- 1Department of Obstetrics & Gynecology, Shaare Zedek Medical Center, Affiliated with the Hebrew University School of Medicine, Jerusalem, Israel
- 2Division of Clinical Pharmacy, Institute for Drug Research, School of Pharmacy, Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel
- 3Department of Cardiology, Hadassah University Hospital, Jerusalem, Israel
Background: Uteroplacental insufficiency associated disorders, such as preeclampsia, fetal growth restriction and obstetrical antiphospholipid syndrome, share pathophysiology and risk factors with cardiovascular diseases treated with statins.
Objective: To evaluate pregnancy outcomes among women with uteroplacental insufficiency disorders who were treated with statins.
Search Strategy: Electronic databases were searched from inception to January 2022
Selection Criteria: Cohort studies and randomized controlled trials.
Data collection and analysis: Pooled odds ratios were calculated using a random-effects model; meta-regression was utilized when applicable.
Main Results: The analysis included ten studies describing 1,391 women with uteroplacental insufficiency disorders: 703 treated with pravastatin and 688 not treated with statins. Women treated with pravastatin demonstrated significant prolongation of pregnancy (mean difference 0.44 weeks, 95%CI:0.01–0.87, p = 0.04, I2 = 96%) and less neonatal intensive care unit admissions (OR = 0.42, 95%CI: 0.23–0.75, p = 0.004, I2 = 25%). In subgroup analysis, prolongation of pregnancy from study entry to delivery was statistically significant in cohort studies (mean difference 8.93 weeks, 95%CI:4.22–13.95, p = 0.00) but not in randomized control studies. Trends were observed toward a decrease in preeclampsia diagnoses (OR = 0.54, 95%CI:0.27–1.09, p = 0.09, I = 44%), perinatal death (OR = 0.32, 95%CI:0.09–1.13, p = 0.08, I2 = 54%) and an increase in birth weight (mean difference = 102 g, 95%CI: -14–212, p = 0.08, I2 = 96%). A meta-regression analysis demonstrated an association between earlier gestational age at initiation of treatment and a lower risk of preeclampsia development (R2 = 1).
Conclusion: Pravastatin treatment prolonged pregnancy duration and improved associated obstetrical outcomes in pregnancies complicated with uteroplacental insufficiency disorders in cohort studies.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/ identifier CRD42020165804 17/2/2020.
Introduction
Preeclampsia (PET) and fetal growth restriction (FGR) are serious complications of pregnancy that increase the morbidity and mortality of both foetuses and parturients. Both, PET and FGR, may be a result of placental insufficiency (Chaddha et al., 2004). additionally, abnormal placentation and its associated disorders may be encountered among women with obstetric antiphospholipid syndrome (APLAS); 20%–30% of pregnancies with APLAS are complicated with PET, FGR or foetal losses (Abou-Nassar et al., 2011). The spectrum of uteroplacental insufficiency disorders results from various factors, including poor trophoblast uterine invasion early in pregnancy, impaired transformation of the uterine spiral arteries to high capacity-low impedance vessels, abnormalities in the development of chorionic villi, endothelial dysfunction and pathologic changes in the antiangiogenic environment. (Gaiser, 2005; Ilekis et al., 2016) PET or FGR occurring early in the course of pregnancy might prompts early delivery, which can lead to neonatal death and disability arising from prematurity, in addition to maternal morbidity which may be associated with PET.
The role of statins in treating and preventing cardiovascular diseases is well established (Brugts et al., 2009); nonetheless, their potential benefit in treating multiple non-cardiovascular conditions is currently under investigation (He et al., 2018). Specifically, endothelial dysfunction in PET, FGR and APLAS is associated with abnormalities in lipid profile, high levels of triglycerides and oxidative stress (Roberts and Cooper, 2001). Statins inhibit HMG-CoA reductase, leading to reduced plasma cholesterol levels (Tsujita, 1990). Statins also have antioxidant, anti-inflammatory, anti-thrombogenic and vasodilating effects (Laufs et al., 1997; Wolfrum et al., 2003; Jain and Ridker, 2005; Bu et al., 2011; Violi et al., 2013). Statins regulate sFlt-1 which is a key antiangiogenic factor associated with the development of preeclampsia through its affect on Hmox-1 (Cudmore et al., 2007), the preclinical evidence supporting the use of statins in the treatment of PET was demonstrated in several animal models (Saad et al., 2014).
Current clinical knowledge regarding the efficacy of statins in the treatment of PET is inadequate. Individual studies presented contradicting results (Gajzlerska-Majewska et al., 2018). Accordingly, current guidelines do not recommend the usage of statins in the prevention or treatment of uteroplacental insufficiency disorders (ACOG Practice Bulletins, 2020).
In this systematic review and meta-analysis, we aimed to assess pregnancy outcomes associated with pravastatin treatment, in cases of primary prevention (pregnancies at high risk of development of uteroplacental insufficiency disorders) and secondary prevention (pregnancies complicated with PET/FGR/APLAS).
Materials and methods
Search strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (2020) framework guidelines (PRISMA) (Page et al., 2021) and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. (Supplementary Table S1, S2) (Stroup et al., 2021).
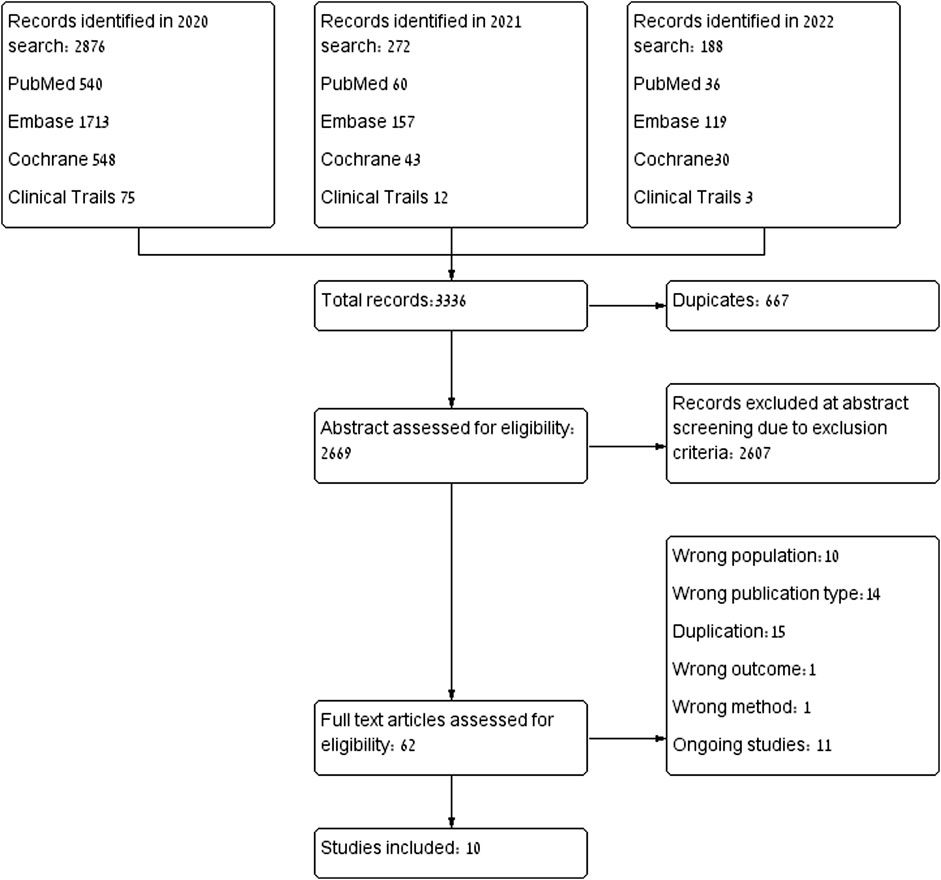
We conducted three systematic database searches: the first included articles from 1953 to February 2020, the second included articles published from the last search to April 2021 and the third included articles published from the last search to January 2022. The search included PubMed/Medline, Embase, Clinical Trials Registry Clinicaltrials.gov and The Cochrane Library. Language restrictions were not set.
