
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 19 October 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1024955
This article is part of the Research Topic Neuroprotective Mechanisms by Phytochemicals in Neurological Disorders View all 9 articles
Neurological diseases are complex diseases affecting the brain and spinal cord, with numerous etiologies and pathogenesis not yet fully elucidated. Tripterygium wilfordii Hook. F. (TWHF) is a traditional Chinese medicine with a long history of medicinal use in China and is widely used to treat autoimmune and inflammatory diseases such as systemic lupus erythematosus and rheumatoid arthritis. With the rapid development of modern technology, the two main bioactive components of TWHF, triptolide and celastrol, have been found to have anti-inflammatory, immunosuppressive and anti-tumor effects and can be used in the treatment of a variety of diseases, including neurological diseases. In this paper, we summarize the preclinical studies of triptolide and celastrol in neurological diseases such as neurodegenerative diseases, brain and spinal cord injury, and epilepsy. In addition, we review the mechanisms of action of triptolide and celastrol in neurological diseases, their toxicity, related derivatives, and nanotechnology-based carrier system.
Neurological diseases are disorders affecting the brain and spinal cord, which are caused by many endogenous and exogenous factors (Mehndiratta and Aggarwal, 2021). These functional or organic disorders cause patients to exhibit many corresponding clinical symptoms, such as dementia, pain and muscle strength disorders, which place a tremendous burden on the patients and society (Borsook, 2012). With the accelerated aging of the population, the incidence of neurological diseases is gradually increasing, and it is becoming a global problem. However, due to the complexity of neurological diseases, the pathogenesis of neurological diseases is not yet clear and effective treatments are limited (Yang et al., 2022a). Studies have shown that many factors are involved in the pathogenesis of neurological diseases (Liu Q. et al., 2022; Delport and Hewer, 2022; Wu et al., 2022). A growing number of studies have suggested that inflammation and immunity play an important role in the development of neurological diseases (Perry et al., 2010; Schiavone and Trabace, 2017; Jiang et al., 2018). Therefore, the investigation of the role of inflammation and immunity in neurological diseases, as well as the related therapeutic approaches, may contribute to the effective treatments of neurological diseases.
Tripterygium wilfordii Hook. F (TWHF) is a perennial woody vine, which grows mainly in Eastern and Southern China, Japan, and Korea (Brinker et al., 2007). As a classical traditional Chinese medicine, TWHF has a history of over 2,000 years of medicinal use (Li and Hao, 2019). TWHF has a wide range of mechanisms of action and has been used in a variety of diseases, especially autoimmune and inflammatory diseases such as rheumatoid arthritis and dermatomyositis (Wang et al., 2016). Currently, more than 100 components have been extracted from TWHF, of which triptolide and celastrol play an important role in the core bioactivity of TWHF, the anti-inflammatory and antioxidant (Figure 1) (Li and Hao, 2019). Triptolide is an important diterpenoid isolated from TWHF and was first extracted for medicinal research in 1972 (Kupchan et al., 1972). Celastrol is derived from a wide variety of natural plants and accounts for a large percentage of the extracts of the roots of TWHF (Lange et al., 2017). Studies have found that triptolide and celastrol are therapeutically effective in preclinical and clinical studies in a variety of diseases, which may be related to their anti-inflammatory and antioxidant effects (Gao et al., 2021; Schiavone et al., 2021). Because of their small molecular weight and lipophilicity, they can cross the blood-brain barrier and work in the brain, so they have been studied in many neurological diseases (Cleren et al., 2005; Lu et al., 2010; De Angelis et al., 2020), and their roles in neurological diseases are the focus of this review (Table 1).
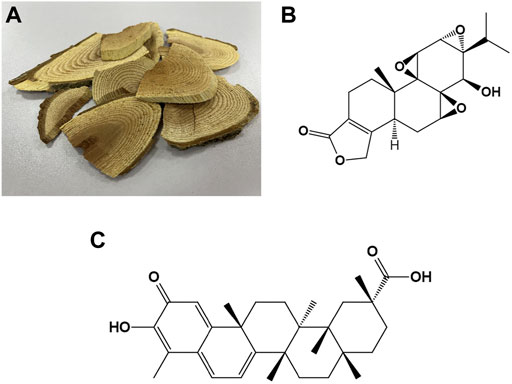
FIGURE 1. The photograph of woody roots of Tripterygium wilfordii Hook. f. and the chemical structures of triptolide and celastrol (A) The photograph of woody roots of Tripterygium wilfordii Hook. f. (B) Chemical structure of triptolide. (C) Chemical structure of celastrol.
In this review, we review the progress of research focusing on the therapeutic potential of triptolide and celastrol in neurological diseases, possible mechanisms of action of them, their toxicity and derivatives, and nanotechnology-based carrier system. This will contribute to the further understanding and in-depth studies of the therapeutic potential of these two components.
Alzheimer’s disease (AD) is a common neurodegenerative disease that can lead to symptoms such as dementia and affect people worldwide (Andrieu et al., 2015). The main pathological features of AD include the formation of extracellular pathological plaques (formed by the accumulation of amyloid-beta (Aβ) peptides) and the presence of intracellular neurofibril entanglements (formed by aggregations of hyperphosphorylated Tau proteins) (Montine et al., 2012). The etiology of AD is not yet fully understood, and a growing body of research suggests an important role for neuroinflammation in the pathology of AD (Dá Mesquita et al., 2016; Wang et al., 2017; Jiang et al., 2022). For example, studies have found that impairment of synaptic function in AD is associated with increased neuroinflammation (Dá Mesquita et al., 2016). Considering the anti-inflammatory and antioxidant effects of TWHF, many studies have reported the therapeutic effects of bioactive components of TWHF on AD in recent years.
Triptolide is one of the important bioactive components of TWHF and it exerts neuroprotective and neurotrophic effects in AD (Li and Hao, 2019). Moreover, it has a small molecular weight and lipophilicity, which makes it have a better performance in the treatment of AD (Shui et al., 2010). The neuroprotective effects of triptolide on AD have been reported in both in vivo and in vitro studies. Wan et al. found that triptolide (intraperitoneal injection) could attenuate the degeneration of dendritic spines in hippocampal neurons in AD rats injected with Aβ 1-40 (Wan et al., 2014). Beside that, a study found that in the amyloid precursor protein/presenilin 1 (APP/PS1) double transgenic AD murine model, peripherally administered triptolide improved cognitive function, accompanied by reduced neuroinflammation and Aβ deposition, and that reduction of Aβ deposition may be associated with enhanced Aβ degradation (Cheng et al., 2014). In another study, in the APP/PS1 model of AD, intraperitoneally injected triptolide was found to reduce astrocyte proliferation and microglial activation in the hippocampal region, which was also related to the formation of pathological plaques and neurofibrillary tangles (Li et al., 2016; Villegas-Llerena et al., 2016). Furthermore, studies have revealed that intraperitoneal injection with tirptolide improved spatial memory deficits in APP/PS1 models of AD, involving inhibition of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) (Wang et al., 2014), and inhibition of inflammatory responses and mitogen-activated protein kinase (MAPK)s activity (Cui et al., 2016). In addition, Bakshi et al. reported that knockout of C-X-C chemokine receptor type 2 (CXCR2) inhibited Aβ production in the APP/PS1 model of AD, further elucidating the mechanism of Aβ production in AD (Bakshi et al., 2011). In vitro experiments revealed that in SH-SY5Y cells, triptolide could exert neuroprotective effects by inhibiting CXCR2 activity and reducing Aβ production (Wang et al., 2013b). These suggest that CXCR2 may play an important role in Aβ production. A variety of cells are involved in the development of AD, of which microglia, as important cells in the neuroinflammatory process, were reported in previous studies. Jiao et al. found that in Aβ 1-42-treated cultured rat microglia, triptolide inhibited tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) expression levels and thus exerted neuroprotective effects (Jiao et al., 2008). Furthermore, triptolide was reported to exert neuroprotective effects in neuronal PC12 cells. A previous study suggested that triptolide decreased the level of reactive oxygen species (ROS), thus attenuating apoptosis in PC12 cells stimulated by glutamate (He et al., 2003). In consistence with this, Gu et al. also found the attenuation of triptolide on apoptosis of PC12 cells (Gu et al., 2004). Beside that, Xu et al. demonstrated the neuroprotective effects of triptolide in Aβ 25-35-treated differentiated PC12 cells, which involves autophagy pathway and inhibition of oxidative stress (Xu et al., 2015; Xu et al., 2016). In view of the clinical use of triptolide, a recent study used nanoparticles loaded with triptolide to treat Aβ 1-42-treated differentiated PC12 cells and showed that this treatment reduced oxidative stress and consequently significantly inhibited cytotoxicity (Jia et al., 2021). The above experimental studies have shown that triptolide exerts neuroprotective effects in AD, which provides a theoretical basis for its clinical application.
In addition to neuroprotective effects, the neurotrophic effects of triptolide in AD has also been studied. It was found that primary astrocytes from rats treated with triptolide showed increased synthesis and release of nerve growth factor (NGF), a key neurotrophic factor that is essential for nerve cells (Xue et al., 2007). Moreover, in a cellular model of AD, treatment with triptolide increased the expression of synaptophysin in hippocampal neurons (Nie et al., 2012). Synaptophysin is an important protein of synaptic vesicle membrane and is mainly expressed at the presynaptic endings of the nervous system (García-Mesa et al., 2022). Recently, a study reported that intraperitoneally injected triptolide increased hippocampal neuroligin-1 (a cell adhesion protein, located on the excitatory postsynaptic membrane) expression in the APP/PS1 model of AD through epigenetic mechanisms (Lu et al., 2019). These studies demonstrate the neurotrophic effects of triptolide on AD models.
Above data suggest that triptolide exerts therapeutic effects on AD by the following mechanisms: 1) inhibiting Aβ deposition by inhibiting BACE1 or CXCR2 in neurons, 2) inhibiting MAPKs activity and attenuating neuroinflammation in microglia, 3) decreasing level of ROS and inhibiting oxidative stress in neurons, 4) attenuating apoptosis and stabilizing mitochondrial membrane potential in neurons, 5) enhancing autophagy in neurons, 6) exerting neurotrophic effects by promoting expression of NGF (in astrocytes), synaptophysin (in neurons), and neuroligin-1.
Celastrol, one of the important bioactive components extracted from TWHF, has been the subject of many studies for the treatment of AD. Allison et al. reported that lipopolysaccharide (LPS) rat model intraperitoneally administered with celastrol exhibited improved performance in memory and learning activity tests (Allison et al., 2001). Moreover, subcutaneous treatment with celastrol was found to attenuate the accumulation of pathological plaque and microglial activation in the double transgenic Tg PS1/APPsw (human APP695sw mutation and the presenilin-1 mutation M146L) AD mice (Paris et al., 2010). In addition, the diabetes mellitus rat model showed reduced cognitive function and increased amyloid substance, similar to the alterations shown in the AD model, and celastrol treatment (intraperitoneal injection) inhibited these alterations (Liao et al., 2018). Recently, a study found that intraperitoneally administered celastrol improved learning and memory deficits in Aβ 25-35-microinjected rats (Xiao et al., 2021). Further, this study suggests that the neuroprotective effect of celastrol is associated with inhibition of nuclear factor-kappaB (NF-kB) activity, improved synaptic function and increased glucose metabolism. A recent study proposed that celastrol mixed in regular feed could reduce the accumulation of neurofibrillary tangles in the brains of two common animal models of AD (P301S microtubule associated protein tau mice and 3XTg mice) by activating transcription factor EB-mediated autophagy and lysosome biogenesis, thereby attenuating memory deficits in AD mice. Similarly, these effects of celastrol were also found in N2a cells (Yang et al., 2022b).
Beside that, other related in vitro experiments have further explored the possible mechanisms involved in the neuroprotective effects of celastrol on AD. Low doses of celastrol have been reported to inhibit the production of TNF-α and IL-1β by human monocytes and macrophages, the expression of major histocompatibility complex II (MHC II) molecules of microglia, and the production of inducible nitric oxide synthase (iNOS) in endothelial cells (Allison et al., 2001). It is well known that NF-kB is an important regulator in inflammatory responses. Paris et al. found that celastrol could attenuate NF-kB activity in HEK293 cells; moreover, they also demonstrated the inhibition of celastrol on Aβ production in 7 W CHO cells overexpressing wild-type human APP, which is related to the inhibition of BACE-1 (β-site APP cleaving enzyme) (Paris et al., 2010). BACE-1 is a major β-secretase, exerting effects on proteolysis of the β-APP to obtain Aβ peptides (Vassar et al., 1999). Furthermore, using molecular docking and in vitro assays, a study found that celastrol exerts neuroprotective effects in IMR-32 cells by inhibiting inhibitor κB kinase (IKK), which is the converging point in the activation of NF-κB (Veerappan et al., 2017). Moreover, it was reported that celastrol inhibited the production of Aβ in LPS-treated H4-APP cells, attenuated NF-kB activity and suppressed cyclooxygenase-2 (COX-2) expression. In addition, the effects of celastrol on increased levels of heat shock protein (Hsp) 70 and Bcl-2 expression have been reported (Zhao et al., 2014). Heat shock proteins are a series of proteins that have effects on folding of proteins. Among them, Hsp27 and Hsp70 are thought to have neuroprotective effects and their low levels are associated with AD (Chow et al., 2014). It was found that celastrol induced the increase in the expression of Hsp27 and Hsp70 in mature brain cortical cultures (Chow et al., 2013). However, in another study, celastrol was found to have no effect on the expression of Hsp70 in Aβ 1-42-treated SH-SY5Y cells, but could inhibit the expression of Hsp90 (Cao et al., 2018). This suggests that celastrol can exert neuroprotective effects through different pathways.
These studies reveal the mechanisms of therpaeutic effects of celastrol on AD: 1) inhibiting NF-kB activity and attenuating neuroinflammation in microglia and neurons, 2) reducing accumulation of neurofibrillary tangles by activating transcription factor EB-mediated autophagy and lysosome biogenesis, 3) inhibiting Aβ production by inhibition of BACE-1 in neurons, 4) increasing the expression of Hsp70 in mature brain cortical cultures while decreasing the expression of Hsp90 in neurons.
Parkinson’s disease (PD), a movement disorder, is the second most common neurodegenerative disease worldwide, mainly affecting older people (Kalia and Lang, 2016). Its main pathological feature is the progressive loss of dopaminergic neurons in the substantia nigra (Dauer and Przedborski, 2003). The main clinical manifestations of PD are resting tremor, bradykinesia, rigidity and postural abnormalities (Postuma et al., 2015). In addition, non-motor symptoms such as autonomic abnormalities, olfactory impairment and cognitive deficits also play an important role in the diagnosis of PD (Martinez-Martin et al., 2011). The pathogenesis of PD is not yet fully understood. Studies have shown that α-synuclein transmission is important in the pathogenesis of PD, which has made α-synuclein a hot topic in the field of PD research in recent years (Kurowska et al., 2011; Ganguly et al., 2022). Moreover, research on neuroinflammation in the pathogenesis of PD has also received widespread attention (Jenner and Olanow, 2006; Arena et al., 2022). As mentioned earlier, TWHF has anti-inflammatory properties. In recent years, there are many studies on the therapeutic effects of its extracts on PD.
