- School of Medicine, Pingdingshan University, Pingdingshan, China
The great success of cisplatin as a chemotherapeutic agent considerably increased research efforts in inorganic biochemistry to identify more metallic drugs having the potential of treating lung cancer. Metal coordination centres, which exhibit a wide range of coordination numbers and geometries, various oxidised and reduced states and the inherent ligand properties offer pharmaceutical chemists a plethora of drug structures. Owing to the presence of C=N and C=S bonds in a thiosemicarbazone Schiff base, N and S atoms in its hybrid orbital has lone pair of electrons, which can generate metal complexes with different stabilities with most metal elements under certain conditions. Such ligands and complexes play key roles in the treatment of anti-lung cancer. Research regarding metallic anti-lung cancer has advanced considerably, but there remain several challenges. In this review, we discuss the potential of thiosemicarbazone Schiff base complexes as anti-lung cancer drugs, their anti-cancer activities and the most likely action mechanisms involving the recent families of copper, nickel, platinum, ruthenium and other complexes.
Introduction
Lung cancer is a malignant tumour that develops from the bronchial mucosa or glands of the lung, which poses the greatest threat to human health and lives, exhibiting the fastest increase rate in terms of morbidity and mortality (Brody, 2014). In 2020, ∼19.3 million new cancer cases and approximately 10 million cancer-related deaths were reported worldwide (Sung et al., 2021). Lung cancer remains the leading cause of cancer-related death, engendering an estimated 1.8 million deaths (18%) with an estimated 2.2 million new cases (11.4%) in 2020 (Sung et al., 2021). The dramatic increase in the cases of lung cancer and multidrug-resistant infections has necessitated the search for novel treatment options and strategies (Tsai et al., 2018). New small-molecule anti-cancer agents Exhibit great potentials. However, the frequent occurrence of multidrug resistance (MDR) in lung cancer warrants the development of specific agents that can overcome MDR (Barrera-Rodriguez and Fuentes, 2015). Metal-based drugs are structurally stable and have unique three-dimensional configurations, which can be effectively used to treat multidrug-resistant infections (Rostan et al., 2021).
Thiosemicarbazone Schiff bases have garnered a lot of interest due to their multifunctional metal-chelating properties, inherent biological activities and structural flexibility (Matesanz et al., 2021a). It was established that thiosemicarbazides were metal chelators before the discovery of their anti-tumour activity. In addition to the presence of the C=N and C=S bonds in their own structure, which is favourable for metal coordination, it possesses a flexible thiourea structure that can introduce different substituents or functional groups (Scarim and Chin, 2022a). Especially for various heterocyclic ligands, introducing more heteroatoms enriches thiosemicarbazone’s coordination mode and enhances its coordination ability (Stefani et al., 2013). They utilize the N–N–S donor system of ligands to form stable complexes with transition metals. Many studies reported that these complexes exhibited higher anti-tumour activity than ligands in vitro and in vivo and were extensively employed in the field of anti-tumour research (Summers, 2019a). Metal complexes are biologically and chemically diverse, unlike their corresponding ligands. This diversity is not only reflected in the metal and its oxidation state but also in the diversity of ligands that form coordination bonds with it and the different coordination modes (Matesanz et al., 2021a). Many literatures report that thiosemicarbazone ligands and their metal complexes have significant antitumor activity against lung cancer, liver cancer, colon cancer, breast cancer, neuroma and other tumors (Onodera et al., 2007; Lovejoy et al., 2012; Maqbool et al., 2020; Dharmasivam et al., 2022). Among many cancers, lung cancer has been the most common disease affecting human health. Thiosemicarbazone ligands have been reported to exhibit significant antitumor activity against lung tumor xenografts and have no significant toxicological effects, both intravenously and orally routes (Lovejoy et al., 2012). Therefore, it is necessary to review the research progress of thiosemicarbazone metal complexes in the treatment of lung cancer. We hope that this review guides researchers to better understand the application of metal-based agents in anti-lung cancer treatment and provide new ideas for the design and research of anti-tumour agents.
Thiosemicarbazone ligands in a clinical trial
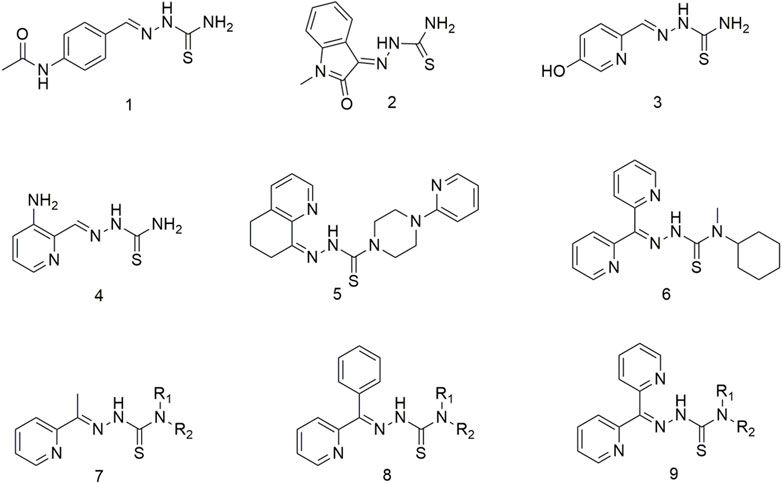
The thiosemicarbazone compounds used in clinical research include acetamidobenzaldehyde semicarbazide (1), N-methylisatin thiosemicarbazone (2), 5-hydroxy-2-methylcarbamate acylpyridine thiosemicarbazide (3), 3-aminopyridine-2-carbaldehyde thiosemicarbazide (4), COTI-2 (5), bis-2-pyridyl ketone 4-cyclohexyl-4-methyl-3-thiosemicarbazide (6) (Scheme 1) (Summers, 2019a; Matesanz et al., 2021a; Scarim and Chin, 2022a). Triapine is the most prevalent anti-cancer agent and has been included in over 20 clinical trials for treating multiple cancers, including pancreatic cancer, non-small cell lung cancer (NSCLC), leukaemia and myeloproliferative diseases (Scarim and Chin, 2022a). However, some tumour types respond poorly to Triapine, developing serious side effects, such as hyperhemoglobinemia (Yu et al., 2006; Yu et al., 2009). Among this class of compounds, the 2-acetylpyridine thiosemicarbazone series (7) demonstrated significant anti-cancer activity (Onodera et al., 2007). Initial studies have shown that thiosemicarbazone can inhibit ribonucleotide reductase activity and further impede DNA synthesis, finally exerting its anti-cancer effect (Kowol et al., 2009; Yu et al., 2011). Further studies have shown that the anti-tumour activity of thiosemicarbazone is due to the joint action of multiple mechanisms (de Siqueira et al., 2019a). Thiosemicarbazone ligands have been widely reported to inhibit cellular iron uptake and affect cellular iron metabolism, and up-regulation of the downstream regulatory gene 1 of the metastasis suppressor protein N-myc (Serda et al., 2014; Malarz et al., 2018). Moreover, compound 9 causes DNA damage and inhibition of selective cellular topoisomerase IIɑ (Rao et al., 2009). This kind of a ligand is a good metal chelator, and the complexes formed by metal coordination exhibit redox activity and can catalyse hydrogen peroxide formation in vivo to generate reactive oxygen species (ROS) (Mckenzie-Nickson et al., 2016; Matesanz et al., 2021a). Through extensive structure-activity relationship studies, a new series of 2-benzoylpyridine thiosemicarbazone (8) incorporated 2-benzoylpyridine moieties in the structural backbone of previous arylhydrazone (Stefani et al., 2013). These compounds exhibited potent anti-lung cancer and anti-metastatic activities (Wu et al., 2006; Mahale et al., 2015). A series of di-2-pyridyl ketone thiosemicarbazone (9) were developed (Lovejoy et al., 2012) to improve the efficacy and safety of these potential anti-tumour drugs. This new compound exhibits markedly selective activity in vivo against human lung cancer (DMS-53) xenografts via the intravenous and oral routes (Lovejoy et al., 2012). Importantly, these analogues did not cause cardiotoxicity in tumour-bearing nude mice at high doses (Wangpu et al., 2016). To overcome MDR, di-2-pyridyl ketone thiosemicarbazone analogues target lysosomes by ‘hijacking’ the MDR pump p-glycoprotein (Pgp), which has potent anti-tumour and anti-metastatic activities both in vitro and in vivo (Wangpu et al., 2016). However, high doses of di-2-pyridyl ketone thiosemicarbazone may cause cardiac fibrosis, which warrants the design and synthesis of new thiosemicarbazone compounds. In second-generation thiosemicarbazone compounds wherein the terminal H of N4 was substituted with an alkyl group, compound 6 showed potential application prospects in antitumor (Lovejoy et al., 2012). Compound 6 exhibits higher anti-tumour activity in vivo and demonstrates considerable improvement in tolerability when administered orally or intravenously compared with first-generation thiosemicarbazone compounds (Stariat et al., 2013; Maqbool et al., 2020; Dharmasivam et al., 2022). Thiosemicarbazones combine with intracellular copper (Cu) ions to produce stable Cu complexes once they are inside the lysosome. These complexes generate ROS through the redox cycle, causing the lysosomal membrane to collapse (LMP), ultimately inducing apoptosis (Zhao et al., 2017; Gu et al., 2019).
