
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 24 November 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1040235
This article is part of the Research TopicEfficacy and Mechanism of Herbal Medicines and Their Functional Compounds in Preventing and Treating Cardiovascular Diseases and Cardiovascular Disease Risk FactorsView all 15 articles
YiQiFuMai injection (YQFM), derived from Shengmai Powder, is wildly applied in the treatment of cardiovascular diseases, such as coronary heart disease and chronic cardiac insufficiency. YiQiFuMai injection is mainly composed of Radix of Panax ginseng C.A. Mey. (Araliaceae), Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae), and Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae), and Triterpene saponins, steroidal saponins, lignans, and flavonoids play the vital role in the potency and efficacy. Long-term clinical practice has confirmed the positive effect of YiQiFuMai injection in the treatment of heart failure, and few adverse events have been reported. In addition, the protective effect of YiQiFuMai injection is related to the regulation of mitochondrial function, anti-apoptosis, amelioration of oxidant stress, inhibiting the expression of inflammatory mediators, regulating the expression of miRNAs, maintaining the balance of matrix metalloproteinases/tissue inhibitor of metalloproteinases (MMP/TIMP) and anti-hypoxia.
Heart failure whose incidence rate rises year by year, is the late stage of cardiovascular disease with high mortality and rehospitalization rates. The latest epidemiological survey showed that the prevalence of heart failure in China was 1.3% (an estimated 13.7 million patients), which had increased by 44% in the past 15 years (Hao et al., 2019). And the mortality rates of heart failure within 30 days, 1 year and 5 years after hospitalization were 10.4%, 22% and 42.3% respectively. The rehospitalization rates within 1 year among patients with acute and chronic heart failure were 43.9% and 31.9% respectively (Loehr et al., 2008; Maggioni et al., 2013). As the last battlefield for the prevention and treatment of cardiovascular diseases, heart failure has caused the heavy economic burden to society and joined the rank of grievous public health problems. So far, pharmacotherapy aiming at blocking the renin angiotensin aldosterone system and sympathetic nervous system is the cornerstone in the treatment of heart failure. And new drugs have earned a place, such as angiotensin receptor neprilysin inhibitor (ARNI) and sodium glucose cotransporter two inhibitor (SGLT2i) (McDonagh et al., 2021). Although great progress has realized in the prevention and treatment of heart failure, the overall prognosis is still poor, and the 5-year survival rate is equivalent to that of some malignant tumors (Stewart et al., 2001). Exploring effective and thorough strategy for the treatment of heart failure remains to be done.
Traditional Chinese Medicine, based on the concept of holism, differentiates syndromes and gives treatment in a Multi-target and individualized way, and its advantages lie in increasing exercise tolerance, improving the quality of life, elevating cardiac function, delaying myocardial remodeling, reducing mortality and rehospitalization rates (Zhang and Li, 2014; Sun et al., 2016). Shengmai Powder, first described in a classic named ‘Yi Xue Qi Yuan’, is composed of Radix of Panax ginseng C.A. Mey. (Araliaceae), Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae), and Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae), which has effects of replenishing qi, recovering pulse, nourishing yin, and promoting body fluid production (Zhang, 1978). It is commonly applied in the treatment of heart failure, coronary heart disease, hypertension, and viral myocarditis (Wu, 1997). Chinese patent medicines derived from Shengmai Powder include YiQiFuMai injection (YQFM), Shengmai injection, Shenmai injection, Shengmai San, Shengmai Yin, Shengmai capsule, etc. Studies have demonstrated the cardioprotective effects of Shengmai-related formulas, inccluding improving cardiac function, ameliorating ventricular remodeling, suppressing inflammation, reducing collagen deposition, and inhibiting apoptosis (Cao Z. H. et al., 2019; Yin et al., 2020).
YQFM, the product of Traditional Chinese Medicine combined with modern pharmaceutical technology, not only retains the effecy of Shengmai Powder, but also takes effect more quicky (Du et al., 2021). It is the only powder injection traditional Chinese medicine cardiotonic approved for listing by the state at present (Fu et al., 2020). YQFM is mainly comprised of Radix of Panax ginseng C.A. Mey. (Araliaceae), Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae), and Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae). Modern pharmacological studies have shown that, as the main active substance of Radix of Panax ginseng C.A. Mey. (Araliaceae), ginsenosides effectively inhibited myocardial hypertrophy, improved myocardial ischemia, promoted vascular regeneration and inhibited apoptosis (Wang W. et al., 2016). Research has found that Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae) guarded cardiovascular system by resisting myocardial ischemia, arrhythmia, thrombosis and improving microcirculation (Fan et al., 2020). The study showed that Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae) acted on various signal pathways to protect myocardial cells from inflammation, apoptosis, oxidative stress, atherosclerosis and other advert effects (Xing et al., 2021). Therefore, YQFM can improve heart function and alleviate heart failure by reducing myocardial ischemia-reperfusion injury, antioxidant stress, regulating ventricular remodeling, and reducing the release of inflammatory factors (Ju et al., 2018). In clinical practice, it is mainly used for the treatment of heart failure, coronary heart disease, angina pectoris and other cardiovascular diseases (Zhang et al., 2021). In 2007, China Food and Drug Administration approved YQFM for the treatment of cardiovascular diseases, including coronary heart disease, exertional angina pectoris and chronic cardiac insufficiency. And YQFM is recommended for acute exacerbation of heart failure in the ‘expert consensus on diagnosis and treatment of chronic heart failure with integrated traditional Chinese and Western medicine’ (Chinese Association of Integrative Medicine, 2016). Research has showed that YQFM significantly reduced the level of N-terminal pro-B-type natriuretic peptide (NT-proBNP), improved cardiac function, and relieved symptoms and signs in patients with acute heart failure (Wang et al., 2021). This review summarized the components, mechanisms and clinical evidences of YQFM in the treatment of heart failure in order to provide a theoretical basis for clinical practice.
