
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 10 October 2022
Sec. Inflammation Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.1009527
This article is part of the Research TopicAnti-inflammatory Immunopharmacology in the Prevention and Treatment of Major Chronic DiseasesView all 12 articles
Since the outbreak of Coronavirus disease (COVID-19) in 2019, it has spread rapidly across the globe. Sleep disorders caused by COVID-19 have become a major concern for COVID-19 patients and recovered patients. So far, there’s no effective therapy on this. Traditional Chinese therapy (TCT) has a great effect on sleep disorders, with rare side effects and no obvious withdrawal symptoms. The cholinergic anti-inflammatory pathway, a neuroregulatory pathway in the central nervous system that uses cholinergic neurons and neurotransmitters to suppress inflammatory responses, has been reported to be associated with sleep disorders and psychiatric symptoms. Many studies have shown that TCT activates the cholinergic anti-inflammatory pathway (CAP), inhibits inflammation, and relieves associated symptoms. Therefore, we believe that TCT may be a potential therapeutic strategy to alleviate sleep disorders induced by COVID-19 through CAP. In this review, we analyzed the relationship between cytokine storm induced by Coronavirus and sleep disorders, explained the influence of CAP on sleep disorders, discussed the TCT’s effect on CAP, and summarized the treatment effect of TCT on sleep disorders. Based on these practical researches and theoretical basis, we propose potential strategies to effectively improve the sleep disorders caused by COVID-19.
An outbreak of pneumonia was caused by a novel coronavirus in Wuhan, Hubei Province province, China, at the end of 2019. Since then, the novel coronavirus has spread rapidly to different countries and regions and has evolved into a major international public health emergency. Clinically, the symptoms of the Coronavirus disease (COVID-19) in 2019 range from asymptomatic to mild symptoms such as fever, fatigue, and cough to severe acute respiratory distress syndrome (ARDS) (Chen et al., 2020; Kwenandar et al., 2020; Zhou et al., 2021). In addition, the COVID-19 pandemic has led to an epidemic of mental illnesses, such as insomnia, depression and anxiety, and symptoms of post-traumatic stress (Guo et al., 2020; Akıncı and Melek Başar, 2021; Huang et al., 2021). Recently, a systematic review of 10 studies using the Pittsburgh Sleep Quality Index (PSQI) questionnaire to assess sleep quality found that about a quarter of COVID-19 survivors was diagnosed with sleep disorders (Cheng et al., 2021a). Sleep disorders were the most common neuropsychiatric symptoms in patients 14–182 days after recovery from COVID-19 (Ding and Yao, 2020). The severity of COVID-19 was closely related to the intensity of the virus and the body’s inflammatory responses (Mehta et al., 2020). In severe cases, an excessive inflammatory response, known as namely, the “cytokine storm,” occurs due to the release of high levels of proinflammatory cytokines and chemokines produced by inflammatory cells. Cytokine storms can lead to multiple organ failures and even death (Channappanavar and Perlman, 2017). While many drugs are effective in relieving symptoms associated with COVID-19 (Polack et al., 2020; Doroftei et al., 2021), there have been relatively rare evidence-based assessments and interventions for mental health disorders (Lai et al., 2020).
Traditional Chinese therapy has been used in epidemic treatment for thousands of years. From smallpox and ancient plagues to avian influenza, Middle East Respiratory Syndrome (MERS), and Severe Acute Respiratory Syndrome (SARS), Chinese have extensive experience in treating infections with Traditional Chinese therapy (TCT) (Chen and Nakamura, 2004; Hsu et al., 2006; Lin et al., 2017). Traditional Chinese therapy includes acupuncture, massage, Chinese herbal medicine, ear acupuncture, moxibustion and so on. The common treatment options of TCT including acupuncture, Chinese herbal medicine and taVNS have been summarized in this paper to reveal the most promising three treatment methods. Acupuncture, Chinese herbal medicine, and transcutaneous auricular vagal nerve stimulation (taTNS) have also been explored as complementary treatments for sleep disorders, and with great effect (Lu et al., 2022; Luan et al., 2022). As a result, TCT has the potential to treat sleep disorders and psychiatric symptoms caused by COVID-19.
The cholinergic anti-inflammatory pathway (CAP) represents a neurological mechanism that suppresses inflammatory responses and was first discovered by Tracey KJ in 2000. They found that parasympathetic nervous system activity affects circulating tumor necrosis factor (TNF) concentrations and shock response to endotoxemia, a so-called “cholinergic anti-inflammatory pathway” (Qin et al., 2017). Activation of CAP is also considered a therapeutic strategy for respiratory diseases (Lv et al., 2022) and has the potential to be a promising therapeutic intervention for COVID-19 infection. The active ingredient in Chinese herbal medicine has been reported to inhibit proinflammatory cytokines and prevent cytokine storms (Dai et al., 2021; Yang et al., 2022). In addition, The World Health Organization (WHO) recommends acupuncture for 16 inflammatory diseases, and some clinical practice guidelines recommend acupuncture for multiple inflammatory diseases (Yang et al., 2016a; Wang et al., 2018a). TaVNS, derived from Chinese ear acupuncture, stimulate the auricle branch of the vagus nerve to activate CAP, which helps reduce inflammation. Several clinical and laboratory studies have also found that taVNS significantly improve and relieve inflammatory reactions (Baptista et al., 2020; Go et al., 2022a). Therefore, TCT has a high potential for treating inflammatory response symptoms caused by the novel coronavirus. In this review, we aim to analyze and summarize if TCT will be a promising strategy for the treatment of treating sleep disorders caused by COVID-19.
The keywords “sleep disorders” was searched in PubMed and web of science from 1986 to 2022. A secondary search was conducted by screening the list of articles that met the inclusion criteria. The keywords were COVID-19, cholinergic anti-inflammatory pathway, acupuncture, taVNS and Chinese herbal medicine. The obtained articles were screened, and irrelevant title or abstract was excluded. Finally, we organized the tables, drew the figures and wrote the text to summarize the traditional Chinese therapy in treating sleep disorders caused by COVID-19 through the cholinergic anti-inflammatory pathway.
Cytokines storm is essentially an immune system overreaction to infection. As the novel coronavirus enters the lungs, its S-protein specifically recognizes the host angiotensin-converting enzyme 2 receptor in alveolar epithelial type II cells. Upon binding, the host serine protease TMPRSS2 breaks down the S protein, allowing the virus to fuse with the cell membrane, and then the novel coronavirus enters the host cell (Wan et al., 2020). The host activates an immune response to clear the virus. In the early stages, virus infection causes the absorption and activation of various inflammatory cells in the lungs, releasing large amounts of cytokines and inflammatory chemokines. The TNF-α and IL-1β and other early active cytokines are rapidly secreted and peak within a few hours. Subsequently, anti-inflammatory cytokines are secreted to regulate the inflammatory response, allowing the body to eliminate harmful stimuli while maintaining cellular homeostasis. However, when the pro-inflammatory balance is disrupted, early reactive cytokines can further trigger the activation and release of a range of cytokines such as IL-2, IL-6, IL-8, IL-12, and inflammatory chemokines, leading to “cascading” effects and the uncontrolled inflammatory responses (Mehta et al., 2020). A retrospective multicenter study involving 150 COVID-19 patients suggests that virus-activated “cytokines storm syndrome” may be associated with COVID-19 mortality (Koralnik and Tyler, 2020). Meanwhile, elevated levels of serum IL-2, TNF-α, IL-7, granulocyte colony-stimulating factor, and interferon-gamma-induced protein 10 were correlated with the severity of COVID-19 (Mehta et al., 2020). Plasma levels of IL-2, IL-7, TNF-α, and other pro-inflammatory cytokines were elevated in COVID-19 patients, and levels of various inflammatory cytokines were higher in (ICU) patients than in non-ICU patients (Huang et al., 2020). Clinical studies have found that severe COVID-19 patients often experience this cytokine storm. Not only can it lead to acute lung injury, but it can also progress to multiple organs, including the central nervous system and peripheral nervous system organs (Gimeno et al., 2009; Koralnik and Tyler, 2020). Table 1 summarized the different neurological symptoms induced by COVID-19. COVID-19 patients experienced many different neurological symptoms during their illness, such as headaches, post-traumatic stress disorder (PTSD), sleep disorders, and depressive symptoms (Arnold et al., 2021; Xu et al., 2021). A previous study found that blocking the biological effects of the cytokines IL-1 and TNF can reduce the amount of non-REM sleep or NREM sleep rebound after sleep deprivation. On the other hand, increasing the supply of these cytokines promoted and inhibits NREM sleep volume and intensity. These findings suggested that both IL-1 and TNF are involved in the homeostatic regulation of sleep (Krueger and Majde, 1995; Zhang et al., 2020). In addition, anti-inflammatory cytokines IL-4, IL-10, and IL-13 were reported to reduce NREM sleep amount in rabbits (Kushikata et al., 1999; Kubota et al., 2000; Opp, 2005), while anti-inflammatory cytokines IFN-γ, IL-2, IL-6, and IL-15 promoted NREM sleep in animal models (Kubota et al., 2001a; Kubota et al., 2001b; Hogan et al., 2003). A clinical study found circulating levels of IL-1, TNF, and IL-6 peak during sleep or early morning (Lange et al., 2010; Chavan et al., 2017). Studies have shown that injecting healthy volunteers with IL-6 prolongs the NREM phase, leading to subjective fatigue and elevated CRP levels (Ranjbaran et al., 2007). In summary, high levels of inflammatory cytokines could lead to sleep disorders during COVID-19.