Triptolide, as one of the main bioactive components of TWHF exerting anti-inflammatory effects, has been the subject of many in vivo and in vitro studies for its therapeutic effects on PD. Zhou et al. developed an inflammatory rat model by LPS intranigral injection and investigated the therapeutic effects of triptolide by intraperitoneal treatment with triptolide for 24 consecutive days. The results showed that triptolide treatment protected dopaminergic neurons and reduced the expression of pro-inflammatory cytokines, such as TNF-α and IL-1β, thereby exerting neuroprotective effects (Zhou et al., 2005). In line with this, in another inflammatory rat model using LPS induction, triptolide treatment protected dopaminergic neurons and inhibited microglial activation (Li G. et al., 2006). Moreover, a study investigated the neuroprotective effects of triptolide in hemiparkinsonian rats using a 1-methyl-4-phenyl pyridinium (MPP+)-induced rat model. They found that intraperitoneally injected triptolide improved behavioral performance in this model and which may be associated with protection of dopaminergic neurons and inhibition of microglial activation (Gao et al., 2008).
Similar results have been reported in vitro experiments. In primary mesencephalic neuron/glia mixed culture, Li et al. reported the inhibition of triptolide on the decrease in [3H]dopamine uptake and loss of tyrosine hydroxylase-immunoreactive neurons induced by LPS, and underlying mechanism may be related to the inhibition of microglial activation (Li et al., 2004). In this study, they also found that treatment with triptolide inhibited the overproduction of TNF-α and NO. In addition, a study also found that triptolide inhibited TNF-α and NO production in primary microglial cultures (Zhou et al., 2003). Furthermore, a research used a novel extraction method to obtain extracts from TWHF and found that the extracts protected dopaminergic neurons in rat mesencephalic neuron-glia cultures induced by LPS, inhibited microglial activation and TNF-α and NO expression (Liu et al., 2010). Most of the triptolide in the extracts obtained by this new extraction method was converted to tripchlorolide, an analogue of triptolide (Li B. et al., 2006). The therapeutic effects of tripchlorolide on PD are currently being studied in vitro and in vivo. For example, tripchlorolide was found to exert neuroprotective and neurotrophic effects on dopaminergic neurons by promoting axonal elongation and protecting dopaminergic neurons, as well as increasing brain-derived neurotrophic factor (BDNF) mRNA expression (Li et al., 2003). In another study, tripchlorolide was reported to protect dopaminergic neurons, inhibit the overproduction of TNF-α and IL-2, and thus exerting neuroprotective effects in rat models of PD (Cheng et al., 2002). Beside that, In a mouse model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD, intraperitoneally treated with tripchlorolide improved behavioral performance, protected dopaminergic neurons and inhibited astroglial responses, thereby exerting neuroprotective effects (Hong et al., 2007). Recently, it has been shown that the inhibitory effect of triptolide on microglial activation during PD treatment may be achieved by targeting the microRNA155–5p/SHIP1 pathway or by upregulating metabotropic glutamate receptor 5 (intraperitoneal injection) (Huang et al., 2018; Feng et al., 2019). In addition, enhanced autophagy in neurons, which in turn promotes the clearance of α-synuclein, is one of the possible mechanisms of triptolide to treat PD (Hu et al., 2017).
The above studies illustrate the therapeutic effects of triptolide and tripchlorolide on PD models, which is associated with the following mechanisms: 1) attenuating neuroninflammation in microglia and neurons, 2) promoting the clearance of α-synuclein in neurons by enhancing autophagy, 3) exerting neurotrophic effects by incresing the expression of BDNF mRNA in neurons. These suggest that triptolide and tripchlorolide may be potential candidates for clinical treatment of PD.
Considering the anti-inflammatory effects of celastrol, there are many studies focusing on its therapeutic effects on PD. Faust et al. reported that in the Drosophila DJ-1A model of PD, celastrol inhibited the reduction of dopaminergic neurons and increased brain dopamine content, thereby exerting neuroprotective effects in the PD model (Faust et al., 2009). Beside that, the therapeutic effects of celastrol have also been reported in mouse models of MPTP-induced PD. A previous study reported that intraperitoneally treated with celastrol attenuated the loss of dopaminergic neurons, increased Hsp70 within dopaminergic neurons, and decreased the levels of NF-kB and TNF-α (Cleren et al., 2005). Furthermore, a recent study suggested that the neuroprotective effects of celastrol (intraperitoneal injection) on MPTP-induced PD mouse models was associated with activation of mitophagy (Lin et al., 2019). However, Zhang et al. reported some contradictory results when they found that celastrol inhibited PTEN-induced kinase 1 (PINK1)-dependent mitophagy (Zhang et al., 2019). This may be due to the complicated mechanisms of action of celastrol. Moreover, the therapeutic mechanisms of celastrol has been further explored. Using the adeno-associated virus (AAV)-mediated human α-synuclein overexpression PD model and the MPTP-induced PD mouse model, a team of researchers found that intraperitoneally injected with celastrol can improve motor deficits and thus exert neuroprotective effects by modulating the nuclear factor-erythroid 2-related factor 2 (Nrf2)- Nod-like receptor family, pyrin-domain-containing 3 (NLRP3)-caspase-1 pathway (Zhang et al., 2021). However, a study found that celastrol (intraperitoneal treatment) did not exert neuroprotective effects in Wistar rats in the presence of lactacystin-induced ubiquitin-proteasome system (UPS) inhibition (Konieczny et al., 2014). Consistent with this, celastrol also did not exert neuroprotective effects in SH-SY5Y cells and mouse primary cortical neurons, which incubated with lactacystin. Moreover, the therapeutic effects of celastrol can be found in the rotenone-induced SH-SY5Y model of PD. For example, Deng et al. reported the protection of celastrol on rotenone-induced SH-SY5Y, involving the induction of autophagy (Deng et al., 2013). Further study suggests that this protective effect may also involve preservation of mitochondrial function and inhibition of p38 MAPK (Choi et al., 2014). Recently, a study focused on the role of celastrol on dendritic cells (DCs), and the results demonstrated that celastrol could mediate antigen trafficking in DCs, thus attenuating α-synuclein-specific T cell responses (Ng et al., 2022), and this may be beneficial to the treatment of PD.
These data suggest that mechanisms of therapeutic effects of celastrol on PD: 1) increasing the expression of Hsp70 in neurons, 2) attenuating neuroinflammation, 3) activating mitophagy, 4) inducing autophagy in neurons, 5) preserving mitochondrial function and inhibiting p38 MAPK, 6) mediating antigen trafficking in DCs, thus attenuating α-synuclein-specific T cell responses.
Multiple sclerosis (MS) is a demyelinating, neurodegenerative disease that occurs mainly in women. Its main pathological features are early demyelination and late neurodegeneration (Dobson and Giovannoni, 2019). The pathogenesis of MS is not well understood, while it is generally regarded as an autoimmune disease in which autoimmunity and neuroinflammation play a critical role (Segal, 2019). There is currently no cure for MS, and many studies have investigated the therapeutic effects of extracts of TWHF, which has anti-inflammatory properties, on it.
To date, there is no cellular model that simulates the onset of MS well; therefore, most basic research on MS has been conducted using animal models. Among the many animal models, the experimental autoimmune encephalomyelitis (EAE) is the most widely used animal model that simulates the onset of MS (Gehrmann et al., 1993). Several studies have shown that triptolide and its analogues can exert neuroprotective effects against EAE. For example, a previous study reported the neuroprotective effects of triptolide on EAE models of C57 BL/6 mice, and results showed that intraperitoneally treated with triptolide could delay the onset of EAE, attenuate the degree of inflammation and demyelination, and improve behavirol deficits; moreover, the inhibition of NF-kB-DNA binding activity was also found (Wang et al., 2008). In addition, Fu et al. found that intraperitoneally injected with (5R)-5-hydroxytriptolide (LLDT-8), one of the derivatives of triptolide, could suppress the severity of EAE models of C57 BL/6 mice by inhibiting T-cell activation (Fu et al., 2006). The therapeutic effects of triptolide (oral administration) was also found in the EAE model of SJL/J mice, involving increased expression levels of Hsp70 and stabilisation of the NF-kB/inhibitor-kappa-B alpha (IkBα) complex (Kizelsztein et al., 2009). In addition to the EAE model, there is also a cuprizone induced toxic model that can be used to study MS, which is primarily used to study damage to oligodendrocytes and myelin sheaths during MS (Abakumova et al., 2015). A recent study demonstrated that, in cuprizone induced toxic models, triptolide administered intraperitoneally improved behavioral deficits and attenuated neuroinflammation by inhibiting NF-kB activation and promoting intrinsic myelin repair (Sanadgol et al., 2018). Together, these related studies demonstrate that inhibiting NF-kB activity and attenuating neuroinflammation are crucial in therapeutic effects of triptolide on MS.
There are also basic studies on the therapeutic effects of celastrol on MS. It was shown that in a relapsing-remitting EAE rat model, celastrol (intraperitoneal injection) inhibited relapses and reduced clinical scores by modulating the Th1/Th2 cytokines profile (increased IL-10 expression while reduced TNF-α expression), inhibiting NF-κB and Toll-like receptor 2 (TLR2) expression, and reducing CD3+ T lymphocytic count (O'Brien et al., 2001; Abdin and Hasby, 2014). MS is a classic autoimmune disease in which the imbalance between Th17 and Treg cells is important in the pathophysiology, as evidenced by the promotion of pathological Th17 responses, and the inhibition of Treg responses (Holmøy, 2008; Di Mitri et al., 2015). Therefore, several studies have further investigated the effects of celastrol on T cells in EAE. For example, Wang et al. reported that intraperitoneally treated with celastrol exerted neuroprotective effects on EAE by inhibiting Th17 cell responses and attenuating cytokine production in bone-marrow derived dendritic cells (Wang et al., 2015). Moreover, another study suggested that the effect of celastrol (intraperitoneal treatment) on T-cell responses is mediated through the MAPK pathway, in which serum/glucocorticoid regulated kinase 1 (SGK1) expression is suppressed, yet BDNF expression is increased (Venkatesha and Moudgil, 2019). In addition, a study using an EAE rat model found that intraperitoneally injected with celastrol inhibited the expression of iNOS and NF-kB and attenuated MS and optic neuritis in the models (Yang et al., 2017). Similar to triptolide, NF-kB is also a key signaling pathway in therapeutic effects of celastrol on MS. Besides that, celastrol can modulate Th1/Th2 cytokines profile and inhibit Th17 cell responses through the MAPK pathway, thus attenuating neuroinflammation.
Huntington’s diseases (HD) is a fatal, progressive neurodegenerative disease. It is caused by excesively expanded CAG repeats in the gene and is an autosomal dominant disorder (Rai et al., 2019). Clinical manifestations of HD include motor dysfunction, cognitive impairment and psychiatric disturbances (Ross and Tabrizi, 2011). Currently, HD cannot be completely cured. Some studies have found that celastrol has therapeutic effects on HD, but there are no reports of triptolide in HD. Cleren et al. reported the neuroprotection of celastrol (intraperitoneal injection) on 3-nitropropionic acid-induced HD rat models, and they found that celastrol could decrease striatal lesion voulme, increase the expression of Hsp70 in the striata, and attenuate astrogliosis (Cleren et al., 2005). Considering the neuroprotective effects of heat shock proteins and the previously mentioned relationship between celastrol and heat shock proteins, another study explored the therapeutic effects of celastrol and its effect on the heat shock response in cellular models of HD. The results showed that celastrol could inhibit polyglutamine aggregation by inducing heat shock factor 1 (HSF1) and then increasing the expression of Hsp70 (Zhang and Sarge, 2007). In addition, a study used a HdhQ111/Q111 knock-in mouse-derived striatal cell line to investigate the effects of celastrol and found that celastrol inhibited mutant huntingtin aggregation, and reversed the abnormal cellular localization of full-length mutant huntingtin (Wang et al., 2005).
Amyotrophic lateral sclerosis (ALS), a neurodegenerative disease, is an common adult motor neuron disease that can lead to progressive muscle atrophy and even death. Although the pathogenesis of ALS has not been fully elucidated, many studies have shown that the genetic component plays an important role in its development. Current treatment for ALS is mainly symptomatic, while there are some basic studies showing the therapeutic effects of celastrol in ALS models. As with HD, the therapeutic effect of triptolide in ALS has not been reported. A study using celastrol in a G93A-superoxide dismutase 1 (SOD1) transgenic mouse model of ALS found that orally administrated celastrol delayed disease onset, improved motor deficits, and increased the number of neurons, promoted Hsp70 expression, and reduced TNF-α and iNOS levels in the spinal cord (Kiaei et al., 2005). In addition, celastrol was found in vitro experiments to exert neuroprotective effects in primary motoneurons induced by staurosporin or H2O2 by activating the heat shock response (i.e. increasing Hsp70 expression) (Kalmar and Greensmith, 2009). Similar neuroprotective effects were found in another in vitro study. Specifically, Li et al. used H2O2 to treat G93A-SOD1 transfected NSC34 cells as a cellular model of ALS and found that celastrol reduced cell death, involving activation of mitogen-activated protein kinase (MEK)/extracellular regulated protein kinases (ERK) and phosphoinositide 3-kinase (PI3K)/serine-threonine kinase (AKT) signaling pathways (Li et al., 2017).
In conclusion, the therapeutic effects of celastrol in HD and ALS are closely related to heat shock proteins, especially Hsp70, although other mechanisms such as inhibition of astrogliosis exist.
With an ageing population, the incidence of stroke is increasing, placing a huge burden on society and families. Of these, ischemic stroke is the most common type of stroke and is the third leading cause of disability worldwide (Benjamin et al., 2018; Meng et al., 2021). The therapeutic time window for ischemic stroke is narrow and there are significant financial and care costs for the treatment and rehabilitation of patients with ischemic stroke, so it is important that patients suffering with ischemic stroke are treated promptly and effectively (Sun et al., 2020). Currently, the main treatment options for ischemic stroke episodes include chemical thrombolysis (intravenous) and mechanical thrombectomy (intra-arterial) (Moussaddy et al., 2018). However, these treatments lead to a classic pathophysiological process known as ischemia/reperfusion injury. During cerebral ischemia/reperfusion injury, multiple pathophysiological mechanisms are involved and interact with each other, including oxidative stress, inflammatory responses, neuronal apoptosis and autophagy (Meng et al., 2021). Therefore, many studies have used models of cerebral ischemia/reperfusion injury to conduct research related to cerebral ischemia.