YQFM is mainly comprised of Radix of Panax ginseng C.A. Mey. (Araliaceae), Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae), and Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae). Triterpene saponins are the main components of Radix of Panax ginseng C.A. Mey. (Araliaceae). In addition, steroidal saponins, flavonoids, and carbohydrates are the main components of Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae) while lignans compounds are the main components of Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae) (Table 1). With the development of analytical methods such as high-performance liquid chromatography (HPLC), liquid-mass spectrometry (LMS) and metabolomics, these new technologies have been applied to explore the chemical components of YQFM. For instance, Zhou et al. have identified the components of QYFM with the help of liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS), including ginsenoside Rf, Rb1, Rb2, Rb3, Rd, Rg3, 20 (S), ginsenoside F2, and Schisandrin B (Zhou et al., 2009). Liu et al. have identified 21 saponins in YQFM by HPLC coupled with quadrupole time-of-flight tandem mass spectrometry (HPLC-Q-TOF-MS), and among them, 13 saponins were reported for the first time (Liu et al., 2018). Furthermore, Wang et al. have determined the contents of fructose, glucose, sucrose, and maltose in YQFM by HPLC-evaporative light scattering and electrospray (Wang Y. et al., 2020). Liu et al. not only identified 65 compounds of YQFM by ultrafast liquid chromatography-ion trap time-of-flight MS (UFLC-IT-TOF/MS) but also quantitatively analyzed 21 compounds, including three Ophiopogon japonicus, 15 ginsenosides, and three lignans (Liu et al., 2016). Zhou et al. have determined 145 compounds of YQFM by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF/MS), including 20 flavonoids, 24 lignans, 27 saponins, 15 carbohydrates, 38 organic acids, and 22 sterols, peptides, and esters (Zhou et al., 2018). Additionally, using saikosaponin A as the internal reference, Zhou et al. detected nine ginsenosides in the plasma of Wistar rats after the intravenous injection of YQFM by LC-electrospray ionization (ESI)-MS (Zhou et al., 2011). Zhang et al. reported eight ginsenosides and four lignans detected by LC-ESI-MS/MS in the plasma of Beagle dogs after intravenous injection of YQFM (Zhang et al., 2018). According to the research of Zheng et al., 10 ginsenosides were identified by UFLC-MS/MS in the plasma of rats with chronic heart failure after the intervention of YQFM (Zheng et al., 2018). From the view of chemical components, pharmacodynamics, network pharmacology, pharmacokinetics, and properties, Li et al. has demonstrated quality markers of YQFM, including ginsenosides (Rb1, Rg1, Rf, Rh1, Rc, Rb2, Ro, and Rg3), Ophiopogon saponin C, phiogenin-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside, pennogenin-3-O-α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyl-(1→4)-β-d-glucopyranoside, fructose, and schisandrin, representing the transfer and change of main substances during the preparation of YQFM, which are the main medicinal chemical components of YQFM (Li D. K. et al., 2019).
Heart failure is a chronic and progressive disease, and myocardial remodeling is a critical factor in the initiation and progression of heart failure.
In HF mice induced by the permanent coronary artery ligation (CAL) with the intervention of YQFM for 2 weeks (0.13 g/kg, 0.26 g/kg, and 0.53 g/kg) showed that YQFM (0.53 g/kg) improved left ventricular function and ameliorated structural injury. It was reported that YQFM restrained the activity of serum lactic dehydrogenase (LDH) and creatine kinase (CK), lowered the levels of serum malondialdehyde (MDA), amino-terminal pro-peptide of pro-collagen type III (PIIINP), NT-proBNP, and myocardial hydroxyproline (HYP). And YQFM appears to reduce oxidation stress, suppress myocardial collagen deposition and fibrosis, and ameliorate cardiac remodeling by the of the blocking effect on mitogen-activated protein kinases (MAPKs) signaling pathway (Pang et al., 2017). Wistar rats were subjected to abdominal aortic coarctation to establish a chronic heart failure model. And the indications for successful modeling was determined by LVEF% ≤ 60% at 8 weeks after the operation. After 14 days of continuous treatment with YQFM (520, 260 and 1,040 mg/kg), the results indicated that YQFM increased the left ventricular posterior wall in the systolic (LVPWs), ejection fraction (EF), fractional shortening (FS), reduced left ventricular end-systolic diameter (LVESD), the levels of serum brain natriuretic peptide (BNP) and copeptin (CPP), whereby improving cardiac function and delaying ventricular remodeling in rats (Zhang et al., 2015).
SD rats were given the intervention of YQFM (0.28, 0.55 and 1.10 g/kg) via tail vein injection for 7 days followed by the intraperitoneal injection of doxorubicin (25 mg/kg) for 5 days to establish acute myocardial injury model. The results manifested that YQFM alleviated doxorubicin-induced myocardial injury and improved cardiac function in rats by reducing the serum levels of LDH, CK, and AST, decreasing left ventricular end-diastolic diameter (LVEDD), and elevating FS (Wang XD. et al., 2020). Vitro experiment has also confirmed the cardioprotective effects of YQFM that the application of YQFM (5 mg/ml) boosted the viability of H9c2 cells exposed to H2O2 (0.2 mmol/L, 5 h) (Li et al., 2020).
ICR mice were treated with different concentrations of YQFM (0.13, 0.26 and 0.53 g/kg, intraperitoneal) for 2 weeks after CAL. The results showed that YQFM redressed mitochondrial dysfunction by normalizing mitochondrial morphology, increasing mitochondrial membrane potential (Δψm), inhibiting the generation of reactive oxygen species (ROS), up-regulating the expression of mitochondrial fusion protein 2 (Mfn2), and reducing the phosphorylation of dynamin-related protein 1 (Drp1), which was related to reduction in NADPH oxidase 2 (NOX2), p67phox, NOX4, calcium voltage-gated channel subunit α1C (CACNA1C) and phosphorylation of calmodulin dependent protein kinase II (p-CaMKII) (Zhang et al., 2019).
In HF mouse models induced by CAL, after the intraperitoneal injection of YQFM (0.13 g/kg, 0.26 g/kg, 0.53 g/kg) for 14 days, the results showed that the levels of serum creatine kinase-MB (CK-MB), aspartate aminotransferase (AST), interleukin-6 (IL-6), troponin, myosin, and myoglobin were down-regulated, and the omentin level elevated. And the study indicated that YQFM improved left ventricular systolic function and suppressed apoptosis on account of boosting the expression of phosphatidylinositol 3-kinase (PI3K), the phosphorylation of protein kinase B (Akt) and adenine monophosphate activated protein kinase (AMPK) and inhibiting the phosphorylation of p38, C-Jun Kinase enzyme (JNK), and extracellular signal-regulated kinase 1/2 (ERK1/2) (Li F. et al., 2019). In vitro experiments, compared with the control group, namely injured H9c2 cells induced by doxorubicin (0.3 μmol/L), the intervention of YQFM (125, 625, 3,125 μg/ml) reduced cytotoxicity, increased cell viability, inhibited the activity of LDH, elevated adenosine triphosphate (ATP) content and restored mitochondrial membrane potential, which played an anti-apoptotic effect (Zeng et al., 2018). Furthermore, the application of YQFM(2.5 mg/ml) on H9c2 cells significantly boosted cell viability and ATP content in apoptotic cells induced by tert-butyl hydroperoxide, and enhanced the phosphorylation of Akt. It also ameliorated the extent of hypertrophy in H9c2 cells induced by angiotensin II (0.1 μM) and elevated the expression of atrial natriuretic peptide (ANP) mRNA (Zhao C. et al., 2018).