Thiosemicarbazone copper complexes
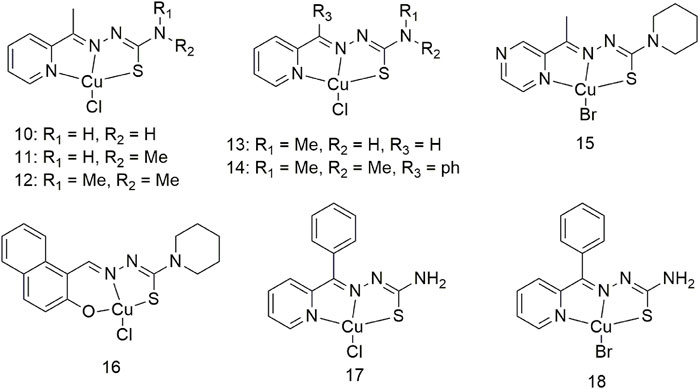
Three Cu (II) complexes (10–12, Scheme 2) were synthesised and characterised to show that thiosemicarbazone Cu (II) complexes demonstrate strong anti-tumour activity (Qi et al., 2020). The anti-proliferative activity of complex 12 on the human lung cancer cell line A549 was the highest (0.20 ± 0.04 mM) of all the complexes. The lipophilicity of thiosemicarbazone ligands is closely related to the anti-proliferative activity of Cu (II) complexes on lung cancer cells Studies regarding the cellular mechanism of such Cu (II) complexes have shown that they promote apoptosis by catalysing hydrogen peroxide to generate intracellular ROS. Researchers proposed to develop two Cu (II) complexes based on the His242 residue of the IIA subdomain of the human serum albumin (HSA) carrier (13 and 14, Scheme 2) (Qi et al., 2016) to improve the delivery efficiency, anti-cancer activity and selectivity of anti-cancer metal preparations in vivo. Complex 13 binds to HSA subdomain IIA by hydrophobic interaction, while complex 14 binds to HSA by coordinating with His242. The cytotoxicity of complex 14 toward the A549 cell line (0.15 ± 0.01 μM) was significantly higher than that of cisplatin (17.36 ± 0.25 μM), and it significantly inhibited the growth of A549 tumours in nude mice. The anti-tumour activity against human large cell lung cancer cells (NCI-H460) of complexes 15 and 16 increased more than 40-fold compared with the ligands and exhibited significant pro-apoptotic activity at nanomolar concentrations (Scheme 2). Complexes 15 and 16 mediate significant antitumor activity by generating reactive oxygen species (ROS) through the redox cycle. (Scheme 2). Excess ROS can lead to the dissipation of mitochondrial membrane potential and promote the release of mitochondrial apoptotic factors. Complexes 17 and 18 also show effective oxidative cleavage of supercoiled DNA in the presence of hydrogen peroxide and exhibit good anti-tumour activity against the NCI-H460 cell line with IC50 values in the range of 0.08–1.98 μM, which are higher than cis-Platinum (Pt) is 83 times lower (Scheme 2) (Liu et al., 2016).
By assessing the structure–activity relationship of six Cu (II) complexes (19–24, Scheme 3), researchers confirmed the multi-target ability of complex 22 on proteins and DNA (Wang et al., 2018). These six Cu complexes showed higher cytotoxicity against lung cancer cells (A549 and NCI-H460) than the ligands. The toxicity of complexes 19–24 toward the A549 cell line (≤13.69 ± 0.97 μM) was significantly higher than that of cisplatin (17.36 ± 0.75 μM). Importantly, in the A549 cell line, the cytotoxicity of the Cu (II) complex was enhanced by 1.0–3.0-fold upon binding to HSA. These six complexes inhibit cancer cell growth mainly by targeting DNA and apoptosis-related proteins in tumours. Forming a complex with HSA can improve the delivery efficiency of Cu complexes and impart a stronger ability to inhibit tumour growth.
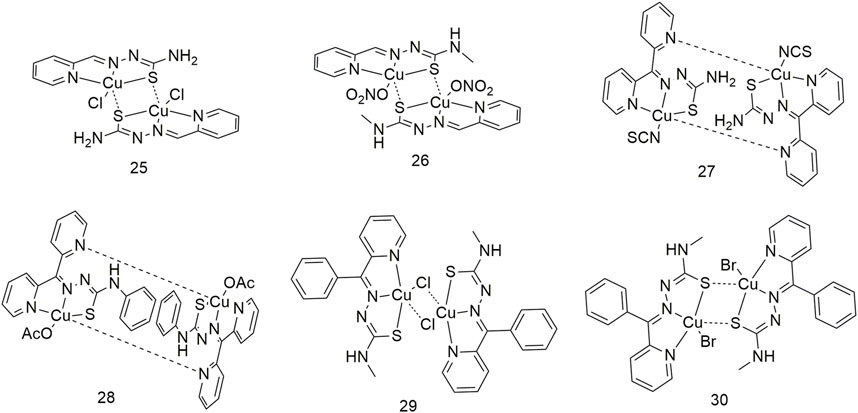
It is essential to conduct research regarding Cu complexes to produce a new generation of metallic anti-cancer drugs. Therefore, four dinuclear Cu (II) complexes (25–28, Scheme 4) were synthesised using Schiff base thiosemicarbazides (Qi et al., 2015). The anti-proliferative activity of these four Cu complexes on lung cancer cells (A549 and NCI-H460) was stronger than that of the ligands. Among them, the cytotoxic activity of complex 28 was the highest, with IC50 values of 0.507 ± 0.021 μM and 0.235 ± 0.010 μM for the A549 and NCI-H460 cell lines, respectively. Binuclear structures and their cytotoxicity are also shown in complexes 29 and 30 (Scheme 4); IC50 values are at the nanomolar level and 83 times lower than cisplatin. Their cytotoxicity is significantly more enhanced than that of the corresponding ligands (Liu et al., 2016).
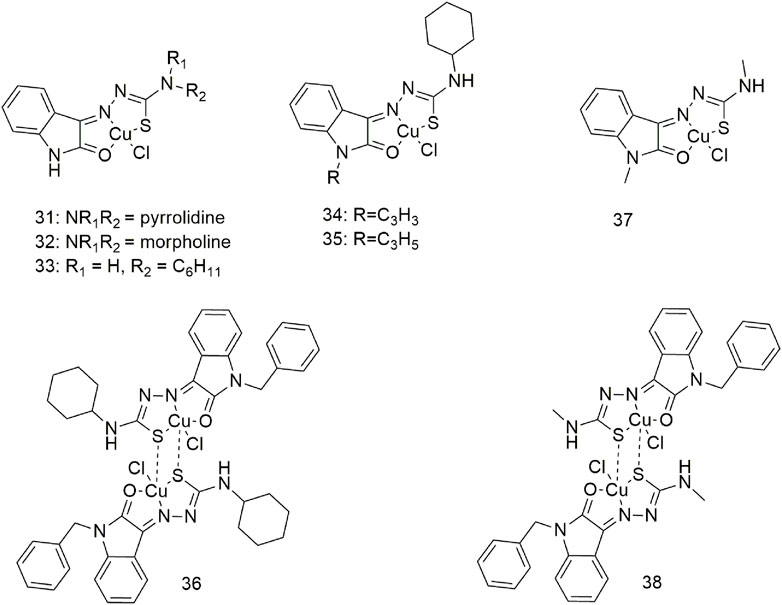
Three complexes (31–33, Scheme 5) with broad-spectrum anti-tumour activity are formed when Cu (II) coordinates with 5-methoxyisatin thiosemicarbazone ligands with different N-terminal substituents; the IC50 value of complex 31 for the A549 cell line is 17.88 ± 0.16 μM (Aneesrahman et al., 2019). Complexes 34–36 act as an intercalating agent toward DNA and as a binding agent toward bovine serum albumin (BSA), (Scheme 5) (Mathiyan et al., 2016). The in vitro cytotoxicity study of complex 36 showed good anti-proliferative activity against the A549 cell line (Mathiyan et al., 2016). Molecular docking studies have shown that complexes 37 and 38 formed hydrogen bonds and hydrophobic interactions with the tyrosinase kinase receptor (Scheme 5) (Haribabu et al., 2021). These complexes can generate morphological changes in the A549 cell line based on fluorescence microscopy and show cytotoxic activity against the A549 cell line with IC50 values of 59.50 and 29.51 µM, respectively.
