- 1Department of Pharmacy, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, China
- 3Department of Digestion, The Third Xiangya Hospital of Central South University, Changsha, China
- 4National Clinical Research Center for Geriatric Disorders (XIANGYA), Xiangya Hospital, Central South University, Changsha, China
Objective: In animals, Helicobacter pylori (Hp)-induced gastric injury is accompanied by a decrease in the activity of the cysteine/glutamate transporter (xCT), which regulates extracellular glutamate levels. However, the impact of xCT activity in patients with Hp infection remains unclear. This study aims to investigate variations of xCT activity in the gastric mucosa of patients with Hp infection and to provide a clinical basis for identifying targets related to Hp infection.
Methods: Our study included a total of 67 patients with gastritis, which consisted of 44 Hp-negative and 23 Hp-positive peptic ulcer cases. The inclusion criteria used to select patients were as follows: gastric histology was determined with a gastroscope, antral biopsies were taken for urease tests, and pathology and culture were performed for analysis of Hp-colonization. The clinical characteristics of the patients were obtained, the expressions of microRNAs and xCT protein were detected using immune histochemical analysis, and the concentration of glutamate in their gastric secretion was determined.
Results: The findings revealed that xCT expression was significantly lower in Hp-positive patients as compared to Hp-negative individuals, which was accompanied by a decrease in glutamate concentration in gastric juice. We also discovered a high expression of microRNAs that have been shown to negatively regulate xCT expression, in Hp-positive patients.
Conclusion: Reduced xCT activity in patients may play an important role in gastric ulcers caused by Hp infection. Our findings suggest that the microRNA/xCT pathway could be a potential treatment target for Hp-infection-related ulcers.
1 Introduction
Helicobacter pylori (Hp) is one of the most common pathogens causing chronic gastritis and gastric cancer (FitzGerald and Smith, 2021) and is classified as a clear human carcinogen in the 15th carcinogenic report issued by the US Department of Health and Human Services. The rate of Hp infection in the global natural population is over 50%. In China, the rate ranges between 40–90%, with an average of 59% (Kamboj et al., 2017). Hp usually colonizes in the mucus layer of the gastric epithelium, causing chronic active gastritis. If left untreated, however, long-term infection with Hp may even lead to gastric cancer. As a result, early treatment of Hp-related gastritis is critical. Antibacterial drugs combined with proton pump inhibitors are currently the main drug strategy for Hp infection. However, the widespread use of antimicrobial regimens has the potential to exacerbate resistance problems (de Brito et al., 2019). Future research on pathogenic mechanisms and alternative treatment targets, therefore, requires immediate attention.
The pathophysiological mechanism of Hp-induced peptic ulcer is complex and not fully understood. On the one hand, Hp is thought to colonize gastric mucosa cells and secrete virulence proteins to induce oxidative stress or inflammatory response, resulting in increased production of various inflammatory factors that can impair gastric mucosa barrier function (Feng et al., 2020; Lu et al., 2020). On the other hand, Hp can inhibit the secretion of endogenous active substances (such as nitric oxide, prostaglandin, epidermal growth factor, etc.) that have a protective effect on the stomach (Echizen et al., 2016; AbdelAziz et al., 2021). Recent research has found that glutamate, an important endogenous active substance, can protect against acute gastric injury induced by multiple factors, including cold-restraint stress (Chen et al., 2001), deoxynivalenol (Wu et al., 2014), and non-steroidal anti-inflammatory drugs (NSAIDs) (Du et al., 2014). However, its potential role in long-term chronic infection remains unknown. Our previous animal studies revealed that Hp-induced gastric injury was associated with reduced activity of xCT, a regulator of extracellular glutamate levels (Du et al., 2014; Du et al., 2020). However, the xCT activity in Hp-infection patients has not been elaborated. In this study, we explored xCT activity and its upstream regulation by microRNA in Hp-infected patients. Through our findings, we intended to provide a clinical basis for identifying Hp infection-associated targets.
2 Materials and methods
2.1 Ethics approval and informed consent statement
The Clinical Research Project was approved by the Institutional Review Board (IRB) of Third Xiangya Hospital, Central South University, Hunan, China (approval number 2015-S109). Because the patients were spread across the country over relatively long distances, we recorded their responses after oral informed consent over the phone.
