- 1Experiment Center, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Office of Academic Affairs, Shandong University of Traditional Chinese Medicine, Jinan, China
- 4Second Affiliated Hospital, Shandong University of Traditional Chinese Medicine, Jinan, China
- 5United Front Work Department, Shandong University of Traditional Chinese Medicine, Jinan, China
L-tryptophan metabolism is involved in the regulation of many important physiological processes, such as, immune response, inflammation, and neuronal function. Indoleamine 2, 3-dioxygenase 1 (IDO1) is a key enzyme that catalyzes the first rate-limiting step of tryptophan conversion to kynurenine. Thus, inhibiting IDO1 may have therapeutic benefits for various diseases, such as, cancer, autoimmune disease, and depression. In the search for potent IDO1 inhibitors, natural quinones were the first reported IDO1 inhibitors with potent inhibitory activity. Subsequently, natural compounds with diverse structures have been found to have anti-IDO1 inhibitory activity. In this review, we provide a summary of these natural IDO1 inhibitors, which are classified as quinones, polyphenols, alkaloids and others. The overview of in vitro IDO1 inhibitory activity of natural compounds will help medicinal chemists to understand the mode of action and medical benefits of them. The scaffolds of these natural compounds can also be used for further optimization of potent IDO1 inhibitors.
1 Introduction
1.1 Tryptophan metabolism and indoleamine 2, 3-dioxygenase 1
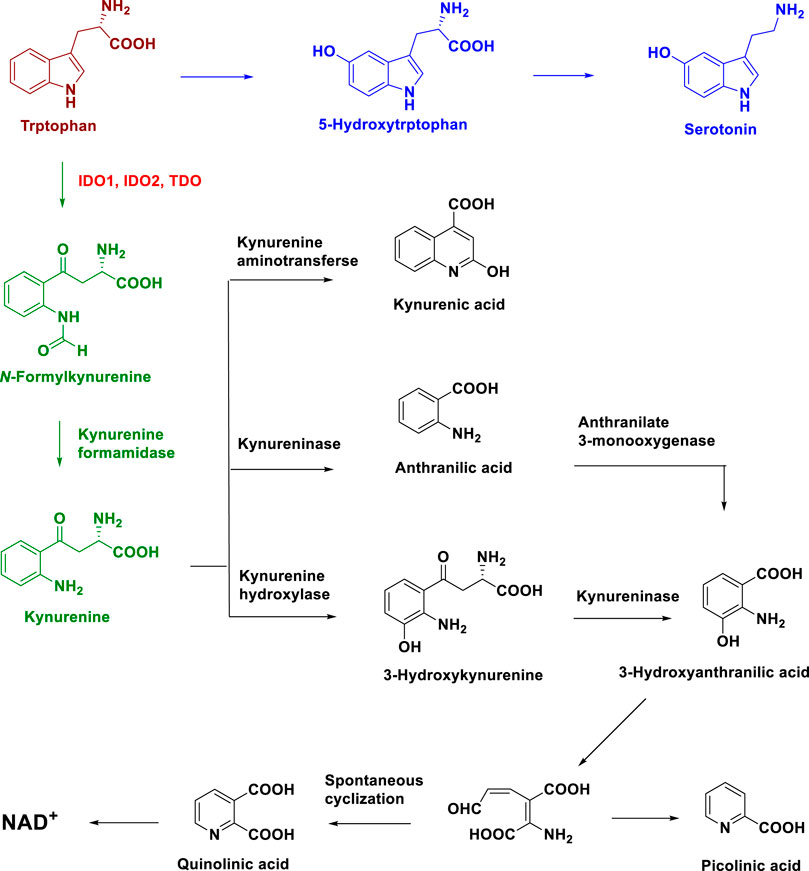
L-Tryptophan (L-Trp) is an essential amino acid, and the normal concentration range of L-Trp in human plasma is 50–100 μM (Wang et al., 2015; Cervenka et al., 2017; Barreto et al., 2018; Platten et al., 2019). L-Trp is important as a protein building block and in the synthesis of several important bioactive metabolites (Wang et al., 2015; Cervenka et al., 2017; Barreto et al., 2018; Platten et al., 2019). However, humans cannot produce L-Trp and must obtain it from food (Barreto et al., 2018). The metabolism of L-Trp occurs via the serotonin pathway and kynurenine (Kyn) pathway (Figure 1) (Barreto et al., 2018). The Kyn pathway metabolizes 95% of L-Trp. In this pathway, L-Trp is oxidized, breaking the 2, 3-double bond of the indole ring to form N-formylkynurenine, which is rapidly converted to Kyn by Kyn formamidase. Next, Kyn is metabolized to kynurenic acid and 3-hydroxy-o-aminobenzoic acid, and 3-hydroxy-o-aminobenzoic acid is used to produce NAD+ (Figure 1). L-Trp consumption and Kyn production are key to immune system regulation under both physiological and disease conditions (Maddison and Giorgini, 2015; Barreto et al., 2018; Odunsi et al., 2022). Recent studies have continued to highlight the importance of L-Trp metabolism in immune regulation, neuronal function, and ageing (Sorgdrager et al., 2019; Platten et al., 2021; Krupa et al., 2022; Merlo et al., 2022; Ouyang et al., 2022; Salminen, 2022).
In the Trp to Kyn metabolic pathway, the oxidation of Trp to N-formylkynurenine is the initial and rate-limiting step, which can be catalyzed by the tryptophan dioxygenase isozymes indoleamine 2, 3-dioxygenase 1 (IDO1), indoleamine 2, 3-dioxygenase 2 (IDO2), and tryptophan 2, 3-dioxygenase (TDO) (Vécsei et al., 2013; Dounay et al., 2015; Dolšak et al., 2021). IDO1, IDO2, and TDO have differences in structure, tissue distribution, and substrate specificity. IDO1 is encoded by the IDO1 gene on human chromosome eight and is widely present in the lung, intestine, colon, kidney, spleen, pancreas, central nervous system, macrophages, and microglia (Tone et al., 1990; Takikawa, 2005; Lewis-Ballester et al., 2017; Santos et al., 2022). IDO1 shows a broad substrate specificity for L-Trp (Km = 20 μM), D-Trp, 5-hydroxy Trp, tryptamine, serotonin, and other Trp analogues (Pantouris et al., 2014). IDO2 is encoded by the IDO2 gene on chromosome eight and is mainly distributed in the kidney, liver, and reproductive organs (Ball et al., 2007; Bakmiwewa et al., 2012; Fukunaga et al., 2012). The enzymatic activity of IDO2 is low, and the Km of IDO2 for L-Trp is around 6.8 mM (Fukunaga et al., 2012). It is speculated that IDO2 might be more involved in cell signaling rather than functioning as a tryptophan dioxygenase (Fukunaga et al., 2012). TDO is encoded by the TDO2 gene on chromosome four and is mostly distributed in the liver with highly specific enzymatic activity for L-Trp (Km = 190 μM) (Löb et al., 2009; Pham et al., 2019). In summary, the three tryptophan dioxygenase enzymes show different substrate activities. The catalytic activity order of these three enzymes for L-Trp is IDO1 (Km = 20 μM) > TDO (Km = 190 μM) > IDO2 (Km = 6.8 mM) (Dolšak et al., 2021).
