- 1Key Laboratory of Theory of TCM, Ministry of Education of China, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Institute of Traditional Chinese Medicine Literature and Culture, Shandong University of Traditional Chinese Medicine, Jinan, China
Salvia miltiorrhiza Bunge (Lamiaceae) is a perennial herb widely found in China since ancient times with a high economic and medicinal value. Salvianolic acid B (Sal-B) is an important natural product derived from Salvia miltiorrhiza and this review summarizes the anticancer activity of Sal-B. Sal-B inhibits tumor growth and metastasis by targeting multiple cell signaling pathways. This review aims to review experimental studies to describe the possible anticancer mechanisms of Sal-B and confirm its potential as a therapeutic drug.
Introduction
Salvia miltiorrhiza Bunge (Lamiaceae) is a traditional Chinese herb that has long been used to effectively promote blood circulation and remove blood stasis (Bachheti et al., 2022; Huang et al., 2022). It has been widely used in China for thousands of years to treat various diseases, including coronary heart disease, myocardial infarction, angina pectoris, and atherosclerosis (Wang et al., 2017; Jia et al., 2019; Jin et al., 2022a). Scientists have researched and developed the Fufang Danshen Dripping Pill, a major innovation in TCM, to facilitate administration and absorption by humans (Hu and Wang, 2019). It is also the first compound TCM drug to pass second-phase United States Food and Drug Administration human clinical trials (Xu et al., 2014).
To date, dozens of lipophilic compounds of Salvia miltiorrhiza Bunge (Lamiaceae) have been identified, such as tanshinones IVI, and salvianphenol A (Jin et al., 2022b), as well as hydrophilic compounds including salvianolic acid A (Sal-A), salvianolic acid B (Sal-B), and protocatechuic aldehyde (Chang et al., 2022; Zhou et al., 2022). The most abundant hydrophilic compounds in Salvia are the phenolic acids (Hong et al., 2022; Jayusman et al., 2022), which are important for human health and are attracting increasing attention (Li et al., 2022). Sal-B exhibits anticancer activity in a variety of cell lines, including prostate, breast, liver, and head and neck squamous cell cancers.
This review summarizes advances in the extensive literature elucidating the antitumor effects and mechanisms of Sal-B compounds in various cancers, providing a reference for research and clinical application. Although several researchers have analyzed the important role of salvianolic acid B in the treatment and prevention of cardiovascular diseases, the anticancer properties of salvianolic acid have not been summarized. Therefore, this review aims to present the anticancer potential of salvianolic acid B and its mechanism of action to provide more information regarding this natural component of herbal medicine.
Cancer and chemoprevention
The main cause of cancer development is the dysfunction of autophagy encoded by various genes, such as tumor suppressors, anti-apoptotic proteins, and growth factors, which allow unrestricted cell proliferation (Chen et al., 2022a; Shen et al., 2022). Patients with early-stage cancer are at risk of distant metastasis. Furthermore, multiple cellular and genetic alterations in the normal epithelium take many years to occur, leading to malignant changes. Therefore, the development of effective, less toxic, and affordable novel pharmacological agents to prevent cancer development is important.
Chemoprevention is a powerful method to prevent or slow down cancer progression (Acquaviva et al., 2022). In addition, many herbal medicines and related active compounds with potent anticancer activity, such as matrine and honokiol have been used as prophylactic agents. Current studies have shown that the anticancer properties of matrine are closely related to inhibition of proliferation and induction of apoptosis. Matrine induces apoptosis in U937 cells and K562 cells through a cytochrome c-triggered caspase-activated mitochondrial pathway (Zhou et al., 2014) but induces toxicity in mouse hepatocytes and its mechanism of action is dependent on reactive oxygen species (ROS) (Liu et al., 2020). Honokiol inhibits the growth and induced apoptosis in HNSCC cell lines and enhanced the growth inhibition and anti-invasive activity of erlotinib, a tyrosine kinase inhibitor (TKI) targeting EGFR (Leeman-Neill et al., 2010). However, these promising activities did not translate into clinical success despite these herbal active ingredients having tremendous potential medicinal properties.
Salvianolic acid B
Sal-B is the most abundant and biologically active hydrophilic component of Salvia miltiorrhiza. According to the Chinese Pharmacopoeia (National Pharmacopoeia Committee, 2020), Sal-B is one of the important reference components for the quality standard of the traditional Chinese medicine Salvia miltiorrhiza. Sal-B contains seven phenolic hydroxyl radicals which have antioxidant activity (Wang et al., 2007) and its structure is shown in Figure 1. Sal-B is of increasing interest to researchers due to its preventive and therapeutic value for cancer as well as cardiovascular and neurodegenerative diseases (Liu et al., 2000). The mechanism is mainly due to its anti-inflammatory and antioxidant properties, regulation of apoptosis, and inhibition of platelet aggregation (Zhao et al., 2011). Sal-B also has therapeutic effects on a variety of cancers, such as lung carcinoma, breast cancer, oral squamous cell carcinoma, head and neck carcinoma, hepatocellular cancer, and glioma cancer cell lines (Kiemlian Kwee, 2016; Khan et al., 2020; Guan et al., 2022).
Sal-B inhibits human hepatocellular carcinoma cell viability
Hepatocellular carcinoma is a major cause of mortality (Riaz et al., 2022; Xia et al., 2022), and hepatitis B and C viruses are major contributors to hepatocellular carcinoma. Unfortunately, most HCC patients are diagnosed at a late stage, thus surgery is not a treatment option (Khafaga et al., 2022; Wei et al., 2022). Traditional chemotherapy is important for cancer patients who are unable to undergo surgery but some current chemotherapy drugs have low response rates and side effects in hepatocellular carcinoma, so there is an urgent need to develop new drugs (Chun, 2022; Udoh et al., 2022). Recently, the anticancer effects of Sal-B have been demonstrated in human cancer cell lines and in vitro studies have shown that Sal-B induces cell death and promotes apoptosis.