Middle cerebral artery occlusion (MCAO) is a common animal model of cerebral ischemia, and many studies have demonstrated the therapeutic effects of triptolide on MCAO. For example, Wei et al. found that triptolide improved neural function, attenuated neuronal apoptosis, and suppressed infiltration of neutrophils in rat with focal ischemia/reperfusion (Wei et al., 2004). Moreover, since inflammatory responses play an important role in cerebral ischemia/reperfusion injury, several studies further explored the effect of triptolide on NF-kB, an essential regulator of inflammatory responses, in MCAO, and reported the inhibitory effect of triptolide on NF-kB activity (Jin et al., 2015; Bai et al., 2016a; Bai et al., 2016b). In addition, Zhang et al. found that triptolide inhibited NF-kB/p53 upregulated modulator of apoptosis (PUMA) signaling pathway, which in turn exerts neuroprotective effects against MCAO (Zhang et al., 2016). This inhibition has also been reported in cellular models. A previous study, using MCAO rats and oxygen-glucose deprivation (OGD) SH-SY5Y cells, found that triptolide inhibited NF-kB and p38 MAPK signaling pathways to exert neuroprotection (Hao et al., 2015). In addition to its effects on NF-kB activity, there are several studies showing that triptolide exerts protective effects against cerebral ischemia by affecting autophagy. For example, Yang et al. found the upregulation of autophagy and downregulation of apoptosis in MCAO treated with triptolide (Yang et al., 2015). In a further study, the team found that the downregulation of apoptosis by triptolide in MCAO was through activation of the PI3K/AKT/mammalian target of rapamycin (mTOR) signaling pathway (Li et al., 2015). Beside that, neurobehavioral function in MCAO was improved by the combined use of the colony-stimulating factor 1 receptor inhibitor and triptolide, involving mechanisms including activation of autophagy and BDNF-AKT signaling pathways (Du et al., 2020). Pan et al. also reported the therapeutic effects of triptolide on MCAO. In their experimental results, triptolide could improve neurobehavioral scores, reduce brain damage, exert anti-inflammatory and antioxidant effects (reduce levels of malondialdehyde (MDA) and ROS, increase superoxide dismutase (SOD) level), and these effects were associated with inhibition of Wnt/β-catenin signaling pathway (Pan and Xu, 2020). In addition, right unilateral common carotid artery occlusion (rUCCAO), which simulates chronic cerebral hypoperfusion, can also be used for cerebral ischemia related research (Yoshizaki et al., 2008). A recent study using rUCCAO and OGD reported the therapeutic effects of triptolide in vitro and in vivo, including alleviating white matter injury, protecting against oligodendrocyte apoptosis directly, and inhibiting microglial inflammation indirectly, and the related mechanism was to increase the phosphorylation of the Src/AKT/GSK 3β singnaling pathway (Wan et al., 2022).
Take together, triptolide exerts neuroprotective effects on cerebral ischemia by the following mechanisms: 1) inhibiting NF-kB signaling pathway, involving inhibition of NF-kB/PUMA signaling pathway and inhibition of p38 MAPK/NF-kB signaling pathway, 2) enhancing autophagy, 3) inhibiting apoptosis by activating PI3K/AKT/mTOR signaling pathway, 4) upregulating BDNF-AKT signaling pathways, 5) exerting antioxidant effects by inhibiting Wnt/β-catenin signaling pathway, 6) inhibiting oligodendrocyte apoptosis by upregulating the phosphorylation of the Src/AKT/GSK 3β pathway.
Many studies have also demonstrated the therapeutic effects of celastrol on cerebral ischemia and related mechanisms. To study the therapeutic effects of celastrol on cerebral ischemia, Li et al. used a permanent middle cerebral artery occlusion (pMCAO) model (Li et al., 2012). It is worth noting that the process of pMCAO does not involve reperfusion, that is, it does not belong to the ischemia/reperfusion injury model, while it is also an important model for cerebral ischemia research. Their results indicated that celastrol improved neurological function and reduced infarct volume in pMCAO possibly through suppressing the expression of NF-kB, p-c-Jun, and p-(c-Jun N-terminal kinase) JNK. In another study, the transient global cerebral ischemia reperfusion (tGCI/R) model was used, and the investigators obtained similar neuroprotective effects as in the previous pMCAO study. The investigators also found that celastrol could suppress the expression of pro-inflammatory cytokines and MDA and elevate the levels of glutathione (GSH) and SOD in tGCI/R. Moreover, these protective effects of celastrol could be mediated by inhibition of high mobility group protein 1 (HMGB1)/NF-kB signaling pathway (Zhang et al., 2020). Further studies investigated the association between celastrol and HMGB1/NF-kB. Liu et al. found that under conditions of MCAO or OGD, celastrol could directly bind to HMGB1 and in turn inhibit the binding of HMGB1 to its downstream inflammatory components; moreover, celastrol could also target NF-kB and inhibit its activity (Liu D. D. et al., 2021). As important immune cells, microglia/macrophages play an important role in regulating inflammatory response and are involved in the pathophysiology of cerebral ischemia (Hu et al., 2012). Studies have shown that microglia/macrophages have different phenotypes, with M1 microglia releasing neurotoxic substances that exacerbate brain damage, and M2 microglia exerting neuroprotective effects by producing neurotrophic substances and removing debris (Brifault et al., 2015). A study reported neuroprotective effects of celastrol in MCAO and further found in OGD that this neuroprotection was through an IL-33/ST2 axis-mediated M2 microglia/macrophage polarization (Jiang et al., 2018). In addition, glycolysis plays a role in the pathogenesis of cerebral ischemia, and some studies have found that inhibition of excessive glycolysis can protect neurons against OGD (Burmistrova et al., 2019; Vojinovic et al., 2020). Chen et al. reported that celastrol inhibited hypoxia inducible factor-1α (HIF-1α)/pyruvate dehydrogenasekinase1 (PDK1), thus attenuating glycolysis and exerting neuroprotection (Chen et al., 2022). Besides that, studies of long noncoding RNAs (LncRNAs) have received much attention in recent years. A recent study focused on the role of LncRNAs-AK005401 in the treatment of MCAO with celastrol, and showed that celastrol exerts neuroprotective effects through inhibition of AK005401/MAP3K12 and activation of PI3K/AKT (Wang et al., 2021). In another study, researchers analyzed the profiles of LncRNAs and mRNAs following celastrol treatment of MCAO by RNA-sequencing and bioinformatic analysis and experimentally verified the significantly different LncRNAs and mRNAs (Liu J. et al., 2022). Liu et al. used ultra-performance liquid chromatography-tandem mass spectrometry (UPLC/MS) to perform lipidomic analysis after celastrol treatment of MCAO and obtained some differentially expressed components (Liu M. et al., 2021). These studies have provided a theoretical basis for subsequent in-depth investigations.
Above studies suggest the mechanisms of therapeutic effects of celastrol on cerebral ischemia: a). attenuating neuroinflammation by inhibiting HMGB1/NF-kB signaling pathway, inhibiting the phosphorylation of the JNK, or promoting an IL-33/ST2 axis-mediated M2 microglia/macrophage polarization, b). suppressing glycolysis by inhibiting HIF-1α/PDK1, c). inhibiting AK005401/MAP3K12 and activating PI3K/AKT signaling pathway.
Traumatic brain injury (TBI) is a type of brain trauma that can lead to long-term disability and possibly secondary neurodegenerative disorders (Sivanandam and Thakur, 2012; Humphreys et al., 2013). The role of neuroinflammation in the pathophysiology of TBI cannot be ignored. Numerous studies have reported the damaging effects of neuroinflammation on brain cells in TBI (Kunz et al., 2002; Strauss, 2008). The two main bioactive components of TWHF, triptolide and celastrol, have been reported to have anti-inflammatory and therapeutic effects in models of TBI. For example, a study found that triptolide improved neurological deficits and attenuated contusion volume, edema, and cell apoptosis in TBI-induced brains. Moreover, it also reported the decreased expressions of pro-inflammatory cytokines while the increased level of anti-inflammatory cytokines (Lee et al., 2012). In addition, celastrol was also found to improve neurobehavioral functions and protect neurons in TBI, and the relevant mechanisms involved the induction of Hsp70/Hsp110 expression (Eroglu et al., 2014).
Spinal cord injury (SCI) is also a neurological disease that can lead to long-term disability, with a high mortality and varying clinical symptoms depending on the degree of lesions (Coyoy-Salgado et al., 2019). In terms of pathology, SCI can be divided into primary, secondary and chronic phases, with oxidative stress and inflammation playing an important role in the pathophysiology of it (Fehlings and Tator, 1995; McDonald and Sadowsky, 2002). Su et al. showed that triptolide could promote spinal cord repair in SCI by attenuating astrogliosis and inflammation, and that attenuation of astrogliosis is via inhibition of the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway (Su et al., 2010). In addition to the modulation of astrocytes, modulation of microglia has also been reported in the neuroprotective effects of triptolide in SCI. A study found that in SCI, the neuroprotection of celastrol on spinal cord injury was achieved by targeting the miR-96/IKKβ/NF-κB pathway and thus inhibiting microglial activation (Huang et al., 2019). Moreover, enhanced autophagy and inhibition of MAPK/ERK1/2 signaling pathway were also found to be involved in the promotion of spinal cord injury repair by triptolide in SCI (Zhu et al., 2020). Furthermore, the neuroprotection of celastrol on SCI was also reported. For example, an in vitro study found that celastrol significantly reduced motorneuron death. As mentioned above, celastrol plays an important role in the induction of Hsp70 in diseases, and there are similar results in the study of SCI, that is, celastrol can play a neuroprotective role by inducing the expression of Hsp70. However, the limited protection of celastrol on the lumbar motor network was found in this study, suggesting that the therapeutic effects of celastrol in SCI are limited (Petrović et al., 2019). In addition, Dai et al. investigated the effects of celastrol on pyroptosis in SCI (Dai et al., 2019). Pyroptosis is a form of cell death that is distinct from necrosis and apoptosis and is associated with immunity and inflammation (Shi et al., 2015). They investigated the effects and mechanisms of celastrol in SCI rats and LPS/adenosine triphosphate (ATP)-induced BV2 cells and found that celastrol attenuated the activation of microglia in the spinal cord, inhibited the expression of pyroptosis-related molecules (NLRP3, caspase-1 and gasdermin D (GSDMD)) and inflammatory cytokines, and increased the levels of anti-inflammatory cytokines. Beside that, their study found that celastrol inhibited the expression of NF-kB, one of the upstream promoters of pyroptosis (Li et al., 2021). These suggest that the protective effects of celastrol against SCI is achieved, at least in part, through the inhibition of pyroptosis. These data suggest that triptolide and celastrol exert therapeutic effects by the following mechanisms: a). attenuating neuroinflammation by targeting miR-96/IKKβ/NF-κB, NF-kB/NLRP3/caspase-1 or MAPK/ERK1/2 pathway, or inducing the expression of Hsp70, b). enhancing autophagy, c). attenuating astrogliosis via inhibiting JAK2/STAT3 pathway.
Epilepsy is a chronic neurological disease with various clinical manifestations, and its typical clinical manifestation is epileptic seizures. As a common brain disorder, epilepsy affects more than 70 million people worldwide, adversely affecting the quality of life of patients (Khan et al., 2020). The etiology of epilepsy is not fully understood. Studies have shown that multiple factors are involved in the pathogenesis of epilepsy, such as genes, infections, structures and immunity (Singh and Trevick, 2016). Abnormal neuronal discharge in the brain is thought to be an important cause of epileptic seizures in epilepsy patients. Such abnormal discharge is associated with loss of neurons, dysfunction of glial cells, and changes in neurotransmitters or neuromodulation. Further, recurrent abnormal discharge of neurons in the brain can lead to neuronal abnormalities and synaptic remodelling, exacerbating epilepsy (He et al., 2021). Currently, drugs such as carbamazepine, phenytoin sodium and valproic acid are commonly used to treat epilepsy. However, their use has some side effects, whereas natural medicines have fewer side effects and are safer. Therefore, research on natural medicines for epilepsy is of clinical importance. Triptolide and celastrol have been found to have potential therapeutic implications in the studies of epilepsy.
Kainic acid is often used to induce epileptic seizures. Pan et al. induced epileptic seizures in rats by intracarotid subcutaneous injection of kainic acid, and in the treated rats, triptolide (15 μg/kg) was administered intraperitoneally daily. The results showed that triptolide had a protective effect on neurons in epileptic rats, and this was associated with increased expression of neuron kv1.1 in the CA3 region of the hippocampus (Pan et al., 2012). Glial cells play an important role in the pathogenesis of epilepsy, among which microglia are innate immune cells in the central nervous system (CNS), and the expression of MHC II in microglia increases under inflammatory conditions, which can activate CD4+ T cells and promote the inflammatory response (Nakahara et al., 2010). In epileptic rats, this was related to neuronal death (Shaw et al., 1994). An in vitro study used kainic acid-stimulated BV2 microglia to investigate the effect of triptolide on microglia. They found that microglia treated with triptolide for 2 h resulted in inhibition of microglial activation (morphology). Furthermore, treatment with triptolide significantly inhibited MHC II expression in microglia, and further promoter predictions and experimental results showed that this inhibition correlated with the inhibition of AP-1/class II transactivator (Sun et al., 2018).
In addition, several studies have also reported on the therapeutic effects of celastrol on epilepsy. Study has reported that researchers used a multiple-hit rat model to explore the therapeutic effects of celastrol on infantile spasms. Considering the anti-inflammatory effects of celastrol and the important role of inflammation and immunity in epilepsy, their findings found that celatrol could have a therapeutic effect on the disease by inhibiting NF-kB (Schiavone et al., 2021). Moreover, a study found that in a disease model of epilepsy, N-methyl-d-aspartate receptor-mediated activation of nicotinamide adenine dinucleotide phosphate oxidase (NOX) induces rapid release of H2O2, which is associated with epileptic seizures. In hippocampal slices and kainic acid-induced rats, treatment with celatrol inhibited NOX activation and rapid H2O2 release, thereby alleviating epileptic seizures (Malkov et al., 2019). However, a study reported the conflicting results, demonstrating that celastrol increases activation of microglia in hippocampal CA1 and CA3 regions and reduces postkindling seizure thresholds (von Rüden et al., 2019). This makes the effects of celastrol on epilepsy even more complex and requires more experiments to explore.
In conclusion, the research on the therapeutic mechanisms of triptolide and celastrol in epilepsy is still not deep enough, and more in-depth studies are needed to further clarify their mechanisms.
