
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 06 October 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1012265
Rosa laevigata Michx. is an ethnic medicine that have strong biological activities used in the traditional medicine system for the treatment of diabetes, nephropathy, myocardial damage, oxidative damage, liver damage and so on. Currently, The Chinese herb R. laevigata Michx. can be divided into two important medicines: Fructus R. laevigata and Radix R. laevigata, from which approximately 148 chemical components have been isolated, including flavonoids, lignans, polyphenols, steroids, triterpenoids, tannins as well as other components. Pharmacological studies have already confirmed that both of these herbs have antioxidant, anti-inflammatory, antiviral and anti-tumor activities, as well as renal protective, immunomodulatory, lipid-lowering, cardiovascular protective, bacteriostatic, and other pharmacological effects. Toxicological tests and quality control studies revealed the safety and nontoxicity of R. laevigata Michx. Therefore, this paper systematically summarizes the traditional uses, botanical, phytochemical, and pharmacology as well as the quality control and toxicology of Fructus and Radix, which in order to provide a comprehensive reference for its continued research.
Rosa laevigata Michx. belongs to the Rosaceae family and is a widely used plant in China. Its different parts are used as herbs in Chinese medicine, its flowers, leaves and stems have different applications, but the main parts used are its fruits and roots, which are two herbs with so much important applications in Chinese medicine. R. laevigata is divided into many types of herbs according to its different medicinal parts, and among these the two most used are: Fructus R. laevigata and Radix R. laevigata.
One of these herbs is Fructus R. laevigata (JinYingZi) which is the dried ripe fruit of the Rosaceae plant that is often called Prickly Elm. It was first recorded in the Shu Ben Cao (during 935-960 AD) written by Han Baosheng and now included in the Chinese Pharmacopoeia. It belongs to the kidney, bladder and large intestine meridian. It has the effects of consolidating the essence and shrinking of urine, consolidating the collapse and stopping belt as well as astringent intestines and stopping diarrhea. It is mainly used for spermatorrhea, frequent enuresis, metrorrhagia and diarrhea (National Pharmacopoeia Committee, 2020).
Another one of these herbs is Radix R. laevigata (JinYingGen) which is also known as Tuogudan. It was first recorded in the Ri Hua Zi Ben Cao and it is flat and non-toxic. It is used to strengthen the essence and astringe the intestines as well as the treatment of spermatorrhea, enuresis, dysentery, diarrhea, metrorrhagia, uterine prolapse, hemorrhoids, scalding and so on (Tan et al., 2010).
Studies have shown that the different parts of the herbs lead to small differences in their efficacy. The extracts of Radix R. laevigata can treat age-related urinary incontinence by causing changes in bladder forcing muscles and bladder capacity during the filling phase (Tianjin No.3 Hospital, 2004). Fructus R. laevigata can treat the symptoms of kidney deficiency, its administration can improve kidney function as well as improve any difficulties in urination and edema in men and women. It also can reduce the frequent night urination. In addition, fructus R. laevigata can improve the function of the gastrointestinal tract, promoting the peristalsis of the intestines, increasing food digestion and reducing the accumulation of harmful substances in the intestinal tract. It also plays a role in elimination of the stool and combats diarrhea and it can reduce the occurrence of gastrointestinal diseases. In terms of chemical composition, both Fructus and Radix are similar. Triterpenoids are almost the same, while the flavonoids, tannins and other components are somewhat different. The corresponding pharmacological activities are also slightly different, with anti-inflammatory, antioxidant, anti-tumor and immunological activities allotted to both medicinal herbs, while other pharmacological activities are different. Fructus R. laevigata and Radix R. laevigata are also different in their applications and, each has its own focus. The Ministry of Health of China has rated Fructus R. laevigata as a new food resource, which has been developed into a third generation of wild fruit food. Therefore, Fructus R. laevigata is widely used in food ingredients, such as its addition when developing fruit juices, fruit wines and yogurt (Li et al., 2022). In addition, a brown pigment for use as a food additive can also be extracted from Fructus. It is also used as raw material for Chinese medicine to treat pelvic inflammation and diabetic cataract, while Radix R. laevigata is used as raw material in Sanjin tablets and gynecological Qianjin tablets to treat gynecological infections. In addition, and it is also used as the main raw material of Guangdong herbal tea.
At present, there are an ever-growing list of reports on Fructus R. laevigata and Radix R. laevigata. Therefore, this review respectively summarizes the chemical composition, pharmacological effects, and quality control as well as the extraction, separation and processing of Fructus R. laevigata and Radix R. laevigata in order to provide a basis for subsequent research on these two important Chinese medicines.
The available information on Rosa laevigata Michx. was collected from scientific databases published from 1989 up to 2021. Information on R. laevigata was obtained from published sources, including monographs on medicinal plants, ancient and modern recorded classics, the Chinese Pharmacopoeia and electronic databases, such as Web of Science, PubMed, CNKI, Wanfang DATA, Google Scholar, Baidu Scholar and Flora of China (FOR). The search terms used for this review included “Rosa laevigata Michx.”, “Fructus Rosa laevigata”, “The root of Rosa laevigata”, “Radix Rosae laevigate” and “Rosaceae” all of which are accepted names and synonyms, “botanical characterization”, “compounds”, “traditional uses”, “pharmacology”, “toxicology”, “quality standard”, “extraction and purification” and “applications”. No language restrictions were applied during the search.
Fructus Rosa laevigata Michx. is the dried ripe fruit of Rosaceae plant family. Flora of China describes it as an evergreen climbing shrub that is approximately 5 m tall and the branchlets are stout with a glabrous stem and having thin stripes. The leaflets are leathery, usually three but more rarely can be five and are 5–10 cm long with petioles. The leaves are elliptical and ovate, obovate or lanceolate-ovate, measuring approximately 2–6 cm long and 1.2–3.5 cm wide. The apex of each leaf is acute or rounded with a sparsely caudate-acuminate and the margin is sharply serrated. The coloration is bright green when viewed from above and yellow-green from below with glabrous. The petiolules and leaf rachis have prickles and glandular hairs. The stipules are free or the base are united with petiole presenting in a lance shape and the margins are finely toothed with glandular tips and are caducous. The flowers are solitary in the leaf axils and are 5–7 cm in diameter, whereas the pedicel are 1.8–2.5 cm long and can occasionally be up to 3 cm. Additionally, the pedicels and calyx tubes are densely glandular and hairy, and these become acicular as the fruit grows. The spals are ovate-lanceolate with a leaf-like apex and the margin are pinnately lobed or entire which often with spiny and glandular hairs. The inner surface is densely pilose and it is slightly shorter than the petals which are white and are broadly obovate. The apex is retuse. There are numerous stamens and carpels, and styles are free and hairy and much shorter than stamens. The purple-brown fruit is pear-shaped, obovate or sparsely sub-globose. The outer layer is densely covered with prickly hairs and the pedicel of fruit is approximately 3 cm long with persistent, sepals. The flowers can be collected in early summer (April to June) and the seeds are collected in July to November (Editorial Board of Flora of China, Chinese Academy of Sciences, 2004).
Roseceae are widely distributed in the temperate and subtropical regions of the northern hemisphere, and their centres are localized in Central and South-West Asia. Asia has the largest number of wild rose species and the longest history of their existence. China is the modern distribution centre of Rosaceae plants, and R. laevigata is mainly found in south-western, southern, central and eastern China. They grow in area such as Shaanxi, Anhui, Jiangxi, Jiangsu, Zhejiang, Hubei, Hunan, Guangdong, Guangxi, Taiwan, Fujian, Sichuan, Yunnan and Guizhou. This plant is usually found in sunny mountain fields, field margins and streamside, and it is generally found in the mountains at altitudes of 200–1600 m (Liu X. G. et al., 2013). R. laevigata is also found in Tibet, mainly in the sunny mountains of southern Tibet at altitudes of 1500–3500 m. R. laevigata produced in this region is influenced by the special climatic conditions and is of better medicinal quality than the mainland varieties (Liu et al., 2010a).
Rosa laevigata Michx. was first published in the Shu Ben Cao(Han, Five Dynasties) written by Han Baosheng during 935-960 AD (Later Shu of the Five Dynasties) (Han et al., 935-960 AD). Its description in this auspicious work was: “R. laevigata is found everywhere. The flowers are white. The seeds resemble quince but are small and yellow with spines. It is often used in Chinese traditional medicine.” Dream Pool Essays (Shen et al., the end of the 11th century), which was written at the end of the 11th century, mentioned the following: “R. laevigata, stop the ejaculation, to take its warm and astringent, the world with gold poppy, to be its red ripe, take the juice and boil the paste to use, a big mistake, red is the taste of sweet, boil the paste is all broken astringent taste, all lose the nature. Nowadays we should pick the half yellow leaves, and these should be dried and pounded before use.” It is also written in the Compendium (Li et al., the middle of the Ming Dynasty) (written in the middle of the Ming Dynasty, during the Jiajing period) that “If one takes R. laevigata for no reason, in order to obtain a quick desire, one should not; if one takes them if one’s essence is not solid, there is no blame.” Written by Huangfu Zhong between 1,368 and 1644 AD, the Guide to Famous Doctors (Huangfu et al., 1368-1644 AD)states that “R. laevigata is useful for treating dream loss and spermatorrhea”. There are also many local stories that include the effects of R. laevigata. For example, the Min Dong Materia Medica (The Editorial Committee of Eastern Fujian Materia Medica, 1962) mentions that R. laevigata can be used to treat hypospermia and spermatorrhea in men and leucorrhoea in women, as well as to treat pubic erections. The Quanzhou Materia Medica (Quanzhou Municipal Science and Technology Commission et al., 1961)also records that R. laevigata can cure frequent urination, polyuria, incontinence of urine, and also the chronic deficiencies diarrhea and dysentery.
Radix R. laevigata has also been included in many local chronicles: the Lingnan Herb Journal (Guangdong Institute of Traditional Chinese Medicine et al., 1961) records that it can cure spermatorrhea and treat stomach pains. The Hunan Medicinal Journal (Hunan Institute of Traditional Chinese Medicine, 2017) mentions that the roots of R. laevigata is a remedy for children’s enuresis, for diarrhoea, and as a treatment for bruises. It is also described in the Jiangxi Folk Herbal Recipes (Gong et al., 1963) as a remedy for women’s leaks and for fire and soup injuries, as well as for recurrent fires in the lower limbs. In the Min Dong Materia Medica (Editorial Committee of Eastern Fujian Materia Medica., 1962), the roots of R. laevigata are used to treat lumbar spinal pain and rheumatic joint pain. The Chinese Medicine in Guangdong (Guangdong Provincial Department of Health, 1959) documents its effectiveness in treating a prolapsed uterus. A summary of the prescription names, sources of prescriptions and formulas for R. laevigata and its roots is given in Supplementary Table S1.
This shows that the Chinese patent medicines which have R. laevigata as one of the main ingredients include Shuiluerwei pill, Zhuangyaojianshen pills, Zhuangyaojianshen tablets, ancient Chinese health essences, ancient Chinese health tablets and so on. Chinese patent medicines with the roots of R. laevigata as the main ingredient include Gynecological Qian Jin Tablets, San Jin Tablets, Gynecological Qian Jin Capsules, Jin Ji Capsules and Guangdong herbal tea. The sales of these products support the Chinese economy to approximately US $5 billion.
The four representative prescription formulations that existed in traditional applications have been optimized with modern technology to produce the widely used proprietary Chinese mediciens (pCms) of today, namely Guangdong Herbal Tea Granules, Gynecology Qianjin Tablets, Sanjin Tablets and Jin Ji Capsules.
Guangdong Herbal Tea Granules (State Drug Certification Z44020615) are granules, which are manufactured by the Guangzhou Wanglaoji Pharmaceutical Co. They are used for clearing away damp-heat, relieving summer heat and producing body fluids containing waste products harmful to the person. They are also used for treating colds of the four seasons, fever and sore throat as well as damp-heat stagnation, dry mouth and redness of the urine.
Gynecology Qianjin Tablets (State Drug Certification Z43020027) are manufactured by the Zhuzhou Qianjin Pharmaceutical Co. These are used for clearing away heat, tonifying Qi and resolving blood stasis. For heat and stagnation caused by these symptoms that originate in the abdomen, manifesting as profuse, yellowish and thick, foul-smelling discharge, abdominal pain, lumbosacral pain and fatigue. These are also useful for treating chronic pelvic inflammatory disease, endometritis and chronic cervicitis with the above symptoms. As a Qianjin Pharmaceutical’s flagship product,“Gynecological Qianjin Tablets” still accounts for a disproportionately large share of the main business sales revenue, accounting for more than 80% of its sales revenues, and it is the “only product” of note from this company.
