
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 October 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1009612
 Yong-Shuai Wang1†
Yong-Shuai Wang1† Wei Wang2†
Wei Wang2† Sai Zhang1†
Sai Zhang1† Shen-Yu Zhang1
Shen-Yu Zhang1 Ai-Zong Shen3
Ai-Zong Shen3 Wei Wang4
Wei Wang4 Hua-Chuan Song1
Hua-Chuan Song1 Huan-Zhang Yao1
Huan-Zhang Yao1 Rui-Peng Song1
Rui-Peng Song1 Fan-Zheng Meng1
Fan-Zheng Meng1 Lei Li5
Lei Li5 Bjoern Nashan6
Bjoern Nashan6 Ji-Zhou Wang1,7*‡
Ji-Zhou Wang1,7*‡ Lian-Xin Liu1,7*‡
Lian-Xin Liu1,7*‡Purpose: To investigate the clinical efficacy of avatrombopag, an oral thrombopoietin receptor agonist, versus subcutaneous recombinant human thrombopoietin (rh-TPO) in the treatment of severe thrombocytopenia (TCP) associated with chronic liver disease (CLD).
Methods: Clinical data of 250 patients with severe TCP associated with CLD were collected in a single hospital from January 2019 to January 2022. The main parameters measured were the therapeutic response rate, changes in platelets (PLTs), and adverse events. Propensity score matching (PSM) was used to avoid possible selection bias.
Results: After PSM, a total of 154 patients were enrolled in the study: 77 in the avatrombopag group and 77 in the rh-TPO group. There was no statistically significant difference between the two groups in the effect of increasing the PLT count (Waldχ2 = 1.659, p = 0.198; Waldχ2 = 0.220, p = 0.639). In addition, no interaction between time and different medications was found (Waldχ2 = 0.540, p = 0.910; Waldχ2 = 1.273, p = 0.736). Interestingly, in the subgroup analysis, both before and after PSM, avatrombopag showed better clinical efficacy than rh-TPO in the treatment of TCP associated with CLD in Child‒Pugh Class A (88.89% vs. 63.41%, p =0.003; 81.33% vs. 61.76%, p = 0.043). Fewer patients reported dizziness in the avatrombopag group than in the rh-TPO group both before and after PSM (7.8% vs. 25.0%; 7.8% vs. 24.7%, p < 0.05).
Conclusion: Both before and after PSM, avatrombopag showed better clinical efficacy than rh-TPO in the treatment of TCP associated with CLD in Child‒Pugh Class A and showed a lower incidence of dizziness in all patients.
Thrombocytopenia (TCP) is the most common hematological complication in patients with chronic liver disease (CLD) (Qamar et al., 2009). Due to the decrease in the platelet count, these patients usually have a higher risk of bleeding during invasive procedures, resulting in increased postoperative complications and mortality (Giannini et al., 2010; Forkin et al., 2018). Traditionally, a growing number of studies have been suggesting that prophylactic platelet (PLT) transfusion before or after invasive procedures can improve the hemostatic potential and reduce the risk of bleeding in CLD patients with severe TCP (platelet count <50 × 109/L) (Maan et al., 2015; Mitchell et al., 2016). This threshold is used as a standard for clinical care and invasive procedures (Scharf, 2021).
Thrombopoietin (TPO) is an important physiological regulator of thrombopoiesis and megakaryocyte maturation, which increases the number of PLTs in the blood by stimulating the production of endogenous functional PLTs. Accumulating studies have revealed that TPO is a therapeutic target that stimulates PLT production (Qureshi et al., 2016; Gilreath et al., 2021).
When considering cost-effectiveness, analysis of increasing PLT values to the same level using traditional PLT infusion may be less costly than TPO agonists. Many centers still use traditional PLT transfusion. However, PLT transfusion may be associated with serious complications, including transfusion reactions and the transmission of infectious agents, which can be fatal in rare cases, and alloimmunization can occur in patients who have received multiple blood product transfusions because recipients form antibodies against donor platelets (Basili et al., 2018; Armstrong et al., 2020; Elsaid et al., 2020; Karlström et al., 2022). In addition, platelet transfusions have only a short-term effect, while TPO agonists have a longer effect. Thus, new and efficient drugs targeting this issue need to be developed and explored in clinical practice.