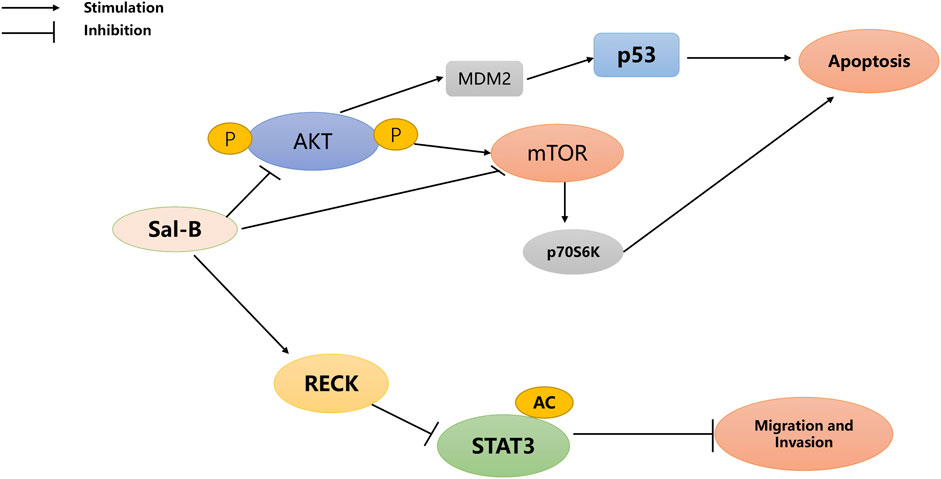
Fu et al. (2021) found a key factor in the ability of Sal-B to induce cell death is the promotion of autophagy and apoptosis in tumor cells. Moreover, Gong et al. showed that Sal-B-induced cell death was associated with AKT/mTOR signaling inhibition (Gong et al., 2016). Teng et al. suggested that Sal-B could be a potential anticancer agent for the treatment of HCC (Teng et al., 2021) (Figure 2). Furthermore, Hillmer et al. reported that Sal-B specifically bound to mortalin and increased the degradation of mortalin proteasomes through ubiquitination, thereby upregulating RECK, inhibiting STAT3, and finally inhibiting the migration and invasion of HCC cells (Hillmer et al., 2016).
(Reversion-inducing cysteine-rich protein with Kazal motifs, RECK; Signal transducer and activator of transcription 3, STAT3; protein kinase B, AKT; mammalian target of rapamycin, mTOR; murine double minute2, MDM2).
A high potency against breast cancer
Breast cancer is the most common female cancer (Di Modica et al., 2022; Zhang H. et al., 2022), with a mortality rate second only to that of lung cancer, especially since the International Agency for Research on Cancer recently found that the mortality rate for breast cancer has now gradually surpassed that of lung cancer (Pearanpan et al., 2022; Yamashita and Kufe, 2022). Triple-negative breast cancer is highly malignant and difficult to treat (Hacking et al., 2022; Montalvo-Castro and Salinas-Jazmín, 2022). Although chemotherapy, radiation therapy, and systemic immunotherapy have led to longer survival, some patients with advanced breast cancer develop metastatic cancer (Gautam et al., 2022; Karn et al., 2022). Furthermore, the development and research of novel drugs to control metastasis remain a great challenge.
Sha et al. revealed that Sal-B can potently inhibit the growth of cultured triple-negative breast cancer cells via a ceramide-mediated pathway (Sha et al., 2018). Sal-B enhances apoptosis and reduces cell proliferation in TNBC by regulating ceramide glycosylase. Moreover, Sal-B has certain therapeutic advantages over current chemotherapeutic drugs (Murugan et al., 2016) as it is less toxic and dose-dependently induced apoptosis of MCF-7 in breast cancer cells (Katary et al., 2019). Furthermore, Qian et al. demonstrated that Sal-B reduced the level of the oxidative stress marker malondialdehyde and increased the plasma level of the antioxidant marker glutathione (GSH), thereby significantly reducing the tumor volume and increasing the median overall survival of solid cancer cells in mice (Qian et al., 2022). Ding et al. synthesized an FA-PEG-TiO2 nanocarrier to load Cur and Sal-B, as it acts synergistically with curcumin for an antitumor effect (Ding et al., 2016). The anticancer mechanism of Sal-B is summarized in Figure 3.
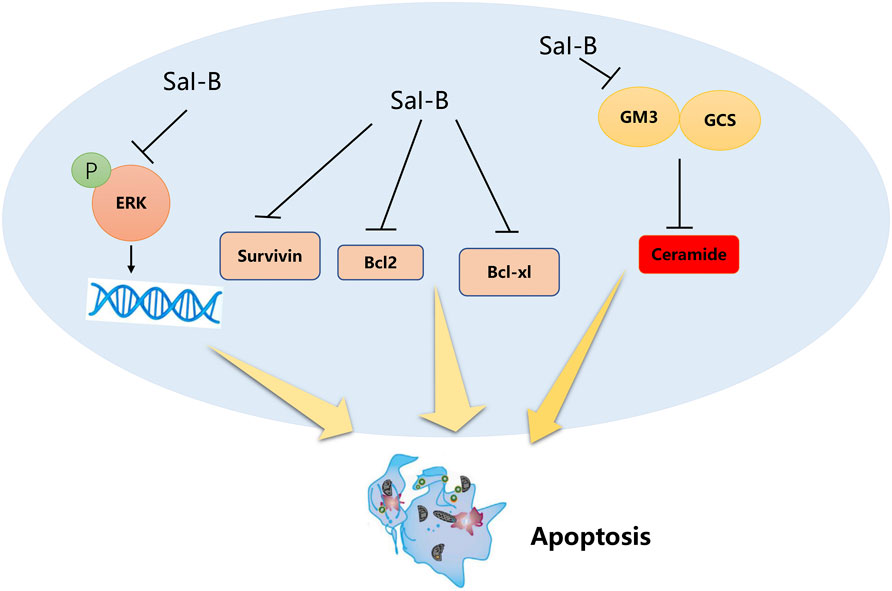
FIGURE 3. Sal-B inhibits Bcl-2, Bcl-xl, Survivin, glucosylceramide, and GM3 synthase expression, inducing ceramide-mediated apoptosis in breast cancer cells (Extracellular regulated protein kinases, ERK; B-cell lymphoma-2, Bcl-2; B-cell lymphoma–extra-large, Bcl-xl; ganglioside M3, GM3; glucosylceramide, GCS).
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) are a scourge on human health (Johnson et al., 2020), and the main strategy to reduce its morbidity and mortality is the early diagnosis (Solomon et al., 2018). The chronic inflammatory microenvironment induces the transformation of normal cells into cancer cells (Yokota et al., 2020; Aulakh et al., 2022), the proliferation of tumor cells, and gene mutations (Park et al., 2022). Current treatments for HNSCC have limited therapeutic outcomes, thus it is important to develop novel pharmacological agents.
