
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 12 January 2023
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1030693
Background: Pulmonary arterial hypertension (PAH) is an incurable pulmonary disease that might result in right heart failure and death. Treatment guidelines recommend upfront or sequential combination therapy for patients with PAH. Recently, several PAH-targeted medications have been approved in Taiwan. This study aimed to investigate treatment patterns and medication adherence in real-world settings.
Method: This was a new-user design study on patients treated with PAH-specific medication between 1 January 2014, and 31 December 2019. Data were extracted from the National Health Insurance Research Database. Medication adherence was evaluated by the proportion of days covered (PDC). Adherence was defined as PDC ≥ .8. Statistical analyses were performed to compare the study outcomes. Logistic regression analysis was performed to identify the association between baseline characteristics and adherence. P < .05 indicated statistical significance.
Results: A total of 1,900 patients with PAH were identified, and 75.3% of them were females. The mean (standard deviation (SD)) age was 57.2 (17.5) years. Only 23 (1.2%) patients began the initial combination therapy. A total of 148 (7.8%) patients switched their initial treatment to another treatment, and 159 (8.4%) patients had sequential combination therapy. The most common combination therapy was endothelin receptor antagonist (ERA) plus phosphodiesterase-5 inhibitor (PDE5i), mostly macitentan plus sildenafil, for initial or sequential combination. The mean (SD) PDC was .71 (.33), and 1,117 (58.8%) patients were adherent. A significant difference in mean PDC was observed between initial ERA users and PDE5i users (p < .0001). No factor was significantly associated with medication adherence.
Conclusion: Patients with PAH mostly initiated sildenafil as monotherapy, and macitentan was added as a sequential combination therapy. The initial ERA and combination groups showed higher medication adherence. Further investigations are needed to identify other factors associated with adherence.
Pulmonary arterial hypertension (PAH) is a rare, progressive, and incurable pulmonary vascular disease characterized by pulmonary microvascular remodeling, resulting in higher pulmonary vascular resistance and right ventricular hypertrophy. This progressive condition can result in right heart failure and death if left untreated (Hassoun, 2021). In Taiwan, the estimated incidence of PAH was 501 cases per million from 1999 to 2011 (Chang et al., 2016). The treatment guidelines recommend oral monotherapy, including endothelin receptor antagonists (ERA), phosphodiesterase-5 inhibitors (PDE5i), and soluble guanylate cyclase stimulators (sGCs), or oral combination therapy for patients with low or intermediate risk and combination therapy, including intravenous prostacyclin analogs (PCA), for patients with high risk (Galiè et al., 2015a). Several PAH-specific medications have been approved and reimbursed for PAH by the Taiwan Food and Drug Administration and Taiwan National Health Insurance (TNHI) since 2015. However, utilization of these PAH-targeted treatments for patients with PAH is unclear in Taiwan. Furthermore, medication adherence and persistence are important issues for managing chronic diseases. Factors associated with PAH medication adherence remain controversial. Thus, this study aimed to investigate the treatment patterns and medication adherence of PAH-specific medications and identify any factors associated with medication adherence among patients with PAH.
This was a new-user design and 1-year follow-up cohort study. All data were extracted from the National Health Insurance Research Database (NHIRD), a single health insurance claims database covering more than 99.6% of the general population in Taiwan. The healthcare information in the database includes outpatients (emergency room utilization is part of the outpatient data), hospitalizations, dental services, and prescription drugs. The study period was from 1 January 2013, to 31 December 2020. This study was approved by the Institutional Review Board (IRB) of Kaohsiung Medical University Hospital (IRB number: KMUHIRB-E(I)-20220061).
Patients with PAH were identified using 1) the International Classification of Diseases, 9th or 10th Revision, Clinical Modification (ICD-9/10-CM) diagnosis codes of pulmonary hypertension (PH) and diseases associated with PAH, 2) ICD-9/10-CM diagnosis codes of chronic thromboembolic pulmonary hypertension (CTEPH)-related conditions, and 3) TNHI claim codes of PAH-targeted medication. The first prescription date of any PAH-targeted medication was defined as the index date. The prescribed medication within 30 days after the index date was defined as the index treatment.
