- Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Postoperative urinary retention (POUR) is a common and disruptive complication following total joint arthroplasty (TJA). The aim of this study is to investigate whether doxazosin can decrease the incidence of POUR and promote recovery under the setting of modern enhanced recovery after TJA.
Methods: In this randomized placebo-controlled trial, patients over 35 years of age undergoing primary unilateral TJA were recruited. Patients received doxazosin (4 mg once) or placebo 2 h before surgery. The primary outcome of interest was the development of POUR, which was diagnosed when patients with a urine volume over 400 ml or overflow incontinence. Postoperative recovery was assessed in terms of hospital length of stay after surgery, daily ambulation distance, visual analogue scale (VAS) pain score and opioid consumption.
Results: A total of 170 male patients were equally randomized into Doxazosin group (mean age 54.2 ± 13.7 years, range 36–88 years) and Placebo group (mean age 54.6 ± 13.9 years, range 38–81 years). The POUR rate was significant lower in Doxazosin group (17.6%) than in Placebo group (36.5%) (p = .006). The mean LOS in the Doxazosin group was 3.1 ± 1.1 days compared to 3.6 ± 1.7 days in the Placebo group (p = .030). Doxazosin group had a longer daily mobilization distance than Placebo group on postoperative day 1 (26.8 ± 11.1 vs. 22.8 ± 9.7; p = .015). Postoperative pain assessed by VAS score and opioid usage was comparable between two groups.
Conclusion: Our results support the routine use of prophylactic doxazosin in male patients to decrease POUR rate and promote postoperative recovery under the setting of modern enhanced recovery after TJA.
Introduction
Postoperative urinary retention (POUR) is common following the total joint arthroplasty (TJA), with reported rates ranging from 20.0% to 46.3% in the literatures (Bjerregaard et al., 2015; Scholten et al., 2018; Schubert et al., 2019; Bracey et al., 2021). As a disruptive complication, POUR can lead to overdistension of the bladder in 44% of cases, which may ultimately result in urologic injury and infection (Baldini et al., 2009). Besides, POUR may lead to the increase in urea and creatinine, which can even result in serious cardiovascular problems, especially in the elderly population. Since indwelling or intermittent urinary catheterization is the only effective management for POUR, the risk of catheter-related complications, including urinary tract infection, bacteremia and even periprosthetic joint infection, can be further increased (Pulido et al., 2008; Nicolle, 2014). Besides, POUR and urinary catheterization are risk factors of increased postoperative pain, reduced early mobilization and prolonged hospital length of stay (LOS) (Pivec et al., 2021; Bracey et al., 2022). POUR severely affects the postoperative recovery after TJA, which is unacceptable for both patients and surgeons under the current setting of modern enhanced recovery after TJA.
Although POUR is far from a new phenomenon and its importance has been entirely highlighted, very few approaches have developed to mitigate its occurrence (Cha et al., 2020; Bracey et al., 2022). Pharmacologic intervention has been considered to be the most effective and direct way to prevent POUR and reduce the use of catheterization after surgery. Alpha adrenoreceptor blockers, such as tamsulosin, doxazosin and prazosin, are the first-line drugs for the lower urinary tract symptoms treatment. These medications can decrease the resistance of the urethra by decreasing the tension of smooth muscle in the prostate and bladder neck, which are rich in alpha1-adrenergic receptors. As a result, alpha adrenoreceptor blockers are proposed to reduce the incidence of POUR after TJA (Balderi and Carli, 2010).
A retrospective study recruiting 559 TJA male patients found that patients who received alpha adrenoreceptor blockers perioperatively had significantly lower risk of POUR and reduced LOS (Schubert et al., 2020). Though some randomized controlled trials have compared the efficacy of tamsulosin and placebo to prevent POUR after TJA, the results seemed controversial (Schubert et al., 2019; Choi et al., 2021). Schubert et al. found no difference in POUR incidence between prophylactic tamsulosin and placebo after TJA (4), while Choi et al. (2021) drew an opposite conclusion. Although Choi et al. (2021)’s study is more recent than Schubert et al. (2019)‘s study, both investigations were of high quality and cannot be ignored. Besides, the perioperative managements in these trials, such as placing indwelling catheters intraoperatively, did not conform to the concept of modern enhanced recovery after TJA and may contribute to the development of POUR and confound the results (Choi et al., 2021).