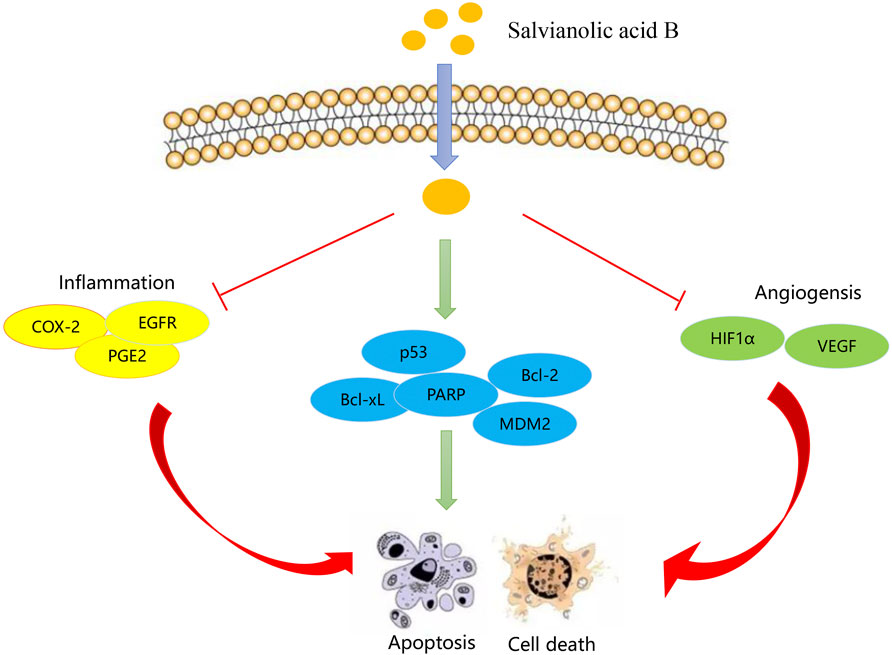
There is growing evidence that Sal-B is a promising chemotherapeutic agent for HNSCC (Kan et al., 2014). Cao et al. (2012) showed that Sal-B can block angiogenesis, thus preventing the transformation of normal epithelial cells into cancer cells and Zhao et al. (2010) found that Sal-B inhibited COX-2 expression in HNSCC cells of different origins. In addition, Sal-B can sequentially inhibit the COX-2/PGE2/EGFR pathway to induce apoptosis (Figure 4) (Hao et al., 2009). Phospholipid complex-loaded nanoparticles (PLC-NPs) encapsulating Sal-B serve as potential carriers in HNSCC (HN13, HN30) cells and Leuk1 cells, inducing apoptosis and cell cycle arrest, increasing the biological activity of Sal-B in vivo (Liu et al., 2007). Nano formulations encapsulate Sal-B within the backbone structure to enhance targeting and increase the drug bioavailability to improve the anticancer potential (Chen et al., 2022b; Liu et al., 2022). However, there are still few studies on the anti-head neck squamous cell carcinoma activity of Sal-B.
(Cyclooxygenase-2, COX-2; epidermal growth factor receptor, EGFR; Prostaglandin (PG) E2, PGE2; poly ADP-ribose polymerase, PARP; Hypoxia-Inducible Factor 1-Alpha, HIF-1α; vascular endothelial growth factor, VEGF).
Inhibitory effect on oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) has become a pressing medical problem worldwide and is the sixth most common malignancy (Panarese et al., 2019; Togni et al., 2022). Surgery and chemotherapy are the conventional treatments for OSCC, with chemotherapy being the most effective (Han et al., 2021; Siquara da Rocha et al., 2022). However, the severe side effects and resistance to chemotherapy are the biggest obstacles to treatment (Abdelmeguid et al., 2021). Therefore, there is an urgent need to find novel strategies and drugs.
A recent study reported the cytotoxic effect of Sal-B on OSCC by inhibiting tumor angiogenesis. Sal-B induced growth inhibition in OSCC cell lines but had limited effects on premalignant cells. Zhou et al. (2006) showed changes in the expression of 17 genes in Sal-B-treated OSCC cells, among which, HIF-1a, TNFα, and MMP9 were inhibited, whereas THBS2 was upregulated (Yang et al., 2011). Sal-B also inhibited the PI3K/AKT/HIF-1α signaling pathway and regulated abnormal glucose metabolism to prevent normal cell carcinogenesis (Wei et al., 2018).
A therapeutic effect in non-small cell lung cancer
Lung cancer is one of the most prevalent and deadly malignancies (Herbst et al., 2018), with annually increasing morbidity and mortality rates (Pennell et al., 2019). Non-small cell lung cancer (NSCLC) is the most prevalent and aggressive type of lung cancer (Broderick, 2020) and comprises three main subtypes, large cell lung carcinoma, squamous cell lung carcinoma, and adenocarcinoma (Babuta et al., 2022; Oronsky et al., 2022). Currently, small molecule TKIs and immunotherapy are among the first-line treatments for NSCLC that have improved survival rates in some patients (Koulouris et al., 2022; Sinjab et al., 2022). However, the overall mortality and morbidity of NSCLC patients remain high, especially in advanced stages. Traditional Chinese medicine has significant efficacy in adjuvant therapy and improving the prognosis of NSCLC patients (Zhang R. et al., 2022).
Zhang S. X. et al. (2022) showed that Sal-B regulates β-catenin and E-cadherin, thereby inhibiting the migration and invasion of cancer cells and inactivating EMT. Sal-B down-regulated the expression of PKM2, LDHA, and GLUT1, affecting glucose uptake, lactate production, enolase activity, cellular ATP levels, and regulating cellular metabolic reprogramming in NSCLC (Chen B. et al., 2022). Yang et al. (2021) showed that Sal-B attenuates NSCLC metastasis through metabolic reprogramming independent of PKM2, revealing its therapeutic promise in the treatment of NSCLC. Han et al. concluded that Sal-B inhibited TGF-β1 and thus induced EMT and migration in A549 cells, hindered cell cycle progression, and promoted cell autophagy and apoptosis. In addition, Sal-B altered the phosphorylation of the MAPK signaling pathway and Smad2/3, especially Smad3 in the junctional region, leading to a decrease in the protein expression of PAI-1 in TGF-β1-stimulated A549 cells (Han et al., 2022). In conclusion, these results suggest that Sal-B has an inhibitory effect on NSCLC by blocking the activation of the MAPK and Smad2/3 signaling pathways, therefore, Sal-B may be a potential therapeutic candidate for NSCLC.
Induces apoptosis in human glioma
Human malignant gliomas are aggressive and infiltrate the limited space of the intracranial cavity and are also common in the central nervous system (Chen et al., 2017; El Khayari et al., 2022). Glioblastomas are highly destructive malignant brain tumors (GBM; World Health Organization grade IV glioma) (Ludwig and Kornblum, 2017; Tsitlakidis et al., 2020), with a high proliferation rate and are highly aggressive. Current treatment is surgical resection, local irradiation, and conventional chemotherapy with temozolomide (TMZ) (Chandrasekar et al., 2022). According to recent studies, the overall median survival of GBM patients is short despite the use of multimodal therapy (Thakur et al., 2022).
Wang et al. (2013) found that Sal-B significantly reduces the viability of U87 cells in a dose- and time-dependent manner. Sal-B also enhances the production of ROS in U87 cells to induce apoptosis (Feng et al., 2022) and dose-dependently increases the phosphorylation of p38 MAPK and p53 (Byun et al., 2018; Chen et al., 2018). In conclusion, Sal-B could be a promising natural component in the treatment of hemangioma cells.
Reduces drug resistance in gastric cancer cells
Gastric cancer also poses a great threat to human health (Correa, 2013; Guggenheim and Shah, 2013. The conventional clinical treatment remains surgery and chemotherapy (Cosma et al., 2022) but the development of resistance to chemotherapeutic drugs leads to failed recovery in most patients (Jelski and Mroczko, 2022; Sobczak and Kędra, 2022). Therefore, research and development of new drugs are key to improving drug efficacy and prolonging patient survival.