CAP is a neuroregulatory pathway in the central nervous system that uses cholinergic neurons and neurotransmitters to suppress systemic inflammatory responses. It releases acetylcholine through the vented ending of efferent vagal endings and binds to α7 nicotinic acetylcholine receptor (α7nAChR) on macrophages and other immune cells, inhibiting macrophage activation and inhibiting the release of pro-inflammatory factors such as TNF-α, IL-1β, IL-6 (Pavlov et al., 2003; Pavlov and Tracey, 2015). The α7nAChR plays a key role in regulating immune responses and oxidative stress in the central and peripheral nervous systems (Ren et al., 2017), participating in processes of learning, memory consolidation, movement, and attention (Fucile et al., 2003; Park et al., 2007). α7nAChR agonist PHA-543613 reduces inflammatory damage and enhances anti-inflammatory factors and antioxidant enzymes (Xue et al., 2019). Furthermore, when the vagus nerve is electrically stimulated, the axon terminals secrete large amounts of ACh, further activating anti-inflammatory pathways in various inflammatory cells (Go et al., 2022b). Experimental results show that stimulation of the distal vagus nerve transection can prevent the elevation of liver and blood TNF caused by septic shock (Song et al., 2008). In addition, an animal model found that electrical stimulation of the vagus nerve and administration of cholinergic neurotransmitter acetylcholine inhibited levels of pro-inflammatory factor TNF-α and reduced inflammatory responses, which were exacerbated by vagotomy (Bonaz et al., 2016). Vagus nerve stimulation (VNS) also significantly reduced levels of pro-inflammatory cytokines IL-6 and IL-1β, as well as the proportion of microglia and macrophages in mice stimulated by lipopolysaccharides (Meneses et al., 2016). In summary, stimulating the vagus nerve or activating α7nAChR effectively inhibits the development of inflammation, and Figure 1 depicted the molecular mechanisms by which CAP attenuates inflammation.
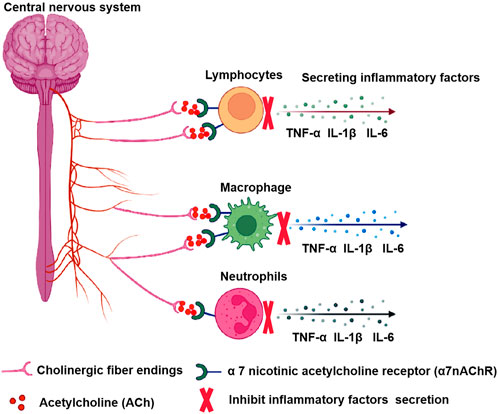
FIGURE 1. The molecular mechanisms of the CAP. The central nervous system releases ACh through efferent vagal endings and binds to α7nAChR on various inflammatory cells, inhibiting the release of pro-inflammatory factors.
Recently, activation of CAP has also been considered a strategy for the treatment of respiratory diseases (Yamada and Ichinose, 2018). The α7nAChR has been shown to activate lung resident immune cells such as alveolar macrophages, epithelial cells, and activated neutrophils, as well as slow local inflammatory responses and reduce lung injury. In the mice model of acute lung injury, VNS prevents lung injury by lung injury autonomic nervous system imbalance and activating α7nAChR through CAP (dos Santos et al., 2011; Yang et al., 2014; Liu et al., 2017a). A cohort clinical study reported the role of α7nAChR in regulating inflammatory response and oxidative stress in the chronic sleep deprivation model. Stimulation of α7nAChR contributes to adverse reactions caused by sleep deprivation. α7nAChR as a biomarker of hippocampal inflammation and oxidative stress after chronic sleep deprivation (Xue et al., 2019). Therefore, targeting CAP with VNS may be a promising treatment for lung injury and sleep disorders caused by COVID-19.
The CAP pathway is an important component of the cholinergic system that connects the nervous system to the immune system and acts as an anti-inflammatory agent through the ACh and vagus nerve (Liu et al., 2015). The cholinergic system has been reported to regulate sleep cycles (Jasper and Tessier, 1971). Studies have shown that acetylcholine plays an important role in wakefulness and breathing in people with sleep apnea (Otuyama et al., 2013). Meng et al. (2021) also found that daytime sleepiness and high blood pressure are associated with sympathetic-vagus nerve imbalance, which may be associated with decreased plasma ACh level. A clinical trial found that short sleepers responded significantly less to ACh forearm blood flow response than normal sleepers (Stockelman et al., 2021). Another study showed that ozone-induced abnormal sleep loss is associated with decreased ACh levels in the medial preoptic region rats (Alfaro-Rodríguez and González-Piña, 2005). Dexzopicclone is one of the most commonly used sleeping drugs in the clinic. It has a sedative, hypnotic effect and partially suppresses pedunculopontine tegmental (PPT) neurons by enhancing gamma-aminobutyric acid. One study reported inhibition of dextran, which reduced the release of the PPT-neuron terminals ACh in the pontine reticular formation and promoted sleep (Hambrecht-Wiedbusch et al., 2010). Cao Q Neurotransmitter test showed that saponins promoted sleep by increasing levels of acetylcholine, acetylcholine laterodorsal tegmental, and acetylcholine in PPT in mice (Cao et al., 2016). Studies have shown that cholinergic neuronal antagonists can block the activation of α7nAChR and increase the wakefulness-associated state induced by cholinergic stimulation (Zant et al., 2016). Nyctinastic herbs decoction (NHD) can prolong para-chlorophenylalanine (PCPA)-induced insomnia in mice, sleep duration, sleep quality, and depressive state was improved, and the mechanism was that the level of ACh attenuated the insomnia effect of PCPA (Yang et al., 2021). These results suggest that ACH levels play a significant role in sleep disturbance.
Some studies have found that α7nAChR plays a key role in the pathophysiology of sleep disorders and may represent a target for the treatment and control of sleep disorders (Saint-Mleux et al., 2004; Xue et al., 2019). TCT has been used in China for more than 2000 years to treat insomnia, such as acupuncture, taVNS, and Chinese herbal medicine (Sarris, 2012). However, the underlying biological mechanisms are largely unknown. Some studies have shown that they can affect CAP, suppress inflammation, and may have sleep relief (Liu et al., 2020b; Liu et al., 2021). Table 2 summarized the impact of the three most common TCT effects on CAP and their advantages and disadvantages.