In chronic heart failure models induced by the ligation of rats’ left anterior descending coronary artery, after the treatment of YQFM (100 mg/kg/d) for 8 weeks, UPLC-Q-TOF-MS combined with nuclear factor kappa-B (NF-κB) active luciferase reporter analyzed potential anti-inflammatory components. It was further demonstrated that YQFM reduced the size of myocardial infarction, improved cardiac function, and inhibited the expression of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), NF-κB, IL-6, and interleukin-1β (IL-1β). And eight potential anti-inflammatory components have been confirmed, including ginsenosides Rb1, Rg1, Rf, Rh1, Rc, Rb2, Ro and Rg3 (Xing et al., 2013).
To investigate the anti-hypoxia effect of the extraction of YQFM, an animal model of chronic intermittent hypoxia was constructed, treated with YQFM (1.4, 2.8, and 5.5 g/kg/d) for 28 days, and betaloc (0.1516 g/kg/d) served as the positive control. The results manifested that YQFM reversed endothelial cell swelling and cardiac vacuolation, improved myocardial hypoxia tolerance and attenuated myocardial damage by increasing EF and stroke volume (SV), inhibiting the activity of CK and LDH, reducing MDA content, and boosting superoxide dismutase (SOD) (Feng et al., 2016).
In ICR mice that were given intraperitoneal injection with isoproterenol (0.02 g/kg/d) for 3 days followed by YQFM (1.352, 0.676 and 0.338 g/kg/d), the results showed that the serum levels of MDA, CK, and LDH and the activity of myeloperoxidase (MPO) decreased, while the serum SOD level elevated, indicating that YQFM exerted great cardioprotective effect (Wang et al., 2013).
In Wistar rats with chronic heart failure that underwent abdominal aorta contraction, after the intervention of QYFM (520 mg/kg, 260 mg/kg, 1,040 mg/kg) for 14 days, there were significant changes of contents in myocardium, including MMP-2, MMP-3, MMP-9, TIMP-1, TIMP-2. The study reported that YQFM decreased the levels of MMP-2, MMP-3, and MMP-9, and elevated the levels of TIMP-1 and TIMP-2, whereby improving cardiac function and delaying ventricular remodeling (Zhang et al., 2016).
In chronic heart failure models established by the ligation of rats’ the left anterior descending coronary artery, after the administration of YQFM (YQFM, 5 mg/kg/d, ip) for 28 days, the differential expression of microRNAs was studied via rat miRNA microarray and bioinformatics analysis. And the results manifested that YQFM increased left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS), decreased left ventricular diameter, and boosted cardiac output (CO) by down-regulating the expression of miR-219a-2-3p, miR-466c-5p, and miR-702-5p, and up-regulating the expression of miR-21-3p, miR-216b-5p, miR-381-3p, and miR-542-3p (Zhao Y. et al., 2018).
Taken together, YQFM reveals great efficacy on delaying myocardial remodeling and improving cardiac function in injured myocardium induced by coronary artery ligation, abdominal aortic constriction, chronic intermittent hypoxia, and doxorubicin. The underlying mechanisms lie in the regulation of mitochondrial function, anti-apoptotic effect on cardiomyocytes, anti-oxidative effect, anti-inflammation, the modulation of the expression of miRNAs, maintenance of MMP/TIMP balance, and anti-hypoxic effect (Table 2).
A clinical study involving 1,134 patients with coronary heart disease and heart failure performed by 35 research centers, who were treated with YQFM (5.2 g/d) for 14 days, revealed that the application of YQFM on routine treatment for heart failure contributed to the reduction of Lee’s heart failure score, Minnesota heart failure quality of life score, and cardiothoracic ratio, elevated SV, CO, EF, and FS, and decreased LVESD. Therefore, YQFM exerted great efficacy on improving the cardiac pumping performance and quality of life of patients and reversing ventricular remodeling (Sun et al., 2012). In addition, research has evaluated the clinical efficacy of YQFM combined with western medicine via stress echocardiography. A study involving 52 patients with ischemic heart failure showed that, compared with the conventional treatment group, YQFM combined with conventional treatment increased EF and early diastolic peak flow velocity/late diastolic peak flow velocity (E/A) value and reduced early mitral filling velocity/early diastolic mitral annular velocity (E/e') ratio and NT-proBNP levels, indicating that YQFM could improve cardiac function (Hu et al., 2014). Two randomized controlled trials concerning 60 elderly patients with chronic heart failure have illustrated that YQFM combined with basic treatment effectively reduced serum NT-proBNP levels and increased EF and 6-min walking distance (6 MWD) compared to basic treatment alone, suggesting the positive effects of YQFM on cardiac function and exercise tolerance (Zhang et al., 2015; Yang et al., 2016). In another randomized controlled trial involving 108 patients with ischemic cardiomyopathy and heart failure, the treatment group was given YQFM combined with Qiliqiangxin Capsules, while the control group was given Qiliqiangxin Capsules alone. And the results manifested that the application of YQFM lowered serum NT-proBNP levels and elevated EF, CO, and 6 MWD, indicating that YQFM combined with Qiliqiangxin Capsules enhanced clinical efficacy, improved clinical symptoms, and promoted the recovery of cardiac function (Jiang et al., 2018). In a study including 103 patients with chronic heart failure and atrial fibrillation, based on the conventional treatment, the control group was given rosuvastatin while the treatment group was given rosuvastatin and YQFM. Compared to the control group, there were significant improvement of cardiac function and 6 MWD performance, increased FS and EF, and reduction in the level of NT-proBNP, LVEDD, the recurrence rate of atrial fibrillation, and risk of permanent atrial fibrillation in the treatment group (Su and Wang, 2018). In a randomized controlled trial of 118 patients with coronary heart disease and chronic heart failure, the application of YQFM combined with atorvastatin improved cardiac function and delayed ventricular remodeling by decreasing LVEDD, lowering the levels of NT-proBNP, soluble CD40 (sCD40) and soluble CD146 (sCD146), and increasing nitric oxide (NO) levels (Li Y. et al., 2019). Furthermore, in a randomized controlled study involved 40 patients who underwent cardiac valve replacement surgery, based on the cardiac rehabilitation, the treatment group received YQFM immediately after surgery. And the results showed that YQFM effectively improved exercise tolerance and 6 MWD performance (Zhang et al., 2020).
Collectively, YQFM exerts great efficacy in the treatment of heart failure, including improving cardiac function, inhibiting ventricular remodeling, and elevating the quality of life. However, the quality of clinical trials of YQFM in the treatment of heart failure remains relatively low. Thus large-sample, multi-center, randomized controlled trials and real world research are vital to provide the evidence-based data (Table 3).