The search strategies incorporated index terms (Mesh) and free text words for the search concepts: pravastatin, atorvastatin, rosuvastatin, pregnancy combined by “AND”; and in each domain, the terms were combined by “OR” (Supplementary Table S3) The first domain contained terms on statins (including synonyms and abbreviations such as HMG-CoA reductase inhibitors), the second domain related to pregnancy.
The detailed protocol is documented online in the International Prospective Register of Systematic Reviews Registry (CRD42020165804). Because this study was a review and meta-analysis, Helsinki board approval was waived.
Data sources and searches
In the search strategy, we included randomized controlled trials (RCTs), non-randomized controlled clinical trials, prospective and retrospective comparative cohort studies, and case-control studies. Every study that included women treated with statins during pregnancy was analysed. Duplicated reports, case reports, case series, cross-sectional studies, pharmacokinetic studies in healthy adults, animal studies, reviews, expert opinion, editorials, letters to the editor, comments, and studies with a high risk of bias were excluded.
Study selection and data extraction
Two investigators (AH and NT) independently identified and extracted articles for potential inclusion, using the Rayyan QCRI web application for systematic reviews (Ouzzani et al., 2016). Disagreements were resolved by referral to a third reviewer (B.H.R.). The full texts of the resulting references were retrieved and analysed. If more than one study published data from the same cohort, we included the report with the higher quality according to the Risk of bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Newcastle-Ottawa Quality Assessment Scale- NOS) to avoid overlap (GA Wells et al., 1932).
Exposure to statins during pregnancy was defined as exposure to any dose and in any trimester of pregnancy. Pregnancies that were defined as high risk for future development of uteroplacental insufficiency (Primary prevention) disorders and those that had developed uteroplacental insufficiency disorders (Secondary prevention) were included. The spectrum of uteroplacental insufficiency disorders included: PET, early-onset FGR (<28w), and women with APLAS who developed PET or FGR. Multiple pregnancies were excluded in patient-level data to avoid bias. The primary outcome included prolongation of pregnancy from study entry to delivery. The secondary outcomes included neonatal outcomes such as admission to the neonatal intensive care unit (NICU), birth weight and perinatal death, and the maternal occurrence of a new diagnosis of PET. Perinatal death was defined as stillbirth or death in the first month of life. Data were extracted from the included studies by a single reviewer and subsequently evaluated by the second reviewer. For studies that did not report the outcomes, we contacted the authors and requested the missing data.
Quality assessment and the risk of bias
The risk of bias and the quality of the observational studies were assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) (GA Wells et al., 1932). The scale is based on eight criteria and provides a score ranging from 0 (high risk of bias) to 9 stars (low risk of bias). A 5-star rating and below was designated high risk of bias, six to seven stars intermediate risk of bias, and eight to nine stars low risk of bias. Randomized controlled studies were evaluated by the Cochrane Collaboration’s Risk of Bias Tool (Higgins et al., 2011). Summary assessments of the risk of bias were derived for each study. Assessments were carried out independently by two investigators (AH and NT).
Data synthesis and analysis
Meta-analysis and meta-regression were performed using Comprehensive Meta-Analysis software. Random-effect pooled odds ratios (ORs) were calculated with the corresponding 95% confidence intervals (CIs) to summarize the results overall and within subgroups. Heterogeneity was assessed by using the I2 statistic. We performed subgroup analyses by study type (RCT, cohort). Random-effects meta-analysis was used to pool analyses. Meta-regression analyses were performed to evaluate whether differences in pravastatin dosage and timing of treatment initiation modified the association between exposure to pravastatin and outcomes, and to explain the heterogeneity in the estimated effect size. The data were analyzed using the Comprehensive Meta-Analysis software.
Publication bias
Assessment of publication bias by visual inspection of the funnel plot was planned, provided the analysis would include at least ten studies.
Results
Study selection
The database search yielded 3,336 citations: PubMed (n = 636), Embase (n = 1989), Cochrane Library (n = 621), clinicaltrials.gov (n = 90). In total, 667 were identical duplicates, and excluded. After abstract assessment, 62 articles were extracted for full-text review. Ten articles dropped out because they included normal pregnancies without any risk factors, nor signs or symptoms that indicated placental insufficiency. (Ofori et al., 2007; Taguchi et al., 2008; Colvin et al., 2010; Winterfeld et al., 2013; Bateman et al., 2015; McGrogan et al., 2017; Botha et al., 2018; Lee et al., 2018). One study that had examined only long-term outcomes was excluded because there were no other studies that examined the same outcome (Costantine, 2022) and another was excluded due to a lack of an appropriate control group (Kupferminc et al., 2021). Fourteen articles were excluded due to publication type or study design (Caroli-Bosc et al., 2001; Pollack et al., 2005; Toleikyte et al., 2011; RuysTitia et al., 2014; Daud et al., 2017; Desai et al., 2017; Kadioglu et al., 2020; Akbar et al., 2021). Studies involving animals or human placenta were excluded, due to a lack of standardization in studies investigating biomolecular markers, those studies or results were excluded as well, finally, only clinical studies were included. The selection process is illustrated in Figure 1. Ultimately, ten studies were included in the analysis, with a total of 1,391 pregnant women. Of these, 703 (50.5%) were treated with pravastatin and 688 (49.5%) were not treated with statins. All ten studies described high treatment adherence.