Chen et al. (2020) reported that Sal-B decreases tumor cell viability, promotes ROS production, induces apoptosis, as well as reduces migration, invasion, and EMT of AGS and AGS/DDP cells. Sal-B also regulates proliferation, EMT, and apoptosis to reduce the resistance to DDP via the AKT/mTOR pathway in DDP-resistant gastric cancer cells (Wang et al., 2021) (Figure 1). Therefore, Sal-B could be a potential antidrug-resistant agent to chemotherapy in gastric cancer. Tao et al. found that Sal-B exhibited superior inhibitory activities on neutrophil extracellular traps formation and significantly attenuated the levels of citrullinated histone H3 (citH3), a biomarker for neutrophil extracellular traps formation (Tao et al., 2018). Violi and Pignatelli (2015) demonstrated that Sal-B modulated the enzymatic cascade involved in NET formation and could disrupt NET formation at the earlier stage by blocking the activities of myeloperoxidase (MPO) and NADPH oxidase (NOX), respectively.
Inhibition of cancer metastasis
Metastasis remains the greatest difficulty in cancer treatment and is associated with more than half of cancer-related deaths (Seyfried and Huysentruyt, 2013). EMT is the main factor involved in cancer cell metastasis, in which the most prominent role is played by the signals released by the mesenchymal cells that make up normal tissue or neoplastic tissue (tumor neointima) (Xu et al., 2005). In addition, matrix metalloproteinases, such as MMP-2/-9, induce metastasis in cancer cells (Mehner et al., 2014). Therefore, mediators targeting these essential metastases have the potential to prevent metastasis and overcome the invasiveness of cancer cells. Sal-B can block metastasis by inducing EMT markers such as E-cadherin, but additional studies should explore the effects of Sal-B on other EMT markers such as ZEB-1, ZEB-2, and TCF3 (Seyfried and Huysentruyt, 2013; Mehner et al., 2014).
The comparison of different pathway in different cancers
Salvianolic acid B has been shown to inhibit a number of cancers. Although different cancers occur by different mechanisms, the treatment of some cancers has similar pathways. In the treatment of hepatocellular carcinoma, gastric cancer and oral squamous cell carcinoma, the PIK3/AKT/mTOR pathway has an important role in the development of these cancers. By inhibiting this pathway, Salvianolic acid B can exert effective anti-tumour effects. In addition, in the treatment of non-small cell lung cancer and glioblastoma, salvianolic acid B inhibits the growth and differentiation of tumour cells by modulating the MAPK pathway to cut off intracellular signaling. The similarity of the treatment mechanisms of different cancers has implications for the treatment of cancer.
Conclusion
Cancer is a malignant disease that affects human health and currently, various methods are available to slow down or stop cancer progression, such as surgery, chemotherapy, radiotherapy, and immunotherapy. However, these methods are associated with limitations such as tumor drug resistance and the specificity of the tumor location. In recent years, increasing attention has been paid to Chinese medicine in health protection, prevention, and treatment of diseases, as Chinese medicines can prevent the occurrence and development of various malignant diseases. Salvia miltiorrhiza Bunge (Lamiaceae) has been widely used in Chinese medicine for over 2000 years and contains several chemical constituents with unique biological effects. In particular, Sal-B induces apoptosis in cancer cells, such as lung, liver, stomach, glioma, and breast cancers by promoting ROS production and regulating energy metabolism but its exact effects on cancer need to be further investigated in vivo. Elucidating the correlation between Sal-B targets and its role in regulating energy metabolism homeostasis will facilitate further research on its antitumor mechanism, thus providing a scientific basis for further clinical research and application.
Author contributions
S-SG conceived and designed the original manuscript and classified the pharmacological literatures; Z-GW reviewed the content of the article and suggested changes. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National High Technology Research and Development Program of the Ministry of Science and Technology (863 Program), No.2013AA093001. Qi Huang Scholars Support Project and National Key Research and Development Program of China (SQ2017YFC170600).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Sal-B: salvianolic acid B; Sal-A: salvianolic acid A; TCM: Traditional Chinese medicine; HCC: Hepatocellular carcinoma; HNSCC: Head and neck squamous cell carcinoma; OSCC: Oral squamous cell carcinoma; ROS: Reactive oxygen species; PKM2: Pyruvate kinase isozyme typeM2; EMT: Epithelial-mesenchymal transition; RECK: Reversion-inducing cysteine-rich protein with Kazal motifs; STAT3: Signal transducer and activator of transcription 3; AKT: protein kinase B; mTOR: mammalian target of rapamycin; MDM2: murine double minute2; ERK: Extracellular regulated protein kinases; Bcl-2: B-cell lymphoma-2; Bcl-xl: B-cell lymphoma–extra-large; GM3: ganglioside M3; GCS: glucosylceramide; COX-2: Cyclooxygenase-2; EGFR: epidermal growth factor receptor; PGE2: prostaglandin (PG) E2; PARP: poly ADP-ribose polymerase; HIF-1α: Hypoxia-Inducible Factor 1-Alpha; VEGF: vascular endothelial growth factor; NETs: neutrophil extracellular traps.