Acupuncture is one of the most popular complementary and alternative therapies. The efficacy of acupuncture in the treatment of inflammatory diseases has been widely reported (Li et al., 2007; Liu et al., 2022). Its anti-inflammatory effect is mainly achieved by activating the vagus nerve (Liu et al., 2013; Yu, 2022). It is performed by anatomically stimulating acupuncture points near the vagus nerve or its cutaneous branches in the ear, mastoid, and occipital regions (da Silva and Dorsher, 2014) and can be operated by manual or electrical stimulation (electroacupuncture) at different acupoints. In recent years, acupuncture has been widely recognized worldwide for its anti-inflammatory effects mediated by CAP. Acupuncture of the ear branch, which is mainly located in the stud and dorsal part of ear branch the ear, has been proven to directly affect vagus nerve activity or regulate the parasympathetic nerve (Nosadini et al., 1986; Gao et al., 2008; Imai et al., 2008; Imai et al., 2009; La Marca et al., 2010). Previous studies have found that the protective effects of electroacupuncture on the intestinal barrier are primarily associated with CAP and the reduction of inflammatory cytokines (Borovikova et al., 2000; Baek et al., 2005). In addition, acupuncture has a neuroregulatory effect on the plant nervous system and can play a role in regulating the balance of the autonomic nervous system clinically. In a mouse model of endotoxemia, Borovikova et al. found that stimulation of the vagus nerve by electroacupuncture inhibits inflammatory mediators produced by macrophages in a concentration-dependent manner (Li et al., 2015). In animal models of arthritis, electroacupuncture inhibits the production of inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF through choline and reduces inflammatory pain (Cai et al., 2019; Zhou et al., 2019). Auricular acupuncture and electroacupuncture “Zusanli” (ST36) inhibited the expression of the pro-inflammatory factors TNF-α and IL-6 in rat models of endotoxemia through the cholinergic anti-inflammatory pathway (Zhao et al., 2012). In addition, electroacupuncture ST36 increased local acetylcholine transferase, promotes ACh transcription and synthesis, inhibits NF-κB expression in lung tissue, and stimulates local CAP in the lung. In another study of LPS-induced systemic infections in animals, ST36 electroacupuncture activated the vagus nerve pathway that connects the spleen, reducing the production of TNF in the spleen (Lim et al., 2016). Low-intensity electroacupuncture at ST36 acupoint in the hindlimb can effectively reduce persistent systemic inflammation (Liu et al., 2020c). Studies have also shown that electroacupuncture “Hegu” (LI4) activates muscarinic acetylcholine receptor signals in the brain through somatic afferent, and then activates the efferent vagus nerve and splenic nerve, exerting an anti-inflammatory effect, reducing TNF, IL-1β, and IL-6 levels and improving survival rate in endotoxemia model rats (Song et al., 2012). In ischemic stroke, seven consecutive days of electroacupuncture on GV20 and GV24 also increase the expression of α7nAChR in hippocampal neurons and decreased the levels of proinflammatory cytokines TNF-α and IL-1β, leading to impaired learning and memory impairment (Liu et al., 2017b). These results suggested that acupuncture’s CAP-mediated anti-inflammatory effects may improve neurological symptoms and may be an effective treatment for sleep disorders caused by COVID-19.
It is worth noting that the anti-inflammatory effect of electroacupuncture is related to acupoint selection, stimulation intensity, body condition, etc. To optimize the stimulation parameters and improve the efficacy and safety of acupuncture therapy, it is worth clinical research to investigate the stimulation intensity of electroacupuncture in driving different autonomic nerve pathways.
TaVNS comes from ear acupuncture. The ear is thought to be directly or indirectly connected to 12 meridians (six yang and six yin) (Round et al., 2013). Neuroanatomical evidence confirmed that the outer ear is the only region of the body where the vagus nerve sensory endings are located (Peuker and Filler, 2002). Recent clinical and animal experiments have shown that percutaneous auricular point vagus nerve stimulation can increase the excitability of efferent vagus nerve excitability, and increase the ACh release and CAP activation by stimulating the cochlea region. ACh binding to α7nAChR resulted in reduced secretion of inflammatory cytokines TNF, IL-1β, and IL-6 (Andersson and Tracey, 2012; Pavlov and Tracey, 2012; Kaczmarczyk et al., 2017). One study found that taVNS inhibited the expression of TNF-α, IL-1β, IL-6, and NF-kB p65 in endotoxemia rat serum through α7nAChR-mediated CAP (Jiang et al., 2018). The results implied that taVNS are a novel neurostimulation therapy with immunomodulatory and anti-inflammatory effects that may be beneficial for sleep disorders caused by inflammation caused by COVID-19.
A large number of Chinese herbal medicine preparations for the treatment of lung diseases have an excellent effect. Activation of CAP is a theoretical basis for traditional Chinese treatment of COVID-19 infection. Berberine is an acetylcholinesterase inhibitor whose main active ingredient is derived from the Chinese herbal medicine Coptis Chinensis (Cho et al., 2006). Berberine has a neuroprotective effect by inhibiting acetylcholinesterase activity, increasing ACh levels and a7nAChR expression, thus regulating CAP, suppressing inflammation, and improving abnormal oxidative stress and cholinergic function (Li et al., 2016; Wang et al., 2019).
Jiao-Tai-Wan contains two kinds of Chinese herbal medicine: Coptis Chinensis and cinnamon. The Coptis Chinensis alkaloid is the most important component in Coptis Chinensis, possessing a variety of medicinal values. Studies have shown that berberine has antibacterial, antioxidant, cardiac, neuroprotective, and spasmodic effects (Ji and Shen, 2011; Park et al., 2012; Li et al., 2019). Cinnamon’s main active ingredient is cinnamon, which has anti-inflammatory, antioxidant, and neuroprotective effects (Yang et al., 2016b). Another study has found that Jiao-Tai-Wan activates the cholinergic pathway and improves cognitive function by reducing acetylcholinesterase activity and increasing acetylcholinesterase content (Wang et al., 2018b).
Pharmacological studies have shown that dandelions have antimicrobial, antiviral, anticancer, antioxidant, anti-inflammatory, and anti-allergic effects (He et al., 2011; Ovadje et al., 2011; Ovadje et al., 2012; Qian et al., 2014; Wang, 2014; Ma et al., 2015; Ovadje et al., 2016; Jedrejek et al., 2017; Rehman et al., 2017; Ding and Wen, 2018; Liu et al., 2018; Rahmat and Damon, 2018). Extract from Dandelion: ethyl acetate extract (EAED). Experimental results of precontraction of the tracheal ring in mice induced by high K + and ACH stimulation showed that EAED could inhibit the high concentration of Ca2+ caused by high potassium and acetylcholine. Improve airway hyperresponsiveness and reduce airway inflammation (Zhao et al., 2020). In addition, in an in vivo study, EAED effectively reduced ACh-induced respiratory resistance in healthy and asthmatic mice.
NHD is a traditional Chinese medicine prescription composed of albizzia bark, nocturnal vine, lily, and Lanzhi. A study of insomnia rodents induced by PCPA found that NHD has a good sedative effect in reducing exercise distance, prolonging sleep time, improving sleep quality, and improving depression, as. NHD effectively inhibits CNS excitability and relieves PCPA-induced insomnia by reducing dopamine, noradrenaline, and ACh levels (Yang et al., 2021). Figure 2 summarized the effect of multiple TCT on CAP.
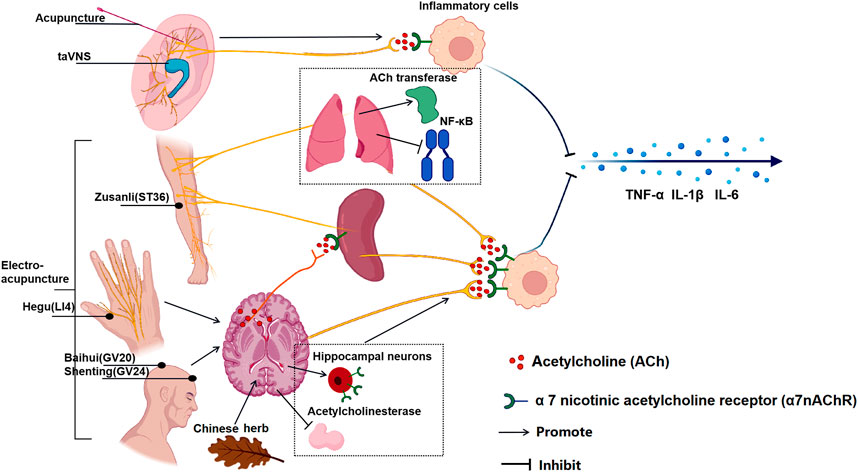
FIGURE 2. The effect of multiple TCT on CAP. Acupuncture and taVNS stimulating in ear branch can activate CAP and the reduce inflammatory cytokines; Auricular acupuncture and electroacupuncture “Zusanli” (ST36) increased the activity of local acetylcholine transferase, promoted the transcription and synthesis of ACh, inhibiting the expression of NF-κB in lung tissue and stimulating the CAP in the lung; Moreover, electroacupuncture ST36 activated the vagus nerve pathway connected to the spleen and reduced the production of TNF in the spleen; Electroacupuncture “Hegu” (LI4) activates muscarinic acetylcholine receptor signals in the brain, and then activates the efferent vagus nerve and splenic nerve to activate CAP; Electroacupuncture at “Baihui" (GV20) and “Shenting” (GV24) increase the expression of α7nAChR in hippocampal neurons; The chinese medcine Jiao-Tai-Wan reduced the activity of acetylcholinesterase (AChE) and increase the content of ACh. The ultimate effect of TCT is to activate CAP to reduce the production of inflammatory factors in effector organs to inhibit inflammation.
