- 1Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Rehabilitation Medicine, The People’s Hospital of Honghu, Honghu, China
- 3Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objective: To investigate the efficacy and safety of dexmedetomidine (DEX) for postoperative patient controlled intravenous analgesia (PCIA).
Measurements: Two investigators independently searched Pubmed, Embase, Scopus, Cochrane Library and CBM for randomized controlled trials of DEX for PCIA.
Main results: Thirty-seven studies with a total of 5,409 patients were included in this meta-analysis. Compared with analgesics alone, DEX for PCIA reduced pain score at 24 h [mean difference (MD) = −0.70; 95% confidence interval (CI): −0.85, −0.54; p < 0.00001, I2 = 90%] and 48 h postoperatively (MD = −0.43; 95% CI: −0.52, −0.34; p < 0.00001, I2 = 96%). Moreover, DEX reduced analgesics consumption during the first 24 h [standardized mean difference (SMD) = −0.25; 95% CI: −0.34, −0.16; p < 0.00001, I2 = 91%] and the number of resuscitation analgesics administered [odds ratio (OR) = 0.54; 95% CI: 0.44, 0.66; p < 0.00001, I2 = 72%]. Furthermore, DEX improved patient satisfaction (OR = 3.55; 95% CI: 2.36, 5.35; p < 0.00001, I2 = 60%), and reduced incidence of side effects, such as postoperative nausea and vomiting (PONV) (OR = 0.47; 95% CI: 0.39, 0.57; p < 0.00001, I2 = 59%) and pruritus after surgery (OR = 0.45; 95% CI: 0.30, 0.68; p = 0.0001, I2 = 0%). Besides, DEX attenuates inflammatory cytokine levels, such as IL-6 (MD = −5.73; 95% CI: −8.34, −3.12; p < 0.00001, I2 = 91%) and TNF-α (MD = −0.63; 95% CI: −0.76, −0.50; p < 0.00001, I2 = 89%). Finally, DEX increased the risk of bradycardia (OR = 1.66; 95% CI: 1.12, 2.45; p = 0.01, I2 = 15%), but the complication of hypotension did not differ between the two groups (OR = 1.30; 95% CI: 0.84, 2.04; p = 0.25, I2 = 0%).
Conclusion: DEX is used for postoperative PCIA analgesia, which can significantly improve the analgesic effect, effectively control postoperative inflammatory response, reduce the dosage and adverse reactions of analgesics, and improve postoperative patient satisfaction. Of course, the impact of the immunosuppressive effect of DEX on the prognosis of patients needs further study.
Systematic review registration: CRD42022340933, https://www.crd.york.ac.uk/prospero/.
Introduction
Patient controlled analgesia (PCA) is currently the most commonly used and ideal method for postoperative analgesia, with rapid onset of action, no analgesic blind spots, relatively stable blood concentrations, and timely control of burst pain by pulse (bolus) dose, meanwhile, it has the advantages of individualized medication and high patient satisfaction, and is suitable for moderate to severe pain after surgery (Pamela and Ronaldo, 2010) Patient controlled intravenous analgesia (PCIA) means that when the patient feels pain, press the start button (bolus) in the PCA pump to inject a set dose of drugs intravenously into the body through a computer-controlled micropump, which commonly used analgesics include opioids (morphine, oxycodone, hydromorphone, sufentanil, hydrocodone, fentanyl, butorfenol, desocine, etc.), tramadol or flurbiprofen axetil, ketorolac, etc. (Jeffrey, 2005). However, the single-drug application of PCIA often limits their widespread use due to poor pain control or the obvious adverse reactions associated with increasing the dose of analgesic drugs, for example, increasing the dosage of opioids in order to improve the analgesic effect often causes adverse reactions such as itching, vomiting, nausea and even respiratory depression (Motamed, 2022). Therefore, PCIA is based on analgesic drugs and is compatible with another adjuvant drug, which can improve the analgesic effect and reduce adverse drug reactions through synergistic effects, has become a popular PCA analgesic program worldwide (Song et al., 2013).
Dexmedetomidine (DEX) is a new type of highly selective α2 adrenergic receptor agonist with sedative, analgesic, anxiolytic and sympathetic inhibitory effects, and no obvious respiratory depression. Multiple meta-analyses have shown that DEX application can significantly reduce the amount of sedative and analgesic drugs, reduce the occurrence of adverse reactions of general anesthesia drugs, and provide better sedative and analgesic effects (Tsaousi et al., 2018; Wang et al., 2018). However, most of the above meta-analyses focus on the application of DEX in general anesthesia, its duration of action is relatively short, and the efficacy and safety of long-term use of DEX in PCIA is still confusing, worthy of attention.
Currently, there are limited studies evaluating the advantages or disadvantages of DEX in PCIA from multicenter large randomized controlled trials. Therefore, it is necessary to conduct a meta-analysis to evaluate the safety and efficacy of DEX in PCIA to provide guidance for clinical anesthesia practice.
Methods
The protocol of this meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42022340933. The date of registration was 16 July 2022.
Searching strategy
Two researchers searched PUBMED, EMBASE, Scopus, Cochrane library and CBM databases independently. The mesh and keywords used for the searches included: “dexmedetomidine,” “patient controlled intravenous analgesia,” and “randomized controlled trial,” and we used an advanced search and limited it to Title/Abstract. The latest search was completed on 17 November 2022, and see the annex for the detailed search strategy. Furthermore, the investigators scanned references of these articles to prevent missing articles.
Study selection
Original studies included were based on PICOS (patient, intervention, comparison, outcome, and study design) as follows, P: postoperative patient received PCIA; I: the use of DEX for PCIA; C: DEX versus controls; O: pain score related indicators (visual analogue scale, VAS; numerical rating scale, NRS; verbal rating scale, VRS; Wong-Baker faces pain scale revision, FPS-R), sedation score, side effects, and so on; S: only randomized controlled trials (RCTs) were included. Studies with following characteristics were excluded: 1) use of DEX for PCIA in non-surgical patients; 2) incomplete and duplicate publications; 3) data cannot be retrieved or converted; 4) non English or Chinese articles.
Data extraction
The two reviewers screened the literature independently. First, duplicate articles were deleted through Endnote (version X7.7.1) and manual methods. Then, articles that met the inclusion criteria were selected by reading the title, abstract, or even full text. Further, relevant data were extracted from the included articles according to the pre designed data extraction table, and cross checked. The pre designed data table includes the name of the first author, the year of publication, the sample size of each group of patients, the interventions of experimental and control group, and the type of surgery. And the following data from the included studies will be extracted for further statistical analysis: 1) pain score at different time points after operation; 2) analgesics consumption during the first 24 postoperative hours; 3) patient satisfaction; 4) the adverse reactions (nausea, vomiting, pruritus, hypotension and bradycardia); 5) sedation scores at different time points postoperatively; 6) changes in inflammatory signals (IL-6 and TNF-α).
Assessment of risk of bias
Two reviewers independently read and evaluated the methodological validity of all eligible studies using the Cochrane Handbook v5.0.2. When disagreements arose, they were resolved through joint consultations. If necessary, a third researcher assisted in the decision. The following information was evaluated: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. They were rated as “high risk of bias,” “uncertain risk of bias,” and “low risk of bias,” respectively.
Statistical analysis
All included RCTs were quantitatively analyzed using Review Manager (version 5.3; Cochrane Collaboration, Copenhagen, Denmark) software, and Stata 12.0 (StataCorp, College Station, TX, United States) was used for Begg’s test. Binary outcomes were computed as odds ratios (OR) with 95% confidence interval (95% CI), and continuous outcomes were calculated as the mean difference (MD) or the standardized mean difference (SMD) with 95% CI. In terms of heterogeneity analysis: a fixed-effects model was used to analyze non-significant heterogeneity data (I2 < 50). Otherwise, the random effects model is used for calculation. If the heterogeneity is significant, look for possible influencing factors of heterogeneity, and try to exclude the influence of heterogeneity through subgroup analysis, sensitivity analysis and other methods.