The inclusion criteria included patients with ≥1 claim for PAH-targeted medication between 1 January 2014, and 31 December 2019 (identification period), and patients with ≥1 claim for PH- or PAH-associated diseases within 6 months before or 1 month after the index date (eligibility period). The exclusion criteria included patients with ≥1 claim for CTEPH-related conditions (e.g., pulmonary embolism or deep vein thrombosis) within the eligibility period and their index treatment including riociguat, previous PAH-specific medication user before 1 January 2014 (washout period, a 1-year period where no PAH-targeted medication prescribed before the index date), patients with <2 claims for PAH-targeted medication during the follow-up period, age under 20 years on the index date, and missing data on gender or age. The follow-up period was censor, death, or 1 year after index date.
All data of sociodemographic information, including age, gender, level of National Health Insurance (NHI) premiums, urbanization level of residence, and residential area, were collected from the “Registry for Beneficiaries” dataset.
The patients’ genders were identified as either male or female. The exact age on the index date was calculated by subtracting the year of birth from the year of the index date. Patients were divided into three age groups (20–39, 40–64, and ≥65 years). NHI premiums were categorized into ten groups based on the patients’ monthly income. Patients with NHI premiums ≤22,800 (NTD) were classified as low income, and those >22,800 (NTD) were classified as high income according to the minimum wage in Taiwan (Chen et al., 2020).
More than 350 townships were categorized into a 7-level development stratification (Liu et al., 2006). Levels 1–2, 3–4, and 5–7 were classified as urban, suburban, and rural, respectively, according to a previous study (Cheng et al., 2019). The residential area of each patient was classified as northern, central, southern, and western areas.
The baseline comorbidities of PAH cohorts were collected from “Inpatient Expenditures by Admission” and “Ambulatory Care Expenditures by Visits” datasets and identified using the modified Elixhauser Comorbidity Index (Elixhauser et al., 1998; Moore et al., 2017). These comorbidities were defined as having ≥2 outpatient or ≥1 inpatient claims with ICD-9/10-CM diagnosis codes of these comorbidities within a year before and including the index date. The number of Elixhauser comorbidities was calculated and was categorized into three levels (0, 1-2, and ≥3).
The clinical examination records, including echocardiography, chest X-ray, and right heart catheterization (RHC), were collected from “Inpatient Expenditures by Admission” and “Ambulatory Care Expenditures by Visits” datasets and were identified using ICD-9-CM diagnosis codes, ICD-10 Procedure Coding System (ICD-10-PCS) codes, and TNHI claim codes (defined as having ≥1 claim within the eligibility period).
All the data on study outcomes were collected from the “Inpatient Expenditures by Admission,” “Ambulatory Care Expenditures by Visits,” “Details of Inpatient Orders,” and “Details of Ambulatory Care Orders” datasets.
Treatment with one category of PAH-targeted medication was defined as monotherapy, and treatment with two or more categories of PAH-targeted medication overlapping more than one day was defined as combination therapy. The index regimen was identified as the PAH-targeted medication used by the patients within 30 days after and including the index date. Treatment with other kinds of PAH-targeted medication different from the initial treatment was defined as switching. Any PAH-targeted medication added to the initial treatment was defined as a sequential combination. Stopping using PAH-targeted medication for more than 90 days (grace period) after running out of pills was defined as discontinuation. The 90-day interval for the treatment pattern of the Sankey diagram was based on the grace period. Day 0 indicates the initial/index treatment. Day 90, 180, and 270 indicates the first regimen within 90–179, 180–269, and 270–359 days after the index date.
Adherence to the initial monotherapy was evaluated using the proportion of days covered (PDC) calculated by dividing the number of days of available medication by the number of days of the follow-up period (Nau, 2012). This study presented two definitions of the denominator for PDC calculation. First, PDC1 was defined as the number of days of available medication divided by 365 days (1-year follow-up). Second, PDC2 was defined as the number of days of available medication divided by the number of days from the index date to the treatment modification, treatment discontinuation, or end of the last prescription (Prieto-Merino et al., 2021). PDC for the initial combination therapy was based on possession of any medication, which meant days covered by at least one medication in the regimen (Basak et al., 2014). PDC ≥ .8 was defined as adherent.
Categorical variables were presented as numbers and percentages, whereas continuous variables were presented as means and standard deviations (SD) or median and interquartile range (IQR). Kruskal–Wallis H tests were performed to analyze PDC1 or PDC2 between the four index treatment groups, and the Chi-square or Fisher’s exact tests were performed to analyze the frequency of medical adherence (PDC≥.8 and PDC<.8) between four index group. Treatment patterns were analyzed descriptively and illustrated using Sankey diagrams. Logistic regression analysis was performed to assess the factors associated with adherence, and goodness of fit statistics for the logistic regression by C-statistic and Hosmer-Lemeshow test.
