
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 07 December 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1015048
This article is part of the Research TopicGinsenosides: Exploring Their Pharmacodynamic Potential and Pharmacokinetics as Adjuvant MedicinesView all 8 articles
Purpose: We aimed to evaluate the effects of Panax notoginseng preparations (PNP) containing Panax Notoginseng Saponins (PNS) or Panaxatriol Saponin (PTS) on platelet aggregation and coagulation in the adjuvant treatment of coronary heart disease (CHD) and ischemic stroke (IS).
Methods: Randomized controlled trials (RCTs) comparing the combination of PNP and aspirin (ASA) versus ASA alone for CHD or IS were searched in eight databases. Subgroup analysis was performed according to saponin category. When statistical heterogeneity was significant, sensitivity analysis was performed using the leave-one-out approach. Funnel plot, Egger’ test, and Begg’ test was adopted to detect publication bias.
Results: Twenty RCTs involving 2216 patients were analyzed. Compared with ASA alone, PNP plus ASA had a stronger inhibitory effect on in PAgR [PNS, WMD = −6.10 (−7.25, −4.95), p < 0.00001; PTS, WMD = −3.53 (−4.68, −2.38), p < 0.00001]; PNS plus ASA better reduced FIB [WMD = −0.43 (−0.49, −0.36)] and DD [WMD = −0.59 (−0.67, −0.51), p < 0.00001], while PLT (p = 0.07) and PT (p = 0.34) were not significantly different; PTS plus ASA better prolonged PT [WMD = 1.90 (1.47, 2.32), p < 0.00001] and PT-INR [WMD = 0.22 (0.11, 0.32), p < 0.0001], whereas no significant difference in DD (p = 0.1) and bleeding-related events (positive fecal occult blood, p = 0.96; upper gastrointestinal bleeding, p = 0.67; subcutaneous hemorrhage, p = 0.51; bulbar conjunctival hemorrhage, p = 0.51; hematuria, p = 0.58). There was no significant difference between PNP plus ASA and ASA alone in terms of gastrointestinal side effect (PNS, p = 0.65; PTS, p = 0.56) and urticaria (PNS, p = 0.57; PTS, p = 0.55).
Conclusion: PNP combined with ASA might produce stronger antiplatelet aggregation and anticoagulation effects without increasing bleeding risk, gastrointestinal side effects, and urticaria compared with ASA alone.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, identifier CRD42022339234.
Panax notoginseng (Sanqi), the root of Panax notoginseng (Burk.) F. H. Chen, is a traditional Chinese medicine with a long history of medicinal use in China. Panax notoginseng contains more than 200 metabolites, including saponins, polysaccharides, dencichine, flavonoids, and fatty acids (Wang M M et al., 2016), which can be mainly classified into two categories: saponins and non-saponins, and saponins are not only the main metabolites of Panax notoginseng, but also representative components which are considered to be responsible for the botanical drug’s activity (Peng et al., 2018; National Pharmacopeia Commission, 2020). As an important part of Chinese pharmaceutical standards, the Chinese Pharmacopoeia records two saponins extracted from Panax notoginseng, namely Panax Notoginseng Saponins (PNS) and Panaxatriol Saponin (PTS), where PNS is the total saponins made from the main root or rhizome of Panax notoginseng and PTS is a processed extract of the dried root and rhizome of Panax notoginseng (National Pharmacopeia Commission, 2020). PNS has anti-platelet, anti-coagulant and inhibits platelet aggregation and thrombosis (Wang T et al., 2016; Shen et al., 2017; Liu et al., 2021). PTS has anti-platelet (Qi et al., 2016; Xu et al., 2021), pro-angiogenic and cerebral perfusion enhancing effects (Hui et al., 2017). Panax notoginseng preparations (PNP) containing PNS include Xuesaitong tablet, Xuesaitong soft capsule, Xuesaitong injection and Xueshuantong injection, while PNP containing PTS include Sanqi Tongshu capsule. As another pharmaceutical form of Panax notoginseng, PNP containing the two saponins (PNS or PTS) mentioned above has been widely used in China for the treatment of coronary heart disease (CHD) and ischemic stroke (IS) (He et al., 2011; Long et al., 2020; Lyu et al., 2020).
Arterial thrombosis is the result of clot formation during atherosclerotic plaque rupture and is the predominant pathological process in CHD and IS (Koupenova et al., 2017). Increased platelet aggregation is thought to be an important pathogenesis of cardiovascular diseases such as angina pectoris and IS and is key to thrombosis (Wu and Hoak, 1975; Rauch et al., 2001). In the secondary prevention of atherosclerotic cardiovascular disease, antiplatelet therapy is an integral part of reducing ischemic events. Antiplatelet therapy is effective in reducing the incidence of adverse vascular events, but at the cost of increased risk of bleeding (Tomaniak et al., 2019; Ha et al., 2021; Pechlivani et al., 2021). The most commonly used antiplatelet agent is aspirin (Mason et al., 2005). The interaction between PNP and aspirin (ASA) is an important clinical concern. However, evidence-based medical evidence on the effects of the combination of PNP and ASA on platelet aggregation and coagulation is as of yet lacking.
The present meta-analysis comprehensively collected studies of PNP adjuvant therapy for CHD and IS, focusing on whether PNP combined with ASA produces stronger antiplatelet and anticoagulant efficacy and whether the combination increases the risk of bleeding, thus providing evidence for the safe and rational clinical use of PNP for CHD and IS.