Results
Characteristics and risk of bias of eligible trials
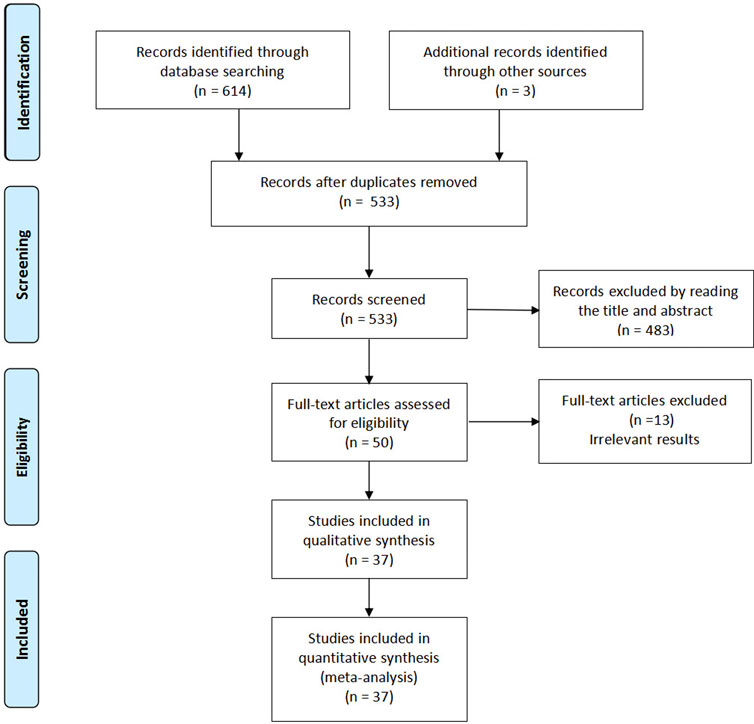
Our research flow chart is shown in Figure 1. A total of 37 RCTs were identified, including 5,409 patients. The risk of bias assessment was fully consistent between the two reviewers and showed moderate overall study quality. As shown in Figure 2, the risk of bias map was created using ReviewManager 5.3 software.

FIGURE 2. The risk of bias assessment of the included studies. (A) Risk bias of summary, (B) Risk bias of graph. Note: There were no high risk of bias found in these studies.
Study characteristics
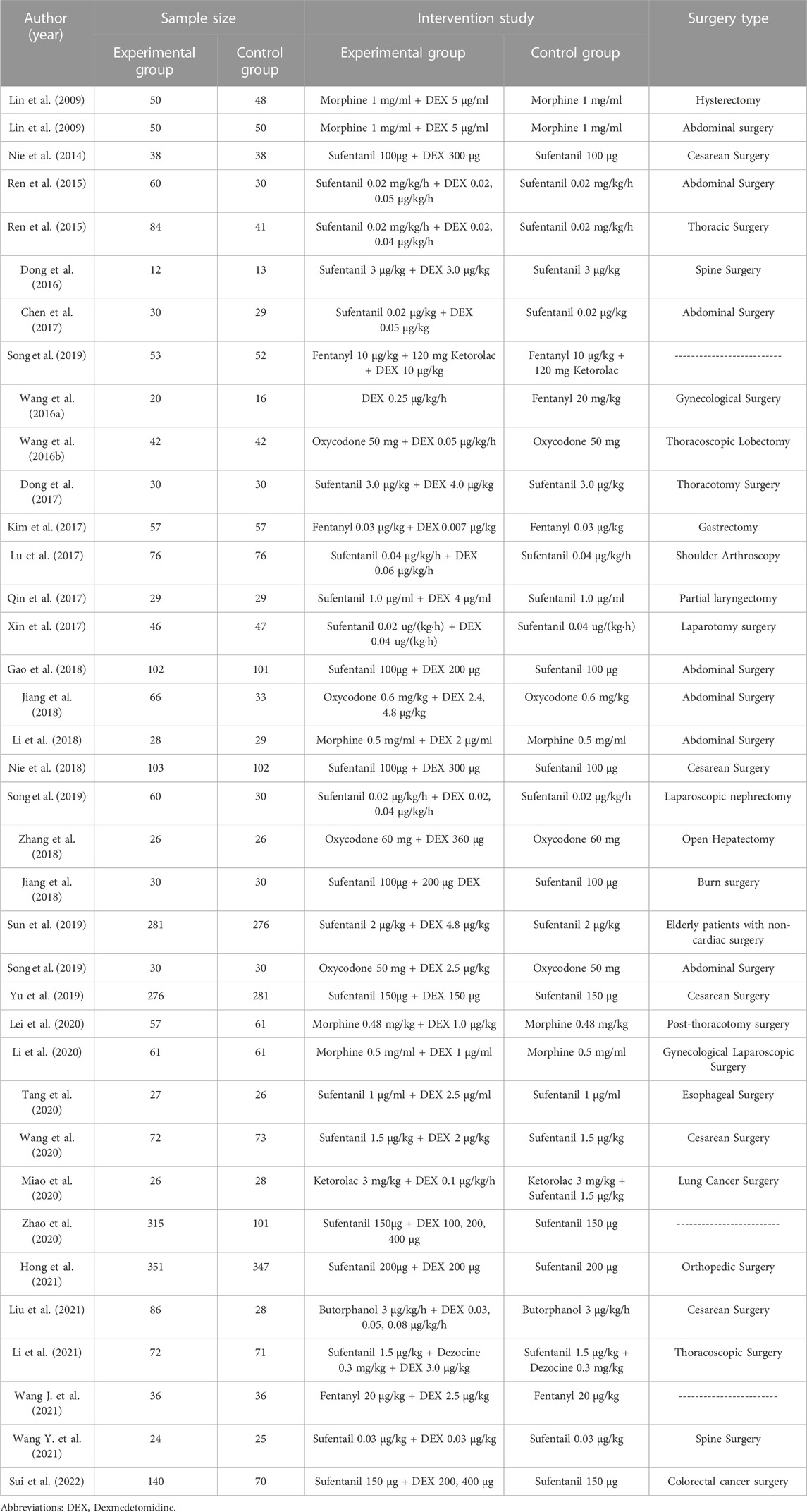
The study characteristics are presented in Table 1. The included studies were first published in 2008 and the sample sizes ranged from 12 to 351 patients. In the 37 studies included in this meta-analysis, PCIA were used in abdominal surgeries (seven studies), cesarean surgeries (five studies), laparoscopic surgery (two studies), spine surgeries (two studies), shoulder arthroscopy (one study), and other procedures (20 study).
Pain score for postoperative patients at different time points
At least seven trials reported pain score for postoperative patients at different time points (0–4 h, 4–8 h, 24 h, 48 h), while three trials reported 12 h pain score after surgery (Figure 3). Significant heterogeneity was observed among the studies in the pooled analysis at 4–8 h (p = 0.003, I2 = 70%), 24 h (p < 0.00001, I2 = 90%) and 48 h (p < 0.00001, I2 = 96%). There was no statistical difference in the results at 0–4 h (MD = −0.02; 95% CI: −0.12, 0.09; p = 0.75), 4–8 h (MD = −0.14; 95% CI: −0.32, 0.04; p = 0.12) and 12 h (MD = −0.22; 95% CI: −0.47, 0.03; p = 0.08), however, the pain score of the patients in the DEX group was significantly lower than that in the control group postoperative 24 h (MD = −0.70; 95% CI: −0.85, −0.54; p < 0.00001) and postoperative 48 h (MD = −0.43; 95% CI: −0.52, −0.34; p < 0.00001). The above results show that, compared with a single analgesics, DEX for PCIA can significantly prolong the postoperative analgesia time and reduce postoperative pain in patients.