References
Abdelmeguid, A. S., Silver, N. L., Boonsripitayanon, M., Glisson, B. S., Ferrarotto, R., Gunn, G. B., et al. (2021). Role of induction chemotherapy for oral cavity squamous cell carcinoma. Cancer 127, 3107–3112. doi:10.1002/cncr.33616
Acquaviva, R., Malfa, G. A., Loizzo, M. R., Xiao, J., Bianchi, S., and Tundis, R. (2022). Advances on natural abietane, labdane and clerodane diterpenes as anti-cancer agents: Sources and mechanisms of action. Mol. (Basel, Switz. 27, 4791. doi:10.3390/molecules27154791
Aulakh, S. S., Silverman, D. A., Young, K., Dennis, S. K., and Birkeland, A. C. (2022). The promise of circulating tumor DNA in head and neck cancer. Cancers 14, 2968. doi:10.3390/cancers14122968
Babuta, J., Hall, Z., and Athersuch, T. (2022). Dysregulated metabolism in EGFR-TKI drug resistant non-small-cell lung cancer: A systematic review. Metabolites 12, 644. doi:10.3390/metabo12070644
Bachheti, R. K., Worku, L. A., Gonfa, Y. H., Zebeaman, M., DeeptiPandey, D. P., Bachheti, A., et al. (2022). Prevention and treatment of cardiovascular diseases with plant phytochemicals: A review. Evid. Based. Complement. Altern. Med. 2022, 5741198. doi:10.1155/2022/5741198
Broderick, S. R. (2020). Adjuvant and neoadjuvant immunotherapy in non-small cell lung cancer. Thorac. Surg. Clin. 30, 215–220. doi:10.1016/j.thorsurg.2020.01.001
Byun, H. S., Zhou, W., Park, I., Kang, K., Lee, S. R., Piao, X., et al. (2018). C-27-carboxylated oleanane triterpenoids up-regulate TRAIL DISC assembly via p38 MAPK and CHOP-mediated DR5 expression in human glioblastoma cells. Biochem. Pharmacol. 158, 243–260. doi:10.1016/j.bcp.2018.10.019
Cao, W., Guo, X. W., Zheng, H. Z., Li, D. P., Jia, G. B., and Wang, J. (2012). Current progress of research on pharmacologic actions of salvianolic acid B. Chin. J. Integr. Med. 18, 316–320. doi:10.1007/s11655-012-1052-8
Chandrasekar, G., Bansal, V. S., Panigrahi, M., and Kitambi, S. S. (2022). An overview of targets and therapies for glioblastoma multiforme. J. Cancer Res. Ther. 18, 591–598. doi:10.4103/jcrt.jcrt_1324_21
Chang, Y. T., Chung, M. C., Chang, C. H., Chiu, K. H., Shieh, J. J., and Wu, M. J. (2022). Anti-EMT and anti-fibrosis effects of protocatechuic aldehyde in renal proximal tubular cells and the unilateral ureteral obstruction animal model. Pharm. Biol. 60, 1198–1206. doi:10.1080/13880209.2022.2088809
Chen, B., Huang, C., Zhang, Y., Tang, X., Li, S., Wang, Q., et al. (2020). Salvia bowleyana Dunn root is a novel source of salvianolic acid B and displays antitumor effects against gastric cancer cells. Oncol. Lett. 20, 817–827. doi:10.3892/ol.2020.11611
Chen, B., Lei, S., Yin, X., Fei, M., Hu, Y., Shi, Y., et al. (2022c). Mitochondrial respiration inhibition suppresses papillary thyroid carcinoma via PI3K/Akt/FoxO1/Cyclin D1 pathway. Front. Oncol. 12, 900444. doi:10.3389/fonc.2022.900444
Chen, R., Smith-Cohn, M., Cohen, A. L., and Colman, H. (2017). Glioma subclassifications and their clinical significance. Neurotherapeutics 14, 284–297. doi:10.1007/s13311-017-0519-x
Chen, W., Wang, N., Li, R. C., Xu, G. F., Bao, G., Jiang, H. T., et al. (2018). Salvianolic acid B renders glioma cells more sensitive to radiation via Fis-1-mediated mitochondrial dysfunction. Biomed. Pharmacother. = Biomedecine Pharmacother. 107, 1230–1236. doi:10.1016/j.biopha.2018.08.113
Chen, Y., Hu, M., Wang, S., Wang, Q., Lu, H., Wang, F., et al. (2022b). Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-β1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int. J. Pharm. 623, 121953. doi:10.1016/j.ijpharm.2022.121953
Chen, Y., Wu, F. H., Wu, P. Q., Xing, H. Y., and Ma, T. (2022a). The role of the tumor microbiome in tumor development and its treatment. Front. Immunol. 13, 935846. doi:10.3389/fimmu.2022.935846
National Pharmacopoeia Committee (2020). Chinese Pharmacopoeia. Beijing China Medical Science and Technology Press, 1088.
Chun, K. H. (2022). Molecular targets and signaling pathways of microRNA-122 in hepatocellular carcinoma. Pharmaceutics 14, 1380. doi:10.3390/pharmaceutics14071380
Correa, P. (2013). Gastric cancer: Overview. Gastroenterol. Clin. North Am. 42, 211–217. doi:10.1016/j.gtc.2013.01.002
Cosma, L. S., Schlosser, S., Tews, H. C., Müller, M., and Kandulski, A. (2022). Hereditary diffuse gastric cancer: Molecular genetics, biological mechanisms and current therapeutic approaches. Int. J. Mol. Sci. 23, 7821. doi:10.3390/ijms23147821
Di Modica, M., Arlotta, V., Sfondrini, L., Tagliabue, E., and Triulzi, T. (2022). The link between the microbiota and HER2+ breast cancer: The new challenge of precision medicine. Front. Oncol. 12, 947188. doi:10.3389/fonc.2022.947188
Ding, L., Li, J., Huang, R., Liu, Z., Li, C., Yao, S., et al. (2016). Salvianolic acid B protects against myocardial damage caused by nanocarrier TiO2; and synergistic anti-breast carcinoma effect with curcumin via codelivery system of folic acid-targeted and polyethylene glycol-modified TiO2 nanoparticles. Int. J. Nanomedicine 11, 5709–5727. doi:10.2147/IJN.S107767
El Khayari, A., Bouchmaa, N., Taib, B., Wei, Z., Zeng, A., and El Fatimy, R. (2022). Metabolic rewiring in glioblastoma cancer: EGFR, IDH and beyond. Front. Oncol. 12, 901951. doi:10.3389/fonc.2022.901951
Feng, S. W., Chang, P. C., Chen, H. Y., Hueng, D. Y., Li, Y. F., and Huang, S. M. (2022). Exploring the mechanism of adjuvant treatment of glioblastoma using temozolomide and metformin. Int. J. Mol. Sci. 23 (15), 8171. doi:10.3390/ijms23158171
Fu, Y., Xiao, Z., Tian, X., Liu, W., Xu, Z., Yang, T., et al. (2021). The novel Chinese medicine JY5 formula alleviates hepatic fibrosis by inhibiting the notch signaling pathway. Front. Pharmacol. 12, 671152. doi:10.3389/fphar.2021.671152
Gautam, N., Elleson, K. M., Ramamoorthi, G., and Czerniecki, B. J. (2022). Current state of cell therapies for breast cancer. Cancer J. 28, 301–309. doi:10.1097/PPO.0000000000000607
