- 1Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 2Department of Reproductive Biology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 3Department of Physiology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 4Stem Cell Biology Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Polycystic ovary syndrome (PCOS) is a multifactorial metabolic and most common endocrine disorder that its prevalence, depending on different methods of evaluating PCOS traits, varies from 4% to 21%. Chronic low-grade inflammation and irregular apoptosis of granulosa cells play a crucial role in the pathogenesis of PCOS infertility. Mesenchymal stem cells (MSCs)-derived exosomes and extracellular vesicles (EVs) are lipid bilayer complexes that act as a means of intercellular transferring of proteins, lipids, DNA and different types of RNAs. It seems that this nanoparticles have therapeutic effects on the PCOS ovary such as regulating immunity response, anti-inflammatory (local and systemic) and suppress of granulosa cells (GCs) apoptosis. Although there are few studies demonstrating the effects of exosomes on PCOS and their exact mechanisms is still unknown, in the present study we reviewed the available studies of the functions of MSC-derived exosome, EVs and secretome on apoptosis of granulosa cells and inflammation in the ovary. Therefore, the novel cell-free therapeutic approaches for PCOS were suggested in this study.
Introduction
Polycystic ovary syndrome (PCOS) is a multifactorial metabolic disease and common endocrine condition that leads to increased production of androgens, decreased production of the estrogens and progesterone and consequences of infertility (Xu and Qiao, 2022). Common biochemical hallmarks of polycystic ovary syndrome are the absence of ovulation with high levels of androgens, luteinizing hormone (LH), luteinizing hormone/follicle-stimulating hormone ratios while follicle-stimulating hormone (FSH) remains normal or low (Eid et al., 2005). The global prevalence of this disorder varies from 4% to 21% depending on different methods of evaluating PCOS traits and diagnostic criteria (Lizneva et al., 2016), which can be recognized as the most common cause of infertility or failed birth in recent years (Rotterdam, 2004; Lizneva et al., 2016). It seems the principal ovarian consequences of PCOS are growth arrest in the early antral follicles and abnormal folliculogenesis (Franks et al., 2008). Although the current treatments include various gonadotropin (Artini et al., 1996), clomiphene citrate (Legro et al., 2007) and metformin (Sam and Dunaif, 2003), but, it has been pointed out that each of these treatments have various advantages and disadvantages (Elnashar et al., 2006; Legro et al., 2007). Therefore, alternative and non-invasive treatments improving follicle growth, resumption of oocyte maturation and different leading factors of PCOS are needed. There is evidence that mesenchymal stem cells (MSCs) have anti-inflammatory, fibrogenesis inhibiting, antioxidant, and regenerative effects (Zhao et al., 2019). These roles can give to the MSCs a potential therapeutic application in various abnormalities such as the female reproductive disorders (Mutlu et al., 2015; He et al., 2018).
In addition to intercellular interactions such as autocrine, paracrine or endocrine signaling, recently, extracellular vesicles (EVs) as a new tool for intercellular communication has attracted the attention of researchers. Although, some researchers consider the secretion of EVs as a mechanism of the cell to dispose of useless molecules (Van der Pol et al., 2012). But using the extracellular vesicles, various active biomolecules including nucleic acids, proteins and lipids can be transferred from origin cells to target cells (György et al., 2011; Koniusz et al., 2016). Precise characterization of the EVs content has opened up their promising applications in diagnosis and therapy, as well as the development of innovative drug delivery systems (Barile and Vassalli, 2017). According to their biosynthesis mechanism and size, those can be divided in to microvesicles (50–3000 nm), exosomes (40–100 nm) and apoptotic bodies (800–5000 nm) (Yamamoto et al., 2016). Origin-based contents, genetic materials and ability to content shuttling to other cells make exosomes as an attractive research subject for manipulating the functions of different cells locally and/or remotely (Han et al., 2016). In various physiological and pathological processes including reproduction, gametogenesis, embryogenesis and differentiation, exosomes are secreted by most cell types into the extracellular environment and have been detected in various body fluids (Raposo and Stoorvogel, 2013; Machtinger et al., 2016) so that they act as a means of transferring proteins, lipids, DNA and diversity of RNA species between cells (Barile and Vassalli, 2017). The presence of EVs in reproductive bio-fluids such as follicular fluid and ovarian fluid shows their role in the intercellular communication necessary for the proper functioning of the reproductive system (Machtinger et al., 2016). Considering the positive role of MSC-derived exosomes, the goal of this study is to review the available reports on their role in treatments of the various reproductive processes and present a potential role of the exosome in in vitro maturation of oocyte and the improve of infertility in PCOS women.
Mesenchymal stem cells-derived exosomes
Growing evidence from a various experimental and clinical trials support the effectiveness of MSCs on treating different diseases such as renal fibrosis, cardiovascular disorders, neurological diseases and female reproductive disorders (Du and Taylor, 2009; Goradel et al., 2018; Liang et al., 2018; Liu et al., 2018; Sneddon et al., 2018); these cells can be harvested from the varieties of tissues including bone marrow, umbilical cord, adipose tissue, placental tissue, menstrual blood and dental pulp (Priester et al., 2020).
In spite of the therapeutic potential of MSCs, large-scale MSC expansion for clinical use is limited owing to the cells’ capacity to divide in culture for a limited number of passages. Also, the cells could be associated with some challenges including difficulty of their transportation, transplant rejection and commercialization (Mendt et al., 2019). Therefore, in the recent decades, great efforts have been taken to find alternatives to reducing problems of MSCs usage while preserving their positive properties.