Meta-analysis evaluating clinical efficacy of YQFM combined with conventional western medicine in the treatment of heart failure has revealed that the intervention of YQFM increased EF, CO and 6 MWD, shortened LVEDD and LVESD, and reduced the serum levels of NT-proBNP and BNP, suggesting that on the basis of conventional western medicine treatment, YQFM further improved cardiac function and the quality of life (Wang XL. et al., 2016; Lian et al., 2016; Zhou et al., 2016; Xiong et al., 2017; Xie and Dai, 2019; Fan et al., 2021). Whereas, lacking of the calculation of sample size, details concerned the western medicine treatment and endpoint indicators, unclear random method, and non-uniform dosages and periods of YQFM weaken the quality of the clinical evidence (Table 4).
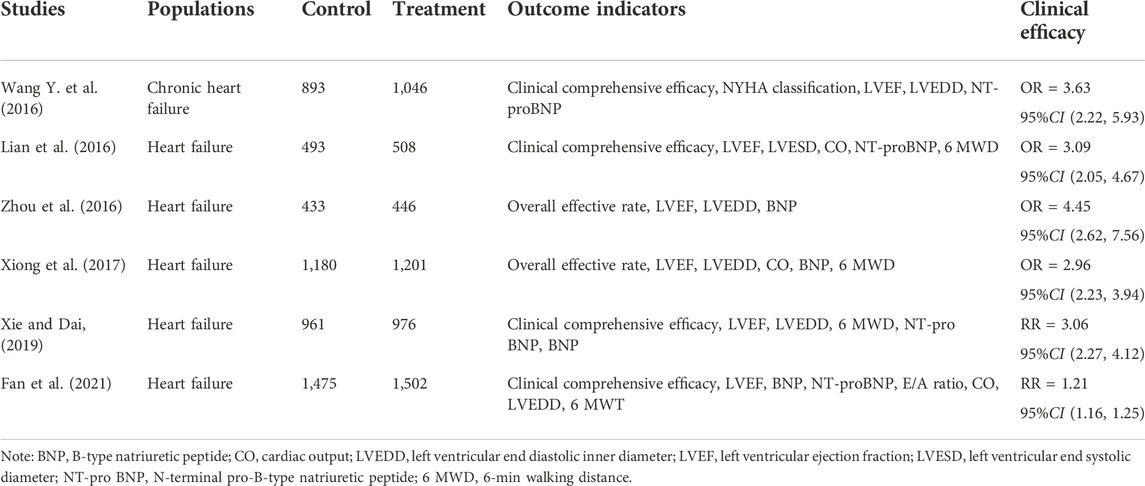
TABLE 4. Meta-analyse and systematic reviews of YiQiFuMai injection in the treatment of heart failure.
Li et al. analyzed 240 patients with coronary heart disease who received YQFM in retrospect, and the results showed that none of the patients had the symptoms or signs of hepatic and renal injury. It was merely reported one case of pharyngeal pain with the injection of YQFM infusion, and the symptom subsided gradually after the withdrawn of YQFM (Li et al., 2018). An analysis involving 998 patients treated with YQFM (>70 years old accounting for 50.69%) exhibited that the incidence of untoward effects was 0.2%, and most of these symptoms and signs were transient without additional treatment (Li K. et al., 2019). Wang et al. analyzed the safety of YQFM on 106 elderly patients (≥80 years old) with cardiovascular diseases, and it was found that YQFM had little impact on the levels of serum alanine aminotransferase (ALT), AST, total bilirubin (TBil), and creatinine (Cr), and only one case (accounting for 0.94%) reported mild palpitation and precordial discomfort (Wang et al., 2018). Sun et al. retrospectively analyzed the performance of YQFM on 2,476 hospitalized patients by the prescription automatic screening system. The study reported that 31 cases had adverse reactions (accounting for 1.25%) that mainly manifested as general damage and skin lesion, such as rash, pruritus, chills, high fever, etc. Additionally, most adverse reactions occurred within 7 days after the medication (Sun et al., 2019). Cao et al. conducted a prospective, large-sample, multi-center analysis involving 44 medical institutions, including 10,767 hospitalized patients treated with YQFM. The research reported 19 cases of adverse reactions (0.176%), and among them, skin lesion was the main one (36.98%). At the same time, it seemed that adverse reactions were more common in the group with higher infusion rate (p < 0.05), indicating that immoderate infusion rate was one of the risk factors for untoward effects (Cao H. B. et al., 2019). Ma et al. analyzed 51 pieces of clinical research involving 7,824 patients who were given YQFM. There were no serious adverse event, and only 23 study mentioned 42 cases of mildly untoward effects in total. Among them, adverse cardiovascular events, adverse neural damage, skin lesion, and gastrointestinal reactions accounted for 28.6%, 20.4%, 22.5%, and 20.3% respectively (Ma et al., 2015). In vitro experiments, YQFM not only inhibited the autonomic contraction of the isolated intestine, but also supressed the spasm of isolated intestine triggered by acetylcholine (Ach) and histamine (His). In vivo experiments the blue staining rate and the levels of His and 5-hydroxytryptamine (5-HT) in mice administered with low-dose YQFM were within the normal range without evident pulmonary injury and auricularinfection. By contrast, only when the mice were given 3.43 times the clinical equivalent dose of YQFM, there were mild increase in the blue staining rate and the levels of His and 5-hydroxytryptamine (5-HT) and inflammation, indicating that YQFM had few allergic reactions within a proper dosage range (Gu et al., 2018). Clinical studies have also verified that adverse reactions of YQFM mainly correlated with inappropriate prescription, including beyond indications and dosages, unnecessary treatment, contraindications, and excessive quantities of solvent, etc (Zhao C. et al., 2018). Though YQFM reveals great safety and efficacy, it is worth noting that following the instructions strictly is the key to avoid adverse events.
Heart failure, the cumulative effect and endpoint of various cardiac abnormalities, eventually leads to the decline of cardiac pump function, putting up with the challenge to the exploration of effective strategy for the prevention and treatment of heart failure (Committee of Exports on Rational Drug Use National Health and Family Planning Commission of The People’Republic of China and Chinese Pharmacists Association, 2019). Long-term clinical practice verified that Traditional Chinese medicine exerts the composite effect through the multi-target and multi-link ways (Zhang RP., 2015). At present, there are plenty of fundamental research and clinical trials on YQFM, involving pharmacodynamic components, pharmacological effects, clinical application and quality markers. Research has demonstrated that YQFM improved cardiac function, inhibited ventricular remodeling, exerted great anti-inflammatory and anti-oxidative effect, regulated mitochondrial function, thereby improving the quality of life of patients with heart failure (Du et al., 2021). YQFM is widely used in the treatment of heart failure, with definite clinical efficacy and fewer adverse reactions, which provides a reference for rational clinical drug use (Figure 1).