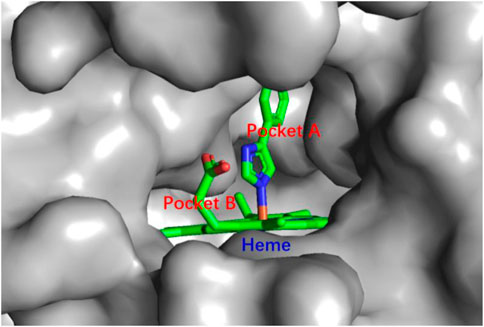
IDO1 is a heme-containing enzyme composed of 403 amino acids. More than 60 human IDO1 crystal structures have been deposited in the Protein Data Bank (PDB) since the crystal structure of IDO1 was first reported in 2006 (PDB ID: 2D0T) (Sugimoto et al., 2006; Maddison and Giorgini, 2015). The crystal structure of IDO1 contains hydrophobic pockets A and B in the active site, with heme at the bottom of pocket A. The inhibitor ligand of PI was also included in the crystal structure (Figure 2) (Sugimoto et al., 2006). In addition, the JK loop forms the front entrance of the active site, which allows the substrate and inhibitors to enter (Lewis-Ballester et al., 2017). Interestingly, studies also revealed that the phosphorylation of two tyrosine residues of IDO1: Tyr115 and Tyr253 regulates the functions of this enzyme (Albini et al., 2017).
2 Indoleamine 2, 3-dioxygenase 1 and its functions
2.1 Immune tolerance in tumors
In tumor microenvironments, tumor killer cells, such as, effector T cells, natural killer cells, are often inhibited and induced to apoptosis to prevent the killer activity, and immune tolerance cells, such as, Treg cells, myeloid-derived suppressor cells, are often activated and promoted to the proliferation (Wu and Dai, 2017; Arneth, 2019). These combined effects create an immunosuppressive microenvironment suitable for tumor growth and lead to tumor immune escape. Research revealed that IDO1 is important in creating the immunosuppressive microenvironment (Ling et al., 2014; Munn and Mellor, 2016; Heidari et al., 2020; Gouasmi et al., 2022; Huang et al., 2022; Zhang et al., 2022). Under physiological conditions, IDO1 is usually expressed at a low level in various tissues. However, IDO1 is overexpressed in many cancers, such as, breast, colorectal, gastric, lung, and endometrial cancers, and IDO1 overexpression is also associated with poor survival rates (Uyttenhove et al., 2003; Dolusić et al., 2011; Heidari et al., 2020; Odunsi et al., 2022).
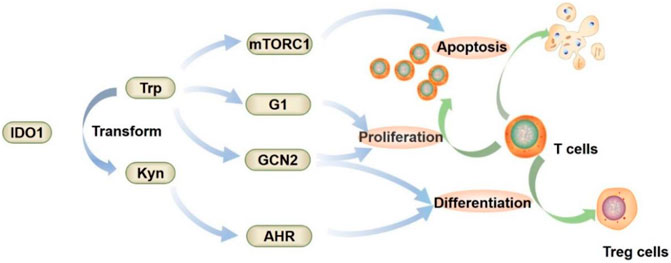
IDO1 mediates tumor immune escape via three main downstream pathways (Figure 3) (Ling et al., 2014; Munn and Mellor, 2016; Heidari et al., 2020). In the first, tryptophan is depleted by the overexpression of IDO1, which increases the degradation of Trp and the production of Kyn. This causes the imbalance between Trp/Kyn in the tumor microenvironment (Liu and Wang, 2009; Zhai et al., 2020). The decrease in Trp and the increase in Kyn prevents T lymphocytes from maturing. IDO1 downstream metabolites, such as, Kyn, kynurenine acid (KA) and 3OH-Kyn, are toxic and can inhibit the functions of T cells, B cells and NK cells. Moreover, Kyn and its’ downstream metabolites can activate aryl hydrocarbon receptor (AHR), which will result in the creating the immunosuppressive microenvironment (Huang et al., 2022; Zhang et al., 2022). The second is the GCN2 pathway. General control nonderepressible 2 (GCN2) is a serine/threonine-protein kinase (Munn et al., 2005). There is a domain in GCN2 that binds to uncharged tRNA to sense amino acid deficiencies. When the level of Trp in cells is low, uncharged tRNAs accumulate in cells and activate GCN2. The activated GCN2 eventually reduces the proliferation of T cells and promotes the differentiation of Treg cells. The third is the mechanistic target of rapamycin complex 1 (mTORC1) pathway. mTORC1 regulates various cellular process (Metz et al., 2012). The level of amino acids strongly affects mTORC1 activity. When the Trp level is decreased, mTORC1 is inhibited, which eventually induces T cell autophagy.
2.2 Inflammation
Inflammation is a normal response to injury or infection and is initiated by the innate immune system to clear away damaged cells (Medzhitov, 2008; Sorgdrager et al., 2019; Esmaeili and Hajavi, 2022). The hallmark of inflammation is the accumulation of various primary inflammatory cells in tissues, which overexpress various cytokines, growth factors, and enzymes. Thus, inflammation is crucial in maintaining health. However, if the tissue remains inflamed for a long time, inflammation can also damage healthy tissue and induce secondary repair, including fibrosis. Proinflammatory cytokines, such as interferon gamma, and other inflammatory signaling molecules, including the lipid mediator prostaglandin E2 and lipopolysaccharide pathogen particles, induce the overexpression of IDO1 (Baumgartner et al., 2019; Alves de Souza et al., 2022). The activation of IDO1 in response to these inflammatory factors induces immune tolerance and eventually controls hyperinflammation (Zhai et al., 2020). The mechanism of IDO1 involvement in inflammation regulation occurs via two main pathways (Heidari et al., 2020; Ogbechi et al., 2020; Gargaro et al., 2022). In the first, the overexpression of IDO1 consumes and depletes the intracellular Trp, thereby mediating immune tolerance. Several metabolites of IDO1 are known to toxic to immune cells and inhibit the regular functions of various immune cells (Huang et al., 2022; Zhang et al., 2022). In the second, the overexpression of IDO1 causes the accumulation of Kyn, which activates the aryl hydrocarbon receptor. This IDO1/Kyn/aryl hydrocarbon receptor signaling pathway regulates T cell activation, induces the differentiation of Treg cells, and changes the immunogenicity of antigen-presenting cells, which eventually has an anti-inflammatory effect (Romani et al., 2008; Sorgdrager et al., 2019). Interestingly, an imbalance in the Kyn/Trp ratio is often observed in inflammation-related disease, including infections and autoimmune disorders (Schröcksnadel et al., 2006; Huang et al., 2020). In summary, IDO1 is overexpressed in response to inflammation and suppresses the immune system to control inflammation.
2.3 Depression
Depression is a mental disorder that has a complicated mechanism (Barreto et al., 2018). Although different hypotheses have been proposed to explain the pathophysiology of depression, the monoaminergic hypothesis, which proposes that depression stems from low levels of the monoamine serotonin (5-hydroxytryptamine) in the brain, has become the basis for developing antidepressant drugs (Healy and Leonard, 1987; Badawy and Morgan, 1991). Serotonin is produced from the metabolism of L-Trp. Less than 5% of L-Trp is processed to synthesize serotonin, and the other 95% of L-Trp in the plasma is consumed by IDO1 to produce Kyn. Trp levels are much lower in the brains of depressed patients than in non-depressed people, and the levels of Trp are clearly associated with the symptoms of depression (Badawy and Morgan, 1991; Platten et al., 2021). Furthermore, depressed patients have high plasma levels of pro-inflammatory cytokines, such as interferon gamma. In an animal model of depression, the activation of IDO1 and increased levels of Kyn have also been measured. Because IDO1 activity is closely related to Trp levels, the activation of IDO1 and the involvement of the immune system and inflammation in depression suggest that the inhibition of IDO1 could be a target for discovering antidepressant drugs (Romani et al., 2008; Huang et al., 2020).