The pathogenesis of neurological diseases is complex and not yet fully elucidated. However, studies have shown that neuroinflammation is involved in the development of neurological diseases and that excessive neuroinflammation exacerbates the severity of the diseases (Ransohoff, 2016). Triptolide and celastrol, two important bioactive components extracted from TWHF, play an important role in attenuating neuroinflammation, thus reducing the severity of neurological diseases. In addition to neuroinflammation, triptolide and celastrol also exert neuroprotection against neurological diseases via neurotrophic effects and other mechanisms. In this process, regulation of neurons, microglia and astrocytes is involved (Figures 2, 3). Considering the important role of neuroinflammation and neurotrophy and the existence of numerous related studies, we will next review the studies about neuroinflammation and neurotrophy first, and finally other related mechanisms of neuroprotection.
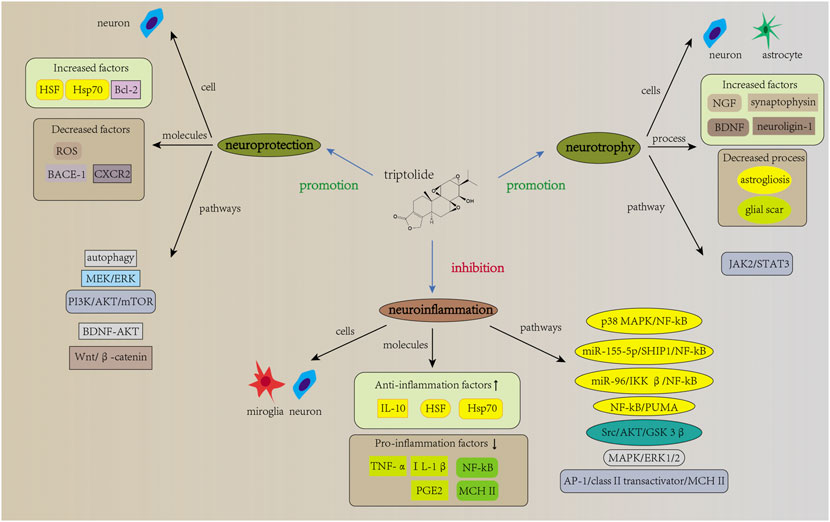
FIGURE 2. Mechanisms of action of triptolide in neurological diseases. The mechanisms of action of triptolide in neurological diseases involves neuroinflammation, neurotrophic effects, and other neuroprotective effects. It attenuates neuroinflammation and regulate inflammation levels in microglia and neuron, as evidenced by increased expression of anti-inflammatory factors and decreased expression of pro-inflammatory factors. Signaling pathways such as NF-kB are involved in this regulation. In addition, current studies have reported neurotrophic effects of triptolide for neurological diseases. Triptolide increases the expression levels of NGF, synaptophysin, neuroligin-1, and BDNF mRNA, and it also inhibits astrogliosis and excessive glial scar, which is associated with the regulation of JAK2/STAT3 signaling pathway. Furthermore, triptolide can act directly on neurons and thus exert neuroprotective effects by affecting other signaling pathways such as autophagy and apoptosis.
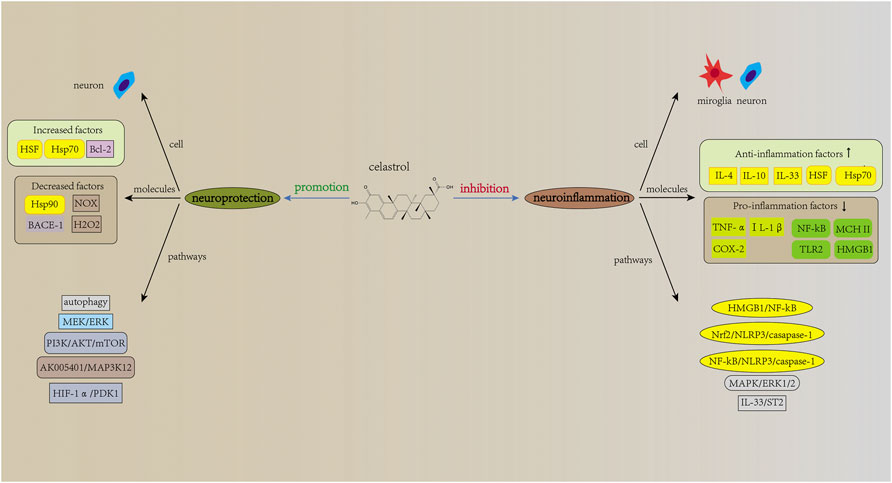
FIGURE 3. Mechanisms of action of celastrol in neurological diseases. Similar to triptolide, celastrol has therapeutic potential in neurological diseases, with related mechanisms of action including inhibition of neuroinflammation and promotion of neuroprotection. The NF-kB signaling pathway is also involved in the action of celastrol, and celastrol can also act directly on neurons, thereby exerting neuroprotective effects. Notably, although many studies have identified neurotrophic effects of triptolide, similar results have not been reported in studies related to celastrol.
Inflammation is the body’s response to endogenous and exogenous stimuli and it is important for the body’s self-protection. However, excessive inflammation can lead to dysregulation of inflammation and aggravate the severity of the diseases (Leite et al., 2022). Neuroinflammation has an important role in the pathophysiology of neurological diseases, and triptolide and celastrol have been reported to play a role in attenuating neuroinflammation and thus alleviating neurological diseases. For example, in vivo experiments, triptolide and celastrol inhibited neuroinflammation and attenuated the loss of dopaminergic neurons, thus improving the clinical performance of PD animal models (Zhou et al., 2005; Zhang et al., 2021). Further studies revealed that these effects were associated with the inhibition of microglial activation (Li G. et al., 2006). Microglia are innate immune cells of the CNS and are involved in the regulation of neuroinflammation (Young and Denovan-Wright, 2021). Studies have shown that triptolide and celastrol can inhibit the expression of pro-inflammatory molecules (TNF-α, IL-1β, MHC II, COX-2, and prostaglandin E2 (PGE2)) and promote the expression of anti-inflammatory cytokines (IL-4, IL-10 and IL-33) in microglia (Allison et al., 2001; O'Brien et al., 2001; Jiao et al., 2008; Jiang et al., 2018; Sun et al., 2018). Many signaling pathways mentioned have been found to be involved in the process (shown in Figures 2, 3). Among these, the NF-kB signaling pathway, a classical inflammation-related signaling pathway, has been the focus of much research. For example, in animal models of MS, triptolide and celastrol have been reported to reduce neuroinflammation and thereby improve behavioral deficits in model animals by inhibiting NF-kB activity (O'Brien et al., 2001; Wang et al., 2008). In addition to neurodegenerative diseases, the inhibition of NF-kB activity by triptolide and celastrol has also been reported in studies on cerebral ischemia and spinal cord injury (Bai et al., 2016a; Huang et al., 2019; Liu D. D. et al., 2021). In these studies, the inhibition of NF-kB activity will eventually lead to the decrease in the expression levels of pro-inflammatory molecules, while the related studies about downstream of NF-kB are not clear. A study using SCI rat models and LPS + ATP-induced BV2 cells showed that celastrol inhibits the activity of NF-kB, which in turn inhibits the expression of its downstream, NLRP3 inflammasome-related pathway, exerting anti-inflammatory effects (Dai et al., 2019). This reveals that the inhibition of NF-kB activity by celastrol leads to the decrease in the level of pyroptosis, thereby reducing the severity of the disease. In addition, many studies have reported the effects of triptolide and celastrol on Hsp70. Heat shock proteins are molecular chaperones that can assist in the proper folding of newly synthesized proteins and increase induced by stress (Agashe and Hartl, 2000). Kizelsztein et al. found that triptolide increased the expression level of Hsp70, stabilized the NF-kB/IkBα complex, which in turn inhibited neuroinflammation and exerted neuroprotective effects on the EAE models (Kizelsztein et al., 2009). Furthermore, by using LPS-stimulated PC12 cells, Geng et al. found that triptolide inhibited NF-kB activity and phosphorylation of p38, ERK1/2 and AKT, thus reducing the expression of COX-2 and PGE2 (Geng et al., 2012). This shows that in terms of inhibiting neuroinflammation, in addition to targeting microglia, triptolide can also directly target neurons and reduce the level of inflammation in neurons. Similar results can also be found in related studies of celastrol. For example, it was found that celastrol inhibited NF-kB activity and attenuated inflammation levels in HEK293 cells and LPS-treated H4-APP cells (Paris et al., 2010; Zhao et al., 2014). Moreover, in OGD-treated rat primary cortical neurons, celastrol was reported to directly bind to HMGB1 and inhibit NF-kB activity, thus exerting anti-inflammatory effect (Liu D. D. et al., 2021). The above studies demonstrate that triptolide and celastrol can exert neuroprotective effects on neurological diseases by inhibiting neuroinflammation, and their inhibition of neuroinflammation can be achieved by targeting both microglia and neurons.
In addition to inhibiting neuroinflammation, neurotrophy is also important to achieve the neuroprotective effects of TWHF extracts on neurological diseases. A previous study reported that triptolide promotes the synthesis and release of NGF from rats primary astrocytes (Xue et al., 2007). This suggests that triptolide can exert neurotrophic effects by affecting astrocytes. However, it did not reveal that whether triptolide can affect astrogliosis and glial scars. Astrocytes are important glial cells within the CNS that can play supportive and nutritional roles for neurons. In the case of injury, astrocyte activation leads to astrogliosis and eventually glial scars formation (Nash et al., 2011). In the early stages of injury, the glial scars separate the injured tissue from the surrounding area and protect the spared tissue (Rolls et al., 2009). However, in the late stage, glial scars prevent axonal regeneration and impair the recovery of function (Yiu and He, 2006). Therefore, it is important to study the effects of triptolide on astrogliosis and glial scar in the injured area. Further study using SCI rat models and LPS-stimulated primary astrocytes revealed that triptolide can inhibit astrogliosis and glial scars formation by affecting the JAK2/STAT3 pathway, which in turn promotes spinal cord repair (Su et al., 2010). Moreover, triptolide and tripchlorolide were also found to increase synaptophysin expression in hippocampal neurons, or BDNF mRNA expression in primary mesencephalic neurons (Li et al., 2003; Nie et al., 2012). Recently, an in vivo study found that triptolide and tripchlorolide increased the expression of neuroligin-1 in the hippocampal region of AD murine models (Lu et al., 2019). These data demonstrate the neurotrophic effects of triptolide in neurological diseases; however, no neurotrophic effect of celastrol has been reported in neurological diseases, and further research is needed.
Besides that, triptolide and celastrol can also exert neuroprotection against neurological diseases through other mechanisms. Polypeptides misfolding is considered as an important pathogenetic mechanism in neurodegenerative diseases (Sweeney et al., 2017). As mentioned earlier, heat shock proteins act as important molecular chaperones to assist in the proper folding of newly synthesized polypeptides. Therefore, targeting heat shock proteins is one of the effective approaches to treat neurodegenerative diseases. It was found that celastrol increases the expression of Hsp70 in neurons, which in turn exerts neuroprotective effects in neurodegenerative diseases (Cleren et al., 2005; Zhang and Sarge, 2007; Kalmar and Greensmith, 2009; Zhao et al., 2014). Besides that, celastrol was also found to increase the expression of Hsp70 in TBI mouse models and SCI spinal cords models, thus exerting neuroprotective effects (Eroglu et al., 2014; Petrović et al., 2019). In addition to increasing the expression of Hsp70, triptolide and celastrol can also exert neuroprotective effects through the following mechanisms: a). decreasing level of oxidative stress. He et al. reported that triptolide inhibited the formation of ROS and the decrease of mitochondrial membrane potential in glutamate-treated PC12 cells (He et al., 2003), b). inhibiting apoptosis. Triptolide inhibits apoptosis by activating the PI3K/AKT/mTOR signaling pathway (Li et al., 2015), c). inducing autophagy. It was reported that triptolide exerts neuroprotection by inducing autophagy in Aβ 25-35-treated differentiated PC12 cells (Xu et al., 2015). Besides that, in MN9D cells, triptolide was found to enhance autophagy in neuronal cells, thus promoting the clearance of α-synuclein (Hu et al., 2017). Moreover, research has shown that celastrol induces autophagy and exerts neuroprotection in rotenone-induced SH-SY5Y cells (Deng et al., 2013), d). attenuating glycolysis. Celastrol attenuates glycolysis and exerts neuroprotection by inhibiting HIF-1α/PDK1 pathway (Chen et al., 2022), e). suppressing NOX activation and rapid H2O2 release. Celastrol inhibits NOX activation and prevents fast H2O2 release, which in turn attenuates epileptic seizure (Malkov et al., 2019). Several pathways involved in the neuroprotective effects are shown in Figures 2, 3.
Although extracts of TWHF can be used to treat a variety of diseases, their toxicity limits clinical application (Li et al., 2014). Among them, triptolide and celastrol have been reported to have hepatotoxicity, nephrotoxicity, cardiotoxicity, reproductive toxicity and hematological toxicity (Table 2) (Bai et al., 2021; Cheng et al., 2021). In addition, poor solubility and bioavailability have also limited the clinical application of triptolide and celastrol, and research on related derivatives (Figure 4) and nanotechnology-based carrier system could improve this situation.
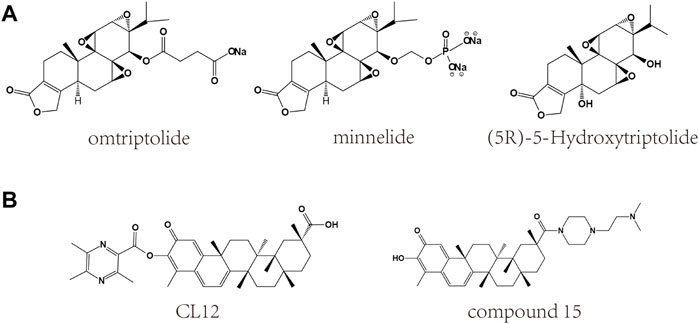
FIGURE 4. Chemical structures of derivatives of triptolide and celastrol. (A) Chemical structures of derivatives of triptolide. (B) Chemical structures of derivatives of celastrol.
Triptolide is one of the important TWHF extracts and is widely used in therapeutic studies for a variety of diseases. However, it has some toxicity and may cause serious side effects, which limits its clinical application. Its toxicity may be related to the epoxide structure covalently binding to and breaking biological macromolecules in the body (Xu et al., 2013). The liver, as an important metabolic organ in the body, has received widespread attention in the toxic damage caused by triptolide. It was found that the administration of triptolide (1 mg/kg) in animal models caused necrosis of hepatocytes with inflammatory cell infiltration, suggesting a possible role of inflammation in triptolide-induced liver injury (Wang et al., 2013a). This hypothesis is supported by another study which reported liver damage in animal models following triptolide administration, as well as inhibition of mitochondrial respiratory chain and increased levels of oxidative stress (Fu et al., 2011). In addition, it has been suggested that mitochondrial dysfunction and mitochondrial autophagy may also be involved in triptolide-induced liver injury (Hasnat et al., 2019). In addition to hepatotoxicity, nephrotoxicity of triptolide has also been reported. An in vivo experiment found that the administration of triptolide (1 mg/kg) to rats induced renal damage and increased level of oxidative stress (Yang et al., 2012). Besides that, triptolide-induced kidney damage was also found in a collagen-induced arthritis rat model (Shen et al., 2019). Moreover, triptolide has been reported to cause reproductive toxicity to both male and female reproductive systems. For example, Ma et al. reported that triptolide can disrupt testicular structure and inhibit spermatogenesis in male mice, which may be associated with downregulation of peroxisome proliferator-activated receptor (PPAR), leading to abnormal energy and lipid metabolism (Ma et al., 2015). As for reproductive toxicity in females, triptolide was found to reduce the expression of estradiol and progesterone in female rats, and the cyclic adenosine monophosphate/protein kinase A pathway may be involved (Zhang et al., 2012a; Zhang et al., 2012b).