Characteristics of the studies
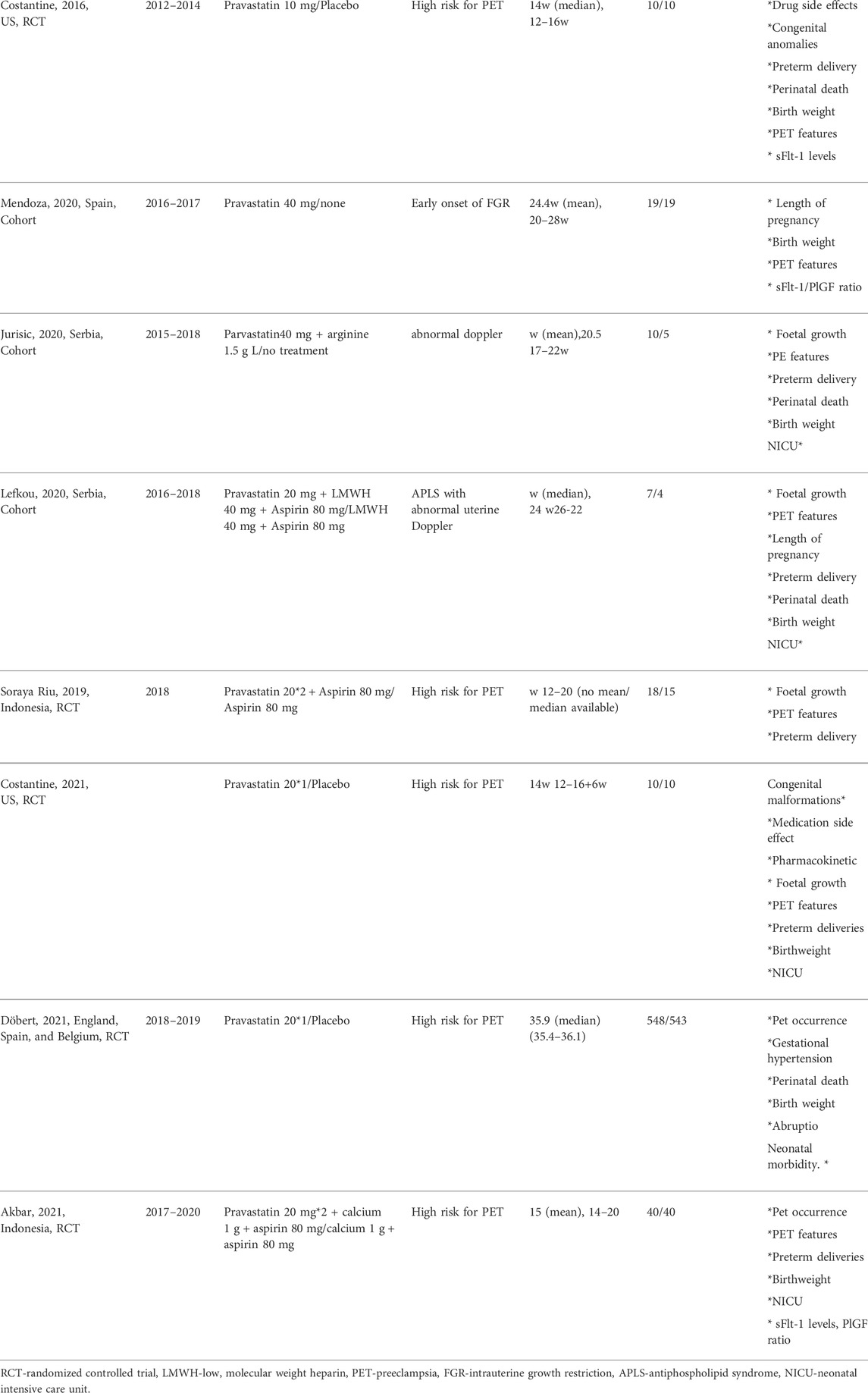
The studies were published between 2016 and 2021 and originated from the United States (n = 2), Indonesia (n = 2), Serbia (n = 2), United Kingdom (n = 1), Spain (n = 1), Greece (n = 1), and multicentre over Europe (n = 1). Four (Lefkou et al., 2016; Lefkou et al., 2020; Mendoza et al., 2020; Jurisic et al., 2021) were cohort studies and six were RCTs (Costantine et al., 2016; Ahmed et al., 2020; Deviana et al., 2020; Dobert et al., 2021; Akbar et al., 2021; Costantine et al., 2021). Studies included women with uteroplacental insufficiency defined as early onset of PET between gestational age 24 and 31 weeks (Lefkou et al., 2016) (Ahmed et al., 2020) (Lefkou et al., 2020), early onset of FGR (Mendoza et al., 2020) (secondary prevention, n = 4) or high risk for developing PET (primary prevention, n = 6). In the primary prevention group, high risk was defined differently; in two studies as a history of severe PET in a prior pregnancy that required delivery before 34 weeks gestation (Costantine et al., 2016), (Costantine et al., 2021), in another study, as a history of previous PET and birth before 37 weeks of gestation or at least two main risk factors for PET (Deviana et al., 2020). An additional study defined high risk pregnancy as having a past poor obstetric history and abnormal placental Dopplers (Jurisic et al., 2021). Abnormal placental Dopplers were defined as uterine artery pulsatility index above the 95th percentile. One study selected women with at least 20% risk for developing PET based on the presence of minimally 2 independent clinical risk factors or abnormal doppler velocimetry index (Akbar et al., 2021) and another (Dobert et al., 2021) selected women with high risk for PET based on a survival-time model of Bayes theorem (Wright et al., 2020). In the secondary prevention group, two studies defined uteroplacental insufficiency as a pregnancy with an APLAS that developed PET or FGR associated with abnormal placental Dopplers (Lefkou et al., 2016; Lefkou et al., 2020) and one study included early onset of PET without proven APLAS (Ahmed et al., 2020). One study also included pregnant women with an early onset of FGR, diagnosed earlier than 28 weeks of pregnancy (Mendoza et al., 2020).
Although the search algorithm included all types of statins, only pravastatin was used in the studies included. Dosing: The dosage of pravastatin differed between studies: 40 mg in five studies, 20 mg in four studies and 10 mg in one study. The comparator treatment included aspirin together with low molecular weight heparin in two studies, aspirin alone in one study, placebo in four studies and no treatment in two studies. Timing of pravastatin initiation: Most studies initiated treatment with pravastatin in the second trimester, but in two studies a minority of the patients were diagnosed and started treatment in their third trimester (Lefkou et al., 2016; Ahmed et al., 2020) and one large RCT included only women in their late third trimester (Dobert et al., 2021). Table 1 displays a summary of the key characteristics of the included RCTs and cohort studies.
Quality assessment
The overall risk of bias among the four nonrandomized studies according to the Newcastle-Ottawa Quality Assessment Scale (NOS) was 7.5. The overall risk of bias of the six randomized controlled studies evaluated by the Cochrane Collaboration’s Risk of Bias Tool was low. The risk of bias assessment is summarized in Supplementary Figures S1, S2.
As ten studies were included in the meta-analysis, we were able to test for funnel plot asymmetry to assess possible publication bias (Higgins et al., 2019). Visual inspection of the funnel plots revealed no indication of publication bias, Egger test also indicates no statistical significance in asymmetry (p = 0.5). (Supplementary Figures S3).
Synthesis of results
Pregnancy prolongation
Eight studies (Costantine et al., 2016; Lefkou et al., 2016; Ahmed et al., 2020; Lefkou et al., 2020; Mendoza et al., 2020; Akbar et al., 2021; Costantine et al., 2021; Jurisic et al., 2021) compared the difference in prolongation of pregnancy from study entry to delivery between women treated with pravastatin and women not treated. Pravastatin treatment was associated with a significant prolongation of pregnancy from study entry to delivery of 0.44 weeks (mean difference 0.44 weeks, 95% CI:0.01–0.87, p = 0.04, I2 = 96%). In a subgroup analysis, prolongation of pregnancy from study entry to delivery was statistically significant in cohort studies (mean difference 8.93 weeks, 95% CI:4.22–13.95, p = 0.00) but not in RCT studies (mean difference 0.37 weeks, 95%CI: (−0.06)−0.80, p = 0.09) (Figure 2).
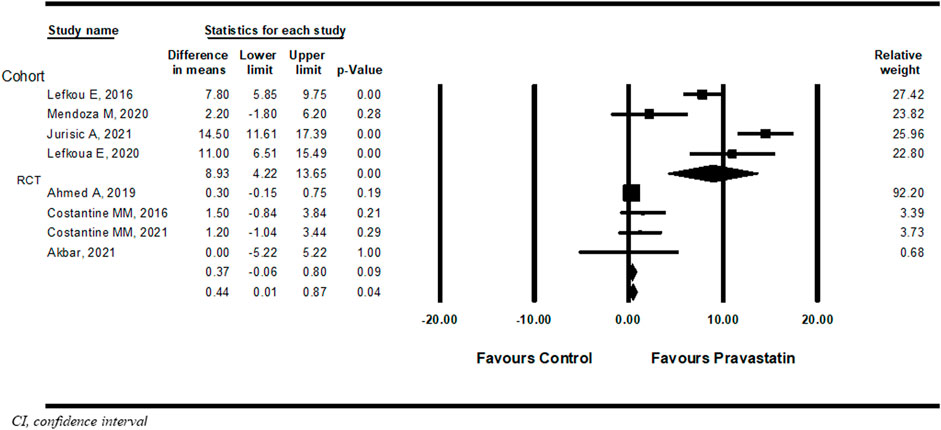
FIGURE 2. Meta-analysis results of the association of pravastatin treatment with a mean difference (weeks) in prolongation of pregnancy from study entry to delivery.
