
Despite the widespread recognition that ADEs are common in elderly patients and extensive epidemiological studies being conducted in Western countries (Field et al., 2004; Passarelli et al., 2005; Alhawassi et al., 2014; Friedman et al., 2015), the prevalence of ADEs and their characteristics have not been well described in the Asian population, including those in South Korea (Leendertse et al., 2010). Moreover, although a few studies have estimated the prevalence of ADEs in South Korea using medical chart reviews and spontaneous reporting (Koo, 2009; Shin et al., 2009; Yu et al., 2015), these studies lack generalizability because the study populations were limited. Several studies have suggested that claims data provide a complementary and alternative method for detecting ADEs with other monitoring systems, such as chart reviews, voluntary reporting, and computerized surveillance (Hougland et al., 2006; Miguel et al., 2013; Kuklik et al., 2017; Digmann et al., 2019). South Korea has a single National Health Insurance program; all populations are covered under this program, approximately 50 million people. The Health Insurance Review and Assessment (HIRA) database contains not only individual insurance information but various health information, including diseases, symptoms, and prescribed medication (Kim et al., 2017). It provides healthcare coverage to all outpatient and inpatient services. Therefore, we conducted this population-based study using a National Health Insurance database to assess the prevalence of ADEs in elderly patients and identify the types of ADEs that occurred in South Korea. We compared the annual prevalence of ADEs and examined patterns of prevalence by sex, age group (65–69, 70–74, 75–79, and ≥80 years), and type of insurance.
Materials and methods
Data source
We conducted a descriptive, retrospective study using Health Insurance Review and Assessment Service-National Patient Sample (HIRA-NPS) claims data from 2015 to 2016. The HIRA-NPS claims data are available from the Health Insurance Review and Assessment Service through a formal request for research purposes (Kim et al., 2017). The HIRA-NPS data are designed to approximate a 3% stratified sample (approximately 1,400,000 persons) of the entire population enrolled in the National Health Insurance (NHI) or Medical Aid (MA) program each year (Kim H et al., 2014). The Patient Sample data was generated systematically by probabilistic sample extraction method using stratified sampling with a total of 32 strata based on sex (2 strata) and age (16 strata) (Kim H et al., 2014). South Korea has a government-run mandatory national health security program consisting of NHI and MA program enrollees. The NHI program is a wage-based, contributory insurance program covering approximately 96% of the population, while the MA program is a government-subsidized public assistance program for low-income and medically indigent individuals (Song, 2009). The patient sample database confirmed the representativeness of the entire South Korean population through a validity test (Kim et al., 2013).
The HIRA-NPS data are cross-sectional, and different patients were selected for the sample data each year for their privacy; therefore, it is not possible to follow an individual over the years (Kim et al., 2017). The data contain each patient’s unique encrypted identification number, age, sex, type of insurance, diagnosis, and prescription drugs, which provide valuable resources for healthcare service research (Kim L et al., 2014b). Diagnoses were encoded in accordance with the International Classification of Diseases, 10th Revision (ICD-10).
Study participants and definition of adverse drug events
To be included in this study, participants with ADEs needed to be aged ≥65 years and have at least one NHI or MA claim record of outpatient, inpatient, or emergency department services with an ADE diagnosis code from the HIRA-NPS in 2015–2016. According to the prevalence-based approach, patients had both new and pre-existing cases of ADEs each year (Kim Y et al., 2013).
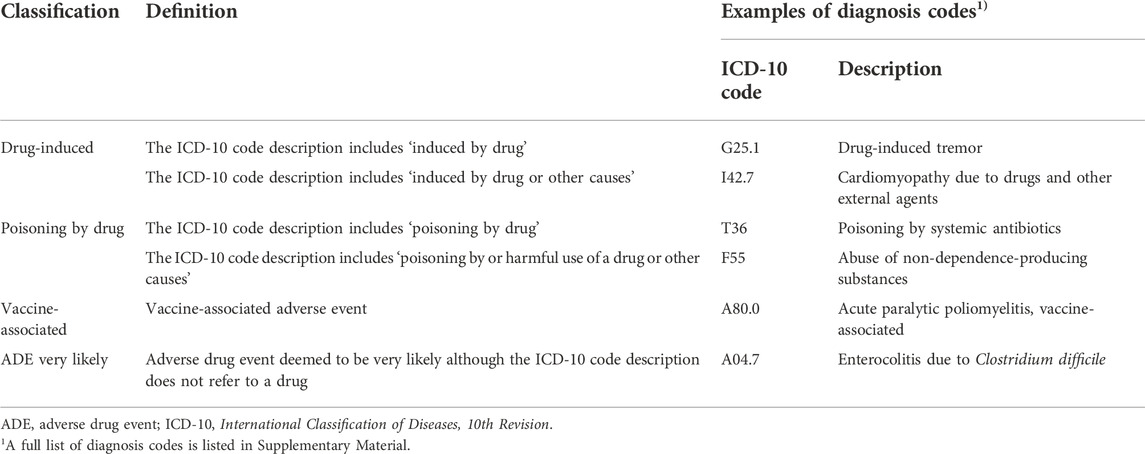
We selected diagnosis codes for ADEs from a previous systematic review to identify ADEs in the claims data (Hohl et al., 2014). The ADE diagnosis codes include the phrase or meaning ‘drug-induced,’ ‘poisoning by drug,’ and ‘vaccine-associated.’ These codes directly describe the drug’s relevance to a symptom or disease.