Celastrol, one of the main bioactive components extracted from TWHF, has anti-inflammatory, antioxidant, and anti-tumour effects and is therefore used in therapeutic studies for many diseases. However, its toxicity has limited further research in the clinical field. A study reported that celsatrol caused liver damage in rats, which may be related to the inhibition of the activity of cytochrome P450s by celsastrol (Sun et al., 2014). Hepatotoxicity of celastrol was also found in an in vitro experiment (rat primary hepatocytes) (Jin et al., 2019). Furthermore, celastrol-induced cardiotoxicity has also been reported. Wang et al. reported that severe edema could be found in the pericardial sac after celastrol treatment (1.5 μM) in zebrafish embryos (Wang et al., 2011). Beside that, the cardiotoxicity of celastrol was found in HEK 293 cells, which may be associated with the inhibition of K+ channels (hERG and Kir2.1) activity (Sun et al., 2006). Moreover, Kusy et al. found that celastrol regulated the hematopoietic cell subsets, thus impairing the development of erythrocytes and B cells, and this further caused hematological toxicity in animal models (Kusy et al., 2012).
Considering the toxicity of triptolide and celastrol, many methods have been used to reduce their toxicity for clinical use. Generally speaking, four methods are usually used including the following: 1). structural modifications, 2). change in processing method, 3). change of the method of administration, and 4). combination of other drugs for co-treatment (Wang et al., 2016). Of these, “structural modification” means investigating derivatives of triptolide and celastrol in order to reduce toxicity, increase efficacy and facilitate clinical application. Structure-activity relationships are important in structural modifications. According to the structure-activity relationship, the derivatives of triptolide obtained after structural modification mainly include omtriptolide (PG490-88) (Kitzen et al., 2009), minnelide (Rivard et al., 2014) and (5R)-5-Hydroxytriptolide (LLDT-8) (Guo et al., 2019). In addition, derivatives of celastrol have also been reported. Celastrol is a triterpenoid quinone methide, and the relevant structural modifications are mainly the C-29 carboxyl group and the A/B ring modification (Bai et al., 2021). Studies have reported that these derivatives can reduce toxicity, increase bioavailability and be more beneficial for clinical applications in a variety of diseases (Zheng et al., 2013; Li and Hao, 2019).
With the advancement of nanoscience, nano-sized carriers are being used for drug loading and the nanotechnology-based carrier system can improve the effectiveness of triptolide and celastrol and reduce their toxicity. Studies have demonstrated that triptolide can be encapsulated to form triptolide-loaded nanomaterials, thus decreasing excessive accumulation of triptolide in normal tissues and reducing its toxicity. A study used triptolide-loaded liposomes, which linked anti-carbonic anhydrase IX antibody (an enzyme overexpressed on the surface of lung cancer cells) on the surface, to improve the treatment effects of triptolide on lung cancer cells (Lin et al., 2017). In addition, inflammation-targeted amphiphilic galactosyl dextran-retinal nanoparticles were used to encapsulate triptolide and the all-trans retinal, which is not only conducive to the drug delivery, but also can exert the synergistic effect of the drugs, thereby significantly enhancing the anti-inflammatory effect (Li et al., 2020). Similar to triptolide, celastrol can also be loaded by nanomaterials, which in turn reduces toxicity, increases bioavailability and enhances therapeutic efficacy. Studies have shown that celastrol can be loaded by various types of nanomaterials, such as liposomes, lipid nanoparticles, and exosomes, and the nanomaterials loaded with celastrol are used to treat various diseases (Guo et al., 2021). However, studies on nanotechnology-based carrier system of triptolide and celastrol are mainly about cancer therapy, and there are fewer studies related to neurological diseases (Liaw et al., 2021; Geng et al., 2022). Therefore, more studies are needed to confirm the efficacy of triptolide-loaded or celastrol-loaded nanomaterials for neurological diseases.
Neurological diseases can affect the brain and spinal cord and cause the corresponding clinical symptoms, which place a greater burden on the patient and society. Therefore, the treatment of neurological diseases is urgent and important. The pathogenesis of neurological diseases is complex and most of them are not due to a single factor; therefore, treatment based on a single-factor etiology is ineffective. Traditional Chinese medicine has a long history of medicinal use in China, with a wide range of targets, and is used for the treatment of many diseases. With the development of modern technology, the research of phytochemical research has gained tremendous progress. Extracts of TWHF (traditional Chinese medicine), triptolide and celastrol, have attracted great interest from researchers because of their structural characteristics and multi-target effects, and have been used in preclinical studies for a variety of neurological diseases involving neurodegenerative diseases, brain and spinal cord injuries, and epilepsy.
Although triptolide and celastrol have excellent potential therapeutic effects, poor water solubility, low oral bioavailability, imprecision of mechanism of action and potential toxicity limit their clinical application. Therefore, many methods have been used to address these problem, such as structural modifications, change in processing method, change of the method of administration, combination of other drugs for co-treatment, and the use of nanotechnology-based carrier system. In addition, to date, there have been no clinical trials to validate the therapeutic effects of triptolide and celastrol in neurological diseases to translate preclinical studies into clinical studies. Considering the potent therapeutic effects of triptolide and celastrol in neurological diseases, further studies on the pathogenesis of neurological diseases, and the mechanism of action, structure-activity relationships and pharmacokinetics of triptolide and celastrol would help to enhance their clinical application. Moreover, the development of nanotechnology can also facilitate the full utilization of these compounds.
JF was responsible to conceive, design, and revise this review. YC searched and collected the related references and wrote the original manuscript. XJ completed the figures and tables.
This review was supported by the Outstanding Scientific Fund of Shengjing Hospital (to JF), Key Research and Development Program of Liaoning Province (No. 2020JH2/10300047 to JF), and National Natural Science Foundation of China (No. 81771271 to JF).
We gratefully thank members of Feng’s lab for their intellectual input.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abakumova, T. O., Kuz'kina, A. A., Zharova, M. V., Pozdeeva, D. A., Gubskii, I. L., Shepeleva, , et al. (2015). Erratum to: Cuprizone model as a tool for preclinical studies of the efficacy of multiple sclerosis diagnosis and therapy. Bull. Exp. Biol. Med. 159 (3), 411. doi:10.1007/s10517-015-2976-8
Abdin, A. A., and Hasby, E. A. (2014). Modulatory effect of celastrol on Th1/Th2 cytokines profile, TLR2 and CD3+ T-lymphocyte expression in a relapsing-remitting model of multiple sclerosis in rats. Eur. J. Pharmacol. 742, 102–112. doi:10.1016/j.ejphar.2014.09.001
Agashe, V. R., and Hartl, F. U. (2000). Roles of molecular chaperones in cytoplasmic protein folding. Semin. Cell Dev. Biol. 11 (1), 15–25. doi:10.1006/scdb.1999.0347
Allison, A. C., Cacabelos, R., Lombardi, V. R., Alvarez, X. A., and Vigo, C. (2001). Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 25 (7), 1341–1357. doi:10.1016/s0278-5846(01)00192-0
Andrieu, S., Coley, N., Lovestone, S., Aisen, P. S., and Vellas, B. (2015). Prevention of sporadic alzheimer's disease: Lessons learned from clinical trials and future directions. Lancet. Neurol. 14 (9), 926–944. doi:10.1016/s1474-4422(15)00153-2
Arena, G., Sharma, K., Agyeah, G., Krüger, R., Grünewald, A., and Fitzgerald, J. C. (2022). Neurodegeneration and neuroinflammation in Parkinson's disease: A self-sustained loop. Curr. Neurol. Neurosci. Rep. 42 (8), 427–440. doi:10.1007/s11910-022-01207-5
Bai, S., Hu, Z., Yang, Y., Yin, Y., Li, W., Wu, L., et al. (2016a). Anti-inflammatory and neuroprotective effects of triptolide via the NF-κB signaling pathway in a rat MCAO model. Anat. Rec. 299 (2), 256–266. doi:10.1002/ar.23293
Bai, S., Sun, Y., Wu, L., Wu, Z., and Fang, M. (2016b). Tripotolide ameliorates inflammation and apoptosis induced by focal cerebral ischemia/reperfusion in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban. 45 (5), 493–500. doi:10.3785/j.issn.1008-9292.2016.09.07
Bai, X., Fu, R. J., Zhang, S., Yue, S. J., Chen, Y. Y., Xu, D. Q., et al. (2021). Potential medicinal value of celastrol and its synthesized analogues for central nervous system diseases. Biomed. Pharmacother. 139, 111551. doi:10.1016/j.biopha.2021.111551
Bakshi, P., Margenthaler, E., Reed, J., Crawford, F., and Mullan, M. (2011). Depletion of CXCR2 inhibits γ-secretase activity and amyloid-β production in a murine model of Alzheimer's disease. Cytokine 53 (2), 163–169. doi:10.1016/j.cyto.2010.10.008
Benjamin, E. J., Virani, S. S., Callaway, C. W., Chamberlain, A. M., Chang, A. R., Cheng, S., et al. (2018). Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation 137 (12), e67–e492. doi:10.1161/cir.0000000000000558
Brifault, C., Gras, M., Liot, D., May, V., Vaudry, D., and Wurtz, O. (2015). Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 46 (2), 520–528. doi:10.1161/strokeaha.114.006864
Brinker, A. M., Ma, J., Lipsky, P. E., and Raskin, I. (2007). Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 68 (6), 732–766. doi:10.1016/j.phytochem.2006.11.029
Burmistrova, O., Olias-Arjona, A., Lapresa, R., Jimenez-Blasco, D., Eremeeva, T., Shishov, D., et al. (2019). Targeting PFKFB3 alleviates cerebral ischemia-reperfusion injury in mice. Sci. Rep. 9 (1), 11670. doi:10.1038/s41598-019-48196-z
Cao, F., Wang, Y., Peng, B., Zhang, X., Zhang, D., and Xu, L. (2018). Effects of celastrol on Tau hyperphosphorylation and expression of HSF-1 and HSP70 in SH-SY5Y neuroblastoma cells induced by amyloid-β peptides. Biotechnol. Appl. Biochem. 65 (3), 390–396. doi:10.1002/bab.1633
Chen, M., Liu, M., Luo, Y., Cao, J., Zeng, F., Yang, L., et al. (2022). Celastrol protects against cerebral ischemia/reperfusion injury in mice by inhibiting glycolysis through targeting HIF-1α/PDK1 Axis. Oxid. Med. Cell. Longev. 2022, 7420507. doi:10.1155/2022/7420507
Cheng, S., LeBlanc, K. J., and Li, L. (2014). Triptolide preserves cognitive function and reduces neuropathology in a mouse model of Alzheimer's disease. PLoS One 9 (9), e108845. doi:10.1371/journal.pone.0108845
Cheng, X. X., Li, F. Q., Huang, M., Wang, X. M., and Han, J. S. (2002). Protective effect of tripchlorolide on dopaminergic neurons in partially lesioned rat model of Parkinson's disease. Yao Xue Xue Bao 37 (5), 339–342.