3 Natural indoleamine 2, 3-dioxygenase 1 inhibitors
Because IDO1 is an important immune checkpoint modulator, it is important in tumor immune escape, and thus is an important therapeutic target in cancer therapy. Over the past decade, there has been intense interest in developing IDO1 inhibitors in both academic institutes and the pharmaceutical industry. A variety of IDO1 inhibitors have been found via methods including high-throughput screening, rational design, and natural compound screening (John et al., 2010). Natural compounds are an important source of pharmacological agents. In the early stages of IDO1 inhibitor discovery before 2010, natural compounds contributed much important structural information for the rational design of IDO1 inhibitors (Delfourne, 2012). Next, we summarize and analyze natural compounds derived IDO1 inhibitors.
3.1 Quinones
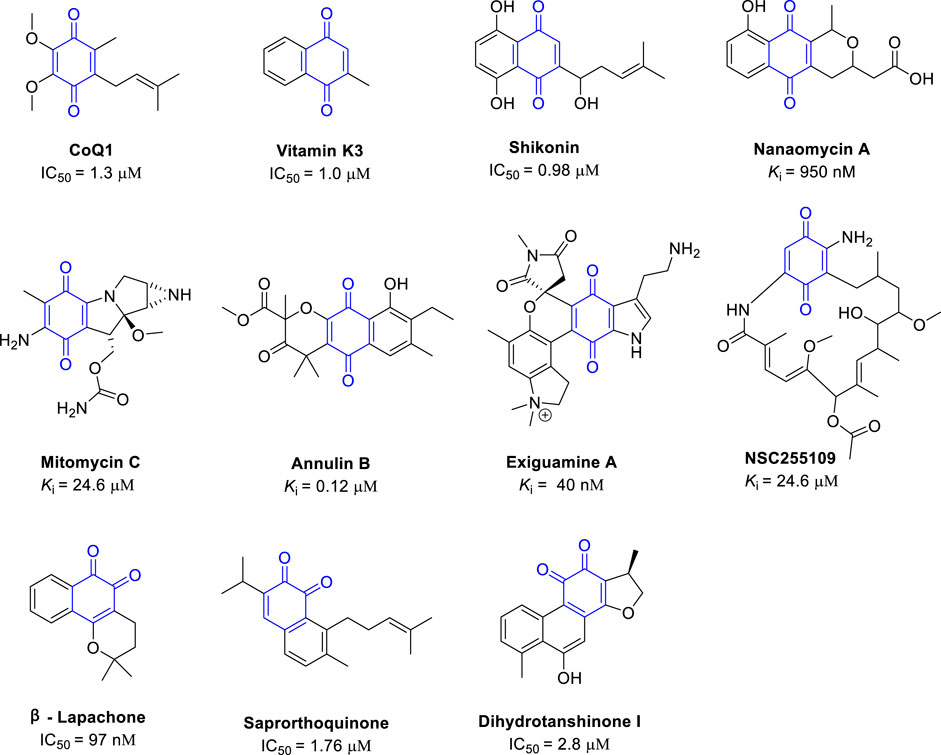
Natural quinones are classified by structure as benzoquinones, naphthoquinones, anthraquinones, and phenanthraquinones. According to the position of the carbonyl group, quinones can also be divided into 1, 2-quinones and 1,4-quinones (Zhang et al., 2021). Natural quinones were one of the first types of IDO inhibitors discovered (Figure 4) (Pereira et al., 2006), and most natural quinones have some IDO1 inhibitory activity. The quinone moiety usually occupies binding pocket A of the IDO1 active center, and can bind to the heme iron at the bottom of the pocket. In this part, we summarize the reported natural quinones with substantial IDO1 inhibitory activity, the structure of these quinones were shown in Figure 4.
Coenzyme Q (CoQ), also called ubiquinone, is a coenzyme family, that is, ubiquitous in cells and membranes in animals and bacteria and has important functions in cell metabolism, including in the mitochondrial respiratory chain (Turunen et al., 2004). CoQ1 is a member of the CoQ family and contains the core 1, 4-benzoquinone scaffold. CoQ1 is a moderate IDO1 inhibitor with an IC50 of 1.3 μM. Because CoQ1 is a simple quinone, it has been modified to improve the IDO1 inhibition activity (Ding et al., 2019). Another important natural quinone, vitamin K3, also called menadione, contains the naphthoquinone scaffold. Menadione has some important pharmacological functions, including the regulation of cell proliferation and cell growth, and has moderate IDO1 inhibitory activity with an IC50 of 1.0 μM (Kumar et al., 2008). However, substituting the methyl side chain in menadione with a long lipid chain to form vitamin K1 abrogates the IDO1 inhibitory activity. Due to their structure simplicity and activity, CoQ1 and menadione are suitable as lead compounds for further structural optimization to improve the IDO1 inhibitory activity.
Screening of natural compound libraries has revealed several natural compounds with interesting IDO1 inhibitory activity. Shikonin A, which is usually isolated from Radix Arnebiae and has anti-inflammatory activity attributed to the inhibition of caspase 1. Shikonin A inhibits IDO1 activity in a dose-dependent manner with an IC50 of 0.98 μM. Given the potency of IDO1 inhibition, it is likely that the anti-inflammatory activity of shikonin A also partially arises from IDO1 inhibition (Guo et al., 2020). The 1, 4-quinone antibiotic nanaomycin has been report to have the activity to inhibit DNA methyltransferase 3B (IC50 = 500 nM). Nanaomycin also found with the IDO1 inhibitory activity (Ki ∼ 950 nM) (Pantouris and Mowat, 2014). Lastly, the 1, 4-quinone mitomycin C also has moderate IDO1 inhibitory activity (Ki = 24.2 μM).
In 2006, the Andersen group found that the MeOH extract of the northeastern Pacific marine hydroid, Garveia annulata had IDO1 inhibitory activity. Further separation of the crude yielded a series of quinones with potent IDO1 inhibitory activity, of which annulin B was the most potent (Ki = 0.12 μM) (Pereira et al., 2006). This was the first reported natural IDO1 inhibitor. The Andersen group also collected the marine sponge Neopetrosia exigua in Papua New Guinea and found that the methanol extract had IDO1 inhibitory activity. The alkaloid exiguanine A isolated from the MeOH extract is one of the most potent natural IDO inhibitors (Ki = 41 nM) (Carr et al., 2008), and thus was selected to be optimized further (Dong et al., 2021). The Mowat group screened about 2800 natural compounds from the National Cancer Institute for IDO and TDO inhibitors and found several other natural compounds with potent IDO1 inhibitory activity. NSC255109 (17-aminodemethoxygeldanamycin) inhibits IDO1 with Ki of around 1.4 μM (Pantouris and Mowat, 2014), and this compound contains the 1, 4-benzoquinone scaffold as part of a cyclized structure.