Gong, L., Di, C., Xia, X., Wang, J., Chen, G., Shi, J., et al. (2016). AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 49, 2538–2548. doi:10.3892/ijo.2016.3748
Guan, Y., Li, L., Kan, L., and Xie, Q. (2022). Inhalation of salvianolic acid B prevents fine particulate matter-induced acute airway inflammation and oxidative stress by downregulating the LTR4/MyD88/NLRP3 pathway. Oxid. Med. Cell. Longev. 2022, 5044356. doi:10.1155/2022/5044356
Guggenheim, D. E., and Shah, M. A. (2013). Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 107, 230–236. doi:10.1002/jso.23262
Hacking, S. M., Yakirevich, E., and Wang, Y. (2022). From immunohistochemistry to new digital ecosystems: A state-of-the-art biomarker review for precision breast cancer medicine. Cancers 14, 3469. doi:10.3390/cancers14143469
Han, G., Wang, Y., Liu, T., Gao, J., Duan, F., Chen, M., et al. (2022). Salvianolic acid B acts against non-small cell lung cancer A549 cells via inactivation of the MAPK and Smad2/3 signaling pathways. Mol. Med. Rep. 25, 184. doi:10.3892/mmr.2022.12700
Han, L., Cheng, J., and Li, A. (2021). hsa_circ_0072387 suppresses proliferation, metastasis, and glycolysis of oral squamous cell carcinoma cells by downregulating miR-503-5p. Cancer biother. Radiopharm. 36, 84–94. doi:10.1089/cbr.2019.3371
Hao, Y., Xie, T., Korotcov, A., Zhou, Y., Pang, X., Shan, L., et al. (2009). Salvianolic acid B inhibits growth of head and neck squamous cell carcinoma in vitro and in vivo via cyclooxygenase-2 and apoptotic pathways. Int. J. Cancer 124 (9), 2200–2209. doi:10.1002/ijc.24160
Herbst, R. S., Morgensztern, D., and Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature 553, 446–454. doi:10.1038/nature25183
Hillmer, E. J., Zhang, H., Li, H. S., and Watowich, S. S. (2016). STAT3 signaling in immunity. Cytokine Growth Factor Rev. 31, 1–15. doi:10.1016/j.cytogfr.2016.05.001
Hong, M., Yu, J., Wang, X., Liu, Y., Zhan, S., Wu, Z., et al. (2022). Tea polyphenols as prospective natural attenuators of brain aging. Nutrients 14, 3012. doi:10.3390/nu14153012
Hu, Y., and Wang, J. (2019). Interactions between clopidogrel and traditional Chinese medicine. J. Thromb. Thrombolysis 48, 491–499. doi:10.1007/s11239-019-01945-3
Huang, X., Jin, L., Deng, H., Wu, D., Shen, Q. K., Quan, Z. S., et al. (2022). Research and development of natural product tanshinone I: Pharmacology, total synthesis, and structure modifications. Front. Pharmacol. 13, 920411. doi:10.3389/fphar.2022.920411
Jayusman, P. A., Nasruddin, N. S., Mahamad Apandi, N. I., Ibrahim, N., and Budin, S. B. (2022). Therapeutic potential of polyphenol and nanoparticles mediated delivery in periodontal inflammation: A review of current trends and future perspectives. Front. Pharmacol. 13, 847702. doi:10.3389/fphar.2022.847702
Jelski, W., and Mroczko, B. (2022). Molecular and circulating biomarkers of gastric cancer. Int. J. Mol. Sci. 23, 7588. doi:10.3390/ijms23147588
Jia, Q., Zhu, R., Tian, Y., Chen, B., Li, R., Li, L., et al. (2019). Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 58, 152871. doi:10.1016/j.phymed.2019.152871
Jin, Z., Chenghao, Y., and Cheng, P. (2022b). Anticancer effect of tanshinones on female breast cancer and gynecological cancer. Front. Pharmacol. 12, 824531. doi:10.3389/fphar.2021.824531
Jin, Z., Zhao, H., Luo, Y., Li, X., Cui, J., Yan, J., et al. (2022a). Identification of core genes associated with the anti-atherosclerotic effects of Salvianolic acid B and immune cell infiltration characteristics using bioinformatics analysis. BMC Complement. Med. Ther. 22, 190. doi:10.1186/s12906-022-03670-6
Johnson, D. E., Burtness, B., Leemans, C. R., Lui, V., Bauman, J. E., and Grandis, J. R. (2020). Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 6, 92. doi:10.1038/s41572-020-00224-3
Kan, S., Cheung, W. M., Zhou, Y., and Ho, W. S. (2014). Enhancement of doxorubicin cytotoxicity by tanshinone IIA in HepG2 human hepatoma cells. Planta Med. 80, 70–76. doi:10.1055/s-0033-1360126
Karn, V., Sandhya, S., Hsu, W., Parashar, D., Singh, H. N., Jha, N. K., et al. (2022). CRISPR/Cas9 system in breast cancer therapy: Advancement, limitations and future scope. Cancer Cell. Int. 22, 234. doi:10.1186/s12935-022-02654-3
Katary, M. A., Abdelsayed, R., Alhashim, A., Abdelhasib, M., and Elmarakby, A. A. (2019). Salvianolic acid B slows the progression of breast cancer cell growth via enhancement of apoptosis and reduction of oxidative stress, inflammation, and angiogenesis. Int. J. Mol. Sci. 20, 5653. doi:10.3390/ijms20225653
Khafaga, A. F., Mousa, S. A., Aleya, L., and Abdel-Daim, M. M. (2022). Three-dimensional (3D) cell culture: A valuable step in advancing treatments for human hepatocellular carcinoma. Cancer Cell. Int. 22, 243. doi:10.1186/s12935-022-02662-3
Khan, H., Ullah, H., Castilho, P., Gomila, A. S., D'Onofrio, G., Filosa, R., et al. (2020). Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 60 (16), 2790–2800. doi:10.1080/10408398.2019.1661827
Kiemlian Kwee, J. (2016). Yin and yang of polyphenols in cancer prevention: A short review. Anticancer. Agents Med. Chem. 16 (7), 832–840. doi:10.2174/1871520616666151116124549
Koulouris, A., Tsagkaris, C., Corriero, A. C., Metro, G., and Mountzios, G. (2022). Resistance to TKIs in EGFR-mutated non-small cell lung cancer: From mechanisms to new therapeutic strategies. Cancers 14, 3337. doi:10.3390/cancers14143337
Leeman-Neill, R. J., Cai, Q., Joyce, S. C., Thomas, S. M., Bhola, N. E., Neill, D. B., et al. (2010). Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin. Cancer Res. 16 (9), 2571–2579. doi:10.1158/1078-0432.CCR-10-0333
Li, M., Zheng, Y., Zhao, J., Liu, M., Shu, X., Li, Q., et al. (2022). Polyphenol mechanisms against gastric cancer and their interactions with gut microbiota: A review. Curr. Oncol. 29, 5247–5261. doi:10.3390/curroncol29080417
Liu, C. L., Xie, L. X., Li, M., Durairajan, S. S., Goto, S., and Huang, J. D. (2007). Salvianolic acid B inhibits hydrogen peroxide-induced endothelial cell apoptosis through regulating PI3K/Akt signaling. PloS one 2, e1321. doi:10.1371/journal.pone.0001321