Furthermore, to minimize the underestimation of the prevalence of ADEs, we added ‘ADE very likely’ codes to comprehensively capture ADEs from the claim records (Hohl et al., 2014). Diagnosis codes associated with ‘ADE very likely’ do not refer to a drug in the diagnosis code description. However, they are probably associated with drug use, according to a causality assessment by clinical experts in a previous study (Hohl et al., 2014). For the analyses, 586 codes, together with sub-codes, were used to identify ADEs, including ‘drug-induced,’ ‘poisoning by drug,’ ‘vaccine-associated,’ and ‘ADE very likely.’ The diagnosis codes and descriptions of the ADEs are presented in Table 1 and Supplementary Table S1, respectively.
Statistical analysis
We estimated the annual prevalence of ADE as the number of patients with ADEs divided by the number of the entire HIRA-NPS population each year. The results were expressed as frequency and percentage (%). To evaluate whether the ADE prevalence had changed annually, the differences in the prevalence of ADEs between 2015 and 2016 were analyzed using the Cochran–Armitage trend test.
To better understand patient characteristics associated with the occurrence of ADEs, we also calculated the age-and sex-specific prevalence in each year and compared the prevalence stratified by sex (male, female), age group (65–69, 70–74, 75–79, and ≥80), and type of insurance (NHI, MA) between 2015 and 2016. In order to compare the sex differences, we calculated the female-to-male ratio of prevalence by age group. Chi-square tests were used to compare differences in prevalence between the sexes.
Additionally, we identified the characteristics of ADE each year and compared the differences between the sexes. To determine the frequent types of ADEs, the frequency of each diagnosis code to define ADEs was calculated annually in 2015 and 2016. If a diagnosis code was given repeatedly to a patient, the code was considered to be claimed only once.
Sensitivity analysis was performed to compare the two approaches in defining the patients with ADE. The patients in the base-case group have the diagnosis codes of ‘drug-induced,’ ‘poisoning by drug,’ and ‘vaccine-associated.’ The patients in the extended definition group included base-case patients and those who have the diagnosis codes of ‘ADE very likely.’ The chi-square test was used to compare the results of the base-case and extended definition analyses.
Statistical analysis was performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, United States). Statistical significance was set at p ≤ 0.05.
Results
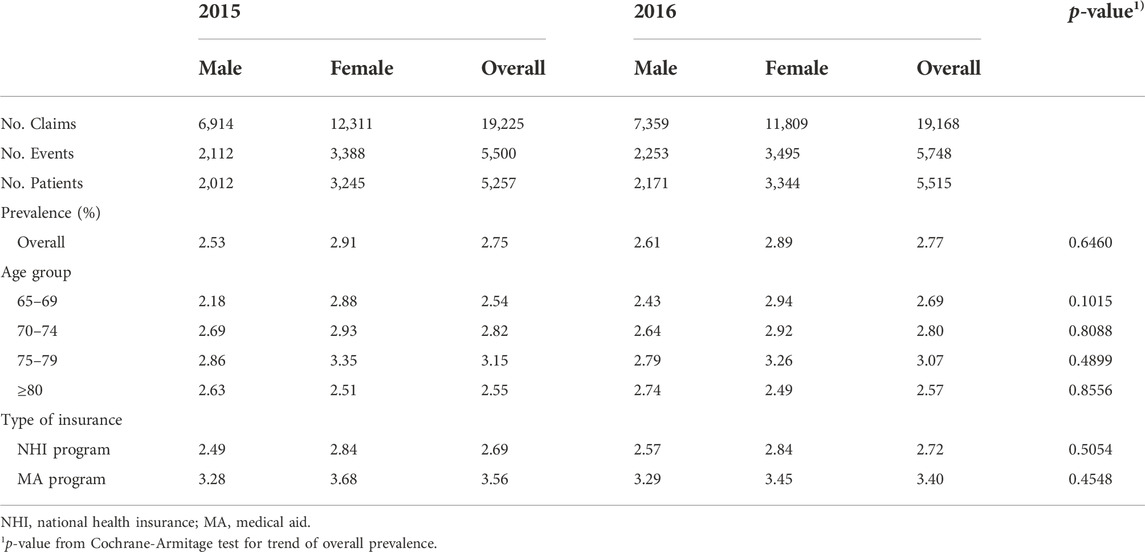
Based on the base-case analysis, 5,257 and 5,515 patients aged 65 years and older were identified as having ADEs in 2015 and 2016, respectively (Table 2). The number of adverse events identified in the claims records was 5,500 and 5,748 in 2015 and 2016, respectively.