Doxazosin gastrointestinal therapeutic system and tamsulosin are the most frequently prescribed two alpha adrenoreceptor blockers, and doxazosin gastrointestinal therapeutic system has been proven to be more efficient than tamsulosin in treating lower urinary tract symptoms (Guo and Tang, 2021). As a result, we conducted this randomized placebo-controlled trial to investigate whether doxazosin can decrease the rate of POUR and promote recovery under the setting of modern enhanced recovery after TJA.
Materials and methods
The Ethical Committee of our institution approved this randomized controlled clinical trial. The registration number of our study at the Chinese Clinical Trial Registry was ChiCTR2200056992. Written informed consents were obtained from all participants prior to the intervention.
Participants recruitment and randomization
Male patients over 35 years of age who were scheduled for primary unilateral total hip/knee arthroplasty from February 2022 to June 2022 were included in this study. Exclusion criteria included American Society of Anesthesiologists (ASA) status IV, use of preoperative or intraoperative urinary catheter, allergy or intolerance to doxazosin, exposure to alpha-blocking medication or 5-alpha reductase inhibitor medication or anticholinergic medications within 30 days prior to the surgery, impaired liver/renal function, opioid dependence, a history of hypotension, a history of myocardial infarction and a history of gastrointestinal strictures.
An independent research assistant performed the randomization using a computer-generated randomization table. The randomization allocation was concealed in sealed opaque envelopes with consecutive numbers. Indistinguishable tablets containing either doxazosin or placebo were prepared by a dedicated study nurse. After envelopes were opened, patients received a single dose of doxazosin (4 mg once, doxazosin gastrointestinal therapeutic system) or placebo 2 h before surgery. Patients, surgeons and investigators were all blinded to the allocation until the data analysis was completed.
Perioperative management
All surgeries were performed by two senior surgeons under general anesthesia without the preoperative or intraoperative use of urinary catheter. Sufentanil .5 μg/kg, midazolam .04 mg/kg, propofol 1–2 mg/kg and cistracurium 2 μg/kg intravenously were used for anesthesia induction, and a following continuous intravenous infusion of .1–.3 μg/(kgmin) of remifentanil, 2–5 mg/(kgh) of propofol and inhalation of sevoflurane were used for anesthesia maintenance (Ding et al., 2020). After surgery, the development of POUR was monitored by the clinical symptoms and bladder ultrasound scans. Patients who failed to void within 4 h after surgery or had clinical symptoms, including suprapubic discomfort, distention symptoms or urinary incontinence, were assessed with the urine volumes. Patients with urine volumes over 400 ml were encouraged to get off the bed and void, and patients still unable to void were managed with a straight catheterization. Patients with urine volumes less than 400 ml had repeated assessment in 2 hours. All participants accepted the same perioperative multimodal analgesic protocol as previous reported (Ding et al., 2022a).
Outcomes
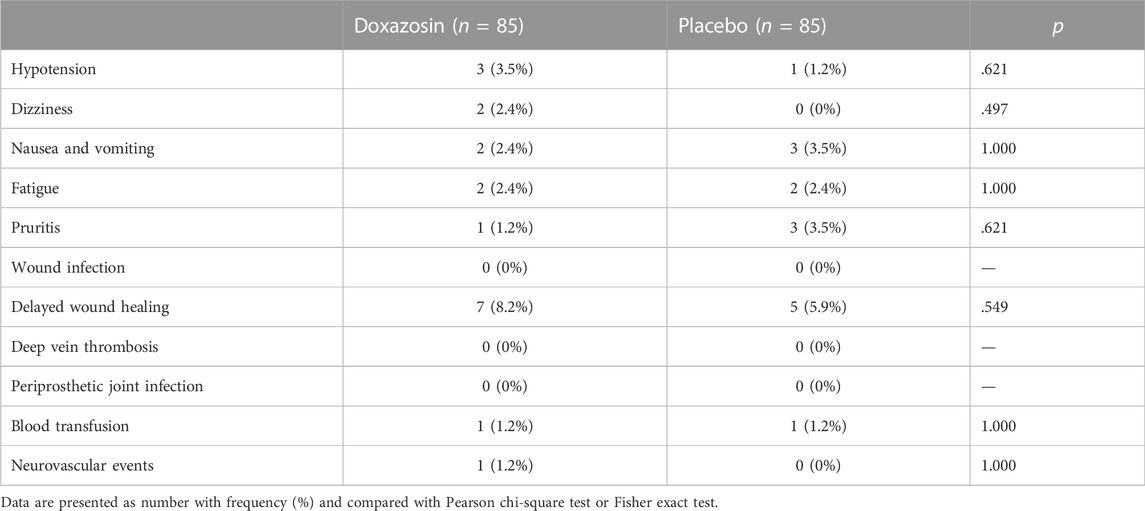
The primary outcome of interest was the development of POUR, which was diagnosed when patients with a urine volume over 400 ml or overflow incontinence. Postoperative recovery was assessed in terms of hospital LOS after surgery, daily ambulation distance, visual analogue scale (VAS) pain score, hospitalization opioid consumption, blood pressure and C-reactive protein. Urinary tract infection and other complications including hypotension, dizziness, nausea and vomiting, fatigue, pruritis, wound infection, delayed wound healing and venous thrombosis, were also recorded (Luo et al., 2018). Patients were followed up until discharge.