MSCs are a massive source for exosome production and are used in various research fields due to their greater availability and high proliferative ability (Cheng et al., 2017; Cheng et al., 2021). Exosomes, which are lipid bilayer nanoparticles that secrete into the microenvironment from various types of cells especially mesenchymal stem cells that offer promising therapeutic potential. In addition to other bioactive molecules that we have detailed in our previous study (Izadi et al., 2021), exosomes have various types of signaling molecules such as mRNA and miRNA (Valadi et al., 2007). Higher biological stability, easier storage, easier penetration into target tissues and low immunogenicity are some of the considerable advantages that make exosomes more useful compared to their source cells for medical applications (El Andaloussi et al., 2013; Zhang et al., 2016). Exosomes secreted from different cells have almost similar protein molecules with biological activities including immune modulation, regeneration, and tissue repair and angiogenesis promotion. Exhibiting the same activities in all MSC-derived exosomes may be related to the existence of a common protein signature (van Balkom et al., 2019). Additionally, some types of MSCs secrete exosomes with unique characteristics (Tang et al., 2021). Rising evidence suggests that MSCs-derived exosomes have immunomodulation, anti-inflammatory (Urbanelli et al., 2015; Izadi et al., 2021) and anti-apoptosis effects (Fu et al., 2020; Wen et al., 2020), therapeutic potential of female reproductive disorders (Liao et al., 2021; Zohrabi et al., 2022).
Potential applications of MSCs-derived exosomes in PCOS patient
Many studies have shown that chronic low-grade, increase in pro-inflammatory cytokines, decrease of anti-inflammatory cytokines, insulin resistance, hypersensitivity of Helper T-cells (Th1); Th1-type immunity and the ratio of Th1 to Th2 cells, as well as Th1 cytokines such as IFN-γ and IL-2 are increased during immune reactions in PCOS patients (Qin et al., 2016) and hyperandrogenism play a crucial roles in PCOS pathogenesis (González et al., 2014a). Moreover, it has been reported that chronic inflammation in PCOS can lead to poor oocyte quality, ovarian dysfunction, disrupts oocyte development, and affect endometrial receptivity (Velez et al., 2021).
Few recent studies have reported that human umbilical cord mesenchymal stem cells (huMSCs) therapy can improve ovarian dysfunction by the systemic immunomodulation and local immune response in the ovary of PCOS patients (Xie et al., 2019). In a letrozole-induced PCOS mouse model, the beneficial effect of human bone marrow derived mesenchymal stem cells (BM-hMSCs)on the partial restoration of ovaries, the number of corpora lutea, and antral follicles has been reported (Chugh et al., 2021a). Notably, an increasing number of studies have discovered that the communication between MSCs and target tissue such as ovarian microenvironment is through the exosomes and secretome (Harrell et al., 2019; Xu et al., 2019). Also, another study reported that huMSCs--derived exosomes ameliorates the granulosa cells immune response through the inhibition of NF-κB signaling pathway in the PCOS (Zhao et al., 2022).
Recently, a study showed that MSCs-derived exosomes cause the decreased concentration of IL-1β and TNF-α, while the secretion of TGF-β increased in in vitro culture of mononuclear cells. Also, it demonstrated that MSCs-derived exosomes can increase Th2 (Th2-related anti-inflammatory cytokine such as IL-10 that is reduced in PCOS patients) and Treg and decrease Th1 (Chen et al., 2016). Apoptosis plays the key role in follicular atresia and cyclic growth and regression of follicles in the human ovary (Tilly, 1996). It has been reported that factors involved in the induction of apoptosis in the ovaries (Jansen et al., 2004) and also the number of atretic follicles increase in PCOS patients (Laven et al., 2001). EVs derived from huMSCs have also shown anti-apoptotic and fertility recovery effects and promoted secretive functions of granulosa cells in induced POI mice (Liu et al., 2020). Also it can reduce ovarian damage and protect GCs through anti-apoptotic and anti-inflammatory effects and improve ovarian function in chemotherapy-induced POF mice (Deng et al., 2021).
Therefore, exosome as a novel cell-free therapeutic strategy can be used promisingly in diseases of inflammatory origin by maintaining the immune balance (Chen et al., 2016).
The effects of bioactive compounds in the MSC-derived exosomes and secretome
Although recently there have been many studies on exosomes as a novel avenue for female infertility treatment, precise mechanisms of MSCs-derived exosomes on female reproductive diseases are also unclear. Given that chronic inflammation is associated with the pathogenesis of PCOS, there is also a positive feedback loop between inflammation, androgen production and metabolic disorders in PCOS (González et al., 2014b; Fox et al., 2019); Since, near to 50% of PCOS patients show high secretion of androgens (Marti et al., 2017; McAllister et al., 2019), therefore, the main strategy to treat PCOS can be suppression of androgen secretion (McAllister et al., 2019).
It has been reported that cytokine IL-10 that is found in secretome improves fertility through the suppressing androgen secretion by ovarian theca cells and reducing inflammation (Chugh et al., 2021b). Bone morphogenetic proteins (BMPs) are multifunctional growth factors that play an important role in folliculogenesis and female fertility; these proteins are secreted by BM-hMSCs (Yoshino et al., 2011). The theca cells in the ovary proliferate rapidly and increased androgen production in PCOS (Bremer, 2010; Zhang et al., 2012), it has been reported that BMP-2 can inhibit the proliferation of different cells in vitro (Hardwick et al., 2004; Chen et al., 2012; Zhang et al., 2012). Another study showed that BMP-2 can treat hyperandrogenemia in PCOS by suppressing steroidogenesis (Chugh et al., 2021a). Therefore, BMP-2 may improve the hyper-androgenemia in PCOS.