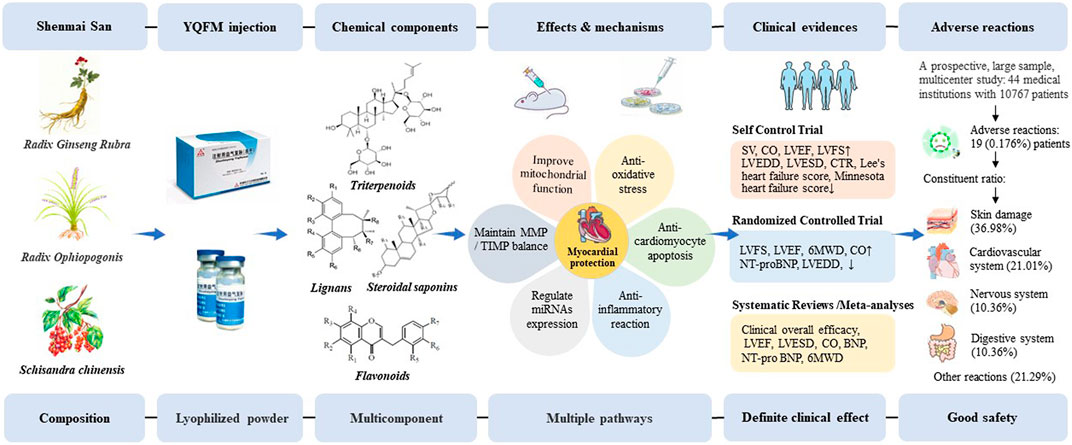
FIGURE 1. The process of YQFM in treating heart failure. BNP, brain natriuretic peptide; CO, cardiac output; CTR, calcitonin receptor; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVFS, left ventricular fractional shortening; MMP/TIMP, matrix metalloproteinases/tissue inhibitor of metalloproteinases; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SV, stroke volume; 6MWD, 6-min walking distance.
However, there are great gaps referring to the dose-effect relationship, pharmacological targets and mechanism of YQFM in the treatment of cardiovascular diseases waiting to be filled in. The multi-component and multi-target characteristics of Traditional Chinese Medicine raise the bar for the exploration of pharmacological mechanism of YQFM in the treatment of heart failure. At present, the pharmacological effects of different components of YQFM on heart failure remains unclear, and the research merely focuses on the study of ginsenosides. However, there are few studies on the pharmacological effects, mechanisms and targets of the two traditional Chinese medicines of Radix of Ophiopogon japonicus (Thunb.) Ker Gawl (Liliaceae), and Fructus of Schisandra chinensis (Turcz.) Baill (Schisandraceae), as well as the important active components of Ophiopogonins, Ophiopogon japonicus polysaccharide and Schizandrin A. What’s more, in some studies the dosage of YQFM is not adaptive for the clinical treatment, resulting in the mismatch between clinical practice and basic science. In the future, research ought to reveal the targets and related signaling pathways of YQFM and differ active components so as to provide scientific guidance for the application of YQFM in clinical practice. Besides, owning to the relatively low quality of clinical trials on YQFM in the treatment of heart failure, large-scale, multi-center, high-quality randomized controlled clinical trials and real world studies are badly in need. Finally, although there are few reports about the adverse effects of YQFM, non-standard prescription still an annoying epidemic in the clinical practice. Therefore, it is necessary to improve the legal system of drug reevaluation and the post-marketing supervision to guard the safety and efficacy of the application of drugs, so as to improve the efficacy of YQFM in the treatment of heart failure.
SL wrote the manuscript. YW and WZ searched and reviewed literature. HS conceived and designed the manuscript.
This work was supported by the China Postdoctoral Science Foundation (2022M710473) and National Key R&D Program of China (2017YFC1700400).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Cao, H. B., Hao, C. Y., Bi, J. F., Luo, R. Z., Xie, A. L., Xie, W., et al. (2019). Clinical safety monitoring study of yiqi fumai lyophilized injection. Drug Eval. Res. 42, 467–471. doi:10.7501/j.issn.1674-6376.2019.03.015
Cao, Z. H., Pan, J. H., Li, N., Qu, X. B., and Han, D. (2019). Modern pharmacological effect and mechanism of Shengmai san. Chin. J. Exp. Tradit. Med. Form. 25, 212–218. doi:10.13422/j.cnki.syfjx.20192208
Chinese Association of Integrative MedicineDoctor Society of integrative MedicineChinese Medical Doctor Association (2016). Expert consensus on Integrated Traditional Chinese and Western medicine diagnosis and treatment of chronic heart failure. Chin. J. Integr. Tradit. West Med. 36, 133–141. doi:10.7661/CJIM.2016.02.0133
Committee of Exports on Rational Drug Use National Health and Family Planning Commission of The People’Republic of ChinaChinese Pharmacists Association (2019). Guidelines for rational use of drugs of heart failure(2 edition). Chin. J. Front. Med. Sci. 11, 1–78. doi:10.12037/YXQY.2019.07-01
Du, H., Meng, Z. P., Yuan, J., Wan, M. X., Zhang, Y. X., Li, Z., et al. (2021). Pharmacological effects and clinical research progress of Yiqi Fumai Lyophilized Injection on cardiovascular diseases. Drug Eval. Res. 44, 2300–2307. doi:10.7501/j.issn.1674-6376.2021.11.002
Fan, G. H., Xing, Z. Y., Liu, M. L., Chen, S. Q., and Wang, M. X. (2021). Systematic evaluation and sequential analysis of efficacy and safety of yiqi fumai(lyophilization) injection in treatment of heart failure. J. Pract. Tradit. Chin. Intern Med. 35, 8–18. doi:10.13729/j.issn.1671-7813.Z20200508
Fan, M. M., Zhang, J. Y., Zhang, X. L., and Wang, S. (2020). Research progress on chemical components and pharmacological action of Radix ophiopogonis. Inf. Tradit. Chin. Med. 37, 130–134. doi:10.19656/j.cnki.1002-2406.200118
Feng, Y. Q., Ju, A. C., Liu, C. H., Wang, T., Yu, B. Y., and Qi, J. (2016). Protective effect of the extract of Yi-Qi-Fu-Mai preparation on hypoxia-induced heart injury in mice. Chin. J. Nat. Med. 14, 401–406. doi:10.1016/S1875-5364(16)30035-8
Fu, C. G., Liu, L. T., and Huang, M. Y. (2020). Chinese experts consensus on clinical applicat ion of Shengmai lnjection. Chin. J. Integr. Tradit. West Med. 40, 1430–1438. doi:10.7661/j.cjim.202001009.045
Gu, Y., Ju, A. C., Man, S. L., Wan, X. M., Li, D. K., and Gao, W. Y. (2018). Evaluating safety of yiqi fumai lyophilized injection. Drug Eval. Res. 41, 399–404. doi:10.7501/j.issn.1674-6376.2018.03.008