Thiosemicarbazone ruthenium complexes
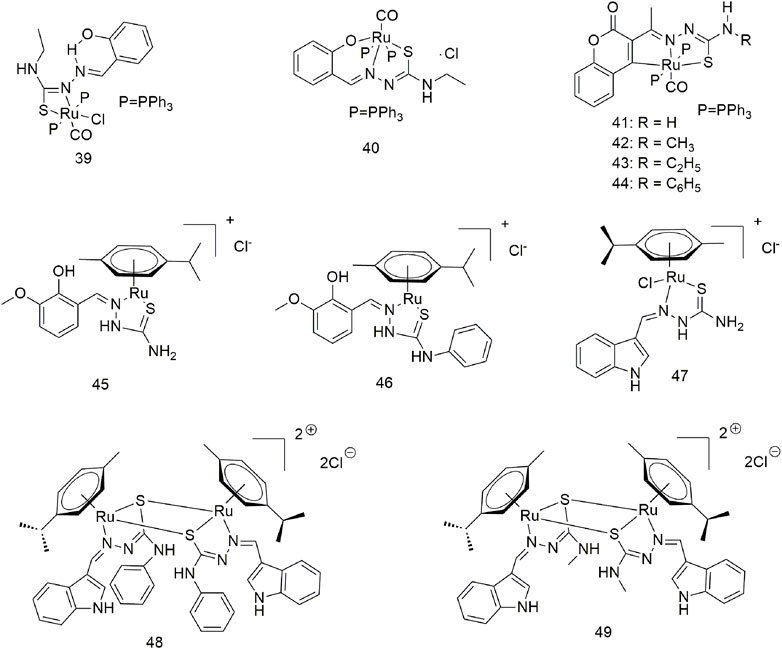
Salicylaldehyde-4-ethylthiosemicarbazide was reacted with [RuHCl(CO) (PPh3)3] to produce complexes 39 and 40 (Scheme 6) (Kalaivani et al., 2014). Complexes 39 (20 ± 1.10 μM) and 40 (17 ± 0.93 μM) showed higher IC50 values for the A549 cell line compared with standard cisplatin (25 ± 1.11 μM). Complexes 39 and 40 are absorbed intracellularly and bind to the protein lysozyme, releasing CO and generating cytotoxicity. Four new divalent organoRu complexes (41–44, Scheme 6) were synthesised by modifying different groups at the N4 position of 3-acetylcoumarin-4-substituted thiosemicarbazide (Kalaiarasi et al., 2018). These complexes are more active than cisplatin and nontoxic to the human normal keratinocyte cell line HaCaT. The activity of complex 43 was optimal due to the N-terminal modification of thiourea with a more electron-donating ethyl group. Anna Gatti et al. reported the structures of two semi-sandwich aromatic Ru (II) thiosemicarbazide complexes (45 and 46, Scheme 6) with remarkable anti-tumour proliferative activity (Gatti et al., 2018). It forms mononuclear (47) and dinuclear complexes (48 and 49) with [RuCl2(p-cymene)]2 in different reactions using indole thiosemicarbazide as a ligand, which is water soluble (Scheme 6) (Haribabu et al., 2018; Haribabu et al., 2021). These complexes exhibit significant cytotoxic activity against the A549 cell line via apoptosis, but not normal cells (L929). Most likely due to its higher DNA/protein binding affinity, binuclear Ru (η6-p-cymene) complex shows better activity than Mono-Ru (η6-p-cymene) complex. Complex 49 (IC50 = 11.5 μM) was significantly more active on the A549 cell line than homogeneous cisplatin (IC50 = 21.3 μM).
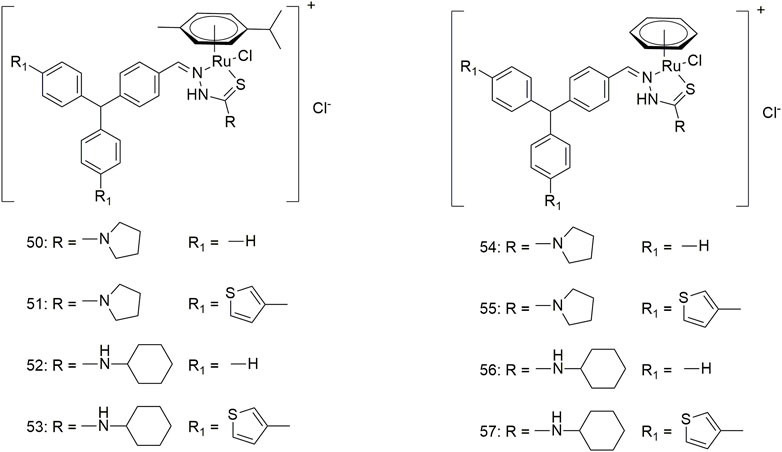
Eight triarylamine-thiosemicarbazides Ru (II)-arene semi-sandwich complexes (50–57, Scheme 7) have been reported to bind DNA/HSA, generating significant cytotoxic activity (Muralisankar et al., 2019). The anti-tumour activity of these Ru (II) complexes on the A549 cell line was significantly different. The IC50 values of complexes 50, 52 and 57 were greater than 100 μM, while complex 54 showed optimal anti-tumour activity (7.24 ± 5.4 μM). Furthermore, the treatment of the A549 cell line with complex 54 (10 μM) for 24 h produced significant changes in cell morphology, nuclear condensation and cell shrinkage, suggesting that cell death occurs via apoptosis under the action of this complex.
Thiosemicarbazone nickel (II) complexes
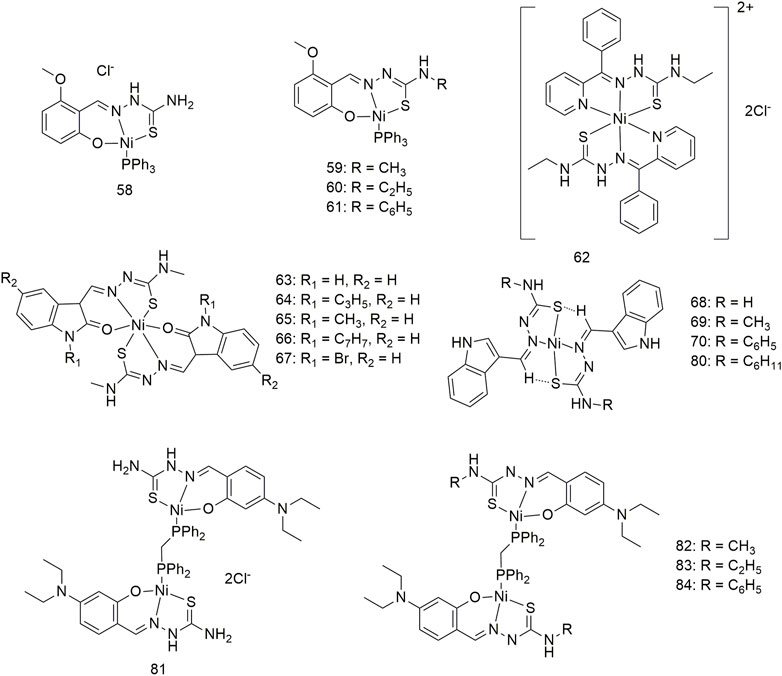
Complexes 58–61 bind to calf thymus (CT)-DNA in an intercalated manner and interact with BSA in a statically quenched manner (Scheme 8) (Kalaivani et al., 2014a). Complexes 58–61 show significant anti-proliferative activity (IC50, 27–30 μM) in the case of the A549 cell line under certain experimental conditions. The anti-tumour mechanisms of these complexes may be ROS-super-generation and lipid peroxidation, resulting in decreased mitochondrial membrane potential, caspase-3 activation and DNA fragmentation. Therefore, complexes 58–61 can cause apoptosis in the A549 cell line in a mitochondria-mediated manner and inhibit the migration and metastasis of lung cancer cells. Additionally, complex 62 interacts with CT-DNA and does not require any external force to cleave the DNA (Scheme 8) (Mathiyan et al., 2016). Complex 62 can bind to BSA and change the secondary structure of the protein. Complex 62 exhibits significant activity against the A549 cell line with an IC50 value of 80.10 μM and is less toxic to the L929 cell line. Complexes 63–67 can interact with CT-DNA and BSA, cleave DNA without external factors and change the secondary structure of proteins (Scheme 8) (Haribabu et al., 2015). These five complexes have significant activity (IC50 < 30 μM) against the A549 cell line, with complex 64 showing an IC50 value of less than 0.1 μM. Complexes 68–80 show high antioxidant activity and antihemolytic activity against 2-2-diphenyl-1-picrylhydrazyl (DPPH) free radicals (Haribabu et al., 2017). The four complexes shows excellent anti-tumour activity (Scheme 8) (IC50, 45.1–57.2 μM) against lung cancer (A549) and low toxicity (IC50 > 600 μM) against mouse embryonic fibroblasts (L929). Intercalation interactions of four binuclear Ni (II) complexes (81–84) with DNA were confirmed by ethylene bromide shift studies and DNA viscosity measurements (Scheme 8) (Kalaiarasi et al., 2020). The interaction mechanism of complexes 81–84 with BSA is static. The four binuclear Ni (II) complexes exhibited significant cytotoxicity against the A549 cell line with lower IC50 values (4.97–6.44 μM) than the standard metal drug cisplatin (IC50, 31.08 ± 0.79 μM).