Sample size calculation and statistical analysis
The sample size was calculated depending on the primary outcome of POUR incidence. According to previous publications, the POUR rate of male patients after TJA was around 40%, and a reduction of 50% (POUR rate of 20% in experiment group) can be regarded as a clinically meaningful difference (Bjerregaard et al., 2015; Scholten et al., 2018). Therefore, at least a sample size of 79 patients in each group would be needed, with a 2-sided alpha level of 5% and a power of 80%.
The normality of data was measured with histograms and quantile-quantile plots. Continuous data with normal distribution were analyzed using the Student’s t-test. Continuous data with skewed distribution were compared using the Mann-Whitney U test. Categorical data are expressed as the number and percentage and were analyzed using Pearson chi-square test or Fisher exact test as appropriate. The level of significance was set at p-value <.05. Statistical analysis was performed using SPSS v22.0 (IBM, Armonk, NY).
Results
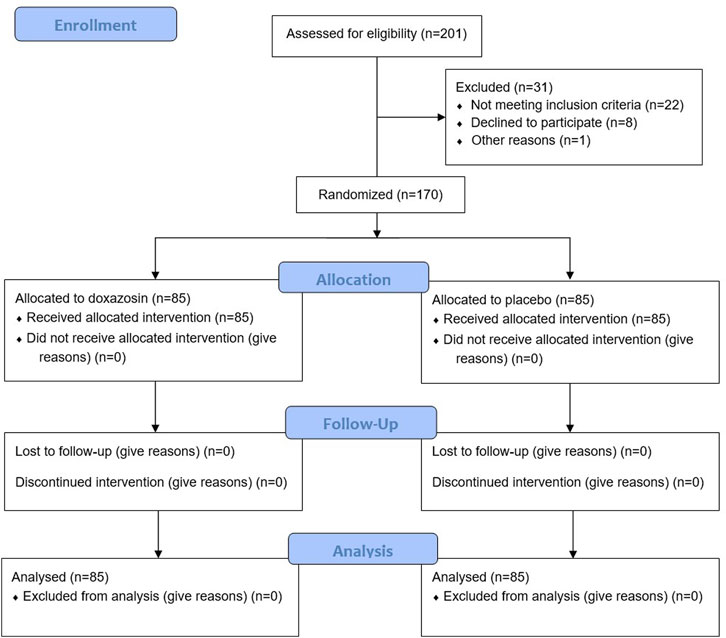
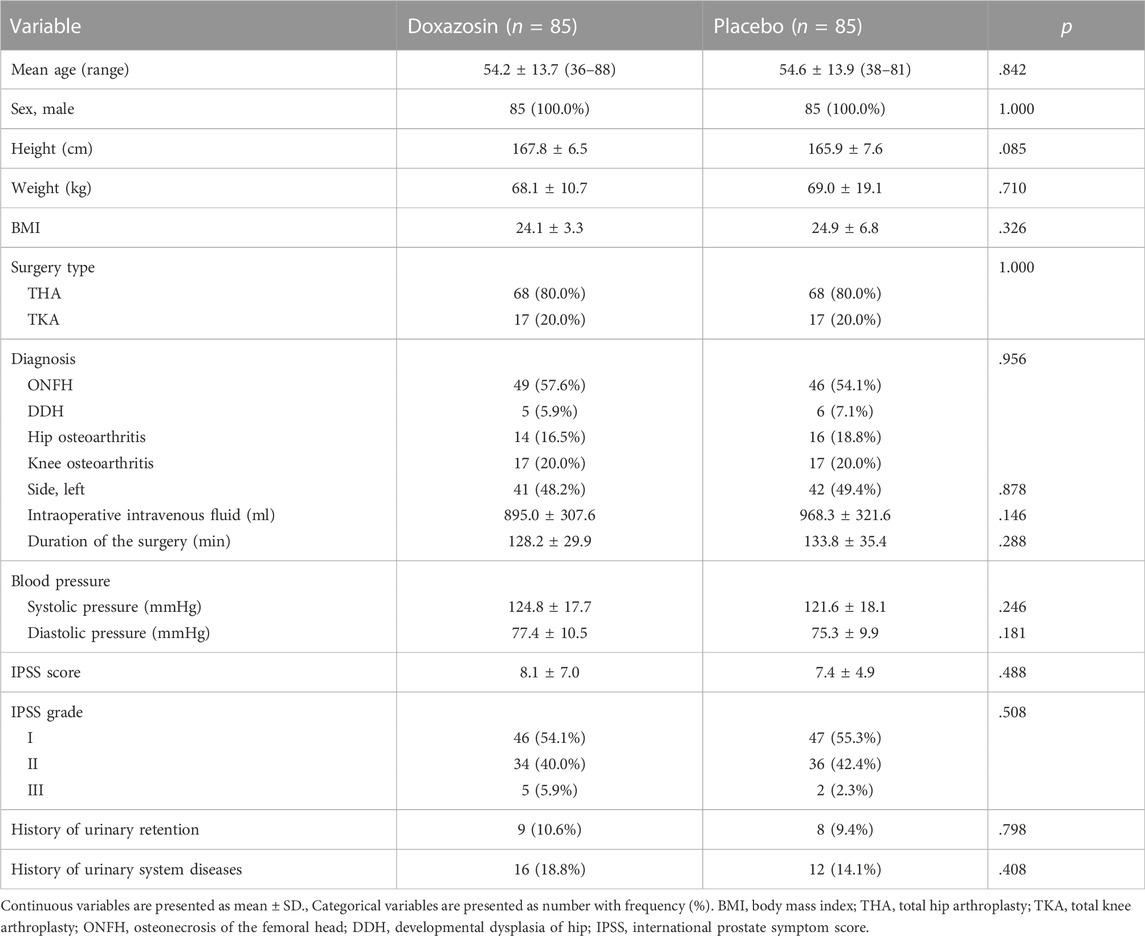
A total of 201 male patients were assessed for eligibility, of whom 31 patients were excluded for reasons (Figure 1). The remaining 170 patients were equally randomized into Doxazosin group and Placebo group. No patients dropped out of the study during the follow-up. The demographics of the enrolled patients showed no significant difference between two groups (Table 1).