Next, we describe IDO1 inhibitors with a 1, 2-quinone scaffold. β-Lapachone, which was first extracted from the lapacho tree (Tabebuia avellanedae), has anticancer activity, and the proposed mechanism is the activation of a non-caspase proteolytic pathway. However, β-lapachone is also a potent IDO1 inhibitor with an IC50 of around 97 nM (Medzhitov, 2008), and thus β-lapachone is also likely to alter the tumor immune environment, contributing to the clearance of tumor cells. Saprorthoquinone, which was isolated from the traditional Chinese medicine, Salvia prionitis Hance, is cytotoxic and inhibits IDO1 with an IC50 of 1.76 μM (Lin et al., 2020). Similarly, dihydrotanshinone I, which was isolated from the traditional Chinese medicine Radix Salviae Miltiorrhizae, is cytotoxic against many types of cancer cells and inhibits IDO1 with an IC50 of 2.8 µM (Guo et al., 2020).
In summary, from the structure-activity relationship of the quinones and IDO1, we found that 1, 2- and 1, 4-quinone core moieties bind well to the heme in the active center of IDO1. Due to the structural priority and potent IDO1 inhibitory activity, many efforts have been dedicated to optimize quinones derived inhibitors, and any further substitutions to the quinone moiety need to be careful evaluated so that they improve rather than disrupt the binding activity (Austin et al., 2014; Carvalho et al., 2014; Centko et al., 2014; Blunt et al., 2015; Shiokawa et al., 2016; Feng et al., 2018; Pan et al., 2018; Yang et al., 2018; Zhang et al., 2018; Zhao et al., 2019; Kong et al., 2020). Considering the IDO1 inhibitory potency of these quinones, their pharmacological benefits, such as anti-inflammatory and anticancer activities, may arise partially from their interaction with IDO1.
3.2 Polyphenols
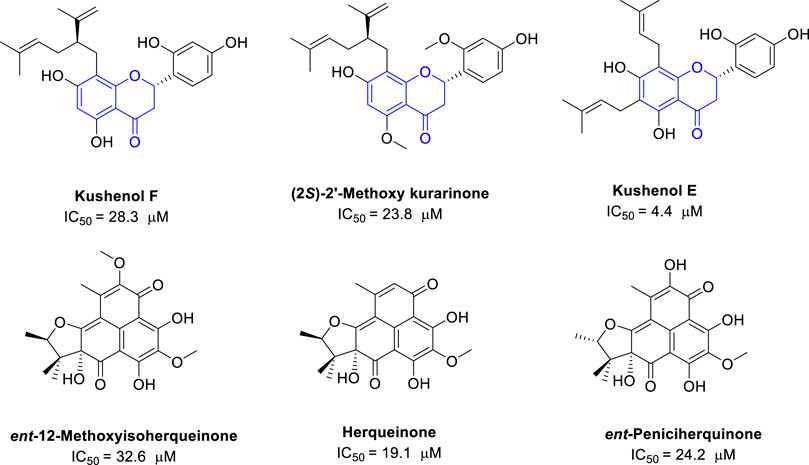
Polyphenols are a large family of natural compounds with various interesting biological activities. Some polyphenols were also found with potential anti-IDO1 activities. The plant Sophora flavescens, which contains many flavonoids, is used in traditional Chinese medicine to treat cancers. Screening for IDO1 inhibitors identified three flavonoids isolated from S. flavescens with moderate inhibition activity. Kushenol E has the most potent IDO1 inhibitory activity with an IC50 of 4.4 μM, followed by (2S)-2′-methoxy kurarinone (IC50 = 23.8 μM) and kushnol F (IC50 = 28.3 μM) (Kwon et al., 2019) (Figure 5). In addition, a Korean group also reported the polyphenols from the Hawaiian volcanic associated fungus Penicillium herquei FT729. Among them, herqueinone has an IC50 of 19.1 μM against IDO1, peniciherquinone inhibits IDO1 with IC50 of 24.2 μM, and ent-12-methoxyisoherqueinone has an IC50 of 32.6 μM against IDO1 (Yu et al., 2022). So far, the polyphenols only reported with moderate IDO1 inhibitory activities.
3.3 Alkaloids
Alkaloids are a structurally diverse class of naturally occurring bases containing at least one nitrogen atom. In this section, we list the alkaloids in the order of their structural simplicity (Figure 6). First, we describe two alkaloids that have some structural similarity to 1, 4-quinones. Tryptanthrin (indolo [2,1-b]quinazolin-6,12-dione) has been extracted from the Chinese medicinal plants Polygonum tinctorium and Isatis tinctoria, and it has various pharmacological activities, including cytotoxicity against several parasites and microorganisms, and it inhibits IDO1 with an IC50 of 7.15 μM (Yang et al., 2013). Tryptanthrin also has two carbonyl groups that point in opposite directions, which is structurally similar to 1, 4-quinones. In addition, NSC111041 was one of several natural compounds with interesting IDO1 inhibitory activity identified by the Mowat group among about 2800 compounds from the National Cancer Institute during a screening campaign for IDO and TDO inhibitors. NSC111041 inhibits IDO1 with Ki of 4.3 μM (Delfourne, 2012; Pantouris et al., 2014). In NSC111041, one of the quinone carbonyl groups is replaced with an imino group (Dolušić et al., 2013), and thus NSC111041 is structurally similar to 1, 4-quinones.
A library of indole analogues were screened for IDO1 inhibitors. Brassinin is an indole-based natural product with reported antifungal and anticancer activity, Brassinin has moderate to low IDO1 inhibitory activity (Ki = 97.7 µM) (Gaspari et al., 2006). Another indole-derived alkaloid is PQA26, which was isolated from the medicinal deciduous tree, Picrasma quassioides (D. Don) Benn, that is, widely grown in south China. The dry branches are used in traditional Chinese medicine for heat clearing, detoxification, and eliminating dampness. A virtual screening method suggested that PQA26 had IDO1 inhibition activity, and moderate IDO1 inhibitory activity (IC50 = 32 μM) was confirmed experimentally (Wang et al., 2019). 3-Deazaguanine was also identified as having IDO1 inhibitory activity in the micromolar range (Ki = 21.4 μM) (Pantouris and Mowat, 2014).
Berberine is a bitter, yellow natural compound, which is isolated from plants in the berberis genus and is an important ingredient in the traditional Chinese medicine, Oren-gedoku-to. Berberine can help to maintain a normal body weight and normal blood sugar levels. Furthermore, berberine has IDO1 inhibitory activity with an IC50 of 9.3 µM. Medicinal chemists have optimized the IDO1 inhibition activity of berberine further (Yu et al., 2010; Wang et al., 2018). Lately, a research group from China reported their isolation of fourteen novel and known alkaloids from the rhizomes of Sinomenium acutum. Among these alkaloids, lysicamine show an IDO1 inhibitory activity with IC50 values of 6.22 ± 0.26 μM (Bi et al., 2022).
Aminophenoxazinones are a group of natural dyes that includes actinomycines, which have antibiotic activity. Some aminophenoxazinones have IDO1 inhibitory activity. For example, cinnabarinic acid is a potent IDO1 inhibitor with an IC50 of 0.46 μM (Pasceri et al., 2013). Several alkaloids contain the cinnabarinic acid moiety. For example, plectosphaeroic acids A–C were isolated from the fungus Plectosphaerella cucumerina, which was cultured from marine sediment from Barkley Sound, British Columbia. These three alkaloids also contained the cinnabarinic acid moiety in their structures, and they are IDO1 inhibitors with an IC50 of 2 μM (Carr et al., 2009). The alkaloid stereoisomers albogrisin D and albogrisin D′ were isolated from Streptomyces albogriseolus MGR072 collected from a mangrove reserve in Fujian Province, China. These compounds have similar IDO1 inhibitory activities with IC50 values of around 10 μM (Gao et al., 2019).