NICU admission
Nine studies (Costantine et al., 2016; Lefkou et al., 2016; Ahmed et al., 2020; Lefkou et al., 2020; Mendoza et al., 2020; Akbar et al., 2021; Costantine et al., 2021; Dobert et al., 2021; Jurisic et al., 2021) examined NICU admission with or without pravastatin treatment. Pravastatin treatment was associated with a significantly decreased risk of NICU admission compared to women not treated with pravastatin (OR = 0.42, 95%CI: 0.23–0.75, p = 0.004, I2 = 25%). In a subgroup analysis, NICU admission was statistically significant both in cohort studies (OR 0.09, 95%CI:0.01–0.68, p = 0.02) and in RCT studies (OR 0.48, 95%CI:0.0.23–0.75, p = 0.02) (Figure 3).
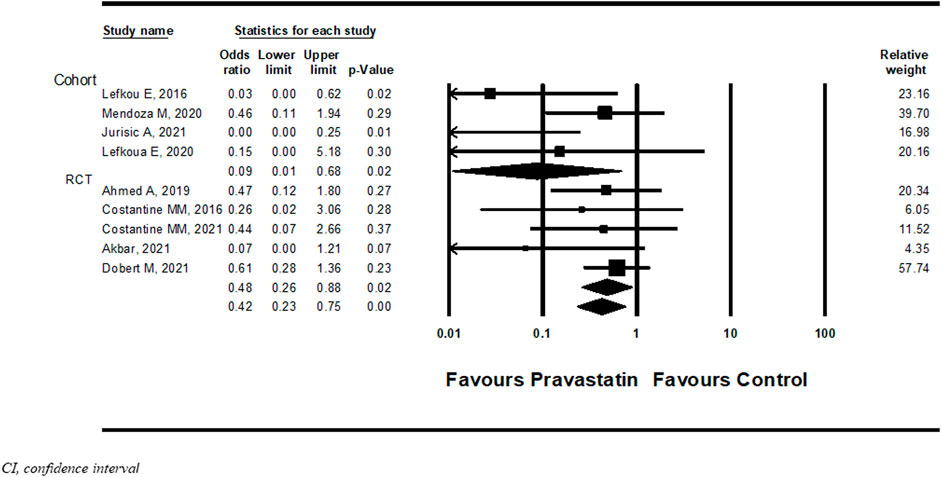
FIGURE 3. Meta-analysis results of the association of pravastatin treatment with admission to the neonatal intensive care unit.
Perinatal death
Ten studies (Dobert et al., 2021; Costantine et al., 2016; Lefkou et al., 2016; Ahmed et al., 2020; Deviana et al., 2020; Lefkou et al., 2020; Mendoza et al., 2020; Akbar et al., 2021; Costantine et al., 2021; Jurisic et al., 2021) examined the association between pravastatin and perinatal death. Although statistical significance was not reached, not in the overall analysis and not in subgroup analysis according to study type, there was a trend towards a decrease in the risk of perinatal death in new-borns of women treated with pravastatin (OR = 0.32, 95%CI: 0.09–1.13, p = 0.08, I2 = 54%), (Figure 4).
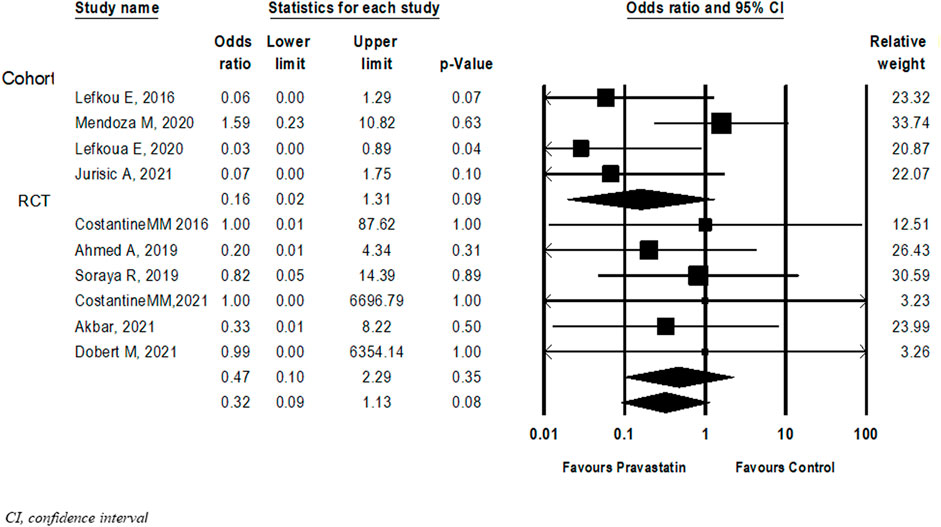
FIGURE 4. Meta-analysis results of Odds ratios for perinatal death following pravastatin treatment versus a control group.
Birth weight
Eight (Costantine et al., 2016; Lefkou et al., 2016; Ahmed et al., 2020; Deviana et al., 2020; Lefkou et al., 2020; Mendoza et al., 2020; Costantine et al., 2021; Jurisic et al., 2021) studies compared birth weight between new-borns of women treated with pravastatin and those who were not. The difference in birth weight following pravastatin treatment was not statistically significant (Mean difference = 102 g, 95%CI: −14–212, p = 0.08, I2 = 96%). In a subgroup analysis, the difference in birthweight following pravastatin treatment was statistically significant in cohort studies (mean difference 1492g, 95%CI: 258–2,725, p = 0.02) but not in RCT studies (mean difference 89 g, 95%CI: (-26)-204, p = 0.13). (Figure 5).
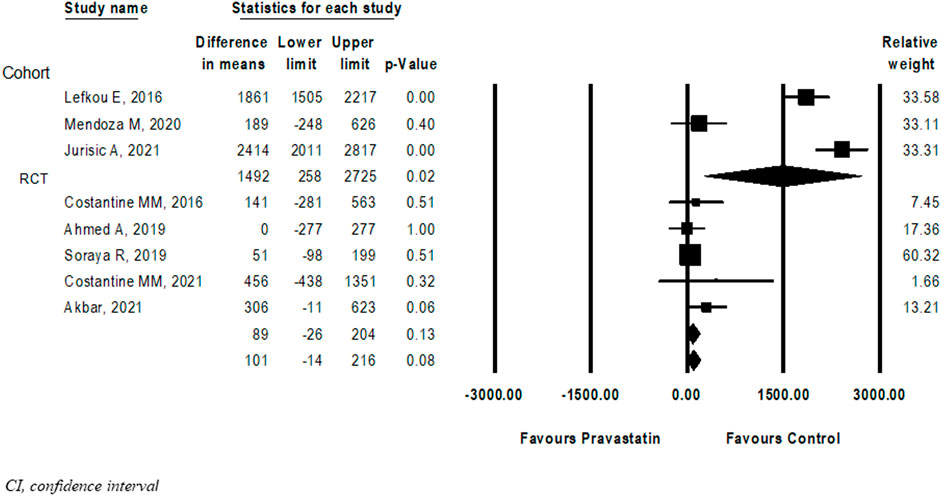
FIGURE 5. Meta-analysis results of the Differences in birth weight (grams) following pravastatin treatment versus a control group.