Recombinant human thrombopoietin (rh-TPO) is generally used for treating thrombocytopenia caused by chemotherapy and immune thrombocytopenia (Vadhan-Raj et al., 2003; Yu et al., 2020). There are no prospective clinical trials to confirm its use in CLD, but multicenter real-world studies have demonstrated its efficacy and safety in CLD patients with TPO (Feng et al., 2022). Avatrombopag, as a novel oral TPO receptor agonist, has been approved recently as an alternative treatment for PLT in the treatment of invasive surgery for CLD patients with severe TCP. Phases I, II, and III studies demonstrated the efficacy and safety of avatrombopag in patients with severe TCP related to CLD (Terrault et al., 2014; Kuter and Allen, 2018; Terrault et al., 2018). Since both drugs are used in this condition, a subsequent issue arises: whether the two drugs can increase platelet count before invasive surgical procedures, whether oral and subcutaneous injections have the same efficacy, and whether there is a difference in adverse drug events effects. However, to date, few studies have focused on the comparison between the efficacy and safety of avatrombopag and rh-TPO.
Thus, in the present study, we analyzed the clinical data of patients with TCP associated with CLD before and after propensity score matching (PSM) to explore the efficacy of avatrombopag compared with rh-TPO in the real-world study.
A total of 250 patients with TCP associated with CLD receiving avatrombopag or rh-TPO treatment were collected retrospectively in our hospital from January 2019 to January 2022. A total of 13 patients were excluded, and 237 patients were included based on the inclusion and exclusion criteria (Figure 1). The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (ID: 2022-RE-016). The written informed consent form signed by the patient was exempted due to the retrospective nature of the study.
Inclusion criteria: 1) TCP associated with CLD, 2) treatment with avatrombopag or rh-TPO, and 3) complete clinical data.
Exclusion criteria: 1) combined with malignant hematological diseases, such as lymphoma and leukemia, 2) combined with major cardiovascular disease, 3) receiving other PLT-containing therapy simultaneously, and 4) missing clinical data (Terrault et al., 2018).
Treatment criteria: CLD patients with severe TCP need to have a platelet count above 50 × 109/L before invasive surgical procedures are performed to reduce the risk of bleeding.
Patients in the avatrombopag treatment group: PLT <40 × 109/L, 60 mg/d once per day and PLT from 40 to 50 × 109/L, 40 mg/d once a day for 5 days orally. Patients in the rh-TPO treatment group received a subcutaneous dose of 300 U/(kg·d) once a day and continuous treatment for 1–5 days.
Parameters assessed: 1) PLT change after using drugs: mean PLT counts after 1–5 days, 6–10 days, and 11–15 days of medication and 2) adverse drug events: portal vein thrombosis, headache, dizziness, blood transfusion reaction, hematuria, proteinuria, fever, abdominal pain, diarrhea, indigestion, fatigue, nausea, and peripheral tissue edema.
Outcome indicators: reaching the increased level of PLT counts ≥50 × 109/L on the 15th day of evaluation after drug administration and no PLT infusion or no occurrence of emergency bleeding risk (Terrault et al., 2018). Therapeutic response rate equaled the number of patients with an effective endpoint/the number of patients included in each group ×100%.
SPSS statistical software (version 26.0) was used for the data analysis. The baseline population was described as the mean ± standard deviation (SD) or median (min, max). Anderson–Darling normality test was used for the normal distribution test. Normal distribution data were compared between the two groups by a t-test. Skew distribution data were compared between the two groups by the Mann–Whitney U test. Count data are expressed as absolute numbers or percentages and were compared between groups using the χ2 test or Fisher’s exact probability method. Repeated measurement data in-point comparisons between groups were done using generalized estimator equations. ROC diagnostic curves were used for platelet count before treatment and outcome indicators. p < 0.05 was considered statistically significant. The propensity score matching (PSM) method was used to adjust the baseline variables between the avatrombopag and rh-TPO groups to draw more accurate conclusions and avoid possible selection bias. Multivariate logistic regression analysis was used to determine the propensity score for each patient based on sex, age, body mass index (BMI), premedication PLT, pathological diagnosis, chronic hepatitis B infection, Child‒Pugh grade, and types of surgical procedure, with 1:1 matching and propensity score by nearest neighbor matching. After PSM, 77 patients were included in each group, and the detailed clinical parameters are summarized in Table 1.