2.2 Patient selection
This study included a total of 67 patients (44 Hp-negative and 23 Hp-positive gastric ulcer cases) who were diagnosed with gastritis during an endoscopic examination at the Third Xiangya Hospital between December 2015 and February 2016. Patients were enrolled in the study only if they met the following criteria: 1) belonged to the age group 18–75 years; 2) had not taken any NSAIDs or antibiotics 2 weeks before the study; 3) had taken a C13 isotope respiration test; and 4) had their gastric histology, urease and Hp-colonization determined. The following criteria were used to exclude patients: 1) a history of gastric or duodenal surgery; 2) active cancer, any acute medical or terminal illness. 3) long-term adverse life history, such as chronic alcohol or tobacco use; and 4) missing clinical indicators. The study protocol was approved by the Ethics Committee of our institution. All patients gave informed consent over the phone before participating in the study. Clinical data on patient characteristics, such as gender, age, position of the ulcer, pathologic grade, and Hp detection results were obtained.
2.3 Endoscopic examination and Helicobacter pylor infection detection
The patients took the 13C-urea breath test before an endoscopic examination. Three specimens were collected during endoscopy from the ulcer margin to perform Hp-colonization analysis, rapid urease test, and histological examination respectively. This was followed by the collection of gastric juice (10 ml) under gastroscopy. A positive Hp infection was confirmed from at least two of three diagnostic tests, namely rapid urease, 13C-urea breath test, or Giemsa stain. A negative result in all three tests defined the absence of infection.
2.4 Histology
Tissue sections were stained with hematoxylin and eosin to assess activity, inflammation, atrophy, and intestinal metaplasia (Figure 1), and graded on a scale of 0, 1, 2, and 3, corresponding to none, mild, moderate, and severe, respectively, accordingly to the updated Sydney system (Toyoshima et al., 2022). Hp colonization in the stomach was measured using Giemsa staining.
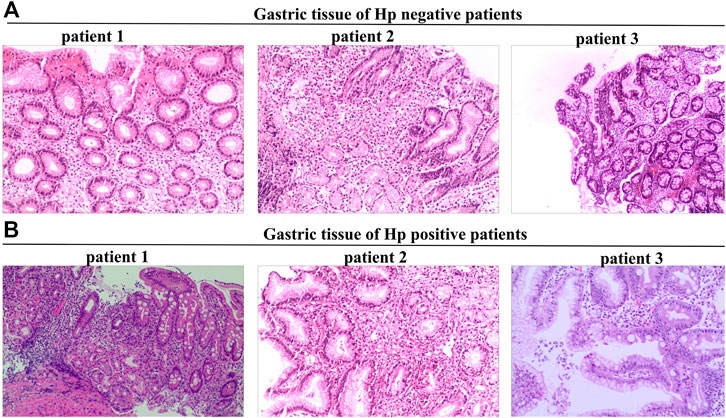
FIGURE 1. Tissue sections stained with hematoxylin-eosin were used to assess activity, inflammation, atrophy, and intestinal metaplasia. (A), Gastric tissue of Hp negative patients. (B), Gastric tissue of Hp positive patients.
2.5 Immunohistochemistry and in situ hybridization
Tissue sections were used to detect the xCT level by immunohistochemistry (IHC), while miRNA expression was determined using in situ hybridization. Acetate buffer (pH 6.0) was used as the immersion solution for the antigen pre-treatment step in IHC staining, while rabbit polyclonal antibody and the Envision (DAKO) polymer detection system were used for staining. Tissue sections were incubated overnight at 4°C with xCT (1:200) antibody, followed by 1-h incubation with anti-rabbit secondary antibody (1:200). In situ hybridization was performed using the miRCURY LNA miRNA ISH Kit (Exiqon) and images were acquired using a microscope (Olympus) with a ×20 objective lens.