Thiosemicarbazone platinum (II), gold (I) and silver (I) complexes
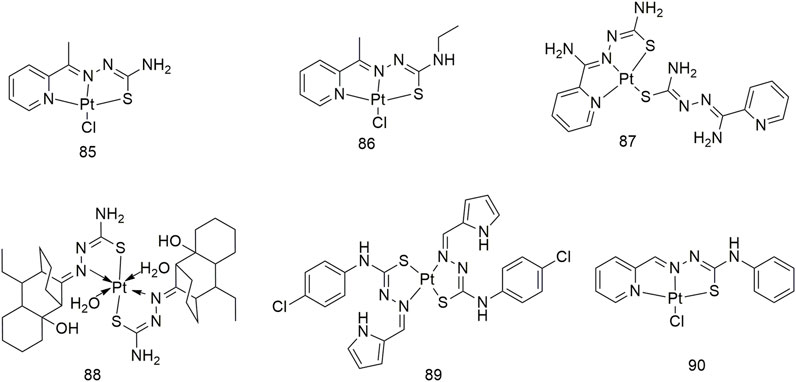
Cisplatin has become a widely used anti-cancer drug in clinics. Current research shows the action mechanism of involves the hydrolysis of cisplatin in the human body, removal of two chlorine atoms and binding to two purines on the genomic DNA and twisting the DNA double helix structure, thereby inhibiting DNA replication and transcription and ultimately causing cancer cell apoptosis (Pahontu et al., 2016a). The quenching mechanism of HSA by three thiosemicarbazide Pt (II) complexes (85–87) may be a static binding mode and impact the microenvironment of tryptophan residues in HSA (Scheme 9) (Lin et al., 2017). These three complexes show significant anti-proliferative activity (IC50, 2.8–9.6 μM) against the NCI-H460 cell line. Among them, the IC50 value of complex 87 toward the NCI-H460 cell line was 2.8 ± 1.1 μM, which was significantly higher than that of cisplatin (IC50, 5.2 ± 2.2 μM). After 48 h of exposure, complex 88 reduced the proliferation rate of NCI-H1573 lung adenocarcinoma cells by 16.46% (Scheme 9) (Pahontu et al., 2016a). Complex 89 is a Pt (II) complex with α-n heterocyclic thiosemicarbazide as ligand, with an IC50 value of 79 ± 4 μM for the A549 cell line after 72 h of incubation (Scheme 9) (Matesanz et al., 2018). The IC50 values of complex 90 against the A549 cell line were 146.6, 101.7 and 69.28, respectively, after 24, 48 and 72 h of treatment (Scheme 9) (Salehi et al., 2022).
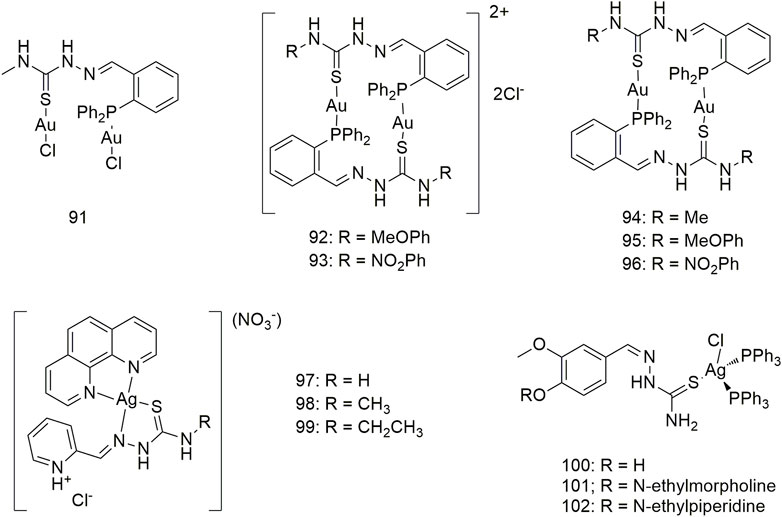
Six phosphothiosemicarbazide gold (Au) (I) binuclear complexes (91–96, Scheme 10) were prepared using synthetic methods. In terms of in vitro cytotoxic activity, the cytotoxic of these Au (I) complexes against the NCI-H460 cell line and MRC5 (normal human lung fibroblasts) was investigated and their IC50 values were compared with that of cisplatin (Lessa et al., 2011). Complexes 91 and 94 strongly inhibited thioredoxin reductase activity. Compounds 97–99 not only exhibited good in vitro anti-proliferative activity against the A549 cell line with IC50 values of 1.49–2.64 μM but also showed less cytotoxicity toward human breast non-tumour cells (MCF-10A) (Silva et al., 2020). The complexes 100–102 showed obvious cytotoxic activity against the A549 cell line, and the IC50 values of 48 h incubation were 7.48 ± 0.21, 8.15 ± 1.21 and 6.46 ± 0.51 μM, respectively, which were higher than the cell activity of cisplatin (IC50, 23.36 ± 0.42 μM) (Scheme 10).
Other classes of thiosemicarbazone metal complexes
Complexes 103 and 104 are two novel pyrazolylthiourea Pd (II) complexes that bind to CT DNA and cleave supercoiled DNA (pUC19) (Scheme 11) (Haribabu et al., 2020). Complexes 103 and 104 show cytotoxic IC50 values of 130.3 and 117.2 μM for the A549 cell line, respectively, in vitro and are less toxic to normal human lung (IMR90) cells (IC50 > 133 μM). Three kinds of clamp-type Pd (II) complexes (105–107, Scheme 11) were synthesised by reacting PdCl2 with thiourea ligands, which can bind to CT-DNA and BSA. Spectroscopic evidence shows an intercalation pattern between DNA and Pd (II) complexes. The binding mode is further confirmed by CD spectroscopy, suggesting that the binding occurs in a non-grooved mode. Changes in the protein secondary structure by the complex were confirmed by simultaneous fluorescence spectroscopy studies. Spectral evidence indicates that the complex shows good binding properties to proteins. Complex 105–107 cleaved pUC19 plasmid DNA without external reagents. These complexes exhibit significant in vitro cytotoxicity against the A549 cell line, suggesting that they can kill cancer cells even at low concentrations. The IC50 of complex 107 toward the A549 cell line is 22.92 μM. Complex 108 significantly inhibits the colony formation and migration abilities of the A549 cell line and can induce apoptosis (Scheme 11) (Ouyang et al., 2017). The anti-cancer activity of complex 108 is much higher than that of its parent ligand, and it generates no obvious toxicity toward non-cancerous human lung fibroblasts (HLF). In vivo experiments proved that complex 108 shows an obvious inhibitory effect on the growth of the A549-xenografted tumour in tumour-bearing mice and has no adverse effects on the mouse body weight and liver. Complex 109 effectively inhibits the proliferation and migration of the A549 and H460 cell lines and induces cell apoptosis (Scheme 11) (Ruizhuo et al., 2016). Complex 109 has IC50 values of 16.41 ± 0.93 μM and 20.04 ± 1.28 μM for the A549 and H460 cell lines, respectively, and demonstrates low cytotoxicity against normal HLF (IC50, 117.16 ± 5.96 μM). These results suggest that thiosemicarbazone complexes with bismuth (Bi) (III) may be an interesting and effective anti-lung cancer drug candidate. The coordination of Bi complexes 110–117 is a thiosemicarbazone containing a quinoline group (Scheme 11) (Scaccaglia et al., 2022). Among them, complexes 113 and 114 show significant inhibitory activities against the A549 cell line with IC50 values of 5.05 ± 1.79 μM and 46.96 ± 16.66 μM, respectively. Complex 111 exhibits significant inhibitory activity against the A549 cell line (IC50, 14.0 ± 1.1 μM) (Abyar et al., 2019). This complex exerts inhibitory activity on the A549 cell line in a cell cycle-dependent manner and induces caspase-mediated apoptosis and caspase-independent cell death. The anti-tumour activity of complex 119 (IC50, 18.9 µM) on the A549 cell line was significantly higher than that of the ligand (IC50, 265.3 µM) (Ming et al., 2015). Through groove binding, two cobalt (II) complexes (120 and 121, Scheme 11) bind to DNA, and the binding capacity of complex 121 is stronger than that of complex 120. Complex 121 shows higher anti-tumour (A549) activity than complex 120. Although the inhibitory activity of complex 121 on A549 cells was inferior to that of cisplatin, its inhibitory efficacy on A549/CDDP in drug-resistant cells was higher than that of cisplatin (Xiaorui et al., 2013).