All statistical analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina, United States). All the statistical analyses were two-tailed, and a p-value ≤ .05 was considered significant.
A total of 5,181 patients were prescribed at least one PAH-specific medication between 1 January 2014, and 31 December 2019. After applying the exclusion criteria, 1,900 patients were involved in the analysis. Figure 1 shows the study population selection.
A total of 1,900 new and sustained users were included in the investigation of baseline characteristics. The mean (SD) age was 57.2 (17.5) years, with a predominance of female patients (n = 1,430; 75.3%). The percentage of patients aged 40–64 years was slightly higher than those aged ≥65 years (n = 839; 44.1% vs. n = 693; 35.5%). Most patients lived in the urban (n = 1,131; 59.5%) and northern areas (n = 882; 46.4%). The proportion of insurance premiums was similar between low-income and high-income groups (n = 949; 49.9% vs. n = 951; 50.1%). Over 90% of the patients had performed echocardiography (n = 1,867; 98.3%) and chest X-ray (n = 1,786; 94.0%), but only one-third of them had conducted RHC (n = 679; 35.7%). Most patients had 3 or more comorbidities (n = 1,205; 63.4%). Table 1 shows the baseline characteristics.
Initial monotherapy was more common than initial combination therapy (n = 1,877; 98.8% vs. n = 23; 1.2%). Of the 1,877 patients with initial monotherapy, 94.8% (n = 1,779) received PDE5i/sGCs, followed by ERA and PCA (n = 82; 4.4% and n = 16; .8%). Moreover, sildenafil was the most popular medication prescribed as initial monotherapy (n = 1,764; 94.0%). During the 1-year follow-up period, the proportion of dual or triple combination therapy increased from 1.2% (n = 23) to 8.6% (n = 163).
Of those treated with ERA initially, 81.7% (n = 67) remained on the same regimen 90 days after the index date. Similar results were also observed in the initial PDE5i/sGCs treatment and initial PCA treatment groups (n = 1,349; 75.8% and n = 13; 81.3%). After one year of follow-up, more than a half of the patients remained initial treatment (ERA 69.5%, PDE5i/sGCs 57.1%, and PCA 68.8%, respectively). Figure 2 shows the treatment patterns from the index date to 365 days after the index date.
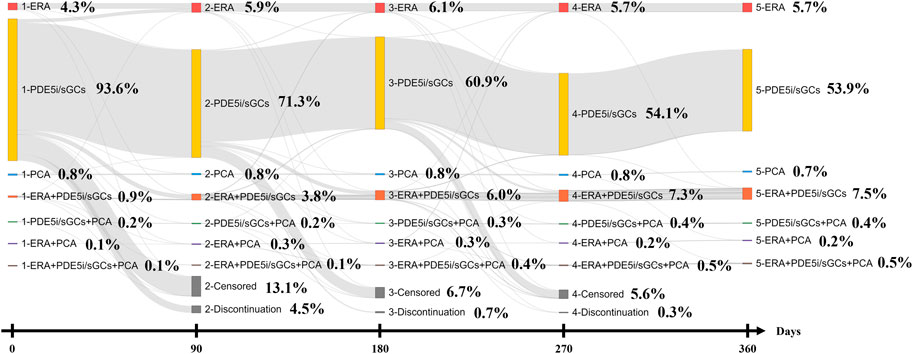
FIGURE 2. Treatment patterns of Sankey diagram (The 90 days interval for Sankey diagram was based on the grace period. Day 0 indicates the initial/index treatment. Day 90, 290, 270 indicates the first regimen within 90–179, 180–269, and 270–359 days after the index date).
Of the 1,900 patients, 148 (7.8%) changed their initial treatment. Of these patients, 84.5% (n = 125) were treated with sildenafil, and 52.7% (n = 78) switched medication from PDE5i to ERA monotherapy. Macitentan (n = 47; 31.8%) was the most popular ERA for switching, followed by bosentan (n = 30; 20.3%) and ambrisentan (n = 4; 2.7%).
Sequential combination therapy was typically dual therapy, with 142 (89.3%) patients being treated with ERA plus PDE5i combination. Only 7 (4.4%) patients had triple therapy as a second regimen.
The mean (SD) and median (IQR) of PDC1 for all patients were .71 (.33) and .89 (.45–.98), which were lower than those of PDC2 (.89 (.15), and .96 (.84–1.00)). Additionally, the percentage of adherent patients (PDC ≥.8) was 58.8% (n = 1,117) in PDC1, which was lower than that in PDC2 (n = 1,526; 80.3%). The goodness of fit of the logistic regression model showed fitting the data moderately well for PDC1 (c-statistic = .568; Hosmer-Lemeshow test p = .4405; Supplementary eTables S1, S2) and PDC 2 (c-statistic = .577; Hosmer-Lemeshow test p = .2651; Supplementary eTables S3, S4).