Sanjin Tablets (State Drug Certification Z45021645) are manufactured by the Guilin Sanjin Pharmaceutical Co. They are used for clearing away heat and detoxifying harmful toxins in the body, clearing away damp-heat and promoting drenching, and benefiting the kidneys. They are also used for treating pyorrhea and redness in the urine, dripping and astringent pain, frequent urination due to humidity and heat in the lower jiao as well as acute and chronic pyelonephritis, cystitis and urinary tract infections associated with the above symptoms. By the end of 2015, “Sanjin Tablets” had established long-term stable customer relationships with more than 500 distributors and more than 2,300 hospitals of Grade IIA or above as well as more than 21,000 pharmacies. They occupied the herbal markets in more than 30 provinces and cities across the country, with leading levels of quality, technology, efficacy and other indicators always being assured. This product has proven to have outstanding qualities and it has remained on the market for more than 30 years.
Jin Ji Capsules (State Drug Certification Z45020293) are manufactured by the Guangxi Lingfeng Pharmaceutical Co. Its functions include clearing away heat and detoxifying harmful toxins, invigorating the spleen and clearing away damp-heat, promoting circulation and activating circulation of the blood. In addition, for adnexitis, endometritis and pelvic inflammatory disease caused by damp-heat infiltration.
These proprietary Chinese medicines are mainly made of Radix R. laevigata, are household names in China and have a pharmaceutical output value of several billion RMB in the country.
So far 148 components have been isolated from Rosa laevigata Michx. of which (82 and 68 compounds are from Fructus R. laevigata and Radix R. laevigata and 15 and six are new, respectively. In addition, 12 identical chemical components are present in both herbs) and these include triterpenes, flavonoids, tannins, lignans, phenolics and other compounds. The chemical constituents that have been identified are listed in Table 1 and their corresponding structures are diagrammatically shown in Figures 1–7.
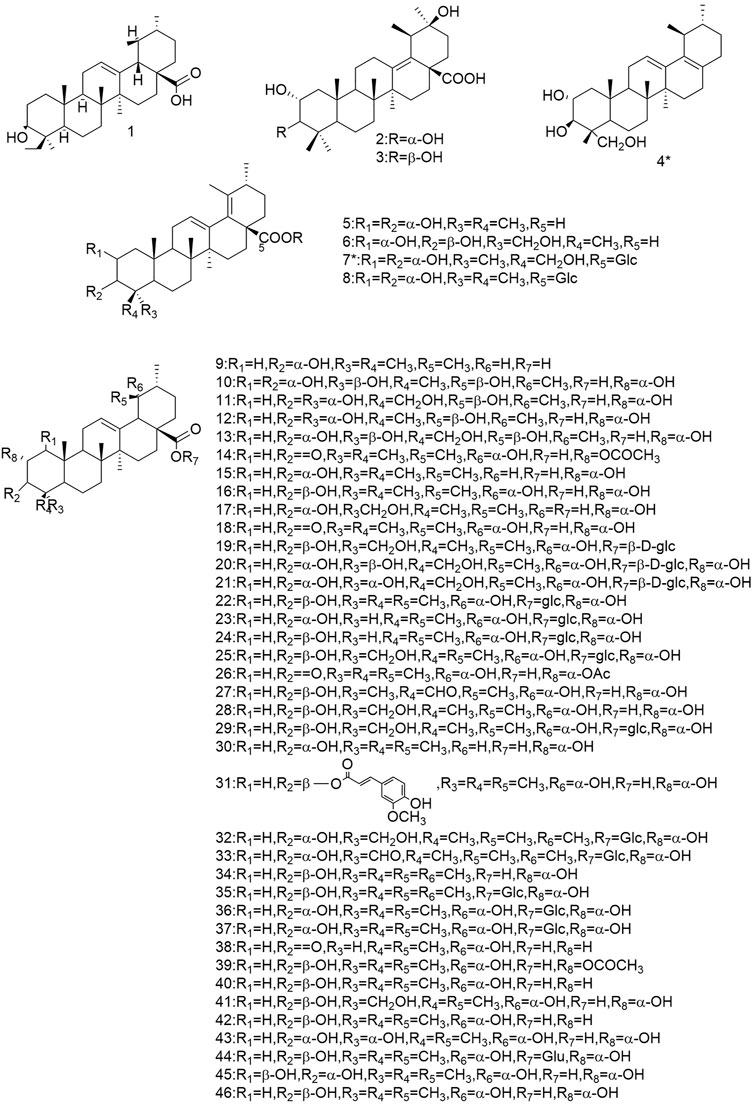
FIGURE 1. Diagrammatic representation of the structural formulas of triterpenoids of compounds found in R. laevigata Michx.
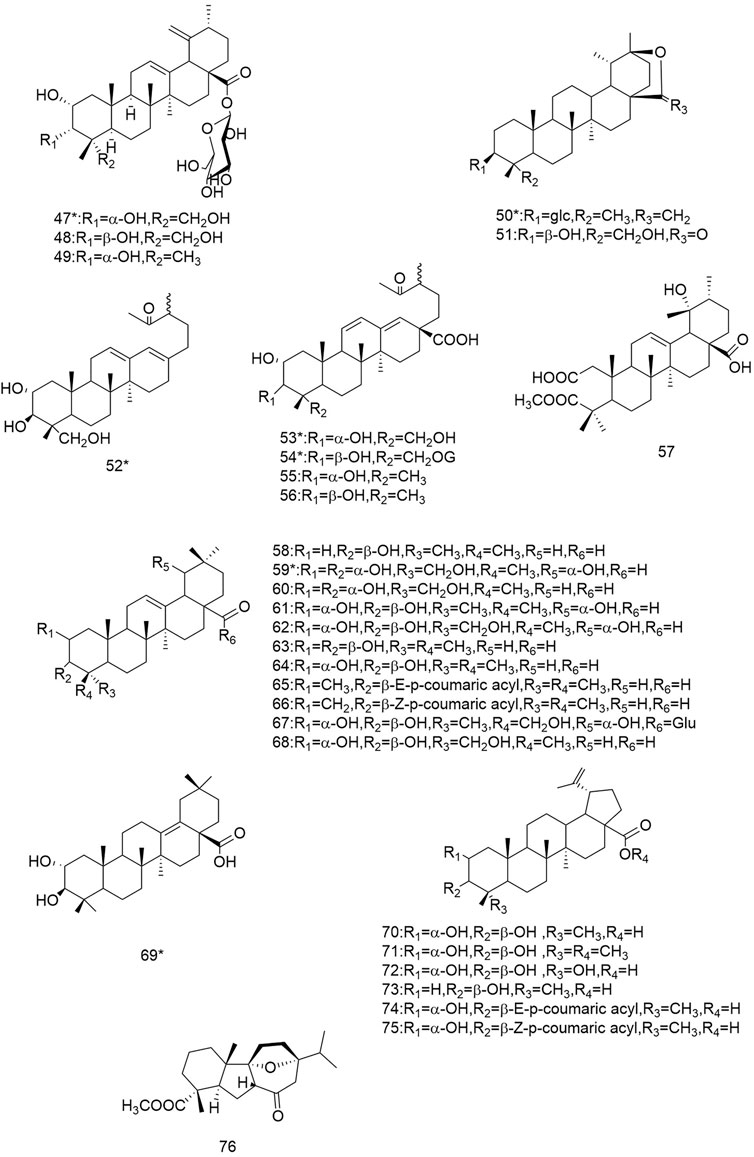
FIGURE 2. Diagrammatic representation of the structural formulas of triterpenoids of compounds found in R. laevigata Michx.
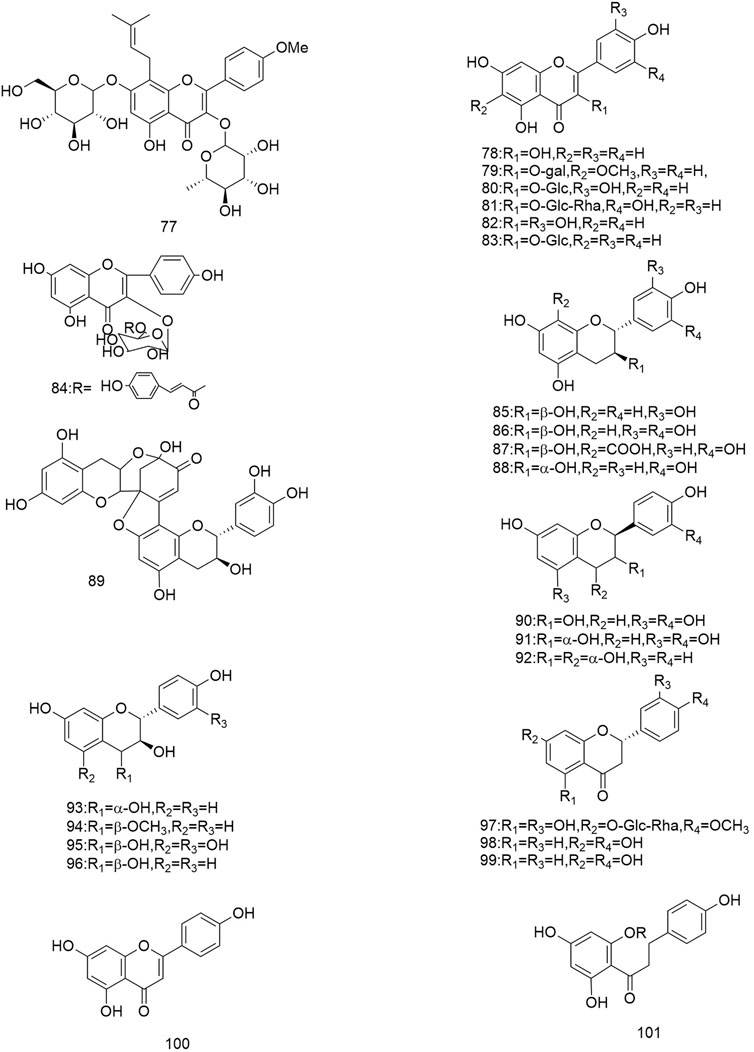
FIGURE 3. Diagrammatic representation of the structural formulas of flavonoids of compounds found in R. laevigata Michx.
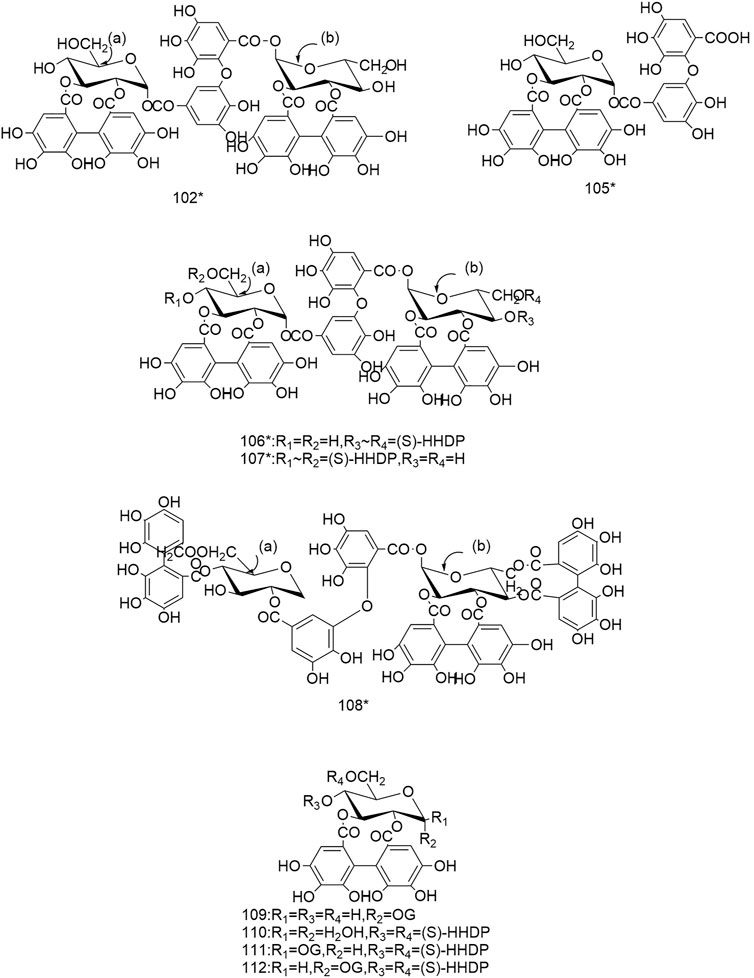
FIGURE 4. Diagrammatic representation of the structural formulas of tannins of compounds found in R. laevigata Michx.