Our meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Page et al., 2021). We registered the protocol with the International Prospective Register of Systematic Reviews under the registration number: CRD42022339234.
A comprehensive search of eight databases including CNKI, Wanfang, VIP, CBM, PubMed, Embase, Web of Science, and the Cochrane library was conducted from inception to 1 June 2022, with no language restrictions. References for relevant reviews and meta-analyses were checked to track relevant studies that might be eligible. PubMed search strategy is displayed in Table 1.
We selected these studies based on the following inclusion criteria: 1) randomized controlled trials (RCTs); 2) patients with CHD and IS; 3) antiplatelet regimen of PNP plus ASA in the intervention group and ASA alone in the control group; 4) studies reporting one or more of six outcomes: platelet aggregation rate (PAgR), platelet count (PLT), prothrombin time (PT), prothrombin time-based international normalized ratio (PT-INR), fibrinogen (FIB), and D-dimer (DD).
Exclusion criteria: 1) required data were not obtained; 2) no outcome measures of interest; 3) no full text or conference abstracts; 4) duplicate publications or a second publication of the same trial.
Two reviewers (LD and YZ) independently reviewed the titles and abstracts of the studies according to the eligibility criteria. If potential studies met the criteria, further full-text evaluation was required. Studies that remained controversial would be arbitrated by a third researcher (YJ). We used NoteExpress software (Version 3.2) to manage the retrieved records.
Two reviewers (LD and YZ) extracted the following data independently of each other: first author, year of publication, characteristics of patients (sample, proportion of male and mean age), disease, antiplatelet regimen, primary outcome (PAgR, PLT, PT, PT-INR, FIB, and DD) and secondary outcome (upper gastrointestinal bleeding, bulbar conjunctival hemorrhage, hematuria, subcutaneous hemorrhage, positive fecal occult blood, gastrointestinal side effects, urticaria), and then cross-checked the extracted data.
Two reviewers (LD and YZ) independently assessed the risk of bias of the studies according to the Cochrane risk-of-bias tool (ROB 2) (Sterne et al., 2019). We judged the studies to be at low risk of bias, with some concerns or high risk of bias, and any disagreements should be adjudicated by a third reviewer (YJ).
We adopted Review Manager (Version 5.4, The Cochrane Collaboration, Copenhagen, 2020), R software (Version 4.0.1; R Foundation for Statistical Computing, Vienna, Austria), and Stata/SE (Version 15.1; StataCorp, College Station, Texas, United States) to analyze data and generate figures. If heterogeneity was low (p values ≥0.1 and I2 < 50%), a fixed-effects model was adopted, otherwise a random-effects model was adopted. For continuous and dichotomous data, we calculated their weighted mean difference (WMD) and risk ratio (RR), respectively. Considering the possible differences in the effects of the two saponins (PNS and PTS), we divided PNP into two categories according to the saponin category and performed subgroup analysis. Sensitivity analysis was performed using the leave-one-out approach to test the stability and reliability of the findings. When more than ten studies reported the outcome, the presence of publication bias was detected by funnel plot, Egger’ test and Begg’ test.
A total of 993 records were identified from the eight databases. After removing 323 duplicate records, the titles and abstracts of 670 records were screened, and then 605 records were excluded. We evaluated the full text of 65 potentially eligible studies. Finally, 20 (Zhou et al., 2008; Zong et al., 2009; Zhang et al., 2010; Li, 2011; Zhong et al., 2011; Piao, 2012; Zhang, 2013; Cheng et al., 2014; Yang, 2014; Yu et al., 2014; Yan and Cui, 2015; Hu, 2016; Wu, 2016; Ma et al., 2017; Shen, 2017; Chen et al., 2018; Xue, 2018; Sun, 2019; Li, 2021; Wang et al., 2021) studies were identified. Figure 1 shows the detailed screening process.
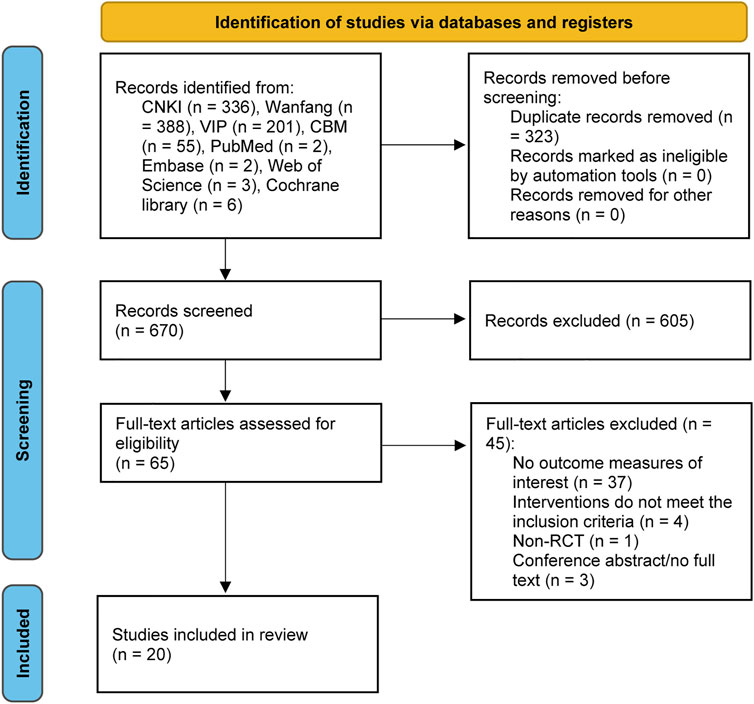
FIGURE 1. PRISMA flow diagram of literature selection and identification. PRISMA, Preferred reporting items for systematic reviews and meta-analysis.