3.4 Others
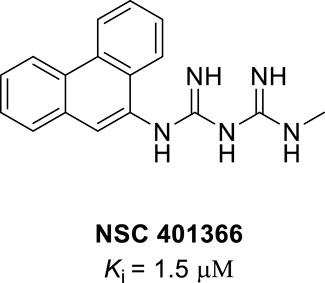
NSC401366 (N-methyl-N″-9-phenanthrylimidodicarbonimidic diamide) is a natural anthracene compound with potent IDO1 inhibition activity, which has a different structure from other IDO1 inhibitors (Figure 7). NSC401366 was discovered by screening the methanol extracts of marine organisms and has potent IDO1 inhibitory activity with a Ki of 1.5 μM (Vottero et al., 2006).
4 Conclusion
The balance in the levels of Trp and Kyn in plasma regulates several important physiological process, such as immune activation and immune tolerance. IDO1 is a key metabolic enzyme responsible for the metabolism of Trp to Kyn. The activation of IDO1 changes the concentration ratio between Trp and Kyn, and thus IDO1 is closely related to several important physiological disorders, including cancer, inflammation, and depression. Due to the importance of IDO1, IDO1 inhibitors have been the focus of intense interest in the pharmaceutical industry (Chen et al., 2021). Several candidate IDO1 inhibitors (Platten et al., 2019; Chen et al., 2021), including indoximod, navoximod, epacadostat, and linrodostat, have entered the clinical research stage for cancer immunotherapy.
Nature is an important source from which many therapeutic agents are obtained. Traditional medicine exploits the unique mode of actions of natural compounds to mitigate functional disorders. Modern medicine is exploring the mechanisms of many traditional medicines to optimize the pharmacological activity of these traditional medicines and mitigate the side effects. This kind of research is difficult because of the complexity of the mechanisms and unknown combined effects. However, it is helpful to begin by demonstrating the modes of action of individual natural compounds. Based on the structure-activity information, scientists can understand and analyze the pharmacological benefits of traditional medicine.
Thus, in this review we have summarized the interactions between IDO1, an important metabolic enzyme, and a group of natural compounds that have IDO1 inhibition activity. We have listed all the natural compounds reported so far to have moderate to strong IDO1 inhibitory activity, and the quinones are the most promising of these compounds. This finding provides structure-activity information that will help medicinal chemists to understand the pharmaceutical benefits of natural compounds (Ianni et al., 2022) and to design potent IDO1 inhibitors.
Author contributions
MR provides the main idea of the article; ML (2nd author) and YT wrote and revised the article, and they have the same contribution; ML (3rd author) and YC collected and sorted out the data.
Funding
This work was supported by 1) Key Technology Research and Development Program of Shan-dong, China (2021CXGC010511); 2) Project for development of TCM Science and Technology in Shandong Province (2019-0231) (M-2022145).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albini, E., Rosini, V., Gargaro, M., Mondanelli, G., Belladonna, M. L., Pallotta, M. T., et al. (2017). Distinct roles of immunoreceptor tyrosine-based motifs in immunosuppressive indoleamine 2, 3-dioxygenase 1. J. Cell. Mol. Med. 21 (1), 165–176. doi:10.1111/jcmm.12954
Alves de Souza, T. M., Fernandes-Santos, C., Araujo da Paixao de Oliveira, J., Tome, L. C. T., Fiestas-Solorzano, V. E., Nunes, P. C. G., et al. (2022). Increased indoleamine 2, 3-dioxygenase 1 (Ido-1) activity and inflammatory responses during chikungunya virus infection. Pathogens 11 (4), 444. doi:10.3390/pathogens11040444
Arneth, B. (2019). Tumor microenvironment. Med. Kaunas. Lith. 56 (1), E15. doi:10.3390/medicina56010015
Austin, C. J., Kahlert, J., Issa, F., Reed, J. H., Smith, J. R., Ioppolo, J. A., et al. (2014). The first indoleamine-2, 3-dioxygenase-1 (Ido1) inhibitors containing carborane, Dalton Trans., 43, 10719–10724. doi:10.1039/c4dt00444b
Badawy, A. A., and Morgan, C. J. (1991). Effects of acute paroxetine administration on tryptophan metabolism and disposition in the rat. Br. J. Pharmacol. 102 (2), 429–433. doi:10.1111/j.1476-5381.1991.tb12190.x
Bakmiwewa, S. M., Fatokun, A. A., Tran, A., Payne, R. J., Hunt, N. H., and Ball, H. J. (2012). Identification of selective inhibitors of indoleamine 2, 3-dioxygenase 2. Bioorg. Med. Chem. Lett. 22 (24), 7641–7646. doi:10.1016/j.bmcl.2012.10.010
Ball, H. J., Sanchez-Perez, A., Weiser, S., Austin, C. J., Astelbauer, F., Miu, J., et al. (2007). Characterization of an indoleamine 2, 3-dioxygenase-like protein found in humans and mice. Gene 396 (1), 203–213. doi:10.1016/j.gene.2007.04.010
Barreto, F. S., Chaves Filho, A. J. M., de Araújo, M., de Moraes, M. O., de Moraes, M. E. A., Maes, M., et al. (2018). Tryptophan catabolites along the indoleamine 2, 3-dioxygenase pathway as a biological link between depression and cancer. Behav. Pharmacol. 29, 165–180. doi:10.1097/FBP.0000000000000384
Baumgartner, R., Forteza, M. J., and Ketelhuth, D. F. J. (2019). The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 122, 154148. doi:10.1016/j.cyto.2017.09.004
Bi, R., Yang, X. N., Zhou, H. F., Peng, L. Y., Liu, J. X., and Zhao, Q. S. (2022). Eleven undescribed alkaloids from the rhizomes of Sinomenium acutum and their Ido1 and TDO inhibitory activities. Phytochemistry 200, 113244. doi:10.1016/j.phytochem.2022.113244
Blunt, C. E., Torcuk, C., Liu, Y., Lewis, W., Siegel, D., Ross, D., et al. (2015). Synthesis and intracellular redox cycling of natural quinones and their analogues and identification of indoleamine-2, 3-dioxygenase (Ido) as potential target for anticancer activity. Angew. Chem. Int. Ed. Engl. 54 (30), 8740–8745. doi:10.1002/anie.201503323
Carr, G., Chung, M. K., Mauk, A. G., and Andersen, R. J. (2008). Synthesis of indoleamine 2, 3-dioxygenase inhibitory analogues of the sponge alkaloid exiguamine A. J. Med. Chem. 51 (9), 2634–2637. doi:10.1021/jm800143h
Carr, G., Tay, W., Bottriell, H., Andersen, S. K., Mauk, A. G., and Andersen, R. J. (2009). Plectosphaeroic acids A, B, and C, indoleamine 2, 3-dioxygenase inhibitors produced in culture by a marine isolate of the fungus Plectosphaerella cucumerina. Org. Lett. 11 (14), 2996–2999. doi:10.1021/ol900972j