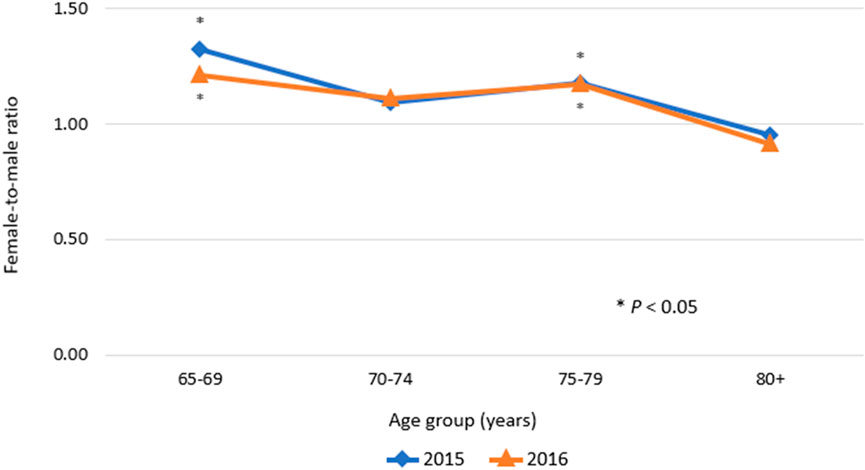
The prevalence of ADEs was 2.75% and 2.77% in 2015 and 2016, respectively. There was no significant difference in overall prevalence between calendar years. In all age groups and types of insurance, the trends in the annual prevalence were quite similar. In both 2015 and 2016, a higher prevalence was observed with increasing age, with the peak prevalence observed in the age group of 75–79 years and a higher prevalence in females. The female-to-male ratio of prevalence of ADEs was significantly higher than 1.0 in the age group of 65–69 years and the age group of 75–79 years (p < 0.05; Figure 1). In contrast, in the over-80 age group, a higher prevalence of ADEs was observed in males compared with females. In addition, in both men and women, the prevalence of ADEs was higher in MA program enrollees compared with NHI program enrollees.
During the study period, the most common ADEs were ‘allergy, unspecified,’ followed by ‘other drug-induced secondary parkinsonism,’ and ‘generalized skin eruption due to drugs and medicaments’ (Table 3). The patterns in the characteristics and frequencies of ADEs were comparable to 2015 and 2016. Notably, ‘other drug-induced secondary parkinsonism,’ the second most common ADE, illustrated a higher distribution in females than in males (Figure 2).
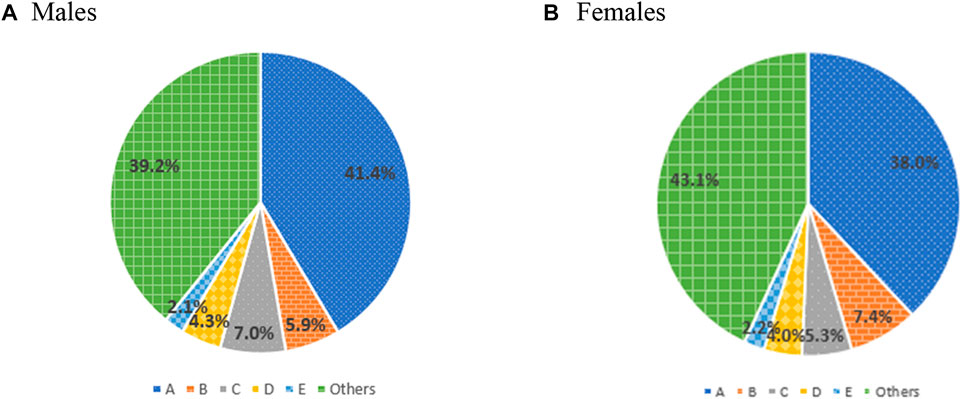
FIGURE 2. Prevalence of frequent type of adverse drug events by sex. A, allergy, unspecified; B, other drug-induced secondary parkinsonism; C, generalized skin eruption due to drugs and medicaments; D, allergic contact dermatitis due to drugs in contact with skin; E, unspecified adverse event of drug or medicament.
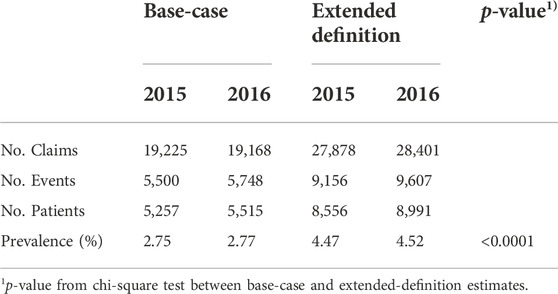
Using the extended definition of ADEs to minimize underestimation, the prevalence of ADEs increased significantly (p < 0.0001; Table 4). According to the extended definition analysis, the prevalence of ADE was 4.47% and 4.52% in 2015 and 2016, respectively, which increased by approximately 60% compared to the estimates of base-case analysis.
Discussion
In this study, we estimated the prevalence of ADEs in people aged 65 years or older in South Korea using the nationally representative claims data. We also examined whether the prevalence of ADEs changed by comparing the prevalence each year and identified the types of ADEs that occurred during the study period.