The mean (SD) of PDC1 and PDC2 of the initial ERA treatment group was significantly higher than that of the initial PDE5i/sGCs treatment group (.88 (.18) vs. .70 (.33); p < .0001 and .94 (.11) vs. .89 (.15); p = .0001). Besides, the mean (SD) of PDC2 of the initial combination group was significantly higher than that of the PDE5i/sGCs group (.96 (.06) vs. .89 (.15); p = .0156). Similar results were found in the percentage of adherent patients between initial treatment groups (p < .05) (Table 2).
The logistic regression analysis showed no significant association between good adherence (PDC1 ≥ .8) and baseline and clinical characteristics, including age (odds ratio (OR) .99; 95% CI .98–1.00; p = .1585), age group (OR 1.09; 95% CI .74–1.59; OR 1.02; 95% CI .53–1.93; p = .7538), gender (OR 1.12; 95% CI .90–1.39; p = .3044), index treatment (OR 2.66; 95% CI .97–7.29; p = .0571), diagnosis group (OR .91; 95% CI .75–1.12; p = .3765), comorbidity index (OR 1.39; 95% CI .80–2.42; OR 1.35; 95% CI .78–2.34; p = .5061), insurance premium (OR 1.12; 95% CI .93–1.35; p = .2276), urbanization level of residence (OR .85; 95% CI .60–1.20; OR .89; 95% CI .63–1.26; p = .6428), and residential area (OR .78; 95% CI .41–1.48; OR .59; 95% CI .31–1.12; OR .88; 95% CI .47–1.66). Furthermore, results showed no association between adherence with PDC2 and baseline and clinical characteristics (Table 3).
This new-user design and 1-year follow-up cohort study analyzed a nationwide and population-based claims database in Taiwan to investigate the sociodemographic and clinical characteristics among patients with PAH and evaluate the treatment patterns, medication adherence, and persistence of PAH-specific medications in real-world settings.
This study showed that most patients initiated PDE5i/sGCs as monotherapy, especially sildenafil, which is consistent with findings reported by Burger et al. (2018), Studer et al. (2019), and Studer et al. (2020) (Burger et al., 2018; Studer et al., 2019; Studer et al., 2020). However, a study from the Japanese registry revealed 31.5% of 108 treatment-naїve patients received upfront dual or triple combination therapy. The higher proportion of index combination therapy might result from 65.7% of patients classified as World Health Organization functional class (WHO-FC) III-IV, and 41.2% of index combination therapy included intravenous epoprostenol (Tamura et al., 2017).
Upfront combination therapy was recommended based on the positive results from the AMBITION trial. This trial demonstrated that the combination of ambrisentan and tadalafil significantly reduced the time to composite clinical failure compared with monotherapy (hazard ratio (HR), .50; 95% CI, .35–.72; p < .001) (Galiè et al., 2015b). However, differences in initial treatment for patients with PAH were observed between the guideline recommendations and the findings of this study and other studies. Wissmüller et al. (2022) reported five main reasons for taking a single medication among 182 patients managed at experienced PH care centers, including 1) failure to escalate due to intolerability, 2) being at low risk and having a favorable response to monotherapy, 3) having mild PAH syndrome, 4) being older and having multiple comorbidities, and 5) lack of satisfactory evidence of combination therapy for some subgroups of PAH (HIV, portal hypertension, and CHD) (Wissmüller et al., 2022). In this study, the mean (SD) age of patients with PAH was 57.5 (17.8) years, and only one-fifth of them were aged <40 years. Moreover, 64.3% of these patients had three or more comorbidities that might reduce the attempts to initiate combination therapy. Another reason is related to the predominance of initial monotherapy in Taiwan. Unlike most PAH-specific medications, physicians do not need prior authorization to prescribe sildenafil for patients with PAH. Furthermore, the combination therapy was not recommended by TNHI unless the disease progressed or the treatment outcome failed. Our findings revealed that whether to initiate monotherapy or combination therapy depended on the disease severity and the local reimbursement rule.