Hao, G., Wang, X., Chen, Z., Zhang, L., Zhang, Y., Wei, B., et al. (2019). Prevalence of heart failure and left ventricular dysfunction in China: The China hypertension survey, 2012-2015. Eur. J. Heart Fail. 21, 1329–1337. doi:10.1002/ejhf.1629
Hu, M. F., Kang, W. Q., Hu, X. Y., Zhang, H., and Song, D. L. (2014). An assessment of the curative effect of yiqi fumai (lyophilized) in the treatment of ischemic diastolic heart failure. Chin. Med. Pharm. 4, 7–11. doi:10.3969/j.issn.2095-0616.2014.16.003
Jiang, W. J., Zhao, Z. H., and Luo, J. (2018). Clinical observation on the treatment of ischemic cardiomyopathy and heart failure by injecting yiqi fumai (Freeze-dried) and Qiliqiangxin capsule. Hebei J. Tradit. Chin. Med. 40, 703–706. doi:10.3969/j.issn.1002-2619.2018.05.014
Ju, A. C., Luo, R. Z., Qin, X. P., Su, X. Q., Li, D. K., Zhou, D. Z., et al. (2018). Pharmacological effects and clinical research progress of Yiqi Fumai Lyophilized lnjection. Drug Eval. Res. 41, 354–364. doi:10.7501/j.issn.1674-6376.2018.03.002
Li, D. K., Su, X. Q., Li, Z., Li, L., Wan, M. X., Zhou, X. Q., et al. (2019). Study on quality marker of yiqi fumai lyophilized injection. Chin. Tradit. Herb. Drug 50, 290–298. doi:10.7501/j.issn.0253-2670.2019.02.004
Li, F., Pang, L. Z., Zhang, L., Zhang, Y., Zhang, Y. Y., Yu, B. Y., et al. (2019). YiQiFuMai powder injection ameliorates chronic heart failure through cross-talk between adipose tissue and cardiomyocytes via up-regulation of circulating adipokine omentin. Biomed. Pharmacother. 119, 109418. doi:10.1016/j.biopha.2019.109418
Li, K., Liu, Y. X., Shi, B. S., Peng, L. H., and Tao, J. Y. (2019). Analysis of yiqi fumai lyophilized injection application and adverse reaction. Drug Eval. Res. 42, 2075–2078. doi:10.7501/j.issn.1674-6376.2019.10.032
Li, K., Shi, B. S., Peng, L. H., and Tao, J. Y. (2018). Effect of Yiqi Fumai Lyophilized Injection on liver and kidney function in clinical application. Drug Eval. Res. 41, 425–427. doi:10.7501/j.issn.1674-6376.2018.03.013
Li, X. F., Wang, X. D., Wan, M. X., Li, Z., Li, D. K., Zhang, Y. W., et al. (2020). Establishment and preliminary verification of protective model of H9C2 cell injury induced by H2O2by Yiqi Fumai Lyophilized Injection. Drug Eval. Res. 43, 1510–1514. doi:10.7501/j.issn.1674-6376.2020.08.007
Li, Y., Jia, X. W., Liu, S. H., Feng, C. N., and Feng, H. P. (2019). Effect of yiqi fumai injection combined with atorvastatin on chronic heart failure patients with coronary heart disease and its effects on sCD40, sCD146 and PAPP-A. Chin. Arch. Tradit. Chin. Med. 37, 1225–1228. doi:10.13193/j.issn.1673-7717.2019.05.049
Lian, B. T., Li, Z. Z., Chen, J. C., Cai, Y. Y., and Ye, X. Q. (2016). Systematic review of yiqi fumai injection for patients with heart failure. Chin. J. Exp. Tradit. Med. Form. 22, 215–220. doi:10.13422/j.cnki.syfjx.2016080215
Liu, C., Ju, A., Zhou, D., Li, D., Kou, J., Yu, B., et al. (2016). Simultaneous qualitative and quantitative analysis of multiple chemical constituents in YiQiFuMai injection by ultra-fast liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Molecules 21, 640. doi:10.3390/molecules21050640
Liu, H. Y., Xu, Y. Q., Ouyang, T., Liu, W. L., Zhang, Y. M., and Xiao, X. H. (2018). Identification of saponins in yiqi fumai freeze-dried powder for injection by HPLC-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Form. 24, 7–12. doi:10.13422/j.cnki.syfjx.2018050007
Loehr, L. R., Rosamond, W. D., Chang, P. P., Folsom, A. R., and Chambless, L. E. (2008). Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am. J. Cardiol. 101, 1016–1022. doi:10.1016/j.amjcard.2007.11.061
Ma, N., Hou, Y. Z., Wang, X. L., and Mao, J. Y. (2015). Literature-based study of adverse effects of Yi-Qi-Fu-Mai sterile powder. Chin. J. New Drugs 24, 1197–1200.
Maggioni, A. P., Dahlström, U., Filippatos, G., Chioncel, O., Crespo Leiro, M., Drozdz, J., et al. (2013). EURObservational research programme: Regional differences and 1-year follow-up results of the heart failure pilot survey (ESC-HF pilot). Eur. J. Heart Fail. 15, 808–817. doi:10.1093/eurjhf/hft050
McDonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A., Böhm, M., et al. (2021). 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42, 3599–3726. doi:10.1093/eurheartj/ehab368
Pang, L. Z., Ju, A. C., Zheng, X. J., Li, F., Song, Y. F., Zhao, Y., et al. (2017). YiQiFuMai Powder Injection attenuates coronary artery ligation-induced myocardial remodeling and heart failure through modulating MAPKs signaling pathway. J. Ethnopharmacol. 202, 67–77. doi:10.1016/j.jep.2017.02.032
Stewart, S., MacIntyre, K., Hole, D. J., Capewell, S., and McMurray, J. J. (2001). More 'malignant' than cancer? Five-year survival following a first admission for heart failure. Eur. J. Heart Fail. 3, 315–322. doi:10.1016/s1388-9842(00)00141-0
Su, H. Y., and Wang, K. L. (2018). Clinical effect of Yiqi Fumai Injection combined with statins in the treat-ment of chronic heart failure complicated with atrial fibrillation. Chin. Med. Her. 15, 70–73. CNKI:SUN:YYCY.0.2018-35-017.