2.6 Determination of glutamate concentration by high-performance liquid chromatography
First, 10 ml of gastric juice was centrifuged at 12000r/min for 3 min and the supernatant was collected. Next, to determine the glutamate content of gastric juice by High-Performance Liquid Chromatography (HPLC), a 10 μL sample or biological reference standard was mixed into 100 μL derivative reagent (a mixture of phthalaldehyde, boric acid buffer, and β-mercaptoethanol). The solution was allowed to react for 3 min at room temperature, transferred to a 2 ml autosampler vial, and analyzed by HPLC. The mobile phase consisted of 0.05 mol/L sodium acetate (pH 6.8), methanol, and, tetrahydrofuran (82:17:1, V/V/V). The flow rate of the column was 1 ml/min at room temperature. The excitation and absorption wavelengths were 338 nm and 430 nm, respectively.
2.7 Statistical analysis
All data were expressed as mean ± standard deviations (mean ± SD). The statistical data from all cases were organized using Microsoft Excel. For statistical analysis, SPSS21.0 was used. The counting data were expressed by frequency (n%) and χ2test. The t-test was used to analyze all factors, and p < 0.05 was considered statistically significant.
3 Results
3.1 Clinical characteristics of the patients enrolled in the study
This study included 44 Hp-negative and 23 Hp-positive peptic ulcer cases. The 44 Hp-negative peptic ulcer patients included 22 males and 22 females, with an average age of 51.8 years (51.80 ± 11.1). The 23 Hp-negative peptic ulcer patients included 14 males and nine females, with an average age of 45.6 years (45.60 ± 10.7). There was no difference in complications between the two groups. In the Hp-positive group, 56.5% of patients had antral ulcers (n = 13), 34.8% had corpus ulcers (n = 8) and 8.7% had multiple site ulcers (n = 2). In the Hp-negative group, 61.4% of patients had antral ulcers (n = 27), 25.0% had corpus ulcers (n = 11) and 13.6% had multiple site ulcers (n = 6) (Table 1).
There was no significant difference in gender, site of ulcer, or grade of pathologic score between the Hp-negative and Hp-positive patients (Table 1), except in age (χ2/t = 2.194, p < 0.05). No cases of intestinal metaplasia were recorded.
3.2 Decreased xCT activity in Helicobacter pylor-positive patients
Results of IHC staining showed that the level of xCT protein was significantly decreased in Hp-positive patients (Figure 2A). We also detected the concentration of glutamate in gastric secretion, which reflects the activity of xCT. Also, the concentration of glutamate in the Hp-positive group was significantly lower as compared to the Hp-negative group (Figure 2B), which indicates differences in xCT activities between these patient groups.
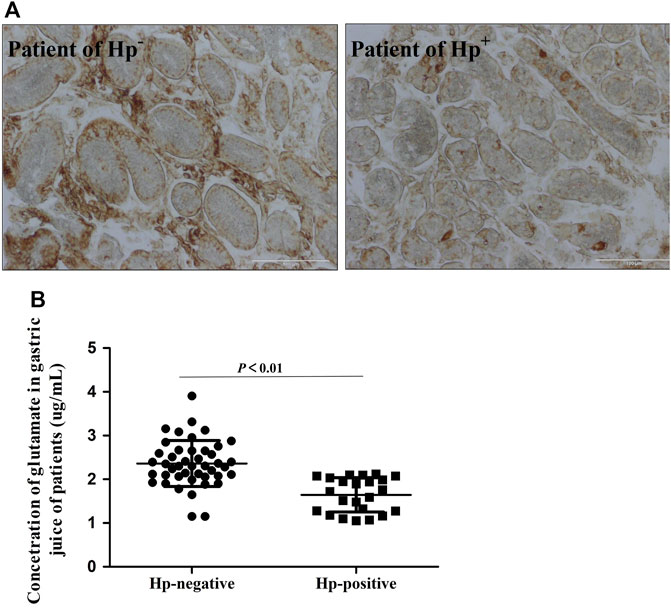
FIGURE 2. Decreased xCT activity in Hp infection patients. (A). Immunohistochemistry staining for xCT antigen in gastro of Hp infection patients. (B). The concentration of glutamate in gastric secretion by HPLC. Data are means ± standard deviations. N = 23–44. Hp is short for Helicobacter pylori.