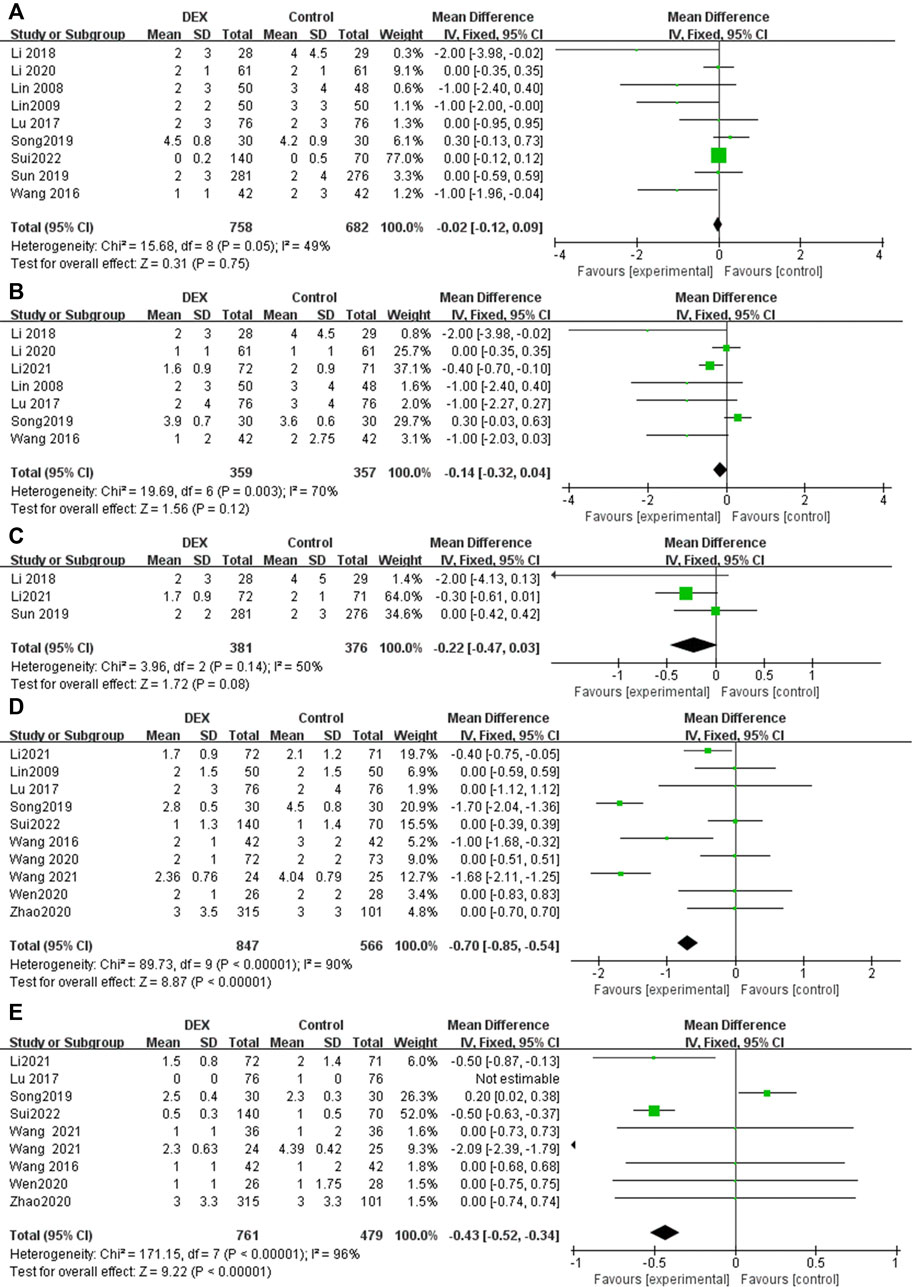
FIGURE 3. Forest plot for pain score in postoperative PCIA patients at different time points. (A) Pain score at 0–4 h, (B) Pain score at 4–8 h, (C) Pain score at 12 h, (D) Pain score at 24 h, (E) Pain score at 48 h.
Ramsay score at postoperative 24 h
Five RCTs reported the ramsay score at 24 h postoperatively, with low heterogeneity among the results (I2 = 0%), and the fixed effects model was used for meta-analysis (Figure 4). The results showed that there was no statistical difference in the ramsay score between the two groups (MD = 0.08; 95% CI −0.01, 0.18; p = 0.10, Figure 4A).
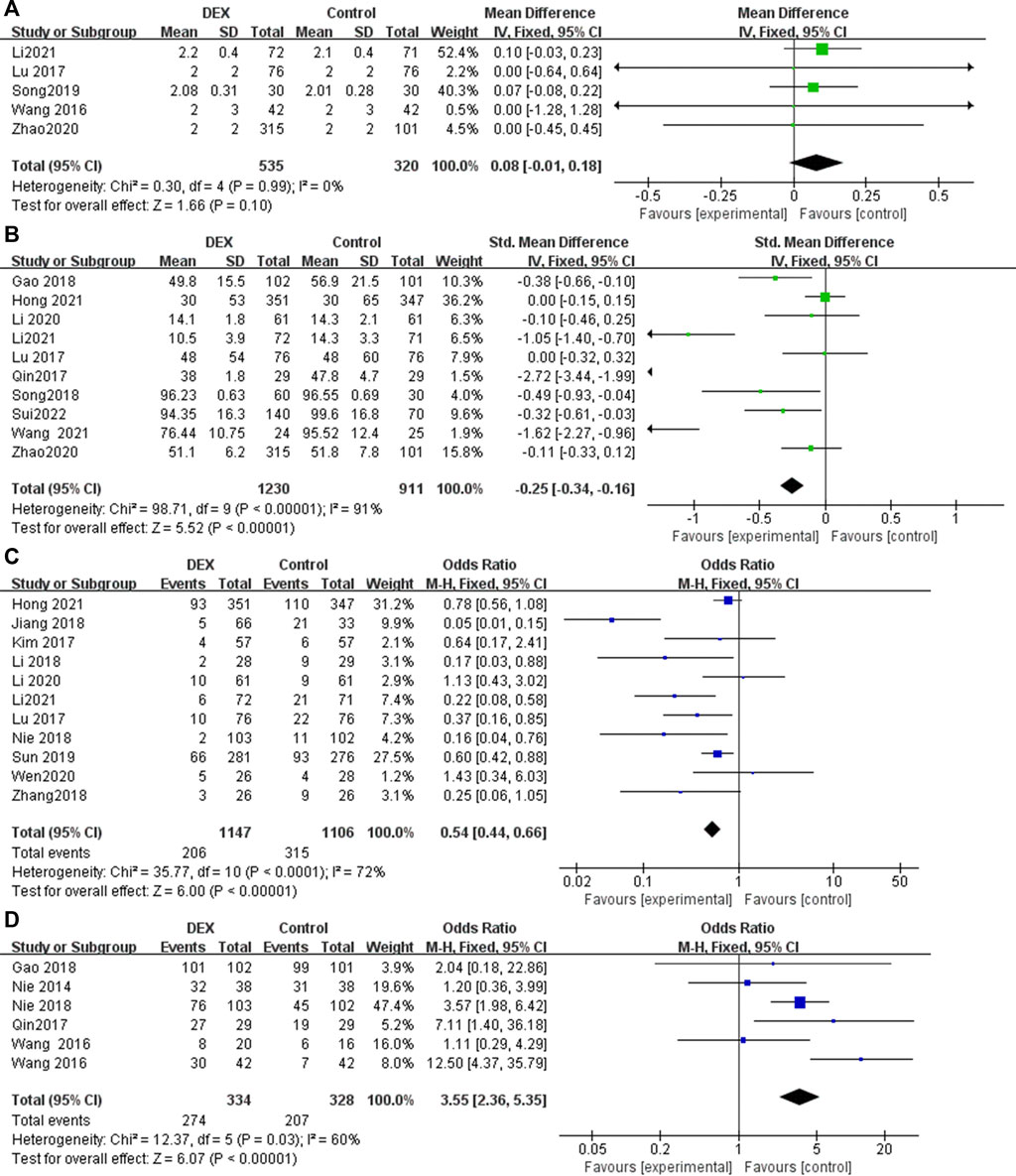
FIGURE 4. Forest plot for sedation, analgesic drug consumption, rescue analgesia and patient satisfaction in postoperative PCIA patients. (A) Ramsay score at 24 h postoperatively, (B) Total consumption of analgesics within 24 h, (C) Use of rescue analgesics, (D) Patient satisfaction.
Total consumption of analgesics within 24 h
Ten studies reported supplemental analgesics consumption during the first 24 h postoperatively. The pooled results indicated that patients receiving DEX for postoperative PCIA exhibited a significant reduction in total supplemental analgesics consumption at 24 h postoperatively, compared with patients receiving analgesics alone (SMD = −0.25; 95% CI: −0.34, −0.16; p < 0.00001, I2 = 91%, Figure 4B).
Use of rescue analgesics
Eleven studies reported the number of times rescue analgesics were used. The pooled results indicated that patients receiving DEX for postoperative PCIA exhibited a significant reduction in use of rescue analgesics, compared with patients receiving analgesics alone (OR = 0.54; 95% CI: 0.44, 0.66; p < 0.00001, I2 = 72%, Figure 4C).
Patient satisfaction
Six studies reported patient satisfaction with PCIA use. Postoperative PCIA patients using DEX were significantly more satisfied than patients using analgesics alone (OR = 3.55; 95% CI: 2.36, 5.35; p < 0.00001, I2 = 60%, Figure 4D).