Based on the HIRA-NPS database, the base-case analysis in our study found that the estimated prevalence of ADEs for those aged 65 years or above in 2015 and 2016 were relatively lower than those reported in other countries. A study using data from a national survey in the United States reported that visits to emergency departments and outpatient clinics related to ADEs were 48.8 per 1,000 persons between 2001 and 2005 (Bourgeois et al., 2010). A systematic review that included fourteen observational studies reported that the prevalence of ADR, a subset of ADE, was 11.0%, ranging from 5.8% to 46.3% (Alhawassi et al., 2014). However, our estimates are significantly higher compared with a previous study that reported ADE prevalence among patients aged 65 years and older who visit an emergency department in a tertiary-care hospital in South Korea was 0.45% (Lee, 2015).
Based on a previous systematic review of sixty-eight studies (Beijer and de Blaey, 2002), our results supported that the prevalence of ADEs increases with age. This is in line with a recent systematic review of thirty-three studies that reported patients aged ≥65 years showed the highest prevalence of ADEs (Insani et al., 2021). Because elderly patients usually have many underlying diseases leading to polypharmacy, they are at risk of ADEs (Hajjar et al., 2007; Atella et al., 2019; Khezrian et al., 2020). According to a previous study, the potential preventability of hospital admission related to medication in elderly patients was approximately twice that in younger patients (Lghoul-Oulad Saïd et al., 2020). Therefore, elderly patients are imperative target populations for effective intervention strategies to prevent ADEs.
Concerning sex differences, we observed that females had a significantly higher prevalence of ADEs than males. This finding is consistent with the results of several other studies (Martin et al., 1998; Zopf et al., 2008; Bourgeois et al., 2010; Kane-Gill et al., 2010; Hofer-Dueckelmann et al., 2011; Wu et al., 2012). For example, an observational study including all patients admitted to an internal hospital in Austria over 6 months reported that more females than males experienced ADEs, particularly elderly (10% vs 6%, p < 0.005) (Hofer-Dueckelmann et al., 2011). The potential reasons for the differences in ADE prevalence by sex can be explained by differences in pharmacokinetics, pharmacodynamics, and drug utilization patterns (Tran et al., 1998; Zucker and Prendergast, 2020).
Patients enrolled in the MA program had a higher prevalence of ADEs than those in the NHI program. This might be related to excessive healthcare resource use and polypharmacy among MA program enrollees (Kim H. et al., 2014; Suh et al., 2014). Previous studies comparing individuals with NHI coverage and those with MA coverage for healthcare utilization revealed that MA program enrollees showed more frequent outpatient visits and hospital admissions (Lee et al., 2020). In addition, Kim et al. reported significant associations between polypharmacy and the lower-income MA population (Kim L et al., 2014). A possible reason for the excessive use of medical services and polypharmacy in the MA program enrollment is that they are not required to provide co-payments for almost all healthcare utilization (Suh et al., 2014; Lee et al., 2020). Furthermore, previous studies have reported that polypharmacy is a significant risk factor for ADEs because of the increased possibility of drug-drug interactions and inappropriate drug use (Onder et al., 2002; Field et al., 2004; Steinman et al., 2006; Rashed et al., 2012). Therefore, quality improvement, such as drug utilization review programs, is recommended to prevent meaningful drug-drug interactions and duplicate prescriptions (Aparasu et al., 2005).
In the present study, allergy and skin manifestations were the most frequent ADEs identified in the claims data. This finding is consistent with a previous result based on a spontaneous report conducted in South Korea (Shin et al., 2009). However, the characteristics of ADEs in our study differed from those in other countries. For example, in a retrospective study examining ADR-related hospital admissions at a single hospital in Thailand, ‘drug-induced neutropenia’ was the most common (Siltharm et al., 2017). Another study conducted in England, Germany, and the United States revealed that ‘Enterocolitis due to Clostridium difficile’ was the most frequent type of ADEs (Stausberg, 2014).
Notably, the second most common ADE was ‘other drug-induced secondary parkinsonism,’ which more frequently occurred in females. An observational study in South Korea reported that females and the elderly showed a high prevalence and incidence of drug-induced secondary parkinsonism (Han et al., 2019). Antipsychotics and gastrointestinal motility drugs are frequently associated with drug-induced secondary parkinsonism (Shin and Chung, 2012; López-Sendón et al., 2013; Kim et al., 2019; Kim and Suh, 2019). It is essential to bear in mind that physicians and other healthcare providers frequently overlook the presence of drug-induced parkinsonism because it is challenging to differentiate drug-induced parkinsonism from idiopathic Parkinson’s disease (Hansen et al., 1992). Recovery after the withdrawal of causal drugs may take several years, and clinical deficits might be progressive and persistent in some cases. Therefore, based on our results, effective intervention to prevent drug-induced secondary parkinsonism would be a critical component of ADE management for the elderly.