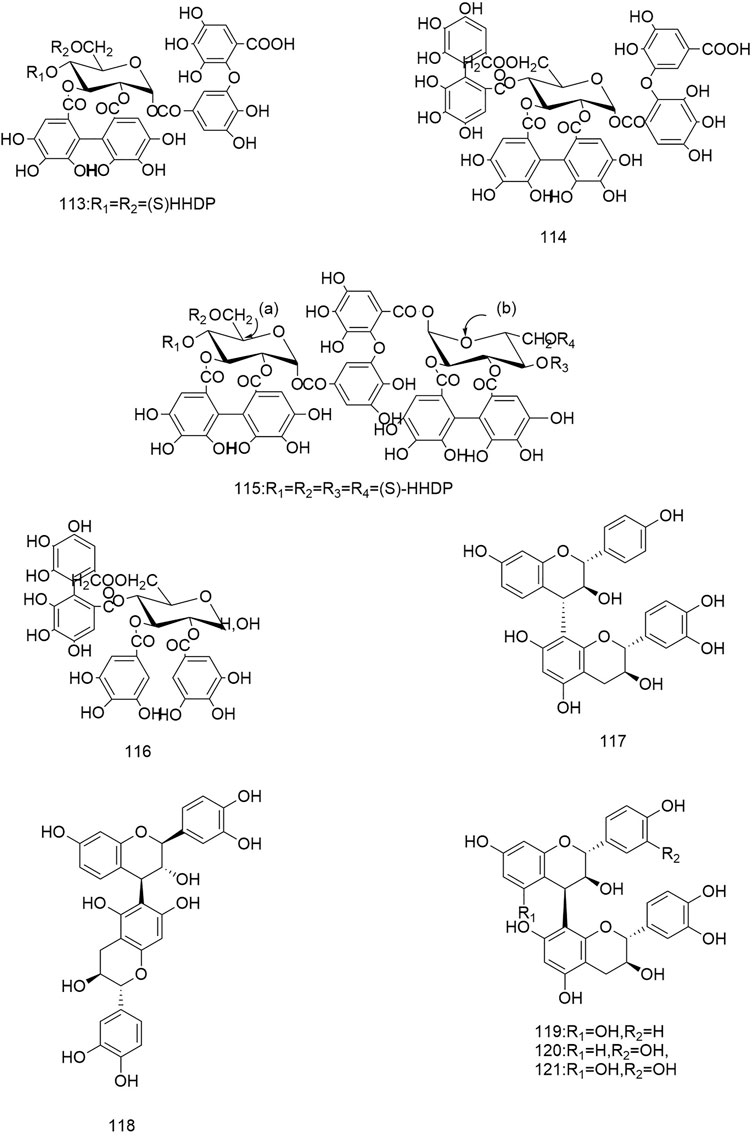
FIGURE 5. Diagrammatic representation of the structural formulas of tannins of compounds found in R. laevigata Michx.
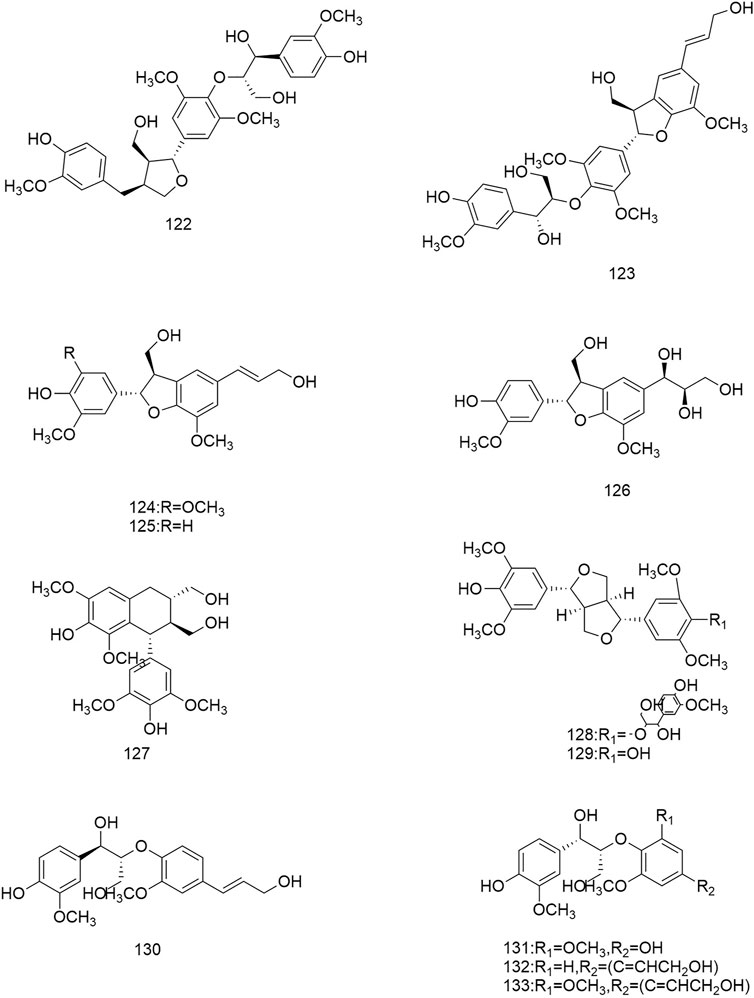
FIGURE 6. Diagrammatic representation of the structural formulas of others of compounds found in R. laevigata Michx.
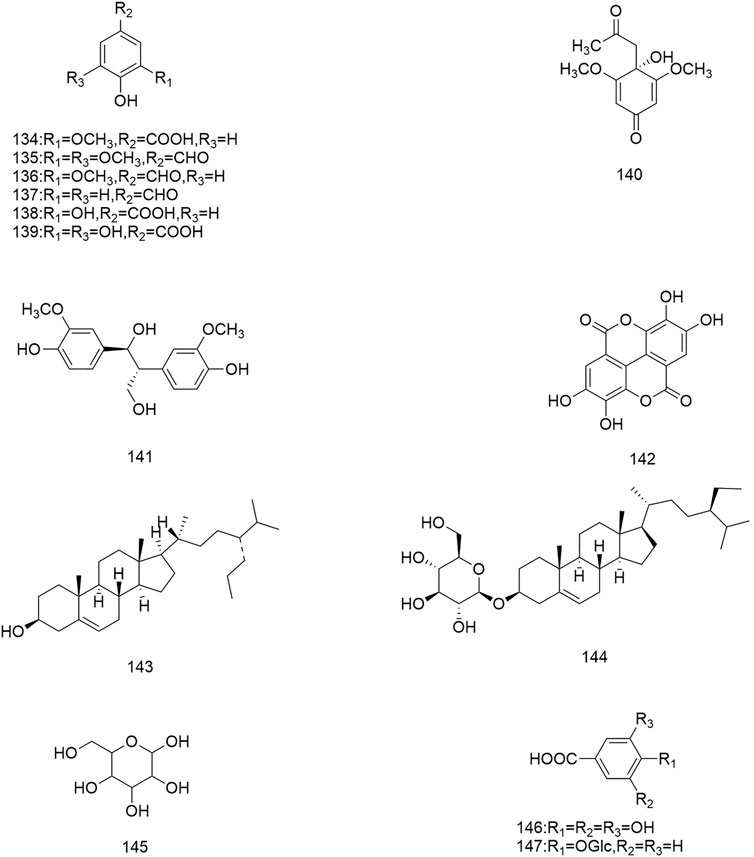
FIGURE 7. Diagrammatic representation of the structural formulas of others of compounds found in R. laevigata Michx.
Among these, the triterpenes are its main active ingredients and are characteristic components of this herb.
The triterpenoids and their derivatives isolated from this species are divided into three types: the ursane, the oleanolane and lupine-types, of which the uresane-type accounts for the majority. To date, 76 triterpenoids have been identified from Fructus R. laevigata and Radix R.laevigata, with 57 (1-57) of the ursane-type, 12 (58-69) of the oleanane-type and 6 (70-75) of the lupine-type. In addition, there is one other conformation of triterpene (76), of which there are a total of 10 novel compounds. There are: 3β-[(R-l-arabinopyranosyl)oxy]-20β-hydroxyursan-28-oic acidδ-lactone (4), 2ɑ,3β,23-trihydroxy-12,17-dien-28-norursane (6), 2ɑ,3β,23-trihydroxy-19-oxo-18,19-seco-12,17-dien-28-norursane (7), 2ɑ,3ɑ,23-trihydroxy-19-oxo-18,19-seco-urs-11.13 (18)-dien-28-oic acid (8), 18,19-split ring-2α,3β,23α-trihydroxy-19-carbonyl-ursane-11.13 (18)-diene-28-carboxylic acid (9), 2α,3α,24-trihydroxy-urs-12,18-dien-28-oic acid-β-d-glucopyranosyl ester (14), 2α,3α,23-trihydroxy-urs-12.19 (29)-dien-28-oic acid-β-d-glucopyranosyl ester (16), 19α-OH-3β-E-feruloyl corosolic acid (42), 2α,3α,19α,23-tetrahydroxyolean-12-en-28-oic acid 59) and 2α,3β-dihydrox-yolean-13 (18)-en-28-oic acid (69).
The flavonoids are widely found in natural plants and can be isolated from Fructus R. laevigata and Radix R. laevigata, and about 25 compounds have so far been isolated. These flavonoids are subdivided into flavonols (77-84), flavan-3-ols (85-91), flavan-3,4-diols (92-96), flavanones (97-99), flavonoids (100) and one other conformation of flavonoids (101). Of these 25 compounds, two are new and these are (+)-catechin-8-acetic acid (87) and guibourtacacidine 4-methyl ether (94).
The extraction and isolation of the total flavonoids from Fructus R. laevigata and Radix R. laevigata have been a particular focus of attention and most of these components have already been identified.
To date a variety of tannins have been isolated from Fructus R. laevigata and Radix R. laevigata, including dimeric ellagitannins (102-104), hydrolysable tannins (105-108), condensed tannins (117-121) and a few other tannins (109-116). Among these seven newly discovered tannins have been identified and these are: tannin E-G (102-104), laevigatin A-D (105-108).
In addition to the three groups mentioned above, Fructus R. laevigata and Radix R. laevigata also contain lignans (122,131), phenols (123-130,132-137,140-142), catechins (138), benzoic acid derivatives (139), sterols (143-144), stilbene compounds (146-147), polysaccharide 148) and other compounds glucose 145).
Pharmacological studies have shown that Fructus Rosa laevigata and Radix Rosa laevigata have a variety of pharmacological activities, including antioxidant activity and renal protection. Moreover, they play a role in immune regulation and have hypolipidemic, anti-inflammatory, cardiovascular protective and antibacterial activities. In addition, Rosa laevigata Michx. also has an important role in combating diabetes and it has antiviral and anti-tumour activities. The pharmacological effects of R. laevigata and its monomers are summarized in Table 2.
The antioxidant activity exhibited by R. laevigata depends on its ability to scavenge free radicals and cause the reduction of metal ions. Studies have shown that Fructus R. laevigata is rich in total flavonoids (TFs), a component that is closely associated with antioxidant activity. This suggests that TFs in R. laevigata may be a rich source of natural antioxidants for the prevention and treatment of diseases caused by oxidative stress such as cardiovascular disease (Li et al., 2021). Liu et al. (2010b) found that TFs of Fructus R. laevigata have a scavenging effect on 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydroxyl and superoxide anion radicals and they have a strong reducing ability. Intragastric administration of TFs (25, 50 mg/kg/day) to hyperlipidemic mice for 4 weeks, showed that TFs significantly increased the levels of several antioxidant substances including catalase (CAT), superoxide dismutase (SOD), GSH and GPX in the liver. It was also seen that TFs significantly reduce hepatic malondialdehyde (MDA) concentrations in a dose-dependent manner.
Liu et al. (2014, 2019) also found a protective effect of TFs of Fructus R. laevigata against hydrogen peroxide-induced cell damage. The data showed that TFs down-regulated the expression of CYP2E1, iNOS, nuclear factor-kappab (NF-κB), BaK and caspase-3 and significantly reduced the mRNA levels of TNF-α and Fas/Fasl. In addition, Jia et al. (2012) concluded that TFs of Fructus R. laevigata can reduce the expression of fragmented DNA, Bax, Bid and p53 as well as activity the activities of caspases-3 and -9. They also increased the protein expression of procaspase-3 and Bcl-2. It is clear that these three mechanisms of antioxidant activity are similar. In summary, TFs can exert antioxidant activity by reducing oxidative stress, cell inflammation and apoptosis, and there is a good quantitative relationship between the TF content and antioxidant activity at a range of concentrations.