For patients with sleep disorders of different severity, the best treatment is individualized therapy tailored to each patient’s symptoms which could implement appropriate physical therapy or external treatment. TCT may be an alternative treatment for this problem. A large meta-analysis on the effects of TCT concluded that acupuncture and taVNS therapy could relieve anxiety and alleviate sleep disturbance, and even lower depression level (Wang et al., 2022). Acupuncture and Chinese herbal medicine are most commonly used in the treatment of depression-related insomnia. Studies have verified their efficacy and safety in treating insomnia (Wang et al., 2006; Song, 2007; Liu and Wan, 2010; Huo et al., 2013). Two systematic studies showed that TCT had fewer adverse reactions to insomnia than Western medicine (Yang et al., 2019; Lin et al., 2021). However, there are still some limitations in the studies related to poor methodology in Chinese herbal medicine, such as complex chemical compositions and unclear efficacy that might be the result of the comprehensive action of all drug ingredients, which to some extent restrict clear conclusions. In addition, many other TCT treatments have also improved sleep disorders (Cho et al., 2013; Bergdahl et al., 2017; Karadag et al., 2017; Li et al., 2018; Meng et al., 2018; Jiao et al., 2020; Hu et al., 2021; Jiang et al., 2021; Jiang et al., 2022a; Jiang et al., 2022b). Table 3 summarizes the effects of most TCT treatments on sleep disorders under different conditions. These studies showed that TCT possesses a great effect on sleep disorders treatment, so it should be extremely informative for sleep disorders induced by COVID-19 in the future.
In summary, high-quality sleep contributes to a strong immune system, so it is possible to resist virus invasion and kill the invaded viruses and promote the recovery of physical function. Therefore, it is worth discussing whether TCT is necessary for the treatment of the patient sleep distress. Our review firstly analyzes the reasons for the sleep disorders caused by the novel coronavirus and found that inflammation was the main reason leading to sleep distress in patients. And we reviewed the mechanisms of three common traditional Chinese in inhibiting inflammation through CAP and relieving the sleep or symptoms. We, therefore, propose that TCT may be a potential strategy to take for the treatment of sleep problems due to inflammation caused by COVID-19.
XX and NZ conceived the idea, participated in its design and coordination, and drafted the manuscript. JF, ZW, and ZY searched all relevant references and collected the related information. ZL critically revised the manuscript. All authors read and approved the final manuscript.
This study was funded by the Institutional Foundation of Xi’an Jiaotong University No. xzy012022100, and Institutional Foundation of The First Affiliated Hospital of Xi’an Jiaotong University No. 2021QN-23.
We would like to thank the website of https://www.biorender.com for providing icons and cell morphological elements that used to complete our Figures 1, 2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACh, acetylcholine; ARDS, acute respiratory distress syndrome; AD: Alzheimer’s disease; α7nAChR, α 7 nicotinic acetylcholine receptor; CAP, cholinergic anti-inflammatory pathway; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; EA, electroacupuncture; EAED, ethyl acetate extract of dandelion; ICU, intensive care unit; IL, interleukin; LPS, lipopolysaccharide; NHD, hypnotic decoction; NREM, non-rapid eye movements; PCPA, para-chlorophenylalanine; PPT, pedunculopontine tegmental; PSQI, pittsburgh sleep quality index; PTSD, post-traumatic stress disorder; SARS, severe acute respiratory syndrome; taVNS, transcutaneous auricular vagal nerve stimulation; TCT, traditional Chinese therapy; TMPRSS2, recombinant transmembrane protease, serine 2; TNF, tumor necrosis factor
Akıncı, T., and Melek Başar, H. (2021). Relationship between sleep quality and the psychological status of patients hospitalised with COVID-19. Sleep. Med. 80, 167–170. doi:10.1016/j.sleep.2021.01.034
Alfaro-Rodríguez, A., and González-Piña, R. (2005). Ozone-induced paradoxical sleep decrease is related to diminished acetylcholine levels in the medial preoptic area in rats. Chem. Biol. Interact. 151, 151–158. doi:10.1016/j.cbi.2004.10.001
Andersson, U., and Tracey, K. J. (2012). Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 30, 313–335. doi:10.1146/annurev-immunol-020711-075015
Arnold, D. T., Milne, A., Samms, E., Stadon, L., Maskell, N. A., and Hamilton, F. W. (2021). Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: A case series. Ann. Intern. Med. 174, 1334–1336. doi:10.7326/M21-1976
Baek, Y. H., Choi, D. Y., Yang, H. I., and Park, D. S. (2005). Analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagen-induced arthritis: mediation by cholinergic and serotonergic receptors. Brain Res. 1057, 181–185. doi:10.1016/j.brainres.2005.07.014
Baptista, A. F., Baltar, A., Okano, A. H., Moreira, A., Campos, A., Fernandes, A. M., et al. (2020). Applications of non-invasive neuromodulation for the management of disorders related to COVID-19. Front. Neurol. 11, 573718. doi:10.3389/fneur.2020.573718
Bergdahl, L., Broman, J. E., Berman, A. H., Haglund, K., von Knorring, L., and Markström, A. (2017). Sleep patterns in a randomized controlled trial of auricular acupuncture and cognitive behavioral therapy for insomnia. Complement. Ther. Clin. Pract. 28, 220–226. doi:10.1016/j.ctcp.2017.06.006
Bonaz, B., Sinniger, V., and Pellissier, S. (2016). Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 594, 5781–5790. doi:10.1113/JP271539
Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., Watkins, L. R., et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. doi:10.1038/35013070
Cai, L., Lu, K., Chen, X., Huang, J. Y., Zhang, B. P., and Zhang, H. (2019). Auricular vagus nerve stimulation protects against postoperative cognitive dysfunction by attenuating neuroinflammation and neurodegeneration in aged rats. Neurosci. Lett. 703, 104–110. doi:10.1016/j.neulet.2019.03.034
Cao, Q., Jiang, Y., Cui, S. Y., Tu, P. F., Chen, Y. M., Ma, X. L., et al. (2016). Tenuifolin, a saponin derived from Radix Polygalae, exhibits sleep-enhancing effects in mice. Phytomedicine 23, 1797–1805. doi:10.1016/j.phymed.2016.10.015
Channappanavar, R., and Perlman, S. (2017). Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 39, 529–539. doi:10.1007/s00281-017-0629-x