SCHEME 11. Structures of palladium (II) (103–107), bismuth (III) (108–117), chromium (III) (10–18) and cobalt (II) (119–121)complexes of thiosemicarbazone.
Complexes 122–133 were derived from four thiosemicarbazone Schiff bases (Scheme 12). Except for complex 124, which has no experimental data, and complex 127, which is inert, other complexes demonstrate significant anti-lung cancer (H460) activity with IC50 values less than 3.2 μM (Yusof et al., 2020). Two types of gallium (Ga) (III) complexes (134–137, Scheme 12) with ligand/Ga (III) ratios of 2:1 and 1:1 exhibit significant anti-proliferative activity against the NCI-H460 cell line, and their IC50 values are between 0.72 and 0.43 μM. The anti-proliferative activity of Ga (III) complexes with a metal/ligand ratio of 1:1 (complex 135) was significantly higher than that of the 1:2 (complex 137) (Qi et al., 2017). Complexes 135 and 137 significantly promote the release of cytochrome C from mitochondria and increase the activities of caspase-3 and -9 in the NCI-H460 cell line. Both types of Ga (III) complexes inhibited the G1/S transition better than ligands alone. Five 1:1 ligand/Ga (III) complexes (138–142, Scheme 12) exhibit significant anti-proliferative activity against lung cancer (A549) with IC50 values between 0.37 and 2.32 μM. The structure–activity relationship results showed that modifying the lipophilic groups present on the ligands significantly improves its anti-proliferative activity, and this biological activity was further enhanced after coordination with Ga. The anti-cancer (A549) activities (IC50, 0.46–1.41 μM) of the Ga (III) complexes 143–148 all exceeded those of the corresponding metal-free ligands (Cao et al., 2019). These Ga (III) complexes are non-toxic to normal hepatocytes and exhibit strong selectivity for tumour cells. The anti-tumour (A549) mechanism of Ga (III) complexes produces intracellular ROS, disrupts mitochondrial membrane potential and ultimately promotes apoptosis.
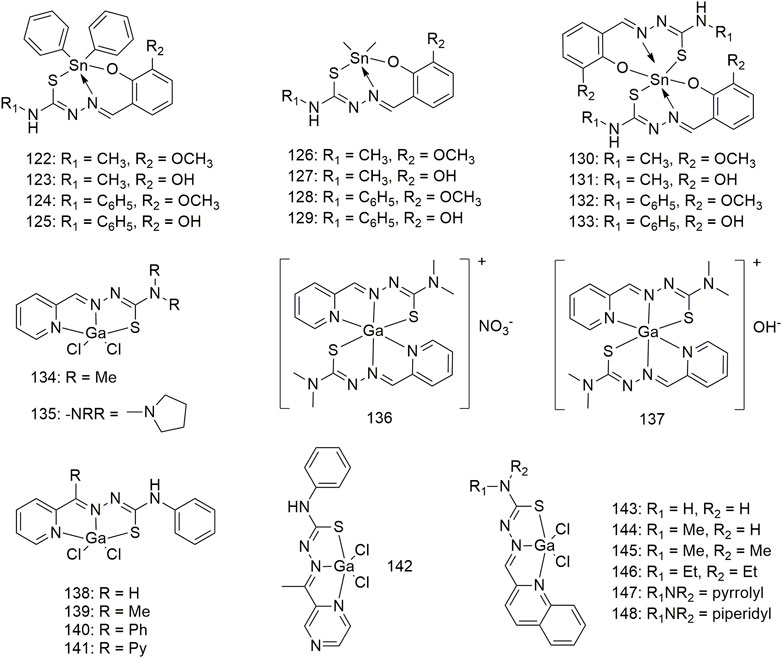
SCHEME 12. Structures of tin (II) (122–133) and gallium (III) (134–148) complexes of thiosemicarbazone.
Copper, nickel and zinc complexes of the same ligand
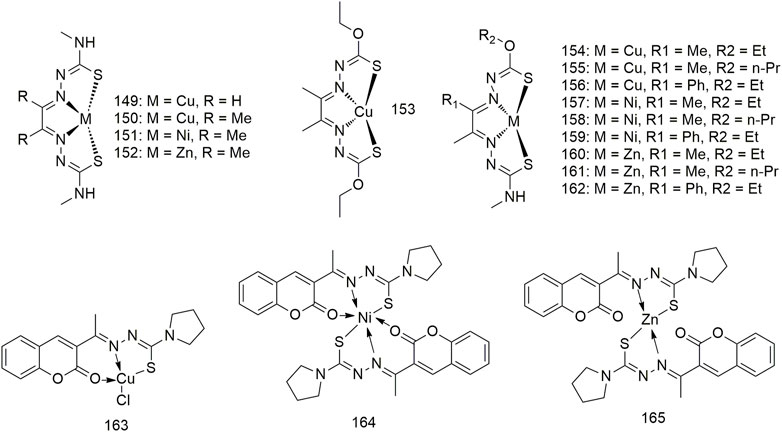
A series of hybrid ligands derived from 4-methyl-3-thiosemicarbazide and hydrazinecarbothioic acid O-alkyl ester ligands chelate with Cu, Ni and zinc (Zn) to form complexes 149–162 (Scheme 13) (Andres et al., 2020). Cu derivatives 154 and 156 exhibit strong anti-cancer selectivity, having GI50 values of less than 100 nM against the A549 lung adenocarcinoma cells, and at least 20-fold lower GI50 activity against IMR90 (>2.0 μM) non-malignant lung fibroblasts. While Ni complexes exhibit much lower anti-tumour activity (>5.3 μM) compared with Cu complexes, Zn complexes show weaker activity (0.055–7.6 μM). Zn and Ni complexes demonstrate little cancer selectivity. The complexes Cu (II) (163), Ni (II) (164) and Zn (II) (165) interacts with CT-DNA via intercalation binding as well as with BSA (Rahman et al., 2017). The activity of these complexes on the A549 cell line was lower than in the other cell lines, in which complex 165 exhibits moderate cytotoxicity against the A549 cell line with an IC50 value of 200 μg/ml.
Discussion
Recently, the increase in the number of patients with lung cancer and the emergence of tumour resistance, the demand for metal compounds that treat cancer continues to grow, not only because cancer is difficult to cure but also because metal-based compounds with remarkable antitumor activity. Metal-based compounds show high cytotoxicity in vitro. Furthermore, the substitution of ligands and modification of existing chemical structures have produced a variety of metal-based compounds, some of which show higher tumour cytotoxicity, low toxicity toward non-tumour cells and better pharmacokinetics.
Metal complexes, such as Cu, Zn, Au, silver (Ag), Pt and Ru, exhibit higher anti-tumour activity than metal-free ligands. Cancer cells have a greater demand for Cu than normal cells, and Cu metabolism is strongly linked to angiogenesis and metastasis (Santini et al., 2014; Shobha et al., 2018; Kannappan et al., 2021). Therefore, since the 1970s, various ligands that form complexes with Cu, such as thiosemicarbazides, imidazoles and phosphines, have been proposed as potential anti-cancer drugs. Furthermore, Cu salts and complexes have been shown to inhibit tumour cell proliferation (Santini et al., 2014). In fact, these Cu complexes allow Cu to undergo redox cycling between the reduced monovalent and oxidised divalent states under the action of cellular oxidants and reductants (Balsa et al., 2021). Cu complexes show redox activity, engender intracellular ROS overload and promote apoptosis through the mitochondrial apoptotic pathway. Many ruthenium (Ru) complexes have been synthesised and evaluated as possible cancer therapeutics over the past two decades (Gatti et al., 2018). The activity of Ru (III) compounds depends on in vivo reduction to more reactive Ru (II) substances, which has led to increased interest in organometallic Ru (II) arene complexes in octahedral configurations, where aromatics stabilise Ru in Ru oxidation states (Haribabu et al., 2018). Some semi-sandwiched Ru (II) arene complexes exhibit notable anti-cancer activity in vitro and in vivo (Gatti et al., 2018). The activity of Ru complex cells is significantly higher than that of cisplatin and the former shows tumour selectivity. This may be due to the good DNA/protein binding affinity of the Ru complex. Ni (II) complexes exhibit obvious inhibitory activity on the A549 cell line and less toxicity on the L929 cell line. Ni (II) complexes interact with CT-DNA and BSA, cleave DNA on the absence of any external factors and change the secondary structure of protein. Nickel can form rich molecular geometries, ensuring the formation of complexes with improved properties (Bisceglie et al., 2019). This facilitates improved drug properties without any increase in cellular resistance or negative side effects from the drugs. Thiosemicarbazone Pt (II), Au (I) and Ag (I) complexes were significantly more active on lung cancer cells than cisplatin. Owing to their similar structural characteristics and coordination chemistry with Pt, palladium (Pd) complexes have been thoroughly investigated for anti-cancer activity similar to that of their Pt analogues. These metal complexes have complicated and diverse action mechanisms and anti-tumour activities, which compensate for the deficiencies in the existing drugs. However, these studies are only at the basic research stage. Overall, a rational design of anti-tumour drugs based on other metal classes is yet to be achieved, and there is a lack of unique metal complexes. Although thiosemicarbazone-based metal complexes have been used in the laboratory and have exhibited good outcomes on lung cancer cells and tumour-bearing nude mice, achieving a clear distinction between therapeutic and toxic doses is a major challenge.