Before PSM, characteristics including sex, age, BMI (kg/m2), mean PLT count before medication, disease type, chronic hepatitis B infection, splenomegaly, Child–Turcotte–Pugh Class grade, and type of invasive surgical procedures were compared between the avatrombopag and rh-TPO groups. There were significant differences in sex, BMI, and mean platelet count between the two groups before medication (p < 0.05). After PSM, there were no significant statistical differences in any of the abovementioned characteristics between the two groups (p > 0.05). The two groups were comparable (Table 1).
The median changes in platelet count before and after treatment in the avatrombopag and rh-TPO groups were compared (Figure 2A). The effective rate was 75.18% (106/141) in the avatrombopag group and 68.75% (66/96) in the rh-TPO group, and there was no statistical difference between the two groups (p = 0.276).
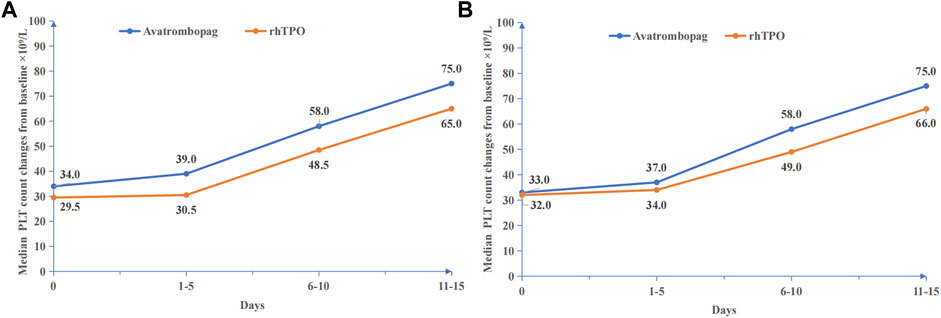
FIGURE 2. Platelet count changes before and after medication. (A) Before PSM, median PLT count changes from baseline ×109/L. (B) After PSM, median PLT count changes from baseline ×109/L.
In patients with ineffective response, six patients received platelet transfusions in the avatrombopag group and eight patients received platelet transfusions in the rh-TPO group. There was no significant difference in the proportion of patients receiving platelet transfusion between the two groups (p = 0.191).
The PLT count showed a time-dependent increase in the treatment duration of both drugs (Waldχ2 = 176.413, p < 0.001). However, no statistically significant difference between the avatrombopag group and the rh-TPO group in the treatment of severe TCP related to CLD was observed with the increased PLT count (Waldχ2 = 1.659, p = 0.198). In addition, no interaction between time and different medications was found (Waldχ2 = 0.540, p = 0.910) (Figure 2A).
Before PSM (Table 2), in Child‒Pugh Class A, the effective rate was 88.89% in the avatrombopag group and 63.41% in the rh-TPO group. There was a significant difference in the effective rate between the two groups (p = 0.003). In Child‒Pugh Class B, the effective rate was 76.60% in the avatrombopag group and 72.00% in the rh-TPO group. There was no significant difference in the effective rate between the two groups (p = 0.417). In addition, in Child‒Pugh Class C, the effective rate was 55.00% in the avatrombopag group and 80.00% in the rh-TPO group. There was no significant difference in the effective rate between the two groups (p = 0.088).
Regardless of whether before or after PSM, the best threshold of PLT was 26.5 × 109/L by the ROC diagnosis curve (Figures 3A and B). Thus, according to the baseline level of platelets, they were divided into PLT ≤25 × 109/L and 26–50 × 109/L to compare the drug effectiveness of the two groups (Table 3). When PLT ≤25 × 109/L, the effective rate was 55.17% in the avatrombopag group and 53.85% in the rh-TPO group. There was no significant difference in effective rate between the two groups (p = 0.914). When PLT ranged from 26 to 50 × 109/L, the effective rate was 80.36% in the avatrombopag group and 78.95% in the rh-TPO group. There was no significant difference in effective rate between the two groups (p = 0.829). However, the efficiency of the avatrombopag group with PLT ≤25 × 109/L was significantly lower than that with PLT ranging from 26 ×1 09/L to 50 × 109/L (55.17% vs. 80.36%). Moreover, the efficiency of the rh-TPO group was significantly lower when PLT ≤25 × 109/L than when PLT ranged from 26 to 50 × 109/L (53.85% vs. 78.95%).