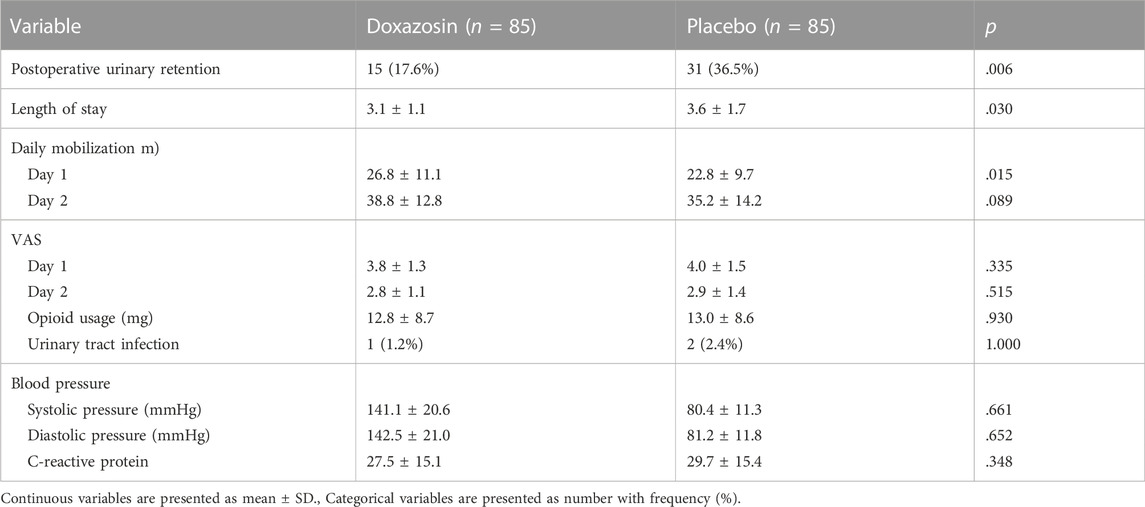
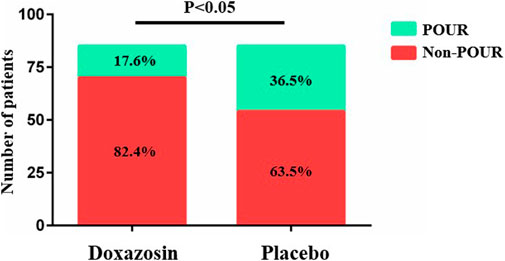
The results regarding POUR and postoperative recovery are presented in Table 2. A total of 46 patients (27.1%) developed POUR in the entire cohort of patients, and the POUR rate was significantly lower in Doxazosin group (15 out of 85; 17.6%) than in Placebo group (31 out of 85; 36.5%) (p = .006) (Figure 2). Three patients received repeated catheterization in Doxazosin group and seven patients needed repeated catheterization in Placebo group. All the catheters were discontinued prior to discharge. The mean LOS in the Doxazosin group was 3.1 days compared to 3.6 days in the Placebo group (p = .030). Doxazosin group had a longer daily mobilization distance than Placebo group on postoperative day 1 (26.8 ± 11.1 vs. 22.8 ± 9.7; p = .015). Postoperative pain assessed by VAS score and opioid usage was comparable between two groups. The rates of urinary tract infection were very low both in Doxazosin group and Placebo group, with no statistical significance (1.2% vs. 2.4%; p = 1.000).
Table 3 shows the incidence of possible adverse events related to doxazosin and postoperative complications. No significant difference was observed in adverse events between groups, such as hypotension, dizziness, nausea and vomiting, fatigue and pruritis. The rates of other postoperative complications were also similar between groups. All adverse events and complications were successfully treated without cessation or patient death.
Discussion
POUR, a common and disruptive complication following TJA, can severely affect the postoperative recovery after TJA and increase the risk of other postoperative complications (Bracey et al., 2022). There remains no efficient approach to mitigating POUR and avoiding the use of indwelling or intermittent urinary catheterization for the treatment of POUR. The most valuable finding of this randomized, double-blind, placebo-controlled trial was that doxazosin 4 mg once (2 h before surgery) can decrease the rate of POUR, increase ambulation distance and reduce hospital LOS. Our results support the routine use of prophylactic single-dose doxazosin in male patients to decrease the rate of POUR and promote postoperative recovery under the setting of modern enhanced recovery after TJA.
In this study, the overall POUR rate in the entire cohort of patients was 27.1% (17.6% in Doxazosin group and 36.5% in Placebo group), which accords with the previous publications and confirms the importance of prevention for this high-incidence complication (Bjerregaard et al., 2015; Scholten et al., 2018; Pivec et al., 2021). It is worth mentioning that the rate of POUR after TJA can be highly variable in the literatures, from 5.1% to 46.3% (Bjerregaard et al., 2015; Scholten et al., 2018; Pivec et al., 2021). The reason for the varied incidence may be that the definitions of POUR were quite different among studies. Some studies used bladder scan to assess the volume of urine in the bladder, and POUR was diagnosed if urine volume exceeds 400 ml (Scotting et al., 2019; Bracey et al., 2021). Some studies relied on the subjective patient reports and the clinical symptoms, such as suprapubic discomfort, distention symptoms and urinary incontinence, to define POUR (Santini et al., 2019; Abdul-Muhsin et al., 2020). It is obvious that bladder scan has higher accuracy and sensitivity than subjective patient reports, and investigations performing bladder scan would expectedly have higher incidences of developing POUR.