There are also several flavonoid quercetin and proanthocyanidins that have antioxidant activity and their scavenging effects of free radicals are also strong. In addition, quercetin has some inhibitory effects on linoleic acid peroxidation, and proanthocyanidins have inhibitory effect on lipid oxidation (Xiao et al., 2010; Chen et al., 2013). Of course, the flavonoids are not the only compounds to have antioxidant activities in R. laevigata, and the others are the total phenols. Meng et al. (2012) found that total phenols have strong scavenging ability for 2.2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals. They also have strong copper ion reduction and metal chelating abilities, Moreno et al. (2007), Apak et al. (2004) and, Du et al. (2009) investigated the ABTS scavenging, copper ion reducing, and metal chelating abilities, respectively. Their results showed a correlation between total phenolic content and antioxidant activity.
From a review of the relevant literature, it san be seen that polysaccharides in Fructus R. laevigata also have antioxidant activity. When the protection rate of vegetable and animal oils was approximately 50%, the mass concentration of polysaccharides was 2.8 and 5.9ng/ml, respectively. The scavenging of hydroxyl radicals by polysaccharides was 4.7 ng/ml when the mass concentration of R. laevigata was at 50%. The scavenging rate of superoxide anion radicals by the same concentration of polysaccharides of Fructus R. laevigata was only 23%. In addition the reduction ability of polysaccharides of R. laevigata to Fe3+ was also found to be very strong. The results showed that the antioxidant activity of Fructus R. laevigata had a good quantitative relationship with the polysaccharide content. However, a single approach cannot fully demonstrate the extent of the antioxidant capacity of a substance. Therefore, it is not always straightforward to correlate the antioxidant capacity of different substances (Wang Y. M. et al., 2016).
Fructus R. laevigata is believed to be able to improve kidney health. There are many studies that have verified the kidney-protective effects of this herb. Zhou et al. (2012) investigated the effects of R. laevigata on renal oxidative stress in streptozotocin-induced diabetic rats. The results showed that R. laevigata significantly improved the renal dysfunction of these diabetic rats. The mechanism involves an increase in SOD and total antioxidant activities and a reduction in the levels of MDA and reactive oxygen species (ROS). The expression of nuclear factor-κB p65 and monocyte chemotactic protein-1 was inhibited at the protein and mRNA levels, respectively, while the expression of IκBα protein was increased. Zhao et al. (2016) found that TFs in R. laevigata have a significant nephron-protective effect on ischemia-reperfusion injury (IRI) by affecting the Sirt1/Nrf2/NF-κB signaling pathway. After treatment with R. laevigata, SOD activity and total antioxidant power increased and the levels of MDA and ROS decreased in rats with kidney injury, achieving an overall reduction in kidney injury. Thus, the effect of adminishtration of R. laevigata extracts on kidney damage is mainly dependent on Sirt 1.
In recent years, a number of studies have shown that the ethanol extracts of Fructus R. laevigata can have some immunological activity. Zhan et al. (2020) identified a novel acidic polysaccharide, PPRLMF-2, which recognizes pattern recognition receptors (PRRs) of macrophages and enhances their immunomodulatory activity by activating MAPKs and NF-κB signaling pathways. The mechanism of action is that PPRLMF-2 can significantly increase the phagocytosis and the secretion of cytokines in murine RAW264.7 cells lines. In addition, SR, GR, Toll-like receptor-2 (TLR-2) and Toll-like receptor-4 (TLR-4) are the major PRRs that upregulate the expression of p-Extracellular signal-regulated kinase (p-ERK), p-c-Jun N-terminal kinase (p-JNK), phospho-NF-κB p38 (p-p38) and phospho-NF-κB p65 (p-p65). In addition, Gao et al. (2018) also found the presence of polyhydroxy triterpenoids which have anti-acetylcholinesterase activity in extracts from R. laevigata. Experiments showed that two pure polyhydroxytriterpenes, 1 and 2, were isolated by the authors and these showed potent anti-acetylcholinesterase activities. Diao et al. (2014) studied the effects of fermented R. laevigata on the immune performance of weaned piglets. They showed that fermented R. laevigata could significantly increase the levels of immunoglobulin IgA, IgM and IgG in these animals (the most suitable concentration was 0.2%) and this improved the immunity of the weaned piglets.
Peng et al. (2014) performed a study on the immunomodulatory activity of Radix and Rhizome R. laevigata of different origins. Their results showed that the hydroextracts of Radix R. laevigata had some specific immunosuppressive activities. It was concluded that different medicinal parts of R. laevigata different origins had similar immunological activities without any significant differences.
TFs components of Fructus R. laevigata have received more attention as lipid-lowering. Zhang et al. (2013a) investigated the effects of TFs in R. laevigata on non-alcoholic fatty liver disease (NAFLD) induced by high-fat diets in animal studies. The results suggest that TFs inhibit hepatic fat accumulation by suppressing the expression of some key molecules in the fatty acid synthesis pathway and promoting the β-oxidation fatty acids. They also showed that TFs did not achieve lipid-lowering activity by inhibiting cholesterol synthesis. In addition, Liu Y. et al. (2010) also found that TFs had strong hypolipidemic activity and the levels were governed partly by enhancing the antioxidant system.
A review of other related literature revealed that the polysaccharides in R. laevigata also have hypo-lipidemic activity. Zhang et al. (2020) found that low molecular polysaccharides extracted from the fruits of Rosa laevigata (RLPs) reduced serum lipid levels and increased those of HDL cholesterol. After further study, the results showed that RLPs (with molecular weights of 9,004, 8,761 and 7,571 Da) exhibited significant hypolipidemic effects in high-fat diet-induced muscle, and they did so by integrating with the PPPAR signaling pathway to improve lipid metabolism dysfunction. The study also illustrated that high doses of RLPs havd stronger hypo-lipidemic effects, and these were more positive when compared to that of polymeric polysaccharides. Another paper also investigated the hypo-lipidemic activity of the polysaccharide, RLP-2 (21.5 kDa) (Yu et al., 2013). They found that the mechanism of action in both studies were similar, and further studies determined that the strength of the activities of these polysaccharides verified the effects of molecular weight on the hypolipidemic activity. In addition, the total polyphenols and saponins in R. laevigata also have been shown to have some hypo-lipidemic activity (Dong et al., 2019; Li J. et al., 2019). However, because the mechanism of lipid synthesis is complex and it has been difficult to identify a specific major factor or mechanism to explain the hypo-lipidemic activity of R. laevigata, so further studies still need to be performed.
Among the studies on the anti-inflammatory activity of Fructus R. laevigata, triterpenoids have received the most attention. Yan et al. (2013) investigated the anti-inflammatory activity of triterpenoids by using the LPS macrophage-induced luciferase assay. Binding of NF-κB to the κB site can control cytokine expression, and the mechanism by which triterpenoids inhibit NK-κB activation may be determined by performing NF-κB luciferase assays in NF-κB-luc293 cells. The triterpenoids 4, 9, 11, and 12 in this study were found to have inhibitory effects on cytokine release in LPS-stimulated mouse macrophages RAW264.7. It was also found that compound 12 had a much stronger anti-inflammatory activity than compound 11. This was probably, due to the presence of a hydroxyl group at C-23, which may have greatly reduced the release of inflammatory factors. Compound 12 showed moderate inhibition of the transcriptional activity of NF-κB with an IC50 of 23.21 μM. Its inhibitory activities against TNFα-release, IL-1β-release, IL-6-release and IL-10-release were 14.32, 8.53, 8.04 and 10.38μM, respectively. The presence of glucoside in this compound may have caused an increase in the anti-inflammatory activity.
Several scholars have also found anti-inflammatory effects of R. laevigata in animal disease models. Ko et al. (2020) found that R. laevigata inhibited the expression levels of MAPK/NF-κB pathways and its downstream signal COX-2 in PM10-induced A549 cells in an induced lung inflammatory disease model. In this dhtudy, R. laevigata was shown to reduce the pro-inflammatory factors activated by PM10. The results in the literature suggest that R. laevigata alleviates PM10-induced lung inflammation, and the mechanism may be that pretreatment with this herb medicine inhibits PM10-induced activation of MAPK phosphorylation and suppresses nuclear translocation of NF-κB p65. In contrast, in a mouse model of chronic inflammation, Lee et al. (2020) investigated whether R. laevigata exhibited anti-inflammatory effects associated with acute asthma in both in vitro and in vivo experiments. Their results showed that after RLM pretreatment, which significantly inhibited EGF-induced NF-κB activity and COX-2 expression levels in A549 cells, inflammatory cells, IgE secretion and related substances were reduced in the model in a dose-dependent manner to reduce allergic airway inflammation. The anti-inflammatory mechanism seen may act through the inhibition of IgE and related cytokines.
It was not only Fructus R. laevigata which had anti-inflammatory activity, but also Radix R. laevigata. Its chemical composition is high in tannins, which play an important role as anti-inflammatory agents (Zou and Qiu, 2011). Zou and Qiu (2011) established a model and found Radix had a better anti-inflammatory effect by measuring its effect on NO release from lipopolysaccharide-induced peritoneal macrophages in mice, and this effect was related to the inhibition of NO release.
The protective effects of Fructus R. laevigata on cardiovascular and cerebrovascular are mainly due to the antioxidant and anti-inflammatory activities of its active ingredients. For example, Luo et al. (2014) found that R. laevigata could increase the mRNA expression levels of CuZn-SOD protein as well as glutathion peroxidase (GSH-PX), catalase (CAT) and superoxide dismutase (SOD) activities in myocardial tissue. High doses of R. laevigata reduced myocardial apoptosis induced by adriamycin and enhanced Bcl-2 gene expression and decreased Bax levels. This confirmed the protective effects of R. laevigata on adriamycin-induced myocardial injury. Liu et al. (2010a) found that high doses of oral TFs reduced total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C).
Jia et al. (2012) demonstrated the protective effects of TFs in R. laevigata against hydrogen peroxide-induced injury in human umbilical vein endothelial cells. Qu et al. (2015) established a myocardial infarction (MI) rat model to study the ability of the active ingredients of R. laevigata in the treatment of this condition. The results showed oral administration of the active ingredients to MI-induced animals gradually restored the decline in cardiac function caused by MI and induced the myocardial regeneration and replacement of necrotic heart tissues. The authors hypothesized that the mechanism of myocardial regeneration induced by the active ingredients of R. laevigata may be the multiple properties of the active ingredients in anti-inflammatory and anti-oxidative stress. This promoted cell survival and prevented IRI. However, the action of the specific ingredients and the specific mechanisms involved are still unclear and need to be further investigated. Chen et al. (2014) found that medium and high concentrations of TFs significantly reduced whole blood viscosity in rats and had some inhibitory effects on platelet aggregation, thus exerting antithrombotic effects. Additionally, Luo et al. (2014) found that R. laevigata could improve doxorubicin (DOX)-induced myocardial toxicity and that co-administration with cloxacin (LOS) provided better myocardial protection. This mechanism may also be related to the anti-inflammatory and antioxidant effects of this plant.
One of the many pharmacological effects of Fructus R. laevigata, such as its antibacterial effect, also plays an important role in daily life. Staphylococcus aureus, Bacillus subtilis and Escherichia coli are considered to be serious modern day hazards and these can affect food hygiene and safety. Liu et al. (2009) preliminarily studied the inhibitory effect of extracts of polysaccharides and flavonoids from the fruits of R. laevigata on these three species of bacteria. The results showed that both extracts had some antibacterial effects, with the polysaccharide having the best antibacterial effect on E. coli (MICs of polysaccharides of R. laevigata on these three bacteria were 25, 50 and 3.13 mg/ml, respectively). This was followed by S. aureus, while the inhibitory effect of flavonoids was exactly the opposite (MICs of flavonoids for these three bacteria were 3.13, 12.5 and 6.25 mg/ml, respectively). Both the polysaccharides and flavonoids showed the weakest inhibition with B. subtilis. However, the relationship between its inhibitory mechanism and its specific structural composition needs to be further investigated. A review of the relevant literature revealed that the roots and stems of R. laevigata are also known to possess antibacterial activities. The acetone extract of R. laevigata had an inhibitory effect on Streptococcus mutans which usually exist in the oral cavity, and the inhibitory effect of this extract was mainly derived from the tannin component of R. laevigata (Li, 2006).