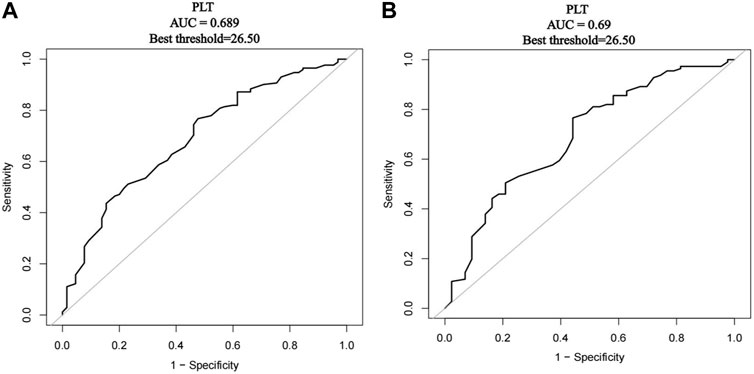
FIGURE 3. Receiver operating characteristics (ROC) curve of platelet count diagnosed with outcome indicators. (A) Before PSM, the ROC diagnostic curve of platelet count diagnosed with outcome indicators. (B) After PSM, the ROC diagnostic curve of platelet count diagnosed with outcome indicators.
Before PSM (Table 4), except for the incidence of dizziness, there was no significant difference in the incidence of adverse drug events, including portal vein thrombosis, abdominal pain, fatigue, indigestion, headache, nausea, diarrhea, and peripheral edema, between these two groups (p > 0.05). Fewer patients reported dizziness and pyrexia in the avatrombopag group than in the rh-TPO group (7.8% vs. 25.0%, 9.2% vs. 21.9%, both p < 0.05). In addition, there were no serious bleeding events in the short term after medication.
The median changes in PLT count before and after treatment in the avatrombopag and rh-TPO groups are shown in Figure 2B. The effective rate was 75.32% (58/77) in the avatrombopag group and 68.83% (53/77) in the rh-TPO group, and there was no statistical difference between the two groups (p = 0.369).
In patients with ineffective response, three patients received platelet transfusions in the avatrombopag group and four patients received platelet transfusions in the rh-TPO group. There was no significant difference in the proportion of patients receiving platelet transfusion between the two groups (p = 1.000).
The PLT count showed a time-dependent increase in the treatment duration of both drugs (Waldχ2 = 142.210, p < 0.001). However, no statistically significant difference between the avatrombopag group and the rh-TPO group in the treatment of severe TCP related to CLD was observed in the effect of increasing PLT count (Waldχ2 = 0.220, p = 0.639). In addition, no interaction between time and different medications was found (Waldχ2 = 1.273, p = 0.736) (Figure 2B).
After PSM (Table 2), in Child–Pugh Class A, the effective rate was 83.33% in the avatrombopag group and 61.76% in the rh-TPO group. There was a significant difference in the effective rate between the two groups (p = 0.043). In Child‒Pugh Class B, the effective rate was 69.57% in the avatrombopag group and 72.00% in the rh-TPO group. There was no significant difference in the effective rate between the two groups (p = 0.853). In Child‒Pugh Class C, the effective rate was 66.67% in the avatrombopag group and 77.78% in the rh-TPO group. There was no significant difference in the effective rate between the two groups (p = 0.711).
After PSM, according to the baseline level of platelets, they were also divided into PLT ≤25 × 109/L and 26–50 × 109/L to compare the drug effectiveness of the two groups (Table 3). When PLT ≤25 × 109/L, the effective rate was 52.63% in the avatrombopag group and 51.85% in the rh-TPO group. There was no significant difference in effective rate between the two groups (p = 0.911). When PLT ranged from 26 to 50 × 109/L, the effective rate was 82.76% in the avatrombopag group and 78.00% in the rh-TPO group. There was no significant difference in the effective rate between the two groups (p = 0.958). However, the efficiency of the avatrombopag group with PLT ≤25 × 109/L was significantly lower than that with PLT ranging from 26 × 109/L to 50 × 109/L (52.63% vs. 82.76%). Moreover, the efficiency of the rh-TPO group was significantly lower when PLT ≤25 × 109/L than when PLT ranged from 26 to 50 × 109/L (51.85% vs. 78.00%).