Liu, J., Shen, H. M., and Ong, C. N. (2000). Salvia miltiorrhiza inhibits cell growth and induces apoptosis in human hepatoma HepG(2) cells. Cancer Lett. 153 (1-2), 85–93. doi:10.1016/s0304-3835(00)00391-8
Liu, J., Zhao, Y., Xia, J., and adn Qiu, M. (2020). Matrine induces toxicity in mouse liver cells through an ROS-dependent mechanism. Res. Vet. Sci. 132, 308–311. doi:10.1016/j.rvsc.2020.07.006
Liu, W., Zhou, Z., Zhu, L., Li, H., and Wu, L. (2022). Chemopreventive efficacy of salvianolic acid B phospholipid complex loaded nanoparticles against experimental oral carcinogenesis: Implication of sustained drug release. Ann. Transl. Med. 10, 244. doi:10.21037/atm-21-4457
Ludwig, K., and Kornblum, H. I. (2017). Molecular markers in glioma. J. Neurooncol. 134, 505–512. doi:10.1007/s11060-017-2379-y
Mehner, C., Hockla, A., Miller, E., Ran, S., Radisky, D. C., and Radisky, E. S. (2014). Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 5 (9), 2736–2749. doi:10.18632/oncotarget.1932
Montalvo-Castro, R. E., and Salinas-Jazmín, N. (2022). Relationship between the expression of complement inhibitory proteins and therapeutic efficacy of antibodies in breast cancer. Gac. Med. Mex. 158, 141–149. doi:10.24875/GMM.M22000657
Murugan, K., Dinesh, D., Kavithaa, K., Paulpandi, M., Ponraj, T., Alsalhi, M. S., et al. (2016). Hydrothermal synthesis of titanium dioxide nanoparticles: Mosquitocidal potential and anticancer activity on human breast cancer cells (MCF-7). Parasitol. Res. 115, 1085–1096. doi:10.1007/s00436-015-4838-8
Oronsky, B., Abrouk, N., Caroen, S., Lybeck, M., Guo, X., Wang, X., et al. (2022). A 2022 update on extensive stage small-cell lung cancer (SCLC). J. Cancer 13, 2945–2953. doi:10.7150/jca.75622
Panarese, I., Aquino, G., Ronchi, A., Longo, F., Montella, M., Cozzolino, I., et al. (2019). Oral and oropharyngeal squamous cell carcinoma: Prognostic and predictive parameters in the etiopathogenetic route. Expert Rev. Anticancer Ther. 19, 105–119. doi:10.1080/14737140.2019.1561288
Park, J. C., Krishnakumar, H. N., and Saladi, S. V. (2022). Current and future biomarkers for immune checkpoint inhibitors in head and neck squamous cell carcinoma. Curr. Oncol. 29, 4185–4198. doi:10.3390/curroncol29060334
Pearanpan, L., Nordin, F. J., Siew, E. L., Kumolosasi, E., Mohamad Hanif, E. A., Masre, S. F., et al. (2022). A cell-based systematic review on the role of annexin A1 in triple-negative breast cancers. Int. J. Mol. Sci. 23, 8256. doi:10.3390/ijms23158256
Pennell, N. A., Arcila, M. E., Gandara, D. R., and West, H. (2019). Biomarker testing for patients with advanced non-small cell lung cancer: Real-World issues and tough choices. Am. Soc. Clin. Oncol. Educ. Book. 39, 531–542. doi:10.1200/EDBK_237863
Qian, C., Yang, C., Tang, Y., Zheng, W., Zhou, Y., Zhang, S., et al. (2022). Pharmacological manipulation of Ezh2 with salvianolic acid B results in tumor vascular normalization and synergizes with cisplatin and T cell-mediated immunotherapy. Pharmacol. Res. 182, 106333. doi:10.1016/j.phrs.2022.106333
Riaz, F., Wei, P., and Pan, F. (2022). Fine-tuning of regulatory T cells is indispensable for the metabolic steatosis-related hepatocellular carcinoma: A review. Front. Cell. Dev. Biol. 10, 949603. doi:10.3389/fcell.2022.949603
Seyfried, T. N., and Huysentruyt, L. C. (2013). On the origin of cancer metastasis. Crit. Rev. Oncog. 18 (1-2), 43–73. doi:10.1615/critrevoncog.v18.i1-2.40
Sha, W., Zhou, Y., Ling, Z. Q., Xie, G., Pang, X., Wang, P., et al. (2018). Antitumor properties of Salvianolic acid B against triple-negative and hormone receptor-positive breast cancer cells via ceramide-mediated apoptosis. Oncotarget 9, 36331–36343. doi:10.18632/oncotarget.26348
Shen, S., Tong, Y., Luo, Y., Huang, L., and Gao, W. (2022). Biosynthesis, total synthesis, and pharmacological activities of aryltetralin-type lignan podophyllotoxin and its derivatives. Nat. Prod. Rep. 39, 1856–1875. doi:10.1039/d2np00028h
Sinjab, A., Rahal, Z., and Kadara, H. (2022). Cell-by-Cell: Unlocking lung cancer pathogenesis. Cancers 14 (14), 3424. doi:10.3390/cancers14143424
Siquara da Rocha, L. O., Souza, B., Lambert, D. W., and Gurgel Rocha, C. A. (2022). Cell-in-Cell events in oral squamous cell carcinoma. Front. Oncol. 12, 931092. doi:10.3389/fonc.2022.931092
Sobczak, M., and Kędra, K. (2022). Biomedical polyurethanes for anti-cancer drug delivery systems: A brief, comprehensive review. Int. J. Mol. Sci. 23, 8181. doi:10.3390/ijms23158181
Solomon, B., Young, R. J., and Rischin, D. (2018). Head and neck squamous cell carcinoma: Genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol. 52, 228–240. doi:10.1016/j.semcancer.2018.01.008
Tao, L., Xu, M., Dai, X., Ni, T., Li, D., Jin, F., et al. (2018). Polypharmacological profiles underlying the antitumor property of Salvia miltiorrhiza root (danshen) interfering with NOX-dependent neutrophil extracellular traps. Oxid. Med. Cell. Longev. 2018, 4908328. doi:10.1155/2018/4908328
Teng, M., Hu, C., Yang, B., Xiao, W., Zhou, Q., Li, Y., et al. (2021). Salvianolic acid B targets mortalin and inhibits the migration and invasion of hepatocellular carcinoma via the RECK/STAT3 pathway. Cancer Cell. Int. 21, 654. doi:10.1186/s12935-021-02367-z
Thakur, S., Kumar, N., Salunke, P., Ahuja, C., and Madan, R. (2022). A randomized study of short course (one week) radiation therapy with or without temozolomide in elderly and/or frail patients with newly diagnosed glioblastoma (GBM). Asian pac. J. Cancer Prev. 23 (7), 2317–2323. doi:10.31557/APJCP.2022.23.7.2317
Togni, L., Caponio, V., Zerman, N., Troiano, G., Zhurakivska, K., Lo Muzio, L., et al. (2022). The emerging impact of tumor budding in oral squamous cell carcinoma: Main issues and clinical relevance of a new prognostic marker. Cancers 14, 3571. doi:10.3390/cancers14153571