Carvalho, C., Siegel, D., Inman, M., Xiong, R., Ross, D., and Moody, C. J. (2014). Benzofuranquinones as inhibitors of indoleamine 2, 3-dioxygenase (Ido). Synthesis and biological evaluation. Org. Biomol. Chem. 12 (17), 2663–2674. doi:10.1039/c3ob42258e
Centko, R. M., Steinø, A., Rosell, F. I., Patrick, B. O., de Voogd, N., Mauk, A. G., et al. (2014). Indoleamine 2, 3-dioxygenase inhibitors isolated from the sponge xestospongia vansoesti: Structure elucidation, analogue synthesis, and biological activity. Org. Lett. 16 (24), 6480–6483. doi:10.1021/ol503337f
Cervenka, I., Agudelo, L. Z., and Ruas, J. L. (2017). Kynurenines: Tryptophan's metabolites in exercise, inflammation, and mental health, Science, 357. eaaf9794. doi:10.1126/science.aaf9794
Chen, S., Tan, J., and Zhang, A. (2021). The ups, downs and new trends of Ido1 inhibitors. Bioorg. Chem. 110, 104815. doi:10.1016/j.bioorg.2021.104815
Delfourne, E. (2012). Marine natural products and other derivatives as potent indoleamine 2, 3-dioxygenase inhibitors. Mini Rev. Med. Chem. 12 (10), 988–996. doi:10.2174/138955712802762374
Ding, Y., Tang, F., Xue, X., Luo, J., Hussain, M., Huang, Y., et al. (2019). Rational design, synthesis and biological evaluation of ubiquinone derivatives as Ido1 inhibitors. Bioorg. Chem. 89, 102870. doi:10.1016/j.bioorg.2019.03.044
Dolšak, A., Gobec, S., and Sova, M. (2021). Indoleamine and tryptophan 2, 3-dioxygenases as important future therapeutic targets. Pharmacol. Ther. 221, 107746. doi:10.1016/j.pharmthera.2020.107746
Dolušić, E., Larrieu, P., Meinguet, C., Colette, D., Rives, A., Blanc, S., et al. (2013). Indoleamine 2, 3-dioxygenase inhibitory activity of derivatives of marine alkaloid tsitsikammamine A. Bioorg. Med. Chem. Lett. 23 (1), 47–54. doi:10.1016/j.bmcl.2012.11.036
Dolusić, E., Larrieu, P., Moineaux, L., Stroobant, V., Pilotte, L., Colau, D., et al. (2011). Tryptophan 2, 3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J. Med. Chem. 54 (15), 5320–5334. doi:10.1021/jm2006782
Dong, J., Pan, X., Yang, Y., Zhang, G., Xiao, Z., and Liu, Z. (2021). Design, synthesis and biological evaluation of exiguamine A analogues as Ido1 inhibitors. Eur. J. Med. Chem. 223, 113631. doi:10.1016/j.ejmech.2021.113631
Dounay, A. B., Tuttle, J. B., and Verhoest, P. R. (2015). Challenges and opportunities in the discovery of new therapeutics targeting the kynurenine pathway. J. Med. Chem. 58 (22), 8762–8782. doi:10.1021/acs.jmedchem.5b00461
Esmaeili, S. A., and Hajavi, J. (2022). The role of indoleamine 2, 3-dioxygenase in allergic disorders. Mol. Biol. Rep. 49 (4), 3297–3306. doi:10.1007/s11033-021-07067-5
Feng, X., Qiu, X., Huang, H., Wang, J., Xu, X., Xu, P., et al. (2018). Palladium(II)-Catalyzed reaction of lawsones and propargyl carbonates: Construction of 2, 3-furanonaphthoquinones and evaluation as potential indoleamine 2, 3-dioxygenase inhibitors. J. Org. Chem. 83 (15), 8003–8010. doi:10.1021/acs.joc.8b00872
Fukunaga, M., Yamamoto, Y., Kawasoe, M., Arioka, Y., Murakami, Y., Hoshi, M., et al. (2012). Studies on tissue and cellular distribution of indoleamine 2, 3-dioxygenase 2: The absence of Ido1 upregulates Ido2 expression in the epididymis. J. Histochem. Cytochem. 60 (11), 854–860. doi:10.1369/0022155412458926
Gao, D., Zhou, T., Da, L. T., Bruhn, T., Guo, L. L., Chen, Y. H., et al. (2019). Characterization and nonenzymatic transformation of three types of alkaloids from Streptomyces albogriseolus MGR072 and discovery of inhibitors of indoleamine 2, 3-dioxygenase. Org. Lett. 21 (21), 8577–8581. doi:10.1021/acs.orglett.9b03149
Gargaro, M., Scalisi, G., Manni, G., Briseno, C. G., Bagadia, P., Durai, V., et al. (2022). Indoleamine 2, 3-dioxygenase 1 activation in mature cDC1 promotes tolerogenic education of inflammatory cDC2 via metabolic communication. Immunity 55 (6), 1032–1050.e14. doi:10.1016/j.immuni.2022.05.013
Gaspari, P., Banerjee, T., Malachowski, W. P., Muller, A. J., Prendergast, G. C., DuHadaway, J., et al. (2006). Structure-activity study of brassinin derivatives as indoleamine 2, 3-dioxygenase inhibitors. J. Med. Chem. 49 (2), 684–692. doi:10.1021/jm0508888
Gouasmi, R., Ferraro-Peyret, C., Nancey, S., Coste, I., Renno, T., Chaveroux, C., et al. (2022). The kynurenine pathway and cancer: Why keep it simple when you can make it complicated. Cancers (Basel) 14 (11), 2793. doi:10.3390/cancers14112793
Guo, W., Yao, S., Sun, P., Yang, T. B., Tang, C. P., Zheng, M. Y., et al. (2020). Discovery and characterization of natural products as novel indoleamine 2, 3-dioxygenase 1 inhibitors through high-throughput screening. Acta Pharmacol. Sin. 41 (3), 423–431. doi:10.1038/s41401-019-0246-4
Healy, D., and Leonard, B. E. (1987). Monoamine transport in depression: Kinetics and dynamics. J. Affect. Disord. 12 (2), 91–103. doi:10.1016/0165-0327(87)90001-2
Heidari, F., Ramezani, A., Erfani, N., and Razmkhah, M. (2020). Indoleamine 2, 3-dioxygenase: A professional immunomodulator and its potential functions in immune related diseases. Int. Rev. Immunol. 41, 346–363. doi:10.1080/08830185.2020.1836176
Huang, X., Zhang, F., Wang, X., and Liu, K. (2022). The role of indoleamine 2, 3-dioxygenase 1 in regulating tumor microenvironment. Cancers (Basel) 14 (11), 2756. doi:10.3390/cancers14112756
Huang, Y. S., Ogbechi, J., Clanchy, F. I., Williams, R. O., and Stone, T. W. (2020). Ido and kynurenine metabolites in peripheral and CNS disorders. Front. Immunol. 11, 388. doi:10.3389/fimmu.2020.00388
Ianni, F., Volpi, C., Moretti, S., Blasi, F., Mondanelli, G., Varfaj, I., et al. (2022). In-depth characterization of phenolic profiling of Moraiolo extra-virgin olive oil extract and initial investigation of the inhibitory effect on Indoleamine-2, 3-Dioxygenase (Ido1) enzyme. J. Pharm. Biomed. Anal. 213, 114688. doi:10.1016/j.jpba.2022.114688
John, S., Thangapandian, S., Sakkiah, S., and Lee, K. W. (2010). Identification of potent virtual leads to design novel indoleamine 2, 3-dioxygenase inhibitors: Pharmacophore modeling and molecular docking studies. Eur. J. Med. Chem. 45 (9), 4004–4012. doi:10.1016/j.ejmech.2010.05.057