Because ADEs are expressed as various signs, symptoms, or diseases, it is difficult to identify an ADE based on the diagnosis codes from the claims record. Therefore, to improve the detection of ADE cases, two approaches by different ADE definitions were used in this study: the base-case and extended definition groups. From the base-case analysis of patients, the number of patients identified according to the extended definition of ADEs increased significantly. In a previous study, the reported prevalence of ADEs varied depending on the operational definition of events, as well as specific aspects such as the study setting, study population, and data collection methods (Leendertse et al., 2010). Stausberg and Hasford studied the prevalence of ADEs using more broad definitions of diagnosis codes, including ‘ADE likely’ and ‘ADE possible,’ which were less associated with drug use than ‘ADE very likely.’ According to their definitions of ADEs, the prevalence of drug-related hospital admission and hospitalization considerably differed, ranging from less than 1%–37.6% (Stausberg and Hasford, 2011). However, data estimates are possibly uncertain because validity and reliability could not be assessed owing to limited information from the claims data; thus, careful interpretation is needed to understand these results.
To the best of our knowledge, this study provides the first comprehensive estimate of the prevalence of ADEs in the elderly in the Asian population using claims data. Our results are representative because the HIRA-NPS claims data provide reliability, and valid information for the entire population of South Korea. Furthermore, various ADEs are identified through the broader focus of adverse events, including the consequences of inappropriate drug use, even though our results are conservative because study participants are limited due to our operational definitions of ADEs.
This study had several limitations, including the potential underestimation of ADEs. First, not all ADEs could be identified due to the limitations of the diagnosis codes. Not all ADEs can be searched using the codes, including the phrases ‘drug-induced,’ ‘poisoning by drug,’ ‘vaccine-associated,’ or even ‘ADE very likely,’ because ADE codes could not cover all potential illnesses or symptoms caused by drugs. Moreover, patient-reported adverse events or abnormalities in laboratory results related to drugs were not recorded because of the limited clinical information available in the claims data. Second, physician under-reporting could account for the low estimate of ADE prevalence. Although physicians are obligated to monitor patients’ ADEs during their practice, a significant proportion do not report ADEs (Dormann et al., 2003). Several reasons for not reporting ADEs include a lack of time due to stressful environments, uncertainty about the drug causing the ADE, difficulty in accessing reporting systems, and lack of awareness of the need to record that ADEs have occurred (Hazell and Shakir, 2006). Third, we included only study participants with at least one claim-encoded ADE diagnosis code. However, most ADEs are mild; thus, a substantial number of patients may not seek medical care for minor signs or symptoms caused by drugs. Fourth, the possible drugs associated with ADEs could not be determined using claims data because of the limited clinical information available and the retrospective study design. Fifth, the 2-year study period may be insufficient to understand any trends in ADE prevalence. Sixth, we did not analyze ADE prevalence in different clinical settings. Further studies are needed to understand the prevalence of ADE in outpatient, inpatient, and emergency departments. Lastly, it was not recent data that we used in this study. Therefore, our study results may not reflect current estimates. However, after 2016, several diagnosis codes classified as sensitive information were not provided in the HIRA-NPS database. Therefore, we used the 2015 and 2016 database, the most recent database that we could fully identify all diagnosis codes.
Conclusion
This study using the representative claims data provided comprehensive estimates of ADE prevalence and characteristics among the elderly in South Korea. Due to the extended life expectancy, the prevalence of ADEs is expected to grow continuously. The results of our study suggest that more efforts will be needed to prevent ADE in the elderly. National healthcare policy, such as regulatory intervention for polypharmacy and educational program, is required to reduce ADE for vulnerable people, especially in MA program enrollees. To effectively prevent and manage ADEs, further studies are needed to explore potential drugs that cause ADEs.
Data availability statement
The datasets presented in this article are not readily available because this study used the Health Insurance Review and Assessment Service-National Patient Sample (HIRA-NPS) 2015 and 2016 database in South Korea. Data were obtained from the HIRA with permission.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Pusan National University (PNU IRB/2020_152_HR). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, EC, SK, and HS; methodology, EC, SK, and HS; software, EC and SK; validation, EC and SK; formal analysis, EC and SK; investigation, EC, SK, and HS; resources, EC and HS; data curation, EC and SK; writing—original draft preparation, EC, SK, and HS; writing—review and editing, SK and HS; visualization, EC, SK, and HS; supervision, HS; project administration, HS; funding acquisition, HS All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant (21153MFDS601) from Ministry of Food and Drug Safety in 2022. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1F1A1069526).