Tsitlakidis, A., Aifantis, E. C., Kritis, A., Tsingotjidou, A. S., Cheva, A., Selviaridis, P., et al. (2020). Mechanical properties of human glioma. Neurol. Res. 42, 1018–1026. doi:10.1080/01616412.2020.1796381
Udoh, U. S., Rajan, P. K., Nakafuku, Y., Finley, R., and Sanabria, J. R. (2022). Cell autophagy in NASH and NASH-related hepatocellular carcinoma. Int. J. Mol. Sci. 23, 7734. doi:10.3390/ijms23147734
Violi, F., and Pignatelli, P. (2015). Clinical application of NOX activity and other oxidative biomarkers in cardiovascular disease: A critical review. Antioxid. Redox Signal. 23, 514–532. doi:10.1089/ars.2013.5790
Wang, J., Ma, Y., Guo, M., Yang, H., and Guan, X. (2021). Salvianolic acid B suppresses EMT and apoptosis to lessen drug resistance through AKT/mTOR in gastric cancer cells. Cytotechnology 73, 49–61. doi:10.1007/s10616-020-00441-4
Wang, L., Ma, R., Liu, C., Liu, H., Zhu, R., Guo, S., et al. (2017). Salvia miltiorrhiza: A potential red light to the development of cardiovascular diseases. Curr. Pharm. Des. 23, 1077–1097. doi:10.2174/1381612822666161010105242
Wang, X., Morris-Natschke, S. L., and Lee, K. H. (2007). New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med. Res. Rev. 27 (1), 133–148. doi:10.1002/med.20077
Wang, Z. S., Luo, P., Dai, S. H., Liu, Z. B., Zheng, X. R., and Chen, T. (2013). Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell. Mol. Neurobiol. 33, 921–928. doi:10.1007/s10571-013-9958-z
Wei, J., Wu, J., Xu, W., Nie, H., Zhou, R., Wang, R., et al. (2018). Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway. Cell. Death Dis. 9, 599. doi:10.1038/s41419-018-0623-9
Wei, L., Wang, Z., Jing, N., Lu, Y., Yang, J., Xiao, H., et al. (2022). Frontier progress of the combination of modern medicine and traditional Chinese medicine in the treatment of hepatocellular carcinoma. Chin. Med. 17, 90. doi:10.1186/s13020-022-00645-0
Xia, H., Huang, Z., Wang, Z., Liu, S., Zhao, X., You, J., et al. (2022). Glucometabolic reprogramming: From trigger to therapeutic target in hepatocellular carcinoma. Front. Oncol. 12, 953668. doi:10.3389/fonc.2022.953668
Xu, H., Wang, D., Peng, C., Huang, X., Ou, M., Wang, N., et al. (2014). Rabbit sera containing compound danshen dripping pill attenuate leukocytes adhesion to TNF-alpha-activated human umbilical vein endothelial cells by suppressing endothelial ICAM-1 and VCAM-1 expression through NF-kappaB signaling pathway. J. Cardiovasc. Pharmacol. 63, 323–332. doi:10.1097/FJC.0000000000000046
Xu, X., Wang, Y., Chen, Z., Sternlicht, M. D., Hidalgo, M., and Steffensen, B. (2005). Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 65 (1), 130–136. doi:10.1158/0008-5472.130.65.1
Yamashita, N., and Kufe, D. (2022). Addiction of cancer stem cells to MUC1-C in triple-negative breast cancer progression. Int. J. Mol. Sci. 23, 8219. doi:10.3390/ijms23158219
Yang, S. H., Wu, H., Yi, Z. J., and Lai, X. (2021). The PKM2 activator TEPP-46 attenuates MCD feeding-induced nonalcoholic steatohepatitis by inhibiting the activation of Kupffer cells. Eur. Rev. Med. Pharmacol. Sci. 25, 4017–4026. doi:10.26355/eurrev_202106_26043
Yang, Y., Ge, P. J., Jiang, L., Li, F. L., and Zhu, Q. Y. (2011). Modulation of growth and angiogenic potential of oral squamous carcinoma cells in vitro using salvianolic acid B. BMC Complement. Altern. Med. 11, 54. doi:10.1186/1472-6882-11-54
Yokota, T., Homma, A., Kiyota, N., Tahara, M., Hanai, N., Asakage, T., et al. (2020). Immunotherapy for squamous cell carcinoma of the head and neck. Jpn. J. Clin. Oncol. 50, 1089–1096. doi:10.1093/jjco/hyaa139
Zhang, H., Tang, J., Cao, Y., and Wang, T. (2022). Salvianolic acid B suppresses non-small-cell lung cancer metastasis through PKM2-independent metabolic reprogramming. Evid. Based. Complement. Altern. Med. 2022, 9302403. doi:10.1155/2022/9302403
Zhang, R., Meng, J., Yang, S., Liu, W., Shi, L., Zeng, J., et al. (2022). Recent advances on the role of ATGL in cancer. Front. Oncol. 12, 944025. doi:10.3389/fonc.2022.944025
Zhang, X., Guo, Q., Li, C., Liu, R., Xu, T., Jin, Z., et al. (2022). Immortal time bias-corrected effectiveness of traditional Chinese medicine in non-small cell lung cancer (C-evid): A prospective cohort study. Front. Oncol. 12, 845613. doi:10.3389/fonc.2022.845613
Zhao, Y., Guo, Y., and Gu, X. (2011). Salvianolic Acid B, a potential chemopreventive agent, for head and neck squamous cell cancer. J. Oncol. 2011, 534548. doi:10.1155/2011/534548
Zhao, Y., Hao, Y., Ji, H., Fang, Y., Guo, Y., Sha, W., et al. (2010). Combination effects of salvianolic acid B with low-dose celecoxib on inhibition of head and neck squamous cell carcinoma growth in vitro and in vivo, 3. Philadelphia, Pa: Cancer prevention research, 787–796. doi:10.1158/1940-6207.CAPR-09-0243
Zhou, H., Xu, M., Gao, Y., Deng, Z., Cao, H., Zhang, W., et al. (2014). Matrine induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol. Cancer 13, 59. doi:10.1186/1476-4598-13-59
Zhou, Y., Xu, W., Liu, A., Tao, Y., Wang, Q., Yang, Y., et al. (2022). Protective effect of salvianolic acid A against N-methyl-N-Nitrosourea-Induced retinal degeneration. Evid. Based. Complement. Altern. Med. 2022, 1219789. doi:10.1155/2022/1219789
Keywords: TCM, traditional Chinese medicine, salvianolic acid B, anticancer activity, apoptosis, mechanism
Citation: Guo S-S and Wang Z-G (2022) Salvianolic acid B from Salvia miltiorrhiza bunge: A potential antitumor agent. Front. Pharmacol. 13:1042745. doi: 10.3389/fphar.2022.1042745
Received: 12 September 2022; Accepted: 14 October 2022;
Published: 25 October 2022.
Edited by:
Muhammad Riaz, Shaheed Benazir Bhutto University, PakistanReviewed by:
Lizhen Huang, South China University of Technology, ChinaFeng Zhang, Nanjing University of Chinese Medicine, China
Copyright © 2022 Guo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Guo Wang, emhlbmd1b3dAMTI2LmNvbQ==
 Sha-Sha Guo
Sha-Sha Guo Zhen-Guo Wang
Zhen-Guo Wang