IL-6 at postoperative 24 h
Two studies reported IL-6 at postoperative 24 h. Compared with patients who received PCIA alone with analgesics or placebo, patients who added DEX significantly decreased the inflammatory cytokine IL-6 at 24 h after surgery (MD = −5.73; 95% CI: −8.34, −3.12; p < 0.00001, I2 = 91%, Figure 5A).

FIGURE 5. Forest plot for inflammatory levels in postoperative PCIA patients. (A) IL-6 at 24 h postoperatively, (B) TNF-α at 24 h postoperatively.
TNF-α at postoperative 24 h
Two studies reported TNF-α at postoperative 24 h. Compared with patients who received PCIA alone with analgesics or placebo, patients who added DEX significantly decreased the inflammatory cytokine TNF-α at 24 h after surgery (MD = −0.63; 95% CI: −0.76, −0.50; p < 0.00001, I2 = 89%, Figure 5B).
Postoperative nausea and vomiting
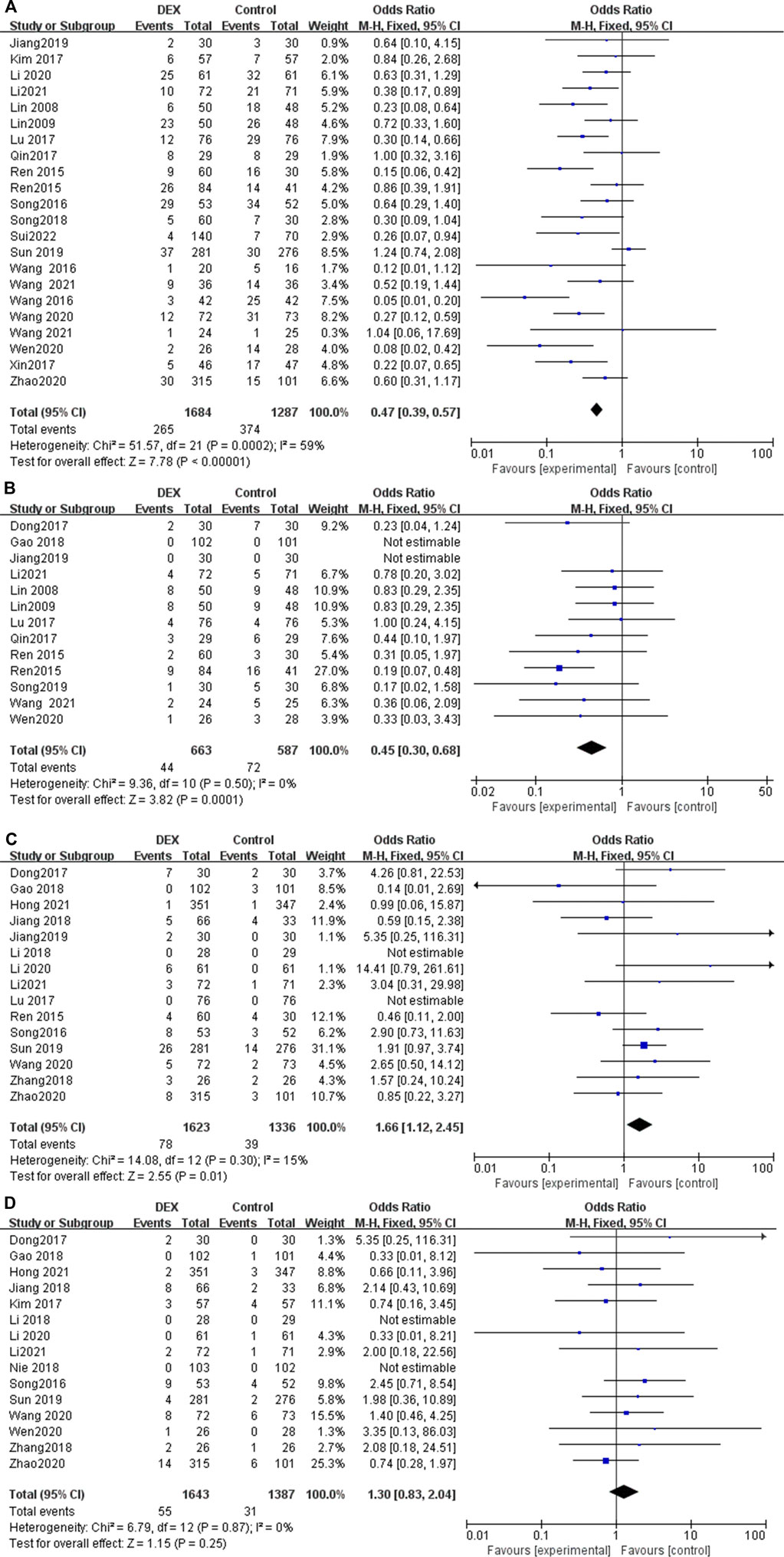
Twenty-two studies reported the incidence of nausea and vomiting in patients undergoing PCIA. The results show that the use of DEX for PCIA can significantly reduce the incidence of postoperative nausea and vomiting, compared with PCIA alone with analgesics (OR = 0.47; 95% CI: 0.39, 0.57; p < 0.00001, I2 = 59%, Figure 6A).
Pruritus after surgery
Thirteen trials reported the incidence of pruritus after using PCIA. Heterogeneity among the studies was insignificant in the pooled analysis (I2 = 0%). Our meta-analysis found that the incidence of pruritus was significantly lower in the PCIA group with the addition of DEX compared to PCIA with analgesics alone (OR = 0.45; 95% Cl: 0.30, 0.68; p = 0.0001, Figure 6B).
Bradycardia after surgery
Fifteen RCTs reported the incidence of bradycardia after using PCIA, with low heterogeneity among the results (I2 = 15%). The incidence of bradycardia in the DEX group was slightly higher than that in the control group (OR = 1.66; 95% CI: 1.12, 2.45; p = 0.01, Figure 6C).
Hypotension after surgery
Fifteen RCTs reported the incidence of hypotension after using PCIA, with low heterogeneity among the results (I2 = 0%). The results showed that there was no statistical difference in the incidence of hypotension after using PCIA between the two groups (OR = 1.30; 95% CI 0.83, 2.04; p = 0.25, Figure 6D).
Sensitivity analysis and subgroup analysis
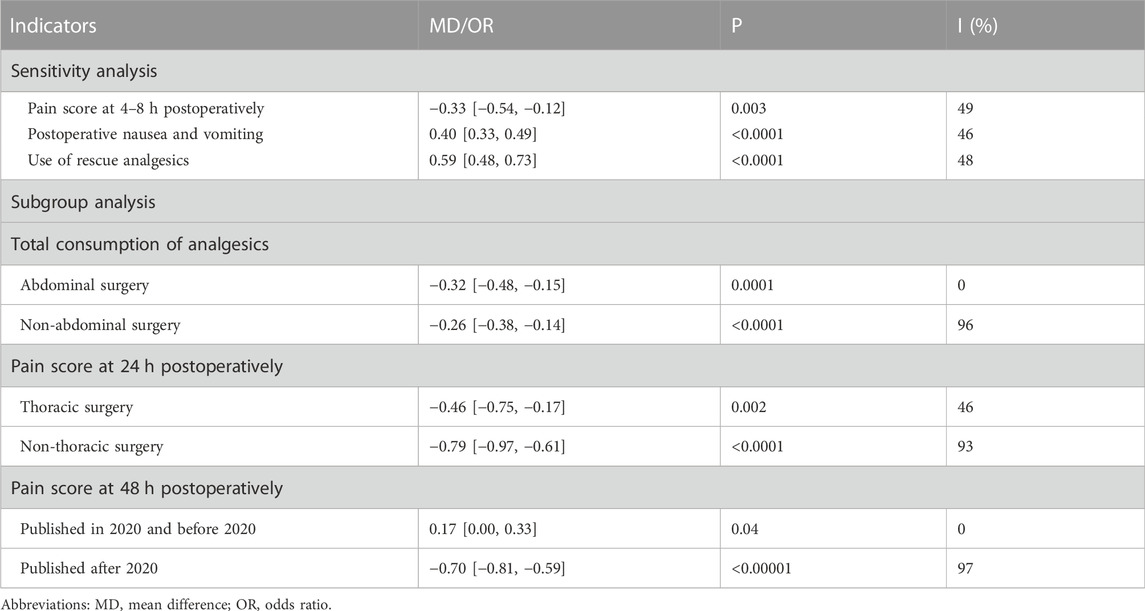
Sensitivity analysis and subgroup analysis were used to find sources of heterogeneity, and minimize the impact of heterogeneity on the stability of results. The heterogeneity of some indicators (4–8 h pain score, PONV, use of rescue analgesics) were significantly reduced among other studies after removal of one studies, while the total consumption of analytics, 24 h and 48 h pain score reduce heterogeneity to some extent through subgroup analysis, as shown in Table 2. For indicators that total consumption of analgesics within 24 h after surgery, PONV, 24 h and 48 h pain score, the result of sensitivity or subgroup analysis were consistent with previous results. By sensitivity analysis, there was a statistically significant difference between the two groups for 4–8 h pain score, showing that the DEX group was significantly lower than the control group, contrary to previous results.