The characteristics of the 20 studies are detailed in Table 2. The year of publication of the 20 studies ranged from 2008 to 2021. A total of 2216 patients were included in the 20 studies, 1110 in the PNP plus ASA group and 1106 in the ASA group, all of whom were middle-aged and elderly. In 15 studies (Zong et al., 2009; Zhang et al., 2010; Zhong et al., 2011; Zhang, 2013; Cheng et al., 2014; Yang, 2014; Yu et al., 2014; Hu, 2016; Wu, 2016; Ma et al., 2017; Chen et al., 2018; Xue, 2018; Sun, 2019; Li, 2021; Wang et al., 2021), the component of PNP was PNS, whereas in 5 studies (Zhou et al., 2008; Li, 2011; Piao, 2012; Yan and Cui, 2015; Shen, 2017), the component of PNP was PTS.
The results of the risk of bias assessment are presented in Figures 2, 3. Six RCTs (Zhou et al., 2008; Cheng et al., 2014; Wu, 2016; Ma et al., 2017; Xue, 2018; Wang et al., 2021) showed low risk on randomization process. One RCT (Wang et al., 2021) showed low risk on deviations from intended intervention. All RCTs showed low risk on missing data. Two RCTs (Ma et al., 2017; Wang et al., 2021) showed low risk on outcome measurement. Only one RCT (Wang et al., 2021) showed low risk on selection of reported result.
The meta-analysis results of PAgR and PLT are shown in Figure 4.
Thirteen studies (Zhou et al., 2008; Zong et al., 2009; Zhang et al., 2010; Li, 2011; Zhong et al., 2011; Piao, 2012; Zhang, 2013; Cheng et al., 2014; Yan and Cui, 2015; Wu, 2016; Shen, 2017; Chen et al., 2018; Wang et al., 2021) included 14 sets of data comparing PAgR in the PNP plus ASA group and the ASA group. There was moderate heterogeneity in the 14 sets of data (p = 0.07, I2 = 38%), so we performed a subgroup analysis according to saponin category. After subgroup analysis, the moderate heterogeneity of PAgR was eliminated. The results of the subgroup analysis were as follows, and eight studies (Zong et al., 2009; Zhang et al., 2010; Zhong et al., 2011; Zhang, 2013; Cheng et al., 2014; Wu, 2016; Chen et al., 2018; Wang et al., 2021) compared the PAgR of the PNS plus ASA group with that of the ASA group. The PAgR of the PNS plus ASA group was lower than that of the ASA group [WMD = −6.10 (−7.25, -4.95), p < 0.00001], with no heterogeneity among the eight studies (p = 0.43, I2 = 0%). Five studies (Zhou et al., 2008; Li, 2011; Piao, 2012; Yan and Cui, 2015; Shen, 2017) included six sets of data comparing PAgR in the PTS plus ASA group and the ASA group. PAgR of the PTS plus ASA group was lower than that of the ASA group [WMD = −3.53 (−4.68, −2.38), p < 0.00001], with no heterogeneity among the six sets of data (p = 0.48, I2 = 0%).
Five studies (Cheng et al., 2014; Yang, 2014; Wu, 2016; Sun, 2019; Li, 2021) compared PLT between the PNS plus ASA group and the ASA group. There was no significant difference in PLT between the PNS plus ASA group and the ASA group [WMD = 4.68 (−0.47, 9.83), p = 0.07], and no heterogeneity among the five studies (p = 0.99, I2 = 0%).
The meta-analysis results of PT, PT-INR, FIB, and DD are shown in Figure 5.