Lai et al. (2009) determined the bacterial inhibitory effects of the roots and stems polysaccharides of R. laevigata using the punch-hole method and an agar diffusion technique. The results showed that the polysaccharides in the roots and stems of R. laevigata inhibited Staphylococcus albicans, Staphylococcus citricola, S. aureus, Klebsiella pneumoniae and Bacillus dysenteriae in a dose-dependent manner. There were also no significant differences in the inhibitory effects of extracts from the two sites. In addition, Lai et al. (2012) also investigated the differences in the antibacterial effects between extracts obtained from using different polar solvents of the roots. The results showed that the aqueous and alcoholic extracts of Radix R. laevigata had better antibacterial effects, mainly due to the higher content of flavonoids, tannins and triterpenoids in these two extracts.
Huang and Liu. (2017) studied the effects of two flavonoid active components of Fructus R. laevigata, rutin and quercetin, on the proliferation of human hepatocellular carcinoma cells cultured in vitro. Both flavonoids were found to exert some inhibitory effect on the proliferative effects of human hepatoma cells BEL-7402 cultured in vitro, with rutin and quercetin having IC50s of 29.91 ± 3.05 and 7.625 ± 2.02 μmol/L, respectively. There were also less toxic to normal human hepatocytes cultured in vitro HL-7702, with IC50s of 29.91 ± 3.05 and 35.54 ± 4.37 μmol/L, respectively. Others also investigated the in vitro antitumor activity of polysaccharides from R. laevigata and found that these compounds had some inhibitory effects on human hepatocellular carcinoma cells cultured in vitro (Huang and Liu, 2015). The inhibitory effect on the proliferation of human hepatocellular carcinoma cells, BEL-7402, cultured in vitro, had an IC50 of (12.43 ± 1.95) μmol/L. There was a lower toxicity to normal human hepatocytes, HL- 7,702, in vitro, with an IC50 of (31.04 ± 3.68) μmol/L. Therefore, it was also concluded that the polysaccharide compounds in R. laevigata also had some anti-tumor activity.
However, polysaccharides in Radix R. laevigata had no significant therapeutic effect when used directly as an anti-tumour drug. However, when combined with 5-Fu, it had a significant potentiation and toxicity reduction effect (Feng and Tian, 2011), suggesting that polysaccharides in the root of R. laevigata may act as a potential anti-tumour adjuvant.
Liu et al. (2017a) studied the direct inactivation, prophylaxis and post-viral penetration inhibition of respiratory syncytial virus (RSV), herpes simplex virus-1 (HSV-1), coxsackie B5 (COX-B5) and enterovirus 71 (EV71) by different extracts of Fructus R. laevigata. Their results showed that Fructus R. laevigata had no preventive effect and no post-penetration inhibition against these viruses. The direct inactivation of RSV by ethyl acetate extract was good with a therapeutic index (TI) of 19.333, which was comparable to that of the positive control, ribavirin (TI of 19.760). The n-butanol extract was able to directly inactivate COX-B5 with a TI of 16.622 and the positive control, ribavirin, with a TI of 17.562. The alcohol extract was most effective against HSV-1 with a TI of 18.922 and the positive control, acyclovir, with a TI of 23.742, while there was no direct inactivation of EV71. However, when using the sonicated of R. laevigata in acetone, methanol and ethanol it was found that the 60% sonicated ethanol extract was relatively more effective against RSV and EV71 than the other two (Li N. et al., 2019).
Liu et al. (2017b) also investigated the in vitro antiviral activity of polysaccharide in R. laevigata and studied their inhibitory effects on RSV, COX-B5 and EV71. They showed that the polysaccharides had in vitro anti-RSV, COX-B5 and EV71 activities, and the anti-RSV and COX-B effects were better than those of the positive control drug ribavirin, with TI of 80.895 and 41.541, respectively. Also some studies have shown that a combination of polysaccharides of R. laevigata and adriamycin has a significant potentiation and toxicity reduction effect, which could significantly increase the tumour inhibition rate. In addition, some studies have shown that the active ingredients of R. laevigata against HSV is a polyhydroxyl pigment (Luo et al., 1989). However, the active ingredients against other viruses have not yet been identified and further research is still needed in this area.
Diabetes is now a “very common” disease and is increasingly being researched and the active use of herbal medicines to treat diabetes and its related diseases are increasing. Liu et al. (2015) investigated the effects of Fructus R. laevigata on the production of ROS and the mitochondrial membrane potential (MMP) in lens epithelial cells under high glucose conditions by establishing a cell model of SRA01/04 in diabetic cataract. The results showed that under high glucose conditions, R. laevigata inhibited ROS production and increased MMP by inducing HO-1 expression. This effect was mediated by the PI3K/AKt and Nrf2/ARE pathways, which are lost if one of these pathways when they are inhibited. Thus it was concluded that R. laevigata can play an important role in the treatment of diabetic cataract.
In a study by Zhou et al. (2014), Fructus R. laevigata was found to inhibit the apoptosis of rat diabetic cataract lens epithelial cells by increasing the Bcl-2/Bax expression ratio, and thus delaying the onset and development of diabetic cataract. In addition, Kumar et al. (2021) used in vitro DNS glucose uptake and western blotting analysis to study the hypoglycaemic effects of ethanolic extracts of R. laevigata and its derivative subfractions (in water, n-butanol, ethyl acetate and n-hexane). They studied the effects of its main bioactive compound, glutarone, on normal and high glucose-induced insulin-resistant hepatic HepG2 cells. The results showed that the ethanolic extract of R. laevigata and its derivative sub-fractions significantly increased glucose uptake in hepatocytes, and the bioactive glutamatergic ingredients significantly increased glucose uptake in insulin resistant cells and promoted insulin sensitivity. This led them to conclude that R. laevigata had an important role in hypoglycaemia and that it can also be effective in improving the hyperglycaemic and hyperlipidemic states of patients with type Ⅱ diabetes (Deng and Zhang, 2018).
The liver is the largest metabolic organ in the body and is associated with a large number of diseases related to its damage and hepatotoxicity. Many studies have revealed that the mechanism of action of Fructus R. laevigata in protecting liver tissues is most closely linked to their anti-inflammatory activity, antioxidant capacity and resistance to oxidative stress. Generally, the main components that exert hepatoprotective effects are the TFs. For example, Zhang et al. (2013b) studied the protective effect of TFs on CCl4-induced hepatotoxicity in mice and found that pretreatment with TFs reduced the expression of CYP2E1, iNOS, NF-κB, Bak, Caspase-3, TNF-ɑ and Fas/FasL, and upregulated the levels of Bcl-2. This reduced the incidence of liver lesions and improved the abnormalities of hepatocytes, thus achieving a protective effect on the liver. In addition, TFs significantly reduced the CCl4-induced increase in aspartate aminotransferase (AST) and alanine transferase (ALT) activities. The TFs of R. laevigata also exerted protective effects against lipopolysaccharide (LPS)-induced liver injury in mice by modulating FXR signaling (Dong et al., 2018). They also significantly reduced serum ALT, AST, total triglycerides (TG), total cholesterol (TC) levels and the relative liver weight, resulting in improvement of pathological changes in the liver.
TFs could also significantly reduce tissue MDA levels and increased SOD and GSH-Px levels, and a mechanistic study showed that these compounds significantly increased nuclear erythroid-like factor 2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1), NAD(P)H dehydrogenase (quinone 1) (NQO1), glutamate-cysteine ligase catalytic (GCLC) subunit and glutamate-cysteine ligase regulatory (GCLM) subunit expression levels. They also decreased the expression levels of ECH-like associated protein 1 (Keap1). Additionally, TFs significantly inhibited the nuclear translocation of NF-κB and subsequently reduced the expression levels of interleukin (IL)-1β, IL-6, high mobility group box 1(HMGB-1) and cyclooxygenase-2 (COX-2) by activating FXR and forkhead box O3 (FOXO3a) against inflammation. They could notably reduce the expression levels of sterol regulatory element binding protein-1c (SREBP-1c), acetyl coenzyme a carboxylase-1 (ACC1), fatty acid synthase (FASN) and stearoyl coenzyme A desaturase 1 (SCD1). These compounds increased the expression levels of carnitine palmitoyltransferase 1 (CPT1) by activating FXR to regulate lipid metabolism. In addition to TFs, the total saponins (TSs) in Fructus R. laevigata also had a protective effect against CCl4-induced acute liver injury in mice. A study showed that pretreatment with these compounds significantly reduced the protein expression levels of CYP2E1, ATF6, GRP78, EIF2, COX-2, NF-κB, p53, Caspase-9 and cytokeratin 18 and the phosphorylation levels of MAPKs. They also significantly reduced the gene expression of TNF-ɑ, IL-6, Fas/FasL and Bax. Like TFs, TSs also upregulated Bcl-2 expression and also reduced serum AST and ALT activities, therefore exerting a hepatoprotective effect by these mechanisms (Dong et al., 2013).
Dong et al. (2015) found a protective effect of TSs against CCl4-induced hepatic fibrosis. The mechanism may be that TSs act as an anti-fibrotic agent by down-regulating matrix metalloproteinases for matrix degradation and also by modulating the signaling pathways related to TGFβ/Smad, focal adhesion kinase 1 (FAK), phosphatidylinositol three kinase (PI3K) protein kinase B (Akt)-p70S6 kinase and MAPKs. Liu et al. (2010b) found that TFs of Fructus R. laevigata also had a protective effect against paracetamol-induced liver injury. Dong et al. (2014) investigated the effects of TSs in R. laevigata on liver injury together with acetaminophen and showed that TSs exerted a protective effect against acetaminophen-induced liver injury by inducing autophagy and anti-inflammatory effects as well as apoptotic cell death.
Xie et al. (2001) found strong scavenging effects of Fructus R. laevigata extracts on NO2- under pH acidic conditions that simulated gastric juice production. It has also been found that TFs and the polysaccharide, TNBS, from Fructus R. laevigata exerted mucosal protective effects in mice with Crohn’s disease by reducing the weight/length ratios of their colons. The TNF-ɑ and IFN-γ levels in the colonic tissues were also reduced (Bian et al., 2018). An aqueous extract of Fructus R. laevigata was also found to play an important role in the urinary system, which may be of benefit in reflex incontinence (Lu et al., 1995). In addition, it was shown that a compounded extract of Fructus R. laevigata could effectively treat white diarrhoea in piglets in a dose-dependent manner (Xiang et al., 2015).
One study also found that the alcoholic extract of Radix R. laevigata exhibited significant tolerance to hypoxia and prolonged the survival periods of mice after its administration (Huang and Liu, 2015).
It is well known that many herbs have therapeutic effects as well as adverse reactions. Relatively few toxicity studies have been reported for Rosa laevigata Michx., but as it becomes more widely used, such issues should receive more attention and raise serious questions about its safety in clinical settings. Zhang et al. (2012) studied the subchronic toxicity of TFs obtained from R. laevigata in a 90-day subchronic toxicity study in rats given oral doses of 500, 1,000 and 2000 mg/kg/day of TFs. Toxicity assessment of TFs based on ophthalmic examination, body weight, feed and water consumption, urinalysis, haematology, clinical biochemistry and pathology were performed in rats. No signs of toxicity of TFs were observed at doses of 500 and 1,000 mg/kg/day, while a decrease in platelet count and an increase in cardiomyocyte voids were found in rats at a dose of 2000 mg/kg/day compared to the control group. In the 2000 mg/kg/day dose group, the relative weight of cardiomyocytes increased significantly in male rats, while the absolute and relative weight of the adrenal glands decreased significantly in female rats at doses of 1,000 and 2000 mg/kg/day. The analysis concluded that at a dose of 1,000 mg/kg/day, the TFs caused only mild side-effects in both male and female rats. This led to the selection of a dose of 500 mg/kg/day for rats as the no visible adverse effect level (NOAEL) for R. laevigata.
Li and Feng. (1990) conducted the first toxicological evaluation test on the brown pigment found in R. laevigata. The Ames test is one used for assessment of acute toxicity and the mouse testicular chromosome test that is a mouse bone marrow micronucleus test for harmful elements such as lead and arsenic were performed. The test results showed that the brown pigment was non-toxic, and all other tests were negative, thus concluding that brown pigment in R. laevigata is a safe natural pigment. Pang et al. (2006) investigated the mutagenic effect of R. laevigata on mice by performing the bone marrow micronucleus sperm deformation and sperm non-programmed DNA (UDS) tests. The results showed that within the dose range used in the experiments, R. laevigata did not cause an increase in the frequency of bone marrow micronuclei and hourly sperm malformations in mice. In addition, no induction effect on male mouse germ cells UDS were observed, indicating that R. laevigata caused no genetic damage on mice and that is a safer herbal medicine.