Maternal outcomes
Six studies (Costantine et al., 2016; Deviana et al., 2020; Mendoza et al., 2020; Akbar et al., 2021; Costantine et al., 2021; Dobert et al., 2021) described a new diagnosis of PET after initiation of pravastatin treatment five of them were RCT and one cohort. Among women treated with pravastatin as opposed to those who were not treated, a nonsignificant decrease in further development of PET was demonstrated (OR = 0.54, 95%CI: 0.27–1.09, p = 0.09, I = 44%) (Figure 6) Dobert et al. (2021) specifically selected women in their late third trimester. A sensitivity analysis that excluded this study showed a significant decrease in the risk for developing PET (OR = 0.37, 95%CI:0.18–0.74, p = 0.01, I = 0%) (Figure 7). A further sensitivity analysis that excluded the cohort study of Mendoza et al. still showed a significant decrease in the risk for developing PET (OR = 0.32, 95%CI:0.14–0.73, p = 0.01) (Supplementary Figure S14).
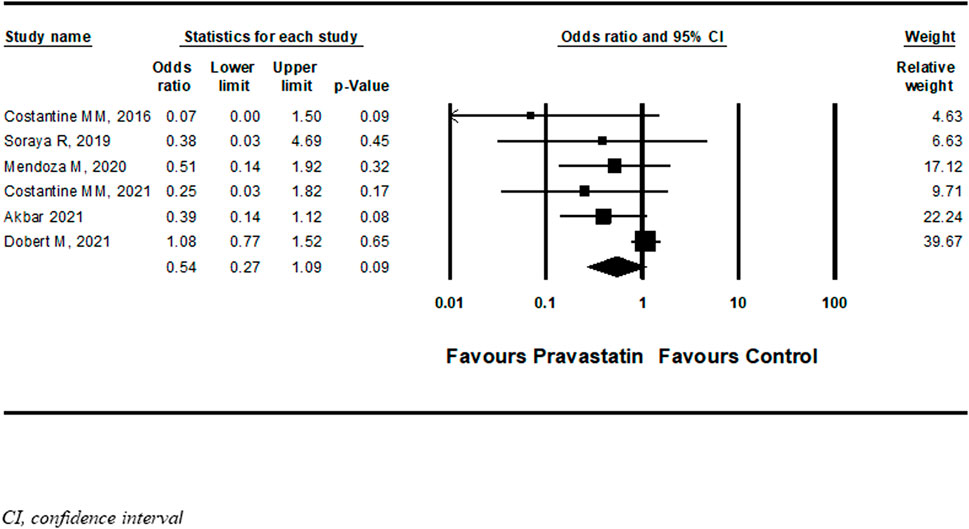
FIGURE 6. Meta-analysis results of the association of pravastatin treatment with new diagnoses of preeclampsia.
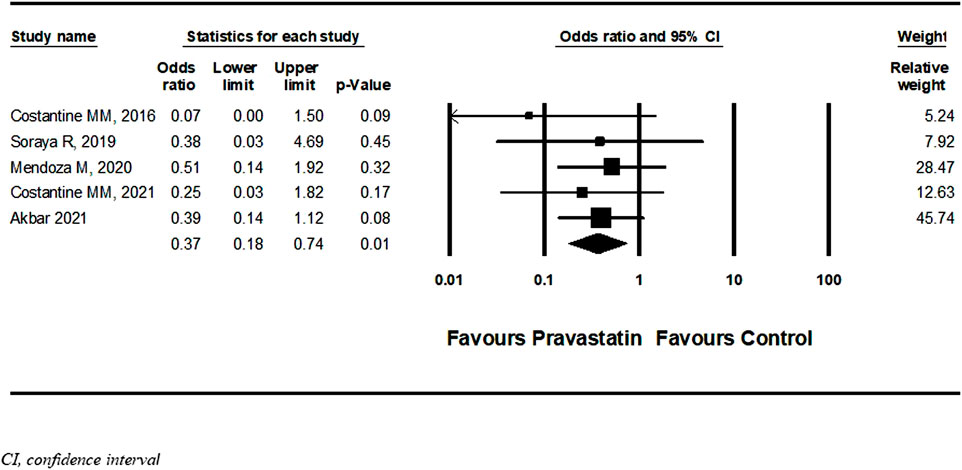
FIGURE 7. Sensitivity meta-analysis of the association of pravastatin treatment with new diagnoses of preeclampsia.
Meta-regression and subgroup analysis
Due to the high heterogeneity found in the analyses, a meta-regression analysis was conducted. A meta-regression revealed a statistically significant association between early gestational age at initiation of pravastatin treatment and a decreased risk for further development of PET (R2 = 1) and NICU admission (R2 = 0.33). No associations were not found between higher pravastatin dose and prolongation of pregnancy from study entry to delivery, birth weight, perinatal death or NICU admission. No associations were not found between earlier gestational age at initiation of pravastatin treatment to prolongation of pregnancy from study entry to delivery, birth weight or perinatal death (Supplementary Figure S4–S13).
Subgroup analysis did not reveal a difference in the odds ratio for pregnancy prolongation, NICU admission, perinatal death and birth weight between studies that used pravastatin for primary vs secondary prevention (Supplementary Table S4).
Discussion
Main findings
In this meta-analysis and meta-regression, pravastatin usage, both for primary and secondary prevention of uteroplacental insufficiency disorders was associated with a significant prolongation of pregnancy, mean of 0.44 weeks, from study entry to delivery. Prematurity is the main cause of neonatal mortality and morbidity as well as late childhood morbidity. (Harrison and Goldenberg, 2016; Jurisic et al., 2021) Preventing preterm births has been delineated as one of the most urgent goals in current obstetrics. Among Pravastatin users, prolonging pregnancy has most probably resulted in the significant decrease observed in NICU admission, and the trends towards decreased perinatal death and increase in neonatal birth weight. In addition, in a sensitivity analysis that included women who had been treated in second trimester, new onset of PET was less common under pravastatin treatment.
Comparison with existing literature
The similarities in pathophysiology and risk factors shared by uteroplacental insufficiency disorders and cardiovascular disease have prompted the search for treatments of uteroplacental insufficiency with different agents used to treat vascular disease (Rezai et al., 2021; Saif et al., 2021; Saito et al., 2021).The underlying mechanism of both conditions involves vascular endothelial dysfunction and endothelial inflammation. The American Heart Association included a history of PET as a risk factor for future cardiovascular disease (Mosca et al., 2011). This association might relate to the shared risk factors for cardiovascular diseases preceding pregnancy, such as obesity, hypertension, diabetes, and dyslipidaemia (Roberts and Cooper, 2001) or alternatively, to the metabolic and vascular changes that PET itself induces (Craici et al., 2008).