After PSM (Table 4), except for the incidence of dizziness, there was no significant difference in the incidence of adverse drug events, including portal vein thrombosis, abdominal pain, pyrexia, fatigue, indigestion, headache, nausea, diarrhea, and peripheral edema, between these two groups (p > 0.05). Fewer patients reported dizziness in the avatrombopag group than in the rh-TPO group (7.8% vs. 24.7%, p = 0.005). In addition, there were no serious bleeding events in the short term after medication.
At present, rh-TPO, glucocorticoids, avatrombopag, and lusutrombopag are commonly used in the clinical treatment of CLD-related TCP. Rh-TPO is a full-length glycosylated TPO purified by gene recombination technology. Rh-TPO and endogenous TPO have similar pharmacological effects in increasing PLT by activating C-MPL (Demetri, 2000). However, problems with the clinical use of rh-TPO include subcutaneous administration and an inability to predict an increase in PLT count. Therefore, new improved drugs or alternative drugs are constantly being explored and developed. Avatrombopag is a novel, oral, small-molecule TPO-R agonist (Terrault et al., 2014; Terrault et al., 2018). It ultimately promotes megakaryocyte proliferation/differentiation and predictably increases the PLT count through the activation of the JAK-STAT and SHC-Ras-Raf-ERK signaling pathways and, therefore, could be used as an alternative to PLT infusion (Bussel et al., 2014; Kuter and Allen, 2018; Długosz-Danecka et al., 2019). Avatrombopag only increases the number of PLT in patients and does not enhance PLT activation (Michelson et al., 2018; Kuter, 2022). To date, there have been few real-world studies of these two drugs in the treatment of TCP associated with CLD, let alone any comparisons between the two drugs.
Two global, multicenter, randomized, double-blind, placebo-controlled phase II (E5501-G000-202, NCT00914927) and phase III clinical trials (ADAPT-1, ADAPT-2) evaluated the efficacy and safety of avatrombopag in patients with severe TCP related to CLD who underwent invasive procedures or surgery. The results suggest that avatrombopag can be used to treat severe TCP related to CLD with high efficacy and safety (Terrault et al., 2014; Terrault et al., 2018). A nationwide multicenter, real-world study has reported the efficacy and safety of rh-TPO in the treatment of TCP caused by cirrhosis (Feng et al., 2022). At the same time, an ethnic sensitivity analysis concluded that avatrombopag was effective and safe in the management of thrombocytopenia in Chinese patients with CLD. Ethnicity does not appear to influence the efficacy and safety of avatrombopag (Lu et al., 2022). Practice Guidelines of the Central European Hepatologic Collaboration (CEHC) on the use of TPO receptor agonists in patients with CLD undergoing invasive procedures were reported. A consensus was agreed that TPO-RAs should be considered for raising PLT count in CLD patients undergoing scheduled abdominal surgery, high-bleeding risk dentistry, endoscopic polypectomy, endoscopic variceal ligation, liver biopsy, liver surgery, liver transplantation, and percutaneous ablation, but it was also agreed that they are less beneficial or not necessary for endoscopy without intervention and paracentesis (Flisiak et al., 2021). Our study included patients with these high-risk procedures, and there were no serious bleeding events in the short term after medication.