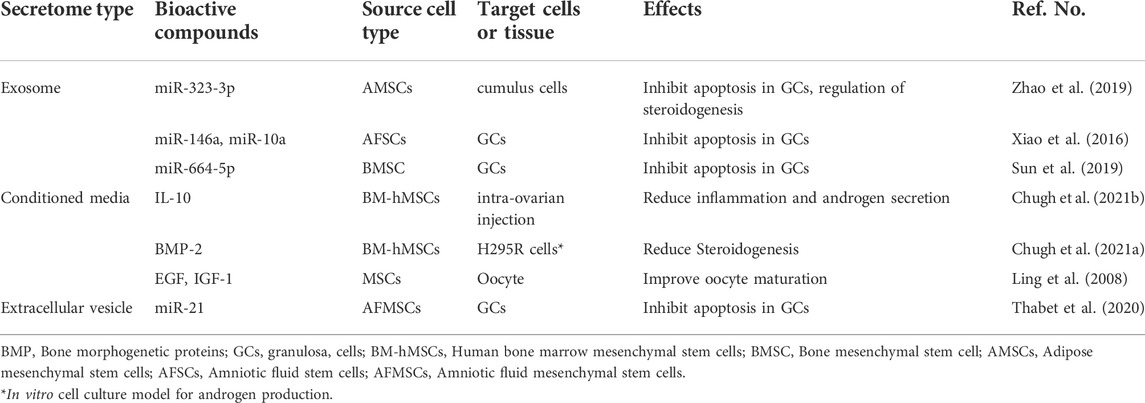
In PCOS and other ovarian disorders, the effect of exosome therapy has been reported to affect apoptosis by delivering genetic material such as miR-323-3p miR-146a and miR-10a (Xiao et al., 2016; Zhao et al., 2019), miR-664-5p (Sun et al., 2019), and miR-21(90).
Mesenchymal stem cells have the ability to secrete a large number of growth factors such as fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF-1), VEGF, TGF-β, and EGF (Labouyrie et al., 1999; Izumida et al., 2005; Yoon et al., 2010) which may have the effect of reinitiate meiosis and improve oocyte maturation (Ling et al., 2008). A clinical trial in which the retrograde injection method was used to transplant MSCs based on a collagen scaffold into the ovaries of patients with some ovarian disorders suggests that EVs can be transferred by intra-ovarian injection (Ding et al., 2018). The biologically active molecules and their effects are summarized in Table 1 and Figure 1.
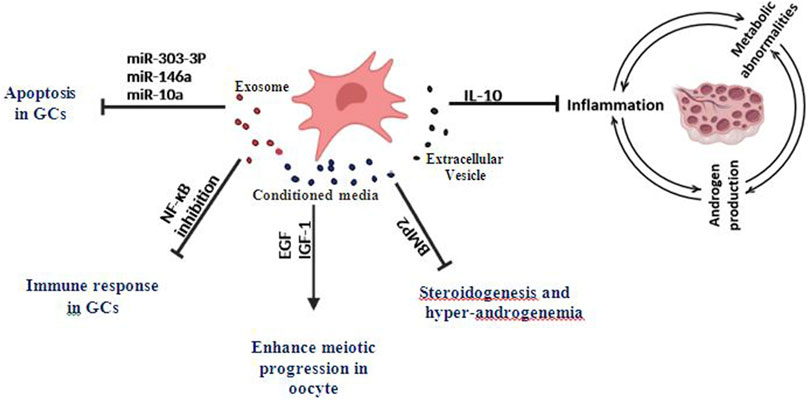
FIGURE 1. Effects of MSCs-derived exosomes, EVs, and Conditioned media on inflammation and granulosa cells apoptosis in PCOS and various ovarian disorders.
Potential applications of MSCs-derived exosomes in enrichment IVM culture medium
Although in vitro fertilization (IVF) is an effective treatment for infertility in PCOS women, it is also associated with an increased risk of ovarian hyperstimulation syndrome (OHSS) (Shalom-Paz et al., 2012). Therefore, minimizing the risk of ovarian stimulation while providing an acceptable fertility success rate should be the focus of treatment efforts. Currently, to prevent OHSS, immature oocytes are collected from small antral follicles within unstimulated or very little stimulated ovaries then these oocytes are matured in vitro. Patients with PCOS could potentially benefit from IVM, as it reduces the risk of OHSS as well as costs (Shalom-Paz et al., 2012; Ho et al., 2019). But since the IVM and success rate of fertilization of oocytes matured in vitro is not satisfactory, therefore, to overcome these limitations faced by IVM, several studies have been conducted that focus on effects of cultural media containing various additives for improving oocyte quality (Jee et al., 2008; Ben-Ami et al., 2011; Blanco et al., 2011; Ishizuka et al., 2013; Sánchez et al., 2015). Primarily, an optimal culture medium is needed to increase the efficiency of IVM, which can be achieved by better understanding the molecular events that trigger oocyte maturation (Chian et al., 2004). Before ovulation, the LH surge triggers a cascade of cellular and molecular events in the ovarian follicle including resumption of oocyte meiosis, cumulus expansion, follicular wall rupture, and cumulus-oocyte mass extrusion (Richards et al., 2002). Despite mural granulosa cells and external theca cells expressing high LH receptors, oocyte and cumulus cells express little or no LH receptors and therefore do not respond to LH exposure in vitro (PENG et al., 1991). Therefore, it seems the effects of LH on cumulus-oocytes may be through the release of the paracrine mediators from granulosa cells (Conti et al., 2006). Also, recently, it was reported that LH stimulation of isolated human granulosa cells causes the increase of EGF-like growth factors (Ben-Ami et al., 2006; Ben-Ami et al., 2009). Several experimental studies in animals and cell culture have demonstrated that EGF and IGF-1 can improve maturation in cumulus surrounded (Sakaguchi et al., 2000; Sakaguchi et al., 2002) and denuded oocytes as well as in vitro which is similar to what happens in vivo (Das et al., 1991; Lonergan et al., 1996).