3.3 Higher expression of miR-30b and miR-27a in Helicobacter pylor-positive patients
The microRNAs MiR-30b and miR-27a have been reported to downregulate xCT expression. However, the variations of these two miRNAs in patients with gastritis are not known. Figure 3A shows a lower expression of miRNA-27a in Hp-negative patients but a higher expression in Hp-positive patients. Similar expression patterns were observed for miR-30b (Figure 3B). These results suggested that variation of miRNA/xCT expression may contribute to Hp infection in clinical settings.
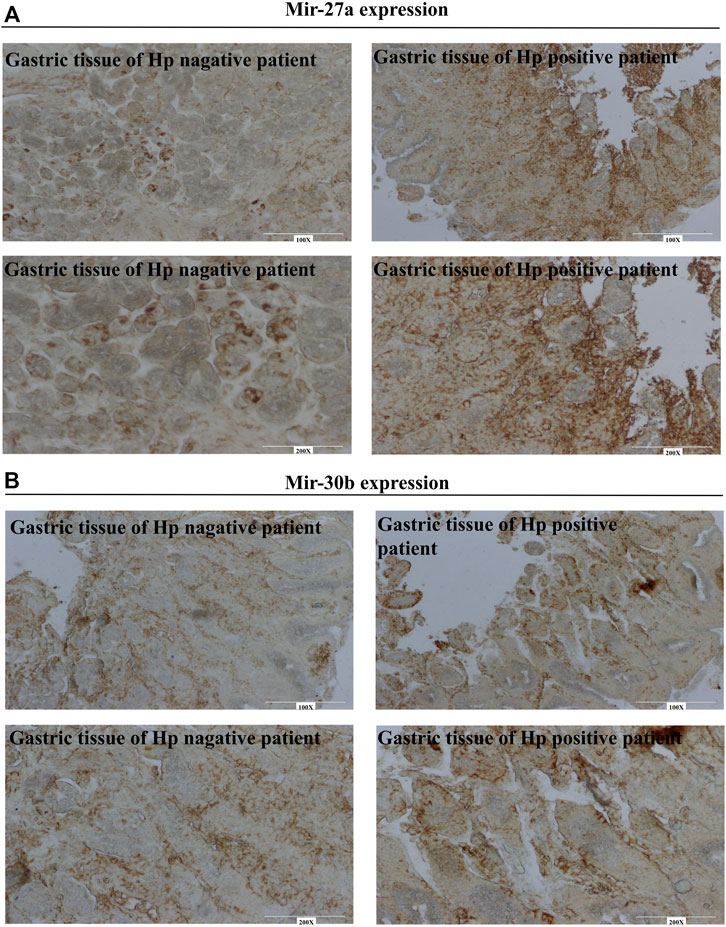
FIGURE 3. Identifying of miR-27a and miR-30b in gastro of patients using in situ hybridization. (A) MiR-27a expression in gastro of Hp negative and positive patients. (B) MiR-30b expression in gastro of Hp negative and positive patients.
3.4 Correlation between glutamate concentration and pathological grades
For Hp-positive patients, ulcers were classified according to their anatomical location, where 56.5% of cases were antral ulcers (n = 13), 34.8% of cases were corpus ulcers (n = 8), and 8.7% cases were multiple site ulcers (n = 2). There was no significant difference between the antrum and corpus of the ulcers. We also analyzed the correlation of glutamate concentration with pathological grades. Moreover, no correlation was observed between glutamate level and pathological grade (Table 2).
4 Discussion
Glutamate is a non-essential amino acid with significant physiological functions. In the past decades, researchers have discovered a critical role of glutamate in the central nervous system, where it has been recognized as one of the most ubiquitous excitatory neurotransmitters (Platt, 2007; Moroz et al., 2021). Recent studies have also shown evidence of glutamate expression in peripheral organs (Rose, 2002; Valdivielso et al., 2020; Zhang et al., 2021), especially in the stomach (Iijima et al., 2008). It participates in gastrointestinal functions, including regulating oxidative reactions, immune responses, and, barrier function. Abnormal glutamate signaling may lead to gastrointestinal diseases such as gastritis or ulcers. The bacteria Helicobacter pyroli and NSAIDs are recognized as the two major causes of gastritis or ulcers. It has been found that decreased glutamate is directly associated with gastric damage in these two gastric ulcer models (Du et al., 2014; Du et al., 2020). Several animal studies have concentrated on the role of xCT/glutamate in gastric ulcers. For example, in cold-restraint animals, glutamate acts on the ionic receptor NMDA to inhibit gastric acid secretion and protects mucous cells (Chen et al., 2001). In our recent study, we also found that reduced xCT activity was involved in an aspirin-induced acute gastric injury, while exogenously supplied glutamate exhibited protective effects on aspirin-induced gastric mucosa injury. The glutamate pathway also protected the gastrointestinal mucosa against Hp infection. However, the majority of these findings are based on animal studies, with no human clinical data available. In this study, we found that the expression of xCT in Hp-positive patients was significantly lower than that in negative individuals and that variations in xCT expression were accompanied by a reduction of glutamate concentration in gastric juice. Furthermore, we discovered an increase in the expression of miRNAs known to negatively regulate xCT (Drayton et al., 2014; Du et al., 2020). These findings suggested that the miRNA/xCT pathway could be a potential treatment target for Hp infection-related ulcers.