Chavan, S. S., Pavlov, V. A., and Tracey, K. J. (2017). Mechanisms and therapeutic relevance of neuroimmune communication. Immunity 46, 927–942. doi:10.1016/j.immuni.2017.06.008
Chen, Z., and Nakamura, T. (2004). Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother. Res. 18, 592–594. doi:10.1002/ptr.1485
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study. Lancet 395, 507–513. doi:10.1016/S0140-6736(20)30211-7
Cheng, P., Casement, M. D., Kalmbach, D. A., Castelan, A. C., and Drake, C. L. (2021). Digital cognitive behavioral therapy for insomnia promotes later health resilience during the coronavirus disease 19 (COVID-19) pandemic. Sleep 44 (4), zsaa258. doi:10.1093/sleep/zsaa258
Cheng, P., Casement, M. D., Kalmbach, D. A., Castelan, A. C., and Drake, C. L. (2021). Digital cognitive behavioral therapy for insomnia promotes later health resilience during the coronavirus disease 19 (COVID-19) pandemic. Sleep 44, zsaa258. doi:10.1093/sleep/zsaa258
Cho, K. M., Yoo, I. D., and Kim, W. G. (2006). 8-hydroxydihydrochelerythrine and 8-hydroxydihydrosanguinarine with a potent acetylcholinesterase inhibitory activity from Chelidonium majus L. Biol. Pharm. Bull. 29, 2317–2320. doi:10.1248/bpb.29.2317
Cho, M. Y., Min, E. S., Hur, M. H., and Lee, M. S. (2013). Effects of aromatherapy on the anxiety, vital signs, and sleep quality of percutaneous coronary intervention patients in intensive care units. Evid. Based. Complement. Altern. Med. 2013, 381381. doi:10.1155/2013/381381
da Silva, M. A., and Dorsher, P. T. (2014). Neuroanatomic and clinical correspondences: acupuncture and vagus nerve stimulation. J. Altern. Complement. Med. 20, 233–240. doi:10.1089/acm.2012.1022
Dai, Y., Qiang, W., Gui, Y., Tan, X., Pei, T., Lin, K., et al. (2021). A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci. Bull. 66, 884–888. doi:10.1016/j.scib.2021.01.005
Ding, A., and Wen, X. (2018). Dandelion root extract protects NCM460 colonic cells and relieves experimental mouse colitis. J. Nat. Med. 72, 857–866. doi:10.1007/s11418-018-1217-7
Ding, X., and Yao, J. (2020). Peer education intervention on adolescents' anxiety, depression, and sleep disorder during the COVID-19 pandemic. Psychiatr. Danub. 32 (3-4), 527–535. doi:10.24869/psyd.2020.527
Doroftei, B., Ciobica, A., Ilie, O. D., Maftei, R., and Ilea, C. (2021). Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics 11, 579. doi:10.3390/diagnostics11040579
dos Santos, C. C., Shan, Y., Akram, A., Slutsky, A. S., and Haitsma, J. J. (2011). Neuroimmune regulation of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 183, 471–482. doi:10.1164/rccm.201002-0314OC
Elder, G. J., Alfonso-Miller, P., Atkinson, W., Santhi, N., and Ellis, J. G. (2020). Testing an early online intervention for the treatment of disturbed sleep during the COVID-19 pandemic (sleep COVID-19): structured summary of a study protocol for a randomised controlled trial. Trials 21, 704. doi:10.1186/s13063-020-04644-0
Fu, W., Wang, C., Zou, L., Guo, Y., Lu, Z., Yan, S., et al. (2020). Psychological health, sleep quality, and coping styles to stress facing the COVID-19 in Wuhan, China. Transl. Psychiatry 10, 225. doi:10.1038/s41398-020-00913-3
Fucile, S., Renzi, M., Lax, P., and Eusebi, F. (2003). Fractional Ca(2+) current through human neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium 34, 205–209. doi:10.1016/s0143-4160(03)00071-x
Gao, X. Y., Zhang, S. P., Zhu, B., and Zhang, H. Q. (2008). Investigation of specificity of auricular acupuncture points in regulation of autonomic function in anesthetized rats. Auton. Neurosci. 138, 50–56. doi:10.1016/j.autneu.2007.10.003
Gimeno, D., Kivimäki, M., Brunner, E. J., Elovainio, M., De Vogli, R., Steptoe, A., et al. (2009). Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the whitehall II study. Psychol. Med. 39, 413–423. doi:10.1017/S0033291708003723
Go, Y. Y., Ju, W. M., Lee, C. M., Chae, S. W., and Song, J. J. (2022). Different transcutaneous auricular vagus nerve stimulation parameters modulate the anti-inflammatory effects on lipopolysaccharide-induced acute inflammation in mice. Biomedicines 10, 247. doi:10.3390/biomedicines10020247
Go, Y. Y., Ju, W. M., Lee, C. M., Chae, S. W., and Song, J. J. (2022). Different transcutaneous auricular vagus nerve stimulation parameters modulate the anti-inflammatory effects on lipopolysaccharide-induced acute inflammation in mice. Biomedicines 10, 247. doi:10.3390/biomedicines10020247
Guo, J., Feng, X. L., Wang, X. H., and van Ijzendoorn, M. H. (2020). Coping with COVID-19:exposure to COVID-19 and negative impact on livelihood predict elevated mental health problems in Chinese adults. Int. J. Environ. Res. Public Health 17, 3857. doi:10.3390/ijerph17113857
Hambrecht-Wiedbusch, V. S., Gauthier, E. A., Baghdoyan, H. A., and Lydic, R. (2010). Benzodiazepine receptor agonists cause drug-specific and state-specific alterations in EEG power and acetylcholine release in rat pontine reticular formation. Sleep 33, 909–918. doi:10.1093/sleep/33.7.909
He, W., Han, H., Wang, W., and Gao, B. (2011). Anti-influenza virus effect of aqueous extracts from dandelion. Virol. J. 8, 538. doi:10.1186/1743-422X-8-538
Hogan, D., Morrow, J. D., Smith, E. M., and Opp, M. R. (2003). Interleukin-6 alters sleep of rats. J. Neuroimmunol. 137, 59–66. doi:10.1016/s0165-5728(03)00038-9
Hsu, C. H., Hwang, K. C., Chao, C. L., Chang, S. G., Ho, M. S., and Chou, P. (2006). Can herbal medicine assist against avian flu? Learning from the experience of using supplementary treatment with Chinese medicine on SARS or SARS-like infectious disease in 2003. J. Altern. Complement. Med. 12, 505–506. doi:10.1089/acm.2006.12.505
Hu, J., Teng, J., Wang, W., Yang, N., Tian, H., Zhang, W., et al. (2021). Clinical efficacy and safety of traditional Chinese medicine Xiao Yao San in insomnia combined with anxiety. Med. Baltim. 100, e27608. doi:10.1097/MD.0000000000027608
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi:10.1016/S0140-6736(20)30183-5
Huang, C., Huang, L., Wang, Y., Li, X., Ren, L., Gu, X., et al. (2021). 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232. doi:10.1016/S0140-6736(20)32656-8
Huo, Z. J., Guo, J., and Li, D. (2013). Effects of acupuncture with meridian acupoints and three Anmian acupoints on insomnia and related depression and anxiety state. Chin. J. Integr. Med. 19, 187–191. doi:10.1007/s11655-012-1240-6