Publication bias
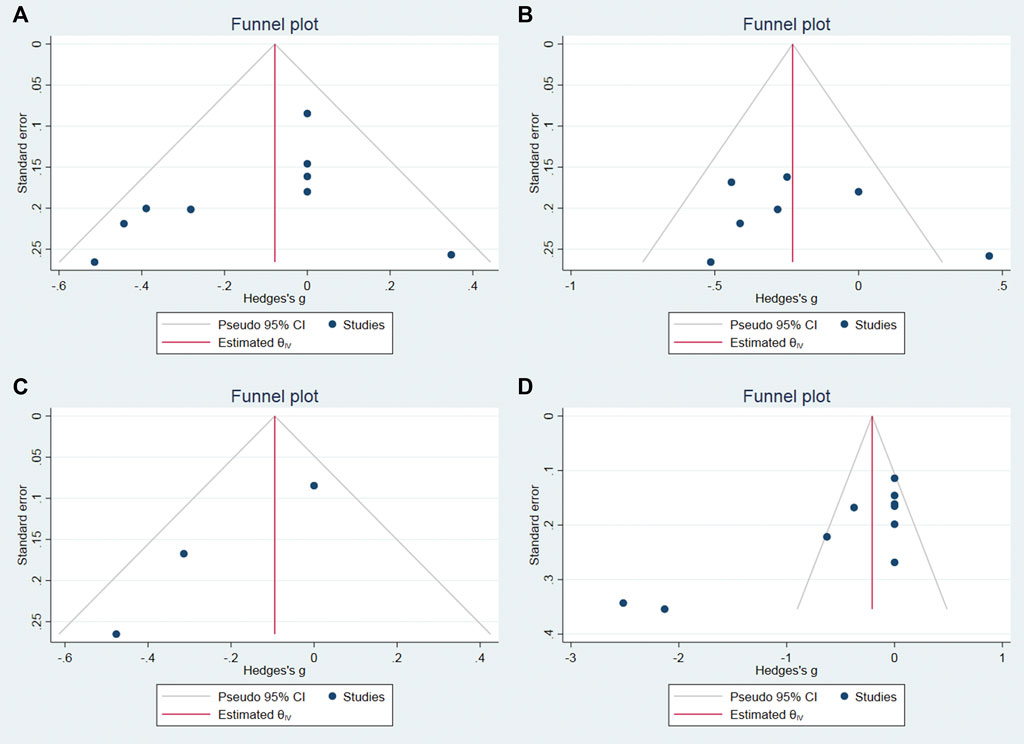
We used Stata software to assess the publication bias of the main results. The p values of begg’s test on the 0–4 h, 4–8 h, 12 h, and 24 h pain score were 0.0763, 1, 0.2963, 0.0013, respectively. Therefore, we believe that the risk of publication bias is low in this meta-analysis, and the funnel plot is shown in Figure 7.
Discussion
This meta-analysis of quantitative studies of DEX for postoperative PCIA shows that DEX improved the analgesic effect, reduces total analgesics consumption, and reduced the analgesic drug-related adverse reactions. Compared with the control group, the pain score in the DEX group were significantly reduced at 24 and 48 h after surgery. In addition, DEX can also significantly improve patient satisfaction and reduce the time of analgesic rescue needs. Also, the analgesics related side effects in the DEX group, such as nausea, vomiting and itching after surgery, were significantly reduced. Moreover, DEX could reduce the levels of inflammatory cytokines such as IL-6 and TNF-α. Ultimately, DEX increased the risk of bradycardia, with no difference in hypotensive complications between the two groups. Based on the results of this meta-analysis, we summarized the specific role of DEX in PCIA, as shown in Table 3.
The significant pharmacological effects of DEX are due to the activation of α2-ARs. DEX can activate presynaptic α2-ARs, inhibit the release of norepinephrine through a negative feedback mechanism, and stop pain signaling (Feng et al., 2019; Liu et al., 2021). The unique “conscious calming” effect of DEX is primarily related to the nucleus locus coeruleus in the brain. Compared with analgesics, it has superior properties, especially in arousal sedation, mild analgesia, and a lower risk of respiratory depression (Hall et al., 2000). In this study, DEX, as an adjuvant for analgesics, could enhance postoperative analgesia and improve patient satisfaction.
PCA is one of the accepted methods for the management of postoperative pain. A cochrane review concluded that PCA was associated with better postoperative pain scores and increased patient satisfaction compared with traditional modes of analgesic drug administration (Abrolat et al., 2018). Intravenous PCA has been widely used for postoperative analgesia over the past few decades (Hadi et al., 2006; Kim et al., 2017).
Pain can be assessed by self-assessment scales, behavioural tests and physiological measures. One-dimensional pain scales, which are quick to assess, concise and easy for patients to understand, are the most commonly used types of pain assessment scales in clinical practice and include the VAS, NRS, VRS, and FPS-R. Of the 37 studies included in our meta-analysis, Sun et al. (2019) and Li et al. (2018) used the NRS, and Lin et al. (2009) used the VRS to assess patients’ postoperative pain, while the remaining studies all used the VAS to assess pain. This meta-analysis found that the addition of DEX resulted in lower pain scores than analgesia alone at 24 and 48 h postoperatively. An interesting phenomenon found in this study is that DEX is not significantly better than analgesics in reducing the pain score of patients 0–12 h after surgery. Intravenous analgesics given prior to switching on the PCIA pump can greatly mask the synergistic analgesic effect of adjuvants in the early postoperative period. There are several potential mechanisms related to the analgesic effect of DEX. Many studies have shown that DEX mainly exerts analgesic effects through three levels: it acts on the locus coeruleus α2AR at the level above the spinal cord, activates the descending noradrenergic inhibitory system, and then enhances the inhibitory synaptic transmission in the dorsal horn of the spinal cord and relieves pain; at the level of the spinal cord, it binds to α2AR in the dorsal horn of the spinal cord, causing cell membrane superization, thereby inhibiting the upward conduction of pain signals; at the peripheral level, it inhibits Ad fibers and C fibers, preventing them from transmitting nociceptive information to the spinal cord (Grape et al., 2019; Bozorgi et al., 2021).
Our study found no statistical difference between DEX and analgesia alone in the RSS sedation score, which may be related to the lower dose of DEX for PCIA. Relevant studies have confirmed that surgery, anxiety, pain and other strong stimuli are associated with sympathetic nervous system excitation. DEX can activate the central locus coeruleus receptor, reduce sympathetic tone, and reduce the release of epinephrine and norepinephrine, resulting in a sedative effect (Qiu et al., 2016).
The total amount of analgesics supplemented in the DEX group 24 h after surgery was less than that in the control group, and the times of use of rescue analgesics in the DEX group was also less than that in the control group. This indicates that the addition of DEX to PCIA exerts a better analgesic effect, and has a synergistic effect with analgesic drugs. Analgesics-related complications were significantly reduced, while patient satisfaction was improved in the DEX group, compared with PCIA with analgesics alone. These changes may be explained as follows: 1) patients receiving DEX for PCIA used lower doses of analgesic drug; 2) DEX reduces noradrenergic activity by activating presynaptic α2 receptors in the locus coeruleus or reducing sympathetic outflow, which may cause postoperative nausea and vomiting (Choi et al., 2017).
IL-6 is a lymphokine produced by activated T cells and fibroblasts. It can make B cell precursors become antibody-producing cells, cooperate with colony-stimulating factors, can promote the growth and differentiation of original bone marrow-derived cells, and enhance the lysis function of natural killer cells (Tanaka et al., 2014; Tanaka et al., 2018). Th2 cells mainly secrete IL-4 and IL-6. DEX group reduced the release of IL-6, polarize Th2 cells to Th1 cells and relieve the suppression of cellular immunity caused by surgical stress. TNF-α is a substance that can cause hemorrhagic necrosis in various tumors appears in serum. It can kill or inhibit tumor cells, promote cell proliferation and differentiation, which is a key pro-inflammatory cytokine (Robinson et al., 2020). The DEX group reduces the release of TNF-α, thereby exerting an anti-inflammatory effect.