Kong, K. M., Zhang, J. W., Liu, B. Z., Meng, G. R., and Zhang, Q. (2020). Discovery of 5-(pyridin-3-yl)-1H-indole-4, 7-diones as indoleamine 2, 3-dioxygenase 1 (Ido1) inhibitors. Bioorg. Med. Chem. Lett. 30 (4), 126901. doi:10.1016/j.bmcl.2019.126901
Krupa, A., Krupa, M. M., and Pawlak, K. (2022). Indoleamine 2, 3 dioxygenase 1-the potential link between the innate immunity and the ischemia-reperfusion-induced acute kidney injury? Int. J. Mol. Sci. 23 (11), 6176. doi:10.3390/ijms23116176
Kumar, S., Malachowski, W. P., DuHadaway, J. B., LaLonde, J. M., Carroll, P. J., Jaller, D., et al. (2008). Indoleamine 2, 3-dioxygenase is the anticancer target for a novel series of potent naphthoquinone-based inhibitors. J. Med. Chem. 51 (6), 1706–1718. doi:10.1021/jm7014155
Kwon, M., Ko, S. K., Jang, M., Kim, G. H., Ryoo, I. J., Son, S., et al. (2019). Inhibitory effects of flavonoids isolated from Sophora flavescens on indoleamine 2, 3-dioxygenase 1 activity. J. Enzyme Inhib. Med. Chem. 34 (1), 1481–1488. doi:10.1080/14756366.2019.1640218
Lewis-Ballester, A., Pham, K. N., Batabyal, D., Karkashon, S., Bonanno, J. B., Poulos, T. L., et al. (2017). Structural insights into substrate and inhibitor binding sites in human indoleamine 2, 3-dioxygenase 1. Nat. Commun. 8 (1), 1693. doi:10.1038/s41467-017-01725-8
Lin, Y., Zhang, H., Niu, T., Tang, M. L., and Chang, J. (2020). Discovery of novel indoleamine 2, 3-dioxygenase 1 (Ido1) and histone deacetylase 1 (HDAC1) dual inhibitors derived from the natural product saprorthoquinone. Mol. (Basel, Switz. 25 (19), E4494. doi:10.3390/molecules25194494
Ling, W., Zhang, J., Yuan, Z., Ren, G., Zhang, L., Chen, X., et al. (2014). Mesenchymal stem cells use Ido to regulate immunity in tumor microenvironment. Cancer Res. 74 (5), 1576–1587. doi:10.1158/0008-5472.CAN-13-1656
Liu, X. Q., and Wang, X. (2009). Indoleamine 2, 3-dioxygenase in tumor induced tolerance. Chin. Med. J. 122 (24), 3072–3077.
Löb, S., Königsrainer, A., Rammensee, H. G., Opelz, G., and Terness, P. (2009). Inhibitors of indoleamine-2, 3-dioxygenase for cancer therapy: Can we see the wood for the trees? Nat. Rev. Cancer 9 (6), 445–452. doi:10.1038/nrc2639
Maddison, D. C., and Giorgini, F. (2015). The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 40, 134–141. doi:10.1016/j.semcdb.2015.03.002
Medzhitov, R. (2008). Origin and physiological roles of inflammation. Nature 454 (7203), 428–435. doi:10.1038/nature07201
Merlo, L. M. F., Peng, W., and Mandik-Nayak, L. (2022). Impact of Ido1 and Ido2 on the B cell immune response. Front. Immunol. 13, 886225. doi:10.3389/fimmu.2022.886225
Metz, R., Rust, S., Duhadaway, J. B., Mautino, M. R., Munn, D. H., Vahanian, N. N., et al. (2012). Ido inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel Ido effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 1 (9), 1460–1468. doi:10.4161/onci.21716
Munn, D. H., and Mellor, A. L. (2016). Ido in the tumor microenvironment: Inflammation, counter-regulation, and tolerance. Trends Immunol. 37 (3), 193–207. doi:10.1016/j.it.2016.01.002
Munn, D. H., Sharma, M. D., Baban, B., Harding, H. P., Zhang, Y., Ron, D., et al. (2005). GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2, 3-dioxygenase. Immunity 22 (5), 633–642. doi:10.1016/j.immuni.2005.03.013
Odunsi, K., Qian, F., Lugade, A. A., Yu, H., Geller, M. A., Fling, S. P., et al. (2022). Metabolic adaptation of ovarian tumors in patients treated with an Ido1 inhibitor constrains antitumor immune responses. Sci. Transl. Med. 14 (636), eabg8402. doi:10.1126/scitranslmed.abg8402
Ogbechi, J., Clanchy, F. I., Huang, Y. S., Topping, L. M., Stone, T. W., and Williams, R. O. (2020). Ido activation, inflammation and musculoskeletal disease. Exp. Gerontol. 131, 110820. doi:10.1016/j.exger.2019.110820
Ouyang, L., Yu, C., Xie, Z., Su, X., Xu, Z., Song, P., et al. (2022). Indoleamine 2, 3-dioxygenase 1 deletion-mediated kynurenine insufficiency in vascular smooth muscle cells exacerbates arterial calcification. Circulation 145 (24), 1784–1798. doi:10.1161/CIRCULATIONAHA.121.057868
Pan, L., Zheng, Q., Chen, Y., Yang, R., Yang, Y., Li, Z., et al. (2018). Design, synthesis and biological evaluation of novel naphthoquinone derivatives as Ido1 inhibitors. Eur. J. Med. Chem. 157, 423–436. doi:10.1016/j.ejmech.2018.08.013
Pantouris, G., and Mowat, C. G. (2014). Antitumour agents as inhibitors of tryptophan 2, 3-dioxygenase. Biochem. Biophys. Res. Commun. 443 (1), 28–31. doi:10.1016/j.bbrc.2013.11.037
Pantouris, G., Serys, M., Yuasa, H. J., Ball, H. J., and Mowat, C. G. (2014). Human indoleamine 2, 3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2, 3-dioxygenase-1. Amino acids 46 (9), 2155–2163. doi:10.1007/s00726-014-1766-3
Pasceri, R., Siegel, D., Ross, D., and Moody, C. J. (2013). Aminophenoxazinones as inhibitors of indoleamine 2, 3-dioxygenase (Ido). Synthesis of exfoliazone and chandrananimycin A. J. Med. Chem. 56 (8), 3310–3317. doi:10.1021/jm400049z
Pereira, A., Vottero, E., Roberge, M., Mauk, A. G., and Andersen, R. J. (2006). Indoleamine 2, 3-dioxygenase inhibitors from the northeastern pacific marine hydroid Garveia annulata. J. Nat. Prod. 69 (10), 1496–1499. doi:10.1021/np060111x
Pham, K. N., Lewis-Ballester, A., and Yeh, S. R. (2019). Structural basis of inhibitor selectivity in human indoleamine 2, 3-dioxygenase 1 and tryptophan dioxygenase. J. Am. Chem. Soc. 141 (47), 18771–18779. doi:10.1021/jacs.9b08871
Platten, M., Friedrich, M., Wainwright, D. A., Panitz, V., and Opitz, C. A. (2021). Tryptophan metabolism in brain tumors - Ido and beyond. Curr. Opin. Immunol. 70, 57–66. doi:10.1016/j.coi.2021.03.005
Platten, M., Nollen, E. A. A., Röhrig, U. F., Fallarino, F., and Opitz, C. A. (2019). Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 18 (5), 379–401. doi:10.1038/s41573-019-0016-5
Romani, L., Fallarino, F., De Luca, A., Montagnoli, C., D'Angelo, C., Zelante, T., et al. (2008). Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451 (7175), 211–215. doi:10.1038/nature06471