Author contributions
X-GB, YZ, and JQ contributed to conceptualizing, writing the original draft manuscript, and reviewing and editing the manuscript.
Funding
This work was supported by the Henan Engineering Technology Research Center of Funiu Mountain Medicinal Resource Utilization and Molecular Medicine, Key Science and Technology Research Projects in Henan Provincial (No. 202102310476), Key scientific research project of henan Province (22A530005), Youth Fund of Pingdingshan College (No. PXY-QNJJ-202110) and Pingdingshan College PhD Startup Fund under Grant (No. PXY-BSQD-202003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abyar, S., Khandar, A. A., Salehi, R., Abolfazl, H. S., Alizadeh, E., Mahkam, M., et al. (2019). In vitro nephrotoxicity and anticancer potency of newly synthesized cadmium complexes. Sci. Rep. 9 (1), 14686. doi:10.1038/s41598-019-51109-9
Andres, S. A., Bajaj, K., Vishnosky, N. S., Peterson, M. A., Mashuta, M. S., Buchanan, R. M., et al. (2020). Synthesis, characterization, and biological activity of hybrid thiosemicarbazone-alkylthiocarbamate metal complexes. Inorg. Chem. 59 (7), 4924–4935. doi:10.1021/acs.inorgchem.0c00182
Aneesrahman, K. N., Ramaiah, K., Rohini, G., Stefy, G. P., Bhuvanesh, N. S. P., and Sreekanth, A. (2019). Synthesis and characterisations of copper (II) complexes of 5-methoxyisatin thiosemicarbazones: Effect of N-terminal substitution on DNA/protein binding and biological activities. Inorganica Chim. Acta 492, 131–141. doi:10.1016/j.ica.2019.04.019
Balsa, L. M., Baran, E. J., and Leon, I. E. (2021). Copper complexes as antitumor agents: In vitro and in vivo evidences. Curr. Med. Chem. 28. E-pub ahead of print. doi:10.2174/0929867328666211117094550
Barrera-Rodriguez, R., and Fuentes, J. M. (2015). Multidrug resistance characterization in multicellular tumour spheroids from two human lung cancer cell lines. Cancer Cell Int. 15, 47. doi:10.1186/s12935-015-0200-6
Bisceglie, F., Orsoni, N., Pioli, M., Bonati, B., Tarasconi, P., Rivetti, C., et al. (2019). Cytotoxic activity of copper(ii), nickel(ii) and platinum(ii) thiosemicarbazone derivatives: Interaction with DNA and the H2A histone peptide. Metallomics 11 (10), 1729–1742. doi:10.1039/c9mt00166b
Cao, W., Qi, J., Qian, K., Tian, L., Cheng, Z., and Wang, Y. (2019). Structure-activity relationships of 2quinolinecarboxaldehyde thiosemicarbazone gallium(III) complexes with potent and selective anticancer activity. J. Inorg. Biochem. 191, 174–182. doi:10.1016/j.jinorgbio.2018.11.017
de Siqueira, L., de Moraes, G. P., de Lima, F. L., de Melo, R. M., and Leite, A. (2019a). Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 170, 237–260. doi:10.1016/j.ejmech.2019.03.024
Dharmasivam, M., Azad, M. G., Afroz, R., Richardson, V., Jansson, P. J., and Richardson, D. R. (2022). The thiosemicarbazone, DpC, broadly synergizes with multiple anti-cancer therapeutics and demonstrates temperature- and energy-dependent uptake by tumor cells. Biochim. Biophys. Acta. Gen. Subj. 1866 (8), 130152. doi:10.1016/j.bbagen.2022.130152
Gatti, A., Habtemariam, A., Romero-Canelon, I., Song, J. I., Heer, B., Clarkson, G. J., et al. (2018). Half-sandwich arene ruthenium(II) and osmium(II) thiosemicarbazone complexes: Solution behavior and antiproliferative activity. Organometallics 37 (6), 891–899. doi:10.1021/acs.organomet.7b00875
Gu, S., Yu, P., Hu, J., Liu, Y., Li, Z., Qian, Y., et al. (2019). Mitochondria-localizing N-heterocyclic thiosemicarbazone copper complexes with good cytotoxicity and high antimetastatic activity. Eur. J. Med. Chem. 164, 654–664. doi:10.1016/j.ejmech.2019.01.014
Haribabu, J., Alajrawy, O. I., Jeyalakshmi, K., Balachandran, C., Krishnan, D. A., Bhuvanesh, N., et al. (2021). N-substitution in isatin thiosemicarbazones decides nuclearity of Cu(II) complexes - spectroscopic, molecular docking and cytotoxic studies. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 246, 118963. doi:10.1016/j.saa.2020.118963
Haribabu, J., Balachandran, C., Tamizh, M. M., Arun, Y., Bhuvanesh, N., Aoki, S., et al. (2020). Unprecedented formation of palladium(II)-pyrazole based thiourea from chromone thiosemicarbazone and [PdCl2(PPh3)2]: Interaction with biomolecules and apoptosis through mitochondrial signaling pathway. J. Inorg. Biochem. 205, 110988. doi:10.1016/j.jinorgbio.2019.110988
Haribabu, J., Jeyalakshmi, K., Arun, Y., Bhuvanesh, N., Perumal, P. T., and Karvembu, R. (2017). Synthesis of Ni(II) complexes bearing indole-based thiosemicarbazone ligands for interaction with biomolecules and some biological applications. J. Biol. Inorg. Chem. 22 (4), 461–480. doi:10.1007/s00775-016-1424-1
Haribabu, J., Jeyalakshmi, K., Arun, Y., Bhuvanesh, N. S. P., Perumal, P. T., and Karvembu, R. (2015). Synthesis, DNA/protein binding, molecular docking, DNA cleavage and in vitro anticancer activity of nickel(II) bis(thiosemicarbazone) complexes. RSC Adv. 5 (57), 46031–46049. doi:10.1039/C5RA04498G
Haribabu, J., Sabapathi, G., Tamizh, M. M., Balachandran, C., Bhuvanesh, N. S. P., Venuvanalingam, P., et al. (2018). Water-soluble Mono- and binuclear Ru(η6-p-cymene) complexes containing indole thiosemicarbazones: Synthesis, DFT modeling, biomolecular interactions, and in vitro anticancer activity through apoptosis. Organometallics 37 (8), 1242–1257. doi:10.1021/acs.organomet.8b00004
Kalaiarasi, G., Dharani, S., Rajkumar, S., Lynch, V. M., and Prabhakaran, R. (2020). Binuclear Ni(II) complexes containing ONS donor Schiff base ligands: Preparation, spectral characterization, X-ray crystallography and biological exploration. J. Inorg. Biochem. 211, 111176. doi:10.1016/j.jinorgbio.2020.111176
Kalaiarasi, G., Rajkumar, S. R. J., Dharani, S., Fronczek, F. R., Muthukumar, N. M. S. A., and Prabhakaran, R. (2018). Cyclometallated ruthenium(ii) complexes with 3-acetyl-2[H]-chromene-2-one derived CNS chelating ligand systems: Synthesis, X-ray characterization and biological evaluation. New J. Chem. 42 (1), 336–354. doi:10.1039/C7NJ02877F
Kalaivani, P., Prabhakaran, R., Dallemer, F., Vaishnavi, E., Poornima, P., Padma, V. V., et al. (2014a). Synthesis, structural characterization, DNA/Protein binding and in vitro cytotoxicity of isomeric ruthenium carbonyl complexes. J. Organomet. Chem. 762, 67–80. doi:10.1016/j.jorganchem.2014.04.003
Kalaivani, P., Saranya, S., Poornima, P., Prabhakaran, R., Dallemer, F., Vijaya, P. V., et al. (2014). Biological evaluation of new nickel(II) metallates: Synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur. J. Med. Chem. 82, 584–599. doi:10.1016/j.ejmech.2014.05.075
Kannappan, V., Ali, M., Small, B., Rajendran, G., Elzhenni, S., Taj, H., et al. (2021). Recent advances in repurposing disulfiram and disulfiram derivatives as copper-dependent anticancer agents. Front. Mol. Biosci. 8, 741316. doi:10.3389/fmolb.2021.741316