Since the only treatment option for POUR is urethral catheterization, and either intermittent or indwelling catheterization can impede the postoperative recovery and increase risk of complications, researchers have been focusing on the prevention of POUR. Alpha adrenoreceptor blockers have been considered to be able to prevent the occurrence of POUR based on the mechanism of decreasing the tension of smooth muscle in the urethra and their use in the treatment of benign prostate hyperplasia. A well-designed double-blinded randomized controlled trial used tamsulosin to prevent POUR in male patients after TJA. They found an approximately 30% decrease of POUR rate in the tamsulosin group, but it did not reach statistically significant (Schubert et al., 2019). On the contrary, our study found doxazosin could lower the risk of POUR in male patients over 35 years old, who are at higher risk of developing POUR (Fernandez et al., 2014; Ziemba-Davis et al., 2019). The superior pharmacokinetic profile and drug delivery rate of doxazosin gastrointestinal therapeutic system used in this study may explain the differences in the findings (Guo and Tang, 2021). Besides, our results were in accordance with a recent randomized controlled study (Choi et al., 2021) and two earlier prospective studies (Tammela et al., 1987; Petersen et al., 1991) confirming the preventive effect of alpha adrenoreceptor blockers for POUR.
Enhancing the postoperative recovery at early stage is the critical point of modern TJA (Ding et al., 2022b). Early mobilization ability and LOS are the most frequently used outcomes to measure the postoperative recovery effect after TJA (Soffin and YaDeau, 2016). Our results showed that doxazosin could increase the mobilization on the first postoperative day and decrease LOS when compared to the placebo. POUR has been demonstrated to impact postoperative recovery (Pivec et al., 2021; Bracey et al., 2022), and remarkably, doxazosin could promote the recovery through decreasing the risk of POUR. The results were similar to a propensity-matched retrospective cohort study which found the cohort taking alpha adrenoreceptor blockers had a 12.1% decreased risk of POUR and a one-day reduced LOS. Besides, no significant side effect of doxazosin, such as hypotension, dizziness, nausea and vomiting, fatigue and pruritis, was observed in this study, possibly due to the low clinical dosage and short-term treatment of doxazosin.
Several limitations existed in our study. Firstly, the perioperative management, especially the urinary tract management, can be quite variable across different institutions, which may affect the generalizability of our results. Secondly, all patients in this study received general anesthesia, which was reported to be at lower risk of POUR than spinal anesthesia in general surgery (Brouwer et al., 2021). General anesthesia may influence the incidence of POUR and thus influence the preventive effect of doxazosin on POUR in this study. Thirdly, patients were asked to void prior to surgery and we did not assess the urine volume prior to surgery. Measurement of postvoid residual prior to surgery may better estimate the incidence of preoperative urinary retention.
Conclusion
In conclusion, prophylactic single-dose doxazosin reduced the incidence of POUR, increased ambulation distance and reduced hospital LOS after TJA. Our results support the routine use of prophylactic doxazosin in male patients to decrease the rate of POUR and promote postoperative recovery under the setting of modern enhanced recovery after TJA.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZD and JC: conceptualization, data curation, methodology, software, and writing–original draft; CH: formal Analysis, methodology, software and writing–review and editing. HW: investigation, conceptualization, project administration, validation and visualization, and writing—review. ZZ: conceptualization, funding acquisition, resources, supervision, and writing—review. All authors read and approved the final manuscript.
Funding
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18039) and Regional Innovation and Cooperation program of Science and Technology Department of Sichuan Province (No. 2021YFQ0028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul-Muhsin, H. M., Jakob, N., Cha, S., Zhang, N., Schwartz, A., Navaratnam, A., et al. (2020). Incidence, outcomes, and prediction of postoperative urinary retention after a nonurologic procedure. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 4 (5), e1900149. doi:10.5435/JAAOSGlobal-D-19-00149
Balderi, T., and Carli, F. (2010). Urinary retention after total hip and knee arthroplasty. Minerva Anestesiol. 76 (2), 120–130.