Given that each oocyte is surrounded by cumulus granulosa, mural granulosa, theca cells and follicular fluid to form ovarian follicles as reproductive units, therefore, the oocyte can be affected by each of these components (Di Pietro, 2016). However, new exosome-based therapeutic approaches in PCOS are few. Recently, regulation of steroidogenesis, promotion of cell growth and inhibition of apoptosis in the cumulus cells by exosomal miR-323-3p has been reported in the women with PCOS (Zhao et al., 2019). Also, animal studies revealed beneficial effects of EVs (Liao et al., 2021), exosomes derived from amniotic fluid stem cells (Xiao et al., 2016) and bone mesenchymal stem cells (Sun et al., 2019) on various ovarian disorders and fertility recovery. These nanoparticles inhibit apoptosis in the damaged granulosa cells through the delivery of miR-146a, miR-10a (Xiao et al., 2016), miR-664-5p (Sun et al., 2019), and miR-21(90). Studies have shown that exosomes derived from huMSCs can increased of Bcl-2 and caspase-3 whereas decreased the expression of Bax, cleaved caspase-3, and cleaved poly (ADP-ribose) polymerase (PARP) to attenuation of cisplatin-induced ovarian granulosa cell apoptosis in vitro (Sun et al., 2017; Zhang et al., 2020). In addition, BM-hMSCs conditioned media could regulate the steroidogenesis, inhibit androgen secretion and suppress inflammatory pathways in a cellular model (Chugh et al., 2021a; Chugh et al., 2021b). Moreover, it has been reported that in vitro maturation of mouse oocytes with or without cumulus cells can be improved by its co-culture with conditioned medium of MSCs (Ling et al., 2008). Therefore, According to the beneficial effects of MSCs-derived exosomes, EVs and secretome can have the potential to optimize the culture media for oocyte maturation in PCOS.
Conclusion
Although the pathogenesis of PCOS is still controversial and remains unclear, several studies implicate chronic inflammation in the pathogenesis of PCOS and others implicate irregular granulosa cell apoptosis in PCOS infertility. In this study, we present a promising opportunity to develop novel cell-free therapy approaches to restore fertility in PCOS condition. According to the recent studies, MSCs-derived exosomes, EVs and secretomes inhibit inflammation and apoptosis, regulate steroidogenesis and inhibit androgen production in in vitro as well as in vivo. Consequently, it is worthwhile to challenge the effectiveness and efficiency of the exosomes in enriched culture media for improving oocyte development as well as PCOS treatment.
Author contributions
MI: study design, investigation, and writing original draft. MR: helping on writing the manuscript and validation of data and revising. AA: helping on writing the first draft of the manuscript. MK: helping on writing the first draft of the manuscript. BA: supervisor, validation of data, and revising the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Stem Cell Biology Research Center.
Acknowledgments
We thank Ali-Mohammad Abdoli for all of his support as the manager of the Yazd Reproductive Sciences Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Artini, P. G., De Micheroux, A., and D’Ambrogio, G. (1996). Growth hormone cotreatment with gonadotropins in ovulation induction. J. Endocrinol. Invest. 19 (11), 763–779. doi:10.1007/BF03347881
Barile, L., and Vassalli, G. (2017). Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 174, 63–78. doi:10.1016/j.pharmthera.2017.02.020
Ben-Ami, I., Armon, L., Freimann, S., Strassburger, D., Ron-El, R., and Amsterdam, A. (2009). EGF-like growth factors as LH mediators in the human corpus luteum. Hum. Reprod. 24 (1), 176–184. doi:10.1093/humrep/den359
Ben-Ami, I., Freimann, S., Armon, L., Dantes, A., Strassburger, D., Friedler, S., et al. (2006). PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: New insights into the coordination between PGE2 and LH in ovulation. Mol. Hum. Reprod. 12 (10), 593–599. doi:10.1093/molehr/gal068
Ben-Ami, I., Komsky, A., Bern, O., Kasterstein, E., Komarovsky, D., and Ron-El, R. (2011). In vitro maturation of human germinal vesicle-stage oocytes: Role of epidermal growth factor-like growth factors in the culture medium. Hum. Reprod. 26 (1), 76–81. doi:10.1093/humrep/deq290
Blanco, M., Demyda, S., Moreno, M. M., and Genero, E. (2011). Developmental competence of in vivo and in vitro matured oocytes: A review. Biotechnol. Mol. Biol. Rev. 6 (7), 155–165.
Bremer, A. A. (2010). Polycystic ovary syndrome in the pediatric population. Metab. Syndr. Relat. Disord. 8 (5), 375–394. doi:10.1089/met.2010.0039
Chen, A., Wang, D., Liu, X., He, S., Yu, Z., and Wang, J. (2012). Inhibitory effect of BMP-2 on the proliferation of breast cancer cells. Mol. Med. Rep. 6 (3), 615–620. doi:10.3892/mmr.2012.962
Chen, W., Huang, Y., Han, J., Yu, L., Li, Y., Lu, Z., et al. (2016). Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 64 (4), 831–840. doi:10.1007/s12026-016-8798-6
Cheng, L., Zhang, K., Wu, S., Cui, M., and Xu, T. (2017). Focus on mesenchymal stem cell-derived exosomes: Opportunities and challenges in cell-free therapy. Stem Cells Int. 2017, 6305295. doi:10.1155/2017/6305295
Cheng, L., Zhang, K., Wu, S., Cui, M., and Xu, T. (2021). Focus on mesenchymal stem cell-derived exosomes: Opportunities and challenges in cell-free therapy focus on mesenchymal stem cell-derived exosomes: Opportunities and challenges in cell-free therapy. Stem Cells Int.