Cheng, Y., Zhao, Y., and Zheng, Y. (2021). Therapeutic potential of triptolide in autoimmune diseases and strategies to reduce its toxicity. Chin. Med. 16 (1), 114. doi:10.1186/s13020-021-00525-z
Choi, B. S., Kim, H., Lee, H. J., Sapkota, K., Park, S. E., Kim, S., et al. (2014). Celastrol from 'Thunder God Vine' protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson's disease. Neurochem. Res. 39 (1), 84–96. doi:10.1007/s11064-013-1193-y
Chow, A. M., Tang, D. W., Hanif, A., and Brown, I. R. (2013). Induction of heat shock proteins in cerebral cortical cultures by celastrol. Cell Stress Chaperones 18 (2), 155–160. doi:10.1007/s12192-012-0364-0
Chow, A. M., Tang, D. W., Hanif, A., and Brown, I. R. (2014). Localization of heat shock proteins in cerebral cortical cultures following induction by celastrol. Cell Stress Chaperones 19 (6), 845–851. doi:10.1007/s12192-014-0508-5
Cleren, C., Calingasan, N. Y., Chen, J., and Beal, M. F. (2005). Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J. Neurochem. 94 (4), 995–1004. doi:10.1111/j.1471-4159.2005.03253.x
Coyoy-Salgado, A., Segura-Uribe, J. J., Guerra-Araiza, C., Orozco-Suárez, S., Salgado-Ceballos, H., Feria-Romero, I. A., et al. (2019). The importance of natural antioxidants in the treatment of spinal cord injury in animal models: An overview. Oxid. Med. Cell. Longev. 2019, 3642491. doi:10.1155/2019/3642491
Cui, Y. Q., Wang, Q., Zhang, D. M., Wang, J. Y., Xiao, B., Zheng, Y., et al. (2016). Triptolide rescues spatial memory deficits and amyloid-β aggregation accompanied by inhibition of inflammatory responses and MAPKs activity in APP/PS1 transgenic mice. Curr. Alzheimer Res. 13 (3), 288–296. doi:10.2174/156720501303160217122803
Dá Mesquita, S., Ferreira, A. C., Sousa, J. C., Correia-Neves, M., Sousa, N., and Marques, F. (2016). Insights on the pathophysiology of Alzheimer's disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev. 68, 547–562. doi:10.1016/j.neubiorev.2016.06.014
Dai, W., Wang, X., Teng, H., Li, C., Wang, B., and Wang, J. (2019). Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int. Immunopharmacol. 66, 215–223. doi:10.1016/j.intimp.2018.11.029
Dauer, W., and Przedborski, S. (2003). Parkinson's disease: Mechanisms and models. Neuron 39 (6), 889–909. doi:10.1016/s0896-6273(03)00568-3
De Angelis, M., Schriever, S. C., Kyriakou, E., Sattler, M., Messias, A. C., Schramm, K. W., et al. (2020). Detection and quantification of the anti-obesity drug celastrol in murine liver and brain. Neurochem. Int. 136, 104713. doi:10.1016/j.neuint.2020.104713
Delport, A., and Hewer, R. (2022). The amyloid precursor protein: A converging point in alzheimer's disease. Mol. Neurobiol. 59 (7), 4501–4516. doi:10.1007/s12035-022-02863-x
Deng, Y. N., Shi, J., Liu, J., and Qu, Q. M. (2013). Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem. Int. 63 (1), 1–9. doi:10.1016/j.neuint.2013.04.005
Di Mitri, D., Sambucci, M., Loiarro, M., De Bardi, M., Volpe, E., Cencioni, M. T., et al. (2015). The p38 mitogen-activated protein kinase cascade modulates T helper type 17 differentiation and functionality in multiple sclerosis. Immunology 146 (2), 251–263. doi:10.1111/imm.12497
Dobson, R., and Giovannoni, G. (2019). Multiple sclerosis - a review. Eur. J. Neurol. 26 (1), 27–40. doi:10.1111/ene.13819
Du, X., Gao, F., Chen, S., Botchway, B. O. A., Amin, N., Hu, Z., et al. (2020). Combinational pretreatment of colony-stimulating factor 1 receptor inhibitor and triptolide upregulates BDNF-akt and autophagic pathways to improve cerebral ischemia. Mediat. Inflamm. 2020, 8796103. doi:10.1155/2020/8796103
Eroglu, B., Kimbler, D. E., Pang, J., Choi, J., Moskophidis, D., Yanasak, N., et al. (2014). Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J. Neurochem. 130 (5), 626–641. doi:10.1111/jnc.12781
Faust, K., Gehrke, S., Yang, Y., Yang, L., Beal, M. F., and Lu, B. (2009). Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson's disease. BMC Neurosci. 10, 109. doi:10.1186/1471-2202-10-109
Fehlings, M. G., and Tator, C. H. (1995). The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 132 (2), 220–228. doi:10.1016/0014-4886(95)90027-6
Feng, Y., Zheng, C., Zhang, Y., Xing, C., Cai, W., Li, R., et al. (2019). Triptolide inhibits preformed fibril-induced microglial activation by targeting the MicroRNA155-5p/SHIP1 pathway. Oxid. Med. Cell. Longev. 2019, 6527638. doi:10.1155/2019/6527638
Fu, Q., Huang, X., Shu, B., Xue, M., Zhang, P., Wang, T., et al. (2011). Inhibition of mitochondrial respiratory chain is involved in triptolide-induced liver injury. Fitoterapia 82 (8), 1241–1248. doi:10.1016/j.fitote.2011.08.019
Fu, Y. F., Zhu, Y. N., Ni, J., Zhong, X. G., Tang, W., Zhou, R., et al. (2006). (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide derivative, prevents experimental autoimmune encephalomyelitis via inhibiting T cell activation. J. Neuroimmunol. 175 (1-2), 142–151. doi:10.1016/j.jneuroim.2006.03.011
Ganguly, U., Singh, S., Chakrabarti, S., Saini, A. K., and Saini, R. V. (2022). Immunotherapeutic interventions in Parkinson's disease: Focus on α-Synuclein. Adv. Protein Chem. Struct. Biol. 129, 381–433. doi:10.1016/bs.apcsb.2021.11.010
Gao, J. P., Sun, S., Li, W. W., Chen, Y. P., and Cai, D. F. (2008). Triptolide protects against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats: Implication for immunosuppressive therapy in Parkinson's disease. Neurosci. Bull. 24 (3), 133–142. doi:10.1007/s12264-008-1225-9
Gao, J., Zhang, Y., Liu, X., Wu, X., Huang, L., and Gao, W. (2021). Triptolide: Pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics 11 (15), 7199–7221. doi:10.7150/thno.57745
García-Mesa, Y., García-Piqueras, J., Cuendias, P., Cobo, R., Martín-Cruces, J., Feito, J., et al. (2022). Synaptophysin is a selective marker for axons in human cutaneous end organ complexes. Ann. Anat. 243, 151955. doi:10.1016/j.aanat.2022.151955
Gehrmann, J., Gold, R., Linington, C., Lannes-Vieira, J., Wekerle, H., and Kreutzberg, G. W. (1993). Microglial involvement in experimental autoimmune inflammation of the central and peripheral nervous system. Glia 7 (1), 50–59. doi:10.1002/glia.440070110
Geng, Y., Fang, M., Wang, J., Yu, H., Hu, Z., Yew, D. T., et al. (2012). Triptolide down-regulates COX-2 expression and PGE2 release by suppressing the activity of NF-κB and MAP kinases in lipopolysaccharide-treated PC12 cells. Phytother. Res. 26 (3), 337–343. doi:10.1002/ptr.3538
Geng, Y., Xiang, J., Shao, S., Tang, J., and Shen, Y. (2022). Mitochondria-targeted polymer-celastrol conjugate with enhanced anticancer efficacy. J. Control. Release 342, 122–133. doi:10.1016/j.jconrel.2022.01.002
Gu, M., Zhou, H. F., Xue, B., Niu, D. B., He, Q. H., and Wang, X. M. (2004). Effect of Chinese herb Tripterygium wilfordii Hook F monomer triptolide on apoptosis of PC12 cells induced by Abeta1-42. Sheng Li Xue Bao 56 (1), 73–78.
Guo, L., Zhang, Y., and Al-Jamal, K. T. (2021). Recent progress in nanotechnology-based drug carriers for celastrol delivery. Biomater. Sci. 9 (19), 6355–6380. doi:10.1039/d1bm00639h
Guo, S., Liu, J., Jiang, T., Lee, D., Wang, R., Zhou, X., et al. (2019). (5R)-5-Hydroxytriptolide (LLDT-8) induces substantial epigenetic mediated immune response network changes in fibroblast-like synoviocytes from rheumatoid arthritis patients. Sci. Rep. 9 (1), 11155. doi:10.1038/s41598-019-47411-1
Hao, M., Li, X., Feng, J., and Pan, N. (2015). Triptolide protects against ischemic stroke in rats. Inflammation 38 (4), 1617–1623. doi:10.1007/s10753-015-0137-x
Hasnat, M., Yuan, Z., Naveed, M., Khan, A., Raza, F., Xu, D., et al. (2019). Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: A novel mechanism in triptolide-induced hepatotoxicity. Cell Biol. Toxicol. 35 (3), 267–280. doi:10.1007/s10565-018-9447-8
He, L. Y., Hu, M. B., Li, R. L., Zhao, R., Fan, L. H., He, L., et al. (2021). Natural medicines for the treatment of epilepsy: Bioactive components, pharmacology and mechanism. Front. Pharmacol. 12, 604040. doi:10.3389/fphar.2021.604040
He, Q., Zhou, H., Xue, B., Niu, D., and Wang, X. (2003). Neuroprotective effects of Tripterygium Wilforddi Hook F monomer T10 on glutamate induced PC12 cell line damage and its mechanism. Beijing Da Xue Xue Bao Yi Xue Ban. 35 (3), 252–255.
Holmøy, T. (2008). The immunology of multiple sclerosis: Disease mechanisms and therapeutic targets. Minerva Med. 99 (2), 119–140.
Hong, Z., Wang, G., Gu, J., Pan, J., Bai, L., Zhang, S., et al. (2007). Tripchlorolide protects against MPTP-induced neurotoxicity in C57BL/6 mice. Eur. J. Neurosci. 26 (6), 1500–1508. doi:10.1111/j.1460-9568.2007.05766.x
Hu, G., Gong, X., Wang, L., Liu, M., Liu, Y., Fu, X., et al. (2017). Triptolide promotes the clearance of α-synuclein by enhancing autophagy in neuronal cells. Mol. Neurobiol. 54 (3), 2361–2372. doi:10.1007/s12035-016-9808-3
Hu, X., Li, P., Guo, Y., Wang, H., Leak, R. K., Chen, S., et al. (2012). Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43 (11), 3063–3070. doi:10.1161/strokeaha.112.659656
Huang, Y. Y., Zhang, Q., Zhang, J. N., Zhang, Y. N., Gu, L., Yang, H. M., et al. (2018). Triptolide up-regulates metabotropic glutamate receptor 5 to inhibit microglia activation in the lipopolysaccharide-induced model of Parkinson's disease. Brain Behav. Immun. 71, 93–107. doi:10.1016/j.bbi.2018.04.006
Huang, Y., Zhu, N., Chen, T., Chen, W., Kong, J., Zheng, W., et al. (2019). Triptolide suppressed the microglia activation to improve spinal cord injury through miR-96/ikkβ/NF-κB pathway. Spine (Phila Pa 1976) 44 (12), E707–e714. doi:10.1097/brs.0000000000002989
Humphreys, I., Wood, R. L., Phillips, C. J., and Macey, S. (2013). The costs of traumatic brain injury: A literature review. Clin. Outcomes Res. 5, 281–287. doi:10.2147/ceor.S44625
Jenner, P., and Olanow, C. W. (2006). The pathogenesis of cell death in Parkinson's disease. Neurology 66, S24–S36. doi:10.1212/wnl.66.10_suppl_4.s24
Jia, L., Nie, X. Q., Ji, H. M., Yuan, Z. X., and Li, R. S. (2021). Multiple-coated PLGA nanoparticles loading triptolide attenuate injury of a cellular model of alzheimer's disease. Biomed. Res. Int. 2021, 8825640. doi:10.1155/2021/8825640
Jiang, M., Liu, X., Zhang, D., Wang, Y., Hu, X., Xu, F., et al. (2018). Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization. J. Neuroinflammation 15 (1), 78. doi:10.1186/s12974-018-1124-6
Jiang, Y., Kang, Y., Liu, J., Yin, S., Huang, Z., and Shao, L. (2022). Nanomaterials alleviating redox stress in neurological diseases: Mechanisms and applications. J. Nanobiotechnology 20 (1), 265. doi:10.1186/s12951-022-01434-5
Jiao, J., Xue, B., Zhang, L., Gong, Y., Li, K., Wang, H., et al. (2008). Triptolide inhibits amyloid-beta1-42-induced TNF-alpha and IL-1beta production in cultured rat microglia. J. Neuroimmunol. 205 (1-2), 32–36. doi:10.1016/j.jneuroim.2008.08.006
Jin, C., Wu, Z., Wang, L., Kanai, Y., and He, X. (2019). CYP450s-Activity relations of celastrol to interact with triptolide reveal the reasons of hepatotoxicity of Tripterygium wilfordii. Molecules 24 (11), 2162. doi:10.3390/molecules24112162
Jin, X. Q., Ye, F., Zhang, J. J., Zhao, Y., and Zhou, X. L. (2015). Triptolide attenuates cerebral ischemia and reperfusion injury in rats through the inhibition the nuclear factor kappa B signaling pathway. Neuropsychiatr. Dis. Treat. 11, 1395–1403. doi:10.2147/ndt.S82052
Kalia, L. V., and Lang, A. E. (2016). Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat. Rev. Neurol. 12 (2), 65–66. doi:10.1038/nrneurol.2015.249
Kalmar, B., and Greensmith, L. (2009). Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects. Cell. Mol. Biol. Lett. 14 (2), 319–335. doi:10.2478/s11658-009-0002-8
Khan, A. U., Akram, M., Daniyal, M., Akhter, N., Riaz, M., Akhtar, N., et al. (2020). Awareness and current knowledge of epilepsy. Metab. Brain Dis. 35 (1), 45–63. doi:10.1007/s11011-019-00494-1
Kiaei, M., Kipiani, K., Petri, S., Chen, J., Calingasan, N. Y., and Beal, M. F. (2005). Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2 (5), 246–254. doi:10.1159/000090364
Kitzen, J. J., de Jonge, M. J., Lamers, C. H., Eskens, F. A., van der Biessen, D., van Doorn, L., et al. (2009). Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur. J. Cancer 45 (10), 1764–1772. doi:10.1016/j.ejca.2009.01.026
Kizelsztein, P., Komarnytsky, S., and Raskin, I. (2009). Oral administration of triptolide ameliorates the clinical signs of experimental autoimmune encephalomyelitis (EAE) by induction of HSP70 and stabilization of NF-kappaB/IkappaBalpha transcriptional complex. J. Neuroimmunol. 217 (1-2), 28–37. doi:10.1016/j.jneuroim.2009.08.017
Konieczny, J., Jantas, D., Lenda, T., Domin, H., Czarnecka, A., Kuter, K., et al. (2014). Lack of neuroprotective effect of celastrol under conditions of proteasome inhibition by lactacystin in in vitro and in vivo studies: Implications for Parkinson's disease. Neurotox. Res. 26 (3), 255–273. doi:10.1007/s12640-014-9477-9
Kunz, T., Marklund, N., Hillered, L., and Oliw, E. H. (2002). Cyclooxygenase-2, prostaglandin synthases, and prostaglandin H2 metabolism in traumatic brain injury in the rat. J. Neurotrauma 19 (9), 1051–1064. doi:10.1089/089771502760341965
Kupchan, S. M., Court, W. A., Dailey, R. G., Gilmore, C. J., and Bryan, R. F. (1972). Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc. 94 (20), 7194–7195. doi:10.1021/ja00775a078
Kurowska, Z., Englund, E., Widner, H., Lindvall, O., Li, J. Y., and Brundin, P. (2011). Signs of degeneration in 12-22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson's disease. J. Park. Dis. 1 (1), 83–92. doi:10.3233/jpd-2011-11004
Kusy, S., Ghosn, E. E., Herzenberg, L. A., and Contag, C. H. (2012). Development of B cells and erythrocytes is specifically impaired by the drug celastrol in mice. PLoS One 7 (4), e35733. doi:10.1371/journal.pone.0035733
Lange, B. M., Fischedick, J. T., Lange, M. F., Srividya, N., Šamec, D., and Poirier, B. C. (2017). Integrative approaches for the identification and localization of specialized metabolites in Tripterygium roots. Plant Physiol. 173 (1), 456–469. doi:10.1104/pp.15.01593
Lee, H. F., Lee, T. S., and Kou, Y. R. (2012). Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respir. Physiol. Neurobiol. 182 (1), 1–8. doi:10.1016/j.resp.2012.01.016
Leite, J. A., Cavalcante-Silva, L. H. A., Ribeiro, M. R., de Morais Lima, G., Scavone, C., and Rodrigues-Mascarenhas, S. (2022). Neuroinflammation and neutrophils: Modulation by ouabain. Front. Pharmacol. 13, 824907. doi:10.3389/fphar.2022.824907
Li, B., Bai, J., Yang, G., Li, Z., Wang, L., and Chen, Y. (2006). A novel method to convert triptolide into tripchlorolide in Tripterygium wilfordii. Phytochem. Anal. 17 (2), 129–133. doi:10.1002/pca.896
Li, F. Q., Cheng, X. X., Liang, X. B., Wang, X. H., Xue, B., He, Q. H., et al. (2003). Neurotrophic and neuroprotective effects of tripchlorolide, an extract of Chinese herb Tripterygium wilfordii Hook F, on dopaminergic neurons. Exp. Neurol. 179 (1), 28–37. doi:10.1006/exnr.2002.8049
Li, F. Q., Lu, X. Z., Liang, X. B., Zhou, H. F., Xue, B., Liu, X. Y., et al. (2004). Triptolide, a Chinese herbal extract, protects dopaminergic neurons from inflammation-mediated damage through inhibition of microglial activation. J. Neuroimmunol. 148 (1-2), 24–31. doi:10.1016/j.jneuroim.2003.10.054
Li, G., Ma, R., and Xu, Y. (2006). Protective effects of triptolide on the lipopolysaccharide-mediated degeneration of dopaminergic neurons in substantia nigra. Zhongguo Zhong Xi Yi Jie He Za Zhi 26 (8), 715–718.