The ability of DEX to reduce plasma levels of TNF-α and IL-6 to reduce the inflammatory response has been demonstrated in several studies (Taniguchi et al., 2004; Qiao et al., 2009; Tasdogan et al., 2009). However, the immunosuppressive effect of DEX on patients is also noteworthy. Su et al. (2018) found that DEX induced proliferation of monocytic myeloid-derived suppressor cells (M-MDSC), a cell population with a strong pro-angiogenic capacity, in lung cancer patients after surgery, and that DEX expanded M-MDSC in mice and promoted tumour metastasis by increasing VEGF production. Smith et al. (2014) similarly focused on the suppressive effects of DEX on the immune system. Interestingly, Lee et al. (2021) used the immunosuppressive effect of DEX to mitigate allograft rejection and prolong graft survival in mice. However, overall, DEX immunosuppression-related articles are scarce, there is currently insufficient attention to its immunosuppressive effects, and more high-quality studies are needed in the future to assess the impact of the immunosuppressive effects of DEX on patient prognosis.
Hypotension and bradycardia were considered major concerns regarding the safety of adding DEX to postoperative intravenous PCA (Carollo et al., 2008), and therefore DEX should be used with caution in patients with severe bradycardia and hypotension. In addition, in patients with stroke or coronary artery disease, the hypotensive or bradycardic effect of DEX is strongly associated with prognosis (Sousa et al., 2021). When used at clinically recommended concentrations, DEX produces dose-dependent hypotension and bradycardia due to inhibition of sympathetic neurotransmission and a decrease in sympathetic tone, this effect may also be caused by baroreceptor reflexes and mediated by enhanced vagal activity (Ebert et al., 2000; Paris and Tonner, 2005; Frolich et al., 2011). In this study, the risk of bradycardia was higher in the DEX group than in the control group, but the risk of hypotension was not statistically different between the two groups, of course, none of the included patients did not require medical correction for the reduced heart rate. However, studies have shown that DEX can inhibit corticotropin-stimulated corticosterone release after prolonged or high-dose use (Maze et al., 1991), but the studies we included did not observe the effect of DEX in PCIA on this indicator, and more clinical studies are necessary to verify in the future. In addition, rebound hypertension and tachycardia after abrupt discontinuation of DEX, changes in tolerability, and the possibility of discontinuation syndrome are also of concern (Feng et al., 2019).
In the past, there were two meta-analyses on the effectiveness and safety of DEX for PCAI. Chen et al. (2020) found that DEX combined with tramadol for PCIA was better than tramadol alone in terms of analgesic effect and safety. Peng et al. (2017) found that DEX was an effective adjuvant to opioid PCIA, which could reduce postoperative pain, opioid demand and opioid related adverse events of patients. Apart from the tramadol and pure opioid agonists (Morphine, Sufentanil, Fentanyl, Oxycodone), our meta-analysis also included studies of butorphanol and ketorolac for PCIA. And our meta-analysis included the largest number of studies (28) of DEX for PCIA to date. In addition, our meta focused on the effect of DEX in PCIA on the postoperative inflammatory response of patients, the area that has not been the focus of previous meta-analyses. Thus, our meta-analysis provides a comprehensive assessment of the efficacy and safety of DEX in PCIA, providing a reference for the clinical application of DEX in PCIA.
There are several limitations in our meta-analysis. First, we may have missed some studies that met the inclusion criteria and had to exclude some studies because the full text was not available. Second, this meta-analysis included several different types of analgesic drugs (morphine, sufentanil, fentanyl, oxycodone, tramadol, etc.) that may affect pain relief outcomes and adverse events. Third, some of our results have large heterogeneity, which may lead to biased results and requires further study. The reasons for the high heterogeneity may be related to different analgesic drugs used in PCIA, different doses of DEX, and different PCIA parameter settings. Finally, for the larger heterogeneity results, although we tried subgroup analysis and sensitivity analysis, we were still unable to reduce their heterogeneity.
Conclusion
Compared with analgesics or placebo alone, DEX combined with postoperative PCIA can achieve better analgesia and patient satisfaction, while reducing analgesics consumption and the occurrence of analgesics-related adverse events.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
RC and SS designed the study and conducted the data analysis. YL checked the final data. XD and MD screened the articles. RC drafted the manuscript and performed data interpretation. YW and YL revised the manuscript. All authors significantly contributed to the paper and read and approved the final manuscript.
Funding
This work was supported by the Wu Jieping Medical Foundation (No. 320.6750.2020-21-12).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrolat, M., Eberhart, L. H. J., Kalmus, G., Koch, T., and Nardi-Hiebl, S. (2018). Patient-controlled analgesia (PCA): An overview about methods, handling and new modalities. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 53 (4), 270–280. doi:10.1055/s-0043-104665
Bozorgi, H., Zamani, M., Motaghi, E., and Eslami, M. (2021). Dexmedetomidine as an analgesic agent with neuroprotective properties: Experimental and clinical aspects. J. Pain Palliat. Care Pharmacother. 35 (3), 215–225. doi:10.1080/15360288.2021.1914280
Carollo, D. S., Nossaman, B. D., and Ramadhyani, U. (2008). Dexmedetomidine: A review of clinical applications. Curr. Opin. Anaesthesiol. 21 (4), 457–461. doi:10.1097/ACO.0b013e328305e3ef
Chen, P., Chen, F., Lei, J., and Zhou, B. (2020). Efficacy and safety of dexmedetomidine combined with tramadol for patient-controlled intravenous analgesia in Chinese surgical patients: A systematic review and meta-analysis. Med. Baltim. 99 (3), e18825. doi:10.1097/MD.0000000000018825
Chen, Z., Tang, R., Zhang, R., Jiang, Y., and Liu, Y. (2017). Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J. Clin. Anesth. 36, 118–122. doi:10.1016/j.jclinane.2016.10.022
Choi, E. K., Seo, Y., Lim, D. G., and Park, S. (2017). Postoperative nausea and vomiting after thyroidectomy: A comparison between dexmedetomidine and remifentanil as part of balanced anesthesia. Korean J. Anesthesiol. 70, 299–304. doi:10.4097/kjae.2017.70.3.299
Dong, C. S., Lu, Y., Zhang, J., Sun, P., Yu, J. M., Wu, C., et al. (2016). The optimal dose of dexmedetomidine added to an sufentanil-based analgesic regimen for postoperative pain control in spine surgery: A probit analysis study. Medicine 95 (39), e4776. doi:10.1097/MD.0000000000004776
Dong, C. S., Zhang, J., Lu, Q., Sun, P., Yu, J. M., Wu, C., et al. (2017). Effect of Dexmedetomidine combined with sufentanil for post- thoracotomy intravenous analgesia:a randomized, controlled clinical study. BMC Anesthesiol. 17 (1), 33. doi:10.1186/s12871-017-0324-4
Ebert, T. J., Hall, J. E., Barney, J. A., Uhrich, T. D., and Colinco, M. D. (2000). The effects of increasing plasma concentrations of Dexmedetomidine in humans. Anesthesiology 93 (2), 382–394. doi:10.1097/00000542-200008000-00016
Feng, M., Chen, X., Liu, T., Zhang, C., Wan, L., and Yao, W. (2019). Dexmedetomidine and sufentanil combination versus sufentanil alone for postoperative intravenous patient-controlled analgesia: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 19 (1), 81. doi:10.1186/s12871-019-0756-0
Frolich, M. A., Arabshahi, A., Katholi, C., Prasain, J., and Barnes, S. (2011). Hemodynamic characteristics of midazolam, propofol, and dexmedetomidine in healthy volunteers. J. Clin. Anesth. 23 (3), 218–223. doi:10.1016/j.jclinane.2010.09.006
Gao, Y., Deng, X., Yuan, H., Leng, Y., Zhang, T., Xu, X., et al. (2018). Patient-controlled intravenous analgesia with combination of dexmedetomidine and sufentanil on patients after abdominal operation: A prospective, randomized, controlled, blinded, multicenter clinical study. Clin. J. Pain 34 (2), 155–161. doi:10.1097/AJP.0000000000000527
Grape, S., Kirkham, K. R., Frauenknecht, J., and Albrecht, E. (2019). Intra-operative analgesia with remifentanil vs. dexmedetomidine: A systematic review and meta-analysis with trial sequential analysis. Anaesthesia 74 (6), 793–800. doi:10.1111/anae.14657
Hadi, M. A., Kamaruljan, H. S., Saedah, A., and Abdullah, N. M. (2006). A comparative study of intravenous patient-controlled analgesia morphine and tramadol in patients undergoing major operation. Med. J. Malays. 61, 570–576.