Chian, R-C., Buckett, W. M., and Tan, S-L. (2004). In-vitro maturation of human oocytes. Reprod. Biomed. Online 8 (2), 148–166. doi:10.1016/s1472-6483(10)60511-1
Chugh, R. M., Park, H.-s., El Andaloussi, A., Elsharoud, A., Esfandyari, S., Ulin, M., et al. (2021). Mesenchymal stem cell therapy ameliorates metabolic dysfunction and restores fertility in a PCOS mouse model through interleukin-10. Stem Cell. Res. Ther. 12 (1), 1–19. doi:10.1186/s13287-021-02472-w
Chugh, R. M., Park, H-s., Esfandyari, S., Elsharoud, A., Ulin, M., and Al-Hendy, A. (2021). Mesenchymal stem cell-conditioned media regulate steroidogenesis and inhibit androgen secretion in a PCOS cell model via BMP-2. Int. J. Mol. Sci. 22 (17), 9184. doi:10.3390/ijms22179184
Conti, M., Hsieh, M., Park, J-Y., and Su, Y-Q. (2006). Role of the epidermal growth factor network in ovarian follicles. Mol. Endocrinol. 20 (4), 715–723. doi:10.1210/me.2005-0185
Das, K., Stout, L. E., Hensleigh, H. C., Tagatz, G. E., Phipps, W. R., and Leung, B. S. (1991). Direct positive effect of epidermal growth factor on the cytoplasmic maturation of mouse and human oocytes. Fertil. Steril. 55 (5), 1000–1004. doi:10.1016/s0015-0282(16)54313-1
Deng, T., He, J., Yao, Q., Wu, L., Xue, L., Wu, M., et al. (2021). Human umbilical cord mesenchymal stem cells improve ovarian function in chemotherapy-induced premature ovarian failure mice through inhibiting apoptosis and inflammation via a paracrine mechanism. Reprod. Sci. 28 (6), 1718–1732. doi:10.1007/s43032-021-00499-1
Di Pietro, C. (2016). Exosome-mediated communication in the ovarian follicle. J. Assist. Reprod. Genet. 33 (3), 303–311. doi:10.1007/s10815-016-0657-9
Ding, L., Yan, G., Wang, B., Xu, L., Gu, Y., Ru, T., et al. (2018). Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci. China. Life Sci. 61 (12), 1554–1565. doi:10.1007/s11427-017-9272-2
Du, H., and Taylor, H. S. (2009). Reviews: Stem cells and female reproduction. Reprod. Sci. 16 (2), 126–139. doi:10.1177/1933719108329956
Eid, G. M., Cottam, D. R., Velcu, L. M., Mattar, S. G., Korytkowski, M. T., Gosman, G., et al. (2005). Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 1 (2), 77–80. doi:10.1016/j.soard.2005.02.008
El Andaloussi, S., Mäger, I., Breakefield, X. O., and Wood, M. J. (2013). Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12 (5), 347–357. doi:10.1038/nrd3978
Elnashar, A., Abdelmageed, E., Fayed, M., and Sharaf, M. (2006). Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: A prospective placebo-controlled study. Hum. Reprod. 21 (7), 1805–1808. doi:10.1093/humrep/del053
Fox, C. W., Zhang, L., Sohni, A., Doblado, M., Wilkinson, M. F., Chang, R. J., et al. (2019). Inflammatory stimuli trigger increased androgen production and shifts in gene expression in theca-interstitial cells. Endocrinology 160 (12), 2946–2958. doi:10.1210/en.2019-00588
Franks, S., Stark, J., and Hardy, K. (2008). Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 14 (4), 367–378. doi:10.1093/humupd/dmn015
Fu, D., Jiang, H., Li, C., Gao, T., Liu, M., and Li, H. (2020). MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 24 (19), 10107–10117. doi:10.26355/eurrev_202010_23230
González, F., Sia, C. L., Bearson, D. M., and Blair, H. E. (2014). Hyperandrogenism induces a proinflammatory TNFα response to glucose ingestion in a receptor-dependent fashion. J. Clin. Endocrinol. Metab. 99 (5), E848–E854. doi:10.1210/jc.2013-4109
González, F., Sia, C. L., Shepard, M. K., Rote, N. S., and Minium, J. (2014). The altered mononuclear cell-derived cytokine response to glucose ingestion is not regulated by excess adiposity in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 99 (11), E2244–E2251. doi:10.1210/jc.2014-2046
Goradel, N. H., Hour, F. G., Negahdari, B., Malekshahi, Z. V., Hashemzehi, M., Masoudifar, A., et al. (2018). Stem cell therapy: A new therapeutic option for cardiovascular diseases. J. Cell. Biochem. 119 (1), 95–104. doi:10.1002/jcb.26169
György, B., Szabó, T. G., Pásztói, M., Pál, Z., Misják, P., Aradi, B., et al. (2011). Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68 (16), 2667–2688. doi:10.1007/s00018-011-0689-3
Han, C., Sun, X., Liu, L., Jiang, H., Shen, Y., Xu, X., et al. (2016). Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016, 7653489. doi:10.1155/2016/7653489
Hardwick, J. C., Van Den Brink, G. R., Bleuming, S. A., Ballester, I., Van Den Brande, J. M., Keller, J. J., et al. (2004). Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology 126 (1), 111–121. doi:10.1053/j.gastro.2003.10.067
Harrell, C. R., Fellabaum, C., Jovicic, N., Djonov, V., Arsenijevic, N., and Volarevic, V. (2019). Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8 (5), 467. doi:10.3390/cells8050467