Sun, J., Qi, X. F., Zhang, L., Chen, X. M., and Ma, P. Z. (2019). A post-marketing surveillance study on patients using Yiqi Fumai Injection (freeze-dried) in a hospital. Chin. J. Hosp. Pharm. 39, 97–100. doi:10.13286/j.cnki.chinhosppharmacyj.2019.01.22
Sun, L. F., An, D. Q., and Guo, L. L. (2016). Advantages and characteristics of traditional Chinese medicine treatment of heart failure. J. Emerg. Tradit. Chin. Med. 25, 452–456. doi:10.3969/j.issn.1004-745X.2016.03.025
Sun, L. J., Zheng, X. K., and Hao, C. Y. (2012). Multicentre clinical research of Yiqifumai Injection in treatment of coronaryheart disease heart failure. Chin. Mod. Med. 19, 7–10. doi:10.3969/j.issn.1674-4721.2012.17.003
Wang, L. L., Zhuge, X., and Lv, X. (2018). Safety of yiqi fumai lyophilized injection in elderly patients. Drug Eval. Res. 41, 422–424. doi:10.7501/j.issn.1674-6376.2018.03.012
Wang, W., Su, G. Y., Hu, W. Q., and Zhao, Y. Q. (2016). Research progress in pharmacological effects of ginsenoside on cardiovascular diseases in last decade. Chin. Tradit. Herb. Drug 47, 3736–3741. doi:10.7501/j.issn.0253-2670.2016.20.029
Wang, X. L., Ma, N., Hou, Y. Z., and Mao, J. Y. (2016). Meta-analysis of the curative effect of Yiqi Fumai for injection (freeze-dried) combined with Western medicine in the treatment of chronic heart failure. J. Tradit. Chin. Med. 57, 391–395. doi:10.13288/j.11-2166/r.2016.05.008
Wang, X. D., Wan, M. X., Zuo, N., Xu, Q., Li, Z., Li, D. K., et al. (2020). Rationality of Yiqi Fumai Lyophilized Injection against doxorubicin inducedacute myocardial injury in rats. Drug Eval. Res. 43, 1522–1527. doi:10.7501/j.issn.1674-6376.2020.08.009
Wang, Y., Liu, Y. X., Yue, H. S., Xu, W. Y., Cao, J. M., Jin, H. Y., et al. (2020). Comparison between charged aerosol detector and evaporative light scattering detector for analysis of sugar in Zhusheyong Yiqi Fumai and study on accuracy of methods. Chin. J. Chin. Mater. Med. 45, 5511–5517. doi:10.19540/j.cnki.cjcmm.20200221.306
Wang, Y. Q., Liu, C. H., Zhang, J. Q., Zhu, D. N., and Yu, B. Y. (2013). Protective effects and active ingredients of yi-qi-fu-mai sterile powder against myocardial oxidative damage in mice. J. Pharmacol. Sci. 122, 17–27. doi:10.1254/jphs.12261fp
Wang, Y. Y., Kong, J. J., Yuan, L., Zhu, B., Xi, X. W., Ran, Y. L., et al. (2021). Clinical observation on yiqi fumai lnjection in the treatment of acute heart failure(qi-yin deficiency syndrome). Chin. J. Integr. Med. Cardio -/Cerebrova Dis. 19, 2142–2145. doi:10.12102/j.issn.1672-1349.2021.13.004
Wu, H. Y. (1997). Pharmacological study and clinical application of Shengmai powder in the prevention and treatment of cardiovascular diseases. Chin. Tradit. Pat. Med. 19, 2. CNKI:SUN:ZCYA.0.1997-03-021.
Xie, N., and Dai, X. H. (2019). Meta analysis on curative effects of yiqi fumai injection (lyophilization) for heart failure. Chin. J. Integr. Med. Cardio -/Cerebrova Dis. 17, 1499–1503. doi:10.12102/j.issn.1672-1349.2019.10.016
Xing, L., Jiang, M., Dong, L., Gao, J., Hou, Y., Bai, G., et al. (2013). Cardioprotective effects of the YiQiFuMai injection and isolated compounds on attenuating chronic heart failure via NF-κB inactivation and cytokine suppression. J. Ethnopharmacol. 148, 239–245. doi:10.1016/j.jep.2013.04.019
Xing, N. N., Qu, H. D., Ren, W. C., and Ma, W. (2021). Main chemical constituents and modern pharmacological action of schisandrae chinensis Fructus: A review. Chin. J. Exp. Tradit. Med. Form. 27, 210–218. doi:10.13422/j.cnki.syfjx.20211407
Xiong, Y., Xu, C., Chen, J. Y., and Yang, Y. (2017). Meta-analysis of Yiqi Fumai for Injection (lyophilized) in the treatment of heart failure. J. Shenyang Pharm. Univ. 34, 428–435. doi:10.14066/j.cnki.cn21-1349/r.2017.05.013
Yang, Y. J., Zhang, Y., Lv, J., Xing, K., and Tian, G. (2016). Treatment of 30 cases of senile coronary heart disease complicated with chronic heart failure by yiqi fumai injection. Shanxi J. Tradit. Chin. Med. 37, 1325–1326. doi:10.3969/j.issn.1000-7369.2016.10.027
Yin, K. K., Gou, X. B., Wan, M. X., Zhang, Y. X., Li, D. K., Kou, J. P., et al. (2020). Research progress on pharmacological effects of Shengmai prescription on heart failure. Drug Eval. Res. 43, 1501–1505. doi:10.7501/j.issn.1674-6376.2020.08.005
Zeng, Y. J., Zhao, X. C., Wan, M. X., Li, D. K., Li, Z., Song, M. Z., et al. (2018). Protective effect of yiqi fumai lyophilized injection against doxorubicin induced cardiotoxicity in H9c2 (2-1) cell. Drug Eval. Res. 41, 380–385. doi:10.7501/j.issn.1674-6376.2018.03.005
Zhang, C. X., Wang, S. Y., Zhao, T. F., and Zhang, M. (2020). Effect of Yiqi Fumai for injection (freeze-dried) on 6-min walking test distance of patients after cardiac valve replacement. Chin. J. Integr. Med. Cardio -/Cerebrova Dis. 18, 462–465. doi:10.12102/j.issn.1672-1349.2020.03.021
Zhang, F., Qiao, X., Lu, H., Zhang, S., Du, W., and Xiao, X. (2018). Application of a sensitive and specific LC-ESI-MS/MS method for the simultaneous quantification of twelve bioactive components in dog plasma for an intravenous pharmacokinetic study of Yiqifumai Injection in beagle dogs. Biomed. Chromatogr. 32, e4256. doi:10.1002/bmc.4256
Zhang, L., Su, X. Q., Li, D. K., Yue, H. S., Ju, A. C., Yang, F., et al. (2021). Verification of quality marker in Yiqi Fumai Lyo ophilized Injection based on clinical efficacy. Chin. Tradit. Herb. Drug 52, 5741–5750.