Baldini, G., Bagry, H., Aprikian, A., and Carli, F. (2009). Postoperative urinary retention: Anesthetic and perioperative considerations. Anesthesiology 110 (5), 1139–1157. doi:10.1097/ALN.0b013e31819f7aea
Bjerregaard, L. S., Bogø, S., Raaschou, S., Troldborg, C., Hornum, U., Poulsen, A. M., et al. (2015). Incidence of and risk factors for postoperative urinary retention in fast-track hip and knee arthroplasty. Acta Orthop. 86 (2), 183–188. doi:10.3109/17453674.2014.972262
Bracey, D. N., Barry, K., Khanuja, H. S., and Hegde, V. (2022). Postoperative urinary retention in modern rapid recovery total joint arthroplasty. J. Am. Acad. Orthop. Surg. 30 (10), 443–447. doi:10.5435/JAAOS-D-21-00963
Bracey, D. N., Hegde, V., Pollet, A. K., Johnson, R. M., Jennings, J. M., and Miner, T. M. (2021). Incidence and predictive risk factors of postoperative urinary retention after primary total knee arthroplasty. J. arthroplasty 36 (7), S345–S350. doi:10.1016/j.arth.2021.02.043
Brouwer, T. A., van Roon, E. N., Rosier, P., Kalkman, C. J., and Veeger, N. (2021). Postoperative urinary retention: Risk factors, bladder filling rate and time to catheterization: An observational study as part of a randomized controlled trial. Perioper. Med. Lond. Engl. 10 (1), 2. doi:10.1186/s13741-020-00167-z
Cha, Y. H., Lee, Y. K., Won, S. H., Park, J. W., Ha, Y. C., and Koo, K. H. (2020). Urinary retention after total joint arthroplasty of hip and knee: Systematic review. J. Orthop. Surg. (Hong Kong) 28 (1), 2309499020905134. doi:10.1177/2309499020905134
Choi, C. I., Kim, J. K., Choo, M. S., Lee, S. H., Chang, J. D., and Han, J. H. (2021). Preventive effects of tamsulosin for postoperative urinary retention after lower limb arthroplasty: A randomized controlled study. Investigative Clin. urology 62 (5), 569–576. doi:10.4111/icu.20200523
Ding, Z., Li, J., Xu, B., Cao, J., Li, H., and Zhou, Z. (2022). Preoperative high sleep quality predicts further decrease in length of stay after total joint arthroplasty under enhanced recovery short-stay program: Experience in 604 patients from a single team. Orthop. Surg. 14 (9), 1989–1997. doi:10.1111/os.13382
Ding, Z. C., Li, H., Huang, C., Yuan, M. C., Cao, J., Wang, H. Y., et al. (2022). Significant analgesic benefits of perioperative duloxetine in patients who have depressive symptoms undergoing total hip arthroplasty: A randomized controlled trial. J. arthroplasty. doi:10.1016/j.arth.2022.10.007
Ding, Z. C., Xu, B., Liang, Z. M., Wang, H. Y., Luo, Z. Y., and Zhou, Z. K. (2020). Limited influence of comorbidities on length of stay after total hip arthroplasty: Experience of enhanced recovery after surgery. Orthop. Surg. 12 (1), 153–161. doi:10.1111/os.12600
Fernandez, M. A., Karthikeyan, S., Wyse, M., and Foguet, P. (2014). The incidence of postoperative urinary retention in patients undergoing elective hip and knee arthroplasty. Ann. R. Coll. Surg. Engl. 96 (6), 462–465. doi:10.1308/003588414X13946184902523
Guo, J., and Tang, R. (2021). Efficacy and tolerability of doxazosin gastro-intestinal therapeutic system versus tamsulosin in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: A systematic review and meta-analysis. Medicine 100 (33), e26955. doi:10.1097/MD.0000000000026955
Luo, Z. Y., Wang, H. Y., Wang, D., Zhou, K., Pei, F. X., and Zhou, Z. K. (2018). Oral vs intravenous vs topical tranexamic acid in primary hip arthroplasty: A prospective, randomized, double-blind, controlled study. J. arthroplasty 33 (3), 786–793. doi:10.1016/j.arth.2017.09.062
Nicolle, L. E. (2014). Catheter associated urinary tract infections. Antimicrob. Resist. Infect. control 3, 23. doi:10.1186/2047-2994-3-23