Imai, K., Ariga, H., Chen, C., Mantyh, C., Pappas, T. N., and Takahashi, T. (2008). Effects of electroacupuncture on gastric motility and heart rate variability in conscious rats. Auton. Neurosci. 138, 91–98. doi:10.1016/j.autneu.2007.11.003
Imai, K., Ariga, H., and Takahashi, T. (2009). Electroacupuncture improves imbalance of autonomic function under restraint stress in conscious rats. Am. J. Chin. Med. 37, 45–55. doi:10.1142/S0192415X0900662X
Jasper, H. H., and Tessier, J. (1971). Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science 172, 601–602. doi:10.1126/science.172.3983.601
Jedrejek, D., Kontek, B., Lis, B., Stochmal, A., and Olas, B. (2017). Evaluation of anti-oxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem. Biol. Interact. 262, 29–37. doi:10.1016/j.cbi.2016.12.003
Ji, H. F., and Shen, L. (2011). Berberine: a potential multipotent natural product to combat alzheimer's disease. Mol. (Basel, Switz. 16, 6732–6740. doi:10.3390/molecules16086732
Jiang, Y., Cao, Z., Ma, H., Wang, G., Wang, X., Wang, Z., et al. (2018). Auricular vagus nerve stimulation exerts antiinflammatory effects and immune regulatory function in a 6-OHDA model of Parkinson's disease. Neurochem. Res. 43, 2155–2164. doi:10.1007/s11064-018-2639-z
Jiang, L., Ouyang, J., and Du, X. (2021). Effects of traditional Chinese medicine exercise therapy on cancer-related fatigue, anxiety and sleep quality in cancer patients: A protocol for systematic review and network meta-analysis. Med. Baltim. 100, e27681. doi:10.1097/MD.0000000000027681
Jiang, N., Li, Y. J., Wang, M. D., Huang, H., Chen, S., Liu, X., et al. (2022). The cognitive-enhancing effects of dendrobium nobile lindl extract in sleep deprivation-induced amnesic mice. Front. Psychiatry 12, 596017. doi:10.3389/fpsyt.2021.596017
Jiang, T., Zhang, Q., Yuan, F., Zhang, F., and Guo, J. (2022). Efficacy of acupuncture and its influence on the emotional network in adult insomnia patients: protocol for a randomized controlled clinical trial. Trials 23, 11. doi:10.1186/s13063-021-05913-2
Jiao, Y., Guo, X., Luo, M., Li, S., Liu, A., Zhao, Y., et al. (2020). Effect of transcutaneous vagus nerve stimulation at auricular concha for insomnia: A randomized clinical trial. Evid. Based. Complement. Altern. Med. 2020, 6049891. doi:10.1155/2020/6049891
Kaczmarczyk, R., Tejera, D., Simon, B. J., and Heneka, M. T. (2017). Microglia modulation through external vagus nerve stimulation in a murine model of Alzheimer’s disease. J. Neurochem. 146, 76–85. doi:10.1111/jnc.14284
Karadag, E., Samancioglu, S., Ozden, D., and Bakir, E. (2017). Effects of aromatherapy on sleep quality and anxiety of patients. Nurs. Crit. Care 22, 105–112. doi:10.1111/nicc.12198
Koralnik, I. J., and Tyler, K. L. (2020). COVID-19: a global threat to the nervous system. Ann. Neurol. 88, 1–11. doi:10.1002/ana.25807
Krueger, J. M., and Majde, J. A. (1995). Cytokines and sleep. Int. Arch. Allergy Immunol. 106, 97–100. doi:10.1159/000236827
Kubota, T., Fang, J., Kushikata, T., and Krueger, J. M. (2000). Interleukin-13 and transforming growth factor-beta1 inhibit spontaneous sleep in rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R786–R792. doi:10.1152/ajpregu.2000.279.3.R786
Kubota, T., Brown, R. A., Fang, J., and Krueger, J. M. (2001). Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1004–R1012. doi:10.1152/ajpregu.2001.281.3.R1004
Kubota, T., Majde, J. A., Brown, R. A., and Krueger, J. M. (2001). Tumor necrosis factor receptor fragment attenuates interferon-γ-induced non-REM sleep in rabbits. J. Neuroimmunol. 119, 192–198. doi:10.1016/s0165-5728(01)00382-4
Kushikata, T., Fang, J., and Krueger, J. M. (1999). Interleukin-10 inhibits spontaneous sleep in rabbits. J. Interferon Cytokine Res. 19, 1025–1030. doi:10.1089/107999099313244
Kwenandar, F., Japar, K. V., Damay, V., Hariyanto, T. I., Tanaka, M., Lugito, N. P. H., et al. (2020). Coronavirus disease 2019 and cardiovascular system: A narrative review. Int. J. Cardiol. Heart Vasc. 29, 100557. doi:10.1016/j.ijcha.2020.100557
La Marca, R., Nedeljkovic, M., Yuan, L., Maercker, A., and Ehlert, U. (2010). Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin. Sci. 118, 537–546. doi:10.1042/CS20090264
Lai, J., Ma, S., Wang, Y., Cai, Z., Hu, J., Wei, N., et al. (2020). Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open 3, e203976. doi:10.1001/jamanetworkopen.2020.3976
Lange, T., Dimitrov, S., and Born, J. (2010). Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sci. 1193, 48–59. doi:10.1111/j.1749-6632.2009.05300.x
Li, Y. Q., Zhu, B., Rong, P. J., Ben, H., and Li, Y. H. (2007). Neural mechanism of acupuncture-modulated gastric motility. World J. Gastroenterol. 13, 709–716. doi:10.3748/wjg.v13.i5.709
Li, J., Li, J., Chen, R., and Cai, G. (2015). Targeting NF-κΒ and TNF-α activation by electroacupuncture to suppress collagen-induced rheumatoid arthritis in model rats. Altern. Ther. Health Med. 21, 26–34.
Li, F., Zhao, Y. B., Wang, D. K., Zou, X., Fang, K., and Wang, K. F. (2016). Berberine relieves insulin resistance via the cholinergic anti-inflammatory pathway in HepG2 cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 36, 64–69. doi:10.1007/s11596-016-1543-5
Li, L. C., Xing, H. J., Liang, Y., Hu, Y. H., An, X., He, X. X., et al. (2018). Comparison of therapeutic effects between thermosensitive moxibustion and medication in the treatment of insomnia of liver-qi stagnation pattern. Acupunct. Res. 43, 573–575. doi:10.13702/j.1000-0607.170765
Li, W., Yin, N., Tao, W., Wang, Q., Fan, H., and Wang, Z. (2019). Berberine suppresses IL-33-induced inflammatory responses in mast cells by inactivating NF-κB and p38 signaling. Int. Immunopharmacol. 66, 82–90. doi:10.1016/j.intimp.2018.11.009
Lim, H. D., Kim, M. H., Lee, C. Y., and Namgung, U. (2016). Anti-inflammatory effects of acupuncture stimulation via the vagus nerve. Plos One 11, e0151882. doi:10.1371/journal.pone.0151882
Lin, S. C., Ho, C. T., Chuo, W. H., Li, S., Wang, T. T., and Lin, C. C. (2017). Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 17, 144. doi:10.1186/s12879-017-2253-8
Lin, Y. H., Chen, C., Zhao, X., Mao, Y. F., Xiang, G. X., Yang, M. Q., et al. (2021). Efficacy and safety of banxia formulae for insomnia: A systematic review and meta-analysis of high-quality randomized controlled trials. Evid. Based. Complement. Altern. Med. 2021, 8833168. doi:10.1155/2021/8833168
Liu, Q. X., and Wan, H. (2010). Treatment of 60 cases of insomnia with depression by the combination of Wu Ling Capsule and acupuncture. JGMCM 25, 2269–2270.