Acknowledgments
We used Health Insurance Review and Assessment Service-National Patient Sample 2015 (HIRA-NPS-2015-0199) and 2016 (S20211228748); however, we declare that the results do not reflect the positions of either the Health Insurance Review and Assessment Service or the Ministry of Health and Welfare in Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1047387/full#supplementary-material
References
Alhawassi, T. M., Krass, I., Bajorek, B. V., and Pont, L. G. (2014). A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin. Interv. Aging 9, 2079–2086. doi:10.2147/CIA.S71178
Aparasu, R. R., Mort, J. R., and Brandt, H. (2005). Polypharmacy trends in office visits by the elderly in the United States, 1990 and 2000. Res. Soc. Adm. Pharm. 1, 446–459. doi:10.1016/j.sapharm.2005.06.004
Atella, V., Piano Mortari, A., Kopinska, J., Belotti, F., Lapi, F., Cricelli, C., et al. (2019). Trends in age-related disease burden and healthcare utilization. Aging Cell 18, e12861. doi:10.1111/acel.12861
Bates, D. W., Cullen, D. J., Laird, N., Petersen, L. A., Small, S. D., Servi, D., et al. (1995). Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Jama 274, 29–34. doi:10.1001/jama.1995.03530010043033
Bates, D. W., Larizgoitia, I., Prasopa-Plaizier, N., and Jha, A. K. (2009). Global priorities for patient safety research, Bmj 338, 775. doi:10.1136/bmj.b1775
Bates, D. W., Spell, N., Cullen, D. J., Burdick, E., Laird, N., Petersen, L. A., et al. (1997). The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. Jama 277, 307–311. doi:10.1001/jama.277.4.307
Beijer, H. J., and de Blaey, C. J. (2002). Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm. World Sci. 24, 46–54. doi:10.1023/a:1015570104121
Bourgeois, F. T., Shannon, M. W., Valim, C., and Mandl, K. D. (2010). Adverse drug events in the outpatient setting: An 11-year national analysis. Pharmacoepidemiol Drug Saf. 19, 901–910. doi:10.1002/pds.1984
Bouvy, J. C., De Bruin, M. L., and Koopmanschap, M. A. (2015). Epidemiology of adverse drug reactions in europe: A review of recent observational studies. Drug Saf. 38, 437–453. doi:10.1007/s40264-015-0281-0
Classen, D. C., Pestotnik, S. L., Evans, R. S., Lloyd, J. F., and Burke, J. P. (1997). Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. Jama 277, 301–306. doi:10.1001/jama.277.4.301
Digmann, R., Thomas, A., Peppercorn, S., Ryan, A., Zhang, L., Irby, K., et al. (2019). Use of Medicare administrative claims to identify a population at high risk for adverse drug events and hospital use for quality improvement. J. Manag. Care Spec. Pharm. 25, 402–410. doi:10.18553/jmcp.2019.25.3.402
Dormann, H., Criegee-Rieck, M., Neubert, A., Egger, T., Geise, A., Krebs, S., et al. (2003). Lack of awareness of community-acquired adverse drug reactions upon hospital admission : Dimensions and consequences of a dilemma. Drug Saf. 26, 353–362. doi:10.2165/00002018-200326050-00004
Field, T. S., Gurwitz, J. H., Harrold, L. R., Rothschild, J., DeBellis, K. R., Seger, A. C., et al. (2004). Risk factors for adverse drug events among older adults in the ambulatory setting. J. Am. Geriatr. Soc. 52, 1349–1354. doi:10.1111/j.1532-5415.2004.52367.x
Friedman, B. W., Cisewski, D. H., Holden, L., Bijur, P. E., and Gallagher, E. J. (2015). Age but not sex is associated with efficacy and adverse events following administration of intravenous migraine medication: An analysis of a clinical trial database. Headache 55, 1342–1355. doi:10.1111/head.12697
Hajjar, E. R., Cafiero, A. C., and Hanlon, J. T. (2007). Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 5, 345–351. doi:10.1016/j.amjopharm.2007.12.002
Han, S., Kim, S., Kim, H., Shin, H.-W., Na, K.-S., and Suh, H. S. (2019). Prevalence and incidence of Parkinson's disease and drug-induced parkinsonism in Korea. BMC Public Health 19, 1328–1329. doi:10.1186/s12889-019-7664-6
Hansen, T. E., Brown, W. L., Weigel, R. M., and Casey, D. E. (1992). Underrecognition of tardive dyskinesia and drug-induced parkinsonism by psychiatric residents. Gen. Hosp. Psychiatry 14, 340–344. doi:10.1016/0163-8343(92)90069-m
Hazell, L., and Shakir, S. A. (2006). Under-reporting of adverse drug reactions : A systematic review. Drug Saf. 29, 385–396. doi:10.2165/00002018-200629050-00003
Hofer-Dueckelmann, C., Prinz, E., Beindl, W., Szymanski, J., Fellhofer, G., Pichler, M., et al. (2011). Adverse drug reactions (ADRs) associated with hospital admissions - elderly female patients are at highest risk. Int. J. Clin. Pharmacol. Ther. 49, 577–586. doi:10.5414/cp201514