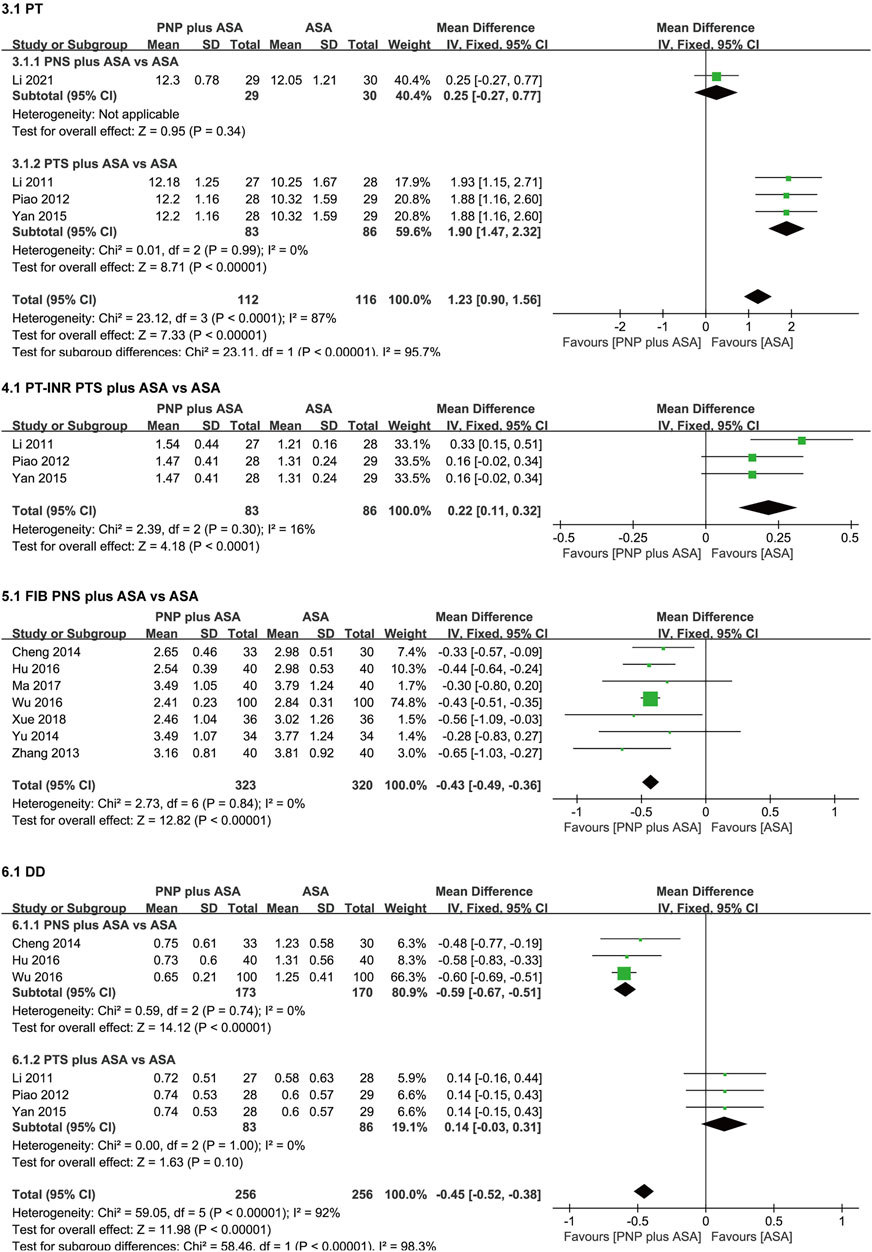
FIGURE 5. Forest plots of PT, PT-INR, FIB, and DD. PT, prothrombin time; PT-INR, prothrombin time-based international normalized ratio; FIB, fibrinogen; DD, D-dimer.
Four studies (Li, 2011; Piao, 2012; Yan and Cui, 2015; Li, 2021) compared PT in the PNP plus ASA group and the ASA group. Considerable heterogeneity existed in the four studies (p < 0.0001, I2 = 87%), so we performed a subgroup analysis according to saponin category. After subgroup analysis, considerable heterogeneity in PT was eliminated. Subgroup analysis were as follows, one study (Li, 2021) compared PT between the PNS plus ASA group and the ASA group. There was no significant difference in PT between the PNS plus ASA group and the ASA group [WMD = 0.25 (-0.27, 0.77), p = 0.34]. Three studies (Li, 2011; Piao, 2012; Yan and Cui, 2015) compared PT between the PTS plus ASA group and the ASA group. PT in the PTS plus ASA group was higher than that in the ASA group [WMD = 1.90 (1.47, 2.32), p < 0.00001], with no heterogeneity among the three studies (p = 0.99, I2 = 0%).
Three studies (Li, 2011; Piao, 2012; Yan and Cui, 2015) compared PT-INR between the PTS plus ASA group and the ASA group. PT-INR in the PTS plus ASA group was higher than that in the ASA group [WMD = 0.22 (0.11, 0.32), p < 0.0001], with no heterogeneity among the three studies (p = 0.30, I2 = 16%).
Seven studies (Zhang, 2013; Cheng et al., 2014; Yu et al., 2014; Hu, 2016; Wu, 2016; Ma et al., 2017; Xue, 2018) compared FIB between the PNS plus ASA group and the ASA group. FIB in the PNS plus ASA group was lower than that in the ASA group [WMD = −0.43 (−0.49, −0.36), p < 0.00001], with no heterogeneity among the seven studies (p = 0.84, I2 = 0%).
Six studies (Li, 2011; Piao, 2012; Cheng et al., 2014; Yan and Cui, 2015; Hu, 2016; Wu, 2016) compared DD in the PNP plus ASA group and the ASA group. There was considerable heterogeneity in the six studies (p < 0.00001, I2 = 92%), so we performed a saponin category-based subgroup analysis. After subgroup analysis, considerable heterogeneity in DD was eliminated. Subgroup analysis were as follows, three studies (Cheng et al., 2014; Hu, 2016; Wu, 2016) compared DD in the PNS plus ASA group and the ASA group. DD in the PNS plus ASA group was lower than that in the ASA group [WMD = −0.59 (−0.67, −0.51), p < 0.00001], and there was no heterogeneity in the three studies (p = 0.74, I2 = 0%). Three studies (Li, 2011; Piao, 2012; Yan and Cui, 2015) compared DD between the PTS plus ASA group and the ASA group. There was no significant difference in DD between the PTS plus ASA group and the ASA group [WMD = 0.14 (−0.03, 0.31), p = 0.1], and there was no heterogeneity among the three studies (p = 1, I2 = 0%).