Liu, R. P., Fang, J. L., Rong, P. J., Zhao, Y., Meng, H., Ben, H., et al. (2013). Effects of electroacupuncture at auricular concha region on the depressive status of unpredictable chronic mild stress rat models. Evid. Based. Complement. Altern. Med. 2013, 789674. doi:10.1155/2013/789674
Liu, F., Li, Y., Jiang, R., Nie, C., Zeng, Z., Zhao, N., et al. (2015). miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp. Lung Res. 41, 261–269. doi:10.3109/01902148.2015.1004206
Liu, Y., Tao, T., Li, W., and Bo, Y. (2017). Regulating autonomic nervous system homeostasis improves pulmonary function in rabbits with acute lung injury. BMC Pulm. Med. 17, 98. doi:10.1186/s12890-017-0436-0
Liu, J., Li, C., Peng, H., Yu, K., Tao, J., Lin, R., et al. (2017). Electroacupuncture attenuates learning and memory impairment via activation of α7nAChR-mediated anti-inflammatory activity in focal cerebral ischemia/reperfusion injured rats. Exp. Ther. Med. 14, 939–946. doi:10.3892/etm.2017.4622
Liu, Q., Zhao, H., Gao, Y., Meng, Y., Zhao, X. X., and Pan, S. N. (2018). Effects of dandelion extract on the proliferation of rat skeletal muscle cells and the inhibition of a lipopolysaccharide-induced inflammatory reaction. Chin. Med. J. 131, 1724–1731. doi:10.4103/0366-6999.235878
Liu, K., Zhang, W., Yang, Y., Zhang, J., Li, Y., and Chen, Y. (2020). Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 39, 101166. doi:10.1016/j.ctcp.2020.101166
Liu, Z., Qin, G., Mana, L., Dong, Y., Huang, S., Wang, Y., et al. (2020). GAPT regulates cholinergic dysfunction and oxidative stress in the brains of learning and memory impairment mice induced by scopolamine. Brain Behav. 10, e01602. doi:10.1002/brb3.1602
Liu, S., Wang, Z. F., Su, Y. S., Ray, R. S., Jing, X. H., Wang, Y. Q., et al. (2020). Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron 108, 436–450. doi:10.1016/j.neuron.2020.07.015
Liu, S., Wang, Z., Su, Y., Qi, L., Yang, W., Fu, M., et al. (2021). A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 598, 641–645. doi:10.1038/s41586-021-04001-4
Liu, S., Wang, Z., Su, Y., Qi, L., Yang, W., Fu, M., et al. (2022). Author correction: a neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 601, E9. doi:10.1038/s41586-021-04290-9
Lu, Y., Zhu, H., Wang, Q., Tian, C., Lai, H., Hou, L., et al. (2022). Comparative effectiveness of multiple acupuncture therapies for primary insomnia: a systematic review and network meta-analysis of randomized trial. Sleep. Med. 93, 39–48. doi:10.1016/j.sleep.2022.03.012
Luan, X., Zhang, X., and Zhou, Y. (2022). The role and clinical observation of traditional Chinese medicine in relieving senile insomnia: A systematic review and meta-analysis. Biomed. Res. Int. 2022, 9484095. doi:10.1155/2022/9484095
Lv, Z. Y., Shi, Y. L., Bassi, G. S., Chen, Y. J., Yin, L. M., Wang, Y., et al. (2022). Electroacupuncture at ST36 (zusanli) prevents T-cell lymphopenia and improves survival in septic mice. J. Inflamm. Res. 15, 2819–2833. doi:10.2147/JIR.S361466
Ma, C., Zhu, L., Wang, J., He, H., Chang, X., Gao, J., et al. (2015). Anti-inflammatory effects of water extract of Taraxacum mongolicum hand-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 168, 349–355. doi:10.1016/j.jep.2015.03.068
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. LANCET 395, 1033–1034. doi:10.1016/S0140-6736(20)30628-0
Meneses, G., Bautista, M., Florentino, A., Diaz, G., Acero, G., Besedovsky, H., et al. (2016). Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J. Inflamm. 13, 33. doi:10.1186/s12950-016-0140-5
Meng, F., Gong, W. J., Liao, Y. X., Xu, H. W., and Wang, X. (2018). Effect of auricular intradermal needling combined with erjian (HX6, 7i) bloodletting on sleep quality and neuroendocrine level in patients with perimenopausal insomnia. Chin. Acupunct. Moxibust 38, 575–579. doi:10.13703/j.0255-2930.2018.06.002
Meng, Z., Sun, B., Chen, W., Zhang, X., Huang, M., and Xu, J. (2021). Depression of non-neuronal cholinergic system may play a role in Co-occurrence of subjective daytime sleepiness and hypertension in patients with obstructive sleep apnea syndrome. Nat. Sci. Sleep. 13, 2153–2163. doi:10.2147/NSS.S339038
Nosadini, A., Costantini, M., Ambrosio, F., Zaninotto, G., Ruol, A., Tremolada, C., et al. (1986). Lower esophageal sphincter and acupuncture: electromanometric evaluation in esophageal achalasia. Dig. Surg. 3, 243–245. doi:10.1159/000171737
Otuyama, L. J., Rizzi, C. F., Piovezan, R. D., Werli, K. S., Brasil, E. L., Sukys-Claudino, L., et al. (2013). The cholinergic system may play a role in the pathophysiology of residual excessive sleepiness in patients with obstructive sleep apnea. Med. Hypotheses 81, 509–511. doi:10.1016/j.mehy.2013.06.024
Ovadje, P., Chatterjee, S., Griffin, C., Tran, C., Hamm, C., and Pandey, S. (2011). Selective induction of apoptosis through activation of caspase-8 in human leukemia cells (Jurkat) by dandelion root extract. J. Ethnopharmacol. 133, 86–91. doi:10.1016/j.jep.2010.09.005
Ovadje, P., Chochkeh, M., Akbari-Asl, P., Hamm, C., and Pandey, S. (2012). Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas 41, 1039–1047. doi:10.1097/MPA.0b013e31824b22a2
Ovadje, P., Ammar, S., Guerrero, J. A., Arnason, J. T., and Pandey, S. (2016). Dandelion root extract affects colorectal cancer proliferation and survival through the activation of multiple death signalling pathways. Oncotarget 7, 73080–73100. doi:10.18632/oncotarget.11485
Park, H. J., Lee, P. H., Ahn, Y. W., Choi, Y. J., Lee, G., Lee, D. Y., et al. (2007). Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur. J. Neurosci. 26, 79–89. doi:10.1111/j.1460-9568.2007.05636.x
Park, D., Lee, H. J., Joo, S. S., Bae, D. K., Yang, G., Yang, Y. H., et al. (2012). Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp. Neurol. 234, 521–526. doi:10.1016/j.expneurol.2011.12.040
Passos, L., Prazeres, F., Teixeira, A., and Martins, C. (2020). Impact on mental health due to COVID-19 pandemic: Cross-sectional study in Portugal and Brazil. Int. J. Environ. Res. Public Health 17, E6794. doi:10.3390/ijerph17186794
Pavlov, V. A., and Tracey, K. J. (2012). The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 8, 743–754. doi:10.1038/nrendo.2012.189
Pavlov, V. A., and Tracey, K. J. (2015). Neural circuitry and immunity. Immunol. Res. 63, 38–57. doi:10.1007/s12026-015-8718-1
Pavlov, V. A., Wang, H., Czura, C. J., Friedman, S. G., and Tracey, K. J. (2003). The cholinergic anti‐inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9, 125–134. doi:10.1007/bf03402177
Peuker, E. T., and Filler, T. J. (2002). The nerve supply of the human auricle. Clin. Anat. 15, 35–37. doi:10.1002/ca.1089
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., et al. (2020). Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615. doi:10.1056/NEJMoa2034577
Qian, L., Zhou, Y., Teng, Z., Du, C. L., and Tian, C. (2014). Preparation and antibacterial activity of oligosaccharides derived from dandelion. Int. J. Biol. Macromol. 64, 392–394. doi:10.1016/j.ijbiomac.2013.12.031
Qin, Z., Wan, J. J., Sun, Y., Wu, T., Wang, P. Y., Du, P., et al. (2017). Nicotine protects against DSS colitis through regulating microRNA-124 and STAT3. J. Mol. Med. 95 (2), 221–233. doi:10.1007/s00109-016-1473-5
Rahmat, L. T., and Damon, L. E. (2018). The use of natural health products especially papaya leaf extract and dandelion root extract in previously untreated chronic myelomonocytic leukemia. Case Rep. Hematol. 2018, 7267920. doi:10.1155/2018/7267920
Ranjbaran, Z., Keefer, L., Stepanski, E., Farhadi, A., and Keshavarzian, A. (2007). The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm. Res. 56, 51–57. doi:10.1007/s00011-006-6067-1
Rehman, G., Hamayun, M., Iqbal, A., Khan, S. A., Khan, H., Shehzad, A., et al. (2017). Effect of methanolic extract of dandelion roots on cancer cell lines and AMP-activated protein kinase pathway. Front. Pharmacol. 8, 875. doi:10.3389/fphar.2017.00875
Ren, C., Tong, Y. L., Li, J. C., Lu, Z. Q., and Yao, Y. M. (2017). The protective effect of alpha 7 nicotinic acetylcholine receptor activation on critical illness and its mechanism. Int. J. Biol. Sci. 13, 46–56. doi:10.7150/ijbs.16404
Round, R., Litscher, G., and Bahr, F. (2013). Auricular acupuncture with laser. Evid. Based Complement. Altern. Med. 2013, 984763. doi:10.1155/2013/984763
Saint-Mleux, B., Eggermann, E., Bisetti, A., Bayer, L., Machard, D., Jones, B. E., et al. (2004). Nicotinic enhancement of the noradrenergic inhibition of sleep-promoting neurons in the ventrolateral preoptic area. J. Neurosci. 24, 63–67. doi:10.1523/JNEUROSCI.0232-03.2004
Sarris, J. (2012). Chinese herbal medicine for sleep disorders: poor methodology restricts any clear conclusion. Sleep. Med. Rev. 16, 493–495. doi:10.1016/j.smrv.2012.06.004
Song, X. M., Li, J. G., Wang, Y. L., Hu, Z. F., Zhou, Q., Du, Z. H., et al. (2008). The protective effect of the cholinergic anti-inflammatory pathway against septic shock in rats. Shock 30, 468–472. doi:10.1097/SHK.0b013e31816d5e49
Song, J. G., Li, H. H., Cao, Y. F., Lv, X., Zhang, P., Li, Y. S., et al. (2012). Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology 116, 406–414. doi:10.1097/ALN.0b013e3182426ebd
Song, Q. (2007). 56 cases of correlation between acupuncture baihui EX-HN1 treatment of depression insomnia. Cap. Med. 48, 48–49.