Inflammation is one of the most important mechanisms of Hp-associated gastritis. On the one hand, during an infection, immune cells are recruited to the infection site to initiate an inflammatory response. On the other hand, the secretion of virulence proteins directly activates the NF-κB pathway and stimulates the secretion of inflammatory factors (Lahner et al., 2018; Yang et al., 2021). Clinical studies have demonstrated higher expression of IL-1β in patients with Hp infection (Koosirirat et al., 2010). Animal studies have also shown that multiple inflammatory factors mediate a Hp infection, with IL-1β and IL-17 over secretion being the most obvious (Semper et al., 2014). Some inflammatory factors (such as IL-1β, TNF-α, etc.) have also been proven to affect glutamate transport to other tissues (such as nerve cells, macrophages, etc.) (Ye et al., 2013). For example, IL-1β significantly reduces the expression of glutamate transporter EAAT2 in astrocytes and also inhibits EAAT1 expression in Purkinje cells (Mandolesi et al., 2013). Previous studies have also demonstrated that inflammatory responses can inhibit the activity of xCT (Du et al., 2014; Wu et al., 2014; Shi et al., 2016). Based on these studies, we wanted to look into the causes behind decreased xCT activity for inflammatory regulation in Hp-infection patients.
MicroRNAs are single-stranded small RNA molecules that are 19–25 nucleotides in length and silence the expression of target genes through complete or incomplete pairing with the 3′-UTR of the target gene. On being stimulated by an external stimulus, such as a microbial infection, the expression of some miRNAs rapidly changes in the host, affecting the expression of target proteins and regulating the inflammatory immune response process. For example, in Hp-induced gastric injury, the expression of mir-155/146b was upregulated, weakening the bacterial clearance from the host (Cheng et al., 2015). Hp contributes to chronic inflammation by increasing the expression of miR-328, which targets CD44. (Ishimoto et al., 2015). It has been reported that miRNAs also regulate the expression of xCT. MiR-27a, for example, negatively regulates xCT, thereby mediating cisplatin resistance in patients (Drayton et al., 2014). In a Hp-infection animals, miR-30b/27a has been proven to negatively regulate xCT gene expression in luciferase reporter assays (Du et al., 2020). Our clinical findings showed that the expression of miR-27a and miR-30b was significantly increased in Hp-positive patients, which is consistent with previous animal studies. Both the literature and our results suggested that the upregulation of miR-30b/27a is involved in the process of Hp infection. However, the mechanism by which Hp causes these miRNA alterations remains unclear and warrants future studies.
In conclusion, we found that patients with Hp infection exhibit reduced expression of xCT and glutamate release, which may play an important role in gastric ulcer induction by the bacteria. Our findings support previous animal studies and suggest that the miRNA/xCT pathway could be a potential treatment target for Hp infection-related ulcers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of Third Xiangya Hospital, Central South University, Hunan, China (approval number 2015-S109). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JD and W-QL designed this experiment. LW, FL, W-QL and YL carried out the experiment. JD and FL collected the clinic cases. W-QL and LW detected these samples. JD, LW, W-QL, and Y-JL analyzed the data and wrote this manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81703592 to JD, No. 82173911 to W-QL, and No. 81573486 to Y-JL) and the Natural Science Foundation of Hunan province (2019JJ50934 to JD). It is also supported by Scientific Research Project of Hunan Provincial Health Commission (No. 202113050843). Research Project established by Chinese Pharmaceutical Association Hospital Pharmacy department (No. CPA-Z05-ZC-2021-002).