In the present real-world study, two important results were found. First, the treatment response rate was 75.18% (106/141) in the avatrombopag group and 68.75% (66/96) in the rh-TPO group. Both before and after PSM, there was no statistically significant difference in treatment efficiency between the two groups. However, in the treatment of Child‒Pugh Class A patients both before and after PSM, the effective rate of the avatrombopag group was higher than that of the rh-TPO group, with a statistically significant difference between the two groups (88.89% vs. 63.41%; 81.33% vs. 61.76%). The use of avatrombopag in the treatment of TCP associated with CLD in Child‒Pugh Class A may achieve better clinical efficacy and significantly reduce the risk of invasive procedures. We thought that this might be because of the way in which it was administered: avatrombopag is administered orally, while rh-TPO is administered subcutaneously. In patients with better liver function, the more effective the drug is absorbed through the digestive system, the better the platelet count will be. For patients with severe liver disease, oral medication is far less effective than injection (Pereira et al., 2005). The clinical response rate of avatrombopag was lower than that of the two phase III clinical trials (75.18% vs. 88.1%/87.9%), which may be due to the difference in the patient populations selected: few patients with liver function in the stage of Child‒Pugh Class C were included, and patients with hepatocellular carcinoma (Barcelona-Clinic Liver Cancer staging classification C or D) were excluded from these clinical trials (Terrault et al., 2014; Terrault et al., 2018). Given the actual clinical situation, these patients excluded from prospective clinical trials will also have application scenarios of the abovementioned drugs in the real world. Second, the efficacy rates of avatrombopag and rh-TPO in patients with initial PLTs >25 × 109/L were higher than those in patients with initial PLTs ≤25 × 109/L both before and after PSM (55.17% vs. 80.36%, 53.85% vs. 78.95%; 55.56% vs. 81.36%, 53.85% vs. 76.47%). Considering the results of ROC analysis (the best threshold of PLT was 26.5 × 109/L both before and after PSM) and the convenience for clinical use, we set the cutoff value as 25 × 109/L.
Regarding safety, there were no serious systemic adverse events caused in the two groups. The most common complications were abdominal pain, fatigue, dizziness, and fever. There was no significant difference in the incidence of abdominal pain, pyrexia, fatigue, indigestion, nausea, diarrhea, peripheral edema, or other adverse events between the two groups. However, the incidence of dizziness in the rh-TPO group was higher than that in the avatrombopag group both before and after PSM (25.0% vs. 7.8%; 24.7% vs. 7.8%). The incidence of abdominal pain and fatigue was higher than that in clinical trials (22.75 vs. 10.3%; 19.9% vs. 6.7%), and there were no significant differences in other adverse events. This may be because we included more patients with Child‒Pugh Class C, whereas clinical trials rarely included such patients (Terrault et al., 2018). These patients have abdominal pain and fatigue symptoms, and it can be difficult to distinguish whether the drug causes these symptoms. In addition, with respect to the delivery route, patients treated with rh-TPO require subcutaneous injection, while patients treated with avatrombopag require oral administration, which is more convenient and safer.
There are still some limitations in our study. First, compared with the prospective clinical trials, it is difficult to monitor patients’ PLT counts dynamically each day due to various issues such as ethics and patient compliance in a real-world study. Second, the change in PLT counts was only directly observed up to 14 days after medication due to the urgency and need for surgical treatment of the patient’s disease, especially in cases where the PLT counts met the minimum requirement for invasive surgery or related treatment (>50 × 109/L). Thus, a long-term result of efficacy and safety needs to be observed in future studies if conditions permit. Third, a limited number of cases were included in the study due to the short time that avatrombopag has been on the market. All of these issues will be focused on and updated further in our future studies.
Taken together, to the best of our knowledge, this is the first real-world study focusing on the comparison of efficacy and safety between avatrombopag and rh-TPO. Our findings proved that avatrombopag showed better clinical efficacy than rh-TPO in the treatment of TCP associated with CLD in Child‒Pugh Class A and showed a lower incidence of dizziness in all patients. The effective rates of avatrombopag and rh-TPO in patients with initial PLTs >25 × 109/L were higher than those in patients with initial PLTs ≤25 × 109/L. In the future, a large cohort or prospective clinical study still needs to be performed to further verify the abovementioned findings.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China (ID: 2022-RE-016). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Guarantor of the article: J-ZW; study design: Y-SW; manuscript draft and analysis: Y-SW; analysis: WW (second author (Department of Oncology)); data acquisition: SZ, S-YZ, and WW (sixth author (Department of Pathology)), A-ZS, LL, H-CS, H-ZY, F-ZM, and R-PS; and critical revisions: BN and L-XL.
This work was partly supported by the National Natural Science Foundation of China (Grant Number 82170618).