Hall, J. E., Uhrich, T. D., Barney, J. A., Arain, S. R., and Ebert, T. J. (2000). Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth. Analg. 90 (3), 699–705. doi:10.1097/00000539-200003000-00035
Hong, H., Zhang, D. Z., Li, M., Wang, G., Zhu, S. N., Zhang, Y., et al. (2021). Impact of dexmedetomidine supplemented analgesia on delirium in patients recovering from orthopedic surgery: A randomized controlled trial. BMC Anesthesiol. 21 (1), 223. doi:10.1186/s12871-021-01441-3
Jeffrey, A. G. (2005). Patient-controlled analgesia. Anesth. Analg. 101 (5), S44–S61. doi:10.1213/01.ANE.0000177102.11682.20
Jiang, Z., Zhou, G., Song, Q., Bao, C., Wang, H., and Chen, Z. (2018). Effect of intravenous oxycodone in combination with different doses of dexmedetomdine on sleep quality and visceral pain in patients after abdominal surgery: A randomized study. Clin. J. Pain 34 (12), 1126–1132. doi:10.1097/AJP.0000000000000645
Kim, N. Y., Kwon, T. D., Bai, S. J., Noh, S. H., Hong, J. H., Lee, H., et al. (2017). Effects of dexmedetomidine in combination with fentanyl-based intravenous patient-controlled analgesia on pain attenuation after open gastrectomy in comparison with conventional thoracic epidural and fentanyl-based intravenous patient-controlled analgesia. Int. J. Med. Sci. 14 (10), 951–960. doi:10.7150/ijms.20347
Lee, C. F., Cheng, C. H., Hung, H. C., Lee, J. C., Wang, Y. C., Wu, T. H., et al. (2021). Sedative and immunosuppressive effects of dexmedetomidine in transplantation. Pharm. (Basel) 14 (8), 825. doi:10.3390/ph14080825
Lei, P. F., Wang, J., Gao, S., Du, B., Wang, H., Li, W. C., et al. (2020). Impact of post-thoracotomy analgesia with dexmedetomidine and morphine on immunocytes: a randomized clinical trial. Braz. J. Anesthesiol. 70 (2), 153–158. doi:10.1016/j.bjan.2019.12.017
Li, H. J., Li, C. J., Wei, X. N., Hu, J., Mu, D. L., and Wang, D. X. (2018). Dexmedetomidine in combination with morphine improves postoperative analgesia and sleep quality in elderly patients after open abdominal surgery: A pilot randomized control trial. PLoS One 13 (8), e0202008. doi:10.1371/journal.pone.0202008
Li, H. J., Liu, S., Geng, Z. Y., and Li, X. Y. (2020). Adding dexmedetomidine to morphine-based analgesia reduces early postoperative nausea in patients undergoing gynecological laparoscopic surgery: A randomized controlled trial. BMC Anesthesiol. 20 (1), 11. doi:10.1186/s12871-019-0928-y
Li, Q., Yao, H., Xu, M., and Wu, J. (2021). Dexmedetomidine combined with sufentanil and dezocine-based patient-controlled intravenous analgesia increases female patients' global satisfaction degree after thoracoscopic surgery. J. Cardiothorac. Surg. 16 (1), 102. doi:10.1186/s13019-021-01472-4
Lin, T. F., Yeh, Y. C., Lin, F. S., Wang, Y. P., Lin, C. J., Sun, W. Z., et al. (2009). Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br. J. Anaesth. 102 (1), 117–122. doi:10.1093/bja/aen320
Liu, S., Peng, P., Hu, Y., Liu, C., Cao, X., Yang, C., et al. (2021). The effectiveness and safety of intravenous dexmedetomidine of different concentrations combined with butorphanol for post-caesarean section analgesia: A randomized controlled trial. Drug Des. devel. Ther. 15, 689–698. doi:10.2147/DDDT.S287512
Lu, J., Chen, G., Zhou, H., Zhou, Q., Zhu, Z., and Wu, C. (2017). Effect of parecoxib sodium pretreatment combined with dexmedetomidine on early postoperative cognitive dysfunction in elderly patients after shoulder arthroscopy: A randomized double blinded controlled trial. J. Clin. Anesth. 41, 30–34. doi:10.1016/j.jclinane.2017.06.004
Maze, M., Virtanen, R., Daunt, D., Banks, S. J., Stover, E. P., and Feldman, D. (1991). Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis : In vivo and in vitro studies. Anesth. Analg. 73 (2), 204–208. doi:10.1213/00000539-199108000-00015
Miao, Z., Wu, P., Wang, J., Zhou, F. C., Lin, Y., Lu, X. Y., et al. (2020). Whole-Course Application of Dexmedetomidine Combined with Ketorolac in Nonnarcotic Postoperative Analgesia for Patients with Lung Cancer Undergoing Thoracoscopic Surgery: A Randomized Control Trial. Pain. Physician. 23 (2), E185–E193.
Motamed, C. (2022). Clinical update on patient-controlled analgesia for acute postoperative pain. Pharm. (Basel) 10 (1), 22. doi:10.3390/pharmacy10010022
Nie, Y., Liu, Y., Luo, Q., and Huang, S. (2014). Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: A randomised, placebo-controlled study. Eur. J. Anaesthesiol. 31 (4), 197–203. doi:10.1097/EJA.0000000000000011
Nie, Y., Tu, W., Shen, X., Yu, W., Yu, Y., Song, X., et al. (2018). Dexmedetomidine added to sufentanil patient-controlled intravenous analgesia relieves the postoperative pain after cesarean delivery: A prospective randomized controlled multicenter study. Sci. Rep. 8 (1), 9952. doi:10.1038/s41598-018-27619-3
Pamela, P. P., and Ronald, D. M. (2010). Current and developing methods of patient-controlled analgesia. Anesthesiol. Clin. 28 (4), 587–599. doi:10.1016/j.anclin.2010.08.010
Paris, A., and Tonner, P. H. (2005). Dexmedetomidine in anaesthesia. Curr. Opin. Anaesthesiol. 18 (4), 412–418. doi:10.1097/01.aco.0000174958.05383.d5
Peng, K., Zhang, J., Meng, X. W., Liu, H. Y., and Ji, F. H. (2017). Optimization of postoperative intravenous patient-controlled analgesia with opioid-dexmedetomidine combinations: An updated meta-analysis with trial sequential analysis of randomized controlled trials. Pain Physician 20 (7), 569–595. doi:10.36076/ppj/2017.7.569
Qiao, H., Sanders, R. D., Ma, D., Wu, X., and Maze, M. (2009). Sedation improves early outcome in severely septic Sprague Dawley rats. Crit. Care 13 (4), R136. doi:10.1186/cc8012
Qin, M. J., Chen, K. Z., Liu, T. J., and Shen, X. (2017). Dexmedetomidine in combination with sufentanil for postoperative analgesia after partial laryngectomy. BMC. Anesthesiol. 17 (1), 66. doi:10.1186/s12871-017-0363-x
Qiu, P., Xiao, J., Wang, K., Gong, S., Yong, Y. E., Xu, J. Q., et al. (2016). The effects of analgesic and sedative on the heart rate variability and plasma catecholamine in patients after severe brain injury. J. Of. Fujian. Med. Univ. 50 (1), 31–37.
Ren, C., Chi, M., Zhang, Y., Zhang, Z., Qi, F., and Liu, Z. (2015). Dexmedetomidine in postoperative analgesia in patients undergoing hysterectomy: A CONSORT-prospective, randomized, controlled trial. Med. Baltim. 94 (32), e1348. doi:10.1097/MD.0000000000001348
Robinson, P. C., Liew, D. F. L., Liew, J. W., Monaco, C., Richards, D., Shivakumar, S., et al. (2020). The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med 1 (1), 90–102. doi:10.1016/j.medj.2020.11.005
Smith, M. A., Hibino, M., Falcione, B. A., Eichinger, K. M., Patel, R., and Empey, K. M. (2014). Immunosuppressive aspects of analgesics and sedatives used in mechanically ventilated patients: An underappreciated risk factor for the development of ventilator-associated pneumonia in critically ill patients. Ann. Pharmacother. 48 (1), 77–85. doi:10.1177/1060028013510698
Song, J., Wei, N., Zhang, J., and Wang, G. (2019). Effect of dexmedetomidine combined with oxycodone patient-controlled intravenous analgesia on the levels of inflammatory cytokine in patients with rectal cancer. Pak. J. Pharm. Sci. 32 (3), 1381–1385.