Acknowledgments
Special acknowledgement to Prof. Li Xiao-Hui for his advice and help in the design of the paper and Li Ming-Liang’s support for clinical data collection. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JP declared a shared parent affiliation with the authors at the time of the review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbdelAziz, E. Y., Tadros, M. G., and Menze, E. T. (2021). The effect of metformin on indomethacin-induced gastric ulcer: Involvement of nitric oxide/Rho kinase pathway. Eur. J. Pharmacol. 892, 173812. doi:10.1016/j.ejphar.2020.173812
Chen, S. H., Lei, H. L., Huang, L. R., and Tsai, L. H. (2001). Protective effect of excitatory amino acids on cold-restraint stress-induced gastric ulcers in mice: Role of cyclic nucleotides. Dig. Dis. Sci. 46, 2285–2291. doi:10.1023/a:1011991721640
Cheng, S. F., Li, L., and Wang, L. M. (2015). miR-155 and miR-146b negatively regulates IL6 in Helicobacter pylori (cagA+) infected gastroduodenal ulcer. Eur. Rev. Med. Pharmacol. Sci. 19, 607–613.
de Brito, B. B., da Silva, F. A. F., Soares, A. S., Pereira, V. A., Santos, M. L. C., Sampaio, M. M., et al. (2019). Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 25, 5578–5589. doi:10.3748/wjg.v25.i37.5578
Drayton, R. M., Dudziec, E., Peter, S., Bertz, S., Hartmann, A., Bryant, H. E., et al. (2014). Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin. Cancer Res. 20, 1990–2000. doi:10.1158/1078-0432.CCR-13-2805
Du, J., Li, X. H., Liu, F., Li, W. Q., Gong, Z. C., and Li, Y. J. (2020). Role of the outer inflammatory protein A/Cystine-Glutamate transporter pathway in gastric mucosal injury induced by Helicobacter pylori. Clin. Transl. Gastroenterol. 11, e00178. doi:10.14309/ctg.0000000000000178
Du, J., Li, X. H., Zhang, W., Yang, Y. M., Wu, Y. H., Li, W. Q., et al. (2014). Involvement of glutamate-cystine/glutamate transporter system in aspirin-induced acute gastric mucosa injury. Biochem. Biophys. Res. Commun. 450, 135–141. doi:10.1016/j.bbrc.2014.05.069
Echizen, K., Hirose, O., Maeda, Y., and Oshima, M. (2016). Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 107, 391–397. doi:10.1111/cas.12901
Feng, G. J., Chen, Y., and Li, K. (2020). Helicobacter pylori promote inflammation and host defense through the cagA-dependent activation of mTORC1. J. Cell. Physiol. 235, 10094–10108. doi:10.1002/jcp.29826
FitzGerald, R., and Smith, S. M. (2021). Capturing chromosome conformation. Methods Mol. Biol. 2283, 1–7. doi:10.1007/978-1-0716-0664-3_1
Iijima, J., Horie, S., Hasegawa, R., Yasui, H., and Takami, S. (2008). Immunohistochemical and morphologic basis for glutamate signaling in the rat stomach. Biol. Pharm. Bull. 31, 1838–1840. doi:10.1248/bpb.31.1838
Ishimoto, T., Izumi, D., Watanabe, M., Yoshida, N., Hidaka, K., Miyake, K., et al. (2015). Chronic inflammation with Helicobacter pylori infection is implicated in CD44 overexpression through miR-328 suppression in the gastric mucosa. J. Gastroenterol. 50, 751–757. doi:10.1007/s00535-014-1019-y
Kamboj, A. K., Cotter, T. G., and Oxentenko, A. S. (2017). Helicobacter pylori: The past, present, and future in management. Mayo Clin. Proc. 92, 599–604. doi:10.1016/j.mayocp.2016.11.017
Koosirirat, C., Linpisarn, S., Changsom, D., Chawansuntati, K., and Wipasa, J. (2010). Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int. Immunopharmacol. 10, 815–818. doi:10.1016/j.intimp.2010.04.021