Kowol, C. R., Trondl, R., Heffeter, P., Arion, V. B., Jakupec, M. A., Roller, A., et al. (2009). Impact of metal coordination on cytotoxicity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (triapine) and novel insights into terminal dimethylation. J. Med. Chem. 52 (16), 5032–5043. doi:10.1021/jm900528d
Lessa, J. A., Guerra, J. C., de Miranda, L. F., Romeiro, C. F., Da, S. J., Mendes, I. C., et al. (2011). Gold(I) complexes with thiosemicarbazones: Cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. J. Inorg. Biochem. 105 (12), 1729–1739. doi:10.1016/j.jinorgbio.2011.09.008
Lin, X. D., Liu, Y. H., Xie, C. Z., Bao, W. G., Shen, J., and Xu, J. Y. (2017). Three Pt(ii) complexes based on thiosemicarbazone: Synthesis, HSA interaction, cytotoxicity, apoptosis and cell cycle arrest. RSC Adv. 7 (42), 26478–26486. doi:10.1039/C7RA04443G
Liu, Y. H., Li, A., Shao, J., Xie, C. Z., Song, X. Q., Bao, W. G., et al. (2016). Four Cu(ii) complexes based on antitumor chelators: Synthesis, structure, DNA binding/damage, HSA interaction and enhanced cytotoxicity. Dalton Trans. 45 (19), 8036–8049. doi:10.1039/c6dt00451b
Lovejoy, D. B., Sharp, D. M., Seebacher, N., Obeidy, P., Prichard, T., Stefani, C., et al. (2012). Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J. Med. Chem. 55 (16), 7230–7244. doi:10.1021/jm300768u
Mahale, S., Bharate, S. B., Manda, S., Joshi, P., Jenkins, P. R., Vishwakarma, R. A., et al. (2015). Antitumour potential of BPT: A dual inhibitor of cdk4 and tubulin polymerization. Cell Death Dis. 6, e1743. doi:10.1038/cddis.2015.96
Malarz, K., Mrozek-Wilczkiewicz, A., Serda, M., Rejmund, M., Polanski, J., and Musiol, R. (2018). The role of oxidative stress in activity of anticancer thiosemicarbazones. Oncotarget 9 (25), 17689–17710. doi:10.18632/oncotarget.24844
Maqbool, S. N., Lim, S. C., Park, K. C., Hanif, R., Richardson, D. R., Jansson, P. J., et al. (2020). Overcoming tamoxifen resistance in oestrogen receptor-positive breast cancer using the novel thiosemicarbazone anti-cancer agent, DpC. Br. J. Pharmacol. 177 (10), 2365–2380. doi:10.1111/bph.14985
Matesanz, A. I., Herrero, J. M., and Quiroga, A. G. (2021a). Chemical and biological evaluation of thiosemicarbazone-bearing heterocyclic metal complexes. Curr. Top. Med. Chem. 21 (1), 59–72. doi:10.2174/1568026620666201022144004
Matesanz, A. I., Jimenez, F. E., Ruiz, M. C., Balsa, L. M., Navarro, R. C., León, I. E., et al. (2018). Mononuclear Pd(ii) and Pt(ii) complexes with an α-N-heterocyclic thiosemicarbazone: Cytotoxicity, solution behaviour and interaction versus proven models from biological media. Inorg. Chem. Front. 5 (1), 73–83. doi:10.1039/C7QI00446J
Mathiyan, M., Jebiti, H., Nattamai, S. P. B., Ramasamy, K., and Anandaram, S. (2016). Synthesis, X-ray crystal structure, DNA/protein binding, DNA cleavage and cytotoxicity studies of N(4) substituted thiosemicarbazone based copper(II)/nickel(II) complexes. Inorg. Chim. Acta. 449, 82–95. doi:10.1016/j.ica.2016.04.043
Mckenzie-Nickson, S., Bush, A. I., and Barnham, K. J. (2016). Bis(thiosemicarbazone) metal complexes as therapeutics for neurodegenerative diseases. Curr. Top. Med. Chem. 16 (27), 3058–3068. doi:10.2174/1568026616666160216155746
Ming, X. L., Jing, Z., Zi, L. W., and Jing, P. W. (2015). Synthesis, crystal structure and antitumor study of a cobalt(II) complex of the 2-acetylpyrazine thiosemicarbazone. Z. für Naturforsch. B 63 (1), 1–5. doi:10.1515/znb-2008-0101
Muralisankar, M., Dheepika, R., Haribabu, J., Balachandran, C., Aoki, S., Bhuvanesh, N., et al. (2019). Design, synthesis, DNA/HSA binding, and cytotoxic activity of half-sandwich Ru(II)-Arene complexes containing triarylamine-thiosemicarbazone hybrids. ACS Omega 4 (7), 11712–11723. doi:10.1021/acsomega.9b01022
Onodera, K., Kasuga, N. C., Takashima, T., Hara, A., Amano, A., Murakami, H., et al. (2007). Synthesis, reaction and structure of a highly light-stable silver(I) cluster with an Ag4S4N4 core having a tridentate 4N-morpholyl 2-acetylpyridine thiosemicarbazone ligand: Use of water-soluble silver(I) carboxylates as a silver(I) source. Dalton Trans. 33, 3646–3652. doi:10.1039/b702427d
Ouyang, R., Yang, Y., Tong, X., Feng, K., Yang, Y., Tao, H., et al. (2017). Potent anticancer activity of a new bismuth (III) complex against human lung cancer cells. J. Inorg. Biochem. 168, 18–26. doi:10.1016/j.jinorgbio.2016.12.006
Pahontu, E., Paraschivescu, C., Ilies, D. C., Poirier, D., Oprean, C., Paunescu, V., et al. (2016a). Synthesis and characterization of novel Cu(II), Pd(II) and Pt(II) complexes with 8-Ethyl-2-hydroxytricyclo(7.3.1.0(2, 7))tridecan-13-one-thiosemicarbazone: Antimicrobial and in vitro antiproliferative activity. Molecules 21 (5), E674. doi:10.3390/molecules21050674
Qi, J., Deng, J., Qian, K., Tian, L., Li, J., He, K., et al. (2017). Novel 2-pyridinecarboxaldehyde thiosemicarbazones Ga(III) complexes with a high antiproliferative activity by promoting apoptosis and inhibiting cell cycle. Eur. J. Med. Chem. 134, 34–42. doi:10.1016/j.ejmech.2017.04.009
Qi, J., Liang, S., Gou, Y., Zhang, Z., Zhou, Z., Yang, F., et al. (2015). Synthesis of four binuclear copper(II) complexes: Structure, anticancer properties and anticancer mechanism. Eur. J. Med. Chem. 96, 360–368. doi:10.1016/j.ejmech.2015.04.031
Qi, J., Wang, X., Liu, T., Kandawa, S. M., Wang, Y., and Zheng, X. (2020). Synthesis, antiproliferative activity and mechanism of copper(II)-thiosemicarbazone complexes as potential anticancer and antimicrobial agents. J. Coord. Chem. 73 (7), 1208–1221. doi:10.1080/00958972.2020.1768378
Qi, J., Yao, Q., Tian, L., and Wang, Y. (2018). Piperidylthiosemicarbazones Cu(II) complexes with a high anticancer activity by catalyzing hydrogen peroxide to degrade DNA and promote apoptosis. Eur. J. Med. Chem. 158, 853–862. doi:10.1016/j.ejmech.2018.09.034
Qi, J., Zhang, Y., Gou, Y., Zhang, Z., Zhou, Z., Wu, X., et al. (2016). Developing an anticancer copper(II) pro-drug based on the His242 residue of the human serum albumin carrier IIA subdomain. Mol. Pharm. 13 (5), 1501–1507. doi:10.1021/acs.molpharmaceut.5b00938
Rahman, K. A., Haribabu, J., Balachandran, C., Bhuvanesh, N. S., Karvembu, R., and Sreekanth, A. (2017). Copper, nickel and zinc complexes of 3-acetyl coumarin thiosemicarbazone: Synthesis, characterization and in vitro evaluation of cytotoxicity and DNA/protein binding properties. Polyhedron 135, 26–35. doi:10.1016/j.poly.2017.06.044
Rao, V. A., Klein, S. R., Agama, K. K., Toyoda, E., Adachi, N., Pommier, Y., et al. (2009). The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res. 69 (3), 948–957. doi:10.1158/0008-5472.CAN-08-1437
Rostan, S., Mahler, G., and Otero, L. (2021). Selenosemicarbazone metal complexes as potential metal-based drugs. Curr. Med. Chem. 29. E-pub ahead of print. doi:10.2174/0929867329666211222115035