Petersen, M. S., Collins, D. N., Selakovich, W. G., and Finkbeiner, A. E. (1991). Postoperative urinary retention associated with total hip and total knee arthroplasties. Clin. Orthop. Relat. Res. 269 (269), 102–108. doi:10.1097/00003086-199108000-00016
Pivec, R., Wickes, C. B., and Austin, M. S. (2021). Pre-Operative urodynamic assessment has poor predictive value for developing post-operative urinary retention. J. arthroplasty 36 (6), 1904–1907. doi:10.1016/j.arth.2021.01.056
Pulido, L., Ghanem, E., Joshi, A., Purtill, J. J., and Parvizi, J. (2008). Periprosthetic joint infection: The incidence, timing, and predisposing factors. Clin. Orthop. Relat. Res. 466 (7), 1710–1715. doi:10.1007/s11999-008-0209-4
Santini, A. J., Jakaraddi, C. A., Polydoros, F., and Metikala, S. (2019). Validity of the International Prostate Symptoms Score in predicting urinary retention after joint replacement. J. Orthop. Surg. (Hong Kong) 27 (3), 2309499019868670. doi:10.1177/2309499019868670
Scholten, R., Kremers, K., van de Groes, S. A. W., Somford, D. M., and Koëter, S. (2018). Incidence and risk factors of postoperative urinary retention and bladder catheterization in patients undergoing fast-track total joint arthroplasty: A prospective observational study on 371 patients. J. arthroplasty 33 (5), 1546–1551. doi:10.1016/j.arth.2017.12.001
Schubert, M. F., Thomas, J. R., Gagnier, J. J., McCarthy, C. M., Lee, J. J., Urquhart, A. G., et al. (2019). The aahks clinical research award: Prophylactic tamsulosin does not reduce the risk of urinary retention following lower extremity arthroplasty: A double-blinded randomized controlled trial. J. arthroplasty 34 (7), S17–S23. doi:10.1016/j.arth.2019.03.039
Schubert, M. F., Thomas, J. R., Yashar, J., Lee, J. J., Urquhart, A. G., Gagnier, J. J., et al. (2020). Post-operative urinary retention after lower extremity arthroplasty and the peri-operative role of selective alpha-1 adrenergic blocking agents in adult male patients: A propensity-matched retrospective cohort study. Int. Orthop. 44 (1), 39–44. doi:10.1007/s00264-019-04420-z
Scotting, O. J., North, W. T., Chen, C., and Charters, M. A. (2019). Indwelling urinary catheter for total joint arthroplasty using epidural anesthesia. J. arthroplasty 34 (10), 2324–2328. doi:10.1016/j.arth.2019.05.047
Soffin, E. M., and YaDeau, J. T. (2016). Enhanced recovery after surgery for primary hip and knee arthroplasty: A review of the evidence. Br. J. Anaesth. 117 (3), iii62–iii72. doi:10.1093/bja/aew362
Tammela, T., Kontturi, M., and Puranen, J. (1987). Prevention of postoperative urinary retention after total hip arthroplasty in male patients. Ann. Chir. Gynaecol. 76 (3), 170–172.
Keywords: doxazosin, postoperative urinary retention, total joint arthroplasty, postoperative recovery, randomized controlled trial
Citation: Ding Z, Cao J, Huang C, Zhou K, Wang H and Zhou Z (2023) Prophylactic doxazosin reduces urinary retention and promotes recovery after total joint arthroplasty: A randomized controlled trial. Front. Pharmacol. 13:1016203. doi: 10.3389/fphar.2022.1016203
Received: 10 August 2022; Accepted: 16 December 2022;
Published: 09 January 2023.
Edited by:
Anis Ahmad, University of Miami Health System, United StatesReviewed by:
Jason Schwalb, Henry Ford Hospital, United StatesMartin Muñoz-Ortega, Autonomous University of Aguascalientes, Mexico
Sakıp Erturhan, University of Gaziantep, Turkiye
Copyright © 2023 Ding, Cao, Huang, Zhou, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongke Zhou, em9uZ2tlMjAxN0AxNjMuY29t; Kai Zhou, emhvdWthaXdzaEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Zichuan Ding
Zichuan Ding Jian Cao†
Jian Cao† Chao Huang
Chao Huang Zongke Zhou
Zongke Zhou