Lahner, E., Carabotti, M., and Annibale, B. (2018). Treatment of Helicobacter pylori infection in atrophic gastritis. World J. Gastroenterol. 24, 2373–2380. doi:10.3748/wjg.v24.i22.2373
Lu, Y., Rong, J., Lai, Y., Tao, L., Yuan, X., and Shu, X. (2020). The degree of Helicobacter pylori infection affects the state of macrophage polarization through crosstalk between ROS and HIF-1α. Oxid. Med. Cell. Longev. 2020, 5281795. doi:10.1155/2020/5281795
Mandolesi, G., Musella, A., Gentile, A., Grasselli, G., Haji, N., Sepman, H., et al. (2013). Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J. Neurosci. 33, 12105–12121. doi:10.1523/JNEUROSCI.5369-12.2013
Moroz, L. L., Nikitin, M. A., Poličar, P. G., Kohn, A. B., and Romanova, D. Y. (2021). Evolution of glutamatergic signaling and synapses. Neuropharmacology 199, 108740. doi:10.1016/j.neuropharm.2021.108740
Platt, S. R. (2007). The role of glutamate in central nervous system health and disease-a review. Vet. J. 173, 278–286. doi:10.1016/j.tvjl.2005.11.007
Rose, C. (2002). Increased extracellular brain glutamate in acute liver failure: Decreased uptake or increased release? Metab. Brain Dis. 17, 251–261. doi:10.1023/a:1021945515514
Semper, R. P., Mejías-Luque, R., Groß, C., Anderl, F., Müller, A., Vieth, M., et al. (2014). Helicobacter pylori-induced IL-1β secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J. Immunol. 193, 3566–3576. doi:10.4049/jimmunol.1400362
Shi, J., He, Y., Hewett, S. J., and Hewett, J. A. (2016). Interleukin 1β regulation of the system xc- substrate-specific subunit, xCT, in primary mouse astrocytes involves the RNA-binding protein HuR. J. Biol. Chem. 291, 1643–1651. doi:10.1074/jbc.M115.697821
Toyoshima, O., Nishizawa, T., Yoshida, S., Matsuno, T., Odawara, N., Toyoshima, A., et al. (2022). Consistency between the endoscopic kyoto classification and pathological updated Sydney system for gastritis: A cross-sectional study. J. Gastroenterol. Hepatol. 37, 291–300. doi:10.1111/jgh.15693
Valdivielso, J. M., Eritja, À., Caus, M., and Bozic, M. (2020). Glutamate-gated NMDA receptors: Insights into the function and signaling in the kidney. Biomolecules 10, E1051. doi:10.3390/biom10071051
Wu, M., Xiao, H., Ren, W., Yin, J., Tan, B., Liu, G., et al. (2014). Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS One 9, e100591. doi:10.1371/journal.pone.0100591
Yang, J. Y., Kim, J. B., Lee, P., and Kim, S. H. (2021). Evodiamine inhibits Helicobacter pylori growth and Helicobacter pylori-induced inflammation. Int. J. Mol. Sci. 22, 3385. doi:10.3390/ijms22073385
Ye, L., Huang, Y., Zhao, L., Li, Y., Sun, L., Zhou, Y., et al. (2013). IL-1β and TNF-α induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. J. Neurochem. 125, 897–908. doi:10.1111/jnc.12263
Keywords: gastric ulcer, xCT, glutamate, HP, micrornas
Citation: Wang L, Li W-Q, Liu F, Li Y-J and Du J (2022) Decreased xCT activity in patients associated with Helicobacter pylori infection. Front. Pharmacol. 13:1021655. doi: 10.3389/fphar.2022.1021655
Received: 17 August 2022; Accepted: 21 November 2022;
Published: 05 December 2022.
Edited by:
Jun Peng, School of Pharmaceutic Science, Central South University, ChinaReviewed by:
Ahmad Karkhah, Babol University of Medical Sciences, IranSuzy Munir Salama, University of Malaya, Malaysia
Copyright © 2022 Wang, Li, Liu, Li and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Du, eGlhbmd5YWR1amllQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Ling Wang1†
Ling Wang1† Wen-Qun Li
Wen-Qun Li Jie Du
Jie Du