The meta-analysis results of adverse reactions are shown in Figure 6; Table 3.
There were no significant differences between the PTS plus ASA group and the ASA group in terms of bleeding-related events [positive fecal occult blood (p = 0.96); upper gastrointestinal bleeding (p = 0.67); subcutaneous hemorrhage (p = 0.51); bulbar conjunctival hemorrhage (p = 0.51); hematuria (p = 0.58)]. There were no significant differences between the PNP plus ASA group and the ASA group in terms of gastrointestinal side effects (PNS, p = 0.65; PTS, p = 0.56) and urticaria (PNS, p = 0.57; PTS, p = 0.55).
Although moderate or considerable heterogeneity between studies reporting PAgR, PT, and DD was eliminated after subgroup analysis, we still performed sensitivity analysis using the leave-one-out approach to verify the stability and reliability of the findings and found that all pooled results were robust.
Thirteen studies (Zhou et al., 2008; Zong et al., 2009; Zhang et al., 2010; Li, 2011; Zhong et al., 2011; Piao, 2012; Zhang, 2013; Cheng et al., 2014; Yan and Cui, 2015; Wu, 2016; Shen, 2017; Chen et al., 2018; Wang et al., 2021) included 14 sets of data comparing the PAgR of PNP plus ASA and ASA alone, so we tested for publication bias and found no evidence of publication bias according to funnel plot, Egger’ test and Begg’ test (Figure 7, Egger’s test, p = 0.462; Begger’s test, p = 1.000). Studies reporting other outcomes were not adequate, so we were unable to perform the test.
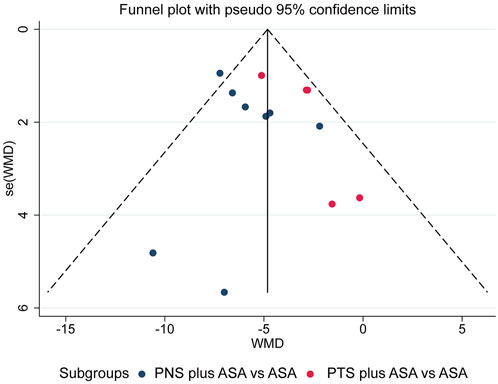
FIGURE 7. Funnel plot of comparison of PAgR between PNS plus ASA group or PTS plus ASA group and ASA group. PAgR, platelet aggregation rate; PNS, Panax Notoginseng Saponins; ASA, aspirin; PTS, Panaxatriol Saponin.
The meta-analysis included 20 studies with 2216 patients. To sum up, our analysis showed that PNP (PNS or PTS) plus ASA had a significantly stronger inhibitory effect on PAgR than ASA alone; PNS plus ASA was better than ASA alone in reducing FIB and DD, while there was no significant difference in PLT and PT; PTS plus ASA was better than ASA alone in prolonging PT and PT-INR, while there was no significant difference in DD; in terms of adverse reactions, there was no significant difference between PTS plus ASA and ASA alone in terms of bleeding-related events, nor was there a significant difference between PNP (PNS or PTS) plus ASA and ASA alone in terms of gastrointestinal side effect and urticaria.
Our meta-analysis focused on the effects of the combination of PNP and ASA on platelet aggregation and coagulation and demonstrated that the combination had synergistic antiplatelet and anticoagulant efficacy. It was found that ASA increases the absorption of PNS by disrupting tight junction proteins to open intercellular space (Tian et al., 2018), while PNS also promotes the absorption of ASA in the gastrointestinal tract (Tian et al., 2017), in addition, a recent study (Wang et al., 2021) showed that the combination of PNS and ASA enhanced the antiplatelet effect of ASA via the AA/COX-1/TXB2 pathway, which may partially explain the synergistic effect of the combination of PNP and ASA. Notably, there was no significant difference in bleeding-related events between the PTS plus ASA group and the ASA group. The combination of ASA with other antiplatelet or anticoagulant drugs produces a stronger antithrombosis effect, but also a higher risk of bleeding (Kozieł and Lip, 2019). Therefore, it is necessary to balance the pros and cons and to rationalize the use of antiplatelet drugs in people at high risk of bleeding. Our results suggested that the combination of PNP, of which PTS is the main component, with ASA did not increase the risk of bleeding, which may be beneficial for patients with atherothrombotic disease at high risk of bleeding, and the underlying mechanisms need to be further explored in basic research. Results of this meta-analysis also showed that the combination of PNP and ASA did not increase gastrointestinal side effects. Aspirin can cause gastric mucosal damage, and in some patients, the cardiovascular benefits of low-dose aspirin may be offset by gastrointestinal risks (Sostres and Lanas, 2011). In vivo studies in rats with myocardial infarction have shown that the combination of PNS and ASA can alleviate ASA-related gastrointestinal side effects via the AA/PG pathway. Although PNP was not found to reduce the gastrointestinal side effects of aspirin in this meta-analysis, it at least did not increase such side effects. Results of this meta-analysis may provide clinicians and medical professionals with evidence-based medical evidence when choosing PNP as an adjunct to the treatment of CHD or IS.