The authors thank BN and L-XL for their help in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
TCP, thrombocytopenia; CLD, chronic liver disease; rh-TPO, recombinant human thrombopoietin; PLT, platelet; PSM, propensity score matching; TPO, thrombopoietin; BMI, body mass index; TACE, transcatheter arterial chemoembolization.
Armstrong, N., Büyükkaramikli, N., and Penton, H. (2020). Avatrombopag and lusutrombopag for thrombocytopenia in people with chronic liver disease needing an elective procedure: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 24 (51), 1–220. doi:10.3310/hta24510
Basili, S., Raparelli, V., and Napoleone, L. (2018). Platelet count does not predict bleeding in cirrhotic patients: Results from the PRO-LIVER study. Am. J. Gastroenterol. 113 (3), 368–375. doi:10.1038/ajg.2017.457
Bussel, J. B., Kuter, D. J., and Aledort, L. M. (2014). A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood 123 (25), 3887–3894. doi:10.1182/blood-2013-07-514398
Demetri, G. D. (2000). Pharmacologic treatment options in patients with thrombocytopenia. Semin. Hematol. 37 (2 Suppl. 4), 11–18. doi:10.1016/s0037-1963(00)90048-9
Długosz-Danecka, M., and Zdziarska, J. (2019). Avatrombopag for the treatment of immune thrombocytopenia. Expert Rev. Clin. Immunol. 15 (4), 327–339. doi:10.1080/1744666X.2019.1587294
Elsaid, M. I., Rustgi, V. K., and Loo, N. (2020). The burden associated with thrombocytopenia and platelet transfusions among patients with chronic liver disease. J. Med. Econ. 23 (4), 378–385. doi:10.1080/13696998.2019.1699563
Feng, R., Liu, Y., and Zhu, X. L. (2022). Recombinant human thrombopoietin increases platelet count in severe thrombocytopenic patients with hepatitis B-related cirrhosis: Multicentre real-world observational study. J. Viral Hepat. 29 (5), 306–316. doi:10.1111/jvh.13655
Flisiak, R., Antonov, K., and Drastich, P. (2021). Practice guidelines of the central European hepatologic collaboration (CEHC) on the use of thrombopoietin receptor agonists in patients with chronic liver disease undergoing invasive procedures. J. Clin. Med. 10 (22), 5419. Published 2021 Nov 19. doi:10.3390/jcm10225419
Forkin, K. T., Colquhoun, D. A., and Nemergut, E. C. (2018). The coagulation profile of end-stage liver disease and considerations for intraoperative management. Anesth. Analg. 126 (1), 46–61.
Giannini, E. G., Greco, A., and Marenco, S. (2010). Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin. Gastroenterol. Hepatol. 8 (10), 899–e109. doi:10.1016/j.cgh.2010.06.018
Gilreath, J., Lo, M., and Bubalo, J. (2021). Thrombopoietin receptor agonists (TPO-RAs): Drug Class considerations for pharmacists. Drugs 81 (11), 1285–1305. doi:10.1007/s40265-021-01553-7
Karlström, C., Gryfelt, G., and Schmied, L. (2022). Platelet transfusion improves clot formation and platelet function in severely thrombocytopenic haematology patients. Br. J. Haematol. 196 (1), 224–233. doi:10.1111/bjh.17820
Kuter, D. J., and Allen, L. F. (2018). Avatrombopag, an oral thrombopoietin receptor agonist: Results of two double-blind, dose-rising, placebo-controlled phase 1 studies. Br. J. Haematol. 183 (3), 466–478. doi:10.1111/bjh.15574
Kuter, D. J. (2022). The structure, function, and clinical use of the thrombopoietin receptor agonist avatrombopag. Blood Rev. 53, 100909. doi:10.1016/j.blre.2021.100909
Lu, J., Jamieson, B. D., and Hui, A. M. (2022). Avatrombopag ethnic sensitivity analysis in chronic liver disease and thrombocytopenia patients: Individual-level pooled analysis. Ther. Adv. Gastroenterol. 15, 17562848221105976. Published 2022 Jun 30. doi:10.1177/17562848221105976