Numerous clinical studies have confirmed the multiple therapeutic benefits resulting from the pleiotropic effects of statins (Davignon, 2004; Ray and Cannon, 2005; Wang et al., 2008). Statins improve endothelial dysfunction by protecting vascular endothelium and stimulating its regeneration and its angiogenesis. The antioxidant action of statins relates to their antithrombotic and vasodilating activities and inhibition of free radical formation. The anti-inflammatory action of statins results from their impact on the immune response, which is expressed as increased blood level inflammatory markers and mediators. The latter include C-reactive protein, L-, E- and P-selectin, intercellular and vascular adhesion molecule (Davignon, 2004; Brownfoot et al., 2015; Zeisler et al., 2016). SFlt-1 is one of the key antiangiogenic circulating factors associated with the development of preeclampsia. Its levels are elevated in pregnant women weeks before the clinical onset of preeclampsia (Zeisler et al., 2016). Previous studies had demonstrated a reduction in levels of sFlt-1 by Hmox, both in vitro (Cudmore et al., 2007) and in mice models (Rezai et al., 2021). Hmox-1 as well as sFlt-1 pathways are regulated by Statins. Placental studies and a case series (Brownfoot et al., 2015) indicated that sFlt-1 levels decrease after statin therapy, but the clinical application of this finding is unclear. Our meta-analysis had four studies that investigated the pravastatin effect on sFlt-1, with contradicting results: Ahmed et al. (2020) reported a non-significant reduction in sFlt-1 levels. Mendoza et al. (2020) reported that in women with early-onset FGR, treatment with pravastatin was associated with significant improvement in sFlt-1/Placental Growth Factor (PIGF) ratio before and during pravastatin treatment. Akbar et al. (2021) reported a significant decrease in sFlt-1 levels before and after pravastatin treatment, and only Costantine et al. (2016) reported no difference at all in sFlt-1 in the umbilical cord between groups.
The safety profile of statin exposure during pregnancy is not well defined. United States Food and Drug Administration (FDA) labelled recommends against the use of statins during pregnancy, based on animal data showing teratogenic potential at high doses (Edison and Muenke, 2004). Therefore, the current practice encompasses the advice to discontinue statins when trying to conceive. However, due to subsequent case registries that did not demonstrate an association between congenital anomalies and statin exposure (Vahedian-Azimi et al., 2021), the FDA recently requested a revision to the information about pregnancy usage of the entire class of statins. The clinical benefits of statins in individuals with familial hypercholesterolemia or cardiovascular diseases should be considered, together with the growing evidence of statins’ potential benefit in preventing and treating uteroplacental insufficiency-associated disorders. Accordingly, their benefit may fairly overcome their controversial risk.
Strengths and limitations
To the best of our knowledge, this is the largest and the only study to pool results of pravastatin for uteroplacental insufficiency-associated disorders. This meta-analysis included all available published data and conducted a systematic analysis according to accepted guidelines. Six RCT’s were included, and all ten studies described high treatment adherence.
As four studies included in this meta-analysis were retrospective, selection bias should be considered. We performed a quality assessment for the risk of bias using the NOS, which resulted in a low probability of selection bias. Due to the small numbers of studies and events, our results should be interpreted with caution, and further studies are required. The cohorts included in this meta-analysis were geographically diverse. This can potentially broaden the generalizability of our results.
The potential weakness of this analysis rests in the high heterogeneity among the studies, and the inclusion of only six RCTs’ with a small number of patients. The favourable outcomes of this meta-analysis may be driven by “cohort” studies and their inherited biases, as the women included in the control groups represented very specific populations. Yet, to address this heterogeneity, a sub-group analysis and random effect model was used. High heterogeneity was also viewed in dosage regimes, treatment indications and the lack of standardization. To address the high heterogeneity, we conducted a meta-regression for pravastatin dosage, study type and early vs. late initiation of pravastatin treatment. Although the analyses of perinatal death and birth weight showed trends toward better outcomes, these trends were not statistically significant, this might a be attributed to the small numbers of precipitants.
While the pathophysiology of uteroplacental dysfunction lies in inadequate trophoblast invasion in the early stages of pregnancy (Roberts and Cooper, 2001), all the studies that investigated pravastatin therapy started in the second to the third trimester and the largest study (Dobert et al., 2021) included only women in late third trimester which might downgrade the beneficial effect of the treatment, earlier initiation of therapy might yield different results from those described. Biomolecular markers, mainly sFlt1 or sFlt-1/PlGF ratio, were measured in few studies. In this meta-analysis we had decided not to address this issue as the reported results may be biased. The bias may be attributed to lack of standardization of measurements performed over various periods of time and to the exclusion of women who delivered after the first measurement from the analysis. In addition, no data were found in the literature on other potent statins such as atorvastatin and rosuvastatin (Stone et al., 2013).
In this analysis, we pooled studies that adjusted for confounders including pre-existing diabetes, a baseline score of biomolecule markers and laboratory together with studies that did not perform any adjustments, this (Jurisic et al., 2021), (Lefkou et al., 2016), (Mendoza et al., 2020) raises the possibility that our results may not reflect the true effect size and may be susceptible to sources of bias.
Conclusion
This meta-analysis suggests that treatment with pravastatin for both primary and secondary prevention of uteroplacental insufficiency disorder may prolong pregnancy duration. An individualized assessment and a personalized approach are advised when pravastatin usage is considered.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study protocol. AH, NT performed the literature search, and BH. Literature screening, and data extraction, were performed by AH, RR, NT, and BH. Risk of bias evaluation was performed by AH and NT. AH and BH conducted the analyses. AH, and RR wrote the draft of the manuscript, and BH and all authors commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1021548/full#supplementary-material
References
Abou-Nassar, K., Carrier, M., Ramsay, T., and Rodger, M. A. (2011). The association between antiphospholipid antibodies and placenta mediated complications: A systematic review and meta-analysis. Thromb. Res. 128 (1), 77–85. doi:10.1016/j.thromres.2011.02.006
ACOG Practice Bulletins (2020). Clinical management guidelines for obstetrician – gynecologists. Obstetrics Gynecol. 133 (76), 168–186. [Internet]Available at: https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2020/07/diagnosis-and-management-of-vulvar-skin-disorders.
Ahmed, A., Williams, D. J., Cheed, V., Middleton, L. J., Ahmad, S., Wang, K., et al. (2020). Pravastatin for early-onset pre-eclampsia: A randomised, blinded, placebo-controlled trial. BJOG 127 (4), 478–488. doi:10.1111/1471-0528.16013
Akbar, M. I. A., Yosediputra, A., Pratama, R. E., Fadhilah, N. L., Sulistyowati, S., Amani, F. Z., et al. (2021). INOVASIA study: A randomized open controlled trial to evaluate pravastatin to prevent preeclampsia and its effects on sFlt1/PlGF levels. Am. J. Perinatol. 47. doi:10.1055/a-1673-5603
Bateman, B. T., Hernandez-Diaz, S., Fischer, M. A., Seely, E. W., Ecker, J. L., Franklin, J. M., et al. (2015). Statins and congenital malformations: Cohort study. BMJ (Online). 350, h1035. doi:10.1136/bmj.h1035
Botha, T. C., Pilcher, G. J., Wolmarans, K., Blom, D. J., and Raal, F. J. (2018). Statins and other lipid-lowering therapy and pregnancy outcomes in homozygous familial hypercholesterolaemia: A retrospective review of 39 pregnancies. [cited 2021 Jul 1]. doi:10.1016/j.atherosclerosis.2018.05.038
Brownfoot, F. C., Tong, S., Hannan, N. J., Binder, N. K., Walker, S. P., Cannon, P., et al. (2015). Effects of pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension 66 (3), 687–697. doi:10.1161/HYPERTENSIONAHA.115.05445
Brugts, J. J., Yetgin, T., Hoeks, S. E., Gotto, A. M., Shepherd, J., Westendorp, R. G. J., et al. (2009). The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ (Online) 339 (7711), b2376. doi:10.1136/bmj.b2376
Bu, D. X., Griffin, G., and Lichtman, A. H. (2011). Mechanisms for the anti-inflammatory effects of statins. Curr. Opin. Lipidol. 22 (3), 165–170. doi:10.1097/MOL.0b013e3283453e41
Caroli-Bosc, F-X. X., Le Gall, P., Pugliese, P., Delabre, B., Caroli-Bosc, C., Demarquay, J. F., et al. (2001). Role of fibrated and HMG-CoA reductase inhibitors in gallstone formation. Disgestive Dis. Sci. 46.