Hohl, C. M., Karpov, A., Reddekopp, L., Doyle-Waters, J., and Stausberg, J. (2014). ICD-10 codes used to identify adverse drug events in administrative data: A systematic review. J. Am. Med. Inf. Assoc. 21, 547–557. doi:10.1136/amiajnl-2013-002116
Hougland, P., Xu, W., Pickard, S., Masheter, C., and Williams, S. D. (2006). Performance of international classification of diseases, 9th revision, clinical modification codes as an adverse drug event surveillance system. Med. Care 44, 629–636. doi:10.1097/01.mlr.0000215859.06051.77
Insani, W. N., Whittlesea, C., Alwafi, H., Man, K. K., Chapman, S., and Wei, L. (2021). Prevalence of adverse drug reactions in the primary care setting: A systematic review and meta-analysis. PLoS One 16, e0252161. doi:10.1371/journal.pone.0252161
Kane-Gill, S. L., Van Den Bos, J. V., and Handler, S. M. (2010). Adverse drug reactions in hospital and ambulatory care settings identified using a large administrative database. Ann. Pharmacother. 44, 983–993. doi:10.1345/aph.1M726
Khezrian, M., McNeil, C. J., Murray, A. D., and Myint, P. K. (2020). An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 11, 2042098620933741. doi:10.1177/2042098620933741
Kim, H, H., Shin, J., Kim, M., and Park, B. (2014). Prevalence and predictors of polypharmacy among Korean elderly. PLoS One 9, e98043. doi:10.1371/journal.pone.0098043
Kim, J.-A., Yoon, S., Kim, L.-Y., and Kim, D.-S. (2017). Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 32, 718–728. doi:10.3346/jkms.2017.32.5.718
Kim, L, L., Kim, J.-A., and Kim, S. (2014). A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol. Health 36, e2014008. doi:10.4178/epih/e2014008
Kim, L., Sakong, J., Kim, Y., Kim, S., Kim, S., Tchoe, B., et al. (2013). Developing the inpatient sample for the National Health Insurance claims data. Health Policy Manag. 23, 152–161. doi:10.4332/kjhpa.2013.23.2.152
Kim, S., Cheon, S.-M., and Suh, H. S. (2019). Association between drug exposure and occurrence of parkinsonism in Korea: A population-based case-control study. Ann. Pharmacother. 53, 1102–1110. doi:10.1177/1060028019859543
Kim, S., and Suh, H. S. (2019). Current status of parkinsonism-related adverse events and associated drugs in Korea. J. Patient Saf. 15, e56–e59. doi:10.1097/PTS.0000000000000373
Kim Y, S. S., Park, J., Jung, Y., Kim, J., and Lee, Tj, (2013). Costing methods in healthcare. Seoul, South Korea: National Evidence-based Healthcare collaborating agency.
Koo, (2009). Effects of adverse drug reactions detected using monitoring program on the length of stay and charges in the hospital setting. Seoul, South Korea: Seoul National University.
Kuklik, N., Stausberg, J., and Jöckel, K.-H. (2017). Adverse drug events in German hospital routine data: A validation of international classification of diseases, 10th revision (ICD-10) diagnostic codes. PLoS One 12, e0187510. doi:10.1371/journal.pone.0187510
Lee, D. W., Jang, J., Choi, D.-W., Jang, S.-I., and Park, E.-C. (2020). The effect of shifting medical coverage from National Health Insurance to Medical Aid type I and type II on health care utilization and out-of-pocket spending in South Korea. BMC Health Serv. Res. 20, 979–1010. doi:10.1186/s12913-020-05778-2
Lee, J. (2015). Adverse drug reactions related emergency department visits. Seoul, South Korea: Sungkyunkwan University.
Leendertse, A. J., Visser, D., Egberts, A. C., and van den Bemt, P. M. (2010). The relationship between study characteristics and the prevalence of medication-related hospitalizations: A literature review and novel analysis. Drug Saf. 33, 233–244. doi:10.2165/11319030-000000000-00000
Lghoul-Oulad Saïd, F., Hek, K., Flinterman, L. E., Herings, R. M., Warlé-van Herwaarden, M. F., de Bie, S., et al. (2020). Prevalence and incidence rate of hospital admissions related to medication between 2008 and 2013 in The Netherlands. Pharmacoepidemiol Drug Saf. 29, 1659–1668. doi:10.1002/pds.5122
López-Sendón, J., Mena, M. A., and G De Yébenes, J. (2013). Drug-induced parkinsonism. Expert Opin. drug Saf. 12, 487
Martin, R. M., Biswas, P. N., Freemantle, S. N., Pearce, G. L., and Mann, R. D. (1998). Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: Analysis of 48 cohort studies. Br. J. Clin. Pharmacol. 46, 505–511. doi:10.1046/j.1365-2125.1998.00817.x
Miguel, A., Azevedo, L. F., Lopes, F., Freitas, A., and Pereira, A. C. (2013). Methodologies for the detection of adverse drug reactions: Comparison of hospital databases, chart review and spontaneous reporting. Pharmacoepidemiol Drug Saf. 22, 98–102. doi:10.1002/pds.3348
Nebeker, J. R., Barach, P., and Samore, M. H. (2004). Clarifying adverse drug events: A clinician's guide to terminology, documentation, and reporting. Ann. Intern Med. 140, 795–801. doi:10.7326/0003-4819-140-10-200405180-00009