Stockelman, K. A., Bain, A. R., Dow, C. A., Diehl, K. J., Greiner, J. J., Stauffer, B. L., et al. (2021). Regular aerobic exercise counteracts endothelial vasomotor dysfunction associated with insufficient sleep. Am. J. Physiol. Heart Circ. Physiol. 320, H1080–H1088. doi:10.1152/ajpheart.00615.2020
Tarsitani, L., Vassalini, P., Koukopoulos, A., Borrazzo, C., Alessi, F., Di Nicolantonio, C., et al. (2021). Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J. Gen. Intern. Med. 36, 1702–1707. doi:10.1007/s11606-021-06731-7
Wan, Y., Shang, J., Graham, R., Baric, R. S., and Li, F. (2020). Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 94, 001277-20. doi:10.1128/JVI.00127-20
Wang, J., Jiang, J. F., and Wang, L. L. (2006). Clinical observation on governor vessel Daoqi method for treatment of dyssomnia in the patient of depression. Chin. Acupuncture&Moxibusion. 26, 328–330.
Wang, H., Yang, G., Wang, S., Zheng, X., Zhang, W., and Li, Y. (2018). The most commonly treated acupuncture indications in the United States: A cross-sectional study. Am. J. Chin. Med. 46, 1387–1419. doi:10.1142/S0192415X18500738
Wang, X. C., Xu, Y. M., Li, H. Y., Wu, C. Y., Xu, T. T., Luo, N. C., et al. (2018). Jiao-tai-wan improves cognitive dysfunctions through cholinergic pathway in scopolamine-treated mice. Biomed. Res. Int. 2018, 3538763. doi:10.1155/2018/3538763
Wang, K., Chen, Q., Wu, N., Li, Y., Zhang, R., Wang, J., et al. (2019). Berberine ameliorates spatial learning memory impairment and modulates cholinergic anti-inflammatory pathway in diabetic rats. Front. Pharmacol. 10, 1003. doi:10.3389/fphar.2019.01003
Wang, J., Chen, Y., Zhai, X., Chu, Y., Liu, X., and Ma, X. (2022). Visualizing research trends and identifying hotspots of traditional Chinese medicine (TCM) nursing technology for insomnia: A 18-years bibliometric analysis of web of science core collection. Front. Neurol. 13, 816031. doi:10.3389/fneur.2022.816031
Wang, H. B. (2014). Cellulase-assisted extraction and antibacterial activity of polysaccharides from the dandelion Taraxacum officinale. Carbohydr. Polym. 103, 140–142. doi:10.1016/j.carbpol.2013.12.029
Weiner, L., Berna, F., Nourry, N., Severac, F., Vidailhet, P., and Mengin, A. C. (2020). Efficacy of an online cognitive behavioral therapy program developed for healthcare workers during the COVID-19 pandemic: the REduction of STress (REST) study protocol for a randomized controlled trial. Trials 21, 870. doi:10.1186/s13063-020-04772-7
Xu, Z., Zhang, D., Xu, D., Li, X., Xie, Y. J., Sun, W., et al. (2021). Loneliness, depression, anxiety, and post-traumatic stress disorder among Chinese adults during COVID-19: A cross-sectional online survey. Plos one 16, e0259012. doi:10.1371/journal.pone.0259012
Xue, R., Wan, Y., Sun, X., Zhang, X., Gao, W., and Wu, W. (2019). Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front. Immunol. 10, 2546. doi:10.3389/fimmu.2019.02546
Yamada, M., and Ichinose, M. (2018). The cholinergic anti-inflammatory pathway:an innovative treatment strategy for respiratory diseases and their comorbidities. Curr. Opin. Pharmacol. 40, 18–25. doi:10.1016/j.coph.2017.12.003
Yang, X., Zhao, C., Gao, Z., and Su, X. (2014). A novel regulator of lung inflammation and immunity: pulmonary parasympathetic inflammatory reflex. QJM 107, 789–792. doi:10.1093/qjmed/hcu005
Yang, G., Hu, R. Y., Deng, A. J., Huang, Y., and Li, J. (2016). Effects of electro-acupuncture at zusanli, guanyuan for sepsis patients and its mechanism through immune regulation. Chin. J. Integr. Med. 22, 219–224. doi:10.1007/s11655-016-2462-9
Yang, D., Liang, X. C., Shi, Y., Sun, Q., Liu, D., Liu, W., et al. (2016). Anti-oxidative and anti-inflammatory effects of cinnamaldehyde on protecting high glucose-induced damage in cultured dorsal root ganglion neurons of rats. Chin. J. Integr. Med. 22, 19–27. doi:10.1007/s11655-015-2103-8
Yang, X. Q., Liu, L., Ming, S. P., Fang, J., and Wu, D. N. (2019). Tian Wang bu xin dan for insomnia: A systematic review of efficacy and safety. Evid. Based. Complement. Altern. Med. 2019, 4260801. doi:10.1155/2019/4260801
Yang, Y., Wu, Y., Xu, P., Guo, F., Guo, F., and Yang, B. (2021). Nyctinastic herbs decoction improves para-chlorophenylalanine-induced insomnia by regulating the expression level of neurotransmitters. Ann. Transl. Med. 9, 1524. doi:10.21037/atm-21-4462
Yang, Z., Liu, Y., Wang, L., Lin, S., Dai, X., Yan, H., et al. (2022). Traditional Chinese medicine against COVID-19: Role of the gut microbiota. Biomed. Pharmacother. 149, 112787. doi:10.1016/j.biopha.2022.112787
Yu, X. (2022). Potential value of electroacupuncture in the treatment of gastrointestinal symptoms of COVID-19. Inflamm. Bowel Dis. 28, e21. doi:10.1093/ibd/izab223
Zant, J. C., Kim, T., Prokai, L., Szarka, S., McNally, J., McKenna, J. T., et al. (2016). Cholinergic neurons in the basal forebrain promote wakefulness by actions on neighboring non-cholinergic neurons:An opto-dialysis study. J. Neurosci. 36, 2057–2067. doi:10.1523/JNEUROSCI.3318-15.2016
Zhang, J., Lu, H., Zeng, H., Zhang, S., Du, Q., Jiang, T., et al. (2020). The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav. Immun. 87, 49–50. doi:10.1016/j.bbi.2020.04.031
Zhao, Y. X., He, W., Jing, X. H., Liu, J. L., Rong, P. J., Ben, H., et al. (2012). Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid. Based Complement. Altern. Med. 2012, 627023. doi:10.1155/2012/627023
Zhao, P., Liu, J., Ming, Q., Tian, D., He, J., Yang, Z., et al. (2020). Dandelion extract relaxes mouse airway smooth muscle by blocking VDLCC and NSCC channels. Cell Biosci. 10, 125. doi:10.1186/s13578-020-00470-8
Zhou, L., Filiberti, A., Humphrey, M. B., Fleming, C. D., Scherlag, B. J., Po, S. S., et al. (2019). Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp. Physiol. 104, 28–38. doi:10.1113/EP087351
Keywords: traditional Chinese therapy, cholinergic anti-inflammatory pathway, sleep disorders, coronavirus disease 2019, cytokine storms
Citation: Xie X, Zhang N, Fu J, Wang Z, Ye Z and Liu Z (2022) The potential for traditional Chinese therapy in treating sleep disorders caused by COVID-19 through the cholinergic anti-inflammatory pathway. Front. Pharmacol. 13:1009527. doi: 10.3389/fphar.2022.1009527
Received: 02 August 2022; Accepted: 23 September 2022;
Published: 10 October 2022.
Edited by:
Dongdong Sun, Nanjing University of Chinese Medicine, ChinaReviewed by:
Alina Gonzalez-Quevedo, Instituto de Neurología y Neurocirugía, CubaCopyright © 2022 Xie, Zhang, Fu, Wang, Ye and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Liu, emhpanVuX2xpdUB4anR1ZmguZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.