Chaddha, V., Viero, S., Huppertz, B., and Kingdom, J. (2004). Developmental biology of the placenta and the origins of placental insufficiency. Semin. Fetal Neonatal Med. 9 (5), 357–369. doi:10.1016/j.siny.2004.03.006
Colvin, Lyn, Slack-Smith, Linda, FionaStanley, J. C. B., and Bower, C. (2010). Linking a pharmaceutical claims database with a birth defects registry to investigate birth defect rates of suspected teratogens. Pharmacoepidemiol. Drug Saf. 19, 1137–1150. doi:10.1002/pds.1995
Costantine, M. M., Cleary, K., Hebert, M. F., Ahmed, M. S., Brown, L. M., Ren, Z., et al. (2016). Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: A pilot randomized controlled trial. Am. J. Obstet. Gynecol. 214 (6), e1–e720. doi:10.1016/j.ajog.2015.12.038
Costantine, M. M. (2022). Long-term child follow-up of the pravastatin for prevention of preeclampsia pilot trials. Am. J. Obstet. Gynecol. 226 (1), S74–S75. doi:10.1016/j.ajog.2021.11.142
Costantine, M. M., West, H., Wisner, K. L., Caritis, S., Clark, S., Venkataramanan, R., et al. (2021). A randomized pilot clinical trial of pravastatin versus placebo in pregnant patients at high risk of preeclampsia. Am. J. Obstet. Gynecol. 225 (6), 666.e1–666.e15. doi:10.1016/j.ajog.2021.05.018
Craici, Iasmina, and Wagner, StevenVDG (2008). Preeclampsia and future cardiovascular risk: Formal risk factor or failed stress test? Ther. Adv. Cardiovasc. Dis. 2 (4), 249–259. doi:10.1177/1753944708094227
Cudmore, M., Ahmad, S., Al-Ani, B., Fujisawa, T., Coxall, H., Chudasama, K., et al. (2007). Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115, 1789–1797. doi:10.1161/CIRCULATIONAHA.106.660134
Daud, A. N. A., Bergman, J. E. H., Oktora, M. P., Kerstjens-Frederikse, W. S., Groen, H., Bos, J. H., et al. (2017). Maternal use of drug substrates of placental transporters and the effect of transporter-mediated drug interactions on the risk of congenital anomalies. PLoS One 12 (3), 01735300–e173614. doi:10.1371/journal.pone.0173530
Davignon, J. (2004). Beneficial cardiovascular pleiotropic effects of statins. Circulation 109 (23 Suppl. L), 39–43. doi:10.1161/01.CIR.0000131517.20177.5a
Desai, R. J., Rothman, K. J., Bateman, B. T., Hernandez-Diaz, S., and Huybrechts, K. F. (2017). A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 28 (2), 249–257. doi:10.1097/EDE.0000000000000595
Deviana, S. R., Sunarno, I., Lukas, E., Al, W., and Amalia, R. (2020). The effect of pravastatin on endothelin-1 levels and pregnancy outcomes in women who have a high risk for preeclampsia: A randomized control trial. Enferm. Clin. 30, 499–505. doi:10.1016/j.enfcli.2019.07.147
Dobert, M., Varouxaki, A. N., Mu, A. C., Syngelaki, A., Ciobanu, A., Akolekar, R., et al. (2021). Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation 144, 670–679. doi:10.1161/CIRCULATIONAHA.121.053963
Edison, R. J., and Muenke, M. (2004). Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am. J. Med. Genet. A 131A (3), 287–298. doi:10.1002/ajmg.a.30386
Ga Wells, B. S., Shea, B., O’Connell, D., Peterson, J., Welch, V., Robertson, J. M. L., et al. (1932). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Round Table 22, 461–478.
Gaiser, R. (2005). Circulating angiogenic factors and the risk of preeclampsia. Surv. Anesthesiol. 49 (1), 14–15. doi:10.1097/01.sa.0000151206.53344.39
Gajzlerska-Majewska, W., Bomba-Opon, D. A., and Wielgos, M. (2018). Is pravastatin a milestone in the prevention and treatment of preeclampsia? J. Perinat. Med. 46 (8), 825–831. doi:10.1515/jpm-2017-0109
Harrison, M. S., and Goldenberg, R. L. (2016). Global burden of prematurity. Semin. Fetal Neonatal Med. 21 (2), 74–79. doi:10.1016/j.siny.2015.12.007
He, Y., Li, X., Gasevic, D., Brunt, E., McLachlan, F., Millenson, M., et al. (2018). Statins and multiple noncardiovascular outcomes: Umbrella review of meta-Analyses of observational studies and randomized controlled trials. Ann. Intern. Med. 169 (8), 543–553. doi:10.7326/M18-0808
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Online) 343 (7829), d5928–d5929. doi:10.1136/bmj.d5928
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane handbook for systematic reviews of interventions. Cochrane Handb. Syst. Rev. Interventions, 1–694.
Ilekis, J. v., Tsilou, E., Fisher, S., Abrahams, V. M., Soares, M. J., Cross, J. C., et al. (2016). Placental origins of adverse pregnancy outcomes: Potential molecular targets: An executive workshop summary of the eunice kennedy shriver national institute of child health and human development. Am. J. Obstet. Gynecol. 215 (1), S1–S46. doi:10.1016/j.ajog.2016.03.001
Jain, M. K., and Ridker, P. M. (2005). Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 4 (12), 977–987. doi:10.1038/nrd1901
Jurisic, A., Jurisic, Z., Lefkou, E., and Girardi, G. (2021). Pravastatin plus L-arginine prevents adverse pregnancy outcomes in women with uteroplacental vascular dysfunction. Vasc. Pharmacol. 137, 106824. doi:10.1016/j.vph.2020.106824
Kadioglu, M., Gun, E., Erkoseoglu, I., Cavusoglu, I., Kalyoncu, N. I., and Yaris, E. (2020). The outcomes of statin exposure in pregnancy. Reprod. Toxicol. 97, 16. [Internet. doi:10.1016/j.reprotox.2020.04.058
Kupferminc, Michael J., Kliger, Chagit, Rimon, Eli, Asher-Landsberg, Jessica, and Avital Skornick-Rapaport, R. G& Y. Y. Pravastatin is useful for prevention of recurrent severe placenta-mediated complications – a pilot s _ Enhanced Reader.pdf. 2021.