Onder, G., Pedone, C., Landi, F., Cesari, M., Della Vedova, C., Bernabei, R., et al. (2002). Adverse drug reactions as cause of hospital admissions: Results from the Italian group of pharmacoepidemiology in the elderly (GIFA). J. Am. Geriatr. Soc. 50, 1962–1968. doi:10.1046/j.1532-5415.2002.50607.x
Passarelli, M. C. G., Jacob-Filho, W., and Figueras, A. (2005). Adverse drug reactions in an elderly hospitalised population: Inappropriate prescription is a leading cause. Drugs Aging 22, 767–777. doi:10.2165/00002512-200522090-00005
Pedrós, C., Quintana, B., Rebolledo, M., Porta, N., Vallano, A., and Arnau, J. M. (2014). Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur. J. Clin. Pharmacol. 70, 361–367. doi:10.1007/s00228-013-1630-5
Rashed, A. N., Wong, I. C., Cranswick, N., Tomlin, S., Rascher, W., and Neubert, A. (2012). Risk factors associated with adverse drug reactions in hospitalised children: International multicentre study. Eur. J. Clin. Pharmacol. 68, 801–810. doi:10.1007/s00228-011-1183-4
Shin, H.-W., and Chung, S. J. (2012). Drug-induced parkinsonism. J. Clin. Neurol. 8, 15–21. doi:10.3988/jcn.2012.8.1.15
Shin, Y. S., Lee, Y. W., Choi, Y. H., Park, B., Jee, Y. K., Choi, S. K., et al. (2009). Spontaneous reporting of adverse drug events by Korean regional pharmacovigilance centers. Pharmacoepidemiol Drug Saf. 18, 910–915. doi:10.1002/pds.1796
Siltharm, C., Pattanaprateep, O., Pongcharoensuk, P., Jeanpeerapong, N., and Thavorncharoensap, M. (2017). Detection of adverse drug reaction (ADR)-related hospital admissions: A pilot study using administrative database for ADR monitoring in Thailand. Pharm. Sci. Asia 44 (3), 142–153. doi:10.29090/psa.2017.03.142
Song, Y. J. (2009). The South Korean health care system. Jmaj 52, 206–209. doi:10.3345/kjp.2009.52.7.752
Stausberg, J., and Hasford, J. (2011). Drug-related admissions and hospital-acquired adverse drug events in Germany: A longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. BMC Health Serv. Res. 11, 134–139. doi:10.1186/1472-6963-11-134
Stausberg, J. (2014). International prevalence of adverse drug events in hospitals: An analysis of routine data from England, Germany, and the USA. BMC Health Serv. Res. 14, 125–129. doi:10.1186/1472-6963-14-125
Steinman, M. A., Landefeld, C., Rosenthal, G. E., Berthenthal, D., Sen, S., and Kaboli, P. J. (2006). Polypharmacy and prescribing quality in older people. J. Am. Geriatr. Soc. 54, 1516–1523. doi:10.1111/j.1532-5415.2006.00889.x
Suh, H. S., Kang, H.-Y., Kim, J., and Shin, E. (2014). Effect of health insurance type on health care utilization in patients with hypertension: A national health insurance database study in Korea. BMC Health Serv. Res. 14, 570–612. doi:10.1186/s12913-014-0570-9
Tran, C., Knowles, S. R., Liu, B. A., and Shear, N. H. (1998). Gender differences in adverse drug reactions. J. Clin. Pharmacol. 38, 1003–1009. doi:10.1177/009127009803801103
Wu, C., Bell, C. M., and Wodchis, W. P. (2012). Incidence and economic burden of adverse drug reactions among elderly patients in ontario emergency departments: A retrospective study. Drug Saf. 35, 769–781. doi:10.1007/BF03261973
Yu, Y. M., Shin, W. G., Lee, J.-Y., Choi, S. A., Jo, Y. H., Youn, S. J., et al. (2015). Patterns of adverse drug reactions in different age groups: Analysis of spontaneous reports by community pharmacists. PloS one 10, e0132916. doi:10.1371/journal.pone.0132916
Zopf, Y., Rabe, C., Neubert, A., Hahn, E. G., and Dormann, H. (2008). Risk factors associated with adverse drug reactions following hospital admission: A prospective analysis of 907 patients in two German University hospitals. Drug Saf. 31, 789–798. doi:10.2165/00002018-200831090-00007
Keywords: adverse drug event, prevalence, drug safety, elderly, female, diagnosis codes
Citation: Choi E, Kim S and Suh HS (2022) Exploring the prevalence and characteristics of adverse drug events among older adults in South Korea using a national health insurance database. Front. Pharmacol. 13:1047387. doi: 10.3389/fphar.2022.1047387
Received: 18 September 2022; Accepted: 22 November 2022;
Published: 01 December 2022.
Edited by:
Jean-Marie Boeynaems, Université libre de Bruxelles, BelgiumReviewed by:
Nadeem Irfan Bukhari, University of the Punjab, PakistanBrian Godman, University of Strathclyde, United Kingdom
Copyright © 2022 Choi, Kim and Suh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hae Sun Suh, aGFlc3VuLnN1aEBraHUuYWMua3I=
 Eunkyeong Choi
Eunkyeong Choi Siin Kim
Siin Kim Hae Sun Suh
Hae Sun Suh