Li, J., and Hao, J. (2019). Treatment of neurodegenerative diseases with bioactive components of Tripterygium wilfordii. Am. J. Chin. Med. 47 (04), 769–785. doi:10.1142/s0192415x1950040x
Li, J. M., Zhang, Y., Tang, L., Chen, Y. H., Gao, Q., Bao, M. H., et al. (2016). Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer's disease. Neural Regen. Res. 11 (9), 1492–1498. doi:10.4103/1673-5374.191224
Li, P., Yang, X., Yang, Y., He, H., Chou, C. K., Chen, F., et al. (2020). Synergistic effect of all-trans-retinal and triptolide encapsulated in an inflammation-targeted nanoparticle on collagen-induced arthritis in mice. J. Control. Release 319, 87–103. doi:10.1016/j.jconrel.2019.12.025
Li, W., Yang, Y., Hu, Z., Ling, S., and Fang, M. (2015). Neuroprotective effects of DAHP and Triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activation. Front. Neuroanat. 9, 48. doi:10.3389/fnana.2015.00048
Li, X. J., Jiang, Z. Z., and Zhang, L. Y. (2014). Triptolide: Progress on research in pharmacodynamics and toxicology. J. Ethnopharmacol. 155 (1), 67–79. doi:10.1016/j.jep.2014.06.006
Li, Y., He, D., Zhang, X., Liu, Z., Zhang, X., Dong, L., et al. (2012). Protective effect of celastrol in rat cerebral ischemia model: Down-regulating p-JNK, p-c-Jun and NF-κB. Brain Res. 1464, 8–13. doi:10.1016/j.brainres.2012.04.054
Li, Y. H., Liu, S. B., Zhang, H. Y., Zhou, F. H., Liu, Y. X., Lu, Q., et al. (2017). Antioxidant effects of celastrol against hydrogen peroxide-induced oxidative stress in the cell model of amyotrophic lateral sclerosis. Sheng Li Xue Bao 69 (6), 751–758.
Li, Y., Xia, Y., Yin, S., Wan, F., Hu, J., Kou, L., et al. (2021). Targeting microglial α-synuclein/TLRs/NF-kappaB/NLRP3 inflammasome Axis in Parkinson's disease. Front. Immunol. 12, 719807. doi:10.3389/fimmu.2021.719807
Liao, W. T., Xiao, X. Y., Zhu, Y., and Zhou, S. P. (2018). The effect of celastrol on learning and memory in diabetic rats after sevoflurane inhalation. Arch. Med. Sci. 14 (2), 370–380. doi:10.5114/aoms.2016.63740
Liaw, K., Sharma, R., Sharma, A., Salazar, S., Appiani La Rosa, S., and Kannan, R. M. (2021). Systemic dendrimer delivery of triptolide to tumor-associated macrophages improves anti-tumor efficacy and reduces systemic toxicity in glioblastoma. J. Control. Release 329, 434–444. doi:10.1016/j.jconrel.2020.12.003
Lin, C., Wong, B. C. K., Chen, H., Bian, Z., Zhang, G., Zhang, X., et al. (2017). Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci. Rep. 7 (1), 1097. doi:10.1038/s41598-017-00957-4
Lin, M. W., Lin, C. C., Chen, Y. H., Yang, H. B., and Hung, S. Y. (2019). Celastrol inhibits dopaminergic neuronal death of Parkinson's disease through activating mitophagy. Antioxidants (Basel) 9 (1), 37. doi:10.3390/antiox9010037
Liu, D. D., Luo, P., Gu, L., Zhang, Q., Gao, P., Zhu, Y., et al. (2021). Celastrol exerts a neuroprotective effect by directly binding to HMGB1 protein in cerebral ischemia-reperfusion. J. Neuroinflammation 18 (1), 174. doi:10.1186/s12974-021-02216-w
Liu, J., Guo, X., Yang, L., Tao, T., Cao, J., Hong, Z., et al. (2022). Effect of celastrol on LncRNAs and mRNAs profiles of cerebral ischemia-reperfusion injury in transient middle cerebral artery occlusion mice model. Front. Neurosci. 16, 889292. doi:10.3389/fnins.2022.889292
Liu, M., Chen, M., Luo, Y., Wang, H., Huang, H., Peng, Z., et al. (2021). Lipidomic profiling of ipsilateral brain and plasma after celastrol post-treatment in transient middle cerebral artery occlusion mice model. Molecules 26 (14), 4124. doi:10.3390/molecules26144124
Liu, Q., Li, Q., Zhang, R., Wang, H., Li, Y., Liu, Z., et al. (2022). circ-Pank1 promotes dopaminergic neuron neurodegeneration through modulating miR-7a-5p/α-syn pathway in Parkinson's disease. Cell Death Dis. 13 (5), 477. doi:10.1038/s41419-022-04934-2
Liu, Y., Chen, H. L., and Yang, G. (2010). Extract of Tripterygium wilfordii Hook F protect dopaminergic neurons against lipopolysaccharide-induced inflammatory damage. Am. J. Chin. Med. 38 (4), 801–814. doi:10.1142/s0192415x10008251
Lu, L., Li, F., and Wang, X. (2010). Novel anti-inflammatory and neuroprotective agents for Parkinson's disease. CNS Neurol. Disord. Drug Targets 9 (2), 232–240. doi:10.2174/187152710791012035
Lu, X., Yang, B., Yu, H., Hu, X., Nie, J., Wan, B., et al. (2019). Epigenetic mechanisms underlying the effects of triptolide and tripchlorolide on the expression of neuroligin-1 in the hippocampus of APP/PS1 transgenic mice. Pharm. Biol. 57 (1), 453–459. doi:10.1080/13880209.2019.1629463
Ma, B., Qi, H., Li, J., Xu, H., Chi, B., Zhu, J., et al. (2015). Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study. Toxicology 336, 84–95. doi:10.1016/j.tox.2015.07.008
Malkov, A., Ivanov, A. I., Latyshkova, A., Bregestovski, P., Zilberter, M., and Zilberter, Y. (2019). Activation of nicotinamide adenine dinucleotide phosphate oxidase is the primary trigger of epileptic seizures in rodent models. Ann. Neurol. 85 (6), 907–920. doi:10.1002/ana.25474
Martinez-Martin, P., Rodriguez-Blazquez, C., Kurtis, M. M., and Chaudhuri, K. R. (2011). The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov. Disord. 26 (3), 399–406. doi:10.1002/mds.23462
McDonald, J. W., and Sadowsky, C. (2002). Spinal-cord injury. Lancet 359, 417–425. doi:10.1016/s0140-6736(02)07603-1
Mehndiratta, M. M., and Aggarwal, V. (2021). Neurological disorders in India: Past, present, and next steps. Lancet. Glob. Health 9 (8), e1043–e1044. doi:10.1016/s2214-109x(21)00214-x
Meng, H., Jin, W., Yu, L., Xu, S., Wan, H., and He, Y. (2021). Protective effects of polysaccharides on cerebral ischemia: A mini-review of the mechanisms. Int. J. Biol. Macromol. 169, 463–472. doi:10.1016/j.ijbiomac.2020.12.124
Montine, T. J., Phelps, C. H., Beach, T. G., Bigio, E. H., Cairns, N. J., Dickson, D. W., et al. (2012). National institute on aging-alzheimer's association guidelines for the neuropathologic assessment of alzheimer's disease: A practical approach. Acta Neuropathol. 123 (1), 1–11. doi:10.1007/s00401-011-0910-3
Moussaddy, A., Demchuk, A. M., and Hill, M. D. (2018). Thrombolytic therapies for ischemic stroke: Triumphs and future challenges. Neuropharmacology 134, 272–279. doi:10.1016/j.neuropharm.2017.11.010
Nakahara, H., Konishi, Y., Beach, T. G., Yamada, N., Makino, S., and Tooyama, I. (2010). Infiltration of T lymphocytes and expression of icam-1 in the hippocampus of patients with hippocampal sclerosis. Acta histochem. cytochem. 43 (6), 157–162. doi:10.1267/ahc.10022
Nash, B., Ioannidou, K., and Barnett, S. C. (2011). Astrocyte phenotypes and their relationship to myelination. J. Anat. 219 (1), 44–52. doi:10.1111/j.1469-7580.2010.01330.x
Ng, L., Wang, X., Yang, C., Su, C., Li, M., and Cheung, A. K. L. (2022). Corrigendum: Celastrol downmodulates alpha-synuclein-specific T cell responses by mediating antigen trafficking in dendritic cells. Front. Immunol. 13, 907993. doi:10.3389/fimmu.2022.907993
Nie, J., Zhou, M., Lü, C., Hu, X., Wan, B., Yang, B., et al. (2012). Effects of triptolide on the synaptophysin expression of hippocampal neurons in the AD cellular model. Int. Immunopharmacol. 13 (2), 175–180. doi:10.1016/j.intimp.2012.03.021
O'Brien, N. C., Charlton, B., Cowden, W. B., and Willenborg, D. O. (2001). Inhibition of nitric oxide synthase initiates relapsing remitting experimental autoimmune encephalomyelitis in rats, yet nitric oxide appears to be essential for clinical expression of disease. J. Immunol. 167 (10), 5904–5912. doi:10.4049/jimmunol.167.10.5904
Pan, W., and Xu, Z. (2020). Triptolide mediates Wnt/β-catenin signalling pathway to reduce cerebral ischemia-reperfusion injury in rats. Folia Neuropathol. 58 (4), 324–333. doi:10.5114/fn.2020.102435
Pan, X., Zou, S. F., Zeng, C. X., Cui, J. H., Hu, B., and Li, Y. W. (2012). Effects of tripterolide on kv1.1 expression of voltage gated potassium channels in epileptic rats. J. Intern. Neurol. Neurosurg. 39 (2), 108–113. doi:10.7666/d.y2150841
Paris, D., Ganey, N. J., Laporte, V., Patel, N. S., Beaulieu-Abdelahad, D., Bachmeier, C., et al. (2010). Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer's disease. J. Neuroinflammation 7, 17. doi:10.1186/1742-2094-7-17
Perry, V. H., Nicoll, J. A., and Holmes, C. (2010). Microglia in neurodegenerative disease. Nat. Rev. Neurol. 6 (4), 193–201. doi:10.1038/nrneurol.2010.17
Petrović, A., Kaur, J., Tomljanović, I., Nistri, A., and Mladinic, M. (2019). Pharmacological induction of Heat Shock Protein 70 by celastrol protects motoneurons from excitotoxicity in rat spinal cord in vitro. Eur. J. Neurosci. 49 (2), 215–231. doi:10.1111/ejn.14218
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30 (12), 1591–1601. doi:10.1002/mds.26424
Rai, S. N., Singh, B. K., Rathore, A. S., Zahra, W., Keswani, C., Birla, H., et al. (2019). Quality control in huntington's disease: A therapeutic target. Neurotox. Res. 36 (3), 612–626. doi:10.1007/s12640-019-00087-x
Ransohoff, R. M. (2016). How neuroinflammation contributes to neurodegeneration. Science 353 (6301), 777–783. doi:10.1126/science.aag2590
Rivard, C., Geller, M., Schnettler, E., Saluja, M., Vogel, R. I., Saluja, A., et al. (2014). Inhibition of epithelial ovarian cancer by Minnelide, a water-soluble pro-drug. Gynecol. Oncol. 135 (2), 318–324. doi:10.1016/j.ygyno.2014.08.031
Rolls, A., Shechter, R., and Schwartz, M. (2009). The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10 (3), 235–241. doi:10.1038/nrn2591
Ross, C. A., and Tabrizi, S. J. (2011). Huntington's disease: From molecular pathogenesis to clinical treatment. Lancet. Neurol. 10 (1), 83–98. doi:10.1016/s1474-4422(10)70245-3
Sanadgol, N., Golab, F., Mostafaie, A., Mehdizadeh, M., Khalseh, R., Mahmoudi, M., et al. (2018). Low, but not high, dose triptolide controls neuroinflammation and improves behavioral deficits in toxic model of multiple sclerosis by dampening of NF-κB activation and acceleration of intrinsic myelin repair. Toxicol. Appl. Pharmacol. 342, 86–98. doi:10.1016/j.taap.2018.01.023
Schiavone, S., Morgese, M. G., Tucci, P., and Trabace, L. (2021). The therapeutic potential of celastrol in central nervous system disorders: Highlights from in vitro and in vivo approaches. Molecules 26 (15), E3993. doi:10.3390/molecules24213993
Schiavone, S., and Trabace, L. (2017). Inflammation, stress response, and redox dysregulation biomarkers: Clinical outcomes and pharmacological implications for psychosis. Front. Psychiatry 8, 203. doi:10.3389/fpsyt.2017.00203
Segal, B. M. (2019). The diversity of encephalitogenic CD4+ T cells in multiple sclerosis and its animal models. J. Clin. Med. 8 (1), 120. doi:10.3390/jcm8010120
Shaw, J. A., Perry, V. H., and Mellanby, J. (1994). MHC class II expression by microglia in tetanus toxin-induced experimental epilepsy in the rat. Neuropathol. Appl. Neurobiol. 20 (4), 392–398. doi:10.1111/j.1365-2990.1994.tb00985.x
Shen, Q., Wang, J., Yuan, Z., Jiang, Z., Shu, T., Xu, D., et al. (2019). Key role of organic cation transporter 2 for the nephrotoxicity effect of triptolide in rheumatoid arthritis. Int. Immunopharmacol. 77, 105959. doi:10.1016/j.intimp.2019.105959
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526 (7575), 660–665. doi:10.1038/nature15514
Shui, G., Wan, Y., Jiang, C., Zhang, H., Chen, P., Wang, C., et al. (2010). Progress in Tripterygium wilfordiiand its bioactive components in the field of pharmacodynamics and pharmacology. Zhongguo Zhong Yao Za Zhi 35 (4), 515–520. doi:10.4268/cjcmm20100425
Singh, A., and Trevick, S. (2016). The epidemiology of global epilepsy. Neurol. Clin. 34 (4), 837–847. doi:10.1016/j.ncl.2016.06.015
Sivanandam, T. M., and Thakur, M. K. (2012). Traumatic brain injury: A risk factor for alzheimer's disease. Neurosci. Biobehav. Rev. 36 (5), 1376–1381. doi:10.1016/j.neubiorev.2012.02.013
Strauss, K. I. (2008). Antiinflammatory and neuroprotective actions of COX2 inhibitors in the injured brain. Brain Behav. Immun. 22 (3), 285–298. doi:10.1016/j.bbi.2007.09.011