Salminen, A. (2022). Role of indoleamine 2, 3-dioxygenase 1 (Ido1) and kynurenine pathway in the regulation of the aging process. Ageing Res. Rev. 75, 101573. doi:10.1016/j.arr.2022.101573
Santos, H., Matheus, L. H. G., Silva, A., Dalmazzo, S. V., Santos, A. A., Santos, L., et al. (2022). Indoleamine 2, 3-dioxygenase-1 expression is changed during bladder cancer cell invasion. Int. J. Tryptophan Res. 15, 11786469211065612. doi:10.1177/11786469211065612
Schröcksnadel, K., Wirleitner, B., Winkler, C., and Fuchs, D. (2006). Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 364 (1-2), 82–90. doi:10.1016/j.cca.2005.06.013
Shiokawa, Z., Kashiwabara, E., Yoshidome, D., Fukase, K., Inuki, S., and Fujimoto, Y. (2016). Discovery of a novel scaffold as an indoleamine 2, 3-dioxygenase 1 (Ido1) inhibitor based on the pyrrolopiperazinone alkaloid, longamide B. ChemMedChem 11 (24), 2682–2689. doi:10.1002/cmdc.201600446
Sorgdrager, F. J. H., Naudé, P. J. W., Kema, I. P., Nollen, E. A., and Deyn, P. P. (2019). Tryptophan metabolism in inflammaging: From biomarker to therapeutic target. Front. Immunol. 10, 2565. doi:10.3389/fimmu.2019.02565
Sugimoto, H., Oda, S., Otsuki, T., Hino, T., Yoshida, T., and Shiro, Y. (2006). Crystal structure of human indoleamine 2, 3-dioxygenase: Catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 103 (8), 2611–2616. doi:10.1073/pnas.0508996103
Takikawa, O. (2005). Biochemical and medical aspects of the indoleamine 2, 3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 338 (1), 12–19. doi:10.1016/j.bbrc.2005.09.032
Tone, S., Takikawa, O., Habara-Ohkubo, A., Kadoya, A., Yoshida, R., and Kido, R. (1990). Primary structure of human indoleamine 2, 3-dioxygenase deduced from the nucleotide sequence of its cDNA. Nucleic Acids Res. 18 (2), 367. doi:10.1093/nar/18.2.367
Turunen, M., Olsson, J., and Dallner, G. (2004). Metabolism and function of coenzyme Q. Biochim. Biophys. Acta 1660 (1-2), 171–199. doi:10.1016/j.bbamem.2003.11.012
Uyttenhove, C., Pilotte, L., Théate, I., Stroobant, V., Colau, D., Parmentier, N., et al. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2, 3-dioxygenase. Nat. Med. 9 (10), 1269–1274. doi:10.1038/nm934
Vécsei, L., Szalárdy, L., Fülöp, F., and Toldi, J. (2013). Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 12 (1), 64–82. doi:10.1038/nrd3793
Vottero, E., Balgi, A., Woods, K., Tugendreich, S., Melese, T., Andersen, R. J., et al. (2006). Inhibitors of human indoleamine 2, 3-dioxygenase identified with a target-based screen in yeast. Biotechnol. J. 1 (3), 282–288. doi:10.1002/biot.200600001
Wang, N., Zhang, J., Li, Q., Xu, H., Chen, G., Li, Z., et al. (2019). Discovery of potent indoleamine 2, 3-dioxygenase (Ido) inhibitor from alkaloids in Picrasma quassioides by virtual screening and in vitro evaluation. Fitoterapia 133, 137–145. doi:10.1016/j.fitote.2019.01.005
Wang, Q., Liu, D., Song, P., and Zou, M. H. (2015). Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. 20, 1116–1143. doi:10.2741/4363
Wang, Y. X., Pang, W. Q., Zeng, Q. X., Deng, Z. S., Fan, T. Y., Jiang, J. D., et al. (2018). Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting Ido1. Eur. J. Med. Chem. 143, 1858–1868. doi:10.1016/j.ejmech.2017.10.078
Wu, T., and Dai, Y. (2017). Tumor microenvironment and therapeutic response. Cancer Lett. 387, 61–68. doi:10.1016/j.canlet.2016.01.043
Yang, R., Chen, Y., Pan, L., Yang, Y., Zheng, Q., Hu, Y., et al. (2018). Design, synthesis and structure-activity relationship study of novel naphthoindolizine and indolizinoquinoline-5, 12-dione derivatives as Ido1 inhibitors. Bioorg. Med. Chem. 26 (17), 4886–4897. doi:10.1016/j.bmc.2018.08.028
Yang, S., Li, X., Hu, F., Li, Y., Yang, Y., Yan, J., et al. (2013). Discovery of tryptanthrin derivatives as potent inhibitors of indoleamine 2, 3-dioxygenase with therapeutic activity in Lewis lung cancer (LLC) tumor-bearing mice. J. Med. Chem. 56 (21), 8321–8331. doi:10.1021/jm401195n
Yu, C. J., Zheng, M. F., Kuang, C. X., Huang, W. D., and Yang, Q. (2010). Oren-gedoku-to and its constituents with therapeutic potential in Alzheimer's disease inhibit indoleamine 2, 3-dioxygenase activity in vitro. J. Alzheimers Dis. 22 (1), 257–266. doi:10.3233/JAD-2010-100684
Yu, J. S., Jeong, S. Y., Li, C., Oh, T., Kwon, M., Ahn, J. S., et al. (2022). New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2, 3-dioxygenase 1 (Ido1). Arch. Pharm. Res. 45 (2), 105–113. doi:10.1007/s12272-022-01372-8
Zhai, L., Bell, A., Ladomersky, E., Lauing, K. L., Bollu, L., Sosman, J. A., et al. (2020). Immunosuppressive Ido in cancer: Mechanisms of action, animal models, and targeting strategies. Front. Immunol. 11, 1185. doi:10.3389/fimmu.2020.01185
Zhang, H., Liu, W., Liu, Z., Ju, Y., Xu, M., Zhang, Y., et al. (2018). Discovery of indoleamine 2, 3-dioxygenase inhibitors using machine learning based virtual screening. MedChemComm 9 (6), 937–945. doi:10.1039/c7md00642j
Zhang, L., Zhang, G., Xu, S., and Song, Y. (2021). Recent advances of quinones as a privileged structure in drug discovery. Eur. J. Med. Chem. 223, 113632. doi:10.1016/j.ejmech.2021.113632
Zhang, Y., Hu, Z., Zhang, J., Ren, C., and Wang, Y. (2022). Dual-target inhibitors of indoleamine 2, 3 dioxygenase 1 (Ido1): A promising direction in cancer immunotherapy. Eur. J. Med. Chem. 238, 114524. doi:10.1016/j.ejmech.2022.114524
Keywords: indoleamine 2, 3-dioxygenase 1, tryptophan, kynurenine, IDO1 inhibitors, natural compounds
Citation: Tan Y, Liu M, Li M, Chen Y and Ren M (2022) Indoleamine 2, 3-dioxygenase 1 inhibitory compounds from natural sources. Front. Pharmacol. 13:1046818. doi: 10.3389/fphar.2022.1046818
Received: 20 September 2022; Accepted: 24 October 2022;
Published: 04 November 2022.
Edited by:
Ciriana Orabona, University of Perugia, ItalyReviewed by:
Samina Bano, University of Karachi, PakistanEleonora Panfili, University of Perugia, Italy
Copyright © 2022 Tan, Liu, Li, Chen and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Ren, bXJybUAxNjMuY29t
†These authors have contributed equally to this work
 Ying Tan1†
Ying Tan1† Meng Ren
Meng Ren