Ruizhuo, O., Yang, Y., Xiao, T., Yaoqin, Y., Huihong, T., Tianyu, Z., et al. (2016). Potential anti-cancer activity of a novel Bi(III) containing thiosemicarbazone derivative. Inorg. Chem. Commun. 73, 138–141. doi:10.1016/j.inoche.2016.10.020
Salehi, R., Abyar, S., Ramazani, F., Khandar, A. A., Hosseini-Yazdi, S. A., White, J. M., et al. (2022). Enhanced anticancer potency with reduced nephrotoxicity of newly synthesized platin-based complexes compared with cisplatin. Sci. Rep. 12 (1), 8316. doi:10.1038/s41598-022-11904-3
Santini, C., Pellei, M., Gandin, V., Porchia, M., Tisato, F., and Marzano, C. (2014). Advances in copper complexes as anticancer agents. Chem. Rev. 114 (1), 815–862. doi:10.1021/cr400135x
Scaccaglia, M., Rega, M., Bacci, C., Giovanardi, D., Pinelli, S., Pelosi, G., et al. (2022). Bismuth complex of quinoline thiosemicarbazone restores carbapenem sensitivity in NDM-1-positive Klebsiella pneumoniae. J. Inorg. Biochem. 234, 111887. doi:10.1016/j.jinorgbio.2022.111887
Scarim, C. B., and Chin, C. M. (2022a). Recent trends in drug development for the treatment of adenocarcinoma breast cancer: Thiazole, triazole, and thiosemicarbazone analogues as efficient scaffolds. Anticancer. Agents Med. Chem. 22 (12), 2204–2240. doi:10.2174/1871520621666211201152815
Serda, M., Kalinowski, D. S., Rasko, N., Potuckova, E., Mrozek-Wilczkiewicz, A., Musiol, R., et al. (2014). Exploring the anti-cancer activity of novel thiosemicarbazones generated through the combination of retro-fragments: Dissection of critical structure-activity relationships. PLoS One 9 (10), e110291. doi:10.1371/journal.pone.0110291
Shobha, D. C., Thulasiram, B., Aerva, R. R., and Nagababu, P. (2018). Recent advances in copper intercalators as anticancer agents. J. Fluoresc. 28 (5), 1195–1205. doi:10.1007/s10895-018-2283-7
Silva, D., Becceneri, A. B., Santiago, J., Gomes, N. J., Ellena, J., Cominetti, M. R., et al. (2020). Silver(I) complexes of 3-methoxy-4-hydroxybenzaldehyde thiosemicarbazones and triphenylphosphine: Structural, cytotoxicity, and apoptotic studies. Dalton Trans. 49 (45), 16474–16487. doi:10.1039/d0dt01134g
Stariat, J., Kovarikova, P., Kucera, R., Klimes, J., Kalinowski, D. S., Richardson, D. R., et al. (2013). Identification of in vitro metabolites of the novel anti-tumor thiosemicarbazone, DpC, using ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Anal. Bioanal. Chem. 405 (5), 1651–1661. doi:10.1007/s00216-012-6562-x
Stefani, C., Jansson, P. J., Gutierrez, E., Bernhardt, P. V., Richardson, D. R., and Kalinowski, D. S. (2013). Alkyl substituted 2'-benzoylpyridine thiosemicarbazone chelators with potent and selective anti-neoplastic activity: Novel ligands that limit methemoglobin formation. J. Med. Chem. 56 (1), 357–370. doi:10.1021/jm301691s
Summers, K. L. (2019a). A structural chemistry perspective on the antimalarial properties of thiosemicarbazone metal complexes. Mini Rev. Med. Chem. 19 (7), 569–590. doi:10.2174/1389557518666181015152657
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tsai, H. I., Jiang, L., Zeng, X., Chen, H., Li, Z., Cheng, W., et al. (2018). DACHPt-loaded nanoparticles self-assembled from biodegradable dendritic copolymer polyglutamic acid-b-D-alpha-tocopheryl polyethylene glycol 1000 succinate for multidrug resistant lung cancer therapy. Front. Pharmacol. 9, 119. doi:10.3389/fphar.2018.00119
Wang, J., Gou, Y., Zhang, Z., Yu, P., Qi, J., Qin, Q., et al. (2018). Developing an anticancer copper(II) multitarget pro-drug based on the His146 residue in the IB subdomain of modified human serum albumin. Mol. Pharm. 15 (6), 2180–2193. doi:10.1021/acs.molpharmaceut.8b00045
Wangpu, X., Lu, J., Xi, R., Yue, F., Sahni, S., Park, K. C., et al. (2016). Targeting the metastasis suppressor, N-myc downstream regulated gene-1, with novel di-2-pyridylketone thiosemicarbazones: Suppression of tumor cell migration and cell-collagen adhesion by inhibiting focal adhesion kinase/paxillin signaling. Mol. Pharmacol. 89 (5), 521–540. doi:10.1124/mol.115.103044
Wu, S., Guo, W., Teraishi, F., Pang, J., Kaluarachchi, K., Zhang, L., et al. (2006). Anticancer activity of 5-benzylidene-2-phenylimino-1, 3-thiazolidin-4-one (BPT) analogs. Med. Chem. 2 (6), 597–605. doi:10.2174/1573406410602060597
Xiaorui, F., Juanjuan, D., Rui, M., Yun, C., Xiaoyi, Y., Jianliang, Z., et al. (2013). Cobalt(II) complexes with thiosemicarbazone as potential antitumor agents: Synthesis, crystal structures, DNA interactions, and cytotoxicity. J. Coord. Chem. 66 (24), 4268–4279. doi:10.1080/00958972.2013.867030
Yu, Y., Kalinowski, D. S., Kovacevic, Z., Siafakas, A. R., Jansson, P. J., Stefani, C., et al. (2009). Thiosemicarbazones from the old to new: Iron chelators that are more than just ribonucleotide reductase inhibitors. J. Med. Chem. 52 (17), 5271–5294. doi:10.1021/jm900552r
Yu, Y., Suryo, R. Y., Hawkins, C. L., and Richardson, D. R. (2011). The potent and novel thiosemicarbazone chelators di-2-pyridylketone-4, 4-dimethyl-3-thiosemicarbazone and 2-benzoylpyridine-4, 4-dimethyl-3-thiosemicarbazone affect crucial thiol systems required for ribonucleotide reductase activity. Mol. Pharmacol. 79 (6), 921–931. doi:10.1124/mol.111.071324
Yu, Y., Wong, J., Lovejoy, D. B., Kalinowski, D. S., and Richardson, D. R. (2006). Chelators at the cancer coalface: Desferrioxamine to triapine and beyond. Clin. Cancer Res. 12 (23), 6876–6883. doi:10.1158/1078-0432.CCR-06-1954
Yusof, E. N. M., Page, A. J., Sakoff, J. A., Simone, M. I., Veerakumarasivam, A., Tiekink, E. R., et al. (2020). Tin(IV) compounds of tridentate thiosemicarbazone Schiff bases: Synthesis, characterization, in-silico analysis and in vitro cytotoxicity. Polyhedron 189, 114729. doi:10.1016/j.poly.2020.114729
Zhao, Y., Guo, C., Wang, L., Wang, S., Li, X., Jiang, B., et al. (2017). A novel fluorinated thiosemicarbazone derivative- 2-(3, 4-difluorobenzylidene) hydrazinecarbothioamide induces apoptosis in human A549 lung cancer cells via ROS-mediated mitochondria-dependent pathway. Biochem. Biophys. Res. Commun. 491 (1), 65–71. doi:10.1016/j.bbrc.2017.07.042
Keywords: thiosemicarbazone, schiff base, metal complexes, anti-lung cancer, chemotherapy
Citation: Bai X-G, Zheng Y and Qi J (2022) Advances in thiosemicarbazone metal complexes as anti-lung cancer agents. Front. Pharmacol. 13:1018951. doi: 10.3389/fphar.2022.1018951
Received: 14 August 2022; Accepted: 13 September 2022;
Published: 27 September 2022.
Edited by:
Yi Gou, Guilin Medical University, ChinaReviewed by:
Ruo Wang, Shanghai Jiao Tong University, ChinaRobert Musiol, University of Silesia in Katowice, Poland
Copyright © 2022 Bai, Zheng and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunyun Zheng, eXVueXVuemhlbmdAcGRzdS5lZHUuY24=; Jinxu Qi, amlueHVxaUBwZHN1LmVkdS5jbg==
 Xian-Guang Bai
Xian-Guang Bai Yunyun Zheng
Yunyun Zheng Jinxu Qi
Jinxu Qi