This study investigated the second regimen for those who had treatment modifications during the one-year follow-up period and reported 307 (16.2%) patients changed their initial treatment. Over half of the patients (51.8%) added another PAH-specific medication to the index regimen. Most of the regimens were ERA plus PDE5i (96.9%), especially macitentan plus sildenafil (47.8%), followed by bosentan plus sildenafil (32.1%). The results are aligned with the guideline’s recommendation of sequential combination therapy and are similar to the findings from US databases (Burger et al., 2018; Studer et al., 2019; Studer et al., 2020).
Several randomized controlled trials (RCTs) have been conducted to evaluate the difference in long-term event-free survival as the primary outcome between combination therapy and monotherapy (Pulido et al., 2013; Galiè et al., 2015b; McLaughlin et al., 2015; Sitbon et al., 2015). A meta-analysis of those RCTs demonstrated a significant risk reduction for clinical worsening among patients treated with combination therapy compared with monotherapy (risk ratio (RR) .65; 95% CI .58–.72; p < .00001) (Lajoie et al., 2016). This finding supported sequential combination therapy for patients with PAH treated with monotherapy. Although several network meta-analysis studies revealed inconsistent conclusions about which monotherapy or combination therapy was the best regimen, ERA and PDE5i combination therapy might be one of the better options (Lin et al., 2018; Wang et al., 2018; Petrovič and Locatelli, 2020; Fu et al., 2021).
The SERAPHIN trial investigated the long-term composite outcomes of patients adding macitentan to the baseline treatment regimen, including sildenafil, and observed a significant risk reduction in the macitentan group (HR .55; 97.5% CI .39–.76; p < .001) (Pulido et al., 2013). A meta-analysis focusing on the effectiveness of ERA plus PDE5i combination, including bosentan plus sildenafil, reported that combination therapy significantly improved outcomes, including 6-minute walking distance (mean difference 15.64; 95% CI 2.67–28.61; p = .02) and time to clinical worsening (OR .56; 95% CI .41–.76; p = .0002), compared with monotherapy (Kirtania et al., 2019).
In this study, no significant association between adherence and baseline or clinical characteristics was found by logistic regression analysis. This finding is consistent with several studies that reported that age, gender, and socioeconomic status were not associated with medication adherence (Waxman et al., 2013; Kjellström et al., 2020). A previous study used the 8-item Morisky Medication Adherence Scale (MMAS-8) questionnaire to evaluate the factors and reported that older age, single-use medication, and higher comorbidities or co-medication had a positive association with adherence (Grady et al., 2018). However, the internal reliability testing showed that Cronbach’s alpha coefficient was .53 (less than the acceptable cut-off value of .7).
Although no significant factors were reported in this study, some differences were found between the initial treatment groups in PDC1 and PDC2. Several reasons should be considered to understand the difference between groups. First, the approval frequencies of medication administration for PAH were different between ERA and PDE5i/sGCs. The frequencies for the ERA group were ambrisentan QD, bosentan BID, and macitentan QD, while those for the PDE5i/sGCs group were sildenafil TID, tadalafil QD, and riociguat TID (Galiè et al., 2015a; Narechania et al., 2020). Since tadalafil was not approved in Taiwan, the medication administration frequency was lower in the ERA group than in the PDE5i/sGCs group (Burks et al., 2018). Although the impact of medication administration frequency on adherence between ERA and PDE5i/sGCs was not investigated, a study focusing on the factors associated with adherence in PDE5i users showed that tadalafil users had better adherence than sildenafil users due to the simpler frequency of medication administration (OR 2.59; 95% CI 1.60–4.22) (Waxman et al., 2013). Moreover, Grady et al. reported that higher administration frequency had a negative impact on medication adherence among patients with PAH (OR .39; 95% CI .24–.65) (Grady et al., 2018). Our study found higher medication adherence due to the lower administration frequency in the ERA group. Second, better clinical outcomes make patients more willing to take medication regularly (Burks et al., 2018). Besides, several meta-analyses demonstrated that upfront or sequential combination therapy improved clinical outcomes, such as 6-minute walking distance 6 MWD and time to clinical worsening, compared to monotherapy (Zhu et al., 2012; Lajoie et al., 2016; Pan et al., 2018; Kirtania et al., 2019). Third, those who had upfront or sequential combination therapy for PAH might have more severe disease status, such as WHO-FC III-IV, at the beginning or have disease progression. Thus, with increased awareness of the severity of the disease or knowledge of the disease itself, patients are more likely to take medication on demand and cooperate with the healthcare team (Burks et al., 2018; Ivarsson et al., 2018).
To the best of our knowledge, this research is the first population-based cohort study investigating PAH-specific medication prescribing patterns and evaluating medication adherence and persistence among patients with PAH in Taiwan.