The factors affecting the variation in the constituent composition of Rosa laevigata Michx. are mainly related to geographical distribution. The polysaccharides and TFs in R. laevigata varies greatly from different locations and this can range from 30.5 to 42.7% and from 3.1 to 5.3%, respectively (Shi, 2012). In Fructus R. laevigata, the polysaccharides and TFs are in negative correspondence, and in general, the herb containing high polysaccharide contents will have a relatively low TF content (Zhang et al., 2014). In addition, Lin and Zhou. (2009) determined the tannin content in the fruits of R. laevigata according to the method of the Chinese Pharmacopoeia (I) 2005 edition and concluded that the concentration ranged from 10.20 to 29.76%. Their experiments also showed that the tannin content in R. laevigata also varies according to the geographical environment. Xu et al. (2002) determined the content of triterpenoid acids in R. laevigata from different origins by HPLC, and they showed that the levels of triterpenoid organic acids in the herbs from different origins differed greatly.
National Pharmacopoeia Committee, 2020 edition stipulates that the polysaccharides obtained from R. laevigata should not be less than 25.0% with respect to the anhydrous glucose (C6H12O6) content.
National Pharmacopoeia Committee, 2020 edition stipulates that the moisture content of R. laevigata should not exceed 18.0% and the total ash content should not exceed 5.0%.
By conducting thermos-gravimetric analysis (TGA) studies on four different parts of R. laevigata including the roots, stems, pulp and seeds, Zhu et al. (2009) analyzed the characteristic parameters of TGA pyrolysis of different parts of the plant. They also derived the pattern of influence of the rate of temperature rise and species characteristics on the pyrolysis of R. laevigata. In addition, their study also showed the ease of decomposition (from easy to difficult) of four parts: seeds, pulp, stem and roots.
Chen et al. (2011) investigated the variation of odour fingerprints of R. laevigata at different time-periods, and then conducted principal component analysis (PCA) and discriminant factor analysis (DFA) on the odour response values measured by using electronic nose (EN) sensors. They also performed statistical quality control (SQC) analysis. The experimental results showed that the combined use of EN, PCA and DFA for odour analysis can be used for quality control of R. laevigata.
In the case of Radix R. laevigata, this herb is relatively scarce and is not included in the pharmacopoeia. There is a lack of complete quality standards for it, and therefore there are only a few studies regarding its content determination. Nevertheless, a review of the relevant literature revealed that the amounts of gallic acid and catechin in radix of R. laevigata and concoction products from different origins was determined by HPLC. The results showed that the content of gallic acid and catechin in the root of R. laevigata from different origins varied significantly, but all the samples contained more catechin than gallic acid (Wei et al., 2021a). In addition, Tan (2012) determined the content of polysaccharides in the roots, stems and fruits of R. laevigata from different origins by taking UV spectrophotometry measurement. The results showed that the polysaccharide content of the roots and stems of R. laevigata from different origins were lower than the fruits. From this they concluded that there were also differences in the polysaccharide content of different parts of R. laevigata.
Wei et al. (2021b) adopted ICP-MS analysis in order to compare the content of metal elements in roots of R. laevigata and its associated products from different origins. The results showed that the most abundant macronutrients were Al and Fe, with the concentration of Al reaching 33.9–270 mg/kg in its concoction. Other trace elements found were B, Ba, Mn, Zn and Sr Theis method provides a necessary reference for the development of better quality standards for Radix R. laevigata.
Since Radix R. laevigata is not included in the pharmacopoeia, Su et al. (2012) stipulated through experimental studies, that the moisture content should not exceed 14.0% and the total ash content should not exceed 6.0%, of which the acid insoluble ash content should not exceed 2.0%. The results of this experiment provide a scientific basis for better quality control of Radix R. laevigata.
Flavonoids and polysaccharides are the main components and active compounds in Rosa laevigata Michx., and the optimization studies with respect to their extraction are essential to ensure the efficacy and quality control of this herbal medicine.
Li et al. (2009) studied the extraction process of flavonoids in R. laevigata by using a warm water extraction method. They also used ethanol and acetone for extractions, and then compared the three methods. They found acetone was better than ethanol which was better than warm water for extraction the constituents. The best extraction conditions were obtained by an orthogonal experiment using 60% ethanol, in a 70°C water bath, at a solid-liquid ratio of 1:150. The best extraction conditions were obtained by using the acetone method: acetone concentration 60%, water bath temperature 50°C and a solid-liquid ratio 1:50. Jiang et al. (2015) used the Box-Behnken response surface method to optimize the extraction process of R. laevigata, and screened out the best extraction process to be: 60% ethanol concentration, 13:1 material-to-liquid ratio, 2 h extraction time and 2 times extraction.
Shang et al. (2018) used the water extraction method to optimize the extraction process of R. laevigata formula granules. Their preferred extraction process was eight times the amount of water and two reflux extractions for 2 hours each time. The average extraction rate of polysaccharides from R. laevigata was calculated to be 22.17%.
Yi et al. (2011) studied the extraction of TFs from the roots of R. laevigata by using ethanol. Then they optimized the process conditions for obtaining TFs by using the orthogonal test method. The results showed that the extraction conditions for TFs from the roots of R. laevigata were best with 80% ethanol, 50°C extraction, reflux for 2.5 h and a material-liquid ratio of 1:50, and the dissolution rate of TFs was measured as 33.90%. Wu et al. (2011) conducted a comparative study of four different resins to obtain the best macroporous adsorbent resin for the purification of TFs from the roots of R. laevigata and optimized this process. The results showed that AB-8 was the best macroporous adsorbent resin for the purification of TFs from the root of R. laevigata. The best process for the separation and purification was 20.0 ml of 0.200 mg/ml sample solution with the AB-8 resin as the adsorbent and 50% ethanol as the eluent. The purification rate of TFs from the roots of R. laevigata could reach 205.40% under these optimal conditions.
1) In terms of chemical composition: most of the current research is focused on the study of flavonoids and triterpenes, and there is relatively little work on other active ingredients such as phenols, tannins and polysaccharides. Therefore, there is a need to strengthen the research on the other ingredients in order to improve the progress of the research on the chemical composition of Fructus R. laevigata and Radix R. laevigata.
2) In terms of the pharmacology: as mentioned above, most of the research today is on flavonoids and triterpenes, so there is a lot of pharmacological studies corresponding to these, while the pharmacological activities of other components are less developed. Moreover, the current pharmacological studies on R. laevigata and its roots are mainly focused on its antioxidant and anti-inflammatory activities, and the many other pharmacological effects that are derived from these two activities, such as renal and cardiovascular protection. Therefore, the mechanism of action has to be studied in depth. In addition, the anti-tumour activity, which is widely studied today, is rarely addressed, which leads to the conclusion that research in this area should be strengthened to provide a scientific basis for the development of new drugs.
3) In terms of quality control: While Fructus R. laevigata has been included in the Chinese Pharmacopoeia, Radix R. laevigata is not yet included. Although there are studies on its quality standards, there is still a need to continue to explore its quality standards in depth and to include Radix R. laevigata in the pharmacopoeia at an the earliest possible date.
4) In terms of toxicity studies: the toxicity studies on R. laevigata are too limited at present and the only study that has produced data is a sub-chronic toxicity study on rats with the TFs of R. laevigata. There are no data to support the few other studies and no toxicity studies on other components have so far emerged. Therefore, extensive toxicity studies should be conducted on this aspect to study the toxic effects of the various components of Fructus R. laevigata and Radix R. laevigata to determine their side-effects, acute toxicity and chronic toxicity to provide a scientific basis for the clinical use of these herbs.
5) In terms of concoction processing: at present, the edible development of R. laevigata is limited to pure fruit juice drinks, compound drinks and fruit wine brewing. Further exploration is needed in terms of its food promotion and value innovation.
As one of the herbs of medicinal and food origin, Rosa laevigata Michx. has strong biological activities of its active chemical components, which has led to its wide use in clinical and daily life. The Chinese National Ministry of Health has rated it as a new food resource and it has now developed into a third generation wild fruit food. Therefore, it is widely used in food ingredients, such as the development of fruit juices, fruit wines and yoghurt. In addition, a brown pigment that can be used as a food additive can also be extracted from R. laevigata. Pharmacological studies have shown that R. laevigata has the effect of improving the gastrointestinal tract, promoting intestinal peristalsis, increasing the digestion of food, reducing the accumulation of harmful substances in the intestinal tract, playing a role in eliminating persistent stools and also reducing the occurrence of gastrointestinal diseases. It is also used as a raw material in Chinese medicines for the clinical treatment of pelvic inflammatory disease, diabetic cataract, etc. The roots of R. laevigata are used as raw materials in San Jin tablets and gynaecological Qian Jin tablets for the treatment of diseases related to gynaecological infections. R. laevigata and its roots also have antioxidant, renal protective, immunomodulatory, hypo-lipidemic, anti-inflammatory, antiviral, anti-tumour, cardiovascular protective and antibacterial activities. In addition, it also plays an important role in diabetes mellitus. Chemical composition studies have shown that R. laevigata and its roots are rich in triterpenoids, flavonoids and polysaccharides, which are inextricably linked to its diverse pharmacological activities. As more and more scholars continue to study Fructus and Radix Rosae laevigatae in depth, these two herbs have a very promising future in the pharmaceutical, health-care and food industry markets.
Designed the paper, X-XQ, Y-YH, and J-QY; collected literatures on phytochemistry, X-XQ and LC; collected literatures on pharmacology and toxicology, X-XQ and Y-YH; revised the review critically for important intellectual content, X-XQ and Y-YH; wrote the original manuscript and revised the review, X-XQ. All authors have read and agreed to published version of the manuscript.
This work was financially supported by the key project of Guangxi Natural Science Foundation (2020GXNSFDA297022).and Doctoral Research Initiation Project for Guangxi University of Chinese Medicine (2018BS048).
The authors thank Dev Sooranna of Imperial College London for editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1012265/full#supplementary-material
Apak, R., Guculu, K. G., Ozyurek, M., and Karademir, S. E. (2004). Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC. J. Agric. Food Chem. 52, 7970–7981. doi:10.1021/jf048741x
Bi, W., Li, Q., Gong, W. H., Xu, Y. C., Jiang, H. L., and Lei, H. M. (2008). Chemical compositions of rosa laevigata michx. J. J. Beijing Universify Traditional Chin. Med. 32 (02), 110–111.
Bian, C., Liu, X., Zhang, Y., Xing, J., Ayiguzhali, R., Zhang, K. K., et al. (2018). The protective effect of Rosa laevigata Fructus extract on the intestinal mucosa of mice colitis. J. J. njiang Med. Univ. 41 (05), 597–601.
Chen, C. P., Fang, S. Y., Peng, C., and Chen, N. D. (2014). Effect of total flavonoids from Rosa laevigata on whole blood viscosity and platelet aggregation in rats. J. J. west Anhui Univ. 30 (02), 72–75.
Chen, L. H., Tan, L. Y., Xiao, B., He, C. Z., and Zhu, J. (2013). Comparative study on antioxidant activities of proanthocyanidins between rosa laevigata and grape skin. J. Chem. Industry For. Prod. 33 (03), 78–84.
Chen, S. J., Mao, J., Han, B. X., and Chen, N. F. (2011). Scent analysis of Rosa laevigata through metal oxide sensor array electronic nose. Rev. Bras. Farmacogn. 21 (6), 1150–1154. doi:10.1590/s0102-695x2011005000127
Dai, H. N., Ma, G. X., Zou, J. M., Zhong, X. Q., Zhou, Y. L., Huang, X. Y., et al. (2016). Triterpenoids from roots of Rosa laevigata. J. Chin. Herb. Med. 47 (03), 374–378.
Deng, C., and Zhang, H. W. (2018). Inhibitory effects of schisandra chinensis, rosa laevigata and cornus officinalis on glycometabolism in type 2 diabetes model rats. J. Nat. Prod. Res. Dev. 30, 568–574.
Diao, X. P., Chen, M. X., Ye, Y. J., Wang, L. J., and Wu, H. Z. (2014). Efect of fermented Rosa laevigata Michx on growth and immunefunction of weaned piglets. J. J. Northeast Agric. Univ. 45 (06), 67–73.