This is the first meta-analysis to focus on the potential interaction of PNP combined with ASA in platelet aggregation and coagulation. In addition to this, in previous meta-analyses (Shang et al., 2013; Song et al., 2017; Duan et al., 2018; Gao et al., 2019), only PNP with PNS as the main active component and not PNP with PTS as the main active component were included, but our meta-analysis included PNP with both two saponins as the main active components and subgroup analyses were performed according to the saponin category.
However, some limitations of this meta-analysis should be noted. First, most of the included literatures were in Chinese and only one was in English, and the possibility of language bias could not be excluded. Second, the methodological quality of the literatures included in the analysis were not high, which might reduce the reliability of our findings to some extent. Third, some of the outcomes of the meta-analysis involved only one of the two saponin, PTS or PNS, such as PLT, PT-INR, and FIB, especially for bleeding-related events, which indicate that the current attention to these areas is still insufficient.
Preliminary evidence from our meta-analysis shows that compared with ASA alone, combined with PNP might produce stronger anti-platelet aggregation and anticoagulation effects without increasing the risk of bleeding, gastrointestinal side effects, and urticaria. However, given the potential biases of included studies, the evidence may be limited and requires further evaluation. Therefore, such preliminary studies need to pay more attention to randomization, blinding and biased reporting to provide evidence of higher methodological quality.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
LD and YJ devised the paper. LD and YZ identified studies, conducted data collection and extraction. YJ performed validation of data. LD and YZ analyzed all the data. LD completed the first draft. KC provided guidelines for this meta-analysis.
Study was supported by National Natural Science Foundation of China (81373822) and CACMS Innovation Fund (CI 2021A00908).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chen, L., Chen, S. X., Yang, M., Liu, B. D., and Sun, S. T. (2018). Analysis on the clinical value of notoginseng total saponin combined with aspirin in the treatment of cerebral infarction for 120 cases. Chin. Med. Mod. Distance Educ. China 16, 54–56. doi:10.3969/j.issn.1672-2779.2018.10.023
Cheng, M. X., Li, H. Y., and Qi, S. S. (2014). Effect of Xuesaitong for injection on thrombosis in acute cerebral infarction. Chin. J. Exp. Traditional Med. Formulae 20, 196–200. doi:10.13422/j.cnki.syfjx.2014100196
Duan, L., Xiong, X., Hu, J., Liu, Y., and Wang, J. (2018). Efficacy and safety of oral panax notoginseng saponins for unstable angina patients: A meta-analysis and systematic review. Phytomedicine 47, 23–33. doi:10.1016/j.phymed.2018.04.044
Gao, Y., Lyu, J., Xie, Y. M., and Sun, M. H. (2019). [Effictiveness and safety of Xueshuantong injection in treatment of unstable angina pectoris: A systematic review and meta-analysis of randomized controlled trials]. China J. Chin. Materia Medica 44, 4366–4378. doi:10.19540/j.cnki.cjcmm.20190724.501
Ha, A. C. T., Bhatt, D. L., Rutka, J. T., Johnston, S. C., Mazer, C. D., and Verma, S. (2021). Intracranial hemorrhage during dual antiplatelet therapy: JACC review topic of the week. J. Am. Coll. Cardiol. 78, 1372–1384. doi:10.1016/j.jacc.2021.07.048
He, L., Chen, X. Y., Zhou, M. K., Zhang, D. P., Yang, J., Yang, M., et al. (2011). Radix/rhizoma notoginseng extract (sanchitongtshu) for ischemic stroke: A randomized controlled study. Phytomedicine 18, 437–442. doi:10.1016/j.phymed.2010.10.004
Hu, H. (2016). Clinical observation of Xueshuantong injection in the treatment of cerebral apoplexy. China Health Care & Nutr. 26, 228–229.
Hui, Z., Sha, D. J., Wang, S. L., Li, C. S., Qian, J., Wang, J. Q., et al. (2017). Panaxatriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement. Altern. Med. 17, 70. doi:10.1186/s12906-017-1579-5
Koupenova, M., Kehrel, B. E., Corkrey, H. A., and Freedman, J. E. (2017). Thrombosis and platelets: An update. Eur. Heart J. 38, 785–791. doi:10.1093/eurheartj/ehw550
Kozieł, M., and Lip, G. Y. H. (2019). Estimating individual lifetime benefit and bleeding risk of adding oral anticoagulation to aspirin for patients with stable cardiovascular disease: Directions from COMPASS? Eur. Heart J. 40, 3779–3781. doi:10.1093/eurheartj/ehz517
Li, T. (2021). Clinical observation of Stroke secondary prevention combined with Xuesaitong in the early recovery of acute cerebral infarction. Yunnan, China: Yunnan University of Chinese Medicine. [master’s thesis]. [Yunnan].
Li, X. M. (2011). Clinical study of Panax notoginseng saponins jointing aspirin’s anti-thrombosis treatment. Shandong, China: Shandong University of traditional Chinese Medicine. [master’s thesis]. [Shandong].