The study population was identified from the NHIRD. All patients treated with PAH-targeted medication will be enrolled in the database. Namely, the findings from the current study could represent the clinical practice of PAH treatment in real-world settings.
However, this study has some limitations. First, the examination results, including echocardiography or RHC, were unavailable to confirm the diagnosis of PAH in the NHIRD. To ascertain the true diagnosis of patients with PAH, not only ICD-9/10-CM codes of PH- or PAH-associated diseases and NHI claim codes of PAH-specific medication were used, but also the specialty of the physician or enrollment of catastrophic illness registry were identified. The identification algorithm was validated by a previous study with a positive predictive value of 32.5–78.0% (Sprecher et al., 2020; Gillmeyer et al., 2021). Additionally, physicians need prior authorization to prescribe PAH-targeted medication, which means that patients’ medical charts would be reviewed before using most PAH-specific medications, except for sildenafil. Second, several baseline or clinical characteristics, such as smoking history, drinking habit, education status, hemodynamic data, and PH severity, could not be identified from the NHIRD. Thus, the association between medication adherence and these factors were not analyzed.
This study provided an overview of patients with PAH who were newly treated with PAH-specific medication and showed that most patients were aged 40–64 years, with a dominance of females. Almost all patients (98.8%) initiated monotherapy, and sildenafil was mostly prescribed. Only one-fifth of the patients underwent treatment switching or sequential combination, while the treatment regimen did not change in most patients at the end of the follow-up period. Of those who underwent sequential combination, nearly 90% were treated with ERA plus PDE5i combination.
The PDC was .89, and the adherent rate was 80.3%. Medication adherence was higher in the index ERA and combination groups than in the PDE5i/sGCs group. No factor was significantly associated with the proportion of adherent patients. Thus, further comprehensive investigations are needed to identify any other factors associated with adherent PAH patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
This study was approved by the IRB of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20220061). Because patients’ data could not be identified, the requirement for informed consent was waived.
CY-T and CY-C designed the research; CY-T implemented the research, analyzed statistics and drafted the manuscript; CW-S and CY-C interpreted data and revised the manuscript; HL-L and CY-C obtained funding. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The research reported in this publication was supported by a grant from Kaohsiung Medical University (KMU-S109032) and the Zuoying Branch of Kaohsiung Armed Forces General Hospital (KAFGH-ZY-A-110028).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/https://www.frontiersin.org/articles/10.3389/fphar.2022.1030693/full#supplementary-material
Basak, R., McCaffrey, D. J., Bentley, J. P., Przybyla, S. M., West-Strum, D., and Banahan, B. F. (2014). Adherence to multiple medications prescribed for a chronic disease: A methodological investigation. J. Manag. Care Spec. Pharm. 20 (8), 815–823. doi:10.18553/jmcp.2014.20.8.815
Burger, C. D., Ozbay, A. B., Lazarus, H. M., Riehle, E., Montejano, L. B., Lenhart, G., et al. (2018). Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the United States. J. Manag. Care Spec. Pharm. 24 (8), 834–842. doi:10.18553/jmcp.2018.17391
Burks, M., Stickel, S., and Galiè, N. (2018). Pulmonary arterial hypertension: Combination therapy in practice. Am. J. Cardiovasc Drugs 18 (4), 249–257. doi:10.1007/s40256-018-0272-5
Chang, W. T., Weng, S. F., Hsu, C. H., Shih, J. Y., Wang, J. J., Wu, C. Y., et al. (2016). Prognostic factors in patients with pulmonary hypertension-A nationwide cohort study. J. Am. Heart Assoc. 5 (9), e003579. doi:10.1161/JAHA.116.003579
Chen, C. Y., Wu, W. T., Wang, Y. L., and Liao, K. M. (2020). Statins for the treatment of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Front. Pharmacol. 11, 613761. doi:10.3389/fphar.2020.613761