Maan, R., de Knegt, R. J., and Veldt, B. J. (2015). Management of thrombocytopenia in chronic liver disease: Focus on pharmacotherapeutic strategies. Drugs 75 (17), 1981–1992. doi:10.1007/s40265-015-0480-0
Michelson, A. D., Smolensky Koganov, E., and Forde, E. E. (2018). Avatrombopag increases platelet count but not platelet activation in patients with thrombocytopenia resulting from liver disease. J. Thromb. Haemost. 16 (12), 2515–2519. doi:10.1111/jth.14295
Mitchell, O., Feldman, D. M., and Diakow, M. (2016). The pathophysiology of thrombocytopenia in chronic liver disease. Hepat. Med. 8, 39–50. Published 2016 Apr 15. doi:10.2147/HMER.S74612
Pereira, S. P., Rowbotham, D., and Fitt, S. (2005). Pharmacokinetics and efficacy of oral versus intravenous mixed-micellar phylloquinone (vitamin K1) in severe acute liver disease. J. Hepatol. 42 (3), 365–370. doi:10.1016/j.jhep.2004.11.030
Qamar, A. A., Grace, N. D., and Groszmann, R. J. (2009). Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin. Gastroenterol. Hepatol. 7 (6), 689–695. doi:10.1016/j.cgh.2009.02.021
Qureshi, K., Patel, S., and Meillier, A. (2016). The use of thrombopoietin receptor agonists for correction of thrombocytopenia prior to elective procedures in chronic liver diseases: Review of current evidence. Int. J. Hepatol. 2016, 1802932. doi:10.1155/2016/1802932
Scharf, R. E. (2021). Thrombocytopenia and hemostatic changes in acute and chronic liver disease: Pathophysiology, clinical and laboratory features, and management. J. Clin. Med. 10 (7), 1530. Published 2021 Apr 6. doi:10.3390/jcm10071530
Terrault, N., Chen, Y. C., and Izumi, N. (2018). Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology 155 (3), 705–718. doi:10.1053/j.gastro.2018.05.025
Terrault, N. A., Hassanein, T., and Howell, C. D. (2014). Phase II study of avatrombopag in thrombocytopenic patients with cirrhosis undergoing an elective procedure. J. Hepatol. 61 (6), 1253–1259. doi:10.1016/j.jhep.2014.07.007
Vadhan-Raj, S., Patel, S., and Bueso-Ramos, C. (2003). Importance of predosing of recombinant human thrombopoietin to reduce chemotherapy-induced early thrombocytopenia. J. Clin. Oncol. 21 (16), 3158–3167. doi:10.1200/JCO.2003.08.003
Yu, Y., Wang, M., and Hou, Y. (2020). High-dose dexamethasone plus recombinant human thrombopoietin vs high-dose dexamethasone alone as frontline treatment for newly diagnosed adult primary immune thrombocytopenia: A prospective, multicenter, randomized trial. Am. J. Hematol. 95 (12), 1542–1552. doi:10.1002/ajh.25989
Keywords: avatrombopag, thrombocytopenia (TCP), chronic liver disease (CLD), recombinant human thrombopoietin (rh-TPO), propensity scorematching (PSM), efficacy
Citation: Wang Y-S, Wang W, Zhang S, Zhang S-Y, Shen A-Z, Wang W, Song H-C, Yao H-Z, Song R-P, Meng F-Z, Li L, Nashan B, Wang J-Z and Liu L-X (2022) Clinical efficacy of avatrombopag and recombinant human thrombopoietin in the treatment of chronic liver disease-associated severe thrombocytopenia: A real-world study. Front. Pharmacol. 13:1009612. doi: 10.3389/fphar.2022.1009612
Received: 03 August 2022; Accepted: 13 September 2022;
Published: 04 October 2022.
Edited by:
Guoxun Chen, The University of Tennessee, Knoxville, United StatesReviewed by:
Mohamed A. Yassin, Hamad Medical Corporation, QatarCopyright © 2022 Wang, Wang, Zhang, Zhang, Shen, Wang, Song, Yao, Song, Meng, Li, Nashan, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Zhou Wang, d2FuZ2pvZUB1c3RjLmVkdS5jbg==; Lian-Xin Liu, bGl1bHhAdXN0Yy5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID: Ji-Zhou Wang, orcid.org/0000-0002-3609-0445; Lian-Xin Liu, orcid.org/0000-0002-3535-6467
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.