Su, Z., Yuan, Y., Cao, L., Zhu, Y., Gao, L., Qiu, Y., et al. (2010). Triptolide promotes spinal cord repair by inhibiting astrogliosis and inflammation. Glia 58 (8), 901–915. doi:10.1002/glia.20972
Sun, H., Liu, X., Xiong, Q., Shikano, S., and Li, M. (2006). Chronic inhibition of cardiac Kir2.1 and HERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J. Biol. Chem. 281 (9), 5877–5884. doi:10.1074/jbc.M600072200
Sun, M., Tang, Y., Ding, T., Liu, M., and Wang, X. (2014). Inhibitory effects of celastrol on rat liver cytochrome P450 1A2, 2C11, 2D6, 2E1 and 3A2 activity. Fitoterapia 92, 1–8. doi:10.1016/j.fitote.2013.10.004
Sun, T., Wang, P., Deng, T., Tao, X., Li, B., and Xu, Y. (2020). Effect of panax notoginseng saponins on focal cerebral ischemia-reperfusion in rat models: A meta-analysis. Front. Pharmacol. 11, 572304. doi:10.3389/fphar.2020.572304
Sun, Z., Du, M., Lu, Y., and Zeng, C. Q. (2018). Effects of triptolide on the expression of MHC II in microglia in kainic acid-induced epilepsy. Mol. Med. Rep. 17 (6), 8357–8362. doi:10.3892/mmr.2018.8891
Sweeney, P., Park, H., Baumann, M., Dunlop, J., Frydman, J., Kopito, R., et al. (2017). Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 6, 6. doi:10.1186/s40035-017-0077-5
Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis, P., et al. (1999). Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741. doi:10.1126/science.286.5440.735
Veerappan, K., Natarajan, S., Ethiraj, P., Vetrivel, U., and Samuel, S. (2017). Inhibition of IKKβ by celastrol and its analogues - an in silico and in vitro approach. Pharm. Biol. 55 (1), 368–373. doi:10.1080/13880209.2016.1241809
Venkatesha, S. H., and Moudgil, K. D. (2019). Celastrol suppresses experimental autoimmune encephalomyelitis via MAPK/SGK1-regulated mediators of autoimmune pathology. Inflamm. Res. 68 (4), 285–296. doi:10.1007/s00011-019-01219-x
Villegas-Llerena, C., Phillips, A., Garcia-Reitboeck, P., Hardy, J., and Pocock, J. M. (2016). Microglial genes regulating neuroinflammation in the progression of Alzheimer's disease. Curr. Opin. Neurobiol. 36, 74–81. doi:10.1016/j.conb.2015.10.004
Vojinovic, D., Kalaoja, M., Trompet, S., Fischer, K., Shipley, M. J., Li, S., et al. (2020). Association of circulating metabolites in plasma or serum and risk of stroke: Meta-analysis from seven prospective cohorts. Neurology 96 (8), e1110–e1123. doi:10.1212/wnl.0000000000011236
von Rüden, E. L., Wolf, F., Gualtieri, F., Keck, M., Hunt, C. R., Pandita, T. K., et al. (2019). Genetic and pharmacological targeting of heat shock protein 70 in the mouse amygdala-kindling model. ACS Chem. Neurosci. 10 (3), 1434–1444. doi:10.1021/acschemneuro.8b00475
Wan, B., Hu, X., Nie, J., Zhou, M., Yang, B., Li, Y., et al. (2014). Effects of triptolide on degeneration of dendritic spines induced by Aβ1-40 injection in rat hippocampus. Neurol. Sci. 35 (1), 35–40. doi:10.1007/s10072-013-1463-0
Wan, Y. S., You, Y., Ding, Q. Y., Xu, Y. X., Chen, H., Wang, R. R., et al. (2022). Triptolide protects against white matter injury induced by chronic cerebral hypoperfusion in mice. Acta Pharmacol. Sin. 43 (1), 15–25. doi:10.1038/s41401-021-00637-0
Wang, C., Wan, H., Li, M., and Zhang, C. (2021). Celastrol attenuates ischemia/reperfusion-mediated memory dysfunction by downregulating AK005401/MAP3K12. Phytomedicine. 82, 153441. doi:10.1016/j.phymed.2020.153441
Wang, J., Gines, S., MacDonald, M. E., and Gusella, J. F. (2005). Reversal of a full-length mutant huntingtin neuronal cell phenotype by chemical inhibitors of polyglutamine-mediated aggregation. BMC Neurosci. 6, 1. doi:10.1186/1471-2202-6-1
Wang, J., Gu, B. J., Masters, C. L., and Wang, Y. J. (2017). A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 13 (11), 703. doi:10.1038/nrneurol.2017.147
Wang, J., Jiang, Z., Ji, J., Wang, X., Wang, T., Zhang, Y., et al. (2013a). Gene expression profiling and pathway analysis of hepatotoxicity induced by triptolide in Wistar rats. Food Chem. Toxicol. 58, 495–505. doi:10.1016/j.fct.2013.04.039
Wang, J., Shi, Z. Q., Xu, X., Xin, G. Z., Chen, J., Qi, L. W., et al. (2013b). Triptolide inhibits amyloid-β production and protects neural cells by inhibiting CXCR2 activity. J. Alzheimers Dis. 33 (1), 217–229. doi:10.3233/jad-2012-120841
Wang, Q., Meng, J., Dong, A., Yu, J. Z., Zhang, G. X., and Ma, C. G. (2016). The pharmacological effects and mechanism of Tripterygium wilfordii Hook F in central nervous system Autoimmunity. J. Altern. Complement. Med. 22 (7), 496–502. doi:10.1089/acm.2016.0004
Wang, Q., Xiao, B., Cui, S., Song, H., Qian, Y., Dong, L., et al. (2014). Triptolide treatment reduces Alzheimer's disease (AD)-like pathology through inhibition of BACE1 in a transgenic mouse model of AD. Dis. Model. Mech. 7 (12), 1385–1395. doi:10.1242/dmm.018218
Wang, S., Liu, K., Wang, X., He, Q., and Chen, X. (2011). Toxic effects of celastrol on embryonic development of zebrafish (Danio rerio). Drug Chem. Toxicol. 34 (1), 61–65. doi:10.3109/01480545.2010.494664
Wang, Y., Cao, L., Xu, L. M., Cao, F. F., Peng, B., Zhang, X., et al. (2015). Celastrol ameliorates EAE induction by suppressing pathogenic T cell responses in the peripheral and central nervous systems. J. Neuroimmune Pharmacol. 10 (3), 506–516. doi:10.1007/s11481-015-9598-9
Wang, Y., Mei, Y., Feng, D., and Xu, L. (2008). Triptolide modulates T-cell inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. Res. 86 (11), 2441–2449. doi:10.1002/jnr.21683
Wei, D. M., Huang, G. Z., Zhang, Y. G., and Rao, G. X. (2004). Influence of triptolide on neuronal apoptosis in rat with cerebral injury after focal ischemia reperfusion. Zhongguo Zhong Yao Za Zhi 29 (11), 1089–1091, 1116.
Wu, X. Y., Liao, B. Y., Xiao, D., Wu, W. C., Xiao, Y., Alexander, T., et al. (2022). Encapsulation of bryostatin-1 by targeted exosomes enhances remyelination and neuroprotection effects in the cuprizone-induced demyelinating animal model of multiple sclerosis. Biomater. Sci. 10 (3), 714–727. doi:10.1039/d1bm01142a
Xiao, Y., Wang, X., Wang, S., Li, J., Xu, X., Wang, M., et al. (2021). Celastrol attenuates learning and memory deficits in an alzheimer's disease rat model. Biomed. Res. Int. 2021, 5574207. doi:10.1155/2021/5574207
Xu, L., Qiu, Y., Xu, H., Ao, W., Lam, W., and Yang, X. (2013). Acute and subacute toxicity studies on triptolide and triptolide-loaded polymeric micelles following intravenous administration in rodents. Food Chem. Toxicol. 57, 371–379. doi:10.1016/j.fct.2013.03.044
Xu, P., Li, Z., Wang, H., Zhang, X., and Yang, Z. (2015). Triptolide inhibited cytotoxicity of differentiated PC12 cells induced by amyloid-beta₂₅₋₃₅ via the autophagy pathway. PLoS One 10, e0142719. doi:10.1371/journal.pone.0142719
Xu, P., Wang, H., Li, Z., and Yang, Z. (2016). Triptolide attenuated injury via inhibiting oxidative stress in Amyloid-Beta25-35-treated differentiated PC12 cells. Life Sci. 145, 19–26. doi:10.1016/j.lfs.2015.12.018
Xue, B., Jiao, J., Zhang, L., Li, K. R., Gong, Y. T., Xie, J. X., et al. (2007). Triptolide upregulates NGF synthesis in rat astrocyte cultures. Neurochem. Res. 32 (7), 1113–1119. doi:10.1007/s11064-006-9253-1
Yang, C., Li, S., Huang, X., Chen, X., Shan, H., Chen, X., et al. (2022a). Silk fibroin hydrogels could Be therapeutic biomaterials for neurological diseases. Oxid. Med. Cell. Longev. 2022, 2076680. doi:10.1155/2022/2076680
Yang, C., Su, C., Iyaswamy, A., Krishnamoorthi, S. K., Zhu, Z., Yang, S., et al. (2022b). Celastrol enhances transcription factor EB (TFEB)-mediated autophagy and mitigates Tau pathology: Implications for Alzheimer's disease therapy. Acta Pharm. Sin. B 12 (4), 1707–1722. doi:10.1016/j.apsb.2022.01.017
Yang, F., Ren, L., Zhuo, L., Ananda, S., and Liu, L. (2012). Involvement of oxidative stress in the mechanism of triptolide-induced acute nephrotoxicity in rats. Exp. Toxicol. Pathol. 64 (7-8), 905–911. doi:10.1016/j.etp.2011.03.013
Yang, H., Liu, C., Jiang, J., Wang, Y., and Zhang, X. (2017). Celastrol attenuates multiple sclerosis and optic neuritis in an experimental autoimmune encephalomyelitis model. Front. Pharmacol. 8, 44. doi:10.3389/fphar.2017.00044
Yang, Y., Gao, K., Hu, Z., Li, W., Davies, H., Ling, S., et al. (2015). Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia. Mediat. Inflamm. 2015, 120198. doi:10.1155/2015/120198
Yiu, G., and He, Z. (2006). Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7 (8), 617–627. doi:10.1038/nrn1956
Yoshizaki, K., Adachi, K., Kataoka, S., Watanabe, A., Tabira, T., Takahashi, K., et al. (2008). Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp. Neurol. 210 (2), 585–591. doi:10.1016/j.expneurol.2007.12.005
Young, A. P., and Denovan-Wright, E. M. (2021). The dynamic role of microglia and the endocannabinoid system in neuroinflammation. Front. Pharmacol. 12, 806417. doi:10.3389/fphar.2021.806417
Zhang, B., Song, C., Feng, B., and Fan, W. (2016). Neuroprotection by triptolide against cerebral ischemia/reperfusion injury through the inhibition of NF-κB/PUMA signal in rats. Ther. Clin. Risk Manag. 12, 817–824. doi:10.2147/tcrm.S106012
Zhang, B., Zhong, Q., Chen, X., Wu, X., Sha, R., Song, G., et al. (2020). Neuroprotective effects of celastrol on transient global cerebral ischemia rats via regulating HMGB1/NF-κB signaling pathway. Front. Neurosci. 14, 847. doi:10.3389/fnins.2020.00847
Zhang, C., Wang, R., Liu, Z., Bunker, E., Lee, S., Giuntini, M., et al. (2019). The plant triterpenoid celastrol blocks PINK1-dependent mitophagy by disrupting PINK1's association with the mitochondrial protein TOM20. J. Biol. Chem. 294 (18), 7472–7487. doi:10.1074/jbc.RA118.006506
Zhang, C., Zhao, M., Wang, B., Su, Z., Guo, B., Qin, L., et al. (2021). The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of Celastrol in Parkinson's disease. Redox Biol. 47, 102134. doi:10.1016/j.redox.2021.102134
Zhang, J., Jiang, Z., Mu, X., Wen, J., Su, Y., and Zhang, L. (2012a). Effect of triptolide on progesterone production from cultured rat granulosa cells. Arzneimittelforschung. 62 (6), 301–306. doi:10.1055/s-0032-1309041
Zhang, J., Liu, L., Mu, X., Jiang, Z., and Zhang, L. (2012b). Effect of triptolide on estradiol release from cultured rat granulosa cells. Endocr. J. 59 (6), 473–481. doi:10.1507/endocrj.ej11-0407
Zhang, Y. Q., and Sarge, K. D. (2007). Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J. Mol. Med. 85 (12), 1421–1428. doi:10.1007/s00109-007-0251-9
Zhao, Y., Zhao, H., Lobo, N., Guo, X., Gentleman, S. M., and Ma, D. (2014). Celastrol enhances cell viability and inhibits amyloid-β production induced by lipopolysaccharide in vitro. J. Alzheimers Dis. 41 (3), 835–844. doi:10.3233/jad-131799
Zheng, Y., Zhang, W. J., and Wang, X. M. (2013). Triptolide with potential medicinal value for diseases of the central nervous system. CNS Neurosci. Ther. 19 (2), 76–82. doi:10.1111/cns.12039
Zhou, H. F., Liu, X. Y., Niu, D. B., Li, F. Q., He, Q. H., and Wang, X. M. (2005). Triptolide protects dopaminergic neurons from inflammation-mediated damage induced by lipopolysaccharide intranigral injection. Neurobiol. Dis. 18 (3), 441–449. doi:10.1016/j.nbd.2004.12.005
Zhou, H. F., Niu, D. B., Xue, B., Li, F. Q., Liu, X. Y., He, Q. H., et al. (2003). Triptolide inhibits TNF-alpha, IL-1 beta and NO production in primary microglial cultures. Neuroreport 14 (7), 1091–1095. doi:10.1097/01.wnr.0000073682.00308.47
Keywords: neurological diseases, traditional Chinese medicine, triptolide, celastrol, neuroprotection, toxicity, derivatives
Citation: Cui Y, Jiang X and Feng J (2022) The therapeutic potential of triptolide and celastrol in neurological diseases. Front. Pharmacol. 13:1024955. doi: 10.3389/fphar.2022.1024955
Received: 22 August 2022; Accepted: 07 October 2022;
Published: 19 October 2022.
Edited by:
Hareram Birla, The State University of New Jersey, United StatesReviewed by:
Hagera Dilnashin, Banaras Hindu University, IndiaCopyright © 2022 Cui, Jiang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Feng, anVhbmZlbmdAY211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.