Laufs, U., la Fata, V., and Liao, J. K. (1997). Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J. Biol. Chem. 272 (50), 31725–31729. doi:10.1074/jbc.272.50.31725
Lee, M. S., Hekimian, A., Doctorian, T., and Duan, L. (2018). Statin exposure during first trimester of pregnancy is associated with fetal ventricular septal defect. Int. J. Cardiol. 269, 111–113. doi:10.1016/j.ijcard.2018.07.002
Lefkou, E., Mamopoulos, A., Dagklis, T., Vosnakis, C., Rousso, D., and Girardi, G. (2016). Pravastatin improves pregnancy outcomes in obstetric antiphospholipid syndrome refractory to antithrombotic therapy. J. Clin. Invest. 126 (8), 2933–2940. doi:10.1172/JCI86957
Lefkou, E., Varoudi, K., Pombo, J., Jurisic, A., Jurisic, Z., Contento, G., et al. (2020). Triple therapy with pravastatin, low molecular weight heparin and low dose aspirin improves placental haemodynamics and pregnancy outcomes in obstetric antiphospholipid syndrome in mice and women through a nitric oxide-dependent mechanism. Biochem. Pharmacol. 182, 114217. doi:10.1016/j.bcp.2020.114217
McGrogan, A., Snowball, J., and Charlton, R. A. (2017). Statins during pregnancy: A cohort study using the general practice research database to investigate pregnancy loss. Pharmacoepidemiol. Drug Saf. 26 (7), 843–852. doi:10.1002/pds.4176
Mendoza, M., Ferrer-Oliveras, R., Bonacina, E., Garcia-Manau, P., Rodo, C., Carreras, E., et al. (2020). Evaluating the effect of pravastatin in early-onset fetal growth restriction: A nonrandomized and historically controlled pilot study. Am. J. Perinatol. 1 (212), 1472–1479. doi:10.1055/s-0040-1713651
Mosca, L., Benjamin, E. J., Berra, K., Bezanson, J. L., Dolor, R. J., Lloyd-Jones, D. M., et al. (2011). Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: A guideline from the American Heart association. Circulation 123 (11), 1243–1262. doi:10.1161/CIR.0b013e31820faaf8
Ofori, B., Rey, E., and Bérard, A. (2007). Risk of congenital anomalies in pregnant users of statin drugs. Br. J. Clin. Pharmacol. 64 (4), 496–509. doi:10.1111/j.1365-2125.2007.02905.x
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 5 (1), 210–10. doi:10.1186/s13643-016-0384-4
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71–1. doi:10.1136/bmj.n71
Pollack, P. S., Shields, K. E., Burnett, D. M., Osborne, M. J., Cunningham, M. L., and Stepanavage, M. E. (2005). Pregnancy outcomes after maternal exposure to simvastatin and lovastatin. Birth Defects Res. A Clin. Mol. Teratol. 73 (11), 888–896. doi:10.1002/bdra.20181
Ray, K. K., and Cannon, C. P. (2005). The potential relevance of the multiple lipid-independent (Pleiotropic) effects of statins in the management of acute coronary syndromes. J. Am. Coll. Cardiol. 46 (8), 1425–1433. doi:10.1016/j.jacc.2005.05.086
Rezai, H., Ahmad, S., Alzahrani, F. A., Sanchez-Aranguren, L., Dias, I. H., Agrawal, S., et al. (20212020). MZe786, a hydrogen sulfide-releasing aspirin prevents preeclampsia in heme oxygenase-1 haplodeficient pregnancy under high soluble flt-1 environment Redox Biol. 38, 101768. doi:10.1016/j.redox.2020.101768
Roberts, J. M., and Cooper, D. W. (2001). Pathogenesis and genetics of pre-eclampsia. Lancet 357 (9249), 53–56. doi:10.1016/s0140-6736(00)03577-7
Ruys Titia, P. E., Maggioni, Aldo, Johnson, Mark R., Sliwa, Karen, Tavazzi, Luigi, Schwerzmann, Markus, et al. (2014). Cardiac medication during pregnancy, data from the ROPAC. Int. J. Cardiol. 177, 124–128. doi:10.1016/j.ijcard.2014.09.013
Saad, A. F., Kechichian, T., Yin, H., Sbrana, E., Longo, M., Wen, M., et al. (2014). Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod. Sci. 21 (1), 138–145. doi:10.1177/1933719113492207
Saif, J., Ahmad, S., Rezai, H., Litvinova, K., Sparatore, A., Alzahrani, F. A., et al. (2021). MZe786, a hydrogen sulfide-releasing aspirin prevents preeclampsia in heme oxygenase-1 haplodeficient pregnancy under high soluble flt-1 environment. Redox Biol. 38, 101768. doi:10.1016/j.redox.2020.101768
Saito, S., Takagi, K., Moriya, J., Kobayashi, T., Kanayama, N., Sameshima, H., et al. (2021). A randomized phase 3 trial evaluating antithrombin gamma treatment in Japanese patients with early-onset severe preeclampsia (KOUNO-TORI study): Study protocol. Contemp. Clin. Trials 107, 106490. doi:10.1016/j.cct.2021.106490
Stone, N. J., Robinson, J. G., Lichtenstein, A. H., Bairey Merz, C. N., Blum, C. B., Eckel, R. H., et al. (2013). 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American Heart association task force on practice guidelines. Circulation 129, 1–45. doi:10.1161/01.cir.0000437738.63853.7a
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2021). Meta-analysis of observational studies.
Taguchi, N., Rubin, E. T., Hosokawa, A., Choi, J., Ying, A. Y., Moretti, M. E., et al. (2008). Prenatal exposure to HMG-CoA reductase inhibitors: Effects on fetal and neonatal outcomes. Reprod. Toxicol. 26 (2), 175–177. doi:10.1016/j.reprotox.2008.06.009
Toleikyte, I., Retterstøl, K., Leren, T. P., and Iversen, P. O. (2011). Pregnancy outcomes in familial hypercholesterolemia: A registry-based study. Circulation 124 (15), 1606–1614. doi:10.1161/CIRCULATIONAHA.110.990929
Tsujita, Y. (1990). HMG-CoA reductase inhibitors. J. Jpn. Atheroscler. Soc. 18 (2), 165–171. doi:10.5551/jat1973.18.2_165
Vahedian-Azimi, A., Makvandi, S., Banach, M., Reiner, Ž., and Sahebkar, A. (2021). Fetal toxicity associated with statins: A systematic review and meta-analysis. Atherosclerosis 327, 59–67. doi:10.1016/j.atherosclerosis.2021.05.006
Violi, F., Calvieri, C., Ferro, D., and Pignatelli, P. (2013). Statins as antithrombotic drugs. Circulation 127 (2), 251–257. doi:10.1161/CIRCULATIONAHA.112.145334
Wang, C. Y., Liu, P. Y., and Liao, J. K. (2008). Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 14 (1), 37–44. doi:10.1016/j.molmed.2007.11.004
Winterfeld, U., Allignol, A., Panchaud, A., Rothuizen, L. E., Merlob, P., Cuppers-Maarschalkerweerd, B., et al. (2013). Pregnancy outcome following maternal exposure to statins: A multicentre prospective study. BJOG 120 (4), 463–471. doi:10.1111/1471-0528.12066
Wolfrum, S., Jensen, K. S., and Liao, J. K. (2003). Endothelium-dependent effects of statins. Arterioscler. Thromb. Vasc. Biol. 23 (5), 729–736. doi:10.1161/01.ATV.0000063385.12476.A7
Wright, D., Wright, A., and Nicolaides, K. H. (2020). The competing risk approach for prediction of preeclampsia. Am. J. Obstet. Gynecol. 223 (1), 12–23. doi:10.1016/j.ajog.2019.11.1247
Keywords: statins, pravasatin, preeclampsia, IUGR, uteroplacental, fetal growth restriction
Citation: Hirsch A, Rotem R, Ternovsky N and Hirsh Raccah B (2022) Pravastatin and placental insufficiency associated disorders: A systematic review and meta-analysis. Front. Pharmacol. 13:1021548. doi: 10.3389/fphar.2022.1021548
Received: 17 August 2022; Accepted: 19 October 2022;
Published: 09 November 2022.
Edited by:
Venkata Kashyap Yellepeddi, The University of Utah, United StatesReviewed by:
Adi Y. Weintraub, Soroka Medical Center, IsraelUma Fogueri, Loxo Oncology, Inc., United States
Copyright © 2022 Hirsch, Rotem, Ternovsky and Hirsh Raccah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayala Hirsch, YXlhbGE0NkBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Ayala Hirsch
Ayala Hirsch Reut Rotem
Reut Rotem Natali Ternovsky
Natali Ternovsky Bruria Hirsh Raccah
Bruria Hirsh Raccah