He, Y., Chen, D., Yang, L., Hou, Q., Ma, H., and Xu, X. (2018). The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell. Res. Ther. 9 (1), 263–267. doi:10.1186/s13287-018-1008-9
Ho, V. N., Braam, S. C., Pham, T. D., Mol, B. W., and Vuong, L. N. (2019). The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum. Reprod. 34 (6), 1055–1064. doi:10.1093/humrep/dez060
Ishizuka, Y., Nishimura, M., Matsumoto, K., Miyashita, M., Takeo, T., Nakagata, N., et al. (2013). The influence of reduced glutathione in fertilization medium on the fertility of in vitro–matured C57BL/6 mouse oocytes. Theriogenology 80 (5), 421–426. doi:10.1016/j.theriogenology.2013.07.002
Izadi, M., Marvast, L. D., Rezvani, M. E., Zohrabi, M., Aliabadi, A., Mousavi, S. A., et al. (2021). Mesenchymal stem-cell derived exosome therapy as a potential future approach for treatment of male infertility caused by Chlamydia infection. Front. Microbiol. 12, 785622. doi:10.3389/fmicb.2021.785622
Izumida, Y., Aoki, T., Yasuda, D., Koizumi, T., Suganuma, C., Saito, K., et al. (2005). Hepatocyte growth factor is constitutively produced by donor-derived bone marrow cells and promotes regeneration of pancreatic β-cells. Biochem. Biophys. Res. Commun. 333 (1), 273–282. doi:10.1016/j.bbrc.2005.05.100
Jansen, E., Laven, J. S., Dommerholt, H. B., Polman, J., van Rijt, C., van den Hurk, C., et al. (2004). Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol. Endocrinol. 18 (12), 3050–3063. doi:10.1210/me.2004-0074
Jee, B. C., Han, S. H., Moon, J. H., Suh, C. S., Kim, S. H., and Group SnucoMARTS, (2008). Influence of well defined protein source on in vitro maturation of human oocyte: Human follicular fluid versus human serum albumin. Fertil. Steril. 89 (2), 348–352. doi:10.1016/j.fertnstert.2007.02.052
Koniusz, S., Andrzejewska, A., Muraca, M., Srivastava, A. K., Janowski, M., and Lukomska, B. (2016). Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front. Cell. Neurosci. 10, 109. doi:10.3389/fncel.2016.00109
Labouyrie, E., Dubus, P., Groppi, A., Mahon, F. X., Ferrer, J., Parrens, M., et al. (1999). Expression of neurotrophins and their receptors in human bone marrow. Am. J. Pathol. 154 (2), 405–415. doi:10.1016/S0002-9440(10)65287-X
Laven, J. S., Imani, B., Eijkemans, M. J., de Jong, F. H., and Fauser, B. C. (2001). Absent biologically relevant associations between serum inhibin B concentrations and characteristics of polycystic ovary syndrome in normogonadotrophic anovulatory infertility. Hum. Reprod. 16 (7), 1359–1364. doi:10.1093/humrep/16.7.1359
Legro, R. S., Barnhart, H. X., Schlaff, W. D., Carr, B. R., Diamond, M. P., Carson, S. A., et al. (2007). Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 356 (6), 551–566. doi:10.1056/NEJMoa063971
Liang, N., Trujillo, C. A., Negraes, P. D., Muotri, A. R., Lameu, C., and Ulrich, H. (2018). Stem cell contributions to neurological disease modeling and personalized medicine. Prog. Neuropsychopharmacol. Biol. Psychiatry 80, 54–62. doi:10.1016/j.pnpbp.2017.05.025
Liao, Z., Liu, C., Wang, L., Sui, C., and Zhang, H. (2021). Therapeutic role of mesenchymal stem cell-derived extracellular vesicles in female reproductive diseases. Front. Endocrinol. 12, 665645. doi:10.3389/fendo.2021.665645
Ling, B., Feng, D., Zhou, Y., Gao, T., Wei, H., and Tian, Z. (2008). Effect of conditioned medium of mesenchymal stem cells on the in vitro maturation and subsequent development of mouse oocyte. Braz. J. Med. Biol. Res. 41, 978–985. doi:10.1590/s0100-879x2008005000053
Liu, B., Ding, F., Hu, D., Zhou, Y., Long, C., Shen, L., et al. (2018). Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell. Res. Ther. 9 (1), 7–14. doi:10.1186/s13287-017-0760-6
Liu, C., Yin, H., Jiang, H., Du, X., Wang, C., Liu, Y., et al. (2020). Extracellular vesicles derived from mesenchymal stem cells recover fertility of premature ovarian insufficiency mice and the effects on their offspring. Cell. Transpl. 29, 0963689720923575. doi:10.1177/0963689720923575
Lizneva, D., Suturina, L., Walker, W., Brakta, S., Gavrilova-Jordan, L., and Azziz, R. (2016). Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 106 (1), 6–15. doi:10.1016/j.fertnstert.2016.05.003
Lonergan, P., Carolan, C., Van Langendonckt, A., Donnay, I., Khatir, H., Mermillod, P., et al. (1996). Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol. Reprod. 54 (6), 1420–1429. doi:10.1095/biolreprod54.6.1420
Machtinger, R., Laurent, L. C., and Baccarelli, A. A. (2016). Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 22 (2), 182–193. doi:10.1093/humupd/dmv055