Liu, L., Zhang, Q. L., Xiao, S. L., Sun, Z. X., Ding, S. L., Chen, Y., et al. (2021). Inhibition of shear-induced platelet aggregation by Xueshuantong via targeting Piezo1 channel-mediated Ca2+ signaling pathway. Front. Pharmacol. 12, 606245. doi:10.3389/fphar.2021.606245
Long, W. J., Liao, H. L., Huang, X., Liu, Q. Q., Tang, Y. Q., Lu, L. M., et al. (2020). Efficacy and safety of high-dose Xueshuantong injection (lyophilised) in reducing the incidence of major adverse cardiovascular events in patients with unstable angina: A protocol of a randomised, parallel-arm, controlled, double-blind and multicentre clinical trial based on dual antiplatelet therapy. BMJ Open 10, e038074. doi:10.1136/bmjopen-2020-038074
Lyu, J., Xie, Y. M., Sun, M. H., and Zhang, L. D. (2020). Efficacy and safety of Xueshuantong injection on acute cerebral infarction: Clinical evidence and GRADE assessment. Front. Pharmacol. 11, 822. doi:10.3389/fphar.2020.00822
Ma, W. X., Wang, B. P., Zhang, Y., and Ke, B. X. (2017). Clinical trial of Xueshuantong injection combined with aspirin in patients with recurrent acute cerebral infarction. Chin. J. Clin. Pharmacol. 33, 554–557. doi:10.13699/j.cnki.1001-6821.2017.06.020
Mason, P. J., Jacobs, A. K., and Freedman, J. E. (2005). Aspirin resistance and atherothrombotic disease. J. Am. Coll. Cardiol. 46, 986–993. doi:10.1016/j.jacc.2004.08.070
National Pharmacopeia Commission (2020). Pharmacopeia of the people’s Republic of China. 2020 Edition. Beijing: China Medical Science and Technology Press.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T, C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pechlivani, N., Kearney, K. J., and Ajjan, R. A. (2021). Fibrinogen and antifibrinolytic proteins: Interactions and future therapeutics. Int. J. Mol. Sci. 22, 12537. doi:10.3390/ijms222212537
Peng, M., Yi, Y. X., Zhang, T., Ding, Y., and Le, J. (2018). Stereoisomers of saponins in panax notoginseng (Sanqi): A review. Front. Pharmacol. 9, 188. doi:10.3389/fphar.2018.00188
Piao, M. S. (2012). Clinical observation of Panax notoginseng saponins jointing aspirin’s treatment of cerebral infarction. Shandong, China: Shandong University of traditional Chinese Medicine. [master’s thesis]. [Shandong].
Qi, H. Y., Huang, Y. L., Yang, Y., Dou, G. J., Wan, F., Zhang, W. W., et al. (2016). Anti-platelet activity of panaxatriol saponins is mediated by suppression of intracellular calcium mobilization and ERK2/p38 activation. BMC Complement. Altern. Med. 16, 174. doi:10.1186/s12906-016-1160-7
Rauch, U., Osende, J. I., Fuster, V., Badimon, J. J., Fayad, Z., and Chesebro, J. H. (2001). Thrombus formation on atherosclerotic plaques: Pathogenesis and clinical consequences. Ann. Intern. Med. 134, 224–238. doi:10.7326/0003-4819-134-3-200102060-00014
Shang, Q. H., Xu, H., Liu, Z. L., Chen, K. J., and Liu, J. P. (2013). Oral panax notoginseng preparation for coronary heart disease: A systematic review of randomized controlled trials. Evid. Based. Complement. Altern. Med. 2013, 940125. doi:10.1155/2013/940125
Shen, D. J. (2017). Clinical observation on 123 cases of cerebral infarction treated with Panax notoginseng saponins combined with aspirin. Fujian Med. J. 39, 97–99. doi:10.3969/j.issn.1002-2600.2017.02.035
Shen, Q., Li, J., Zhang, C. X., Wang, P. B., Mohammed, A., Ni, S. S., et al. (2017). Panax notoginseng saponins reduce high-risk factors for thrombosis through peroxisome proliferator-activated receptor -γ pathway. Biomed. Pharmacother. 96, 1163–1169. doi:10.1016/j.biopha.2017.11.106
Song, H. Y., Wang, P. L., Liu, J. G., and Wang, C. L. (2017). Panax notoginseng preparations for unstable angina pectoris: A systematic review and meta-analysis. Phytother. Res. 31, 1162–1172. doi:10.1002/ptr.5848
Sostres, C., and Lanas, A. (2011). Gastrointestinal effects of aspirin. Nat. Rev. Gastroenterol. Hepatol. 8, 385–394. doi:10.1038/nrgastro.2011.97
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, 14898. doi:10.1136/bmj.l4898
Sun, Q. S. (2019). Study on the effect of Xuesaitong injection on platelet parameters and hemorheology in 336 cases of ischemic cerebral infarction. J. North Pharm. 16, 71–72. doi:10.3969/j.issn.1672-8351.2019.01.055
Tian, Z., Pang, H., Du, S., Lu, Y., Zhang, L., Wu, H., et al. (2017). Effect of Panax notoginseng saponins on the pharmacokinetics of aspirin in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1040, 136–143. doi:10.1016/j.jchromb.2016.12.007
Tian, Z., Pang, H., Zhang, Q., Du, S., Lu, Y., Zhang, L., et al. (2018). Effect of aspirin on the pharmacokinetics and absorption of panax notoginseng saponins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1074-1075, 25–33. doi:10.1016/j.jchromb.2017.12.033