Cheng, B. R., Chang, H. T., Lin, M. H., Chen, T. J., Chou, L. F., and Hwang, S. J. (2019). Rural-urban disparities in family physician practice patterns: A nationwide survey in taiwan. Int. J. Health Plann Manage 34 (1), e464–e473. doi:10.1002/hpm.2662
Elixhauser, A., Steiner, C., Harris, D. R., and Coffey, R. M. (1998). Comorbidity measures for use with administrative data. Med. Care 36 (1), 8–27. doi:10.1097/00005650-199801000-00004
Fu, W., He, W., Li, Y., Chen, Y., Liang, J., Lei, H., et al. (2021). Efficacy and safety of novel-targeted drugs in the treatment of pulmonary arterial hypertension: A bayesian network meta-analysis. Drug Deliv. 28 (1), 1007–1019. doi:10.1080/10717544.2021.1927243
Galiè, N., Barberà, J. A., Frost, A. E., Ghofrani, H. A., Hoeper, M. M., McLaughlin, V. V., et al. (2015). Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 373 (9), 834–844. doi:10.1056/NEJMoa1413687
Galiè, N., Humbert, M., Vachiery, J. L., Gibbs, S., Lang, I., Torbicki, A., et al. (2015). 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): Endorsed by: Association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur. Heart J. 37 (1), 67–119. doi:10.1093/eurheartj/ehv317
Gillmeyer, K. R., Nunez, E. R., Rinne, S. T., Qian, S. X., Klings, E. S., and Wiener, R. S. (2021). Development and validation of algorithms to identify pulmonary arterial hypertension in administrative data. Chest 159 (5), 1986–1994. doi:10.1016/j.chest.2020.12.010
Grady, D., Weiss, M., Hernandez-Sanchez, J., and Pepke-Zaba, J. (2018). Medication and patient factors associated with adherence to pulmonary hypertension targeted therapies. Pulm. Circ. 8 (1), 2045893217743616. doi:10.1177/2045893217743616
Hassoun, P. M. (2021). Pulmonary arterial hypertension. N. Engl. J. Med. 385 (25), 2361–2376. doi:10.1056/NEJMra2000348
Huang, W. C., Hsu, C. H., Sung, S. H., Ho, W. J., Chu, C. Y., Chang, C. P., et al. (2018). 2018 TSOC guideline focused update on diagnosis and treatment of pulmonary arterial hypertension. J. Formos. Med. Assoc. 118 (12), 1584–1609. doi:10.1016/j.jfma.2018.12.009
Ivarsson, B., Hesselstrand, R., Rådegran, G., and Kjellstrom, B. (2018). Adherence and medication belief in patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension: A nationwide population-based cohort survey. Clin. Respir. J. 12 (6), 2029–2035. doi:10.1111/crj.12770
Kirtania, L., Maiti, R., Srinivasan, A., and Mishra, A. (2019). Effect of combination therapy of endothelin receptor antagonist and phosphodiesterase-5 inhibitor on clinical outcome and pulmonary haemodynamics in patients with pulmonary arterial hypertension: A meta-analysis. Clin. Drug Investig. 39 (11), 1031–1044. doi:10.1007/s40261-019-00841-1
Kjellström, B., Sandqvist, A., Hjalmarsson, C., Nisell, M., Nasman, P., and Ivarsson, B. (2020). Adherence to disease-specific drug treatment among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. ERJ Open Res. 6 (4), 00299–02020. doi:10.1183/23120541.00299-2020
Lajoie, A. C., Lauzière, G., Lega, J. C., Lacasse, Y., Martin, S., Simard, S., et al. (2016). Combination therapy versus monotherapy for pulmonary arterial hypertension: A meta-analysis. Lancet Respir. Med. 4 (4), 291–305. doi:10.1016/S2213-2600(16)00027-8
Lin, H., Wang, M., Yu, Y., Qin, Z., Zhong, X., Ma, J., et al. (2018). Efficacy and tolerability of pharmacological interventions for pulmonary arterial hypertension: A network meta-analysis. Pulm. Pharmacol. Ther. 50, 1–10. doi:10.1016/j.pupt.2017.11.002
Liu, C-Y., Hung, Y-T., Chuang, Y-L., Chen, Y-J., Weng, W-S., Liu, J-S., et al. (2006). Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 4 (1), 1–22.
McLaughlin, V., Channick, R. N., Ghofrani, H. A., Lemarie, J. C., Naeije, R., Packer, M., et al. (2015). Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur. Respir. J. 46 (2), 405–413. doi:10.1183/13993003.02044-2014
Moore, B. J., White, S., Washington, R., Coenen, N., and Elixhauser, A. (2017). Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ elixhauser comorbidity index. Med. Care 55 (7), 698–705. doi:10.1097/MLR.0000000000000735
Narechania, S., Torbic, H., and Tonelli, A. R. (2020). Treatment discontinuation or interruption in pulmonary arterial hypertension. J. Cardiovasc Pharmacol. Ther. 25 (2), 131–141. doi:10.1177/1074248419877409
Nau, D. P. (2012). Proportion of days covered (PDC) as a preferred method of measuring medication adherence, 6. Springfield, VA: Pharmacy Quality Alliance, 25.