Song, J. W., Shim, J. K., Song, Y., Yang, S. Y., Park, S. J., and Kwak, Y. L. (2013). Effect of ketamine as an adjunct to intravenous patient-controlled analgesia, in patients at high risk of postoperative nausea and vomiting undergoing lumbar spinal surgery. Br. J. Anaesth. 111 (4), 630–635. doi:10.1093/bja/aet192
Sui, X., Wang, Y., Jin, M. X., Li, K., Jiang, G., Song, A. L., et al. (2022). The effects of dexmedetomidine for patient-controlled analgesia on postoperative sleep quality and gastrointestinal motility function after surgery: A prospective, randomized, double-blind, and controlled trial. Front. Pharmacol. 13, 990358. doi:10.3389/fphar.2022.990358
Sousa, G. C., Fernandes, M. V., Cruz, F. F., Antunes, M. A., da, Silva. C. M., Takyia, C., et al. (2021). Comparative effects of dexmedetomidine and propofol on brain and lung damage in experimental acute ischemic stroke. Sci. Rep. 11 (1), 23133. doi:10.1038/s41598-021-02608-1
Su, X., Fan, Y., Yang, L., Huang, J., Qiao, F., Fang, Y., et al. (2018). Dexmedetomidine expands monocytic myeloid-derived suppressor cells and promotes tumour metastasis after lung cancer surgery. J. Transl. Med. 16 (1), 347. doi:10.1186/s12967-018-1727-9
Sun, Y., Jiang, M., Ji, Y., Sun, Y., Liu, Y., and Shen, W. (2019). Impact of postoperative dexmedetomidine infusion on incidence of delirium in elderly patients undergoing major elective noncardiac surgery: A randomized clinical trial. Drug Des. devel. Ther. 13, 2911–2922. doi:10.2147/DDDT.S208703
Tanaka, T., Narazaki, M., and Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6 (10), a016295. doi:10.1101/cshperspect.a016295
Tanaka, T., Narazaki, M., and Kishimoto, T. (2018). Interleukin (IL-6) immunotherapy. Cold Spring Harb. Perspect. Biol. 10 (8), a028456. doi:10.1101/cshperspect.a028456
Tang, C., Hu, Y., Zhang, Z., Wei, Z., Wang, H., Geng, Q., et al. (2020). Dexmedetomidine with sufentanil in intravenous patient-controlled analgesia for relief from postoperative pain, inflammation and delirium after esophageal cancer surgery. Biosci. Rep. 40 (5), BSR20193410. doi:10.1042/BSR20193410
Taniguchi, T., Kidani, Y., Kanakura, H., Takemoto, Y., and Yamamoto, K. (2004). Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit. Care Med. 32 (6), 1322–1326. doi:10.1097/01.ccm.0000128579.84228.2a
Tasdogan, M., Memis, D., Sut, N., and Yuksel, M. (2009). Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. J. Clin. Anesth. 21 (6), 394–400. doi:10.1016/j.jclinane.2008.10.010
Tsaousi, G. G., Pourzitaki, C., Aloisio, S., and Bilotta, F. (2018). Dexmedetomidine as a sedative and analgesic adjuvant in spine surgery: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 74 (11), 1377–1389. doi:10.1007/s00228-018-2520-7
Wang, J., Cui, L., Fan, L., and Wang, J. (2021). Clinical effect of different drugs and infusion techniques for patient-controlled analgesia after spinal tumor surgery: A prospective, randomized, controlled clinical trial. Clin. Ther. 43 (6), 1020–1028. doi:10.1016/j.clinthera.2021.03.019
Wang, X., Liu, N., Chen, J., Xu, Z., Wang, F., and Ding, C. (2018). Effect of intravenous dexmedetomidine during general anesthesia on acute postoperative pain in Adults: A systematic review and meta-analysis of randomized controlled trials. Clin. J. Pain 34 (12), 1180–1191. doi:10.1097/AJP.0000000000000630
Wang, X., Liu, W., Xu, Z., Wang, F., Zhang, C., Wang, B., et al. (2016a). Effect of dexmedetomidine alone for intravenous patient-controlled analgesia after gynecological laparoscopic surgery: A consort-prospective, randomized, controlled trial. Medicine 95 (19), e3639. doi:10.1097/MD.0000000000003639
Wang, X., Wang, K., Wang, B., Jiang, T., Xu, Z., Wang, F., et al. (2016b). Effect of oxycodone combined with dexmedetomidine for intravenous patient-controlled analgesia after video-assisted thoracoscopic lobectomy. J. Cardiothorac. Vasc. Anesth. 30 (4), 1015–1021. doi:10.1053/j.jvca.2016.03.127
Wang, Y., Fang, X., Liu, C., Ma, X., Song, Y., and Yan, M. (2020). Impact of intraoperative infusion and postoperative PCIA of dexmedetomidine on early breastfeeding after elective cesarean section: A randomized double-blind controlled trial. Drug Des. devel. Ther. 14, 1083–1093. doi:10.2147/DDDT.S241153
Wang, Y., Lei, Y., Gu, Y., Kong, X., Bian, Z., and Ji, F. (2021). Effect of dexmedetomidine on CD4+ T cells and programmed cell death protein-1 in postoperative analgesia: A prospective, randomized, controlled study. Minerva Anestesiol. 87 (4), 423–431. doi:10.23736/S0375-9393.20.14581-4
Xin, J., Zhang, Y. B., Zhou, L., Liu, F., Zhou, X. S., Liu, B., et al. (2017). Effect of dexmedetomidine infusion for intravenous patient-controlled analgesia on the quality of recovery after laparotomy surgery. Oncotarget. 8 (59), 100371–100383. doi:10.18632/oncotarget.22232
Yu, H. Y., Wang, S. Y., Quan, C. X., Fang, C., Luo, S. C., Li, D. Y., et al. (2019). Dexmedetomidine alleviates postpartum depressive symptoms following cesarean section in Chinese women: A randomized placebo-controlled study. Pharmacotherapy 39 (10), 994–1004. doi:10.1002/phar.2320
Zhang, B., Wang, G., Liu, X., Wang, T. L., and Chi, P. (2018). The opioid-sparing effect of perioperative dexmedetomidine combined with oxycodone infusion during open hepatectomy: A randomized controlled trial. Front. Pharmacol. 8, 940. doi:10.3389/fphar.2017.00940
Zhao, W. S., Hu, Y. A., Chen, H., Wang, X. F., Wang, L. P., Wang, Y., et al. (2020). The effect and optimal Dosage of Dexmedetomidine Plus Sufentanil for Postoperative Analgesia in Elderly Patients With Postoperative Delirium and Early Postoperative Cognitive Dysfunction: A single-center, Prospective, Randomized, Double-Blind, Controlled Trial. Front. Neurosci. 14, 549516. doi:10.3389/fnins.2020.549516
Keywords: dexmedetomidine, patient controlled intravenous analgesia, postoperative analgesia, randomized controlled trail, meta-analysis
Citation: Chen R, Sun S, Li Y, Dou X, Dai M, Wu Y and Lin Y (2022) Efficacy and safety evaluation of dexmedetomidine for postoperative patient controlled intravenous analgesia: A systematic review and meta-analysis. Front. Pharmacol. 13:1028704. doi: 10.3389/fphar.2022.1028704
Received: 26 August 2022; Accepted: 29 November 2022;
Published: 12 December 2022.
Edited by:
Teijo I. Saari, University of Turku, FinlandReviewed by:
Ali Saffaei, Shahid Beheshti University of Medical Sciences, IranAijun Shan, Jinan University, China
Copyright © 2022 Chen, Sun, Li, Dou, Dai, Wu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wu, d3V5YW41MTUxQGh1c3QuZWR1LmNu; Yun Lin, ZnJhbmtsaW55dW5AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
 Rui Chen
Rui Chen Shujun Sun1†
Shujun Sun1† Xiaoke Dou
Xiaoke Dou Maosha Dai
Maosha Dai Yun Lin
Yun Lin