Ding, Y., Huang, Y. L., Liu, J. L., Wang, L., Yan, X. J., Li, D. P., et al. (2017). Chemical constituents from the roots of Rosa laevigata. J. Guihaia. 37 (2), 255–259.
Dong, D. S., Qi, Y., Xu, L. N., Yin, L. H., Xu, Y. W., Han, X., et al. (2019). Correction: Total saponins from Rosa laevigata Michx fruit attenuates hepatic steatosis induced by high-fat diet in rats. Food Funct. 10, 5240–5241. doi:10.1039/c9fo90039j
Dong, D. S., Xu, L. N., Han, X., Qi, Y., Xu, Y. W., Yin, L. H., et al. (2014). Effects of the total saponins from rosa laevigata michx fruit against acetaminophen-induced liver damage in mice via induction of autophagy and suppression of inflammation and apoptosis. Molecules 19 (6), 7189–7206. doi:10.3390/molecules19067189
Dong, D. S., Yin, L. H., Qi, Y., Xu, L. N., and Peng, J. Y. (2015). Protective effect of the total saponins from rosa laevigata michx fruit against carbon tetrachloride-induced liver fibrosis in rats. Nutrients 7 (6), 4829–4850. doi:10.3390/nu7064829
Dong, D. S., Zhang, S., Yin, L. H., Tang, X. Q., Xu, Y. W., Han, X., et al. (2013). Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 62, 120–130. doi:10.1016/j.fct.2013.08.050
Dong, L., Han, X., Tao, X., Xu, L., Xu, Y., Fang, L., et al. (2018). Protection by the total flavonoids from Rosa laevigata michx fruit against lipopolysaccharide-induced liver injury in mice via modulation of FXR signaling. Foods 7 (6), 88. doi:10.3390/foods7060088
Du, G. R., Li, M. J., Ma, F. W., and Liang, D. (2009). Antioxidant capacity and the relationship with polyphenol and Vitamin C in Actinidia fruits. Food Chem. x. 113, 557–562. doi:10.1016/j.foodchem.2008.08.025
Editorial Board of Flora of China, Chinese Academy of Sciences (2004). Flora reipublicae popularis sinicae. Science Press, 448.
Feng, C. N., and Tian, S. Y. (2011). Separating and purifying polysaccharides from root of Cherokee rose and its in vivo effect of antitumor activity. J. Chin. J. Exp. Traditional Med. Formulae 17 (06), 209–212.
Gao, P. Y., Han, T., Jin, M., Li, D. Q., Jiang, F. Y., Zhang, L. X., et al. (2018). Extraction and isolation of polyhydroxy triterpenoids from Rosa laevigata Michx. fruit with anti-acetylcholinesterase and neuroprotection properties. RSC Adv. 8 (67), 38131–38139. doi:10.1039/c8ra07930g
Gao, P. Y., Ling, Z. L., Ying, P., Piao, S. J., Zeng, N., Lin, H. W., et al. (2010). Triterpenes from fruits of Rosa laevigata. Biochem. Syst. Ecol. 38 (3), 457–459. doi:10.1016/j.bse.2010.03.014
Guangdong Institute of Traditional Chinese Medicine (1961). Lingnan Herbal Medicine. Shanghai: Shanghai Science and Technology Press.South China Institute of botany co-editor
Guangdong Provincial Department of Health (1959). Guangdong traditional Chinese medicine jinfang anthology episode 1. Guangzhou: Guangdong People's Publishing House.
Huang, Y. L., and Liu, Y. (2015). Experimental study on the antineoplastic effect of polysaccharide from fructus Rosae laevigatae in vitro. J. Genomics Appl. Biol. 34, 1848–1851.
Huang, Y. L., and Liu, Y. (2017). Preliminary antitumor activity study of flavonoids from extracts of rosa laevigata. J. Genomics Appl. Biol. 36, 4007–4011.
Hunan Institute of Traditional Chinese Medicine (2017). Hunan pharmaceutical journal. Changsha: Hunan Science and Technology Press, 348.04
Jia, Y. N., Ji, L., Zhang, S., Xu, L. N., Yin, L. H., Li, L., et al. (2012). Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 50 (9), 3133–3141. doi:10.1016/j.fct.2012.06.047
Jiang, Y. C., Deng, C., and Li, J. L. (2015). Optimal process of effective constituents from Fructus Rosa Laevigatae made with honey by Box-Behnken response surface method. J. Cent. South Pharm. 13 (09), 938–942.
Ko, H. M., Choi, S. H., Kim, Y., An, E. J., Lee, S. H., Kim, K., et al. (2020). Effect of rosa laevigata on PM10-induced inflammatory response of human lung epithelial cells. Evidence-Based Complementary Altern. Med. 2020, 1–9. doi:10.1155/2020/2893609
Kumar, K. J. S., Li, C., Tseng, Y. H., and Wang, S. Y. (2021). Fruits of Rosa laevigata and its bio-active principal sitostenone facilitate glucose uptake and insulin sensitivity in hepatic cells via AMPK/PPAR-γ activation. Phytomedicine Plus 1 (4), 100109. doi:10.1016/j.phyplu.2021.100109
Lai, Y. X., Wang, Y., Pan, C. S., and Tian, S. Y. (2009). Bacteriostatic action of polysaccharides of the root and stem of rosa laevigata. J. Chin. Pharm. 20 (24), 1857–1858.
Lai, Y. X., Yan, H. J., Tian, S. Y., and Yu, J. H. (2012). Study on antibacterial effects of radix Rosae laevigatae extract. J. Pharm. Today. 22 (09), 531–533.
Lee, S. H., Choi, S. H., Lee, I. S., Kim, Y., An, E. J., and Jang, H. J. (2020). Anti-inflammatory effect of Rosa laevigata extract on in vitro and in vivo model of allergic asthma via the suppression of IgE and related cytokines. Mol. Cell. Toxicol. 16 (2), 119–127. doi:10.1007/s13273-019-00063-8
Li, B. L., Yuan, J., and Wu, J. W. (2021). A review on the phytochemical and pharmacological properties of Rosa laevigata: A medicinal and edible plant. Chem. Pharm. Bull. 69 (5), 421–431. doi:10.1248/cpb.c20-00743
Li, H., Fang, W., Wang, Z., and Chen, Y. (2022). Physicochemical, biological properties, and flavour profile of rosa roxburghii tratt, pyracantha fortuneana, and rosa laevigata michx fruits: A comprehensive review. Food Chem. 366, 130509. doi:10.1016/j.foodchem.2021.130509
Li, H. Y., and Feng, X. (1990). Extraction and toxicological evaluation of Brown pigment from Rosa laevigata. J.Chemistry ang Industry For. Prod. 10 (03), 195–201.
Li, H. Z. (2006). Inhibitory effect of acetone extract of Rosa laevigata on oral Streptococcus mutans. J. Med. World. (05), 126–127.
Li, J., Zhu, X., Luan, J. J., and Zhang, X. P. (2019). Anti EV71 and RSV virus analysis of ultrasonic extracts of Rosa laevigata in acetone, methanol and ethanol. J. SHANDONG Chem. Ind. 48 (05), 88–89+91.
Li, N., Chen, Q., Liu, H. N., Pei, G., and He, G. X. (2019). Effect of total polyphenols from Rosa laevigata. J. Chin. Tradit. Pat. Med. 41 (11), 2773–2776.
Li, N. M., Zhong, X. H., Li, L. N., and Yuan, Z. J. (2009). Study on extraction technology of flavonoids in rose laevigata michx. J. Hunan Agric. Sci. (03), 79–82.
Li, X., Cao, W., Shen, Y., Li, N., Dong, X. P., Wang, K. J., et al. (2012). Antioxidant compounds from Rosa laevigata fruits. Food Chem. x. 130 (3), 575–580. doi:10.1016/j.foodchem.2011.07.076
Li, X. R., and Wei, L. X. (1997). [Chemical components from rosa laevigata michx]. J. Chin. J. traditional Chin. Med. 22 (5), 298–299.
Li, Y. L., Dai, H. N., Ma, G. X., Zhang, W., Wu, T. Y., Wang, Y. Q., et al. (2017). A new triterpenic acid from the roots of Rosa laevigata. J.Acta Pharm. Sin. 52 (3), 425–429.
Lin, Y. Y., and Zhou, C. H. (2009). Determination of tannin in Rosa laevigata from different producing areas. J. HUNAN J. TRADITIONAL Chin. Med. 25 (1), 95–97.
Liu, M., Xu, Y. W., Han, X., Liang, C., Yin, L. H., Xu, L. N., et al. (2014). Potent effects of flavonoid-rich extract from Rosa laevigata Michx fruit against hydrogen peroxide-induced damage in PC12 cells via attenuation of oxidative stress, inflammation and apoptosis. Molecules 19, 11816–11832. doi:10.3390/molecules190811816
Liu, M., Xu, Y. W., Han, X., Liang, C., Yin, L. H., Xu, L. N., et al. (2019). Correction: Liu, M., et al. Potent Effects of Flavonoid-Rich Extract from Rosa laevigata Michx Fruit against Hydrogen Peroxide-Induced Damage in PC12 Cells via Attenuation of Oxidative Stress, Inflammation and Apoptosis. Molecules. 19, 11816-11832. J. Molecules (Basel, Switzerland) 24 (23), 4228.
Liu, X. G., Li, J. L., Gao, P. Y., and Wu, Z. Y. (2013b). Recent advances in research on edible rosa laevigata michx. J. Food Sci. 34 (11), 392–398.
Liu, X. G., Zhang, W. C., Jin, M., Wu, Z. Y., Mou, X. K., and Gao, P. Y. (2013a). Isolation and identification of triterpenoids from the fruit of Rosa laevigata Michx. J. J. Shenyang Pharm. Univ. 30 (11), 851–857.
Liu, X. R., Gu, Z. Z., and Zhu, M. J. (2009). Study on antimicrobial effects from Rosa laevigata’s fruits. J. Sci. Technol. Food Industry 30 (07), 168–169+333.
Liu, X. W., Hou, L., and Cui, Q. H. (2017a). Study on extraction optimization and in vitro antiviral activity of Polysaccharide from Rosa laevigata. J. J. Chin. Med. Mater. 40 (07), 1679–1682.
Liu, X. W., Tian, J. Z., and Yuan, Q. (2017b). Antiviral activity of different solvent extractions of Rosa laevigata Michx. J. Chin. J. Pharm. Anal. 37 (12).
Liu, Y. H., Tian, Y. G., Long, H., Li, G., Liu, Z. X., and Wei, H. (2018). A new seco-triterpene from roots of Rosa laevifgata. J. Chin. Traditional Herb. Drugs 49 (24), 5740–5745.
Liu, Y., Li, J. L., Gao, P. Y., and Wu, Z. Y. (2010c). Research progress on medicinal and edible homogeny wilding rosa laevigata mickx. J. Anhui Agri. Sci. 38 (14), 7276–7280.
Liu, Y., Luo, W., Luo, X., Yong, Z., and Zhong, X. (2015). Effects of Rosa laevigata Michx. extract on reactive oxygen species production and mitochondrial membrane potential in lens epithelial cells cultured under high glucose. Int. J. Clin. Exp. Med. 8 (9), 15759–15765.
Liu, Y. T., Lu, B. N., and Peng, J. Y. (2010a). Hepatoprotective activity of the total flavonoids from Rosa laevigata Michx fruit in mice treated by paracetamol. Food Chem. x. 125 (2), 719–725. doi:10.1016/j.foodchem.2010.09.080
Liu, Y. T., Lu, B. N., Xu, L. N., Yin, L. H., Wang, X. N., Peng, J. Y., et al. (2010b). The antioxidant activity and hypolipidemic activity of the total flavonoids from the fruit of Rosa laevigata Michx. Nat. Sci. (Irvine). 2 (3), 175–183. doi:10.4236/ns.2010.23027
Lu, Y., Sun, Z. G., Xu, H. Q., and Zhang, S. W. (1995). Effect of water extract of Rosa laevigata on urinary system. J. Chin. Traditional Herb. Drugs 26 (10), 529–531+557+560.
Luo, W. M., Liu, Y. F., Liu, H., and Luo, X. Y. (2014). Rosa laevigata michx combined losartan against chronic cardiotoxicity induced with doxorubicin in rats. J. J. Hubei Med. Coll. 33 (01), 11–14.
Luo, W. M. (2014). Study on the effect and mechanism of myocardial injury protection of chinaberry by adriamycin. Taihe hospital, shiyan city, Hubei province, 05–13.