Marti, N., Bouchoucha, N., Sauter, K-S., and Flück, C. E. (2017). Resveratrol inhibits androgen production of human adrenocortical H295R cells by lowering CYP17 and CYP21 expression and activities. PloS one 12 (3), e0174224. doi:10.1371/journal.pone.0174224
McAllister, J. M., Han, A. X., Modi, B. P., Teves, M. E., Mavodza, G. R., Anderson, Z. L., et al. (2019). miRNA profiling reveals miRNA-130b-3p mediates DENND1A variant 2 expression and androgen biosynthesis. Endocrinology 160 (8), 1964–1981. doi:10.1210/en.2019-00013
Mendt, M., Rezvani, K., and Shpall, E. (2019). Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transpl. 54 (2), 789–792. doi:10.1038/s41409-019-0616-z
Mutlu, L., Hufnagel, D., and Taylor, H. S. (2015). The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol. Reprod. 92 (6), 138. doi:10.1095/biolreprod.114.126771
Peng, X-R., Hsueh, A. J., Lapolt, P. S., Bjersing, L., and Ny, T. (1991). Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology 129 (6), 3200–3207. doi:10.1210/endo-129-6-3200
Priester, C., MacDonald, A., Dhar, M., and Bow, A. (2020). Examining the characteristics and applications of mesenchymal, induced pluripotent, and embryonic stem cells for tissue engineering approaches across the germ layers. Pharmaceuticals 13 (11), 344. doi:10.3390/ph13110344
Qin, L., Xu, W., Li, X., Meng, W., Hu, L., Luo, Z., et al. (2016). Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome: Analysis by flow cytometry. Eur. J. Obstet. Gynecol. Reprod. Biol. 197, 136–141. doi:10.1016/j.ejogrb.2015.12.003
Raposo, G., and Stoorvogel, W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell. Biol. 200 (4), 373–383. doi:10.1083/jcb.201211138
Richards, J. S., Russell, D. L., Ochsner, S., and Espey, L. L. (2002). Ovulation: New dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 64 (1), 69–92. doi:10.1146/annurev.physiol.64.081501.131029
Rotterdam, E. (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81, 19–25. doi:10.1016/j.fertnstert.2003.10.004
Sakaguchi, M., Dominko, T., Leibfried-Rutledge, M., Nagai, T., and First, N. (2000). A combination of EGF and IGF-I accelerates the progression of meiosis in bovine follicular oocytes in vitro and fetal calf serum neutrali zes the acceleration effect. Theriogenology 54 (8), 1327–1342. doi:10.1016/s0093-691x(00)00439-8
Sakaguchi, M., Dominko, T., Yamauchi, N., Leibfried-Rutledge, M., Nagai, T., and First, N. (2002). Possible mechanism for acceleration of meiotic progression of bovine follicular oocytes by growth factors in vitro. Reproduction 123 (1), 135–142. doi:10.1530/rep.0.1230135
Sam, S., and Dunaif, A. (2003). Polycystic ovary syndrome: Syndrome XX? Trends Endocrinol. Metab. 14 (8), 365–370. doi:10.1016/j.tem.2003.08.002
Sánchez, F., Romero, S., De Vos, M., Verheyen, G., and Smitz, J. (2015). Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum. Reprod. 30 (6), 1396–1409. doi:10.1093/humrep/dev083
Shalom-Paz, E., Holzer, H., Son, W-Y., Levin, I., Tan, S. L., and Almog, B. (2012). PCOS patients can benefit from in vitro maturation (IVM) of oocytes. Eur. J. Obstet. Gynecol. Reprod. Biol. 165 (1), 53–56. doi:10.1016/j.ejogrb.2012.07.001
Sneddon, J. B., Tang, Q., Stock, P., Bluestone, J. A., Roy, S., Desai, T., et al. (2018). Stem cell therapies for treating diabetes: Progress and remaining challenges. Cell. stem Cell. 22 (6), 810–823. doi:10.1016/j.stem.2018.05.016
Sun, B., Ma, Y., Wang, F., Hu, L., and Sun, Y. (2019). miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell. Res. Ther. 10 (1), 360–369. doi:10.1186/s13287-019-1442-3
Sun, L., Li, D., Song, K., Wei, J., Yao, S., Li, Z., et al. (2017). Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci. Rep. 7 (1), 1–13. doi:10.1038/s41598-017-02786-x
Tang, Y., Zhou, Y., and Li, H-J. (2021). Advances in mesenchymal stem cell exosomes: A review. Stem Cell. Res. Ther. 12 (1), 71–12. doi:10.1186/s13287-021-02138-7
Thabet, E., Yusuf, A., Abdelmonsif, D. A., Nabil, I., Mourad, G., and Mehanna, R. A. (2020). Extracellular vesicles miRNA-21: A potential therapeutic tool in premature ovarian dysfunction. Mol. Hum. Reprod. 26 (12), 906–919. doi:10.1093/molehr/gaaa068
Tilly, J. L. (1996). Apoptosis and ovarian function. Rev. Reprod. 1 (3), 162–172. doi:10.1530/ror.0.0010162
Urbanelli, L., Buratta, S., Sagini, K., Ferrara, G., Lanni, M., and Emiliani, C. (2015). Exosome-based strategies for diagnosis and therapy. Recent Pat. CNS Drug Discov. 10 (1), 10–27. doi:10.2174/1574889810666150702124059
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., and Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell. Biol. 9 (6), 654–659. doi:10.1038/ncb1596