Pan, J., Lei, L., and Zhao, C. (2018). Comparison between the efficacy of combination therapy and monotherapy in connective tissue disease associated pulmonary arterial hypertension: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 36 (6), 1095–1102.
Petrovič, M., and Locatelli, I. (2020). Comparative effectiveness of pulmonary arterial hypertension drugs in treatment-naive patients: A network meta-analysis. J. Comp. Eff. Res. 9 (1), 7–22. doi:10.2217/cer-2019-0037
Prieto-Merino, D., Mulick, A., Armstrong, C., Hoult, H., Fawcett, S., Eliasson, L., et al. (2021). Estimating proportion of days covered (PDC) using real-world online medicine suppliers' datasets. J. Pharm. Policy Pract. 14 (1), 113. doi:10.1186/s40545-021-00385-w
Pulido, T., Adzerikho, I., Channick, R. N., Delcroix, M., Galie, N., Ghofrani, H. A., et al. (2013). Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 369 (9), 809–818. doi:10.1056/NEJMoa1213917
Sitbon, O., Channick, R., Chin, K. M., Frey, A., Gaine, S., Galie, N., et al. (2015). Selexipag for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 373 (26), 2522–2533. doi:10.1056/NEJMoa1503184
Sprecher, V. P., Didden, E. M., Swerdel, J. N., and Muller, A. (2020). Evaluation of code-based algorithms to identify pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients in large administrative databases. Pulm. Circ. 10 (4), 2045894020961713. doi:10.1177/2045894020961713
Studer, S., Hull, M., Pruett, J., Elliott, C., Tsang, Y., and Drake, W. (2020). Retrospective database analysis of treatment patterns among patients with pulmonary arterial hypertension. Pulm. Ther. 6 (1), 79–92. doi:10.1007/s41030-019-00106-4
Studer, S., Hull, M., Pruett, J., Koep, E., Tsang, Y., and Drake, W. (2019). Treatment patterns, healthcare resource utilization, and healthcare costs among patients with pulmonary arterial hypertension in a real-world US database. Pulm. Circ. 9 (1), 2045894018816294. doi:10.1177/2045894018816294
Tamura, Y., Kumamaru, H., Satoh, T., Miyata, H., Ogawa, A., Tanabe, N., et al. (2017). Effectiveness and outcome of pulmonary arterial hypertension-specific therapy in Japanese patients with pulmonary arterial hypertension. Circ. J. 82 (1), 275–282. doi:10.1253/circj.CJ-17-0139
Wang, S., Yu, M., Zheng, X., and Dong, S. (2018). A Bayesian network meta-analysis on the efficacy and safety of eighteen targeted drugs or drug combinations for pulmonary arterial hypertension. Drug Deliv. 25 (1), 1898–1909. doi:10.1080/10717544.2018.1523257
Waxman, A., Chen, S. Y., Boulanger, L., Watson, J. A., and Golden, G. (2013). Factors associated with adherence to phosphodiesterase type 5 inhibitors for the treatment of pulmonary arterial hypertension. J. Med. Econ. 16 (2), 298–306. doi:10.3111/13696998.2012.756399
Wissmüller, M., Xanthouli, P., Benjamin, N., Grünig, E., Richter, M. J., Gall, H., et al. (2022). Profiles and treatment patterns of patients with pulmonary arterial hypertension on monotherapy at experienced centres. ESC Heart Fail 9 (5), 2873–2885. doi:10.1002/ehf2.13804
Keywords: pulmonary arterial hypertension, treatment patterns, medication adherence, pharmacoepidemiogy, proportion of days covered
Citation: Tsai C-Y, Shen C-W, Lai H-L and Chen C-Y (2023) Adherence and treatment patterns of disease-specific drugs among patients with pulmonary arterial hypertension: A nationwide, new-user cohort study. Front. Pharmacol. 13:1030693. doi: 10.3389/fphar.2022.1030693
Received: 29 August 2022; Accepted: 21 December 2022;
Published: 12 January 2023.
Edited by:
Christos Kontogiorgis, Democritus University of Thrace, GreeceReviewed by:
Yunshan Cao, Gansu Provincial Hospital, ChinaCopyright © 2023 Tsai, Shen, Lai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chung-Yu Chen, amsyOTc1NTI1QGhvdG1haWwuY29t; Hsuan-Lin Lai, aHN1YW5saW4wMzMxQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.