Luo, X. Z., Xie, C. Y., Zheng, M. S., and Chen, B. R. (1989). Observations on anti-herpes simplex virus effect of fructus Rosae laevigata eye drops and on its clinical benefit. J. Ophthalmic Res. 7 (01), 47–48.
Meng, J. F., Fang, Y. L., Gao, J. S., Qiao, L. L., Zhang, A., Guo, Z. J., et al. (2012). Phenolics composition and antioxidant activity of wine produced from spine grape ( vitis davidii foex) and Cherokee rose ( rosa laevigata michx.) fruits from south China. J. Food Sci. 77 (1), 8–14. doi:10.1111/j.1750-3841.2011.02499.x
Moreno, J., Peinado, J., and Peinado, R. A. (2007). Antioxidant activity of musts from Pedro Ximénez grapes subjected to off-vine drying process. Food Chem. x. 104, 224–228. doi:10.1016/j.foodchem.2006.11.028
National Pharmacopoeia Committee (2020). Chinese pharmacopoeia. Beijing: China Medical Science and Technology Press, 232.
Pang, H. M., Zhu, Y. Z., and Gao, J. C. (2006). Mutagenic effect of Rosa laevigata on mice. J. J. Toxicol. 20, 345.
Peng, H. Y., Shou, X. Y., Wang, T., Zhao, G. L., Zhang, Z., Jiang, Z. Z., et al. (2014). Study on immunomodulatory activity of root and rhizome of Rosa laevigata from different habitats. J.Chinese Traditional Herb. Drugs 45 (13), 1903–1906.
Qu, H. Y., Feng, Z., Li, Z. Y., Li, C. S., Tang, M. F., Zhou, Z., et al. (2015). Induction of substantial myocardial regeneration by an active fraction of the Chinese herb Rosa laevigata Michx. BMC Complement. Altern. Med. 15 (1), 359. doi:10.1186/s12906-015-0795-0
Quanzhou Municipal Science and Technology Commission (1961). Quanzhou municipal health bureau. Quanzhou Institute of Medical Sciences.
Shang, Y. Y., Hu, H., and Liu, Y. C. (2018). Optimization of the extraction technology of rosa laevigata formula granules and study on its quality standard and fingerprint. J. China Pharm. 29 (14), 1922–1927.
Su, Y. Y., Guo, Q. L., Tian, S. Y., and Lu, L. G. (2012). Studies on quality standard of radix Rosae laevigatae. J. Chin. J. Exp. Traditional Med. Formulae 18 (15), 109–111.
Tan, H. T. (2012). Comparison on the content of polysaccharide in different parts of Rosae Laevigatae Michx.from various sources. J. Glob. Tradit. Chin. Med. 5, 266–268.
Tan, N. X., Guo, Q. L., Tian, S. Y., and M, Q. X. (2010). The summary of medicinal history and modern research on the root of Rosa laevigata. J. Asia-Pacific Tradit. Med. 6 (12), 167–169.
The Editorial Committee of Eastern Fujian Materia Medica (1962). Ming Dong Materia Medica, collection 1. Fujian Province Min Dong Ben Cao Editorial Committee.
Tianjin No.3 Hospital (2004). ). Clinical observation and experimental study on the treatment of geriatric urinary incontinence with the extracts of Radix R. laevigata. Tianjin Municipal Health Bureau, 06–10.
Wang, J. Y., Zhang, G. L., Cheng, D. L., and Wu, F. E. (2001). The chemical constitute from rosa laevigata michx. J. Res. Dev. Nat. Prod. 13 (01), 21–23.
Wang, X. J., Zhang, L., Chen, L. H., and Zeng, Z. X. (2016). Investigation on antioxidant activity of polysaccharides from wild Rosae Laevigata Fructus in Western Hunan province. J. Chin. J. Pharm. Anal. 36 (03), 438–443.
Wang, Y. M., Su, X. J., Fu, Y., Wang, Z. F., and Zhang, C. X. (2016). The chemical constituents from the roots of Rosa laevigata Michx.(Ⅰ). J. ACTA Sci. Nat. Univ. SUNYATSENI. 55 (02), 76–80+84.
Wei, X. Y., Deng, Q., Lu, X. L., Lu, X. L., Shu, K., Zhuo, S., et al. (2021a). Difference analysis on contents of gallic acid and catechin in roots of Rosa laevigata and its processed products from different habitats of Guangxi. J/OL. Guangxi plants 1-14, 12–13.
Wei, X. Y., Deng, Q., and Shu, K. (2021b). Determination of 22 metal elements in the roots of Rosa laevigata and its processed products from different habitats of- Guangxi by ICP-MS. J. Guihaia. Jul. 41 (7), 1209–1218.
Wu, G. Q., Yi, Y. H., Zou, M. Q., Tian, S. Y., and Zhao, Z. Y. (2011). Study on the purification process of total flavonoids from root of rosa laevigata Michx.by macroporous adsorption resin. J. Med. Plant. 2 (07), 39–41.
Wu, X. W., Gao, P. Y., Li, L. Z., Lu, Y., and Song, S. J. (2009). Studies on the chemical constituents of Rosa laevigata Michx fruits. J. J. Pharm. Pract. 27 (3), 183–185.
Xiang, Z. L., Shan, H. Z., CHai, F. H., and Wang, H. Y. (2015). Therapeutic effect of compound extract of Rosa laevigata on piglet white dysentery. J. Jilin Animal Husb. Veterinary Med. 36 (05), 31–32+35.
Xiao, K. J., Xiong, P., and Wang, J. (2010). Studies on extraction and antioxidative activity of rosa laevigata michx big pigment. J. China Food Addit. 22 (01), 87–91+111.
Xie, M. X., Ding, X. W., and Chen, J. Q. (2001). Study on the scavenging effect of Rosa laevigata extract on NO2-. J. Guangzhou Food Sci. Technol. (02), 52–55.
Shi, J. L. (2012). Comparison of polysaccharide and total flavonoids contents in Rosa laevigata from different habitats. J. China Pharm. 15 (5), 648–650.
Xu, Y. C., Ma, C. H., Wei, L. X., Zhou, W. X., Yuan, W. S., and Lei, X. L. (2002). Determination of triterpene acids in Rosa laevigata by HPLC. J. J. Beijing Univ. TCM 25 (4), 42–44.
Yan, G. Q., Li, S. P., Hu, J., Zhai, X. Y., Ma, W., Li, N., et al. (2014). Phenolic constituents from the roots of Rosa laevigata (Rosaceae). Biochem. Syst. Ecol. 52, 23–26. doi:10.1016/j.bse.2013.09.006
Yan, M., Zhu, Y., Zhang, H. J., Jiao, W. H., Han, B. N., Liu, Z. X., et al. (2013). Anti-inflammatory secondary metabolites from the leaves of Rosa laevigata. Bioorg. Med. Chem. 21 (11), 3290–3297. doi:10.1016/j.bmc.2013.03.018
Ye, P., Mao, Q., and Guo, Y. H. (1993). Isolation and identification of triterpenoids from Rosa laevigata Michx. J. J. Guiyang Coll. traditional Chin. Med. 15 (04), 62–64+61.
Yi, Y. H., Wu, G. Q., and Wang, L. J. (2011). Study on ultrasonic extraction of total saponins of root of Rosa Laevigate Wichx. J. Sci. Technol. Food Industry 32 (5), 270–272.
Yoshida, Takashi., Tanaka, Katsumi., Chen, X. M., and Okuda, Takuo. (1989). Dimeric ellagitannins, laevigatins E, F and G, from Rosa laevigata. Phytochemistry 28 (9), 2451–2454. doi:10.1016/s0031-9422(00)98003-8
Yu, C. H., Dai, X. Y., Chen, Q., Zang, J. N., Deng, L. L., Liu, Y. H., et al. (2013). Hypolipidemic and antioxidant activities of polysaccharides from Rosae Laevigatae Fructus in rats. Carbohydr. Polym. 94 (1), 56–62. doi:10.1016/j.carbpol.2013.01.006
Yuan, J. Q., Yang, X. Z., Miao, J. H., Tang, C. P., Ke, C. Q., Zhang, J. B., et al. (2008). New triterpene glucosides from the roots of rosa laevigata michx. Molecules 13, 2229–2237. doi:10.3390/molecules13092229
Zeng, N., Shen, Y., Li, L. Z., Jiao, W. H., Gao, P. Y., Song, S. J., et al. (2011). Anti-inflammatory triterpenes from the leaves of Rosa laevigata. J. Nat. Prod. 74 (4), 732–738. doi:10.1021/np1007922
Zhan, Q. P., Wang, Q., Lin, R. G., He, P., Lai, F. R., Zhang, M. M., et al. (2020). Structural characterization and immunomodulatory activity of a novel acid polysaccharide isolated from the pulp of Rosa laevigata Michx fruit. Int. J. Biol. Macromol. 145, 1080–1090. doi:10.1016/j.ijbiomac.2019.09.201
Zhang, D. P., Ou, G. D., and Huang, G. K. (2014). Comparison of polysaccharides and total flavonoids in Rosa laevigata from different habitats. J. J. China Traditional Chin. Med. Inf. 4 (2), 12–13.
Zhang, S., Lu, B. N., Han, X., Xu, L. N., Qi, Y., Yin, L. H., et al. (2013a). Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 55, 60–69. doi:10.1016/j.fct.2012.12.041
Zhang, S., Zheng, L. L., Dong, D. S., Xu, L. N., Yin, L. H., Qi, Y., et al. (2013b). Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 141 (3), 2108–2116. doi:10.1016/j.foodchem.2013.05.019
Zhang, S., Zheng, L. L., Xu, L. N., Sun, H. J., Li, H., Yao, J. H., et al. (2012). Subchronic toxicity study of the total flavonoids from Rosa laevigata Michx fruit in rats. Regul. Toxicol. Pharmacol. 62 (2), 221–230. doi:10.1016/j.yrtph.2011.12.009
Zhang, X. J., Hu, Y. H., Jin, C. Z., and Wu, W. G. (2020). Extraction and hypolipidemic activity of low molecular weight polysaccharides isolated from rosa laevigata fruits. Biomed. Res. Int. 2020, 2043785. doi:10.1155/2020/2043785
Zhao, L. Y., Xu, L. N., Tao, X. F., Han, X., Yin, L. H., Qi, Y., et al. (2016). Protective effect of the total flavonoids from rosa laevigata michx fruit on renal ischemia-reperfusion injury through suppression of oxidative stress and inflammation. Molecules 21 (7), 952. doi:10.3390/molecules21070952
Zhou, J., He, X. Z., and Xiao, Q. G. (2014). Inhibitive effects of Rosa laevigata on LEC apoptosis of diabetic cataract rats by increasing Bcl-2/Bax expression ratio. J. Rec. Adv. Ophthalmol. 34 (04), 314–318.
Zhou, Y. J., Liao, Q. J., Luo, Y. P., Qing, Z. Q., Zhang, Q. J., and He, G. P. (2012). Renal protective effect of Rosa laevigata Michx. by the inhibition ofoxidative stress in streptozotocin-induced diabetic rats. Mol. Med. Rep. 5 (6), 1548–1554. doi:10.3892/mmr.2012.855
Zhu, G. H., Zhou, X., Yan, Z. H., Xiong, H. H., Huang, D. X., and Zuo, S. (2009). Thermogravimetric analysis of different parts of Rosa laevigata. C. Chin. J. Anal. Chem. 2009, 1.
Zou, T. B., and Qiu, Y. M. (2011). Anti-inflammatory and antidiarrheal effects of the root from Rosa laevigata. J.Chinese J. Veterinary Med. 47 (05), 52–53.
Keywords: Rosa laevigata Michx., Fructus R. laevigata, Radix R. laevigata, traditional medicine, phytochemistry, pharmacology, quality control
Citation: Quan X-X, Huang Y-Y, Chen L and Yuan J-Q (2022) Traditional uses, phytochemical, pharmacology, quality control and modern applications of two important Chinese medicines from Rosa laevigata Michx.: A review. Front. Pharmacol. 13:1012265. doi: 10.3389/fphar.2022.1012265
Received: 05 August 2022; Accepted: 05 September 2022;
Published: 06 October 2022.
Edited by:
Shuai Ji, Xuzhou Medical University, ChinaReviewed by:
Sabyasachi Banerjee, Gupta College of Technological Sciences, IndiaCopyright © 2022 Quan, Huang, Chen and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-Quan Yuan, eWpxZ3hAMTYzLmNvbQ==
†These authors contributed equally to the work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.