van Balkom, B. W., Gremmels, H., Giebel, B., and Lim, S. K. (2019). Proteomic signature of mesenchymal stromal cell‐derived small extracellular vesicles. Proteomics 19 (1-2), 1800163. doi:10.1002/pmic.201800163
Van der Pol, E., Böing, A. N., Harrison, P., Sturk, A., and Nieuwland, R. (2012). Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 64 (3), 676–705. doi:10.1124/pr.112.005983
Velez, L. M., Seldin, M., and Motta, A. B. (2021). Inflammation and reproductive function in women with polycystic ovary syndrome. Biol. Reprod. 104 (6), 1205–1217. doi:10.1093/biolre/ioab050
Wen, Z., Mai, Z., Zhu, X., Wu, T., Chen, Y., Geng, D., et al. (2020). Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell. Res. Ther. 11 (1), 36–17. doi:10.1186/s13287-020-1563-8
Xiao, G-Y., Cheng, C-C., Chiang, Y-S., Cheng, W. T-K., Liu, I., and Wu, S-C. (2016). Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 6 (1), 1–12. doi:10.1038/srep23120
Xie, Q., Xiong, X., Xiao, N., He, K., Chen, M., Peng, J., et al. (2019). Mesenchymal stem cells alleviate DHEA-induced polycystic ovary syndrome (PCOS) by inhibiting inflammation in mice. Stem Cells Int. 2019, 9782373. doi:10.1155/2019/9782373
Xu, S., Liu, C., and Ji, H-L. (2019). Concise review: Therapeutic potential of the mesenchymal stem cell derived secretome and extracellular vesicles for radiation-induced lung injury: Progress and hypotheses. Stem Cells Transl. Med. 8 (4), 344–354. doi:10.1002/sctm.18-0038
Xu, Y., and Qiao, J. (2022). Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): A review of literature. J. Healthc. Eng., 9240569. doi:10.1155/2022/9240569
Yamamoto, S., Azuma, E., Muramatsu, M., Hamashima, T., Ishii, Y., and Sasahara, M. (2016). Significance of extracellular vesicles: Pathobiological roles in disease. Cell. Struct. Funct. 41, 137–143. doi:10.1247/csf.16014
Yoon, B. S., Moon, J-H., Jun, E. K., Kim, J., Maeng, I., Kim, J. S., et al. (2010). Secretory profiles and wound healing effects of human amniotic fluid–derived mesenchymal stem cells. Stem Cells Dev. 19 (6), 887–902. doi:10.1089/scd.2009.0138
Yoshino, O., Shi, J., Osuga, Y., Harada, M., Nishii, O., Yano, T., et al. (2011). The function of bone morphogenetic proteins in the human ovary. Reprod. Med. Biol. 10 (1), 1–7. doi:10.1007/s12522-010-0072-3
Zhang, B., Yeo, R. W. Y., Tan, K. H., and Lim, S. K. (2016). Focus on extracellular vesicles: Therapeutic potential of stem cell-derived extracellular vesicles. Int. J. Mol. Sci. 17 (2), 174. doi:10.3390/ijms17020174
Zhang, J., Ge, Y., Sun, L., Cao, J., Wu, Q., Guo, L., et al. (2012). Effect of bone morphogenetic protein-2 on proliferation and apoptosis of gastric cancer cells. Int. J. Med. Sci. 9 (2), 184–192. doi:10.7150/ijms.3859
Zhang, J., Yin, H., Jiang, H., Du, X., and Yang, Z. (2020). The protective effects of human umbilical cord mesenchymal stem cell-derived extracellular vesicles on cisplatin-damaged granulosa cells. Taiwan. J. Obstet. Gynecol. 59 (4), 527–533. doi:10.1016/j.tjog.2020.05.010
Zhao, Y., Pan, S., and Wu, X. (2022). Human umbilical cord mesenchymal stem cell-derived exosomes inhibit ovarian granulosa cells inflammatory response through inhibition of NF-κB signaling in polycystic ovary syndrome. J. Reprod. Immunol. 152, 103638. doi:10.1016/j.jri.2022.103638
Zhao, Y., Tao, M., Wei, M., Du, S., Wang, H., and Wang, X. (2019). Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed. Biotechnol. 47 (1), 3804–3813. doi:10.1080/21691401.2019.1669619
Keywords: mesenchymal stem cell, exosome, extracellular vesicle, apoptosis, chronic inflammation
Citation: Izadi M, Rezvani ME, Aliabadi A, Karimi M and Aflatoonian B (2022) Mesenchymal stem cells-derived exosomes as a promising new approach for the treatment of infertility caused by polycystic ovary syndrome. Front. Pharmacol. 13:1021581. doi: 10.3389/fphar.2022.1021581
Received: 17 August 2022; Accepted: 28 September 2022;
Published: 10 October 2022.
Edited by:
Reza Shirazi, UNSW Sydney, AustraliaReviewed by:
Ali Falahati, Yazd University, IranKatia Candido Carvalho, Faculty of Medicine, University of São Paulo, Brazil
Copyright © 2022 Izadi, Rezvani, Aliabadi, Karimi and Aflatoonian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Behrouz Aflatoonian, b.aflatoonian@ssu.ac.ir
†ORCID: Mahin Izadi, https://orcid.org/0000-0002-7907-6168; Mohammad Ebrahim Rezvani, https://orcid.org/0000-0001-6146-806X; Behrouz Aflatoonian, https://orcid.org/0000-0002-7250-9813
 Mahin Izadi
Mahin Izadi Mohammad Ebrahim Rezvani
Mohammad Ebrahim Rezvani Ali Aliabadi
Ali Aliabadi Mahdieh Karimi4
Mahdieh Karimi4 Behrouz Aflatoonian
Behrouz Aflatoonian