Tomaniak, M., Chichareon, P., Onuma, Y., Deliargyris, E. N., Takahashi, K., Kogame, N., et al. (2019). Benefit and risks of aspirin in addition to ticagrelor in acute coronary syndromes: A post hoc analysis of the randomized global leaders trial. JAMA Cardiol. 4, 1092–1101. doi:10.1001/jamacardio.2019.3355
Wang, M. M., Xue, M., Xu, Y. G., Miao, Y., Kou, N., Yang, L., et al. (2016). Panax notoginseng saponin is superior to aspirin in inhibiting platelet adhesion to injured endothelial cells through COX pathway in vitro. Thromb. Res. 141, 146–152. doi:10.1016/j.thromres.2016.03.022
Wang T, T., Guo, R. X., Zhou, G. H., Zhou, X. D., Kou, Z. Z., Sui, F., et al. (2016). Traditional uses, botany, phytochemistry, pharmacology and toxicology of panax notoginseng (burk.) F.H. Chen: A review. J. Ethnopharmacol. 188, 234–258. doi:10.1016/j.jep.2016.05.005
Wang, W., Yang, L., Song, L., Guo, M., Li, C., Yang, B., et al. (2021). Combination of Panax notoginseng saponins and aspirin potentiates platelet inhibition with alleviated gastric injury via modulating arachidonic acid metabolism. Biomed. Pharmacother. 134, 111165. doi:10.1016/j.biopha.2020.111165
Wu, K. K., and Hoak, J. C. (1975). Increased platelet aggregates in patients with transient ischemic attacks. Stroke 6, 521–524. doi:10.1161/01.str.6.5.521
Wu, Y. J. (2016). Effect of aspirin combined with Xuesaitong injection on the serum Hcy levels of 100 cases of patients with ischemic stroke. Shanghai Med. Pharm. J. 37, 44–47.
Xu, Z. Y., Xu, Y., Xie, X. F., Tian, Y., Sui, H. J., Sun, Y., et al. (2021). Anti-platelet aggregation of Panax notoginseng triol saponins by regulating GP1BA for ischemic stroke therapy. Chin. Med. 16, 12. doi:10.1186/s13020-021-00424-3
Xue, C. M. (2018). Clinical observation of Xuesaitong combined with aspirin in the treatment of cerebral infarction complicated with middle cerebral artery stenosis. Chin. J. Ethnomedicine Ethnopharmacy 27, 93–95.
Yan, X. Y., and Cui, D. Z. (2015). Clinical observation on treating cerebral infarction with Panax notoginseng saponins combined with aspirin. Clin. J. Chin. Med. 7, 3–5. doi:10.3969/j.issn.1674-7860.2015.22.002
Yang, Y., Zheng, X. F., Chen, J. W., Chen, K., Jiang, S. D., et al. (2014). Oxidative damage to osteoblasts can be alleviated by early autophagy through the endoplasmic reticulum stress pathway-implications for the treatment of osteoporosis. Free Radic. Biol. Med. 22, 10–20. doi:10.1016/j.freeradbiomed.2014.08.028
Yu, Y. C., Wu, S. Z., and Hou, Q. (2014). Clinical study on Xueshuantong combined with aspirin in treatment of senium acute cerebral infarction. Drugs & Clin. 29, 782–785. doi:10.7501/j.issn.1674-5515.2014.07.020
Zhang, H. (2013). Analysis of efficacy and adverse reactions of Xuesaitong injection in the treatment of unstable angina pectoris. Lishizhen Med. Materia Medica Res. 24, 1945–1946. doi:10.3969/j.issn.1008-0805.2013.08.061
Zhang, J. Y., Peng, L., Liu, D. B., and Liu, D. H. (2010). Observation on the efficacy of Panax notoginseng saponins combined with aspirin in preventing recurrence of cerebral infarction. J. Pract. Med. 26, 1424–1425. doi:10.3969/j.issn.1006-5725.2010.08.064
Zhong, S. S., Wang, Y. Z., Yang, H., Wang, S. H., Zhao, Y. H., Liao, H. Y., et al. (2011). Clinical efficacy of saponins combined with aspirin to prevent stroke recurrence. Med. Innovation China 8, 38–39. doi:10.3969/j.issn.1674-4985.2011.32.020
Zhou, B. R., Xu, Z. Q., Liu, Z. F., Guan, H. T., and Wang, R. (2008). [Clinical value of protoparaxotril saporlirs combined with aspirin in the secondary prevention of cerebral infarction]. Chin. J. Integr. Traditional West. Med. 28, 797–800. doi:10.3321/j.issn:1003-5370.2008.09.008
Keywords: panax notoginseng preparation, aspirin, panax notoginseng saponins, panaxatriol saponin, platelet aggregation, coagulation, meta-analysis
Citation: Dai L, Zhang Y, Jiang Y and Chen K (2022) Panax notoginseng preparation plus aspirin versus aspirin alone on platelet aggregation and coagulation in patients with coronary heart disease or ischemic stroke: A meta-analysis of randomized controlled trials. Front. Pharmacol. 13:1015048. doi: 10.3389/fphar.2022.1015048
Received: 09 August 2022; Accepted: 28 November 2022;
Published: 07 December 2022.
Edited by:
Ming Zhang, Jilin University, ChinaReviewed by:
Yong Jia, Jilin University, ChinaCopyright © 2022 Dai, Zhang, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keji Chen, a2pjaGVudmlwQDE2My5jb20=; Yuerong Jiang, amlhbmdfeXVlcm9uZ0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.