Zhang, P. (2015). Advantages, disadvantages, and trend of integrative medicine in the treatment of heart failure. Cell biochem. Biophys. 72, 363–366. doi:10.1007/s12013-014-0466-7
Zhang, P. Y., and Li, Z. G. (2014). Advantages, disadvantages and trends of integrated traditional Chinese and Western medicine in the treatment of heart failure. J. Tradit. Chin. Med. 55, 449–450. doi:10.13288/j.11-2166/r.2014.05.025
Zhang, Q. Y., Wang, B. H., Liu, W. S., and Deng, Y. F. (2015). Effect of yiqi fumai recipe on heart function and heart failure markers in rats with chronic heart failure. Liaoning J. Tradit. Chin. Med. 42, 2233–2235. doi:10.13192/j.issn.1000-1719.2015.11.070
Zhang, Q. Y., Wang, B. H., Liu, W. S., and Deng, Y. F. (2016). Regulative effects of yiqifumai injection on the activity of matrix metalloproteinase in rats with chronic heart failure. Chin. J. Integr. Med. Cardio -/Cerebrova Dis. 14, 825–829. doi:10.3969/j.issn.1672-1349.2016.08.009
Zhang, R. P. (2015). Observation on the curative effect of Yiqi Fumai injection in the treatment of chronic heart failure in the elderly. Chin. J. Integr. Med. Cardio -/Cerebrova Dis. 13, 1647–1648. doi:10.3969/j.issn.1672-1349.2015.14.022
Zhang, Y., Zhang, L., Zhang, Y., Fan, X., Yang, W., Yu, B., et al. (2019). YiQiFuMai powder injection attenuates coronary artery ligation-induced heart failure through improving mitochondrial function via regulating ROS generation and CaMKII signaling pathways. Front. Pharmacol. 10, 381. doi:10.3389/fphar.2019.00381
Zhao, C., Zhai, J. H., Zhang, J., and Zhang, Y. K. (2018). An analysis of off label medication in the elderly inpatients in our hospitai with the use of Yiqi-fumai for Injection. Chin. J. Hosp. Pharm. 38, 2469–2473. doi:10.13286/j.cnki.chinhosppharmacyj.2018.23.18
Zhao, Y., Li, Y., Tong, L., Liang, X., Zhang, H., Li, L., et al. (2018). Analysis of microRNA expression profiles induced by yiqifumai injection in rats with chronic heart failure. Front. Physiol. 9, 48. doi:10.3389/fphys.2018.00048
Zheng, H. R., Chu, Y., Zhou, D. Z., Ju, A. C., Li, W., Li, X., et al. (2018). Integrated pharmacokinetics of ginsenosides after intravenous administration of YiQiFuMai powder injection in rats with chronic heart failure by UFLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1072, 282–289. doi:10.1016/j.jchromb.2017.10.056
Zhou, D. D., Jiang, S. J., Tong, L., Yang, Y. W., Wang, G. L., Ye, Z. L., et al. (2009). Quantitative determination of eight major constituents in the traditional Chinese medicinal Yi-Qi-Fu-Mai preparation by LC. Chroma. 70, 969–974. doi:10.1365/s10337-009-1252-3
Zhou, D., Tong, L., Wan, M., Wang, G., Ye, Z., Wang, Z., et al. (2011). An LC-MS method for simultaneous determination of nine ginsenosides in rat plasma and its application in pharmacokinetic study. Biomed. Chromatogr. 25, 720–726. doi:10.1002/bmc.1508
Zhou, L., Fan, F. F., Patiguli, A., Zheng, L. L., and Xu, H. S. (2016). A Meta-analysis on effectiveness and safety of Yiqifumai Injection in the treatment of heart failure. Chin. Med. Her. 13, 93–96. CNKI:SUN:YYCY.0.2016-22-023.
Zhou, Y. Y., Jiao, Y. T., Wang, Y. S., Hou, Y. Y., Li, D. K., Zhou, D. Z., et al. (2018). Chemical compositions analysis for yiqi fumai lyophilized injection byUPLC-Q-TOF/MS. Drug Eval. Res. 41, 446–450. doi:10.7501/j.issn.1674-6376.2018.03.016
Ach acetylcholine
Akt protein kinase B
ALT alanine aminotransferase
AMPK adenine monophosphate activated protein kinase
ANP atrialnatriureticpeptide
ARNI angiotensin receptor neprilysin inhibitor
AST aspartate aminotransferase
ATP adenosinetriphosphate
BNP brain natriuretic peptide
CACNA1C calcium voltage-gated channel subunit α1C
CAL coronary artery ligation
CK creatine kinase
CK-MB creatine kinase-MB
CO cardiac output
CPP copeptin
Cr creatinine
Drp1 dynamin-related protein one
E/A early diastolic peak flow velocity/late diastolic peak flow velocity
E/e' early mitral filling velocity/early diastolic mitral annular velocity
EF ejection fraction
ERK1/2 extracellular signal-regulated kinase 1/2
FS fractional shortening
His histamine
HPLC high-performance liquid chromatography
HPLC-Q-TOF-MS HPLC coupled with quadrupole time-of-flight tandem mass spectrometry
HYP hydroxyproline
IL-1β interleukin-1β
IL-6 interleukin-6
JNK C-Jun Kinase enzyme
LC-ESI-MS liquid chromatography electrospray ionization mass spectrometry
LDH lactic dehydrogenase
LMS liquid-mass spectrometry
LVEDD left ventricular end-diastolic diameter
LVEF left ventricular ejection fraction
LVESD left ventricular end-systolic diameter
LVPWs left ventricular posterior wall in the systolic
LVFS left ventricular fractional shortening
MAPKs mitogen-activated protein kinases
MDA malondialdehyde
Mfn2 mitochondrial fusion protein two
MMP/TIMP matrix metalloproteinases/tissue inhibitor of metalloproteinases
MPO myeloperoxidase
NF-κB nuclear factor kappa-B
NO nitric oxide
NOX2 NADPH oxidase two
NT-proBNP N-terminal pro-B-type natriuretic peptide
p-CaMKII phosphorylation of calmodulin dependent protein kinase II
PI3K phosphatidylinositol 3-kinase
PIIINP amino-terminal pro-peptide of pro-collagen type III
ROS reactive oxygen species
sCD146 soluble CD146
sCD40 soluble CD40
SGLT2i sodium glucose cotransporter two inhibitor
SOD superoxide dismutase
SV stroke volume
TBil total bilirubin
TNF-α tumor necrosis factor-alpha
UFLC-IT-TOF/MS ultrafast liquid chromatography-ion trap time-of-flight MS
UPLC-Q-TOF/MS ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry
YQFM the YiQiFuMai injection
5-HT 5-hydroxytryptamine
6 MWD 6-min walking distance.
Keywords: heart failure, YiQiFuMai injection, chemical components, clinical evidences, mechanisms
Citation: Lv S, Wang Y, Zhang W and Shang H (2022) The chemical components, action mechanisms, and clinical evidences of YiQiFuMai injection in the treatment of heart failure. Front. Pharmacol. 13:1040235. doi: 10.3389/fphar.2022.1040235
Received: 09 September 2022; Accepted: 14 November 2022;
Published: 24 November 2022.
Edited by:
Qing Yong He, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Hua Yang, China Pharmaceutical University, ChinaCopyright © 2022 Lv, Wang, Zhang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongcai Shang, c2hhbmdob25nY2FpQGZveG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.